Abstract
Ischemia-reperfusion injury occurs when blood supply to an organ is disrupted—ischemia—and then restored—reperfusion—and is commonly found under different pathological settings such as cerebral, myocardial, renal, and hepatic ischemia-reperfusion injuries. Despite apparent differences as to the cause of these diseases, emerging evidence suggests that common signaling pathways, such as exosomes and microRNAs (miRNAs), are involved in this context. Although miRNAs are also found in the extracellular milieu, plenty of miRNAs are found in exosomes and are thus protected from degradation. miRNAs selectively sorted into exosomes potentially regulate specific aspects of the onset and progression of ischemic stroke. Such mechanisms involve the regulation of cell survival, inflammation, angiogenesis, and neurogenesis. Likewise, miRNAs shuttled into exosomes are involved in the pathogenesis of myocardial, renal, and hepatic ischemia-reperfusion injuries. This review will discuss recent evidence on the exosome-facilitated progression of four ischemia-reperfusion conditions, particularly concerning miRNAs within these vesicles. The notion is given to miRNAs participating in more than one of the four conditions, indicating a considerable degree of overlap across ischemia-reperfusion conditions. We will conclude the review by highlighting clinical opportunities of such exosome-derived miRNAs both as biomarkers and as therapeutic targets.
Graphical abstract
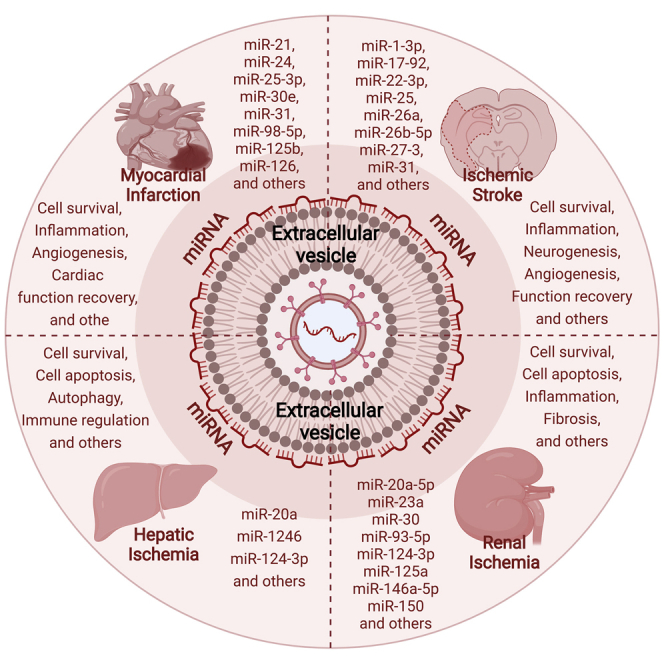
Exosomal miRNAs can exert biological functions via modulating multiple pathogeneses after cerebral, myocardial, renal, and hepatic ischemia-reperfusion injuries, which decisively regulates tissue progression. These miRNAs that appeared in at least two ischemia-reperfusion conditions exhibit a significant degree of overlaps and emerge an excellent consistency as for modes of action.
Introduction
Ischemia-reperfusion injury is a complex pathological process that begins with tissue anoxia and is accompanied by the production of free oxygen radical-induced inflammatory responses.1 Ischemia promotes intracellular and mitochondrial calcium levels via impairing ATPase-dependent ion transport and reducing intracellular pH impair cell volume regulatory mechanisms, further leading to the lysis of organelle and plasma membranes.2,3 Although it salvages the delivery of oxygen and substrates required for aerobic ATP generation and normalizes extracellular pH, reperfusion itself tends to be related to detrimental consequences by inducing paradoxical tissue responses, endoplasmic reticulum (ER) stress, and postischemic capillary no reflow, which amplify ischemic tissue injury.2,3 Among these conditions, cerebral, myocardial, renal, and hepatic ischemia-reperfusion injuries are eminent players since they continue to be among the most frequent causes of debilitating disease and death in medicine.2 Although noticeable pathophysiological discrepancies exist, the gene responses to ischemia-reperfusion in these four disorders show a high degree of similarities, such as protein-coding RNAs and non-coding RNAs (ncRNAs). The emerging recent evidence reveals that the common signaling pathways, exosomal-derived microRNAs (miRNAs/miRs), the most common ncRNAs, as an essential means of intercellular communication, have attracted considerable interest in this context. Exosomes are lipid bilayer-enclosed spheres that serve as the bridges for a critical role in transferring membrane-bound proteins, lipids, and ncRNAs from donors to recipient cells.4 miRNAs are small endogenous ncRNAs that regulate gene expression posttranscriptionally by functioning as endogenous negative gene regulators.5,6 Interestingly, as essential components, miRNAs are selectively enriched into exosomes, and miRNAs shuttled in exosomes can exert biological functions to regulate specific aspects of onset and progression of ischemia-reperfusion injury.7 Owing to aberrantly expressed after ischemia, exosomal miRNAs are revealed to serve as a potential source of biomarkers and novel therapeutic targets.8, 9, 10 This present review is meant as a summary of the latest literature concerning the role of exosome-related miRNA contents in the progression of cerebral, myocardial, renal, and hepatic ischemia-reperfusion injuries. Aspects regarding the cellular and subcellular source of exosomal miRNAs, their cellular targets, and biological effects are assessed, with a particular emphasis on the potential as biomarkers and therapeutic targets.
Brain, heart, liver, and kidney are eminent players for ischemia-reperfusion injury with a tight interaction: Dysfunction following ischemic stroke
Ischemic stroke is an acute cerebrovascular disease characterized by the sudden interruption of blood supply and oxygen, followed by subsequent restoration of blood flow and reoxygenation in a singular part of the brain, contributing to severe disability and death.11 Myocardial ischemia-reperfusion injury in acute myocardial infarction is the most important cause of morbidity and mortality worldwide.12 Renal ischemia-reperfusion occurs in artery stenosis, partial nephrectomy, and most commonly during kidney transplantation, causing severe consequences or organ dysfunction and, as a result, yielding renal failure and ultimate death.13 Hepatic ischemia-reperfusion injury is a significant complication often seen in liver surgery and transplantation.14 Despite cerebral, myocardial, renal, and hepatic ischemia-reperfusion injuries involving diverse pathophysiological processes with respective organs, these four pathological settings have the common feature that is among the most frequent causes of debilitating disease and death globally. Additionally, since brain damage can modify the autonomic and neurohormonal pathways involved in the control of myocardial, renal, and hepatic function, patients affected by ischemic stroke are incredibly vulnerable to severe adverse events on these organs.
Brain-heart interaction: Myocardial dysfunction following ischemic stroke
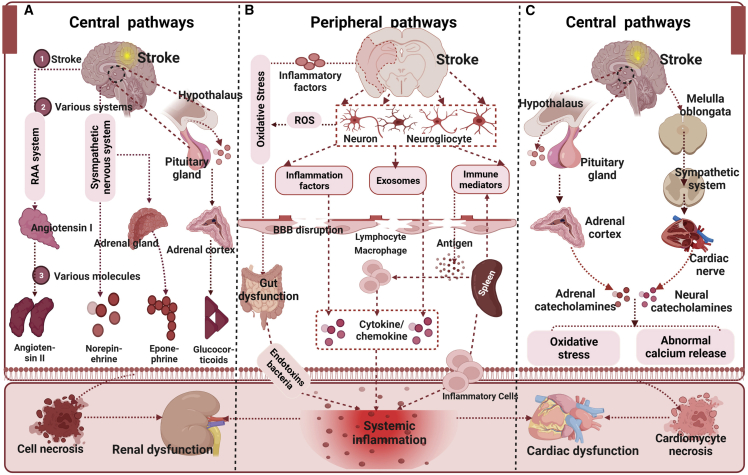
Broadly, since brain damage can regulate the autonomic and neurohormonal pathways associated with the modulation of heart function, ischemic stroke in acute phase-induced myocardial dysfunction are highly vulnerable to mortality, lifelong cardiac failure, or mild and recoverable injury, which are tightly related to the severity of the ischemic stroke and neurological deficits.15 As such, impaired myocardial dysfunction because of severe ischemic stroke is a predictor of worse functional outcomes and secondary complications.16 Following acute ischemic stroke, in the first 24 h, 60%–85% of patients indicate an electrocardiographic abnormality.17 During the first 3 months, 19% of patients with ischemic stroke have at least one severe cardiac adverse event, 28.5% uncover impairment of left ventricular ejection fraction, and 13%–29% induce systolic dysfunction.17 The pathophysiological mechanisms by which ischemic stroke leads to myocardial dysfunction remain unclear. Leading mechanisms primarily involve the hypothalamic-pituitary-adrenal axis,18 immune responses,19 inflammatory responses,19 gut dysbiosis,20 and other risk factors.21 (Figure 1) Regardless of the mechanisms, myocardial dysfunction (such as atherosclerosis and atrial fibrillation) increases the risk of developing acute ischemic stroke, and vice versa, acute ischemic stroke induces a variety of pathways as aforementioned, therefore accelerating patients to develop a myocardial dysfunction.23
Figure 1.
Role of central autonomic network and systemic inflammation in mediating renal and cardiac dysfunction after ischemic stroke
(A) Ischemic stroke activates the hypothalamic-pituitary-adrenal axis, sympathetic nervous system, and the renin-angiotensin-aldosterone system, which regulate hormone and neurotransmitter release, thus resulting in kidney dysfunction. (B) Release of inflammatory factors by injured brain cells and increased oxidative stress can cause blood-brain barrier disruption, stroke-induced gut microbiome dysbiosis can transfer bacterial and endotoxin translocation to the blood, and the spleen can activate the immune cell, thereby leading to systemic inflammation. Systemic inflammation is central in promoting renal and cardiac dysfunction after stroke. (C) Ischemic stroke-induced activation of the hypothalamic-pituitary-adrenal axis and autonomic activation can alter the release of adrenal catecholamines and neural catecholamines, thus resulting in cardiac dysfunction. This image is adapted from a previous study22 published under the Creative Common attribution license.
Brain-kidney interaction: Renal dysfunction following ischemic stroke
Ischemic acute kidney injury (AKI) is a worldwide problem related to promoting morbidity and mortality and can be considered a systemic inflammatory condition that forms within a few days or even hours. A meta-analysis of 12 studies and 4,532,181 patients who had an acute ischemic stroke provided evidence that AKI is a common complication following acute ischemic stroke, with a pooled prevalence incidence of 12.9% of cases suffering AKI.24 This is associated with increased mortality following acute ischemic stroke. Tsagalis et al.25 retrospectively recorded the data of patients hospitalized for a first-ever stroke and indicated that approximately 28% of patients developed moderate or severe renal dysfunction, defined as glomerular filtration rate ≤60 mL/min/1.73 m2. Additionally, among Hispanic patients who had a stroke (90% of patients had an ischemic stroke), 62.5% showed an AKI.22 The AKI rate varies widely due to the inconsistent AKI-defining criteria and coding definitions. Mechanically, the central mechanisms of stroke-induced renal dysfunction may involve the central autonomic network and sympathetic nervous system;22 the peripheral mechanisms include the regulation of immune responses, autoregulation, and the neuroendocrine system, with the help of releasing exosomes.22 (Figure 1) Nevertheless, growing evidence suggests that patients with renal dysfunction have a graded and independent inverse impact on ischemic stroke, with a higher thrombotic complication.26 Renal dysfunction can exacerbate stroke pathogenesis and worsen recovery outcomes.26
Brain-liver interaction: Hepatic dysfunction following ischemic stroke
In patients with cerebrovascular disease and/or stroke, the prevalence of hepatic dysfunction is unknown since only a few data about the prevalence of hepatic dysfunction in these patients have been published. Kim and colleagues investigated 295 patients admitted with acute ischemic stroke compared with 1,942 control subjects, and they indicated that a greater proportion of the stroke group had significant liver fibrosis (>8 kPa) (9.2% versus 1.8%, p < 0.001), as well as a higher severity of fibrosis (odds ratio [OR] = 1.268).27 In a preclinical study, ischemic stroke can induce an immune response. In the liver, the TUNEL+ apoptotic cells, Iba1+ macrophages, CD68+ macrophages, Ki67+ proliferating cells, and interleukin-10 (IL-10)+ anti-inflammatory cells increased and that of CD8α+ T cells decreased after middle cerebral artery occlusion (MCAO).28 Interestingly, an immune-mediated interaction pathway in experimentally caused liver inflammation whereby, activate resident immune cells in the brain (i.e., the microglia) is demonstrated, peripheral circulating monocytes transmigrate into the brain, resulting in the development of sickness behaviors.29
Exosome and miRNA
Exosome: Biogenesis and characteristics
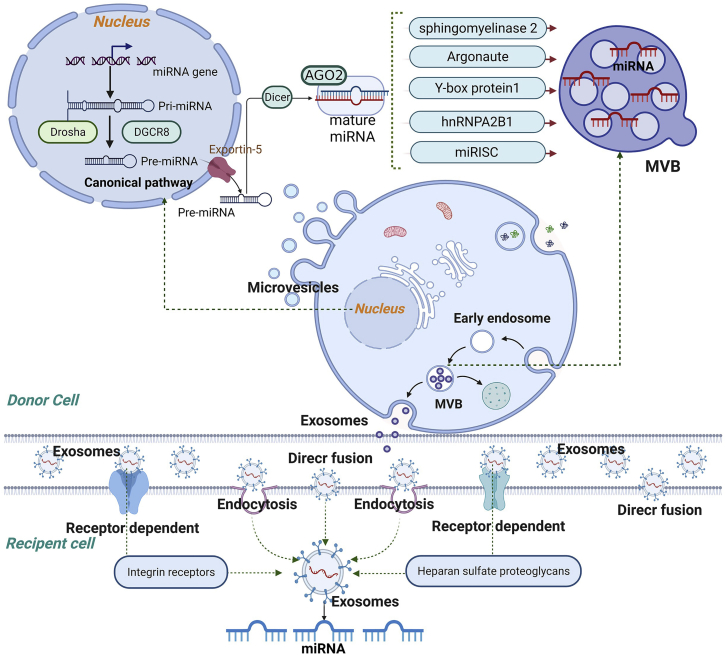
The exosome, with a size of approximately 30–150 nm, is one of the three terminologies (based on their diameter size) of membrane-bound extracellular vesicles (EVs).30,31 Four primary processes, including initiation, endocytosis, multivesicular body (MVB) formation, and secretion of intraluminal vesicles (ILVs), are involved in the formation of exosomes.32 The first invagination of the plasma membrane occurs during endocytosis, which induces the cell membrane to sag inward to form an early endosome’s de novo formation, accumulating ILVs in the lumen.33 Early endosomes tend to mature, leading to the formation of MVBs when a part of the endosomal membrane invaginates and buds into its lumen.34 After that, MVBs either come to the plasma membrane to release ILVs (called exosomes) into the extracellular space or fuse with the lysosome for degradation or autophagosomes to deliver cargos.32,35 The formation of MVBs and ILVs is a tightly regulated process, and the most exhaustive mechanism is the endosomal sorting complex required for transportation (ESCRT)-independent or -dependent pathway.36 After release, exosomes can be extracted via ultracentrifugation with 100,000–200,000 × g. Considerable protein markers have been revealed to characterize exosomes, such as CD63, CD9, CD81, ALIX, TSG101, and others.37,38 As mentioned, exosomes contain a variety of molecular cargos from the donor cells, e.g., RNAs, ncRNAs, DNAs, lipids, and metabolites, which determine the structures, biological properties, and functions of exosomes.
miRNA: Biogenesis and characteristics
The emerging evidence demonstrates that ncRNAs play a vital role in regulating gene expression and contribute to numerous disorders.39 miRNAs, one type of ncRNAs, are much earlier reported (first discovered in Caenorhabditis elegans) and the most discussed, with approximately 18–24 nucleotides in size. It is a family of posttranscriptional gene repressors that has appeared throughout the biology kingdom and have been widely related to the regulation of gene expression by combining with the 3′ untranslated region of the target mRNA sequence, suppressing the mRNA level.40 miRNA biogenesis is initiated by transcription of these miRNA-coding loci by RNA polymerase II,41 as consensus knows, in turn, to form plenty of nucleotide long stem-looped hairpin primary precursors for miRNA. These are known as primary miRNA transcripts or pri-miRNAs,42 which further yield a 70–100 nucleotide long pre-miRNA with the help of Drosha and DCGR8 and then are transported to the cytoplasm via exportin 5 and RAN-GTP.5 Thereafter, the pre-miRNA can produce an RNA complex (the miRNA and related passenger strand), which is then loaded into an RNA-induced silencing complex, of which the major effector is Ago2. Finally, Dicer, a double-stranded-specific RNaseIII enzyme, cleaves double-stranded RNA and cuts the pre-miRNAs into 20–23 nucleotide duplexes, which remain bound to Ago2.43
miRNA loading into exosomes and uptake by recipient cells
Although the miRNA cargos in exosomes, to some extent, present the transcriptomes of various cells, the miRNA profiles derived are substantially different from those originating from their cells of origin, revealing that numerous miRNA species are selectively encapsulated into exosomes.44 Various attempts have been performed to illustrate the mechanisms of loading and sorting miRNA into exosomes. Several pathways for loading miRNAs into exosomes are described. The first one is the sphingomyelinase 2-dependent pathway, which is the first molecule revealed to be associated with miRNA loading into exosomes. The overexpression of sphingomyelinase 2 promotes miRNAs’ sorting into exosome, whereas the inhibition of it shows a contrary result.45 The second pathway is from the inherent structure of miRNA with a 3′ end adenylation and uridylation, which is vital for the recognition by AGO2. miRNAs with an adenylated 3′ end are predominantly uncovered in cells, whereas miRNAs with a uridylated 3′ end are sorted in exosomes, as demonstrated in RNA sequencing research on human B cells and the related exosomes.46 The third sorting pathway involves the sumoylated heterogeneous nuclear ribonucleoprotein (hnRNP; mainly including hnRNPA2B1, hnRNPA1, and hnRNPC)-dependent pathway via binding to miRNAs and facilitating the loading of miRNAs into exosome.47 The fourth pathway is the miRNA-induced silencing complex (miRISC)-associated pathway. The miRISC is mainly composed of miRNA, miRNA-repressible mRNA, GW182, and AGO2. The RNA-binding protein mediates the last sorting pathway. SYNCRIP selectively sorts miRNAs and has a 4-nucleotide motif near the 3′ end, independently of hnRNPA2B1.48 Y-box protein 1 can selectively sort miR-223 into exosomes in HEK-293T cells.49
After intracellular loading of miRNA into exosome and binding to the region of their cells’ membrane and through plasma membrane fusion, ultimately achieving miRNA-containing exosomes release, three potential modes for the uptake of exosomal miRNA are reported. These include the following. (1) Targeting the recipient cells’ surface directly and merging with the cell membrane. (2) Internalizing through endocytic mechanisms, with an association of clathrin- or caveolae-regulated endocytosis, phagocytosis, or macropinocytosis.50 Of note, as a prominent role, however, phagocytosis and macropinocytosis can transmit miRNAs to the lysosome for degradation. After successful uptake, the generation of functional proteins from exosome-derived miRNAs in recipient cells was negligible,44 revealing that endosomal escape is vital for miRNA function and that exosome has a natural function of endosomal escape without a precise mechanism so far. (3) The miRNAs docking in exosomes can be assimilated by recipient cells by directly targeting the corresponding cytomembrane receptors, which subsequently activate or inhibit interrelated signaling pathways.51 These different kinds of receptors could be manipulated on the surface of the exosome to increase their uptake. For instance, targeting the heparan sulfate proteoglycans on the cytomembrane promoted the uptake of exosomes derived from the tumor by endocytosis,52 and various integrin receptors can increase the selective uptake of exosomes by certain specific tumors such as breast and ovarian.44 In addition, the interaction of T cell immunoglobulin and mucin domain-containing protein 4 molecules with phosphatidylserine on exosomal membranes can also increase the intake of exosomes as well as the miRNAs loading in exosomes.53 A brief overview so as to how the communication between cells via exosomal miRNAs is presented in Figure 2.
