Abstract
Aim
Serum levels of Calcitonin Gene-Related Peptide (CGRP)-like immunoreactivity (CGRP-LI) in migraine patients before and after starting treatment with erenumab were measured to evaluate the association with clinical treatment response.
Methods
Blood samples were collected from the cubital fossa before (T0) and 2–4 weeks after (T1) starting treatment with erenumab. Clinical response was monitored using a daily headache e-diary. Serum levels of CGRP-LI, assessed using radioimmunoassay, were compared between T0 and T1, correcting for migraine reduction. In addition, for both T0 and T1, linear regression models were constructed using migraine reduction as outcome and serum CGRP-LI as independent variable, corrected for age, gender and monthly migraine days (MMD) at baseline.
Results
Serum CGRP-LI did not differ between T0 and T1 (p = 0.30). However, there was an interaction between time and reduction in MMD (p = 0.01). Absolute reduction in MMD in the third month after treatment with erenumab was associated with serum CGRP-LI at T1, 2–4 weeks after starting treatment with erenumab (p = 0.003), but not with serum CGRP-LI at T0 (p = 0.24).
Conclusion
Lower serum CGRP-LI 2–4 weeks after starting treatment with erenumab was associated with a higher reduction in migraine days after three months of treatment. Although the underlying mechanisms remain to be determined, this suggests that changes in CGRP levels, shortly after starting erenumab, are important for its clinical effect.
Keywords: CGRP, Serum, Monoclonal antibodies, Migraine
Highlights
• Lower serum levels of calcitonin gene-related peptide (CGRP)-like immunoreactivity (CGRP-LI) in migraine patients at 2–4 weeks after starting treatment with erenumab were associated with better treatment response after three months.
• Early changes in serum CGRP may be important for the clinical effect of erenumab in migraine.
Introduction
Activation of the trigeminovascular system and the subsequent release of calcitonin gene-related peptide (CGRP) play an important role in the pathophysiology of migraine [1]. CGRP levels have been shown to be elevated in the jugular vein during spontaneous migraine attacks [2], while in chronic migraine patients the interictal CGRP levels were also found to be elevated [3]. In addition, infusion of CGRP in migraine patients induces a migraine-like headache, similar to the subject’s spontaneous attack, in approximately 60% of patients [4].
The development of monoclonal antibodies directed against CGRP (eptinezumab, fremanezumab and galcanezumab) or its receptor (erenumab) has been a major advancement in the treatment of migraine. Unfortunately, not all migraine patients can be considered responders to this type of medication. In clinical trials, approximately 50% of migraine patients had 50% reduction in monthly migraine days (MMD) in the last month of treatment or as mean response over several months of treatment. In patients with previous failure to 2–4 prophylactics 30–40% achieved a 50% reduction, with, as expected, a lower placebo response [5–10]. A real-life study in our center in those patients with ≥ 8 MMD and failure on 2–4 prophylactics showed that of all patients 60% had ≥ 30% MMD reduction in at least half of their treatment period (≥ 3/6 months) [11].
Increasing the understanding of the pathophysiological effects of anti-CGRP (receptor) antibodies and uncovering differences between responders and non-responders to this treatment will help to improve migraine care even further. While it has been suggested that serum CGRP decreases when migraine attack frequency decreases [3], another small study suggested an increase in serum CGRP levels after long term blockade of the CGRP receptor with erenumab [12], but no clear underlying mechanisms were proposed. Indeed, a lot is still unknown about the clearance of CGRP, which may be caused by endopeptidases, but in addition possibly also by neuronal reuptake [13].
In the present study, we assessed serum CGRP levels in migraine patients before and 2–4 weeks after starting treatment with erenumab and evaluated the association with the clinical treatment response.
