Abstract
H-NS regulates the flagellar master operon (flhDC) and thus is necessary for flagellation of Escherichia coli. However, the molecular mechanism of its regulation has remained unknown. Genetic screening of a transposon insertion abolishing the H-NS effect revealed a previously unidentified gene, named hdfR, encoding a LysR family protein. Binding of purified HdfR to the flhDC promoter was demonstrated by a DNA mobility shift assay, indicating that HdfR is a transcriptional regulator for the flagellar master operon. Furthermore, the expression of the hdfR gene was shown to be negatively regulated by H-NS.
The Escherichia coli flagellar system consists of more than 40 genes whose products are required for flagellar assembly, function, and sensory signaling (12). Expressions of the genes are regulated in a cascade mode. At the top of the hierarchy is the flhDC operon, encoding the FlhD and FlhC proteins, which are essential for expression of downstream flagellar genes (11). Flagellar expression is affected by various environmental conditions (20), perhaps involving at least some transcriptional regulators. In most cases, flagellar expression is modulated at the transcriptional level of the flhDC operon. The cyclic AMP (cAMP) receptor protein-cAMP complex and the OmpR protein are known to affect the expression of the flhDC operon by binding to its promoter region (21, 23).
H-NS, a nucleoid protein (7, 25), affects the expression of many unrelated genes, including proVWX, bgl (9), appY (1), and fimB (6) of E. coli or Salmonella enterica serovar Typhimurium, and also affects expression of some virulence genes of Salmonella serovar Typhimurium and Shigella spp. (8, 16, 13). The majority of affected genes are negatively regulated by H-NS, although some, including the flagellar regulon, are positively regulated. It has been reported that H-NS-deficient cells are nonflagellated because of reduced transcription of flhDC (4). Although it was assumed that H-NS positively affects flhDC transcription, its regulation has not been clearly demonstrated. In an assay of in vitro transcription of the flhDC operon (23), purified H-NS did not enhance the transcription. Thus, it was suspected that the regulation of flhDC by H-NS might be indirect.
In this study, we isolated an insertion enabling cells to enhance flhDC expression even in the absence of H-NS. The insertion was found in a gene, named hdfR, encoding a LysR family protein, which has a helix-turn-helix DNA-binding motif. HdfR binds to the promoter region of the flhDC operon, and its expression was negatively regulated by H-NS, suggesting that the apparent activation of flhDC transcription by H-NS is mediated through the negative regulator HdfR.
A transposon insertion abolishing the repression of flhDC-lacZ due to an H-NS defect.
Genetic screening employing random transposon insertion was performed to search for a putative mediator involved in the H-NS-dependent regulation of flhDC. As a tool to monitor flhDC expression, the flhDC-lacZ protein fusion contained in bacteriophage λSS10 (21) was used. The lower level of LacZ activity of the protein fusion made it possible to screen for a clone with a distinguishable phenotype on an indicator plate, which was impractical with the transcriptional fusion used later. A pool of random transposon insertions obtained from CP807 (E. coli K-12 ΔlacZ thr leu his met) (21) infected with phage λ::TnphoA132 (tet) (26) was transferred to MS368 (MC4100 flhD+ λSS10 hns::neo) (27) by P1. Among the derivatives of MS368 containing random insertions, a clone showing derepression of flhDC-lacZ even in the absence of H-NS was selected on indicator agar plates (Luria-Bertani agar containing 50 μg of 5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside [X-Gal]/ml) and exhibited a consistent increase in LacZ activity (14) when transferred back to MS368.
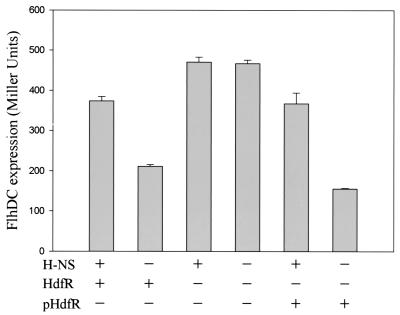
To monitor the transcriptional activities of the flhDC operon, the insertion was transferred to strains MS358 (MC4100 flhD+ λMS205) and MS359 (MS358 hns::neo) lysogenized with λMS205, containing the flhDC-lacZ transcriptional fusion. The λMS205 phage was constructed by subcloning the flhDC region including −544 to +593 from the transcriptional start (23) into pRS415 (promoterless lacZYA; bla), which was double recombined into λRS45 (′lacZAY bla′) using lacZAY and bla homologies (22). A strain with a single prophage, confirmed by PCR (17), was used to monitor the transcriptional activities of the flhDC-lacZ fusion. As shown in Fig. 1, the LacZ activity of MS372 containing the transposon insertion, later designated hdfR::TnphoA132, was higher than that of the parent strain and was not affected by an hns::neo mutation. This indicates that the transposon insertion abolished the hns effect on flhDC transcription.
