Abstract
Background
Information regarding effects of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant strains on clinical manifestations and outcomes of coronavirus disease 2019 (COVID-19) in pregnant women is limited.
Methods
A retrospective observational study was conducted using the data from the nationwide COVID-19 registry in Japan. We identified pregnant patients with symptomatic COVID-19 hospitalized during the study period. The Delta and Omicron variants of concern (VOC) predominant periods were defined as August 1 to December 31, 2021 and January 1 to May 31, 2022, respectively. Clinical characteristics were compared between the patients in the Delta and Omicron VOC periods. In addition, logistic regression analysis was performed to identify risk factors for developing moderate-to-severe COVID-19.
Results
During the study period, 310 symptomatic COVID-19 cases of pregnant women were identified; 111 and 199 patients were hospitalized during the Delta and Omicron VOC periods, respectively. Runny nose and sore throat were more common, and fatigue, dysgeusia, and olfactory dysfunction were less common manifestations observed in the Omicron VOC period. In the multivariable logistic regression analysis, onset during the later stage of pregnancy (OR: 2.08 [1.24–3.71]) and onset during the Delta VOC period (OR: 2.25 [1.08–4.90]) were independently associated with moderate-to-severe COVID-19, whereas two doses of SARS-CoV-2 vaccine were protective against developing moderate-to-severe COVID-19 (OR: 0.34 [0.13–0.84]).
Conclusions
Clinical manifestations of COVID-19 in pregnant women differed between the Delta and Omicron VOC periods. SARS-CoV-2 vaccination was still effective in preventing severe COVID-19 throughout the Delta and Omicron VOC periods.
Keywords: Pregnant women, Coronavirus disease 2019, Severe acute respiratory syndrome coronavirus 2, Variant of concern
1Introduction
Coronavirus disease 2019 (COVID-19) continues to be a major problem worldwide. Over 500 million cases and 6 million deaths were reported as of June 2022 [1]. Along with the spread of the COVID-19 pandemic, reports of COVID-19 in pregnant women have increased [2,3]. It has been reported that pregnant women are more susceptible to severe COVID-19 than non-pregnant women, particularly in the second and third trimester of pregnancy [[4], [5], [6]]. It is also known that premature births increase in pregnant women with COVID-19 [7,8].
Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), the cause of COVID-19, can easily mutate, and many variant strains have arisen so far. Of these, clinically and epidemiologically important strains are categorized as variants of concern (VOC), including the Delta and Omicron VOC [9]. The Delta VOC has been reported to be more infectious and cause more severe COVID-19 than the conventional variant strains [10,11]. The Omicron VOC, which emerged later, is even more contagious than the Delta VOC, but its severity is reported to be lower [12,13]. Furthermore, the effectiveness of the SARS-CoV-2 vaccines has decreased against the Omicron VOC [14,15]. As such, the impact of the predominant variant strains on the clinical characteristics of COVID-19 in the general population is now fairly well understood. It has also been reported that clinical characteristics, such as severity of COVID-19, may differ by ethnicity or socioeconomic factors [16,17]; therefore, it is important to evaluate data regarding the clinical characteristic of COVID-19 in pregnant women from different countries and regions. However, the information regarding the characteristics of maternal COVID-19 during the Delta and Omicron VOC periods is very limited [12,18].
Therefore, we conducted this study to clarify the differences in clinical characteristics of COVID-19 in pregnant women between the Delta and Omicron VOC periods in Japan.
2Patients and methods
2.1Study design, patient population, and purposes of the study
This study is a retrospective observational study using the data from the COVID-19 registry Japan (COVIREGI-JP), the largest nationwide COVID-19 registry in Japan. The details of COVIREGI-JP were described previously [19]. Briefly, 706 institutions across Japan have participated and enrolled 70,000 patients as of June 2022 [20]. Laboratory diagnosed, hospitalized COVID-19 patients in all age groups were included. This study identified from this registry the pregnant patients with symptomatic COVID-19 hospitalized between August 1, 2021 to March 31, 2022. The Delta and Omicron VOC periods were defined as August 1 to December 31, 2021 and January 1 to May 31, 2022, respectively. Information regarding patients’ backgrounds and clinical characteristics, including age, gestational age, SARS-CoV-2 vaccination history, prior exposure to COVID-19 before admission, signs and symptoms at admission, clinical course, treatment, and outcomes were extracted from the database. Asymptomatic cases were excluded from the analyses.
