Abstract
Purpose
To report fertility treatment use and outcomes among patients who use donor sperm for intrauterine insemination (IUI), in vitro fertilization (IVF), and reciprocal IVF (co-IVF).
Methods
This is a retrospective review of patients who used donor sperm at an urban, southeastern academic reproductive center between 2014 and 2020.
Results
Among the 374 patients presenting for care, 88 (23.5%) were single, 188 (50.3%) were in a same-sex female partnership, and 98 (26.2%) had a male partner with a diagnosis of male factor infertility. Most patients did not have infertility (73.2%). A total of 1106 cycles were completed, of which there were 931 IUI cycles, 146 traditional IVF cycles, and 31 co-IVF cycles. Live birth rates per cycle were 11% in IUI, 42% in IVF, and 61% in co-IVF. Of all resulting pregnancies, hypertensive disorders were most commonly experienced (18.0%), followed by preterm delivery (15.3%), neonatal complications (9.5%), gestational diabetes (4.8%), and fetal growth restriction (4.8%). Of the 198 infants born, fifteen (8.3%) required admission to the neonatal intensive care unit and three (1.7%) demised. Pregnancy and neonatal complications were more likely to occur in older patients and patients with elevated body mass index.
Conclusion
The use of donor sperm for fertility treatment is increasing. These data show reassuring live birth rates; however, they also highlight the risks of subsequent pregnancy complications. With the expansion of fertility treatment options for patients, these data assist provider counseling of patients regarding anticipated cycle success rates and possible pregnancy complications.
Keywords: Donor sperm, Intrauterine insemination, In vitro fertilization, Co-IVF, Pregnancy outcome, Lesbian couple
Introduction
Donor sperm use has become widespread in reproductive medicine and assisted reproductive technology (ART). Donor sperm can be used in various fertility treatments, including intrauterine insemination (IUI) and in vitro fertilization (IVF). Its use has increased over time; from 1996 to 2014, donor sperm use in IVF cycles increased from 3.8 to 6.2% of all cycles [1, 2]. Although inseminations are not reported in the USA, it is estimated that almost half of a million women used donor sperm for fertility treatment in 2017 [2]. Patients who use donor sperm are heterogeneous and include single women, lesbian women, transgender men, and couples with male factor infertility. Lesbian women may also use donor sperm for co-IVF, an innovative method of ART that allows simultaneous parenting in lesbian couples wherein one partner is an oocyte source and the other carries the pregnancy [3]. Historically, patients in same-sex female partnerships have been overlooked in the setting of infertility treatments, as some ART centers may not have accepted lesbian, gay, bisexual, or transgender (LGBT) patients and some LGBT patients may not have felt comfortable seeking family-building assistance [4]. However, concurrent with growing acceptance, in 2021, the American Society for Reproductive Medicine released a committee opinion emphasizing that access to reproductive technology should not be limited to patients who meet the heteronormative status quo [5].
Despite the increasing use of donor sperm, outcome data are mostly available on treatment outcomes (e.g., clinical pregnancy and live birth rates) and are primarily in a population using IVF. These studies have found higher clinical pregnancy and live birth rates in IVF cycles with donor sperm use compared to those using non-donor sperm after adjusting for maternal age [1, 6]. Interestingly, the studies available assessing outcomes in IUI cycles have demonstrated similar clinical pregnancy and live birth rates comparing donor and non-donor sperm cycles [7, 8]. Obstetric and perinatal outcomes, such as rates of miscarriage, low birth weight, preterm delivery, and maternal obstetric complications, are not as well studied [1, 7, 9–11].
While several studies have demonstrated that rates of preeclampsia are higher in women who conceive following donor oocyte IVF cycles, few studies have investigated rates of preeclampsia and gestational hypertension in pregnancies following donor sperm IUI and IVF.
[12–14]. In addition, there are little data on rates of other common pregnancy complications, such as gestational diabetes and fetal growth restriction, as well as short-term and long-term neonatal outcomes.
Regarding the LGBT patient population specifically, current research on the use of donor sperm IUI and IVF is still limited and with conflicting results [3, 15–18]. While some of the literature shows higher pregnancy and live birth rates in lesbian women undergoing IUI and fresh IVF cycles compared to heterosexual couples, other existing studies demonstrate no difference. Additionally, most of the research in the LGBT population consists of small sample sizes. Co-IVF is largely under-researched, and although the existing literature shows tremendous promise with high pregnancy and live birth rates, these studies are small with the largest study capturing 121 couples [15, 19]. As lesbian couples’ utilization of fertility treatments increases over time [20], it becomes abundantly important to broaden our knowledge of this patient population as well as the efficacy and practicality of the fertility treatments pursued.
This study aims to contribute to the existing body of literature and fill the knowledge gap regarding donor sperm use and fertility treatments for lesbian couples, with a specific focus on donor sperm IUI, donor sperm IVF, and donor sperm co-IVF obstetric and perinatal outcomes.
