Abstract
Introduction
Pemphigus vulgaris (PV) is a rare autoimmune disease that causes painful blistering. Interleukin-15 (IL-15) as a member of the immunoregulatory cytokines family is associated with the development of the chronic inflammatory or autoimmune disease. There is not much information available in the literature on the exact role IL-15 plays in PV.
Objectives
The goal of this study was to evaluate the serum levels of IL-15 in patients with PV and assess the association of IL-15 with anti-desmoglein antibodies and the severity of the disease.
Methods
Fifty-three individuals affected with active PV and 38 age- and gender-matched healthy controls were participated in this study. Disease severity was assessed using Autoimmune Bullous Skin Disorder Intensity Score (ABSIS). Serum levels of IL-15 (pg/mL) and anti-desmoglein antibodies (Dsg1, 3) were determined.
Results
In the patient group, IL-15 serum levels were statistically higher than those in the control group (3.71 ± 1.5 vs. 0.79 ± 1.03, P < 0.001). A positive correlation was found between serum levels of IL-15 and ABSIS (r = 0.5, P = 0.04). We found no significant correlation between serum concentrations of IL-15 and antidesmoglein antibodies (Dsg1 or Dsg3).
Conclusions
An increase in serum level of IL-15 in patients with PV and its relationship with disease severity suggest that this cytokine possibly contributes to the pathogenesis of the disease and targeting IL-15 will likely provide a new insight into the treatment of this disease.
Keywords: pemphigus vulgaris, interleukin-15, anti-desmoglein, IL-15, ABSIS
Introduction
Pemphigus vulgaris (PV) is a rare autoimmune disorder that causes painful blisters and erosions on the skin and mucosa [1]. PV is caused by environmental and genetic factors that lead to immunological impairments. Auto-antibodies against the desmosomal adhesion proteins of epidermal keratinocytes, desmoglein3 and/or desmoglein 1, lead to the loss of epidermal cell adhesion and development of erosive lesions [1,2]. To date, different cytokines, such as osteopontin, interleukin (IL)-4 and IL-21 have been suggested to be associated with disease pathogenesis and severity [3–5]. These cytokines contribute to rising proinflammatory responses and production of autoantibodies through differentiation of naïve T-cells into effector T-ells, increased B-cell responses, and favors a class switch to IgG4. Interfering with the function of them using monoclonal antibodies, such as dupilumab for IL-4/IL-4 receptor could be probably effective in treating some PV patients [6,7]. In contrast, regulatory cytokines, such as TGF-β and IL-35-dependent mechanisms could mediate restoration of self-tolerance, which had been broken in patients with autoimmune diseases [8,9]. However, the role of some less studied cytokines, such as IL-15 has remained controversial. To our best knowledge, 3 studies have assessed the serum levels of IL-15 in PV patients. Two have reported increased serum levels of IL-15 and one suggested suppressed production of IL-15 as compared to the healthy controls [10–12].
IL-15 is a glycoprotein cytokine produced by multiple cell types including monocytes, macrophages, dendritic cells, fibroblasts, and epithelial cells. It is well known that IL-15 contributes to the survival and proliferation of T-cell [13]. It also increases the proliferation of B lymphocytes and promotes their differentiation into the plasma cells [13,14]. Recent studies have indicated that inhibiting IL-15 with various methods may be the goal of appropriate treatment to reduce inflammation [14].
Objectives
IL-15 is involved in the pathogenesis of a variety of autoimmune diseases such as Sjogren disease, Behcet disease, systemic lupus erythematous (SLE) and rheumatoid arthritis (RA) [3,15]_ENREF_14. There has been little information about the exact role of IL-15 in pemphigus pathogenesis or how IL-15 production relates to the severity of pemphigus.
In this regard, the goal of this study was to assess the serum levels of IL-15 in patients with PV and to find out whether IL-15 levels were associated with severity of their disease.