FIG. 1.
Effects of hns and hdfR mutations on the transcription of the flhDC operon. β-Galactosidase activities expressed from the strains containing the flhDC-lacZ transcriptional fusion in λMS205 were measured in cells grown in TB medium (1% tryptone, 0.25% NaCl) at 35°C to an optical density at 600 nm of 0.4 to 0.5. Strains used were MS358 (λMS205 hns+ hdfR+), MS359 (MS358 hns), MS372 (MS358 hdfR), MS373 (MS358 hns hdfR), MS372/pMS272ΔH (hdfR/pHdfR), and MS373/pMS272ΔH (hns hdfR/pHdfR). The activities of β-galactosidase are presented in Miller units (14), with standard deviations (error bars) estimated from three independent samples.
The insertion was found in a novel gene named hdfR.
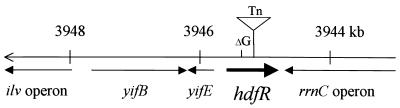
The site of the transposon insertion was identified by an inverse PCR. After digestion of chromosomal DNA with Sau3A1, PCR amplification was performed for the ligated DNA with a pair of outwardly directed primers complementary to the regions in the transposon. The DNA amplified was sequenced to search for homology in the E. coli genome database. The result revealed an insertion site in the putative gene yifA at 84 min (Fig. 2). In the E. coli genome database for strain MG1655, yifA is located next to another gene, pssR, named under the presumption that it locates in the same region where pssR1 was mapped (24). The pssR1 strain was isolated as a mutant exhibiting an elevated expression of pssA, encoding the phosphatidylserine synthase.
FIG. 2.
Chromosomal location of the hdfR gene. The hdfR gene was previously divided into two ORFs, pssR and yifA, by E. coli genome sequencing for strain MG1655. Numbers indicate nucleotide positions, in kilobases. Arrows indicate directions of the ORFs. The nucleotide and deduced amino acid sequences of the hdfR gene have been submitted to GenBank (accession no. AF25103). ΔG indicates the location of the missing guanine nucleotide in the reported sequence (accession no. AE000453). The insertion site of TnphoA132 (Tn) is also indicated.
The GenBank report for pssR (accession no. AAC77484) predicts an open reading frame (ORF) encoding a protein of 133 amino acids which contains a helix-turn-helix DNA-binding motif found in the LysR family proteins. However, the PssR protein was unusually small compared to other LysR family proteins of about 300 amino acids (18). Moreover, pssR is overlapped (157 bp) with the neighboring yifA gene. Thus, we suspected that there might be a sequencing error around that region. When we sequenced the junction between pssR and yifA, we found an additional G just after the 80th codon of pssR in our strains derived from W3110 or MC4100. Accordingly, the pssR and yifA genes merged into a single ORF of 279 amino acids, which was named hdfR, for hns-dependent flhDC regulator. The predicted size of the protein matches well with the band on a sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel (data not shown). In this protein, the helix-turn-helix motif is found in the region between residues 17 and 47. The site of transposon insertion was found immediately after amino acid residue 168 (Fig. 2). Recently, GenBank filed an hdfR homologue (STMD1.99 [accession no. AF233324]) in Salmonella serovar Typhimurium LT2, encoding a 282-amino-acid protein (82% identical to the E. coli HdfR).
In order to confirm that the insertion effect is solely due to the disruption of the hdfR gene, a complementation experiment was conducted with the hdfR-containing plasmid. The DNA fragment containing hdfR originated from the Kohara library (10), in which the gene is expressed by its own promoter. Introduction of the plasmid into strains containing hdfR::TnphoA restored the normal level of flhDC-lacZ transcription (Fig. 1, pHdfR), although the plasmid expression of HdfR exhibits a copy effect, as seen in the rightmost bar of Fig. 1.
Flagellar regulation by hdfR seems independent of membrane phospholipid.
As described previously, hdfR was found in a region where pssR, which is involved in the expression of pssA, was mapped. On the other hand, it was reported that the pssA and psd genes, encoding enzymes for the synthesis of phosphatidylethanolamine (PE), are required for the expression of the flhDC operon (19). Thus, one might suspect that flagellar regulation and phospholipid synthesis might be associated at either the genetic or the physiological level.