Materials and methods
Patient selection
This is a retrospective cohort study that includes all patients who used anonymous donor sperm for fertility treatment, including IUI, traditional IVF, and co-IVF, between 2014 and 2020 at a single urban academic reproductive center in the southeastern United States. Approval from the Institutional Review Board was obtained. Donor sperm users were identified via andrology records. Patient demographics, cycle characteristics, and pregnancy and delivery-specific information were collected. Cycles using donor oocytes were excluded from the study.
Variables
Demographic information abstracted from medical records included patient age, parity, race, body mass index, and infertility diagnosis. Markers of ovarian reserve, including antral follicle count (AFC) and anti-Mullerian hormone (AMH) level, were collected if available. IUI cycle characteristics analyzed were age at the time of treatment, medications used, sperm total motile count, number of follicles, endometrial thickness, and the total number of cycles completed. IVF and co-IVF cycle characteristics analyzed were the cycle ovarian stimulation protocol, number of oocytes retrieved, number of cleavage stage and blastocyst embryos, number of embryos transferred, frozen versus fresh transfer, and number of cycles and embryo transfers completed. For co-IVF specifically, we also collected demographic information on the gestational partner. Sperm for all cycles was provided through anonymous donation via sperm banks. Obstetric information collected included gestational age at delivery, infant weight, mode of delivery, pregnancy complications, placental complications, and neonatal complications. Patients who did not receive obstetric care or deliver within the same hospital system as the reproductive center were contacted to obtain the pregnancy, delivery, and neonate information.
Outcomes
The primary outcome of the study was the live birth rate per cycle. Live birth was defined as a live infant born after 24 weeks gestation. Secondary outcomes of the study include rates of clinical pregnancy, biochemical pregnancy, first-trimester loss, and pregnancy of unknown location or ectopic pregnancy. Clinical pregnancy was defined as the presence of a gestational sac on transvaginal ultrasound, and biochemical pregnancy was defined as a positive beta human chorionic gonadotropin result with no ultrasound findings of a pregnancy. Additional outcomes measured were the incidence of multiple gestations; preterm deliveries; cesarean deliveries; postpartum hemorrhage (PPH); pregnancy complications such as hypertensive disorders of pregnancy, gestational diabetes mellitus (GDM), fetal growth restriction (FGR), and preterm pre-labor rupture of membranes (PPROM); placental complications such as placental abruption, previa, or accreta spectrum; and neonatal complications, such as neonatal demise and number of days of required observation in the neonatal intensive care unit (NICU).
Statistical analysis
Our primary independent variables included treatment type (IUI, IVF, co-IVF) and relationship status (same-sex partner, male partner, single). Our primary outcome variables included pregnancy outcomes and complications, including pregnancy and placental complications. A multivariate logistic regression analysis was performed to control for age, race, and body mass index (BMI). The multivariate regression models were run both as single-level models and as multilevel models to account for potential clustering given that a single patient could have contributed multiple cycles to our database. All statistical tests were two-sided with a type I error set at 0.05. All analyses were performed using IBM SPSS Statistics version 28.
Results
Patient demographics
In total, 374 patients who used donor sperm between 2014 and 2020 were included in this study. The use of donor sperm for IUI and IVF cycles increased over time with 52 cycles in 2014 to 189 cycles in 2020 (Fig. 1). Demographic characteristics of our cohort are demonstrated in Table 1. Of all the patients, 88 (23.5%) were single, 188 (50.3%) were in a same-sex female partnership, and 98 (26.2%) had a male partner with a diagnosis of male factor infertility. The mean age of patients was 38.9 (standard deviation (SD) 5.5) years, and the mean BMI of patients was 28.9 (SD 7.0) kg/m2. Most patients identified as Caucasian (51.6%), followed by Black or African American (36.9%), Asian (8.6%), or other races (3.5%). Thirteen (3.5%) patients identified as Hispanic ethnicity. The majority of patients were nulliparous (69.8%) and did not have a history of infertility (73.3%).
Fig. 1.
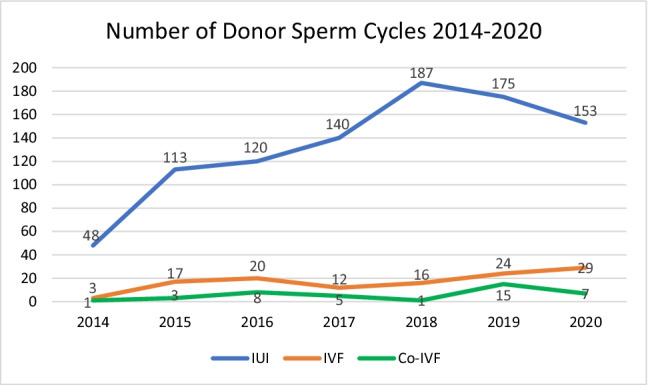
Trends in donor sperm use for IUI and IVF between 2014 and 2020
Table 1.
