Abstract
Episodic memory deficits have increasingly been recognized as a cognitive feature of depression. To quantify these deficits and determine how they are moderated by various tasks (e.g., stimulus valence) and participant (e.g., age, depression diagnosis) variables, we conducted a three-level meta-analysis on 995 effect sizes derived from 205 studies with 236 unique comparisons between depressive and control groups on episodic memory measures. Overall, depression was associated with small to moderate deficits in episodic memory, Hedges’ g = −0.36, 95% CI [−0.41 to −0.31]. Effects were larger in older age, in diagnosed compared to subthreshold depression, and in those taking medication for depression; effects did not differ between those with current and remitted symptoms. Stimulus valence moderated the effects, such that depression-related deficits were particularly pronounced for positive and neutral stimuli, but not for negative stimuli. Educational attainment served as a sort of protective factor, in that at higher levels of education, depressed group performance was more similar to that of controls. These findings confirm the episodic memory deficits in depression but highlight the important differences in the size of these deficits across a number of task- and participant-related variables.
Keywords: episodic memory, major depression, subthreshold depression, aging, meta-analysis
Depression is a serious public health issue. In 2019, 19.4 million adults reported experiencing at least one major depressive episode in the past year in the United States alone, and of those, 67.5% were associated with severe impairment in their daily lives (Substance Abuse and Mental Health Services Administration, 2020). Major depressive disorder (MDD) is highly heterogenous (Ostergaard et al., 2011). Impairments resulting from depression can resonate through all areas of individuals’ lives, affecting relationships, work and school performance, and even day-to-day leisure activities (see Papakostas et al., 2004; Sheehan et al., 2017, for reviews). Although much of the research is rightfully focused on the telltale signs of changes in mood and well-being, depression is also often accompanied by impairments in cognitive functioning (see Austin et al., 2001; Cambridge et al., 2018, for reviews). In the present meta-analysis, we focus on one of the more prominent of these impairments, namely, the deficit in episodic memory functioning.
Episodic Memory Deficits in Depression
Episodic memory is the richly detailed memory for not only the events we experience but also the associated details such as the contexts in which the events occur. As is common in the field (e.g., Atkinson & Shiffrin, 1968), we view episodic memory as distinct from working memory or short-term memory, and thus exclude tests for the latter two constructs from our analysis. Likewise, like Tulving (1972, 2002), we consider episodic memory distinct from semantic memory, or memory for general knowledge; thus, we exclude tests for the latter as well. The best measure of episodic memory is retrieval performance after a delay, while episodic encoding is associated with additional neural processes beyond those used in delayed measures, such as processing speed and executive functioning (Casaletto et al., 2017). However, because studies have often used immediate retrieval as a measure of episodic memory encoding, we include these measures in our operationalization of episodic memory in this meta-analysis. Our aim in focusing on episodic memory is not to understate the interconnectedness of episodic memory and other cognitive domains frequently impaired in depression, such as executive functioning (Dotson et al., 2020). An analysis of all of these domains is beyond the scope of a single meta-analysis. However, by better defining the relationship between depression and episodic memory, we hope to encourage further research into common mechanisms that may underly a wider range of cognitive deficits.
Converging evidence suggests that episodic memory may be disrupted in depression (Ahern & Semkovska, 2017; Bora et al., 2013; Burt et al., 1995; Kindermann & Brown, 1997; Lee et al., 2012; McDermott & Ebmeier, 2009; Rock et al., 2014). Previous meta-analyses have consistently found that relative to healthy controls, individuals with depression tend to show reduced memory performance. Researchers have in turn proposed a range of theoretical explanations for impaired memory in depression. For example, recall is generally found to be more affected than is recognition (Bora et al., 2013; Burt et al., 1995), in line with the resource allocation theory. This hypothesis posits that depression, or more specifically, the maladaptive ruminative thinking patterns that are a feature of depression (Nolen-Hoeksema et al., 2008), occupy a portion of the depressed individual’s limited cognitive resources, thereby reducing the resources available to perform other cognitive tasks (Ellis & Ashbrook, 1988; see Gotlib & Joormann, 2010, for review). The claim of the resource allocation view is that, because depression reduces one’s cognitive capacity, the greatest deficits should be observed in more effortful processes, such as generating one’s own retrieval cues as required in recall tasks, resulting in particularly deep deficits in these types of tasks. This is not, however, universally found. Notably, in their meta-analysis, Kindermann and Brown (1997) obtained the opposite pattern: Recognition deficits were larger than those in free, cued, and incidental recall, though this meta-analysis was restricted to studies investigating depression in older adults (sample Mage ≥ 55).
Another possibility is that depression and memory deficits could both arise from a common factor, such as chronic stress. The social signal transduction theory of depression argues that the upregulation of proinflammatory cytokines in response to stressful life events can drive the onset of depressive symptoms (Slavich & Irwin, 2014). At the same time, increased inflammation has been linked to neuro-degeneration and associated memory impairments seen in depression and increasing age (Hurley & Tizabi, 2013). The combined emotional, cognitive, and somatic effects of inflammation would consequently manifest concomitantly. If this were the case, then treatment with anti-inflammatory agents might prove effective at simultaneously lessening the severity of depression and memory deficits.
Depression may also develop in response to cognitive deficits like impaired memory. A recent population-based longitudinal study identified mild cognitive impairment (MCI) as a potential risk factor for the onset of depression, suggesting that declining cognitive function could itself give rise to the negative feelings characteristic of depression (Mirza et al., 2017). Although Mirza and colleagues looked specifically at MCI in individuals 55 and older, it is also possible that younger individuals experience a period of cognitive decline prior to the onset of clinical depression.
The above findings suggest that age is potentially an important factor to consider when exploring memory deficits in depression. In typical aging, greater deficits are often observed for recall than recognition, and this deficit is often ascribed to a decrease in cognitive resources (Danckert & Craik, 2013; Rhodes et al., 2019). Additionally, older adults frequently demonstrate a decreased ability to spontaneously self-initiate effective encoding and retrieval operations. This deficit is often seen as a sign of resource allocation difficulty, brought on by a decrease in overall cognitive resources (see Craik & Rose, 2012, for review)—paralleling the deficit that is also frequently reported in depression (Channon & Green, 1999; Elderkin-Thompson et al., 2007; Hertel, 2000; Joormann et al., 2009; Taconnat et al., 2010). A few meta-analyses have investigated whether age moderates the relationship between depression and memory, with conflicting conclusions. Burt et al. (1995) found that recall and recognition were more impaired earlier in life. Similarly, Kindermann and Brown (1997) found larger depression-related memory impairments in studies that contained individuals younger than age 45. Others (Ahern & Semkovska, 2017; Bora et al., 2013) have not found a modulating effect of age, though Bora et al. (2013) did find that older age of depression onset was associated with more severe verbal memory deficits. One benefit of using a large sample size and a wide age range, as we did in the current meta-analysis, is that we were able to explore the effects of participant age at the time of study and age of depression onset as separate moderating variables. Although the use of cross-sectional data necessarily limits our ability to arbitrate between different causal theories of depression and memory deficits, moderator analyses can help provide preliminary support for one theory over others.
Though several meta-analyses have assessed memory deficits in depression, the characteristics of the samples differ greatly, making it difficult to discern the patterns of these deficits. For example, Ahern and Semkovska (2017) restricted their meta-analysis to studies with first-episode MDD, while Burt et al. (1995) included studies with unipolar and bipolar participants, and Bora et al. (2013) analyzed only studies with euthymic MDD participants. In the current meta-analysis, we excluded bipolar depression, as episodic memory has more often been assessed in unipolar depression in the literature; additionally, this reduced diagnostic variance across studies, allowing us to more clearly delineate the effects of depression proper.
One of the most noteworthy omissions in previous meta-analyses of memory performance in depression is that none have included studies in which cases of subthreshold depression (i.e., depressive symptoms that do not meet clinical criteria for MDD diagnosis) were represented in the samples. In a meta-analysis assessing cognitive control deficits in depression, Dotson et al. (2020) found similar deficits between those with clinical and subthreshold depression in their overall sample; in a subsample where the mean age was 39 or older, those with a clinical diagnosis performed significantly worse than those with subthreshold symptoms. While the effects of clinical versus subthreshold depression on episodic memory have not been previously investigated in a meta-analysis, it is feasible that memory performance may differ as a function of clinical diagnosis due to the reliance of episodic memory on underlying cognitive control processes (also frequently referred to as executive functions), such as attention allocation and information monitoring (see Duarte & Dulas, 2020; Gliebus, 2018, for reviews). Subthreshold depression is quite prevalent in the population, with estimates ranging from 1.4% to 17.2%, and is associated with increased risk of developing major depression (Cuijpers & Smit, 2004; Karsten et al., 2011; Tuithof et al., 2018). Furthermore, while rates of clinical depression decrease with age, rates of subthreshold depression increase (see Polyakova et al., 2014; Szymkowicz et al., 2019, for reviews). For these reasons, among others, it is crucial to understand the extent to which memory deficits observed in MDD are also present in subclinically depressed samples.
That said, it is also worth acknowledging the limitations of a binary distinction between clinical and subthreshold depression, as defined by the Diagnostic and Statistical Manual of Mental Disorders (DSM). Although the DSM has proven a useful means of diagnosing depression over the past several decades, the symptom cutoffs defined therein are necessarily artificial. At the same time, a single measure of DSM-defined symptomatology can belie the heterogeneity of depression across individuals; two individuals may receive the same diagnosis while having very different manifestations of the disorder (Ostergaard et al., 2011). Recent work has suggested that a dimensional classification scheme could better characterize the nature of depression (Hankin, 2019). Such a model might include a broad indicator of psychopathology (e.g., the p factor proposed by Caspi et al., 2014) to account for the overlap between disorders with high comorbidity—such as depression and anxiety disorders—as well as a set of internalizing factors and specific symptoms that separately account for depression (Hankin, 2019).
There are several outstanding questions regarding variables that may impact the relationship between depression and memory. In the following sections, we describe our four main questions and detail how we address each in the current meta-analysis.
Is the Pattern of Episodic Memory Deficits in Depression Consistent Across the Adult Lifespan?
There are a number of reasons to believe that depression-related memory deficits may be magnified in older age. For one, episodic memory is dependent on underlying cognitive control functions, and these functions are impaired in both normal aging and depression. For memories to be encoded accurately, one often needs to selectively attend to relevant information and inhibit irrelevant details, such as conversations of nearby customers when having a conversation with one’s dining partner (James et al., 2016; Kuhl & Chun, 2014). For memory retrieval, one may need to monitor the retrieved details with respect to the current context and decision criteria (e.g., “Did I hear this story from my son or daughter?”; Barredo et al., 2015; Benjamin, 2007). Both aging (Campbell et al., 2012; Jacoby et al., 2005; Kray & Ferdinand, 2014; Rey-Mermet & Gade, 2018) and depression (Snyder, 2013; Wagner et al., 2012; Yang et al., 2017; Zaninotto et al., 2016; Zetsche et al., 2018) have been associated with deficits in these kinds of cognitive control functions, and there is evidence for a synergistic effect such that older adults with MDD show the greatest deficits (Dotson et al., 2020).
Another reason to believe that age and depression may have interactive effects on memory is that similar patterns of neural degeneration have been observed in depression and healthy, as well as pathological, aging. In their comprehensive review of structural changes in healthy and pathological aging, Lockhart and DeCarli (2014) describe a linear decline in prefrontal cortex (PFC) volume with advancing age. Hippocampal volume, on the other hand, remains relatively stable until the sixth decade of life, at which point it decreases rapidly. Volumetric studies of depression have delivered similar results, indicating atrophy in both the hippocampus (Butters et al., 2008; Byers & Yaffe, 2011; Juan & Adlard, 2019; Leonard & Myint, 2009; Videbech & Ravnkilde, 2004) and PFC (Hurley & Tizabi, 2013; Leonard & Myint, 2009). Given the importance of the PFC in cognitive control (see Banich, 2009, for review; Braver, 2012; Gratton et al., 2018) and the key role of the hippocampus in episodic memory (de Flores et al., 2015; Eichenbaum et al., 2007), such declines could contribute to the cognitive deficits observed in depression and aging.
Some longitudinal studies have posited a link between depression and accelerated cognitive aging. For example, depressive symptoms are predictive of higher rates of incident cognitive impairment (Köhler et al., 2010; Wilson et al., 2014); steeper declines in Mini-Mental State Examination (MMSE) scores (Rapp et al., 2011); and worse performance over time on tests of verbal memory, prospective memory, verbal fluency, and attention (Gale et al., 2012). Together, these results suggest that depression may move forward the point at which cognitive declines characteristic of aging appear. In the current meta-analysis, we assess the moderating effect of age to investigate whether episodic memory deficits are consistent across the adult lifespan, or if they increase as a function of age.
What Are the Effects of Valence on Episodic Memory in Depression?
One way in which memory impairments in normal aging are unlike those typically seen in depression is in emotional memory. Specifically, while older adults, relative to younger adults, have been shown to exhibit better memory for positive than negative events (Carstensen & Mikels, 2005; Mather, 2012; Thomas & Hasher, 2006), negative events are typically better remembered in depression (e.g., Marchetti et al., 2018). Mood-congruency effects in memory—wherein an individual’s affective state biases them toward stimuli of the same valence—are well documented in past research (Bower, 1981; Matt et al., 1992; Van Vleet et al., 2019). Depressed participants frequently exhibit impaired memory performance for positive and neutral stimuli both in episodic (Marchetti et al., 2018) and autobiographical (Young et al., 2012) memory tests. The current meta-analysis includes valence as a moderator variable to assess its impact on memory in depression across different task demands.
Are Depression-Related Memory Deficits Tied to One’s Current Depressive State?
If memory impairments in depression are linked to the current depressive state, impairments would be expected to vary as a function of symptom severity regardless of diagnosis, and for memory performance to improve once symptoms have remitted. If, on the other hand, memory deficits are independent of depressive state, one would expect those deficits to be more pronounced in clinically diagnosed depression than in subthreshold depression, and that they would persist following treatment into symptom remission. Currently, these remain open questions and we address them in this meta-analysis in the following ways.
Are Memory Deficits Similar Across Clinical and Subthreshold Depression?
Studies have shown negative associations between total depressive symptoms and gray matter volume in the hippocampus (O’Shea et al., 2018), as well as other areas known to be affected in depression such the PFC (see Besteher et al., 2020, for review). Additionally, like those with clinical depression (Cao et al., 2012), individuals with subthreshold symptoms show weaker functional connectivity between hippocampal and frontal regions compared to healthy controls (Zhu et al., 2014). Research into whether these abnormalities in subthreshold depression translate into episodic memory deficits, and how those deficits compare to those observed in MDD, has yielded inconsistent results. Some studies have indicated that symptom severity in those with subthreshold depression is negatively correlated with memory performance (Dotson et al., 2008; Elderkin-Thompson et al., 2007) and positively correlated with memory bias (Everaert et al., 2013; Marchetti et al., 2018; Reid et al., 2006), such that those with higher severity show better memory for negative relative to positive material. However, other researchers have not shown such correlations (Jermann et al., 2008; Spalletta et al., 2014), and others have shown that subthreshold depression is not associated with impaired episodic memory relative to controls (Airaksinen et al., 2004). The current meta-analysis will be the first to comprehensively evaluate how episodic memory impairments in subclinical depression compare to those associated with MDD.
Do Memory Deficits Persist Into Remission?
While there are well-attested deficits in a range of cognitive control functions in remitted depression (Hasselbalch et al., 2011; Nakano et al., 2008; Paelecke-Habermann et al., 2005; Preiss et al., 2009; Rock et al., 2014; Smith et al., 2006), findings are less consistent for episodic memory. Some past work has reported memory deficits in participants in the euthymic stage of depression, regardless of age (Airaksinen et al., 2006; Preiss et al., 2009; Sheline et al., 1999), while one meta-analysis found deficits were greater in those with remitted late-onset depression (defined as onset after age 50–65, depending on the study) than in those with early-onset (Bora et al., 2013). By contrast, other researchers have reported minimal memory deficits in remitted depressives compared to healthy controls (Rock et al., 2014). Given the mixed results of previous research on patients in the euthymic phase of depression, it remains unclear which elements of episodic memory impairment persist into remission. To address this uncertainty, we considered current depression status as a moderator variable in the present meta-analysis.
Does Memory Improve With Depression Treatment?
As discussed, some studies have suggested that memory impairments in depression are dependent on the current depressive state, such that memory performance improves following successful treatment (Douglas & Porter, 2009; Russo et al., 2015). However, it is unclear whether these improvements vary as a function of the type of treatment. For example, while some have concluded that patients taking antidepressants experience greater cognitive control deficits than unmedicated patients (see Snyder, 2013, for review), the results are inconsistent with respect to memory performance. Some researchers have found poorer memory in unmedicated compared to medicated participants (Burt et al., 1995), others have found medication to be negatively associated with memory (Lee et al., 2012), and some have found no relationship between medication status and memory performance (Ahern & Semkovska, 2017; Kindermann & Brown, 1997). In the current meta-analysis, we investigate the moderating effect of antidepressant use on episodic memory performance.
Are There Factors That Protect Against the Negative Effects of Depression on Memory?
Some studies have found that education may serve as a protective factor: Higher educational attainment is associated with fewer memory deficits among patients with depression (Mackin et al., 2014; Murphy & O’Leary, 2010). However, some researchers have indicated that more years of education predict greater memory deficits in late-life depression (O’Shea et al., 2015), while others have found no significant relationship between education and general cognitive function (Bhalla et al., 2006; Siddarth et al., 2021). Some evidence has indicated an overall advantage for females on episodic memory tasks (see Asperholm et al., 2019 for meta-analysis and review); however, women are also twice as likely as men to experience MDD and report higher severity of symptoms (see Altemus et al., 2014, for review). Previous meta-analyses have not found a moderating effect of gender on memory in remitted depression (Bora et al., 2013) or on cognitive control in current depression (Snyder, 2013). Whether gender moderates depressive effects on episodic memory specifically has yet to be assessed in a meta-analysis. The current meta-analysis aims to determine whether factors like education and gender help to buffer against the negative effects of depression on episodic memory performance.
The Current Meta-Analysis
The last meta-analysis to explore memory impairments in depressed adults across a variety of memory tasks was published over two decades ago (Burt et al., 1995). Meta-analyses published since then have investigated deficits in cognitive functioning, not specifically episodic memory, across a narrow range of neuropsychological tests in specific patient populations, such as those in their first episode of clinically diagnosed MDD (Ahern & Semkovska, 2017; Bora et al., 2013; Burt et al., 1995; Lee et al., 2012; McDermott & Ebmeier, 2009; Rock et al., 2014). The goal of the current meta-analysis is to address the open questions outlined above by identifying both task-related (e.g., type of memory task, type of material used, stimuli valence) and participant (e.g., age, diagnosis) variables that moderate the effects of depression on episodic memory. To do this, we conducted a three-level meta-analysis using 995 effect sizes from 205 studies with 236 unique comparisons between depressive and healthy-control groups. Our meta-analysis is the first to use a three-level model to assess episodic memory deficits in depression. One reason to prefer multilevel modeling of effect sizes is that this analysis allows for the inclusion of multiple effect sizes for each study (Cheung, 2014), as opposed to averaging these effects as is necessary in traditional meta-analysis. Episodic memory studies often contain multiple effect sizes (e.g., measures of immediate and delayed recall and recognition), and the use of multilevel modeling then improves the ability to examine our moderators of interest.
Method
The annotated data set and R code used in this meta-analysis are available on the Open Science Framework: https://osf.io/t8ynd/?view_only=05c342695faa476795218c6a31ecfec5
Selection of Studies
We conducted a systematic review and meta-analysis following the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines (Moher et al., 2009). Two of the authors independently searched the electronic databases Pubmed and PsycINFO from the year 1980 to November 2020 for studies that investigated the association between depression and episodic memory. The year 1980 was selected because this was the year the DSM-III (American Psychiatric Association, 1980) introduced the term “major depressive disorder” and outlined the diagnostic criteria, which have remained essentially the same in newer editions. Limiting the search to human population, adults ages 18 and older, we used the terms (a) “depress*” or “MDD,” and (b) “episodic” in combination with “memory,” “encoding,” or “retrieval”; “recollection”; “recall”; “recognition”; “source memory”; “subsequent memory”; or “associative memory” to search titles and abstracts of published studies. The asterisk allows for the inclusion of alternate endings (e.g., searching for “depress*” would return matches for “depression,” “depressive,” and “depressed”). A subsequent search was conducted by examining the references of the eligible articles to identify additional studies. Our database search was not limited to studies published in the English language.
Though we included clinical trials in our database searches, we also searched clinicaltrials.gov for completed studies with results of depression (or depressive disorders or depressed mood) in adults involving memory. To capture unpublished literature, we searched ProQuest Dissertations and Theses Global using the same search terms described above. Additionally, we made calls for unpublished data in APA Division 20: Adult Development and Aging, APA Division 40: Society for Clinical Neuropsychology, and the International Neuropsychological Society.
Eligibility was determined by examining first the title and abstract then the full text of each article. Reviews and meta-analyses were excluded. Inclusion criteria were as follows: (a) use of sample diagnosed with unipolar depression (either in a current or remitted state) or with depressive symptoms as measured by a standardized questionnaire; (b) inclusion of a healthy, control comparison group; and (c) reporting of at least one objective measure of episodic memory performance for both the depressed and control groups (means and standard deviations, or sufficient statistical data such as F or t statistics to be able to calculate effect sizes). Longitudinal studies were included if baseline assessments were reported for depressed and control groups; only these baseline assessments were used in the analyses. Because major medical or psychological comorbidities could have a confounding influence on memory performance, we excluded studies in which any member of the depressed or control sample was reported to have dementia, Alzheimer’s disease, MCI, brain injury, Parkinson’s disease, history of stroke, human immunodeficiency virus (HIV), cancer, diabetes, multiple sclerosis, attention deficit hyperactivity disorder (ADHD), schizophrenia, alcohol or substance abuse, personality disorders, posttraumatic stress disorder (PTSD), bipolar disorder, psychotic symptoms of depression, or postpartum depression. Generalized anxiety was not exclusionary due to the high comorbidity of depression and anxiety. Studies conducted in residential care facilities and inpatient settings were included if the sample met all other criteria. In cases where data from the same or overlapping groups of participants were reported in separate records, the most recently published study and/or that with the largest sample size was retained to prevent duplication of effect sizes. The interrater agreement for the inclusion/exclusion categorization of records was .96. Disagreements in decisions to include or exclude particular records were discussed and reconciled.
After deleting duplicate records, 2,938 records were screened (2,355 published and 583 unpublished). Of these, 533 were deemed eligible for the full-text search based on titles and abstracts. When full texts of potentially eligible records were not available online (72 in total), we attempted to contact the corresponding author; however, current author contact information could not be located for 49 of the records (particularly for dissertations, theses, and older articles). We received full texts from 15 of the 23 authors contacted. Corresponding authors were also contacted when records lacked sufficient detail to determine a record’s eligibility (e.g., when samples from separate records were suspected to overlap). Five out of six authors responded to requests for this information. Through this search process we identified 205 records (176 published, 29 unpublished) that met all criteria (see Figure 1).
Figure 1. Flowchart Illustrating the Stages of Study Selection for Inclusion in the Meta-Analysis.
Data Extraction and Variable Coding
Two of the authors independently extracted data from eligible records and entered those into a spreadsheet created by the first author. All data were then compared and discrepancies were noted. The interrater agreement for data extraction and variable coding was .97. The first author referenced the full text of the records to rectify these discrepancies.
The following variables, when available, were extracted for each record: publication details, study characteristics, place of study, sample recruitment site (e.g., local communities, outpatient settings), study design (e.g., cross-sectional), diagnostic status (i.e., the proportion of individuals in the depressed group who had received a formal diagnosis of MDD1), depression status (the proportion of individuals in the depressed group who were currently depressed vs. remitted, see Footnote 1), symptom severity (name of depression questionnaire and score of individuals in the depressed group on that assessment), episode (the proportion of individuals in the depressed group with recurrent depression vs. first-episode depression, see Footnote 1), age of onset, time since diagnosis, medication status (the proportion of individuals in the depressed group who were taking medication for depression, see Footnote 1), sample size, age range, mean age for depressed and control groups, sex/gender2 (the proportion of females in the sample), mean years of education, intelligence quotient (IQ) information (IQ test name and mean score for depressed and control groups), and whether groups were matched for age and education. When only age of onset or time since diagnosis was provided, one was calculated from the other using the mean age of individuals in the depression group. Table S1 (Supplemental Material) presents these details for each study included in the meta-analysis.
Episodic memory measures were coded to indicate the type of memory test (recall, recognition); the presence of a delay between study and test (immediate, short delay, long delay); for recall: whether the task was cued (free, cued); the stimulus materials (words, high verbalizable stimuli, low verbalizable stimuli); the valence of the stimuli (neutral, positive, negative); the memory outcome measure, accuracy (e.g., hits, items correctly recalled), discrimination (d′, Pr), commission errors (e.g., false alarms, intrusions); and, when applicable, whether the measure assessed familiarity or recollection. For test delay, immediate was defined as memory tests that occurred right after encoding, with no break or intervening task, for example, list learning trials on the California Verbal Learning Test (CVLT). Long delay was defined as memory tests that occurred more than 20 min after encoding, consistent with neuropsychological tests (Delis et al., 2000; Wechsler, 2009). Short delay was defined as test delays that fell between immediate and long delay—after a brief intervening task (e.g., a mathematical distractor task as in MacQueen et al., 2003, or recall of a distractor word list like in the CVLT) or after a short break (the shortest being the 3-min delay in the Rey–Osterrieth complex figure task). For stimulus material, high verbalizable stimuli were defined as those that lend themselves toward verbal descriptions that are fairly consistent across participants, for example, pictures of objects, images from the International Affective Picture System (IAPS); low verbalizable stimuli were defined as those that cannot be easily described verbally, such as abstract designs or spatial locations (Golby et al., 2001). For valence, positive, negative, and neutral were defined using the classifications provided by the authors (see Supplemental Material, Table S2 for classification methods).
It should be noted that while Voyer et al. (2021) argue that Trial 1 (or List 1) on the CVLT reflects short-term or working memory rather than episodic memory, others do not (e.g., Chapman et al., 2006; Lundervold et al., 2019; Raffard et al., 2016). The main reason is that the list length for the CVLT far exceeds the typical short-term memory span, and it would tap primary or short-term memory only when participants elect to start their recall at the end of the list, which they are not instructed to do. Thus, while Trial 1 may be contaminated with short-term memory, it is unlikely to be a pure measure of this construct. We tested this contamination empirically by examining the 10 studies that provided details for both Trial 1 recall and final trial recall by dummy coding this variable and testing it as a moderator of the effect sizes. Results showed a nonsignificant effect of trial (β = −0.10, p = .214). That said, it is still important to note that the boundary between short-term/working memory and episodic memory is still unclear and not necessarily agreed upon, and the two may sometimes be difficult to distinguish (Asperholm et al., 2019).
