Abstract
Background
Clinical onset of multiple sclerosis (MSpostvacc) and myelin-oligodendrocyte-glycoprotein-antibody-associated disease (MOGADpostvacc) has been reported in association with SARS-CoV-2-vaccination. There is uncertainty as to whether this is causality (denovo disease) or temporal coincidence (manifestation of a preexisting, subclinical neuroinflammation).
Objectives
Comparing the clinical characteristics of MSpostvacc-patients versus patients with MS (PwMS) whose clinical onset occurred independently of vaccination (MSreference).
Methods
Consecutive patients with clinical onset ≤30 days after SARS-CoV-2-vaccination were included. Clinical data, cerebrospinal fluid (CSF) parameters and magnetic resonance imaging (MRI) as well as optical coherence tomography (OCT) data were compared to an age- and sex-matched MSreference-cohort.
Results
We identified 5 MSpostvacc and 1 MOGADpostvacc patients who developed their clinical onset ≤ 30 days after SARS-CoV-2-vaccination. Clinical characteristics, CSF, MRI and OCT parameters from MSpostvacc patients were comparable to the MSreference cohort and showed evidence of preexisting subclinical CNS disease. The single case with MOGADpostvacc clearly differed from PwMS in higher CSF cell counts, remission of MRI lesions during follow-up, and absence of oligoclonal bands.
Conclusions
Our case series indicates that MSpostvacc patients showed a rather typical initial manifestation in temporal association with SARS-CoV-2-vaccination and harbored preexisting subclinical neuroinflammation. This argues against the denovo development of MS in this cohort.
Keywords: Multiple Sclerosis (MS), Myelin oligodendrocyte glycoprotein antibody-related disease (MOGAD), SARS-CoV-2- (COVID-19) vaccination, Demyelinating disease
1. Introduction
The coronavirus disease 2019 (COVID-19) pandemic in the context of the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) outbreak in 2019 poses a serious global public health threat [Zhu et al., 2020]. One solution to this disease is to immunize the population worldwide through vaccination. Different vaccines are currently available to prevent SARS-CoV-2 infection and serious disease progression, their safety and effectiveness have been repeatedly reported [Pormohammad et al., 2021]. Central nervous system (CNS) demyelinating disorders (DD) after SARS-CoV-2 infection as well as following COVID-19 vaccination have been described [Ismail & Salama, 2022], including onset of multiple sclerosis (MS) [Havla et al., 2022] or cases of myelin oligodendrocyte glycoprotein antibody-associated disease (MOGAD) [Mumoli et al., 2022, Dams et al., 2022, Simone et al., 2021, Francis et al., 2022, Jarius et al., 2022]. So far, no increased risk for relapse activity could be observed in MS patients following the first and second dose of COVID-19 vaccination [Epstein et al., 2022]. Based on the extremely high vaccination numbers worldwide (over 12 billion vaccine doses administered by July 2022 [Our world in data (2022) COVID-19 vaccination doses administered 2022]) compared to singular events, it continues to seem more likely that it may be temporal coincidence rather than causality. Nevertheless, some severe cases with immediate onset of MS relapses shortly after COVID-19 vaccination have been reported [Maniscalco et al., 2021].
At the time of first clinical onset, it is usually difficult to tell whether the onset of CNS-DD occurred causally or coincidentally after SARS-CoV-2 vaccination. Also, in the absence of previous diagnostic workup, it is not possible to say with certainty whether the onset is an expression of a denovo development of a previously not existing disease or merely the first clinical manifestation of a preexisting, up to now subclinical neuroinflammation. With this case study we investigated whether patients with clinical onset of CNS-DD in temporal association with SARS-CoV-2 vaccination (MSpostvacc) showed already at the time of the first symptoms evidence of preexisting subclinical inflammatory CNS disease and whether the clinical characteristics of the MSpostvacc cohort (phenotype, cerebrospinal fluid (CSF) parameters, magnetic resonance imaging (MRI), optical coherence tomography (OCT)) would differ from a typical early MS cohort (MSreference).
2. Material & methods
2.1. Study population
The outpatient clinic of the Institute of Clinical Neuroimmunology of the LMU Hospital is a transregional special outpatient clinic for the treatment of neuroimmunological diseases. Included were all consecutive patients (N=6; MS: 5, MOGAD: 1) in the period from January 2020 and September 2021 with the first manifestation of CNS-DD ≤ 30 days after SARS-CoV-2 vaccination (1st or 2nd dose of vaccination) without previous SARS-CoV-2 virus infection and without history for previous neurological symptoms. Routine clinical data were retrieved by chart review and included demographic data, medical history, clinical scores such as the Expanded Disability Status Scale (EDSS) [Kurtzke, 1983], and laboratory values including CSF parameters, data on SARS-CoV-2 vaccination as well as MRI and OCT data. In addition, all patients were seen and investigated at least once in our department of neurology and/or at our outpatient clinic. To compare clinical characteristics from patients with onset of CNS-DD after SARS-CoV2 vaccination (MSpostvacc), we chose as a control group an existing, well-characterized cohort with early MS (MSreference). MS diagnosis was made according to the 2017 McDonald criteria [Thompson et al., 2018], and MOGAD diagnosis was made according to the recently proposed criteria [Jarius et al., 2018].
All patients gave written informed consent (no. 163-16). All procedures were performed according to the Helsinki Declaration (1964, as revised 2013) and with national ethical standards for experiments on humans.
2.2. Magnetic resonance imaging (MRI)
A standard MRI was done as part of routine clinical practice on a 1.5 or 3 Tesla scanner. All patients received an MRI of the brain and imaging of the entire spinal cord, with the exception of patient 1, who did not receive an MRI of the cervical spine. The contrast agent gadolinium (Gd) was used in five brain and three spine MRIs. The contrast agent gadolinium (Gd) was used in five brain and three spine MRIs. T2-weighted (T2w) images were evaluated by a specialized neuroradiologist (HZ) for location, number and configuration of inflammatory lesions.