Methods
Patients
To evaluate the serum levels of IL-15, 53 individuals with active PV and 38 healthy individuals were enrolled in this study. Among 53 patients, 37 cases were newly diagnosed while 16 cases were presented with relapse during minimal therapy (5–10 mg prednisolone per day). PV was diagnosed based on clinical evidence, histopathologic findings, Direct Immune-Fluorescence examination and the detection of serum autoantibodies by ELISA. Participants with any history of inflammatory/autoimmune diseases, hematologic and solid malignancies, viral hepatitis, and HIV were excluded. This study was approved by the ethical committee of our skin research center (ethical code: IR.SBMU.SRC.REC.1395.41). Prior to enrollment in the study, all participants provided written informed consent.
Clinical and Laboratory Data
All the patients enrolled in the study had an active disease, defined as the development of at least 3 de novo blisters/erosions, which do not heal spontaneously within one week. The Autoimmune Bullous Skin Disorder Intensity Score (ABSIS) was used to assess the severity of the disease. The ABSIS provides a maximum score of 206 (150 points for skin involvement, 11 points for oral involvement, and 45 points for subjective oral discomfort) [16]. The score is determined by calculating the percentage of blisters involvement and erosions on the skin, along with weighting factors at the stage of the blistering and erosions, respectively. Discomfort during eating and drinking is also considered [16]._ENREF_16
Cytokine Measurements
For evaluating the level of IL-15 and anti-desmogleins (Dsg), 10 ccs venous blood of each participant was collected. The blood samples were centrifuged to gather the serums. Then, the serums were frozen at −80°C. After gathering all the samples, levels of IL-15 were measured by an ELISA kit (Diaclone, France), according to the manufacturer’s instructions. Using the ELISA method (Euroimmun AG) anti-Dsg 1 and 3 antibodies were measured in patients. Values ≥ 20 of relative units per milliliter (RU/ml) were considered positive.
Statistical Analysis
Findings were respectively expressed as mean ± standard deviation or as number (percentage) for continuous variables and categorical data, respectively. Independent samples t test and non-parametric Mann–Whitney U test were applied to compare the means of continuous variables and chi squared test was used for categorical variables. Analysis of variance (ANOVA test) was used for comparing means between variables. To determine if there was a linear relationship between variables, Pearson correlation testing was performed. We used the statistical package of SPSS version 16.0.0. (SPSS Inc.) To analyze the data. The level of significance was considered as P values less than 0.05.
Results
Patient Characteristics
Fifty-three patients with PV (35 females and 18 males) and 38 healthy individuals (24 women and 14 men) were enrolled in the study. The control group was frequency-matched to cases by gender and age. The mean ages of patients and control subjects were equal to 45.62 ± 12.27 and 44.21 ± 13.15, respectively. The baseline demographics and clinical characteristics of the participants have been presented in Table 1. The two groups were comparable in age, gender, and comorbidities (Table 1).
Table 1.
Baseline demographics and clinical characteristics of patients with pemphigus and healthy controls.
| Characteristic | PV patients (N = 53) | Healthy controls (N = 38) | P |
|---|---|---|---|
|
| |||
| Gender | |||
| Female | 35 (66.0% ) | 24 (63.2%) | 0.70 |
| Male | 18 (34.0% ) | 14 (36.8% ) | |
|
| |||
| Age, Years | |||
| Mean ± SD | 45.62 ± 12.27 | 44.21 ± 13.15 | 0.60 |
| Median (range) | 46 (21 – 72 ) | 45.50 (25 – 73) | |
|
| |||
| Time until diagnosis or exacerbation, month | |||
| Mean ± SD | 2.6 ±2.3 | — | |
|
| |||
| Type of pemphigus involvement | |||
| Mucosal | 18 (34%) | ||
| Cutaneous | 7 (13.2%) | — | |
| Mucocutaneous | 28 (52.8%) | ||
|
| |||
| ABSIS, Mean ± SD; | |||
| Total score a | 36.88 ± 24.92 | — | |
|
| |||
| Anti-Dsg1 Antibody (RU/ml) | |||
| Mean ±SD | 238.68 ± 47.70 | — | |
|
| |||
| Anti-Dsg3 Antibody (RU/ml) | |||
| Mean ±SD | 729.63 ± 99.05 | — | |
|
| |||
| IL-15 (pg/ml) | |||
| Mean ± SD | 3.71 ± 1.5 | 0.79 ± 1.03 | < 0.001 |
ABSIS = Autoimmune Bullous Skin Disorder Intensity Score; Dsg = Desmoglein; IL-15 = Interleukin-15;; SD = standard deviation.