Methods
Participants
All patients that started treatment with erenumab in the Leiden Headache Center, a national referral centre, were invited to participate. They were all diagnosed with migraine, episodic or chronic, with or without aura, by a neurology resident in consultation with a neurologist with headache expertise or by a neurologist with a headache expertise, according to the ICHD-3 criteria [14]. None of the patients had a second primary headache disorder. Only tension type headache was allowed, as this is common in patients with chronic migraine [14]. Given the restricted availability of erenumab, all patients had at least 8 migraine days per month, and failed on at least 4 migraine prophylactics (meaning being ineffective, discontinued because of side effects or being contraindicated), including at least a betablocker, candesartan, valproate and topiramate. None of the patients had medication overuse headache.
Approval for this study was obtained from the LUMC Medical Ethical Committee and all participants gave written informed consent.
Treatment
Patients were treated with erenumab 70 mg, administered subcutaneously once every four weeks. No additional prophylactic treatment was used.
Headache diary
The clinical response to erenumab was monitored using a validated daily headache e-diary [11, 15, 16]. This diary contains questions on the presence of headache, headache characteristics, accompanying symptoms and the use of acute migraine medication. In case of a headache, an automated algorithm based on the ICHD-3 criteria determined whether it was a migraine day. Additionally, days on which a triptan was taken, as well as aura without headache symptoms, were also counted as migraine days. Patients started this diary at least 4 weeks before starting treatment (the baseline period). In line with clinical trials [7], the clinical response was assessed by comparing MMD in week 9–12 (i.e. after three doses of erenumab) to that in the 4 week pre-treatment baseline observation period. A month is defined as 28 days (4 weeks).
Serum CGRP assays
Patients were invited to the hospital before starting treatment with erenumab (T0) and 2–4 weeks (after Tmax, but before the second dosing) after starting treatment with erenumab (T1). At both time points blood samples were collected from the antecubital vein, while subjects rested in a sitting position. The blood was then allowed to clot and was centrifuged at room temperature for 20 min at 622 g/2000 rpm to separate serum. Samples were then immediately stored at -80 °C in aliquots of 500 µL until analyzed.
For radioimmunoassay (RIA), a commercial kit (CGRP (Human) - RIA Kit (Phoenix pharmaceuticals, Burlingame, California, United States), detection range 0.53–660 pmol/l), was used following manufacturers’ instructions to measure CGRP-like immunoreactivity (CGRP-LI) levels. Biochemical assays were performed by an experienced lab technician who was blinded to the patient identity, study day and treatment effect of erenumab. All samples were analyzed in the same laboratory, under the same environmental conditions, and using the same batch for samples from different patients and different study days, to avoid a possible batch effect. Samples with values outside the detection range were set on the limits of the detection range.
Statistics
Sample size was based on the available data. Baseline characteristics, including, sex, age, headache diagnosis and baseline headache measures were summarized using means and standard deviations or frequencies and proportions. For each patient the clinical response to erenumab was determined by calculating the absolute reduction in migraine days in the third month (week 9–12) after initiating treatment compared to the baseline month (4 weeks before starting treatment).
As serum CGRP-LI levels were highly skewed, a log transformation was applied, and these log-transformed values of CGRP-LI levels were used in all statistical analyses. However, for the sake of clarity, in the result section CGRP-LI levels are presented, without log transformation, as medians with interquartile ranges. To relate our CGRP measurements to measurements performed earlier by others, CGRP-LI levels at T0 were related to age and sex, with a Pearson correlation and an independent t-test, respectively. Comparisons between T0 and T1 were made using a repeated measurements model, with absolute reduction in monthly migraine days added as a covariate to assess the relation between change in serum CGRP-LI and change in migraine frequency.
To investigate the predictive value of serum CGRP-LI for the clinical response, two linear regression models were made with absolute migraine reduction as the outcome variable and with sex, age, migraine days at baseline as covariates. In our primary analysis, log serum CGRP-LI at T0, and in our secondary analysis log serum CGRP-LI at T1, was added as an independent variable.
