Abstract
Objectives
Multiple sclerosis (MS) is the most common neurological disease that causes disability in the nervous system, which reduces the quality of life (QoL). Studies have shown positive effects of therapeutic exercise with supplementation on motor- and cognitive function, fatigue, and QoL in individuals with MS. The purpose of this study was to investigate the effect of home-based aerobic training (AT) and vitamin D (Vit D) supplementation on fatigue and QoL in patients with MS during the COVID-19 outbreak.
Equipment and methods
We recruited 40 females (20–40 years) with MS (EDSS: 3–5). The subjects were then randomly assigned to one of four groups: AT (n = 9; 50–75 percent, 20–40 minutes per day, three days per week), Vit D supplementation (Vit D; n = 9; 50,000 IU one day per week), AT plus Vit D supplementation (AT + Vit D; n = 10), and sedentary control (C; n = 10 placebo). The data were analyzed using paired t-test and one-way analysis of variance and Tukey's post hoc test using SPSS 26 at a significance level of P < 0.05.
Results
After eight weeks of intervention, fatigue grade markedly reduced in the AT + Vit D, AT, and Vit D groups); However, fatigue increased in the control group. QoL increased significantly in AT + Vit D, AT, and Vit D compared to C. Also, the results show that the AT + Vit D had significantly higher QoL than AT and Vit D. These findings suggest that therapeutic AT and Vit D supplementation effectively reduces fatigue and improves the QoL in female MS patients.
Keywords: Exercise, Vitamin D, Fatigue, Quality of life, MS
Résumé
Objectifs
La sclérose en plaques (SEP) est la maladie neurologique la plus courante, et elle entraîne des troubles neurologiques qui impactent négativement la qualité de vie (QdV). Des études ont montré des effets positifs de l’exercice associé à une supplémentation sur les fonctions motrices et cognitives, la fatigue et la qualité de vie chez les personnes atteintes de SEP. Le but de cette étude était d’étudier l’effet d’un entraînement aérobie réalisé à domicile (AT) et d’une supplémentation en vitamine D (Vit D) sur la fatigue et la qualité de vie de patientes atteintes de SEP pendant l’épidémie de COVID-19.
Matériel et méthodes
Nous avons recruté 40 femmes (20 à 40 ans) atteintes de SEP (EDSS: 3–5). Les sujets ont ensuite été répartis au hasard dans l’un des quatre groupes: AT (n = 9; 50–75 %, 20–40 minutes par jour, trois jours par semaine), supplémentation en vitamine D (Vit D ; n = 9 ; 50 000 UI un jour par semaine), AT plus vitamine D (AT + Vit D ; n = 10) et contrôle sédentaire (C ; n = 10 placebo). Les données ont été analysées à l’aide d’un test t pour valeurs appariesé, d’une analyse de variance à un facteur complété par un test post hoc de Tukey à l’aide de SPSS 26 avec un niveau de signification fixé à p < 0,05.
Résultats
Après huit semaines d’intervention, le degré de fatigue est nettement réduit dans les groupes AT + Vit D, AT et Vit D) ; Par contre, la fatigue a augmenté dans le groupe témoin. La qualité de vie a augmenté de manière significative dans AT + Vit D, AT et Vit D par rapport à C. De plus, les résultats montrent que l’AT + Vit D avait une qualité de vie significativement meilleure que AT et Vit D. Ces résultats suggèrent que la supplémentation thérapeutique en AT et Vit D réduit la fatigue et améliore la qualité de vie chez les femmes atteintes de SEP.
Mots clés: Exercice, Vitamine D, Fatigue, Qualité de vie, SEP
Practice points.
-
•
Vitamin D (Vit D) and aerobic tainting (AT) produced favorable changes in fatigue and quality of life.
-
•
Vit D increased the beneficial effects of AT on the fatigue and quality of life.
-
•
Combined AT + Vit D can be beneficial for the primary prevention of multiple sclerosis
1. Introduction
Multiple sclerosis (MS) is one of the most common immune-mediated chronic disorders of the central nervous system. The immune system attacks the myelin that covers nerve fibers, resulting in involvement [1] and destruction of the myelin sheath in the brain and spinal cord and eventually MS [2]. While the prevalence of MS varies significantly across the globe, it is estimated that between 2 and 2.5 million people suffer from the disease [3]. According to a Cameron study, Northern Europe had 250 MS patients per 100,000, while Japan had only 6 per 100,000 [4]. In 2011, the prevalence of MS in Canada was estimated at 290 per 100,000 [5]. In the Middle East, Iran was one of the most highly MS prevalent countries [6] with 88 cases per 100,000 [7]. In October 2016, the Iranian MS Society reported approximately 70,000 MS cases [8]. Although environmental and genetic factors increase the risk of MS, the exact etiology of the MS is unknown [9]. With a three-fold higher prevalence in women than in men, approximately 70% of patients were female, with the disease primarily affecting women between 20 and 40 [10]. The incidence of MS at young ages has several consequences on the Quality of Life (QoL) and functional capacity, including fatigue, weakness of limbs, and movement disorders that threaten the independence and ability of the individual. It also increases depression and reduces the QoL [11]. Fatigue affects 75% of MS patients, which is the most crucial factor in reducing personal independence and QoL in MS patients and the most common cause for patients not participating in physical activity programs [12]. There hasn’t been enough research on fatigue's important effects on other MS symptoms [13].