Values are reported as numbers (%) unless otherwise specified.
Total score is sum of objective and subjective scores.
Serum Levels of IL-15
PV patients had significantly higher levels of IL-15 than controls. The mean serum levels of IL-15 ± SD in PV patients were 3.71 ± 1.5 pg/ml, while for healthy controls were 0.79 ± 1.03 pg/ml, which was statistically different (P < 0.001). Figure 1 demonstrates the IL-15 levels in these two groups. In patients with PV, no significant correlations were detected between IL-15 levels and anti-Dsg1 (r = 0.06, P = 0.6) or anti-Dsg3 (r = 0.006, P = 0.9) antibodies. Serum levels of IL-15 were positively correlated with PV severity according to total ABSIS (r = 0.5, P = 0.04).
Figure 1.
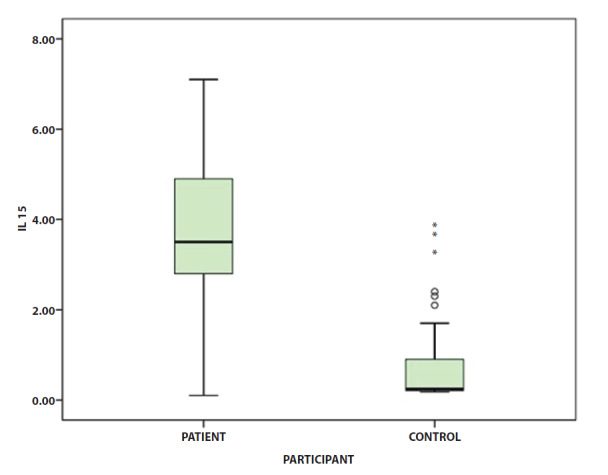
Serum IL-15 concentrations (pg/ml) in patients with pemphigus in comparison to healthy controls. Overall, IL-15 levels in the pemphigus group were higher than that of the control group (P < 0.001).
IL-15 = Interleukin-15.
In the patients group, there was no significant difference in the mean levels of serum IL-15 between men and women or between patients with cutaneous, mucosal, or mucocutaneous forms of the disease (P = 0.9 and 0.09, respectively). There was a significant correlation between pemphigus severity (ABSIS score) and anti- Dsg3 (r = 0.3, P = 0.01) and anti-Dsg1 levels (r = 0.6, P < 0.001)._ENREF_15
Conclusions
In this study, we have evaluated plasma levels of IL-15 in patients with PV in the active phase of the disease and searched for a potential relationship between levels of IL-15 and the severity of the disease. We have found that levels of IL-15 are significantly higher in patients compared to healthy controls. Our study group results were comparable to those previously reported [10,11].
Approximately two decades ago, D’auria et al assessed the levels of IL-15 in the serum sample of patients affected with three different bullous dermatoses ( 5 with bullous pemphigoid, 15 with PV and 15 with pemphigus erythematosus) [10]. They showed a higher level of IL-15 serum in all the dermatosis as compared with healthy subjects. They also showed a significant correlation between the number of lesions and IL-15 serum levels [10]._ENREF_16 Additionally, Ameglio et al showed increased levels of interleukin 15 in the serum of 15 PV patients with active disease [11]. In contrast, in a recently conducted study, Timoteo et al with the evaluation of 20 PV patients and 20 healthy controls revealed a significantly lower serum level of IL-15 in patients with PV than in control group patients [12]. In their study the study population consisted of non-active patients under pharmacologic therapy, then their results can be confounded by potential suppressive effects of immunosuppressants on IL-15 secretions.
