Abstract
Background
Vaccinations have reduced severe burden of COVID-19 and allowed for lifting of non-pharmaceutical interventions. However, with immunity waning alongside emergence of more transmissible variants of concern, vaccination strategies must be examined.
Methods
Here we apply a SARS-CoV-2 transmission model to identify preferred frequency, timing, and target groups for vaccine boosters to reduce public health burden and health systems risk. We estimated new infections and hospital admissions averted over 2 years through annual or biannual boosting of those eligible (those who received doses one and two) who are (1) most vulnerable (60+ or living with comorbidities) or (2) those 5+, at universal (98% of eligible) or lower coverage (85% of those 50+ or with comorbidities and 50% of 5–49 year olds) representing moderate vaccine fatigue and/or hesitancy. We simulated three emerging variant scenarios: (1) no new variants; (2) 25% more infectious and immune-evading Omicron-level severity variants emerge annually and become dominant; (3) emerge biannually. We further explored the impact of varying seasonality, variant immune-evading capacity, infectivity, severity, timing, and vaccine infection blocking assumptions.
Results
To reduce COVID-19-related hospitalisations over the next 2 years, boosters should be provided for all those eligible annually 3–4 months ahead of peak winter whether or not new variants of concern emerge. Only boosting those most vulnerable is unlikely to ensure reduced stress on health systems. Moreover, boosting all eligible better protects those most vulnerable than only boosting the vulnerable group. Conversely, while this strategy may not ensure reduced stress on health systems, as an indication of cost-effectiveness, per booster dose more hospitalisations could be averted through annual boosting of those most vulnerable versus all eligible, since those most vulnerable are more likely to seek hospital care once infected, whereas increasing to biannual boosting showed diminishing returns. Results were robust when key model parameters were varied. However, we found that the more frequently variants emerge, the less the effect boosters will have, regardless of whether administered annually or biannually.
Conclusions
Delivering well-timed annual COVID-19 vaccine boosters to all those eligible, prioritising those most vulnerable, can reduce infections and hospital admissions. Findings provide model-based evidence for decision-makers to plan for administering COVID-19 boosters ahead of winter 2022–2023 to help mitigate the health burden and health system stress.
Supplementary Information
The online version contains supplementary material available at 10.1007/s40121-022-00683-z.
Keywords: COVID-19, SARS-CoV-2, Vaccination, Booster, Immunity, Modelling
Summary Points
| As COVID-19-related immunity wanes and more transmissible SARS-CoV-2 variants of concern continue to emerge, future vaccination booster strategies must be examined. |
| We applied an individual-based COVID-19 transmission model to identify the preferred frequency, timing, and target groups for vaccine boosters to reduce the public health burden and health systems risk. |
| We found that COVID-19 boosters should be provided for all those eligible 3–4 months ahead of peak winter season at least over the next 2 years whether or not new variants of concern emerge. |
| Conversely, since those most vulnerable (those aged 60 years and older or those living with comorbidities) are most likely to require hospitalisation following SARS-CoV-2 infection, we found that more hospitalisations could be averted per booster dose through annual boosting of those most vulnerable versus boosting all eligible; however, only boosting the vulnerable group is unlikely to ensure reduced stress on health systems. |
| Future booster strategies must also consider the vaccine-induced immune-evading capacity of existing and new variants to first-generation COVID-19 vaccines and to next-generation vaccines as they become available. |
Introduction
Between December 2019 and May 2022, 529 million confirmed cases of COVID-19 and > 6.29 million COVID-19-related deaths were reported [1]. As of May 2022, > 11.9 billion COVID-19 vaccine doses have been administered worldwide, but as SARS-CoV-2 variants continue to spread, increasing global vaccine coverage will be crucial. Rollout strategies for first and second vaccine doses are being combined with subsequent booster doses to bolster immunity levels. Rapid viral evolution and the relatively short period that COVID-19 vaccines have been available have limited our ability to observe vaccine efficacy and duration over long periods. For example, studies have reported effectiveness against symptomatic disease from the Omicron variant following a booster dose with an mRNA vaccine to resume to levels similar to those following dose 2 [2, 3]. This is reassuring for those who received their booster dose and are thus benefiting from a lower probability of becoming infected and developing COVID-19-related disease, but highlights the need for a longer-term vaccination strategy that accounts for waning immunity, particularly with the likelihood of the emergence of new immune-evading variants of concern.
Prior to Omicron subvariants, new SARS-CoV-2 variants had emerged and become dominant about every 6 months [4], and while the overall risk of severe disease or death from Omicron infection is lower than from the previously dominant Delta variant [5], Omicron subvariants are more infectious and their rapid spread is still causing health system stress. Long-COVID may also continue to contribute to the public health burden. Furthermore, variants of the Omicron lineage are continuing to evolve and make gains by eroding host immunity. If this continues, then SARS-CoV-2 epidemics may reach steady state and become endemic similar to influenza. In this case, however, when population-wide immunity declines, circulating variants with immune-evading mutations could become dominant and may potentially be more severe, and periodic waves may arise [4]. This emphasises the urgency for everyone to receive at least one COVID-19 vaccine and highlights the need for strategic delivery of vaccine booster doses.
Using our individual-based SARS-CoV-2 transmission model, OpenCOVID, we simulated a variety of vaccination strategies and model assumptions to identify the preferred frequency, timing, and target population for future vaccine booster doses in light of potential emerging viral variants. Model outcomes are used to assess the impact of different booster strategies on public health outcomes and health system risks.