The primary purpose of the study was to describe the clinical and epidemiological differences of COVID-19 in symptomatic pregnant women hospitalized between the Delta and Omicron VOC predominant periods. The secondary purpose was to assess the effect of SARS-CoV-2 vaccination and the type of predominant variant strains on COVID-19 severity.
2.2Definition of outcomes
In this study, patients were divided into two groups, namely, the mild and moderate-to-severe groups. The moderate-to-severe group was defined as the presence of one or more of the following [4]: clinical condition at the time of admission (respiratory rate ≥ 24 breaths/minute or oxygen saturation ≤ 94% on ambient air, or need of supplemental oxygen administration), the requirement of noninvasive oxygen supports (including nasal cannula, face mask, reservoir mask, high-flow oxygen device, biphasic positive airway pressure, and continuous positive airway pressure), need for mechanical ventilation, need for extracorporeal membrane oxygenation (ECMO), intensive care unit (ICU), and death. The patients who did not meet these criteria were categorized as mild.
2.3Statistical analysis
Categorical variables were described as numbers and percentages, and continuous variables were described as median and interquartile range (IQR). To compare two groups, the chi-square test for categorical variables and Mann-Whitney U test for continuous variables were used as univariable analysis. To determine the risk factors for developing moderate-to-severe COVID-19, a logistic regression analysis was performed. In addition to SARS-CoV-2 vaccination history and type of VOC, potential confounders including age, gestational age, underlying diseases, and smoking history were entered as covariates. In addition to the total cohort analysis, a subgroup analysis including only patients during the Omicron VOC period was performed to investigate the characteristics of the latest circulating Omicron strain alone, which has been associated with less severe disease. The results of multivariable analysis were expressed as odds ratio (OR) and 95% confidence interval (CI). All statistical analyses were conducted using the statistical software R version 4.1.3.
2.4Ethics
This study was performed with permission of the ethics committees of the National Center for Global Health and Medicine (NCGM-G-003494-0) and the National Center for Child Health and Development (NCCHD-2022-052).
3Results
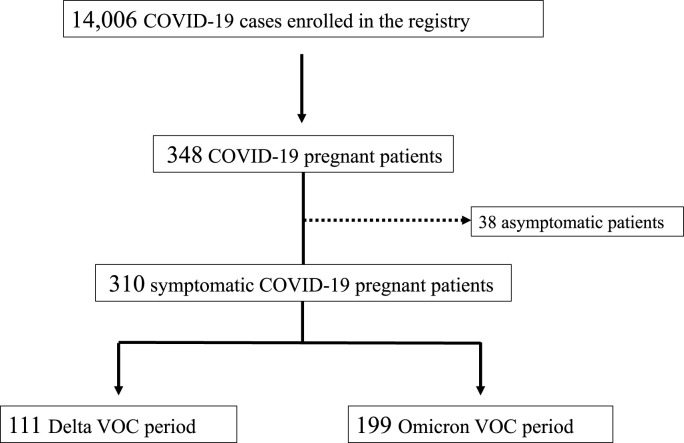
During the study period, 14,006 COVID-19 cases were enrolled into the registry. Among them, we found 348 cases of pregnant women with COVID-19. After excluding 38 asymptomatic patients, 310 symptomatic patients were identified; 111 and 199 patients were hospitalized during the Delta and Omicron VOC periods, respectively (Fig. 1 ). Patient characteristics are summarized in Table 1 . The median (IQR) age was 30 (26–35). The numbers and percentages of infections that occurred during the first, second, and third trimesters were 40 (13.0%), 98 (31.9%), and 169 (55.0%), respectively. The most common underlying disease was bronchial asthma (n = 17, 5.5%), followed by obesity (n = 10, 5.5%) and diabetes mellitus (n = 6, 1.9%). Exposure within 14 days prior to admission was recognized in 197 (64.0%), and more than half of the patients contracted COVID-19 from their family (n = 158, 51.0%). These characteristics were similar between the Delta and Omicron VOC periods. For the SARS-CoV-2 vaccination history, 132 (42.6%) patients had received two doses of SARS-CoV-2 vaccine, and the majority of these patients were hospitalized in the Omicron VOC period (n = 4, 3.6% in the Delta VOC period and n = 128, 64.3% in the Omicron VOC period).
Fig. 1.
Patient selection flow diagram
VOC, variant of concern
Table 1.
