Abstract
Bone marrow stromal cells (MSCs) are regulated by the chemical and physical features of a biomaterial surface. When grown on titanium (Ti) and Ti alloy surfaces like titanium-aluminum-vanadium (Ti6Al4V) with specific topographies that mimic the micro, meso, and nanoscale features of an osteoclast resorption pit, they undergo a rapid change in cell shape to assume a columnar morphology typical of a secretory osteoblast. These cells exhibit markers associated with an osteoblast phenotype, including osteocalcin and osteopontin and they secrete factors associated with osteogenesis, including bone morphogenetic protein 2 (BMP2), vascular endothelial growth factor (VEGF), and neurotrophic semaphorins. The pathway involves a shift in integrin expression from α5β1 to α2β1 and signaling via Wnt5a rather than Wnt3a. Conditioned media from these cultures can stimulate vasculogenesis by human endothelial cells as well as osteoblastic differentiation of MSCs not grown on the biomimetic substrate, suggesting that the surface could promote osteogenesis in vivo through similar mechanisms. In vivo studies using a variety of animal models confirm that implants with biomimetic surfaces result in improved osseointegration compared to Ti implants with smooth surfaces, as do meta-analyses comparing clinical performance of implant surface topographies.
Keywords: Osseointegration, Bone marrow stromal cells, MSCs, Osteoblasts, Osteoprogenitor cells, Titanium, BMP2, Semaphorin, Osteogenesis, Wnt5a, Nano
INTRODUCTION
Bone-facing non-resorbable implant components capable of bone ingrowth provide implant stability through the formation of a mechanical interlock. Over two decades, refinements have focused on pore size, pore configuration, modulus of elasticity at the interface with bone, and degree of micromotion during initial bone ingrowth [1]. The advent of spine interbody fusion devices opened the door to examine the possibility that modifications to the surface at the microscale would improve bone formation and osseointegration of the implant. The dental implant industry provided a large literature on clinical success of a variety of surface designs, particularly on titanium implants [2,3]. The basic science information underlying the clinical studies indicated that surfaces that had a microstructure resembling an osteoclast resorption pit supported the more robust osteogenic response based on a number of outcomes, including osteoblast differentiation of bone marrow stromal cells (MSCs) and osteoprogenitor cells [4]. These studies, described below, also examined the mechanisms involved in the osteogenic response, enabling the application of this literature to orthopaedic implants manufactured using titanium and its alloys [5,6].
BIOLOGY OF BONE HEALING AND THE IMPACT OF IMPLANT SURFACE TOPOGRAPHY
Bone must be able to respond to a variety of loading conditions, requiring it to be metabolically active, continuously remodeling in response to mechanical stimulation, systemic factors like hormones, and local factors produced by cells present within the tissue. Bone tissue consists of a mineralized type I collagen matrix; osteoblast-lineage cells that synthesize, calcify, and maintain the matrix; osteoclasts that resorb the matrix and prepare it for subsequent rounds of formation; and osteocytes, the most abundant bone cell, that coordinate the activity of osteoblasts and osteoclasts and may also resorb bone matrix [7]. Bone tissue also contains a complex vascular network together with its associated nerves, as well as immune lineage cells, including monocytes, macrophages and lymphocytes [8,9].
When an implant is placed in bone, fluid at the surgical site adsorbs onto the surface. The affinity for, and conformation of proteins on the surface are determined by its physical and chemical properties. One of the constituents in the wound fluid, fibronectin, adsorbs to the surface and provides binding sites for the alpha-5, beta-1 (α5β1) integrins present in MSCs. The clot that forms between the bone bed and the implant surface also contains a complex fibrillar network that enables MSCs to migrate to the site [10]. Monocytes and macrophages are also present at the site and recent studies indicate that Ti substrates that have a complex microscale topography similar to an osteoclast resorption pit and have a hydrophilic surface chemistry support the pro-healing macrophage M2 phenotype rather than the pro-inflammatory M1 phenotype [9,11]. The MSCs produce factors that modulate the response of immune cells within the environment and the immune cells produce factors that recruit additional MSCs and immune cells [12,13].