Methods
Model Framework
Our individual-based model, OpenCOVID, is a dynamic stochastic discrete-time model of SARS-CoV-2 transmission and COVID-19 disease progression and response [6]. The model simulates viral transmission between infectious and susceptible individuals that come in contact through an age-structured, small-world network. The probability of transmission in each exposure stage is influenced by the infectiousness of the infected individual, the immunity of the susceptible individual (acquired through previous infection and/or through vaccination), and a background seasonality pattern (reflecting a larger proportion of contacts being in closer contact indoors as the temperatures became cooler). Infectiousness is a function of variant infectivity and time since infection. Once infected, a latency period is followed by a pre-symptomatic stage, after which an individual can experience asymptomatic, mild, or severe disease. Severe cases can lead to hospitalisation, intensive care unit admission, and ultimately death (see Table S1 in the Electronic Supplementary Material for further details on prognosis probabilities by age following infection with SARS-CoV-2 Omicron variant). Recovery after infection leads to development of immunity. This immunity is assumed to wane over time as illustrated in Figure S1, with the risk of new infection depending on the probability of exposure and properties of existing and potential novel SARS-COV-2 variants (i.e., infectiousness and immune evading profile). Briefly, the model captures immunity decay for naturally acquired and vaccine-induced infection and seasonality (as illustrated in the Electronic Supplementary Material Figures S1 and S2, respectively); infection states and non-pharmaceutical interventions (NPIs) can be included (as further described in the “Methods” section “Non-pharmaceutical Interventions”). They can be tailored to a specific setting or as investigated here an archetypal setting with Re fitted to 1.0 in spring (as further described in the “Methods” section “Model Parameterisation and Initialisation”) in the absence of any NPIs assuming immunity from natural infection and vaccination is similar to profiles for Europe in early 2022 during the first Omicron wave. A detailed description of the OpenCOVID model including model equations is provided in [6]. Open access source code for the OpenCOVID model used in this study is publicly available at https://github.com/SwissTPH/OpenCOVID/tree/manuscript_june_2022/src, with all figure and output codes available at https://zenodo.org/record/6878852#.Yv53t3ZBw2xMI2w. Ethics approval and consent to participate in this study is not applicable.
Model Parameterisation and Initialisation
We simulate an 850-day period, approximately 2 years and 4 months from spring in year 1 to summer in year 3. We apply a global demographic distribution [7] to a simulated population of 100,000 people and assume 40% of the population had previously been infected with SARS-CoV-2 at least once over the 2-year simulation period.
The model was fitted to allow an archetypal setting with an effective reproduction number, , of 1.0 at the start of the simulation period, representing the shoulder season between winter and summer in year 1. This represents the global trend for case numbers when the Omicron variant emerged and became the dominant variant in late 2021. Using an average number of daily contacts the model was fitted to this of 1.0 in early spring 2022. This inherently captures the effect of any non-pharmaceutical interventions that were in place at that time. To reflect the element of chance that naturally occurs in model transmission dynamics, 100 random stochastic simulations were performed for each scenario with 95% prediction intervals presented.
Seasonality
Simulations represent an archetypal setting approximately representative of Northern hemisphere seasonality. Seasonality is assumed to follow a scaled cosine function. It oscillates between minimum seasonal forcing during the peak summer period and maximum forcing during peak winter months as illustrated in Figure S2 of the Electronic Supplementary Material. As people tend to remain indoors during the winter, the probability of transmission per contact is increased during winter months. The best estimate for the seasonal scaling factor was taken from a previous study assessing the COVID-19 epidemic in Switzerland [6]. This previous study simultaneously fit this seasonal scaling factor parameter along with several other parameters to align the OpenCOVID model to epidemiological data. To assess the effect of the seasonal scaling factor on model outcomes, this value was subjected to a sensitivity analysis.
Non-pharmaceutical Interventions
To examine the sole effect of vaccinations on the spread of SARS-CoV-2 and COVID-19 disease progression, we did not explicitly model any NPIs such as physical distancing and facemask usage and lockdowns in future scenarios. However, since a proportion of the population will likely continue to wear masks in some situations such as on public transport in certain settings, this protective effect was captured indirectly by using the number of effective network contacts to calibrate the model to the effective reproduction number at the start of the simulation period.
Vaccination Strategy
In this analysis, we simulate the impact of the first-generation mRNA vaccine [here we modelled vaccine profile for the Pfizer/BioNTech (BNT162b2)], which was developed with specificity to the dominant SARS-CoV-2 variant at the time, the Alpha variant. Vaccines have a two-fold effect; first, they provide protection against new infection through development of immunity. Second, once infected, vaccines reduce the probability of developing severe symptoms, leading to reduced hospitalisations, intensive care unit admissions, and potentially death.
Individuals who previously received primary vaccination (doses one and two) prior to the start of the simulation period were considered eligible to receive booster doses. Default universal coverage of booster doses was set to 98% coverage of those eligible. We assume 1500 booster doses per 100,000 people per day (1.5% of the simulated population) could be administered based on achievable vaccine delivery rates across several countries [8]. Doses were administered sequentially by descending age and comorbidity risk group as described in the “Scenario Design” section of the “Methods.”
Following administration of each booster dose, vaccine-induced immunity is assumed to immediately peak at 85% [3] before exponentially waning to 15% with a half-life of 105 days (based on longer-term waning for dose 2 from reference [2]) (see Figure S1 in the Electronic Supplementary Material for details). The infection-blocking component of the vaccine was assumed to represent 80% of the overall 85% vaccine efficacy, with the remaining 5% attributed to preventing infections from progressing to severe disease. This infection-blocking value was subjected to a sensitivity analysis. Vaccine protection is modelled in the context of naturally acquired immunity following SARS-CoV-2 infection, whereby natural immunity is assumed to reach peak immunity of 95% aligned with findings from Chivese et al. [9]. Before waning exponentially to 20% in 600 days [10].
Variant Properties
At the time of writing, Omicron was the dominant SARS-CoV-2 variant worldwide, with subvariants BA.1, BA.2, BA.4, BA.5, and BA.2.12.1 having emerged along the Omicron lineage, having replaced the previously dominant Delta variant (B.1.617.2). Based on previous global trends, we assume conservatively that new variants of concern will emerge ahead of the winter season at 6 or 12 month intervals. We assume a variant profile with 25% higher infectivity than the previously dominant variant (a transmission multiplication factor of 1.25 per exposure), with immune evasion properties of 25%, but with the same severity as the Omicron variant. The probability that immunologically naïve individuals who become infected will develop severe disease is dependent on variant severity, but also the individuals’ age and comorbidity status, with further details described in [11]. To assess the effect of variant properties on model outcomes, values for variant infectivity, severity, and immune-evading capacity were subjected to a sensitivity analysis.
Scenario Design
Scenarios were designed to model the impact of vaccine booster doses administered at different frequencies, coverage levels, and timings and to different target groups. A baseline scenario was established whereby the primary series vaccination (doses one and two) was implemented prior to the simulation period for those 5 years and older, with no booster doses given during the simulations. Baseline vaccination coverage is 93% of those most vulnerable (those 60 years and older following WHO reports [12] or persons with comorbidities), 90% of 50–59 year olds, 80% of 30–49 year olds, and 75% of 5–29 year olds.
