Abstract
Whereas prone positioning of intubated patients suffering from acute respiratory distress syndrome represents the standard of care, proning non-intubated patients, so-called “awake prone positioning (APP),” has only recently gained popularity and undergone scientific evaluation. In this review, we summarize current evidence on physiological and clinical effects of APP on patients' centered outcomes, such as intubation and mortality, the safety of the technique, factors and predictors of success, practical issues for optimal implementation, and future areas of research. Current evidence supports using APP among patients suffering from acute hypoxemic respiratory failure due to COVID-19 and undergoing advanced respiratory support, such as high-flow nasal cannula, in an intensive care unit setting. Healthcare teams should aim to prone patients at least 8 h daily. Future research should focus on optimizing the tolerance of the technique and comprehensively evaluating benefits in other patient populations.
Keywords: Respiratory distress syndrome, Intensive care units, Prone position, Intubation
Introduction
Hypoxemic respiratory failure, especially acute respiratory distress syndrome (ARDS), is one of the leading causes of mechanical ventilation requirement and mortality in patients with coronavirus disease 2019 (COVID-19).[1,2] Average COVID-19-associated mortality has reached 17% among hospitalized patients, with a significant geographical variation in a meta-analysis of 42 studies involving 423,117 patients worldwide.[3] A number of death risk factors have been reported, including older age,[2], [3], [4] male sex,[3,5] lower partial pressure of oxygen (PaO2)/fraction of inspired oxygen (FiO2) ratio,[5] comorbidity, such as chronic obstructive pulmonary disease and type 2 diabetes,[3,5] intensive care unit (ICU) admission,[1] and aberrant cytokine storm.[1,6] The incidence of hypoxemic respiratory failure/ARDS has been reported to be as high as 30%,[1,2] and >50% of patients with COVID-19 admitted to ICU need oxygen supplementation or respiratory support upon admission.[7] Whereas placing intubated patients in the prone position represents the standard of care and is associated with reduced mortality, placing non-intubated patients in the prone position, so-called “awake prone positioning (APP),” has only recently gained scientific interest after being implemented merely as an innovative rescue technique when clinicians faced the first COVID-19 pandemic waves. Here, we summarize the current knowledge and evidence on physiological effects, benefits, and potential harms associated with APP, the best way to practically implement it, and further research perspectives on the matter.
Physiological and Clinical Effects of APP
The effect of APP on oxygenation improvement
Early case series studies have shown a reduction of respiratory rate and improvement in pulse oximetric saturation(SpO2), PaO2, and PaO2/FIO2 during APP,[8,9] which have been confirmed by subsequent cohort studies.[10], [11], [12], [13], [14] This improvement in oxygenation and clinical presentation usually happens in the early stage of APP (from 30 min to 6 h of the first episode). However, several observational studies have reported no improvement in oxygenation after APP.[15,16] Notably, patients in the prone group in these observational studies have been generally less severe (lower acute physiology and chronic health evaluation [APACHE] II scores) compared with patients in the supine positioning group. As for randomized controlled studies, several studies with a small sample size have found no significant improvement in oxygenation in patients with APP.[17], [18], [19], [20] A meta-trial with 1126 patients (567 in APP vs. 559 in standard care) has shown a significant effect of APP on reducing the respiratory rate and improving oxygenation.[21] Seven currently available meta-analyses have concluded that APP improves oxygenation in patients with COVID-19-induced acute hypoxemic respiratory failure.[22], [23], [24], [25], [26], [27], [28]
The effect of APP on reducing the need for intubation
During the first wave of the pandemic, large cohort studies have reported that the need for intubation and invasive mechanical ventilation in patients with COVID-19 admitted to ICU and out-of-ICU was about 80% and 26%, respectively.[7,29] Efforts have been undertaken to reduce the need for intubation due to its association with higher mortality and the use of scarce resources in the pandemic setting.[2,7] Early observational studies have shown controversial effects of APP on the need for intubation,[[10], [11], [12], [13], [14], [15], [16],[30], [31], [32]] while early randomized controlled studies with small sample sizes have not been able to detect the differences in intubation induced by APP.[[17], [18], [19], [20],33] However, from observational studies, it is interesting to note that a significant reduction in the need for intubation through APP implementation has been found in most of the studies in which patients received predominantly high-flow nasal cannula (HFNC).[11,[30], [31], [32]] The largest randomized evidence published as an international multi-centered meta-trial, including patients hospitalized with acute hypoxemic respiratory failure due to COVID-19 pneumonia and requiring HFNC treatment, has demonstrated that APP significantly reduced the risk of intubation by 7%.[21] Moreover, meta-analyses and systematic reviews of non-randomized controlled studies have also shown the effect of APP on reducing the risk of intubation,[22] except for a meta-analysis conducted by Pavlov et al.[25] at early pandemic, and including only observational studies.[27] The introduction of randomized controlled studies offsets this effect.[24,34] Interpretation of these meta-analyses should be cautious because different inclusion criteria have been used, and eventually, different studies have been included for the final analysis as the evidence evolved, comprising various combinations of randomized and non-randomized studies published at different time points. It would be important to dissect the studies based on the study design and perform subgroup analyses on different patient groups to identify the cluster of patients that could benefit most from APP. In this regard, a recent large meta-analysis of 1985 patients from 10 randomized controlled studies and 2669 patients from 19 non-randomized controlled studies has confirmed the benefit of APP in reducing the need for intubation based on both randomized controlled studies and non-randomized controlled studies.[35] Moreover, it has further revealed that this benefit is more robust in patients undergoing advanced respiratory support (e.g., HFNC and non-invasive ventilation [NIV]) and in ICU settings at enrollment.