Figure 2.
The brief sorting mechanism of exosomal miRNA and uptake by recipient cells
The biogenesis of miRNA involves transcription of a pri-miRNA, formation of pre-miRNA, translocation to the cytoplasm, and maturation of the miRNA. miRNAs containing different RNA motifs can be loaded into multivesicular bodies (MVBs) via different RNA-binding proteins. MVBs can either follow a degradation pathway fusing with lysosomes or release the intraluminal vesicles as exosomes to the extracellular space. Recipient cells can uptake exosomal miRNAs by three pathways: direct fusion, endocytosis, and receptor signaling.
Roles of exosomal miRNAs in ischemic stroke
The role of exosomal miRNAs derived from brain-derived cells
Neurons and neurogliocytes (oligodendrocytes, astrocytes, microglia) are vital players in maintaining the homeostasis of the microenvironment. Upon a stroke condition, ischemia and reperfusion can result in anaerobic metabolism and develop the release of pro-inflammatory cytokines from neurogliocytes and neurons, promoting neuroprotection or further neurotoxicity within the brain ischemia area.54 Neurogliocytes, which survive after ischemia and play a key role in response to ischemia, are the primary components of the peri-lesions environment and have been implicated in poststroke immune modulation.55 An ongoing ischemic insult, excitotoxicity stress neurons, and inflammation through releasing “find-me” signals (e.g., ATP), exposing “eat-me” signals (e.g., phosphatidylserine), and binding to opsonin can induce neurogliocytes (e.g., microglia) to phagocytose such neurons.56 Activation of these cells elicits the release of plentiful potential exosomes into the extracellular space, further boosting function in neurovascular repair, inflammatory regulation, and cell preservation. Additionally, specific types of exosomal miRNAs act as neuroprotective players under ischemic conditions through interaction with the effects downstream.
Microglia, the principal immune cells of the brain, are immediately activated and migrate toward the location of the lesion after ischemia.57 Microglia exacerbate brain damage or support endogenous brain repair,58 correlating with distinct phenotypes, as indicated by the pro-inflammatory M1 and the anti-inflammatory M2 types.59 Exosomal miRNAs (miR-124,60,61 miR-26a,60 miR-137,63 miR-424-5p61) derived from microglia are involved in supporting neuronal survival, angiogenesis, and neurological recovery and inhibiting glial scar formation. For example, exosomal miR-124 released from M2 microglia (such as IL-4-polarized microglia) unregulated after stroke in the penumbra region exerts neuroprotection via increasing neural survival and attenuating neural deficits, apoptosis, and glial scar formation.62,63 miR-26a and miR-13762,63 similarly shuttled in M2 exosome and increased angiogenesis by promoting endothelial cell tube formation and neurological recovery via attenuating neuronal apoptosis, respectively. In contrast, miR-424-5p from hypoxic microglia induces significant brain microvascular endothelial cell damage by modulating the FGF2/STAT3 pathway.61
As such, normoxic (miR-17-5p,64 miR-34c,65 miR-361,67 miR-190b66) and hypoxic (miR-92b-3p,67 miR-7670-3p,67 miR-29a68) astrocyte-derived exosomal miRNAs have been uncovered to improve neuronal survival and poststroke functional recovery and inhibit inflammation. Concerning neuronal survival, exosomal miRNAs, such as miR-92b-3p and miR-361,67,69 can not only directly promote neuronal viability but also those such as miR-7670-3p67 and miR-190b66 indirectly inhibit autophagy and apoptosis-induced cell death. Regarding neuroinflammatory regulation, exosomal miRNAs can suppress inflammation both in vitro and in vivo (miR-17-5p,64 miR-29a68). The regulation of poststroke functional recovery seems to be broader, as indicated by the improvements in scores of various neurological functions.67,69
Cortical neurons, as well, can release exosomes from their somatodendritic compartments to modulate poststroke immune response (such as inflammation) and vascular remodeling (such as vascular integrity) via further secreting miRNAs. Exosomal miR-181c-3p derived from cortical neurons exerts protective effects on astrocyte neuroinflammation via downregulation of CXCL1 using an MCAO rat model.70 Additionally, neurons can transfer miR-132, a highly conserved and neuron-enriched miRNA, via secreting exosomes to endothelial cells to maintain brain vascular integrity by monitoring the expression of vascular endothelial cadherin.71
Endothelial cells, the eminent cellular component of the blood-brain barrier (BBB), develop the interface between circulating blood and CNS to maintain homeostasis by transferring exosomal miRNAs. For example, brain endothelial cell exosomes improve functional motor recovery and are pivotal for altering synaptic plasticity and function via transmitting miR-126-3p specifically, whereby they promote neurite outgrowth and suppress PC12 cell apoptosis.72 Axonal application of exosomes significantly elevates miRNAs that promote axonal growth via inhibiting proteins in distal axons and parent somata. Mechanistically, they alter axonal growth by regulating miR-19a, miR-27a, miR-195, and miR-298.73 In serum exosomes of patients who have had acute ischemic stroke, they play a crucial role in regulating cell survival and neuroinflammation by altering subtypes of microglia. Moreover, they have the potential to exert or enhance these effects via transmitting miR124-3p,74 miR-126,76 and miR-27-3P.75
The role of exosomal miRNA derived from mesenchymal stem cells (MSCs)
MSC-derived exosomes are emerging to be an appealing therapeutic tool for ischemic stroke, with the MSC-derived properties and the characteristics of effortless storage, lower immunogenicity, higher safety profile, and nature delivery vehicles. Current research indicates that MSC exosomes promote poststroke recovery due to their ability to modulate the expression of recipient cell protein, alter cell properties involved in stroke, and mediate restorative effects, such as cell survival, inflammation, neurogenesis, and angiogenesis, via miRNA transfer.
Cell survival
A great deal of miRNAs enveloped into MSC exosomes, such as miR-1-3p, miR-22-3p, miR-25, miR-26a, miR-26b-5p, miR-31, miR-126, miR-132, miR-138-5p, miR-146a-5p, miR-206, miR-223-3p, and miR-542-3p, were demonstrated to improve neuronal, astrocytic, oligodendrocytic, and microglial survival by downregulating target genes including KDM6B, p53, KLF9, CH25H, TRAF6, ACVR2B, LCN2, TLR4, RMRP, or CysLT2R.38,76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87 Autophagy is also associated with the promotion of cell death via leading to cellular accumulation of toxic metabolites or cellular self-degradation.88 Exosomal transfer of miRNAs from MSCs can reduce cell death by inhibiting excessive autophagy. For example, Kuang et al. illustrated that miR-25-3p loading into MSC exosomes protected primary neurons exposed to oxygen-glucose deprivation against injury by improved autophagic flux.38
Inflammation and neurogenesis
Concerning inflammation and neurogenesis, similar to those mentioned earlier, a total of 7 miRNAs, miR-26b-5p, miR-126, miR-138-5p, miR-138-5p, miR-221-3, miR-223-3p, and miR-542-3p, produced from MSC exosomes can suppress neuroinflammation of neurons, microglia, and astrocytes via inhibiting CH25H, LCN2, RMRP, ATF3, CysLT2R, and TLR4, further causing the downregulation of the TLR4, SMAD2, IRAK1/TRAF6, and PI3K/Akt/mTOR pathways.76, 77, 78,80,83,87 Transfer of miR-124 and miR-17-92 released from MSC exosomes and miR-26a enveloped into exosomes derived from urine stem cells can improve neurogenesis after stroke directly or indirectly by HDAC6 downregulation.89, 90, 91
Angiogenesis
Likewise, in preclinical studies, angiogenic effects have been uncovered for exosomes to mediate miR-181b and miR-210 transfer from MSCs to brain microvascular endothelial cells, mechanistically, through TRPM7 and TIMP3 downregulation and HIF1α, integrin-β3, VEGF, and CD34 elevation.92,93 Additionally, Gregorius et al.94 evaluated the effects of MSC exosome on microvascular remodeling. The results showed that exosomes from hypoxic, instead of normoxic, MSCs improved endothelial proliferation, migration, and tube formation in vitro and accelerated microvascular densities, microvascular length, and branching point densities in vivo.94 Of note, hypoxic conditions altered a distinct set of miRNAs in MSC exosomes related to angiogenesis, with three being increased (miR-126-3p, miR-140-5p, let-7c-5p) and three decreased (miR-186-5p, miR-370-3p, miR-409-3p), altogether suggesting that hypoxic preconditioning enhances the restorative effects of MSCs via altering master gene levels of miRNAs enveloped into exosomes.94
More interaction effects and related molecular targets under ischemic stroke are summarized in Table 1and Figure 3.38,63,65,67,69,70,74, 75, 76, 77, 78, 79, 80, 81, 82, 83, 84, 85, 86, 87,89, 90, 91, 92, 93,95, 96, 97, 98, 99, 100, 101, 102
Table 1.
Preclinical studies evaluating the effect of exosomal miRNAs in ischemic stroke
| miRNA | Expression | Donor cell | Recipient cell | Exosome isolation | Main function |
|---|---|---|---|---|---|
| miR-1-3p82 | upregulation | ucMSCs | primary neurons | ultracentrifugation | improve the cell viability and suppress apoptosis of neurons |
| miR-17-9289 | upregulation | MSCs | neurons, glial cells | ultracentrifugation | improve neurological function and enhance oligodendrogenesis, neurogenesis, and neurite remodeling/neuronal dendrite plasticity |
| miR-22-3p81 | upregulation | ADSCs | primary neurons | ultracentrifugation | improve neuronal survival by promoting the anti-apoptotic signaling cascade |
| miR-2538 | upregulation | ADSCs | primary neurons | ultracentrifugation | inhibit autophagic flux and protect primary neurons from OGD injury |
| miR-26a84 | upregulation | ADSCs | primary neurons | ultracentrifugation | arrest neuronal damage by disrupting the KLF9-mediated suppression on TRAF2/KLF2 axis |
| miR-26a90 | upregulation | USCs | NSCs | ultracentrifugation | promote both proliferation and neuronal differentiation of NSCs |
| miR-26b-5p85 | upregulation | ucMSCs | SH-SY5Y, PC12, primary microglia | ultracentrifugation | inhibit neuronal apoptosis induced by M1 microglia polarization following OGD |
| miR-27-3p75 | upregulation | patient serum | BV2 microglia | ultracentrifugation | aggravate cerebral injury, impede behavior recovery, and promote microglia activation and inflammatory cytokine expressions |
| miR-3186 | upregulation | ADSCs | primary neurons | kit | reduce infarct volume and neuronal cell apoptosis after stroke |
| miR-34c65 | upregulation | ASs | N2a | ultracentrifugation | promote proliferation and inhibit apoptosis of N2a cells stimulated with OGD |
| miR-92b-3p67 | upregulation | primary ASs | primary neurons | ultracentrifugation | attenuate OGD-induced neuron death and apoptosis |
| miR-9895 | upregulation | primary neurons | primary microglia | ultracentrifugation | inhibit platelet-activating factor receptor-mediated microglial phagocytosis to attenuate neuronal death |
| miR-12491 | upregulation | BMSCs | NPCs | ultracentrifugation | ameliorate the brain injury by promoting neurogenesis |
| miR-124-3p74 | downregulation | patient serum | BV2 | Kit | negatively correlate with serum proinflammatory cytokines and the NIHSS and promote the migration in LPS-induced BV2 microglia |
| miR-12463 | upregulation | BV2 | primary ASs | ultracentrifugation | attenuate glial scar formation and astrocyte activation, proliferation, and migration and promote astrocyte to neural progenitor transition |
| miR-12696 | upregulation | patient serum | SH-SY5Y | ultracentrifugation | regulate the cell cycle and promote ischemia/hypoxia tolerance in neurons |
| miR-12697 | upregulation | ECs | ECs, SMCs, ASs | ultracentrifugation | increase axon and myelin density as well as vascular density, arterial diameter, and vessel patency, promoting M2 macrophage polarization |
| miR-12677 | upregulation | ADSCs | neurons, ECs, BV2 | ultracentrifugation | inhibit microglial activation and the expression of inflammatory factors, improve functional recovery, and enhance neurogenesis |
| miR-13279 | upregulation | BMSCs | primary neurons | ultracentrifugation | mitigate neuronal injury by targeting and suppressing Acvr2b expression |
| miR-133b98 | upregulation | BMSCs | neurons, ASs | ultracentrifugation | increase axonal plasticity and neurite remodeling and regulate the CTGF expression in astrocytes |
| miR-13499 | downregulation | BMSCs | OLs | ultracentrifugation | suppress OL apoptosis |
| miR-135a-5p100 | upregulation | M2 microglia | HT-22 | ultracentrifugation | promote the proliferation and inhibit the apoptosis of neuronal cells and the expression of autophagy-related proteins |
| miR-137101 | upregulation | M2 microglia | primary neurons | ultracentrifugation | attenuate neuronal apoptosis, infarct volume, and behavioral deficits |
| miR-138-5p76 | upregulation | BMSCs | primary ASs | kit | promote cell proliferation, inhibit apoptosis of astrocytes injured by OGD, and reduce the expression of inflammatory factors |
| miR-146a-5p80 | upregulation | ucMSCs | BV2 microglia | ultracentrifugation | reduce infarct volume, attenuate behavioral deficits, and ameliorate microglial activation |
| miR-181b93 | upregulation | ADSCs | BMECs | kit | promote the angiogenesis of BMECs after OGD via miRNA-181b/TRPM7 axis |
| miR-181c-3p70 | downregulation | primary neurons | primary ASs | kit | decrease the expression of CXCL1 and inflammatory factors in astrocytes |
| miR-20682 | upregulation | ucMSCs | primary neurons | ultracentrifugation | improve the cell viability and suppress apoptosis of neurons |
| miR-21092 | upregulation | BMSCs | BMECs | ultracentrifugation | promote VEGF expression and angiogenesis |
| miR-221-3p83 | upregulation | BMSCs | primary neurons | ultracentrifugation | attenuate inflammation, pathological changes, and apoptosis in MCAO mice brain tissues and promote the viability and repress apoptosis of OGD-treated neurons |
| miR-223-3p87 | upregulation | MSCs | BV2 | ultracentrifugation | reduce cerebral infarct volume, improve neurological deficits, and promote learning and memorizing abilities |
| miR-36169 | upregulation | primary AS | PC12 | ultracentrifugation | relieve nerve damage caused by ischemia and suppress cell apoptosis |
| miR-542-3p78 | upregulation | MSCs | HA1800 ASs | ultracentrifugation | alleviate OGD-induced cell apoptosis, ROS, and inflammation response |
| miR-1290102 | upregulation | ucMSCs | primary neurons | ultracentrifugation | protect neurons by attenuating apoptosis |
BMSCs, bone marrow-derived mesenchymal stem cells; OGD, oxygen-glucose deprivation; MCAO, middle cerebral artery occlusion; ECs, endothelial cells; SMCs, smooth muscle cells; ADSCs, adipose-derived stem cells; CTGF, connective tissue growth factor; ucMSCs, umbilical cord mesenchymal stem cells; KLF9, Kruppel-like factor; TRAF2, tumor necrosis factor receptor (TNFR)-associated factor 2; OLs, oligodendrocytes; ASs, astrocytes; BMECs, brain microvascular endothelial cells; VEGF, vascular endothelial growth factor; USCs, human urine-derived stem cells; NSCs, neural stem cells; NPCs, neural progenitor cells.
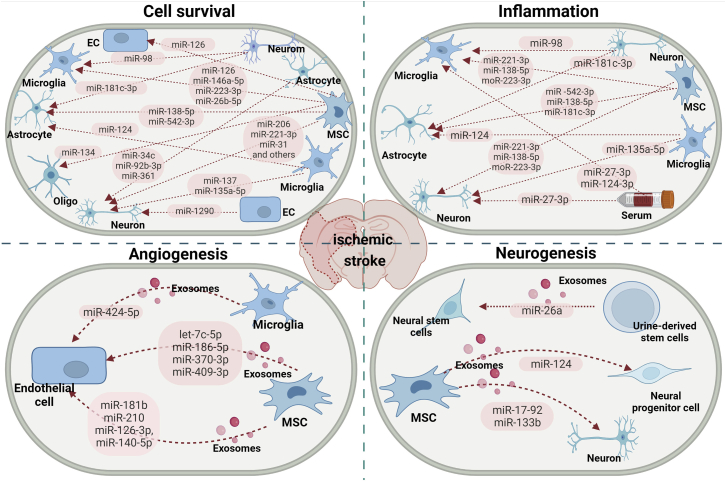
Figure 3.
The involvement of exosome-associated miRNAs in ischemic stroke
Different donor cells, namely neurons, microglia, astrocytes, endothelial cells, serum, and MSCs, can regulate recipient cells by transferring a set of exosome-associated miRNAs, modulating biological behaviors including neuronal survival, inflammation, angiogenesis, and neurogenesis, therefore regulating ischemic stroke progression and recovery.
