Abstract
The sequencing, cloning, and expression of the pfp gene from Dictyoglomus thermophilum, which consists of 1,041 bp and encodes a pyrophosphate-dependent phosphofructokinase, are described. A phylogenetic analysis indicates that the enzyme is closely related to the pyrophosphate-dependent enzyme from Thermoproteus tenax. The recombinant and native enzymes share a high degree of similarity for most properties examined.
Phophofructokinase (PFK) is one of the key enzymes in glycolysis. The textbook version of ATP-dependent PFK (ATP-PFK) is present throughout the domains of Eucarya, Bacteria, and Archaea, although it is not yet known if the single archaeal ATP-PFK recently described is related by sequence (6, 11, 15). Since the first pyrophosphate-dependent PFK (PPi-PFK) was reported by Reeves et al., who identified it in Entamoeba histolytica (21), a large number of PPi-PFKs have been found in higher plants, primitive eukaryotes, and bacteria, and a single example has been found in archaea (14, 17, 22, 25, 28). It has been suggested previously that PPi-PFKs may represent the ancestral PFK because PPi is thought to be an ancient source of metabolic energy utilized before the advent of ATP as the near-universal energy carrier (4, 12, 16). Furthermore, because PPi-PFKs can function more readily than ATP-PFKs in the gluconeogenic direction, they could also have acted as primitive fructose-1,6-bisphosphatases. Dictyoglomus thermophilum Rt46 B.1 is an extremely thermophilic bacterium isolated from a New Zealand hot spring and is deeply rooted near the base of the order Thermotogales (18). The Dictyoglomus PPi-PFK has recently been purified and characterized (8). In this paper, we describe the cloning, phylogeny, and overexpression of the first PPi-PFK from an extremely thermophilic bacterium.
D. thermophilum Rt46 B.1 was obtained from the Thermophile Research Unit Culture Collection, Hamilton, New Zealand, and grown in Dictyoglomus medium (18). The Escherichia coli strain used for cloning and expression experiments was JM109 (Promega Life Sciences), and it was grown at 37°C with vigorous aeration in Luria-Bertani broth supplemented with ampicillin (100 μg/ml). The expression plasmid pKK223-3 was obtained from Pharmacia Biotech. Genomic DNA from D. thermophilum was prepared as described by Sambrook et al. (24). Large-scale plasmid DNA was purified from E. coli by using the alkaline lysis method combined with double cesium chloride gradient purification. Restriction digests, electrophoresis, and Southern blotting were carried out according to standard methods (24). In order to clone the full-length pfp gene from D. thermophilum, a 350-bp fragment was initially amplified, using Ampli Taq Gold polymerase (Perkin-Elmer Cetus), by PCR. The sense and antisense degenerate primers were designed according to the N-terminal sequence of the purified native protein and the internal fructose-6-phosphate (F-6-P) binding conserved sequence, respectively. The PCR product was used to probe genomic DNA of Dictyoglomus strain Rt46 B.1 digested with 16 restriction enzymes. Analysis of the Southern data identified several bands of appropriate sizes within the Sau3AI, RsaI, and Sau96I digests. The inverse-PCR technique was employed, and with each enzyme, a single fragment was amplified for sequencing (fragments were 360, 700, and 2,200 bp, respectively) (7).
Identification of ORF encoding the pfp gene.
The ORF representing the full-length sequence of the D. thermophilum PPi-PFK gene was amplified using a forward primer corresponding to the N terminus (the first seven codons) and containing an upstream EcoRI site (in bold) and a 5′-end spacer (5′-GGA GAA TTC ATG AGT AAA ATG CGT ATT GGT G-3′), and a reverse primer corresponding to the C terminus (the last seven codons) and containing a flanking HindIII site (in bold) and a 5′-end spacer (5′-GC GAG AAG CTT TAC TTA TTA AAG AAA GTT TTT ACG-3′). The complete sequence of 1,233 nucleotides obtained from overlapping inverse-PCR contigs contains an ORF of 1,041 bp with 346 codons, beginning with an ATG and ending with a TAA stop codon. One hairpin sequence downstream of the stop codon which could act as a transcription terminator was found (20). Potential promoter sites at the 5′ end of the coding region were also identified. For example, a Pribnow-like box sequence (TAAAAT) is located 41 nucleotides upstream from the ATG start codon and is similar to the −10 (TATAAT) promoter sequence. In addition, the TTGTCA sequence located 17 bases upstream from the TAAAAT sequence is similar to the −35 (TTGACA) promoter sequence (20). Finally, a potential ribosome binding site (AGGAGG) was also identified and is located 4 nucleotides upstream of the start codon. The codon usage for Dictyoglomus PFK, as expected, reflected the G+C content of the genomic DNA, which is 29.3 mol% (18). For example, among the 43 glycine codons, only 2 were terminated with a C and 3 were terminated with a G. In addition, all codons for phenylalanine, proline, and threonine were terminated with either an A or a U (not shown).