Recently, Alhazzani et al.[36] have published a randomized clinical trial on awake prone position. Of 400 patients enrolled in this study, over 2/3 received HFNC. Although the intubation rate on day 30 was not significantly different between groups of APP and standard care (34.1% vs. 40.5%, respectively, with a hazard ratio of 0.81, 95% confidence interval[CI]: 0.59–1.12, P=0.20), they have reported a lower risk of intubation for patients who received HFNC in their subgroup analyses. The authors have also recognized that the effect size for the primary study outcome (intubation rate on day 30) was imprecise and did not exclude a clinically important benefit.
The effect of APP on mortality
A large retrospective study with 505 patients in the APP group and 322 patients in the control group has shown that APP halved the hospital mortality of COVID-19 patients (control group mortality 37.3% vs. APP group 19.8%)[12]; however, none of the available randomized controlled studies have reported such a significant reduction in mortality by APP.[[19], [20], [21],33,37] Some meta-analyses pooling non-randomized controlled studies and randomized controlled studies together have also demonstrated that APP was associated with lower mortality compared with supine positioning.[24,34] By separating meta-analysis of randomized controlled studies and non-randomized controlled studies, the most recent evidence has found no reduction in mortality when pooling randomized controlled studies data while observing significantly lower mortality in the APP group when pooling non-randomized controlled studies data.[35] Moreover, the aforementioned randomized controlled trial by Alhazzanni et al.[36] has also shown no difference in mortality between APP and control groups. These results call for attention to possible recruitment and publication bias among non-randomized studies. Indeed, the mortality data from the non-randomized controlled studies should be interpreted cautiously because significant differences in patients’ characteristics are noticed in the supine group, such as older age, more ICU patients, and more severe patients classified by a higher sequential organ failure assessment (SOFA), or APACHE II score,[11,12,15,38] which are among the recognized risk factors for COVID-19-associated mortality. In addition, mortality has been exclusively designed as a secondary outcome in all the currently available randomized controlled studies, which might be underpowered to detect a significant difference regarding mortality. Therefore, more randomized controlled studies are needed to reveal the true effect of APP on mortality.
Safety of APP
Adverse events, such as pressure wounds, endotracheal tube dislodgement, and sustained hypotension, and the complexity of prone positioning (PP) maneuvers, including but not limited to the requirement of practical skills, are the major concerns impeding its implementation in mechanically ventilated patients.[39,40] In theory, APP seems easier to manage because patients are conscious without an endotracheal tube, meaning that they could be instructed to change position by themselves and autonomously adjust their position to improve tolerance. Based on the current studies, no severe adverse events have been reported during APP. The most commonly reported adverse events associated with APP are discomfort, skin breakdown, vomiting, central line dislodgement, back pain, and bloating sensation.[13,[17], [18], [19],21,33,36,41] Generally, APP is well tolerated,[9,21,22,37] but discomfort and anxiety have been reported as the most common reasons for intolerance.[37,41] Whereas these side effects can be considered mild, they might still impact patients’ adherence to the technique.
Influential Factors on the Treatment Success of APP
Similar to other therapeutic maneuvers/procedures for the treatment of acute hypoxemic respiratory failure, selection of appropriate patients plays a key role in treatment success. Clinicians need to consider the following factors while assessing the benefits of APP for patients.
Disease severity and respiratory support
Based on the physiological mechanism of APP in improving oxygenation and patient-centered outcomes, a significant effect size is expected to be more easily detected in patients with more severe diseases. In a multicenter randomized controlled study including 248 patients with moderate hypoxemia only (FiO2 <50%), Fralick et al.[42] have not observed any improvement in the composite outcome of death, mechanical ventilation, or worsening in respiratory failure. In another recent non-randomized controlled trial which included 501 patients, most of whom were treated with a low-flow nasal cannula, Qian et al.[43] have observed no clinical benefit. Notably, this study has even reported a potential risk of short-term harm from APP; however, it has not been apparent on day 28. In this study, mortality has been much higher than the intubation rate, meaning that several patients have died without being intubated. Those patients with “do not intubate” orders might have represented a critical confounding factor.
The HFNC has been used for oxygen support in the studies included in the meta-trial by Ehrmann et al.[21] In other randomized controlled studies in which the use of respiratory support devices has been heterogeneous, including low-flow nasal cannula, face masks, and non-rebreathing masks, no significant improvement in intubation rate or mortality has been found.[[17], [18], [19], [20],37,42,43] In line with this, in a recent non-randomized controlled trial including only patients with NIV, Musso et al.[44] have found that early and prolonged APP was associated with decreased intubation and mortality. Also, the subgroup analysis of a recent systematic review and meta-analysis has revealed that the benefits of APP in reducing intubation were limited to patients with advanced respiratory support (HFNC and NIV) at enrollment.[35] Taken together, current evidence does not support the use of APP in COVID-19 patients without the need for advanced respiratory support; however, there is no solid evidence of harm in this subgroup, and the studies performed so far have been underpowered due to the low intubation rate. It is noteworthy to investigate to what extent the combined use of APP and advanced respiratory support play a role in treatment success.