Patient characteristics among all patients undergoing fertility treatment using donor sperm at a single clinic between 2014 and 2020 (n = 374)
| Age in years, mean (SD) | 38.9 (5.5) |
| BMI (kg/m2), mean (SD) | 28.85 (7.0) |
| Normal weight, BMI < 25 | 125 |
| Overweight, BMI 25–29.9 | 96 |
| Obese, BMI > 30 | 133 |
| Race, n (%) | |
| Black or African American | 138 (36.9) |
| White | 193 (51.6) |
| Asian | 20 (5.3) |
| Other races | 32 (8.6) |
| Hispanic ethnicity, n (%) | 13 (3.5) |
| Relationship status, n (%) | |
| Single | 88 (23.5) |
| Same-sex partner | 188 (50.3) |
| Male partner | 98 (26.2) |
| Gravidity, n (%) | |
| 0 | 261 (69.8) |
| 1 | 80 (21.4) |
| 2 | 17 (4.5) |
| 3 | 9 (2.4) |
| 4 + | 7 (1.9) |
| Parity, n (%) | |
| Full term | 39 (10.5) |
| Preterm | 7 (1.9) |
| Losses | 65 (17.4) |
| Living children | 43 (11.5) |
| Fertility treatment type, n (%) | |
| IUI | 298 (80.0) |
| Number of IUI cycles, mean (SD) | 2.91 |
| IVF | 38 (10.2) |
| Co-IVFa | 46 (12.3) |
| Multiple treatment types | 61 (16.3) |
| Infertility type, n (%) | |
| Tubal factor | 6 (1.6) |
| Uterine factor | 5 (1.3) |
| Ovulatory | 4 (1.1) |
| Diminished ovarian reserve | 11 (2.9) |
| Male factor | 65 (17.4) |
| Endometriosis | 1 (0.3) |
| Unexplained fertility | 4 (1.1) |
| No infertility | 274 (73.3) |
a23 couples
Cycle outcomes
A total of 1106 cycles were completed, of which there were 931 IUI cycles, 146 traditional IVF cycles, and 31 co-IVF cycles. Live birth rates per cycle were 11% in IUI, 42% in IVF, and 61% in co-IVF. Clinical pregnancy rates per cycle were 18% in IUI, 50% in IVF, and 77% in co-IVF. Miscarriage rates per cycle were 4% in IUI, 7% in IVF, and 6% in co-IVF. Clinical pregnancy and live birth were more likely to occur following IVF and co-IVF compared to IUI (Table 2). After adjusting for potential confounders, patients who used IVF were approximately 7 times more likely to have a live birth (adjusted odds ratio [aOR] = 6.73; 95% CI: 4.39–10.34, p < 0.001) compared to patients who used IUI (per cycle). Patients who used co-IVF were approximately 14 times more likely (aOR = 14.58; 95% CI: 6.34–33.56, p < 0.001) to have a live birth compared to patients who used IUI (per cycle). There were no significant differences in miscarriage rates among patients using fertility treatment, regardless of the treatment modality used. However, miscarriage rates were higher among all patients aged greater than 35.
Table 2.
Adjusted odds ratios (aOR) for live birth and clinical pregnancy from fertility treatment cycles utilizing donor sperm at a single clinic between 2014 and 2020
| Live birth | Clinical pregnancy | |||
|---|---|---|---|---|
| aOR (95% CI) | P value | aOR (95% CI) | P value | |
| Treatment type (compared to IUI) | ||||
| IVF | 6.73 (4.39–10.34) | < .001 | 6.10 (4.07–9.13) | < .001 |
| Co-IVF | 14.58 (6.34–33.56) | < .001 | 17.11 (7.02–41.77) | < .001 |
| Age groups (compared to < 35 years) | ||||
| 35–37 years | 0.59 (0.39–0.90) | .015 | 0.78 (0.53–1.14) | .196 |
| 38–40 years | 0.64 (0.38–1.08) | .094 | 0.66 (0.41–1.08) | .094 |
| ≥ 41 years | 0.13 (0.06–0.27) | < .001 | 0.26 (0.15–0.44) | < .001 |
| Race (compared to White race) | ||||
| Black | 0.85 (0.57–1.27) | .425 | 0.84 (0.59–1.21) | .352 |
| Asian | 0.54 (0.23–1.27) | .157 | 0.50 (0.23–1.09) | .080 |
| Hispanic ethnicity | 0.67 (0.19–2.37) | .540 | 0.65 (0.23–1.90) | .433 |
| Body mass index categories (compared to normal weight) | ||||
| Overweight | 0.56 (0.36–0.89) | .015 | 0.74 (0.49–1.11) | .140 |
| Obese | 0.82 (0.55–1.24) | .378 | 0.84 (0.58–1.22) | .358 |
| Relationship status (compared to opposite-sex pairs) | ||||
| Single | 1.49 (0.80–2.77) | .214 | 1.36 (0.80–2.32) | .258 |
| Same sex | 1.48 (0.92–2.36) | .104 | 1.06 (0.70–1.60) | .790 |
BMI was found to be a predictor of live birth and clinical pregnancy rates per cycle across all treatment modalities (Table 2). Compared to patients with a normal BMI, patients with a BMI between 25.1 and 30 had similar rates of clinical pregnancy but were half as likely to have a live birth (aOR = 0.56; 95% CI: 0.36–0.89, p = 0.015). A BMI greater than 30 was not significantly associated with the likelihood of having a live birth. Race, ethnicity, and relationship status were not significantly associated with the likelihood of having a live birth or clinical pregnancy.
