Abstract
Background
Despite literature’s evidence about COVID-19 vaccines' safety, concerns have arisen regarding adverse events, including the possible impact on fertility, accentuated by misinformation and anti-vaccine campaigns. The present study aims to answer the question: Is there any impact of COVID-19 vaccines on the fertility of men and women of reproductive age?
Methods
PubMed, Scopus, Web of Science, Cochrane and Embase databases were searched for eligible studies until June 8th, 2022. The search was restricted to articles regarding humans, published in any languages, without additional restrictions. Studies’ quality was assessed by the Newcastle-Ottawa and the Before and After Quality Assessment scales for cohort and pre-post studies, respectively. Random-effect meta-analyses were performed for parameters considered in ≥ 2 studies, calculating means, p-values and 95 % Confidence Intervals (CIs).
Results
Out of 1406 studies screened, 29 were included in the systematic review. These studies, conducted in Israel (34.5 %), USA (24.1 %), Russia (20.7 %) China (10.3 %), Italy (3.5 %), North America (3.5%) and Turkey (3.5 %) were of poor (34.5 %), moderate (58.6 %) and good (6.9 %) quality. Meta-analyses were performed for pre- and post-vaccination sperm progressive motility (44 %, 95 % CI 42 %-62 % vs 43 %, 95 % CI 31 %-59 % p = 0.07) and concentration (50.6 mln/ml, 95 % CI 35.1–72.8 vs 55.4 mln/ml, 95 % CI 37.4–82.2p = 0.12). Biochemical (0.51, 95 % CI 0.40–0.66 vs 0.60, 95 % CI 0.53–0.68p = 0.45) and clinical (0.45, 95 % CI 0.37–0.54 vs 0.47, 95 % CI 0.40–0.55 p = 0.31) pregnancy rate did not differ among vaccinated and not vaccinated groups. Subgroup meta-analyses based on the type of vaccine showed no significant difference: between vaccinated with mRNA vaccines and non-vaccinated regarding biochemical pregnancy rates; pre- and post-vaccination with Gam-COVID-Vac regarding testosterone, FSH and LH levels; pre- and post-vaccination with BNT162b2 vaccines regarding sperm volumes.
Conclusion
Based on the studies published so far, there is no scientific proof of any association between COVID-19 vaccines and fertility impairment in men or women.
Keywords: COVID-19 vaccines, SARS-CoV-2 Vaccine, Fertility, Adverse effects, Male infertility, Female infertility, Reproduction
1. Introduction
Over the course of the first year of the Severe Acute Respiratory Syndrome Coronavirus-2 (SARS-CoV-2) infection spread, the only available measures to tackle it were personal distancing, wearing protection masks, early identifications and isolations of positive patients and their contacts. Later, starting from December 2020, vaccines started to be available as the main tool to fight the pandemic. The COVID-19 pandemic has been accompanied by misinformation and conspiracy theories since the beginning, especially concerning the newly developed vaccines. The latter has been a breeding ground for vaccine opponents [37].
The European Medicines Agency (EMA) [35]and Food and Drug Administration (FDA)[22], [12]issued emergency authorization for COVID-19 vaccines, even if some individuals requested its withhold, claiming that the vaccines posed irreparable harms to the population and possible female infertility risks [45]. The possible mechanism that supposedly linked vaccines to fertility impairment in reproductive age women was hypothesised to be the cross-reactivity with syncytin-1, claiming a similarity between it and spike protein. Syncytin-1 plays an essential role in implantation and its dysfunction might indicate a failed implantation, an early pregnancy loss, or later problems related to abnormal placentation such as preeclampsia. Nevertheless, aminoacidic sequences of the two proteins seem to be different and the cross-reactivity was not observed. In males it was supposed that the vaccine could affect spermatogenesis and sperm parameters, considering that the SARS-CoV-2 virus has been associated to male fertility impairment [11].
Since 2020[16], around 11 billion doses of vaccines have been administered all over the world. Thus far, there has been an important body of literature that has proven short- to medium-term safety profiles and efficacy [5] of these vaccines in preventing COVID-19 infection, hospitalisation and deaths [43].
However, concerns on the possible impact on fertility, accentuated by misinformation and anti-vaccine campaigns [13], continued to circulate on social media and online, corresponding with increased internet searches for topics related to infertility and COVID-19 vaccines [27], [41]. Fear about the possible effects of the vaccines on fertility has been a major driver of COVID-19 vaccine hesitancy [14], with important repercussions on public health.
While increasing numbers of new cases are still being reported in many countries, with new variants spreading fast among the population, vaccination against SARS-CoV-2 remains an important measure to prevent serious infection, and misinformation and doubts regarding the vaccines should be properly addressed, in order to guide the population into safer, informed choices and provide clinicians with evidence based, scientific information. In this context, this systematic review and meta-analysis aims at summarising and assessing available data on the possible impact of COVID-19 vaccines on male and female fertility.
2. Methods
This systematic review and meta-analysis was reported according to the Preferred Reporting Items for Systematic Reviews and meta-Analyses (PRISMA) statement. The protocol of this systematic review was registered to Prospero registration number CRD42022314744.
2.1. Research question
This work aimed to answer the question: is there any impact of COVID-19 vaccines on the fertility of men and women of reproductive age? The research question was structured following the PI/ECOS framework (Population, Intervention/Exposure, Comparison, Outcome, Setting/Time):
-
•
Population: Men and women of reproductive age vaccinated with at least one dose of COVID-19 vaccines;
-
•
Intervention: Vaccination with COVID-19 vaccines;
-
•
Comparison: Men and women of reproductive age not vaccinated against COVID-19 or prior vaccination against COVID-19;
-
•
Outcome: Impact on male (hormonal levels, sperm parameters) and female (pregnancy rate and pregnancy loss rate, antral follicle count, ovarian follicle functions) fertility;
-
•
Setting/Time: All.
2.2. Literature search
The literature search was conducted on PubMed, Scopus, Web of Science, Cochrane and Embase electronic databases using keywords such as: “COVID-19”; “COVID-19 Pandemic”; “SARS Coronavirus 2 Infection”; “COVID-19 Vaccines”; ”COVID-19 Vaccines adverse effects“; “SARS-CoV-2 Vaccine”; “BNT162b2 mRNA vaccine”; “COVID-19 mRNA Vaccine*” OR “mRNA-1273 vaccine”; “Fertility”; “Male Infertility”; “Female Infertility”.
At first, a search string was built for PubMed, using MeSH terms, Boolean operators, and free text words. Afterwards, the string was adapted for being used in the other databases (Supplementary Material 1).
The search was restricted to articles regarding humans, published in any languages, without additional restrictions and was last performed on June 8th, 2022, for all databases. Furthermore, the reference lists of the included studies were hand searched to look for additional studies.
2.3. Study selection and Inclusion/Exclusion criteria
All articles obtained from the search strategy were imported to Rayyan QCRI and duplicates were removed. Two independent reviewers (DZ and ELG) selected the identified studies evaluating title and abstract for each study.
All studies which investigated the impact that COVID-19 vaccines might have on fertility, both in male and in female subjects, were considered pertinent.
Only studies reporting primary data were included in the systematic review.
Case reports, case-series (reporting data for fewer than ten patients), reviews (narrative or systematic), communications, perspectives were excluded.
Articles related to pregnancy outcomes instead of fertility were excluded. Data regarding the effect of SARS-CoV-2 virus on fertility were also excluded. Any disagreements were resolved through discussion among the study team members.
2.4. Data extraction
The full texts of the articles included after the first screening were uploaded on a shared Google Drive file. Two researchers (LP and ELG) performed the data extraction process.