Patient characteristics.
| Variables | Number of cases | Subcategory | Total | Delta VOC period | Omicron VOC period | P value |
|---|---|---|---|---|---|---|
| Case number | 310 | 111 | 199 | |||
| Age (years), median (IQR) | 310 | 30 (26–35) | 31 (27–35) | 30 (26–35) | 0.628 | |
| Body weight, median (IQR) | 301 | 58.0 (53.0–64.0) | 57.6 (53.0–62.3) | 58.0 (53.4–65.0) | 0.333 | |
| Smoking history | 310 | Currently smoking | 15 (4.8) | 5 (4.5) | 10 (5.0) | 0.869 |
| Past smoking | 44 (14.2) | 18 (16.2) | 26 (13.1) | |||
| Never | 222 (71.6) | 77 (69.4) | 145 (72.9) | |||
| Unknown | 29 (9.4) | 11 (9.9) | 18 (9.0) | |||
| Gestational age category | 310 | 1st trimester (0 to < 14 weeks) | 40 (13.0) | 14 (12.6) | 26 (13.3) | 0.807 |
| 2nd trimester (14 to < 28 weeks) | 98 (31.9) | 38 (34.2) | 60 (30.6) | |||
| 3rd trimester (≥28 weeks) | 169 (55.0) | 59 (53.2) | 110 (56.1) | |||
| Unknown | 3 (1.1) | 0 (0.0) | 3 (1.5) | |||
| Underlying disease, number (%) | 310 | Any underlying disease | 33 (10.6) | 12 (10.8) | 21 (10.6) | 0.999 |
| Bronchial asthma | 17 (5.5) | 7 (6.3) | 10 (5.0) | 0.830 | ||
| Obesity | 10 (3.2) | 4 (3.6) | 6 (3.0) | 0.999 | ||
| Diabetes mellitus | 6 (1.9) | 1 (0.9) | 5 (2.5) | 0.577 | ||
| Collagen disease | 3 (1.0) | 0 (0.0) | 3 (1.5) | 0.487 | ||
| Immunosuppressive condition, number (%) | 310 | 1 (0.3) | 0 (0.0) | 1 (0.5) | 0.999 | |
| Exposure within 14 days prior to admission | 310 | Travel abroad | 1 (0.3) | 1 (0.9) | 0 (0.0) | 0.999 |
| Close contact with COVID-19 cases | 197 (64.0) | 76 (69.7) | 121 (60.8) | 0.218 | ||
| Family | 158 (51.0) | 56 (50.5) | 102 (51.3) | 0.986 | ||
| Educational facility | 7 (2.3) | 3 (2.7) | 4 (2.0) | 0.999 | ||
| Nonfamily roommates | 4 (1.3) | 2 (1.8) | 2 (1.0) | 0.943 | ||
| Workplace | 21 (6.8) | 11 (9.9) | 10 (5.0) | 0.160 | ||
| Healthcare facility | 2 (0.6) | 1 (0.9) | 1 (0.5) | 0.999 | ||
| Others | 10 (3.2) | 7 (6.3) | 3 (1.5) | 0.050 | ||
| Number of patients with two doses of SARS-CoV-2 vaccine | 310 | 132 (42.6) | 4 (3.6) | 128 (64.3) | <0.001 |
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory coronavirus type 2; VOC, variant of concern; IQR, interquartile range; NA, not applicable.
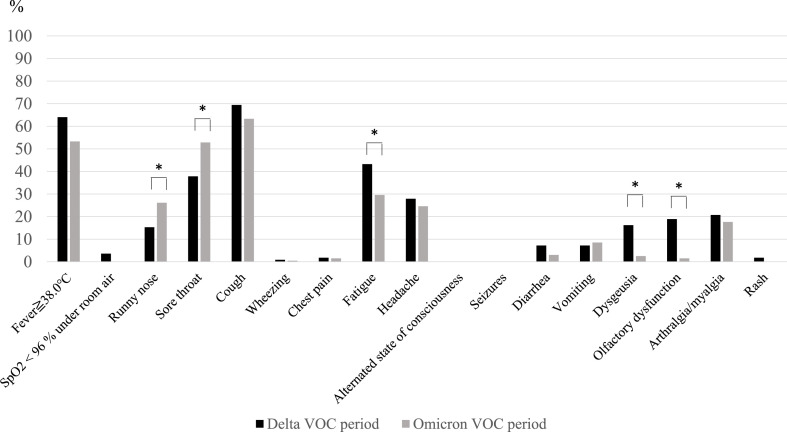
The incidence of symptoms at hospitalization during these periods is shown in Fig. 2 . Runny nose (P = 0.047) and sore throat (P = 0.028) were more common, and fatigue (P = 0.026), dysgeusia (P < 0.001), and olfactory dysfunction (P < 0.001) were less common in the Omicron VOC period than in the Delta VOC period.