2.3. Optical coherence tomography (OCT)
All OCT data were collected as part of routine clinical practice in our outpatient clinic using a spectral-domain OCT with automatic real-time function (SD-OCT) (SPECTRALIS, Heidelberg Engineering, Heidelberg, Germany), layer segmentation was performed by the OCT manufacturer software (Eye Explorer 1.9.10.0 Heidelberg Engineering, Heidelberg, Germany). Data analysis followed the international APOSTEL 2.0 and OSCAR-IB criteria [Aytulun et al., 2021, Schippling et al., 2015]. A 3.5 mm ring scan around the optic nerve (12°, 1536 A-scans, ART ≤ 100) was used to measure the peripapillary retinal nerve fiber layer thickness (pRNFL). Total macular volume (TMV) as well as thickness of the ganglion cell and inner plexiform layer (GCIPL) and inner nuclear layer (INL) were measured on a macular volume scan (20° × 20°, 25 B-scans, ART ≤ 49) using a ø 3 mm ring fixed to the fovea.
3. Results
3.1. MSpostvacc: Case summary of the individual medical records
Five patients (three females, mean age: 33 years) developed with a mean of 8 (range: 1-12) days after SARS-CoV-2 vaccination the first clinical episode of CNS-DD and were diagnosed with MS. For clinical characteristics at the time of clinical onset, please refer to the case descriptions and Table 1 .
Table 1.
Case series characterization. ChAdOx1: Oxford-AstraZeneca (ChAdOx1-S/nCoV-19, AZD1222); BNT162b2: Pfizer-BioN-Tech; CS: glucocorticoid pulse therapy; CSF: cerebrospinal fluid; EDSS: Expanded Disability Status Scale; Gd: gadolinium; na: not applicable; PLEX: plasma exchange. + feature present; - feature not present. * Anti-SARS-CoV-2 IgG (ELISA, Euroimmun AG).
|
MSpostvacc cohort |
MOGADpostvacc patient | |||||
|---|---|---|---|---|---|---|
| Patient 1 | Patient 2 | Patient 3 | Patient 4 | Patient 5 | Patient 6 | |
| Clinical data | ||||||
| 1st COVID-19 vaccination | BNT162b2 | BNT162b2 | BNT162b2 | BNT162b2 | ChAdOx1 | ChAdOx1 |
| Clinical onset after 1st COVID-19 vaccination (days) | 6 | na | 12 | na | 8 | 8 |
| 2nd COVID-19 vaccination | na | BNT162b2 | BNT162b2 | BNT162b2 | ChAdOx1 | BNT162b2 |
| Clinical onset after 2nd COVID-19 vaccination (days) | na | 5 | na | 1 | na | na |
| First EDSS | 3.5 | 1.0 | 1.0 | 2.0 | 2.5 | 2.5 |
| Clinical manifestation | spinal cord | brain/ brainstem |
optic nerve | optic nerve | brain/ brainstem |
brain/ brainstem |
| Acute therapy | 2x CS, PLEX | na | 2x CS | 1x CS | 3x CS | 1x CS |
| EDSS follow-up (weeks after onset) |
3.5 (12) |
3.0 (5) |
1.0 (13) |
3.0 (32) |
3.5 (15) |
1.5 (21) |
| Laboratory data | ||||||
| CSF cell count (per μl) (days after COVID-19 vaccination) |
7 (17) |
4 (20) |
6 (26) |
4 (23) |
7 (84) |
106 (11) |
| CSF-specific OCB | positive | positive | positive | positive | positive | negative |
| AQP4-IgG/MOG-IgG | negative/negative | na/negative | negative/negative | negative/negative | negative/negative | negative/positive |
| Anti-SARS-CoV-2-ELISA (IgG)* (days after vaccination) |
4.6 (18) |
9.6 (35) |
8.2 (62) |
na |
2.7 (48) |
3.6 (37) |
| MRI data | ||||||
| Cerebral MRI | ||||||
| Time interval cMRT to onset (days) | 16 | 9 | 25 | 6 | 81 | 5 |
| MS specific T2w-hyperintens lesions periventricular | + | + | + | + | + | − |
| MS specific T2w-hyperintens lesions (juxta-) cortical | + | − | + | + | + | − |
| MS specific T2w-hyperintens lesions infratentorial | + | + | + | − | + | − |
| unspecific T2w-hyperintens lesions unspecific/subcortical | + | − | + | + | + | + |
| T2w-hyperintens N. opticus | − | − | + | + | − | − |
| T2w-hyperintens lesions spinal | − | − | − | + | − | − |
| Gd-enhancement | − | 2 (pons, corpus callosum) |
na | 1 (juxtacortical) |
1 (periventricular) |
− |
| Number of lesions | >20 | 3 | 7 | confluent | confluent | confluent |
| Spinal MRI | ||||||
| Time interval sMRT to onset (days) | 11 | 27 | 92 | 7 | 75 | 7 |
| Number of T2w-hyperintens lesions cervical spinal cord | na | 0 | 0 | 8 | 0 | 0 |
| Number of T2w-hyperintens lesions thoracic spinal cord | 1 | 0 | 1 | 7 | na | 0 |
| Gd-enhancement | + | na | − | na | na | − |
| Dissemination in time on MRI | + | + | − | + | + | − |
| Dissemination in space on MRI | + | + | + | + | + | na |
Case 1: RMS with dissemination in space and time on MRI and cerebrospinal fluid inflammatory syndrome (pleocytosis, OCBs positive)
A 28-year-old woman presented as an overall first neurological symptom a sensory impairment and a left leg paresis with corresponding acute inflammatory spinal cord lesion at Th6 on MRI six days after the first SARS-CoV-2 vaccination (BNT162b2, Comirnaty©, BioNTech/Pfizer). Additionally, multiple MS-specific, Gd-negative cerebral lesions were detected on MRI. CSF analysis revealed 7 cells/µl and positive oligoclonal bands (OCB). Possible - especially infectious - differential diagnoses were ruled out and diagnosis of relapsing MS according to the McDonald criteria was set. This case was published as a single case [Havla et al., 2022].