A standardised Google Drive spreadsheet was created, in order to extract the following data:
first author, year, country, study design, population, sex, age, vaccine type, number of doses, time vaccination-recruitment, indicator used to measure fertility, main results, limits.
2.5. Data synthesis and Statistical analyses
A descriptive analysis was performed based on data such as population, sex, age, vaccine type, indicator used to measure fertility. Two researchers (ELG, DZ) were involved in the data synthesis process. When not present in the studies, Confidence Intervals (CI) were calculated.
When possible, random-effect meta-analyses were performed for the same outcome (fertility indicator) reported in ≥ 2 studies. The effect measure for most fertility indicator included mean concentrations or rates. First, meta-nalyses were performed for any type of vaccine used, followed by subgroup analysis based on the specific vaccine administered. The inconsistency index (I2) was used to estimate the heterogeneity across the included studies. The heterogeneity between studies was considered low if the I2-value was < 50 %. Statistical significance was considered at p < 0.05. Publication bias was explored through the Egger test, where p < 0.05 indicated significant publication bias [20] and presented through funnel plots. Statistical analyses were performed using the STATA software package v. 15 (Stata Corporation, College 162 Station, TX, USA).
2.6. Quality assessment
The included studies were evaluated, in terms of methodological quality, based on the study design.Newcastle–Ottawa scale was used for cohort studies, in order to assess the following quality parameters: selection of study groups, comparability of study groups and ascertainment of outcome, giving scores that range from 0 to 9. For pre-post studies was used the Before and After Quality Assessment scale, to assess the following quality parameters: clearness of the study objective, description of eligibility criteria, representativeness and enrolment of participants, sample size, intervention description and delivery, outcome measures, blindness, loss to follow-up, statistical methods.
The overall evidence quality was summarised grouping the articles into three categories, based on methodological quality: good (studies that met at least 75 % of the quality criteria), moderate (studies that met between 50 % and 74 % of the quality criteria) and poor (studies that met<50 % of the quality criteria).
3. Results
3.1. Bibliographical search
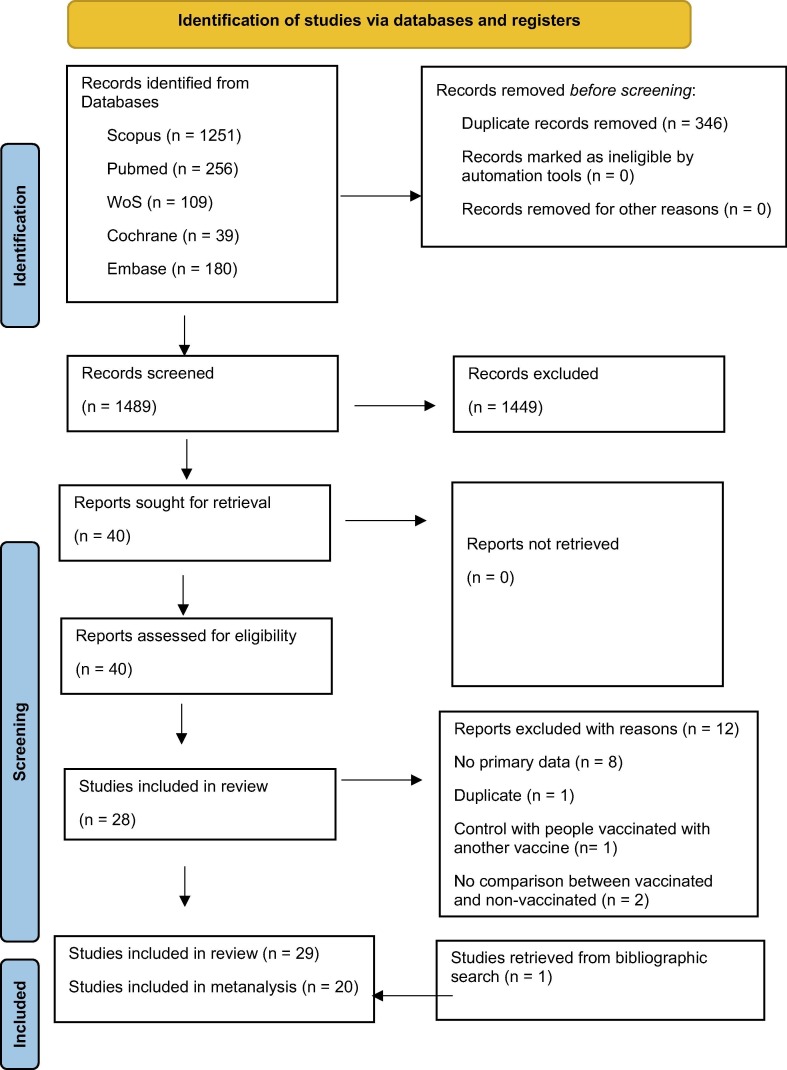
Our searching strategy produced a total of 1489 records, after duplicate removal. After the screening process, 39 articles were considered pertinent for potential inclusion and the full texts were retrieved. Finally, 29 articles [1], [2], [3], [4], [6], [7], [8], [10], [15], [17], [18], [21], [23], [25], [26], [28], [29], [30], [31], [33], [34], [36], [38], [39], [40], [42], [44], [46], [47])were included in the systematic review and 20 articles [2], [3], [4], [6], [7], [18], [21], [23], [25], [26], [30], [33], [34], [36], [38], [39], [40], [42], [46], [47] in the meta-analysis (Fig. 1 ).
Fig. 1.
Flowchart of the screening and selection process.
3.2. Description of the included studies
After the selection process, 29 studies were included (Fig. 1): 10 (34.5 %) were conducted in Israel [4], [6], [7], [8], [28], [29], [33], [34], [39], [40]), 7 (24.1 %) in the USA [2], [3], [10], [23], [26], [30], [31]), 6 (20.7 %) in Russia [1], [15], [17], [18], [21], [38], 3 (10.3 %) in China [25], [46], [47], 1 (3.5 %) in Italy [36], 1 (3.5 %) in North-America (Canada and USA) [44]and 1 (3.5 %) in Turkey [42].
In 16 studies from Israel and USA [2], [3], [4], [6], [7], [8], [10], [23], [28], [29], [30], [31], [33], [34], [39], [40]), as well as the study from Turkey [42], the vaccines investigated were the two mRNA vaccines, BNT162b2 and mRNA-1273 (respectively, known as Pfizer and Moderna); the 6 studies from Russia (20.7 %)[1], [15], [17], [18], [21], [38] investigated the adenovirus-vector vaccine Gam-COVID-Vac (also known as Sputnik V); 3 studies from China (10.3 %)[25], [46], [47]investigated the inactivated vaccine (Sinopharm or Sinovac). In the other 3 studies from Italy, North America and USA, several vaccines were analysed (mRNA and viral vector based)[26], [36], [44].
In total, 15 studies (51.7 %) were conducted on female population [2], [3], [4], [6], [8], [15], [17], [25], [26], [29], [30], [31], [33], [40], [42]), 11 studies (37.9 %) on males [1], [7], [10], [18], [21], [23], [28], [36], [38], [39], [47]and 3 studies (10.3 %) included both populations [34], [44], [47].