Fig. 2.
Incidence of symptoms at hospitalization in the Delta and Omicron VOC periods
* indicates statistical significance
VOC, variant of concern
Among the patients, 52 (16.8%) cases required noninvasive oxygen support. The most commonly used medication type for COVID-19 was steroids (n = 41, 13.2%), followed by remdesivir (n = 27, 8.7%) and casirivimab/imdevimab (n = 4, 1.3%). For severity and outcomes, eight (2.6%) patients required ICU admission, and one patient required invasive mechanical ventilation; however, no patients died. Compared with the Delta VOC period, fewer patients in the Omicron VOC period required noninvasive oxygen support, remdesivir, or steroid administration (Table 2 ).
Table 2.
Comparison of severity, complications, and outcomes between the Delta variant and Omicron VOC periods.
| Variables | Total | Delta VOC period | Omicron VOC period | P value |
|---|---|---|---|---|
| Number of cases | 310 | 111 | 199 | |
| Noninvasive oxygen support (nasal cannula, face mask, reservoir mask, high-flow oxygen device) | 52 (16.8) | 32 (28.8) | 20 (10.1) | <0.001 |
| Invasive mechanical ventilation/ECMO | 1 (0.3) | 0 (0.0) | 1 (0.5) | 0.999 |
| Medications | ||||
| Remdesivir | 27 (8.7) | 21 (18.9) | 6 (3.0) | 0.003 |
| Casirivimab/imdevimab | 4 (1.3) | 4 (3.6) | 0 (0.0) | 0.030 |
| Sotorovimab | 0 (0.0) | 0 (0.0) | 6 (3.0) | 0.030 |
| Nirmatorelvir/ritonavir | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
| Steroids | 41 (13.2) | 32 (28.8) | 9 (4.5) | <0.001 |
| Length of hospital stay (days), median (IQR) | 8 (5–9) | 8 (6–9) | 8 (5–9) | 0.282 |
| ICU admission | 8 (2.6) | 5 (4.5) | 3 (1.5) | 0.222 |
| Death | 0 (0.0) | 0 (0.0) | 0 (0.0) | NA |
VOC, variant of concern; ECMO, extracorporeal membrane oxygenation; IQR, interquartile range.
Table 3 shows the results of univariable and multivariable analyses between the mild and moderate-to-severe COVID-19 groups. In the multivariable logistic regression analysis, later stage of trimester (OR: 2.08 [1.24–3.71]) and the patients in the Delta VOC period (OR: 2.25 [1.08–4.90]) were independently associated with moderate-to-severe COVID-19, and a two-dose regimen of SARS-CoV-2 vaccine was protective for developing moderate-to-severe COVID-19 (OR: 0.34 [0.13–0.84]). Finally, similar analyses were performed, including a subset of patients in the Omicron VOC period (Supplemental Table 1). Later stages of gestation were still significantly associated with moderate-to-severe COVID-19 (OR: 4.10 [1.41–16.92]). The patients with moderate-to-severe COVID-19 less commonly received SARS-CoV-2 vaccinations, but this was not statistically significant (OR: 0.40 [0.15–1.03]).
Table 3.
Univariable and multivariable analyses for identifying risk factors for moderate-to-severe COVID-19 in pregnant women.