Roles of exosomal miRNAs in myocardial infarction
Numerous researchers investigating various exosomal miRNAs revealed a novel insight into crosstalk in ischemic stroke, as mentioned above, and a vital role of miRNAs enveloped in exosomes in myocardial ischemia is also recognized.103, 104, 105 Given that, at the incipient stage (24 h), a set of miRNAs, namely miR-1, miR-21, miR-126, miR-146b, miR-208, and miR-9651, are increased after myocardial infarction, whereas others, such as miR-133, miR-195, and miR-320, are reduced.106,107 Exosomes, in the meantime, could be taken up by neighboring or distant myocardial cells, given their various targets, which they modulate by posttranscriptional regulation of gene expression; miRNAs loaded into exosomes are potent repair factors.108 There is increasing evidence that miRNAs are selectively enriched in exosomes, exerting biological functions via modulating specific aspects of myocardial ischemia and, as such, participating in cardiomyocyte survival, cardiac functional recovery, inflammatory responses, and angiogenesis,109, 110, 111, 112, 113, 114, 115, 116, 117, 118, 119, 120, 121, 122, 123, 124, 125, 126, 127, 128, 129, 130, 131, 132, 133, 134, 135, 136, 137, 138, 139, 140, 141, 142, 143 as indicated in Table 2 and Figure 4 and further discussed in the following sections.
Table 2.
Preclinical studies evaluating the effect of exosomal miRNAs in myocardial infarction
| miRNA | Expression | Donor cell | Recipient cell | Exosome isolation | Main function |
|---|---|---|---|---|---|
| miR-21112 | upregulation | patient serum | cardiomyocytes | kit | reduce the infarct size and cell apoptosis through PDCD4 downregulation |
| miR-21113 | upregulation | HEK293T | cardiomyocytes and HUVECs | ultracentrifugation | reduce PDCD4 expression and attenuate cell apoptosis |
| miR-21-5p111 | upregulation | CTs | HMVECs | ultracentrifugation | suppress apoptosis and promote the survival of HMVECs and angiogenesis |
| miR-24114 | upregulation | BMSCs | cardiomyocytes and H9c2 | ultracentrifugation | inhibit cardiomyocyte apoptosis and improve myocardial function |
| miR-25-3p115 | upregulation | BMSCs | cardiomyocytes | ultracentrifugation | reduce apoptosis and cytokine expression |
| miR-30e116 | upregulation | BMSCs | cardiomyocytes | ultracentrifugation | inhibit LOX1 expression, downregulate the activity of the NF-κB p65/caspase-9 signaling, and ameliorate heart failure |
| miR-31117 | upregulation | ADSCs | HMVECs | ultracentrifugation | promote HMVEC migration and tube formation by targeting FIH1 |
| miR-98-5p142 | upregulation | BMSCs | cardiomyocytes | ultracentrifugation | suppress myocardial enzyme levels, oxidative stress, inflammation response, macrophage infiltration, and infarct size |
| miR-125b118 | upregulation | BMSCs | cardiomyocytes | ultracentrifugation | ameliorate cardiomyocyte apoptosis and cardiac damage |
| miR-126119 | upregulation | ADSCs | cardiomyocytes and EPCs | ultracentrifugation | protect myocardial cells from apoptosis, inflammation, and fibrosis and boost angiogenesis |
| miR-126120 | downregulation | ECs | cardiomyocytes | ultracentrifugation | severe cardiac dysfunction and hypertrophy |
| miR-129121 | upregulation | HUVECs | cardiomyocytes | ultracentrifugation | downregulate TLR4 and disrupt the NF-κB signaling and NLRP3 inflammasome to protect against I/R injury |
| miR-129-5p122 | upregulation | BMSCs | cardiomyocytes | ultracentrifugation | decrease inflammatory cytokine expression, apoptosis, and fibrosis |
| miR-132123 | downregulation | CPCs | ECs | ultracentrifugation | enhance tube formation via downregulating RasGAP-p120 |
| miR-133a-3p124 | upregulation | ucMSCs | cardiomyocytes, H9c2, and HUVECs | ultracentrifugation | promote angiogenesis, inhibit apoptosis, reduce fibrosis, and preserve heart function |
| miR-143125 | upregulation | SMCs | ECs | ultracentrifugation | regulate angiogenesis by reducing the proliferation index of ECs and their capacity to form vessel-like structures |
| miR-143110 | downregulation | patient serum | HUVECs | ultracentrifugation | promote cell proliferation, migration, and tube formation |
| miR-145125 | upregulation | SMCs | ECs | ultracentrifugation | regulate angiogenesis by reducing the proliferation index of ECs and the capacity to form vessel-like structures |
| miR-146a126 | upregulation | ADSCs | cardiomyocytes and H9c2 | ultracentrifugation | suppress apoptosis and inflammatory response |
| miR-150-5p127 | upregulation | BMSCs | cardiomyocytes | ultracentrifugation | decrease Bax expression, alleviate pathological changes of the myocardium, decrease apoptosis rate, and improve cardiac function |
| miR-153-3p128 | downregulation | BMSCs | H9c2 and ECs | ultracentrifugation | reduce the apoptosis by promoting ANGPT1 expression and VEGF/VEGFR2/PI3K/Akt/eNOS pathway activation |
| miR-185129 | upregulation | BMSCs | cardiomyocytes | ultracentrifugation | repress ventricular remolding by inhibiting SOCS2 |
| miR-208b143 | upregulation | patient plasma | HUVECs | ultracentrifugation | suppress cell viability and migration and promote cell apoptosis by regulating Bcl2 and Bax and the FAK/MAPK1/Raf-1 pathway |
| miR-210123 | downregulation | CPCs | cardiomyocytes | ultracentrifugation | inhibit apoptosis by downregulating ephrin A3 and PTP1b |
| miR-210130 | upregulation | BMSCs | cardiomyocytes | ultracentrifugation | increase cardiomyocytes viability, improve heart function, and reduce cardiac fibrosis |
| miR-210131 | downregulation | BMSCs | HUVECs | ultracentrifugation | improve angiogenesis and cardiac function |
| miR-212-5p132 | upregulation | BMSCs | cardiomyocytes | ultracentrifugation | protect against cardiac fibrosis |
| miR-218-5p133 | upregulation | EPCs | cardiomyocytes | ultracentrifugation | promote CF proliferation and inhibit myocardial fibrosis |
| miR-223134 | upregulation | ucMSCs | HUVECs and H9c2 | ultracentrifugation | facilitate angiogenesis of HUVECs, repress inflammatory response and apoptosis, and promote angiogenesis in cardiomyocytes |
| miR-322135 | upregulation | CPCs | HUVECs | ultracentrifugation | promote angiogenesis via the upregulation of Nox2 |
| miR-328-3p136 | upregulation | cardiomyocytes | cardiomyocytes | ultracentrifugation | promote the activation of the caspase pathway and apoptosis |
| miR-338137 | upregulation | MSCs | cardiomyocytes and H9c2 | ultracentrifugation | inhibit H2O2-induced apoptosis and improve cardiac function by regulating MAP3K2/JNK signaling pathway |
| miR-363-3p133 | upregulation | EPCs | cardiomyocytes | ultracentrifugation | promote CF angiogenesis and inhibit myocardial fibrosis |
| miR-486-5p138 | upregulation | BMSCs | CFs and ECs | ultracentrifugation | promote angiogenesis by downregulating fibroblast MMP19 and increase the potency of myocardial repair |
| miR-494-3p140 | upregulation | BMDCs | CMECs | ultracentrifugation | enhance tube formation and promote angiogenesis |
| miR-671140 | upregulation | adMSCs | cardiomyocytes | ultracentrifugation | alleviate fibrosis and cell apoptosis |
| miR-4732-3p141 | upregulation | MSCs | cardiomyocytes and HUVECs | ultracentrifugation | induce angiogenesis and inhibit myofibroblast differentiation and the production of extracellular matrix |
BMSCs, bone marrow-derived mesenchymal stem cells; LOX1, lectin-like oxidized low-density lipoprotein receptor-1; SOCS2, suppressor of cytokine signaling 2; adMSCs, adipose-derived mesenchymal stem cells; HUVECs, human umbilical vein endothelial cells; TLR4, Toll-like receptor 4; NLRP3, NOD-like receptor 3; I/R, ischemia-reperfusion; ADSCs, adipose-derived stem cells; EPCs, endothelial progenitor cells; ucMSCs, umbilical cord mesenchymal stem cells; EGR1, early growth response factor 1; CFs, cardiac fibroblasts; ECs, endothelial cells; HIF1, hypoxia-inducible factor-1; HMVECs, human microvascular endothelial cells; CPCs, cardiac progenitor cells; NOX, NADPH oxidase; MMP19, matrix metalloproteinase 19; BMDCs, bone marrow-derived dendritic cells; CMECs, cardiac microvascular endothelial cells; SMCs, smooth muscle cells; CTs, cardiac telocytes.
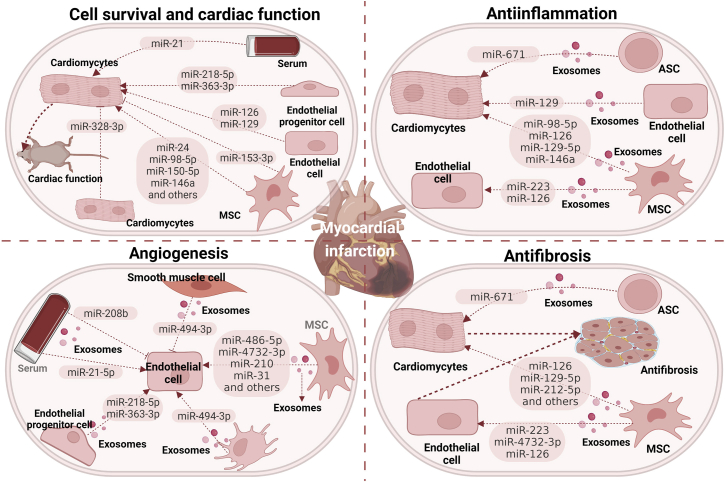
Figure 4.
The involvement of exosome-associated miRNAs in myocardial infarction
The recipient cells can internalize various exosome-associated miRNAs released from different donors, which modulate various biological responses, including cell survival, inflammation, angiogenesis, and fibrosis, thus regulating myocardial infarction progression and recovery. ADSC, adipose tissue-derived mesenchymal stromal cell.
Cell survival and cardiac functional recovery
Preclinical studies mimicking myocardial infarction found that exosomal miRNAs can improve hypoxic cardiomyocyte survival and cardiac function recovery. As such, a series of miRNAs were shuttled via exosomes, including miR-21, miR-98-5p, miR-25, miR-30e, miR-125b, miR-126, miR-4732-3p, miR-146a, miR-185, miR-150-5p, miR-210, miR-212-5p, miR-31, miR-486-5p, miR-338, and miR-671, which, collected from MSCs, cardiac progenitor cells, endothelial cells, endothelial progenitor cells, or patient serum, promoted cardiomyocyte survival by downregulating miRNA targets including PDCD4, FASL, TLR4, PTEN, LOX1, p53, BAK, or SOCS2.112,114,117,118,120,123,124,127,129, 130, 131, 132, 133,137,138,140, 141, 142 In addition to the effect on cardiomyocyte survival, several miRNAs, namely miR-24, miR-98-5p, miR-125b, miR-126, miR-133a-3p, miR-150-5p, miR-338, miR-4732-3p, miR-31, miR-210, and miR-486-5p, loading into exosomes from cardiomyocytes, MSCs, or endothelial cells were identified to promote cardiac function recovery by promoting cardiomyocyte survival, anti-inflammation, angiogenesis, or anti-fibrosis.114,117,118,120,124,127,131,138,141,142 Suggestively, exosomal miRNAs play momentous roles in coordinating responses to cardioprotective effects, i.e., by promoting cardiomyocyte survival and cardiac functional recovery. Notably, not all miRNAs encapsulated in exosome samples have a cardioprotective effect. Exosomal miR-153-3p and miR-328-3p released from ischemic cardiomyocytes and MSCs were identified to aggravate ischemic cardiomyocyte death through ANGPT1 and VEGF/VEGFR/PIK/Akt/eNOS deactivation and caspase-3 activation, respectively.128,136
Inflammation
Anti-inflammatory effects have been reported for exosomes to mediate miR-671, miR-223, miR-98-5p, miR-126, miR-129, miR-129-5p, and miR-146a transfer from MSCs and endothelial cells to endothelial cells or cardiomyocytes, mechanistically, through TGFBR2, SMAD2, NF-kB/P65, S100A9, TLR4, HMGU1, and ERG1 downregulation and PI3K/AKT activation.119,121,125,126,140,142 Herein, MSCs can inhibit inflammation of heart muscle cells and endothelial cells via suppressing inflammasome activation, a multiprotein complex capable of cleaving and producing pro-inflammatory factors.
Angiogenesis
It has been uncovered that increasing the survival of cardiac endothelial cells, ameliorating cardiac angiogenesis, and mediating the recanalization of cardiac collaterals are great therapeutic targets. Secreted miRNAs encapsulated in exosomes derived from cardiomyocytes, MSCs, cardiac progenitor cells, dendritic cells, or patient serum, including miR-486-5p,138 miR-494-3p,139 miR-4732-3p,141 miR-210,109 miR-322,135 miRNA-143,110 miRNA-21-5p,111 and miR-31,117 could protect against myocardial ischemia via promoting cardiac angiogenesis and vascular regeneration, leading to accelerated blood flow to ischemic myocardium. Some miRNAs, such as miR-210, play a role via other effectors directly and indirectly. Overexpression of miR-210 in endothelial cells facilitates capillary-like structure formation and VEGF-driven cell migration. Injection of miR-210 into the myocardium demonstrated the increase of angiogenesis in cardiomyocytes and vascular density in the area around the infarct with a 2-fold increase in VEGF expression levels.109 For another, miR-210 promotes angiogenesis after myocardial infarction via downregulating expression of EFNA3 protein and upregulating hepatocyte growth factor to improve new ventricular remodeling, showing that miR-210 is indirectly concerned by the process of angiogenesis.144,145 Importantly, not all miRNAs encapsulated in exosome samples support angiogenic effects. miR-143 and miR-145 derived from smooth muscle cells and miR-208b derived from patient plasma can be transferred into endothelial cells, further inducing endothelial cell death via mechanisms that cause HKII, integrin-β8, or Bcl2 downregulation and CDKN1A, FAK, RAF1, MAPK1, or Bax upregulation.125,143
Roles of exosomal miRNAs in renal ischemia-reperfusion injury
Ischemia-reperfusion injury is a primary cause of AKI that is linked to high morbidity, mortality, and healthcare costs and for which no effective prevention or management is available except for supportive care in the practice of dialysis. After an ischemic insult in the kidney and blood flow to the kidney, oxidative damage mediated by reactive oxygen species develops various harmful cellular responses, inducing inflammation, apoptosis, endothelial and tubular cell damage, fibrosis, and acute renal dysfunction. These pathological processes play an integral role in the initiation and extension of AKI; hence, inhibition is a potential therapeutic modality to relieve renal ischemia-reperfusion injury. Similar to myocardial infarction and ischemic stroke, due perhaps to the acute nature and severity of injury related to AKI, a series of miRNAs, including miR-16, miR320, miR-101-3p, miR-127-3p, miR-210-3p, miR-126-3p, miR-26b-5p, miR-29a-3p, miR-146a-5p, miR-27a-3p, miR-93-3p, and miR-10a-5p, are downregulated in the serum of patients with AKI. In contrast, others involving miR-494, miR-210, miR-21, miR-21-3p, and miR-192 are upregulated at defined time points.146, 147, 148, 149, 150 Administration of several cell-derived exosomes facilitates miRNA levels in ischemic kidney tissue or serum. Similarly, most studies on AKI injury have previously been performed using tissues or cells that were experimentally exposed to renal ischemia and reperfusion,151, 152, 153, 154, 155, 156, 157, 158, 159, 160, 161, 162, 163, 164, 165, 166, 167, 168, 169, 170, 171, 172, 173, 174, 175, 176 as described in Table 3 and Figure 5 and emphasized in the following sections.
Table 3.
Preclinical studies evaluating the effect of exosomal miRNAs in renal ischemia-reperfusion injury
| miRNA | Expression | Donor cell | Recipient cell | Exosome isolation | Main function |
|---|---|---|---|---|---|
| miR-20a-5p151 | upregulation | HK-2 | TECs | ultracentrifugation | promote TECs’ proliferation and improve mitochondrial functions |
| miR-21156 | upregulation | serum, C2C12 | TECs | ultracentrifugation | anti-inflammatory and anti-apoptotic effects and attenuate sepsis-induced renal injury |
| miR-21157 | upregulation | senescent cells | HPTCs | ultracentrifugation | promote HPTCs’ phenotype transition through enhancing HIF-1α signaling |
| miR-21158 | upregulation | BMDCs | TECs | ultracentrifugation | promote BMDCs’ maturation |
| miR-21159 | upregulation | TECs | kidney, heart, liver, and lungs | ultracentrifugation | decrease apoptosis and reduce proinflammatory cytokines production in kidney, heart, liver, and lungs |
| miR-23a160 | upregulation | TECs | macrophages | ultracentrifugation | activate macrophages to promote tubulointerstitial inflammation via suppression of the ubiquitin editor A20 |
| miR-30152 | upregulation | MSCs | TECs | ultracentrifugation | alleviate mitochondrial fragmentation and DRP1 activation and inhibit mitochondrial apoptotic pathways |
| miR-93-5p153 | upregulation | MSCs | kidney tissue | ultracentrifugation | inhibit apoptosis and inflammation, reduce tissue damage, and promote renal function |
| miR-124-3p154 | upregulation | TECs | HK2 cell | ultracentrifugation | HPC EVs are more effective to attenuate mice renal I/R injury than normoxic EVs |
| miR-125a155 | upregulation | rat kidney tissues | NA | kit | biomarker |
| miR-125b-5p161 | upregulation | ucMSCs | TECs and HK2 | ultracentrifugation | attenuate the cell-cycle arrest and apoptosis of TECs |
| miR-146a-5p163 | upregulation | USCs | HK2 cell | ultracentrifugation | reduce renal tubular injury and inhibit local inflammation and oxidative stress in cells |
| miR-150162 | upregulation | TECs | kidney interstitial fibroblast cells | ultracentrifugation | initiate the activation and proliferation of fibroblasts |
| miR-150-5p164 | upregulation | TECs | kidney fibroblasts | ultracentrifugation | activate fibroblasts and aggravate renal fibrosis |
| miR-199a-3p167 | upregulation | BMSCs | HK-2 cell | ultracentrifugation | inhibit apoptosis, downregulate Sema3A, activate AKT and ERK pathways, and alleviate kidney ischemia injury |
| miR-199a-5p166 | upregulation | BMSCs | TECs | ultracentrifugation | amplify the suppression of ER stress and further protect against I/R injury |
| miR-200a-3p167 | upregulation | MSCs | TECs | ultracentrifugation | suppress inflammatory response, inhibit cell apoptosis, and regulate mitochondrial structure and function |
| miR-216a-5p168 | upregulation | USCs | HK-2 cell | ultracentrifugation | induce apoptosis suppression and functional protection |
| miR-218-5p169 | upregulation | kidney perfusate | PBMCs | ultracentrifugation | modulate immune responses in transplant recipients |
| miR-351155 | upregulation | rat kidney tissues | NA | kit | biomarker |
| miR-374b-5p170 | upregulation | TECs | M1 macrophage | ultracentrifugation | activate a high-level inflammatory response and M1 macrophage reaction |
| miR-486-5p171 | upregulation | ECFCs | ECs, TECs | ultracentrifugation | prevent ischemic kidney injury by targeting phosphatase and tensin homolog and inhibiting ECs apoptosis |
| miR-486-5p172 | upregulation | ECFCs | HUVECs | ultracentrifugation | decrease PTEN, stimulate Akt phosphorylation, and induce potent functional and histologic protection |
| miR-486-5p174 | upregulation | ECFCs | HUVECs | ultracentrifugation | involve interaction of CXCR4 with endothelial cell SDF-1α |
| miR-486-5p173 | upregulation | ECFCs | ECs | ultracentrifugation | inhibit apoptosis of ECs |
| miR-500a-3p175 | downregulation | patient serum | TECs | ultracentrifugation | suppress MLKL expression and attenuate cisplatin-induced programmed cell death and NF-κB-driven renal inflammation in ECs |
| miR-687176 | upregulation | rat kidney tissues | liver tissue | kit | upregulate hepatic tissue inflammation and induce liver tissue injury and apoptosis |
ucMSCs, umbilical cord mesenchymal stem cells; BMSCs, bone marrow-derived mesenchymal stem cells; TECs, tubular epithelial cells; USCs, human urine-derived stem cells; ECFCs, endothelial colony-forming cells; ECs, endothelial cells; PTs, proximal tubules; MLKL, mixed lineage kinase domain-like protein; HPTCs, human proximal tubular cells; PBMCs, peripheral blood mononuclear cells; HPC, hypoxia preconditioning; EVs, extracellular vesicles; I/R, ischemia-reperfusion; ECFCs, endothelial colony-forming cells; ADSCs, adipose-derived stem cells; HUVECs, human umbilical endothelial cells; PTEN, phosphatase and tensin homolog; BMDCs, bone marrow-derived dendritic cells; ER, ER BIP, binding immunoglobulin protein; CXCR4, CXC chemokine receptor type 4; SDF-1α, stromal cell-derived factor-1α; DRP1, dynamin-related protein 1.