Tree construction and phylogenetic comparison.
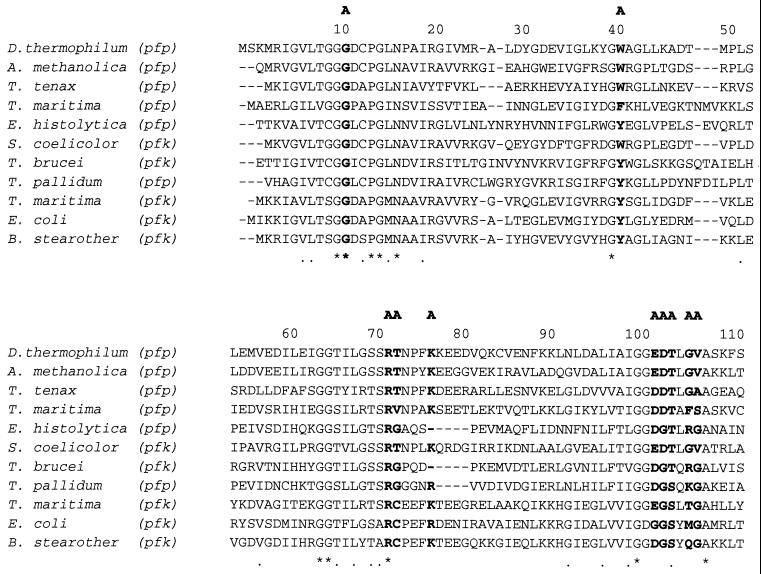
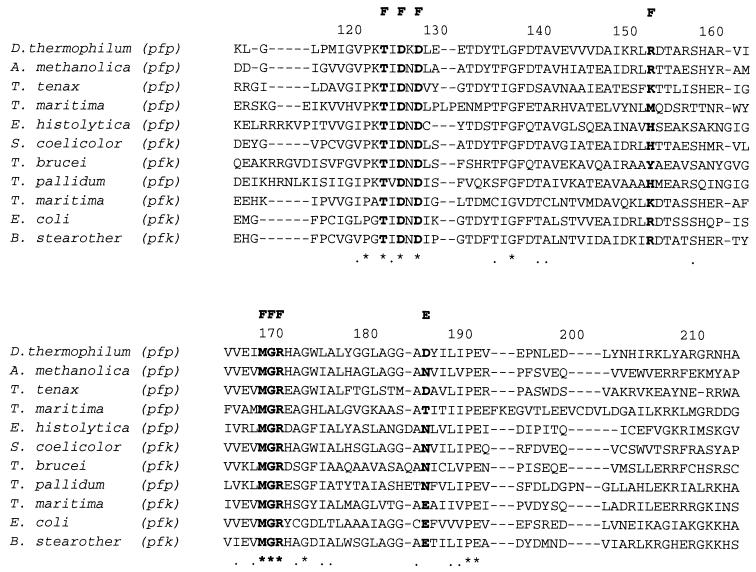
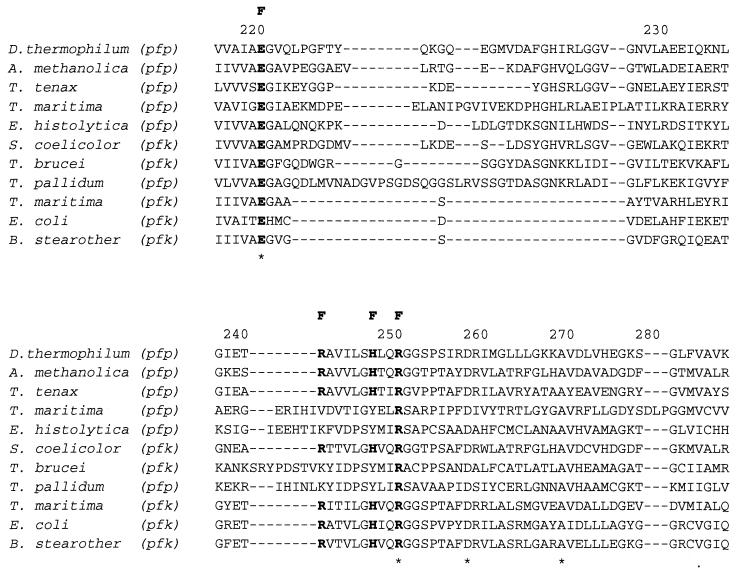
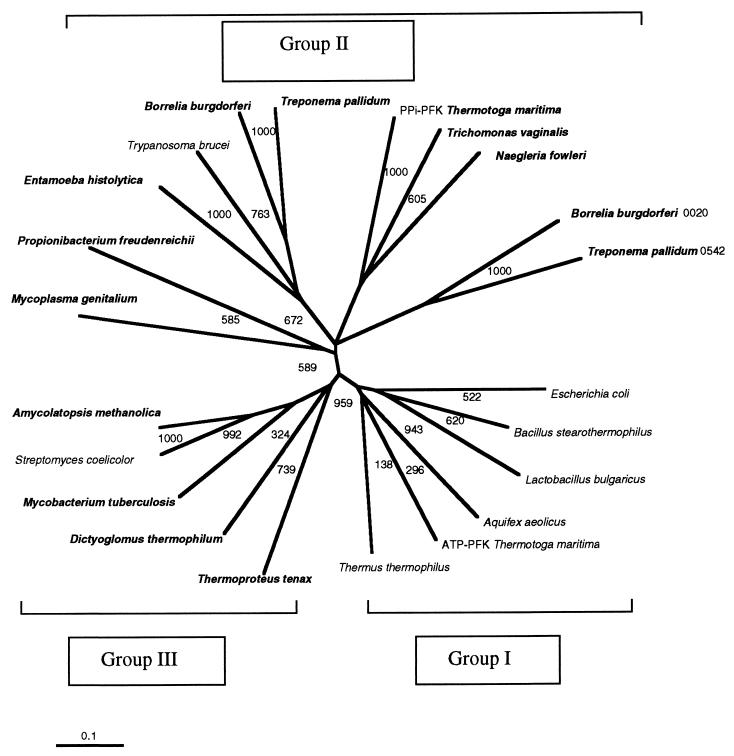
Ten representative amino acid sequences of PFKs from eukaryotes, bacteria, and the crenarcheaon Thermoproteus tenax were retrieved from sequence databases and aligned (1) with the PPi-PFK sequence from D. thermophilum (Fig. 1). The F-6-P binding sites were highly conserved in all PFKs examined. Dictyoglomus PFK has almost the same amino acid residues for F-6-P binding as those from Amycolatopsis methanolica, T. tenax, Streptomyces coelicolor, Bacillus stearothermophilus, and E. coli (9, 23). The PFK from Dictyoglomus also possesses amino acid residues in the phosphoryl binding site identical to those for A. methanolica, T. tenax (PPi-PFK), and S. coelicolor (ATP-PFK). A phylogenetic tree was generated from 22 representative amino acid sequences (Fig. 2). The D. thermophilum sequence is most homologous to the group III PFKs, as defined by Siebers et al. (25), including the PPi-PFKs from T. tenax and A. methanolica, the putative PPi-PFK from Mycobacterium tuberculosis, and the ATP-PFK from S. coelicolor. The full sequence of the Dictyoglomus pfp gene agreed completely with the N-terminal sequence obtained from the purified native protein, except for the lack of the first methionine residue in the native enzyme, which supports our previous suggestion, based on the sequence, that the enzyme has homology with the T. tenax enzyme (8). The data from the alignment of 22 sequences and the phylogenetic tree strongly support the contention that the central carbohydrate metabolism of glycolysis was established before the segregation of the three domains of life. The fact that all of the sequences presented in the phylogenetic tree can be aligned, despite their varying phophoryl donor specificities, demonstrates their homology and therefore, their likely evolution from a single common ancestral sequence (9, 11).
FIG. 1.