All other scenarios are identical to baseline with primary vaccination (doses one and two) administered pre-simulation at baseline coverage, but with first-generation booster doses (three and up) administered during the simulation to 98% of those who previously received the primary series, that is those eligible to receive booster doses, with this referred to as universal booster coverage. Vaccine boosters were administered every 6 or 12 months, starting in year 1 ahead of summer for the biannual booster scenario or ahead of winter for the annual scenario. For all booster scenarios, administering doses to those most vulnerable every 6 months also includes boosting all eligible every 12 months. Lower booster coverage of those eligible was administered first to 85% of those most vulnerable (those 60 years and older or persons with comorbidities), then 85% of individuals 50–59 years of age, followed by 50% of 30–49 year olds, and lastly 50% of 5–29 year olds. Lower booster coverage is an approximate reflection of booster coverage levels in Europe at the time of writing and represents moderate vaccine fatigue and/or hesitancy [8].
Sensitivity Analysis
A sensitivity analysis was conducted to assess the impact of varying key model parameter inputs, as detailed in Table 1, on projected outcomes.
Table 1.
Inputs used for the sensitivity analysis of key parameters
| Parameter | Parameter effect | Best estimate | Lower bound | Upper bound |
|---|---|---|---|---|
| Seasonality-scaling factor (dimensionless)a | Strength of seasonal forcing on infectiousness per exposure | 0.30 [6] | 0.20 | 0.40 |
| Emerging variant severity (dimensionless) | Multiplicative probability of severe disease for each new emerging variant | 1.00 | 0.80 | 1.20 |
| Emerging variant timing | Impact of when novel variants emerge and become dominant | 3.5 months before peak winter | − 1 month | + 1 month |
| Vaccine infection-blocking (%) | Infection-blocking effect of vaccination (remainder is severe disease prevention) | 0.85b | 0.60 | 0.95 |
| Emerging variant immune evasion (%) | Impact of immune evasion on variant infectiousness | 0.25 | 0 | 0.50 |
| Emerging variant infectivity (ratio) | Multiplicative probability of transmission per exposure for each emerging variant | 1.25 | 1.10 | 1.40 |
No effect was observed from varying daily vaccine booster capacity (results not shown) from 1500 (best estimate) to 750 doses (lower bound) and 2250 (upper bound) per day in a simulated population of 100,000 people following default timing to start administering doses
aSee Electronic Supplementary Material Figure S2 for the seasonality profile
bAs shown in Electronic Supplementary Material Figure S1
Results
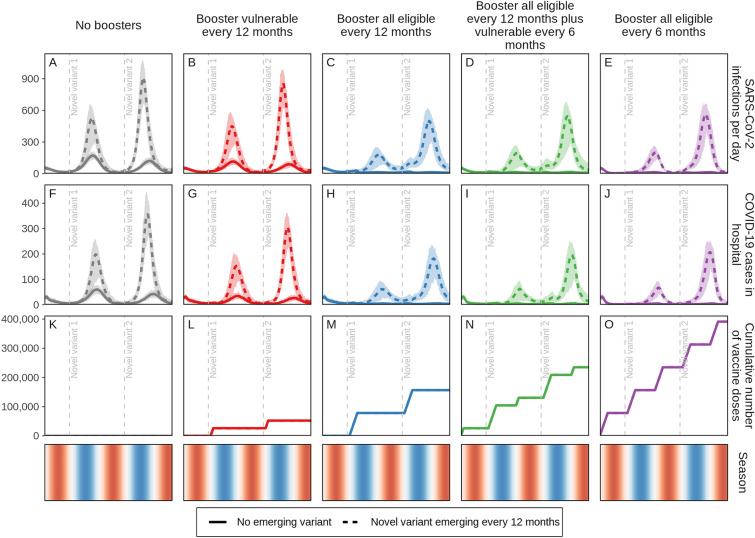
We examined the benefit of targeting booster doses to those eligible to receive boosters who are either most vulnerable (those 60 years and older or persons with comorbidities) or all those eligible (5 years and older) using the described age and risk factor priority scheme in settings with either no new SARS-CoV-2 variants emerging or with new variants 25% more infectious and immune-evading, but with the same level of severity as the Omicron variant emerging annually. We found that the biggest relative impact was from annual boosting of all those eligible, which was consistent with or without new emerging variants (Fig. 1, blue dashed and solid curves, respectively), given the assumptions modelled for waning immunity. In the case where no new variants emerge, boosting only those most vulnerable every 12 months led to a 32% (95% CI 24–40%) reduction in hospital admissions over a 2-year period compared with no boosters. If annual boosting were to be expanded to those eligible, a 70% (95% CI 65–73%) reduction in hospitalisations could be achieved over this period. If boosting frequency were increased to every 6 months and targeted to only those most vulnerable (while continuing to boost those eligible annually), then a 77% (95% CI 74–80%) reduction could be realised, an over two-fold decrease in admissions. Extending biannual boosting to those eligible is projected to have diminishing returns, with an 81% (95% CI 79–83%) reduction in hospitalisations over this period, keeping in mind that the numbers of booster doses required per 100,000 people per year for these four strategies (annual boosting of most vulnerable, eligible annually, vulnerable biannually with eligible annually, and eligible biannually) is 26,000, 78,000, 104,000, and 156,000, respectively, with those most vulnerable representing a third of those eligible to receive boosters.
Fig. 1.