In all analyses a two-sided p-value < 0.05 was considered to indicate significant differences. All statistical analyses were performed using IBM SPSS Statistics for Windows, version 25 (IBM Corp., Armonk, N.Y., USA).
Results
In total, 96 participants started treatment with erenumab. Two patients discontinued treatment before the three month follow-up period ended, and thus were excluded from all analyses. CGRP measurements of 5 patients were missing at follow-up, since one patient was not able to attend the second visit because of a debilitating migraine attack, and four measurements were missing because of COVID-19 measures, when patients were not allowed to come to the hospital for nonurgent (research) issues. These patients were excluded regarding analyses with follow-up measurements. In total, 94 patients were included, of which 79 were women. Baseline characteristics are described in Table 1. At T0, three values were below and one above the detection range. At T1, six values were below and one above the detection range.
Table 1.
Baseline characteristics (n = 94)
| Characteristic | |
|---|---|
| Women, n (%) | 79 (84) |
| Age, mean ± SD (years) | 42 ± 12.6 |
| Migraine without aura, n (%) | 60 (64) |
| Episodic migraine, n (%) | 52 (55) |
| MMD baseline, mean ± SD | 13.7 ± 5.7 |
| MHD baseline, mean ± SD | 16.5 ± 6.1 |
| Failed prophylactics, mean ± SD | 5.0 ± 1.0 |
MMD monthly migraine days, MHD monthly headache days, A month is defined as 28 days. Baseline = 28 days before starting treatment
Baseline comparisons
Serum CGRP-LI at T0 was not significantly different between women (median (IQR) CGRP-LI = 15.1 (8.3–47.8) pmol/l) and men (median (IQR) CGRP-LI = 10.6 (6.3–29.7) pmol/l) (p = 0.12). Serum CGRP-LI at T0 levels were negatively correlated to age (r = -0.26, p = 0.01).
Erenumab
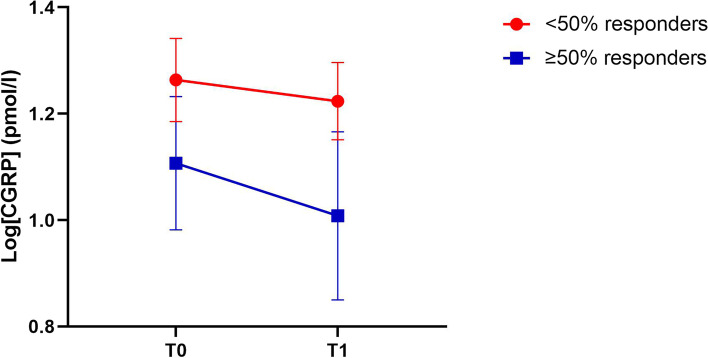
Serum CGRP-LI did not differ between T0, before starting erenumab, (median (IQR) CGRP-LI: 14.1 (8.2–33.9) pmol/l) and T1, after 2–4 weeks treatment (median (IQR) CGRP-LI: 13.8 (7.0–33.1) pmol/l) (F(1, 86) = 1.1, p = 0.30). However, there was an interaction between time and reduction in MMD (F(1, 86) = 6.8, p = 0.01). To visualize the interaction between migraine reduction and change in serum CGRP-LI, we present a line graph, separated for < 50% and ≥ 50% responders (Fig. 1).
Fig. 1.
Change in serum log[CGRP-LI] between T0 and T1 (2–4 weeks after starting erenumab) separated for patients with < 50% and ≥ 50% reduction in monthly migraine day (MMD) reduction after three months of treatment. Data presented as mean ± SEM
Tables 2 and 3 present the β-coefficients and p-values of the linear regression analyses with serum CGRP-LI at T0 and serum CGRP-LI at T1 as predictor for the clinical response. Absolute MMD reduction after three months of treatment with erenumab was associated with serum CGRP-LI at T1 (β = -2.13, p = 0.003), but not with serum CGRP-LI at T0 (β = -0.80, p = 0.24).