15 studies (51.7 %) included In Vitro Fertilization (IVF)/IVG patients (11 studies on females [2], [3], [4], [6], [8], [25], [30], [31], [33], [40], [42], 2 studies on couples [34], [46]) and 2 studies on males [36], [39]). 2 studies from Russia [15], [17]) , 1 from Turkey [42]and 1 from Israel [29]were conducted on healthy women (10.3 %). As for the studies on males, 7 were conducted on healthy men [1], [7], [18], [21], [23], [28], [47], 1 on two comparative groups of healthy and unhealthy men [38], 2 on men undergoing IVG or assisted reproduction technology (ART) technology [36], [39], and 1 on men from a big database [10].
As for the study design, 14 were pre-post studies (48.3 %)[1], [7], [15], [17], [18], [21], [23], [29], [34], [36], [38], [39], [42], [47]) and 15 were cohort studies (51.7 %)[2], [3], [4], [6], [8], [10], [25], [26], [28], [30], [31], [33], [40], [44], [46]. For more detailed information consult Data extraction table-Supplementary Material 2.
3.3. Quality assessment
After the quality assessment of the cohort studies with the Newcastle-Ottawa scale, 13.3 % of the studies resulted to be of good quality (2/15) [10], [25]), 33.3 % resulted to be of moderate quality (5/15) [2], [33], [44], [46], [26]), 53.3 % resulted to be of poor quality (8/15) [3], [4], [6], [8], [28], [30], [31], [40].
After the quality assessment of the pre-post studies with the Before and After Quality Assessment scale, 85.71 % of the studies resulted to be of moderate quality (12/14) [15], [17], [18], [21], [23], [29], [34], [36], [38], [39], [42], [47]), 14.28 % resulted to be of poor quality (2/14) [1], [7].
4. Main results - metanalysis
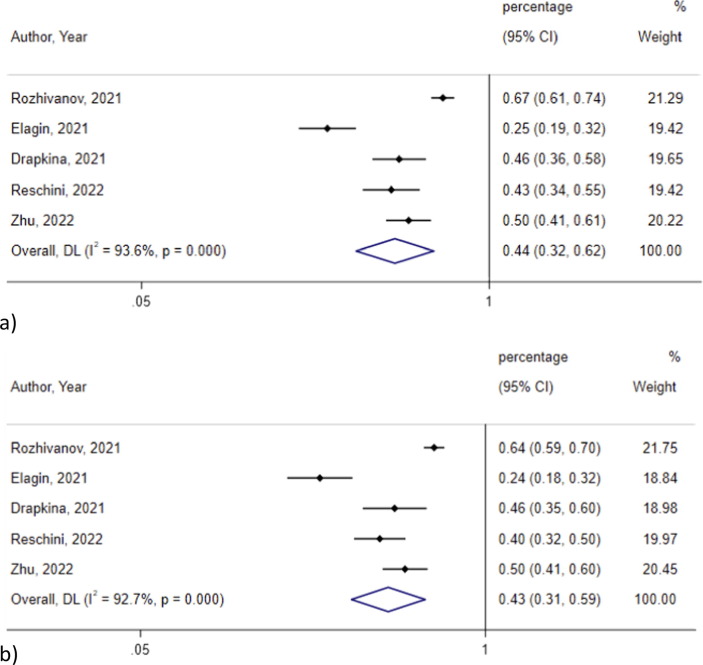
The metanalysis of 5 pre-post studies [18], [21], [36], [38], [47]including a total of 298 males, did not show any significant difference on progressive motility before and after vaccination with any type of COVID-19 vaccines (44 %, 95 % CI 42 %-62 %, I2 = 93.6 % vs 43 %, 95 % CI 31 %-59 %, I2 = 92.7 %; p = 0.07) (Fig. 2 ). There was still no significant difference when conducting a subgroup analysis based on the type of vaccine used (Table 1 ).
Fig. 2.
Progressive motility a) before and b) after vaccination.
Table 1.
Subgroup analysis in groups vaccinated with mRNA vaccines and non-vaccinated.
| Vaccinated |
Non vaccinated |
||||
|---|---|---|---|---|---|
| Study; Subgroup | Rate | 95 % CI | Rate | 95 % CI | p value |
| Biochemical pregnancy rate | |||||
| Aharon, 2021 | 0.756 | 0.637–0.842 | 0.73 | 0.678–0.774 | |
| Morris, 2021 | 0.80 | 0.641–0.90 | 0.739 | 0.638–0.819 | |
| Aizer, 2022 | 0.264 | 0.207–0.327 | 0.288 | 0.211–0.376 | |
| Avraham, 2022 | 0.047 | 0.017–0.099 | 0.098 | 0.053–0.161 | |
| Aharon, 2022 | 0.738 | 0.679–0.798 | 0.749 | 0.718–0.78 | |
| Pooled (95 % CI) | 0.44 | 0.31–0.63 | 0.5 | 0.4–0.62 | 0.496 |
| I2 | 97.10 % | 96.40 % | |||
| Clinical pregnancy rate | |||||
| Aharon, 2021 | 0.634 | 0.509–0.738 | 0.569 | 0.516–0.623 | |
| Morris, 2021 | 0.657 | 0.492–0.792 | 0.625 | 0.521–0.729 | |
| Aizer, 2022 | 0.25 | 0.194–0.313 | 0.264 | 0.167–0.313 | |
| Avraham, 2022 | 0.328 | 0.248–0.417 | 0.331 | 0.252–0.418 | |
| Aharon, 2022 | 0.595 | 0.527–0.663 | 0.637 | 0.602–0.673 | |
| Safrai, 2022 | 0.19 | 0.086–0.341 | 0.19 | 0.086–0.341 | |
| Odeh-Natour, 2022 | 0.440 | 0.224–0.522 | 0.50 | 0.260–0.740 | |
| Pooled (95 % CI) | 0.42 | 0.32–0.56 | 0.43 | 0.33–0.55 | 0.574 |
| I2 | 93.20 % | 95.40 % | |||
| Ongoing pregnancy rate | |||||
| Aharon, 2021 | 0.667 | 0.54–0.765 | 0.561 | 0.507–0.614 | |
| Morris, 2021 | 0.657 | 0.492–0.792 | 0.523 | 0.420–0.624 | |
| Aizer, 2022 | 0.245 | 0.190–0.308 | 0.227 | 0.161–0.305 | |
| Aharon, 2022 | 0.475 | 0.404–0.545 | 0.536 | 0.497–0.574 | |
| Pooled (95 % CI) | 0.48 | 0.33–0.70 | 0.46 | 0.38--0.57 | 0.173 |
| I2 | 95.20 % | 91 % | |||
| Estradiol | |||||
| Author, Year | Estradiol (pMol/l) | 95 % CI | Estradiol (pMol/l) | 95 % CI | |
| Safrai, 2022 | 8869.8 | 3466.2–14273.4 | 6486.6 | 1730.8–11242.4 | |
| Avraham, 2022 | 5896.69 | 5113.34–6680.04 | 6199.54 | 5358.01–7041.07 | |
| Aharon, 2022 | 2559.4 | 1188.2–3930.6 | 2513.7 | 1257.6–3769.8 | |
| Odeh Natour, 2022 | 2070 | 921–2919 | 1637 | 1028–2682 | |
| Pooled (95 % CI) | 4074.25 | 2191.4–7574.7 | 3623.6 | 1859.5–7061.1 | 0.182 |
| I2 | 93.60 % | 92.70 % | |||
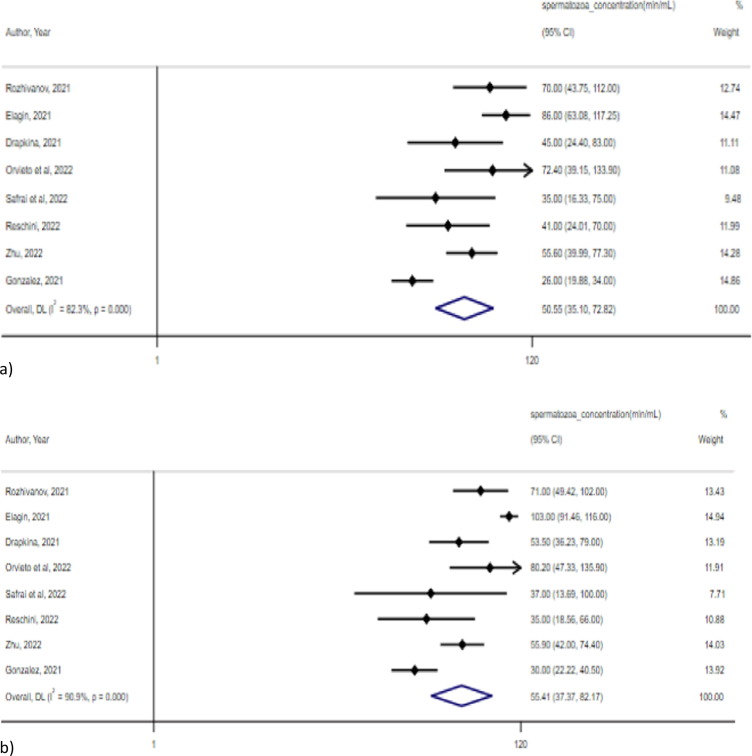
Sperm concentration after vaccination with any type of vaccine did not significantly differ in the metanalysis of 8 pre-post studies, including a total of 451 males [18], [21], [23], [34], [36], [38], [39], [47](50.6 mln/ml, 95 % CI 35.1–72.8 mln/ml, I2 = 82.3 % vs 55.4 mln/ml, 95 % CI 37.4–82.2 mln/ml, I2 = 90.9 %; p = 0.12) (Fig. 3 ).