Duration of APP
In the meta-trial by Ehrmann et al.,[21] only 17% of patients who attained a daily average time on APP ≥8 h/day have had treatment failure (intubation or death), compared to 48% of those with <8 h/day on APP. Similar results have been observed in the single largest randomized controlled study from Mexico, in which the duration of APP has been linearly correlated with the probability of treatment success (r=0.70, P <0.001), and a daily duration of APP ≥8 h/day has predicted success with a sensitivity of 90% and specificity of 87%.[45] However, given the observational nature of the data precluding causal inference, cautious interpretation is warranted. Interestingly, the presence of silent hypoxemia (i.e., hypoxemia without dyspnea) has been associated with a longer duration of APP (12.4 h/day vs. 7.6 h/day, P <0.001), which could partially explain the improved outcomes found in this subgroup. Esperatti et al.[46] have found a similar dose-response effect in a large cohort study from Argentina. They have included 335 patients with confirmed COVID-19 and treated with HFNC and found a progressive reduction in the risk of intubation as the APP duration increased, with a cutoff of ≥6 h/day associated with an odds ratio (OR) of 0.36 (95% CI: 0.2–0.7) for intubation, and a cutoff of ≥8 h/day associated with an OR of 0.37 (95%CI: 0.17–0.8) for mortality. APP might be considered as a cardiopulmonary reserve test in these acutely ill patients, selecting the fittest patients most likely to perform well, but the patient's tolerance relies not only on disease severity and comorbidities but also on patient motivation. Therefore, we suggest clinicians actively encourage patients to use PP, aiming for at least 8 h/day.
Timing of initiating APP
Data on timing from HFNC to initiation of APP are scarce. In a post hoc analysis of a randomized controlled study, early APP has been defined as APP starting within 24 h of HFNC initiation. They have found that patients with late APP (>24 h of HFNC initiation) had higher mortality than patients with early APP (45% vs. 26%, P=0.03).[47] Although these results should be considered exploratory, they are consistent with those obtained in the study by Ibarra-Estrada et al.,[45] in which APP has been initiated at 11.1 h after HFNC initiation. Therefore, we suggest that APP should be initiated as soon as patients are indicated for HFNC treatment.
Predictors of Treatment Success on APP
Once the initiation of APP is decided, it is important to identify patients who eventually will succeed from APP (defined as survival without intubation) as early as possible. Some variables reflecting disease severity at baseline have been identified as potential predictors of outcomes in patients with COVID-19. In the largest study including 212 patients treated with conventional oxygen, patients who survived had an improvement in SpO2, PaO2/FIO2, and respiratory rate after APP sessions, while these variables have not improved in non-survivors.[48] However, the timing of measurements and duration of APP sessions have not been reported.
In the large randomized controlled study by Ibarra-Estrada et al.,[45] several predictors for treatment success have been reported within the first 3 days after admission. First, at the initiation of HFNC, a ROX index of >6.0 (the ROX index is the ratio of SpO2/FiO2 to respiratory rate; it is a clinical score combining a single number of the three main parameters used to monitor a patient with acute hypoxemic respiratory failure) and d-dimer concentrations of <1.4 mg/dL have been associated with APP treatment success. But the variable with the highest area under the receiver operating characteristics curve (AUC) has been the respiratory rate at enrollment with a cutoff of ≤25 breaths/min showing a sensitivity of 90% and a specificity of 77%. Then, an increase in ROX index of ≥1.2 after the first APP session has had a sensitivity of 62% and specificity of 84%. Finally, a decrease in lung ultrasound score (LUS) score by ≥2 points, indicating less severe consolidation, on the third day from randomization, showed a sensitivity of 83% and specificity of 77% for APP success.[45]
As the research regarding predictors of response to APP has been limited to observational studies and a post hoc analysis, it is essential to note that no single variable is accurate enough to justify a solid recommendation regarding decision-making for intubation. In line with these results, it is our common practice to continuously assess the potential benefit of APP in a stepwise fashion: for instance, variables, such as the presence of silent hypoxemia at hospital admission, respiratory rate at the initiation of HFNC, and response in ROX index after the first APP session, can be helpful in decision-making regarding transferring patients to step-down units to avoid ICU overwhelming during pandemic surges, especially if patients show adequate tolerance to long APP sessions. Finally, as long as there are no indications for immediate intubation, all possible measures to increase patient adherence to APP should be taken regardless of the level of care.