Daily projected impact on SARS-CoV-2 infections and COVID-19-related hospital admissions in just over a 2-year period in a population of 100,000 individuals. Those who previously received vaccine doses one and two were eligible to receive a first-generation COVID-19 vaccine booster dose. Individuals who received boosters every 12 or 6 months are aggregated in two groups, (1) those most vulnerable, defined as those 60 years and older or persons with comorbidities, and (2) all eligible subjects 5 years and older. For these groups, either no new variant emerged—and thus the same infectiousness and severity as the Omicron variant was assumed (solid curves)—or 25% more infectious and immune-evading novel variants, with Omicron-level variant severity, emerged annually (dashed curves) with vertical dashed lines indicating initial variant emergence. Shaded areas represent stochastic uncertainty surrounding projections. Cumulative number and timing of vaccine doses are shown for each scenario. Seasonality is illustrated in the bottom row where red shading indicates warmer spring and summer seasons, blue cooler fall and winter seasons, and white the seasonal transition. See Figures S3A–S5B in the Electronic Supplementary Material for details
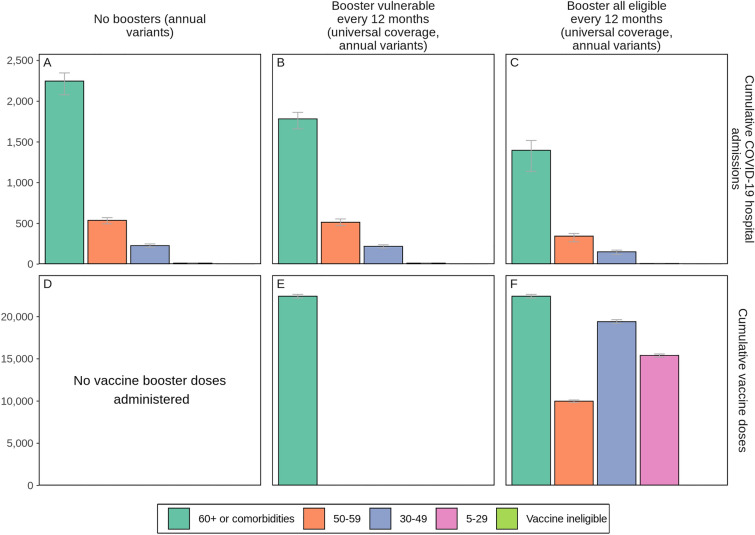
Similar trends were observed when variants that are 25% more infectious and immune evading, but similar in severity as the Omicron variant, emerged annually and became dominant, although overall reductions in hospitalisations were more modest than when no variant emerged. Over the 2-year study period, if variants were to emerge biannually then 15% (95% CI 3–25%) of hospitalisations could be averted if only those most vulnerable were boosted yearly and 27% (95% CI 17–34%) if those eligible were boosted yearly compared with no boosters. Increasing booster frequency to every 6 months could avert 35% (95% CI 28–43%) or 40% (95% CI 31–45%) of COVID-19-related hospitalisations if those most vulnerable or those eligible were boosted, respectively. Importantly, boosting 98% of those eligible (those 5 years and older who received vaccine doses one and two) actually protects those most vulnerable 60 years and older or those with comorbidities more than only boosting this vulnerable group as shown in Fig. 6.
Fig. 6.
Cumulative projected impact of administering COVID-19 vaccine boosters to those most vulnerable or to all those eligible every 12 months on COVID-19-related hospital admissions by age and risk group over a 2-year period in a simulated population of 100,000 individuals when new SARS-CoV-2 variants with 25% more infectiousness and immune-evading capacity than the previously dominant variant but with the same severity as Omicron emerge annually. Cumulative numbers of vaccine doses are shown in panels D-F. Error bars represent the stochastic uncertainty in model projections. See Figure S9 in the Electronic Supplementary Material for details.
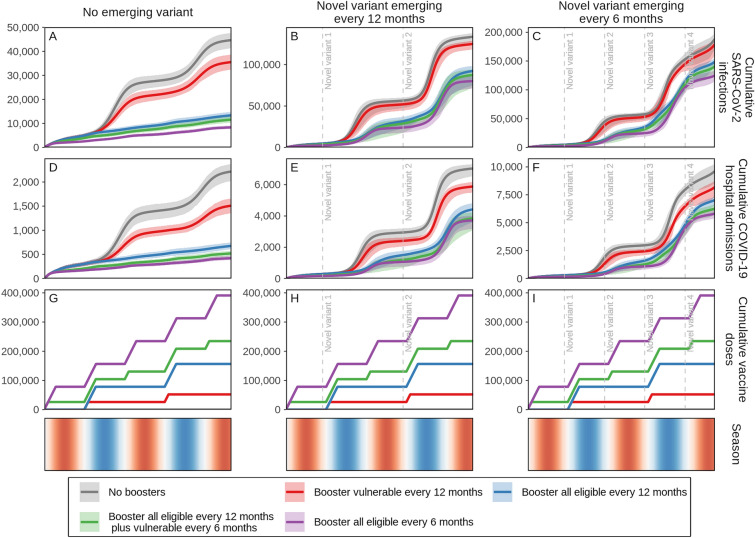
We further explored the effect of a viral variant emerging conservatively every 6 months, assuming each new variant is 25% more immune-evading and 25% more infectious than the previous dominant variant, but with the same level of severity as the Omicron variant (Figures S12A, B in the Electronic Supplementary Material show the prevalence for each variant over time). Under these conditions, we also estimate a substantial benefit of annual boosters for all eligible people followed by diminishing returns if boosting more frequently than once per year. However, the relative differences between these strategies are much less pronounced, as the new variants partially evade any previously acquired immunity (naturally acquired and/or vaccine-induced), leading to more overall SARS-CoV-2 infections. We show the cumulative projected impact on SARS-CoV-2 infections and COVID-19-related hospital admissions with no emerging variant or new variants emerging every 12 or 6 months (Fig. 2). This reinforces that regardless of the presence or frequency of new emerging variants, while there is benefit to boosting those most vulnerable annually ahead of the cooler seasons, administering annual boosters to all those eligible shows the biggest relative gain in infections and hospital admissions averted (Fig. 2, blue curves). Whereas boosting every 6 months is predicted to have the biggest benefit, based on the additional vaccine booster doses needed to boost twice as often (every 6 months) we estimate diminishing returns (Fig. 2, green and purple curves).
Fig. 2.