These surface properties also support osteoblastic differentiation of MSCs. When grown on such a surface, MSCs undergo a change in cell polarity, assuming a columnar morphology rather than being flattened and spread [14]. Their integrin profile changes from predominantly α5β1 to α2β1 and α1β1, which bind RGD and GFOGR motifs in type 1 collagen [10,15]. In addition, they express markers associated with an osteoblast phenotype, including osteocalcin and osteopontin, as well as factors associated with modulation of osteoclast activity such as osteoprotegerin, which is a decoy receptor for RANK ligand, and transforming growth factor beta 1 (TGFβ1) [16,17]. These changes occur rapidly and do not require the addition of any osteogenic media components like dexamethasone or beta-glycerol phosphate [18]. The MSCs also express factors associated with vasculogenesis such as vascular endothelial growth factor-165 (VEGF) and fibroblast growth factor-2 (FGF2), as well as factors associated with neurogenesis such as semaphorin 3a, 3c and 4a [13,19].
Analysis of Ti, titanium-zirconium (TiZr) and titanium-aluminum-vanadium (Ti6Al4V) surfaces that have been generated using various grit blasting/acid etching methods shows that osteoblastic differentiation of MSCs and osteoprogenitor cells is favored by topographies that mimic osteoclast resorption pits created during normal bone remodeling [5,6] (Figure 1). The osteoclast resorption pit has an average width of 30–100 μm, an average depth of 8 μm, and a nanotextured surface averaging 60 nm. Moreover, the pits are not isolated on the surface but are linked to each other via a scalloped border, created as the osteoclast migrates across the bone surface [20].
