Abstract
Glaucoma is the leading cause of global irreversible blindness, necessitating research for new, more efficacious treatment options than currently exist. Trabecular meshwork (TM) cells play an important role in the maintenance and function of the aqueous outflow pathway, and studies have found that there is decreased cellularity of the TM in glaucoma. Regeneration of the TM with stem cells has been proposed as a novel therapeutic option by several reports over the last few decades.
Stem cells have the capacity for self-renewal and the potential to differentiate into adult functional cells. Several types of stem cells have been investigated in ocular regenerative medicine: tissue specific stem cells, embryonic stem cells, induced pluripotent stem cells, and adult mesenchymal stem cells. These cells have been used in various glaucoma animal models and ex vivo models and have shown success in IOP homeostasis and TM cellularity restoration. They have also demonstrated stability without serious side effects for a significant period of time.
Based on current knowledge of TM pathology in glaucoma and existing literature regarding stem cell regeneration of this tissue, we propose a human clinical study as the next step in understanding this potentially revolutionary treatment paradigm. The ability to protect and replace TM cells in glaucomatous eyes could change the field forever.
Keywords: Trabecular meshwork regeneration, stem cells, glaucoma
1.1. Introduction
Glaucoma is a chronic optic neuropathy characterized by changes in the structure of the optic nerve head and progressive loss of retinal ganglion cells (RGCs). Importantly, it is the leading cause of global irreversible blindness, and disease prevalence is estimated to increase to over 100 million people by the year 2040 (Tham et al., 2014). The present and future burden of glaucoma inspires continued research for novel treatment strategies.
Current clinical and surgical management focuses on reducing intraocular pressure (IOP), one of the primary risk factors for the development of glaucoma. IOP is determined by a balance between production and elimination of aqueous humor. There are two pathways for aqueous outflow from the eye: the conventional trabecular outflow pathway and the unconventional uveoscleral pathway. IOP homeostasis via the conventional pathway is a natural process by which pressure changes result in alterations in outflow resistance, effected by trabecular meshwork (TM), ciliary muscle and Schlemm’s canal inner wall cells (Abu-Hassan et al., 2015; Acott et al., 2014). Most ocular hypotensive treatments are given topically, and decrease IOP by reducing the production of aqueous humor (e.g. beta blockers, alpha agonists, carbonic anhydrase inhibitors) or by targeting the uveoscleral pathway (e.g. prostaglandin analogs, alpha agonists). Some newer drugs (e.g. rho kinase inhibitors, prostaglandin analogs) and older drugs (e.g. miotics, sympathomimetics) target the conventional pathway. However, some patients progress despite reaching their lowest safely achievable IOP. Further, adherence to multiple eye drops can be difficult for patients, although improved with the use of combination drops (Barnebey and Robin, 2017; Reardon et al., 2011).