Perinatal outcomes
Of the cycles for which placental and neonatal outcomes were available (n = 189 live births), seventy-four (39.1%) pregnancies had resulting complications, including hypertensive disorders of pregnancy, gestational diabetes, fetal growth restriction, and postpartum hemorrhage (Table 3). Thirty-four (18.0%) patients suffered from hypertensive disorders of pregnancy, including worsening chronic hypertension, gestational hypertension, preeclampsia, eclampsia, and hemolysis, elevated liver enzymes, and low platelet count (HELLP) syndrome. Nine (4.8%) patients had gestational diabetes and nine (4.8%) patients had fetal growth restriction. Two second-trimester losses occurred. One maternal death occurred following an eclamptic seizure, and one intrauterine fetal demise (IUFD) was reported. Regarding delivery complications, fourteen (7.4%) patients had a postpartum hemorrhage. Most pregnancies resulted in a full-term delivery (n = 153, 84.1%); however, preterm delivery occurred in twenty-nine (15.9%) patients. Information regarding gestational age at delivery was unavailable for seven (3.7%) patients. Most preterm deliveries were iatrogenic due to complications of preeclampsia, HELLP syndrome, and FGR; however, almost half of the preterm deliveries (n = 13, 43.3%) occurred due to spontaneous preterm labor or preterm premature rupture of membranes. Multiple gestations occurred in fifteen pregnancies, of which eleven resulted in live births. Of all resulting pregnancies, there were 14 (7.7%) twin gestations and one (0.35%) triplet gestation. Placenta previa was noted in two pregnancies, and placental abruption was noted in two pregnancies. No pregnancies were complicated by placenta accreta. The mean infant weight at delivery for all infants was 3130 (SD 665) g. Of the 198 infants born, fifteen (8.3%) required admission to the NICU and three (1.7%) infants died after delivery.
Table 3.
Obstetric and neonatal outcomes among all live births resulting from fertility treatment utilizing donor sperm that occurred at a single institution between 2014 and 2020, n = 189 life births
| IUI n = 106 |
IVF n = 62 |
Co-IVF n = 21 |
|
|---|---|---|---|
| Pregnancy or placental complications, n = 74 (39.1%)a | |||
| Postpartum hemorrhage | 9 (8.5) | 4 (6.5) | 1 (4.8) |
| Hypertensive disorder | 21 (19.8) | 8 (12.9) | 5 (23.8) |
| Fetal growth restriction | 6 (5.7) | 3 (4.8) | 0 (0.0) |
| Gestational diabetes | 6 (5.7) | 1 (1.6) | 2 (9.5) |
| Preterm pre-labor rupture of membranes | 5 (4.7) | 6 (9.7) | 2 (9.5) |
| Placental abruption | 1 (0.9) | 1 (1.6) | 0 (0.0) |
| Placenta previa | 0 (0.0) | 2 (3.2) | 0 (0.0) |
| Gestational outcome, n (%) | |||
| Preterm (< 37 weeks gestation) | 15 (14.2) | 10 (16.1) | 4 (19.0) |
| Term (≥ 37 weeks gestation) | 84 (79.2) | 52 (83.9) | 17 (81.0) |
| Gestational age at delivery (weeks, SD) | 38.1 (3.2) | 38.1 (3.3) | 38.0 (3.3) |
| Mode of delivery, n (%) | |||
| Normal spontaneous vaginal delivery | 51 (48.1) | 31 (50.0) | 13 (61.9) |
| Cesarean section | 42 (39.6) | 28 (45.2) | 6 (28.6) |
| Operative delivery | 6 (5.7) | 1 (1.6) | 2 (9.5) |
| Neonatal outcomes | |||
| NICU admission, n (%) | 8 (7.5) | 3 (4.8) | 4 (19.0) |
| Neonatal death, n (%) | 2 (1.9) | 0 (0.0) | 0 (0.0) |
| Mean length of NICU admission (days, SD) | 56.1 (99.4) | 6.0 (2.8) | 5.3 (7.5) |
| Mean weight at delivery, grams (SD) | 3113 (658) | 3169 (637) | 3206 (671) |
aComplete information regarding pregnancy outcomes was not available for 22 (11.6%) patients who had a live birth
Rates of pregnancy and placental complications were similar across patients who used IUI, IVF, and co-IVF. BMI influenced rates of pregnancy complications (Table 4). Compared to patients with a normal BMI, overweight patients were approximately three times more likely to have a pregnancy or placental complication (aOR = 3.39; 95% CI: 1.41–8.13, p = 0.006). Similarly, obese patients were approximately three times more likely to have a pregnancy or placental complication (aOR = 2.91; 95% CI: 1.31–6.46, p = 0.009). Neither treatment type nor race was significantly predictive of pregnancy complications.
Table 4.