Cumulative projected impact on SARS-CoV-2 infections and COVID-19-related hospital admissions for just over a 2-year period in a simulated population of 100,000 individuals with those who previously received vaccine doses one and two being eligible to receive first-generation COVID-19 vaccine booster doses. Individuals who received boosters every 12 or 6 months are aggregated in two groups: (1) those most vulnerable, defined as those 60 years and older or persons with comorbidities, and (2) all eligible subjects 5 years and older. For these two groups, scenarios were designed either with no new emerging SARS-CoV-2 variants, assuming the same infectiousness and severity of the Omicron variant (A, D, and G), or with 25% more infectious and immune-evading novel variants with the same severity as the Omicron variant emerging annually (B, E, and H) or biannually (C, F, and I) with initial emergence indicated by vertical dashed lines. Shaded areas represent the stochastic uncertainty surrounding projections. Cumulative number and timing of vaccine doses are shown for each scenario. Seasonality is illustrated in the bottom row where red shading indicates warmer spring and summer seasons, blue cooler fall and winter seasons, and white seasonal transitions. See Figures S6, S7, and S10–S14B in the Electronic Supplementary Material for details
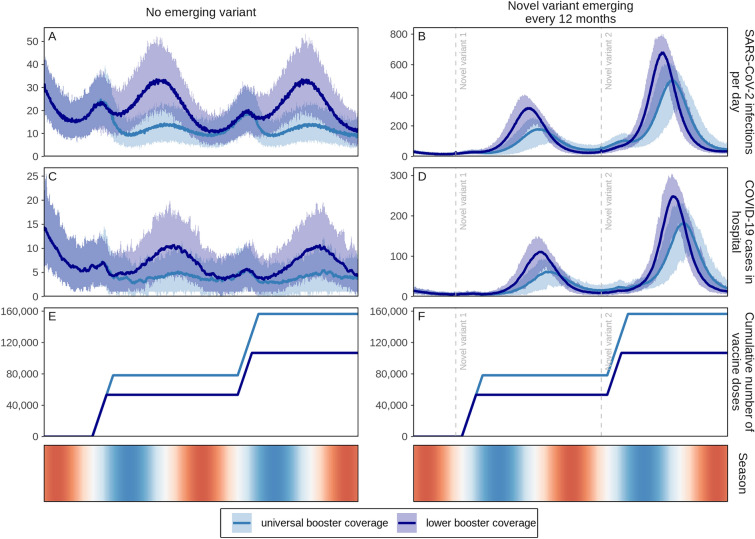
We also examined whether lower booster uptake (85% coverage among those 50 years and older or persons with comorbidities and 50% among those 5–49 years of age) will have a substantial impact on the public health burden over a 2-year period. We found that when no new variants emerge, COVID-19-related hospitalisations could be reduced by 60% (95% CI 53–65%) (Fig. 3C, dark blue curve) at lower booster coverage compared with 70% (95% CI 65–73%) when 98% of those eligible (including 93% of those 60+ or with comorbidities, 90% of 50–59 year olds, 80% of 30–49 year olds, and 75% of 5–29 year olds who received doses one and two) are boosted annually (Fig. 3C, light blue curve). Assuming a new variant emerges annually, we find that hospitalisations could be reduced by 37% (95% CI 30–48%) with universal boosting (98% coverage of those eligible), dropping to 26% (95% CI 18–32%) with lower coverage (Fig. 3D, light and dark blue curves, respectively).
Fig. 3.
Daily projected impact on SARS-CoV-2 infections and COVID-19-related hospital admissions over 2 years in a simulated population of 100,000 individuals with first-generation COVID-19 vaccine boosters administered annually. Boosters were administered at universal coverage (98% of those eligible to receive a booster dose (including 93% of those 60 years and older or persons with comorbidities, 90% of 50–59 year olds, 80% of 30–49 year olds, and 75% of 5–29 year olds who received doses one and two) and lower coverage levels (85% of those eligible who are 50 years and older or who are living with comorbidities and 50% of those eligible 5–49 years of age). For these two groups, either no new SARS-CoV-2 variants emerged, assuming the same infectiousness, immune-evading capacity, and severity as the Omicron variant (A, C, and E), or novel variants with 25% more infectiousness and immune-evading capacity than the previously dominant variant but with the same level of severity as the Omicron variant emerged every 12 months, with initial variant emergence indicated by vertical dashed lines (B, D, and F). Shaded areas represent stochastic uncertainty surrounding projections. Cumulative number and timing of boosters are shown for each scenario. Seasonality is illustrated in the bottom row where red shading indicates warmer spring and summer seasons, blue cooler fall and winter seasons, and white the seasonal transition. See Figure S7 in the Electronic Supplementary Material for cumulative impact
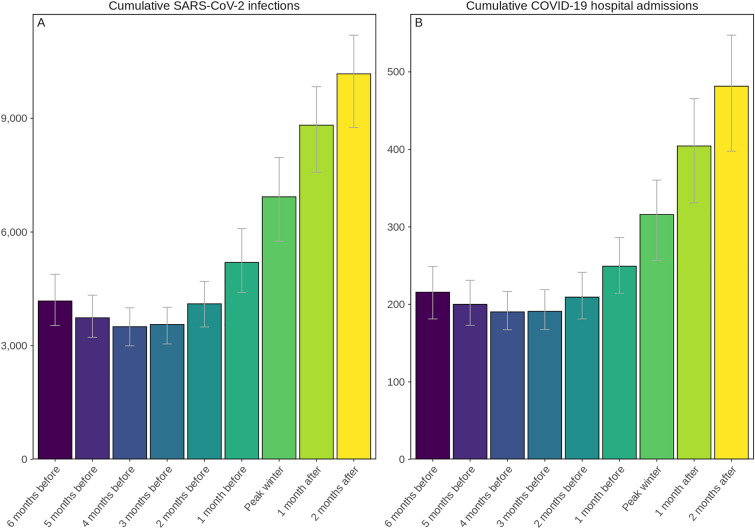
To reduce SARS-COV-2 infections and COVID-19-related hospital admissions when a new variant of concern emerges annually ahead of the winter season, we found that the best time to deliver annual COVID-19 booster doses is 3–4 months before peak winter when people tend to remain indoors most often and their number of contacts increases as does their probability of SARS-CoV-2 transmission (see Fig. 4, and Figure S16A in the Electronic Supplementary Material for details). The seasonality profile as illustrated in Figure S2 of the Electronic Supplementary Material, with the seasonality forcing scaler varied in the sensitivity analysis as shown in Fig. 5, will affect these results. In addition, for this modelling study by default it was assumed booster vaccines were rolled out to 1.5% of the population every day. No effect was observed if the daily boosting capacity was reduced to 0.75% or increased to 2.25% (results not shown in Fig. 5).
Fig. 4.
Projected cumulative annual impact on new SARS-CoV-2 infections (A) and COVID-19-related hospital admissions (B) of delivering vaccine boosters to all those eligible 5 years of age and older from either 6 months before to 2 months after peak winter temperatures assuming a 25% more infectious and immune-evading novel variant but with the same severity as the Omicron variant emerges prior to the fall season. Error bars represent the uncertainty in model projections. See Figures S8, S16A, and S16B in the Electronic Supplementary Material for details
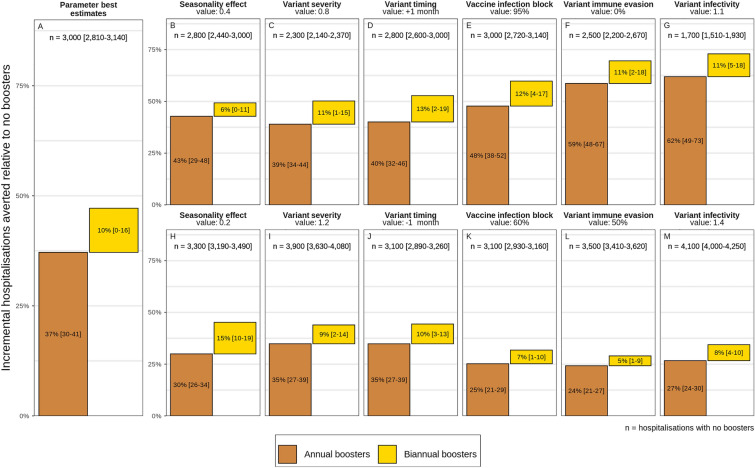
Fig. 5.