Figure 5.
The involvement of exosome-associated miRNAs in renal ischemia-reperfusion injury
Various recipient cells can uptake a series of exosome-associated miRNAs derived from different donor cells, which alter several biological processes, namely cell survival, apoptosis, inflammation, and fibrosis, regulating myocardial infarction progression and recovery. USC, urine-derived stem cells.
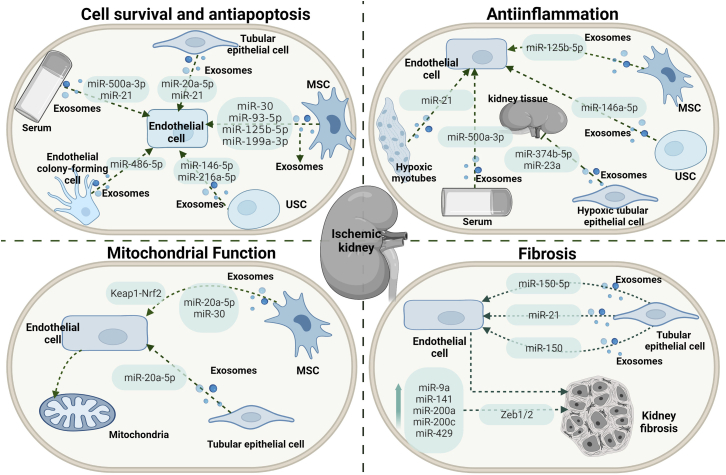
Cell survival and apoptosis
Among various cell death pathways, apoptosis, a category of programmed cell death, accounts for a large proportion of all death by renal ischemia-reperfusion injury, mediated by a crowd of pro- and anti-apoptotic factors promoted by extrinsic and intrinsic pathways via the activation of death receptors and mitochondria.177 A set of exosome-encapsulated miRNAs from various donor cells can be taken into renal tubular epithelial cells and result in efficient silencing or activating of mRNAs to regulate apoptosis, further promoting renal tubular epithelial cell survival. Nephroprotective effects were demonstrated for a great number of exosomal miRNAs, namely miR-125b-5p,161 miR-146a-5p,163 miR-21,158,161 miR-20a-5p,151 miR-486-5p,172,173 miR-500a-3P,175 miR-216a-5p,168 miR-199a-3p,165 miR-30,154 and miR-93-5p,153 which, collected from MSCs, urine-derived stem cells, hypoxic myotubes, HK2, endothelial colony-forming cells, or serum from patients and mice with AKI, protected tubular epithelial cells from apoptosis by downregulating a set of miRNA targets, namely p53, IRAK1, nuclear factor κB (NF-κB), PDCD4/NF-κB, PTEN/AKT, PTEN, Sema3A, or DRP1, or activating several miRNAs downstream, including AKT and ERK.151, 152, 153,156,159,161,163,165,168,172,173,175 In response to renal ischemia-reperfusion injury, mitochondrial fission occurs and can lead to apoptosis and necrosis, suggesting that it appears to be important for the progression of the apoptotic pathway. Interestingly, several exosomal miRNAs are involved in the regulation of mitochondrial dysfunction associated with apoptosis. Cao et al.167 indicated that MSC exosomes that accumulated in the renal tubules during renal ischemia-reperfusion injury enhanced mitochondrial functions by mechanisms that increased miR-200a-3p expression as well as activated the Keap1-Nrf2 pathway. Exosomal miR-30 and miR-20a-5p, respectively, from MSCs and hypoxic renal tubular epithelial cells protect against acute tubular mitochondrial injury associated with apoptosis.151,152
Inflammation
Tubulointerstitial inflammation is a critical pathological feature of AKI and triggers the evolution of interstitial fibrosis. Ischemia promotes tubulointerstitial inflammation due to ischemic tubular epithelial cells activating macrophages to promote tubulointerstitial inflammation in the kidney. Exosomal miRNAs regulate inflammatory responses through a set of miRNA targets. Hence, miR-93-5p, miR-146a-5p, and hsa-miR-500a-3P transferred via exosomes from MSCs, urine-derived stem cells, or serum from patients with AKI repress inflammation activation in the ischemic kidney by downregulating the miRNA target: the MLKL or IRAK1 that further repressed NF-κB.153,163,175 Of note, importantly, not all exosomal miRNAs have anti-inflammatory effects. Injection of the miR-374b-5p- and miR-23a-enriched exosomes from hypoxic tubular epithelial cells into mice renal parenchyma resulted in a high-level inflammatory response and M1 macrophage activation.160,170 Hypoxia-inducible factor 1α (HIF-1α), a vital transcription factor, has been well-documented as an oxygen-sensitive regulator to orchestrate the protective effect in response to ischemia. Interestingly, HIF-1α appears to mediate the secretion of exosomal miR-23a derived from hypoxic tubular epithelial cells via suppression of A20.160 Exosomal miR-21 derived from hypoxic myotubes can be integrated into renal tubular epithelial cells and target the downstream PDCD4/NF-κB and PTEN/AKT pathways, exerting anti-inflammation, whereas HIF-1α siRNA injection reversed the observation.156 Taken together, the HIF-1α-dependent release of miRNA-enriched exosomes is involved in inflammation modulation.
Fibrosis
Renal fibrosis, a standard pathological change in the progression of AKI to chronic disease, is characterized by myofibroblast activation and interstitial extracellular matrix accumulation.178 Tubular epithelial cells can employ exosomes to exert profibrotic effects on an injured kidney through miRNA shuttling. Hence, miR-150-5p-, miR-21-, and miR-150-containing exosomes from tubular epithelial cells initiate the activation and proliferation of fibroblasts directly or via the SOCS1 or PPARα/HIF-1α signaling pathway indirectly.157,162,164 Additionally, a bilateral renal ischemia-reperfusion model can induce an AKI in the early phase, accompanied by renal fibrosis. In the meantime, urinary exosomal miRNAs, namely miR-9a, miR-141, miR-200a, miR-200c, and miR-429, which share Zeb1/2 as a typical target mRNA, are upregulated together, indicating that they may be associated with developing renal fibrosis.155 In summary, tubular epithelial cell-released exosomal miRNAs can be a promising candidate for therapeutic studies in either animal models or further preclinical trials for alleviating progressive kidney fibrosis.
Roles of exosomal miRNAs in hepatic ischemia-reperfusion injury
Hepatic ischemia-reperfusion injury, a significant complication of hemorrhagic shock, resection, and transplantation, has complex pathophysiology, which involves the two interrelated local phases of ischemia-induced cell damage and reperfusion-induced inflammation. As our knowledge of hepatic ischemia-reperfusion injury gets deeper, current research on the pathogenesis and treatment has already focused on miRNAs. Notable miRNAs that are increased during the pathogenesis of hepatic ischemia-reperfusion injury involve miR-122, miR-450b, miR-155, miR-191, miR-370, miR-210, miR-34, miR-297, miR-497-5p, and miR-128-3p, which, in turn, exaggerate ischemia-reperfusion injury via suppression of their target genes,179, 180, 181, 182, 183, 184, 185, 186, 187, 188 whereas others, namely miR-146a, miR-194, miR-140-5p, miR-142-3p, and miR-9-5p, are reduced.189, 190, 191, 192, 193 Studies have demonstrated the renal protective effects of specific miRNAs, namely miR-20a, miR-1246, and miR-124-3p, which are highly expressed in MSC exosomes and exert a regulatory effect by delivering prewrapped miRNAs to recipient cells.194, 195, 196, 197 As discussed previously, MSC-derived exosomal miR-20a is involved in regulating apoptosis during renal ischemia-reperfusion injury. Similarly, miR-20a could alleviate ischemia-reperfusion injury-induced abnormal expression of genes related to apoptosis and autophagy, such as active caspase-3, mTOR, P62, and LC3II.194 Both hepatocytes and liver tissue exhibited significantly downregulated levels of miR-1246, and exosomes containing miR-1246 could modulate T helper 17/regulatory T balance, induce anti-apoptotic and pro-survival effects, and ameliorate ischemia-reperfusion injury-induced hepatic dysfunction in mice.195,196 In addition, exosomes that carry more abundant miR-124-3p can increase the content of miR-124-3p in grafts and have an increased potential to suppress ferroptosis of ischemia/reperfusion-treated hepatocytes by inhibiting prostate six transmembrane epithelial antigen 3. In contrast, exosomes from MSC knocked out for miR-124-3p indicated a reversed effect on ferroptosis.197 Hence, many miRNAs are found in MSCs’ corresponding exosomes (see also Table 4), but the precise signaling cascades regulated under the condition of hepatic ischemia-reperfusion injury are not yet fully known.
Table 4.
Preclinical studies evaluation of the actions of exosomal microRNA in hepatic ischemia-reperfusion injury.
| miRNA | Expression | Donor cell | Recipient cell | Exosome isolation | Main function |
|---|---|---|---|---|---|
| miR-20a194 | upregulation | ucMSCs | LO2, HepG2 | ultracentrifugation | alleviate the abnormal expression of genes related to apoptosis and autophagy (active caspase-3, mTOR, P62, LC3II) |
| miR-1246195 | upregulation | ucMSCs | LO2 | kit | protect hepatocytes against I/R injury via modulating the differentiation of Tregs and Th17 cells |
| miR-1246196 | upregulation | ucMSCs | LO2 | ultracentrifugation | induce anti-apoptotic and pro-survival effects in LO2 cells and ameliorate I/R-induced hepatic dysfunction |
| miR-124-3p197 | upregulation | BMSCs | hepatocytes | ultracentrifugation | reduce ferroptosis of ischemic cells by inhibiting STEAP3 |
ucMSCs, umbilical cord mesenchymal stem cells; I/R, ischemia-reperfusion; BMSCs, bone marrow-derived mesenchymal stem cells; STEAP3, prostate six transmembrane epithelial antigen 3; Tregs, regulatory T cells.
The exosomal miRNAs involved in at least two of four ischemia-reperfusion conditions
Although four disorders yield diverse pathophysiological processes with respective tissues and organs, ischemia-reperfusion is the common feature. Intriguingly, certain same exosomal miRNAs appear to participate in more than one of four ischemia-reperfusion conditions via modulating respective signaling pathways. These overlapped miRNAs exhibit similar protective consequences for disease outcomes. Paying abundant attention to uncovering potential overlapped miRNAs may provide novel insights into tissue remodeling processes and identify targets for ischemia-reperfusion condition therapies. From the above exosomal-miRNA intervention studies, a total of 63 miRNAs have, meanwhile, been identified, for which robust evidence suggests their involvement in more than one of the four ischemia-reperfusion pathophysiological statuses. Of these 63 miRNAs, ischemic stroke condition yields 28 miRNAs, myocardial infarction condition yields 30 miRNAs, and renal ischemia-reperfusion condition yields 19 miRNAs, whereas only three miRNAs are reported in hepatic ischemia-reperfusion condition. Suggestively, these miRNAs have a large degree of overlap under these conditions. A total of 17 miRNAs, namely, miR-20a (miR-20a-5p); miR-21; miR-25 (miR-25-3p); miR-30 (miR-30e); miR-31; miR-98 (miR-98-5p); miR-124 (miR-124-3p); miR-125a (miR-125b and miR-125b-5p); miR-126; miR-132; miR-133b (miR-133a-3p); miR-146a (same as miR-146a-5p); miR-150 (miR-150-5p); miR-210; miR-218-5p; miR-223 (miR-223-3p); and miR-486-5p, have, meanwhile, been uncovered to be involved in more than one of the four conditions. Hence, 9 overlapped miRNAs (known as miR-25, miR-31, miR-98, miR-126, miR-132, miR-133a-3p, miR-146a, miR-210, miR-223) and 8 overlapped miRNAs (miR-21, miR-30, miR-124, miR-125b, miR-146a-5p, miR-218-5p, miR-486-5p) have been identified between ischemic stroke and myocardial infarction and between renal ischemia-reperfusion and myocardial infarction, respectively. Likewise, two miRNAs, namely miR-146a-5p and miR-124 (miR-124-3p), are identified to be involved in three of the four ischemic conditions, whereas none of the miRNAs are shown to be involved in all four conditions.
Of note, regardless of the source of exosomes, miRNAs involved in more than one of four pathophysiological conditions have a large degree of overlaps concerning modes of action. For example, studies reporting an increase in cell survival in one ischemic condition usually had a corresponding effect in three other conditions, such as miR-125, miR-95, miR-126, and miR-146a. As such, miRNAs with roles in the angiogenesis promotion or inflammation inhibition in one condition also demonstrated similar effects in three other conditions, such as miR-126, miR-223, miR-133a-3p, and miR-210. Importantly, diverging effects have been revealed for one miRNA. Perhaps owing to the disparate nature of ischemia and donor cells, inverse effects were reported for myocardial infarction compared with ischemic renal injury in the case of miR-21. This is decreased in mouse hearts after myocardial infarction, while serum and HEK293T cell exosome increased it. miR-21 exosomes efficiently delivered miR-21 into cardiomyocytes, significantly repressed cardiomyocyte apoptosis in both in vivo and in vitro models of myocardial infarction, and reduced the infarct size in mouse hearts after myocardial infarction by reducing PDCD4 expression.112,113 Inversely, miR-21 secreted from senescent cells could facilitate epithelial-to-mesenchymal transition of human proximal tubular cells, further induce kidney fibrosis via the mechanisms that target PPARα protein, and consequently enhance HIF-1α expression.157
Exosome-associated miRNAs as novel potential biomarkers and therapeutic targets
Given the important clinical implications of exosomes for various ischemic conditions, the extracorporeal strategies for specifically targeting exosomes are a promising therapeutic option in managing ischemia. Exosomes, the natural miRNA carriers, have several attributes. Exosomes have high stability, protecting miRNAs from degradation by endogenous RNases. They, in the meantime, are luxuriant in multiple body fluids such as blood, urine, saliva, and cerebrospinal fluids, which are easy to isolate and endow with non-invasive advantages. A plethora of miRNAs are aberrantly expressed after ischemia-reperfusion injury, which is suggested as a biomarker of ischemia. Hence, altering miRNAs derived from exosomes may draw a clue to the progression of ischemia-reperfusion conditions, making them attractive therapeutic targets.
Exosome-associated miRNAs as potential biomarkers
Up- or downregulated levels of exosomal miRNAs detected in ischemia-reperfusion conditions contribute to the diagnosis. Upon stroke conditions, exosomal miR-134, miR-21-5p, miR-30a-5p, miR-223, miR-9, and miR-124, which were collected from the plasma or serum from patients who had a stroke, are upregulated,198, 199, 200, 201 whereas others, namely miR-422a and miR-125b-2-3p, are downregulated.202 Likewise, in myocardial infarction, a set of miRNAs shuttled via exosomes, namely miR-122-5p, miR-126, and miR-21, from serum are increased,203,204 whereas miR-143, miR-204, miR-1915-3p, miR-4507, and miR-3656 are decreased.110,205,206 Of note, miR-21 (miR-21-5p),199,204 an overlapped miRNA, is not only a promising biomarker for diagnosing myocardial infarction and ischemic stroke but distinguishing among hyperacute, subacute, and recovery phase ischemic stroke. The area under the curve is 0.714 for the subacute phase and 0.734 for the recovery phase.199 By comparing exosomal expression patterns at baseline, pretreatment, and posttreatment, correlating with clinical parameters, and combining with continuous follow up, it is possible to predict patient prognosis. miR-223, one of the most highly expressed miRNAs in exosomes of healthy humans, is elevated after the onset of acute ischemic stroke in circulating exosomes, correlates to NIHSS score, and inclines to a poor outcome.200 As such, exosomal miR-134, miR-9, and miR-124 derived from serum from patients who had ischemic stroke correlate positively with NIHSS scores, infarct volumes, and serum concentrations of IL-6.198,201 Suggestively, high expression of exosomal miR-9, miR-124, miR-134, and miR-223 correlate to a worse prognosis.