| Variables | Moderate-to-severe COVID-19 (n = 52) | Mild COVID-19 (n = 258) | OR (95% CI) |
OR (95% CI) |
||
|---|---|---|---|---|---|---|
| Unadjusted | P value | Adjusted | P value | |||
| Age (years), median (IQR) | 32 (26–34) | 30 (26–35) | 1.03 (0.97–1.08) | 0.338 | 1.04 (0.98–1.10) | 0.226 |
| Currently smoking | 1 (1.9) | 14 (5.4) | 0.34 (0.02–1.76) | 0.305 | 0.37 (0.02–2.15) | 0.360 |
| Gestational age category | ||||||
| 1st trimester (0 to < 14 weeks) | 1 (1.9) | 39 (15.1) | 2.06 (1.26–3.57) | 0.006 | 2.08 (1.24–3.71) | 0.008 |
| 2nd trimester (14 to < 28 weeks) | 15 (28.8) | 83 (32.2) | ||||
| 3rd trimester (≥28 weeks) | 36 (69.2) | 133 (51.6) | ||||
| Unknown | 0 (0.0) | 3 (1.2) | ||||
| Underlying disease, number (%) | ||||||
| Any underlying diseasea | 8 (15.4) | 25 (9.7) | 1.69 (0.68–3.86) | 0.229 | 1.59 (0.60–3.90) | 0.327 |
| Bronchial asthma | 4 (7.7) | 13 (5.0) | 1.57 (0.43–4.66) | 0.447 | ||
| Obesity | 3 (5.8) | 7 (2.7) | 2.20 (0.46–8.20) | 0.266 | ||
| Diabetes mellitus | 2 (3.8) | 4 (1.6) | 2.54 (0.35–13.38) | 0.289 | ||
| Collagen disease | 0 (0.0) | 3 (1.2) | NA | 0.987 | ||
| Number of patients with two doses of SARS-CoV-2 vaccine | 9 (17.3) | 123 (47.7) | 0.23 (0.10–0.47) | <0.001 | 0.34 (0.13–0.84) | 0.021 |
| Patients in the Delta VOC period | 32 (61.5) | 79 (30.6) | 3.63 (1.97–6.82) | <0.001 | 2.25 (1.08–4.90) | 0.035 |
COVID-19, coronavirus disease 2019; SARS-CoV-2, severe acute respiratory coronavirus type 2; VOC, variant of concern; IQR, interquartile range; OR, odds ratio; CI, confidential interval; NA, not applicable.
Only “Any underlying disease” was entered into the multivariable analysis.
4Discussion
This study revealed that the clinical characteristics of pregnant COVID-19 patients were different between the Delta and Omicron VOC periods and the overall severity was lower in the latter. In addition, later stages of pregnancy and lack of SARS-CoV-2 vaccination were associated with higher severity throughout both periods.
It is known that the characteristics of COVID-19 in the general population can be different according to the predominant SARS-CoV-2 variant strains. For example, it has been reported that the clinical manifestations of COVID-19 were different in the Delta and Omicron VOC predominant periods; sore throat was more common (70.5% vs. 60.8%) and loss of smell was less common (16.7% vs. 52.7%) in the Omicron VOC period than those in the Delta VOC period [12]. Regarding their severity, many studies showed that COVID-19 caused by the Omicron VOC was less severe than the one caused by the Delta VOC or other variant strains [12,21,22]. A report investigating the clinical characteristics of COVID-19 in pregnant women revealed that pregnant women were less likely to have fever, cough, dyspnea, and myalgia, but more likely to be admitted to the intensive care unit compared with non-pregnant women [5]. However, information regarding the effect of different variant strains on clinical manifestations and severity of COVID-19 in pregnant women has been limited. Our data showed a higher incidence of runny nose and sore throat and lower incidence of fatigue, dysgeusia, and olfactory dysfunction and milder clinical course in the Omicron VOC period than those in the Delta VOC period, which are consistent with previous reports in the general population.
Later stage of pregnancy was identified as a risk factor for developing moderate-to-severe COVID-19 for pregnant women in this study. Our research group previously reported that COVID-19 in pregnant women in the second and third trimesters were more severe than those in the first trimester using data from the COVIREGI-JP in the pre-Delta VOC period [4]. The results of the current study showed that this characteristic has not changed even in the Delta and Omicron VOC periods. This observation suggests that pregnant women, particularly in the later stage of pregnancy, should continue to take greater precautions to avoid COVID-19.