Trabecular cells play several roles in the maintenance and normal function of the conventional aqueous outflow pathway. Researchers have observed that cellularity in the inner and intermediate zones of the TM is decreased in eyes with glaucoma. Interestingly, cellularity in the outer juxtacanalicular zone, the site of most outflow resistance regulation, has been found to be similar in non-glaucomatous and glaucomatous eyes (Alvarado et al., 1984, 1981; Pearson and Martin, 2015). This suggests the existence of a paracrine signaling pathway from the inner/intermediate TM to the site of regulation of resistance, i.e. mainly in the juxtacanalicular (JCT) TM region and Schlemm’s canal endothelial cells. In other words, one interpretation of this data is that paracrine signaling from the uveal and/or corneoscleral meshwork affects JCT cellular function, so that even if JCT cells are present, their function is altered due to cell loss in the corneoscleral meshwork. Identification of such paracrine factors will be essential in the development of other non-cell based therapies. In any case, decreased cellularity of the TM and increased resistance to outflow is associated with ocular hypertension. Mouse models of glaucoma have also demonstrated that TM cell death is associated with IOP elevation and emphasize the importance of TM cell function for aqueous outflow (Epstein, 2012; Mallick et al., 2021; Shepard et al., 2007; Zode et al., 2011), although a model in rats showed an opposite effect (Zhang et al., 2014). Despite these associations, a direct causal link between cellularity and aqueous outflow resistance is not understood mechanistically, although it could perhaps involve fusion of denuded TM beams (Gong and Swain, 2016), which in turn is known to be associated with reduced facility (Li et al., 2021, 2019; Wang et al., 2018, 2017b). Nonetheless, it is known that TM cellularity reduction is associated with outflow facility reduction in a transgenic myocilin mutant mouse model (Xiong et al., 2021; Zhu et al., 2016; Zode et al., 2011) and in an ex vivo saponin perfused human anterior segmental model (Abu-Hassan et al., 2015, p.). Many labs are researching therapies to prevent damage to or to regenerate TM cells (Abu-Hassan et al., 2015; Chang and Goldberg, 2012; Du et al., 2013; Manuguerra-GagnÉ et al., 2013; Roubeix et al., 2015; Xiong et al., 2021; Yun et al., 2018, 2016; Zhou et al., 2020; Zhu et al., 2017, 2016). One proposed approach for achieving this has been the use of stem cell therapy.
Stem cells show much promise for cell-based therapies in glaucoma because of their capacity for self-renewal and potential to differentiate into or to support adult functional cells. While regenerating RGCs has proven difficult due to the complex architecture of the retina and the required length of central nervous system projections, it may be more feasible to restore cells in the trabecular meshwork, an important component of the aqueous humor outflow pathway, with stem cells (Du et al., 2013, 2012). It is proposed that repairing the cellularity of the TM can improve aqueous outflow, prevent RGC apoptosis, and ultimately prevent visual loss due to elevated IOP (Bull et al., 2009; Du et al., 2013, 2012; Johnson et al., 2010; Pearson and Martin, 2015; Yun et al., 2016).
We will discuss current knowledge about the pathology of the TM in glaucoma, how stem cells can be used to regenerate this damaged tissue, and our proposed future use of this technology. The ability to protect and replace TM cells is a potential therapeutic target that could revolutionize the management of glaucoma.
1.2. The Trabecular Meshwork
The TM is histologically divided into three layers: the inner uveal meshwork, the central corneoscleral meshwork, and the outer juxtacanalicular tissue, juxtaposed to the inner wall of Schlemm’s canal (Figure 1) (Llobet et al., 2003). The juxtacanalicular meshwork along with the adjacent inner wall of Schlemm’s canal are thought to be the major sites of outflow resistance (Johnson, 2006). The TM is a pressure sensitive outflow pathway that undergoes configurational changes with variation in intraocular pressure. These changes are inducible and reversible physical responses of the tissue (Johnstone and Grant, 1973). Cell culture studies have revealed that TM cells function as endothelial cells (lining the aqueous channels), phagocytic cells (removing material that may obstruct aqueous outflow), and connective tissue cells (producing extracellular matrix [ECM] components and secreting enzymes) (Polansky et al., 1984, 1979).
FIGURE 1. Schematic diagram of the conventional outflow pathway for aqueous humor drainage.