The measures listed above were investigated as task moderators for the relationship between memory and depression. Note that for all moderator variables, effect sizes were coded for one level of the variable or the other (e.g., recall OR recognition for type of memory test, immediate OR short delay OR long delay for test delay). If a study reported just a single estimate for a battery that spanned multiple moderators of interest—for instance, a series of tests that included both recognition and recall but was reported as a single z-score rather than a separate score for recall and recognition—this estimate was not included in the analysis of that particular moderator, but it was included in all other relevant analyses.
All relevant effect sizes were included, but redundant measures were excluded (e.g., if a study reported performance on individual learning trials for a word-list memory test like the CVLT, the effect sizes for each learning trial were included; if a cumulative score was additionally reported, this measure was used in place of the individual learning trial scores). Estimates of memory bias or strategies (e.g., semantic clustering) were not included. If a study reported both index scores (e.g., verbal memory, immediate memory) and scores on individual subtests from the Wechsler Memory Test, effect sizes were calculated using the subtest scores to more precisely code the variables as outlined above (e.g., recall, immediate). Table S3 (Supplemental Material) presents effect sizes and associated task moderator categorizations for each study included in the meta-analysis.
Meta-Analytic Approach
Means and standard deviations were extracted for all episodic memory measures. Effect sizes for the memory measures reported in each study were calculated as Cohen’s d to compare performance of depressed participants to controls. Cohen’s d was calculated as (M1 − M2)/SDpooled, where M1 is the mean memory performance of the depressed group, M2 is the mean memory performance of the control group, and SDpooled is the pooled standard deviation of the two groups. For error measures in which higher scores indicate worse performance (e.g., false alarms), mean performance of the depressed group was subtracted from mean performance of the control group. Thus, more negative effect sizes reflect lower memory performance among depressed individuals relative to healthy controls. When means and standard deviations were not available, F or t statistics or d values were extracted. Effect sizes were estimated from F or t statistics using an online effect size calculator (Wilson, 2001; Wilson & Lipsey, 2001). In cases where data were presented only in a figure, the WebPlotDigitizer software (Rohatgi, 2019) was used to extract the relevant data. Corresponding authors were contacted to obtain missing information, particularly that which was necessary to calculate effect size. Fourteen of 38 authors responded to these requests. When such information could not be obtained, effect sizes were estimated (this was the case in 56 of the 995 included effect sizes, or 5.6%). In cases where the direction of the effect was known (e.g., when means but no measures of dispersion were provided), we estimated using conservative p values; p = .05 was used when the effect was labeled as significant, and p = .5 when it was labeled as nonsignificant, as in Coles et al. (2019; 27 of 56). If significance information was not provided but the direction of the effect was known, we assumed the effect was nonsignificant and used p = .5 for our calculations (15 of 56). In cases where the direction was not known, the effect size was entered as zero (14 of 56). Hedges’ g correction for small-sample bias (Hedges & Olkin, 1985) was applied as g = d[1 − (3/4 df − 1)], where df represents the degrees of freedom for the entire sample (i.e., the total number of participants in both patient and healthy-control groups, minus two).
We conducted a three-level meta-analysis using the metafor package for R (Viechtbauer, 2010), augmented with the clubSandwich package (Pustejovsky, 2021). Additionally, the dplyr (Wickham et al., 2020) package was used for data manipulation. The three-level approach by itself (here implemented in the rma.mv() function) is more explicitly described in Cheung (2014). In a three-level random effects model, three sources of variance are modeled: sampling variance of the effect sizes, variance between effect sizes from the same study, and variance between effect sizes from different studies. A key advantage of this approach is that it allows one to fit models with dependent effect sizes nested within studies, allowing researchers to use all available information from studies with nonindependent effect sizes. This is in contrast to traditional two-level models, in which researchers may deal with dependencies by averaging effect sizes within a study, which may lower the statistical power because some information in the effect sizes is lost through aggregation; alternatively, they may select only one effect size per study, which in turn limits the questions that can be addressed by the meta-analysis since some effect sizes are excluded. By analyzing individual effect sizes, the three-level model allows one to investigate variation between effect sizes and to test moderator variables to explain this variation while accounting for overlap in information that is contributed by effect sizes from the same study, therefore avoiding artificial inflation of power and an increase in Type I error. To deal with possible dependencies within studies, we deployed cluster-robust variance estimation with small-sample corrections through the coef_test() function in the clubSandwich package as described in Pustejovsky and Tipton (2021).
For each analysis, mean effect size and 95% confidence intervals were computed. Heterogeneity of effect sizes was assessed using the Q statistic, similar to traditional, two-level meta-analyses. When the Q statistic is not significant, it suggests studies differ only as a result of sampling error at the subject level; when the Q statistic is significant, it suggests factors in addition to sampling error are needed to account for variation. A second measure of heterogeneity, I2, describes the percentage of variation across studies that is due to heterogeneity rather than chance, and is calculated as 100% × (Q − df)/Q (Higgins & Thompson, 2002). Unlike traditional methods, however, the I2 statistic, which indexes the degree of heterogeneity, is split over levels. I2 at Level 2 (I(2)2) and Level 3 (I(3)2) indicate the proportion of the total variation of the effect sizes due to Level 2 and Level 3 between-study heterogeneity. For multilevel regression models, τ2 at Level 2 (τI(2)2) indicates the heterogeneity of effects due to differences between measures, while τ2 at Level 3 (τI(3)2) indicates the heterogeneity of effect sizes across studies after controlling for the different types of measures at Level 2. R2 indicates the proportion of estimated heterogeneity at Level 2 (R(2)2) and Level 3 (R(3)2) explained by the predictors.
Moderator Analyses
We investigated a number of moderators as possible sources of between-studies heterogeneity using metaregression analyses (using the maximum likelihood estimate method). The task moderators we examined were test type (recall, recognition), recall type (free, cued), test delay (immediate, short delay, long delay), material (words, high verbalizable stimuli, low verbalizable stimuli), valence (positive, neutral, negative), memory outcome (accuracy, discrimination, commission errors), and memory process (familiarity, recollection). The participant moderators were age, sex, years of education, IQ, diagnostic status, depression status, age of depression onset, time since diagnosis, episode (first episode vs. recurrent), early versus late-onset, symptom severity, medication status, matched age, matched education, and recruitment site. Only studies that reported education as a continuous variable (or provided enough information for years of education to be estimated) were included in the respective analysis. Recruitment site was assessed by comparing samples from patient settings (inpatient, outpatient, and randomized clinical trials) with those from community and university settings. Only studies that recruited depression groups exclusively from one of these two types of settings were included in the respective analysis (e.g., if a study recruited depressed participants from outpatient clinics and through community advertisements, it was not included in the analysis of recruitment site; however, if a study recruited from both inpatient and outpatient settings, it was included and coded for the “patient” category). Studies that did not report information about the moderator of interest were excluded from the respective analyses. Finally, to examine whether published studies had larger effects than unpublished studies, we included a moderator for publication status. Categorical moderators were dummy coded and entered into metaregression equations. For categorical moderators with more than two levels (e.g., valence), two of the levels were used as baselines in separate metaregression equations to allow for comparisons between all levels (e.g., neutral as baseline in one model to allow for comparisons with positive and negative; positive as baseline in another model to allow for comparison with negative).
Results
Descriptive Information
Effect sizes were calculated for each depression subsample with respect to a single healthy-control group, resulting in 236 unique comparisons from 205 articles with a total of 995 effect sizes. The included articles ranged in publication year from 1982 to 2020. These articles reflect research conducted across 30 countries: the United States (33.7%); the United Kingdom (9.8%); Germany (9.3%); Canada (8.3%); France (3.9%); Brazil and the Netherlands (3.4% each); Spain (2.9%); New Zealand, Sweden, and Switzerland (2.4% each); China, Japan, and Taiwan (2.0% each); Hungary and Norway (1.5% each); Belgium, Greece, Italy, and South Korea (1.0% each); Australia, Colombia, Czech Republic, Denmark, Hong Kong, Ireland, Israel, Portugal, Serbia, and Turkey (0.5% each). It was unclear where two studies were conducted. Included studies were published in English, Spanish, French, and Hungarian. The studies included a total of 7,900 depressed participants and 10,121 healthy controls. See Table S4 (Supplemental Material) for descriptive statistics for the sample and moderator variables and the effect size distribution across moderators; See Table 1 for descriptive statistics for continuous moderators across the levels of categorical moderators.
Table 1.
Continuous Across Categorical Moderator Characteristics for Studies Included in the Meta-Analysis
| Continuous moderator | M | Range | j | M | Range | j | M | Range | j |
|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||
| Test type | Recall | Recognition | |||||||
|
| |||||||||
| Age | 47.6 | 18.2–92.3 | 180 | 47.5 | 18.5–92.3 | 106 | |||
| Sex | .680 | .2–1 | 174 | .655 | .2–1 | 100 | |||
| Education | 12.7 | 3.9–17.4 | 113 | 12.5 | 7.1–16.3 | 68 | |||
| Premorbid IQ | 108.2 | 78.9–123.1 | 45 | 108.4 | 90.6–123.1 | 32 | |||
| Diagnostic status | .845 | 0–1 | 188 | .820 | 0–1 | 110 | |||
| Depression status | .807 | 0–1 | 188 | .822 | 0–1 | 110 | |||
| Medication status | .455 | 0–1 | 132 | .380 | 0–1 | 81 | |||
| Symptom severity | 15.5 | .2–33 | 144 | 15.3 | 1.5–32.8 | 82 | |||
| Episode | .628 | 0–1 | 64 | .608 | 0–1 | 36 | |||
| Time since diagnosis | 135.6 | 1.7–477.6 | 57 | 137.7 | 7.1–477.6 | 35 | |||
| Age of depression onset | 37.7 | 15.3–74.1 | 57 | 34.2 | 15.3–71.3 | 35 | |||
| Onset (LOD vs. EOD) | .572 | 0–1 | 28 | .474 | 0–1 | 11 | |||
|
| |||||||||
| Recall type | Cued | Free | |||||||
|
| |||||||||
| Age | 46.3 | 21.1–83.3 | 37 | 47.9 | 18.2–92.3 | 168 | |||
| Sex | .674 | .3–1 | 37 | .676 | .2–1 | 161 | |||
| Education | 13.7 | 7.2–16.0 | 26 | 12.7 | 3.9–17.4 | 108 | |||
| Premorbid IQ | 109.1 | 95.3–117.0 | 13 | 108.3 | 78.9–123.1 | 41 | |||
| Diagnostic status | .873 | 0–1 | 40 | .850 | 0–1 | 173 | |||
| Depression status | .909 | 0–1 | 40 | .791 | 0–1 | 173 | |||
| Medication status | .383 | 0–1 | 31 | .438 | 0–1 | 122 | |||
| Symptom severity | 17.7 | 2.4–28.5 | 31 | 15.2 | .2–33.0 | 132 | |||
| Episode | .551 | 0–1 | 13 | .614 | 0–1 | 60 | |||
| Time since diagnosis | 164.1 | 1.7–477.6 | 18 | 132.2 | 1.7–477.6 | 52 | |||
| Age of depression onset | 34.8 | 15.8–74.1 | 18 | 37.9 | 15.3–74.1 | 52 | |||
| Onset (LOD vs. EOD) | .530 | 0–1 | 7 | .582 | 0–1 | 27 | |||
|
| |||||||||
| Test delay | Immediate | Short delay | Long delay | ||||||
|
| |||||||||
| Age | 49.2 | 18.2–92.3 | 135 | 44.9 | 18.5–84.9 | 88 | 49.5 | 19.5–76.5 | 104 |
| Sex | .662 | .2–1 | 127 | .679 | .2–1 | 87 | .667 | .2–1 | 102 |
| Education | 12.6 | 5.6–17.4 | 89 | 12.7 | 3.9–16.3 | 53 | 13.0 | 5.6–16.5 | 74 |
| Premorbid IQ | 107.9 | 78.9–123.1 | 37 | 109.7 | 90.6–119.9 | 20 | 107.8 | 78.9–123.1 | 34 |
| Diagnostic status | .873 | 0–1 | 139 | .777 | 0–1 | 93 | .889 | 0–1 | 108 |
| Depression status | .791 | 0–1 | 139 | .883 | 0–1 | 93 | .769 | 0–1 | 108 |
| Medication status | .431 | 0–1 | 106 | .408 | 0–1 | 58 | .488 | 0–1 | 86 |
| Symptom severity | 15.6 | .2–32.8 | 109 | 15.9 | 1.5–33.0 | 69 | 15.0 | 1.2–31.2 | 90 |
| Episode | .646 | 0–1 | 50 | .627 | 0–1 | 29 | .602 | 0–1 | 47 |
| Time since diagnosis | 127.5 | 4.7–352.0 | 44 | 152.9 | 1.7–477.6 | 24 | 140.4 | 1.7–477.6 | 40 |
| Age of depression onset | 38.3 | 17.6–74.1 | 44 | 31.3 | 15.8–71.3 | 24 | 36.8 | 15.8–64.8 | 40 |
| Onset (LOD vs. EOD) | .560 | 0–1 | 19 | .496 | 0–1 | 11 | .534 | 0–1 | 19 |
|
| |||||||||
| Stimulus material | Words | High verbalizable | Low verbalizable | ||||||
|
| |||||||||
| Age | 46.7 | 18.2–92.3 | 190 | 50.7 | 19.9–92.3 | 42 | 55.0 | 32.0–76.4 | 44 |
| Sex | .666 | .2–1 | 186 | .664 | .2–1 | 40 | .663 | .2–1 | 42 |
| Education | 12.8 | 3.9–17.4 | 119 | 11.5 | 6.4–16.2 | 26 | 12.2 | 5.6–15.8 | 34 |
| Premorbid IQ | 108.5 | 78.9–123.1 | 48 | 105.2 | 78.9–123.1 | 12 | 104.9 | 78.9–119.9 | 19 |
| Diagnostic status | .835 | 0–1 | 200 | .736 | 0–1 | 44 | .994 | .812–1 | 44 |
| Depression status | .824 | 0–1 | 200 | .849 | 0–1 | 44 | .796 | 0–1 | 44 |
| Medication status | .428 | 0–1 | 139 | .397 | 0–1 | 30 | .527 | 0–1 | 37 |
| Symptom severity | 15.5 | .2–33.0 | 152 | 16.7 | 3.1–32.8 | 30 | 17.2 | 1.2–32.8 | 36 |
| Episode | .609 | 0–1 | 66 | .564 | 0–1 | 12 | .548 | 0–1 | 23 |
| Time since diagnosis | 136.0 | 1.7–477.6 | 59 | 63.1 | 7.1–160.0 | 7 | 118.9 | 4.7–316.8 | 21 |
| Age of depression onset | 37.1 | 15.3–74.1 | 59 | 38.7 | 23.2–71.3 | 7 | 41.4 | 25.0–64.8 | 21 |
| Onset (LOD vs. EOD) | .572 | 0–1 | 28 | .517 | 0–1 | 5 | .561 | 0–1 | 13 |
|
| |||||||||
| Valence | Neutral | Positive | Negative | ||||||
|
| |||||||||
| Age | 47.9 | 18.5–92.3 | 192 | 36.6 | 18.2–84.9 | 72 | 35.8 | 18.2–84.9 | 81 |
| Sex | .665 | .2–1 | 186 | .724 | .3–1 | 69 | .709 | .3–1 | 77 |
| Education | 12.7 | 3.9–17.4 | 129 | 13.2 | 8.1–17.4 | 27 | 13.2 | 8.1–17.4 | 28 |
| Premorbid IQ | 108.7 | 78.9–123.1 | 54 | 104.1 | 90.6–113.0 | 8 | 106.5 | 90.6–118.4 | 10 |
| Diagnostic status | .850 | 0–1 | 200 | .758 | 0–1 | 75 | .696 | 0–1 | 86 |
| Depression status | .829 | 0–1 | 200 | .853 | 0–1 | 75 | .868 | 0–1 | 86 |
| Medication status | .440 | 0–1 | 150 | .436 | 0–1 | 42 | .417 | 0–1 | 44 |
| Symptom severity | 15.9 | .2–33.0 | 149 | 15.4 | 1.5–29.5 | 60 | 14.9 | 1.5–29.5 | 69 |
| Episode | .635 | 0–1 | 70 | .600 | 0–1 | 14 | .560 | 0–1 | 15 |
| Time since diagnosis | 133.8 | 1.7–477.6 | 65 | 99.3 | 22.0–230.6 | 17 | 99.3 | 22.0–230.6 | 17 |
| Age of depression onset | 36.2 | 15.3–74.1 | 65 | 28.1 | 15.3–56.0 | 17 | 28.1 | 15.3–56.0 | 17 |
| Onset (LOD vs. EOD) | .554 | 0–1 | 28 | 1 | — | 2 | 1 | — | 2 |
|
| |||||||||
| Memory outcome | Accuracy | Discrimination | Commission errors | ||||||
|
| |||||||||
| Age | 46.6 | 18.1–92.3 | 208 | 41.3 | 19.4–84.9 | 32 | 44.3 | 18.2–92.3 | 32 |
| Sex | .665 | .2–1 | 186 | .718 | .5–1 | 30 | .652 | .3–1 | 34 |
| Education | 12.7 | 3.9–17.4 | 129 | 13.3 | 10.2–15.9 | 19 | 12.5 | 8.4–16.0 | 15 |
| Premorbid IQ | 108.7 | 78.9–123.1 | 54 | 108.8 | 93.5–118.4 | 14 | 106.7 | 95.3–117.0 | 9 |
| Diagnostic status | .850 | 0–1 | 200 | .713 | 0–1 | 32 | .699 | 0–1 | 38 |
| Depression status | .829 | 0–1 | 200 | .843 | 0–1 | 32 | .958 | 0–1 | 38 |
| Medication status | .440 | 0–1 | 150 | .363 | 0–1 | 21 | .375 | 0–1 | 23 |
| Symptom severity | 15.9 | .2–33.0 | 149 | 15.2 | 2.4–27.5 | 27 | 17.6 | 7.0–28.5 | 24 |
| Episode | .635 | 0–1 | 70 | .467 | 0–1 | 11 | .677 | .5–1 | 4 |
| Time since diagnosis | 133.8 | 1.7–477.6 | 65 | 118.1 | 4.7–477.6 | 13 | 140.6 | 30.7–205.3 | 12 |
| Age of depression onset | 36.2 | 15.3–74.1 | 65 | 28.9 | 15.8–42.3 | 13 | 29.9 | 18.9–41.8 | 12 |
| Onset (LOD vs. EOD) | .554 | 0–1 | 28 | 0 | — | 1 | — | — | 0 |
|
| |||||||||
| Memory process | Familiarity | Recollection | |||||||
|
| |||||||||
| Age | 34.2 | 19.9–47.1 | 9 | 35.5 | 19.9–47.3 | 10 | |||
| Sex | .685 | .571–.813 | 10 | .677 | .571–.813 | 11 | |||
| Education | 14.7 | 13.8–15.5 | 4 | 14.7 | 13.8–15.5 | 4 | |||
| Premorbid IQ | 113.6 | 110.5–118.4 | 6 | 114.0 | 110.5–118.4 | 7 | |||
| Diagnostic status | .5 | 0–1 | 10 | .545 | 0–1 | 11 | |||
| Depression status | .884 | 0–1 | 10 | .894 | 0–1 | 11 | |||
| Medication status | .536 | 0–1 | 6 | .459 | 0–1 | 7 | |||
| Symptom severity | 13.9 | 10.3–19.1 | 9 | 14.7 | 10.3–21.6 | 10 | |||
| Episode | .750 | 0–1 | 4 | .726 | 0–1 | 5 | |||
| Time since diagnosis | 133.3 | 25.2–241.2 | 4 | 133.3 | 25.2–241.2 | 4 | |||
| Age of depression onset | 28.0 | 24.8–35.8 | 4 | 28.0 | 24.8–35.8 | 4 | |||
| Onset (LOD vs. EOD) | — | — | 0 | — | — | 0 | |||
|
| |||||||||
| Publication status | Unpublished | Published | |||||||
|
| |||||||||
| Age | 37.5 | 18.2–84.9 | 34 | 47.4 | 18.5–92.3 | 190 | |||
| Sex | .739 | .3–1 | 30 | .663 | .2–1 | 190 | |||
| Education | 13.1 | 10.7–16.0 | 9 | 12.7 | 3.9–17.4 | 127 | |||
| Premorbid IQ | 113.6 | 106.0–120.8 | 6 | 108.2 | 78.9–123.1 | 49 | |||
| Diagnostic status | .518 | 0–1 | 37 | .867 | 0–1 | 199 | |||
| Depression status | .915 | 0–1 | 37 | .824 | 0–1 | 199 | |||
| Medication status | .245 | 0–.706 | 16 | .463 | 0–1 | 150 | |||
| Symptom severity | 15.0 | 1.5–20.3 | 27 | 15.9 | .2–33.0 | 152 | |||
| Episode | .587 | .3–1 | 7 | .632 | 0–1 | 68 | |||
| Time since diagnosis | 85.3 | 74.6–95.9 | 2 | 133.9 | 1.7–477.6 | 65 | |||
| Age of depression onset | 23.3 | 22.4–24.2 | 2 | 36.7 | 15.3–74.1 | 65 | |||
| Onset (LOD vs. EOD) | .320 | — | 1 | .578 | 0–1 | 28 | |||
|
| |||||||||
| Recruitment site | Community/university setting | Patient setting | |||||||
|
| |||||||||
| Age | 36.9 | 18.2–83.3 | 58 | 48.3 | 22.6–76.5 | 114 | |||
| Sex | .686 | .3–1 | 61 | .666 | .2–1 | 109 | |||
| Education | 13.8 | 8.7–16.5 | 20 | 12.1 | 3.9–16.1 | 82 | |||
| Premorbid IQ | 113.2 | 104.1–123.1 | 9 | 106.8 | 78.9–117.0 | 31 | |||
| Diagnostic status | .450 | 0–1 | 69 | .975 | 0–1 | 114 | |||
| Depression status | .841 | 0–1 | 69 | .838 | 0–1 | 114 | |||
| Medication status | .201 | 0–1 | 34 | .585 | 0–1 | 92 | |||
| Symptom severity | 13.4 | 1.5–33.0 | 49 | 17.5 | 1.2–31.2 | 90 | |||
| Episode | .699 | .5–1 | 17 | .577 | 0–1 | 37 | |||
| Time since diagnosis | 146.0 | 63.6–330.0 | 10 | 110.7 | 1.7–352.0 | 40 | |||
| Age of depression onset | 35.1 | 15.3–56.4 | 10 | 38.2 | 18.9–74.1 | 40 | |||
| Onset (LOD vs. EOD) | .570 | —a | 4 | .621 | 0–1 | 17 | |||
Note. j = number of unique depression-control group comparisons. All continuous moderators are based on information from depression groups. Diagnostic status = proportion diagnosed with major depressive disorder. Depression status = proportion experiencing current (as opposed to remitted) symptoms of depression. Episode = proportion with recurrent episodes of depression (as opposed to first-episode depression). Onset = proportion with late-onset depression (LOD; onset after age ∼50) relative to early-onset depression (EOD). Medication = proportion taking medication for depression. If a study only reported Age of Depression Onset, Time Since Diagnosis was calculated using the mean age (and vice versa).
Range is not reported because the estimated onset was used in all four studies. That is, the exact proportion of participants with LOD versus EOD could not be calculated for these studies, so the proportion was estimated using the mean proportion from all other studies that reported data on this variable.
Overall Effect and Publication Bias
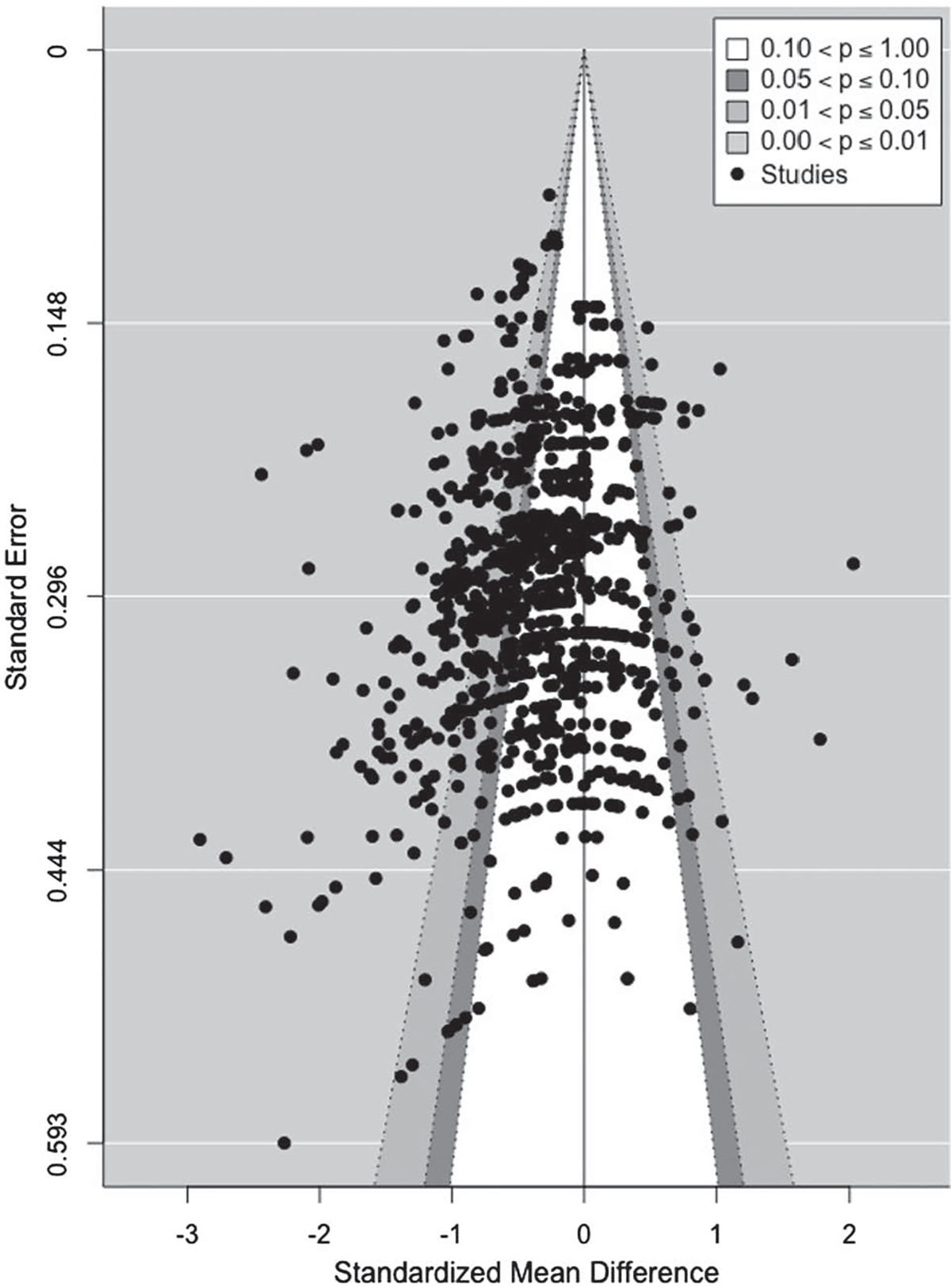
The need for a three-level model was assessed by comparing fit with that of a two-level model. The difference in fit was significant, χ2 = 1,597.74, p < .0001, indicating the three-level model was a better fit for the data. The three-level model yielded an average effect size of −0.36, 95% CI [−0.41 to −0.31]; p < .0001). Heterogeneity was significant, Q(994) = 3,524.93, p < .0001; τ(2)2 = 0.09, p < .0001; τ(3)2 = 0.11, p < .0001; I(2)2 = 0.34 and I(3)2 = 0.41), suggesting the effect sizes were highly variable and thus indicating the need for moderator analyses. Regression analysis on the funnel plot (Figure 2) was conducted using an Egger test appropriate for multilevel meta-analysis (Rodgers & Pustejovsky, 2020). Both the Egger sandwich test and the multi-level meta-analysis (MLMA) Egger test yielded nonsignificant slopes (p = .66 and .79, respectively, using the modified measure of precision described in Pustejovsky & Rodgers, 2019), indicating no evidence for selective reporting.