Multiple alignment of amino acid sequences of PFKs from D. thermophilum and nine other species, carried out by using Clustal W (version 1.6). B. stearother, B. stearothermophilus, pfp and pfk, genes encoding PPi-PFK and ATP-PFK, respectively; A, F, and E, residues involved in binding ATP, F-6-P, and phosphoenolypyruvate (PEP), respectively, for the E. coli ATP-PFK. Asterisks, identical residues; dashes, gaps; dots, highly conserved residues.
FIG. 2.
Phylogenetic relationships of PPi- and ATP-PFKs. The tree is based on distance analysis (neighbor-joining method) of full amino acid sequences of PPi- and ATP-PFKs in the EMBL and Swiss Prot databanks. Bootstrap values are based on 1,000 replicates and are given at each node. All of the PPi-PFKs are bolded. Group I includes Thermus thermophilus, Thermotoga maritima (ATP-PFK), Aquifex aeolicus, Lactobacillus bulgaricus, B. stearothermophilus, and E. coli. Group II includes Treponema pallidum 0542, Borrelia burgdorferi 0020, Naegleria fowleri, Trichomonas vaginalis, Thermotoga maritima (PPi-PFK), Treponema pallidum, B. burgdorferi, Trypanosoma brucei, Entamoeba histolytica, Propionibacterium freudenreichii, and Mycoplasma genitalium. Group III includes Thermoproteus tenax, D. thermophilum, Mycobacterium tuberculosis, A. methanolica, and S. coelicolor.
A number of X-ray crystallographic and site-directed mutagenesis studies have investigated the roles of PFK amino acid residues in substrate binding and catalysis (3, 9, 19, 23), as well as those amino acid residues related to allosteric properties (5, 27). For example, when the Glu−187 of E. coli PFK is replaced by Asp or Leu, the allosteric transition is abolished. The Glu−187 of E. coli PFK is apparently necessary for the protein to undergo the change from the active into the inactive state induced by phosphoenolpyruvate (PEP) (3). In addition, Valdez et al. (26) reported that Arg−25 and Arg−211 of the B. stearothermophilus ATP-PFK are involved in direct binding of PEP and GDP (26). Sequence analysis of the Dictyoglomus PPi-PFK shows that this enzyme has an Asp−187, a Met−25 and an Arg−211, which suggests that this enzyme should be nonallosteric. Biochemical properties reported here and in our earlier study (8) have also confirmed that both the native and recombinant Dictyoglomus PFK enzymes are nonallosteric.
Expression, purification, and characterization of recombinant PPi-PFK.
The ligation mixture containing restriction enzyme-digested plasmid pKK223-3 and PCR product with the full-length Dictyoglomus pfp gene was used to transform E. coli strain JM109 by electroporation (Gene Pulser; Bio-Rad). Potential clones with inserts were examined by restriction digests with EcoRI and HindIII before being grown in 800 ml of Luria-Bertani medium with ampicillin (100 μg/ml) at 30°C. In-frame ligation of the amplified DNA into pKK223-3 plasmid was confirmed by DNA sequencing (not shown). Isopropyl-β-d-thiogalactoside (1.0 mM) was added when the cell culture density reached approximately 0.6 at 595 nm, and enzyme activity was checked hourly after induction for up to 5 h. The conditions for purification and characterization of the recombinant enzyme were essentially the same as those described for the purification of the native enzyme (8). The final yield of the purified recombinant enzyme was 53 mg of protein from 800 ml of induced culture (approximately 5 g [wet weight] of cell pellet), indicating high-level expression of the enzyme (Table 1). A single band was obtained by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) from the purified Dictyoglomus recombinant enzyme (Table 1; Fig. 3). The recombinant and native enzymes had the same estimated molecular weights (approximately 37,000) when both were run on the same SDS-PAGE gel (data not shown). In addition, a comparison of the native and recombinant Dictyoglomus PPi-PFKs enzymes demonstrates that they possess a high degree of similarity (Table 2). Most of the biochemical and the kinetic properties of the recombinant enzyme were very similar to those of the native enzyme (8), including, for example, thermostability and the extreme sensitivity to Cu2+. Aliquots of the recombinant and native enzyme preparations were dialyzed against MilliQ water overnight at 4°C to prepare them for molecular mass analysis using mass spectrometry. Enzyme solution (5 μl) was mixed with 50% formic acid (5 μl) to ionize the proteins prior to mass spectrometric detection in the mobile phase, which was 50:50 methyl cyanide/H2O (continuous flow rate of 0.02 ml/min). The calculated molecular mass for the recombinant enzyme from the gene sequence was 37,445 Da which is similar to the size estimates obtained with SDS-PAGE (estimated molecular weight, 37,000) and mass spectrometry (estimated molecular weight, 38,057). The enzyme is indicated to be a homodimer based on a molecular mass of 74.2 kDa using a large secondary peak from mass spectrometry (10) and 76.7 kDa by gel filtration and comparison with the size estimate obtained by SDS-PAGE.