Table 2.
Linear regression analysis with log-transformed serum CGRP-LI levels (mol/l) T0
| Variable | β (95% CI)1 | p | β (95% CI)2 | p |
|---|---|---|---|---|
| Age | 0.07 (-0.001–0.13) | 0.05 | 0.07 (0.004–0.14) | 0.04 |
| Sex | 1.92 (-0.41–4.25) | 0.10 | 2.50 (0.17–4.82) | 0.04 |
| Migraine days baseline | 0.09 (-0.06–0.24) | 0.24 | 0.11 (-0.04–0.26) | 0.14 |
| Serum CGRP-LI | -1.033 (-2.37–0.30) | 0.13 | -0.80 (-2.16–0.55) | 0.24 |
| N = 94. 1Simple linear regression. 2multiple regression, corrected for all tested variables. CI = confidence interval. T0 = baseline, before starting treatment with erenumab. The outcome is absolute reduction migraine days during month 3 after starting treatment with erenumab compared to baseline. One month is defined as 28 days. | ||||
Table 3.
Linear regression analysis with log-transformed serum CGRP-LI levels (mol/l) T1
| Variable | β (95% CI)1 | p | β (95% CI)2 | p |
|---|---|---|---|---|
| Age | 0.07 (-0.001–0.13) | 0.05 | 0.044 (-0.03 − 0.12) | 0.24 |
| Sex | 1.92 (-0.41–4.25) | 0.10 | 2.979 (0.65–5.31) | 0.01 |
| Migraine days baseline | 0.09 (-0.06–0.24) | 0.24 | 0.10 (-0.06–0.25) | 0.22 |
| Serum CGRP-LI | -2.12 (-3.44 - -0.80) | 0.002 | -2.13 (-3.52 - -0.73) | 0.003 |
| N = 89. 1Simple linear regression. 2multiple regression, corrected for all tested variables. CI = confidence interval. T1 = 2–4 weeks after starting treatment with erenumab. The outcome is absolute reduction migraine days during month 3 after starting treatment with erenumab compared to baseline. One month is defined as 28 days. | ||||
Discussion
Lower serum CGRP-LI levels measured 2–4 weeks after starting treatment with erenumab are associated with a higher migraine reduction after three months. Serum CGRP-LI levels before start of treatment with erenumab were not associated with clinical response.
Previous small studies suggested that chronic migraine patients may have higher serum CGRP levels than episodic migraine patients [2, 3]. It was also suggested that serum CGRP levels in episodic migraine patients with a history of chronification are within the range of episodic migraine CGRP levels [3]. This may suggest that, when migraine attack frequency decreases, spontaneously or due to successful treatment, one might expect to measure lower serum CGRP levels. In contrast, a small proof-of-concept study, in which CGRP levels were measured before, after one month and after six months of treatment with erenumab, suggested an increase [12]. While the association with the clinical response was not described and the sample size was too small (n = 7) to demonstrate statistical significance, it was suggested that serum levels of CGRP did not change in the first month, but tended to increase after six months [12].
The present study focused on identifying a possible early predictor for clinical response to treatment with erenumab. We deliberately chose to measure CGRP-LI early (after 2–4 weeks), as we did in another recent study from our group [17], so we could analyze the association with the clinical response, and at the same time rule out whether changes in CGRP-LI were a secondary effect due to a change in migraine days. Although CGRP-LI levels were not different between T0 and T1, an interaction was found with migraine reduction after three months. In addition, lower serum CGRP-LI levels 2–4 weeks after the first erenumab injection were associated with a larger monthly migraine day reduction after three months, while CGRP-LI levels at T0 were not associated with the clinical response. Moreover, the CGRP-LI levels at T1 were not associated with migraine reduction in the first two months (results not shown). These findings combined suggest that, promptly after starting anti-CGRP treatment, there are relevant changes in serum CGRP-LI that are important for the clinical effect and these changes are not a secondary effect of a decrease in migraine frequency. Interestingly, although the clinical effect of erenumab is already evident in the first month, the monthly migraine days seem to decrease further after the first month, which seems to be in line with what could be expected given the long half-life of the mAbs [18, 19].