Fig. 3.
Spermatozoa concentration a) before and b) after vaccination.
Egger’s test showed no significant publication bias for studies assessing progressive motility before and after vaccination (p = 0.05 and p = 0.08, respectively) and for studies assessing sperm concentration before and after vaccination (p = 0.7 and p = 0.05, respectively).
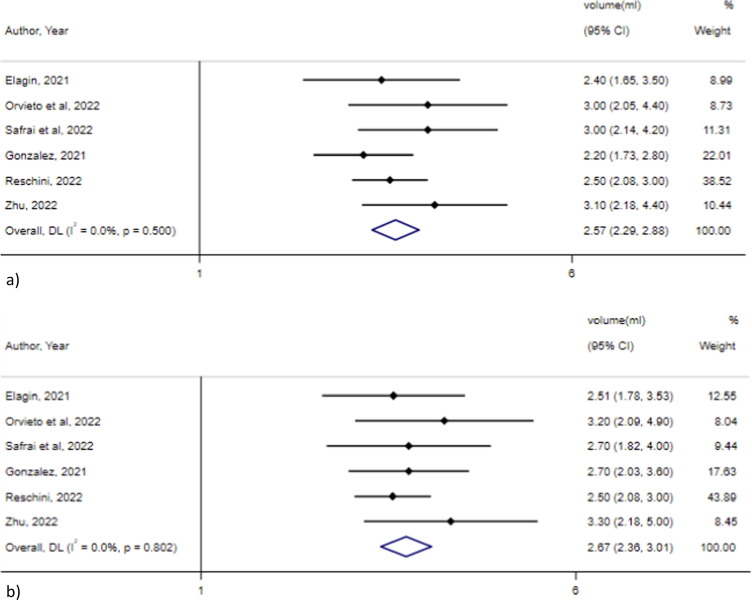
The metanalysis of 6 studies [21], [23], [34], [36], [39], [47], including a total of 346 males, showed no significant difference in the sperm volume before and after vaccination with any type of vaccine (2.6 ml, 95 % CI 2.3–2.9 ml, I2 = 0 %% vs 2.7 ml, 95 % CI 2.4–3.0, I2 = 0 %; p = 0.32). Egger’s test showed no significant publication bias for studies assessing sperm volume before and after vaccination (p = 0.23 and p = 0.07, respectively) (Fig. 4 ).
Fig. 4.
Sperm volume a) before and b) after vaccination.
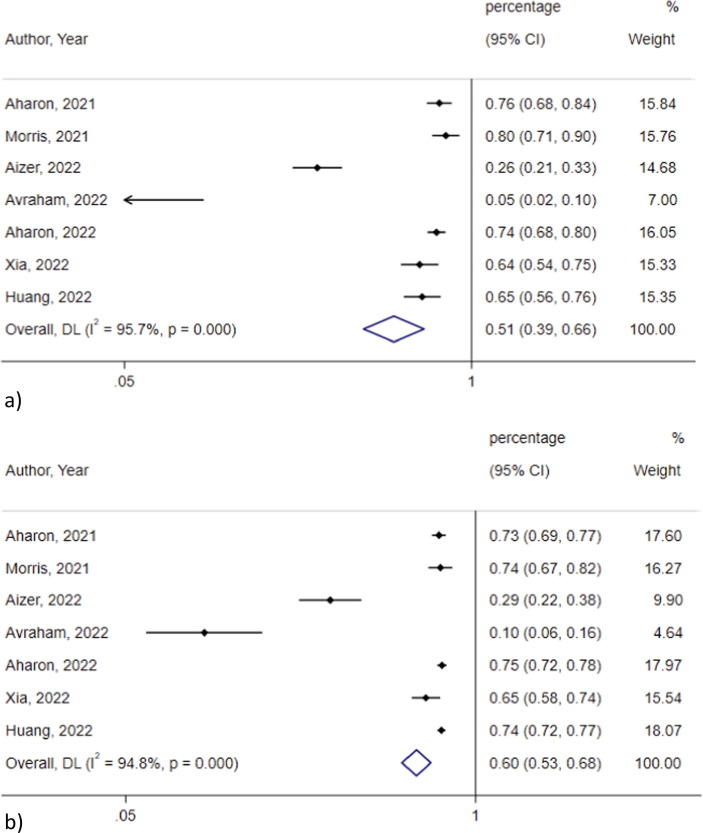
Biochemical pregnancy rate was not significantly different among vaccinated and not vaccinated groups (0.51, 95 % CI 0.40–0.66, I2 = 95.7 % vs 0.60, 95 % CI 0.53–0.68, I2 = 94.8 %; p = 0.45) in 7 studies [2], [3], [4], [6], [25], [31], [46](Fig. 5 ). The heterogeneity decreased after omitting the studies by Avraham 2022, and Aizer 2022 (I2 = 45.6 % and I2 = 15.4 %, respectively), which reported lower biochemical pregnancy rates compared to the rest of the studies. Egger’s test showed significant publication bias for studies assessing biochemical pregnancy rate among vaccinated (p = 0.019) and non-vaccinated subjects (p = 0.01), which lost significance after omitting the study by Avraham 2022, that counted for most of the heterogeneity
Fig. 5.
Biochemical pregnancy rate in the a) vaccinated and b) non vaccinated group.
