Abstract
Background
We aimed to explore the association between long-term exposure to particulate matter ≤ 2.5 µm (PM2.5) and metabolic syndrome (MetS) and its components including fasting blood glucose (FBG), blood pressure, triglyceride (TG), high-density lipoprotein cholesterol (HDL-c) and waist circumference among adults and elderly in south China.
Methods
We surveyed 6628 participants in the chronic disease and risk factors surveillance conducted in 14 districts of Guangdong province in 2015. MetS was defined based on the recommendation by the Joint Interim Societies’ criteria. We used the spatiotemporal land-use regression (LUR) model to estimate the two-year average exposure of ambient air pollutants (PM2.5, PM10, SO2, NO2, and O3) at individual levels. We recorded other covariates by using a structured questionnaire. Generalized linear mixed model was used for analysis.
Results
A 10-μg/m3 increase in the two-year mean PM2.5 exposure was associated with a higher risk of developing MetS [odd ratio (OR): 1.17, 95% confidence interval (CI): 1.01, 1.35], increased risk of fasting blood glucose level.
(OR: 1.18, 95% CI: 1.02, 1.36), and hypertriglyceridemia (OR: 1.36, 95% CI: 1.18, 1.58) in the adjusted/unadjusted models (all P < 0.05). We found significant interaction between PM2.5 and the region, exercise on the high TG levels, and an interaction with the region, age, exercise and grain consumption on FBG (P interaction < 0.05).
Conclusions
Long-term exposure to PM2.5 was associated with MetS, dyslipidemia and FBG impairment. Efforts should be made for environment improvement to reduce the burden of MetS-associated non-communicable disease.
Supplementary Information
The online version contains supplementary material available at 10.1186/s12940-022-00888-2.
Keywords: PM2.5, Metabolic syndrome, Blood pressure, Triglyceride, High-density lipoprotein cholesterol, Fasting blood glucose, Waist circumference
Introduction
Metabolic syndrome (MetS) is a cluster of metabolic disorders including abdominal obesity, hypertension, hypertriglyceridemia, low high-density lipoprotein cholesterol (HDL-c) and hyperglycemia [1]. MetS has been recognized as an urgent public health concern because it affects 20–30% of the global population, of which the standardized prevalence of MetS is around 24.2% in China [2, 3]. Previous studies showed that MetS was associated with an increased risk of cardiovascular diseases (CVDs), diabetes mellitus, cancers and other chronic non-communicable diseases [4, 5]. Evidence suggests that MetS-related adverse health outcomes may be enhanced not only by genetic factors, physical inactivity and unhealthy diet [6–9], but also by environmental pollutant exposure [10, 11], including air pollution.
Accumulating studies have added to the evidence that the inhalation of particulate matte ≤ 2.5 µm (PM2.5) might lead to pulmonary oxidative stress, systemic inflammation, vascular dysfunction and atherosclerosis [12–16]. Previous studies suggested that PM2.5 was the major risk factor for adverse health outcomes including hypertension [12], obesity [13], elevated fasting blood glucose (FBG) [14, 15], waist circumference [16] and dyslipidemia [17], which were crucial components in the diagnosis of MetS. However, the effects of PM2.5 on blood pressure [18, 19], fasting blood glucose [20, 21] and obesity [22–24] still remained inconsistent. Furthermore, the evidence concerning the associations of air pollution and MetS is still scarce. To our knowledge, only a few studies have reported the detrimental effects of long-term exposure to ambient air pollution on MetS [17, 25–28], which were mainly conducted in the developed countries such as Korea, North America or Saudi Arabia [17, 25, 26]. Only two epidemiological studies evaluated the associations between PM2.5 and the prevalence of MetS in the developing countries such as China [27, 28] among adolescents and children [27], and adults and elderly [28]. In addition, the effects of PM2.5 on specific components on MetS in Chinese population was limited based on the prior evidence.
As one of the most developed provinces in southern China, there has been considerable lifestyle and dietary changes during these decades in Guangdong, resulting in the increase of MetS and stroke, coronary heart disease, and cancers [29]. Meanwhile, air pollution has become one of the most severe environmental problem in Guangdong [30]. In the CAPES study, despite a relatively low concentrations of PM, there was a higher risk of the total, cardiovascular and respiratory mortality attributed to PM in Guangzhou (the capital city of Guangdong province), compared with the heavy industry cities in northeastern China, where PM pollution was more severe [31]. The relatively higher concentration of the toxic component including polybrominated diphenyl ethers (PBDEs) found in PM2.5 in southern China [32, 33] might help provide the evidence for the stronger association between PM and mortality.
Considering the current MetS epidemic, the more toxic effect of PM2.5 in south China, the inconsistent effects of PM2.5 on specific components of MetS, and the limited information of the association between PM2.5 and MetS, we explored the effects of ambient PM2.5 pollution on MetS and its components [blood pressure, triglyceride (TG), high-density lipoprotein-cholecsterol (HDL-c), fasting blood glucose (FBG) and waist circumference] in Guangdong, China. To address the knowledge gap, our findings would provide important public health implications which aimed to reduce the detrimental impact of ambient air pollution of PM2.5 on CVDs and MetS in China.
Materials and methods
Study design and participants
This study was conducted using a multistage, probability-based sampling strategy, based on the Chronic Disease and Risk Factors Surveillance in 2015 in Guangdong province, China. 14 surveillance points were randomly selected. Between October 2015 and February 2016, adults aged 18 years who were living in the current residence for at least 6 months were recruited. All participants were interviewed face-to-face by using a structured questionnaire, which has been described previously [34, 35]. In addition, participants underwent anthropometric measurements (blood pressure, fasting glucose, blood pressure, waist circumstance, height and weight) and blood sample collection by the well-trained public health practitioners from the local health stations or community health service centers. The study protocol was approved by the ethics review committee of the National Center for Chronic and Non-Communicable Disease Control and Prevention, China Center for Disease Control and Prevention. All participants were provided written informed consent. Inclusion and exclusion criteria of participants have been reported previously [36].
MetS definition
The diagnosis of MetS [1] was based on the Joint Interim Societies’ definition. Participants were considered to have MetS if they met any three of the five following conditions (1): Elevated TG levels: ≥ 1.7 mmol/l (150 mg/dl) [1]; (2) Decreased HDL-c levels: < 1.0 mmol/l (40 mg/dl) for men; < 1.3 mmol/l (50 mg/dl) for women [1]; (3) Elevated blood pressure [systolic blood pressure (SBP) ≥ 130 or diastolic blood pressure (DBP) ≥ 85 mmHg] [1]; (4) Elevated FBG levels [FBG ≥ 5.6 mmol/L (100 mg/dl)] [1]; (5) Central obesity, defined as an elevated waist circumference according to the WHO criteria: ≥ 90 cm for men; ≥ 80 cm for women [37]. See Table 1 for further details.
Table 1.