Case 2: RMS with dissemination in space and time on MRI and cerebrospinal fluid inflammatory syndrome (OCBs positive)
A 44-year-old woman developed as first clinical symptom paresthesia of the right side of the body and a central oculomotor disorder five days after the second SARS-CoV-2 vaccination (BNT162b2, Comirnaty©, BioNTech/Pfizer). In total, three hyperintense lesions were detectable on cerebral MRI (T2w/FLAIR), two of them showed contrast enhancement (infratentorial/pons and periventricular). 4 cells/µl and CSF specific OCB were found in the CSF and the diagnosis of relapsing MS according to the McDonald criteria was set.
Case 3: RMS with dissemination in space and time on MRI and cerebrospinal fluid inflammatory syndrome (pleocytosis, OCBs positive)
A 26-year-old man was diagnosed with optic neuritis (ON; blurred vision with interfering images, color desaturation) 12 days after the first SARS-CoV-2 vaccination (BNT162b2, Comirnaty©, BioNTech/Pfizer). ON was his first clinical neurological symptom. The diagnosis of relapsing MS was made in the presence of several supra-, infratentorial and spinal lesions on MRI - all of them without contrast enhancement - and of an inflammatory CSF profile (6 cells/μl, positive OCB) (Fig. 1 ).
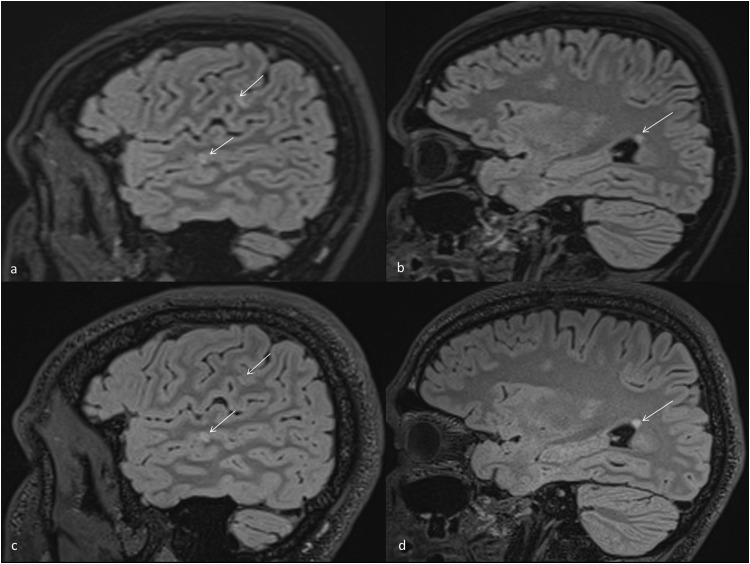
Fig. 1.
Sagittal T2w MRI scans of case 3 (stable MSpostvacc)
a) Sagittal scan with two juxtacortical lesions (arrows). b) Sagittal scan showing one subcortical and one periventricular lesion (arrow). c-d) Follow-up imaging after 2 months without evidence of new lesion (arrows).
Case 4: RMS with dissemination in space and time on MRI and cerebrospinal fluid inflammatory syndrome (OCBs positive)
30-year-old woman reported as first clinical symptom a reduced visual acuity with eye movement pain on the left eye one day after the second SARS-CoV-2 vaccination (BNT162b2, Comirnaty©, BioNTech/Pfizer). Multiple cerebral and one spinal lesion were present on MRI scans. CSF analysis revealed 4 cells/ µl and detection of positive OCB. Relapsing MS was diagnosed.
Case 5: RMS with dissemination in space and time on MRI and cerebrospinal fluid inflammatory syndrome (pleocytosis, OCBs positive)
Eight days after the first vaccination with Oxford-AstraZeneca COVID-19 vaccine (ChAdOx1 nCoV-19; AstraZeneca), a 36-year-old man developed as first clinical symptom a severe depressive syndrome, followed by sensory symptoms of both legs, double vision and a neurogenic bladder/rectal dysfunction. Cerebral MRI showed several large periventricular lesions including two well demarcated cerebellar lesions, two periventricular lesions showed contrast enhancement, spinal cord MRI was unremarkable (Fig. 2 , a-b). CSF analysis detected an increased cell count (7 cells/μl) and specific OCB. Despite two cycles of CS therapy, there was only partial remission of symptoms. 15 weeks later the patient experienced another relapse with worsening of previous symptoms, a new gait disorder and double vision. Follow-up cerebral MRI showed new inflammatory lesions in the pons and the cerebellum on both sides, therefore, CS treatment was administered again (Fig. 2 , c-d).