In our study, the pregnant patients who received two doses of SARS-CoV-2 vaccine were associated with milder severity of COVID-19. There have been many studies that showed efficacy in preventing SARS-CoV-2 infection, hospitalization, and death due to COVID-19 including the Delta or Omicron VOC periods [14,15,[23], [24], [25]]. The favorable safety and efficacy of SARS-CoV-2 vaccines in pregnant women have also been reported [[26], [27], [28], [29]] and SARS-CoV-2 vaccination in pregnant women at any stage of pregnancy has been recommended worldwide, including in Japan [[30], [31], [32]]. Our study results in the total cohort were consistent with previous reports and demonstrated the benefits of SARS-CoV-2 vaccination in pregnant women even in the Delta and Omicron VOC periods. The subset analysis of patients in the Omicron VOC period also showed a protective trend for the vaccine, although this was not statistically significant. This is likely due to the insufficient number of patients in the Omicron VOC period, but further investigation in a larger population is warranted. In the present study, the SARS-CoV-2 vaccination coverage of pregnant women differed greatly, ranging from 3.6% in the Delta VOC period to 64.3% in the omicron VOC period. At the time of the Delta VOC period, SARS-CoV-2 vaccines had already been approved for two doses for adult population [33], and the cause of this difference is unknown. However, we speculate that the higher vaccine efficacy against the Delta VOC [14] prevented a significant proportion of hospitalizations during this period.
This study has several limitations. First, our registry did not include the outcomes of pregnancy. Therefore, we could not investigate about the association with predominant VOCs and pregnancy outcomes. Several meta-analyses revealed the negative impacts of maternal COVID-19 on pregnancy outcomes, such as increasing preterm birth, preeclampsia, and stillbirth [[34], [35]]. However, the information regarding the effect of predominant VOCs on fetal outcomes is still limited and further study is warranted to reveal the unreported effects in Japan. Second, we had no individual data regarding the type of VOCs in our patients. Therefore, the effect of VOCs on clinical characteristics could not be assessed directly. However, surveillance data from the National Institute of Infectious Diseases indicated that more than 95% of variant strains detected in Japan during our defined Delta and Omicron VOC periods were the Delta and the Omicron VOC, respectively [36]. Therefore, we believe that the impact of the lack of sequence information for the VOCs was minimal. Lastly, as the registry only includes inpatients, it could be biased if the indications for hospitalization have changed in each period. For example, more severely ill patients may have been selectively admitted during the Omicron VOC period, when the number of patients was higher, which may have affected the interpretation of severity in both periods.
In conclusion, the clinical characteristics of COVID-19 in pregnant women differed by the period of predominant VOCs. To achieve better management of COVID-19, the information on the clinical features of COVID-19 in pregnant women should continue to be updated in preparation for any new VOC emerging in the future.
5Authorship statement
KS contributed to conceptualizing and designing the study and drafted the manuscript. ST and TA contributed to perform statistical analysis, and revising of the manuscript. NM, YA, SS, and NI contributed to data collection, and revising of the manuscript. TF, MY, N Ozawa, YI and IM contributed to the revised the manuscript. N Ohmagari contributed to the revised the manuscript, and supervised the study. All authors meet the ICMJE authorship criteria. All authors approved the final manuscript as submitted and agree to be accountable for all aspects of the work.
Declaration of competing interest
K. Shoji received payment for lectures from Mitsubishi Tanabe Pharma, Astellas, AbbVie GK, Biomerieux Japan, Nippon Becton Dickinson Company, Ltd., VIATRIS and Gilead. S. Tsuzuki received payment for supervising medical articles from Gilead Sciences, Inc. The other authors declare that they do not have any conflicts of interests directly associated with the study.
Acknowledgements
This work was partly supported by the Ministry of Health, Labour and Welfare (MHLW) Research on Emerging and Re-emerging Infectious Diseases and Immunization Program Grant Number 19HA1003 and Repository of Data and Biospecimen of Infectious Disease Program (REBIND).
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.jiac.2022.09.005.