Projected percentage incremental hospitalisations averted over a 2-year period by annual (brown bars) and biannual (yellow bars) COVID-19 vaccine boosters for those eligible under varying seasonality, emerging variant severity, variant timing, vaccine infection-blocking efficacy, emerging variant immune-evading capacity, and emerging variant infectivity from best estimate values as listed in Table 1. See Figures S15 and S17 in the Electronic Supplementary Material for details
For all key parameters that were varied, annual boosting consistently provides the majority of reductions of COVID-19-related hospital admissions over the 2-year study period. Higher variant infectivity and higher immune-evading capacity most reduced the effect of boosters (see Fig. 5L, M). The largest relative effect of biannual boosting was observed under lower seasonal effect (see Fig. 5H), in which case biannual boosting is responsible for half the percentage of hospitalisations averted [15% (95% CI 10–19%)] compared with annual boosters [30% (95% CI 26–34%)].
As an indication of cost-effectiveness, we found that more hospitalisations could be averted per booster dose through annual boosting of those most vulnerable versus all those eligible over the study period (see Figure S17 in the Electronic Supplementary Material for details). This can be attributed to the higher likelihood of those most vulnerable needing hospitalisation following SARS-CoV-2 infection, in this case those 60 years of age and older or those living with comorbidities, compared with those less vulnerable to infection. This finding was robust even when key model parameters were varied, again showing diminishing returns per dose when boosting frequency is increased to every 6 months. However, only boosting those most vulnerable (60+ or with comorbidities) may not be sufficient to prevent exceeding health system capacity thresholds (Fig. 6).
Discussion
To reduce COVID-19-related hospitalisation admissions over the next 2 years, our model-based evidence showed that vaccine boosters should be provided for all those eligible (those who previously received two doses) at least 3–4 months ahead of the winter season whether or not new variants of concern emerge and become dominant. At the time of writing, our findings were consistent with a commentary from the chair of global public health at the University of Edinburgh, who stated that, based on data for the UK, the country should be planning the roll-out of boosters to their entire population this autumn [13]. Consistent with our study, a review also found that booster vaccinations should be prioritised to high-risk groups, such as the elderly and people with immunodeficiency, but that not everyone needs to receive a booster dose 6 months after dose two [14]. We found that, under our current assumptions for naturally acquired and vaccine-induced immunity waning, only boosting those most vulnerable (defined as those 60 years of age or older or people living with comorbidities) may be insufficient to ensure reduced stress on health systems, whereas increasing the frequency of boosters to every 6 months (to those most vulnerable or all those eligible) showed diminishing returns. Importantly, we showed that boosting all those eligible protects those most vulnerable more than only boosting this vulnerable group, with important vaccine strategy implications for the forthcoming Northern 2022 winter. Moreover, given the early and large influenza epidemic that has been ongoing in Australia over their winter season, which has led to increased health system stress, it is even more critical to re-evaluate vaccination strategies now. All efforts should be made to reduce COVID-19-related stress on health systems ahead of the anticipated influenza season in the Northern hemisphere. These results are robust when key model parameters were varied. However, for all results, the more frequently variants with mounting infectiousness emerge, the less effect vaccine boosters will have, regardless of whether boosters are administered annually or biannually.
Resources needed to administer booster doses more often and more extensively should be measured against the predicted benefit to public health and health systems capacity, as well as any potential health risks associated with ongoing booster administration. These include dose supply (including global equity in distribution), effort, and cost required to increase the frequency. Should the number of COVID-19-related hospital admissions averted per booster dose administered be used as a health savings proxy metric for cost-effectiveness, then more hospitalisations could be averted per booster dose through annual boosting of those most vulnerable versus all eligible even when assumption values for variant infectivity, immune-evading capacity, vaccine infection blocking, seasonality effect, variant severity, and variant timing are increased or decreased within assumed bounds, and under assumptions for waning immunity and study settings. However, only boosting those most vulnerable may be insufficient to prevent health system risk.
Vaccination hesitancy and fatigue must also factor into this assessment and be considered as part of vaccine booster campaigns and messaging; lessons can be learned from seasonal influenza strategies. Even if there were no challenges surrounding vaccine hesitancy and repeated booster fatigue, we cannot boost indefinitely against SARS-CoV-2 infection and COVID-19 disease. Regardless, evidence from this study suggests that more frequent boosting to every 6 months has diminishing returns on investment (see Fig. 2). Should booster doses not be available or not be accepted particularly as more infectious and potentially more severe subvariants emerge, hospital admissions may once again overwhelm health systems. This compounded with the potential for double epidemics with influenza and respiratory syncytial virus (as has been reported in Australia [15]) escalates the need for stronger preparedness for such eventualities given the high mutation rate of SARS-CoV-2 [16]. Therefore, it is prudent for countries to continue targeting and supporting vaccination boosting campaigns for all. Since it is anticipated that more infectious and immune-escaping variants will continue to emerge, existing vaccines may become insensitive to new variants, which reinforces the need for effective adapted and possibly multivalent vaccines with longer durations, coupled with plans for adjusted and improved strategies over the years.
Vaccine Targets and Inequity of Vaccine Distribution
While the WHO target to fully vaccinate (with two doses) 40% of the world’s population against SARS-CoV-2 by the end of 2021 was missed, coverage reached 60% by the end of May, which left only a month remaining to achieve the 70% mid-2022 coverage target [17]. While it is important to consider scaling up booster strategies, the WHO warns that broad-based COVID-19 booster programmes, including delivering additional booster vaccinations to groups at lower risk of developing severe disease may prolong the global pandemic by diverting supply to nations with already high coverage and therefore away from nations with low coverage, giving the virus more chance to spread and mutate [18]. Given that 2.7 billion people had still not even received their first dose of the COVID-19 vaccine as of the end of May 2022 [19], WHO experts have urged prioritising first doses over boosters [18]. Similarly, Gavi, the Vaccine Alliance, advocates for the global community to focus on ensuring every adult receives at least one dose in 2022 [19].