Criteria for clinical diagnosis of the metabolic syndrome
| Conditions | Recommended threshold | |
|---|---|---|
| For Men | For women | |
| Elevated TG levels | ≥ 1.7 mmol/l (150 mg/dl) | ≥ 1.7 mmol/l (150 mg/dl) |
| Decreased HDL-c levels | < 1.0 mmol/l (40 mg/dl) for males | < 1.3 mmol/l (50 mg/dl) |
| Elevated blood pressure | Elevated blood pressure | Elevated blood pressure |
| Elevated FBG levels | FBG ≥ 5.6 mmol/l (100 mg/dl) | FBG ≥ 5.6 mmol/l (100 mg/dl) |
| Central obesity | waist circumference ≥ 90 cm | waist circumference ≥ 80 cm |
Participants were considered to have MetS if they meet any three of the five following conditions (1): Elevated TG levels: ≥ 1.7 mmol/l (150 mg/dl); (2) Decreased HDL-c levels: < 1.0 mmol/l (40 mg/dl) for men; < 1.3 mmol/l (50 mg/dl) for women; (3) Elevated blood pressure (SBP ≥ 130 or DBP ≥ 85 mmHg); (4) Elevated FBG levels [FBG ≥ 5.6 mmol/l (100 mg/dl)]; (5) Central obesity was defined as elevated waist circumference: ≥ 90 cm for men; ≥ 80 cm for women
Assessment of long-term exposure to air pollution
We used the spatiotemporal land-use regression (LUR) model to estimate the two-year average exposure of ambient air pollutants including PM2.5, particulate matter < 10 µm (PM10), sulfur dioxide (SO2), nitrogen dioxide (NO2) and ozone (O3) at individual levels. The details of the data and prediction process has been published previously [38], which were as follows:
The spatiotemporal LUR model was built with the following predictors: population density, road length, land-use data (farmland, blue space, living land, and green space), and ambient visibility. Two smooth temporal basis functions were analyzed to estimate the secular trend of air pollution. The R2 was 88.86% with the root mean square error (RMSE) of 5.65%, based on the findings of the tenfold cross-validation.
Residence address was extracted from the questionnaire and included into the model to forecast the weekly average air pollution between April 2013 and December 2016.
The two-year averaged air pollutant concentrations before the investigation date were estimated for each individual.
Covariates
The following covariates were incorporated to examine the potential confounding and mediating effects: age, sex (man and woman), race (Han and minority), region (urban and rural), occupation (physical work and non-physical work), education level (none, primary school education, middle school education, university education or higher), marital status (none, primary school education, middle school education and university education or higher), household income (< 30, 30–50, 50–100, 100–200 and ≥ 200 × 1000 RMB), weight change in the past year (an increase of > 2.5 kg, unchanged < 2.5 kg, a decease of > 2.5 kg and unclear), alcohol consumption, exercise, family history of diabetes (no and yes), exercise (no and yes), alcohol consumption (no and yes), passive smoking (no and yes), cigarette smoking (non-smoker and smoker), biomass fuel use (no and yes), body-mass index (BMI) (under weight, normal and overweight/obese), grain consumption, vegetable and fruit consumption and red meat consumption. The definition of the covariates is summerized in E-Table 1 [34, 35, 39, 40].
Statistical analysis
We analyzed the characteristics between the groups with MetS and without MetS, by demonstrating the mean and standard deviation for continuous variables and frequencies for categorical variables. The t-test was performed to analyze the distribution of continuous variables, and when indicated, appropriate transformation was applied. A contingency table and Chi-squared test was performed for analyzing the frequencies of categorical variables. The normality and equality of variance was assessed by using the Shapiro–Wilk’s test and Bartlett’s test, respectively. The odds ratios (ORs) and 95% confidence intervals (95%CIs) were calculated for determining the association between ambient air pollutant exposure to PM2.5 and the presence of MetS and its components by using the generalized linear mixed model, based on the three stepwise models to confirm the validity of findings. Family was treated as random effect by calculating the intraclass correlation coefficient (ICC). We compared the Akaike's information criterion value of these three models to avoid over-fitting. The magnitude of collinearity was assessed based on the variance inflation factor (VIF). The VIF of 5 or greater indicated collinearity among the variables. Variables with the evidence of a significant collinearity were excluded from the model. The Spearman’s rank correlation test was used to determine the relationship between pollutants. Strong, moderate, and weak correlations were defined as the coefficients (rs) greater than 0.60, 0.30 to 0.60, and less than 0.30, respectively. Since strong and moderate correlation was identified between PM2.5 and other pollutant models, we only applied the single pollutant model (PM2.5) to avoid covariance. We further stratified the study participants by the region, sex, age, cigarette smoking, alcohol consumption, exercise, BMI, grain consumption, vegetable and fruit consumption and red meat consumption, to study the significant associations between PM2.5 and MetS, high TG and FBG in each stratum. We also included the interaction terms in the generalized linear mixed effect models to test the interactions between PM2.5 and MetS, high TG and FBG in each subgroup. All statistical analyses were performed with R software (version 4.0.2). The threshold of statistical significance for P value was set to be 0.05.
Results
A total of 8991 participants were included in this study, among whom 1157 had missing key variables, 252 had previously been diagnosed as having CVDs, 954 had taken measures to control blood pressure, blood glucose, and lipids. Therefore, 6628 participants were included in the final analysis, with a mean age of 50.1 years. Table 2 shows the demographic characteristics of the participants. 1691 of the participants were diagnosed as having MetS, and 4937 without. Participants with MetS were more likely to have poorer education, lower household income, less exercise and higher BMI as compared with participants without MetS (Table 2).
Table 2.