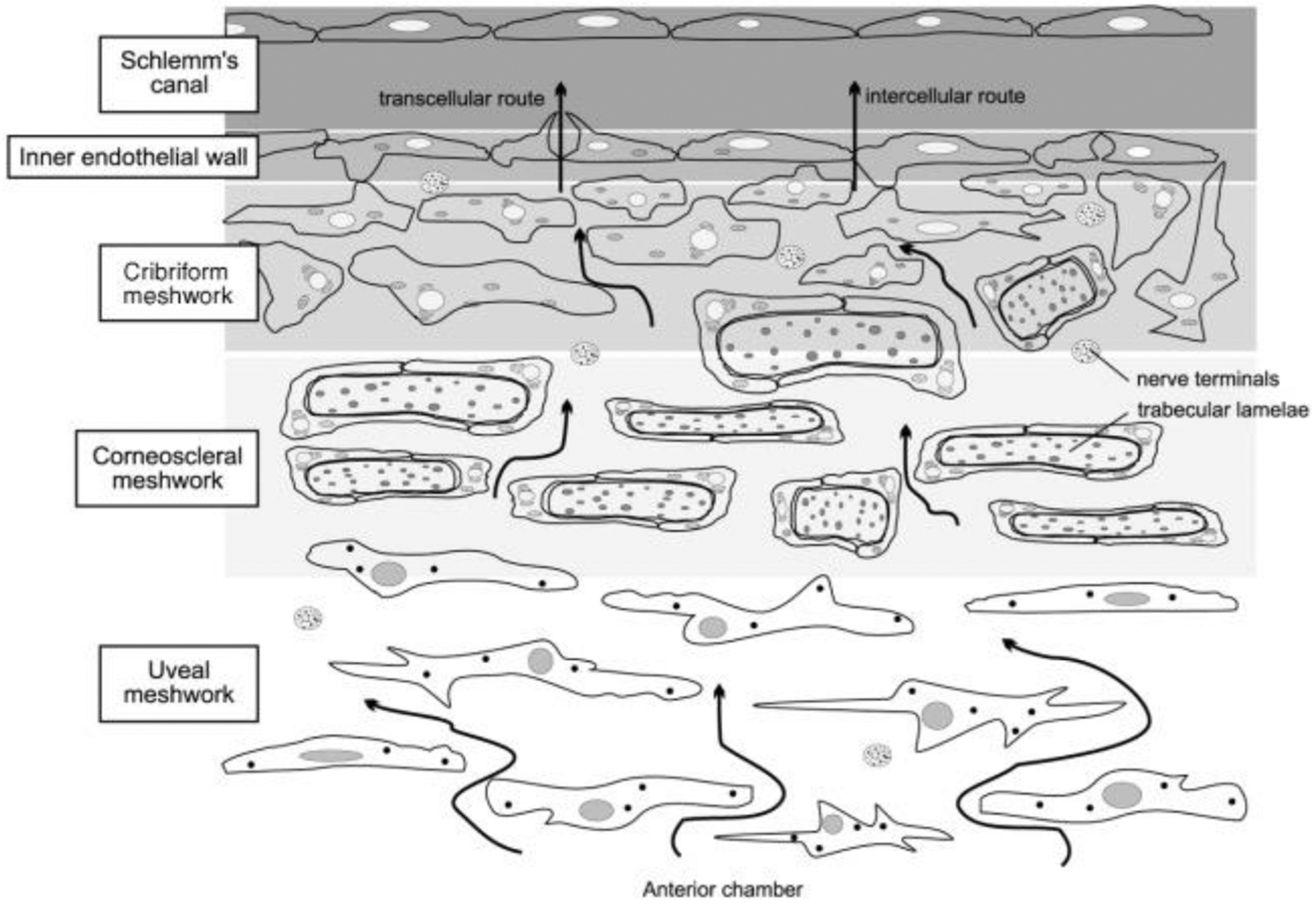
The arrows schematically indicate the flow patterns aqueous humor, i.e. from the anterior chamber, across the trabecular meshwork (TM) toward Schlemm’s canal. The regions of the trabecular meshwork (TM), from inner to outer are: the uveal meshwork, corneoscleral meshwork, and the juxtacanalicular or cribriform meshwork. Aqueous humor flows through the intercellular spaces of the TM and crosses the inner wall of Schlemm’s canal via two different routes: intercellular and transcellular. Resistance to aqueous humor flow increases progressively from the inner TM to the juxtacanalicular region, where intercellular spaces narrow and the cribiform TM and inner wall of Schlemm’s canal interact. Adapted from Llobet et al, Physiology, 2003, 18: 205–209 with permission (license number 1139632-1).