Fatigue is more than a simple symptom that can negatively impact various aspects of life (i.e., employment, socialization, and coping with illness) and daily life activities [14]. Using inefficient coping approaches causes elevated stress and disease severity in MS patients with low QoL [15]. Non-pharmacological approaches (e.g., exercise, meditation, nutrition therapy, herbal and medicinal supplements, energy therapy, and relaxation) could be used as a complementary, not alternative therapy to reduce the symptoms (fatigue, pain, spasms, etc.) and increase the QoL of MS patients [16]. Some studies have considered exercise and physical activity as important methods to help control MS symptoms and complications [17]. Due to the possible effects of physical activity on increasing body temperature, patients were advised to refrain from exercising in the past (17). Recently, studies have shown that therapeutic exercise (aerobics, stretching, and stretching exercises) with moderate intensity or other rehabilitation techniques can improve fatigue and physical function in MS patients [18], [19]. Several studies have shown the positive effects of regular aerobic exercise on reducing symptoms, including muscle spasms, fatigue, back pain, increased QoL, and improved mood [20], [21], [22], [23]. Additionally, Vitamin D (Vit D) supplementation effectively manages and treats autoimmune diseases [24]. In general, Vit D is an immune regulator with an anti-inflammatory activity that causes anti-inflammatory responses by binding to the Vit D receptor in various immune system cells [25]. Vit D deficiency can impair muscle function and lead to sarcopenia and, consequently, decreased muscle strength [26]. Some studies suggest that Vit D deficiency is related to reduced finger muscle strength and function in adult girls [27]. Convincing evidence has suggested that Vit D might be associated with normal skeletal muscle development and optimizing muscle strength and performance [28]. Epidemiological studies, genetics, and animal models have shown the possibility of the nerve regeneration process following Vit D supplementation [29].
The COVID-19 outbreak has also increased concerns and reduced QoL, especially in MS patients. In addition, due to quarantine and the fear of contracting COVID-19, MS patients’ engagement in sports clubs and physical activities has decreased. Given the potential effect of Aerobic Training (AT) and the effect mentioned above of Vit D, the question arises as to whether combined Vit D + AT effectively improves fatigue and QoL of MS patients during the COVID-19 outbreak?
2. Materials and methods
2.1. Participants and study design
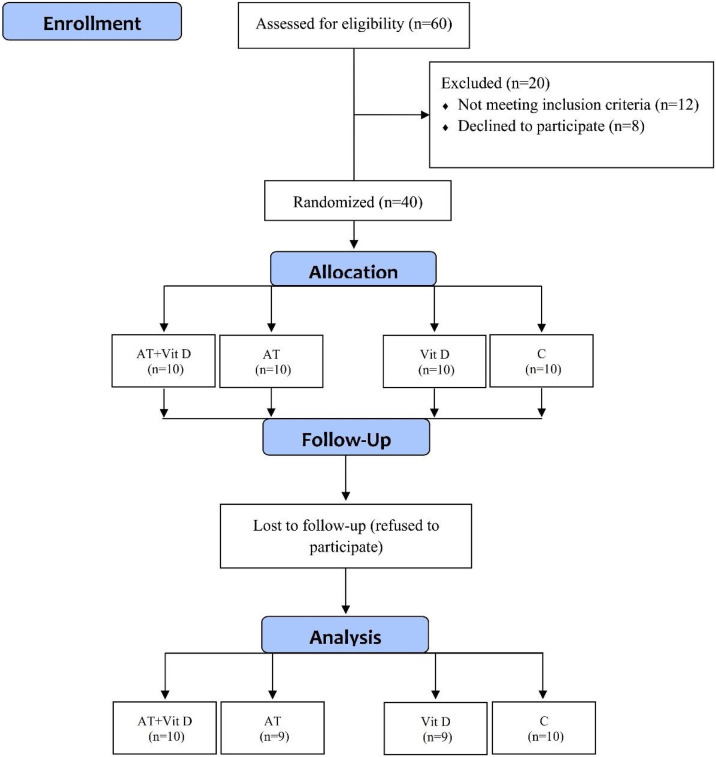
This clinical trial study investigated the effects of 8-week home-based AT and Vit D supplementation fatigue and QoL in patients with MS. As detailed in Fig. 1 , the study population consisted of 60 participants (aged 20–40 years) from the Kermanshah MS center with a disability scale of 3–5.
Figure 1.
Flow chart of the study population. AT + Vit D: aerobic training + vitamin D supplement; AT: aerobic training group; Vit D: vitamin D supplement; C: the control group.
In this study, 40 women with MS (20–40 years) in Kermanshah were selected using purposive and available sampling method based on G. POWER 3.1 software with a statistical power of 99%, the effect size of 95%, and significance level at 0.05.
Inclusion criteria included: at least two years MS history, disability scale of 3-5, lack of regular exercise, no history of other diseases, no smoking, and no immunosuppressive drug consumption. Exclusion criteria included a history of heart disease, hypertension, orthopedic disorders, and diabetes, consuming other drugs in addition to MS medications that might affect the individual's response to the intervention. Also, other criteria were lack of regular exercise training and testing sessions, muscle injuries, inability to perform the exercise, COVID-19 infection, and severe relapses during the study period.
The Ethics Committee approved the trial (IR.RAZI.REC.1400.002) of the Kermanshah Razi University. Written informed consent was obtained from all participants, including agreement of the patients to participate as volunteers and feasibility to leave the study. Forty subjects were randomly assigned into four equal (n = 10) groups; AT + Vit D, AT, Vit D, and control (C) using the lottery method.
2.2. Intervention
AT experimental groups exercised at home under online supervision three times per week for two months. All training sessions were carried out under the supervision of exercise physiologists.
2.3. AT
Walking aerobics exercise was performed at home under online supervision. The subjects were instructed to do waking aerobics three times a week, starting with 20 min at 50% of HRmax per session, and to increase their total weekly walking time up to 40 min at 70% of HRmax per session [30] which was recommended by the MS Association of America (MSAA) [31] (Table 1 ). Every session included 10 min of warm-up and 10 min of cooling down period. The walking program was designed to be of moderate intensity.
Table 1.
Aerobic training protocol.
| Variables | Week |
|||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | |
| Intensity (HRmax) | 50–55% | 50–55% | 55–60% | 55–60% | 60–65% | 60–65% | 65–70% | 65–70% |
| Time (min) | 20 | 25 | 25 | 30 | 30 | 35 | 35 | 40 |
| Borg scale | 10 | 10 | 10 | 11 | 11 | 11 | 12 | 12 |
At the outset, the HRmax formula was used to determine the target heart rate [HRmax = 220− age] [32]. The participants learned to count pulse rate and to monitor heart rate in an instructional session using the pulse palpation method. To assure that the desired heart rate (exercise intensity) was achieved and maintained during the walking aerobics phase, the 6–20 rating of perceived exertion (RPE) scale was considered [33] (Table 1). This walking program was continued for eight weeks.