Appendix A. Supplementary data
The following is the Supplementary data to this article:
References
- 1.World Health Organization . COVID-19 weekly epidemiological update. Edition 101. 2022. https://www.who.int/publications/m/item/weekly-epidemiological-update-on-covid-19---20-july-2022 published 20 July 2022. Available at: [Google Scholar]
- 2.Badr D.A., Picone O., Bevilacqua E., et al. Severe acute respiratory syndrome coronavirus 2 and pregnancy outcomes according to gestational age at time of infection. Emerg Infect Dis. 2021;27:2535–2543. doi: 10.3201/eid2710.211394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Metz T.D., Clifton R.G., Hughes B.L., et al. Association of SARS-CoV-2 infection with serious maternal morbidity and mortality from obstetric complications. JAMA. 2022;327:748–759. doi: 10.1001/jama.2022.1190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Shoji K., Tsuzuki S., Akiyama T., et al. 2022 Jan 17. Clinical characteristics and outcomes of COVID-19 in pregnant women: a propensity score matched analysis of the data from the COVID-19 Registry Japan. [Online ahead of print] ciac028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Allotey J., Stallings E., Bonet M., et al. Clinical manifestations, risk factors, and maternal and perinatal outcomes of coronavirus disease 2019 in pregnancy: living systematic review and meta-analysis. BMJ. 2020 Sep 1;370:m3320. doi: 10.1136/bmj.m3320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Zambrano L.D., Ellington S., Strid P., et al. Update: characteristics of symptomatic women of reproductive age with laboratory-confirmed SARS-CoV-2 infection by pregnancy status - United States, january 22-october 3, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1641–1647. doi: 10.15585/mmwr.mm6944e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chinn J., Sedighim S., Kirby K.A., et al. Characteristics and outcomes of women with COVID-19 giving birth at US academic centers during the COVID-19 pandemic. JAMA Netw Open. 2021;4 doi: 10.1001/jamanetworkopen.2021.20456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Woodworth K.R., Olsen E.O., Neelam V., et al. Birth and infant outcomes following laboratory-confirmed SARS-CoV-2 infection in pregnancy - SET-NET, 16 jurisdictions, March 29-october 14, 2020. MMWR Morb Mortal Wkly Rep. 2020;69:1635–1640. doi: 10.15585/mmwr.mm6944e2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.World Health Organization Tracking SARS-CoV-2 variants. https://www.who.int/activities/tracking-SARS-CoV-2-variants Available at:
- 10.Public Health England SARS-CoV-2 variants of concern and variants under investigation in England Technical briefing 14. https://assets.publishing.service.gov.uk/government/uploads/system/uploads/attachment_data/file/991343/Variants_of_Concern_VOC_Technical_Briefing_14.pdf Available at:
- 11.Fisman D.N., Tuite A.R. Evaluation of the relative virulence of novel SARS-CoV-2 variants: a retrospective cohort study in Ontario, Canada. CMAJ (Can Med Assoc J) 2021;193 doi: 10.1503/cmaj.211248. E1619-e25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Menni C., Valdes A.M., Polidori L., et al. Symptom prevalence, duration, and risk of hospital admission in individuals infected with SARS-CoV-2 during periods of omicron and delta variant dominance: a prospective observational study from the ZOE COVID Study. Lancet. 2022;399:1618–1624. doi: 10.1016/S0140-6736(22)00327-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Del Águila-Mejía J., Wallmann R., Calvo-Montes J., et al. Secondary attack rate, transmission and incubation periods, and serial interval of SARS-CoV-2 omicron variant, Spain. Emerg Infect Dis. 2022;28:1224–1228. doi: 10.3201/eid2806.220158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Collie S., Champion J., Moultrie H., et al. Effectiveness of BNT162b2 vaccine against omicron variant in South Africa. N Engl J Med. 2022;386:494–496. doi: 10.1056/NEJMc2119270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Thompson M.G., Natarajan K., Irving S.A., et al. Effectiveness of a third dose of mRNA vaccines against COVID-19-associated emergency department and urgent care encounters and hospitalizations among adults during periods of delta and omicron variant predominance - VISION network, 10 states, August 2021-january 2022. MMWR Morb Mortal Wkly Rep. 2022;71:139–145. doi: 10.15585/mmwr.mm7104e3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mackey K., Ayers C.K., Kondo K.K., et al. Racial and ethnic disparities in COVID-19-related infections, hospitalizations, and deaths : a systematic review. Ann Intern Med. 2021;174:362–373. doi: 10.7326/M20-6306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escobar G.J., Adams A.S., Liu V.X., et al. Racial disparities in COVID-19 testing and outcomes : retrospective cohort study in an integrated Health system. Ann Intern Med. 2021;174:786–793. doi: 10.7326/M20-6979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Birol Ilter P., Prasad S., Mutlu M.A., et al. Maternal and perinatal outcomes of SARS-CoV-2 infection in unvaccinated pregnancies during Delta and Omicron waves. Ultrasound Obstet Gynecol. 