Basic characteristics of participants by metabolic syndrome
| Characteristics | Total (n = 6628) | Metabolic Syndrome | p | |
|---|---|---|---|---|
| Event (n = 1691) | Non-Event (n = 4937) | |||
| Age (year), mean (SD) | 50.12 (14.73) | 54.09 (12.83) | 48.76 (15.09) | < 0.001* |
| Sex, n (%) | < 0.001* | |||
| Man | 2955 (44.6) | 677 (40.0) | 2278 (44.6) | |
| Women | 3673 (55.4) | 1014 (60.0) | 2659 (55.4) | |
| Race, n (%) | 0.570 | |||
| Han | 6562 (99.0) | 1672 (98.9) | 4890 (99.0) | |
| Minority | 66 (1.0) | 19 (1.1) | 47 (1.0) | |
| Region, n (%) | 0.092 | |||
| Urban | 3613 (54.5) | 892 (52.7) | 2721 (55.1) | |
| Rural | 3015 (45.5) | 799 (47.3) | 2216 (44.9) | |
| Occupation, n (%) | 0.273 | |||
| Physical work | 5070 (76.5) | 1310 (77.5) | 3760 (76.2) | |
| Non-physical work | 1558 (23.5) | 381 (22.5) | 1177 (23.8) | |
| Educational level, n (%) | < 0.001* | |||
| None | 836 (12.6) | 303 (17.9) | 533 (10.8) | |
| Primary school education | 2246 (33.9) | 625 (37.0) | 1621 (32.8) | |
| Middle school education | 2905 (43.8) | 656 (38.8) | 2249 (45.6) | |
| University education or higher | 641 (9.7) | 107 (6.3) | 534 (10.8) | |
| Marriage status, n (%) | < 0.001* | |||
| Unmarried | 357 (5.4) | 46 (2.7) | 311 (6.3) | |
| Married | 5968 (90.0) | 1544 (91.3) | 4424 (89.6) | |
| Widowed or divorced | 303 (4.6) | 101 (6.0) | 202 (4.1) | |
| Household income (× 1000 RMB) | 0.038* | |||
| < 30 | 1029 (15.5) | 261 (15.4) | 768 (15.6) | |
| 30 ≤ Household income < 50 | 1171 (17.7) | 321 (19.0) | 850 (17.2) | |
| 50 ≤ Household income < 100 | 1218 (18.4) | 280 (16.6) | 938 (19.0) | |
| 100 ≤ Household income < 200 | 514 (7.8) | 122 (7.2) | 392 (7.9) | |
| ≥ 200 | 171 (2.6) | 34 (2.0) | 137 (2.8) | |
| Refuse to answer or don't know | 2525 (38.1) | 673 (39.8) | 1852 (37.5) | |
| Behaviors factors | ||||
| Cigarette smoking | 0.003* | |||
| Nonsmoker | 4428 (66.8) | 1180 (69.8) | 3248 (65.8) | |
| Smoker | 2200 (33.2) | 511 (30.2) | 1689 (34.2) | |
| Alcohol consumption, n (%) | 0.027* | |||
| No | 3929 (59.3) | 1041 (61.6) | 2888 (58.5) | |
| Yes | 2699 (40.7) | 650 (38.4) | 2049 (41.5) | |
| Exercise, n (%) | 0.019* | |||
| No | 5479 (82.7) | 1430 (86.6) | 4049 (82.0) | |
| Yes | 1149 (17.3) | 261 (15.4) | 888 (18.0) | |
| Family history of diabetes mellitus, n (%) | 0.524 | |||
| No | 6222 (93.9) | 1582 (93.6) | 4640 (94.0) | |
| Yes | 406 (6.1) | 109 (6.4) | 297 (6.0) | |
| Weight change in the past 12 months, n (%) | 0.221 | |||
| Increase in > 2.5 kg | 609 (9.2) | 164 (9.7) | 445 (9.0) | |
| Unchanged (< 2.5 kg) | 4743 (71.6) | 1217 (72.0) | 3526 (71.4) | |
| Decease in > 2.5 kg | 596 (9.0) | 132 (7.8) | 464 (9.4) | |
| Unclear | 680 (10.3) | 178 (10.5) | 502 (10.2) | |
| Household air pollution exposure | ||||
| Passive smoking, n (%) | 0.598 | |||
| No | 1531 (32.0) | 393 (31.2) | 1138 (32.3) | |
| Yes | 3250 (68.0) | 868 (68.8) | 2382 (67.7) | |
| Biomass fuel | 0.323 | |||
| No | 5136 (77.5) | 1325 (78.4) | 3811 (77.2) | |
| Yes | 1492 (22.5) | 366 (21.6) | 1126 (22.8) | |
| Grain consumption(g/daily), medium (IQR) | 400.00 (376.90) | 394.39 (371.73) | 400.00 (380.00) | 0.212 |
| Vegetable and Fruit consumption (g/daily), medium (IQR) | 308.00 (298.35) | 308.00 (293.33) | 308.33 (299.00) | 0.891 |
| Red Meat consumption (g/daily), medium (IQR) | 71.43 (95.96) | 53.57 (96.29) | 80.00 (115.86) | 0.019* |
| Ambient air pollution exposure (μg/m3), mean (SD) | ||||
| PM2.5 | 37.2 (4.8) | 37.4 (4.5) | 37.1 (4.8) | 0.040* |
| PM10 | 55.4 (5.0) | 55.2 (4.8) | 55.5 (5.1) | 0.041* |
| SO2 | 16.1 (3.8) | 16.2 (3.9) | 16.1 (3.8) | 0.227 |
| NO2 | 26.0 (12.6) | 25.3 (11.4) | 26.2 (12.9) | 0.007* |
| O3 | 56.2 (6.4) | 56.7 (5.9) | 56.1 (6.5) | 0.005* |
| Anthropometry | ||||
| BMI (kg/m2), mean (SD) | 23.04 (3.36) | 25.65 (3.21) | 22.15 (2.92) | < 0.001* |
| BMI category, n (%) | < 0.001* | |||
| Under weight | 504 (56.4) | 15 (0.9) | 489 (9.9) | |
| Normal | 3741 (7.6) | 504 (29.8) | 3237 (65.6) | |
| Overweight/ Obese | 2383 (36.0) | 1172 (69.3) | 1211 (24.5) | |
| MetS, n (%) | 1691 (25.5) | 1691 (100.0) | - | |
| Central obesity, n (%) | 2038 (30.7) | 1236 (73.1) | 802 (16.2) | < 0.001* |
| High TG, n (%) | 1379 (20.8) | 998 (59.0) | 381 (7.7) | < 0.001* |
| Low HDL-c, n (%) | 2759 (41.6) | 1325 (78.4) | 1434 (29.0) | < 0.001* |
| Hypertension, n (%) | 3339 (50.4) | 1430 (84.6) | 1909 (38.7) | < 0.001* |
| High FBG, n (%) | 1606 (24.2) | 920 (54.4) | 686 (13.9) | < 0.001* |
BMI Body-mass index, FBG Fasting blood glucose, HDL-c High-density lipoprotein cholesterol, IQR Inter Quartile Range, n Number, MetS Metabolic syndrome, NO2 Nitrogen dioxide, O3 Ozone, PM2.5 Particulate matter ≤ 2.5 µm, PM10 Particulate matter < 10 µm, Red meat beef, pork, lamb, SD Standard deviation, SO2 Sulfur dioxide, TG Triglyceride
∗:P < 0.05
Table 3 demonstrates the descriptive statistics of air pollution concentrations in 14 district surveillance points, as well as their pairwise correlations. The range concentration of PM2.5, PM10, SO2, NO2, and O3 were 27.99 to 46.96 µg/m3, 42.17 to 67.33 µg/m3, 9.31 to 22.28 µg/m3, 7.94 to 62.68 µg/m3, 40.54 to 68.83 µg/m3, respectively. The mean concentration of PM2.5 and PM10 exceeded the World Health Organization (WHO) air quality guidelines,, which respective recommended values was 5 µg/m3, 15 µg/m3 and 10 µg/m3 [41] in the surveillance points in this study. In general, the air pollutants were highly or moderately correlated with each other (rs ranged from -0.35 to 0.75).
Table 3.
Summary statistics and Spearman correlations of 2-year mean air pollutants
| Summary statistics | Spearman correlation coefficients | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Mean | Median | Minimum | Maximum | IQR | PM2.5 | PM10 | SO2 | NO2 | O3 | |
| PM2.5 (μg/m3) | 37.17 | 38.30 | 27.99 | 46.96 | 8.84 | 1.00 | 0.71* | 0.52* | 0.60* | -0.49* |
| PM10 (μg/m3) | 55.43 | 55.09 | 42.17 | 67.33 | 7.58 | 1.00 | 0.63* | 0.75* | -0.51* | |
| SO2 (μg/m3) | 16.12 | 15.92 | 9.31 | 22.28 | 5.44 | 1.00 | 0.37* | -0.35* | ||
| NO2 (μg/m3) | 25.98 | 23.07 | 7.94 | 62.68 | 18.07 | 1.00 | -0.68* | |||
| O3 (μg/m3) | 56.23 | 56.96 | 40.54 | 68.83 | 7.38 | 1.00 | ||||
PM2.5 Particulate matter ≤ 2.5 µm, PM10 Particulate matter < 10 µm, SO2 Sulfur dioxide, NO2 Nitrogen dioxide, O3 Ozone
Note: Spearman correlation coefficients, *:P < 0.05
Table 4 shows the adjusted odds ratios of metabolic syndrome and its components with 10-μg/m3 increase in PM2.5. For all participants, PM2.5 was positively associated with MetS. Results of the single pollutant model showed that each 10 μg/m3 increase in two years of exposure to PM2.5 was associated with a 1.17-fold (95% CI: 1.01–1.35) higher risk of MetS (P < 0.05, model 3). In the analysis of other components of MetS, each 10 μg/m3 increase in the two-year mean exposure of PM2.5 was associated with high TG and high FBG, with a respective odd ratio (OR) of 1.36 (95% CI: 1.18–1.58) and 1.18 (95% CI: 1.02–1.36) in the single-pollutant model (P < 0.05, model 3). No association was observed between ambient PM2.5 exposure and central obesity, low HDL-c, and hypertension. Results in model 1 to model 3 were not changed materially, suggesting that the results were robust (Table 4).