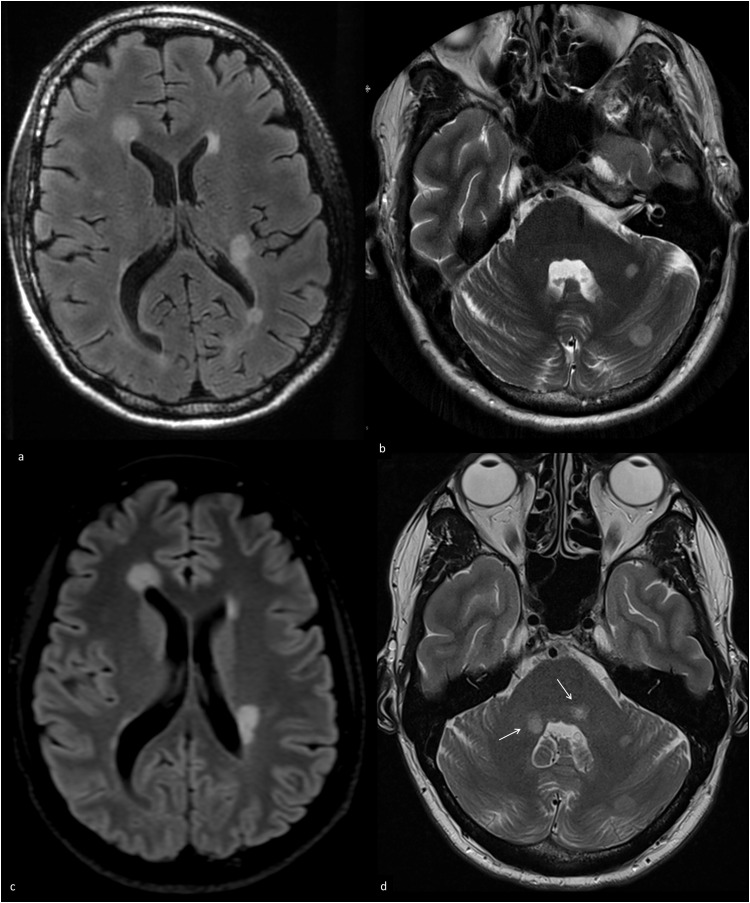
Fig. 2.
Axial T2w and FLAIR MRI scans of case 5 (active MSpostvacc)
a-b) Initial FLAIR (a) and T2w (b) MRI with MS-typical periventricular and infratentorial hyperintensities. c-d) Follow-up MRI after 1 month with new, well demarcated infratentorial lesions in the middle cerebellar peduncle on both sides (arrows).
3.2. MOGADpostvacc: Case summary of the medical record
Case 6: MOGAD with brain lesions and cerebrospinal fluid inflammatory syndrome (pleocytosis, OCBs negative)
A 38-year-old woman noticed nine days after the first Oxford-AstraZeneca COVID-19 vaccine (ChAdOx1 nCoV-19; AstraZeneca) as the first neurological manifestation overall severe fatigue, headache, a central oculomotor dysfunction with double vision and urinary retention (initial EDSS 2.5). A sinus vein thrombosis was excluded, but cerebral MRI revealed multiple fluffy supra- and infratentorial, non-contrast enhancing lesions, spinal MRI was unremarkable (Fig. 3 , a-c). 12 days after onset, CSF analysis showed an increased cell count of 106/μl (60% lymphocytes, 25% granulocytes) without detection of CSF-specific OCB nor intrathecal immunoglobulin synthesis. Anti-MOG-IgG was detected in serum by live-cell based assay (1:10240) and by an immunofluorescence test (IFT, 1:100++), and the diagnosis of MOGAD was confirmed. Therapy with high-dose CS (1g each for 5 days) resulted in good clinical improvement and long-term immunotherapy was not initiated. Pleocytosis decreased in CSF (39 cells per μl) 21 days after onset. 99 days after the first vaccination, the patient received a second SARS-CoV-2 vaccination (BNT162b2, Comirnaty©, BioNTech/Pfizer) without any worsening of neurological symptoms. On follow-up (five months after onset), the patient showed stable mild neurological abnormalities (EDSS 1.5) but complete remission of the MRI lesions (Fig. 3 , d-f) while anti-MOG-IgG was still positive (IFT: 1:100++) in serum.
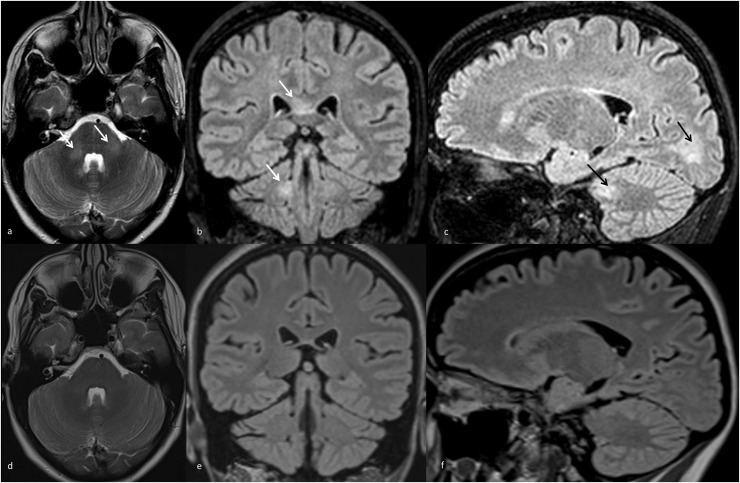
Fig. 3.
T2w and FLAIR MRI scans of case 6 (MOGADpostvacc)
a) Axial (T2w), b) coronal, c) sagittal scans (both FLAIR) showing poorly demarcated hyperintensities at the corpus callosum, the cerebellum, cerebellar peduncles, and fluffy white matter lesions in the occipital lobes (arrows). d-f) Follow-up MRI after 4 months without evidence of residual or new lesions.
3.3. MSreference: cohort description
The reference cohort (n = 76) is an early MS cohort independently recruited at our center after the initial event and before initiation of a disease modifying therapy. All routine clinical data (MRI, OCT, CSF, clinical score, medical history) from this cohort are standardized collected during routine clinical visits. From the MS cohort, three age- and sex-matched MS patients per MSpostvacc-patient were randomly selected as a control cohort (MSreference). The mean age at diagnosis was 33 years; 9 women, 6 men were included. The disease characteristics of the MSreference cohort compared to the MS/MOGADpostvacc patients are summarized in Table 2 .
Table 2.