Figure 1.
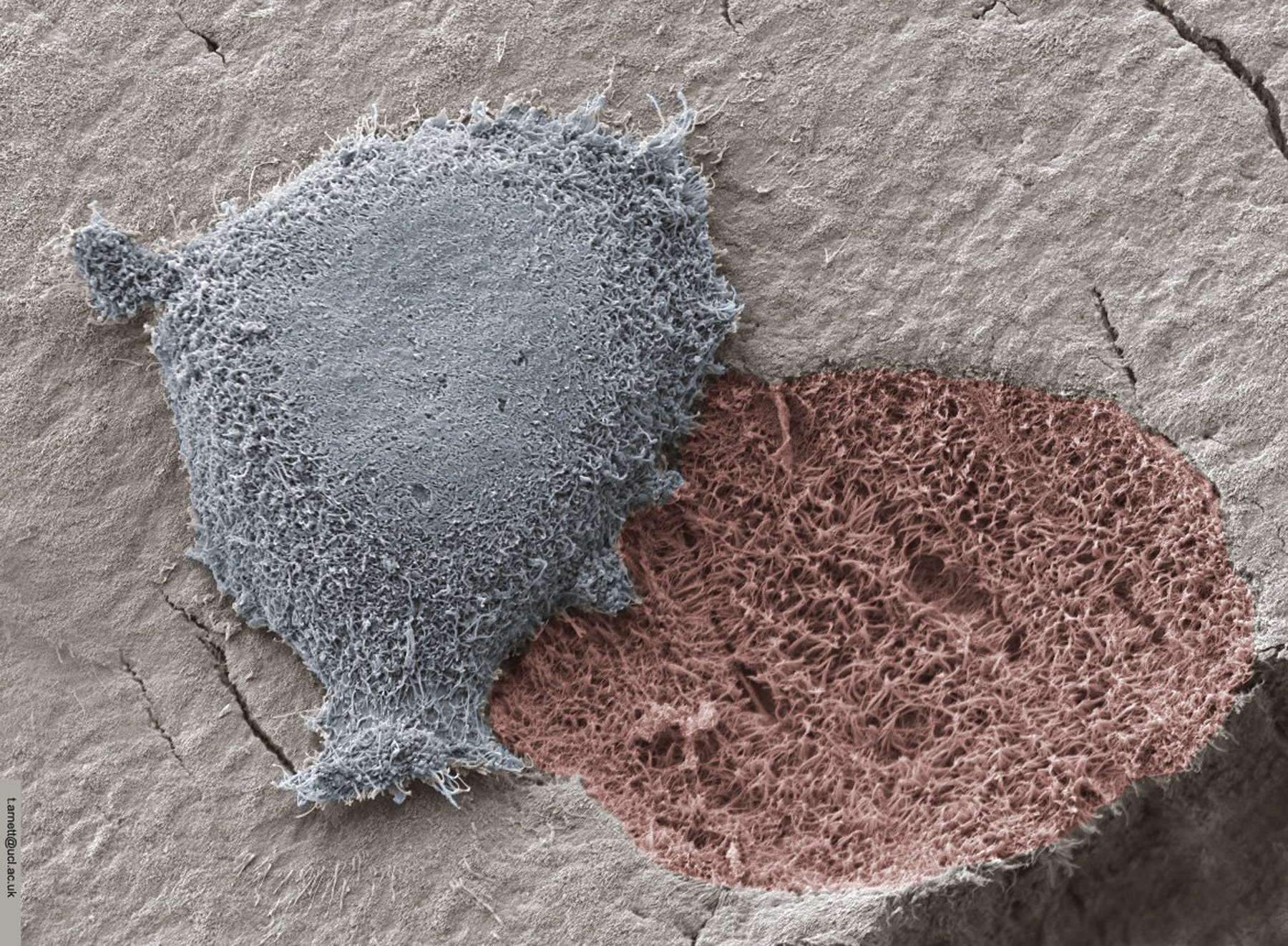
Osteoclast resorbing a bone surface and leaving exposed organic matrix. Image courtesy of Prof Tim Arnett, University College London.
The most effective biomimetic surfaces have irregular closely spaced microscale pits overlaid with microscale, mesoscale, and nanoscale textures that are shaped like pointed isosceles triangles [5], reminiscent of osteoclasts resorption pits with the mesoscale and nanoscale structures on the resorption pit surface. When MSCs and osteoprogenitor cells are cultured on these surfaces, they produce high levels of BMP2 [21] and express receptors for BMP2, indicating that they are inducing the osteoblast phenotype via autocrine and paracrine mechanisms [22]. This hypothesis is supported by the observation that addition of anti-BMP2 [22] antibodies to the cultures blocks the effect of the surface on osteoblast differentiation. In addition, they produce Wnt11 [23], causing a shift from producing Wnt3a to Wnt5a (Figure 2).
Figure 2.
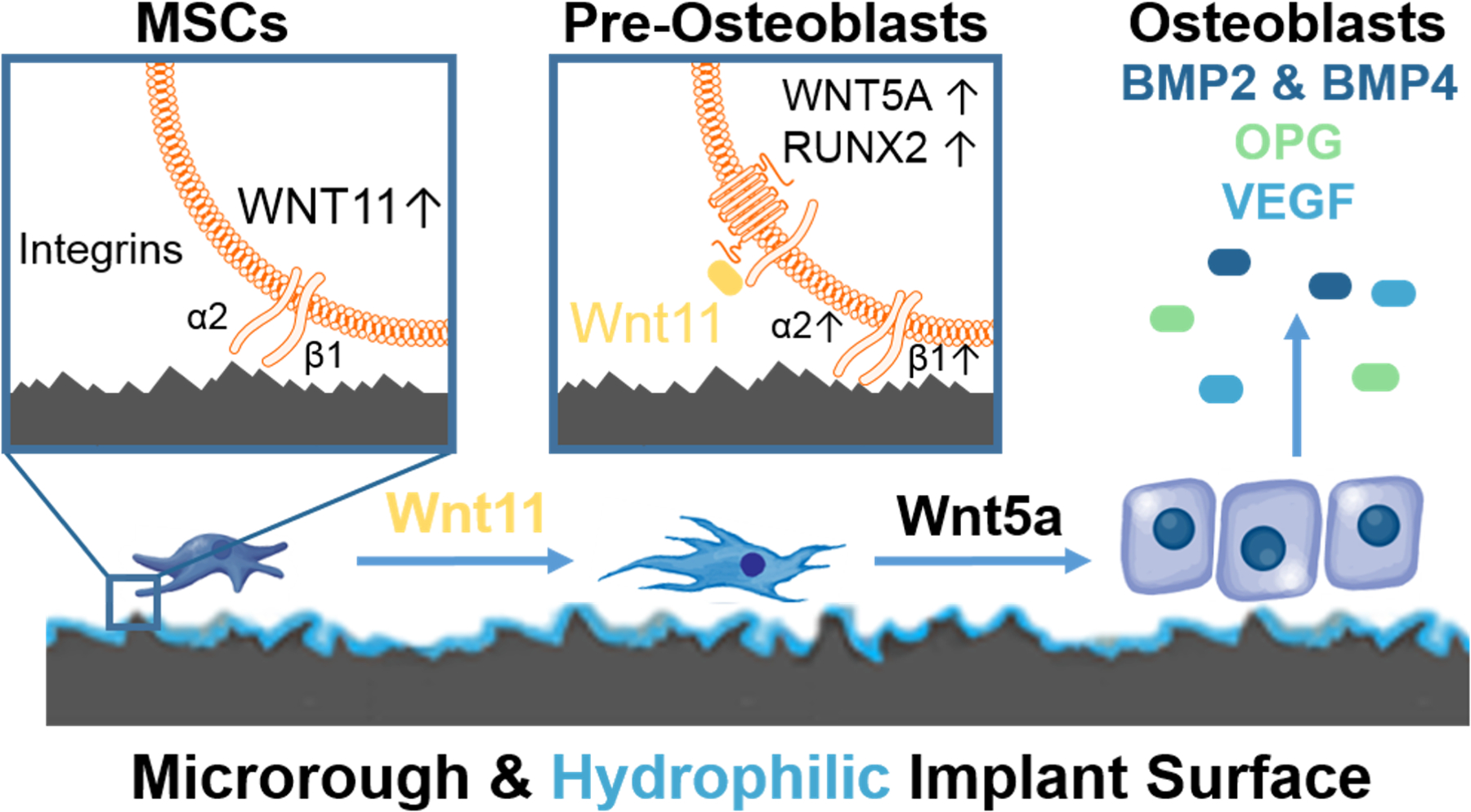
Mechanisms involved in the regulation of osteoblastic differentiation of bone marrow stromal cells (MSCs) on microstructured Ti-based implant surfaces. MSCs migrate to the implant environment and attach via α5β1 integrin binding to fibronectin. MSCs begin to sense the implant architecture shifting to production of α2β1 integrin binding to collagen type 1 and upregulate non-canonical Wnt11 (left panel). Wnt11 acts internally and externally of the cell to increase the number of pre-osteoblasts in the implant