With age, TM cell density decreases and the trabecular beams (the TM cell basement membrane) thickens. Scanning electron microscope studies of trabecular tissues from older eyes demonstrate enlarged trabecular cells adjacent to acellular connective tissue beams. The decline in cellularity of the meshwork was demonstrated to be slightly more pronounced in the filtration region than the non-filtration region in histologic studies (Alvarado et al., 1981). The loss of TM cellularity is further accelerated in eyes with primary open angle glaucoma (POAG) (Alvarado et al., 1984; Kahook et al., 2012). However, we are unaware of studies of changes in TM cellularity in other forms of glaucoma, and this represents an important knowledge gap. Age and disease-related decrease in TM cells, abnormal ECM accumulation, and increased stiffness may result in increased outflow resistance and thus increased IOP (Yun et al., 2016). Repopulating and/or regenerating non-functional TM cells with stem cells to repair outflow facility is a potential therapy that has been investigated in vitro and in animal studies with favorable results (Abu-Hassan et al., 2015; Du et al., 2013; Manuguerra-GagnÉ et al., 2013; Roubeix et al., 2015; Xiong et al., 2021; Yun et al., 2018, 2016; Zhou et al., 2020; Zhu et al., 2017, 2016). Here we define a “non-functional TM cell” as a cell that fails to participate in maintaining a normal IOP and a homeostatic response, which may be characterized by one or more of: decreased ability to phagocytose, decreased contractile ability, altered cytokine production, and/or failure to respond to normal IOP-associated mechanical stimuli, such as stretch.
1.3. Resident Stem Cells in the Trabecular Meshwork
Adult stem cells maintain and repair various tissues in the human body. These multipotent stem cells can self-renew and generate specialized cell types within the tissue of origin. They are present in bone marrow, brain, heart, adipose tissue, and skeletal tissue, among others (Verfaillie, 2002; Zhang et al., 2018). In 1982, an unusual population of trabecular meshwork cells termed “Schwalbe’s line cells” was discovered. These cells are located at Schwalbe’s Line, the transitional area between the corneal endothelium and the anterior non-filtering portion of the TM, also known as the “insert” or non-filtering region, and comprise about 2–5% of the total TM cell population (Braunger et al., 2014; Raviola, 1982; Yun et al., 2016). Immunostaining studies that showed stem cell markers in the TM and in the transition zone first described by Raviola provided more direct evidence for the existence of these stem cells (Whikehart et al., 2005), and it was hypothesized that the insert zone is a source for TM cell renewal (Kelley et al., 2009). Subsequently, Yun et al. and Braunger et al. showed label-retaining stem cells in the insert region of the TM in mice and monkeys, respectively (Braunger et al., 2014; Yun et al., 2016). Immunostaining of human tissue reported that cells expressing the stem cell markers ABCG2 and P75 were present mainly in the insert region, consistent with this region being a location of TM stem cells (Sundaresan et al., 2019).
Trabecular meshwork stem cells (TMSCs) have been studied, characterized, and/or isolated by culture in several groups (Acott and Bacon, 1989; Castro and Du, 2019; Du et al., 2012; Gonzalez et al., 2006; Kelley et al., 2009; Nadri et al., 2013; Yun et al., 2018). In 1989, it was discovered that laser trabeculoplasty in human post-mortem eyes produced an approximately six-fold increase in cell division within a specialized population of TM cells located anterior to the filtering portion of the meshwork, similar to those observed in 1982 (Acott and Bacon, 1989; Raviola, 1982). By two weeks post-laser, these cells had migrated into and repopulated the laser burn sites (Acott and Bacon, 1989). This provided further evidence that resident stem cells exist in the TM and suggested that they can be stimulated to repopulate an area of damaged tissue. Importantly, control eyes that did not receive laser showed a low but significant basal level of cell division in the same area of the meshwork, indicating that these cells play a role in normal repopulation of the TM if/when TM cells are lost. However, due to the simple fact that there is a reduction in TM cellularity in POAG eyes and with age, this ability to repopulate must be impaired with age and in certain conditions. Immunostaining studies on human eyes indicate that TMSC number is reduced with age (Sundaresan et al., 2019) and in POAG patients (Sundaresan et al., 2021), supporting the hypothesis that the loss of TM cellularity is associated with diminished cell repopulation capacity in addition to TM cell apoptosis and senescence in glaucomatous eyes (Baleriola et al., 2008; He et al., 2008; Liton et al., 2005). Nevertheless, we still do not currently understand all the factors that control cell repopulation ability, and this is a key research area.