2.4. Diet and vit D supplementation
To attend the training program, the participants filled two questionnaires. First, a questionnaire was used to assess participants’ readiness, including demographic data, health status, and physical activity. A second questionnaire was a detailed semi-quantitative food frequency questionnaire customized to the Iranian population and composed common food items, serving sizes, and meals, as designed to record and analyze 3-day food recalls before and at the end of the intervention. The food processor nutritionist four software (FPN4) was used to determine the food intake and macronutrient consumption (protein, fat, and carbohydrates). A day before collecting blood samples in the pre and post-test, subjects were asked to consume the same food and macronutrient composition. In general, the subjects’ diet consisted of 55% carbohydrates, 30% fat, and 15% protein.
In this study, both the AT + Vit D group and Vit D group received 50,000 units of Vit D supplement (made by the Zahravi Pharmaceutical Company in Iran) once per week for eight weeks [24], [34]. The C and AT groups received a placebo weekly (made by the Zahravi Pharmaceutical Company, Iran) with the same shape, color, smell, and tastes as a Vit D supplement for eight weeks.
2.5. Anthropometric measurements and body composition
Subjects were familiarized with the study process three days before the start of the intervention and at the end of the study, and primary measurements such as anthropometric parameters and body composition were recorded. Height was measured to the nearest 0.5 cm using a stadiometer (DETECTO, Model 3PHTROD-WM, USA), and Waist circumference was measured to the nearest 0.5 cm with a non-elastic tape measure. Also, the fat mass of the whole body, BMI, and weight of any patient was determined using INBODY test using bioelectric impedance analysis (Zeus 9.9 PLUS: Jawon Medical Co., Ltd., Kungsang Bukdo, South Korea). The body composition measurements were recorded at the beginning and the end of the study early in the morning between 8–9 am after at least 12 hours of fasting overnight and after emptying the bladder to minimize the effect of water consumption on the results. Subjects were asked not to participate in intensive physical activity 48 hours before the test and refrain from taking diuretic drugs and diuretics.
2.6. Fatigue and QoL
Fatigue was assessed using the validated Persian version Modified Fatigue Impact Scale (MFIS) [35], recommended by the MS Council for Clinical Practice Guidelines. The MFIS is a multi-dimensional scale that was derived from the initial 40-item fatigue impact scale. It is intended to investigate the various aspects of fatigue by evaluating its impact on physical, cognitive, and psychosocial functions. The overall score of the MFIS is composed of nine items for physical status (pMFIS), ten items for cognitive level (cMFIS), and two items for psychosocial function status (psMFIS). The QoL in the studied cohort was assessed using the MSQOL-54 (MSQOL-54) questionnaire, a disease-specific inventory that was previously validated in an Iranian population [36]. This scale has the advantage of assaying both healthy individuals and MS patients’ QoL. The MSQOL-54 has a high test-retest reliability and internal consistency, and evidence of content and construct validity. It contains a 36-item generic module derived from the SF-36 questionnaire and an 18-item module related to the specific clinical condition. The questions were administered on the first and last days of the interventional program, respectively. All the performed scales were translated and validated from the source language into Persian [35], [36]. The questionnaires were completed in the same order starting with MSIF followed by MSQOL-54. Each subscale and composite score ranged from 0 to 100, with a higher score indicating better QoL. Composite scores were extracted by weighted sums of subscale scores.
2.7. Statistical analysis
All statistical analyses were performed using the SPSS statistical software (version 26; SPSS Inc., Chicago, IL, USA) was used at a significant level of P< 0.05. The Shapiro–Wilk's test was used for evaluating the normality of distribution. ANOVA and t-test were used to compare the mean hepatic risk factors between and within groups, respectively. Tukey's post hoc test was used if significant differences were found.
3. Results
The mean age, height, bodyweight, and serum 25-OH-Vit D of patients are presented in Table 2 . The findings on the scores of MFIS and their between-group comparison are presented in Table 2. Based on the results of the t-test, there was a significant difference in the mean of serum 25-OH-Vit D in the post-test compared to the pre-test. Meanwhile, serum 25-OH-Vit D increased significantly in all intervention groups after eight weeks, except for C. After the intervention, AT + Vit D, AT, and Vit D increased serum 25-OH-Vit D significantly compared to the C. The results indicate a significant difference in serum 25-OH-Vit D between AT + Vit D with AT, while no significant difference was found between AT + Vit D and AT with Vit D (Table 2). According to the results of the t-test, there were significant differences in the mean of scores of MFIS in the post-test compared to the pre-test. After eight weeks, scores of MFIS significantly decreased in all intervention groups, while in the C, these variables increased significantly (Table 2). The results of one-way ANOVA showed a significant difference in scores of MFIS between the groups. Tukey's post hoc test results show that scores of MFIS were significant in AT + Vit D, AT, and Vit D compared to C. Also, a significant difference in the scores of MFIS was observed between AT + Vit D and AT compared to Vit D. The results indicated no significant difference in the scores of MFIS in AT + Vit D compared to AT alone (Table 2).
Table 2.
Mean ± SD anthropometric indices, serum 25-OH-vitamin D, and scores of MFIS after and before the intervention among the groups.