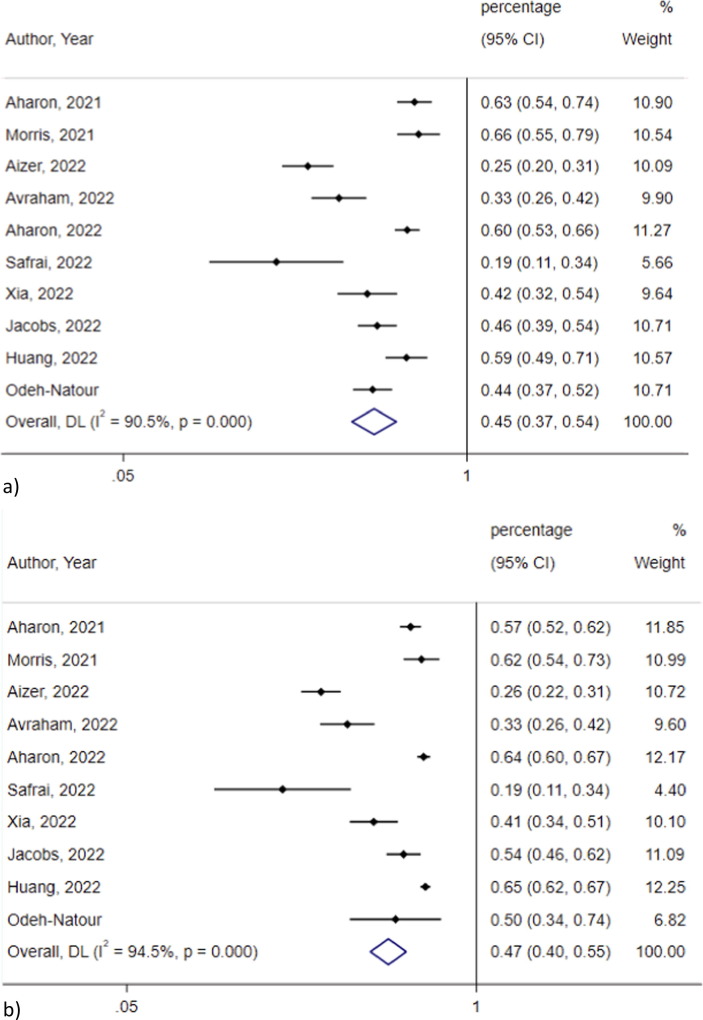
Clinical pregnancy rate did not significantly differ between vaccinated and non-vaccinated women in the metanalysis of 10 studies [2], [3], [4], [6], [25], [26], [31], [33], [40], [46](0.45, 95 % CI 0.37–0.54, I2 = 90.5 % vs 0.47, 95 % CI 0.40–0.55, I2 = 94.5 %; p = 0.31) (Fig. 6 ). The heterogeneity was not significantly lower after omitting studies one by one. Egger’s test showed significant publication bias for studies assessing clinical pregnancy rate among vaccinated (p = 0.01) and non-vaccinated subjects (p = 0.03).
Fig. 6.
Clinical pregnancy rate in the a) vaccinated and b) non vaccinated group.
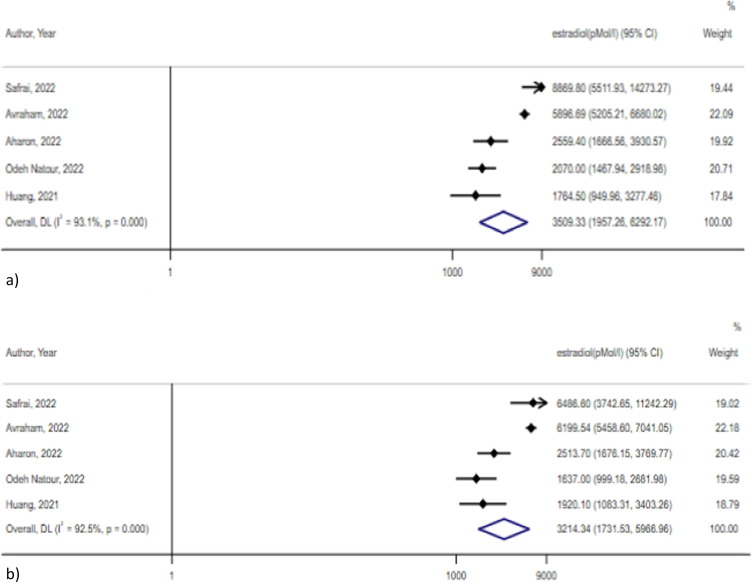
Estradiol levels did not significantly differ between vaccinated and non-vaccinated women in the metanalysis of 5 studies [3], [6], [25], [33], [40](3509.3 pMol/L, 95 % CI 1957.3–6292.2 pMol/L, I2 = 93.1 % vs 3214.3 pMol/L, 95 % CI 1731.5–5967 pMol/L, I2 = 92.5 %; p = 0.38) (Fig. 7 ). Egger’s test showed no significant publication bias for studies assessing estradiol levels among vaccinated and non-vaccinated subjects (p = 0.24 and p = 0.1, respectively).
Fig. 7.
Estradiol levels in the a) vaccinated and b) non vaccinated group.
5. Subgroup analysis based on the type of vaccines
5.1. mRNA vaccines: BNT162b2 and mRNA-1273
The metanalysis of studies that assessed the effect of mRNA vaccines on the fertility of women who were going through IVG, showed no significant difference on biochemical [2], [3], [4], [6]), [31]), clinical pregnancy rate [2], [3], [4], [6], [31], [33], [40] and ongoing pregnancy rate [2], [3], [4], [31]between the vaccinated and not vaccinated groups (Table 1).
5.2. BNT162b2 vaccine
The meta-analyses of included studies that assessed the effect of BNT162b2 vaccine on the fertility of men who were going through IVF, showed no significant difference on Sperm volume [34], [39](3 ml. 95 % CI 2.33–3.83, I2 = 0 % vs 2.92 ml, 95 % CI 2.19–3.9, I2 = 0 %; p = 0.56], Spermatozoa concentration [34], [39](52.2 mln/ml, 95 % CI 25.7–106.04 mln/ml, I2 = 52.8 % vs 61.39 mln/ml, 95 % CI 29.35–126.25 mln/ml, I2 = 44.9 %; p = 0.17) and Progressive motility (62 %, 95 % CI 55–70 %, I2 = 0 % vs 63 %, 95 % CI 59–67 %, I2 = 0 %; p = 0.75) before and after vaccination [7], [34].
5.3. Gam-COVID-Vac (Sputnik V)
Testosterone levels, spermatozoa concentrations, progressive motility, normal forms, FSH and LH levels were not significantly different before and after the administration of Gam-COVID-Vac in the metanalysis performed [18], [21], [38](Table 2 ).
Table 2.
Subgroup analysis in groups before and after vaccination with Gam-COVID-Vac vaccine.