environment. This method is achieved by increasing production Wnt5a, and RUNX2 transcription in the nucleus (middle panel). These pre-osteoblasts mature into osteoblasts that product robust concentrations of bone morphogenetic protein 2 and 4, osteoprotegerin (OPG), and vascular endothelial growth factor (VEGF) necessary for bone apposition and mineralization (right panel).
Recent work has shown that semaphorins are also involved in mediating the effects of surface topography on osteoblastic differentiation of MSCs. Semaphorin 3A (sema3A) can work independently of Wnt3a, Wnt5a, and BMP2 to enhance osteoblast differentiation, with activation of sema3A occurring alongside of Wnt5A [19]. Addition of anti-sema3A antibodies to cultures of MSCs grown on microtextured Ti substrates blocks effect of the surface on osteoblastic differentiation of MSCs, indicating the important role that these factors play in the process.
These results also imply that factors produced by MSCs on the surface could regulate the osteoblast differentiation of MSCs and osteoprogenitor cells not on the surface and this is exactly what co-culture experiments show to be the case [22]. Addition of anti-BMP2 antibodies to MSC cultures blocks the stimulatory effect of the conditioned media on osteoblastic differentiation of MSCs not on the biomimetic surface. As noted above, growth on an osteoclast resorption pit biomimetic surface modulates factors produced by MSCs that regulate inflammation, vasculogenesis, bone remodeling, and neurogenesis [24,25]. This suggests that they might generate an osteoinductive milieu around the implant surface in vivo.