TABLE 1.
Purification of the recombinant PPi-PFK from D. thermophilum Rt46 B.1
| Step | Total activity (U) | Total protein (mg) | Sp act (U/mg) | Purification (fold) | Yield (%) |
|---|---|---|---|---|---|
| Heated supernatant | 757 | 2,300 | 0.33 | 1 | 100 |
| Phenyl-Sepharose | 516 | 329 | 1.6 | 4.8 | 68 |
| Q-Sepharose | 363 | 79 | 4.6 | 14 | 48 |
| Red dye 120 | 328 | 53 | 6.2 | 19 | 43 |
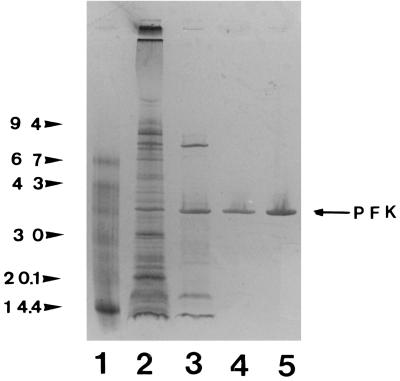
FIG. 3.
SDS-PAGE gel (silver-stained) of fractions obtained during purification of the recombinant Dictyoglomus PPi-PFK. Lane 1, molecular mass markers, as follows: phosphorylase b (molecular mass, 94 kDa), bovine serum albumin (67 kDa), ovalbumin (43 kDa), carbonic anhydrase (30 kDa), soybean trypsin inhibitor (20.1 kDa), and α-lactalbumin (14.4 kDa); lane 2, cell extract following heat treatment; lanes 3 through 5, fractions obtained after purification through phenyl-Sepharose, Q-Sepharose, and red dye 120 ligand, respectively. Lanes 2, 3, 4, and 5 contained 3.0, 1.0, 0.75, and 0.75 μg of protein, respectively.
TABLE 2.
Comparison of some properties of native and recombinant D. thermophilum PPi-PFK
| PFK | Mg2+ (mM) | pI | Mol wta | Forward pH optimum |
Km (mM)
|
Half-life (min) at 80°Cb | % Activity in response to 1 μm Cu2+ | |
|---|---|---|---|---|---|---|---|---|
| F-6-P | PPi | |||||||
| Recombinant | 1–3 | 4.3 | 37,000 | 5.8 | 0.136 | 0.116 | 165 | 40 |
| Native | 1–3 | 4.4 | 37,000 | 5.9 | 0.127 | 0.082 | 170 | 38 |
Determined by SDS-PAGE.
Native and recombinant enzymes (150 μg ml−1) were incubated at 80°C in buffer containing 50 mM MOPS, 2 mM MgCl2, and 0.02% Triton X-100, pH 6.4, under a mineral oil overlay for different lengths of time. Residual activity at 50°C was then assayed and compared to that of the unheated control.
PFK is present in most organisms, and its slowly evolving nature makes it a valuable genotypic marker enabling the exploration of the origins of glycolysis and of life. Within this context, Mertens (13) has suggested that the role of PPi-PFK is that of a glycolytic enzyme adapted to anaerobiosis. However, other investigators have pointed out that PPi-PFKs (especially those in Archaea and extremely thermophilic bacteria) are likely to be more ancient than ATP-PFKs (2, 16). The results from the Dictyoglomus PPi-PFK strongly support the latter hypothesis. As seen with the enzyme from T. tenax, the Dictyoglomus enzyme is the first biochemically characterized extremely thermophilic bacterial PPi-dependent enzyme and offers another opportunity to gain insight into the differentiation of PFK substrate specificities and the characteristics of the phenotype of the original ancestral PFK precursor. The group III enzymes are suggested to be of a more ancient origin than group II enzymes, so it follows that the PPi-PFKs from T. tenax and extremely thermophilic bacteria may represent more ancient enzymes than the enzymes in mesophilic bacteria, primitive eukaryotes, and higher plants. In support of this latter contention, in two cases, that of S. coelicolor and Trypanosoma brucei, there is strong sequence evidence for the evolution of ATP-PFKs from PPi-PFKs (2, 15). Finally, it is hoped that the X-ray crystallographic determination of the structure of PPi-PFK from D. thermophilum Rt46 B.1, which is in progress, will help clarify the phylogenetic origins of PFKs.