Comparison between CSF and neuroimaging (MRI, OCT) data of the MSpostvacc and MSreference cohorts
| MSpostvacc | MSreference | ||
|---|---|---|---|
| Clinical data (number of patients/total) | |||
| Clinical symptom during onset due to lesion(s) in the area of Brain Brainstem Optic nerve Spinal chord Brain and Spinal chord |
3/5 (60%) 3/5 (60%) 2/5 (40%) 1/5 (20%) |
7/15 (47%) 4/15 (27%) 5/15 (33%) 1/15 (7%) |
|
| Evidence for other and/or previous autoimmune diseases | 1/5 (20%) | 2/15 (13%) | |
| Family history positive for neuroimmunological disease(s) | 1/5 (20%) | 4/15 (27%) | |
| CSF data (Mean ± SD or n/total) (n) | |||
| Time interval clinical onset to CSF analysis (days) | 27 ± 28(5) | 34 ± 9(13) | |
| Cell count(per µl) | 5.60 ± 1.52(5) | 6.29 ± 7.63(14) | |
| CSF spec. OCB | 5/5 (100%) | 13/14 (93%) | |
| Protein (mg/dl) | 32.20 ± 11.78(5) | 36.48 ± 13.33(12) | |
| Albumin quotient (CSF/Serum) | 4.39 ± 1.58(5) | 5.14 ± 2.23(11) | |
| IgG quotient (CSF/Serum) | 4.22 ± 2.48(5) | 3.69 ± 2.34(12) | |
| IgM quotient(CSF/Serum) | 4.98 ± 8.07(5) | 1.49 ± 1.78(10) | |
| IgA quotient (CSF/Serum) | 1.56 ± 0.83(5) | 2.55 ± 2.47(9) | |
| MRI data (number of patients with T2w-hyperintens lesions/total) | |||
| Number of lesions Cerebral 0 – 5 6 – 10 11 – 20 >20 / confluent Cervical spinal chord 0 1 – 5 >5 Thoracic spinal chord 0 1 – 5 >5 |
1/5 (20%) 1/5 (20%) 0/5 (0%) 2/5 (40%) 3/4 (75%) 0/4 (0%) 1/4 (25%) 1/4 (25%) 2/4 (50%) 1/4 (25%) |
3/15 (20%) 4/15 (27%) 4/15 (27%) 4/15 (27%) 7/15 (47%) 7/15 (47%) 1/15 (7%) 10/15 (67%) 4/15 (27%) 1/15 (7%) |
|
| Cerebral MRI | |||
| Periventricular | 5/5 (100%) | 14/15 (93%) | |
| (Juxta-)cortical | 4/5 (80%) | 13/15 (87%) | |
| Infratentorial | 4/5 (80%) | 9/15 (60%) | |
| Unspecific/subcortical | 4/5 (80%) | 14/15 (93%) | |
| Nervus opticus | 2/5 (40%) | 1/15 (7%) | |
| Spinal | 1/5 (20%) | 3/10 (30%) | |
| Spinal MRI | |||
| Cervical spinal cord | 1/4 (25%) | 6/15 (40%) | |
| Thoracic spinal cord | 3/5 (60%) | 5/15 (30%) | |
| OCT data all eyes | |||
| Number of patients | 5 | 15 | |
| Number of eyes | 10 | 30 | |
| TMV (mm3) | 2.04 ± 0.11 | 2.15 ± 0.09 | |
| GCIPL (μm) | 0.53 ± 0.06 | 0.58 ± 0.05 | |
| INL (μm) | 0.24 ± 0.02 | 0.25 ± 0.02 | |
| pRNFL (μm) | 91.70 ± 10.55 | 96.14 ± 9.26 | |
| OCT data eyes without a history of optic neuritis | |||
| Number of patients | 5 | 15 | |
| Number of eyes | 8 | 24 | |
| TMV (mm3) | 2.05 ± 0.12 | 2.14 ± 0.10 | |
| GCIPL (μm) | 0.55 ± 0.05 | 0.58 ± 0.04 | |
| INL (μm) | 0.23 ± 0.02 | 0.25 ± 0.02 | |
| pRNFL (μm) | 91.50 ± 11.88 | 96.63 ± 8.78 | |
3.4. Comparison between MSpostvacc and MSreference
For details of the clinical characteristics of the groups to be compared at the time of the first clinical symptoms, we refer to Table 2.
MRI data: A comparable CNS affection of inflammatory lesions is demonstrated comparing different anatomical localizations, numbers and configuration between the MSpostvacc and MSreference cohorts based on cerebral and spinal MRI data, except for less cervical spinal cord involvement in MSpostvacc patients.
CSF data: Within the CSF profiles both cohorts revealed a mild pleocytosis, except of one MSreference patient CSF-specific OCB were detectable in all cases.
OCT data: OCT data revealed a reduced mean TMV (2.04 mm3) and mean GCIPL thickness (0.53 μm) in the macular scan as well as a reduced pRNFL thickness (91.70 μm) in MSpostvacc eyes compared to the MSreference cohort (TMV: 2.15 mm3; GCIPL: 0.58 μm; pRNFL: 96.14 μm). This effect is also observed after exclusion of eyes with a history of ON (MSpostvacc: 2; MSreference: 6 eyes) in both groups. A statistical evaluation was not performed due to the small number of cases.
3.5. Comparison between MSpostvacc and MOGADpostvacc
Clinically the MOGADpostvacc patient differed from MSpostvacc by additional systemic symptoms with headache and malaise. In each of the two cohorts, there was one patient with Oxford-AstraZeneca COVID-19 vaccine (ChAdOx1 nCoV-19; AstraZeneca). Both patients who received Oxford-AstraZeneca COVID-19 vaccine (ChAdOx1 nCoV-19; AstraZeneca) experienced bladder dysfunction without evidence for spinal cord lesions.