| Variables | AT + Vit D (n = 10) | AT (n = 9) | Vit D (n = 9) | C (n = 10) | P-valuea |
|---|---|---|---|---|---|
| Age (years) | 27.70 ± 2.68 | 26.77 ± 2.27 | 25.44 ± 2.29 | 28.11 ± 3.62 | 0.563 |
| Height (cm) | 163.40 ± 2.63 | 166.03 ± 2.65 | 162.33 ± 1.50 | 164.20 ± 1.54 | 0.467 |
| Bodyweight (Kg) | 71.90 ± 1.91 | 73.12 ± 1.93 | 71.11 ± 2.02 | 73.01 ± 1.33 | 0.086 |
| Serum 25-OH-Vit D (ng/mL) | |||||
| Before | 25.80 ± 1.81 | 26.55 ± 1.50 | 26.44 ±1.42 | 27.20 ± 3.45 | |
| After | 35.60 ± 1.89 | 29.66 ± 1.65 | 32.88 ± 1.90 | 25.10 ± 1.44 | |
| P† | 0.001* | 0.002* | 0.001* | 0.111 | |
| Δ | 9.8 ± 0.08 μβ | 3.11 ± 0.15β | 6.44 ± 0.48β | −2.1 ± 2.01 | 0.001¥ |
| Physical domain score | |||||
| Before | 17.23 ± 2.03 | 17.33 ± 1.78 | 17.11 ± 2.43 | 17.01 ± 3.23 | |
| After | 15.62 ± 3.12 | 15.96 ± 2.24 | 16.68 ± 1.19 | 17.48 ± 1.04 | |
| P† | 0.001* | 0.001* | 0.039* | 0.059 | |
| Δ | −1.61 ± 1.09€β | −1.37 ± 0.46€β | −0.43 ± 1.24β | 0.47 ± 2.19 | 0.001¥ |
| Cognitive domain score | |||||
| Before | 13.13 ± 1.93 | 13.21 ± 2.03 | 12.98 ± 1.06 | 12.90 ± 0.66 | |
| After | 10.27 ± 2.11 | 10.67 ± 1.28 | 11.97 ± 0.87 | 13.49 ± 1.33 | |
| P† | 0.001* | 0.002* | 0.011* | 0.042* | |
| Δ | −2.86 ± 0.18€β | −2.54 ± 0.75€β | −1.01 ± 0.19β | 0.59 ± 0.67 | 0.001¥ |
| Psychosocial domain score | |||||
| Before | 5.21 ± 0.41 | 5.42 ± 0.24 | 5.11 ± 0.15 | 5.16 ± 0.38 | |
| After | 3.80 ± 0.23 | 3.98 ± 0.83 | 4.66 ± 0.31 | 5.49 ± 0.24 | |
| P† | 0.001* | 0.013* | 0.034* | 0.046* | |
| Δ | −1.41 ± 0.18€β | −1.44 ± 0.59€β | −0.45 ± 0.16β | 0.33 ± 0.14 | 0.001¥ |
| Total MFIS score | |||||
| Before | 35.57 ± 4.20 | 35.96 ± 4.05 | 35.20 ±3.64 | 35.07 ± 4.27 | |
| After | 29.69 ± 5.46 | 30.61 ± 4.35 | 33.31 ± 2.37 | 36.46 ± 2.61 | |
| P† | 0.001* | 0.001* | 0.021* | 0.048* | |
| Δ | −8.88 ± 1.26€β | −5.35 ± 0.3€β | −1.89 ± 1.09β | 1.39 ± 1.66 | 0.001¥ |
AT + Vit D: aerobic training + vitamin D supplement; AT: aerobic training group; Vit D: vitamin D supplement group; C: the control group. *Data analysis was done by the analysis of one-way analysis of variance test followed by post hoc Tukey's test. P†: Statistical analysis was done by paired sample t-test. *: Significantly different in comparison pre- and post within the groups. ¥: Significantly different comparing Δ between groups. μ: Significantly different comparing with AT. €: Significantly different comparing with Vit D. β: Significantly different comparing with C; a: Significant differences between groups.
The findings on the QoL subscales and their between-group comparison are presented in Table 3 . Based on the results of the t-test, there were significant differences in the mean of QoL subscales in the post-test compared to the pre-test. After eight weeks, scores of QoL subscales significantly increased in all intervention groups, while in the C, these variables decreased significantly (Table 3). The results of one-way ANOVA showed a significant difference in scores of QoL subscales between the groups. The results of Tukey's post hoc test show that scores of QoL subscales were significant in AT + Vit D, AT, and Vit D compared to C. Also, when AT + Vit D was compared to AT and Vit D, significant differences in physical health, role limitations due to physical problems, role limitations due to emotional issues, pain, emotional well-being, social function, cognitive function, sexual function, satisfaction with sexual function, and Mental health composite were observed. The results indicate a significant difference in all scores of QoL subscales in AT + Vit D and AT compared to Vit D lone (Table 3).
Table 3.
Mean ± SD of the scores of multiple sclerosis QoL subscales after and before the intervention among the groups.
| Variables | AT + Vit D | AT | Vit D | C | P-valuea |
|---|---|---|---|---|---|
| Physical health | |||||
| Before | 39.13 ± 1.43 | 39.32 ± 2.23 | 38.90 ± 2.10 | 38.85 ± 2.32 | |
| After | 49.04 ± 2.17 | 46.14 ± 1.35 | 41.32 ± 1.54 | 37.06 ± 1.60 | |
| P† | 0.001* | 0.001* | 0.031* | 0.087 | |
| Δ | 9.91 ± 0.74 μ€β | 6.82 ± 0.88€β | 2.42 ± 0.56β | −1.79 ± 0.72€β | 0.001¥ |
| Role limitations due to physical problems | |||||
| Before | 31.67 ± 2.15 | 32.13 ± 2.11 | 31.95 ± 3.01 | 32.32 ± 1.73 | |
| After | 43.21 ± 3.17 | 40.18 ± 1.93 | 36.14 ± 1.19β | 31.73 ± 1.26 | |
| P† | 0.001* | 0.023* | 0.039* | 0.214 | |
| Δ | 11.54 ± 1.02 μ€β | 8.05 ± 0.18€β | 4.19 ± 1.82β | −0.59 ± 0.74 | 0.001¥ |
| Role limitations due to emotional problems | |||||
| Before | 35.23 ± 1.12 | 36.22 ± 2.41 | 35.97 ± 3.07 | 36.04 ± 1.57 | |