Adjusted odds ratios (aOR) for perinatal complications and miscarriage from fertility treatment cycles utilizing donor sperm at a single clinic between 2014 and 2020
| Pregnancy/placental complicationsa | Miscarriage | |||
|---|---|---|---|---|
| aOR (95% CI) | P value | aOR (95% CI) | P value | |
| Treatment type (compared to IUI) | ||||
| IVF | 0.72 (0.34–1.53) | .398 | 1.92 (0.91–4.04) | .088 |
| Co-IVF | 0.51 (0.13–1.99) | .335 | 1.81 (0.39–8.34) | .448 |
| Age groups (compared to < 35 years) | ||||
| 35–37 years | 1.02 (0.45–2.29) | .964 | 2.43 (1.01–5.81) | .046 |
| 38–40 years | 2.51 (0.91–6.91) | .076 | 3.05 (1.17–7.96) | .022 |
| ≥ 41 years | 1.17 (0.27–5.08) | .832 | 2.76 (1.08–7.10) | .035 |
| Race (compared to White race) | ||||
| Black | 0.66 (0.30–1.45) | .297 | 0.90 (0.45–1.80) | .769 |
| Asian | 0.36 (0.03–3.71) | .389 | 0.83 (0.18–3.85) | .769 |
| Hispanic ethnicityb | 0.55 (0.05–6.1) | .629 | - | |
| BMI categories (compared to normal weight) | ||||
| Overweight | 3.39 (1.41–8.13) | .006 | 1.39 (0.67–2.88) | .373 |
| Obese | 2.91 (1.31–6.46) | .009 | 0.83 (0.39–1.74) | .614 |
| Relationship status (compared to opposite-sex pairs) | ||||
| Single | 0.86 (0.27–2.76) | .793 | 0.82 (0.34–1.97) | .659 |
| Same sex | 0.49 (0.19–1.25) | .136 | 0.69 (0.32–1.49) | .342 |
aPregnancy complications included hypertensive disorders of pregnancy, gestational diabetes, fetal growth restriction, preterm delivery, placental complications, and neonatal complications
bCells suppressed for Hispanic ethnicity and miscarriage given zero cell
Discussion
Our study demonstrated an increase in donor sperm fertility treatment cycles between 2014 and 2020 at a single institution. Most donor sperm users choose IUI as their initial treatment modality before pursuing IVF or co-IVF; however, live birth rates were highest (almost sevenfold) in IVF cycles compared to IUI cycles. The most frequent pregnancy complication was hypertensive disorders of pregnancy.
Overall, the total number of patients using donor sperm for conception gradually increased during the study period. Reasons for this include increased efforts to provide more access to fertility care, the growing use of ART among LGBTQ patients, and a higher volume of patients seeking care. Although more patients were utilizing donor sperm over time, the use of donor sperm for IUI cycles did decrease in 2019 and 2020. One possible explanation for this is the temporary suspension of fertility treatment in the spring of 2020 at the beginning of the COVID-19 pandemic [21]. Another possible explanation is that there is increasing insurance coverage for IVF since 2019 at our institution, so more patients are choosing to initiate treatment with IVF rather than first pursuing IUI. Because success rates with IVF are higher than IUI on a per-cycle basis, there are altogether fewer cycles.
We found that patient BMI is predictive of live birth. Specifically, our study findings showed that, compared to women with a BMI < 25, women who are overweight (BMI 25.0–29.9) had lower rates of live birth whereas women who are obese (BMI > 30) had similar rates of live birth. Perhaps one reason for this is that women falling into the category of “obesity” have closer follow-up and more intensive counseling than patients who fall into the “overweight” category. The association of BMI with pregnancy outcomes following ART and IUI have been previously studied with mixed results. While there is literature that demonstrates lower pregnancy and live birth rates among obese patients following IVF cycles [22–25], other studies have suggested that BMI does not affect pregnancy outcomes [26–28]. Most of this research was conducted using analysis of non-donor sperm cycles, and our study contributes further data on the impact of BMI on fertility treatment outcomes in the context of donor sperm cycles.
Our patients who used co-IVF for conception had the highest live birth rates, even after adjusting for age and other potential confounders. Similar promising findings have been demonstrated in prior studies [29, 30] with one particular study showing higher pregnancy, ongoing pregnancy, and live birth rates in co-IVF compared to traditional IVF [30]. There are several reasons that could explain these findings. First, patient selection may play a role. Both patients in these co-maternity cycles may be younger with fewer health problems. Additionally, co-maternity IVF cycles can be quite costly due to several factors, including monitoring for both patients and the possible need for a frozen embryo transfer. Little is known regarding the impact of socioeconomic status on ART outcomes [31]. Second, gestational carriers do not undergo ovarian stimulation. Prior studies have demonstrated that exposure to exogeneous gonadotropins during ovarian stimulation can negatively affect endometrial receptivity, leading to lower pregnancy and live birth rates following fresh embryo transfer compared to frozen embryo transfer in traditional IVF cycles [32–34]. With co-IVF, the gestational parent’s uterus is not exposed to supraphysiologic levels of gonadotropins or estrogen.