Table 4.
Adjusted odd ratios of metabolic syndrome and its components in overall population with 10-μg/m3 increase in PM2.5
| Variables | MetS | Central obesity | High TG | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| AIC | OR (95%CI) | P | AIC | OR (95%CI) | P | AIC | OR (95%CI) | P | |||
| Model 1 | 7522.5 | 1.14(1.01, 1.29) | 0.039* | 8181.9 | 1.04 (0.93, 1.16) | 0.516 | 6757.9 | 1.36 (1.34, 1.38) | < 0.001* | ||
| Model 2 | 5807.8 | 1.17(1.15, 1.19) | < 0.001* | 4075.2 | 1.02 (0.85, 1.23) | 0.806 | 6190.5 | 1.40 (1.21, 1.62) | < 0.001* | ||
| Model 3 | 5807.8 | 1.17(1.01, 1.35) | 0.042* | 4060.1 | 0.98 (0.82, 1.18) | 0.813 | 6183.0 | 1.36 (1.18, 1.58) | < 0.001* | ||
| Variables | Low HDL-c | Hypertension | High FBG | ||||||||
| AIC | OR (95%CI) | P | AIC | OR (95%CI) | P | AIC | OR (95%CI) | P | |||
| Model 1 | 8989.0 | 1.00 (0.90, 1.12) | 0.944 | 9171.0 | 1.04 (0.93, 1.16) | 0.506 | 7302.1 | 1.17 (1.02, 1.35) | 0.023* | ||
| Model 2 | 8358.3 | 0.98 (0.87, 1.11) | 0.784 | 7680.7 | 1.02 (0.90, 1.16) | 0.721 | 6871.9 | 1.15 (1.01, 1.33) | 0.047* | ||
| Model 3 | 8318.3 | 0.99 (0.88, 1.12) | 0.867 | 7658.5 | 1.03 (0.91,1.16) | 0.682 | 6823.2 | 1.18 (1.02, 1.36) | 0.026* | ||
Model 1: Exposure to PM2.5;
Model 2: Model 1 adjusted with age, sex, education, marital status, body mass index, household income;
Model 3: Model 2 adjusted with exercise, cigarette smoking status, biomass fuel, alcohol consumption, red meat consumption
AIC Akaike information criterion, CI Confidence interval, FBG Fasting blood glucose, HDL-c High-density lipoprotein cholesterol, MetS Metabolic syndrome, OR Odd ratio, TG Triglyceride
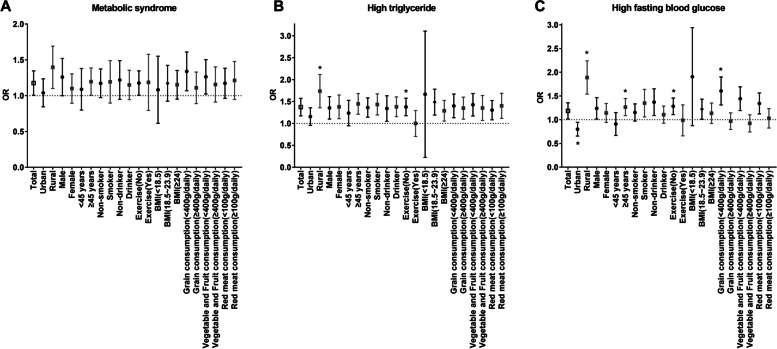
Table 5 shows the subgroup analysis by the region, sex, age, cigarette smoking, alcohol consumption, exercise, BMI, grain consumption, vegetable and fruit consumption and red meat consumption (Table 5, Fig. 1). We did not find statistically significant interactions between PM2.5 and the aforementioned variables for MetS. We observed stronger associations between PM2.5 and high TG levels in subgroups who took less exercise, living in rural area, with statistically significant interactions (Pinteraction < 0.05). In addition, each 10 μg/m3 increase in two-year mean exposure to PM2.5 was associated with 87%, 26%, 59% and 28% and higher risk of high FBG among subgroups living in rural area, ≥ 45 years old, having < 400 g/daily grain intake and less exercise, with statistically significant interactions among these groups (Pinteraction < 0.05).
Table 5.