| Pre vaccination |
Post vaccination |
||||
|---|---|---|---|---|---|
| Study; Subgroup | Testosterone level | 95 % CI | Testosterone level | 95 % CI | p value |
| Testosterone | |||||
| Rozhivanov, 2021 | 16.5 | 14.0–22.1 | 16.7 | 14.0–22.1 | |
| Elagin, 2021 | 22.20 | 17.65–30.63 | 17.25 | 13.00–19.85 | |
| Drapkina, 2021 | 9.60 | 8.30–12.8 | 11.80 | 9.40–13.2 | |
| Pooled (95 % CI) | 15.15 | 9.37–24.48 | 14.88 | 11.18–19.82 | 0.635 |
| I2 | 86.90 % | 89.40 % | |||
| Spermatozoa concentration | Concentration | 95 % CI | Concentration | 95 % CI | |
| Rozhivanov, 2021 | 70.0 | 51.0–112.0 | 71 | 53–102 | |
| Elagin, 2021 | 86.00 | 49.75–117.25 | 103.00 | 59.0–116.0 | |
| Drapkina, 2021 | 45.0 | 25.0–83.0 | 53.5 | 32.0–79.0 | |
| Pooled (95 % CI) | 69.94 | 49.77–98.29 | 75.49 | 49.82–114.4 | 0.098 |
| I2 | 42.70 % | 84.10 % | |||
| Progressive Motility | Progressive Motility | 95 % CI | Progressive Motility | 95 % CI | |
| Rozhivanov, 2021 | 0.67 | 0.6–0.74 | 0.64 | 0.58–0.7 | |
| Elagin, 2021 | 0.2478 | 0.178–0.316 | 0.2376 | 0.159–0.315 | |
| Drapkina, 2021 | 0.46 | 0.325–0.58 | 0.455 | 0.28–0.6 | |
| Pooled (95 % CI) | 0.43 | 0.24–0.77 | 0.41 | 0.23–0.75 | 0.906 |
| I2 | 96.50 % | 95.6 | |||
| Normal forms | Normal forms | 95 % CI | Normal forms | 95 % CI | |
| Rozhivanov, 2021 | 0.09 | 0.06–0.15 | 0.09 | 0.07–0.15 | |
| Elagin, 2021 | 0.07 | 0.05–0.1175 | 0.08 | 0.05–0.15 | |
| Drapkina, 2021 | 0.02 | 0.02–0.03 | 0.02 | 0.020.03 | |
| Pooled (95 % CI) | 0.05 | 0.02–0.13 | 0.05 | 0.02–0.15 | 0.21 |
| I2 | 92 % | 92.1 | |||
| FSH levels | FSH levels | 95 % CI | FSH levels | 95 % CI | |
| Elagin, 2021 | 5.08 | 2.96–7.20 | 4.88 | 2.43–7.33 | |
| Drapkina, 2021 | 4.80 | 3.10–6.40 | 4.80 | 3.20–7.00 | |
| Pooled (95 % CI) | 4.91 | 3.98–6.13 | 4.84 | 3.67–6.38 | 0.75 |
| I2 | 0.00 % | 0.00 % | |||
| LH levels | LH levels | 95 % CI | LH levels | 95 % CI | |
| Elagin, 2021 | 4.05 | 3.23–4.6 | 4.10 | 2.92–5.2 | |
| Drapkina, 2021 | 3.20 | 2.10–4.36 | 4.30 | 2.60–5.5 | |
| Pooled (95 % CI) | 3.76 | 3.04–4.66 | 4.2 | 3.54–4.98 | 0.23 |
| I2 | 47.50 % | 0.00 % | |||
6. Discussion
Published literature has suggested that the SARS-CoV-2 infection can impair male fertility by worsening semen parameters, potentially lowering testosterone levels, and increasing risk of erectile dysfunction [19], [32]. As for female fertility, studies report the detrimental impact that oxidative stress has on the quality of oocytes and embryos, implying that SARS-CoV-2 could alter female fertility, as well as significant, but reversible, menstrual alterations, and slightly modified ovarian reserve and hormonal balance [9].
In this context, the COVID-19 vaccines seem a solution to prevent the infection and its impact on health, including fertility. Nevertheless, COVID-19 vaccination campaign has been associated with distrust and disinformation, particularly regarding any possible repercussions of vaccination on male and female fertility. Against this background, the present systematic review and meta-analysis aimed at summarising and assessing available data on the possible impact of COVID-19 vaccines on male and female fertility.
The included studies that assessed the possible effect of COVID-19 vaccines on female fertility reported no significant worsening of any fertility indicator evaluated. Most of these studies [2], [3], [4], [6], [8], [25], [30], [31], [33], [40], [42]) were conducted among women who were going through IVF and there were no significant detrimental effects of vaccination on trigger day estradiol and progesterone concentrations, serum and follicular fluid estradiol and progesterone, number of oocytes [8], [25], [33], implantation rate([31]), and pregnancy rate [2], [25], [30]. Two studies assessed the levels of AMH in healthy reproductive age women and no significant difference was found in ovarian reserve [15], [29], as well as in serum levels of AMH, FSH, TSH, estradiol [3], [6], [25], [33], [39], antiphospholipid antibodies [15].
As for the impact of COVID-19 vaccines on male fertility, studies conducted among healthy men showed absence of adverse effects of the COVID-19 vaccines on molecular features of semen samples [1]), sperm volume and concentration, motility, morphology, FSH and LH levels [2], [3], [4], [6], [7], [18], [21], [23], [25], [26], [28], [31], [33], [39]. In the study by Carto et al., mRNA vaccines were associated with a decreased risk of developing orchitis and/or epididymitis (OR = 0.568; 95 % CI: 0.497–0.649; p < 0.0001), while prolactin and testosterone levels were significantly lower after vaccination with Gam-COVID-Vac in the study by Elagin et al.
The study by Rozhivanov et al., conducted among men with normozoospermia and pathozoospermia, concluded that vaccination with Gam-COVID-Vac (Sputnik V) had no effect on testosterone levels or quality of ejaculate. Two studies assessing the impact of vaccines on fertility among men undergoing IVF or infertility management showed no differences before and after vaccination regarding sperm volume, concentration and morphology [36], [39].
Couples undergoing IVF did not have any significant changes on mean peak estradiol and progesterone levels, mean number of oocytes, semen volume, sperm concentration or motility before and after mRNA vaccines [34], while fecundability rate and number of pregnancies did not have a significant difference between couples vaccinated with any type of vaccine compared to not vaccinated ones [44].
Subgroup meta-analyses based on the type of vaccine showed no significant difference: between vaccinated with mRNA vaccines and non-vaccinated regarding biochemical pregnancy rates [2], [3], [4], [6], [31]; pre- and post-vaccination with Gam-COVID-Vac regarding testosterone, FSH and LH levels; pre- and post-vaccination with BNT162b2 vaccines regarding sperm volumes [18], [21], [38].
The results of this systematic review and meta-analysis should be considered in the light of some limitations. The follow-up time in the included studies ranged from a minimum of 7 days after the first dose to a maximum of 9 months. Considering that any possible impact of vaccines on fertility could be seen after a certain time period, there is the need for longer time of follow. Furthermore, there is the need for more robust studies, systematic investigation, with more precise eligibility criteria, with appropriate sample size and more representative population, focusing not only on particular ones, such as those going through IVF. Nevertheless, the results of this systematic review and meta-analysis are important, since affirm no effects of COVID-19 vaccines on human fertility, based on the studies published so far, and could serve as a guide for future, well-designed studies. Moreover, these results can assist healthcare professionals (doctors, midwives, nurses) in addressing the doubts and questions of their reproductive-age patients regarding the possible association between COVID-19 vaccines and male or female fertility.
Considering that COVID-19 infection itself may be associated with impaired fertility, COVID-19 vaccination could act as a tool to preserve reproductive function through the prevention of COVID-19 infection. Furthermore, it has been reported that vaccination has other protective effects; for example, vaccinated men are less likely to develop orchitis and/or epididymitis compared to unvaccinated men [10].
In this context, vaccination against SARS-CoV-2 remains an important measure to prevent serious infection and release the burden of this pandemic. Misinformation and doubts regarding the vaccines should be properly addressed, in order to guide the population into safer, informed choices and provide clinicians with evidence based, scientific information [24].
6.1. Conclusions
So far, there is no scientific proof of any association between COVID-19 vaccines and fertility impairment in men or women. Considering that COVID-19 infection could pose a threat to the human reproductive health, vaccination represents an important choice to prevent adverse COVID-19 outcomes.
Authors’ role.