Figure 2. Funnel Plot of Standard Error as a Function of Effect Size (Standardized Mean Difference Between Depression and Control Groups).
Task Moderators
Table 2 contains effect size estimates for each level of each task moderator and the accompanying moderator analyses. Our analyses show that depressed individuals performed worse than controls across all measures except for familiarity and negatively valenced stimuli.
Table 2.
Task Moderator Analyses
| Moderator and levels | j | k | g | β | 95% CI | p | R (2) 2 | R (3) 2 | Q | τ(2)2 | τ(3)2 |
|---|---|---|---|---|---|---|---|---|---|---|---|
|
| |||||||||||
| Test type (recognition vs. recall) | 233 | 988 | — | −0.17 | [−0.27, −0.06] | .002 | .058 | .007 | 3504.2* | 0.08* | 0.11* |
| Recognition | 110 | 342 | −0.24 | — | [−0.32, −0.16] | — | — | — | — | — | — |
| Recall | 188 | 646 | −0.41 | — | [−0.47, −0.34] | — | — | — | — | — | — |
| Recall type (cued vs. free) | 186 | 643 | — | −0.04 | [−0.15, 0.06] | .427 | .009 | .000 | 2429.9* | 0.09* | 0.13* |
| Cued | 40 | 80 | −0.37 | — | [−0.48, −0.25] | — | — | — | — | — | — |
| Free | 173 | 563 | −0.41 | — | [−0.48, −0.35] | — | — | — | — | — | — |
| Test delay | 217 | 921 | — | — | — | — | .000 | .004 | 3238.9* | 0.09* | 0.10* |
| Immediate vs. short delay | 194 | 660 | — | 0.02 | [−0.06, 0.09] | .679 | — | — | — | — | — |
| Immediate vs. long delay | 170 | 644 | — | 0.01 | [−0.04, 0.07] | .665 | — | — | — | — | — |
| Short delay vs. long delay | 168 | 538 | — | −0.004 | [−0.08, 0.07] | .922 | — | — | — | — | — |
| Immediate | 140 | 380 | −0.38 | — | [−0.43, −0.31] | — | — | — | — | — | — |
| Short delay | 92 | 277 | −0.35 | — | [−0.43, −0.28] | — | — | — | — | — | — |
| Long delay | 108 | 261 | −0.36 | — | [−0.42, −0.30] | — | — | — | — | — | — |
| Stimili material | 229 | 978 | — | — | — | — | .000 | .044 | 3482.7* | 0.09* | 0.10* |
| Words vs. high verbal | 223 | 898 | — | 0.06 | [−0.05, 0.17] | .266 | — | — | — | — | — |
| Words vs. low verbal | 206 | 827 | — | −0.10 | [−0.21, 0.01] | .074 | — | — | — | — | — |
| High vs. low verbal | 78 | 231 | — | −0.17 | [−0.30, −0.03] | .024 | — | — | — | — | — |
| Verbal words | 200 | 747 | −0.36 | — | [−0.41, −0.30] | — | — | — | — | — | — |
| Verbal pictures | 44 | 151 | −0.29 | — | [−0.40, −0.19] | — | — | — | — | — | — |
| Nonverbal | 44 | 80 | −0.46 | — | [−0.57, −0.35] | — | — | — | — | — | — |
| Valence | 232 | 984 | — | — | — | — | .071 | .186 | 3470.7* | 0.08* | 0.09* |
| Neutral vs. positive | 231 | 815 | — | 0.03 | [−0.06, 0.12] | .516 | — | — | — | — | — |
| Neutral vs. negative | 231 | 840 | — | 0.33 | [0.23, 0.43] | <.001 | — | — | — | — | — |
| Positive vs. negative | 87 | 313 | — | 0.30 | [0.17, 0.44] | <.001 | — | — | — | — | — |
| Neutral | 200 | 671 | −0.42 | — | [−0.47, −0.36] | — | — | — | — | — | — |
| Positive | 75 | 144 | −0.39 | — | [−0.48, −0.30] | — | — | — | — | — | — |
| Negative | 86 | 169 | −0.08 | — | [−0.18, 0.01] | — | — | — | — | — | — |
| Memory outcome | 236 | 995 | — | — | — | — | .037 | .029 | 3524.9* | 0.09* | 0.10* |
| Accuracy vs. discrimination | 234 | 904 | — | 0.08 | [−0.19, 0.36] | .553 | — | — | — | — | — |
| Accuracy vs. com errors | 222 | 897 | — | 0.22 | [0.08, 0.36] | .004 | — | — | — | — | — |
| Discrimination vs. com errors | 69 | 189 | — | 0.13 | [−0.16, 0.43] | .375 | — | — | — | — | — |
| Accuracy | 220 | 806 | −0.38 | — | [−0.45, −0.32] | — | — | — | — | — | — |
| Discrimination | 32 | 98 | −0.30 | — | [−0.55, −0.05] | — | — | — | — | — | — |
| Errors | 38 | 91 | −0.16 | — | [−0.30, −0.03] | — | — | — | — | — | — |
| Memory process (Fam. vs. Rec.) | 11 | 50 | — | −0.25 | [−0.66, 0.15] | .274 | .146 | .000 | 146.6* | 0.07* | 0.10 |
| Familiarity | 10 | 23 | −0.18 | — | [−0.45, 0.09] | — | — | — | — | — | — |
| Recollection | 11 | 27 | −0.44 | — | [−0.79, −0.09] | — | — | — | — | — | — |
| Publication status (unpub vs. pub) | 236 | 995 | — | −0.19 | [−0.33, −0.06] | .008 | .000 | .050 | 3524.9* | 0.09* | 0.10* |
| Unpublished | 37 | 210 | −0.20 | — | [−0.32, −0.08] | — | — | — | — | — | — |
| Published | 199 | 785 | −0.39 | — | [−0.45, −0.34] | — | — | — | — | — | — |
| Valence in self-reference tasks (positive vs. negative) | 34 | 99 | — | 0.71 | [0.38, 1.04] | <.001 | .293 | 0 | 574.4* | 0.03* | a |
| Positive | 31 | 47 | −0.45 | — | [−0.66, −0.24] | — | — | — | — | — | — |
| Negative | 34 | 52 | 0.26 | — | [0.10, 0.43] | — | — | — | — | — | — |
| Valence in external focus tasks (positive vs. negative) | 57 | 204 | — | 0.12 | [−0.003, 0.24] | .064 | .027 | .002 | 718.4* | 0.11* | 0.06* |
| Positive | 46 | 92 | −0.23 | — | [−0.35, −0.11] | — | — | — | — | — | — |
| Negative | 56 | 112 | −0.11 | — | [−0.21, −0.01] | — | — | — | — | — | — |
Note. The moderator name is in boldface, the specific comparison between levels is in boldface italics. The first variable in the comparisons serves as the baseline (e.g., recognition is the baseline). j = number of unique depression-control group comparisons. k = number of effect sizes. g = Hedges’ g. β coefficients are from metaregression analyses where categorical moderators with two or three levels were dummy coded and entered into the models as predictors. 95% CI corresponds to the β for moderators or g values for individual levels of moderators. p corresponds to the β for moderators. R(2)2 = proportion of estimated heterogeneity explained by the predictors at Level 2. R(3)2 = proportion of estimated heterogeneity explained by the predictors at Level 3. Q = Q statistic on the homogeneity of effect sizes. τ(2)2 = heterogeneity of effects due to differences between measures. τ(3)2 = heterogeneity of effect sizes across studies after controlling for the different types of measures at Level 2. High verbal = high verbalizable stimuli; Low verbal = low verbalizable stimuli; Com errors = commission errors; Fam. = familiarity; Rec. = recollection; Unpub = unpublished; Pub = published. For the levels of the moderators, j does not necessarily add up to the total j for the moderator (nor do the percentages add up to 100) because depression and control group performance may be compared on both recall and recognition, for example, within a single study.
The three-level model indicated there was not enough variance at Level 3, creating a convergence issue. However, rerunning this analysis with a two-level model yielded the same result as the three-level model.
p < .05 for Q and τ2 statistics.
Test Type
There was a significant effect of type of memory test, where the difference between depressed individuals and normal controls was significantly larger in recall than in recognition (β = −0.17, p = .002).
Stimulus Material
Group differences were larger for low verbalizable stimuli than for high verbalizable stimuli (β = −0.17, p = .024). However, differences were not significant between words and high verbalizable stimuli (β = 0.06, p = .266) or between words and low verbalizable stimuli (β = −0.10, p = .074).
Memory Outcome
Poorer performance in the depressed group compared to control was due more to lower accuracy (e.g., hits, items correctly recalled) than to a greater number of commission errors (e.g., false alarms, intrusions). While accuracy did not significantly differ from discrimination (β = 0.08, p = .553), and discrimination did not significantly differ from commission errors (β = 0.13, p = .375), accuracy did significantly differ from commission errors (β = 0.22, p = .004). That is, those with depression were more likely to make errors of omission than errors of commission, indicative of a conservative response bias.
Valence
The effect of valence was significant. While memory performance between positive and neutral stimuli did not differ between groups (β = 0.03, p = .516), memory for negative stimuli was significantly better than that for both neutral and positive stimuli in depressed participants relative to controls (β > 0.30, p < .001).
Some evidence suggests that the manner in which events are encoded (e.g., with reference to the self vs. others) may be an important factor influencing emotional memory in depression (see Wisco, 2009, for review). To examine this, we compared memory performance for positive and negative material in self-referencing tasks (e.g., “Does this adjective describe me?”) versus other kinds of tasks where attention at encoding is externally focused (e.g., “Is this caption compatible with the image?”). Only in self-referencing tasks (β = 0.71, p < .001) but not externally focused tasks (β = 0.12, p = .064) did valence moderate the relationship between depression and memory. As can be seen in the last six rows of Table 2, those with depression showed worse memory compared to controls for positive material in self-referencing tasks (g = −0.45), and for both positive and negative material in externally focused tasks (g = −0.23 and −0.11, respectively). However, in self-referencing tasks, those with depression showed superior memory for negative material relative to controls (g = 0.26).
Nonsignificant Task Moderators
Neither test delay (β < 0.02, p > .665), recall type (β = −0.04, p = .427), nor memory process (β = −0.25, p = .274) significantly moderated the relationship between depression and memory.
Participant Moderators
Table 3 presents the moderator analyses for each of the participant variables.
Table 3.
Participant Moderator Analyses
| Moderator | j | k | β | 95% CI | P | R (2) 2 | R (3) 2 | Q | τ(2)2 | τ(3)2 |
|---|---|---|---|---|---|---|---|---|---|---|
|
| ||||||||||
| Age (linear) | 224 | 943 | −0.007 | [−0.01, −0.005] | <.0001 | .000 | .179 | 3368.9* | 0.08* | 0.09* |
| Age (quadratic) | — | — | 0.0002 | [0.00001, 0.0003] | .041 | .000 | .200 | — | 0.08* | 0.09* |
| Sex | 220 | 901 | −0.17 | [−0.51, 0.17] | .324 | .002 | .000 | 3289.5* | 0.09* | 0.11* |
| Education | 136 | 563 | 0.05 | [0.03, 0.07] | <.001 | .000 | .163 | 2049.1* | 0.08* | 0.10* |
| Premorbid IQ | 55 | 243 | 0.01 | [−0.005, 0.02] | .333 | .001 | .024 | 841.3* | 0.08* | 0.07* |
| Diagnostic status | 236 | 995 | −0.30 | [−0.40, −0.21] | <.001 | .000 | .134 | 3524.9* | 0.09* | 0.09* |
| Depression status | 236 | 995 | −0.06 | [−0.18, 0.07] | .387 | .000 | .002 | 3524.9* | 0.09* | 0.11* |
| Matched age | 216 | 911 | −0.13 | [−0.27, 0.02] | .096 | .000 | .022 | 3226.7* | 0.09* | 0.11* |
| Matched education | 177 | 713 | 0.06 | [−0.11, 0.22] | .504 | .001 | .001 | 2682.5* | 0.10* | 0.11* |
| Medication status | 166 | 661 | −0.24 | [−0.39, −0.09] | .002 | .001 | .091 | 2340.7* | 0.08* | 0.11* |
| Symptom severity—MADRS | 27 | 115 | −0.01 | [−0.02, 0.001] | .101 | .000 | .213 | 307.6* | 0.0003 | 0.05* |
| Symptom severity—HDRS | 83 | 327 | −0.01 | [−0.02, 0.0002] | .053 | .001 | .046 | 972.8* | 0.04* | 0.09* |
| Symptom severity—BDI | 102 | 438 | −0.01 | [−0.02, −0.004] | .009 | .000 | .191 | 1735.8* | 0.18* | 0.05* |
| Symptom severity (HDRS scale) | 179 | 752 | −0.01 | [−0.02, −0.01] | <.001 | .000 | .097 | 2683.6* | 0.10* | 0.07* |
| Episode (first vs. recurrent) | 75 | 298 | −0.03 | [−0.32, 0.26] | .844 | .0003 | .0002 | 1147.7* | 0.06* | 0.11* |
| Time since diagnosis | 67 | 296 | 0.0001 | [−0.001, 0.001] | .851 | .000 | .002 | 1278.3* | 0.08* | 0.12* |
| Age of depression onset | 67 | 296 | −0.01 | [−0.02, −0.01] | <.001 | .000 | .228 | 1278.3* | 0.08* | 0.09* |
| Early- vs. late-onset | 29 | 116 | −0.25 | [−0.71, 0.21] | .301 | .005 | .057 | 357.8* | 0.01 | 0.14* |
| Recruitment site | 183 | 772 | −0.36 | [−0.46, −0.25] | <.001 | .004 | .269 | 3068.0* | 0.11* | 0.08* |
|
| ||||||||||
| Mood-congruency effects (interactions with valence) | ||||||||||
| Age | 221 | 936 | — | — | — | .075 | .255 | 3320.2* | 0.08* | 0.08* |
| Age | −0.01 | [−0.01, −0.003] | <.001 | |||||||
| Valence | 0.33 | [0.05, 0.61] | .029 | |||||||
| Age × Valence | −0.001 | [−0.01, 0.01] | .722 | |||||||
| Depression status | 232 | 984 | — | — | — | .076 | .173 | 3470.7* | 0.08* | 0.09* |
| Depression status | −0.08 | [−0.21, 0.05] | .251 | |||||||
| Valence | 0.30 | [−0.07, 0.66] | .153 | |||||||
| Depression status × Valence | 0.03 | [−0.36, 0.41] | .901 | |||||||
| Diagnostic status | 232 | 984 | — | — | — | .083 | .256 | 3470.7* | 0.08* | 0.08* |
| Diagnostic status | −0.29 | [−0.39, −0.19] | <.001 | |||||||
| Valence | 0.18 | [0.04, 0.31] | .020 | |||||||
| Diagnostic status × Valence | 0.19 | [0.01, 0.40] | .071 | |||||||
Note. j = number of studies/clusters. k = number of effect sizes. β coefficients are from metaregression analyses where continuous moderators were entered in the models as predictors, or categorical moderators (in the case of matched age and matched education) with two levels were dummy coded and entered in the models as predictors. 95% CI corresponds to the β for moderators. p corresponds to the β for moderators. R(2)2 = proportion of estimated heterogeneity explained by the predictors at Level 2. R(3)2 = proportion of estimated heterogeneity explained by the predictors at Level 3. Q = Q statistic on the homogeneity of effect sizes. τ(2)2 = heterogeneity of effects due to differences between measures. τ(3)2 = heterogeneity of effect sizes across studies after controlling for the different types of measures at Level 2. MADRS = Montgomery–Åsberg Depression Rating Scale; HDRS = Hamilton Depression Rating Scale; BDI = Beck Depression Inventory.
p < .05.
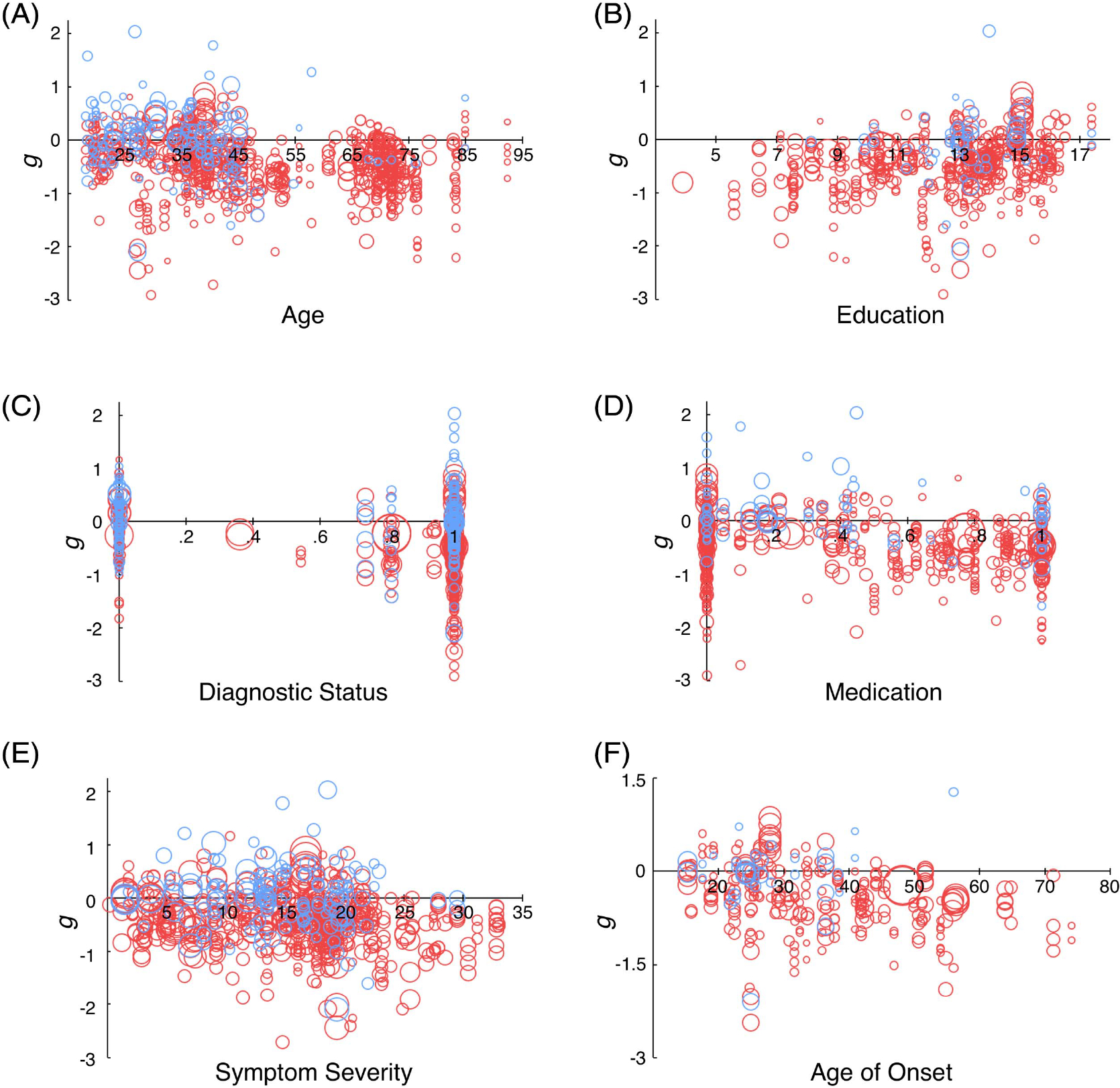
Age
Age showed a significant linear relationship with effect size (β = −0.007, p < .001), such that each year of age was associated with an increase in the depression effect of 0.007 SD units (see Figure 3A). Adding a quadratic component for age to test for nonlinear effects yielded a significant result (β = 0.0002, p = .041), indicating that memory performance in depression decreases from early to midlife, then improves somewhat in later life, yet not back to the level observed in young ages.
Figure 3.
Effect Size as a Function of Age (A), Education (B), Diagnostic Status (C), Medication Status (D), Symptom Severity (E), and Age of Onset (F)
Note. Bubble size corresponds to the sample size of the depression group. Blue = negative stimuli, red = positive and neutral stimuli. All presented variables significantly moderated the relationship between depression and episodic memory. See the online article for the color version of this figure.
Education
As shown in Figure 3B, for each additional year of education, individuals in the depressed group performed 0.05 SD better relative to controls (p < .001). Since some studies reported very low estimates for years of education (e.g., Tam & Lam, 2012, in which the duration of education was reported as 3.9 years in the depression group), we wanted to ensure these studies were not biasing the relationship between education and effect size. We conducted an additional analysis limited to those studies in which depressed participants had at least 10 years of education. Again, this yielded a significant effect, β = 0.05, 95% CI [0.01, 0.09], j = 117, k = 465, p = .009, R(2)2 = .000 and R(3)2 = .082.
Diagnostic Status: Formal MDD Diagnosis Versus Subthreshold Depression
Diagnostic status was found to have a significant effect (β = −0.30, p < .001): The larger the proportion of MDD diagnoses in the depressed sample, the larger the difference between depressed and control groups (see Figure 3C).
Medication Status
Participants in the depression group who were taking medication performed significantly worse than those who were not taking medication, relative to controls (β = −0.24, p = .002; see Figure 3D). To ensure this effect was not confounded by including individuals without diagnoses, we conducted an additional analysis only in those who were diagnosed with MDD. The effect remained significant, β = −0.23, 95% CI [−0.40 to −0.07], j = 149, k = 583, p = .006, R(2)2 = .001 and R(3)2 = .082).
Symptom Severity
The three most widely used inventories to measure depressive symptom severity in the present sample of studies were the Montgomery–Åsberg Depression Rating Scale (MADRS), the Hamilton Depression Rating Scale (HDRS), and the Beck Depression Inventory (BDI). Higher scores on the BDI (β = −0.013, p = .009), but not on the MADRS (β = −0.012, p = .101) or HDRS (β = −0.009, p = .053), were associated with greater memory deficits in the depressed groups compared to the controls. An additional analysis was conducted after transforming all severity scores to the HDRS scale using the formulas described in Heo et al. (2007; HDRS = −1.58 + 0.86 [MADRS]) and Vittengl et al. (2005; HDRS = 0.65 + 0.67 [BDI]).3 This analysis revealed that higher symptom severity was associated with a larger deficit in depressed versus control groups (β = −0.13, p < .001; see Figure 3E).
Age of Depression Onset
Later age of onset was associated with significantly larger differences between depressed individuals and controls (β = −0.01, p < .001; see Figure 3F).
Recruitment Site
The settings from which participants were recruited did significantly moderate the relationship between depression and memory (β = −0.36, p < .001; see Table 3). While those in depression groups showed reduced memory relative to controls across the board, these differences were smaller in groups recruited from community and university settings, g = −0.17, 95% CI [−0.24 to −0.10] than in groups recruited from patient settings, g = −0.53, 95% CI [−0.61 to −0.45].
Nonsignificant Participant Moderators
Depression status (β = −0.06, p = .387),4 sex (β = −0.17, p = .324), premorbid IQ (β = 0.01, p = .333), depressive episode (first vs. recurrent; β = −0.03, p = .844), late versus early-onset (β = −0.25, p = .301), time since diagnosis (β = 0.0001, p = .851), matched age (β = −0.13, p = .096), and matched education (β = 0.06, p = .504) did not significantly influence effect size. Note that for the analysis of premorbid IQ, only studies that used standardized measures of IQ were included. One study (Ladegaard et al., 2014) reported scores on the Danish Adult Reading Test (DART); these scores were converted using the formula described in Hjorthøj et al. (2013): IQ = 128.5 − 0.84 × (DART errors).
Publication Status
A concern in meta-analyses is that effect size estimates may be inflated if unpublished materials are excluded, as effects are often smaller than those found in published literature. In the current meta-analysis, 21% of the effect size estimates were from unpublished material. Our analyses showed that effects from published sources were in fact larger than those from unpublished sources, g = −0.39 95% CI [−0.45, −0.34] and g = −0.20 [−0.32, −0.08], respectively; β = −0.19, p = .008; see Table 2.
Unique Predictive Power of Moderators
Ideally, we would be able to explore the unique predictive power of each moderator variable by simultaneously entering all moderators into a regression equation. Although our sample is relatively large, not all studies reported information for all moderators. However, because some moderators were correlated across studies (correlation tables presented in Supplemental Material, Table S5), we wanted to investigate whether they were accounting for unique variance in effect sizes. To avoid overfitting the model and to ensure sample size was sufficiently large, we only considered moderators with information reported for >75% of the effect sizes (see Table S4, Supplemental Material). Each of these moderators (age, diagnostic status, depression status, symptom severity, publication status, and recruitment site) was analyzed in a model with the other moderators with which it was correlated. For the sake of brevity, select results are presented here, with the statistics for all models presented in Table S6 (Supplemental Material).