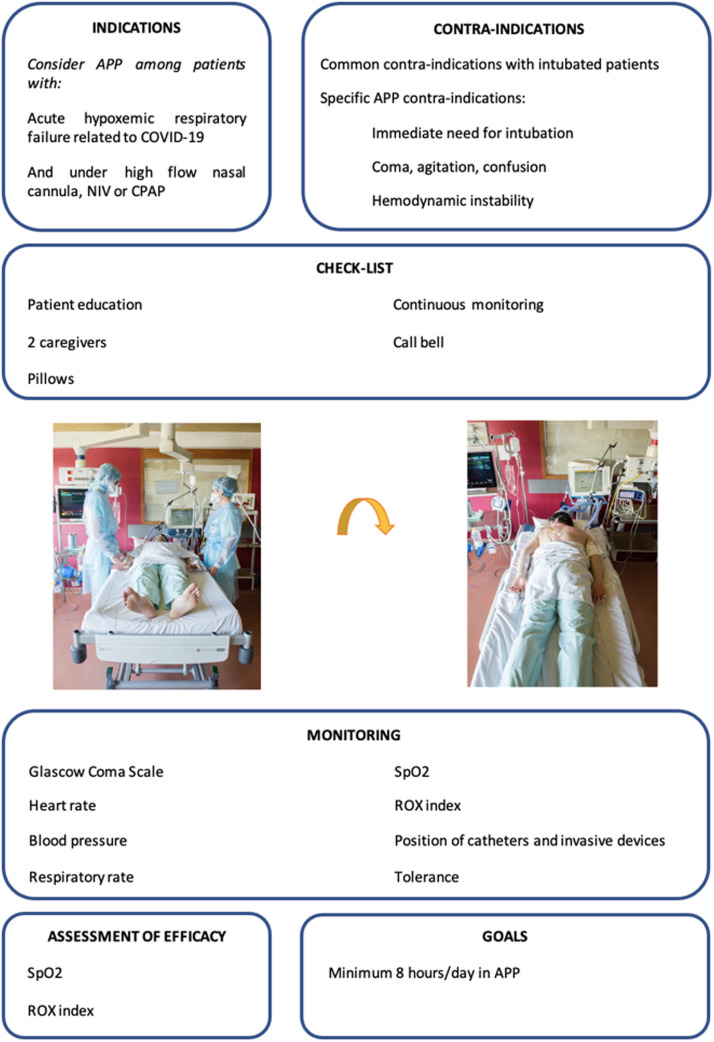
How to Prone a Non-intubated Patient?
In this section, we share our experience and techniques that have been used since the pandemic to place patients in APP with a focus on improving tolerance.
Preparation
First, it is essential to take time to explain the technique of APP and its expected benefits to the patient. After 2 years of the COVID-19 pandemic, patients are usually aware of the poor prognosis of admission to ICU and intubation. Explaining to patients that studies have scientifically proven that APP can reduce intubation rate might be an important source of motivation for patients and the team. An example of a patient education document is available in the Supplemantary Material.
The prone position is ideally supervised by two caregivers, at least for the first session. A physician may be present for the first session, depending on the local organization. However, it is important to note that “self-proning” studies (i.e., patients proning themself without any healthcare team assistance) have been associated with poor adherence and that APP success requires a substantial team effort and motivation.
The oxygenation device (HFNC, NIV, continuous positive airway pressure [CPAP], etc.) is placed on the patient's head. Catheters and other invasive devices must be secured. As a safety measure, FIO2 needs to be increased to 1.0 before proning to provide oxygen reserve for patients. The monitoring electrodes are removed from the anterior part of the chest to avoid skin lesions during prolonged PP sessions. Pulse oximetry should be monitored during the procedure. If there are buttons on the hospital gown, depending on the design, these need to be removed and put back on the patient once in the prone position to prevent pressure sores.
Prone positioning
Some patients can prone by themselves; however, supervision and assistance must be provided to favor adherence to the therapy. Others might need assistance, and in this case, we can follow the four classic steps used for the intubated patient: lateral translation, placement in the lateral position, reversal, and final installation [Figure 1]. The arms can be positioned along the body or on either side of the head, and it is essential to discuss with the patient and try different positions with their cooperation to find the most suitable position.
Figure 1.
How to assist patients with prone positioning. A: Patient in supine position. B: Lateral translation. C: Lateral position. D: Reversal of prone position. E: Replacement of electrodes on the back of the patient. F–I: Different positions of the arms can be proposed. J,K: Pillows can be used to improve the comfort, and the head of the bed can be elevated.
The monitoring electrodes are placed on the back of the patient. The patient should be as comfortable as possible. Pillows of different sizes can be placed under the chest, head, arms, and legs depending on patients’ preferences to improve their comfort. The bed can also be tilted to improve patient comfort. It is necessary to facilitate the patients’ access to their smartphones or anything that can help them relax (music, television, etc.). A calling device needs to be placed in the patient's hand to address their needs during APP.
Analgesics can be administered in case of pain related to the position. Some authors have reported the use of light sedation with benzodiazepines or dexmedetomidine to improve tolerance.[49,50] However, this practice has not been evaluated in a randomized controlled trial. Patients should be instructed and assisted to lie in the prone position for as long and as frequently as possible. Efforts are needed to maximize the duration of the sessions and the total time spent in APP daily. A goal of 8 h minimum per day in APP is advised.
At the end of the APP session, the electrodes are removed from the back. The same steps described above are followed for the reversal of the patient.
Monitoring
Vital signs should be continuously monitored during the APP session. An improvement in oxygenation can be evaluated during the session by measuring SpO2, SpO2:FIO2, and ROX index. Notably, as a pragmatic alternative to PaO2, SpO2 needs to be maintained at 90–97% to have a linear correlation with PaO2. If desaturation occurs, FIO2 should be increased to 1.0, and the physician in charge should be immediately contacted. If desaturation persists, APP should be terminated, and the indication for intubation should be evaluated. The time spent in the APP must be quantified daily.
Proning a non-intubated patient takes time, especially when the sessions are repeated over the day and patients require isolation measures. The involvement and motivation of the entire healthcare team are essential. The technique and its expected effects should be explained to the team ideally during dedicated training. A written procedure should be available in the unit. A memo sheet for healthcare staff is provided in Figure 2.
Figure 2.
Practical memo sheet of APP. APP: Awake prone positioning; CPAP: Continuous positive airway pressure; NIV: Non-invasive ventilation.