Perinatal complications were seen across all fertility treatment modalities, and hypertensive disorders of pregnancy were the most common. Other studies have demonstrated similar findings with higher rates of hypertensive disorders of pregnancy following donor sperm cycles compared to autologous sperm cycles [12, 13]. However, rates of other perinatal complications such as preterm birth and low birth weight are similar among donor sperm and autologous sperm cycles [9, 11, 13]. These findings support an immunologic theory of preeclampsia, which suggests that normal placentation requires an established immune tolerance between the mother and the developing fetus [14]. Abnormalities in the maternal immune response can occur after exposure to novel maternal or paternal antigens, such as donor sperm or donor oocyte. Previous studies that focused on donor oocyte outcomes have found higher rates of hypertensive disorders of pregnancy and fetal growth restriction, supporting this immunologic theory for placental dysfunction [35]. It is important to note that the gestational parent in the co-IVF cycle will technically have both a non-autologous oocyte and donor sperm, which may negatively potentiate poor perinatal outcomes. Scant literature has examined outcomes specifically in co-IVF cycles, which may differ from gestational carrier (GC) cycles given the intimate relationship between the partner carrying the pregnancy and the oocyte source. Literature specifically in GC cycles has demonstrated higher rates of adverse perinatal outcomes, such as hypertensive disorders, gestational diabetes, placental disorders, preterm delivery, low birth weight, and postpartum hemorrhage [36, 37]. More studies with larger sample sizes are needed to further study the perinatal outcomes of co-IVF in order to provide comprehensive patient counseling.
This study has several strengths. We focus on a diverse patient population. Approximately one-half of patients identified as an ethnic minority, which is unique among the existing literature that is primarily comprised of Caucasian study participants. Additionally, most of the patients included in this study did not have known underlying infertility, which is also unique as most of the existing literature on fertility treatments is based on patients with an established infertility diagnosis. This study was completed at a single center allowing for uniformity of fertility treatment protocols and examination of trends at a single center. Lastly, this detailed chart review included patient contact for perinatal outcomes among patients who utilized donor sperm for IUI.
This study also has its limitations. First, it is retrospective in nature, and our cohort does not include a group of patients using non-donor sperm to achieve pregnancy. Thus, it is difficult to draw any definitive conclusions regarding the effects of donor sperm use on obstetric and perinatal outcomes. In looking at previously published literature comparing donor and non-donor sperm cycles, some demonstrate higher live birth rates following donor sperm IVF cycles [6] while others looking particularly at rates of adverse perinatal outcomes have conflicting results [7, 9, 13]. Second, while our study does capture many patients and donor sperm cycles, the number of patients who had a live birth is not large enough to make conclusions regarding perinatal outcomes. Third, the number of patients experiencing a pregnancy complication was small, and we were missing 22 (11.6%) pregnancy outcomes despite several attempts to contact former patients. Thus, our results should be interpreted with caution. Lastly, transgender patients may also use donor sperm to achieve pregnancy, but this patient population was not reflected in our cohort. As with other published literature, co-IVF is still relatively new and under-utilized in our institution, which makes our ability to draw conclusions about its success and feasibility difficult.
In conclusion, many patients are pursuing pregnancy via IUI, IVF, and co-IVF with donor sperm. While most patients who use donor sperm are women in same-sex female partnerships, single women and women in heterosexual partnerships with male factor infertility are also using donor sperm. As more patients are seeking pregnancy with donor sperm, this research will aid in patient counseling regarding treatment choices, anticipated pregnancy and live birth rates, and potential pregnancy and neonatal complications. Further studies are needed to learn why patients choose certain treatment modalities over others, and larger studies are needed to capture more co-IVF cycles, pregnancy complications, and neonatal complications.
Declarations
The authors did not receive support from any organization for the submitted work, and they have no relevant financial or non-financial interests to disclose. This retrospective chart review study involving human participants was in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. The Human Investigation Committee (IRB) of Emory University approved this study. Daniela Diego, Heather Hipp, and Jennifer Kawwass contributed to the study conception and design. Material preparation and data collection were performed by Daniela Diego. Data analysis was performed by Alexandra Medline and Lisa Shandley. The first draft of the manuscript was written by Daniela Diego, and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Gerkowicz SA, Crawford SB, Hipp HS, Boulet SL, Kissin DM, Kawwass JF. Assisted reproductive technology with donor sperm: national trends and perinatal outcomes. Am J Obstet Gynecol. 2018;218(421):e1–e10. doi: 10.1016/j.ajog.2017.12.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arocho R, Lozano EB, Halpern CT. Estimates of donated sperm use in the United States: National Survey of Family Growth 1995–2017. Fertil Steril. 2019;112:718–723. doi: 10.1016/j.fertnstert.2019.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Getrajdman C, Lee JA, Copperman AB. Co-IVF for same-sex female couples. Semin Reprod Med. 2017;35:415–419. doi: 10.1055/s-0037-1605380. [DOI] [PubMed] [Google Scholar]