Exosomes as delivery vehicles for miRNA-based therapy
The use of carrier systems for the delivery of therapeutic payloads to targeted cells or tissues has attracted considerable attention, and, owing to their potential to shuttle cargos, exosomes have gained privilege in nanotherapeutics. Many ischemia-reperfusion injury-suppressive miRNAs are downregulated in cells and corresponding exosomes; thus, one strategy to inhibit the progression of the hypoxic disorder is encapsulating exogenous ischemia-reperfusion injury-suppressive miRNAs into exosomes and transmitting them to recipient cells and tissues. An exosome-based miRNA delivery system with an extensively applicable ability in ischemia-reperfusion injury therapy had been demonstrated; using a lentiviral vector (LV)-modulated approach, MSCs were infected with LV-miR-30e-5p. Afterward, miR-30e-enriched exosomes markedly inhibited LOX1 expression, thereby downregulating the NF-κB p65/caspase-9 signaling activity and ameliorating heart failure after myocardial infarction in rats.116 Likewise, intravenous administration of exosomes overexpressing miR-126 poststroke promoted functional recovery, enhanced neurogenesis, and inhibited inflammation.77 Besides that, inhibition of ischemia-reperfusion injury-upregulated exosomal miRNAs is another valuable method, given that they are commonly increased upon such conditions. As described above, miR-21-containing exosome could facilitate kidney fibrosis via the PPARα-HIF-1α signaling pathway. Nevertheless, inhibition of miR-21, using caloric restriction or caloric restriction mimetics of donor cells, prevented the occurrence of mesenchymal transition in recipient cells. Currently, the delivery of miRNA inhibitors or small interfering RNAs (siRNAs) through exosomes gains much attention, for example as illustrated by Kuang et al. that native MSC exosomes, but not exosomes obtained from MSCs pretreated with anti-miR-25-3p, cause oligonucleotide autophagic flux and cell death by modulating p53-BNIP3 in C57BL/6 mice exposed to cerebral ischemia.38
Conclusion and perspectives of the significance of exosomal miRNAs
Exosomes are paramount in the progression and development of ischemia-reperfusion injury, mainly depending on their cargos. Upon such conditions, miRNAs enveloped in exosomes are revealed to exert biological functions via modulating specific aspects, such as participation in cell survival, inflammation, neurogenesis, angiogenesis, apoptosis, and fibrosis, which decisively regulate tissue progression. Importantly, exosomal miRNAs that appeared in at least two of four conditions exhibit a substantial degree of overlap and have an excellent consistency for modes of action. Circulating, cell-free exosomal miRNAs have great potential as diagnostic or therapeutic biotools, generally serving as an exciting development in ischemia-reperfusion injury management. Nevertheless, major challenges remain to be solved prior to the implementation of exosomal miRNAs as clinical assays in such disorders. A technique to generate pure and homogeneous exosomes and a deeper mechanism underlying the release and cargo machinery in all four conditions must be explored further. Different isolation protocols may lead to isolating subpopulations of exosomes with different miRNAs, proteins, functions, and non-vesicular macromolecules such as lipoproteins.207, 208, 209, 210 Notably, the nuclear RNA exosome complex is eukaryotes’ most versatile RNA-degradation machine.211, 212, 213 Therefore, it is well worth demonstrating the nuclear RNA exosome complex regulation by modulating the levels of exosomal miRNAs. Currently, there are 31 clinical trials associated with miRNAs enveloped in exosomes with diverse states registered at https://clinicaltrials.gov/. Of these 31 trials, 7 are completed, 13 are active and recruiting, 2 are active but not recruiting, 4 are not yet recruiting, 1 is enrolling by invitation, and 4 are unknown. Although preclinical and clinical studies are just on the threshold and more in-depth investigations to uncover the effects and mechanisms of exosomal miRNAs are imperative, the dawn of an exosomal miRNA era is expected with the indefatigable endeavor of researchers.
Acknowledgments
This work was supported by the Tianjin Science and Technology Support Key Project (grant number 20YFZCSY00010) and the Tianjin Key Medical Discipline (specialty) Construction Project (grant number TJYXZDXK-076C).
Author contributions
W.X., Z.W., X.Y., and P.L. designed the manuscript. W.X., Y.Q., Z.W., and X.Y. wrote and drafted the manuscript. Z.W., J.Z., and P.L. revised the manuscript. W.X. and Z.W. prepared the tables and figures. All authors contributed to the article and approved the submitted version.
Declaration of interests
The authors have declared that no competing interests exist.
Contributor Information
Wenqiang Xin, Email: wenqiang.xin@stud.uni-goettingen.de.
Xinyu Yang, Email: yangxinyu@tmu.edu.cn.
Zengguang Wang, Email: wzgforrest@163.com.
References
- 1.Kula-Alwar D., Prag H., Krieg T. Targeting succinate metabolism in ischemia/reperfusion injury. Circulation. 2019;140:1968–1970. doi: 10.1161/circulationaha.119.042791. [DOI] [PubMed] [Google Scholar]
- 2.Kalogeris T., Baines C., Krenz M., Korthuis R. Ischemia/reperfusion. Compr. Physiol. 2016;7:113–170. doi: 10.1002/cphy.c160006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chouchani E., Pell V., James A., Work L., Saeb-Parsy K., Frezza C., Krieg T., Murphy M. A unifying mechanism for mitochondrial superoxide production during ischemia-reperfusion injury. Cell Metab. 2016;23:254–263. doi: 10.1016/j.cmet.2015.12.009. [DOI] [PubMed] [Google Scholar]
- 4.Cai Q., He B., Wang S., Fletcher S., Niu D., Mitter N., Birch P., Jin H. Message in a bubble: shuttling small RNAs and proteins between cells and interacting organisms using extracellular vesicles. Annu. Rev. Plant Biol. 2021;72:497–524. doi: 10.1146/annurev-arplant-081720-010616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ha M., Kim V. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014;15:509–524. doi: 10.1038/nrm3838. [DOI] [PubMed] [Google Scholar]
- 6.Yates L., Norbury C., Gilbert R. The long and short of microRNA. Cell. 2013;153:516–519. doi: 10.1016/j.cell.2013.04.003. [DOI] [PubMed] [Google Scholar]
- 7.Li C., Xu X. Biological functions and clinical applications of exosomal non-coding RNAs in hepatocellular carcinoma. Cell. Mol. Life Sci. 2019;76:4203–4219. doi: 10.1007/s00018-019-03215-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhang C., Yang X., Qi Q., Gao Y., Wei Q., Han S. lncRNA-HEIH in serum and exosomes as a potential biomarker in the HCV-related hepatocellular carcinoma. Cancer Biomark. 2018;21:651–659. doi: 10.3233/cbm-170727. [DOI] [PubMed] [Google Scholar]
- 9.Kalani M., Alsop E., Meechoovet B., Beecroft T., Agrawal K., Whitsett T., Huentelman M., Spetzler R., Nakaji P., Kim S., et al. Extracellular microRNAs in blood differentiate between ischaemic and haemorrhagic stroke subtypes. J. Extracell. Vesicles. 2020;9:1713540. doi: 10.1080/20013078.2020.1713540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ghoreishy A., Khosravi A., Ghaemmaghami A. Exosomal microRNA and stroke: a review. J. Cell. Biochem. 2019;120:16352–16361. doi: 10.1002/jcb.29130. [DOI] [PubMed] [Google Scholar]
- 11.Xiong Y., Wakhloo A.K., Fisher M. Advances in acute ischemic stroke therapy. Circ. Res. 2022;130:1230–1251. doi: 10.1161/circresaha.121.319948. [DOI] [PubMed] [Google Scholar]
- 12.Heusch G., Gersh B.J. The pathophysiology of acute myocardial infarction and strategies of protection beyond reperfusion: a continual challenge. Eur. Heart J. 2017;38:774–784. doi: 10.1093/eurheartj/ehw224. [DOI] [PubMed] [Google Scholar]
- 13.Lerink L.J.S., de Kok M.J.C., Mulvey J.F., Le Dévédec S.E., Markovski A.A., Wüst R.C.I., Alwayn I.P.J., Ploeg R.J., Schaapherder A.F.M., Bakker J.A., et al. Preclinical models versus clinical renal ischemia reperfusion injury: a systematic review based on metabolic signatures. Am. J. Transplant. 2022;22:344–370. doi: 10.1111/ajt.16868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guan Y., Yao W., Yi K., Zheng C., Lv S., Tao Y., Hei Z., Li M. Nanotheranostics for the management of hepatic ischemia-reperfusion injury. Small. 2021;17:e2007727. doi: 10.1002/smll.202007727. [DOI] [PubMed] [Google Scholar]
- 15.Yoshimura S., Toyoda K., Ohara T., Nagasawa H., Ohtani N., Kuwashiro T., Naritomi H., Minematsu K. Takotsubo cardiomyopathy in acute ischemic stroke. Ann. Neurol. 2008;64:547–554. doi: 10.1002/ana.21459. [DOI] [PubMed] [Google Scholar]
- 16.Milionis H., Faouzi M., Cordier M., D'Ambrogio-Remillard S., Eskandari A., Michel P. Characteristics and early and long-term outcome in patients with acute ischemic stroke and low ejection fraction. Int. J. Cardiol. 2013;168:1082–1087. doi: 10.1016/j.ijcard.2012.11.036. [DOI] [PubMed] [Google Scholar]
- 17.Adeoye A., Ogah O., Ovbiagele B., Akinyemi R., Shidali V., Agyekum F., Aje A., Adebayo O., Akinyemi J., Kolo P., et al. Prevalence and prognostic features of ECG abnormalities in acute stroke: findings from the SIREN study among Africans. Glob. Heart. 2017;12:99–105. doi: 10.1016/j.gheart.2017.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barugh A., Gray P., Shenkin S., MacLullich A., Mead G. Cortisol levels and the severity and outcomes of acute stroke: a systematic review. J. Neurol. 2014;261:533–545. doi: 10.1007/s00415-013-7231-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Iadecola C., Anrather J. The immunology of stroke: from mechanisms to translation. Nat. Med. 2011;17:796–808. doi: 10.1038/nm.2399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kelly J., Kennedy P., Cryan J., Dinan T., Clarke G., Hyland N. Breaking down the barriers: the gut microbiome, intestinal permeability and stress-related psychiatric disorders. Front. Cell. Neurosci. 2015;9:392. doi: 10.3389/fncel.2015.00392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Boehme A., Esenwa C., Elkind M. Stroke risk factors, genetics, and prevention. Circ. Res. 2017;120:472–495. doi: 10.1161/circresaha.116.308398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zhao Q., Yan T., Chopp M., Venkat P., Chen J. Brain-kidney interaction: renal dysfunction following ischemic stroke. J. Cereb. Blood Flow Metab. 2020;40:246–262. doi: 10.1177/0271678x19890931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Z., Venkat P., Seyfried D., Chopp M., Yan T., Chen J. Brain-heart interaction: cardiac complications after stroke. Circ. Res. 2017;121:451–468. doi: 10.1161/circresaha.117.311170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Zorrilla-Vaca A., Ziai W., Connolly E., Geocadin R., Thompson R., Rivera-Lara L. Acute kidney injury following acute ischemic stroke and intracerebral hemorrhage: a meta-analysis of prevalence rate and mortality risk. Cerebrovasc. Dis. 2018;45:1–9. doi: 10.1159/000479338. [DOI] [PubMed] [Google Scholar]
- 25.Tsagalis G., Akrivos T., Alevizaki M., Manios E., Stamatellopoulos K., Laggouranis A., Vemmos K. Renal dysfunction in acute stroke: an independent predictor of long-term all combined vascular events and overall mortality. Nephrol. Dial. Transplant. 2009;24:194–200. doi: 10.1093/ndt/gfn471. [DOI] [PubMed] [Google Scholar]
- 26.Chelluboina B., Vemuganti R. Chronic kidney disease in the pathogenesis of acute ischemic stroke. J. Cereb. Blood Flow Metab. 2019;39:1893–1905. doi: 10.1177/0271678x19866733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kim S., Song D., Heo J., Yoo J., Kim B., Park J., Kim D., Ahn S., Kim K., Han K., et al. Liver fibrosis assessed with transient elastography is an independent risk factor for ischemic stroke. Atherosclerosis. 2017;260:156–162. doi: 10.1016/j.atherosclerosis.2017.02.005. [DOI] [PubMed] [Google Scholar]
- 28.Tan C., Wang Z., Zheng M., Zhao S., Shichinohe H., Houkin K. Responses of immune organs after cerebral ischemic stroke. J. Nippon Med. Sch. 2021;88:228–237. doi: 10.1272/jnms.JNMS.2021_88-308. [DOI] [PubMed] [Google Scholar]
- 29.D'Mello C., Swain M. Liver-brain inflammation axis. Am. J. Physiol. Lung Cell Mol. Physiol. 2011;301:G749–G761. doi: 10.1152/ajpgi.00184.2011. [DOI] [PubMed] [Google Scholar]
- 30.Kang M., Jordan V., Blenkiron C., Chamley L. Biodistribution of extracellular vesicles following administration into animals: a systematic review. J. Extracell. Vesicles. 2021;10:e12085. doi: 10.1002/jev2.12085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crescitelli R., Lässer C., Szabó T., Kittel A., Eldh M., Dianzani I., Buzás E., Lötvall J. Distinct RNA profiles in subpopulations of extracellular vesicles: apoptotic bodies, microvesicles and exosomes. J. Extracell. Vesicles. 2013;2 doi: 10.3402/jev.v2i0.20677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Théry C., Zitvogel L., Amigorena S. Exosomes: composition, biogenesis and function. Nat. Rev. Immunol. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 33.Yang E., Wang X., Gong Z., Yu M., Wu H., Zhang D. Exosome-mediated metabolic reprogramming: the emerging role in tumor microenvironment remodeling and its influence on cancer progression. Signal. Transduct. Target Ther. 2020;5:242. doi: 10.1038/s41392-020-00359-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pathania A., Prathipati P., Challagundla K. New insights into exosome mediated tumor-immune escape: clinical perspectives and therapeutic strategies. Biochim. Biophys. Acta Rev. Cancer. 2021;1876:188624. doi: 10.1016/j.bbcan.2021.188624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Barois N., de Saint-Vis B., Lebecque S., Geuze H., Kleijmeer M. MHC class II compartments in human dendritic cells undergo profound structural changes upon activation. Traffic. 2002;3:894–905. doi: 10.1034/j.1600-0854.2002.31205.x. [DOI] [PubMed] [Google Scholar]
- 36.Kowal J., Tkach M., Théry C. Biogenesis and secretion of exosomes. Curr. Opin. Cell Biol. 2014;29:116–125. doi: 10.1016/j.ceb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 37.Jeppesen D., Fenix A., Franklin J., Higginbotham J., Zhang Q., Zimmerman L., Liebler D., Ping J., Liu Q., Evans R., et al. Reassessment of exosome composition. Cell. 2019;177:428–445.e18. doi: 10.1016/j.cell.2019.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Kuang Y., Zheng X., Zhang L., Ai X., Venkataramani V., Kilic E., Hermann D.M., Majid A., Bahr M., Doeppner T.R. Adipose-derived mesenchymal stem cells reduce autophagy in stroke mice by extracellular vesicle transfer of miR-25. J. Extracell. Vesicles. 2020;10:e12024. doi: 10.1002/jev2.12024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mohapatra S., Pioppini C., Ozpolat B., Calin G. Non-coding RNAs regulation of macrophage polarization in cancer. Mol. Cancer. 2021;20:24. doi: 10.1186/s12943-021-01313-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Guo H., Ingolia N., Weissman J., Bartel D. Mammalian microRNAs predominantly act to decrease target mRNA levels. Nature. 2010;466:835–840. doi: 10.1038/nature09267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Lee Y., Kim M., Han J., Yeom K., Lee S., Baek S., Kim V. MicroRNA genes are transcribed by RNA polymerase II. EMBO J. 2004;23:4051–4060. doi: 10.1038/sj.emboj.7600385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cai X., Hagedorn C., Cullen B. Human microRNAs are processed from capped, polyadenylated transcripts that can also function as mRNAs. RNA. 2004;10:1957–1966. doi: 10.1261/rna.7135204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bartel D. MicroRNAs: target recognition and regulatory functions. Cell. 2009;136:215–233. doi: 10.1016/j.cell.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.O'Brien K., Breyne K., Ughetto S., Laurent L., Breakefield X. RNA delivery by extracellular vesicles in mammalian cells and its applications. Nat. Rev. Mol. Cell Biol. 2020;21:585–606. doi: 10.1038/s41580-020-0251-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kosaka N., Iguchi H., Hagiwara K., Yoshioka Y., Takeshita F., Ochiya T. Neutral sphingomyelinase 2 (nSMase2)-dependent exosomal transfer of angiogenic microRNAs regulate cancer cell metastasis. J. Biol. Chem. 2013;288:10849–10859. doi: 10.1074/jbc.M112.446831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Koppers-Lalic D., Hackenberg M., Bijnsdorp I., van Eijndhoven M., Sadek P., Sie D., Zini N., Middeldorp J., Ylstra B., de Menezes R., et al. Nontemplated nucleotide additions distinguish the small RNA composition in cells from exosomes. Cell Rep. 2014;8:1649–1658. doi: 10.1016/j.celrep.2014.08.027. [DOI] [PubMed] [Google Scholar]