Much is still unknown about the effects of blocking the CGRP receptor. Indeed, it does not seem unlikely that serum levels of CGRP would increase due to upregulation after long term blockade of the CGRP receptor [12, 20]. However, interactions between CGRP activity and several other peptides (and/or their receptors) probably induce a more complex cascade of events, that could either increase or decrease serum CGRP. CGRP can act through both the CGRP and the amylin 1 receptors, with unknown effects on further CGRP release [21]. In addition, CGRP release may be indirectly influenced by changing activity of the sympathetic nervous system and endogenous endothelin-1 release, which may modulate CGRP release through the TRPV1 receptor [22, 23]. Lastly, CGRP might regulate its own release through presynaptic mechanisms [24].
A strong feature of our study is the use of a daily e-diary. The time-lock reduces the risk of recall bias, and with the automated algorithm (reduction in) migraine days could be determined accurately. In addition, while CGRP has a short half-life and is rapidly cleared from the blood, all our blood samples were collected under the same circumstances and processed and stored directly after blood draw. The association between CGRP-LI levels and age and sex have been described in the literature before[13, 25]. Although we could only demonstrate a numerical and not statistical difference between men and women (most likely due to a lack of statistical power in our population including only a limited number of men), we did see an association between CGRP-LI levels and age in our samples, supporting the validity of the CGRP assessment [13].
Previous studies demonstrated that CGRP-LI levels measured in the antecubital vein are generally lower than in the jugular vein, and differences between migraine patients and controls are generally smaller in antecubital vein than in jugular vein samples. In our study, this same phenomenon might have caused insufficient power for our comparison between T0 and T1. However, we decided to use the antecubital vein for blood sampling because it is more patient friendly, and because previous studies demonstrated that the antecubital vein is suitable to measure CGRP-LI in migraine patients. Moreover, a lot is still unclear about CGRP-LI measurements in human serum, where CGRP most probably has been degraded into smaller fragments by endogenous peptidases [26]. Therefore, we consider data on CGRP-LI serum levels important within a study, where all samples were treated identically as described above, but we remain cautious about an interpretation of the absolute levels that we measured. A second limitation is that, due to the high migraine frequency in our study population, blood sample collection did not always take place on an interictal day. However, there was no difference between the CGRP-LI levels on migraine days and non-migraine days (data not shown). This is probably due to the fact that all our patients had high frequent episodic or chronic migraine, in whom interictal CGRP-LI levels are most likely already increased [2, 3]. Thirdly, the significant effect of sex on the clinical response needs to be interpreted with caution as there were very few men in our analysis and our study was not powered to determine a difference in effectiveness of monoclonal CGRP-antibodies between men and women. This needs to be investigated in a separate study [27].
Currently, in many countries treatment with anti-CGRP (receptor) antibodies is only available to a subset of patients, namely patients with a high monthly attack frequency and/or who already demonstrated not to respond to multiple preventive treatments. Data from clinical trials and real life data show that not all migraine patients have a successful migraine reduction in response to treatment with anti-CGRP (receptor) antibodies [11 28]. Even though anti-CGRP (receptor) antibodies were specifically developed for the preventive treatment of migraine, it is yet unclear why some patients do not respond and others are responders. Recently, we demonstrated that CGRP-mediated trigeminovascular activity before initiating erenumab partly may explain this clinical response [17]. However, it is of utmost importance to increase the understanding of response to anti-CGRP treatment even further and to uncover reasons for (non-)response. Future studies, in larger patient cohorts, may need to be performed to confirm our results. In addition, future research needs to unravel the exact mechanisms behind the relation between serum CGRP levels and clinical response to erenumab. Finally, measuring CGRP in patients receiving an anti-CGRP antibody might provide additional information on the expectations of effects of this treatments.