Preparedness for Future Waves
While the number of confirmed COVID-19-related deaths may be underreported [20], the healthcare system now has more collective experience treating those with severe disease than it did during previous waves of COVID-19. Medical oxygen supplies and distribution have been strengthened in many countries [21]. In addition, oral antivirals to treat COVID-19 have been authorised for inpatient and home use in several countries as well hospital-administered injectable monoclonal antibodies are now available. These pharmaceutical interventions, together with mask usage and physical distancing measures being observed to varying degrees across settings, and coupled with increasing vaccination rates and natural immunity from previous infection, may make certain settings better positioned to mitigate and respond to the subsequent COVID-19 waves. Nevertheless, with continuing spread of Omicron subvariants, even if Omicron infection causes less severe disease, should cases spike again, a smaller proportion of a large number of cases needing hospitalisation may again lead to health system stress.
While increasing vaccination coverage is a good solution to reducing the burden from COVID-19, a sizable proportion of the population continues to refuse vaccination in high-, middle-, and even low-income settings [22]. Even though testing rates have begun to plateau [23], meaning cases are likely being underreported, ongoing SARS-CoV-2 transmission will inevitably result in the emergence of new SARS-CoV-2 variants or subvariants of concern. This is why it is important to be strategic with COVID-19 vaccination campaigns. As we cannot continue to boost for COVID-19 indefinitely, in effort to avoid vaccination fatigue and to better protect against new variants, second-generation vaccines that are multivalent, meaning they protect better against more than one SARS-CoV-2 variant and will remain more effective, longer, are being developed and approved. Under the booster strategy recommended in this study, more effective (as shown in the sensitivity analysis conducted in this study) and longer lasting second-generation booster vaccines will avert even more infections and hospital admissions than were predicted here using first-generation boosters.
Study Limitations
As with all simulation studies, our results are based on several assumptions and include some limitations. Within our individual-based transmission model, transition between disease states and chance to be infected include probabilities for infection, transmission, and disease severity, based on studies cited in [6]. These probabilities will influence model outcomes and our conclusions on vaccination so sensitivity analysis was conducted on several parameters. First, we identified that Omicron’s potential severity had little effect on the number of new cases projected, also because no impact on viral load was assumed. Higher viral load in the model is associated with increased transmissibility. Second, values higher or lower than 1.0 for the effective reproduction number at the start of the winter period, prior to introduction of Omicron, may influence peak hospital admissions estimates; however, the relative impact of vaccination is not sensitive to this parameter. Third, this study only considers direct admissions to hospital due to COVID-19, but does not consider COVID-19-related admissions due to certain long-COVID health consequences. Therefore, there is the potential for additional health system burden not modelled here. Fourth, we did not model the effect of any NPIs, but we assume re-implementing NPIs could be considered if thresholds for case numbers or health system capacity were reached. We also acknowledge that booster vaccines began to be rolled out as early as fall 2021 in certain countries, whereas we simulated booster administration starting in spring 2022. Last, depending on emerging variant profile and vaccine coverage levels, should naturally acquired and/or vaccine-induced immunity have different protection levels and/or should immunity wane differently (speed, decay type) than what was assumed, then the population would be more or less protected and our projected outcomes may prove to be too pessimistic or too optimistic. We did not model the effect of hybrid immunity, which occurs when an individual experiences both naturally acquired and vaccine-induced immunity. Recent studies have identified hybrid immunity to provide a higher level of protection against infection and severe disease than we assumed in the model [24–26]. Since new SARS-CoV-2 variants of concern, and therefore infections, will continue to emerge, and vaccinations will continue to need to be implemented, the prevalence of hybrid immunity will increase. This will affect population susceptibility, especially in the long term. As more is known about hybrid immunity to SARS-CoV-2 infection and COVID-19 disease, this should be considered as part of future modelling studies and when interpreting these results.
Conclusions
To reduce the public health burden of COVID-19 and the associated health system risks, vaccine-boosting strategies must account for rollout constraints and cost-effectiveness. This study provides much needed, timely quantitative evidence to inform decisions around future boosting strategies.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
The authors acknowledge and thank the members of the Disease Modelling Unit, Swiss Tropical and Public Health Institute, specifically Dr Nakul Chitnis for his input. Simulations were performed at sciCORE (http://scicore.unibas.ch/) the scientific computing facility at the University of Basel.
Funding
Funding for the study was provided by the Botnar Research Centre for Child Health (DZX2165 to MAP), the Swiss National Science Foundation Professorship of MAP (PP00P3_203450), and Swiss National Science Foundation NFP 78 COVID-19 2020 (4079P0_198428 to MAP). Botnar Research Centre for Child Health also funded the jounal's Rapid Service Fee.
Author Contributions
SLK, MAP, and AJS conceived the study with input from EALR and MR. AJS further developed the model with input from SLK, EALR, MR, and MAP. AJS and SLK performed the analyses, prepared the figures, and conducted model and analysis validation. SLK drafted the manuscript together with AJS. All authors contributed to interpreting results and editing the manuscript.
Disclosures
Sherrie L Kelly, Epke A Le Rutte, Maximilian Richter, Melissa A Penny, and Andrew J Shattock have nothing to disclose.
Compliance with Ethics Guidelines
Ethics approval and consent to participate in this study is not applicable.
Consent for Publication
All authors gave final approval for publication. No other consent is required.
Competing Interests
The authors declare that they have no competing interests.
Data Availability
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study. Data informing model parameters are described herein and in Shattock et al. [6] All model and figure code are open access and publicly available at https://github.com/SwissTPH/OpenCOVID/tree/manuscript_june_2022/src and https://zenodo.org/record/6878852#.Yv53t3ZBw2xMI2w. The model and figure code for this study uses version 2.2 of OpenCOVID.
Ethical approval
Ethics approval and consent to participate in this study is not applicable.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.WHO Coronavirus (COVID-19) Dashboard Geneva: WHO; 2022. https://covid19.who.int/. Accessed 28 May 2022.