When included in a model with age, depression status, publication status, and recruitment site, diagnostic status was no longer a significant moderator (β = −0.14, p = .122) of the relationship between depression and memory (age and recruitment site, however, remained significant; β = −0.01, p = .002 and β = −0.23, p = .002, respectively), Diagnostic status likely accounts for shared variance with other moderators. For instance, patient samples are more frequently diagnosed with depression relative to community and university samples. However, diagnostic status and symptom severity were both significant (β = −0.23, p = .002 and β = −0.02, p = .015, respectively) when entered into a model with depression status (β = 0.10, p = .385), suggesting that diagnostic status and symptom severity account for unique variance in the relationship between depression and memory performance.
While medication status was only reported for 66.4% of the effect sizes, a reviewer suggested we assess its unique predictive power given it was significantly correlated with onset age, episode, and diagnostic status (see Table S5, Supplemental Material); episode and diagnostic status may be particularly necessary to examine with medication status as they can be seen as measures of severity (though notably, medication status was not found to correlate with symptom severity). We therefore conducted three additional models with multiple moderators:
Medication status, episode, and diagnostic status.
Medication status, episode, diagnostic status, and age of onset (age of onset was not added to the initial model since it decreased the number of effect sizes from 255 to 167).
Medication status and symptom severity (though these variables were not correlated, we felt this may be an important model to examine).
In the first two models, medication status was the only significant moderator (β = −0.429, p < .001 in Model 1; β = −0.318, p = .033 in Model 2). In the third model, both medication status and symptom severity were significant (β = −0.237, p = .003 and β = −0.013, p = .003, respectively).
Valence × Participant Moderator Interactions
Age-related changes in emotional memory are well-documented in the literature (Reed et al., 2014), but it is unclear whether these changes are impacted in depression. Furthermore, given our interest in exploring whether depressive deficits are tied to one’s current mood state, it is also worth exploring mood-congruency effects in particular. That is, does memory for emotional material differ as a function of diagnosis or current symptoms? To investigate these questions, we conducted additional analyses with age, depression status, and diagnostic status with the addition of valence interaction terms. Because memory did not differ for positive and neutral valence, we used the dummy-coded variable for negative valence, with 1 indicating a negative stimulus and 0 indicating a positive or neutral stimulus. Each model included terms for the participant moderator (e.g., age), valence, and the Moderator × Valence interaction. The lower section of Table 3 presents the results of these analyses. The interactions with age (β = −0.001, p = .722), depression status (β = 0.03, p = .901), and diagnostic status (β = 0.19, p = .071) were all nonsignificant.
Discussion
MDD is not simply a condition of disordered mood, but rather is characterized by wide-ranging symptoms that disrupt daily functioning. Particularly important for daily functioning is episodic memory—the ability to accurately remember personally experienced events. Consistent with previous meta-analyses that have investigated memory, but not episodic memory specifically (Ahern & Semkovska, 2017; Bora et al., 2013; Burt et al., 1995; Lee et al., 2012; McDermott & Ebmeier, 2009; Rock et al., 2014), we found individuals with depression showed an overall episodic memory deficit compared to healthy controls, with a Cohen’s d of 0.36—a small to medium effect. Our large sample of studies and the use of a three-level model allowed for powerful, novel explorations of task and participant variables as moderators of the relationship between depression and memory impairments. We set out to answer four main questions regarding the nature of these impairments. These are described in detail below.
Is the Pattern of Episodic Memory Deficits in Depression Consistent Across the Adult Lifespan?
The finding that participant age predicted greater episodic memory deficits in depression suggests that depression-related disruptions in memory are not uniform across the adult lifespan, but are in fact magnified in later years. It should be noted that the addition of a quadratic component in this analysis was significant, suggesting that studies with middle-aged participants showed the worst performance in depressed groups relative to controls. As can be seen in Figure 3A, however, there was a paucity of studies with midlife individuals. Thus, further work is needed to assess this midlife pattern.
The linear effect of age, however, was quite robust, indicating that age is a significant moderator of the relationship between depression and memory. Our results suggest that at age 20, an individual with depression is expected to perform 0.14 SD below controls; at age 70 the group difference grows to 0.49 SD. As Dotson et al. (2020) noted, age- and depression-related changes may interact to produce a sort of “double jeopardy” for cognitive dysfunction. It is therefore possible that depression accelerates the cognitive declines often observed in aging. The present findings support this idea and are consistent with previous research suggesting that memory impairment is closely linked to functional disability in depressed older adults (Gallo et al., 2003; Yen et al., 2011).
One possibility for this finding of worse performance with increased age is that older individuals may have spent a greater total amount of time in depressive episodes. However, many (Albert et al., 2018; Bearden et al., 2006; Delaloye et al., 2010; Dresler et al., 2010) but not all (Behnken et al., 2010) studies have failed to find correlations between duration of depressive symptoms and memory performance, consistent with the current meta-analysis. It should be noted, however, that “time since diagnosis” does not capture the time spent in depressive episodes, as individuals with depression may spend time in and out of episodes over the course of many years (Möller, 2008). At the same time, the extent to which the “time since diagnosis” measure reflects actual illness duration may be informed by cohort effects. For instance, as social stigma surrounding mental health has been lessening in the relatively recent past, it could be that older adults, relative to their younger counterparts, might underreport depressive symptoms or avoid treatment entirely (Conner et al., 2010). In other words, it could be that at least for some studies, older adults are potentially underreporting their depressive symptoms relative to young adults and/or only those older adults with severe depression are willing to seek treatment, relative to younger adults. Such cohort effects could contribute, at least in part, to the finding of lower performance with increasing age.
Another indicator of longer symptom duration is recurrent depression; however, episode (recurrent vs. first episode) did not significantly moderate the relationship between memory and depression, in samples where all individuals had first-episode depression, g = −0.46, 95% CI [−0.62, −0.31]; in samples where all individuals had recurrent depression, g = −0.44, 95% CI [−0.71, −0.17]. It is possible these effects were obscured by the age of onset effect, in which later age of onset was associated with significantly worse memory performance. This age of onset effect seems relevant to the idea that late-life depression may be an early symptom or risk factor of dementia (Barnes et al., 2012; Byers et al., 2012; Dotson et al., 2008, 2010; Saczynski et al., 2010). Depressive symptoms may also develop in reaction to perceived declines in cognitive functioning (see Byers & Yaffe, 2011, for review). Longitudinal assessments with careful consideration of the timing of symptom development are needed to elucidate this relationship. While we cannot rule out the possibility that some of the cases of late-onset depression in the current meta-analysis include individuals with prodromal dementia, the age of onset effect was reliable across age, suggesting this explanation is unlikely. Collectively, these results support the idea that the age at which an individual is diagnosed with depression is more predictive of memory performance than is symptom duration or recurrence.
Across age, depression was associated with worse performance on almost all episodic memory tasks—recall (both free and cued) and recognition; immediate, short, and long-delayed tests; verbalizable words and pictures and nonverbalizable material; positive and neutral material; and recollection. Recollection, but not familiarity-based memory, was significantly affected in depression. These results support the idea that, like aging, depression has the largest impacts on cognition under conditions in which demands on frontally mediated cognitive control processes are high (see Dotson et al., 2020; Grahek et al., 2019; Snyder, 2013, for reviews), such as strategic retrieval of source-specifying details and subjective recollection of episodic information (e.g., temporal, spatial, internally generated associations; Cohn et al., 2008; Duarte & Dulas, 2020; Duarte et al., 2008; Duarte & Kensinger, 2019).
However, the nonsignificant difference between free and cued recall tasks challenges this idea: free recall requires self-initiated reconstructive processes, whereas in cued recall, the cue provides more environmental support for recovery of the original event details (Craik, 1983). It is worth noting, however, the heterogeneity in the cued recall tasks. In some tasks, like the CVLT, semantic categories (e.g., fruits, tools) are used as cues. In others, such as associative memory tasks, participants are given pairs of items, and one of the items later serves as a retrieval cue for the other; the semantic similarity between cue and item can vary. This is important because age-related associative memory impairments are typically reduced when items are semantically related or can be integrated or unitized (Ahmad et al., 2015; Badham et al., 2012; Naveh-Benjamin et al., 2003). However, an analysis comparing these two types of cued recall tasks showed no significant differences, β = −0.14, 95% CI [−0.40 to 0.12], j = 38, k = 75, p = .299. While we were not able to investigate the benefit of unitization in depression due to few studies reporting such information, this is a question future studies may wish to explore.
It should be noted that the lack of a significant test delay effect in our results suggests that depression is associated with episodic memory impairments as well as impairments in other abilities. As previously mentioned, delayed retrieval tasks are the optimal measures for assessing episodic memory. Immediate retrieval tasks recruit some of the same processes as those used for delayed retrieval, in addition to other processes, including those governing executive functioning and processing speed (Casaletto et al., 2017). Other meta-analyses have shown depression-related deficits in these domains (Dotson et al., 2020; Snyder, 2013), therefore our findings are consistent with the idea that depressive deficits are observed in a variety of cognitive domains.
What Are the Effects of Valence on Episodic Memory in Depression?
While memory performance for positive and neutral material was impaired in those with depression relative to controls, this was not the case with negative stimuli. These findings indicate mood-congruency effects (Bower, 1981; Marchetti et al., 2018; Van Vleet et al., 2019), such that material which is consistent with one’s mood state, or depressive self-schema, is better remembered than that which is inconsistent (Beck, 1967; Disner et al., 2011). Some evidence suggests that mood-congruent effects may be more prevalent in self-referencing tasks (e.g., Bradley et al., 1995; Romero et al., 2014, 2016) compared to externally focused encoding tasks (see Wisco, 2009, for review). This was supported by the current findings: Although individuals with depression exhibited poorer memory than controls for positive material across the two types of tasks, only in self-referencing tasks were their memory better than that of controls for negative material. The self-reference effect has consistently been demonstrated to improve memory performance, as self-referential processing encourages greater elaboration that taps into well-established schemas (Klein & Nelson, 2014; Symons & Johnson, 1997). In individuals with depression, the self-reference effect is stronger for negative material because it is more consistent with their self-schema (LeMoult & Gotlib, 2019).
It is also worth considering the potential confounding effects of stimulus traits besides valence. (Bireta et al., 2021) recently suggested that factors such as word length, arousal, and frequency of use might be responsible for some of the significant results typically attributed to valence in verbal memory tasks. After equating lists of positively and negatively valenced words on a host of linguistic factors, the authors failed to find immediate recall performance differences between the two stimulus sets in a healthy sample. As for the present work, 58 of the studies included in the valence analyses used verbal stimuli. Most, but not all of these studies controlled for at least word length (53.4%) and frequency of use (55.2%; see Table S2, Supplemental Material for details), suggesting that our findings are somewhat robust to stimulus noise. Unfortunately, information on other word qualities was not commonly provided in the included studies. Although beyond the scope of this work, further defining the interaction of depression, valence, and linguistic factors remains an interesting line of inquiry.
One intriguing question for future studies is whether the so-called age-related “positivity effect” is minimized in depression. The positivity effect is illustrated in memory studies when older adults remember more positive than negative information while younger adults tend to show the opposite pattern (Carstensen & Mikels, 2005; Mather, 2012; see Reed et al., 2014, for meta-analysis and review). Older adults engage in emotion regulation strategies to prioritize processing of positive information; however, when these regulatory processes are disrupted, the positivity effect in memory is eliminated (Mather & Knight, 2005). As chronic stressors have been shown to decrease the ability to use these regulation strategies (see Knight & Durbin, 2015, for review), it is feasible that depression later in life may similarly limit their implementation. We were unable to show that mood congruency was affected by age, however, this analysis was likely underpowered as a result of only eight effect sizes associated with negative valence in studies where the mean age was over 55. Thus, the interaction between age and depression on episodic emotional memory biases remains unclear and is a question ripe for future investigation.
Are Depression-Related Memory Deficits Tied to One’s Current Depressive State?
Given our combined findings of greater episodic memory impairments in diagnosed MDD than in subthreshold depression and a nonsignificant difference between those with current and remitted symptoms, we believe memory deficits are not tied to an individual’s depressed mood. It is, however, possible that remitted participants were still depressed relative to controls, as evidenced by significantly higher severity scores on depression inventories (see Table S7, Supplemental Material). Though the scores for all remitted groups were in the range considered “normal” for the various inventories, questions about these cutoff scores have been raised (see Romera et al., 2011; Zimmerman et al., 2004, 2012, for reviews). Furthermore, longitudinal studies have found that episodic memory impairments persist following recovery from depressive symptoms (Airaksinen et al., 2006). It is therefore likely that remission may represent lessening of affective symptoms but not cognitive symptoms. Together, these findings suggest episodic memory impairments in depression are independent of depressed mood.
Consistent with the idea that treated depression may not confer better episodic memory performance, use of depression medication was associated with poorer memory performance. While some meta-analyses have identified positive effects for certain antidepressants on hippocampal neurogenesis (Boldrini et al., 2009; Micheli et al., 2018) and certain memory functions (Prado et al., 2018; Rosenblat et al., 2015), others have not (Deuschle et al., 2004; Nagane et al., 2014; Shilyansky et al., 2016). For example, Neu et al. (2005) found that while depressive symptoms as measured by the HDRS disappeared after treatment with antidepressants; verbal memory impairments persisted even after a 6-month remission period. The persistence of memory difficulties in remitted depression highlights a blind spot in treatment interventions. To date, most pharmacological treatments focus only on alleviating mood-related symptoms. Our findings underscore the necessity for interventions that target both mood and cognitive symptoms in depression.
Antidepressant medications may sometimes contribute to episodic memory deficits in depression, perhaps due to anticholinergic side effects, which have been shown to reduce long-term memory recall, even in healthy, nondepressed participants (Schmitt et al., 2001; see Serretti et al., 2010, for review). Indeed, some of the most commonly reported cognitive side effects of antidepressants include memory impairments (Popovic et al., 2015). Thus, although the current meta-analysis cannot address this question directly, it is possible that memory impairments may be worsened or even induced following antidepressant use in depression.
We would be remiss if we did not consider other potential explanations for the negative association between depression medication use and memory performance. The metaregression approach is only able to identify associations between variables across studies, but it is unable to determine causal relationships or directionality. It is certainly possible that memory disturbances are a particularly motivating factor for individuals to seek treatment. Relatedly, these symptoms may contribute to physician’s perceptions of patients’ depression severity, thereby making it more likely for those individuals who exhibit memory problems to be prescribed medication for depression. Notably, when assessing the unique predictive power of moderators, medication remained a significant predictor of memory deficits in depression when controlling on diagnostic status, episode, onset age, and symptom severity. That said, the data we used necessarily represent a snapshot that cannot speak to other factors relevant to antidepressant treatment. For instance, medication may have been prescribed when subjects had more severe symptoms that had lessened by the experiment date. Because we only had information on present symptom severity available, it was not possible to additionally test the predictive power of depressive symptoms pretreatment. Further, the analyses conducted herein could not consider the specific details of patients’ antidepressant regimens (e.g., length of treatment, types of medications administered, dosage, polypharmacy). Consequently, we do not believe physicians should change their recommendations for medication based purely on these results. Although past work has suggested that acute treatment with antidepressants does not alleviate (nor worsen) verbal memory deficits in depression, the impact of chronic treatment could be different (Shilyansky et al., 2016). Additional experimental work over a longer time period is needed to more precisely define the relationship between depression medication and memory performance.
Are There Factors That Protect Against Memory Impairments in Depression?
In the current meta-analysis, higher educational attainment was associated with less memory impairment. Education is often used as a proxy for cognitive reserve (Anthony & Lin, 2018; Stern, 2002; Yochim & Woodhead, 2018). Cognitive reserve can be viewed as an active process in which the brain attempts to leverage preexisting cognitive processes or new compensatory techniques to counteract the effects of pathology (Stern, 2002; Stern et al., 2018). That is, two individuals with depression may have similar pathology (e.g., reduced hippocampal volume), but the individual with higher cognitive reserve will show fewer cognitive deficits than the one with lower cognitive reserve (Barulli & Stern, 2013).
Similar moderating effects of cognitive reserve have been reported in previous studies (Murphy & O’Leary, 2010; Venezia et al., 2018) and in systematic reviews and meta-analyses (Lee et al., 2012; Opdebeeck et al., 2015), with differences between MDD and control groups diminishing with higher levels of reserve. Similar effects have also been reported in subthreshold depression (McLaren et al., 2015). The protective effects of cognitive reserve have been linked to cognitive networks being (a) more efficient, in that less activation is needed to perform at a similar level as less efficient networks, and (b) having a higher capacity, in that networks can ramp up activation to a higher level in response to increasing task difficulty (Barulli & Stern, 2013; Cabeza et al., 2018). Although IQ correlates with education (see Ritchie & Tucker-Drob, 2018, for review), we did not find IQ to have the same moderating effect as education on memory performance in depression. However, fewer studies reported information about IQ (54 vs. 136 that reported years of education), likely limiting our ability to detect robust effects. More consistent reporting of IQ estimates in future studies is needed. Furthermore, a number of different tests were used to measure IQ. Some, like the National Adult Reading Test, simply measure reading ability, while others like the Wechsler Adult Intelligence Scale measure verbal and nonverbal abilities. The variety of tests, in combination with the relatively small-sample size, was a major limitation of this analysis.
It should be noted that correlates of education such as socioeconomic status (SES), occupation, health-care access, diet, lifestyle factors, etc. could also moderate the relationship between depression and memory impairments. Though the studies included in the current meta-analysis did not provide sufficient detail for us to explore these factors, future research should investigate how they may interactively serve as protective or harmful influences on memory impairments. For example, high education could buffer the impact of poor diet on memory, while low education and poor access to health care could magnify impairments.
Limitations
Like other meta-analyses, some of our results may have been influenced by publication bias and outcome reporting bias (Dwan et al., 2013; John et al., 2012), however, analysis on the funnel plot did not reveal significant evidence for publication bias in the current sample. Selection bias in participant samples is another potential concern, in that individuals selected for depression groups may differ in substantial ways from the population. One concern in particular is that individuals with higher levels of depressive symptomology might elect to not participate in studies but might once remitted; this effect would then reduce the effect sizes. Additionally, the majority of the records included in this work were published in English. Because depression etiology and expression are known to vary based on cultural and social factors (Chentsova-Dutton et al., 2014; Juhasz et al., 2012), it is possible that our results are not entirely generalizable to cultures where English is less dominant. Nonetheless, we hope that by including reports from a wide range of countries we were able to mitigate this issue to some extent.
We were unable to determine whether the quadratic effect of age is indicative of a true dip in memory performance in midlife depression, or if the trend is simply attributable to fewer studies with middle-aged individuals. An additional limitation for the age analysis is that the included studies varied greatly in the age ranges of their samples. For example, while some recruited only young or older adults, others recruited participants across the adult lifespan. By using the mean age of the depressed groups as we did in the current meta-analysis, those studies with wider age range tended to have mean ages that fell into the midlife range (e.g., in Sheline et al., 1999, age range = 23–86, Mage = 52.8). More studies with middle-age and older adults, particularly those that assess emotional memory, are needed to address outstanding questions regarding interactive influences of age and depression on episodic memory.
A limitation in our analyses of participant moderators is that a number of these variables were defined as the proportion of individuals in the samples with particular characteristics (e.g., the proportion of females, the proportion taking medication for depression). To explore, say, sex effects, ideally one would have memory performance means for females and for males. However, it is not common practice for researchers to report performance estimates in this manner unless sex differences are an explicit focus on the study. Additionally, some of our moderators may have not fully captured informative details about the variables. For example, our education moderator represented duration of schooling, which may be less informative than the quality of educational attainment (see Jones et al., 2011, for review). Lastly, while we explored the effects of pharmacological treatment, we were unable to analyze specific classes of antidepressants, polypharmacy, or whether therapy or other nonpharmacological interventions moderate the relationship between depression and memory due to insufficient data.
Potential confounds identified in Table 1 must be taken into consideration. For example, diagnostic status and medication status—variables shown to significantly influence memory performance in depression groups—were much lower in unpublished studies than in published studies and in community/university settings than in patient settings. This is perhaps unsurprising given that most unpublished studies were theses and dissertations that used convenience samples of university students who were more likely to be undiagnosed and thus unmedicated compared to patient samples. It is certainly possible that the differences in unpublished versus published studies and in community/university versus patient samples were driven by diagnostic and medication status variables.
The limitations for laboratory tasks to predict everyday memory functioning (Salthouse, 2012) must also be acknowledged. While we have provided some evidence of memory disruptions in depression, the real-world implications of these disruptions are beyond the scope of the current meta-analysis but are certainly worthy of future investigations. Another drawback, as mentioned in the Results, is that we were limited in our ability to simultaneously investigate sets of moderator variables. Finally, given the cross-sectional nature of the data included in the current meta-analysis, in addition to the limitations of the metaregression approach to determine directionality, we are unable to draw conclusions about the causal links between depression and memory impairments.
Conclusions
Depression has long been recognized as a burdensome mood disorder but only recently have researchers begun appreciating the full toll depression takes on a wide range of cognitive functions. Until now, major questions regarding episodic memory impairments across different types of tasks and materials, as well as the influence of demographic and other participant-related variables, have remained unresolved. We believe our meta-analysis has helped to clarify and paint a more comprehensive picture of the memory disruptions in depression. Based on 995 effect sizes from 205 studies with 236 unique depressed-control group comparisons, we definitively show clear evidence of depression-related deficits in episodic memory across the adult lifespan. These deficits were most pronounced in older age, in diagnosed MDD, in those taking medication for depression, and in those with fewer years of education. Depression status did not influence depressive memory deficits. Memory for positive and neutral material was particularly impacted in depression, while memory for negative material was not. Educational attainment was associated with lower levels of depressive deficits in memory.
We believe our meta-analysis provides a much-needed update to the literature on episodic memory in depression, providing considerable evidence for what is and is not impaired, and what factors moderate those impairments. While some questions were beyond what we were able to investigate, we hope our meta-analysis inspires continued investigations of the challenges faced by those with depression to inform novel treatments that can target the cognitive symptoms, in addition to hallmark affective symptoms, that cause severe disruptions in everyday life.
Supplementary Material
Public Significance Statement.
This meta-analysis demonstrated small to moderate depression-related deficits in episodic memory. Deficits were more pronounced in older age, in clinical depression, and in those receiving pharmacological treatment, while fewer deficits were observed in memory for negative material and with higher educational attainment. By shedding light on depression’s nuanced relationship with episodic memory, the present work encourages a greater consideration of cognitive symptoms when diagnosing and treating depression.
Acknowledgments
This research was supported in part by a Ruth L. Kirschstein National Research Service Award (NRSA) Institutional Research Training Grant from the National Institutes of Health (National Institute on Aging), Grant number 5T32AG000175.
Footnotes
The annotated data set and R code used in this meta-analysis are available on the Open Science Framework: https://osf.io/t8ynd/?view_only=05c342695faa476795218c6a31ecfec5
Supplemental materials: https://doi.org/10.1037/bul0000344.supp
When exact proportions could not be calculated, the mean value was entered. This was done for 8/236 entries for diagnostic status, 5/236 for depression status, 15/75 for episode, and 3/166 for medication status.
The terms “sex” and “gender” are often used interchangeably in the literature. We used sex/gender as recorded by the original researchers: “sex” was reported for 70 of the depression samples, “gender” was reported for 133; for 22 samples, neither term was used (only number or proportion of females and males was reported); 11 samples did not report any sex/gender information. For simplicity, we use the term “sex” to refer to the sex/gender variable in the remainder of the article.
In cases where articles reported both BDI and MADRS scores, we transformed both values then took the average.
Because those with current symptoms included individuals with and without diagnoses, we also assessed depression status just in those who were diagnosed; however, the effect remained nonsignificant, β = −0.14, 95% CI [−0.27 to −0.002], j = 180, k = 736, p = .052, R(2)2 = .000, and R(3)2 = .023.
References
- *Addis DR, Hach S, & Tippett LJ (2016). Do strategic processes contribute to the specificity of future simulation in depression? British Journal of Clinical Psychology, 55(2), 167–186. 10.1111/bjc.12103 [DOI] [PubMed] [Google Scholar]
- Ahern E, & Semkovska M (2017). Cognitive functioning in the first-episode of major depressive disorder: A systematic review and meta-analysis. Neuropsychology, 31(1), 52–72. 10.1037/neu0000319 [DOI] [PubMed] [Google Scholar]
- Ahmad FN, Fernandes M, & Hockley WE (2015). Improving associative memory in older adults with unitization. Neuropsychology, Development, and Cognition. Section B, Aging, Neuropsychology and Cognition, 22(4), 452–472. 10.1080/13825585.2014.980216 [DOI] [PubMed] [Google Scholar]
- *Ai H, Opmeer EM, Veltman DJ, van der Wee NJ, van Buchem MA, Aleman A, & van Tol MJ (2015). Brain activation during emotional memory processing associated with subsequent course of depression. Neuropsychopharmacology, 40(10), 2454–2463. 10.1038/npp.2015.96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Airaksinen E, Larsson M, Lundberg I, & Forsell Y (2004). Cognitive functions in depressive disorders: Evidence from a population-based study. Psychological Medicine, 34(1), 83–91. 10.1017/S0033291703008559 [DOI] [PubMed] [Google Scholar]
- Airaksinen E, Wahlin A, Larsson M, & Forsell Y (2006). Cognitive and social functioning in recovery from depression: Results from a population-based three-year follow-up. Journal of Affective Disorders, 96(1–2), 107–110. 10.1016/j.jad.2006.05.004 [DOI] [PubMed] [Google Scholar]
- *Alalade E, Denny K, Potter G, Steffens D, & Wang L (2011). Altered cerebellar-cerebral functional connectivity in geriatric depression. PLOS ONE, 6(5), Article e20035. 10.1371/journal.pone.0020035 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Albert KM, Potter GG, McQuoid DR, & Taylor WD (2018). Cognitive performance in antidepressant-free recurrent major depressive disorder. Depression and Anxiety, 35(8), 694–699. 10.1002/da.22747 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Alhaj HA, Massey AE, & McAllister-Williams RH (2007). A study of the neural correlates of episodic memory and HPA axis status in drug-free depressed patients and healthy controls. Journal of Psychiatric Research, 41(3–4), 295–304. 10.1016/j.jpsychires.2006.08.008 [DOI] [PubMed] [Google Scholar]
- Altemus M, Sarvaiya N, & Neill Epperson C (2014). Sex differences in anxiety and depression clinical perspectives. Frontiers in Neuroendocrinology, 35(3), 320–330. 10.1016/j.yfrne.2014.05.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Alves GS, Karakaya T, Fußer F, Kordulla M, O’Dwyer L, Christl J, Magerkurth J, Oertel-Knöchel V, Knöchel C, Prvulovic D, Jurcoane A, Laks J, Engelhardt E, Hampel H, & Pantel J (2012). Association of microstructural white matter abnormalities with cognitive dysfunction in geriatric patients with major depression. Psychiatry Research, 203(2–3), 194–200. 10.1016/j.pscychresns.2011.12.006 [DOI] [PubMed] [Google Scholar]
- American Psychiatric Association. (1980). Diagnostic and statistical manual of mental disorders (3rd ed.).