DZ and MLDP conceived the research hypothesis and designed the study. DZ and ELG structured and performed the search strategy. ELG and LP performed the screening, selection process and data extraction. ELG performed the quality assessment. DZ and MLDP reviewed the results obtained from the data extraction process considering the quality assessment of the papers. DZ and ELG performed the metanalysis; DZ, ELG and LP wrote a first draft of the manuscript and MLDP revised the work for important intellectual content. All authors gave the final approval of the version to be published, and agreed on all aspects of the work, especially concerning its accuracy and integrity.
Data availability statement
The data underlying this article are available in the article and in its online supplementary material.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgements
The authors would like to express the highest appreciation to Dr. Anna Shukhovtseva for helping with the translation of articles in Russian.
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.vaccine.2022.09.019.
Appendix A. Supplementary material
The following are the Supplementary data to this article:
Data availability
All data is presented in the manuscript or supplementary material
References
- 1.Adamyan L.V., Vechorko V.I., Elagin V.V., Dashko A.A., Doroshenko D.A., Stepanian A.A., et al. COVID-19 vaccine does not affect male reproductive health (based on RNA-sequencing data) Problemy Reproduktsii. 2021;27(5):8. doi: 10.17116/repro2021270518. [DOI] [Google Scholar]
- 2.Aharon D., Canon C.M., Hanley W.J., Lee J.A., Lederman M.A., Stein D.E., et al. Mrna COVID-19 vaccines do not compromise implantation of euploid embryos. Fertil Steril. 2021;116(3) doi: 10.1016/j.fertnstert.2021.07.215. [DOI] [Google Scholar]
- 3.Aharon D., Lederman M., Ghofranian A., Hernandez-Nieto C., Canon C., Hanley W., et al. In Vitro Fertilization and Early Pregnancy Outcomes After Coronavirus Disease 2019 (COVID-19) Vaccination. Obstet Gynecol. 2022;139(4):490–497. doi: 10.1097/AOG.0000000000004713. [DOI] [PubMed] [Google Scholar]
- 4.Aizer A., Noach-Hirsh M., Dratviman-Storobinsky O., Nahum R., Machtinger R., Yung Y., et al. The effect of coronavirus disease 2019 immunity on frozen-thawed embryo transfer cycles outcome. Fertil Steril. 2022;117(5):974–979. doi: 10.1016/j.fertnstert.2022.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Asghar N., Mumtaz H., Syed A.A., Eqbal F., Maharjan R., Bamboria A., et al. Safety, efficacy, and immunogenicity of COVID-19 vaccines; a systematic review. Immunological Medicine. 2022;1–13 doi: 10.1080/25785826.2022.2068331. [DOI] [PubMed] [Google Scholar]
- 6.Avraham S., Kedem A., Zur H., Youngster M., Yaakov O., Yerushalmi G.M., et al. Coronavirus disease 2019 vaccination and infertility treatment outcomes. Fertil Steril. 2022;117(6):1291–1299. doi: 10.1016/j.fertnstert.2022.02.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Barda S., Laskov I., Grisaru D., Lehavi O., Kleiman S., Wenkert A., et al. The impact of <scp>COVID</scp> -19 vaccine on sperm quality. International Journal of Gynecology & Obstetrics. 2022 doi: 10.1002/ijgo.14135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bentov Y., Beharier O., Moav-Zafrir A., Kabessa M., Godin M., Greenfield C.S., et al. Ovarian follicular function is not altered by SARS–CoV-2 infection or BNT162b2 mRNA COVID-19 vaccination. Hum Reprod. 2021;36(9):2506–2513. doi: 10.1093/humrep/deab182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carp-Veliscu A., Mehedintu C., Frincu F., Bratila E., Rasu S., Iordache I., et al. The Effects of SARS-CoV-2 Infection on Female Fertility: A Review of the Literature. Int J Environ Res Public Health. 2022;19(2):984. doi: 10.3390/ijerph19020984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Carto C., Nackeeran S., Ramasamy R. COVID-19 vaccination is associated with a decreased risk of orchitis and/or epididymitis in men. Andrologia. 2022;54(2) doi: 10.1111/and.14281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Chen F., Zhu S., Dai Z., Hao L., Luan C., Guo Q., et al. Effects of COVID-19 and mRNA vaccines on human fertility. Hum Reprod. 2021;37(1):5–13. doi: 10.1093/humrep/deab238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Comirnaty and Pfizer-BioNTech COVID-19 Vaccine | FDA. (2021, March 2). https://www.fda.gov/emergency-preparedness-and-response/coronavirus-disease-2019-covid-19/comirnaty-and-pfizer-biontech-covid-19-vaccine.
- 13.Diaz P., Reddy P., Ramasahayam R., Kuchakulla M., Ramasamy R. COVID-19 vaccine hesitancy linked to increased internet search queries for side effects on fertility potential in the initial rollout phase following Emergency Use Authorization. Andrologia. 2021;53(9) doi: 10.1111/and.14156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Diaz P., Zizzo J., Balaji N.C., Reddy R., Khodamoradi K., Ory J., et al. Fear about adverse effect on fertility is a major cause of COVID-19 vaccine hesitancy in the United States. Andrologia. 2022;54(4) doi: 10.1111/and.14361. [DOI] [PubMed] [Google Scholar]
- 15.Dolgushina D.N.V., Drapkina D.Y.U.S., Krechetova K.L.V., Ivanets I.T.Y., Menzhinskaya M.I.V., Gus G.A.I., et al. Gam-COVID-Vac (Sputnik V) vaccine has no adverse effect on ovarian reserve in reproductive-age women. Akusherstvo i Ginekologiia. 2021;7_2021:81–86. doi: 10.18565/aig.2021.7.81-86. [DOI] [Google Scholar]
- 16.Dong E., Du H., Gardner L. An interactive web-based dashboard to track COVID-19 in real time. Lancet Infect Dis. 2020;20(5):533–534. doi: 10.1016/S1473-3099(20)30120-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dovgan A., Drapkina Y., Dolgushina N., Menzhinskaya I., Krechetova L., Sukhikh G. Effects of COVID-19 vector vaccine on autoantibody profile in reproductive age women. Bulletin of Russian State Medical University. 2022;2022(2) doi: 10.24075/brsmu.2022.016. [DOI] [Google Scholar]
- 18.Drapkina Yu D Yu S, Dolgushina DNV, Shatylko TSTV, Nikolaeva MNMA., Menzhinskaya IMIV, Ivanets IT Yu, Krechetova LKLV, Gamidov SGSI, Bairamova BGR, & Sukhikh SGT. Gam-COVID-Vac (Sputnik V) vaccine has no adverse effect on spermatogenesis in men. Akusherstvo i Ginekologiia, 7_2021, 88–94. https://doi.org/10.18565/aig.2021.7.88-94.