EFFECT OF SURFACE TOPOGRAPHY ON OSTEOGENESIS IN VIVO
Cell culture provides a method for understanding the mechanisms involved in the response of cells and tissues to surface topography but it does not provide definitive evidence that this affects osseointegration in vitro. Meta analyses of clinical outcomes using various Ti dental implant topographies showed a strong correlation between clinical success and the expression of osteocalcin by cells grown on identical surfaces in vitro [2,3]. To begin to assess whether implant surface design could impact osseointegration in skeletal bone, we have performed a number of studies including the use of grit blasted Ti6Al4V pedicle screws in sheep spine, grit blasted/acid etched Ti and Ti6Al4V screws in rat and rabbit femurs, and grit blasted/acid etched Ti screws in osteoporotic rat femoral bone [26–28]. Animal models have also been used to assess effectiveness of Ti implants with hydrophilic, microtextured surfaces in diseases like diabetes and osteoporosis that compromise healing and bone quality [27]. These studies provided direct correlation between in vitro and in vivo outcomes and confirm the value of the biomimetic topography. We have also investigated the effectiveness of grit blasted/acid etched surfaces on 3D printed Ti6Al4V devices in regenerating alveolar bone sufficiently to support reconstruction of the mandible in humans and once again confirmed the importance of this biomimetic principle in achieving stable osseointegration [26].
ROLE OF NANOTEXTURES IN THE REGULATION OF OSTEOGENESIS
Most studies examining the role of surface topography on osteoblast differentiation have used polymeric constructs on tissue culture polystyrene surfaces to tease out the various contributions of stiffness and shape [15,29]. These studies have relied on the use of osteogenic culture media, which are high in Ca++ and have additives like dexamethasone, which simulates alkaline phosphatase activity, together with a phosphate source like beta glycerol phosphate. Even with these additives, the MSCs or osteoprogenitor cells must form multicellular nodules before they begin to express an osteoblast phenotype and the mineral that they deposit is due at least in part to the high calcium phosphate ion product that is generated by the action of alkaline phosphatase [18]. Numerous studies have shown that these effects are mediated by Wnt3a signaling. In contrast, when cells are cultured on osteoclast resorption pit biomimetic Ti surfaces, they shift from Wnt3a to Wnt5a signaling while still in monolayer [14,19,23]. This shift requires α2β1 integrin signaling and is accompanied by a change in cell shape. Certainly, the polymer models provide valuable insights into MSC regulation, but without the microscale surface topography to underly the nanofeatures, the results must be viewed with caution.
A wide variety of nanomodifications have been applied to implant surfaces to improve clinical outcomes. Some of these modifications are applied to machined surfaces and their effectiveness in vitro is assessed using osteogenic media and only limited assessment of outcome measures [30]. Even when nanofeatures are generated on microstructured topographies resembling an osteoclast resorption pit, the specific shapes, sizes, and crystallinities of the nanostructures result in very different outcomes [5,6,31]. While some of these do support osteoblastic differentiation of MSCs to some extent, the full panoply of outcomes is observed in only a limited subset of modifications [31].
We have demonstrated that specific nanoscale topographies activate pre-osteoblastic cell differentiation [32] through a mechanism involving integrin-mediated focal adhesion kinase [33]. Additionally, these biomimetic nanotopgraphies enhance bone graft osteointegration [34]. More recently we have demonstrated that hydroxyapatite particle density regulates pre-osteoblastic cell differentiation [35,36]. Importantly, when specific nanofeatures are applied to microtextured Ti6Al4V surfaces with a biomimetic osteoclast resorption pit topography, MSCs and pre-osteoblasts display the full panoply of characteristics associated with well differentiated osteoblasts and produce factors that support osteogenesis, vasculogenesis, neurogenesis, and pro-healing immune response [11,21].
Taken together, these studies suggest that specific nanofeatures, as well as their density and the underlying substrate topography, may positively affect osteointegration. Investigators have taken advantage of this observation by applying nano features such as Ti nanotubes or hydroxyapatite crystals to the surface of polymeric materials such as polyether-ether-ketone (PEEK) in order to render them more osteogenic [37–39]. However, the underlying material lacks the microtopography that recapitulates the biomimetic topography that favors osteogenesis. Thus, even with bone ingrowth from the bone bed by creeping substitution, the interface with the implant is not bone outgrowth but fibrous connective tissue [40].
CONCLUSION
Collectively, there is strong preclinical and clinical success supporting the use of implants that possess biomimetic surface topography. Using these surfaces, we have been able to elucidate the behavior of cells as they sense and respond to materials. Understanding these mechanisms is key to predict how the next generation of orthopaedic implants will need to be designed to improve implant longevity, reduce healing time, and reduce biofilm formation.