| After | 53.36 ± 1.45 | 48.65 ± 2.36 | 39.55 ± 1.24 | 35.21 ± 1.42 | |
| P† | 0.001* | 0.001* | 0.034* | 0.471 | |
| Δ | 18.13 ± 0.33 μ€β | 12.43 ± 0.05€β | 3.58 ± 1.83β | −0.83 ± 0.15 | 0.001¥ |
| Pain | |||||
| Before | 65.42 ± 1.26 | 66.03 ± 1.31 | 65.76 ± 2.09 | 66.20 ± 2.13 | |
| After | 74.36 ± 2.01 | 69.37 ± 2.19 | 67.25 ± 1.26 | 65.18 ± 1.39 | |
| P† | 0.001* | 0.035* | 0.049* | 0.327 | |
| Δ | 8.94 ± 0.75 μ€β | 3.34 ± 0.88€β | 1.49 ± 0.83β | −1.02 ± 0.74 | 0.001¥ |
| Emotional well being | |||||
| Before | 41.54 ± 1.19 | 41.12 ± 2.25 | 40.95 ± 2.16 | 42.33 ± 1.70 | |
| After | 49.18 ± 2.11 | 46.13 ± 2.48 | 43.14 ± 1.44 | 42.04 ± 0.98 | |
| P† | 0.001* | 0.017* | 0.028* | 0.514 | |
| Δ | 7.64 ± 0.92 μ€β | 5.01 ± 0.23€β | 2.19 ± 0.72β | −0.29 ± 0.72 | 0.001¥ |
| Energy | |||||
| Before | 30.56 ± 1.42 | 31.26 ± 2.65 | 30.88 ± 2.15 | 31.84 ± 1.38 | |
| After | 38.06 ± 2.24 | 36.77 ± 1.83 | 33.27 ± 1.46 | 30.09 ± 0.78 | |
| P† | 0.001* | 0.001* | 0.037* | 0.491 | |
| Δ | 7.5 ± 0.82€β | 5.51 ± 0.82€β | 2.39 ± 0.69β | 1.75 ± 0.6 | 0.001¥ |
| Health perception | |||||
| Before | 42.56 ± 2.08 | 43.13 ± 1.76 | 43.34 ± 1.96 | 42.33 ± 2.11 | |
| After | 51.12 ± 1.67 | 50.16 ± 1.78 | 47.10 ± 1.21 | 42.08 ± 1.04 | |
| P† | 0.001* | 0.001* | 0.021* | 0.647 | |
| Δ | 8.56 ± 0.41€β | 7.03 ± 0.02€β | 3.76 ± 0.75β | −0.25 ± 1.07 | 0.001¥ |
| Social function | |||||
| Before | 60.44 ± 1.23 | 61.11 ± 2.76 | 61.32 ± 3.04 | 60.88 ± 2.13 | |
| After | 67.20 ± 1.46 | 65.27 ± 1.29 | 63.15 ± 1.36 | 60.55 ± 1.39 | |
| P† | 0.001* | 0.015* | 0.029* | 0.723 | |
| Δ | 6.76 ± 0.23 μ€β | 4.16 ± 1.47€β | 1.83 ± 1.68β | −0.33 ± 0.74 | 0.001¥ |
| Cognitive function | |||||
| Before | 65.23 ± 1.21 | 66.17 ± 1.73 | 65.22 ± 2.06 | 66.27 ± 2.16 | |
| After | 72.20 ± 1.46 | 70.04 ± 1.33 | 68.06 ± 1.05 | 66.02 ± 1.44 | |
| P† | 0.001* | 0.019* | 0.034* | 0.650 | |
| Δ | 6.97 ± 0.25 μ€β | 4.23 ± 0.4€β | 2.84 ± 1.01β | 0.25 ± 0.72 | 0.001¥ |
| Health distress | |||||
| Before | 70.32 ± 1.56 | 71.13 ± 1.21 | 70.66 ± 3.19 | 70.30 ± 2.14 | |
| After | 77.36 ± 2.01 | 76.27 ± 1.29 | 73.45 ± 2.16 | 70.21 ± 2.09 | |
| P† | 0.001* | 0.035* | 0.049* | 0.421 | |
| Δ | 7.04 ± 0.45€β | 5.14 ± 0.08€β | 2.79 ± 1.03β | −0.09 ± 0.05 | 0.001¥ |
| Sexual function | |||||
| Before | 68.22 ± 2.31 | 68.76 ± 1.82 | 69.11 ± 2.34 | 69.30 ± 2.24 | |
| After | 76.36 ± 2.01 | 73.27 ± 1.07 | 71.35 ± 1.34 | 69.21 ± 2.19 | |
| P† | 0.001* | 0.021* | 0.044* | 0.627 | |
| Δ | 8.14 ± 0.3 μ€β | 4.51 ± 0.75€β | 2.24 ± 1β | 0.09 ± 0.05 | 0.001¥ |
| Change in health | |||||
| Before | 43.15 ± 1.38 | 42.67 ± 2.76 | 43.04 ± 1.96 | 42.73 ± 2.33 | |
| After | 47.12 ± 1.57 | 46.30 ± 1.34 | 45.10 ± 0.92β | 42.38 ± 1.24 | |
| P† | 0.001* | 0.027* | 0.043* | 0.501 | |
| Δ | 3.97 ± 0.19€β | 3.63 ± 1.42€β | 2.06 ± 1.04β | −0.35 ± 1.09 | 0.001¥ |
| Satisfaction with sexual function | |||||
| Before | 55.34 ± 2.27 | 56.16 ± 1.64 | 55.77 ± 1.40 | 56.33 ± 2.21 | |
| After | 63.20 ± 1.33 | 60.22 ± 2.30 | 59.14 ± 1.51 | 55.80 ± 1.43 | |
| P† | 0.001* | 0.017* | 0.036* | 0.320 | |
| Δ | 7.86 ± 0.94 μ€β | 4.06 ± 0.66€β | 3.37 ± 0.11β | −0.53 ± 0.18 | 0.001¥ |
| Overall QoL | |||||
| Before | 54.74 ± 1.47 | 55.19 ± 1.54 | 55.47 ± 0.88 | 54.68 ± 1.20 | |
| After | 59.20 ± 1.33 | 58.27 ± 2.36 | 56.13 ± 1.47 | 54.19 ± 1.59 | |
| P† | 0.001* | 0.001* | 0.039* | 0.670 | |
| Δ | 4.46 ± 0.14€β | 3.08 ± 0.82€β | 0.66 ± 0.59β | −0.19 ± 0.39 | 0.001¥ |
| Physical health composite | |||||
| Before | 48.15 ± 2.08 | 48.77 ± 2.76 | 47.14 ± 2.06 | 48.59 ± 1.50 | |
| After | 57.11 ± 1.36 | 56.32 ± 2.31 | 53.16 ± 1.58 | 48.21 ± 2.23 | |
| P† | 0.001* | 0.001* | 0.022* | 0.709 | |
| Δ | 8.96 ± 0.72 €β | 7.55 ± 0.45€β | 6.02 ± 0.48β | −0.38 ± 0.73 | 0.001¥ |
| Mental health composite | |||||
| Before | 53.24 ± 1.30 | 54.17 ± 1.66 | 53.37 ± 1.43 | 54.33 ± 2.48 | |
| After | 62.21 ± 1.40 | 59.73 ± 2.30 | 57.12 ± 1.49 | 53.80 ± 1.43 | |
| P† | 0.001* | 0.027* | 0.034* | 0.420 | |
| Δ | 8.97 ± 0.1 μ€β | 5.56 ± 0.64€β | 3.75 ± 0.06β | −0.53 ± 1.05 | 0.001¥ |
AT + Vit D: aerobic training + vitamin D supplement; AT: aerobic training group; Vit D: vitamin D supplement group; C: the control group. *Data analysis was done by the analysis of one-way analysis of variance test followed by post hoc Tukey's test; P†: Statistical analysis was done by paired sample t-test. *: Significantly different in comparison pre- and post within the groups. ¥: Significantly different comparing Δ between groups. μ: Significantly different comparing with AT. €: Significantly different comparing with Vit D. β: Significantly different comparing with C.