MRI data: MRI showed multiple supra- and infratentorial cerebral lesions in the MOGADpostvacc patient which differed from lesions of patients with diagnosis of MS by a more fluffy appearance and cerebellar lesions were less well demarcated compared to lesion from case 6 (MSpostvacc). In contrast to the MSpostvacc patients - MRI lesions almost completely resolved on follow-up after four months (Fig. 3).
CSF data: CSF analysis revealed a more pronounced elevation of cells (106/µl) without detection of OCB in the MOGADpostvacc patient, while the MSpostvacc cohort showed only mild pleocytosis (mean: 5.60 cells/µl) with detection of OCB in all cases.
OCT data: OCT in MOGADpostvacc showed no evidence of retinal neuroaxonal degeneration at the time of initial manifestation, whereas retinal neuroaxonal degeneration was already detectable at clinical onset in the MSpostvacc cohort.
4. Discussion
Our data suggest that the clinical characteristics at first clinical onset of MS in timed relation (≤30 days) to the SARS-CoV-2 vaccination (MSpostvacc) are comparable to the onset characteristics of a typical early MS cohort (MSreference). Although this is not proof, there is a strong argument that this is not a denovo development of MS but rather the first clinical manifestation of preexisting subclinical CNS inflammation. Our data support a preexisting chronic neuroinflammation with 3 relevant facts, all observed at the time of the first clinical onset: i) evidence of CSF-specific OCBs in all MSpostvacc patients, ii) evidence of spatial or even temporal dissemination on MRI, and iii) evidence of retinal neuroaxonal degeneration on OCT examination.
These data, although collected from a very small cohort, are nonetheless relevant because the published cases of CNS-DD after SARS-CoV-2 vaccination [Havla et al., 2022, Khayat-Khoei et al., 2022] lead to uncertainty about the safety of the vaccines. In the context of other vaccinations, especially the hepatitis B and yellow fever vaccination, an increased risk of an MS disease activity or even MS onset was previously speculated, even if the question of a denovo manifestation remains open [Geier & Geier, 2005, Papeix et al., 2021, Achiron et al., 2021]. However, cohort studies have so far always been able to exclude a statistically significant risk for the initial manifestation of a relapse in chronic inflammatory CNS disease or in the context of MS [Achiron et al., 2021]. These data therefore suggest an expected temporal coincidence rather than a causal relationship [Achiron et al., 2021, Farez & Correale, 2011, Mailand & Frederiksen, 2017, Langer-Gould et al., 2014]. The coincidence may be particularly suspected for MSpostvacc patient 4, in whom a causal association seems immunologically extremely unlikely because of the short interval (1 day) between SARS-CoV-2 vaccination and the onset of MS. But also considering the worldwide ongoing vaccination campaign against the SARS-CoV-2 virus, no causality can be assumed due to the few other individual cases so far.
In MS, it is known that there is a subclinical phase with persistent inflammation even in the period before the initial clinical onset of the disease. This preexisting subclinical disease leads to the fact that firstly in MRI chronic inflammation with demyelination and axonal damage may already be detectable at the time of first clinical onset [Bjornevik et al., 2020, Borgström et al., 2020]. Further, cohort analyses using OCT in patients with early MS have consistently shown that retinal neuroaxonal damage is predominantly present at the initial clinical phase of MS and independent of clinical optic nerve involvement compared with matched healthy controls [Borgström et al., 2020, Petzold et al., 2017, Martinez-Lapiscina et al., 2016, Paul et al., 2021]. Lastly, it is also known that the presence of CSF-specific OCBS i) has a high positive predictive value for MS and ii) is associated with a significantly increased risk of conversion to MS in patients with a clinically isolated syndrome [Calabrese et al., 2021].
Although the size of the case series is too small for statistical analysis, we wanted to evaluate exploratively the alternative hypothesis (H1) that the characteristics at first clinical onset of a presumed denovo MS after SARS-CoV-2 vaccination differ from the characteristics of a classic early MS cohort without vaccination in the temporal association with onset. In summary, we did not find evidence for true denovo development, since at least our cohort overall does not differ from the onset characteristics of a typical early MS cohort (MSreference).
However, the exploratory study also allows for a second clinically relevant analysis. This is to answer the question of whether the onset characteristics of MSpostvacc differ from MOGADpostvacc. Due to the rarity of this disease, we could describe the clinical characteristics at the time of clinical onset of MOGAD in only one patient (MOGADpostvacc) and compared it with the MSpostvacc cohort on the one hand, but also with other published MOGADpostvacc patients on the other hand [Mumoli et al., 2022, Dams et al., 2022, Simone et al., 2021, Francis et al., 2022, Jarius et al., 2022]. Similar to other reported MOGADpostvacc patients our MOGADpostvacc patient showed at the time of the first clinical onset no evidence of preexisting chronic inflammatory CNS disease and no dissemination in time. The MRI lesions did not show any MS-typical pattern and remitted completely during the course, however, they are in line with the reported MOGAD-typical lesions [Calabrese et al., 2021]. There was no pleocytosis and OCT showed no evidence of retinal neuroaxonal degeneration. Onset of MOGAD occurred in the context with ChAdOx1-S/nCoV-19 vaccination in most of the published cases [Jarius et al., 2022] as well as in our MOGADpostvacc patient. The clinical presentation of our patient was also comparable to the already known MOGADpostvacc clinical and paraclinical features [Mumoli et al., 2022, Dams et al., 2022, Simone et al., 2021, Francis et al., 2022, Jarius et al., 2022]. The clinical presentation in our MOGADpostvacc patient showed involvement of the cerebellar peduncle, a good clinical recovery after acute therapy, a stable MOG-IgG detection in the course and despite a resolution of inflammatory lesions in the follow-up MRI.