Future Research on APP
A mean daily APP duration of >8 h/day has been associated with treatment success.[21] Unlike intubated patients undergoing PP, APP time completely depends on patient comfort and tolerance. Future endeavors should be made to improve patient comfort and tolerance under prone position. Friedman et al.[51] have used an electronic wireless device to remind the patients to stay in the prone position in their study, and the effects have remained unknown. Since tolerance to APP might be complicated, involving body habitus, sleeping position, culture, education, etc., a qualitative study to interview patients undergoing APP might find out why patients could not tolerate APP and their feelings about it. A multidisciplinary team from respiratory care, nursing, physiotherapy, wound care, and psychological professionals might help to establish a feasible and effective bundle. Second, how APP works for patients with acute hypoxemic respiratory failure remains unclear. Recently, several studies assessing intubated patients’ responses to PP by electrical impedance tomography (EIT) or chest computed tomography have revealed that the oxygenation improvement was associated with the lung recruitment of dorsal zones and the lung collapse of the ventral zones after PP.[52,53] These findings explain that oxygenation is improved in some but not all patients. Future studies might assess the effects of APP on lung recruitment, particularly the combined use of different respiratory support devices with APP, to explore why the lower risk of intubation has predominantly been found in patients requiring advanced respiratory support, such as HFNC or NIV.[35] Similarly, the effects of APP on patient breathing, specifically the patient's self-inflicted lung injury (P-SILI),[54] are worth investigating. In a recent study by Tonelli et al.,[55] nasal pressure swings have been highly correlated with esophageal pressure swings, which is promising in the utilization during APP. Finally, due to the benefits of easy implementation and no cost, APP can be applied to patients with acute hypoxemic respiratory failure caused by non-COVID-19 pneumonia, with the aim to reduce intubation. Five cohort studies with a small sample size have been published before the COVID-19 pandemic. None of them has demonstrated the benefits of avoiding intubation.[22] Thus, randomized controlled trials assessing whether APP can reduce intubation rate in patients with acute hypoxemic respiratory failure induced by non-COVID-19 pneumonia are highly warranted.[56]
Conclusions
Based on the current knowledge, the prone position should be offered to all patients with COVID-19-induced acute hypoxemic respiratory failure when they require advanced respiratory support (HFNC, NIV, or CPAP) as it is associated with a significant reduction in intubation rates. This should ideally be done in an ICU under continuous monitoring. Beyond this specific population, APP might be considered for less severe patients, given the lack of harm observed in all studies. On a case-by-case basis, APP may be considered in some patients with non-COVID-19 acute hypoxemic respiratory failure.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Conflicts of Interest
Stephan Ehrmann declares consultancies, travel reimbursement and research support from Fisher & Paykel Healthcare and Aerogen Ltd. Jie Li discloses research funding from Fisher & Paykel Healthcare Ltd, Aerogen Ltd, and Rice Foundation, and speaker fees from American Association for Respiratory Care, Aerogen Ltd, Heyer Ltd, and Fisher & Paykel Healthcare Ltd. Jie Li also serves as section editor for Respiratory Care. The other authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgments
Acknowledgments
We thank Carlos Garijo, MD, Léa Rentz, and Aymeric Collot for their help in taking the photos.
Managing Editor: Jingling Bao
Footnotes
Supplementary material associated with this article can be found, in the online version, at doi:10.1016/j.jointm.2022.07.003.
Appendix. Supplementary materials
References
- 1.Huang C, Wang Y, Li X, Ren L, Zhao J, Hu Y, et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet. 2020;395(10223):497–506. doi: 10.1016/S0140-6736(20)30183-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Zhou F, Yu T, Du R, Fan G, Liu Y, Liu Z, et al. Clinical course and risk factors for mortality of adult inpatients with COVID-19 in Wuhan, China: A retrospective cohort study. Lancet. 2020;395(10229):1054–1062. doi: 10.1016/S0140-6736(20)30566-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Dessie ZG, Zewotir T. Mortality-related risk factors of COVID-19: A systematic review and meta-analysis of 42 studies and 423,117 patients. BMC Infect Dis. 2021;21(1):855. doi: 10.1186/s12879-021-06536-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.O'Driscoll M, Ribeiro Dos Santos G, Wang L, Cummings DAT, Azman AS, Paireau J, et al. Age-specific mortality and immunity patterns of SARS-CoV-2. Nature. 2021;590(7844):140–145. doi: 10.1038/s41586-020-2918-0. [DOI] [PubMed] [Google Scholar]