2022;60:96–102. doi: 10.1002/uog.24916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Matsunaga N., Hayakawa K., Terada M., et al. Clinical epidemiology of hospitalized patients with COVID-19 in Japan: report of the COVID-19 REGISTRY Japan. Clin Infect Dis. 2021;73:e3677–e3689. doi: 10.1093/cid/ciaa1470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.National Center for Global Medicine COVID-19 registry Japan. https://covid-registry.ncgm.go.jp/ Available at:
- 21.Abdullah F., Myers J., Basu D., et al. Decreased severity of disease during the first global omicron variant covid-19 outbreak in a large hospital in tshwane, South Africa. Int J Infect Dis. 2022;116:38–42. doi: 10.1016/j.ijid.2021.12.357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maslo C., Friedland R., Toubkin M., et al. Characteristics and outcomes of hospitalized patients in South Africa during the COVID-19 omicron wave compared with previous waves. JAMA. 2022;327:583–584. doi: 10.1001/jama.2021.24868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chodick G., Tene L., Rotem R.S., et al. The effectiveness of the two-dose BNT162b2 vaccine: analysis of real-world data. Clin Infect Dis. 2022;74:472–478. doi: 10.1093/cid/ciab438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Dagan N., Barda N., Kepten E., et al. BNT162b2 mRNA covid-19 vaccine in a nationwide mass vaccination setting. N Engl J Med. 2021;384:1412–1423. doi: 10.1056/NEJMoa2101765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Polack F.P., Thomas S.J., Kitchin N., et al. Safety and efficacy of the BNT162b2 mRNA covid-19 vaccine. N Engl J Med. 2020;383:2603–2615. doi: 10.1056/NEJMoa2034577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brinkley E., Mack C.D., Albert L., et al. COVID-19 vaccinations in pregnancy: comparative evaluation of acute side effects and self-reported impact on quality of life between pregnant and non-pregnant women in the United States. Am J Perinatol. 2022 May 6 doi: 10.1055/s-0042-1748158. [Online ahead of print] [DOI] [PubMed] [Google Scholar]
- 27.Stock S.J., Carruthers J., Calvert C., et al. SARS-CoV-2 infection and COVID-19 vaccination rates in pregnant women in Scotland. Nat Med. 2022;28:504–512. doi: 10.1038/s41591-021-01666-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Fu W., Sivajohan B., McClymont E., et al. Systematic review of the safety, immunogenicity, and effectiveness of COVID-19 vaccines in pregnant and lactating individuals and their infants. Int J Gynaecol Obstet. 2022;156:406–417. doi: 10.1002/ijgo.14008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Goldshtein I., Nevo D., Steinberg D.M., et al. Association between BNT162b2 vaccination and incidence of SARS-CoV-2 infection in pregnant women. JAMA. 2021;326:728–735. doi: 10.1001/jama.2021.11035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.American College of Obstetricians and Gynecologists COVID-19 vaccination considerations for obstetric–gynecologic care. https://www.acog.org/clinical/clinical-guidance/practice-advisory/articles/2020/12/covid-19-vaccination-considerations-for-obstetric-gynecologic-care Practice advisory. Last updated June 3, 2022. Available at:
- 31.Ministry of Health, Labour and Welfare COVID-19 vaccine Q&A. https://www.mhlw.go.jp/stf/covid-19/qa.html Available at:
- 32.UK Health Security Agency Guidance COVID-19 vaccination: a guide on pregnancy and breastfeeding. https://www.gov.uk/government/publications/covid-19-vaccination-women-of-childbearing-age-currently-pregnant-planning-a-pregnancy-or-breastfeeding/covid-19-vaccination-a-guide-for-women-of-childbearing-age-pregnant-planning-a-pregnancy-or-breastfeeding Available at:
- 33.Aizawa Y., Takanashi S., Ogimi C. Updates on coronavirus disease 2019 in children in Japan. Pediatr Infect Dis J. 2022 Jul 18 doi: 10.1097/INF.0000000000003641. [Online ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wei S.Q., Bilodeau-Bertrand M., Liu S., et al. The impact of COVID-19 on pregnancy outcomes: a systematic review and meta-analysis. CMAJ (Can Med Assoc J) 2021;193 doi: 10.1503/cmaj.202604. E540-e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wang X., Chen X., Zhang K. Maternal infection with COVID-19 and increased risk of adverse pregnancy outcomes: a meta-analysis. J Matern Fetal Neonatal Med. 2022:1–8. doi: 10.1080/14767058.2022.2033722. [DOI] [PubMed] [Google Scholar]
- 36.National Institute of Infectious Diseases 20220623_genome_weekly_lineageJAPAN. https://www.mhlw.go.jp/stf/covid-19/kokunainohasseijoukyou.html#h2_1 Available at:
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.