4. Discussion
To the best of our knowledge, this study is the first, single-blind randomized controlled trial specifically designed to assess the effectiveness of combined AT and Vit D supplementation on MS-related fatigue and QoL in patients with MS. The eight weeks of AT and Vit D supplementation significantly improved fatigue, increased QoL, and Vit D levels in favor of combined AT and Vit D. Consistent with the results of the present study, Beckman et al. (2020) reported that increasing Vit D levels reduces fatigue and increases the QoL in MS patients [37], Rezaei et al. (2016), also reported the effectiveness of combined core stability exercise training and Vit D compared with exercise or Vit D supplementation alone [38]. Several mechanisms have been proposed to explain the possible beneficial effects of AT. Increased metabolism during and after AT has been reported as a primary mechanism for improved fatigue in MS patients. Accordingly, AT-induced increased metabolism increases blood flow (oxygen and nutrients delivery), reduces muscle weakness, and ultimately improves the nervous system function [39]. AT enhances muscle oxidation capacity and activates the aerobic biochemical system to generate adaptive responses by increasing oxygen delivery to active skeletal muscles [40]. In general, AT stimulates structural and functional changes in muscles and increases capillary availability, myoglobin storage, mitochondrial function, and oxidative enzymes by the consecutive recruitment of muscle fibers. Therefore, AT in MS patients with more blood supply and muscle efficiency might reduce fatigue [40]. Studies have suggested that AT could improve fatigue probably by managing the immune system, altering inflammatory and anti-inflammatory cytokines, and normalizing the hypothalamic-pituitary-adrenal axis imbalances [41], [42]. AT may also improve QoL by lowering epinephrine, norepinephrine, cholesterol, and triglyceride levels, as well as the immune system, endorphin secretion, and mood [43]. Also, according to the literature, the production of free radicals (e.g., nitric oxide) in MS patients is probably increased due to disruption of the nicotinamide adenine dinucleotide phosphate (NADP + ) system, which intensifies inflammatory responses and, consequently, exacerbates brain damage. Therefore, Vit D might prevent the progression of MS by inhibiting nitric oxide production [43], reducing myostatin expression (a negative regulator of skeletal muscle growth), and increasing myogenic cell differentiation. As a result, studies have shown that Vit D metabolites alter muscle metabolism by boosting protein synthesis, increasing the ratio of type 2 muscle fibers, and improving muscle performance, potentially due to the existence of Vit D receptors in skeletal muscle [28]. Overall, our findings support the hypothesis that Vit D supplementation reduces fatigue and QoL in individuals with Vit D deficiency. This effect could be increased when combined with AT. The limitations of this study include being cross-sectional, the small sample size, and the exposure of the subjects to sunlight.
5. Limitations
The limitations of our study were as follows: First, this was a short-term trial, and it is unknown if longer durations of supplementation could cause further improvements. Second, the impact of different dose levels was not investigated in the current research. Third, the low number of subjects prevents the generalization of the results.
6. Conclusion
Eight weeks of AT and Vit D supplementation in MS patients resulted in a clinically meaningful improved fatigue, enhanced QoL, and increased Vit D levels compared with each AT or Vit D alone and control. Therefore, lifestyle changes can effectively improve the condition of patients with MS. However, due to some contradictory reports, further research using a larger sample size is needed.
Ethical consideration
This study was approved by the ethics committee of Razi University of Kermanshah (IR.RAZI.REC.1400.002) and registered in the Iranian Clinical Trial Registration Center under the code IRCT20201129049525N1.
Funding/Support
The authors declared that the research did not receive any financial grants.
Disclosure of interest
The authors declare that they have no competing interest.
Acknowledgments
This article was a part of MSc thesis at the Department of Exercise Physiology, Faculty of Sport Sciences, Razi University, Kermanshah, Iran. The authors would like to thank the subjects for their willing participation in this study.