Funding:
National Institutes of Health R01 AM 072500–20 (BDB; ZS); AB Dental (Ashdod, Israel) (ZS); Medtronic Spine (Memphis, TN) (BDB; ZS); Institut Straumann AG (Basel, Switzerland) (BDB; ZS); R01 AR068132–20 (HJD), NASA grant 80NSSC18K1473 (HJD) and National Space Biological Research Institute NSBRI/NASA MA02802 (HJD).
Footnotes
Financial Disclosures/Conflicts of Interest:
BDB is a paid consultant for Medtronic Spine (Memphis, TN). BDB is an unpaid consultant for Institut Straumann AG (Basel, Switzerland). MBB, FRN, HJD have no financial disclosures or conflicts of interest. ZS is an unpaid consultant for AB Dental (Ashdod, Israel). AB Dental, Institut Straumann AG, and Medtronic Spine have provided research discs as gifts in kind.
References
- [1].Spector M. Historical review of porous-coated implants. J Arthroplasty 1987;2:163–77. 10.1016/s0883-5403(87)80024-4. [DOI] [PubMed] [Google Scholar]
- [2].Buser D, Janner SFM, Wittneben J, Brägger U, Ramseier CA, Salvi GE. 10-Year survival and success rates of 511 titanium implants with a sandblasted and acid-etched surface: a retrospective study in 303 partially edentulous patients. Clin Oral Implants Res 2012;26:1121–8. 10.1111/j.1708-8208.2012.00456.x. [DOI] [PubMed] [Google Scholar]
- [3].Müller F, Al-nawas B, Storelli S, Quirynen M, Hicklin S, Castro-laza J. Small-diameter titanium grade IV and titanium-zirconium implants in edentulous mandibles : five-year results from a double- blind , randomized controlled trial. BMC Oral Health 2015:1–10. 10.1186/s12903-015-0107-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Albrektsson T, Johansson C. Osteoinduction, osteoconduction and osseointegration. Eur Spine J 2001;10:96–101. 10.1007/s005860100282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Olivares-Navarrete R, Hyzy SL, Berg ME, Schneider JM, Hotchkiss K, Schwartz Z, et al. Osteoblast lineage cells can discriminate microscale topographic features on titanium-aluminum-vanadium surfaces. Ann Biomed Eng 2014;42:2551–61. 10.1007/s10439-014-1108-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [6].Lotz EM, Olivares-Navarrete R, Hyzy SL, Berner S, Schwartz Z, Boyan BD. Comparable responses of osteoblast lineage cells to microstructured hydrophilic titanium–zirconium and microstructured hydrophilic titanium. Clin Oral Implants Res 2017;28:e51–9. 10.1111/clr.12855. [DOI] [PubMed] [Google Scholar]
- [7].Vahidi G, Rux C, Sherk VD, Heveran CM. Lacunar-canalicular bone remodeling: Impacts on bone quality and tools for assessment. Bone 2021;143:115663. 10.1016/j.bone.2020.115663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Salazar VS, Gamer LW, Rosen V. BMP signalling in skeletal development, disease and repair. Nat Rev Endocrinol 2016;12:203–21. 10.1038/nrendo.2016.12. [DOI] [PubMed] [Google Scholar]
- [9].Hotchkiss KM, Reddy GB, Hyzy SL, Schwartz Z, Boyan BD, Olivares-Navarrete R. Titanium surface characteristics, including topography and wettability, alter macrophage activation. Acta Biomater 2016;31:425–34. 10.1016/j.actbio.2015.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [10].Keselowsky BG, Wang L, Schwartz Z, Garcia AJ, Boyan BD. Integrin a5 controls osteoblastic proliferation and differentiation responses to titanium substrates presenting different roughness characteristics in a roughness independent manner. J Biomed Mater Res - Part A 2007;80:700–10. 10.1002/jbm.a. [DOI] [PubMed] [Google Scholar]
- [11].Trindade R, Albrektsson T, Tengvall P, Wennerberg A. Foreign body reaction to biomaterials: on mechanisms for buildup and breakdown of osseointegration. Clin Implant Dent Relat Res 2016;18:192–203. 10.1111/cid.12274. [DOI] [PubMed] [Google Scholar]
- [12].Terheyden H, Lang NP, Bierbaum S, Stadlinger B. Osseointegration - communication of cells. Clin Oral Implants Res 2012;23:1127–35. 10.1111/j.1600-0501.2011.02327.x. [DOI] [PubMed] [Google Scholar]