Conclusion
Lower serum levels of CGRP-LI shortly after starting treatment with erenumab were associated with a higher reduction in migraine days after three months of treatment. While the underlying mechanisms remain to be determined, this suggests that early changes in CGRP-LI levels shortly after starting erenumab, are important for its clinical effect.
Acknowledgements
Not applicable.
Abbreviations
- MMD
Monthly migraine days
- MHD
Monthly headache days
- CGRP
Calcitonin gene-related peptide
- ICHD
International Classification of Headache Disorders
- RIA
Radioimmunoassay
- TRPV1
Transient receptor potential cation channel subfamily V member 1
Authors’ contributions
SdVL, AMvdB and GT contributed to the study design. IG performed the sample assays. SdVL carried out the statistical analyses and wrote the first draft. All authors contributed to the interpretation of the results. All authors read and approved the final manuscript.
Funding
This study was supported by Vici grant 09150181910040 from the Dutch Research Council (AMVDB).
Availability of data and materials
Data not published within the article is available from the corresponding author on reasonable request.
Declarations
Ethics approval and consent to participate
The study was approved by the medical ethics committee of Leiden University Medical Centre.
Consent for publication
Not applicable.
Competing interests
Gisela Terwindt reports consultancy support from Allergan/Abbvie, Lilly, Lundbeck, Novartis, and Teva, and independent support from Dutch Organization for Scientific Research, the Dutch Heart & Brain Foundations, IRRF and Dioraphte. Antoinette MaassenVanDenBrink reports consultancy or industry grant support from Novartis, Lilly and Teva, and independent support from the Dutch Research Council and the Dutch Heart & Brain Foundations. Simone de Vries Lentsch, Ingrid Garrelds and Jan Danser have nothing to disclose.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Gisela M. Terwindt and Antoinette MaassenVanDenBrink contributed equally to this work.
References
- 1.Goadsby PJ, Edvinsson L. Trigeminovascular System and Migraine: studies Characterizing Cerebrovascular and Neuropeptide Changes Seen in Humans and Cat. Annals of neurology. 1993;33:48–56. doi: 10.1002/ana.410330109. [DOI] [PubMed] [Google Scholar]
- 2.Goadsby PJ, Edvinsson L, Ekman R. Vasoactive Peptide Release in the Extracerebral Circulation of Humans During Migraine Headache. Annals of neurology. 1990;28:183–187. doi: 10.1002/ana.410280213. [DOI] [PubMed] [Google Scholar]
- 3.Cernuda-Morollón E, Larrosa D, Ramón C, Vega J, Martínez-Camblor P, Pascual J. Interictal increase of CGRP levels in peripheral blood as a biomarker for chronic migraine. Neurology. 2013;81:1191–1196. doi: 10.1212/WNL.0b013e3182a6cb72. [DOI] [PubMed] [Google Scholar]
- 4.Hansen JM, Hauge AW, Olesen J, Ashina M. Calcitonin gene-related peptide triggers migraine-like attacks in patients with migraine with aura. Cephalalgia. 2010;30(10):1179–1186. doi: 10.1177/0333102410368444. [DOI] [PubMed] [Google Scholar]
- 5.Dodick DW, Ashina M, Brandes JL, Kudrow D, Lanteri-Minet M, Osipova V, et al. ARISE: A Phase 3 randomized trial of erenumab for episodic migraine. Cephalalgia. 2018;38(6):1026–1037. doi: 10.1177/0333102418759786. [DOI] [PubMed] [Google Scholar]
- 6.Skljarevski V, Matharu M, Millen BA, Ossipov MH, Kim BK, Yang JY. Efficacy and safety of galcanezumab for the prevention of episodic migraine: Results of the EVOLVE-2 Phase 3 randomized controlled clinical trial. Cephalalgia. 2018;38(8):1442–1454. doi: 10.1177/0333102418779543. [DOI] [PubMed] [Google Scholar]