- 47.Villarroya-Beltri C., Gutiérrez-Vázquez C., Sánchez-Cabo F., Pérez-Hernández D., Vázquez J., Martin-Cofreces N., Martinez-Herrera D., Pascual-Montano A., Mittelbrunn M., Sánchez-Madrid F. Sumoylated hnRNPA2B1 controls the sorting of miRNAs into exosomes through binding to specific motifs. Nat. Commun. 2013;4:2980. doi: 10.1038/ncomms3980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Santangelo L., Giurato G., Cicchini C., Montaldo C., Mancone C., Tarallo R., Battistelli C., Alonzi T., Weisz A., Tripodi M. The RNA-binding protein SYNCRIP is a component of the hepatocyte exosomal machinery controlling MicroRNA sorting. Cell Rep. 2016;17:799–808. doi: 10.1016/j.celrep.2016.09.031. [DOI] [PubMed] [Google Scholar]
- 49.Shurtleff M., Temoche-Diaz M., Karfilis K., Ri S., Schekman R. Y-box protein 1 is required to sort microRNAs into exosomes in cells and in a cell-free reaction. Elife. 2016;5 doi: 10.7554/eLife.19276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tian T., Zhu Y., Zhou Y., Liang G., Wang Y., Hu F., Xiao Z. Exosome uptake through clathrin-mediated endocytosis and macropinocytosis and mediating miR-21 delivery. J. Biol. Chem. 2014;289:22258–22267. doi: 10.1074/jbc.M114.588046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Fan Q., Yang L., Zhang X., Peng X., Wei S., Su D., Zhai Z., Hua X., Li H. The emerging role of exosome-derived non-coding RNAs in cancer biology. Cancer Lett. 2018;414:107–115. doi: 10.1016/j.canlet.2017.10.040. [DOI] [PubMed] [Google Scholar]
- 52.Christianson H., Svensson K., van Kuppevelt T., Li J., Belting M. Cancer cell exosomes depend on cell-surface heparan sulfate proteoglycans for their internalization and functional activity. Proc. Natl. Acad. Sci. USA. 2013;110:17380–17385. doi: 10.1073/pnas.1304266110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Miyanishi M., Tada K., Koike M., Uchiyama Y., Kitamura T., Nagata S. Identification of Tim4 as a phosphatidylserine receptor. Nature. 2007;450:435–439. doi: 10.1038/nature06307. [DOI] [PubMed] [Google Scholar]
- 54.Mukandala G., Tynan R., Lanigan S., O'Connor J. The effects of hypoxia and inflammation on synaptic signaling in the CNS. Brain Sci. 2016;6 doi: 10.3390/brainsci6010006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Xu S., Lu J., Shao A., Zhang J., Zhang J. Glial cells: role of the immune response in ischemic stroke. Front. Immunol. 2020;11:294. doi: 10.3389/fimmu.2020.00294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Brown G. Neuronal loss after stroke due to microglial phagocytosis of stressed neurons. Int. J. Mol. Sci. 2021;22 doi: 10.3390/ijms222413442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Hu X., Leak R., Shi Y., Suenaga J., Gao Y., Zheng P., Chen J. Microglial and macrophage polarization—new prospects for brain repair. Nat. Rev. Neurol. 2015;11:56–64. doi: 10.1038/nrneurol.2014.207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ma Y., Wang J., Wang Y., Yang G. The biphasic function of microglia in ischemic stroke. Prog. Neurobiol. 2017;157:247–272. doi: 10.1016/j.pneurobio.2016.01.005. [DOI] [PubMed] [Google Scholar]
- 59.Cheng Q., Shen Y., Cheng Z., Shao Q., Wang C., Sun H., Zhang Q. Achyranthes bidentata polypeptide k suppresses neuroinflammation in BV2 microglia through Nrf2-dependent mechanism. Ann. Transl. Med. 2019;7:575. doi: 10.21037/atm.2019.09.07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tian Y., Zhu P., Liu S., Jin Z., Li D., Zhao H., Zhu X., Shu C., Yan D., Dong Z. IL-4-polarized BV2 microglia cells promote angiogenesis by secreting exosomes. Adv. Clin. Exp. Med. 2019;28:421–430. doi: 10.17219/acem/91826. [DOI] [PubMed] [Google Scholar]
- 61.Xie L., Zhao H., Wang Y., Chen Z. Exosomal shuttled miR-424-5p from ischemic preconditioned microglia mediates cerebral endothelial cell injury through negatively regulation of FGF2/STAT3 pathway. Exp. Neurol. 2020;333:113411. doi: 10.1016/j.expneurol.2020.113411. [DOI] [PubMed] [Google Scholar]
- 62.Song Y., Li Z., He T., Qu M., Jiang L., Li W., Shi X., Pan J., Zhang L., Wang Y., et al. M2 microglia-derived exosomes protect the mouse brain from ischemia-reperfusion injury via exosomal miR-124. Theranostics. 2019;9:2910–2923. doi: 10.7150/thno.30879. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Li Z., Song Y., He T., Wen R., Li Y., Chen T., Huang S., Wang Y., Tang Y., Shen F., et al. viaM2 microglial small extracellular vesicles reduce glial scar formation the miR-124/STAT3 pathway after ischemic stroke in mice. Theranostics. 2021;11:1232–1248. doi: 10.7150/thno.48761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Du L., Jiang Y., Sun Y. Astrocyte-derived exosomes carry microRNA-17-5p to protect neonatal rats from hypoxic-ischemic brain damage via inhibiting BNIP-2 expression. Neurotoxicology. 2021;83:28–39. doi: 10.1016/j.neuro.2020.12.006. [DOI] [PubMed] [Google Scholar]
- 65.Wu W., Liu J., Yang C., Xu Z., Huang J., Lin J. Astrocyte-derived exosome-transported microRNA-34c is neuroprotective against cerebral ischemia/reperfusion injury via TLR7 and the NF-κB/MAPK pathways. Brain Res. Bull. 2020;163:84–94. doi: 10.1016/j.brainresbull.2020.07.013. [DOI] [PubMed] [Google Scholar]
- 66.Pei X., Li Y., Zhu L., Zhou Z. Astrocyte-derived exosomes transfer miR-190b to inhibit oxygen and glucose deprivation-induced autophagy and neuronal apoptosis. Cell Cycle. 2020;19:906–917. doi: 10.1080/15384101.2020.1731649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Xu L., Cao H., Xie Y., Zhang Y., Du M., Xu X., Ye R., Liu X. Exosome-shuttled miR-92b-3p from ischemic preconditioned astrocytes protects neurons against oxygen and glucose deprivation. Brain Res. 2019;1717:66–73. doi: 10.1016/j.brainres.2019.04.009. [DOI] [PubMed] [Google Scholar]
- 68.Liu X., Lv X., Liu Z., Zhang M., Leng Y. MircoRNA-29a in astrocyte-derived extracellular vesicles suppresses brain ischemia reperfusion injury via TP53INP1 and the NF-κB/NLRP3 Axis. Cell. Mol. Neurobiol. 2021 doi: 10.1007/s10571-021-01040-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Bu X., Li D., Wang F., Sun Q., Zhang Z. microRNA-361Protective role of astrocyte-derived exosomal in cerebral ischemic-reperfusion injury by regulating the signaling pathway and targeting. Neuropsychiatr. Dis. Treat. 2020;16:1863–1877. doi: 10.2147/ndt.S260748. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 70.Song H., Zhang X., Chen R., Miao J., Wang L., Cui L., Ji H., Liu Y. Cortical neuron-derived exosomal MicroRNA-181c-3p inhibits neuroinflammation by downregulating CXCL1 in astrocytes of a rat model with ischemic brain injury. Neuroimmunomodulation. 2019;26:217–233. doi: 10.1159/000502694. [DOI] [PubMed] [Google Scholar]
- 71.Xu B., Zhang Y., Du X., Li J., Zi H., Bu J., Yan Y., Han H., Du J. Neurons secrete miR-132-containing exosomes to regulate brain vascular integrity. Cell Res. 2017;27:882–897. doi: 10.1038/cr.2017.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gao B., Zhou S., Sun C., Cheng D., Zhang Y., Li X., Zhang L., Zhao J., Xu D., Bai Y. Brain endothelial cell-derived exosomes induce neuroplasticity in rats with ischemia/reperfusion injury. ACS Chem. Neurosci. 2020;11:2201–2213. doi: 10.1021/acschemneuro.0c00089. [DOI] [PubMed] [Google Scholar]
- 73.Zhang Y., Qin Y., Chopp M., Li C., Kemper A., Liu X., Wang X., Zhang L., Zhang Z. Ischemic cerebral endothelial cell-derived exosomes promote axonal growth. Stroke. 2020;51:3701–3712. doi: 10.1161/strokeaha.120.031728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Qi Z., Zhao Y., Su Y., Cao B., Yang J., Xing Q. Serum extracellular vesicle-derived miR-124-3p as a diagnostic and predictive marker for early-stage acute ischemic stroke. Front. Mol. Biosci. 2021;8:685088. doi: 10.3389/fmolb.2021.685088. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ye Z., Hu J., Xu H., Sun B., Jin Y., Zhang Y., Zhang J. Serum exosomal microRNA-27-3p aggravates cerebral injury and inflammation in patients with acute cerebral infarction by targeting PPARγ. Inflammation. 2021;44:1035–1048. doi: 10.1007/s10753-020-01399-3. [DOI] [PubMed] [Google Scholar]
- 76.Deng Y., Chen D., Gao F., Lv H., Zhang G., Sun X., Liu L., Mo D., Ma N., Song L., et al. Exosomes derived from microRNA-138-5p-overexpressing bone marrow-derived mesenchymal stem cells confer neuroprotection to astrocytes following ischemic stroke via inhibition of LCN2. J. Biol. Eng. 2019;13:71. doi: 10.1186/s13036-019-0193-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Geng W., Tang H., Luo S., Lv Y., Liang D., Kang X., Hong W. Exosomes from miRNA-126-modified ADSCs promotes functional recovery after stroke in rats by improving neurogenesis and suppressing microglia activation. Am. J. Transl. Res. 2019;11:780–792. [PMC free article] [PubMed] [Google Scholar]
- 78.Cai G., Cai G., Zhou H., Zhuang Z., Liu K., Pei S., Wang Y., Wang H., Wang X., Xu S., et al. Mesenchymal stem cell-derived exosome miR-542-3p suppresses inflammation and prevents cerebral infarction. Stem Cell Res. Ther. 2021;12:2. doi: 10.1186/s13287-020-02030-w. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 79.Feng B., Meng L., Luan L., Fang Z., Zhao P., Zhao G. Upregulation of extracellular vesicles-encapsulated miR-132 released from mesenchymal stem cells attenuates ischemic neuronal injury by inhibiting Smad2/c-jun pathway Acvr2b suppression. Front. Cell Dev. Biol. 2020;8:568304. doi: 10.3389/fcell.2020.568304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Zhang Z., Zou X., Zhang R., Xie Y., Feng Z., Li F., Han J., Sun H., Ouyang Q., Hua S., et al. Human umbilical cord mesenchymal stem cell-derived exosomal miR-146a-5p reduces microglial-mediated neuroinflammation via suppression of the IRAK1/TRAF6 signaling pathway after ischemic stroke. Aging. 2021;13:3060–3079. doi: 10.18632/aging.202466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Zhang Y., Liu J., Su M., Wang X., Xie C. Exosomal microRNA-22-3p alleviates cerebral ischemic injury by modulating KDM6B/BMP2/BMF axis. Stem Cell Res. Ther. 2021;12:111. doi: 10.1186/s13287-020-02091-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhong Y., Luo L. Exosomes from human umbilical vein endothelial cells ameliorate ischemic injuries by suppressing the RNA component of mitochondrial RNA-processing endoribonuclease via the induction of miR-206/miR-1-3p levels. Neuroscience. 2021;476:34–44. doi: 10.1016/j.neuroscience.2021.08.026. [DOI] [PubMed] [Google Scholar]
- 83.Ai Z., Cheng C., Zhou L., Yin S., Wang L., Liu Y. Bone marrow mesenchymal stem cells-derived extracellular vesicles carrying microRNA-221-3p protect against ischemic stroke via ATF3. Brain Res. Bull. 2021;172:220–228. doi: 10.1016/j.brainresbull.2021.04.022. [DOI] [PubMed] [Google Scholar]
- 84.Hou Z., Chen J., Yang H., Hu X., Yang F. microRNA-26a shuttled by extracellular vesicles secreted from adipose-derived mesenchymal stem cells reduce neuronal damage through KLF9-mediated regulation of TRAF2/KLF2 axis. Adipocyte. 2021;10:378–393. doi: 10.1080/21623945.2021.1938829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Li G., Xiao L., Qin H., Zhuang Q., Zhang W., Liu L., Di C., Zhang Y. Exosomes-carried microRNA-26b-5p regulates microglia M1 polarization after cerebral ischemia/reperfusion. Cell Cycle. 2020;19:1022–1035. doi: 10.1080/15384101.2020.1743912. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 86.Lv H., Li J., Che Y. miR-31 from adipose stem cell-derived extracellular vesicles promotes recovery of neurological function after ischemic stroke by inhibiting TRAF6 and IRF5. Exp. Neurol. 2021;342:113611. doi: 10.1016/j.expneurol.2021.113611. [DOI] [PubMed] [Google Scholar]
- 87.Zhao Y., Gan Y., Xu G., Hua K., Liu D. Exosomes from MSCs overexpressing microRNA-223-3p attenuate cerebral ischemia through inhibiting microglial M1 polarization mediated inflammation. Life Sci. 2020;260:118403. doi: 10.1016/j.lfs.2020.118403. [DOI] [PubMed] [Google Scholar]
- 88.Han B., Zhang Y., Zhang Y., Bai Y., Chen X., Huang R., Wu F., Leng S., Chao J., Zhang J., et al. Novel insight into circular RNA HECTD1 in astrocyte activation via autophagy by targeting MIR142-TIPARP: implications for cerebral ischemic stroke. Autophagy. 2018;14:1164–1184. doi: 10.1080/15548627.2018.1458173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Xin H., Katakowski M., Wang F., Qian J., Liu X., Ali M., Buller B., Zhang Z., Chopp M. MicroRNA cluster miR-17-92 cluster in exosomes enhance neuroplasticity and functional recovery after stroke in rats. Stroke. 2017;48:747–753. doi: 10.1161/strokeaha.116.015204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Ling X., Zhang G., Xia Y., Zhu Q., Zhang J., Li Q., Niu X., Hu G., Yang Y., Wang Y., et al. Exosomes from human urine-derived stem cells enhanced neurogenesis via miR-26a/HDAC6 axis after ischaemic stroke. J. Cell Mol. Med. 2020;24:640–654. doi: 10.1111/jcmm.14774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Yang J., Zhang X., Chen X., Wang L., Yang G. Exosome mediated delivery of miR-124 promotes neurogenesis after ischemia. Mol. Ther. Nucleic Acids. 2017;7:278–287. doi: 10.1016/j.omtn.2017.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Zhang H., Wu J., Wu J., Fan Q., Zhou J., Wu J., Liu S., Zang J., Ye J., Xiao M., et al. Exosome-mediated targeted delivery of miR-210 for angiogenic therapy after cerebral ischemia in mice. J. Nanobiotechnol. 2019;17:29. doi: 10.1186/s12951-019-0461-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Yang Y., Cai Y., Zhang Y., Liu J., Xu Z. Exosomes secreted by adipose-derived stem cells contribute to angiogenesis of brain microvascular endothelial cells following oxygen-glucose deprivation in vitro through MicroRNA-181b/TRPM7 Axis. J. Mol. Neurosci. 2018;65:74–83. doi: 10.1007/s12031-018-1071-9. [DOI] [PubMed] [Google Scholar]
- 94.Gregorius J., Wang C., Stambouli O., Hussner T., Qi Y., Tertel T., Börger V., Mohamud Yusuf A., Hagemann N., Yin D., et al. Small extracellular vesicles obtained from hypoxic mesenchymal stromal cells have unique characteristics that promote cerebral angiogenesis, brain remodeling and neurological recovery after focal cerebral ischemia in mice. Basic Res. Cardiol. 2021;116:40. doi: 10.1007/s00395-021-00881-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Yang J., Cao L., Wang X., Guo W., Guo R., Sun Y., Xue T., Cai Z., Ji J., Cheng H., et al. Neuronal extracellular vesicle derived miR-98 prevents salvageable neurons from microglial phagocytosis in acute ischemic stroke. Cell Death Dis. 2021;12:23. doi: 10.1038/s41419-020-03310-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cui J., Liu N., Chang Z., Gao Y., Bao M., Xie Y., Xu W., Liu X., Jiang S., Liu Y., et al. Exosomal MicroRNA-126 from RIPC serum is involved in hypoxia tolerance in SH-SY5Y cells by downregulating DNMT3B. Mol. Ther. Nucleic Acids. 2020;20:649–660. doi: 10.1016/j.omtn.2020.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Venkat P., Cui C., Chopp M., Zacharek A., Wang F., Landschoot-Ward J., Shen Y., Chen J. MiR-126 mediates brain endothelial cell exosome treatment-induced neurorestorative effects after stroke in type 2 diabetes mellitus mice. Stroke. 2019;50:2865–2874. doi: 10.1161/strokeaha.119.025371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Xin H., Li Y., Liu Z., Wang X., Shang X., Cui Y., Zhang Z., Chopp M. MiR-133b promotes neural plasticity and functional recovery after treatment of stroke with multipotent mesenchymal stromal cells in rats via transfer of exosome-enriched extracellular particles. Stem Cells. 2013;31:2737–2746. doi: 10.1002/stem.1409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xiao Y., Geng F., Wang G., Li X., Zhu J., Zhu W. Bone marrow-derived mesenchymal stem cells-derived exosomes prevent oligodendrocyte apoptosis through exosomal miR-134 by targeting caspase-8. J. Cell. Biochem. 2018 doi: 10.1002/jcb.27519. [DOI] [PubMed] [Google Scholar]
- 100.Liu Y., Li Y., Xiao L., Chen L., Zheng S., Zeng E., Xu C. Extracellular vesicles derived from M2 microglia reduce ischemic brain injury through microRNA-135a-5p/TXNIP/NLRP3 axis. Lab. Invest. 2021;101:837–850. doi: 10.1038/s41374-021-00545-1. [DOI] [PubMed] [Google Scholar]
- 101.Zhang D., Cai G., Liu K., Zhuang Z., Jia K., Pei S., Wang X., Wang H., Xu S., Cui C., et al. Microglia exosomal miRNA-137 attenuates ischemic brain injury through targeting Notch1. Aging. 2021;13:4079–4095. doi: 10.18632/aging.202373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Yue K., Zhang P., Zheng M., Cao X., Cao Y., Zhang Y., Zhang Y., Wu H., Lu Z., Liang L., et al. Neurons can upregulate Cav-1 to increase intake of endothelial cells-derived extracellular vesicles that attenuate apoptosis via miR-1290. Cell Death Dis. 2019;10:869. doi: 10.1038/s41419-019-2100-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Zhang Z., Buller B., Chopp M. Exosomes - beyond stem cells for restorative therapy in stroke and neurological injury. Nat. Rev. Neurol. 2019;15:193–203. doi: 10.1038/s41582-018-0126-4. [DOI] [PubMed] [Google Scholar]