IL-15 is assumed to be a member of the immunoregulatory cytokines family which is primarily produced by monocytes, macrophage, dendritic cells, fibroblast, and epithelial cells.
It is believed that IL-15 overexpression is associated with the development of chronic inflammatory disease or autoimmune disorders. When IL-15 is overexpressed, autoreactive T-cells are survived for longer periods of time, that results in abnormal lymphocyte activation [14]. Furthermore IL-15 is also involved in the activation and proliferation of Natural Killer cells (NKs) [17]. Stern et al have suggested a possible role for NKs in the pathobiology of PV and D’auria et al have shown that the number of circulating natural killer cells is significantly correlated with the concentration of IL-15 in patients with pemphigus [10,18].
According to the mentioned role of IL-15 in the differentiation and development of involved cells in immune responses, the role of this cytokine has been studied in a number of autoimmune diseases. There are some reports showing that the mean levels of IL-15 serum are significantly higher in Behcet disease, SLE and rheumatoid arthritis [19]. Active SLE patients had significantly higher levels of IL-15 serum compared to healthy controls, while it was not directly associated with disease activity [20]. The levels of IL-15 in the serum and synovial fluid of patients with rheumatoid arthritis, were much higher compared to the controls [21]. Interestingly, the levels of IL-15 were related to the disease severity and serum levels of IL-15 were strongly correlated with the levels of rheumatoid factor and anti-CCP [21].
Collectively these findings indicated that IL-15 is a key cytokine in several autoimmune diseases, and raises the possibility that targeting IL15 with various anti-IL15 approaches may provide a new insight for the treatment of such disorders [19]. For instance, in an animal model of human psoriasis (xenograft mouse), the IL-15 blockade led to the psoriasis resolution [22]. In rat models of induced arthritis, weekly administration of small interfering RNA targeting IL-15 reduces the expression of proinflammatory mediators in the inflamed joints, alleviates disease progression and significantly inhibits the clinical, radiologic and histologic features of rheumatoid arthritis [19].
In the case of pemphigus, corticosteroid medications that usually used to suppress auto-antibodies, include delayed and serious side effects [23]. Targeting B-cell by the anti-CD20 molecule (rituximab) is currently the best available agent for the treatment of pemphigus patients not responding to conventional treatments [24]. This drug eliminates peripheral B-cells. B-cell depletion, may not be desirable because B-cells are essential for antibody production, activation of T-cells and complements. Therefore, interrupting the homing of B-cells by blocking the IL-15 pathway might be a suitable alternative to reduce excessive inflammation [14]. Torn et al showed that in patients with RA, the treatment with rituximab significantly decreased IL-15 serum levels as well as IL-15 cellular levels. They concluded that sustained clinical improvement following rituximab treatment was related to IL-15 and the mechanisms by which IL-15 exerts influence on T-cells [25].
Based on our findings, the elevated levels of IL-15 in sera of patients with pemphigus suggest that this cytokine may actually be involved in pathogenesis of pemphigus. The generalizability of these results is subject to certain limitations. For instance, in our study, we did not analyze the serum level of IL-15 after treatment of patients with steroid pulse or rituximab, and we intend to address it in future work. In our study, although we showed there is a positive correlation between serum IL15 levels and disease severity we did not find a direct association between IL-15 serum levels and anti-Dsg levels. It might be due to different mechanisms regarding pemphigus pathogenesis and autoantibody production. It could be speculated that IL-15 might rather be involved in tuning the immune system towards autoimmunity not to directly exert its influence on antibody production.
Taken together our findings suggest that levels of IL-15 in the sera of patients with pemphigus are high, which suggests that this cytokine may have a role in the pathogenesis of pemphigus. Whether the higher level of IL-15 is the cause or result of autoimmune diseases remains to be determined in future studies.