- 7.Reuter U, Goadsby PJ, Lanteri-Minet M, Wen S, Hours-Zesiger P, Ferrari MD, et al. Efficacy and tolerability of erenumab in patients with episodic migraine in whom two-to-four previous preventive treatments were unsuccessful: a randomised, double-blind, placebo-controlled, phase 3b study. The Lancet. 2018;392(10161):2280–2287. doi: 10.1016/S0140-6736(18)32534-0. [DOI] [PubMed] [Google Scholar]
- 8.Ferrari MD, Diener HC, Ning X, Galic M, Cohen JM, Yang R, et al. Fremanezumab versus placebo for migraine prevention in patients with documented failure to up to four migraine preventive medication classes (FOCUS): a randomised, double-blind, placebo-controlled, phase 3b trial. The Lancet. 2019;394(10203):1030–1040. doi: 10.1016/S0140-6736(19)31946-4. [DOI] [PubMed] [Google Scholar]
- 9.Mulleners WM, Kim B-K, Láinez MJA, Lanteri-Minet M, Pozo-Rosich P, Wang S, et al. Safety and efficacy of galcanezumab in patients for whom previous migraine preventive medication from two to four categories had failed (CONQUER): a multicentre, randomised, double-blind, placebo-controlled, phase 3b trial. The Lancet Neurology. 2020;19(10):814–825. doi: 10.1016/S1474-4422(20)30279-9. [DOI] [PubMed] [Google Scholar]
- 10.Dodick DW, Silberstein SD, Bigal ME, Yeung PP, Goadsby PJ, Blankenbiller T, et al. Effect of Fremanezumab Compared With Placebo for Prevention of Episodic Migraine: A Randomized Clinical Trial. JAMA. 2018;319(19):1999–2008. doi: 10.1001/jama.2018.4853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.de Vries Lentsch S, Verhagen IE, van den Hoek TC, MaassenVanDenBrink A, Terwindt GM. Treatment with the monoclonal calcitonin gene-related peptide receptor antibody erenumab: A real-life study. European journal of neurology. 2021;28(12):4194–4203. doi: 10.1111/ene.15075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tringali G, Vollono C, Calabresi P, Navarra P. A proof-of-concept study on CGRP plasma levels of migraineurs during a 6-month treatment with ERENUMAB. The journal of headache and pain. 2020;21(1):124. doi: 10.1186/s10194-020-01193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Russell FA, King R, Smillie SJ, Kodji X, Brain SD. Calcitonin gene-related peptide: physiology and pathophysiology. Physiological reviews. 2014;94(4):1099–1142. doi: 10.1152/physrev.00034.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Headache Classification Committee of the International Headache Society. The International Classification of Headache Disorders, 3rd edition. Cephalalgia. 2018;38:1-211. [DOI] [PubMed]
- 15.van Casteren DS, Verhagen IE, de Boer I, de Vries Lentsch S, Fronczek R, Van Zwet EW, et al. E-diary use in clinical headache practice: A prospective observational study. Cephalalgia. 2021;41(11–12):1161–1171. doi: 10.1177/03331024211010306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.van Casteren DS, Verhagen IE, van der Arend BWH, van Zwet EW, MaassenVanDenBrink A, Terwindt GM. Comparing Perimenstrual and Nonperimenstrual Migraine Attacks Using an e-Diary. Neurology. 2021;97(17):e1661-e1671. doi: 10.1212/WNL.0000000000012723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Vries Lentsch S, Al-Hassany L, Ferrari MD, Terwindt GM, MaassenVanDenBrink A. CGRP-mediated trigeminovascular reactivity in migraine patients treated with erenumab. Journal of neurology, neurosurgery, and psychiatry. Published Online First: 27 January. 2022 doi: 10.1136/jnnp-2021-327992. [DOI] [PubMed] [Google Scholar]