- 104.Khan M., Nickoloff E., Abramova T., Johnson J., Verma S., Krishnamurthy P., Mackie A., Vaughan E., Garikipati V., Benedict C., et al. Embryonic stem cell-derived exosomes promote endogenous repair mechanisms and enhance cardiac function following myocardial infarction. Circ. Res. 2015;117:52–64. doi: 10.1161/circresaha.117.305990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zheng D., Huo M., Li B., Wang W., Piao H., Wang Y., Zhu Z., Li D., Wang T., Liu K. The role of exosomes and exosomal MicroRNA in cardiovascular disease. Front. Cell Dev. Biol. 2020;8:616161. doi: 10.3389/fcell.2020.616161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Ren X., Wu J., Wang X., Sartor M., Jones K., Qian J., Nicolaou P., Pritchard T., Fan G. MicroRNA-320 is involved in the regulation of cardiac ischemia/reperfusion injury by targeting heat-shock protein 20. Circulation. 2009;119:2357–2366. doi: 10.1161/circulationaha.108.814145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Tang Y., Zheng J., Sun Y., Wu Z., Liu Z., Huang G. MicroRNA-1 regulates cardiomyocyte apoptosis by targeting Bcl-2. Int. Heart J. 2009;50:377–387. doi: 10.1536/ihj.50.377. [DOI] [PubMed] [Google Scholar]
- 108.Zheng X., Hermann D., Bähr M., Doeppner T. The role of small extracellular vesicles in cerebral and myocardial ischemia-Molecular signals, treatment targets, and future clinical translation. Stem Cells. 2021;39:403–413. doi: 10.1002/stem.3329. [DOI] [PubMed] [Google Scholar]
- 109.Wang L., Jia Q., Xinnong C., Xie Y., Yang Y., Zhang A., Liu R., Zhuo Y., Zhang J. Role of cardiac progenitor cell-derived exosome-mediated microRNA-210 in cardiovascular disease. J. Cell Mol. Med. 2019;23:7124–7131. doi: 10.1111/jcmm.14562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Geng T., Song Z., Xing J., Wang B., Dai S., Xu Z. Exosome derived from coronary serum of patients with myocardial infarction promotes angiogenesis through the miRNA-143/IGF-IR pathway. Int. J. Nanomed. 2020;15:2647–2658. doi: 10.2147/ijn.S242908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Liao Z., Chen Y., Duan C., Zhu K., Huang R., Zhao H., Hintze M., Pu Q., Yuan Z., Lv L., et al. cdip1Cardiac telocytes inhibit cardiac microvascular endothelial cell apoptosis through exosomal miRNA-21-5p-targeted silencing to improve angiogenesis following myocardial infarction. Theranostics. 2021;11:268–291. doi: 10.7150/thno.47021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Gu H., Liu Z., Li Y., Xie Y., Yao J., Zhu Y., Xu J., Dai Q., Zhong C., Zhu H., et al. Serum-derived extracellular vesicles protect against acute myocardial infarction by regulating miR-21/PDCD4 signaling pathway. Front. Physiol. 2018;9:348. doi: 10.3389/fphys.2018.00348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Song Y., Zhang C., Zhang J., Jiao Z., Dong N., Wang G., Wang Z., Wang L. Localized injection of miRNA-21-enriched extracellular vesicles effectively restores cardiac function after myocardial infarction. Theranostics. 2019;9:2346–2360. doi: 10.7150/thno.29945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Zhang C., Shao K., Liu C., Li C., Yu B. Hypoxic preconditioning BMSCs-exosomes inhibit cardiomyocyte apoptosis after acute myocardial infarction by upregulating microRNA-24. Eur. Rev. Med. Pharmacol. Sci. 2019;23:6691–6699. doi: 10.26355/eurrev_201908_18560. [DOI] [PubMed] [Google Scholar]
- 115.Peng Y., Zhao J., Peng Z., Xu W., Yu G. Exosomal miR-25-3p from mesenchymal stem cells alleviates myocardial infarction by targeting pro-apoptotic proteins and EZH2. Cell Death Dis. 2020;11:317. doi: 10.1038/s41419-020-2545-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Pu L., Kong X., Li H., He X. Exosomes released from mesenchymal stem cells overexpressing microRNA-30e ameliorate heart failure in rats with myocardial infarction. Am. J. Transl. Res. 2021;13:4007–4025. [PMC free article] [PubMed] [Google Scholar]
- 117.Zhu D., Wang Y., Thomas M., McLaughlin K., Oguljahan B., Henderson J., Yang Q., Chen Y., Liu D. Exosomes from adipose-derived stem cells alleviate myocardial infarction via microRNA-31/FIH1/HIF-1α pathway. J. Mol. Cell. Cardiol. 2022;162:10–19. doi: 10.1016/j.yjmcc.2021.08.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Zhu L., Tian T., Wang J., He J., Chen T., Pan M., Xu L., Zhang H., Qiu X., Li C., et al. Hypoxia-elicited mesenchymal stem cell-derived exosomes facilitates cardiac repair through miR-125b-mediated prevention of cell death in myocardial infarction. Theranostics. 2018;8:6163–6177. doi: 10.7150/thno.28021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Luo Q., Guo D., Liu G., Chen G., Hang M., Jin M. Exosomes from MiR-126-overexpressing adscs are therapeutic in relieving acute myocardial ischaemic injury. Cell. Physiol. Biochem. 2017;44:2105–2116. doi: 10.1159/000485949. [DOI] [PubMed] [Google Scholar]
- 120.Chen J., Cui C., Yang X., Xu J., Venkat P., Zacharek A., Yu P., Chopp M. MiR-126 affects brain-heart interaction after cerebral ischemic stroke. Transl. Stroke Res. 2017;8:374–385. doi: 10.1007/s12975-017-0520-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Zheng S., Wang L., Ma H., Sun F., Wen F. microRNA-129 overexpression in endothelial cell-derived extracellular vesicle influences inflammatory response caused by myocardial ischemia/reperfusion injury. Cell Biol. Int. 2021;45:1743–1756. doi: 10.1002/cbin.11614. [DOI] [PubMed] [Google Scholar]
- 122.Wang S., Dong J., Li L., Wu R., Xu L., Ren Y., Hu X. Exosomes derived from miR-129-5p modified bone marrow mesenchymal stem cells represses ventricular remolding of mice with myocardial infarction. J. Tissue Eng. Regen. Med. 2022;16:177–187. doi: 10.1002/term.3268. [DOI] [PubMed] [Google Scholar]
- 123.Barile L., Lionetti V., Cervio E., Matteucci M., Gherghiceanu M., Popescu L., Torre T., Siclari F., Moccetti T., Vassalli G. Extracellular vesicles from human cardiac progenitor cells inhibit cardiomyocyte apoptosis and improve cardiac function after myocardial infarction. Cardiovasc. Res. 2014;103:530–541. doi: 10.1093/cvr/cvu167. [DOI] [PubMed] [Google Scholar]
- 124.Zhu W., Sun L., Zhao P., Liu Y., Zhang J., Zhang Y., Hong Y., Zhu Y., Lu Y., Zhao W., et al. Macrophage migration inhibitory factor facilitates the therapeutic efficacy of mesenchymal stem cells derived exosomes in acute myocardial infarction through upregulating miR-133a-3p. J. Nanobiotechnol. 2021;19:61. doi: 10.1186/s12951-021-00808-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Climent M., Quintavalle M., Miragoli M., Chen J., Condorelli G., Elia L. TGFβ triggers miR-143/145 transfer from smooth muscle cells to endothelial cells, thereby modulating vessel stabilization. Circ. Res. 2015;116:1753–1764. doi: 10.1161/circresaha.116.305178. [DOI] [PubMed] [Google Scholar]
- 126.Pan J., Alimujiang M., Chen Q., Shi H., Luo X. Exosomes derived from miR-146a-modified adipose-derived stem cells attenuate acute myocardial infarction-induced myocardial damage via downregulation of early growth response factor 1. J. Cell. Biochem. 2019;120:4433–4443. doi: 10.1002/jcb.27731. [DOI] [PubMed] [Google Scholar]
- 127.Wu Z., Cheng S., Wang S., Li W., Liu J. BMSCs-derived exosomal microRNA-150-5p attenuates myocardial infarction in mice. Int. Immunopharmacol. 2021;93:107389. doi: 10.1016/j.intimp.2021.107389. [DOI] [PubMed] [Google Scholar]
- 128.Ning W., Li S., Yang W., Yang B., Xin C., Ping X., Huang C., Gu Y., Guo L. Blocking exosomal miRNA-153-3p derived from bone marrow mesenchymal stem cells ameliorates hypoxia-induced myocardial and microvascular damage by targeting the ANGPT1-mediated VEGF/PI3k/Akt/eNOS pathway. Cell. Signal. 2021;77:109812. doi: 10.1016/j.cellsig.2020.109812. [DOI] [PubMed] [Google Scholar]
- 129.Li Y., Zhou J., Zhang O., Wu X., Guan X., Xue Y., Li S., Zhuang X., Zhou B., Miao G., et al. Bone marrow mesenchymal stem cells-derived exosomal microRNA-185 represses ventricular remolding of mice with myocardial infarction by inhibiting SOCS2. Int. Immunopharmacol. 2020;80:106156. doi: 10.1016/j.intimp.2019.106156. [DOI] [PubMed] [Google Scholar]
- 130.Cheng H., Chang S., Xu R., Chen L., Song X., Wu J., Qian J., Zou Y., Ma J. Hypoxia-challenged MSC-derived exosomes deliver miR-210 to attenuate post-infarction cardiac apoptosis. Stem Cell Res. Ther. 2020;11:224. doi: 10.1186/s13287-020-01737-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Wang N., Chen C., Yang D., Liao Q., Luo H., Wang X., Zhou F., Yang X., Yang J., Zeng C., et al. Mesenchymal stem cells-derived extracellular vesicles, via miR-210, improve infarcted cardiac function by promotion of angiogenesis. Biochim. Biophys. Acta, Mol. Basis Dis. 2017;1863:2085–2092. doi: 10.1016/j.bbadis.2017.02.023. [DOI] [PubMed] [Google Scholar]
- 132.Wu Y., Peng W., Fang M., Wu M., Wu M. MSCs-derived extracellular vesicles carrying miR-212-5p alleviate myocardial infarction-induced cardiac fibrosis via NLRC5/VEGF/TGF-β1/SMAD Axis. J. Cardiovasc. Transl. Res. 2022;15:302–316. doi: 10.1007/s12265-021-10156-2. [DOI] [PubMed] [Google Scholar]
- 133.Ke X., Yang R., Wu F., Wang X., Liang J., Hu X., Hu C. Exosomal miR-218-5p/miR-363-3p from endothelial progenitor cells ameliorate myocardial infarction by targeting the p53/JMY signaling pathway. Oxid. Med. Cell. Longev. 2021;2021:5529430. doi: 10.1155/2021/5529430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Yang M., Liao M., Liu R., Zhang Q., Zhang S., He Y., Jin J., Zhang P., Zhou L. Human umbilical cord mesenchymal stem cell-derived extracellular vesicles loaded with miR-223 ameliorate myocardial infarction through P53/S100A9 axis. Genomics. 2022;114:110319. doi: 10.1016/j.ygeno.2022.110319. [DOI] [PubMed] [Google Scholar]
- 135.Youn S., Li Y., Kim Y., Sudhahar V., Abdelsaid K., Kim H., Liu Y., Fulton D., Ashraf M., Tang Y., et al. Modification of cardiac progenitor cell-derived exosomes by miR-322 provides protection against myocardial infarction through Nox2-dependent angiogenesis. Antioxidants. 2019;8 doi: 10.3390/antiox8010018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Huang J., Wang F., Sun X., Chu X., Jiang R., Wang Y., Pang L. Myocardial infarction cardiomyocytes-derived exosomal miR-328-3p promote apoptosis via Caspase signaling. Am. J. Transl. Res. 2021;13:2365–2378. [PMC free article] [PubMed] [Google Scholar]
- 137.Fu D., Jiang H., Li C., Gao T., Liu M., Li H. MicroRNA-338 in MSCs-derived exosomes inhibits cardiomyocyte apoptosis in myocardial infarction. Eur. Rev. Med. Pharmacol. Sci. 2020;24:10107–10117. doi: 10.26355/eurrev_202010_23230. [DOI] [PubMed] [Google Scholar]
- 138.Li Q., Xu Y., Lv K., Wang Y., Zhong Z., Xiao C., Zhu K., Ni C., Wang K., Kong M., et al. Small extracellular vesicles containing miR-486-5p promote angiogenesis after myocardial infarction in mice and nonhuman primates. Sci. Transl. Med. 2021;13 doi: 10.1126/scitranslmed.abb0202. [DOI] [PubMed] [Google Scholar]
- 139.Liu H., Zhang Y., Yuan J., Gao W., Zhong X., Yao K., Lin L., Ge J. Dendritic cell-derived exosomal miR-494-3p promotes angiogenesis following myocardial infarction. Int. J. Mol. Med. 2021;47:315–325. doi: 10.3892/ijmm.2020.4776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Wang X., Zhu Y., Wu C., Liu W., He Y., Yang Q. Adipose-derived mesenchymal stem cells-derived exosomes carry MicroRNA-671 to alleviate myocardial infarction through inactivating the TGFBR2/Smad2 Axis. Inflammation. 2021;44:1815–1830. doi: 10.1007/s10753-021-01460-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Sánchez-Sánchez R., Gómez-Ferrer M., Reinal I., Buigues M., Villanueva-Bádenas E., Ontoria-Oviedo I., Hernándiz A., González-King H., Peiró-Molina E., Dorronsoro A., et al. miR-4732-3p in extracellular vesicles from mesenchymal stromal cells is cardioprotective during myocardial ischemia. Front. Cell Dev. Biol. 2021;9:734143. doi: 10.3389/fcell.2021.734143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Zhang L., Wei Q., Liu X., Zhang T., Wang S., Zhou L., Zou L., Fan F., Chi H., Sun J., et al. Exosomal microRNA-98-5p from hypoxic bone marrow mesenchymal stem cells inhibits myocardial ischemia-reperfusion injury by reducing TLR4 and activating the PI3K/Akt signaling pathway. Int. Immunopharmacol. 2021;101:107592. doi: 10.1016/j.intimp.2021.107592. [DOI] [PubMed] [Google Scholar]
- 143.Jiang W., Song Q., Lu Z., Wang S., Liu T., Wang X., Wang M. Myocardial infarction-associated extracellular vesicle-delivered miR-208b affects the growth of human umbilical vein endothelial cells via regulating CDKN1A. BioMed Res. Int. 2021;2021:9965639. doi: 10.1155/2021/9965639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Fan Z., Qu X., Chu P., Gao Y., Gao X., Chen S., Tian N. MicroRNA-210 promotes angiogenesis in acute myocardial infarction. Mol. Med. Rep. 2018;17:5658–5665. doi: 10.3892/mmr.2018.8620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fasanaro P., D'Alessandra Y., Di Stefano V., Melchionna R., Romani S., Pompilio G., Capogrossi M., Martelli F. MicroRNA-210 modulates endothelial cell response to hypoxia and inhibits the receptor tyrosine kinase ligand Ephrin-A3. J. Biol. Chem. 2008;283:15878–15883. doi: 10.1074/jbc.M800731200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Arvin P., Samimagham H., Montazerghaem H., Khayatian M., Mahboobi H., Ghadiri Soufi F. Early detection of cardiac surgery-associated acute kidney injury by microRNA-21. Bratisl. Lek. Listy. 2017;118:626–631. doi: 10.4149/bll_2017_120. [DOI] [PubMed] [Google Scholar]
- 147.Kang Z., Li Z., Huang P., Luo J., Liu P., Wang Y., Xia T., Zhou Y. Remote ischemic preconditioning upregulates microRNA-21 to protect the kidney in children with congenital heart disease undergoing cardiopulmonary bypass. Pediatr. Nephrol. 2018;33:911–919. doi: 10.1007/s00467-017-3851-9. [DOI] [PubMed] [Google Scholar]
- 148.Lorenzen J., Kielstein J., Hafer C., Gupta S., Kümpers P., Faulhaber-Walter R., Haller H., Fliser D., Thum T. Circulating miR-210 predicts survival in critically ill patients with acute kidney injury. Clin. J. Am. Soc. Nephrol. 2011;6:1540–1546. doi: 10.2215/cjn.00430111. [DOI] [PubMed] [Google Scholar]
- 149.Aguado-Fraile E., Ramos E., Conde E., Rodríguez M., Martín-Gómez L., Lietor A., Candela Á., Ponte B., Liaño F., García-Bermejo M. A pilot study identifying a set of microRNAs as precise diagnostic biomarkers of acute kidney injury. PLoS One. 2015;10:e0127175. doi: 10.1371/journal.pone.0127175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Wu R., Wu Y., Yang L., Deng Y., Chen D. [Value of serum level of microRNA-494 in predicting prognosis of acute renal injury after cardiac surgery in children] Zhonghua Wei Zhong Bing Ji Jiu Yi Xue. 2019;31:1469–1473. doi: 10.3760/cma.j.issn.2095-4352.2019.12.007. [DOI] [PubMed] [Google Scholar]
- 151.Yu W., Zeng H., Chen J., Fu S., Huang Q., Xu Y., Xu A., Lan H., Tang Y. miR-20a-5p is enriched in hypoxia-derived tubular exosomes and protects against acute tubular injury. Clin. Sci. (Lond.) 2020;134:2223–2234. doi: 10.1042/cs20200288. [DOI] [PubMed] [Google Scholar]
- 152.Gu D., Zou X., Ju G., Zhang G., Bao E., Zhu Y. Mesenchymal stromal cells derived extracellular vesicles ameliorate acute renal ischemia reperfusion injury by inhibition of mitochondrial fission through miR-30. Stem Cells Int. 2016;2016:2093940. doi: 10.1155/2016/2093940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Zhang X., Wang N., Huang Y., Li Y., Li G., Lin Y., Atala A., Hou J., Zhao W. Extracellular vesicles from three dimensional culture of human placental mesenchymal stem cells ameliorated renal ischemia/reperfusion injury. Int. J. Artif. Organs. 2022;45:181–192. doi: 10.1177/0391398820986809. [DOI] [PubMed] [Google Scholar]
- 154.Zhang L., Liu H., Xu K., Ling Z., Huang Y., Hu Q., Lu K., Liu C., Wang Y., Liu N., et al. Hypoxia preconditioned renal tubular epithelial cell-derived extracellular vesicles alleviate renal ischaemia-reperfusion injury mediated by the HIF-1α/Rab22 pathway and potentially affected by microRNAs. Int. J. Biol. Sci. 2019;15:1161–1176. doi: 10.7150/ijbs.32004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Sonoda H., Lee B., Park K., Nihalani D., Yoon J., Ikeda M., Kwon S. miRNA profiling of urinary exosomes to assess the progression of acute kidney injury. Sci. Rep. 2019;9:4692. doi: 10.1038/s41598-019-40747-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Pan T., Jia P., Chen N., Fang Y., Liang Y., Guo M., Ding X. Delayed remote ischemic preconditioning ConfersRenoprotection against septic acute kidney injury via exosomal miR-21. Theranostics. 2019;9:405–423. doi: 10.7150/thno.29832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Liu J., Cai G., Ning Y., Wang J., Lv Y., Guo Y., Fu B., Hong Q., Sun X., Chen X. Caloric restriction alleviates aging-related fibrosis of kidney through downregulation of miR-21 in extracellular vesicles. Aging. 2020;12:18052–18072. doi: 10.18632/aging.103591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Liang Y., Zhang T., Jia P., Xu X., Fang Y., Ding X. Interaction between bone marrow-derived dendritic cells and miR-21 of tubular renal epithelial cells under hypoxia. Eur. Rev. Med. Pharmacol. Sci. 2019;23:1641–1651. doi: 10.26355/eurrev_201902_17124. [DOI] [PubMed] [Google Scholar]