Nucleotide sequence accession number.
The Dictyoglomus pfp gene sequence data has been submitted to GenBank under accession number AF268276.
Acknowledgments
We thank the Royal Society Marsden Science Foundation and the University of Waikato for financial support during the course of this study.
We also thank Pat Gread and Cameron Evans for assistance with the mass spectrometry.
REFERENCES
- 1.Altschul S F, Gish W, Miller W, Myers W, Lipman D L. Basic local alignment search tool. J Mol Biol. 1997;215:403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- 2.Alves A M C R, Euverink G J W, Bibb M J, Dijkhuizen L. Identification of ATP-dependent phosphofructokinase as a regulatory step in the glycolytic pathway of the actinomycete Streptomyces coelicolor A3(2) Appl Environ Microbiol. 1997;63:956–961. doi: 10.1128/aem.63.3.956-961.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Auzat I, Le Bras G, Branny P, Torre F D L, Theunissen B, Garel J-R. The role of Glu 187 in the regulation of phosphofructokinase by phosphoenolpyruvate. J Mol Biol. 1994;235:68–72. doi: 10.1016/s0022-2836(05)80014-2. [DOI] [PubMed] [Google Scholar]
- 4.Baltscheffsky H, editor. Origin and evolution of biological energy conversion. New York, N.Y: VCH Publishers; 1996. Energy conversion leading to the origin and early evolution of life: did inorganic pyrophosphate precede adenosine triphosphate? pp. 1–9. [Google Scholar]
- 5.Berger S A, Evans P R. Active-site mutants altering the cooperativity of E. coli phosphofructokinase. Nature. 1990;343:575–576. doi: 10.1038/343575a0. [DOI] [PubMed] [Google Scholar]
- 6.Byrnes W M, Zhu X, Younathan E S, Chang S H. Kinetic characteristics of phosphofructokinase from Bacillus stearothermophilus: MgATP nonallosterically inhibits the enzyme. Biochemistry. 1994;33:3424–3431. doi: 10.1021/bi00177a036. [DOI] [PubMed] [Google Scholar]
- 7.Collins S F, Weissman S M. Directional cloning of DNA fragments at large distances from an initial probe: a circularization method. Genetics. 1984;81:6812–6816. doi: 10.1073/pnas.81.21.6812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ding Y-H R, Ronimus R S, Morgan H W. Purification and properties of the pyrophosphate-dependent phosphofructokinase from Dictyoglomus thermophilum Rt46 B.1. Extremophiles. 1999;3:131–137. doi: 10.1007/s007920050108. [DOI] [PubMed] [Google Scholar]
- 9.Evans P R, Hudson P J. Structure and control of phosphofructokinase from Bacillus stearothermophilus. Nature. 1979;279:500–504. doi: 10.1038/279500a0. [DOI] [PubMed] [Google Scholar]
- 10.Hans V, Heinemann U, Dobson C M, Robinson C V. Detection of a monomeric intermediate associated with dimerization of protein Hu by mass spectrometry. J Am Chem Soc. 1998;120:6427–6428. [Google Scholar]
- 11.Hansen T, Schönheit P. Purification and properties of the first-identified, archaeal, ATP-dependent 6-phosphofructokinase, an extremely thermophilic non-allosteric enzyme, from the hyperthermophilic Desulfurococcus amylolyticus. Arch Microbiol. 2000;173:103–109. doi: 10.1007/s002039900114. [DOI] [PubMed] [Google Scholar]
- 12.Kornberg A. Inorganic polyphosphate: toward making a forgotten polymer unforgettable. J Bacteriol. 1995;177:491–496. doi: 10.1128/jb.177.3.491-496.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Mertens E. Pyrophosphate-dependent phosphofructokinase, an anaerobic glycolytic enzyme? FEBS Lett. 1991;285:1–5. doi: 10.1016/0014-5793(91)80711-b. [DOI] [PubMed] [Google Scholar]