- Anthony M, & Lin F (2018). A systematic review for functional neuroimaging studies of cognitive reserve across the cognitive aging spectrum. Archives of Clinical Neuropsychology, 33(8), 937–948. 10.1093/arclin/acx125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Araujo NB, Moraes HS, Silveira H, Arcoverde C, Vasques PE, Barca ML, Knapskog A-B, Engedal K, Coutinho ESF, Deslandes AC, & Laks J (2014). Impaired cognition in depression and Alzheimer (AD): A gradient from depression to depression in AD. Arquivos de Neuro-Psiquiatria, 72(9), 671–679. 10.1590/0004-282X20140108 [DOI] [PubMed] [Google Scholar]
- *Arnold JF, Fitzgerald DA, Fernández G, Rijpkema M, Rinck M, Eling PA, Becker ES, Speckens A, & Tendolkar I (2011). Rose or black-coloured glasses? Altered neural processing of positive events during memory formation is a trait marker of depression. Journal of Affective Disorders, 131(1–3), 214–223. 10.1016/j.jad.2010.12.011 [DOI] [PubMed] [Google Scholar]
- *Ashare R, Strasser AA, Wileyto EP, Cuevas J, & Audrain-McGovern J (2014). Cognitive deficits specific to depression-prone smokers during abstinence. Experimental and Clinical Psychopharmacology, 22(4), 323–331. 10.1037/a0037072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asperholm M, Högman N, Rafi J, & Herlitz A (2019). What did you do yesterday? A meta-analysis of sex differences in episodic memory. Psychological Bulletin, 145(8), 785–821. 10.1037/bul0000197 [DOI] [PubMed] [Google Scholar]
- Atkinson RC, & Shiffrin RM (1968). Human memory: A proposed system and its control processes. In Spence KW & Spence JT (Eds.), The psychology of learning and motivation: Advances in research and theory (Vol. II, pp. 89–195). Academic Press. [Google Scholar]
- Austin MP, Mitchell P, & Goodwin GM (2001). Cognitive deficits in depression: Possible implications for functional neuropathology. The British Journal of Psychiatry, 178, 200–206. 10.1192/bjp.178.3.200 [DOI] [PubMed] [Google Scholar]
- *Avila R, Moscoso MAA, Ribeiz S, Arrais J, Jaluul O, & Bottino CMC (2009). Influence of education and depressive symptoms on cognitive function in the elderly. International Psychogeriatrics, 21(3), 560–567. 10.1017/S1041610209008928 [DOI] [PubMed] [Google Scholar]
- *Avila R, Ribeiz S, Duran FL, Arrais JP, Moscoso MA, Bezerra DM, Jaluul O, Castro CC, Busatto GF, & Bottino CM (2011). Effect of temporal lobe structure volume on memory in elderly depressed patients. Neurobiology of Aging, 32(10), 1857–1867. 10.1016/j.neurobiolaging.2009.11.004 [DOI] [PubMed] [Google Scholar]
- *Bäckman L, & Forsell Y (1994). Episodic memory functioning in a community-based sample of old adults with major depression: Utilization of cognitive support. Journal of Abnormal Psychology, 103(2), 361–370. 10.1037/0021-843X.103.2.361 [DOI] [PubMed] [Google Scholar]
- *Bäckman L, Hassing L, Forsell Y, & Viitanen M (1996). Episodic remembering in a population-based sample of nonagenarians: Does major depression exacerbate the memory deficits seen in Alzheimer’s disease? Psychology and Aging, 11(4), 649–657. 10.1037/0882-7974.11.4.649 [DOI] [PubMed] [Google Scholar]
- Badham SP, Estes Z, & Maylor EA (2012). Integrative and semantic relations equally alleviate age-related associative memory deficits. Psychology and Aging, 27(1), 141–152. 10.1037/a0023924 [DOI] [PubMed] [Google Scholar]
- *Bai T, Xie W, Wei Q, Chen Y, Mu J, Tian Y, & Wang K (2017). Electroconvulsive therapy regulates emotional memory bias of depressed patients. Psychiatry Research, 257, 296–302. 10.1016/j.psychres.2017.07.069 [DOI] [PubMed] [Google Scholar]
- *Baldwin R, Jeffries S, Jackson A, Sutcliffe C, Thacker N, Scott M, & Burns A (2004). Treatment response in late-onset depression: Relationship to neuropsychological, neuroradiological and vascular risk factors. Psychological Medicine, 34(1), 125–136. 10.1017/S0033291703008870 [DOI] [PubMed] [Google Scholar]
- *Ballmaier M, Narr KL, Toga AW, Elderkin-Thompson V, Thompson PM, Hamilton L, Haroon E, Pham D, Heinz A, & Kumar A (2008). Hippocampal morphology and distinguishing late-onset from early-onset elderly depression. The American Journal of Psychiatry, 165(2), 229–237. 10.1176/appi.ajp.2007.07030506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banich MT (2009). Executive function: The search for an integrated account. Current Directions in Psychological Science, 18(2), 89–94. 10.1111/j.1467-8721.2009.01615.x [DOI] [Google Scholar]
- *Baños RM, Medina PM, & Pascual J (2001). Explicit and implicit memory biases in depression and panic disorder. Behaviour Research and Therapy, 39(1), 61–74. 10.1016/S0005-7967(99)00158-8 [DOI] [PubMed] [Google Scholar]
- Barnes DE, Yaffe K, Byers AL, McCormick M, Schaefer C, & Whitmer RA (2012). Midlife vs late-life depressive symptoms and risk of dementia: Differential effects for Alzheimer disease and vascular dementia. Archives of General Psychiatry, 69(5), 493–498. 10.1001/archgenpsychiatry.2011.1481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barredo J, Öztekin I, & Badre D (2015). Ventral fronto-temporal pathway supporting cognitive control of episodic memory retrieval. Cerebral Cortex (New York, N.Y), 25(4), 1004–1019. 10.1093/cercor/bht291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Barrick EM, & Dillon DG (2018). An ERP study of multidimensional source retrieval in depression. Biological Psychology, 132, 176–191. 10.1016/j.biopsycho.2018.01.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barulli D, & Stern Y (2013). Efficiency, capacity, compensation, maintenance, plasticity: Emerging concepts in cognitive reserve. Trends in Cognitive Sciences, 17(10), 502–509. 10.1016/j.tics.2013.08.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Baudic S, Tzortzis C, Barba GD, & Traykov L (2004). Executive deficits in elderly patients with major unipolar depression. Journal of Geriatric Psychiatry and Neurology, 17(4), 195–201. 10.1177/0891988704269823 [DOI] [PubMed] [Google Scholar]
- *Bazin N, Perruchet P, & Féline A (1996). Mood congruence effect in explicit and implicit memory tasks: A comparison between depressed patients, schizophrenic patients and controls. European Psychiatry, 11(8), 390–395. 10.1016/S0924-9338(97)82575-8 [DOI] [PubMed] [Google Scholar]
- *Bearden CE, Glahn DC, Monkul ES, Barrett J, Najt P, Villarreal V, & Soares JC (2006). Patterns of memory impairment in bipolar disorder and unipolar major depression. Psychiatry Research, 142(2–3), 139–150. 10.1016/j.psychres.2005.08.010 [DOI] [PubMed] [Google Scholar]
- *Beblo T, Kater L, Baetge S, Driessen M, & Piefke M (2017). Memory performance of patients with major depression in an everyday life situation. Psychiatry Research, 248, 28–34. 10.1016/j.psychres.2016.12.013 [DOI] [PubMed] [Google Scholar]
- Beck AT (1967). Depression: Clinical, experimental, and theoretical aspects. Hoeber Medical Division. [Google Scholar]
- *Behnken A, Schöning S, Gerss J, Konrad C, de Jong-Meyer R, Zwanzger P, & Arolt V (2010). Persistent non-verbal memory impairment in remitted major depression—Caused by encoding deficits? Journal of Affective Disorders, 122(1–2), 144–148. 10.1016/j.jad.2009.07.010 [DOI] [PubMed] [Google Scholar]
- *Belanoff JK, Kalehzan M, Sund B, Fleming Ficek SK, & Schatzberg AF (2001). Cortisol activity and cognitive changes in psychotic major depression. The American Journal of Psychiatry, 158(10), 1612–1616. 10.1176/appi.ajp.158.10.1612 [DOI] [PubMed] [Google Scholar]
- Benjamin AS (2007). Memory is more than just remembering: Strategic control of encoding, accessing memory, and making decisions. Psychology of Learning and Motivation, 48, 175–223. 10.1016/S0079-7421(07)48005-7 [DOI] [Google Scholar]
- *Bergfeld IO, Mantione M, Hoogendoorn MLC, Ruhé HG, Horst F, Notten P, van Laarhoven J, van den Munckhof P, Beute G, Schuurman PR, & Denys D (2017). Impact of deep brain stimulation of the ventral anterior limb of the internal capsule on cognition in depression. Psychological Medicine, 47(9), 1647–1658. 10.1017/S0033291717000113 [DOI] [PubMed] [Google Scholar]
- Besteher B, Gaser C, & Nenadic I (2020). Brain structure and subclinical symptoms: A dimensional perspective of psychopathology in the depression and anxiety spectrum. Neuropsychobiology, 79, 270–283. 10.1159/000501024 [DOI] [PubMed] [Google Scholar]
- Bhalla RK, Butters MA, Mulsant BH, Begley AE, Zmuda MD, Schoderbek B, Pollock BG, Reynolds CF III, & Becker JT (2006). Persistence of neuropsychologic deficits in the remitted state of late-life depression. The American Journal of Geriatric Psychiatry, 14(5), 419–427. 10.1097/01.JGP.0000203130.45421.69 [DOI] [PubMed] [Google Scholar]
- *Biedermann SV, Bumb JM, Demirakca T, Ende G, & Sartorius A (2016). Improvement in verbal memory performance in depressed inpatients after treatment with electroconvulsive therapy. Acta Psychiatrica Scandinavica, 134(6), 461–468. 10.1111/acps.12652 [DOI] [PubMed] [Google Scholar]
- *Bien GD (2004). The effects of major depression on the verbal learning and memory performance of elderly outpatients on the California Verbal Learning Test (Publication No. 3134397) [Doctoral dissertation, Pacific Graduate School of Psychology]. [Google Scholar]
- Bireta TJ, Guitard D, Neath I, & Surprenant AM (2021). Valence does not affect serial recall. Canadian Journal of Experimental Psychology, 75(1), 35–47. 10.1037/cep0000239 [DOI] [PubMed] [Google Scholar]
- *Bisol Balardin J, Vedana G, Ludwig A, de Lima DB, Argimon I, Schneider R, Luz C, Schroder N, & Bromberg E (2009). Contextual memory and encoding strategies in young and older adults with and without depressive symptoms. Aging & Mental Health, 13(3), 313–318. 10.1080/13607860802534583 [DOI] [PubMed] [Google Scholar]
- *Boeker H, Schulze J, Richter A, Nikisch G, Schuepbach D, & Grimm S (2012). Sustained cognitive impairments after clinical recovery of severe depression. Journal of Nervous and Mental Disease, 200(9), 773–776. 10.1097/NMD.0b013e318266ba14 [DOI] [PubMed] [Google Scholar]
- Boldrini M, Underwood MD, Hen R, Rosoklija GB, Dwork AJ, John Mann J, & Arango V (2009). Antidepressants increase neural progenitor cells in the human hippocampus. Neuropsychopharmacology, 34(11), 2376–2389. 10.1038/npp.2009.75 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Bolshin LA (2020). Tunnel vision: A novel investigation of the effect of depression on field of view (Publication No. 27831513) [Doctoral dissertation, Fielding Graduate University]. [Google Scholar]
- Bora E, Harrison BJ, Yücel M, & Pantelis C (2013). Cognitive impairment in euthymic major depressive disorder: A meta-analysis. Psychological Medicine, 43(10), 2017–2026. 10.1017/S0033291712002085 [DOI] [PubMed] [Google Scholar]
- *Botelho de Oliveira S, Flórez RN, & Caballero DA. (2012). Consistent declarative memory with depressive symptomatology. Revista Colombiana de Psiquiatría, 41(4), 881–899. 10.1016/S0034-7450(14)60053-6 [DOI] [PubMed] [Google Scholar]
- Bower GH (1981). Mood and memory. American Psychologist, 36(2), 129–148. 10.1037/0003-066X.36.2.129 [DOI] [PubMed] [Google Scholar]
- *Bradley BP, Mogg K, & Williams R (1995). Implicit and explicit memory for emotion-congruent information in clinical depression and anxiety. Behaviour Research and Therapy, 33(7), 755–770. 10.1016/0005-7967(95)00029-W [DOI] [PubMed] [Google Scholar]
- Braver TS (2012). The variable nature of cognitive control: A dual mechanisms framework. Trends in Cognitive Sciences, 16(2), 106–113. 10.1016/j.tics.2011.12.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Brébion G, Smith MJ, & Widlocher D (1997). Discrimination and response bias in memory: Effects of depression severity and psychomotor retardation. Psychiatry Research, 70(2), 95–103. 10.1016/S0165-1781(97)03098-9 [DOI] [PubMed] [Google Scholar]
- *Bremner JD, Vythilingam M, Vermetten E, Vaccarino V, & Charney DS (2004). Deficits in hippocampal and anterior cingulate functioning during verbal declarative memory encoding in midlife major depression. The American Journal of Psychiatry, 161(4), 637–645. 10.1176/appi.ajp.161.4.637 [DOI] [PubMed] [Google Scholar]
- *Bruno D, Nierenberg J, Cooper TB, Marmar CR, Zetterberg H, Blennow K, Hashimoto K, & Pomara N (2017). The recency ratio is associated with reduced CSF glutamate in late-life depression. Neurobiology of Learning and Memory, 141, 14–18. 10.1016/j.nlm.2017.03.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burt DB, Zembar MJ, & Niederehe G (1995). Depression and memory impairment: A meta-analysis of the association, its pattern, and specificity. Psychological Bulletin, 117(2), 285–305. 10.1037/0033-2909.117.2.285 [DOI] [PubMed] [Google Scholar]
- Butters MA, Young JB, Lopez O, Aizenstein HJ, Mulsant BH, Reynolds CF III, DeKosky ST, & Becker JT (2008). Pathways linking late-life depression to persistent cognitive impairment and dementia. Dialogues in Clinical Neuroscience, 10(3), 345–357. 10.31887/DCNS.2008.10.3/mabutters [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers AL, Covinsky KE, Barnes DE, & Yaffe K (2012). Dysthymia and depression increase risk of dementia and mortality among older veterans. The American Journal of Geriatric Psychiatry, 20(8), 664–672. 10.1097/JGP.0b013e31822001c1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byers AL, & Yaffe K (2011). Depression and risk of developing dementia. Nature Reviews. Neurology, 7(6), 323–331. 10.1038/nrneurol.2011.60 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Albert M, Belleville S, Craik FIM, Duarte A, Grady CL, Lindenberger U, Nyberg L, Park DC, Reuter-Lorenz PA, Rugg MD, Steffener J, & Rajah MN (2018). Maintenance, reserve and compensation: The cognitive neuroscience of healthy ageing. Nature Reviews Neuroscience, 19(11), 701–710. 10.1038/s41583-018-0068-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Callahan BL, Laforce R Jr., Dugas M, & Hudon C (2017). Memory for emotional images differs according to the presence of depressive symptoms in individuals at risk for dementia. International Psychogeriatrics, 29(4), 673–685. 10.1017/S1041610216002179 [DOI] [PubMed] [Google Scholar]
- Cambridge OR, Knight MJ, Mills N, & Baune BT (2018). The clinical relationship between cognitive impairment and psychosocial functioning in major depressive disorder: A systematic review. Psychiatry Research, 269, 157–171. 10.1016/j.psychres.2018.08.033 [DOI] [PubMed] [Google Scholar]
- Campbell KL, Grady CL, Ng C, & Hasher L (2012). Age differences in the frontoparietal cognitive control network: Implications for distractibility. Neuropsychologia, 50(9), 2212–2223. 10.1016/j.neuropsychologia.2012.05.025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao X, Liu Z, Xu C, Li J, Gao Q, Sun N, Xu Y, Ren Y, Yang C, & Zhang K (2012). Disrupted resting-state functional connectivity of the hippocampus in medication-naïve patients with major depressive disorder. Journal of Affective Disorders, 141(2–3), 194–203. 10.1016/j.jad.2012.03.002 [DOI] [PubMed] [Google Scholar]
- Carstensen LL, & Mikels JA (2005). At the intersection of emotion and cognition—Aging and the positivity effect. Current Directions in Psychological Science, 14(3), 117–121. 10.1111/j.0963-7214.2005.00348.x [DOI] [Google Scholar]
- Casaletto KB, Marx G, Dutt S, Neuhaus J, Saloner R, Kritikos L, Miller B, & Kramer JH (2017). Is “Learning” episodic memory? Distinct cognitive and neuroanatomic correlates of immediate recall during learning trials in neurologically normal aging and neurodegenerative cohorts. Neuropsychologia, 102, 19–28. 10.1016/j.neuropsychologia.2017.05.021 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Caspi A, Houts RM, Belsky DW, Goldman-Mellor SJ, Harrington H, Israel S, Meier MH, Ramrakha S, Shalev I, Poulton R, & Moffitt TE (2014). The p factor: One general psychopathology factor in the structure of psychiatric disorders? Clinical Psychological Science, 2(2), 119–137. 10.1177/2167702613497473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Cerny BM, Stange JP, Kling LR, Hamlat EJ, O’Donnell LA, Deveney C, & Langenecker SA (2019). Self-reported affective biases, but not all affective performance biases, are present in depression remission. British Journal of Clinical Psychology, 58(3), 274–288. 10.1111/bjc.12217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Channon S, Baker JE, & Robertson MM (1993). Effects of structure and clustering on recall and recognition memory in clinical depression. Journal of Abnormal Psychology, 102(2), 323–326. 10.1037/0021-843X.102.2.323 [DOI] [PubMed] [Google Scholar]
- Channon S, & Green PS (1999). Executive function in depression: The role of performance strategies in aiding depressed and non-depressed participants. Journal of Neurology, Neurosurgery, and Psychiatry, 66(2), 162–171. 10.1136/jnnp.66.2.162 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SB, Gamino JF, Cook LG, Hanten G, Li X, & Levin HS (2006). Impaired discourse gist and working memory in children after brain injury. Brain and Language, 97(2), 178–188. 10.1016/j.bandl.2005.10.002 [DOI] [PubMed] [Google Scholar]
- Chentsova-Dutton YE, Ryder AG, & Tsai J (2014). Understanding depression across cultural contexts. In Gotlib IH & Hammen CL (Eds.), Handbook of depression (pp. 337–354). Guilford Press. [Google Scholar]
- Cheung MW (2014). Modeling dependent effect sizes with three-level meta-analyses: A structural equation modeling approach. Psychological Methods, 19(2), 211–229. 10.1037/a0032968 [DOI] [PubMed] [Google Scholar]
- *Chu CS, Sun IW, Begum A, Liu SI, Chang CJ, Chiu WC, Chen C-H, Tang H-S, Yang C-L, Lin Y-C, Chiu C-C, & Stewart R (2017). The association between subjective memory complaint and objective cognitive function in older people with previous major depression. PLOS ONE, 12(3), Article e0173027. 10.1371/journal.pone.0173027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Clark L, Sarna A, & Goodwin GM (2005). Impairment of executive function but not memory in first-degree relatives of patients with bipolar I disorder and in euthymic patients with unipolar depression. The American Journal of Psychiatry, 162(10), 1980–1982. 10.1176/appi.ajp.162.10.1980 [DOI] [PubMed] [Google Scholar]
- *Cláudio V, Noronha M, & Balola MF (2014). Directed forgetting in major depression. Revista de Psicopatología y Psicología Clínica, 19(2), 105–115. 10.5944/rppc.vol.19.num.2.2014.1306 [DOI] [Google Scholar]
- Cohn M, Emrich SM, & Moscovitch M (2008). Age-related deficits in associative memory: The influence of impaired strategic retrieval. Psychology and Aging, 23(1), 93–103. 10.1037/0882-7974.23.1.93 [DOI] [PubMed] [Google Scholar]
- *Colby CA (1982). Memory deficit in moderately depressed university students (Publication No. NK52956) [Doctoral dissertation, University of Western Ontario]. [Google Scholar]
- Coles NA, Larsen JT, & Lench HC (2019). A meta-analysis of the facial feedback literature: Effects of facial feedback on emotional experience are small and variable. Psychological Bulletin, 145(6), 610–651. 10.1037/bul0000194 [DOI] [PubMed] [Google Scholar]
- Conner KO, Copeland VC, Grote NK, Koeske G, Rosen D, Reynolds CF III, & Brown C (2010). Mental health treatment seeking among older adults with depression: The impact of stigma and race. The American Journal of Geriatric Psychiatry, 18(6), 531–543. 10.1097/JGP.0b013e3181cc0366 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Constant EL, Adam S, Gillain B, Lambert M, Masquelier E, & Seron X (2011). Cognitive deficits in patients with chronic fatigue syndrome compared to those with major depressive disorder and healthy controls. Clinical Neurology and Neurosurgery, 113(4), 295–302. 10.1016/j.clineuro.2010.12.002 [DOI] [PubMed] [Google Scholar]
- *Contador I, Fernandez-Calvo B, Cacho J, Ramos F, & Lopez-Rolon A (2010). Nonverbal memory tasks in early differential diagnosis of Alzheimer’s disease and unipolar depression. Applied Neuropsychology, 17(4), 251–261. 10.1080/09084282.2010.525098 [DOI] [PubMed] [Google Scholar]
- *Contador-Castillo I, Fernández-Calvo B, Cacho-Gutiérrez LJ, Ramos-Campos F, & Hernández-Martín L (2009). Depression in Alzheimer type-dementia: Is there any effect on memory performance. Revista de Neurología, 49(10), 505–510. 10.33588/rn.4910.2009130 [DOI] [PubMed] [Google Scholar]
- *Corrêa MS, Balardin JB, Caldieraro MA, Fleck MP, Argimon I, Luz C, & Bromberg E (2012). Contextual recognition memory deficits in major depression are suppressed by cognitive support at encoding. Biological Psychology, 89(2), 293–299. 10.1016/j.biopsycho.2011.11.001 [DOI] [PubMed] [Google Scholar]
- *Cottencin O, Gruat G, Thomas P, Devos P, Goudemand M, & Consoli SM (2008). Directed forgetting in depression. Journal of the International Neuropsychological Society, 14(5), 895–899. 10.1017/S1355617708081186 [DOI] [PubMed] [Google Scholar]
- Craik FI, & Rose NS (2012). Memory encoding and aging: A neurocognitive perspective. Neuroscience and Biobehavioral Reviews, 36(7), 1729–1739. 10.1016/j.neubiorev.2011.11.007 [DOI] [PubMed] [Google Scholar]
- Craik FIM (1983). On the transfer of information from temporary to permanent memory. Philosophical Transactions of the Royal Society of London. Series B, Biological Sciences, 302(1110), 341–359. 10.1098/rstb.1983.0059 [DOI] [Google Scholar]
- *Croisile B, Astier JL, Beaumont C, & Mollion H (2011). The 5-word test in 37 depressed patients compared with 36 normal controls and 35 patients with mild Alzheimer’s disease [Evaluation de la memoire au moyen du Test des 5 mots chez 37 depressifs compares a 36 temoins et 35 patients ayant une forme legere de maladie d’Alzheimer]. Encephale, 37(2), 127–132. 10.1016/j.encep.2010.10.003 [DOI] [PubMed] [Google Scholar]
- *Crowson JJ Jr. (1997). Self-focus, viewing preference, and memory valence in depression (Publication No. 9827506) [Doctoral dissertation, University of Kansas]. [Google Scholar]
- Cuijpers P, & Smit F (2004). Subthreshold depression as a risk indicator for major depressive disorder: A systematic review of prospective studies. Acta Psychiatrica Scandinavica, 109(5), 325–331. 10.1111/j.1600-0447.2004.00301.x [DOI] [PubMed] [Google Scholar]
- Danckert SL, & Craik FI (2013). Does aging affect recall more than recognition memory? Psychology and Aging, 28(4), 902–909. 10.1037/a0033263 [DOI] [PubMed] [Google Scholar]
- *Daniel BD, Montali A, Gerra ML, Innamorati M, Girardi P, Pompili M, & Amore M (2013). Cognitive impairment and its associations with the path of illness in affective disorders: A comparison between patients with bipolar and unipolar depression in remission. Journal of Psychiatric Practice, 19(4), 275–287. 10.1097/01.pra.0000432597.79019.e2 [DOI] [PubMed] [Google Scholar]
- *Danion JM, Kauffmann-Muller F, Grangé D, Zimmermann MA, & Greth P (1995). Affective valence of words, explicit and implicit memory in clinical depression. Journal of Affective Disorders, 34(3), 227–234. 10.1016/0165-0327(95)00021-E [DOI] [PubMed] [Google Scholar]
- *Dannehl K, Rief W, & Euteneuer F (2019). Effects of cognitive behavioural therapy on verbal learning and memory in major depression: Results of a randomized controlled trial. Clinical Psychology & Psychotherapy, 26(3), 291–297. 10.1002/cpp.2350 [DOI] [PubMed] [Google Scholar]
- de Flores R, La Joie R, & Chételat G (2015). Structural imaging of hippocampal subfields in healthy aging and Alzheimer’s disease. Neuroscience, 309, 29–50. 10.1016/j.neuroscience.2015.08.033 [DOI] [PubMed] [Google Scholar]
- *Degl’Innocenti A, & Bäckman L (1999). Source memory in major depression. Journal of Affective Disorders, 54(1–2), 205–209. 10.1016/S0165-0327(98)00167-0 [DOI] [PubMed] [Google Scholar]
- *Delaloye C, Baudois S, de Bilbao F, Dubois Remund C, Hofer F, Lamon M, Ragno Paquier C, Weber K, Herrmann FR, Giardini U, & Giannakopoulos P (2008). Cognitive impairment in late-onset depression. Limited to a decrement in information processing resources? European Neurology, 60(3), 149–154. 10.1159/000144086 [DOI] [PubMed] [Google Scholar]
- *Delaloye C, Moy G, de Bilbao F, Baudois S, Weber K, Hofer F, Paquier CR, Donati A, Canuto A, Giardini U, von Gunten A, Stancu RI, Lazeyras F, Millet P, Scheltens P, Giannakopoulos P, & Gold G (2010). Neuroanatomical and neuropsychological features of elderly euthymic depressed patients with early- and late-onset. Journal of the Neurological Sciences, 299(1–2), 19–23. 10.1016/j.jns.2010.08.046 [DOI] [PubMed] [Google Scholar]