In order to test whether hdfR is allelic to pssR, whose mutation increases the expression of pssA, we examined the effect of hdfR insertion on the expression of the pssA gene using a PpssA-lacZ fusion on pMS330. The plasmid contained the promoter fragment, including 440 bp upstream to 174 bp downstream from the translation initiation site of pssA. The β-galactosidase activities of pMS330 in MS296 (MC4100 flhD+), MS299 (MS296 hns::neo), MS377 (MS296 hdfR::TnphoA132), and MS380 (MS296 hns::neo hdfR::TnphoA132) were similar within the ranges between 11,300 and 14,300 Miller units (14). This result suggests that the hdfR gene may not regulate pssA and thus differs from pssR. We also measured the proportions of PE among the total cellular phospholipids from the wild type (MS296) and hns mutant (MS299) strains using thin-layer chromatography (15). The two strains contained similar ratios of PE: 71.2% for MS296 and 72.4% for MS299. This implies that the hns effect on the transcription of flhDC is not due to a PE depletion.
Purified HdfR binds to the promoter region of flhDC.
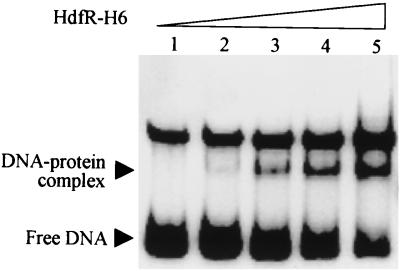
In order to test the possibility of HdfR serving as a transcriptional regulator, a gel shift assay was performed with purified HdfR for the promoter region of the flhDC operon, including −626 to +185 from the transcription start. HdfR with a C-terminal His tag (HdfR-His6) was expressed under the control of the T7 promoter from the pET-HdfR plasmid derived from pET-21b (Novagen). After induction with isopropyl-β-d-thiogalactopyranoside (IPTG) in the BL21(DE3) strain, the amount of the HdfR-His6 protein was estimated to be about 10% of the total soluble fraction that appeared on a Coomassie-stained SDS-polyacrylamide gel. For purification of HdfR with an additional 22 amino acids including the His tag at the C terminus, a His-bind resin (Novagen) was loaded with an addition of 3-[(3-cholamidopropyl)-dimethylammonio]-1-propanesulfonate (CHAPS) (Sigma) to the binding buffer at a concentration of 0.25% because HdfR-His6 bound much better under mildly denaturing conditions than under native conditions. The purified protein exhibits a size of about 35 kDa with more than 98% purity on an SDS-polyacrylamide gel. The binding of purified HdfR-His6 on the DNA fragment shifted mobility on gel electrophoresis (Fig. 3), suggesting that the protein functions as a transcriptional regulator for the flhDC operon.
FIG. 3.
Gel mobility shift of the DNA fragment containing the flhDC promoter by purified HdfR-His6. The probe DNA corresponding to the region from −626 to +185 bases from the transcription start was obtained by PCR and labeled with [γ-32P]ATP using polynucleotide kinase (Boehringer Mannheim). For a 20-μl reaction volume, 1 ng of DNA probe (about 3,000 cpm), 2 μg of sheared salmon sperm DNA, and 0 to 100 ng of purified proteins were used. The buffer contained 50 mM KCl, 20 mM Tris-Cl (pH 7.9), 1 mM dithiothreitol, 10% glycerol, and 125 μg of bovine serum albumin/ml. The whole mixture was stored on ice for 30 min and loaded onto a 5% low-ionic-strength polyacrylamide gel (2). The DNA species are appropriately marked. The unlabeled upper band appears to be a denatured form of the probe generated during the purification step. Amounts of purified HdfR-His6 proteins used are none (lane 1), 10 ng (lane 2), 20 ng (lane 3), 50 ng (lane 4), and 100 ng (lane 5).
Expression of the hdfR gene is negatively regulated by H-NS.
The results so far suggest a possibility that HdfR is a mediator for H-NS-dependent regulation of the flhDC operon. The next question would be how hdfR is regulated by H-NS. We directly examined the expression of hdfR by H-NS by subcloning the fragment of hdfR containing its promoter region (flanked by Sau3AI) to lacZ (preceded by a BamHI site) in pMC1396 (5). The resulting plasmid (pMS274) carries a lacZ translational fusion, whose β-galactosidase activity was increased about twofold by a deletion of hns (hns::neo), from 1,022.3 ± 5.6 Miller units for the wild type (MS296/pMS274) to 2,181.1 ± 20.1 for its hns::neo drivative (MS299/pMS274). This result indicates that H-NS negatively modulates the expression of hdfR, although we still cannot exclude the possibility of indirect interaction between H-NS and the promoter of hdfR.