- 14.Mertens E, Van Schaftingen E, Müller M. Presence of a fructose-2,6-bisphosphate-insensitive pyrophosphate:fructose-6-phosphate phosphotransferase in the anaerobic protozoa Tritrichomonas foetus, Trichomonas vaginalis and Isotricha prostoma. Mol Biochem Parasitol. 1989;37:183–190. doi: 10.1016/0166-6851(89)90150-3. [DOI] [PubMed] [Google Scholar]
- 15.Michels P A M, Chevalier N, Opperdoes F R, Rider M H, Rigden D J. The glycosomal ATP-dependent phosphofructokinase of Trypanosoma brucei must have evolved from an ancestral pyrophosphate-dependent enzyme. Eur J Biochem. 1997;250:698–704. doi: 10.1111/j.1432-1033.1997.00698.x. [DOI] [PubMed] [Google Scholar]
- 16.Morgan H W, Ronimus R S. Pyrophosphate-dependent phosphofructokinase in thermophilic and non-thermophilic microorganisms. In: Adam M W, Wiegel J W, editors. Thermophiles: the key to molecular evolution and the origin of life. London, United Kingdom: Taylor and Francis; 1998. [Google Scholar]
- 17.O'Brien W E, Bowien S, Wood H G. Isolation and characterisation of a pyrophosphate-dependent phosphofructokinase from Propionibacterium shermanii. J Biol Chem. 1975;250:8690–8695. [PubMed] [Google Scholar]
- 18.Patel B K, Morgan H W, Wiegel J, Daniel R M. Isolation of an extremely thermophilic chemoorganotrophic anaerobe similar to Dictyoglomus thermophilum from New Zealand hot springs. Arch Microbiol. 1987;147:21–24. [Google Scholar]
- 19.Poorman R A, Randolph A, Kemp R G, Heinrikson R L. Evolution of phosphofructokinase-gene duplication and creation of new effector sites. Nature. 1984;309:467–469. doi: 10.1038/309467a0. [DOI] [PubMed] [Google Scholar]
- 20.Pribnow D. Nucleotide sequence of an RNA polymerase binding site at an early T7 promoter. Proc Natl Acad Sci USA. 1975;72:184–188. doi: 10.1073/pnas.72.3.784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Reeves R E, South D J, Blytt H J, Warren L G. Pyrophosphate:d-fructose 6-phosphate 1-phosphotransferase. J Biol Chem. 1974;249:7737–7741. [PubMed] [Google Scholar]
- 22.Ronimus R S, Morgan H M, Ding Y-H R. Phosphofructokinase activities within the order Spirochaetales and the characterisation of the pyrophosphate-dependent phosphofructokinase from Spirochaeta thermophila. Arch Microbiol. 1999;172:401–406. doi: 10.1007/s002030050777. [DOI] [PubMed] [Google Scholar]
- 23.Rypniewski W R, Evans P R. Crystal structure of unliganded phosphofructokinase from Escherichia coli. J Mol Biol. 1989;207:805–821. doi: 10.1016/0022-2836(89)90246-5. [DOI] [PubMed] [Google Scholar]
- 24.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 25.Siebers B, Klenk H P, Hensel R. PPi-dependent phosphofructokinase from Thermoproteus tenax, an archaeal descendant of an ancient line in phosphofructokinase evolution. J Bacteriol. 1998;180:2137–2143. doi: 10.1128/jb.180.8.2137-2143.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Valdez B C, Chang S H, Younathan E S. Site-directed mutagenesis at the regulatory site of fructose-6-phosphate-1-kinase from Bacillus stearothermophilus. Biochem Biophys Res Com. 1988;156:537–542. doi: 10.1016/s0006-291x(88)80875-1. [DOI] [PubMed] [Google Scholar]
- 27.Valdez B C, French B A, Younathan E S, Chang S H. Site-directed matagenesis in Bacillus stearothermophilus fructose-6-phosphate 1-kinase. J Biol Chem. 1989;264:131–135. [PubMed] [Google Scholar]
- 28.Yan T-F J, Tao M. Multiple forms of pyrophosphate: d-fructose-6-phosphate 1-phosphotransferase from wheat seedlings. J Biol Chem. 1984;259:5087–5092. [PubMed] [Google Scholar]