- 18.Goadsby PJ, Reuter U, Hallstrom Y, Broessner G, Bonner JH, Zhang F, et al. One-year sustained efficacy of erenumab in episodic migraine: Results of the STRIVE study. Neurology. 2020;95(5):e469-e479. doi: 10.1212/WNL.0000000000010019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.de Vries Lentsch S, Rubio-Beltrán E, MaassenVanDenBrink A. Changing levels of sex hormones and calcitonin gene-related peptide (CGRP) during a woman’s life: Implications for the efficacy and safety of novel antimigraine medications. Maturitas. 2021;145:73–77. doi: 10.1016/j.maturitas.2020.12.012. [DOI] [PubMed] [Google Scholar]
- 20.Tringali G, Navarra P. Anti-CGRP and anti-CGRP receptor monoclonal antibodies as antimigraine agents. Potential differences in safety profile postulated on a pathophysiological basis. Peptides. 2019;116:16–21. doi: 10.1016/j.peptides.2019.04.012. [DOI] [PubMed] [Google Scholar]
- 21.Sonne N, Karsdal MA, Henriksen K. Mono and dual agonists of the amylin, calcitonin, and CGRP receptors and their potential in metabolic diseases. Mol Metab. 2021;46:101109. doi: 10.1016/jmolmet2020101109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Dux M, Babes A, Manchen J, Sertel-Nakajima J, Vogler B, Schramm J, et al. High-dose phenylephrine increases meningeal blood flow through TRPV1 receptor activation and release of calcitonin gene-related peptide. European journal of pain (London, England) 2020;24(2):383–397. doi: 10.1002/ejp.1495. [DOI] [PubMed] [Google Scholar]
- 23.Khodorova A, Richter J, Vasko MR, Strichartz G. Early and late contributions of glutamate and CGRP to mechanical sensitization by endothelin-1. The journal of pain: official journal of the American Pain Society. 2009;10(7):740–749. doi: 10.1016/j.jpain.2009.01.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zheng F, Nixdorf-Bergweiler BE, van Brederode J, Alzheimer C, Messlinger K. Excitatory Effects of Calcitonin Gene-Related Peptide (CGRP) on Superficial Sp5C Neurons in Mouse Medullary Slices. International journal of molecular sciences. 2021;22(7):3794. doi: 10.3390/ijms22073794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Valdemarsson S, Edvinsson L, Hedner P, Ekman R. Hormonal influence on calcitonin gene-related peptide in man: effects of sex difference and contraceptive pills. Scandinavian journal of clinical and laboratory investigation. 1990;50(4):385–388. doi: 10.3109/00365519009091595. [DOI] [PubMed] [Google Scholar]
- 26.Kruuse C, Iversen HK, Jansen-Olesen I, Edvinsson L, Olesen J. Calcitonin gene-related peptide (CGRP) levels during glyceryl trinitrate (GTN)-induced headache in healthy volunteers. Cephalalgia. 2010;30(4):467–474. doi: 10.1111/j.1468-2982.2009.01963.x. [DOI] [PubMed] [Google Scholar]
- 27.MaassenVanDenBrink A, Terwindt GM, Cohen JM, Barash S, Campos VR, Galic M, et al. Impact of age and sex on the efficacy of fremanezumab in patients with difficult-to-treat migraine: results of the randomized, placebo-controlled, phase 3b FOCUS study. The journal of headache and pain. 2021;22(1):152. doi: 10.1186/s10194-021-01336-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ashina M, Tepper S, Brandes JL, Reuter U, Boudreau G, Dolezil D, et al. Efficacy and safety of erenumab (AMG334) in chronic migraine patients with prior preventive treatment failure: A subgroup analysis of a randomized, double-blind, placebo-controlled study. Cephalalgia. 2018;38(10):1611–1621. doi: 10.1177/0333102418788347. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data not published within the article is available from the corresponding author on reasonable request.