Subgroup analysis of the association between per two-year mean 10-μg/m3 increase in PM2.5 and metabolic syndrome, high triglyceride and high fasting blood among adults and elderly
| Variable | MetS | Pinter | High TG | Pinter | High FBG | Pinter |
|---|---|---|---|---|---|---|
| OR (95%CI) | OR (95%CI) | OR (95%CI) | ||||
| Region | 0.054 | 0.004* | < 0.001* | |||
| Urban (n = 3613) | 1.03 (0.85, 1.24) | 1.15 (0.96, 1.36) | 0.79 (0.66, 0.95) * | |||
| Rural (n = 3015) | 1.38 (1.11, 1.70) * | 1.71 (1.37, 2.13) * | 1.87 (1.55, 2.25) * | |||
| Sex | 0.275 | 0.848 | 0.517 | |||
| Men (n = 2955) | 1.24 (1.01, 1.53) * | 1.34 (1.11, 1.62) * | 1.23 (1.02, 1.47) * | |||
| Women (n = 3673) | 1.09 (0.90, 1.31) | 1.36 (1.12, 1.66) * | 1.13 (0.95, 1.35) | |||
| Age | 0.412 | 0.083 | 0.049* | |||
| < 45 years(n = 2316) | 1.07 (0.81, 1.39) | 1.21 (0.96, 1.54) | 0.89 (0.68, 1.16) | |||
| ≥ 45 years(n = 4312) | 1.19 (1.01, 1.39) * | 1.43 (1.22, 1.69) * | 1.26 (1.09, 1.45) * | |||
| Cigarette smoking | 0.793 | 0.637 | 0.081 | |||
| Nonsmoker(n = 4428) | 1.16 (0.98, 1.38) | 1.35 (1.15, 1.59) * | 1.14 (0.98, 1.34) | |||
| Smoker(n = 2200) | 1.17 (0.91, 1.50) | 1.42 (1.20, 1.68) * | 1.33 (1.07, 1.65) * | |||
| Alcohol consumption | 0.156 | 0.195 | 0.261 | |||
| Non-drinker (n = 3929) | 1.20 (0.96, 1.50) | 1.32 (1.06, 1.64) * | 1.35 (1.10, 1.66) * | |||
| Drinker (n = 2699) | 1.14 (0.95, 1.36) | 1.37 (1.16, 1.61) * | 1.10 (0.93, 1.29) | |||
| Exercise | 0.269 | 0.015* | 0.040* | |||
| No (n = 5479) | 1.17 (1.01, 1.35) * | 1.37 (1.18, 1.58) * | 1.28 (1.11, 1.46) * | |||
| Yes (n = 1149) | 1.14 (0.82, 1.60) * | 0.97 (0.72, 1.31) * | 0.95 (0.68, 1.33) | |||
| BMI | 0.795 | 0.681 | 0.349 | |||
| Underweight(n = 504) | 1.02 (0.65, 1.58) | 1.25 (0.48, 3.27) | 1.72 (0.98, 3.02) | |||
| Normal (n = 3741) | 1.16 (0.93, 1.43) | 1.47 (1.20, 1.79) * | 1.21 (1.02, 1.44) * | |||
| Over weight/Obese (n = 2383) | 1.14 (0.96, 1.36) | 1.28 (1.06, 1.53) * | 1.12 (0.93, 1.36) | |||
| Grain consumption | 0.713 | 0.897 | < 0.001* | |||
| < 400 g/daily(n = 3232) | 1.32 (1.08, 1.62) * | 1.38 (1.14, 1.68) * | 1.59 (1.32, 1.91) * | |||
| ≥ 400 g/daily (n = 3396) | 1.09 (0.90, 1.34) | 1.33 (1.11, 1.61) * | 0.96 (0.80, 1.15) | |||
| Vegetable and Fruit consumption | 0.979 | 0.869 | 0.419 | |||
| < 400 g/daily(n = 3858) | 1.25 (1.03, 1.51) * | 1.41 (1.18, 1.69) * | 1.43 (1.20, 1.70) * | |||
| ≥ 400 g/daily (n = 2770) | 1.14 (0.92, 1.41) | 1.36 (1.10, 1.66) * | 0.91 (0.75, 1.11) | |||
| Red Meat consumption | 0.312 | 0.332 | 0.860 | |||
| < 100 g/daily (n = 3970) | 1.16 (0.97, 1.39) | 1.29 (1.09, 1.53) * | 1.33 (1.13, 1.57) * | |||
| ≥ 100 g/daily (n = 2658) | 1.19 (0.96, 1.49) | 1.38 (1.13, 1.70) * | 1.02 (0.83, 1.24) |
AIC Akaike information criterion, BMI Body-mass index, CI Confidence interval, FBG Fasting blood glucose, HDL-C High-density lipoprotein cholesterol, MetS Metabolic syndrome, OR Odd ratio, TG Triglyceride
Adjusted with age, sex, education, marital status, body mass index, household income, exercise, cigarette smoking status, biomass fuel, alcohol consumption, red meat consumption
∗: P < 0.05
Fig. 1.
Associations of long-term PM2.5 exposure with MetS, high TG and high FBG in different stratum (*represents the p value for interaction with significance). A) Metabolic syndrome, B) High triglyceride, C) High fasting blood glucose
Discussion
Understanding the impacts of long-term exposure to ambient PM2.5 on MetS is crucial, because 25.5% of the population had MetS in the studied regions of southern China. This study was conducted to elucidate the key research question regarding whether exposure to ambient PM2.5 would increase the risk of having MetS and confer a detrimental impact on its specific components in Guangdong province. Information regarding the associations between PM2.5 and the prevalence of MetS with its specific components in China remains scarce. Reassuringly, we found that long-term exposure to ambient PM2.5 pollution was significantly associated with an increased risk of MetS. In addition, long-term exposure to PM2.5 increased the risk of high TG and high FBG. Furthermore, the participants living in rural area, aged greater than 45 years, having less exercises and < 400 g/daily grain intake were more susceptible to the adverse effects of ambient PM2.5 exposure.
Although previous studies and the current study were conducted in different geographical areas, with differences in the population characteristics, pollutant concentrations or sources, exposure duration and exposure measurement, it is worth mentioning that positive associations of long-term ambient PM2.5 pollution exposure with MetS remained consistent and that the magnitudes of the effect estimates observed in these studies were comparable. The normative aging study in New York [17] and a cross-sectional study in China [27] found that 10 μg/m3 increase in ambient PM2.5 was associated with a 10% to 31% higher risk of MetS among children, adolescents and elderly population. A nationwide population-based cohort study in Korea showed that each 10 µg/m3 increase in one-year averaged concentration of PM2.5 was associated with a 7% higher risk of MetS in adults [25]. Likewise, the Chinese health study found that each 10 μg/m3 increase in the long-term exposure to PM2.5 was associated with 5% higher risk of MetS in 15,477 adults from 33 communities in northeast China [28]. We have detected the largest magnitude of effect estimates of the association between PM2.5 and MetS in adults. Compared with other heavy industry cities in northeast China, higher risk of total, cardiovascular and respiratory mortality was found in Guangzhou, where the concentration of PM was relatively low [31]. The relatively high concentration of the toxic components (e.g. PBDEs) in PM2.5 detected in southern China [32, 33] might help explain the paradoxically larger effect estimates of the association between PM and total/cardiovascular/respiratory disease mortality and MetS, in the scenario of the lower concentration of PM in Guangdong.
Regarding the complexity of metabolic alterations that constitute MetS, many studies have investigated the association between long- and short-term exposure of PM2.5 and its specific components [15, 17, 21, 42–45]. Several population-based studies have reported harmful effects of ambient PM2.5 on FBG, yet the results were inconsistent. Though Alderete et al. did not identify a statistically significant association between long-term exposure to PM2.5 and FBG in Los Angeles Latino children [21], several other studies investigating the harmful effects of PM2.5 on FBG has supported our findings in different population [15, 42, 43]. The Normative Aging Study found that exposure to high levels of PM2.5 within 28 days was associated with an increased level of FBG [43]. A cross-sectional study revealed a positive association between exposure to PM2.5 and increased FBG among primary school children in China [15]. Few studies have investigated the relationship between PM2.5 and high TG. We are awared of only three studies which were conducted in specific populations or yielded different results from this study. Similar to the results from 587 elderly individuals in the US [17] and 73,117 subjects with known CVDs and risk factors in southern Israel [44], we have identified the adverse impact of PM2.5 on TG. However, none of the significant association was found in the population-based cross-sectional study conducted in northeast China [45]. Similar to the results of Wallwork RS et al. [17], we did not reveal a significant association between PM2.5 and abdominal obesity, low HDL-c and hypertension, which are the essential components of MetS that are often presented as the underlying and/or preceding other components [46] and cardiovascular events [47, 48]. PM2.5 might activate the metabolic mechanisms such as inflammation, which might increase the risk of developing elevated FBG and hypertriglyceridemia without substantially increasing the risk of abdominal obesity, low HDL-c or hypertension.