- 159.Jia P., Wu X., Dai Y., Teng J., Fang Y., Hu J., Zou J., Liang M., Ding X. MicroRNA-21 is required for local and remote ischemic preconditioning in multiple organ protection against sepsis. Crit. Care Med. 2017;45:e703–e710. doi: 10.1097/ccm.0000000000002363. [DOI] [PubMed] [Google Scholar]
- 160.Li Z., Lv L., Tang T., Wang B., Feng Y., Zhou L., Cao J., Tang R., Wu M., Liu H., et al. HIF-1α inducing exosomal microRNA-23a expression mediates the cross-talk between tubular epithelial cells and macrophages in tubulointerstitial inflammation. Kidney Int. 2019;95:388–404. doi: 10.1016/j.kint.2018.09.013. [DOI] [PubMed] [Google Scholar]
- 161.Cao J., Wang B., Tang T., Wen Y., Li Z., Feng S., Wu M., Liu D., Yin D., Ma K., et al. Exosomal miR-125b-5p deriving from mesenchymal stem cells promotes tubular repair by suppression of p53 in ischemic acute kidney injury. Theranostics. 2021;11:5248–5266. doi: 10.7150/thno.54550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Guan H., Peng R., Mao L., Fang F., Xu B., Chen M. Injured tubular epithelial cells activate fibroblasts to promote kidney fibrosis through miR-150-containing exosomes. Exp. Cell Res. 2020;392:112007. doi: 10.1016/j.yexcr.2020.112007. [DOI] [PubMed] [Google Scholar]
- 163.Li X., Liao J., Su X., Li W., Bi Z., Wang J., Su Q., Huang H., Wei Y., Gao Y., et al. miR-146a-5pHuman urine-derived stem cells protect against renal ischemia/reperfusion injury in a rat model via exosomal which targets. Theranostics. 2020;10:9561–9578. doi: 10.7150/thno.42153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Zhou X., Zhao S., Li W., Ruan Y., Yuan R., Ning J., Jiang K., Xie J., Yao X., Li H., et al. Tubular cell-derived exosomal miR-150-5p contributes to renal fibrosis following unilateral ischemia-reperfusion injury by activating fibroblast in vitro and in vivo. Int. J. Biol. Sci. 2021;17:4021–4033. doi: 10.7150/ijbs.62478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Zhu G., Pei L., Lin F., Yin H., Li X., He W., Liu N., Gou X. Exosomes from human-bone-marrow-derived mesenchymal stem cells protect against renal ischemia/reperfusion injury via transferring miR-199a-3p. J. Cell. Physiol. 2019;234:23736–23749. doi: 10.1002/jcp.28941. [DOI] [PubMed] [Google Scholar]
- 166.Wang C., Zhu G., He W., Yin H., Lin F., Gou X., Li X. BMSCs protect against renal ischemia-reperfusion injury by secreting exosomes loaded with miR-199a-5p that target BIP to inhibit endoplasmic reticulum stress at the very early reperfusion stages. FASEB J. 2019;33:5440–5456. doi: 10.1096/fj.201801821R. [DOI] [PubMed] [Google Scholar]
- 167.Cao H., Cheng Y., Gao H., Zhuang J., Zhang W., Bian Q., Wang F., Du Y., Li Z., Kong D., et al. In vivo tracking of mesenchymal stem cell-derived extracellular vesicles improving mitochondrial function in renal ischemia-reperfusion injury. ACS Nano. 2020;14:4014–4026. doi: 10.1021/acsnano.9b08207. [DOI] [PubMed] [Google Scholar]
- 168.Zhang Y., Wang J., Yang B., Qiao R., Li A., Guo H., Ding J., Li H., Ye H., Wu D., et al. Transfer of MicroRNA-216a-5p from exosomes secreted by human urine-derived stem cells reduces renal ischemia/reperfusion injury. Front. Cell Dev. Biol. 2020;8:610587. doi: 10.3389/fcell.2020.610587. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Rutman A., Negi S., Saberi N., Khan K., Tchervenkov J., Paraskevas S. Extracellular vesicles from kidney allografts express miR-218-5p and alter Th17/Treg ratios. Front. Immunol. 2022;13:784374. doi: 10.3389/fimmu.2022.784374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 170.Ding C., Zheng J., Wang B., Li Y., Xiang H., Dou M., Qiao Y., Tian P., Ding X., Xue W. Exosomal MicroRNA-374b-5p from tubular epithelial cells promoted M1 macrophages activation and worsened renal ischemia/reperfusion injury. Front. Cell Dev. Biol. 2020;8:587693. doi: 10.3389/fcell.2020.587693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Livingston M., Wei Q. MicroRNAs in extracellular vesicles protect kidney from ischemic injury: from endothelial to tubular epithelial. Kidney Int. 2016;90:1150–1152. doi: 10.1016/j.kint.2016.08.032. [DOI] [PubMed] [Google Scholar]
- 172.Viñas J., Burger D., Zimpelmann J., Haneef R., Knoll W., Campbell P., Gutsol A., Carter A., Allan D., Burns K. Transfer of microRNA-486-5p from human endothelial colony forming cell-derived exosomes reduces ischemic kidney injury. Kidney Int. 2016;90:1238–1250. doi: 10.1016/j.kint.2016.07.015. [DOI] [PubMed] [Google Scholar]
- 173.Viñas J., Spence M., Porter C., Douvris A., Gutsol A., Zimpelmann J., Campbell P., Burns K. micro-RNA-486-5p protects against kidney ischemic injury and modifies the apoptotic transcriptome in proximal tubules. Kidney Int. 2021;100:597–612. doi: 10.1016/j.kint.2021.05.034. [DOI] [PubMed] [Google Scholar]
- 174.Viñas J., Spence M., Gutsol A., Knoll W., Burger D., Zimpelmann J., Allan D., Burns K. Receptor-Ligand interaction mediates targeting of endothelial colony forming cell-derived exosomes to the kidney after ischemic injury. Sci. Rep. 2018;8:16320. doi: 10.1038/s41598-018-34557-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Jiang L., Liu X., Ma Q., Yang Q., Gao L., Li H., Wang J., Wei B., Wen J., Li J., et al. hsa-miR-500a-3P alleviates kidney injury by targeting MLKL-mediated necroptosis in renal epithelial cells. FASEB J. 2019;33:3523–3535. doi: 10.1096/fj.201801711R. [DOI] [PubMed] [Google Scholar]
- 176.Ashour H., Rashed L., Elkordy M., Abdelwahed O. Remote liver injury following acute renal ischaemia-reperfusion: involvement of circulating exosomal miR-687 and regulation by thymoquinone. Exp. Physiol. 2021;106:2262–2275. doi: 10.1113/ep089765. [DOI] [PubMed] [Google Scholar]
- 177.Green D., Reed J. Mitochondria and apoptosis. Science. 1998;281:1309–1312. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 178.Eddy A. Scraping fibrosis: UMODulating renal fibrosis. Nat. Med. 2011;17:553–555. doi: 10.1038/nm0511-553. [DOI] [PubMed] [Google Scholar]
- 179.Ju C., Wang M., Tak E., Kim B., Emontzpohl C., Yang Y., Yuan X., Kutay H., Liang Y., Hall D., et al. Hypoxia-inducible factor-1α-dependent induction of miR122 enhances hepatic ischemia tolerance. J. Clin. Invest. 2021;131 doi: 10.1172/jci140300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Huang Z., Mou T., Luo Y., Pu X., Pu J., Wan L., Gong J., Yang H., Liu Y., Li Z., et al. Inhibition of miR-450b-5p ameliorates hepatic ischemia/reperfusion injury via targeting CRYAB. Cell Death Dis. 2020;11:455. doi: 10.1038/s41419-020-2648-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Pan W., Wang L., Zhang X., Zhang H., Zhang J., Wang G., Xu P., Zhang Y., Hu P., Zhang X., et al. Hypoxia-induced microRNA-191 contributes to hepatic ischemia/reperfusion injury through the ZONAB/Cyclin D1 axis. Cell Death Differ. 2019;26:291–305. doi: 10.1038/s41418-018-0120-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Tang B., Wang Z., Qi G., Yuan S., Yu S., Li B., Wei Y., Huang Q., Zhai R., He S. MicroRNA-155 deficiency attenuates ischemia-reperfusion injury after liver transplantation in mice. Transpl. Int. 2015;28:751–760. doi: 10.1111/tri.12528. [DOI] [PubMed] [Google Scholar]
- 183.Li L., Li G., Yu C., Shen Z., Xu C., Feng Z., Zhang X., Li Y. A role of microRNA-370 in hepatic ischaemia-reperfusion injury by targeting transforming growth factor-β receptor II. Liver Int. 2015;35:1124–1132. doi: 10.1111/liv.12441. [DOI] [PubMed] [Google Scholar]
- 184.Pan W., Wang H., Zhang X., Xu P., Wang G., Li Y., Huang K., Zhang Y., Zhao H., Du R., et al. miR-210 participates in hepatic ischemia reperfusion injury by forming a negative feedback loop with SMAD4. Hepatology. 2020;72:2134–2148. doi: 10.1002/hep.31221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Kim H., Joe Y., Yu J., Chen Y., Jeong S., Mani N., Cho G., Pae H., Ryter S., Chung H. Carbon monoxide protects against hepatic ischemia/reperfusion injury by modulating the miR-34a/SIRT1 pathway. Biochim. Biophys. Acta. 2015;1852:1550–1559. doi: 10.1016/j.bbadis.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 186.Yue Y., Du Z., Tao J., Shi L. Inhibition of microRNA-297 alleviates THLE-2 cell injury induced by hypoxia/reoxygenation by inhibiting NLRP3 inflammasome activation via sirtuin 3. Can. J. Physiol. Pharmacol. 2022;100:125–133. doi: 10.1139/cjpp-2021-0287. [DOI] [PubMed] [Google Scholar]
- 187.Wu K., Tao G., Xu T., An Y., Yu X., Wang Y., Wang S., Guo W., Ma L. Downregulation of miR-497-5p prevents liver ischemia-reperfusion injury in association with MED1/TIMP-2 axis and the NF-κB pathway. FASEB J. 2021;35:e21180. doi: 10.1096/fj.202001029R. [DOI] [PubMed] [Google Scholar]
- 188.Mou T., Luo Y., Huang Z., Zheng D., Pu X., Shen A., Pu J., Li T., Dai J., Chen W., et al. Inhibition of microRNA-128-3p alleviates liver ischaemia-reperfusion injury in mice through repressing the Rnd3/NF-B axis. Innate Immun. 2020;26:528–536. doi: 10.1177/1753425920928449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Luo Y., Huang Z., Zong K., Cao Z., Peng D., Zhou B., Shen A., Yan P., Wu Z. miR-194 ameliorates hepatic ischemia/reperfusion injury via targeting PHLDA1 in a TRAF6-dependent manner. Int. Immunopharmacol. 2021;96:107604. doi: 10.1016/j.intimp.2021.107604. [DOI] [PubMed] [Google Scholar]
- 190.Jiang W., Kong L., Ni Q., Lu Y., Ding W., Liu G., Pu L., Tang W., Kong L. miR-146a ameliorates liver ischemia/reperfusion injury by suppressing IRAK1 and TRAF6. PLoS One. 2014;9:e101530. doi: 10.1371/journal.pone.0101530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yu Q., Chen S., Tang H., Yang H., Zhang J., Shi X., Li J., Guo W., Zhang S. miR-140-5p alleviates mouse liver ischemia/reperfusion injury by targeting CAPN1. Mol. Med. Rep. 2021;24 doi: 10.3892/mmr.2021.12314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Li Y., Gao M., Xu L., Yin L., Qi Y., Peng J. MicroRNA-142-3p attenuates hepatic ischemia/reperfusion injury via targeting of myristoylated alanine-rich C-kinase substrate. Pharmacol. Res. 2020;156:104783. doi: 10.1016/j.phrs.2020.104783. [DOI] [PubMed] [Google Scholar]
- 193.Duan Y., Meng Y., Gao Z., Wang X., Zhang H. microRNA-9-5p protects liver sinusoidal endothelial cell against oxygen glucose deprivation/reperfusion injury. Open Life Sci. 2021;16:375–383. doi: 10.1515/biol-2021-0042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 194.Zhang L., Song Y., Chen L., Li D., Feng H., Lu Z., Fan T., Chen Z., Livingston M., Geng Q. MiR-20a-containing exosomes from umbilical cord mesenchymal stem cells alleviates liver ischemia/reperfusion injury. J. Cell. Physiol. 2020;235:3698–3710. doi: 10.1002/jcp.29264. [DOI] [PubMed] [Google Scholar]
- 195.Xie K., Liu L., Chen J., Liu F. Exosomal miR-1246 derived from human umbilical cord blood mesenchymal stem cells attenuates hepatic ischemia reperfusion injury by modulating T helper 17/regulatory T balance. IUBMB Life. 2019;71:2020–2030. doi: 10.1002/iub.2147. [DOI] [PubMed] [Google Scholar]
- 196.Xie K., Liu L., Chen J., Liu F. Exosomes derived from human umbilical cord blood mesenchymal stem cells improve hepatic ischemia reperfusion injury via delivering miR-1246. Cell Cycle. 2019;18:3491–3501. doi: 10.1080/15384101.2019.1689480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Wu L., Tian X., Zuo H., Zheng W., Li X., Yuan M., Tian X., Song H. miR-124-3p delivered by exosomes from heme oxygenase-1 modified bone marrow mesenchymal stem cells inhibits ferroptosis to attenuate ischemia-reperfusion injury in steatotic grafts. J. Nanobiotechnol. 2022;20:196. doi: 10.1186/s12951-022-01407-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Zhou J., Chen L., Chen B., Huang S., Zeng C., Wu H., Chen C., Long F. Increased serum exosomal miR-134 expression in the acute ischemic stroke patients. BMC Neurol. 2018;18:198. doi: 10.1186/s12883-018-1196-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Wang W., Li D., Li R., Zhou X., Yu D., Lan X., Li J., Liu J. Diagnosis of hyperacute and acute ischaemic stroke: the potential utility of exosomal MicroRNA-21-5p and MicroRNA-30a-5p. Cerebrovasc. Dis. 2018;45:204–212. doi: 10.1159/000488365. [DOI] [PubMed] [Google Scholar]
- 200.Chen Y., Song Y., Huang J., Qu M., Zhang Y., Geng J., Zhang Z., Liu J., Yang G. Increased circulating exosomal miRNA-223 is associated with acute ischemic stroke. Front. Neurol. 2017;8:57. doi: 10.3389/fneur.2017.00057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 201.Ji Q., Ji Y., Peng J., Zhou X., Chen X., Zhao H., Xu T., Chen L., Xu Y. Increased brain-specific MiR-9 and MiR-124 in the serum exosomes of acute ischemic stroke patients. PLoS One. 2016;11:e0163645. doi: 10.1371/journal.pone.0163645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Li D., Liu J., Wang W., Li R., Yu D., Lan X., Li J. Plasma exosomal miR-422a and miR-125b-2-3p serve as biomarkers for ischemic stroke. Curr. Neurovasc. Res. 2017;14:330–337. doi: 10.2174/1567202614666171005153434. [DOI] [PubMed] [Google Scholar]
- 203.Ling H., Guo Z., Du S., Liao Y., Li Y., Ding C., Song C. Serum exosomal miR-122-5p is a new biomarker for both acute coronary syndrome and underlying coronary artery stenosis. Biomarkers. 2020;25:539–547. doi: 10.1080/1354750x.2020.1803963. [DOI] [PubMed] [Google Scholar]
- 204.Ling H., Guo Z., Shi Y., Zhang L., Song C. Serum exosomal MicroRNA-21, MicroRNA-126, and PTEN are novel biomarkers for diagnosis of acute coronary syndrome. Front. Physiol. 2020;11:654. doi: 10.3389/fphys.2020.00654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Chen Z., Yan Y., Wu J., Qi C., Liu J., Wang J. Expression level and diagnostic value of exosomal NEAT1/miR-204/MMP-9 in acute ST-segment elevation myocardial infarction. IUBMB Life. 2020;72:2499–2507. doi: 10.1002/iub.2376. [DOI] [PubMed] [Google Scholar]
- 206.Su J., Li J., Yu Q., Wang J., Li X., Yang J., Xu J., Liu Y., Xu Z., Ji L., et al. Exosomal miRNAs as potential biomarkers for acute myocardial infarction. IUBMB Life. 2020;72:384–400. doi: 10.1002/iub.2189. [DOI] [PubMed] [Google Scholar]
- 207.Nordin J., Lee Y., Vader P., Mäger I., Johansson H., Heusermann W., Wiklander O., Hällbrink M., Seow Y., Bultema J., et al. Ultrafiltration with size-exclusion liquid chromatography for high yield isolation of extracellular vesicles preserving intact biophysical and functional properties. Nanomedicine. 2015;11:879–883. doi: 10.1016/j.nano.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 208.Van Deun J., Mestdagh P., Sormunen R., Cocquyt V., Vermaelen K., Vandesompele J., Bracke M., De Wever O., Hendrix A. The impact of disparate isolation methods for extracellular vesicles on downstream RNA profiling. J. Extracell. Vesicles. 2014;3 doi: 10.3402/jev.v3.24858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 209.Nawaz M., Camussi G., Valadi H., Nazarenko I., Ekström K., Wang X., Principe S., Shah N., Ashraf N., Fatima F., et al. The emerging role of extracellular vesicles as biomarkers for urogenital cancers. Nat. Rev. Urol. 2014;11:688–701. doi: 10.1038/nrurol.2014.301. [DOI] [PubMed] [Google Scholar]
- 210.Sódar B., Kittel Á., Pálóczi K., Vukman K., Osteikoetxea X., Szabó-Taylor K., Németh A., Sperlágh B., Baranyai T., Giricz Z., et al. Low-density lipoprotein mimics blood plasma-derived exosomes and microvesicles during isolation and detection. Sci. Rep. 2016;6:24316. doi: 10.1038/srep24316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 211.Kilchert C., Wittmann S., Vasiljeva L. The regulation and functions of the nuclear RNA exosome complex. Nat. Rev. Mol. Cell Biol. 2016;17:227–239. doi: 10.1038/nrm.2015.15. [DOI] [PubMed] [Google Scholar]
- 212.Schmid M., Jensen T. The nuclear RNA exosome and its cofactors. Adv. Exp. Med. Biol. 2019;1203:113–132. doi: 10.1007/978-3-030-31434-7_4. [DOI] [PubMed] [Google Scholar]
- 213.Schmidt K., Butler J. Nuclear RNA surveillance: role of TRAMP in controlling exosome specificity. Wiley Interdiscip. Rev. RNA. 2013;4:217–231. doi: 10.1002/wrna.1155. [DOI] [PMC free article] [PubMed] [Google Scholar]