- 4.Carpinello OJ, Jacob MC, Nulsen J, Benadiva C. Utilization of fertility treatment and reproductive choices by lesbian couples. Fertil Steril. 2016;106:1709–13 e4. doi: 10.1016/j.fertnstert.2016.08.050. [DOI] [PubMed] [Google Scholar]
- 5.Ethics Committee of the American Society for Reproductive Medicine. Electronic address aao. Access to fertility treatment irrespective of marital status, sexual orientation, or gender identity: an Ethics Committee opinion. Fertil Steril. 2021;116:326–30. [DOI] [PubMed]
- 6.Bortoletto P, Willson S, Romanski PA, Davis OK, Rosenwaks Z. Reproductive outcomes of women aged 40 and older undergoing IVF with donor sperm. Hum Reprod. 2021;36:229–235. doi: 10.1093/humrep/deaa286. [DOI] [PubMed] [Google Scholar]
- 7.Chen L, Zhu L, Cai C, Yan G, Sun H. Clinical and neonatal outcomes of intrauterine insemination with frozen donor sperm. Syst Biol Reprod Med. 2018;64:240–245. doi: 10.1080/19396368.2018.1453563. [DOI] [PubMed] [Google Scholar]
- 8.Johal JK, Gardner RM, Vaughn SJ, Jaswa EG, Hedlin H, Aghajanova L. Pregnancy success rates for lesbian women undergoing intrauterine insemination. F S Rep. 2021;2:275–281. doi: 10.1016/j.xfre.2021.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Yu B, Fritz R, Xie X, Negassa A, Jindal S, Vega M, et al. The impact of using donor sperm in assisted reproductive technology cycles on perinatal outcomes. Fertil Steril. 2018;110:1285–1289. doi: 10.1016/j.fertnstert.2018.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gibbons WE, Cedars M, Ness RB. Society for Assisted Reproductive Technologies Writing G. Toward understanding obstetrical outcome in advanced assisted reproduction: varying sperm, oocyte, and uterine source and diagnosis. Fertil Steril. 2011;95:1645–9 e1. [DOI] [PubMed]
- 11.Kamath MS, Antonisamy B, Selliah HY, La Marca A, Sunkara SK. Perinatal outcomes following IVF with use of donor versus partner sperm. Reprod Biomed Online. 2018;36:705–710. doi: 10.1016/j.rbmo.2018.03.016. [DOI] [PubMed] [Google Scholar]
- 12.Gonzalez-Comadran M, Urresta Avila J, Saavedra Tascon A, Jimenez R, Sola I, Brassesco M, et al. The impact of donor insemination on the risk of preeclampsia: a systematic review and meta-analysis. Eur J Obstet Gynecol Reprod Biol. 2014;182:160–166. doi: 10.1016/j.ejogrb.2014.09.022. [DOI] [PubMed] [Google Scholar]
- 13.Allen CP, Marconi N, McLernon DJ, Bhattacharya S, Maheshwari A. Outcomes of pregnancies using donor sperm compared with those using partner sperm: systematic review and meta-analysis. Hum Reprod Update. 2021;27:190–211. doi: 10.1093/humupd/dmaa030. [DOI] [PubMed] [Google Scholar]
- 14.Levron Y, Dviri M, Segol I, Yerushalmi GM, Hourvitz A, Orvieto R, et al. The ‘immunologic theory’ of preeclampsia revisited: a lesson from donor oocyte gestations. Am J Obstet Gynecol. 2014;211(383):e1–5. doi: 10.1016/j.ajog.2014.03.044. [DOI] [PubMed] [Google Scholar]
- 15.Bodri D, Nair S, Gill A, Lamanna G, Rahmati M, Arian-Schad M, et al. Shared motherhood IVF: high delivery rates in a large study of treatments for lesbian couples using partner-donated eggs. Reprod Biomed Online. 2018;36:130–136. doi: 10.1016/j.rbmo.2017.11.006. [DOI] [PubMed] [Google Scholar]
- 16.Tarin JJ, Garcia-Perez MA, Cano A. Deficiencies in reporting results of lesbians and gays after donor intrauterine insemination and assisted reproductive technology treatments: a review of the first emerging studies. Reprod Biol Endocrinol. 2015;13:52. doi: 10.1186/s12958-015-0053-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ferrara I, Balet R, Grudzinskas JG. Intrauterine donor insemination in single women and lesbian couples: a comparative study of pregnancy rates. Hum Reprod. 2000;15:621–625. doi: 10.1093/humrep/15.3.621. [DOI] [PubMed] [Google Scholar]
- 18.Nordqvist S, Sydsjo G, Lampic C, Akerud H, Elenis E, Skoog SA. Sexual orientation of women does not affect outcome of fertility treatment with donated sperm. Hum Reprod. 2014;29:704–711. doi: 10.1093/humrep/det445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marina S, Marina D, Marina F, Fosas N, Galiana N, Jove I. Sharing motherhood: biological lesbian co-mothers, a new IVF indication. Hum Reprod. 2010;25:938–941. doi: 10.1093/humrep/deq008. [DOI] [PubMed] [Google Scholar]
- 20.Fiske E, Weston G. Utilisation of ART in single women and lesbian couples since the 2010 change in Victorian legislation. Aust N Z J Obstet Gynaecol. 2014;54:497–499. doi: 10.1111/ajo.12260. [DOI] [PubMed] [Google Scholar]
- 21.American society for reproductive medicine (ASRM) patient management and clinical recommendations during the coronavirus (COVID-19) pandemic. 2020. [cited; Available from https://www.asrm.org/globalassets/asrm/asrm-content/news-and-publications/covid-19/covidtaskforce.pdf.