We describe here a new LysR family protein, HdfR, which is involved in the H-NS-dependent regulation of the flagellar master operon by binding to the upstream region of the operon. The expression of hdfR was negatively controlled by H-NS, which explains the negative effect on flagellation of an H-NS mutation. When H-NS is inactive, the expression of hdfR gene is increased, resulting in an overproduction of HdfR, which will reduce the transcription of flhDC by binding to its upstream region. In a previous report (23), the observation of flhDC activation in vivo by H-NS was not correlated with an in vitro transcription assay. Our model involving HdfR provides an alternative to the previous conjecture that H-NS serves as a direct activator for flhDC. Our model is more consistent with the fact that the modes of H-NS regulation in most cases were characterized as transcriptional silencers. It would be interesting in the near future to reveal the physiological signal to which HdfR is responding. The global nature of transcriptional regulation by the LysR family protein may also apply to the regulation involving HdfR. Therefore, a future study of HdfR is likely to uncover a novel global network comprising the flagellar regulon.
Nucleotide sequence accession number.
The nucleotide and deduced amino acid sequences of the hdfR gene have been submitted to GenBank under accession no. AF25103.
Acknowledgments
We thank T. Mizuno, Bob Simons, and C. Ueguchi for strains and plasmids.
This work was supported in part by the Creative Research Initiative Program.
REFERENCES
- 1.Atlung T, Sund S, Olesen K, Brondsted L. The histone-like protein H-NS acts as a transcriptional repressor for expression of the anaerobic and growth phase activator AppY of Escherichia coli. J Bacteriol. 1996;178:3418–3425. doi: 10.1128/jb.178.12.3418-3425.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Short protocols in molecular biology. 2nd ed. New York, N.Y: John Wiley and Sons, Inc.; 1992. [Google Scholar]
- 3.Bartlett D H, Frantz B B, Matsumura P. Flagellar transcriptional activators FlbB and FlaI: gene sequences and 5′ consensus sequences of operons under FlbB and FlaI control. J Bacteriol. 1988;170:1575–1581. doi: 10.1128/jb.170.4.1575-1581.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bertin P, Terao E, Lee E H, Lejeune P, Colson C, Danchin A, Collatz E. The H-NS protein is involved in the biogenesis of flagella in Escherichia coli. J Bacteriol. 1994;176:5537–5540. doi: 10.1128/jb.176.17.5537-5540.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Casadaban M J. Transposition and fusion of the lac genes to selected promoters in Escherichia coli using bacteriophage lambda and Mu. J Mol Biol. 1976;104:541–555. doi: 10.1016/0022-2836(76)90119-4. [DOI] [PubMed] [Google Scholar]
- 6.Donato G M, Lilivelt M J, Kawula T H. Promoter-specific repression of fimB expression by the Escherichia coli nucleoid-associated protein H-NS. J Bacteriol. 1997;179:6618–6625. doi: 10.1128/jb.179.21.6618-6625.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Durrenberger M, Teana A L, Citro G, Venanzi F, Gualerzi C O, Pon C L. Escherichia coli DNA-binding protein H-NS is localized in the nucleoid. Res Microbiol. 1991;142:373–380. doi: 10.1016/0923-2508(91)90106-k. [DOI] [PubMed] [Google Scholar]
- 8.Harrison J A, Pickard D, Higgins C F, Khan A, Chatfield S N, Ali T, Dorman C J, Hormaeche C E, Dougan G. Role of hns in the virulence phenotype of pathogenic salmonellae. Mol Microbiol. 1994;13:133–140. doi: 10.1111/j.1365-2958.1994.tb00408.x. [DOI] [PubMed] [Google Scholar]
- 9.Higgins C F, Dorman C J, Stirling D A, Waddell L, Booth I R, May G, Bremer E. A physiological role for DNA supercoiling in the osmotic regulation of gene expression in S. typhimurium and E. coli. Cell. 1988;52:569–584. doi: 10.1016/0092-8674(88)90470-9. [DOI] [PubMed] [Google Scholar]
- 10.Kohara Y, Akiyama K, Isono K. The physical map of the whole E. coli chromosome: application of new strategy for rapid analysis and sorting of a large genomic library. Cell. 1987;50:495–508. doi: 10.1016/0092-8674(87)90503-4. [DOI] [PubMed] [Google Scholar]