As seen in other air pollutant studies, the health effects shown in our study were relatively small. However, regarding the broad extent of the exposed population and the continuous nature of exposure, health implications of ambient PM2.5 exposures should be considered at the population level rather than at the individual level [49, 50]. Metabolic risk factors have long been hypothesized as the mediators between air pollutants and CVDs [45, 51, 52]. A previous study showed that participants with an existing metabolic risk factor had a higher risk of CVDs than those without [45]. The results of high TG and high FBG attributed to PM2.5 based on our analyses may help provide the evidence to support these hypotheses. In addition, MetS, high FBG and TG can be translated into adverse health outcomes of CVDs and diabetes mellitus [4, 5]. Participants with type 2 diabetes and hypertriglyceridemia may be more susceptible to the cardiovascular effects of PM2.5 than those without cardiometabolic risk factors. Small differences in the glucose/TG control within the normal range could be translated into the clinically meaningful variation in CVDs and diabetes mellitus risk [53]. These metabolic associations may represent the intermediate factors that help explain the detrimental effect of increased exposure to PM2.5 on CVDs and diabetes mellitus morbidity and mortality. Nevertheless, our findings were not unexpected because air pollution exposure and metabolic risk factors have been closely associated with the heightened inflammatory responses, which is implicated in the development of CVD [52]. Thus, participants with high TG and high FBG might be more susceptible to the detrimental effects of PM2.5, which could help interpret a higher CVD prevalence.
There were limitations regarding the study design and data interpretation. The causality between ambient PM2.5 exposure and MetS and its components cannot be confirmed owning to the cross-sectional study design. Second, data on the secondary MetS diseases were also not fully collected. Although we have excluded participants with CVDs, other diseases including hyperlipidemia and renal hypertension were not available, which might have influenced on the results. Third, the information on multiple food intake was limited regarding the importance of such variable on the etiology of MetS. Furthermore, there could be interactions between PM2.5 and multiple indoor air pollutants (e.g., mold, household fuels, allergens, tobacco smoke, cooking, furniture, paints, cleaning agents) [54], which cannot be readily disentangled.
However, our findings remain robust. We conducted the LUR model to determine PM2.5 exposure at a specific address to safeguard the accuracy of the exposure assessment. Additionally, our association analyses were based on multiple models, with the results not being materially altered. Because the long-term health risk of TG and FBG may be important predictors for future risks of CVDs and diabetes mellitus, efforts should be endeavored to minimize the concentration and exposure to PM2.5 pollution.
Conclusion
In conclusion, this study adds to the comprehensive evidence of the association between long-term exposure to PM2.5 and MetS. Dyslipidemia especially high triglyceride and FBG impairment is strongly associated with PM2.5 levels. However, further prospective studies are needed to confirm our findings.
Supplementary Information
Acknowledgements
We thank National Center for Chronic and Non-Communicable Disease Control and Prevention, Chinese Center for Disease Control and Prevention for their instruction of the program. We thank the working staffs from the local center for disease control and prevention, and the public health practitioners from the local health station or community health service center, for their organization and contribution for the program. We thank prof. Wei-jie Guan for his help in linguistic advice.
Abbreviations
- CVDs
Cardiovascular diseases
- PM2.5
Particulate matter ≤ 2.5 µm
- PM10
Particulate matter < 10 µm
- SO2
Sulfur dioxide
- NO2
Nitrogen dioxide
- O3
Ozone
- MetS
Metabolic syndrome
- FBG
Fasting blood glucose
- TG
Triglyceride
- HDL-c
High-density lipoprotein cholesterol
- LUR
Land-use regression
- AIC
Akaike information criterion
- OR
Odd ratio
- CI
Confidence interval
- PBDEs
Polybrominated diphenyl ethers
- RMSE
Root mean square error
- BMI
Body-mass index
- ICC
Intraclass correlation coefficient
- VIF
Variance inflation factor
- WHO
World Health Organization
Authors’ contributions
Xue-yan Zheng contributed to conceptualization, methodology, formal analysis, interpretation of data, drafting and review of the manuscript. Si-li Tang: contributed to methodology, formal analysis, data curation, visualization and writing original draft of the manuscript. Tao Liu contributed to conceptualization, methodology, software and validation. Ye Wang contributed to investigation, validation and supervision of the interview. Xiao-jun Xu contributed to investigation, validation and supervision of the interview. Ni Xiaocontributed to investigation, validation and supervision of the interview. Yan-jun Xu contributed to investigation, validation and supervision of the interview. Zhao-xuan He contributed to validation and supervision of the interview.. Shu-li Ma contributed to validation and supervision of the interview.. Yu-liang Chen contributed to investigation, validation and supervision of the interview. Rui-lin Meng contributed to investigation, validation and supervision of the interview. Li-feng Lin contributed to conceptualization, methodology, supervision, drafting and review of the manuscript.
Funding
This study was supported by Guangdong provincial medical science and technology research funding (C2021083).
Declarations
Competing interests
We declare no competing interests.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Xue-yan Zheng, Si-li Tang and Tao Liu contributed equally to this work. Li-feng Lin and Rui-ling Meng shared co-corresponding authorship.
Contributor Information
Rui-lin Meng, Email: 931627747@qq.com.
Li-feng Lin, Email: lifenglinGD@163.com.
References
- 1.Alberti KGMM, Eckel RH, Grundy SM, Zimmet PZ, Cleeman JI, Donato KA, et al. Harmonizing the Metabolic Syndrome A Joint Interim Statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120(16):1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 2.O'Neill S, O'Driscoll L. Metabolic syndrome: a closer look at the growing epidemic and its associated pathologies. Obes Rev. 2015;16(1):1–12. doi: 10.1111/obr.12229. [DOI] [PubMed] [Google Scholar]
- 3.Li Y, Zhao L, Yu D, Wang Z, Ding G. Metabolic syndrome prevalence and its risk factors among adults in China: A nationally representative cross-sectional study. PLoS ONE. 2018;13(6):e0199293. doi: 10.1371/journal.pone.0199293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grundy SM. Controversy in clinical endocrinology - Metabolic syndrome: A multiplex cardiovascular risk factor. J Clin Endocrinol Metab. 2007;92(2):399–404. doi: 10.1210/jc.2006-0513. [DOI] [PubMed] [Google Scholar]