In 2012, these TMSCs were further isolated and characterized. Investigators found that TMSCs isolated by clonal growth in vitro were positive for several stem cell markers that primary TM cells lack (Figure 2). Their stem cell character was confirmed by their ability to display phenotypic properties of cornea, adipose, and neural tissue under culture conditions that induce multipotent stem cell differentiation (Du et al., 2012). The multipotency of TMSCs has also been demonstrated in work investigating restoration of RGCs in various retinal conditions. Isolated TMSCs cultured on an amniotic membrane with various induction materials could be cultivated to express various photoreceptor genes which could potentially be used to treat retinal diseases such as age related macular degeneration and retinitis pigmentosa in the future (Nadri et al., 2013). It was also discovered that TMSCs could differentiate into TM cells with phagocytic function that expressed characteristic TM proteins in the presence of aqueous humor or bovine serum culture media (Du et al., 2012). Others demonstrated the stem cell properties of TMSCs after about eight years of cryopreservation. After thawing, all TMSC strains maintained viability and comparable proliferation, maintained stem cell markers at both the protein and gene levels, and could differentiate into osteocytes and neurons but not adipocytes. After induction for TM differentiation, the cells had increased expression of CHI3L1 and AQP1, two important markers for TM cells. More importantly, these induced TM cells were responsive to dexamethasone treatment with increased expression of MYOC and ANGPTL7 (Kumar et al., 2020b) which follows the consensus recommendations for TM cell identification (Keller et al., 2018). Together, these results confirmed that there is a resident population of stem cells in the human trabecular meshwork that can be isolated, expanded, and induced to differentiate into functional TM cells (and other differentiated cells) in vitro (Castro and Du, 2019; Du et al., 2012; Nadri et al., 2013).
FIGURE 2. Differential expression of stem cell (SC) and trabecular meshwork (TM) markers between TMSCs and primary TM cells.
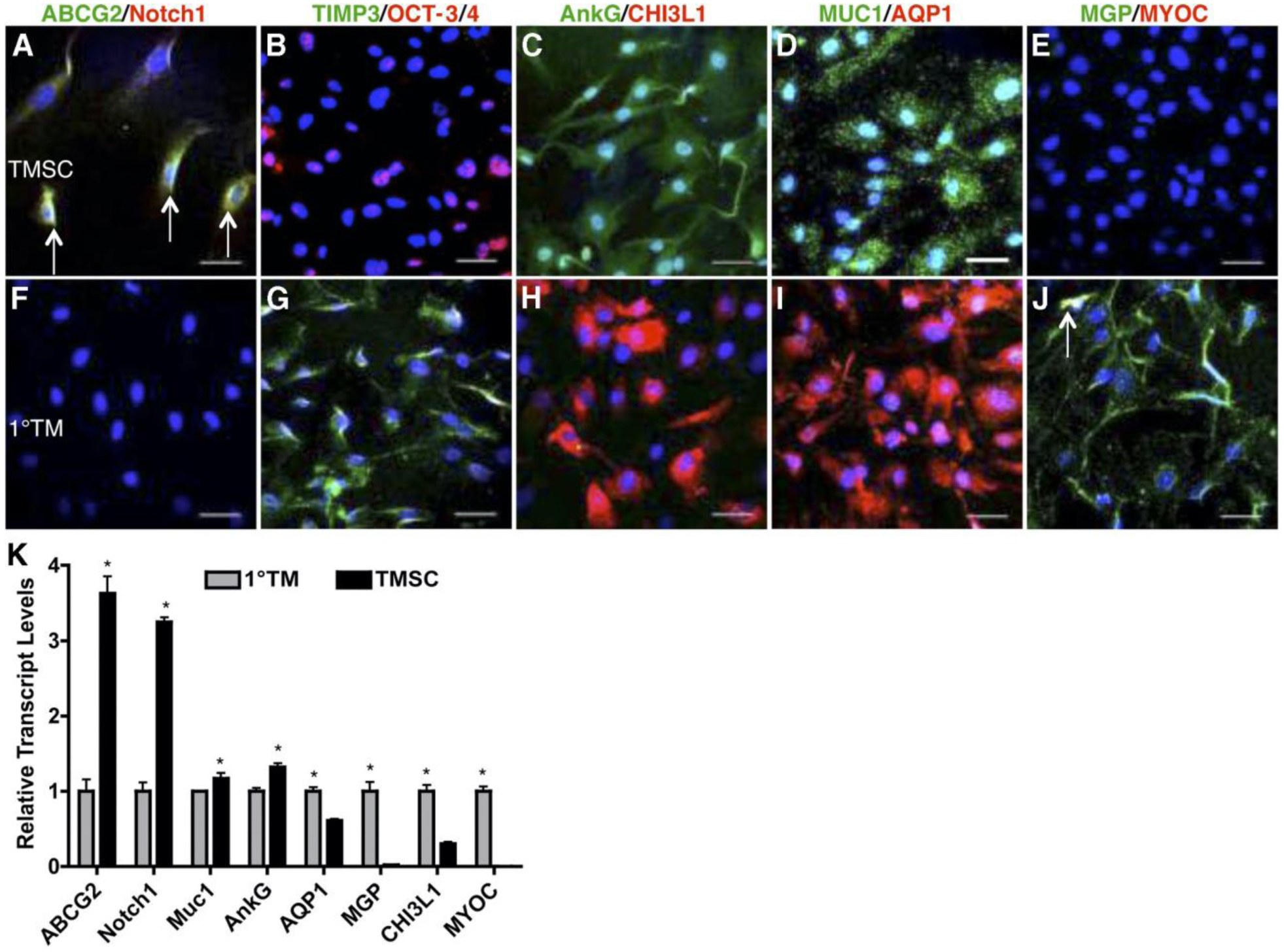
Clonal passaged TMSCs (A–E) and primary TM cells (F–J) were stained with the stem cell markers ABCG2 (green), Notch1 (red), OCT-3/4 (red), AnkG (green), MUC1 (green); and the TM markers TIMP3 (green), CHI3L1 (red), AQP1 (red), MGP (green); and MYOC (red). Arrows in (A) point to ABCG2 and Notch1 double-positive cells. Arrow in (J) points to a MGP and MYOC double-positive cell. Nuclei are stained blue with DAPI. Scale bars: 50 mm. (K) Quantification of expression of stem cell and TM markers from primary TM cells and clonal TMSCs at passage 4 by qRT-PCR. Error bars show SD of triplicate analyses. *P < 0.05 (n = 3, Student’s t-test). Adapted with permission from Du Y et al. Invest Ophthalmol Vis Sci. 2012;53:1566–1575.