To summarise the second analysis of this cohort study, our MOGADpostvacc patient shows a more monophasic MOGAD typical clinical course, except for the lack of spinal lesions in this case, and is similar to the already published individual cases in clinical presentation. As a side note, our patient received the second SARS-CoV-2 vaccination alternatively with BNT162b2 (Comirnaty©, BioNTech/Pfizer) 14 weeks later, without any new clinical-neurological or paraclinical deterioration.
Both analyses of the present cohort study have limitations. Due to the rarity of these events, the detailed clinical characterization of 6 patients with first clinical onset of CNS inflammatory disease is a strength. Nevertheless, our cohort is still too small to generalize the results. Another limitation is the retrospective analysis of the data. Finally, the analyses on the MOGADpostvacc patient are based only on a single patient.
The present cohort study is relevant for vaccination guidance and counseling of patients. At least in our cohort, the clinical onset of MS after vaccination against SARS-CoV-2 does not indicate denovo development of new disease. The first clinical manifestation of preexisting subclinical neuroinflammation is most likely to have a coincidental temporal relationship with vaccination. In contrast, no pre-existing subclinical disease was evident in the single patient with MOGADpostvacc.
Declarations
JAG, HZ, EO, TC report no disclosures relevant to the manuscript.
TK has received speaker honoraria and/or personal fees for advisory boards from Novartis Pharma, Roche Pharma, Alexion/Astra Zeneca and Biogen. The Institution she works for has received grant support for her research from Bayer-Schering AG, Novartis and Chugai Pharma in the past.
JH has received grants for OCT research from the Friedrich-Baur-Stiftung and Merck, personal fees and non-financial support from Celgene, Merck, Alexion, Novartis, Janssen, Roche, Biogen and non-financial support of the Guthy-Jackson Charitable Foundation, all outside the submitted work. JH is partially funded by the German Federal Ministry of Education and Research ((DIFUTURE), Grant Numbers 01ZZ1603[A-D] and 01ZZ1804[A-H]).
CRediT authorship contribution statement
J.A. Gernert: Resources, Project administration, Writing – original draft, Formal analysis. H. Zimmermann: Resources, Visualization, Writing – review & editing. E. Oswald: Resources, Writing – review & editing. T. Christmann: Resources, Writing – review & editing. T. Kümpfel: Supervision, Writing – review & editing, Resources. J. Havla: Supervision, Methodology, Conceptualization, Writing – original draft, Project administration, Resources.
Declaration of Competing Interest
JAG, HZ, EO, TC report no disclosures relevant to the manuscript.
TK has received speaker honoraria and/or personal fees for advisory boards from Novartis Pharma, Roche Pharma, Alexion/Astra Zeneca and Biogen. The Institution she works for has received grant support for her research from Bayer-Schering AG, Novartis and Chugai Pharma in the past.
JH has received grants for OCT research from the Friedrich-Baur-Stiftung and Merck, personal fees and non-financial support from Celgene, Merck, Alexion, Novartis, Janssen, Roche, Biogen and non-financial support of the Guthy-Jackson Charitable Foundation, all outside the submitted work. JH is partially funded by the German Federal Ministry of Education and Research ((DIFUTURE), Grant Numbers 01ZZ1603[A-D] and 01ZZ1804[A-H]).
Acknowledgments
The authors would like to thank Prof. Dr. M. Dieterich and all the neurologists involved for the care of the MSpostvacc patients.
References
- Achiron A, Dolev M, Menascu S, et al. COVID-19 vaccination in patients with multiple sclerosis: what we have learnt by February 2021. Mult. Scler. 2021;27(6):864–870. doi: 10.1177/13524585211003476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aytulun A, Cruz-Herranz A, Aktas O, et al. APOSTEL 2.0 recommendations for reporting quantitative optical coherence tomography studies. Neurology. 2021;97(2):68–79. doi: 10.1212/WNL.0000000000012125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjornevik K, Munger KL, Cortese M, et al. Serum neurofilament light chain levels in patients with presymptomatic multiple sclerosis. JAMA Neurol. 2020;77(1):58–64. doi: 10.1001/jamaneurol.2019.3238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgström M, Tisell A, Link H, Wilhelm E, Lundberg P, Huang-Link Y. Retinal thinning and brain atrophy in early MS and CIS. Acta Neurol. Scand. 2020;142(5):418–427. doi: 10.1111/ane.13282. [DOI] [PubMed] [Google Scholar]
- Calabrese M, Marastoni D, Crescenzo F, Scalfari A. Early multiple sclerosis: diagnostic challenges in clinically and radiologically isolated syndrome patients. Curr. Opin. Neurol. 2021;34(3):277–285. doi: 10.1097/WCO.0000000000000921. [DOI] [PubMed] [Google Scholar]
- Dams L, Kraemer M, Becker J. MOG-antibody-associated longitudinal extensive myelitis after ChAdOx1 nCoV-19 vaccination. Mult. Scler. 2022;28(7):1159–1162. doi: 10.1177/13524585211057512. [DOI] [PubMed] [Google Scholar]
- Epstein S, Xia Z, Lee AJ, et al. Vaccination against SARS-CoV-2 in neuroinflammatory disease: early safety/tolerability data. Mult. Scler. Relat. Disord. 2022;57 doi: 10.1016/j.msard.2021.103433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farez MF, Correale J. Immunizations and risk of multiple sclerosis: systematic review and meta-analysis. J. Neurol. 2011;258(7):1197–1206. doi: 10.1007/s00415-011-5984-2. [DOI] [PubMed] [Google Scholar]