- 5.Grasselli G, Greco M, Zanella A, Albano G, Antonelli M, Bellani G, et al. Risk factors associated with mortality among patients with COVID-19 in intensive care units in Lombardy, Italy. JAMA Intern Med. 2020;180(10):1345–1355. doi: 10.1001/jamainternmed.2020.3539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.COVID-19 Multi-omics Blood ATlas (COMBAT) Consortium Electronic address: julian.knight@well.ox.ac.uk; COvid-19 Multi-omics Blood ATlas (COMBAT) Consortium. A blood atlas of COVID-19 defines hallmarks of disease severity and specificity. Cell. 2022;185(5):916–938. doi: 10.1016/j.cell.2022.01.012. e58. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.COVID-ICU Group on behalf of the REVA Network and the COVID-ICU Investigators Clinical characteristics and day-90 outcomes of 4244 critically ill adults with COVID-19: A prospective cohort study. Intensive Care Med. 2021;47(1):60–73. doi: 10.1007/s00134-020-06294-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Sartini C, Tresoldi M, Scarpellini P, Tettamanti A, Carcò F, Landoni G, et al. Respiratory parameters in patients with COVID-19 after using noninvasive ventilation in the prone position outside the intensive care unit. JAMA. 2020;323(22):2338–2340. doi: 10.1001/jama.2020.7861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Elharrar X, Trigui Y, Dols AM, Touchon F, Martinez S, Prud'homme E, et al. Use of prone positioning in nonintubated patients with COVID-19 and hypoxemic acute respiratory failure. JAMA. 2020;323(22):2336–2338. doi: 10.1001/jama.2020.8255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zang X, Wang Q, Zhou H, Liu S, Xue X. Efficacy of early prone position for COVID-19 patients with severe hypoxia: A single-center prospective cohort study. Intensive Care Med. 2020;46(10):1927–1929. doi: 10.1007/s00134-020-06182-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jouffroy R, Darmon M, Isnard F, Geri G, Beurton A, Fartoukh M, et al. Impact of prone position in non-intubated spontaneously breathing patients admitted to the ICU for severe acute respiratory failure due to COVID-19. J Crit Care. 2021;64:199–204. doi: 10.1016/j.jcrc.2021.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Nieto OR, Escarraman-Martinez D, Guerrero-Gutierrez MA, Zamarron-Lopez EI, Mancilla-Galindo J, Kammar-García A, et al. Awake prone positioning and oxygen therapy in patients with COVID-19: The APRONOX study. Eur Respir J. 2022;59(2) doi: 10.1183/13993003.00265-2021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Padrão EMH, Valente FS, Besen BAMP, Rahhal H, Mesquita PS, de Alencar JCG, et al. Awake prone positioning in COVID-19 hypoxemic respiratory failure: Exploratory findings in a single-center retrospective cohort study. Acad Emerg Med. 2020;27(12):1249–1259. doi: 10.1111/acem.14160. [DOI] [PubMed] [Google Scholar]
- 14.Barker J, Pan D, Koeckerling D, Baldwin AJ, West R. Effect of serial awake prone positioning on oxygenation in patients admitted to intensive care with COVID-19. Postgrad Med J. 2022;98(1159):360–364. doi: 10.1136/postgradmedj-2020-139631. [DOI] [PubMed] [Google Scholar]
- 15.Jagan N, Morrow LE, Walters RW, Klein LP, Wallen TJ, Chung J, et al. The POSITIONED study: Prone positioning in nonventilated coronavirus disease 2019 patients – A retrospective analysis. Crit Care Explor. 2020;2(10):e0229. doi: 10.1097/CCE.0000000000000229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Ferrando C, Mellado-Artigas R, Gea A, Arruti E, Aldecoa C, Adalia R, et al. Awake prone positioning does not reduce the risk of intubation in COVID-19 treated with high-flow nasal oxygen therapy: A multicenter, adjusted cohort study. Crit Care. 2020;24(1):597. doi: 10.1186/s13054-020-03314-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kharat A, Dupuis-Lozeron E, Cantero C, Marti C, Grosgurin O, Lolachi S, et al. Self-proning in COVID-19 patients on low-flow oxygen therapy: A cluster randomised controlled trial. ERJ Open Res. 2021;7(1):00692–02020. doi: 10.1183/23120541.00692-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Taylor SP, Bundy H, Smith WM, Skavroneck S, Taylor B, Kowalkowski MA. Awake prone positioning strategy for nonintubated hypoxic patients with COVID-19: A pilot trial with embedded implementation evaluation. Ann Am Thorac Soc. 2021;18(8):1360–1368. doi: 10.1513/AnnalsATS.202009-1164OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Jayakumar D, Ramachandran Dnb P, Rabindrarajan Dnb E, Vijayaraghavan Md BKT, Ramakrishnan Ab N, Venkataraman Ab R. Standard care versus awake prone position in adult nonintubated patients with acute hypoxemic respiratory failure secondary to COVID-19 infection – A multicenter feasibility randomized controlled trial. J Intensive Care Med. 2021;36(8):918–924. doi: 10.1177/08850666211014480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Johnson SA, Horton DJ, Fuller MJ, Yee J, Aliyev N, Boltax JP, et al. Patient-directed prone positioning in awake patients with COVID-19 requiring hospitalization (PAPR) Ann Am Thorac Soc. 2021;18(8):1424–1426. doi: 10.1513/AnnalsATS.202011-1466RL. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ehrmann S, Li J, Ibarra-Estrada M, Perez Y, Pavlov I, McNicholas B, et al. Awake prone positioning for COVID-19 acute hypoxaemic respiratory failure: A randomised, controlled, multinational, open-label meta-trial. Lancet Respir Med. 2021;9(12):1387–1395. doi: 10.1016/S2213-2600(21)00356-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tan W, Xu DY, Xu MJ, Wang ZF, Dai B, Li LL, et al. The efficacy and tolerance of prone positioning in non-intubation patients with acute hypoxemic respiratory failure and ARDS: A meta-analysis. Ther Adv Respir Dis. 2021;15 doi: 10.1177/17534666211009407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Pb S, Mittal S, Madan K, Mohan A, Tiwari P, Hadda V, et al. Awake prone positioning in non-intubated patients for the management of hypoxemia in COVID-19: A systematic review and meta-analysis. Monaldi Arch Chest Dis. 2021;91(2) doi: 10.4081/monaldi.2021.1623. [DOI] [PubMed] [Google Scholar]