In 2013, investigators discovered that human TMSCs expanded in vitro have the ability to differentiate into TM cells when transplanted in vivo in mouse models. Human TMSCs injected into mouse anterior chambers (AC) successfully integrated into the normal non-diseased mouse TM, did not initiate disorders of the eye, did not elicit an inflammatory response, and were viable within the eye for four months (Du et al., 2013). TMSCs share some characteristics with mesenchymal stem cells, such as similarities in cell surface marker expression (e.g. CD73, CD90, CD166 (Du 2021, IOVS)). Further, they share immunosuppressive properties (Du et al., 2009; Patel et al., 2008), which may explain why xenotransplantation elicited neither immunorejection nor inflammation. The hypothesis that restoring damaged TM with stem cells is a possible approach for regulating aqueous outflow through the TM was then further studied; laser photocoagulation was used to damage half of the TM circumference in mice; then, intracameral human TMSCs (laser-TMSC) versus fibroblasts (laser-Fibro) were introduced. Results showed that TMSCs preferentially homed to, and integrated into, laser-damaged TM (Figure 3). They also helped suppress inflammatory responses, reconstruct TM structure, and maintain normal IOP and outflow facility after laser photocoagulation. This was in contrast to the laser-Fibro mice that had significantly elevated IOP that persisted for up to four weeks. Importantly, integrated TMSCs expressed differentiated TM cell markers (AQP1 and CHI3L1) and expressed the chemokine pair CXCR4/SDF1 that plays a role in chemotaxis of TMSCs and TM cells in vitro (Yun et al., 2018). In a recent paper, human TMSCs were intracamerally transplanted into ocular hypertensive mice carrying a transgenic myocilin Y437H mutation, and were found to reduce IOP, restore outflow facility, increase TM cellularity, and reorganize the extracellular matrix, prevent retinal ganglion cell damage and preserve retinal ganglion cell function (Figure 4) (Xiong et al., 2021). Interestingly, in the myocilin model, repopulation of the TM