- 5.Hanley AJG, Festa A, D'Agostino RB, Wagenknecht LE, Savage PJ, Tracy RP, et al. Metabolic and inflammation variable clusters and prediction of type 2 diabetes - Factor analysis using directly measured insulin sensitivity. Diabetes. 2004;53(7):1773–1781. doi: 10.2337/diabetes.53.7.1773. [DOI] [PubMed] [Google Scholar]
- 6.Carr DB, Utzschneider KM, Hull RL, Kodama K, Retzlaff BM, Brunzell JD, et al. Intra-abdominal fat is a major determinant of the national cholesterol education program adult treatment panel III criteria for the metabolic syndrome. Diabetes. 2004;53(8):2087–2094. doi: 10.2337/diabetes.53.8.2087. [DOI] [PubMed] [Google Scholar]
- 7.Cornier M-A, Dabelea D, Hernandez TL, Lindstrom RC, Steig AJ, Stob NR, et al. The Metabolic Syndrome. Endocr Rev. 2008;29(7):777–822. doi: 10.1210/er.2008-0024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gustat J, Srinivasan Sr Fau Elkasabany A, Elkasabany A Fau Berenson GS, Berenson GS. Relation of self-rated measures of physical activity to multiple risk factors of insulin resistance syndrome in young adults: the Bogalusa Heart Study. J Clin Epidemiol. 2002;55(10):997–1006. doi: 10.1016/S0895-4356(02)00427-4. [DOI] [PubMed] [Google Scholar]
- 9.Ford ES, Giles Wh Fau -Dietz WH, Dietz WH. Prevalence of the metabolic syndrome among US adults: findings from the third National Health and Nutrition Examination Survey. Jama. 2002;287(3):356–9. doi: 10.1001/jama.287.3.356. [DOI] [PubMed] [Google Scholar]
- 10.Park SK, Auchincloss AH, O'Neill MS, Prineas R, Correa JC, Keeler J, et al. Particulate Air Pollution, Metabolic Syndrome, and Heart Rate Variability: The Multi-Ethnic Study of Atherosclerosis (MESA) Environ Health Perspect. 2010;118(10):1406–1411. doi: 10.1289/ehp.0901778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tsai S-S, Lin Y-S, Chen S-T, Chu P-H. Metabolic syndrome positively correlates with the risks of atherosclerosis and diabetes in a Chinese population. Eur J Intern Med. 2018;54:40–45. doi: 10.1016/j.ejim.2018.04.009. [DOI] [PubMed] [Google Scholar]
- 12.Yang BY, Qian Z, Howard SW, Vaughn MG, Fan SJ, Liu KK, et al. Global association between ambient air pollution and blood pressure: A systematic review and meta-analysis. Environ Pollut. 2018;235:576–588. doi: 10.1016/j.envpol.2018.01.001. [DOI] [PubMed] [Google Scholar]
- 13.Mazidi M, Speakman JR. Ambient particulate air pollution (PM2.5) is associated with the ratio of type 2 diabetes to obesity. Sci Rep. 2017;7(1):9144. doi: 10.1038/s41598-017-08287-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ma R, Zhang Y, Sun Z, Xu D, Li T. Effects of ambient particulate matter on fasting blood glucose: A systematic review and meta-analysis. Environ Pollut. 2020;258:113589. doi: 10.1016/j.envpol.2019.113589. [DOI] [PubMed] [Google Scholar]
- 15.Cai L, Wang S, Gao P, Shen X, Jalaludin B, Bloom MS, et al. Effects of ambient particulate matter on fasting blood glucose among primary school children in Guangzhou. China Environmental Research. 2019;176:108541. doi: 10.1016/j.envres.2019.108541. [DOI] [PubMed] [Google Scholar]
- 16.Cao S, Guo Q, Xue T, Wang B, Wang L, Duan X, et al. Long-term exposure to ambient PM2.5 increase obesity risk in Chinese adults: A cross-sectional study based on a nationwide survey in China. Sci Total Environ. 2021;778:145812. doi: 10.1016/j.scitotenv.2021.145812. [DOI] [PubMed] [Google Scholar]
- 17.Wallwork RS, Colicino E, Zhong J, Kloog I, Coull BA, Vokonas P, et al. Ambient Fine Particulate Matter, Outdoor Temperature, and Risk of Metabolic Syndrome. Am J Epidemiol. 2017;185(1):30–39. doi: 10.1093/aje/kww157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang H, Qian J, Zhao H, Wang J, Zhu H, Zhou Y, et al. A study of the association between atmospheric particulate matter and blood pressure in the population. Blood Press. 2016;25(3):169–176. doi: 10.3109/08037051.2015.1111019. [DOI] [PubMed] [Google Scholar]
- 19.Liang R, Zhang B, Zhao X, Ruan Y, Lian H, Fan Z. Effect of exposure to PM2.5 on blood pressure: a systematic review and meta-analysis. J Hypertens. 2014;32(11):2130–41. doi: 10.1097/HJH.0000000000000342. [DOI] [PubMed] [Google Scholar]
- 20.Erqou S, Clougherty JE, Olafiranye O, Magnani JW, Aiyer A, Tripathy S, et al. Particulate Matter Air Pollution and Racial Differences in Cardiovascular Disease Risk. Arterioscler Thromb Vasc Biol. 2018;38(4):935–942. doi: 10.1161/ATVBAHA.117.310305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Alderete TL, Habre R, Toledo-Corral CA- O, Berhane K, Chen Z, Lurmann FW, et al. Longitudinal Associations Between Ambient Air Pollution With Insulin Sensitivity, β-Cell Function, and Adiposity in Los Angeles Latino Children. Diabetes. 2017;66(7):1789–96. doi: 10.2337/db16-1416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Fioravanti S, Cesaroni G, Badaloni C, Michelozzi P, Forastiere F, Porta D. Traffic-related air pollution and childhood obesity in an Italian birth cohort. Environ Res. 2018;160:479–486. doi: 10.1016/j.envres.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 23.Fleisch AF, Luttmann-Gibson H, Perng W, Rifas-Shiman SL, Coull BA, Kloog I, et al. Prenatal and early life exposure to traffic pollution and cardiometabolic health in childhood. Pediatr Obes. 2017;12(1):48–57. doi: 10.1111/ijpo.12106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tamayo-Ortiz M, Tellez-Rojo MM, Rothenberg SJ, Gutierrez-Avila I, Just AC, Kloog I, et al. Exposure to PM2.5 and Obesity Prevalence in the Greater Mexico City Area. Int J Environ Res Public Health. 2021;18(5):2301. doi: 10.3390/ijerph18052301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lee S, Park H, Kim S, Lee E-K, Lee J, Hong YS, et al. Fine particulate matter and incidence of metabolic syndrome in non-CVD patients: A nationwide population-based cohort study. Int J Hyg Environ Health. 2019;222(3):533–540. doi: 10.1016/j.ijheh.2019.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Shamy M, Alghamdi M, Khoder MI, Mohorjy AM, Alkhatim AA, Alkhalaf AK, et al. Association between Exposure to Ambient Air Particulates and Metabolic Syndrome Components in a Saudi Arabian Population. Int J Environ Res Public Health. 2018;15(1):27. doi: 10.3390/ijerph15010027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhang JS, Gui ZH, Zou ZY, Yang BY, Ma J, Jing J, et al. Long-term exposure to ambient air pollution and metabolic syndrome in children and adolescents: A national cross-sectional study in China. Environ Int. 2021;148:106383. doi: 10.1016/j.envint.2021.106383. [DOI] [PubMed] [Google Scholar]