- *Delgado VB, Kapczinski F, & Chaves ML (2012). Memory mood congruency phenomenon in bipolar I disorder and major depression disorder patients. Brazilian Journal of Medical and Biological Research, 45(9), 856–861. 10.1590/S0100-879X2012007500098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delis DC, Kramer JH, Kaplan E, & Ober BA (2000). California Verbal Learning Test—Second edition. Adult version. Manual. Psychological Corporation. [Google Scholar]
- *Denny EB, & Hunt RR (1992). Affective valence and memory in depression: Dissociation of recall and fragment completion. Journal of Abnormal Psychology, 101(3), 575–580. 10.1037/0021-843X.101.3.575 [DOI] [PubMed] [Google Scholar]
- Deuschle M, Kniest A, Niemann H, Erb-Bies M, Colla N, Hamann B, & Heuser I (2004). Impaired declarative memory in depressed patients is slow to recover: Clinical experience. Pharmacopsychiatry, 37(4), 147–151. 10.1055/s-2004-827168 [DOI] [PubMed] [Google Scholar]
- *DeVille DC, Kerr KL, Avery JA, Burrows K, Bodurka J, Feinstein JS, Khalsa SS, Paulus MP, & Simmons WK (2018). The neural bases of interoceptive encoding and recall in healthy adults and adults with depression. Biological Psychiatry: Cognitive Neuroscience and Neuroimaging, 3(6), 546–554. 10.1016/j.bpsc.2018.03.010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Dietsche B, Backes H, Stratmann M, Konrad C, Kircher T, & Krug A (2014). Altered neural function during episodic memory encoding and retrieval in major depression. Human Brain Mapping, 35(9), 4293–4302. 10.1002/hbm.22475 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Dillon DG, Dobbins IG, & Pizzagalli DA (2014). Weak reward source memory in depression reflects blunted activation of VTA/SN and parahippocampus. Social Cognitive and Affective Neuroscience, 9(10), 1576–1583. 10.1093/scan/nst155 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Disner SG, Beevers CG, Haigh EA, & Beck AT (2011). Neural mechanisms of the cognitive model of depression. Nature Reviews Neuroscience, 12(8), 467–477. 10.1038/nrn3027 [DOI] [PubMed] [Google Scholar]
- *Dömötör E, Sarosi A, Balogh G, Szekely A, Hejjas K, Sasvari-Szekely M, & Faludi G (2007). Association of neurocognitive endophenotype and STin2 polymorphism in major depressive disorder [Neurokognitiv endofenotipus es STin2 polimorfizmus asszociacio vizsgalata major depresszioban]. Neuropsychopharmacologia Hungarica, 9(2), 53–62. https://www.ncbi.nlm.nih.gov/pubmed/17970527 [PubMed] [Google Scholar]
- Dotson VM, Beydoun MA, & Zonderman AB (2010). Recurrent depressive symptoms and the incidence of dementia and mild cognitive impairment. Neurology, 75(1), 27–34. 10.1212/WNL.0b013e3181e62124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson VM, McClintock SM, Verhaeghen P, Kim JU, Draheim AA, Syzmkowicz SM, Gradone AM, Bogoian HR, & Wit L (2020). Depression and cognitive control across the lifespan: A systematic review and meta-analysis. Neuropsychology Review, 30(4), 461–476. 10.1007/s11065-020-09436-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dotson VM, Resnick SM, & Zonderman AB (2008). Differential association of concurrent, baseline, and average depressive symptoms with cognitive decline in older adults. The American Journal of Geriatric Psychiatry, 16(4), 318–330. 10.1097/JGP.0b013e3181662a9c [DOI] [PMC free article] [PubMed] [Google Scholar]
- Douglas KM, & Porter RJ (2009). Longitudinal assessment of neuropsychological function in major depression. The Australian and New Zealand Journal of Psychiatry, 43(12), 1105–1117. 10.3109/00048670903279887 [DOI] [PubMed] [Google Scholar]
- *Dowis JD (1982). The effects of depression on recall of negative and positive words and feedback (Publication No. 8318846) [Doctoral dissertation, Indiana University]. [Google Scholar]
- *Drakeford JL, Edelstyn NM, Oyebode F, Srivastava S, Calthorpe WR, & Mukherjee T (2010). Recollection deficiencies in patients with major depressive disorder. Psychiatry Research, 175(3), 205–210. 10.1016/j.psychres.2008.08.010 [DOI] [PubMed] [Google Scholar]
- Dresler M, Kluge M, Genzel L, Schüssler P, & Steiger A (2010). Impaired off-line memory consolidation in depression. European Neuropsychopharmacology, 20(8), 553–561. 10.1016/j.euroneuro.2010.02.002 [DOI] [PubMed] [Google Scholar]
- Duarte A, & Dulas M (2020). Episodic memory decline in aging. In Thomas AK & Gutchess A (Eds.), The Cambridge handbook of cognitive aging: A life course perspective (pp. 200–217). Cambridge University Press. 10.1017/9781108552684.013 [DOI] [Google Scholar]
- Duarte A, Henson RN, & Graham KS (2008). The effects of aging on the neural correlates of subjective and objective recollection. Cerebral Cortex (New York, N.Y.), 18(9), 2169–2180. 10.1093/cercor/bhm243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duarte A, & Kensinger E (2019). Age-related changes in episodic memory. In Samanez-Larkin GR (Ed.), The aging brain: Functional adaptation across adulthood. American Psychological Association. 10.1037/0000143-005 [DOI] [Google Scholar]
- *Dunbar GC, & Lishman WA (1984). Depression, recognition-memory and hedonic tone a signal detection analysis. The British Journal of Psychiatry, 144, 376–382. 10.1192/bjp.144.4.376 [DOI] [PubMed] [Google Scholar]
- Dwan K, Gamble C, Williamson PR, Kirkham JJ, & the Reporting Bias Group. (2013). Systematic review of the empirical evidence of study publication bias and outcome reporting bias—An updated review. PLOS ONE, 8(7), Article e66844. 10.1371/journal.pone.0066844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Edington SW (1987). The effects of depression, context statements, and word pleasantness on recall in college students (Publication No. 8812002) [Doctoral dissertation, Hofstra University]. ProQuest Dissertations & Theses Global. [Google Scholar]
- Eichenbaum H, Yonelinas AP, & Ranganath C (2007). The medial temporal lobe and recognition memory. Annual Review of Neuroscience, 30, 123–152. 10.1146/annurev.neuro.30.051606.094328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Elderkin-Thompson V, Mintz J, Haroon E, Lavretsky H, & Kumar A (2007). Executive dysfunction and memory in older patients with major and minor depression. Archives of Clinical Neuropsychology, 22(2), 261–270. 10.1016/j.acn.2007.01.021 [DOI] [PubMed] [Google Scholar]
- *Ellis AJ, Wells TT, Vanderlind WM, & Beevers CG (2014). The role of controlled attention on recall in major depression. Cognition and Emotion, 28(3), 520–529. 10.1080/02699931.2013.832153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis HC, & Ashbrook PW (1988). Resource allocation model of the effects of depressed mood states on memory. In Fiedler K& Forgas JP (Eds.), Affect, cognition, and social behavior (pp. 25–43). Hogrefe. [Google Scholar]
- *Ellwart T, Rinck M, & Becker ES (2003). Selective memory and memory deficits in depressed inpatients. Depression and Anxiety, 17(4), 197–206. 10.1002/da.10102 [DOI] [PubMed] [Google Scholar]
- *Everaert J, Tierens M, Uzieblo K, & Koster EH (2013). The indirect effect of attention bias on memory via interpretation bias: Evidence for the combined cognitive bias hypothesis in subclinical depression. Cognition and Emotion, 27(8), 1450–1459. 10.1080/02699931.2013.787972 [DOI] [PubMed] [Google Scholar]
- *Fairhall SL, Sharma S, Magnusson J, & Murphy B (2010). Memory related dysregulation of hippocampal function in major depressive disorder. Biological Psychology, 85(3), 499–503. 10.1016/j.biopsycho.2010.09.002 [DOI] [PubMed] [Google Scholar]
- *Fritzsche A, Dahme B, Gotlib IH, Joormann J, Magnussen H, Watz H, Nutzinger DO, & von Leupoldt A (2010). Specificity of cognitive biases in patients with current depression and remitted depression and in patients with asthma. Psychological Medicine, 40(5), 815–826. 10.1017/S0033291709990948 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Fritzsche A, Watz H, Magnussen H, Tuinmann G, Löwe B, & von Leupoldt A (2013). Cognitive biases in patients with chronic obstructive pulmonary disease and depression—A pilot study. British Journal of Health Psychology, 18(4), 827–843. 10.1111/bjhp.12025 [DOI] [PubMed] [Google Scholar]
- Gale CR, Allerhand M, Deary IJ, & the HALCyon Study Team. (2012). Is there a bidirectional relationship between depressive symptoms and cognitive ability in older people? A prospective study using the English Longitudinal Study of Ageing. Psychological Medicine, 42(10), 2057–2069. 10.1017/S0033291712000402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Gallassi R, Di Sarro R, Morreale A, & Amore M (2006). Memory impairment in patients with late-onset major depression: The effect of antidepressant therapy. Journal of Affective Disorders, 91(2–3), 243–250. 10.1016/j.jad.2006.01.018 [DOI] [PubMed] [Google Scholar]
- Gallo JJ, Rebok GW, Tennsted S, Wadley VG, Horgas A, & the Advanced Cognitive Training for Independent and Vital Elderly (Active) Study Investigators. (2003). Linking depressive symptoms and functional disability in late life. Aging & Mental Health, 7(6), 469–480. 10.1080/13607860310001594736 [DOI] [PubMed] [Google Scholar]
- *Gandy KC (2015). An EEG investigation of memory in depression: The effect of cognitive processing (Publication N. 1599118) [Master’s thesis, University of Texas at Arlington]. ProQuest Dissertations & Theses Global. [Google Scholar]
- *Garcia RG, Valenza G, Tomaz CA, & Barbieri R (2016). Relationship between cardiac vagal activity and mood congruent memory bias in major depression. Journal of Affective Disorders, 190, 19–25. 10.1016/j.jad.2015.09.075 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gliebus GP (2018). Memory dysfunction. Continuum (Minneap Minn), 24(3), 727–744. 10.1212/CON.0000000000000619 [DOI] [PubMed] [Google Scholar]
- Golby AJ, Poldrack RA, Brewer JB, Spencer D, Desmond JE, Aron AP, & Gabrieli JD (2001). Material-specific lateralization in the medial temporal lobe and prefrontal cortex during memory encoding. Brain: A Journal of Neurology, 124(9), 1841–1854. 10.1093/brain/124.9.1841 [DOI] [PubMed] [Google Scholar]
- *Golinkoff M (1986). Cognitive impairments in depression (Publication No. 8627802) [Doctoral dissertation, University of Illinois at Chicago]. ProQuest Dissertations & Theses Global. [Google Scholar]
- *Gomez RG, Posener JA, Keller J, DeBattista C, Solvason B, & Schatzberg AF (2009). Effects of major depression diagnosis and cortisol levels on indices of neurocognitive function. Psychoneuroendocrinology, 34(7), 1012–1018. 10.1016/j.psyneuen.2009.01.017 [DOI] [PubMed] [Google Scholar]
- Gotlib IH, & Joormann J (2010). Cognition and depression: Current status and future directions. Annual Review of Clinical Psychology, 6, 285–312. 10.1146/annurev.clinpsy.121208.131305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Gourgouvelis J, Yielder P, & Murphy B (2017). Exercise promotes neuroplasticity in both healthy and depressed brains: An fMRI pilot study. Neural Plasticity, 2017, Article 8305287. 10.1155/2017/8305287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grahek I, Shenhav A, Musslick S, Krebs RM, & Koster EHW (2019). Motivation and cognitive control in depression. Neuroscience and Biobehavioral Reviews, 102, 371–381. 10.1016/j.neubiorev.2019.04.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Grant MM, Thase ME, & Sweeney JA (2001). Cognitive disturbance in outpatient depressed younger adults: Evidence of modest impairment. Biological Psychiatry, 50(1), 35–43. 10.1016/S0006-3223(00)01072-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gratton G, Cooper P, Fabiani M, Carter CS, & Karayanidis F (2018). Dynamics of cognitive control: Theoretical bases, paradigms, and a view for the future. Psychophysiology, 55(3), 1–29. 10.1111/psyp.13016 [DOI] [PubMed] [Google Scholar]
- *Grön G, Bittner D, Schmitz B, Wunderlich AP, & Riepe MW (2002). Subjective memory complaints: Objective neural markers in patients with Alzheimer’s disease and major depressive disorder. Annals of Neurology, 51(4), 491–498. 10.1002/ana.10157 [DOI] [PubMed] [Google Scholar]
- *Hach S, Tippett LJ, & Addis DR (2014). Neural changes associated with the generation of specific past and future events in depression. Neuropsychologia, 65, 41–55. 10.1016/j.neuropsychologia.2014.10.003 [DOI] [PubMed] [Google Scholar]
- *Hahn C, Lim HK, Won WY, Joo SH, Ahn KJ, Jung WS, & Lee CU (2015). Sub-regional volumes changes of the corpus callosum in the drug naive patients with late-onset depression. Progress in NeuroPsychopharmacology & Biological Psychiatry, 56, 46–51. 10.1016/j.pnpbp.2014.07.008 [DOI] [PubMed] [Google Scholar]
- *Hamilton JP, & Gotlib IH (2008). Neural substrates of increased memory sensitivity for negative stimuli in major depression. Biological Psychiatry, 63(12), 1155–1162. 10.1016/j.biopsych.2007.12.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Hammar A, & Ardal G (2013). Verbal memory functioning in recurrent depression during partial remission and remission-brief report. Frontiers in Psychology, 4, Article 652. 10.3389/fpsyg.2013.00652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hankin BL (2019). A choose your own adventure story: Conceptualizing depression in children and adolescents from traditional DSM and alternative latent dimensional approaches. Behaviour Research and Therapy, 118, 94–100. 10.1016/j.brat.2019.04.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Hansson PB, Murison R, Lund A, & Hammar Å (2015). Cognitive functioning and cortisol profiles in first episode major depression. Scandinavian Journal of Psychology, 56(4), 379–383. 10.1111/sjop.12230 [DOI] [PubMed] [Google Scholar]
- *Harrington MO, Nedberge KM, & Durrant SJ (2018). The effect of sleep deprivation on emotional memory consolidation in participants reporting depressive symptoms. Neurobiology of Learning and Memory, 152, 10–19. 10.1016/j.nlm.2018.04.013 [DOI] [PubMed] [Google Scholar]
- *Hart RP, Kwentus JA, Hamer RM, & Taylor JR (1987). Selective reminding procedure in depression and dementia. Psychology and Aging, 2(2), 111–115. 10.1037/0882-7974.2.2.111 [DOI] [PubMed] [Google Scholar]
- Hasselbalch BJ, Knorr U, & Kessing LV (2011). Cognitive impairment in the remitted state of unipolar depressive disorder: A systematic review. Journal of Affective Disorders, 134(1–3), 20–31. 10.1016/j.jad.2010.11.011 [DOI] [PubMed] [Google Scholar]
- Hedges LV, & Olkin I (1985). Statistical methods for meta-analysis. Academic Press. [Google Scholar]
- Heo M, Murphy CF, & Meyers BS (2007). Relationship between the Hamilton Depression Rating Scale and the Montgomery-Asberg Depression Rating Scale in depressed elderly: A meta-analysis. The American Journal of Geriatric Psychiatry, 15(10), 899–905. 10.1097/JGP.0b013e318098614e [DOI] [PubMed] [Google Scholar]
- *Hertel PT (2000). The cognitive-initiative account of depression-related impairments in memory. In Medin D (Ed.), The psychology of learning and motivation (Vol. 39, pp. 47–71). Academic Press. [Google Scholar]
- *Hertel PT, & El-Messidi L (2006). Am I blue? Depressed mood and the consequences of self-focus for the interpretation and recall of ambiguous words. Behavior Therapy, 37(3), 259–268. 10.1016/j.beth.2006.01.003 [DOI] [PubMed] [Google Scholar]
- *Hertel PT, & Mahan A (2008). Depression-related differences in learning and forgetting responses to unrelated cues. Acta Psychologica, 127(3), 636–644. 10.1016/j.actpsy.2007.11.004 [DOI] [PubMed] [Google Scholar]
- *Hertel PT, & Milan S (1994). Depressive deficits in recognition: Dissociation of recollection and familiarity. Journal of Abnormal Psychology, 103(4), 736–742. 10.1037/0021-843X.103.4.736 [DOI] [PubMed] [Google Scholar]
- *Hertel PT, & Rude SS (1991). Depressive deficits in memory: Focusing attention improves subsequent recall. Journal of Experimental Psychology: General, 120(3), 301–309. 10.1037/0096-3445.120.3.301 [DOI] [PubMed] [Google Scholar]
- Higgins JP, & Thompson SG (2002). Quantifying heterogeneity in a meta-analysis. Statistics in Medicine, 21(11), 1539–1558. 10.1002/sim.1186 [DOI] [PubMed] [Google Scholar]
- *Hill PB (1990). Memorial processing in depression: Are there deficits? (Publication No. 9100288) [Doctoral dissertation, Temple University]. ProQuest Dissertations & Theses Global. [Google Scholar]
- Hjorthøj CR, Vesterager L, & Nordentoft M (2013). Test-retest reliability of the Danish Adult Reading Test in patients with comorbid psychosis and cannabis-use disorder. Nordic Journal of Psychiatry, 67(3), 159–163. 10.3109/08039488.2012.691544 [DOI] [PubMed] [Google Scholar]
- *Howe ML, & Malone C (2011). Mood-congruent true and false memory: Effects of depression. Memory, 19(2), 192–201. 10.1080/09658211.2010.544073 [DOI] [PubMed] [Google Scholar]
- *Huang CL (2009). Residual cognitive deficit in adults with depression who recovered after 6-month treatment: Stable versus state-dependent markers. Journal of Clinical Medicine Research, 1(4), 202–206. 10.4021/jocmr2009.10.1266 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Hunt J (1997). Selective memory bias in the processing of weight, shape and food related words in women with bulimia nervosa, depression and female non-clinical controls (Publication No. 27696828) [Doctoral dissertation, Open University]. ProQuest Dissertations & Theses Global. [Google Scholar]
- Hurley LL, & Tizabi Y (2013). Neuroinflammation, neurodegeneration, and depression. Neurotoxicity Research, 23(2), 131–144. 10.1007/s12640-012-9348-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacoby LL, Bishara AJ, Hessels S, & Toth JP (2005). Aging, subjective experience, and cognitive control: Dramatic false remembering by older adults. Journal of Experimental Psychology: General, 134(2), 131–148. 10.1037/0096-3445.134.2.131 [DOI] [PubMed] [Google Scholar]
- James T, Strunk J, Arndt J, & Duarte A (2016). Age-related deficits in selective attention during encoding increase demands on episodic reconstruction during context retrieval: An ERP study. Neuropsychologia, 86, 66–79. 10.1016/j.neuropsychologia.2016.04.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Jayaweera HK, Hickie IB, Duffy SL, Mowszowski L, Norrie L, Lagopoulos J, & Naismith SL (2016). Episodic memory in depression: The unique contribution of the anterior caudate and hippocampus. Psychological Medicine, 46(10), 2189–2199. 10.1017/S0033291716000787 [DOI] [PubMed] [Google Scholar]
- *Jermann F, van der Linden M, & D’Argembeau A (2008). Identity recognition and happy and sad facial expression recall: Influence of depressive symptoms. Memory, 16(4), 364–373. 10.1080/09658210801935413 [DOI] [PubMed] [Google Scholar]
- John LK, Loewenstein G, & Prelec D (2012). Measuring the prevalence of questionable research practices with incentives for truth telling. Psychological Science, 23(5), 524–532. 10.1177/0956797611430953 [DOI] [PubMed] [Google Scholar]
- Jones RN, Manly J, Glymour MM, Rentz DM, Jefferson AL, & Stern Y (2011). Conceptual and measurement challenges in research on cognitive reserve. Journal of the International Neuropsychological Society, 17(4), 593–601. 10.1017/S1355617710001748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joormann J, Hertel PT, LeMoult J, & Gotlib IH (2009). Training forgetting of negative material in depression. Journal of Abnormal Psychology, 118(1), 34–43. 10.1037/a0013794 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juan SMA, & Adlard PA (2019). Ageing and cognition. In Harris J & Korolchuk V (Eds.), Biochemistry and cell biology of ageing: Part II clinical science. Subcellular biochemistry (Vol. 91). Springer. [Google Scholar]
- Juhasz G, Eszlari N, Pap D, & Gonda X (2012). Cultural differences in the development and characteristics of depression. Neuropsychopharmacologia Hungarica, 14(4), 259–265. https://www.ncbi.nlm.nih.gov/pubmed/23269213 [PubMed] [Google Scholar]
- *Kaczmarczyk M, Wingenfeld K, Kuehl LK, Otte C, & Hinkelmann K (2018). Childhood trauma and diagnosis of major depression: Association with memory and executive function. Psychiatry Research, 270, 880–886. 10.1016/j.psychres.2018.10.071 [DOI] [PubMed] [Google Scholar]
- Karsten J, Hartman CA, Smit JH, Zitman FG, Beekman AT, Cuijpers P, Willem van der Does AJ, Ormel J, Nolen WA, & Penninx BW. (2011). Psychiatric history and subthreshold symptoms as predictors of the occurrence of depressive or anxiety disorder within 2 years. The British Journal of Psychiatry, 198(3), 206–212. 10.1192/bjp.bp.110.080572 [DOI] [PubMed] [Google Scholar]
- *Kaschel R, Logie RH, Kazén M, & Della Sala S (2009). Alzheimer’s disease, but not ageing or depression, affects dual-tasking. Journal of Neurology, 256(11), 1860–1868. 10.1007/s00415-009-5210-7 [DOI] [PubMed] [Google Scholar]
- *Kassel MT, Rao JA, Walker SJ, Briceño EM, Gabriel LB, Weldon AL, Avery ET, Haase BD, Peciña M, Considine CM, Noll DC, Bieliauskas LA, Starkman MN, Zubieta J-K, Welsh RC, Giordani B, Weisenbach SL, & Langenecker SA (2016). Decreased fronto-limbic activation and disrupted semantic-cued list learning in major depressive disorder. Journal of the International Neuropsychological Society, 22(4), 412–425. 10.1017/S1355617716000023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Kaymak SU, Demir B, Sentürk S, Tatar I, Aldur MM, & Uluğ B (2010). Hippocampus, glucocorticoids and neurocognitive functions in patients with first-episode major depressive disorders. European Archives of Psychiatry and Clinical Neuroscience, 260(3), 217–223. 10.1007/s00406-009-0045-x [DOI] [PubMed] [Google Scholar]
- *Khatri N (2003). Cognition and depression: Implicit memory in remitted females (Publication No. NQ87048) [Doctoral dissertation, University of Calgary]. ProQuest Dissertations & Theses Global. [Google Scholar]
- Kindermann SS, & Brown GG (1997). Depression and memory in the elderly: A meta-analysis. Journal of Clinical and Experimental Neuropsychology, 19(5), 625–642. 10.1080/01688639708403749 [DOI] [PubMed] [Google Scholar]
- *Kircanski K, Mazur H, & Gotlib IH (2013). Behavioral activation system moderates self-referent processing following recovery from depression. Psychological Medicine, 43(9), 1909–1919. 10.1017/S0033291712002851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klein SB, & Nelson CR (2014). The effects of self-reference on memory: A conceptual and methodological review of inferences warranted by the self-reference effect. In Perfect TJ & Lindsay DS(Eds.), The SAGE handbook of applied memory (pp. 256–272). SAGE Publications. 10.4135/9781446294703.n15 [DOI] [Google Scholar]
- Knight BG, & Durbin K (2015). Aging and the effects of emotion on cognition: Implications for psychological interventions for depression and anxiety. PsyCh Journal, 4(1), 11–19. 10.1002/pchj.84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Knutelska ME (2005). Learning profiles in healthy aging, late-life depression and Alzheimer’s disease: The serial position effect in free recall (Publication No. 3159227) [Doctoral dissertation, City University of New York]. ProQuest Dissertations & Theses Global. [Google Scholar]
- *Koenig AM, DeLozier IJ, Zmuda MD, Marron MM, Begley AE , Anderson SJ, Reynolds CF III, Arnold SE, Becker JT, & Butters MA (2015). Neuropsychological functioning in the acute and remitted States of late-life depression. Journal of Alzheimer’s Disease, 45(1), 175–185. 10.3233/JAD-148006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Köhler S, van Boxtel MP, van Os J, Thomas AJ, O’Brien JT, Jolles J, Jolles J, Verhey FRJ, & Allardyce J (2010). Depressive symptoms and cognitive decline in community-dwelling older adults. Journal of the American Geriatrics Society, 58(5), 873–879. 10.1111/j.1532-5415.2010.02807.x [DOI] [PubMed] [Google Scholar]
- *Korten NC, Penninx BW, Kok RM, Stek ML, Oude Voshaar RC , Deeg DJ, & Comijs HC (2014). Heterogeneity of late-life depression: Relationship with cognitive functioning. International Psychogeriatrics, 26(6), 953–963. 10.1017/S1041610214000155 [DOI] [PubMed] [Google Scholar]
- *Krasnoperova EN (1998). Attentional and memory biases for interpersonal stimuli in clinical depression and anxiety (Publication No. 9908797) [Doctoral dissertation, Stanford University]. ProQuest Dissertations & Theses Global. [Google Scholar]
- Kray J, & Ferdinand NK (2014). Task switching and aging. In Grange JA & Houghton G (Eds.), Task switching and cognitive control (pp. 350–371). Oxford University Press. 10.1093/acprof:osobl/9780199921959.003.0014 [DOI] [Google Scholar]
- Kuhl BA, & Chun MM (2014). Memory and attention. In Nobre AC& Kastner S (Eds.), Oxford library of psychology. The Oxford handbook of attention (pp. 806–836). Oxford University Press. [Google Scholar]
- *Kuiper NA, & Derry PA (1982). Depressed and nondepressed content self-reference in mild depressives. Journal of Personality, 50(1), 67–80. 10.1111/j.1467-6494.1982.tb00746.x [DOI] [PubMed] [Google Scholar]