- Francis A, Palace J, Fugger L. MOG antibody-associated disease after vaccination with ChAdOx1 nCoV-19. Lancet Neurol. 2022;21(3):217–218. doi: 10.1016/S1474-4422(22)00043-6. [DOI] [PubMed] [Google Scholar]
- Geier DA, Geier MR. A case-control study of serious autoimmune adverse events following hepatitis B immunization. Autoimmunity. 2005;38(4):295–301. doi: 10.1080/08916930500144484. [DOI] [PubMed] [Google Scholar]
- Havla J, Schultz Y, Zimmermann H, Hohlfeld R, Danek A, Kümpfel T. First manifestation of multiple sclerosis after immunization with the Pfizer-BioNTech COVID-19 vaccine. J. Neurol. 2022;269(1):55–58. doi: 10.1007/s00415-021-10648-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail II, Salama S. A systematic review of cases of CNS demyelination following COVID-19 vaccination. J. Neuroimmunol. 2022;362 doi: 10.1016/j.jneuroim.2021.577765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarius S, Paul F, Aktas O, et al. MOG encephalomyelitis: international recommendations on diagnosis and antibody testing. J. Neuroinflamm. 2018;15(1):134. doi: 10.1186/s12974-018-1144-2. Published 2018 May 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarius S, Bieber N, Haas J, Wildemann B. MOG encephalomyelitis after vaccination against severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2): case report and comprehensive review of the literature [published online ahead of print, 2022 Jun 23] J. Neurol. 2022:1–15. doi: 10.1007/s00415-022-11194-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khayat-Khoei M, Bhattacharyya S, Katz J, et al. COVID-19 mRNA vaccination leading to CNS inflammation: a case series. J. Neurol. 2022;269(3):1093–1106. doi: 10.1007/s00415-021-10780-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurtzke JF. Rating neurologic impairment in multiple sclerosis: an expanded disability status scale (EDSS) Neurology. 1983;33(11):1444–1452. doi: 10.1212/wnl.33.11.1444. [DOI] [PubMed] [Google Scholar]
- Langer-Gould A, Qian L, Tartof SY, et al. Vaccines and the risk of multiple sclerosis and other central nervous system demyelinating diseases. JAMA Neurol. 2014;71(12):1506–1513. doi: 10.1001/jamaneurol.2014.2633. [DOI] [PubMed] [Google Scholar]
- Mailand MT, Frederiksen JL. Vaccines and multiple sclerosis: a systematic review. J. Neurol. 2017;264(6):1035–1050. doi: 10.1007/s00415-016-8263-4. [DOI] [PubMed] [Google Scholar]
- Maniscalco GT, Manzo V, Di Battista ME, et al. Severe multiple sclerosis relapse after COVID-19 vaccination: a case report. Front. Neurol. 2021;12 doi: 10.3389/fneur.2021.721502. Published 2021 Aug 10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martinez-Lapiscina EH, Arnow S, Wilson JA, et al. Retinal thickness measured with optical coherence tomography and risk of disability worsening in multiple sclerosis: a cohort study. Lancet Neurol. 2016;15(6):574–584. doi: 10.1016/S1474-4422(16)00068-5. [DOI] [PubMed] [Google Scholar]
- Mumoli L, Vescio V, Pirritano D, Russo E, Bosco D. ADEM anti-MOG antibody-positive after SARS-CoV2 vaccination. Neurol. Sci. 2022;43(2):763–766. doi: 10.1007/s10072-021-05761-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Our world in data (2022) COVID-19 vaccination doses administered. https://ourworldindata.org/explorers/coronavirus-data-explorer?zoomToSelection=true&pickerSort=asc&pickerMetric=location&Interval=Cumulative&Relative+to+Population=false&country=~OWID_WRL&Metric=Vaccine+doses&Color+by+test+positivity=false. Accessed July 1, 2022.
- Papeix C, Mazoyer J, Maillart E, et al. Multiple sclerosis: Is there a risk of worsening after yellow fever vaccination? Mult. Scler. 2021;27(14):2280–2283. doi: 10.1177/13524585211006372. [DOI] [PubMed] [Google Scholar]
- Paul F, Calabresi PA, Barkhof F, et al. Optical coherence tomography in multiple sclerosis: a 3-year prospective multicenter study. Ann. Clin. Transl. Neurol. 2021;8(12):2235–2251. doi: 10.1002/acn3.51473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petzold A, Balcer LJ, Calabresi PA, et al. Retinal layer segmentation in multiple sclerosis: a systematic review and meta-analysis. Lancet Neurol. 2017;16(10):797–812. doi: 10.1016/S1474-4422(17)30278-8. [DOI] [PubMed] [Google Scholar]
- Pormohammad A, Zarei M, Ghorbani S, et al. Efficacy and safety of COVID-19 vaccines: a systematic review and meta-analysis of randomized clinical trials. Vaccines (Basel) 2021;9(5):467. doi: 10.3390/vaccines9050467. Published 2021 May 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schippling S, Balk LJ, Costello F, et al. Quality control for retinal OCT in multiple sclerosis: validation of the OSCAR-IB criteria. Mult. Scler. 2015;21(2):163–170. doi: 10.1177/1352458514538110. [DOI] [PubMed] [Google Scholar]
- Simone AM, Monti G, Amidei S, et al. Acute disseminated encephalomyelitis associated with anti-myelin oligodendrocyte glycoprotein (MOG-IGG) antibody in a patient with recent vaccination against SARS-CoV-2. J. Neurol. Sci. 2021;429 doi: 10.1016/j.jns.2021.118167. [DOI] [Google Scholar]
- Thompson AJ, Banwell BL, Barkhof F, et al. Diagnosis of multiple sclerosis: 2017 revisions of the McDonald criteria. Lancet Neurol. 2018;17(2):162–173. doi: 10.1016/S1474-4422(17)30470-2. [DOI] [PubMed] [Google Scholar]
- Zhu N, Zhang D, Wang W, et al. A novel coronavirus from patients with pneumonia in China, 2019. N. Engl. J. Med. 2020;382(8):727–733. doi: 10.1056/NEJMoa2001017. [DOI] [PMC free article] [PubMed] [Google Scholar]