- 2.Andrews N, Stowe J, Kirsebom F, Toffa S, Rickeard T, Gallagher E, et al. COVID-19 vaccine effectiveness against the omicron (B.1.1.529) variant. N Engl J Med. 2022;386:1532–1546. doi: 10.1056/NEJMoa2119451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Khoury DS, Steain M, Triccas JA, Sigal A, Davenport MP, Cromer D. A meta-analysis of early results to predict vaccine efficacy against omicron. medRxiv. 2021 doi: 10.1101/2021.12.13.21267748v2. [DOI] [Google Scholar]
- 4.Callaway E. Are COVID surges becoming more predictable? New Omicron variants offer a hint. Nat News. 2022;605(7909):204–206. doi: 10.1038/d41586-022-01240-x. [DOI] [PubMed] [Google Scholar]
- 5.Nyberg T, Ferguson NM, Nash SG, Webster HH, Flaxman S, Andrews N, et al. Comparative analysis of the risks of hospitalisation and death associated with SARS-CoV-2 omicron (B.1.1.529) and delta (B.1.617.2) variants in England: a cohort study. Lancet. 2022;399(10332):1303–1312. doi: 10.1016/S0140-6736(22)00462-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Shattock AJ, Le Rutte EA, Dünner RP, Sen S, Kelly SL, Chitnis N, et al. Impact of vaccination and non-pharmaceutical interventions on SARS-CoV-2 dynamics in Switzerland. Epidemics. 2022;38:100535. doi: 10.1016/j.epidem.2021.100535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.World Population Prospects Population Division. Geneva. 2019.
- 8.ECDC. The European Surveillance System (TESSy) Stockholm: European Centre for Disease Prevention and Control 2020. https://www.ecdc.europa.eu/en/publications-data/european-surveillance-system-tessy. Accessed 28 May 2022.
- 9.Chivese T, Matizanadzo JT, Musa OAH, Hindy G, Furuya-Kanamori L, Islam N, et al. The prevalence of adaptive immunity to COVID-19 and reinfection after recovery—a comprehensive systematic review and meta-analysis. Pathog Glob Health. 2022;116(4):269–281. doi: 10.1080/20477724.2022.2029301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.CDC . Science brief: SARS-CoV-2 infection-induced and vaccine-induced immunity. Atlanta: CDS; 2021. [Google Scholar]
- 11.Le Rutte EA, Shattock AJ, Chitnis N, Kelly SL, Penny MA. Modelling the impact of Omicron and emerging variants on SARS-CoV-2 transmission and public health burden. Commun Med. 2022;2(93). 10.1038/s43856-022-00154-z. [DOI] [PMC free article] [PubMed]
- 12.WHO. COVID-19: vulnerable and high risk groups. Geneva: WHO; 2022. https://www.who.int/westernpacific/emergencies/covid-19/information/high-risk-groups.
- 13.Sridhar D. Don’t be complacent, another COVID wave is coming. Here’s how we can manage it. Guardian; 2022. https://www.theguardian.com/commentisfree/2022/jun/13/rise-covid-cases-what-we-know-so-far. Accessed 13 June 2022.
- 14.Meng H, Mao J, Ye Q. Booster vaccination strategy: necessity, immunization objectives, immunization strategy, and safety. J Med Virol. 2022;94(6):2369–2375. doi: 10.1002/jmv.27590. [DOI] [PubMed] [Google Scholar]
- 15.Eden JS, Sikazwe C, Xie R, Deng Y-M, Sullivan SG, Michie A, et al. Off-season RSV epidemics in Australia after easing of COVID-19 restrictions. Nat Commun. 2022;13(1):2884. doi: 10.1038/s41467-022-30485-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Amicone M, Borges V, Alves MJ, Isidro J, Zé-Zé L, Duarte S, et al. Mutation rate of SARS-CoV-2 and emergence of mutators during experimental evolution. Evol Med Public Health. 2022;10(1):142–155. doi: 10.1093/emph/eoac010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.WHO . Achieving 70% COVID-19 immunization coverage by mid-2022: statement of the independent allocation of vaccines group (IAVG) of COVAX. Geneva: WHO; 2021. [Google Scholar]
- 18.Geddes L. Prioritise first doses of COVID-19 vaccines over boosters, say WHO experts Geneva: Gavi, the Vaccine Alliance; 2022 [updated 17 January]. https://www.gavi.org/vaccineswork/prioritise-first-doses-covid-19-vaccines-over-boosters-say-who-experts.
- 19.Gavi. The last to receive COVID vaccines are the hardest and most critical to reach [press release]. Geneva: Gavi, the Vaccine Alliance, 2022. https://www.gavi.org/vaccineswork/last-receive-covid-vaccines-are-hardest-and-most-critical-reach.
- 20.Adam D. The pandemic's true death toll: millions more than official counts. Nature. 2022;601(7893):312–315. doi: 10.1038/d41586-022-00104-8. [DOI] [PubMed] [Google Scholar]
- 21.CHAI. With investments over the last two years, CHAI is working with governments to close the medical oxygen gap in over 25 countries: Clinton Health Access Initiative; 2022. https://www.clintonhealthaccess.org/blog/with-investments-over-the-last-two-years-chai-is-working-with-governments-to-close-the-medical-oxygen-gap-in-over-25-countries/. Accessed 21 June 2022.
- 22.Mallapaty S. Researchers fear growing COVID vaccine hesitancy in developing nations. Nature. 2022;601(7892):174–175. doi: 10.1038/d41586-021-03830-7. [DOI] [PubMed] [Google Scholar]
- 23.Our World In Data. Total COVID-19 tests per 1000 people London. 2022. https://ourworldindata.org/grapher/full-list-cumulative-total-tests-per-thousand-map?tab=chart. Accessed 28 May 2022.
- 24.Nordström P, Ballin M, Nordström A. Risk of SARS-CoV-2 reinfection and COVID-19 hospitalisation in individuals with natural and hybrid immunity: a retrospective, total population cohort study in Sweden. Lancet Infect Dis. 2022;22(6):781–790. doi: 10.1016/S1473-3099(22)00143-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Castillo SM, Khaoua H, Courtejoie N. Vaccine-induced and naturally-acquired protection against Omicron and Delta symptomatic infection and severe COVID-19 outcomes, France, December 2021 to January 2022. Euro Surveill. 2022;27(16):2200250. doi: 10.2807/1560-7917.ES.2022.27.16.2200250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altarawneh HN, Chemaitelly H, Ayoub HH, Tang P, Hasan MR, Yassine HM, et al. Effect of prior infection, vaccination, and hybrid immunity against symptomatic BA.1 and BA.2 Omicron infections and severe COVID-19 in Qatar. medRxiv. 2022 doi: 10.1101/2022.03.22.22272745. [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data sharing is not applicable to this article as no datasets were generated or analysed during the current study. Data informing model parameters are described herein and in Shattock et al. [6] All model and figure code are open access and publicly available at https://github.com/SwissTPH/OpenCOVID/tree/manuscript_june_2022/src and https://zenodo.org/record/6878852#.Yv53t3ZBw2xMI2w. The model and figure code for this study uses version 2.2 of OpenCOVID.