- *Kwiatkowski SJ, & Parkinson SR (1994). Depression, elaboration, and mood congruence: Differences between natural and induced mood. Memory & Cognition, 22(2), 225–233. 10.3758/BF03208893 [DOI] [PubMed] [Google Scholar]
- *Ladegaard N, Larsen ER, Videbech P, & Lysaker PH (2014). Higher-order social cognition in first-episode major depression. Psychiatry Research, 216(1), 37–43. 10.1016/j.psychres.2013.12.010 [DOI] [PubMed] [Google Scholar]
- *Lamar M, Charlton R, Zhang A, & Kumar A (2012). Differential associations between types of verbal memory and prefrontal brain structure in healthy aging and late life depression. Neuropsychologia, 50(8), 1823–1829. 10.1016/j.neuropsychologia.2012.04.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Leal SL, Noche JA, Murray EA, & Yassa MA (2017). Disruption of amygdala-entorhinal-hippocampal network in late-life depression. Hippocampus, 27(4), 464–476. 10.1002/hipo.22705 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Lee CY, Wang LJ, Lee Y, Hung CF, Huang YC, Lee MI, & Lee SY (2018). Differentiating bipolar disorders from unipolar depression by applying the Brief Assessment of Cognition in Affective Disorders. Psychological Medicine, 48(6), 929–938. 10.1017/S003329171700229X [DOI] [PubMed] [Google Scholar]
- Lee RS, Hermens DF, Porter MA, & Redoblado-Hodge MA (2012). A meta-analysis of cognitive deficits in first-episode Major Depressive Disorder. Journal of Affective Disorders, 140(2), 113–124. 10.1016/j.jad.2011.10.023 [DOI] [PubMed] [Google Scholar]
- LeMoult J, & Gotlib IH (2019). Depression: A cognitive perspective. Clinical Psychology Review, 69, 51–66. 10.1016/j.cpr.2018.06.008 [DOI] [PubMed] [Google Scholar]
- Leonard BE, & Myint A (2009). The psychoneuroimmunology of depression. Human Psychopharmacology, 24(3), 165–175. 10.1002/hup.1011 [DOI] [PubMed] [Google Scholar]
- *Lim SL, & Kim JH (2005). Cognitive processing of emotional information in depression, panic, and somatoform disorder. Journal of Abnormal Psychology, 114(1), 50–61. 10.1037/0021-843X.114.1.50 [DOI] [PubMed] [Google Scholar]
- *Liu WH, Wang LZ, Zhao SH, Ning YP, & Chan RC (2012). Anhedonia and emotional word memory in patients with depression. Psychiatry Research, 200(2–3), 361–367. 10.1016/j.psychres.2012.07.025 [DOI] [PubMed] [Google Scholar]
- Lockhart SN, & DeCarli C (2014). Structural imaging measures of brain aging. Neuropsychology Review, 24(3), 271–289. 10.1007/s11065-014-9268-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundervold AJ, Halleland HB, Brevik EJ, Haavik J, & Sørensen L (2019). Verbal memory function in intellectually well-functioning adults with ADHD: Relations to working memory and response inhibition. Journal of Attention Disorders, 23(10), 1188–1198. 10.1177/1087054715580842 [DOI] [PubMed] [Google Scholar]
- *Lurie L (1986). The effects of depression and elation on recognition memory (Publication No. NL33045) [Doctoral dissertation, University of West Ontario]. ProQuest Dissertations & Theses Global. [Google Scholar]
- Mackin RS, Nelson JC, Delucchi KL, Raue PJ, Satre DD, Kiosses DN, Alexopoulos GS, & Arean PA (2014). Association of age at depression onset with cognitive functioning in individuals with late-life depression and executive dysfunction. The American Journal of Geriatric Psychiatry, 22(12), 1633–1641. 10.1016/j.jagp.2014.02.006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *MacQueen GM, Campbell S, McEwen BS, Macdonald K, Amano S, Joffe RT, Nahmias C, & Young LT (2003). Course of illness, hippocampal function, and hippocampal volume in major depression. Proceedings of the National Academy of Sciences of the United States of America, 100(3), 1387–1392. 10.1073/pnas.0337481100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *MacQueen GM, Galway TM, Hay J, Young LT, & Joffe RT (2002). Recollection memory deficits in patients with major depressive disorder predicted by past depressions but not current mood state or treatment status. Psychological Medicine, 32(2), 251–258. 10.1017/S0033291701004834 [DOI] [PubMed] [Google Scholar]
- *Maeshima H, Baba H, Nakano Y, Satomura E, Namekawa Y, Takebayashi N, Suzuki T, Mimura M, & Arai H (2012). Residual memory dysfunction in recurrent major depressive disorder—A longitudinal study from Juntendo University Mood Disorder Project. Journal of Affective Disorders, 143(1–3), 84–88. 10.1016/j.jad.2012.05.033 [DOI] [PubMed] [Google Scholar]
- *Mah L, Anderson ND, Verhoeff NPLG, & Pollock BG (2017). Negative emotional verbal memory biases in mild cognitive impairment and late-onset depression. The American Journal of Geriatric Psychiatry, 25(10), 1160–1170. 10.1016/j.jagp.2017.05.005 [DOI] [PubMed] [Google Scholar]
- Marchetti I, Everaert J, Dainer-Best J, Loeys T, Beevers CG, & Koster EHW (2018). Specificity and overlap of attention and memory biases in depression. Journal of Affective Disorders, 225, 404–412. 10.1016/j.jad.2017.08.037 [DOI] [PubMed] [Google Scholar]
- Mather M (2012). The emotion paradox in the aging brain. Annals of the New York Academy of Sciences, 1251(1), 33–49. 10.1111/j.1749-6632.2012.06471.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mather M, & Knight M (2005). Goal-directed memory: The role of cognitive control in older adults’ emotional memory. Psychology and Aging, 20(4), 554–570. 10.1037/0882-7974.20.4.554 [DOI] [PubMed] [Google Scholar]
- Matt GE, Vazquez C, & Campbell WK (1992). Mood-congruent recall of affectively toned stimuli: A meta-analytic review. Clinical Psychology Review, 12(2), 227–255. 10.1016/0272-7358(92)90116-P [DOI] [Google Scholar]
- McDermott LM, & Ebmeier KP (2009). A meta-analysis of depression severity and cognitive function. Journal of Affective Disorders, 119(1–3), 1–8. 10.1016/j.jad.2009.04.022 [DOI] [PubMed] [Google Scholar]
- *McDowall J (1984). Recall of pleasant and unpleasant words in depressed subjects. Journal of Abnormal Psychology, 93(4), 401–407. 10.1037/0021-843X.93.4.401 [DOI] [PubMed] [Google Scholar]
- McLaren ME, Szymkowicz SM, Kirton JW, & Dotson VM (2015). Impact of education on memory deficits in subclinical depression. Archives of Clinical Neuropsychology, 30(5), 387–393. 10.1093/arclin/acv038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Merling AN (1996). The nature of the memory deficit in geriatric depression (Publication No. 9733212) [Doctoral dissertation, Yeshiva University]. ProQuest Dissertations & Theses Global. [Google Scholar]
- Micheli L, Ceccarelli M, D’Andrea G, & Tirone F (2018). Depression and adult neurogenesis: Positive effects of the antidepressant fluoxetine and of physical exercise. Brain Research Bulletin, 143, 181–193. 10.1016/j.brainresbull.2018.09.002 [DOI] [PubMed] [Google Scholar]
- *Michopoulos I, Zervas IM, Pantelis C, Tsaltas E, Papakosta VM, Boufidou F, Nikolaou C, Papageorgiou C, Soldatos CR, & Lykouras L (2008). Neuropsychological and hypothalamic-pituitary-axis function in female patients with melancholic and non-melancholic depression. European Archives of Psychiatry and Clinical Neuroscience, 258(4), 217–225. 10.1007/s00406-007-0781-8 [DOI] [PubMed] [Google Scholar]
- *Milne AM, MacQueen GM, & Hall GB (2012). Abnormal hippocampal activation in patients with extensive history of major depression: An fMRI study. Journal of Psychiatry & Neuroscience, 37(1), 28–36. 10.1503/jpn.110004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirza SS, Ikram MA, Bos D, Mihaescu R, Hofman A, & Tiemeier H (2017). Mild cognitive impairment and risk of depression and anxiety: A population-based study. Alzheimer’s & Dementia, 13(2), 130–139. 10.1016/j.jalz.2016.06.2361 [DOI] [PubMed] [Google Scholar]
- *Mogg K, Bradbury KE, & Bradley BP (2006). Interpretation of ambiguous information in clinical depression. Behaviour Research and Therapy, 44(10), 1411–1419. 10.1016/j.brat.2005.10.008 [DOI] [PubMed] [Google Scholar]
- Moher D, Liberati A, Tetzlaff J, Altman DG, & PRISMA Group. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Annals of Internal Medicine, 151(4), 264–269, W264. 10.7326/0003-4819-151-4-200908180-00135 [DOI] [PubMed] [Google Scholar]
- Möller HJ (2008). Outcomes in major depressive disorder: The evolving concept of remission and its implications for treatment. The World Journal of Biological Psychiatry, 9(2), 102–114. 10.1080/15622970801981606 [DOI] [PubMed] [Google Scholar]
- *Moritz S, Gläscher J, & Brassen S (2005). Investigation of mood-congruent false and true memory recognition in depression. Depression and Anxiety, 21(1), 9–17. 10.1002/da.20054 [DOI] [PubMed] [Google Scholar]
- *Mulligan NW (2011). Implicit memory and depression: Preserved conceptual priming in subclinical depression. Cognition and Emotion, 25(4), 730–739. 10.1080/02699931.2010.500479 [DOI] [PubMed] [Google Scholar]
- Murphy M, & O’Leary E (2010). Depression, cognitive reserve and memory performance in older adults. International Journal of Geriatric Psychiatry, 25(7), 665–671. 10.1002/gps.2404 [DOI] [PubMed] [Google Scholar]
- *Murray LA, Whitehouse WG, & Alloy LB (1999). Mood congruence and depressive deficits in memory: A forced-recall analysis. Memory, 7(2), 175–196. 10.1080/741944068 [DOI] [PubMed] [Google Scholar]
- Nagane A, Baba H, Nakano Y, Maeshima H, Hukatsu M, Ozawa K, Suzuki T, & Arai H (2014). Comparative study of cognitive impairment between medicated and medication-free patients with remitted major depression: Class-specific influence by tricyclic antidepressants and newer antidepressants. Psychiatry Research, 218(1–2), 101–105. 10.1016/j.psychres.2014.04.013 [DOI] [PubMed] [Google Scholar]
- Nakano Y, Baba H, Maeshima H, Kitajima A, Sakai Y, Baba K, Suzuki T, Mimura M, & Arai H (2008). Executive dysfunction in medicated, remitted state of major depression. Journal of Affective Disorders, 111(1), 46–51. 10.1016/j.jad.2008.01.027 [DOI] [PubMed] [Google Scholar]
- *Nash MI (2015). Exploring the effects of depression and physical activity on pattern separation performance (Publication No. 28105375) [Doctoral dissertation, Brigham Young University]. ProQuest Dissertations & Theses Global. [Google Scholar]
- Naveh-Benjamin M, Hussain Z, Guez J, & Bar-On M (2003). Adult age differences in episodic memory: Further support for an associative-deficit hypothesis. Journal of Experimental Psychology: Learning, Memory, and Cognition, 29(5), 826–837. 10.1037/0278-7393.29.5.826 [DOI] [PubMed] [Google Scholar]
- *Nemeth VL, Csete G, Drotos G, Greminger N, Janka Z, Vecsei L, & Must A (2016). The effect of emotion and reward contingencies on relational memory in major depression: An eye-movement study with follow-up. Frontiers in Psychology, 7, Article 1849. 10.3389/fpsyg.2016.01849 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neu P, Bajbouj M, Schilling A, Godemann F, Berman RM, & Schlattmann P (2005). Cognitive function over the treatment course of depression in middle-aged patients: Correlation with brain MRI signal hyperintensities. Journal of Psychiatric Research, 39(2), 129–135. 10.1016/j.jpsychires.2004.06.004 [DOI] [PubMed] [Google Scholar]
- Nolen-Hoeksema S, Wisco BE, & Lyubomirsky S (2008). Rethinking Rumination. Perspectives on Psychological Science, 3(5), 400–424. 10.1111/j.1745-6924.2008.00088.x [DOI] [PubMed] [Google Scholar]
- *Nouchi R, & Kawashima R (2012). Effect of the survival judgment task on memory performance in subclinically depressed people. Frontiers in Psychology, 3, Article 114. 10.3389/fpsyg.2012.00114 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Novaretti TMDS, Radanovic M, & Nitrini R (2012). Screening for cognitive impairment in late onset depression in a Brazilian sample using the BBRC-Edu. Dementia & Neuropsychologia, 6(2), 85–90. 10.1590/S1980-57642012DN06020004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Nussbaum PD (1987). Qualitative aspects of memory performance in depressed versus demented elderly (Publication No. 1332536) [Master’s thesis, University of Arizona]. ProQuest Dissertations & Theses Global. [Google Scholar]
- O’Shea DM, Dotson VM, Woods AJ, Porges EC, Williamson JB, O’Shea A, & Cohen R (2018). Depressive symptom dimensions and their association with hippocampal and entorhinal cortex volumes in community dwelling older adults. Frontiers in Aging Neuroscience, 10, Article 40. 10.3389/fnagi.2018.00040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- O’Shea DM, Fieo RA, Hamilton JL, Zahodne LB, Manly JJ, & Stern Y (2015). Examining the association between late-life depressive symptoms, cognitive function, and brain volumes in the context of cognitive reserve. International Journal of Geriatric Psychiatry, 30(6), 614–622. 10.1002/gps.4192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Olsen EK, Bjorkquist OA, Bodapati AS, Shankman SA, & Herbener ES (2015). Associations between trait anhedonia and emotional memory deficits in females with schizophrenia versus major depression. Psychiatry Research, 230(2), 323–330. 10.1016/j.psychres.2015.09.012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opdebeeck C, Quinn C, Nelis SM, & Clare L (2015). Does cognitive reserve moderate the association between mood and cognition? A systematic review. Reviews in Clinical Gerontology, 25(3), 181–193. 10.1017/S0959259815000155 [DOI] [Google Scholar]
- Ostergaard SD, Jensen SO, & Bech P (2011). The heterogeneity of the depressive syndrome: When numbers get serious. Acta Psychiatrica Scandinavica, 124(6), 495–496. 10.1111/j.1600-0447.2011.01744.x [DOI] [PubMed] [Google Scholar]
- *Ottowitz WE, Deckersbach T, Savage CR, Lindquist MA, & Dougherty DD (2010). Neural correlates of strategic processes underlying episodic memory in women with major depression: A 15O-PET study. The Journal of Neuropsychiatry and Clinical Neurosciences, 22(2), 218–230. 10.1176/jnp.2010.22.2.218 [DOI] [PubMed] [Google Scholar]
- Paelecke-Habermann Y, Pohl J, & Leplow B (2005). Attention and executive functions in remitted major depression patients. Journal of Affective Disorders, 89(1–3), 125–135. 10.1016/j.jad.2005.09.006 [DOI] [PubMed] [Google Scholar]
- *Pantzar A, Laukka EJ, Atti AR, Fastbom J, Fratiglioni L, & Bäckman L (2014). Cognitive deficits in unipolar old-age depression: A population-based study. Psychological Medicine, 44(5), 937–947. 10.1017/S0033291713001736 [DOI] [PubMed] [Google Scholar]
- Papakostas GI, Petersen T, Mahal Y, Mischoulon D, Nierenberg AA, & Fava M (2004). Quality of life assessments in major depressive disorder: A review of the literature. General Hospital Psychiatry, 26(1), 13–17. 10.1016/j.genhosppsych.2003.07.004 [DOI] [PubMed] [Google Scholar]
- *Pedersen A, Küppers K, Behnken A, Kroker K, Schöning S, Baune BT, Rist F, Arolt V, & Suslow T (2009). Implicit and explicit procedural learning in patients recently remitted from severe major depression. Psychiatry Research, 169(1), 1–6. 10.1016/j.psychres.2008.06.001 [DOI] [PubMed] [Google Scholar]
- *Pegg E (2009). Interactions between rumination, depression and cognition (Publication No. 11009571) [Doctoral dissertation, University of Manchester]. ProQuest Dissertations & Theses Global. [Google Scholar]
- *Peterson-Masri A (1996). Differentiating mild depression from mild dementia in nursing home residents using memory and decision-making tasks (Publication No. 9715514) [Doctoral dissertation, Fordham University]. ProQuest Dissertations & Theses Global. [Google Scholar]
- Polyakova M, Sonnabend N, Sander C, Mergl R, Schroeter ML, Schroeder J, & Schönknecht P (2014). Prevalence of minor depression in elderly persons with and without mild cognitive impairment: A systematic review. Journal of Affective Disorders, 152–154, 28–38. 10.1016/j.jad.2013.09.016 [DOI] [PubMed] [Google Scholar]
- *Pomara N, Bruno D, Sarreal AS, Hernando RT, Nierenberg J, Petkova E, Sidtis JJ, Wisniewski TM, Mehta PD, Pratico D, Zetterberg H, & Blennow K (2012). Lower CSF amyloid beta peptides and higher F2-isoprostanes in cognitively intact elderly individuals with major depressive disorder. The American Journal of Psychiatry, 169(5), 523–530. 10.1176/appi.ajp.2011.11081153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Popovic D, Vieta E, Fornaro M, & Perugi G (2015). Cognitive tolerability following successful long term treatment of major depression and anxiety disorders with SSRi antidepressants. Journal of Affective Disorders, 173, 211–215. 10.1016/j.jad.2014.11.008 [DOI] [PubMed] [Google Scholar]
- *Portella MJ, Marcos T, Rami L, Navarro V, Gastó C, & Salamero M (2003). Residual cognitive impairment in late-life depression after a 12-month period follow-up. International Journal of Geriatric Psychiatry, 18(7), 571–576. 10.1002/gps.895 [DOI] [PubMed] [Google Scholar]
- *Porter RJ, Gallagher P, Thompson JM, & Young AH (2003). Neurocognitive impairment in drug-free patients with major depressive disorder. The British Journal of Psychiatry, 182, 214–220. 10.1192/bjp.182.3.214 [DOI] [PubMed] [Google Scholar]
- Prado CE, Watt S, & Crowe SF (2018). A meta-analysis of the effects of antidepressants on cognitive functioning in depressed and nondepressed samples. Neuropsychology Review, 28(1), 32–72. 10.1007/s11065-018-9369-5 [DOI] [PubMed] [Google Scholar]
- *Preiss M, Kucerova H, Lukavsky J, Stepankova H, Sos P, & Kawaciukova R (2009). Cognitive deficits in the euthymic phase of unipolar depression. Psychiatry Research, 169(3), 235–239. 10.1016/j.psychres.2008.06.042 [DOI] [PubMed] [Google Scholar]
- Pustejovsky JE (2021). clubSandwich: Cluster-robust (sandwich) variance estimators with small-sample corrections (Version R package Version0.5.3) [Computer software]. https://cran.r-project.org/web/packages/clubSandwich/index.html
- Pustejovsky JE, & Rodgers MA (2019). Testing for funnel plot asymmetry of standardized mean differences. Research Synthesis Methods, 10(1), 57–71. 10.1002/jrsm.1332 [DOI] [PubMed] [Google Scholar]
- Pustejovsky JE, & Tipton E (2021). Meta-analysis with Robust Variance Estimation: Expanding the range of working models. Prevention Science. Advance online publication. 10.1007/s11121-021-01246-3 [DOI] [PubMed] [Google Scholar]
- Raffard S, Gutierrez LA, Yazbek H, Larue A, Boulenger JP, Lançon C, Benoit M, Faget C, Norton J, & Capdevielle D (2016). Working memory deficit as a risk factor for severe apathy in schizophrenia: A 1-year longitudinal study. Schizophrenia Bulletin, 42(3), 642–651. 10.1093/schbul/sbw002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Ramel W, Goldin PR, Eyler LT, Brown GG, Gotlib IH, & McQuaid JR (2007). Amygdala reactivity and mood-congruent memory in individuals at risk for depressive relapse. Biological Psychiatry, 61(2), 231–239. 10.1016/j.biopsych.2006.05.004 [DOI] [PubMed] [Google Scholar]
- *Rampacher F, Lennertz L, Vogeley A, Schulze-Rauschenbach S, Kathmann N, Falkai P, & Wagner M (2010). Evidence for specific cognitive deficits in visual information processing in patients with OCD compared to patients with unipolar depression. Progress in NeuroPsychopharmacology & Biological Psychiatry, 34(6), 984–991. 10.1016/j.pnpbp.2010.05.008 [DOI] [PubMed] [Google Scholar]
- *Ramponi C, Murphy FC, Calder AJ, & Barnard PJ (2010). Recognition memory for pictorial material in subclinical depression. Acta Psychologica, 135(3), 293–301. 10.1016/j.actpsy.2010.07.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Rao JA, Jenkins LM, Hymen E, Feigon M, Weisenbach SL, Zubieta JK, & Langenecker SA (2016). Differential resting state connectivity patterns and impaired semantically cued list learning test performance in early course remitted major depressive disorder. Journal of the International Neuropsychological Society, 22(2), 225–239. 10.1017/S1355617716000011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Rapp MA, Dahlman K, Sano M, Grossman HT, Haroutunian V, & Gorman JM (2005). Neuropsychological differences between late-onset and recurrent geriatric major depression. The American Journal of Psychiatry, 162(4), 691–698. 10.1176/appi.ajp.162.4.691 [DOI] [PubMed] [Google Scholar]
- Rapp MA, Schnaider-Beeri M, Wysocki M, Guerrero-Berroa E, Grossman HT, Heinz A, & Haroutunian V (2011). Cognitive decline in patients with dementia as a function of depression. The American Journal of Geriatric Psychiatry, 19(4), 357–363. 10.1097/JGP.0b013e3181e898d0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed AE, Chan L, & Mikels JA (2014). Meta-analysis of the age-related positivity effect: Age differences in preferences for positive over negative information. Psychology and Aging, 29(1), 1–15. 10.1037/a0035194 [DOI] [PubMed] [Google Scholar]
- Reid SC, Salmon K, & Lovibond PF (2006). Cognitive biases in childhood anxiety, depression, and aggression: Are they pervasive or specific? Cognitive Therapy and Research, 30(5), 531–549. 10.1007/s10608-006-9077-y [DOI] [Google Scholar]
- *Reppermund S, Ising M, Lucae S, & Zihl J (2009). Cognitive impairment in unipolar depression is persistent and non-specific: Further evidence for the final common pathway disorder hypothesis. Psychological Medicine, 39(4), 603–614. 10.1017/S003329170800411X [DOI] [PubMed] [Google Scholar]
- Rey-Mermet A, & Gade M (2018). Inhibition in aging: What is preserved? What declines? A meta-analysis. Psychonomic Bulletin & Review, 25(5), 1695–1716. 10.3758/s13423-017-1384-7 [DOI] [PubMed] [Google Scholar]
- Rhodes S, Greene NR, & Naveh-Benjamin M (2019). Age-related differences in recall and recognition: A meta-analysis. Psychonomic Bulletin & Review, 26(5), 1529–1547. 10.3758/s13423-019-01649-y [DOI] [PubMed] [Google Scholar]
- *Ridout N (2005). Processing of emotional material in major depression: Cognitive and neuropsychological investigations (Publication No. 10166875) [Doctoral dissertation, University of St. Andrews]. ProQuest Dissertations & Theses Global. [Google Scholar]
- *Ridout N, Dritschel B, Matthews K, McVicar M, Reid IC, & O’Carroll RE (2009). Memory for emotional faces in major depression following judgement of physical facial characteristics at encoding. Cognition and Emotion, 23(4), 739–752. 10.1080/02699930802121137 [DOI] [Google Scholar]
- *Riedel WJ, Schoenmakers E, Vermeeren A, & O’Hanlon JF (1999). The influence of trazodone treatment on cognitive functions in outpatients with major depressive disorder. Human Psychopharmacology, 14(7), 499–508. [DOI] [Google Scholar]
- *Rinck M, & Becker ES (2005). A comparison of attentional biases and memory biases in women with social phobia and major depression. Journal of Abnormal Psychology, 114(1), 62–74. 10.1037/0021-843X.114.1.62 [DOI] [PubMed] [Google Scholar]
- Ritchie SJ, & Tucker-Drob EM (2018). How much does education improve intelligence? A meta-analysis. Psychological Science, 29(8), 1358–1369. 10.1177/0956797618774253 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Ritter E, Després O, Monsch AU, & Manning L (2006). Topographical recognition memory sensitive to amnestic mild cognitive impairment but not to depression. International Journal of Geriatric Psychiatry, 21(10), 924–929. 10.1002/gps.1581 [DOI] [PubMed] [Google Scholar]
- Rock PL, Roiser JP, Riedel WJ, & Blackwell AD (2014). Cognitive impairment in depression: A systematic review and meta-analysis. Psychological Medicine, 44(10), 2029–2040. 10.1017/S0033291713002535 [DOI] [PubMed] [Google Scholar]
- Rodgers MA, & Pustejovsky JE (2020). Evaluating meta-analytic methods to detect selective reporting in the presence of dependent effect sizes. Psychological Methods. Advance online publication. 10.1037/met0000300 [DOI] [PubMed] [Google Scholar]
- Rohatgi A (2019). WebPlotDigitizer (Version 4.2) [Computer software]. https://automeris.io/WebPlotDigitizer
- Romera I, Pérez V, Menchón JM, Polavieja P, & Gilaberte I (2011). Optimal cutoff point of the Hamilton Rating Scale for Depression according to normal levels of social and occupational functioning. Psychiatry Research, 186(1), 133–137. 10.1016/j.psychres.2010.06.023 [DOI] [PubMed] [Google Scholar]
- *Romero N, Sanchez A, & Vazquez C (2014). Memory biases in remitted depression: The role of negative cognitions at explicit and automatic processing levels. Journal of Behavior Therapy and Experimental Psychiatry, 45(1), 128–135. 10.1016/j.jbtep.2013.09.008 [DOI] [PubMed] [Google Scholar]
- *Romero N, Sanchez A, Vázquez C, & Valiente C (2016). Explicit self-esteem mediates the relationship between implicit self-esteem and memory biases in major depression. Psychiatry Research, 242, 336–344. 10.1016/j.psychres.2016.06.003 [DOI] [PubMed] [Google Scholar]