Understanding the role of IL-15 in PV provides a scientific basis for the development of novel therapeutic options for this autoimmune disease. Anti-cytokine therapy is an emerging treatment and considered to be a promising therapy in autoimmune diseases; while There is a need for further clarification to establish whether inhibition of IL-15 action might prove valuable in the treatment of PV.
Footnotes
Competing interests: None.
Authorship: All authors have contributed significantly to this publication.
Funding: The present study was founded by Skin Research Center, Shahid Beheshti University of Medical Sciences.
References
- 1.Schmidt E, Kasperkiewicz M, Joly P. Pemphigus. Lancet. 2019;394(10201):882–894. doi: 10.1016/S0140-6736(19)31778-7. [DOI] [PubMed] [Google Scholar]
- 2.Murrell DF, Peña S, Joly P, et al. Diagnosis and Management of Pemphigus: recommendations by an International Panel of Experts. J Am Acad Dermatol. 2018;82(3):575–585.e1. doi: 10.1016/j.jaad.2018.02.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ketabi Y, Nasiri S, Kheirodin M, Tavakolpour S, Mozafari N. The elevated level of osteopontin in patients with pemphigus vulgaris: A cytokine-like protein with a therapeutic potential. Dermatol Ther. 2019 Jul;32(4):e12973. doi: 10.1111/dth.12973. [DOI] [PubMed] [Google Scholar]
- 4.Tavakolpour S. Interleukin 21 as a new possible player in pemphigus: Is it a suitable target? Int Immunopharmacol. 2016;34:139–145. doi: 10.1016/j.intimp.2016.02.020. [DOI] [PubMed] [Google Scholar]
- 5.Tavakolpour S, Tavakolpour V. Interleukin 4 inhibition as a potential therapeutic in pemphigus. Cytokine. 2016;77:189–195. doi: 10.1016/j.cyto.2015.09.017. [DOI] [PubMed] [Google Scholar]
- 6.Tavakolpour S. Dupilumab: a revolutionary emerging drug in atopic dermatitis and its possible role in pemphigus. Dermatol Ther. 2016;29(5):299. doi: 10.1111/dth.12327. [DOI] [PubMed] [Google Scholar]
- 7.Russo R, Cozzani E, Gasparini G, Parodi A. Targeting Interleukin 4 Receptor α: a New Approach to the Treatment of Cutaneous Autoimmune Bullous Diseases? Dermatol Ther. 2020;33(1):e13190. doi: 10.1111/dth.13190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tavakolpour S, Kheiry F, Mirsafaei HS, Akhlaghdoust M. The possible role of interleukin-35 and its therapeutic potential in pemphigus. Int Immunopharmacol. 2017;42:11–17. doi: 10.1016/j.intimp.2016.11.005. [DOI] [PubMed] [Google Scholar]
- 9.Liu M, Li S, Li MO. TGF-β Control of Adaptive Immune Tolerance: A Break From Treg Cells. Bioessays. 2018;40(11):1800063. doi: 10.1002/bies.201800063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.D’auria L, Bonifati C, Cordiali-Fei P, et al. Increased serum interleukin-15 levels in bullous skin diseases: correlation with disease intensity. Arch Dermatol Res. 1999;291(6):354–356. doi: 10.1007/s004030050421. [DOI] [PubMed] [Google Scholar]
- 11.Ameglio F, D’Auria L, Cordiali-Fei P, et al. Anti-intercellular substance antibody log titres are correlated with serum concentrations of interleukin-6, interleukin-15 and tumor necrosis factor-alpha in patients with Pemphigus vulgaris relationships with peripheral blood neutrophil counts, disease severity and duration and patients’ age. J Biol Regul Homeost Agents. 1999;13(4):220–224. [PubMed] [Google Scholar]