- 24.Fazzini B, Page A, Pearse R, Puthucheary Z. Prone positioning for non-intubated spontaneously breathing patients with acute hypoxaemic respiratory failure: A systematic review and meta-analysis. Br J Anaesth. 2022;128(2):352–362. doi: 10.1016/j.bja.2021.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pavlov I, He H, McNicholas B, Perez Y, Tavernier E, Trump MW, et al. Awake prone positioning in non-intubated patients with acute hypoxemic respiratory failure due to COVID-19. Respir Care. 2021:09191. doi: 10.4187/respcare.09191. rescare. [DOI] [PubMed] [Google Scholar]
- 26.Ponnapa Reddy M, Subramaniam A, Afroz A, Billah B, Lim ZJ, Zubarev A, et al. Prone positioning of nonintubated patients with coronavirus disease 2019 – A systematic review and meta-analysis. Crit Care Med. 2021;49(10):e1001–e1014. doi: 10.1097/CCM.0000000000005086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Chua EX, Zahir SMISM, Ng KT, Teoh WY, Hasan MS, Ruslan SRB, et al. Effect of prone versus supine position in COVID-19 patients: A systematic review and meta-analysis. J Clin Anesth. 2021;74 doi: 10.1016/j.jclinane.2021.110406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kollias A, Kyriakoulis KG, Rapti V, Trontzas IP, Nitsotolis T, Syrigos K, et al. Prone positioning in patients with COVID-19: Analysis of multicenter registry data and meta-analysis of aggregate data. In Vivo. 2022;36(1):361–370. doi: 10.21873/invivo.12711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Franco C, Facciolongo N, Tonelli R, Dongilli R, Vianello A, Pisani L, et al. Feasibility and clinical impact of out-of-ICU noninvasive respiratory support in patients with COVID-19-related pneumonia. Eur Respir J. 2020;56(5) doi: 10.1183/13993003.02130-2020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Fazzini B, Fowler AJ, Zolfaghari P. Effectiveness of prone position in spontaneously breathing patients with COVID-19: A prospective cohort study. J Intensive Care Soc. 2021 doi: 10.1177/1751143721996542. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Vianello A, Turrin M, Guarnieri G, Molena B, Arcaro G, Turato C, et al. Prone positioning is safe and may reduce the rate of intubation in selected COVID-19 patients receiving high-flow nasal oxygen therapy. J Clin Med. 2021;10(15):3404. doi: 10.3390/jcm10153404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Tonelli R, Pisani L, Tabbì L, Comellini V, Prediletto I, Fantini R, et al. Early awake proning in critical and severe COVID-19 patients undergoing noninvasive respiratory support: A retrospective multicenter cohort study. Pulmonology. 2022;28(3):181–192. doi: 10.1016/j.pulmoe.2021.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rosén J, von Oelreich E, Fors D, Jonsson Fagerlund M, Taxbro K, Skorup P, et al. Awake prone positioning in patients with hypoxemic respiratory failure due to COVID-19: The PROFLO multicenter randomized clinical trial. Crit Care. 2021;25(1):209. doi: 10.1186/s13054-021-03602-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Beran A, Mhanna M, Srour O, Ayesh H, Sajdeya O, Ghazaleh S, et al. Effect of prone positioning on clinical outcomes of non-intubated subjects with COVID-19. Respir Care. 2022;67(4):471–479. doi: 10.4187/respcare.09362. [DOI] [PubMed] [Google Scholar]
- 35.Li J, Luo J, Pavlov I, Perez Y, Tan W, Roca O, et al. Awake prone positioning for non-intubated patients with COVID-19-related acute hypoxaemic respiratory failure: A systematic review and meta-analysis. Lancet Respir Med. 2022;10(6):573–583. doi: 10.1016/S2213-2600(22)00043-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Alhazzani W, Parhar KKS, Weatherald J, Al Duhailib Z, Alshahrani M, Al-Fares A, et al. Effect of awake prone positioning on endotracheal intubation in patients with COVID-19 and acute respiratory failure: A randomized clinical trial. JAMA. 2022;327(21):2104–2113. doi: 10.1001/jama.2022.7993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gad GS. Awake prone positioning versus non invasive ventilation for COVID-19 patients with acute hypoxemic respiratory failure. Egypt J Anaesth. 2021;37(1):85–90. doi: 10.1080/11101849.2021.1889944. [DOI] [Google Scholar]
- 38.Loureiro-Amigo J, Suárez-Carantoña C, Oriol I, Sánchez-Díaz C, Coloma-Conde A, Manzano-Espinosa L, et al. Prone position in COVID-19 patients with severe acute respiratory distress syndrome receiving conventional oxygen therapy: A retrospective study. Arch Bronconeumol. 2022;58(3):277–280. doi: 10.1016/j.arbres.2021.05.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Douglas IS, Rosenthal CA, Swanson DD, Hiller T, Oakes J, Bach J, et al. Safety and outcomes of prolonged usual care prone position mechanical ventilation to treat acute coronavirus disease 2019 hypoxemic respiratory failure. Crit Care Med. 2021;49(3):490–502. doi: 10.1097/CCM.0000000000004818. [DOI] [PubMed] [Google Scholar]