- [13].Hu K, Olsen BR. The roles of vascular endothelial growth factor in bone repair and regeneration. Bone 2016;91:30–8. 10.1016/j.bone.2016.06.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Andre P, Wang Q, Wang N, Gao B, Schilit A, Halford MM, et al. The Wnt coreceptor Ryk regulates Wnt/planar cell polarity by modulating the degradation of the core planar cell polarity component Vangl2. J Biol Chem 2012;287:44518–25. 10.1074/jbc.M112.414441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Lai M, Hermann CD, Cheng A, Olivares-Navarrete R, Gittens RA, Bird MM, et al. Role of A2B1 integrins in mediating cell shape on microtextured titanium surfaces. J Biomed Mater Res - Part A 2015;103:564–73. 10.1002/jbm.a.35185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [16].Mustafa K, Rubinstein J, Lopez BS, Arvidson K. Production of transforming growth factor beta1 and prostaglandin E2 by osteoblast-like cells cultured on titanium surfaces blasted with TiO2 particles. Clin Oral Implants Res 2003;14:50–6. [DOI] [PubMed] [Google Scholar]
- [17].Lotz EM, Berger MB, Schwartz Z, Boyan BD. Regulation of osteoclasts by osteoblast lineage cells depends on titanium implant surface properties. Acta Biomater 2018;68:296–307. 10.1016/j.actbio.2017.12.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Langenbach F, Handschel J. Effects of dexamethasone, ascorbic acid and β-glycerophosphate on the osteogenic differentiation of stem cells in vitro. Stem Cell Res Ther 2013;4:117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Lotz EM, Berger MB, Boyan BD, Schwartz Z. Regulation of mesenchymal stem cell differentiation on microstructured titanium surfaces by semaphorin 3A. Bone 2020;134. 10.1016/j.bone.2020.115260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Henriksen K, Bollerslev J, Everts V, Karsdal MA. Osteoclast activity and subtypes as a function of physiology and pathology - Implications for future treatments of osteoporosis. Endocr Rev 2011;32:31–63. 10.1210/er.2010-0006. [DOI] [PubMed] [Google Scholar]
- [21].Boyan BD, Lotz EM, Schwartz Z. (*) Roughness and hydrophilicity as osteogenic biomimetic surface properties. Tissue Eng Part A 2017;23:1479–89. 10.1089/ten.TEA.2017.0048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Berger MB, Bosh KB, Jacobs TW, Joshua Cohen D, Schwartz Z, Boyan BD. Growth factors produced by bone marrow stromal cells on nanoroughened titanium–aluminum–vanadium surfaces program distal MSCs into osteoblasts via BMP2 signaling. J Orthop Res 2020. 10.1002/jor.24869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [23].Boyan BDBD, Olivares-navarrete R, Berger MB, Hyzy SLSL, Schwartz Z. Role of Wnt11 during Osteogenic Differentiation of Human Mesenchymal Stem Cells on Microstructured Titanium Surfaces. Sci Rep 2018;8:8588. 10.1038/s41598-018-26901-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Raines AL, Olivares-Navarrete R, Wieland M, Cochran DL, Schwartz Z, Boyan BD. Regulation of angiogenesis during osseointegration by titanium surface microstructure and energy. Biomaterials 2010;31:4909–17. 10.1016/j.biomaterials.2010.02.071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Bang SM, Moon HJ, Kwon YD, Yoo JY, Pae A, Kwon IK. Osteoblastic and osteoclastic differentiation on SLA and hydrophilic modified SLA titanium surfaces. Clin Oral Implants Res 2014;25:831–7. 10.1111/clr.12146. [DOI] [PubMed] [Google Scholar]