- 28.Yang BY, Qian ZM, Li S, Fan S, Chen G, Syberg KM, et al. Long-term exposure to ambient air pollution (including PM1) and metabolic syndrome: The 33 Communities Chinese Health Study (33CCHS) Environ Res. 2018;164:204–211. doi: 10.1016/j.envres.2018.02.029. [DOI] [PubMed] [Google Scholar]
- 29.Zhou M, Wang H, Zhu J, Chen W, Wang L, Liu S, et al. Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet. 2016;387(10015):251–272. doi: 10.1016/S0140-6736(15)00551-6. [DOI] [PubMed] [Google Scholar]
- 30.Guan W-J, Zheng X-Y, Chung KF, Zhong N-S. Impact of air pollution on the burden of chronic respiratory diseases in China: time for urgent action. Lancet. 2016;388(10054):1939–1951. doi: 10.1016/S0140-6736(16)31597-5. [DOI] [PubMed] [Google Scholar]
- 31.Chen R, Kan H, Chen B, Huang W, Bai Z, Song G, et al. Association of Particulate Air Pollution With Daily Mortality. Am J Epidemiol. 2012;175(11):1173–1181. doi: 10.1093/aje/kwr425. [DOI] [PubMed] [Google Scholar]
- 32.Li J, Liu X, Yu L-L, Zhang G, Li X-D, Lee CSL, et al. Comparing polybrominated diphenyl ethers (PBDEs) in airborne particles in Guangzhou and Hong Kong: sources, seasonal variations and inland outflow. J Environ Monit. 2009;11(6):1185–1191. doi: 10.1039/b900525k. [DOI] [PubMed] [Google Scholar]
- 33.Wang C, Li W, Chen J, Wang H, Li T, Shen G, et al. Summer atmospheric polybrominated diphenyl ethers in urban and rural areas of northern China. Environ Pollut. 2012;171:234–240. doi: 10.1016/j.envpol.2012.07.041. [DOI] [PubMed] [Google Scholar]
- 34.Wang B, He M, Chao A, Engelgau MM, Saraiya M, Wang L, et al. Cervical Cancer Screening Among Adult Women in China, 2010. Oncologist. 2015;20(6):627–634. doi: 10.1634/theoncologist.2014-0303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang L, Peng W, Zhao Z, Zhang M, Shi Z, Song Z, et al. Prevalence and Treatment of Diabetes in China, 2013–2018. JAMA. 2021;326(24):2498–2506. doi: 10.1001/jama.2021.22208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang Y, Wang Y, Wang H, Liu Z, Yu X, Yan J, et al. Prevalence of mental disorders in China: a cross-sectional epidemiological study. The lancet Psychiatry. 2019;6(3):211–224. doi: 10.1016/S2215-0366(18)30511-X. [DOI] [PubMed] [Google Scholar]
- 37.World Health Organization. Waist circumference and waist-hip ratio: report of a WHO expert consultation; August 8–11, 2008; Geneva, Switzerland. Accessed July 15, 2021. http://apps.who.int/iris/bitstream/handle/10665/44583/9789241501491_eng.pdf
- 38.Liu T, Chen X, Xu Y, Wu W, Tang W, Chen Z, et al. Gut microbiota partially mediates the effects of fine particulate matter on type 2 diabetes: Evidence from a population-based epidemiological study. Environ Int. 2019;130:104882. doi: 10.1016/j.envint.2019.05.076. [DOI] [PubMed] [Google Scholar]
- 39.Zheng XY, Tang SL, Guan WJ, Ma SL, Li C, Xu YJ, et al. Exposure to biomass fuel is associated with high blood pressure and fasting blood glucose impairment in females in southern rural China. Environ Res. 2021;199:111072. doi: 10.1016/j.envres.2021.111072. [DOI] [PubMed] [Google Scholar]
- 40.Cai L, Wang S, Gao P, Shen X, Jalaludin B, Bloom MS, et al. Effects of ambient particulate matter on fasting blood glucose among primary school children in Guangzhou. China Environ Res. 2019;176:108541. doi: 10.1016/j.envres.2019.108541. [DOI] [PubMed] [Google Scholar]
- 41.World Health Organization. WHO global air quality guidelines: particulate matter (PM2.5 and PM10), ozone, nitrogen dioxide, sulfur dioxide and carbon monoxide. 2021. Geneva: World Health Organization. Accessed May 1st, 2022 https://apps.who.int/iris/handle/10665/345329. [PubMed]
- 42.Lucht SA, Hennig F, Matthiessen C, Ohlwein S, Icks A, Moebus S, et al. Air Pollution and Glucose Metabolism: An Analysis in Non-Diabetic Participants of the Heinz Nixdorf Recall Study. Environ Health Perspect. 2018;126(4):047001. doi: 10.1289/EHP2561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Peng C, Bind MAC, Colicino E, Kioog I, Byun HM, Cantone L, et al. Particulate Air Pollution and Fasting Blood Glucose in Nondiabetic Individuals: Associations and Epigenetic Mediation in the Normative Aging Study, 2000–2011. Environ Health Perspect. 2016;124(11):1715–21. doi: 10.1289/EHP183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Yitshak Sade M, Kloog I, Liberty IF, Schwartz J, Novack V. The Association Between Air Pollution Exposure and Glucose and Lipids Levels. J Clin Endocrinol Metab. 2016;101(6):2460–2467. doi: 10.1210/jc.2016-1378. [DOI] [PubMed] [Google Scholar]
- 45.Yang BY, Guo Y, Markevych I, Qian Z, Bloom MS, Heinrich J, et al. Association of Long-term Exposure to Ambient Air Pollutants With Risk Factors for Cardiovascular Disease in China. JAMA Netw Open. 2019;2(3):e190318. doi: 10.1001/jamanetworkopen.2019.0318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Despres JP, Lemieux I. Abdominal obesity and metabolic syndrome. Nature. 2006;444(7121):881–887. doi: 10.1038/nature05488. [DOI] [PubMed] [Google Scholar]
- 47.Austin MA, Hokanson Je Fau -Edwards KL, Edwards KL. Hypertriglyceridemia as a cardiovascular risk factor. Am J Cardiol. 1998;81(4A):7B–12B. doi: 10.1016/S0002-9149(98)00031-9. [DOI] [PubMed] [Google Scholar]
- 48.Sowers JR, Epstein M Fau -Frohlich ED, Frohlich ED. Diabetes, hypertension, and cardiovascular disease: an update. Hypertension. 2001;37(4):1053–9. doi: 10.1161/01.HYP.37.4.1053. [DOI] [PubMed] [Google Scholar]
- 49.Rajagopalan S, Brook RD. Air Pollution and Type 2 Diabetes Mechanistic Insights. Diabetes. 2012;61(12):3037–3045. doi: 10.2337/db12-0190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kunzli N, Kaiser R, Medina S, Studnicka M, Chanel O, Filliger P, et al. Public-health impact of outdoor and traffic-related air pollution: a European assessment. Lancet. 2000;356(9232):795–801. doi: 10.1016/S0140-6736(00)02653-2. [DOI] [PubMed] [Google Scholar]
- 51.Giorgini P, Di Giosia P, Grassi D, Rubenfire M, Brook RD, Ferri C. Air pollution exposure and blood pressure: an updated review of the literature. Curr Pharm Des. 2016;22(1):28–51. doi: 10.2174/1381612822666151109111712. [DOI] [PubMed] [Google Scholar]
- 52.Rajagopalan S, Al-Kindi SG, Brook RD. Air pollution and cardiovascular disease: JACC State-of-the-Art Review. J Am Coll Cardiol. 2018;72(17):2054–2070. doi: 10.1016/j.jacc.2018.07.099. [DOI] [PubMed] [Google Scholar]
- 53.Gerstein HC. Glucose: a continuous risk factor for cardiovascular disease. Diabetic medicine: a journal of the British Diabetic Association. 1997;14(Suppl 3):S25–31. doi: 10.1002/(SICI)1096-9136(199708)14:3+<S25::AID-DIA441>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 54.Devien L, Giovannelli J, Cuny D, Matran R, Amouyel P, Hulo S, et al. Sources of household air pollution: The association with lung function and respiratory symptoms in middle-aged adult. Environ Res. 2018;164:140–148. doi: 10.1016/j.envres.2018.02.016. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.