- 22.Sermondade N, Huberlant S, Bourhis-Lefebvre V, Arbo E, Gallot V, Colombani M, et al. Female obesity is negatively associated with live birth rate following IVF: a systematic review and meta-analysis. Hum Reprod Update. 2019;25:439–451. doi: 10.1093/humupd/dmz011. [DOI] [PubMed] [Google Scholar]
- 23.Garcia-Ferreyra J, Carpio J, Zambrano M, Valdivieso-Mejia P, Valdivieso-Rivera P. Overweight and obesity significantly reduce pregnancy, implantation, and live birth rates in women undergoing In Vitro Fertilization procedures. JBRA Assist Reprod. 2021;25:394–402. doi: 10.5935/1518-0557.20200105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.MacKenna A, Schwarze JE, Crosby JA, Zegers-Hochschild F. Outcome of assisted reproductive technology in overweight and obese women. JBRA Assist Reprod. 2017;21:79–83. doi: 10.5935/1518-0557.20170020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kawwass JF, Kulkarni AD, Hipp HS, Crawford S, Kissin DM, Jamieson DJ. Extremities of body mass index and their association with pregnancy outcomes in women undergoing in vitro fertilization in the United States. Fertil Steril. 2016;106:1742–1750. doi: 10.1016/j.fertnstert.2016.08.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Starosta A, Gordon CE, Hornstein MD. Predictive factors for intrauterine insemination outcomes: a review. Fertil Res Pract. 2020;6:23. doi: 10.1186/s40738-020-00092-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Whynott RM, Summers KM, Van Voorhis BJ, Mejia RB. Effect of body mass index on intrauterine insemination cycle success. Fertil Steril. 2021;115:221–228. doi: 10.1016/j.fertnstert.2020.07.003. [DOI] [PubMed] [Google Scholar]
- 28.Huyghe S, Verest A, Thijssen A, Ombelet W. Influence of BMI and smoking on IUI outcome with partner and donor sperm. Facts Views Vis Obgyn. 2017;9:93–100. [PMC free article] [PubMed] [Google Scholar]
- 29.Yeshua A, Lee JA, Witkin G, Copperman AB. Female couples undergoing IVF with partner eggs (co-IVF): pathways to parenthood. LGBT Health. 2015;2:135–139. doi: 10.1089/lgbt.2014.0126. [DOI] [PubMed] [Google Scholar]
- 30.Nunez A, Garcia D, Gimenez-Bonafe P, Vassena R, Rodriguez A. Reproductive outcomes in lesbian couples undergoing reception of oocytes from partner versus autologous in vitro fertilization/intracytoplasmic sperm injection. LGBT Health. 2021;8:367–371. doi: 10.1089/lgbt.2020.0282. [DOI] [PubMed] [Google Scholar]
- 31.Imrie R, Ghosh S, Narvekar N, Vigneswaran K, Wang Y, Savvas M. Socioeconomic status and fertility treatment outcomes in high-income countries: a review of the current literature. Hum Fertil (Camb). 2021;1–11. [DOI] [PubMed]
- 32.Shapiro BS, Daneshmand ST, Restrepo H, Garner FC, Aguirre M, Hudson C. Matched-cohort comparison of single-embryo transfers in fresh and frozen-thawed embryo transfer cycles. Fertil Steril. 2013;99:389–392. doi: 10.1016/j.fertnstert.2012.09.044. [DOI] [PubMed] [Google Scholar]
- 33.Insogna IG, Lanes A, Lee MS, Ginsburg ES, Fox JH. Association of fresh embryo transfers compared with cryopreserved-thawed embryo transfers with live birth rate among women undergoing assisted reproduction using freshly retrieved donor oocytes. JAMA. 2021;325:156–163. doi: 10.1001/jama.2020.23718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Check JH, Katsoff B, Brasile D, Wilson C, Summers-Chase D. Comparison of pregnancy outcome following frozen embryo transfer (ET) in a gestational carrier program according to source of the oocytes. Clin Exp Obstet Gynecol. 2011;38:26–27. [PubMed] [Google Scholar]
- 35.Schwartz KM, Boulet SL, Kawwass JF, Kissin DM. Perinatal outcomes among young donor oocyte recipients. Hum Reprod. 2019;34:2533–2540. doi: 10.1093/humrep/dez213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Woo I, Hindoyan R, Landay M, Ho J, Ingles SA, McGinnis LK, et al. Perinatal outcomes after natural conception versus in vitro fertilization (IVF) in gestational surrogates: a model to evaluate IVF treatment versus maternal effects. Fertil Steril. 2017;108:993–998. doi: 10.1016/j.fertnstert.2017.09.014. [DOI] [PubMed] [Google Scholar]
- 37.Phillips AM, Magann EF, Whittington JR, Whitcombe DD, Sandlin AT. Surrogacy and pregnancy. Obstet Gynecol Surv. 2019;74:539–545. doi: 10.1097/OGX.0000000000000703. [DOI] [PubMed] [Google Scholar]