- 19.Dubin J.M., Bennett N.E., Halpern J.A. The adverse impact of COVID-19 on men’s health. Curr Opin Urol. 2022;32(2):146–151. doi: 10.1097/MOU.0000000000000966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Egger M., Smith G.D., Schneider M., Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315:629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Elagin V.V., Adamyan L.V., Vechorko V.I., Doroshenko D.A., Dashko A.A., Filippov O.S., et al. COVID-19 vaccine and male reproductive health (preliminary data) Problemy Reproduktsii. 2021;27(4):17. doi: 10.17116/repro20212704117. [DOI] [Google Scholar]
- 22.FDA Authorizes Moderna COVID-19 Vaccine | The Medical Letter, Inc. 2021. https://secure.medicalletter.org/w1616a. [PubMed]
- 23.Gonzalez D.C., Nassau D.E., Khodamoradi K., Ibrahim E., Blachman-Braun R., Ory J., et al. Sperm Parameters Before and After COVID-19 mRNA Vaccination. JAMA. 2021;326(3):273. doi: 10.1001/jama.2021.9976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.González-Melado F.J., di Pietro M.L. The vaccine against COVID-19 and institutional trust. Enfermedades Infecciosas y Microbiologia Clinica (English Ed) 2021;39(10):510–515. doi: 10.1016/j.eimce.2021.09.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Huang J., Xia L., Lin J., Liu B., Zhao Y., Xin C., et al. No Effect of Inactivated SARS-CoV-2 Vaccination on in vitro Fertilization Outcomes: A Propensity Score-Matched Study. Journal of Inflammation Research. 2022;15:839–849. doi: 10.2147/JIR.S347729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jacobs E., Summers K., Sparks A., Mejia R. Fresh Embryo Transfer Cycle Characteristics and Outcomes Following In Vitro Fertilization via Intracytoplasmic Sperm Injection Among Patients With and Without COVID-19 Vaccination. JAMA Network Open. 2022;5(4) doi: 10.1001/jamanetworkopen.2022.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kwok A., Qu Y., Howard J., Luk J., Nejat E.J. Differences in anxiety concerning the Covid-19 virus and Covid-19 vaccine between women undergoing infertility treatment and those not pursuing treatment. Fertil Steril. 2021;116(3) doi: 10.1016/j.fertnstert.2021.07.975. [DOI] [Google Scholar]
- 28.Lifshitz D., Haas J., Lebovitz O., Raviv G., Orvieto R., Aizer A. Does mRNA SARS-CoV-2 vaccine detrimentally affect male fertility, as reflected by semen analysis? Reproductive BioMedicine Online. 2022;44(1):145–149. doi: 10.1016/j.rbmo.2021.09.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mohr-Sasson A., Haas J., Abuhasira S., Sivan M., Doitch Amdurski H., Dadon T., et al. The effect of Covid-19 mRNA vaccine on serum anti-Müllerian hormone levels. Hum Reprod. 2022;37(3):534–541. doi: 10.1093/humrep/deab282. [DOI] [PubMed] [Google Scholar]
- 30.Morris R.S. SARS-CoV-2 spike protein seropositivity from vaccination or infection does not cause sterility. F&S Reports. 2021;2(3):253–255. doi: 10.1016/j.xfre.2021.05.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Morris R.S., Morris A.J., Il N. Exposure of ovaries to covid-19 vaccination does not impair fertility. Fertil Steril. 2021;116(3) doi: 10.1016/j.fertnstert.2021.08.027. [DOI] [Google Scholar]
- 32.Nassau D.E., Best J.C., Kresch E., Gonzalez D.C., Khodamoradi K., Ramasamy R. Impact of the SARS-CoV-2 virus on male reproductive health. BJU International. 2022;129(2):143–150. doi: 10.1111/bju.15573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Odeh-Natour R., Shapira M., Estrada D., Freimann S., Tal Y., Atzmon Y., et al. Does mRNA SARS-CoV-2 vaccine in the follicular fluid impact follicle and oocyte performance in IVF treatments? Am J Reprod Immunol. 2022;87(5) doi: 10.1111/aji.13530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Orvieto R., Noach-Hirsh M., Segev-Zahav A., Haas J., Nahum R., Aizer A. Does mRNA SARS-CoV-2 vaccine influence patients’ performance during IVF-ET cycle? Reproductive Biology and Endocrinology. 2021;19(1):69. doi: 10.1186/s12958-021-00757-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Pinho AC. (2020, December 21). EMA recommends first COVID-19 vaccine for authorisation in the EU | European Medicines Agency. https://www.ema.europa.eu/en/news/ema-recommends-first-covid-19-vaccine-authorisation-eu.
- 36.Reschini M., Pagliardini L., Boeri L., Piazzini F., Bandini V., Fornelli G., et al. COVID-19 Vaccination Does Not Affect Reproductive Health Parameters in Men. Front Public Health. 2022;10 doi: 10.3389/fpubh.2022.839967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Romer D., Jamieson K.H. Conspiracy theories as barriers to controlling the spread of COVID-19 in the U.S. Soc Sci Med. 2020;263 doi: 10.1016/j.socscimed.2020.113356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rozhivanov R.V., Mokrysheva N.G. Ejaculate quality and testosterone levels in men vaccinated with Gam-Covid-Vac (Sputnik-V) Problemy Reproduktsii. 2021;27(4):22. doi: 10.17116/repro20212704122. [DOI] [Google Scholar]
- 39.Safrai M., Herzberg S., Imbar T., Reubinoff B., Dior U., Ben-Meir A. The BNT162b2 mRNA Covid-19 vaccine does not impair sperm parameters. Reproductive BioMedicine Online. 2022;44(4):685–688. doi: 10.1016/j.rbmo.2022.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Safrai M., Kremer E., Atias E., Ben-Meir A. Dispelling Damaging Myths: BNT162b2 COVID-19 Vaccine Does Not Afect Fertility and Pregnancy Rate in Women Undergoing IVF Treatment. Reproductive Sciences. 2022;29 [Google Scholar]
- 41.Sajjadi N.B., Nowlin W., Nowlin R., Wenger D., Beal J.M., Vassar M., et al. United States internet searches for “infertility” following COVID-19 vaccine misinformation. Journal of Osteopathic Medicine. 2021;121(6):583–587. doi: 10.1515/jom-2021-0059. [DOI] [PubMed] [Google Scholar]
- 42.Soysal Ç., Yılmaz E. The effect of COVID-19 vaccine on ovarian reserve. Saudi Med J. 2022;43(5):486–490. doi: 10.15537/smj.2022.43.5.20220007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ssentongo P., Ssentongo A.E., Voleti N., Groff D., Sun A., Ba D.M., et al. SARS-CoV-2 vaccine effectiveness against infection, symptomatic and severe COVID-19: a systematic review and meta-analysis. BMC Infect Dis. 2022;22(1):439. doi: 10.1186/s12879-022-07418-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wesselink A.K., Hatch E.E., Rothman K.J., Wang T.R., Willis M.D., Yland J., et al. A Prospective Cohort Study of COVID-19 Vaccination, SARS-CoV-2 Infection, and Fertility. Am J Epidemiol. 2022;191(8):1383–1395. doi: 10.1093/aje/kwac011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wodarg WSD. 2020, December 1. Petition/motion for administrative/regulatory action regarding confirmation of efficacy end points and use of data in connection with the following clinical trial (s): phase III-eudract number: 2020-002641-2. corona-ausschuss.de. . https://www.wodarg.com/app/download/9033912514/ Wodarg_Yeadon_EMA_Petition_Pfizer_Trial_FINAL_ 01DEC2020_signed_with_Exhibits_geschwa%CC%88rzt.pdf? t=1606870652.
- 46.Xia W., Zhao J., Hu Y., Fang L., Wu S. Investigate the effect of COVID-19 inactivated vaccine on sperm parameters and embryo quality in in vitro fertilization. Andrologia. 2022;54(6) doi: 10.1111/and.14483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Zhu H., Wang X., Zhang F., Zhu Y., Du M.-R., Tao Z.-W., et al. Evaluation of inactivated COVID-19 vaccine on semen parameters in reproductive-age males: a retrospective cohort study. Asian Journal of Andrology. 2022;24(5):441. doi: 10.4103/aja202225. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data underlying this article are available in the article and in its online supplementary material.
All data is presented in the manuscript or supplementary material