- 12.Timoteo RP, da Silva MV, Miguel CB, et al. Th1/Th17-related cytokines and chemokines and their implications in the pathogenesis of pemphigus vulgaris. Mediators Inflamm. 2017;2017:7151285. doi: 10.1155/2017/7151285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sisto M, Lorusso L, Lisi S. Interleukin-15 as a potential new target in Sjögren’s syndrome-associated inflammation. Pathology. 2016 Oct;48(6):602–607. doi: 10.1016/j.pathol.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 14.Patidar M, Yadav N, Dalai SK. Interleukin 15: A key cytokine for immunotherapy. Cytokine Growth Factor Rev. 2016;31:49–59. doi: 10.1016/j.cytogfr.2016.06.001. [DOI] [PubMed] [Google Scholar]
- 15.Carroll H, Paunović V, Gadina M. Signalling, inflammation and arthritis: Crossed signals: the role of interleukin-15 and-18 in autoimmunity. Rheumatology (Oxford) 2008;47(9):1269–1277. doi: 10.1093/rheumatology/ken257. [DOI] [PubMed] [Google Scholar]
- 16.Pfütze M, Niedermeier A, Hertl M, Eming R. Introducing a novel Autoimmune Bullous Skin Disorder Intensity Score (ABSIS) in pemphigus. Eur J Dermatol. 2007;17(1):4–11. doi: 10.1684/ejd.2007.0090. [DOI] [PubMed] [Google Scholar]
- 17.Marçais A, Cherfils-Vicini J, Viant C, et al. The metabolic checkpoint kinase mTOR is essential for IL-15 signaling during the development and activation of NK cells. Nat Immunol. 2014;15(8):749–757. doi: 10.1038/ni.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Stern J, Keskin D, Barteneva N, Zuniga J, Yunis E, Ahmed A. Possible role of natural killer cells in pemphigus vulgaris–preliminary observations. Clin Exp Immunol. 2008;152(3):472–481. doi: 10.1111/j.1365-2249.2008.03638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Yang X-K, Xu W-D, Leng R-X, et al. Therapeutic potential of IL-15 in rheumatoid arthritis. Hum Immunol. 2015;76(11):812–818. doi: 10.1016/j.humimm.2015.09.041. [DOI] [PubMed] [Google Scholar]
- 20.Aringer M, Stummvoll G, Steiner G, et al. Serum interleukin-15 is elevated in systemic lupus erythematosus. Rheumatology (Oxford) 2001;40(8):876–881. doi: 10.1093/rheumatology/40.8.876. [DOI] [PubMed] [Google Scholar]
- 21.Pavkova Goldbergova M, Pavek N, Lipkova J, et al. Circulating cytokine pattern and factors describing rheumatoid arthritis: IL-15 as one of the biomarkers for RA? Biomarkers. 2012;17(7):655–662. doi: 10.3109/1354750X.2012.719036. [DOI] [PubMed] [Google Scholar]
- 22.Villadsen LS, Schuurman J, Beurskens F, et al. Resolution of psoriasis upon blockade of IL-15 biological activity in a xenograft mouse model. The Journal of clinical investigation. 2003;112(10):1571–1580. doi: 10.1172/JCI18986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gheisari M, Faraji Z, Dadras MS, et al. Methylprednisolone pulse therapy plus adjuvant therapy for Pemphigus Vulgaris; an analysis of ten years’ experience on 312 patients. Dermatol Ther. 2019;32(5):e13057. doi: 10.1111/dth.13057. [DOI] [PubMed] [Google Scholar]
- 24.Wang H-H, Liu C-W, Li Y-C, Huang Y-C. Efficacy of rituximab for pemphigus: a systematic review and meta-analysis of different regimens. Acta Derm Venereol. 2015;95(8):928–932. doi: 10.2340/00015555-2116. [DOI] [PubMed] [Google Scholar]
- 25.Diaz-Torné C, Ortiz de Juana MA, Geli C, et al. Rituximab-induced interleukin-15 reduction associated with clinical improvement in rheumatoid arthritis. Immunology. 2014;142(3):354–362. doi: 10.1111/imm.12212. [DOI] [PMC free article] [PubMed] [Google Scholar]