References
- 1.Wendebourg M.J., Heesen C., Finlayson M., Meyer B., Pöttgen J., Köpke S. Patient education for people with multiple sclerosis-associated fatigue: a systematic review. PLoS One. 2017;12:e0173025. doi: 10.1371/journal.pone.0173025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Dobryakova E., Genova H.M., DeLuca J., Wylie G.R. The dopamine imbalance hypothesis of fatigue in multiple sclerosis and other neurological disorders. Front Neurol. 2015;6:52. doi: 10.3389/fneur.2015.00052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Mazumder R., Murchison C., Bourdette D., Cameron M. Falls in people with multiple sclerosis compared with falls in healthy controls. PLoS One. 2014;9:e107620. doi: 10.1371/journal.pone.0107620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cameron M.H., Peterson V., Boudreau E.A., Downs A., Lovera J., Kim E., et al. Fatigue is associated with poor sleep in people with multiple sclerosis and cognitive impairment. Mult Scler Int. 2014;2014:1–5. doi: 10.1155/2014/872732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Urits I., Adamian L., Fiocchi J., Hoyt D., Ernst C., Kaye A.D., et al. Advances in the understanding and management of chronic pain in multiple sclerosis: a comprehensive review. Curr Pain Headache Rep. 2019;23:1–11. doi: 10.3390/ph14080831. [DOI] [PubMed] [Google Scholar]
- 6.Heydarpour P., Khoshkish S., Abtahi S., Moradi-Lakeh M., Sahraian M.A. Multiple sclerosis epidemiology in Middle East and North Africa: a systematic review and meta-analysis. Neuroepidemiology. 2015;44:232–244. doi: 10.1159/000431042. [DOI] [PubMed] [Google Scholar]
- 7.Eskandarieh S., Heydarpour P., Elhami S.-R., Sahraian M.A. Prevalence and incidence of multiple sclerosis in Tehran, Iran. Iran J Public Health. 2017;46:699. [PMC free article] [PubMed] [Google Scholar]
- 8.Mousavizadeh A., Dastoorpoor M., Naimi E., Dohrabpour K. Time-trend analysis and developing a forecasting model for the prevalence of multiple sclerosis in Kohgiluyeh and Boyer-Ahmad Province, southwest of Iran. Public Health. 2018;154:14–23. doi: 10.1016/j.puhe.2017.10.003. [DOI] [PubMed] [Google Scholar]
- 9.Sorensen P.S. Multiple sclerosis: pathophysiology revisited. Lancet Neurol. 2005;4:9–10. doi: 10.1016/S1474-4422(04)00948-2. [DOI] [PubMed] [Google Scholar]
- 10.Elhami S.-R., Mohammad K., Sahraian M.A., Eftekhar H. A 20-year incidence trend (1989–2008) and point prevalence (March 20, 2009) of multiple sclerosis in Tehran, Iran: a population-based study. Neuroepidemiology. 2011;36:141–147. doi: 10.1159/000324708. [DOI] [PubMed] [Google Scholar]
- 11.Carroll C.C., Gallagher P.M., Seidle M.E., Trappe S.W. Skeletal muscle characteristics of people with multiple sclerosis. Arch Phys Med Rehabil. 2005;86:224–229. doi: 10.1016/j.apmr.2004.03.035. [DOI] [PubMed] [Google Scholar]
- 12.Morrow S.A., Rosehart H., Johnson A.M. The effect of Fampridine-SR on cognitive fatigue in a randomized double-blind crossover trial in patients with MS. Mult Scler Relat Disord. 2017;11:4–9. doi: 10.1016/j.msard.2016.10.011. [DOI] [PubMed] [Google Scholar]
- 13.Hasanpour-Dehkordi A., Jivad N., Solati K. Effects of yoga on physiological indices, anxiety and social functioning in multiple sclerosis patients: a randomized trial. J Clin Diagn Res. 2016;10:VC01. doi: 10.7860/JCDR/2016/18204.7916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ghazagh M., Zadhasan Z. The effect of group mindfulness-based stress reduction program on the quality of life and fatigue in patients with multiple sclerosis. Avicenna J Nurs Midwifery Care. 2019;27:35–44. http://nmj.umsha.ac.ir/article-1-1877- [Google Scholar]
- 15.Salehpoor G., Rezaei S., Hosseininezhad M. Quality of life in multiple sclerosis (MS) and role of fatigue, depression, anxiety, and stress: a bicenter study from north of Iran. Iran J Nurs Midwifery Res. 2014;19:593. [PMC free article] [PubMed] [Google Scholar]
- 16.Mostert S., Kesselring J. Effects of a short-term exercise training program on aerobic fitness, fatigue, health perception and activity level of subjects with multiple sclerosis. Mult Scler J. 2002;8:161–168. doi: 10.1191/1352458502ms779oa. [DOI] [PubMed] [Google Scholar]
- 17.Hermann B.P., Vickrey B., Hays R.D., Cramer J., Devinsky O., Meador K., et al. A comparison of health-related quality of life in patients with epilepsy, diabetes and multiple sclerosis. Epilepsy Res. 1996;25:113–118. doi: 10.1016/0920-1211(96)00024-1. [DOI] [PubMed] [Google Scholar]
- 18.Motl R.W., McAuley E., Snook E.M. Physical activity and quality of life in multiple sclerosis: possible roles of social support, self-efficacy, and functional limitations. Rehabil Psychol. 2007;52:143. doi: 10.1037/0090-5550.52.2.143. [DOI] [Google Scholar]
- 19.Motl R.W., McAuley E., Snook E.M., Gliottoni R.C. Physical activity and quality of life in multiple sclerosis: intermediary roles of disability, fatigue, mood, pain, self-efficacy and social support. Psychol Health Med. 2009;14:111–124. doi: 10.1080/13548500802241902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Soleimany M., Ziba F.N., Kermani A., Hosseini F. Comparison of sleep quality in two groups of nurses with and without rotation work shift hours. Iran J Nurs. 2007;20:29–38. http://ijn.iums.ac.ir/article-1-266-en.html [Google Scholar]
- 21.Petajan J.H., Gappmaier E., White A.T., Spencer M.K., Mino L., Hicks R.W. Impact of aerobic training on fitness and quality of life in multiple sclerosis. Ann Neurol. 1996;39:432–441. doi: 10.1002/ana.410390405. [DOI] [PubMed] [Google Scholar]