- 40.Guérin C, Albert RK, Beitler J, Gattinoni L, Jaber S, Marini JJ, et al. Prone position in ARDS patients: Why, when, how and for whom. Intensive Care Med. 2020;46(12):2385–2396. doi: 10.1007/s00134-020-06306-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Solverson K, Weatherald J, Parhar K. Tolerability and safety of awake prone positioning COVID-19 patients with severe hypoxemic respiratory failure. Can J Anaesth. 2021;68(1):64–70. doi: 10.1007/s12630-020-01787-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Fralick M, Colacci M, Munshi L, Venus K, Fidler L, Hussein H, et al. Prone positioning of patients with moderate hypoxaemia due to covid-19: Multicentre pragmatic randomised trial (COVID-PRONE) BMJ. 2022;376 doi: 10.1136/bmj-2021-068585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Qian ET, Gatto CL, Amusina O, Dear ML, Hiser W, Buie R, et al. Assessment of awake prone positioning in hospitalized adults with COVID-19: A nonrandomized controlled trial. JAMA Intern Med. 2022;182(6):612–621. doi: 10.1001/jamainternmed.2022.1070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Musso G, Taliano C, Molinaro F, Fonti C, Veliaj D, Torti D, et al. Early prolonged prone position in noninvasively ventilated patients with SARS-CoV-2-related moderate-to-severe hypoxemic respiratory failure: Clinical outcomes and mechanisms for treatment response in the PRO-NIV study. Crit Care. 2022;26(1):118. doi: 10.1186/s13054-022-03937-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Ibarra-Estrada M, Li J, Pavlov I, Perez Y, Roca O, Tavernier E, et al. Factors for success of awake prone positioning in patients with COVID-19-induced acute hypoxemic respiratory failure: Analysis of a randomized controlled trial. Crit Care. 2022;26(1):84. doi: 10.1186/s13054-022-03950-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Esperatti M, Busico M, Fuentes NA, Gallardo A, Osatnik J, Vitali A, et al. Impact of exposure time in awake prone positioning on clinical outcomes of patients with COVID-19-related acute respiratory failure treated with high-flow nasal oxygen: A multicenter cohort study. Crit Care. 2022;26(1):16. doi: 10.1186/s13054-021-03881-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kaur R, Vines DL, Mirza S, Elshafei A, Jackson JA, Harnois LJ, et al. Early versus late awake prone positioning in non-intubated patients with COVID-19. Crit Care. 2021;25(1):340. doi: 10.1186/s13054-021-03761-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dueñas-Castell C, Borre-Naranjo D, Rodelo D, Lora L, Almanza A, Coronell W, et al. Changes in oxygenation and clinical outcomes with awake prone positioning in patients with suspected COVID-19 in low-resource settings: A retrospective cohort study. J Intensive Care Med. 2021;36(11):1347–1353. doi: 10.1177/08850666211049333. [DOI] [PubMed] [Google Scholar]
- 49.Taboada M, Baluja A, Santos LD, González I, Veiras S, Caruezo V, et al. Effectiveness of dexmedetomidine combined with high flow nasal oxygen and long periods of awake prone positioning in moderate or severe COVID-19 pneumonia. J Clin Anesth. 2021;72 doi: 10.1016/j.jclinane.2021.110261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cruz Salcedo EM, Rodriguez LM, Patel J, Seevaratnam AR. Use of dexmedetomidine in early prone positioning combined with high-flow nasal cannula and non-invasive positive pressure ventilation in a COVID-19 positive patient. Cureus. 2020;12(9):e10430. doi: 10.7759/cureus.10430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Friedman E, Franzone J, Ko ER, Corey K, Mock J, Alavian N, et al. Rationale and design of the prone position and respiratory outcomes in non-intubated COVID-19 patients: The “PRONE” study. Contemp Clin Trials. 2021;109 doi: 10.1016/j.cct.2021.106541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Rossi S, Palumbo MM, Sverzellati N, Busana M, Malchiodi L, Bresciani P, et al. Mechanisms of oxygenation responses to proning and recruitment in COVID-19 pneumonia. Intensive Care Med. 2022;48(1):56–66. doi: 10.1007/s00134-021-06562-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Fossali T, Pavlovsky B, Ottolina D, Colombo R, Basile MC, Castelli A, et al. Effects of prone position on lung recruitment and ventilation-perfusion matching in patients with COVID-19 acute respiratory distress syndrome: A combined CT scan/electrical impedance tomography study. Crit Care Med. 2022;50(5):723–732. doi: 10.1097/CCM.0000000000005450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Grieco DL, Menga LS, Eleuteri D, Antonelli M. Patient self-inflicted lung injury: Implications for acute hypoxemic respiratory failure and ARDS patients on non-invasive support. Minerva Anestesiol. 2019;85(9):1014–1023. doi: 10.23736/S0375-9393.19.13418-9. [DOI] [PubMed] [Google Scholar]
- 55.Tonelli R, Cortegiani A, Marchioni A, Fantini R, Tabbì L, Castaniere I, et al. Nasal pressure swings as the measure of inspiratory effort in spontaneously breathing patients with de novo acute respiratory failure. Crit Care. 2022;26(1):70. doi: 10.1186/s13054-022-03938-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Morales-Quinteros L, Schultz MJ, Serpa-Neto A, Antonelli M, Grieco DL, Roca O, et al. Awake prone positioning in nonintubated spontaneous breathing ICU patients with acute hypoxemic respiratory failure (PRONELIFE)-protocol for a randomized clinical trial. Trials. 2022;23(1):30. doi: 10.1186/s13063-021-05991-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.