- *Rosenberg HJ (1984). The effect of mood on recall of positive feedback (Publication No. 8418838) [Doctoral dissertation, Hofstra University]. ProQuest Dissertations & Theses Global. [Google Scholar]
- Rosenblat JD, Kakar R, & McIntyre RS (2015). The cognitive effects of antidepressants in major depressive disorder: A systematic review and meta-analysis of randomized clinical trials. The International Journal of Neuropsychopharmacology, 19(2), Article pyv082. 10.1093/ijnp/pyv082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Roy M (2006). La réactivité du cortisol salivaire de sujets souffrant de dépression majeure exposés à des stimuli émotifs et neutres (Publication No. MR24785) [Doctoral dissertation, McGill University]. ProQuest Dissertations & Theses Global. [Google Scholar]
- Russo M, Mahon K, & Burdick KE (2015). Measuring cognitive function in MDD: Emerging assessment tools. Depression and Anxiety, 32(4), 262–269. 10.1002/da.22297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saczynski JS, Beiser A, Seshadri S, Auerbach S, Wolf PA, & Au R (2010). Depressive symptoms and risk of dementia: The Framingham Heart Study. Neurology, 75(1), 35–41. 10.1212/WNL.0b013e3181e62138 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Saleh A, Potter GG, McQuoid DR, Boyd B, Turner R, MacFall JR, & Taylor WD (2017). Effects of early life stress on depression, cognitive performance and brain morphology. Psychological Medicine, 47(1), 171–181. 10.1017/S0033291716002403 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salthouse T (2012). Consequences of age-related cognitive declines. Annual Review of Psychology, 63, 201–226. 10.1146/annurev-psych-120710-100328 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Sarosi A, Gonda X, Balogh G, Domotor E, Szekely A, Hejjas K, Sasvari-Szekely M, & Faludi G (2008). Association of the STin2 polymorphism of the serotonin transporter gene with a neurocognitive endophenotype in major depressive disorder. Progress in NeuroPsychopharmacology & Biological Psychiatry, 32(7), 1667–1672. 10.1016/j.pnpbp.2008.06.014 [DOI] [PubMed] [Google Scholar]
- *Savaskan E, Müller SE, Böhringer A, Schulz A, & Schächinger H (2008). Antidepressive therapy with escitalopram improves mood, cognitive symptoms, and identity memory for angry faces in elderly depressed patients. The International Journal of Neuropsychopharmacology, 11(3), 381–388. 10.1017/S1461145707007997 [DOI] [PubMed] [Google Scholar]
- Schmitt JA, Kruizinga MJ, & Riedel WJ (2001). Non-serotonergic pharmacological profiles and associated cognitive effects of serotonin reuptake inhibitors. Journal of Psychopharmacology (Oxford, England), 15(3), 173–179. 10.1177/026988110101500304 [DOI] [PubMed] [Google Scholar]
- *Schwartzberg S (1994). Cognitive functioning in depressed elderly patients (Publication No. NN11360) [Doctoral dissertation, University of Alberta]. ProQuest Dissertations & Theses Global. [Google Scholar]
- *Sears CR, Newman KR, Ference JD, & Thomas CL (2011). Attention to emotional images in previously depressed individuals: An eye-tracking study. Cognitive Therapy and Research, 35(6), 517–528. 10.1007/s10608-011-9396-5 [DOI] [Google Scholar]
- *Sejunaite K, Lanza C, & Riepe MW (2018). Everyday false memories in older persons with depressive disorder. Psychiatry Research, 261, 456–463. 10.1016/j.psychres.2018.01.030 [DOI] [PubMed] [Google Scholar]
- Serretti A, Calati R, Goracci A, Di Simplicio M, Castrogiovanni P, & De Ronchi D (2010). Antidepressants in healthy subjects: What are the psychotropic/psychological effects? European Neuropsychopharmacology, 20(7), 433–453. 10.1016/j.euroneuro.2009.11.009 [DOI] [PubMed] [Google Scholar]
- *Sexton CE, McDermott L, Kalu UG, Herrmann LL, Bradley KM, Allan CL, Le Masurier M, Mackay CE, & Ebmeier KP (2012). Exploring the pattern and neural correlates of neuropsychological impairment in late-life depression. Psychological Medicine, 42(6), 1195–1202. 10.1017/S0033291711002352 [DOI] [PubMed] [Google Scholar]
- Sheehan DV, Nakagome K, Asami Y, Pappadopulos EA, & Boucher M (2017). Restoring function in major depressive disorder: A systematic review. Journal of Affective Disorders, 215, 299–313. 10.1016/j.jad.2017.02.029 [DOI] [PubMed] [Google Scholar]
- *Sheline YI, Sanghavi M, Mintun MA, & Gado MH (1999). Depression duration but not age predicts hippocampal volume loss in medically healthy women with recurrent major depression. The Journal of Neuroscience: The Official Journal of the Society for Neuroscience, 19(12), 5034–5043. 10.1523/JNEUROSCI.19-12-05034.1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shilyansky C, Williams LM, Gyurak A, Harris A, Usherwood T, & Etkin A (2016). Effect of antidepressant treatment on cognitive impairments associated with depression: A randomised longitudinal study. The Lancet. Psychiatry, 3(5), 425–435. 10.1016/S2215-0366(16)00012-2 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Shimizu Y, Kitagawa N, Mitsui N, Fujii Y, Toyomaki A, Hashimoto N, Kako Y, Tanaka T, Asakura S, & Kusumi I (2013). Neurocognitive impairments and quality of life in unemployed patients with remitted major depressive disorder. Psychiatry Research, 210(3), 913–918. 10.1016/j.psychres.2013.08.030 [DOI] [PubMed] [Google Scholar]
- Siddarth P, Funes CM, Laird KT, Ercoli L, & Lavretsky H (2021). Predictors of cognitive improvement following treatment for late-life depression. Journal of Geriatric Psychiatry and Neurology, 34(2), 162–168. 10.1177/0891988720915515 [DOI] [PubMed] [Google Scholar]
- *Sisti M (1992). The relationship of depression to incidental and intentional learning (Publication No. 9226045) [Doctoral dissertation, Hofstra University]. ProQuest Dissertations & Theses Global. [Google Scholar]
- Slavich GM, & Irwin MR (2014). From stress to inflammation and major depressive disorder: A social signal transduction theory of depression. Psychological Bulletin, 140(3), 774–815. 10.1037/a0035302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Slife BD, Miura S, Thompson LW, Shapiro JL, & Gallagher D (1984). Differential recall as a function of mood disorder in clinically depressed patients: Between- and within-subject differences. Journal of Abnormal Psychology, 93(4), 391–400. 10.1037/0021-843X.93.4.391 [DOI] [PubMed] [Google Scholar]
- *Sloan LL (1997). Processing strategies and recall performance for narrative passages and word lists of negative and neutral affective valence in depression (Publication No. 9825424) [Doctoral dissertation, University of North Dakota]. ProQuest Dissertations & Theses Global. [Google Scholar]
- Smith DJ, Muir WJ, & Blackwood DH (2006). Neurocognitive impairment in euthymic young adults with bipolar spectrum disorder and recurrent major depressive disorder. Bipolar Disorders, 8(1), 40–46. 10.1111/j.1399-5618.2006.00275.x [DOI] [PubMed] [Google Scholar]
- *Smith KJ, Mullally S, McLoughlin D, & O’Mara S (2014). Validation of the face-name pairs task in major depression: Impaired recall but not recognition. Frontiers in Psychology, 5, Article 92. 10.3389/fpsyg.2014.00092 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snyder HR (2013). Major depressive disorder is associated with broad impairments on neuropsychological measures of executive function: A meta-analysis and review. Psychological Bulletin, 139(1), 81–132. 10.1037/a0028727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Söderlund H, Moscovitch M, Kumar N, Daskalakis ZJ, Flint A, Herrmann N, & Levine B (2014). Autobiographical episodic memory in major depressive disorder. Journal of Abnormal Psychology, 123(1), 51–60. 10.1037/a0035610 [DOI] [PubMed] [Google Scholar]
- *Soderstrom NC, Davalos DB, & Vázquez SM (2011). Metacognition and depressive realism: Evidence for the level-of-depression account. Cognitive Neuropsychiatry, 16(5), 461–472. 10.1080/13546805.2011.557921 [DOI] [PubMed] [Google Scholar]
- Spalletta G, Piras F, Caltagirone C, & Fagioli S (2014). Hippocampal multimodal structural changes and subclinical depression in healthy individuals. Journal of Affective Disorders, 152–154, 105–112. 10.1016/j.jad.2013.05.068 [DOI] [PubMed] [Google Scholar]
- Stern Y (2002). What is cognitive reserve? Theory and research application of the reserve concept. Journal of the International Neuropsychological Society, 8(3), 448–460. 10.1017/S1355617702813248 [DOI] [PubMed] [Google Scholar]
- Stern Y, Gazes Y, Razlighi Q, Steffener J, & Habeck C (2018). A task-invariant cognitive reserve network. NeuroImage, 178, 36–45. 10.1016/j.neuroimage.2018.05.033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Substance Abuse and Mental Health Services Administration. (2020). Key substance use and mental health indicators in the United States: Results from the 2019 National Survey on Drug Use and Health. https://www.samhsa.gov/data/sites/default/files/reports/rpt29393/2019NSDUHFFRPDFWHTML/2019NSDUHFFR090120.htm
- *Swainson R, Hodges JR, Galton CJ, Semple J, Michael A, Dunn BD, Iddon JL, Robbins TW, & Sahakian BJ (2001). Early detection and differential diagnosis of Alzheimer’s disease and depression with neuropsychological tasks. Dementia and Geriatric Cognitive Disorders, 12(4), 265–280. 10.1159/000051269 [DOI] [PubMed] [Google Scholar]
- *Sweeney JA, Kmiec JA, & Kupfer DJ (2000). Neuropsychologic impairments in bipolar and unipolar mood disorders on the CANTAB neurocognitive battery. Biological Psychiatry, 48(7), 674–684. 10.1016/S0006-3223(00)00910-0 [DOI] [PubMed] [Google Scholar]
- Symons CS, & Johnson BT (1997). The self-reference effect in memory: A meta-analysis. Psychological Bulletin, 121(3), 371–394. 10.1037/0033-2909.121.3.371 [DOI] [PubMed] [Google Scholar]
- *Szu-Ting Fu T, Koutstaal W, Poon L, & Cleare AJ (2012). Confidence judgment in depression and dysphoria: The depressive realism vs. negativity hypotheses. Journal of Behavior Therapy and Experimental Psychiatry, 43(2), 699–704. 10.1016/j.jbtep.2011.09.014 [DOI] [PubMed] [Google Scholar]
- Szymkowicz SM, Woods AJ, Dotson VM, Porges EC, Nissim NR, O’Shea A, Cohen RA, & Ebner NC (2019). Associations between subclinical depressive symptoms and reduced brain volume in middle-aged to older adults. Aging & Mental Health, 23(7), 819–830. 10.1080/13607863.2018.1432030 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Taconnat L, Baudouin A, Fay S, Raz N, Bouazzaoui B, El-Hage W, Isingrini M, & Ergis AM (2010). Episodic memory and organizational strategy in free recall in unipolar depression: The role of cognitive support and executive functions. Journal of Clinical and Experimental Neuropsychology, 32(7), 719–727. 10.1080/13803390903512645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Tam CW, & Lam LC (2012). Cognitive and functional impairment in Chinese elderly with late-onset depression. East Asian Archives of Psychiatry, 22(1), 25–30. https://www.ncbi.nlm.nih.gov/pubmed/22447802 [PubMed] [Google Scholar]
- *Tarsia M, Power MJ, & Sanavio E (2003). Implicit and explicit memory biases in mixed anxiety-depression. Journal of Affective Disorders, 77(3), 213–225. 10.1016/S0165-0327(02)00119-2 [DOI] [PubMed] [Google Scholar]
- *Taylor JL, & John CH (2004). Attentional and memory bias in persecutory delusions and depression. Psychopathology, 37(5), 233–241. 10.1159/000080719 [DOI] [PubMed] [Google Scholar]
- Thomas RC, & Hasher L (2006). The influence of emotional valence on age differences in early processing and memory. Psychology and Aging, 21(4), 821–825. 10.1037/0882-7974.21.4.821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Toki S, Okamoto Y, Onoda K, Matsumoto T, Yoshimura S, Kunisato Y, Okada G, Shishida K, Kobayakawa M, Fukumoto T, Machino A, Inagaki M, & Yamawaki S (2014). Hippocampal activation during associative encoding of word pairs and its relation to symptomatic improvement in depression: A functional and volumetric MRI study. Journal of Affective Disorders, 152–154, 462–467. 10.1016/j.jad.2013.07.021 [DOI] [PubMed] [Google Scholar]
- *Toner BB, Garfinkel PE, Jeejeebhoy KN, Scher H, Shulhan D, & Di Gasbarro I (1990). Self-schema in irritable bowel syndrome and depression. Psychosomatic Medicine, 52(2), 149–155. 10.1097/00006842-199003000-00003 [DOI] [PubMed] [Google Scholar]
- *Travis S, Coupland NJ, Silversone PH, Huang Y, Fujiwara E, Carter R, Seres P, & Malykhin NV (2015). Dentate gyrus volume and memory performance in major depressive disorder. Journal of Affective Disorders, 172, 159–164. 10.1016/j.jad.2014.09.048 [DOI] [PubMed] [Google Scholar]
- *Tsaltas E, Kalogerakou S, Papakosta VM, Kontis D, Theochari E, Koutroumpi M, Anyfandi E, Michopoulos I, Poulopoulou C, Papadimitriou G, & Oulis P (2011). Contrasting patterns of deficits in visuospatial memory and executive function in patients with major depression with and without ECT referral. Psychological Medicine, 41(5), 983–995. 10.1017/S0033291710001443 [DOI] [PubMed] [Google Scholar]
- Tuithof M, Ten Have M, van Dorsselaer S, Kleinjan M, Beekman A, & de Graaf R (2018). Course of subthreshold depression into a depressive disorder and its risk factors. Journal of Affective Disorders, 241, 206–215. 10.1016/j.jad.2018.08.010 [DOI] [PubMed] [Google Scholar]
- Tulving E (1972). Episodic and semantic memory. In Tulving E & Donaldson W (Eds.), Organization of memory (pp. 381–403). Academic Press. [Google Scholar]
- Tulving E (2002). Episodic memory: From mind to brain. Annual Review of Psychology, 53, 1–25. 10.1146/annurev.psych.53.100901.135114 [DOI] [PubMed] [Google Scholar]
- *Turner AD (2010). Cortisol levels and neurocognitive performance in depressed patients and healthy controls (Publication No. 3433062) [Doctoral dissertation, Howard University]. ProQuest Dissertations & Theses Global. [Google Scholar]
- *Turner AD, Furey ML, Drevets WC, Zarate C Jr., & Nugent AC (2012). Association between subcortical volumes and verbal memory in unmedicated depressed patients and healthy controls. Neuropsychologia, 50(9), 2348–2355. 10.1016/j.neuropsychologia.2012.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Uekermann J, Daum I, Schlebusch P, Wiebel B, & Trenckmann U (2003). Depression and cognitive functioning in alcoholism. Addiction, 98(11), 1521–1529. 10.1046/j.1360-0443.2003.00526.x [DOI] [PubMed] [Google Scholar]
- *Vakil E, Grunhaus L, Nagar I, Ben-Chaim E, Dolberg OT, Dannon PN, & Schreiber S (2000). The effect of electroconvulsive therapy (ECT) on implicit memory: Skill learning and perceptual priming in patients with major depression. Neuropsychologia, 38(10), 1405–1414. 10.1016/S0028-3932(00)00047-6 [DOI] [PubMed] [Google Scholar]
- *van der Laat M (2004). Individual differences in source monitoring as a function of depression, stimulus valence, and encoding context in a college student sample (Publication No. 3154747) [Doctoral dissertation, Purdue University]. ProQuest Dissertations & Theses Global. [Google Scholar]
- *van Eijndhoven P, van Wingen G, Fernández G, Rijpkema M, Pop-Purceleanu M, Verkes RJ, Buitelaar J, & Tendolkar I (2013). Neural basis of recollection in first-episode major depression. Human Brain Mapping, 34(2), 283–294. 10.1002/hbm.21439 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *van Tol MJ, Demenescu LR, van der Wee NJ, Kortekaas R, Marjan MAN, Boer JAD, Renken RJ, van Buchem MA, Zitman FG, Aleman A, & Veltman DJ (2012). Functional magnetic resonance imaging correlates of emotional word encoding and recognition in depression and anxiety disorders. Biological Psychiatry, 71(7), 593–602. 10.1016/j.biopsych.2011.11.016 [DOI] [PubMed] [Google Scholar]
- Van Vleet T, Stark-Inbar A, Merzenich MM, Jordan JT, Wallace DL, Lee MB, Dawesd HE, Chang EF, & Nahum M (2019). Biases in processing of mood-congruent facial expressions in depression. Psychiatry Research, 275, 143–148. 10.1016/j.psychres.2019.02.076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Vasudev A, Saxby BK, O’Brien JT, Colloby SJ, Firbank MJ, Brooker H, Wesnes K, & Thomas AJ (2012). Relationship between cognition, magnetic resonance white matter hyperintensities, and cardiovascular autonomic changes in late-life depression. The American Journal of Geriatric Psychiatry, 20(8), 691–699. 10.1097/JGP.0b013e31824c0435 [DOI] [PubMed] [Google Scholar]
- Venezia RG, Gorlyn M, Burke AK, Oquendo MA, Mann JJ, & Keilp JG (2018). The impact of cognitive reserve on neurocognitive performance in Major Depressive Disorder. Psychiatry Research, 270, 211–218. 10.1016/j.psychres.2018.09.031 [DOI] [PubMed] [Google Scholar]
- Videbech P, & Ravnkilde B (2004). Hippocampal volume and depression: A meta-analysis of MRI studies. The American Journal of Psychiatry, 161(11), 1957–1966. 10.1176/appi.ajp.161.11.1957 [DOI] [PubMed] [Google Scholar]
- Viechtbauer W (2010). Conducting meta-analyses in R with the metaphor package. Journal of Statistical Software, 36(3), 1–48. 10.18637/jss.v036.i03 [DOI] [Google Scholar]
- Vittengl JR, Clark LA, Kraft D, & Jarrett RB (2005). Multiple measures, methods, and moments: A factor-analytic investigation of change in depressive symptoms during acute-phase cognitive therapy for depression. Psychological Medicine, 35(5), 693–704. 10.1017/S0033291704004143 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voyer D, Saint Aubin J, Altman K, & Gallant G (2021). Sex differences in verbal working memory: A systematic review and meta-analysis. Psychological Bulletin, 147(4), 352–398. 10.1037/bul0000320 [DOI] [PubMed] [Google Scholar]
- *Vythilingam M, Vermetten E, Anderson GM, Luckenbaugh D, Anderson ER, Snow J, Staib LH, Charney DS, & Bremner JD (2004). Hippocampal volume, memory, and cortisol status in major depressive disorder: Effects of treatment. Biological Psychiatry, 56(2), 101–112. 10.1016/j.biopsych.2004.04.002 [DOI] [PubMed] [Google Scholar]
- Wagner S, Doering B, Helmreich I, Lieb K, & Tadić A (2012). A meta-analysis of executive dysfunctions in unipolar major depressive disorder without psychotic symptoms and their changes during antidepressant treatment. Acta Psychiatrica Scandinavica, 125(4), 281–292. 10.1111/j.1600-0447.2011.01762.x [DOI] [PubMed] [Google Scholar]
- *Wang CE, Brennen T, & Holte A (2006). Automatic and effortful processing of self-statements in depression. Cognitive Behaviour Therapy, 35(2), 117–124. 10.1080/16506070500466865 [DOI] [PubMed] [Google Scholar]
- *Watkins PC (1991). Implicit vs explicit mood congruent memory bias in depression (Publication No. 9200097) [Doctoral dissertation, Louisiana State University and Agricultural and Mechanical College]. ProQuest Dissertations & Theses Global. [Google Scholar]
- Wechsler D (2009). Wechsler memory scale-IV administration and scoring manual (4th ed.). Pearson. [Google Scholar]
- *Weiland-Fiedler P, Erickson K, Waldeck T, Luckenbaugh DA, Pike D, Bonne O, Charney DS, & Neumeister A (2004). Evidence for continuing neuropsychological impairments in depression. Journal of Affective Disorders, 82(2), 253–258. 10.1016/j.jad.2003.10.009 [DOI] [PubMed] [Google Scholar]
- *Weisenbach SL, Kassel MT, Rao J, Weldon AL, Avery ET, Briceno EM, Ajilore O, Mann M, Kales HC, Welsh RC, Zubieta J-K, & Langenecker SA (2014). Differential prefrontal and subcortical circuitry engagement during encoding of semantically related words in patients with late-life depression. International Journal of Geriatric Psychiatry, 29(11), 1104–1115. 10.1002/gps.4165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickham H, François R, Henry L, & Müller K (2020). dplyr: A grammar of data manipulation. In (Version R package version 0.8.5; ). https://CRAN.R-project.org/package=dplyr [Google Scholar]
- Wilson DB (2001). Practical meta-analysis effect size calculator. https://www.campbellcollaboration.org/escalc/html/EffectSizeCalculator-Home.php
- Wilson DB, & Lipsey M (2001). Practical meta-analysis. SAGE Publications. [Google Scholar]
- Wilson RS, Capuano AW, Boyle PA, Hoganson GM, Hizel LP, Shah RC, Nag S, Schneider JA, Arnold SE, & Bennett DA (2014). Clinical-pathologic study of depressive symptoms and cognitive decline in old age. Neurology, 83(8), 702–709. 10.1212/WNL.0000000000000715 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wisco BE (2009). Depressive cognition: Self-reference and depth of processing. Clinical Psychology Review, 29(4), 382–392. 10.1016/j.cpr.2009.03.003 [DOI] [PubMed] [Google Scholar]
- *Xie C, Li W, Chen G, Douglas Ward B, Franczak MB, Jones JL, Antuono PG, Li S-J, & Goveas JS (2012). The co-existence of geriatric depression and amnestic mild cognitive impairment detrimentally affect gray matter volumes: Voxel-based morphometry study. Behavioural Brain Research, 235(2), 244–250. 10.1016/j.bbr.2012.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- *Xie H, Jiang D, & Zhang D (2018). Individuals with depressive tendencies experience difficulty in forgetting negative material: Two mechanisms revealed by ERP data in the directed forgetting paradigm. Scientific Reports, 8(1), Article 1113. 10.1038/s41598-018-19570-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y, Cao S, Shields GS, Teng Z, & Liu Y (2017). The relationships between rumination and core executive functions: A meta-analysis. Depression and Anxiety, 34(1), 37–50. 10.1002/da.22539 [DOI] [PubMed] [Google Scholar]
- *Yeh ZT, & Hua MS (2009). Effects of depressive disorder on false memory for emotional information. Depression and Anxiety, 26(5), 456–463. 10.1002/da.20453 [DOI] [PubMed] [Google Scholar]
- Yen YC, Rebok GW, Gallo JJ, Jones RN, & Tennstedt SL (2011). Depressive symptoms impair everyday problem-solving ability through cognitive abilities in late life. The American Journal of Geriatric Psychiatry, 19(2), 142–150. 10.1097/JGP.0b013e3181e89894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yochim BP, & Woodhead EL (2018). Psychology of aging: A biopsychosocial perspective. Springer. [Google Scholar]
- Young KD, Erickson K, & Drevets WC (2012). Match between cue and memory valence during autobiographical memory recall in depression. Psychological Reports, 111(1), 129–148. 10.2466/09.02.15.PR0.111.4.129-148 [DOI] [PubMed] [Google Scholar]
- *Yu KT (2018). Cross-cultural examination of the cognitive theory of depression among individuals of Chinese and Canadian descent (Publication No. 28141616) [Master’s thesis, University of Regina]. ProQuest Dissertations & Theses Global. [Google Scholar]
- *Yuan Y, Zhang Z, Bai F, Yu H, You J, Shi Y, Qian Y, Liu W, & Jiang T (2009). Larger regional white matter volume is associated with executive function deficit in remitted geriatric depression: An optimized voxel-based morphometry study. Journal of Affective Disorders, 115(1–2), 225–229. 10.1016/j.jad.2008.09.018 [DOI] [PubMed] [Google Scholar]
- Zaninotto L, Solmi M, Veronese N, Guglielmo R, Ioime L, Camardese G, & Serretti A (2016). A meta-analysis of cognitive performance in melancholic versus non-melancholic unipolar depression. Journal of Affective Disorders, 201, 15–24. 10.1016/j.jad.2016.04.039 [DOI] [PubMed] [Google Scholar]
- *Zeki Al Hazzouri A, Caunca MR, Nobrega JC, Elfassy T, Cheung YK, Alperin N, Dong C, Elkind MSV, Sacco RL, DeCarli C, & Wright CB (2018). Greater depressive symptoms, cognition, and markers of brain aging: Northern Manhattan Study. Neurology, 90(23), e2077–e2085. 10.1212/WNL.0000000000005639 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zetsche U, Bürkner PC, & Schulze L (2018). Shedding light on the association between repetitive negative thinking and deficits in cognitive control—A meta-analysis. Clinical Psychology Review, 63, 56–65. 10.1016/j.cpr.2018.06.001 [DOI] [PubMed] [Google Scholar]
- Zhu X, Li R, Wang P, & Li J (2014). Aberrant functional connectivity of the hippocampus in older adults with subthreshold depression. PsyCh Journal, 3(4), 245–253. 10.1002/pchj.60 [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Martinez J, Attiullah N, Friedman M, Toba C, Boerescu DA, & Rahgeb M (2012). Further evidence that the cutoff to define remission on the 17-item Hamilton Depression Rating Scale should be lowered. Depression and Anxiety, 29(2), 159–165. 10.1002/da.20870 [DOI] [PubMed] [Google Scholar]
- Zimmerman M, Posternak MA, & Chelminski I (2004). Implications of using different cut-offs on symptom severity scales to define remission from depression. International Clinical Psychopharmacology, 19(4), 215–220. 10.1097/01.yic.0000130232.57629.46 [DOI] [PubMed] [Google Scholar]
- *Zupan Z, Zezelj I, & Andjelkovic I (2017). Memory bias in depression: Effects of self-reference and age. Journal of Social and Clinical Psychology, 36(4), 300–315. 10.1521/jscp.2017.36.4.300 [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