- 22.Ardakani M.D. Effect of aerobic exercise program on quality of life in male patients with multiple sclerosis. JSSU. 2020;28:2971–2981. doi: 10.18502/ssu.v28i8.4454. [DOI] [Google Scholar]
- 23.Stuifbergen A.K., Blozis S.A., Harrison T.C., Becker H.A. Exercise, functional limitations, and quality of life: a longitudinal study of persons with multiple sclerosis. Arch Phys Med Rehabil. 2006;87:935–943. doi: 10.1016/j.apmr.2006.04.003. [DOI] [PubMed] [Google Scholar]
- 24.VanAmerongen B., Dijkstra C., Lips P., Polman C. Multiple sclerosis and vitamin D: an update. Eur J Clin Nutr. 2004;58:1095–1109. doi: 10.1038/sj.ejcn.1601952. [DOI] [PubMed] [Google Scholar]
- 25.Verstuyf A., Carmeliet G., Bouillon R., Mathieu C. Vitamin D: a pleiotropic hormone. Kidney Int. 2010;78:140–145. doi: 10.1038/ki.2010.17. [DOI] [PubMed] [Google Scholar]
- 26.Solomon A., Bouloux P. Modifying muscle mass – the endocrine perspective. J Endocrinol. 2006;191:349–360. doi: 10.1677/joe.1.06837. [DOI] [PubMed] [Google Scholar]
- 27.Ward K.A., Das G., Berry J.L., Roberts S.A., Rawer R., Adams J.E., et al. Vitamin D status and muscle function in post-menarchal adolescent girls. J Clin Endocrinol Metab. 2009;94:559–563. doi: 10.1210/jc.2008-1284. [DOI] [PubMed] [Google Scholar]
- 28.Houston D.K., Tooze J.A., Hausman D.B., Johnson M.A., Nicklas B.J., Miller M.E., et al. Change in 25-hydroxyvitamin D and physical performance in older adults. J Gerontol A Biol Sci Med Sci. 2011;66:430–436. doi: 10.1093/gerona/glq235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mosayebi G., Ghazavi A., Payani M. 2006. The effect of vitamin D3 on the inhibition of experimental autoimmune encephalomyelitis in C57BL/6 mice.http://rjms.iums.ac.ir/article-1-629- [DOI] [PubMed] [Google Scholar]
- 30.Habibi A., Majdinasab N., Ghalvand A. The effects of aerobic exercise on lipid profile and body composition in women with multiple sclerosis. Jundishapur J Chronic Dis Care. 2015;4:1–6. doi: 10.5812/jjcdc.26619. [DOI] [Google Scholar]
- 31.Rae-Grant A., Day G.S., Marrie R.A., Rabinstein A., Cree B.A., Gronseth G.S., et al. Practice guideline recommendations summary: Disease-modifying therapies for adults with multiple sclerosis: Report of the Guideline Development, Dissemination, and Implementation Subcommittee of the American Academy of Neurology. Neurology. 2018;90:777–788. doi: 10.1212/WNL.0000000000005347. [DOI] [PubMed] [Google Scholar]
- 32.Branco B.H.M., de Oliveira Mendes F., Ladeia G.F., Bertolini S.M.M.G., Badilla P.V., Andreato L.V. Maximum heart rate predicted by formulas versus values obtained in graded exercise tests in Brazilian jiu-jitsu athletes. Sport Sci Health. 2020;16:39–45. doi: 10.1007/s11332-019-00570-0. [DOI] [Google Scholar]
- 33.Kalb R., Brown T.R., Coote S., Costello K., Dalgas U., Garmon E., et al. Exercise and lifestyle physical activity recommendations for people with multiple sclerosis throughout the disease course. Mult Scler J. 2020;26:1459–1469. doi: 10.1177/1352458520915629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tuyserkani H., Rezaei M. Role of vitamins D and A in immune system of multiple sclerosis patients: an updated mini-review. J Rev Med Sci. 2020;1:45–48. [Google Scholar]
- 35.Ghajarzadeh M., Jalilian R., Eskandari G., Ali Sahraian M., Reza Azimi A. Validity and reliability of Persian version of Modified Fatigue Impact Scale (MFIS) questionnaire in Iranian patients with multiple sclerosis. Disabil Rehabil. 2013;35:1509–1512. doi: 10.3109/09638288.2012.742575. [DOI] [PubMed] [Google Scholar]
- 36.Ghaem H., Haghighi A.B., Jafari P., Nikseresht A. Validity and reliability of the Persian version of the multiple sclerosis quality of life questionnaire. Neurol India. 2007;55:369. doi: 10.4103/0028-3886.33316. [DOI] [PubMed] [Google Scholar]
- 37.Beckmann Y., Türe S., Duman S.U. Vitamin D deficiency and its association with fatigue and quality of life in multiple sclerosis patients. EPMA J. 2020;11:65–72. doi: 10.1007/s13167-019-00191-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rezaee H., Koushkie Jahromi M., Salesi M., Izadi S. The influence of core stability exercise and vitamin d on some of physical fitness indices in young multiple sclerosis (MS) women. Sport Physiol. 2017;9:17–34. doi: 10.22089/SPJ.2017.2244.1299. [DOI] [Google Scholar]
- 39.De Groot M.H., Phillips S.J., Eskes G.A. Fatigue associated with stroke and other neurologic conditions: implications for stroke rehabilitation. Arch Phys Med Rehabil. 2003;84:1714–1720. doi: 10.1053/S0003-9993(03)00346-0. [DOI] [PubMed] [Google Scholar]
- 40.Kargarfard M., Etemadifar M., Baker P., Mehrabi M., Hayatbakhsh R. Effect of aquatic exercise training on fatigue and health-related quality of life in patients with multiple sclerosis. Arch Phys Med Rehabil. 2012;93:1701–1708. doi: 10.1016/j.apmr.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 41.White L.J., Castellano V. Exercise and brain health--implications for multiple sclerosis: Part II--immune factors and stress hormones. Sports Med. 2008;38:179–187. doi: 10.2165/00007256-200838030-00001. [DOI] [PubMed] [Google Scholar]
- 42.White L.J., Castellano V. Exercise and brain health—implications for multiple sclerosis. Sports Med. 2008;38:91–100. doi: 10.2165/00007256-200838020-00001. [DOI] [PubMed] [Google Scholar]
- 43.Ibrahim M.H., Alloush T.K., Rahim M.K.A., Vitamin D. Level in multiple sclerosis patients. Could vitamin D level be routine investigation for multiple sclerosis patients? Neurosci Med. 2014;5:201. doi: 10.4236/nm.2014.55023. [DOI] [Google Scholar]