- 11.Komeda Y. Fusions of flagellar operons to lactose genes on a Mu lac bacteriophage. J Bacteriol. 1982;150:16–26. doi: 10.1128/jb.150.1.16-26.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macnab R M. Flagella and motility. In: Neidhardt F C, Curtiss III R, Ingraham J L, Lin E C C, Low K B, Magasanik B, Reznikoff W S, Riley M, Schaechter M, Umbarger H E, editors. Escherichia coli and Salmonella: cellular and molecular biology. 2nd ed. Washington, D.C.: ASM Press; 1996. pp. 123–145. [Google Scholar]
- 13.Maurelli A T, Sansonetti P J. Identification of a chromosomal gene controlling temperature-regulated expression of Shigella virulence. Proc Natl Acad Sci USA. 1988;85:2820–2824. doi: 10.1073/pnas.85.8.2820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Miller J H. Experiments in molecular genetics. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1972. [Google Scholar]
- 15.Nishijima M, Raetz C R H. Membrane lipid biogenesis in Escherichia coli: identification of genetic loci for phosphatidylglycerophosphate synthetase and construction of mutants lacking phosphatidylglycerol. J Biol Chem. 1979;254:7837–7844. [PubMed] [Google Scholar]
- 16.O'Byrne C P, Dorman C J. Transcription of the Salmonella typhimurium spv virulence locus is regulated negatively by the nucleoid-associated protein H-NS. FEMS Microbiol Lett. 1994;121:99–105. doi: 10.1111/j.1574-6968.1994.tb07082.x. [DOI] [PubMed] [Google Scholar]
- 17.Powell B S, Court D L, Nakamura Y, Rivas M P, Turnbough C L., Jr Rapid confirmation of single copy lambda prophage integration by PCR. Nucleic Acids Res. 1994;22:5765–5766. doi: 10.1093/nar/22.25.5765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schell M A. Molecular biology of the LysR family of transcriptional regulators. Annu Rev Microbiol. 1993;47:597–626. doi: 10.1146/annurev.mi.47.100193.003121. [DOI] [PubMed] [Google Scholar]
- 19.Shi W, Bogdanov M, Dowhan W, Zusman D R. The pss and psd genes are required for motility and chemotaxis in Escherichia coli. J Bacteriol. 1993;175:7711–7714. doi: 10.1128/jb.175.23.7711-7714.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shi W, Li C, Louise C J, Adler J. Mechanism of adverse conditions causing lack of flagella in Escherichia coli. J Bacteriol. 1993;175:2236–2240. doi: 10.1128/jb.175.8.2236-2240.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shin S, Park C. Modulation of flagellar expression in Escherichia coli by acetyl phosphate and the osmoregulator OmpR. J Bacteriol. 1995;177:4696–4702. doi: 10.1128/jb.177.16.4696-4702.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Simons R W, Houman F, Kleckner N. Improved single and multicopy lac-based cloning vectors for protein and operon fusions. Gene. 1987;53:85–96. doi: 10.1016/0378-1119(87)90095-3. [DOI] [PubMed] [Google Scholar]
- 23.Soutourina B, Kolb A, Krin E, Laurent-Winter C, Rimsky S, Danchin A, Bertin P. Multiple control of flagellum biosynthesis in Escherichia coli: role of H-NS protein and the cyclic AMP-catabolite activator protein complex in transcription of the flhDC master operon. J Bacteriol. 1999;181:7500–7508. doi: 10.1128/jb.181.24.7500-7508.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sparrow C P, Raetz R H. A trans-acting regulatory mutation that causes overproduction of phosphatidylserine synthase in Escherichia coli. J Biol Chem. 1983;258:9963–9967. [PubMed] [Google Scholar]
- 25.Varshavsky A J, Nedospasov S A, Bakayeva V V, Georgiev G P. Histone-like proteins in the purified Escherichia coli deoxyribonucleoprotein. Nucleic Acids Res. 1977;4:2725–2745. doi: 10.1093/nar/4.8.2725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wilmes-Reisenberg M R, Wanner B L. TnphoA and TnphoA′ elements for making and switching fusions for study of transcription, translation, and cell surface localization. J Bacteriol. 1992;174:4558–4575. doi: 10.1128/jb.174.14.4558-4575.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Yamada H, Yoshida T, Tanaka K, Sanakawa C, Mizuno T. Molecular analysis of the Escherichia coli hns gene encoding a DNA-binding protein, which preferentially recognizes curved DNA sequences. Mol Gen Genet. 1991;230:332–336. doi: 10.1007/BF00290685. [DOI] [PubMed] [Google Scholar]