- [26].Cohen DJ, Cheng A, Kahn A, Aviram M, Whitehead AJ, Hyzy SL, et al. Novel Osteogenic Ti-6Al-4V Device For Restoration Of Dental Function In Patients With Large Bone Deficiencies: Design, Development And Implementation. Sci Rep 2016;6:20493. 10.1038/srep20493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Lotz EM, Cohen DJ, Schwartz Z, Boyan BD. Titanium implant surface properties enhance osseointegration in ovariectomy induced osteoporotic rats without pharmacologic intervention. Clin Oral Implants Res 2020;31:374–87. 10.1111/clr.13575. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Reiner T, Armada L, Nunes MA, Muniz E, Tinoco B. Incorporation and remodeling of bone block allografts in the maxillary reconstruction : a randomized clinical trial. Clin Implant Dent Relat Res 2016:180–94. 10.1111/cid.12441. [DOI] [PubMed] [Google Scholar]
- [29].Olivares-Navarrete R, Lee EM, Smith K, Hyzy SL, Doroudi M, Williams JK, et al. Substrate stiffness controls osteoblastic and chondrocytic differentiation of mesenchymal stem cells without exogenous stimuli. PLoS One 2017;12:e0170312. 10.1371/journal.pone.0170312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Abraham CM. A brief historical perspective on dental implants, their surface coatings and treatments. Open Dent J 2014;8:50–5. 10.2174/1874210601408010050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Cheng A, Goodwin WB, deGlee BM, Gittens RA, Vernon JP, Hyzy SL, et al. Surface modification of bulk titanium substrates for biomedical applications via low-temperature microwave hydrothermal oxidation. J Biomed Mater Res A 2018;106:782–96. 10.1002/jbm.a.36280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Lim JY, Hansen JC, Siedlecki CA, Runt J, Donahue HJ. Human foetal osteoblastic cell response to polymer-demixed nanotopographic interfaces. J R Soc Interface 2005;2:97–108. 10.1098/rsif.2004.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Lim JY, Dreiss AD, Zhou Z, Hansen JC, Siedlecki CA, Hengstebeck RW, et al. The regulation of integrin-mediated osteoblast focal adhesion and focal adhesion kinase expression by nanoscale topography. Biomaterials 2007;28:1787–97. 10.1016/j.biomaterials.2006.12.020. [DOI] [PubMed] [Google Scholar]
- [34].Loiselle AE, Wei L, Faryad M, Paul EM, Lewis GS, Gao J, et al. Specific biomimetic hydroxyapatite nanotopographies enhance osteoblastic differentiation and bone graft osteointegration. Tissue Eng Part A 2013;19:1704–12. 10.1089/ten.TEA.2012.0560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Juhl OJ 4th, Merife A-B, Zhang Y, Lemmon CA, Donahue HJ. Hydroxyapatite Particle Density Regulates Osteoblastic Differentiation Through β-Catenin Translocation. Front Bioeng Biotechnol 2020;8:591084. 10.3389/fbioe.2020.591084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Juhl OJ 4th, Latifi SM, Donahue HJ. Effect of carbonated hydroxyapatite submicron particles size on osteoblastic differentiation. J Biomed Mater Res B Appl Biomater 2021. 10.1002/jbm.b.34797. [DOI] [PubMed] [Google Scholar]
- [37].Girasole G, Muro G, Mintz A, Chertoff J. Transforaminal lumbar interbody fusion rates in patients using a novel titanium implant and demineralized cancellous allograft bone sponge. Int J Spine Surg 2013;7:e95–100. 10.1016/j.ijsp.2013.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [38].Jiya TU, Royen TSBJ Van, Resorbable PÁ. Posterior lumbar interbody fusion using non resorbable poly-ether-ether-ketone versus resorbable poly-L-lactide-co-D , L-lactide fusion devices. Clinical outcome at a minimum of 2-year follow-up. Eur Spine J 2011;20:618–22. 10.1007/s00586-010-1568-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Najeeb S, Khurshid Z, Matinlinna JP, Siddiqui F, Nassani MZ, Baroudi K. Nanomodified Peek Dental Implants : Bioactive Composites and Surface Modification — A Review. Int J Dent 2015;2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [40].Yang JJ, Yu CH, Chang B, Yoem JS, Lee JH, Lee C-K. Subsidence and Nonunion after Anterior Cervical Interbody Fusion Using a Stand-Alone Polyetheretherketone ( PEEK ) Cage. Clin Orthop Surg 2011;3:16–23. 10.4055/cios.2011.3.1.16. [DOI] [PMC free article] [PubMed] [Google Scholar]