after MSC delivery seems to be primarily due to resident stem cells, presumably “reactivated” by exogenous MSCs (Zhu et al., 2017). Why the resident progenitor cells cannot repopulate the TM normally is unclear, but we speculate that this may be due to these cells also producing the mutant form of myocilin in this model. Whether endogenous stem cells can repopulate the TM without exogenous stem cell transplantation in other circumstances is unknown and merits further study.
FIGURE 3. Human trabecular meshwork stem cells (TMSCs) home to laser-damaged trabecular meshwork (TM) regions after intracameral injection.
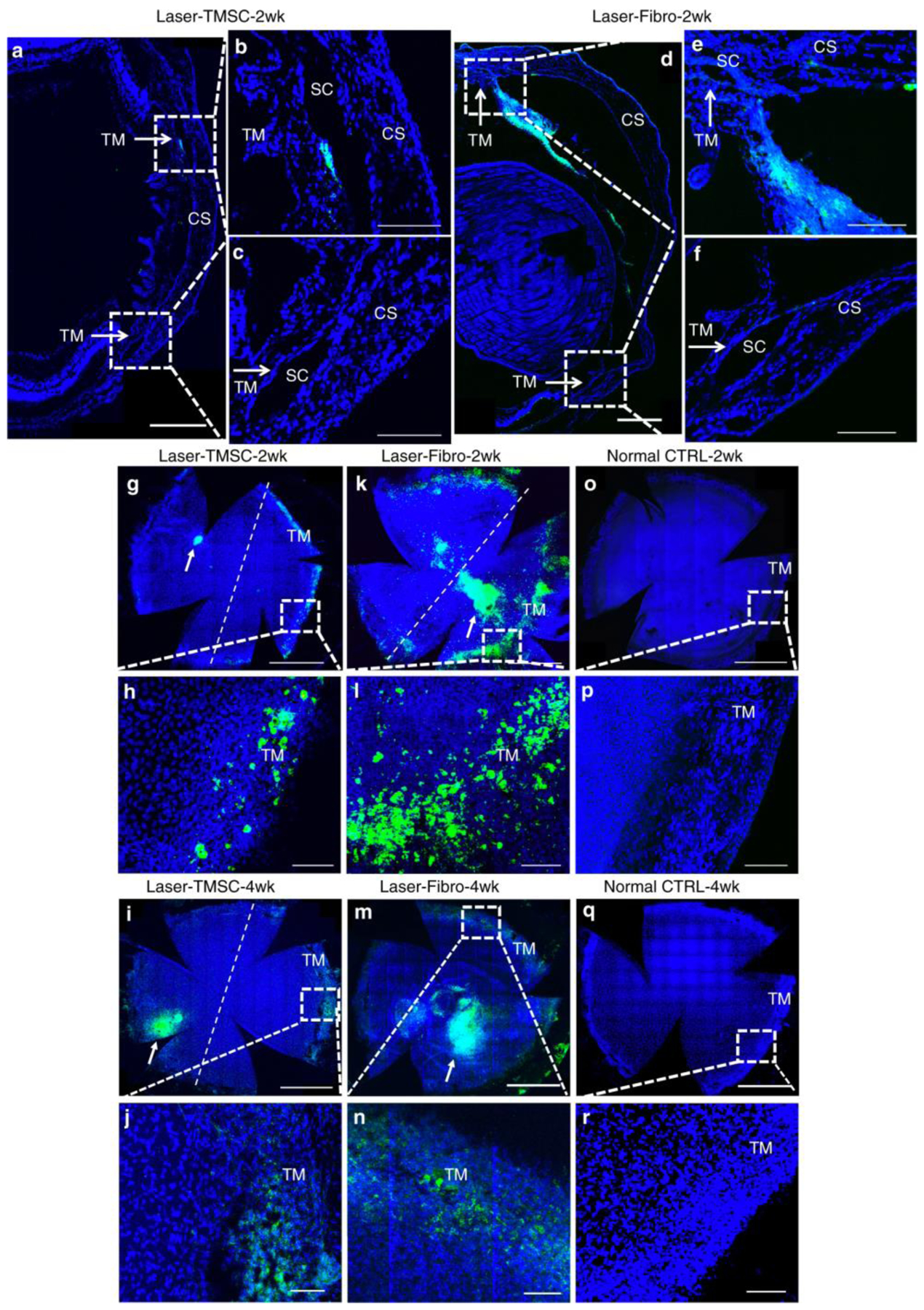
TMSCs and fibroblasts were prelabeled with DiO (green) prior to intracameral injection, following which sections and wholemounts were stained with DAPI (blue). Cryosections (a–f) show localization of injected TMSCs (a–c) or fibroblasts (d–f) 2 weeks after transplantation. Scale bars, 300 μm. b, c, e, f are magnifications of the boxed regions in a, e. b, e show laser-damaged regions, while c, f are unlasered regions. Arrows point to the TM. Scale bars, 100 μm. Wholemounts show distribution of transplanted TMSCs at 2 weeks (g, h) and 4 weeks (i, j), transplanted fibroblasts at 2 weeks (k, l) and 4 weeks (m, n), control eyes without cell transplantation (o–r). The right side of the dotted line is the laser-damaged region, whereas the left side is unlasered region. h, f, p, j, n, r are magnifications of the boxed regions in g, k, o, i, m, q. The green cell clusters on the corneas (white arrows in g, l, k, m) were injected cells healing corneal wounds caused by injection needles. Scale bars, 1 mm (g, k, o, i, m, q); 100 μm (h, f, p, j, n, r). Abbreviations: SC Schlemm’s canal, CS corneal stroma. Adapted from Yun, H., et al. Commun Biol 2018; 1(1): 216 under the Creative Commons Attribution 4.0 International (CCBY4.0) license.
FIGURE 4. Transplanted trabecular meshwork stem cells (TMSCs) reduce intraocular pressure (IOP), increase outflow facility, preserve retinal ganglion cell (RGC) function, and prevent RGC loss in Tg-MyocY437H mice.
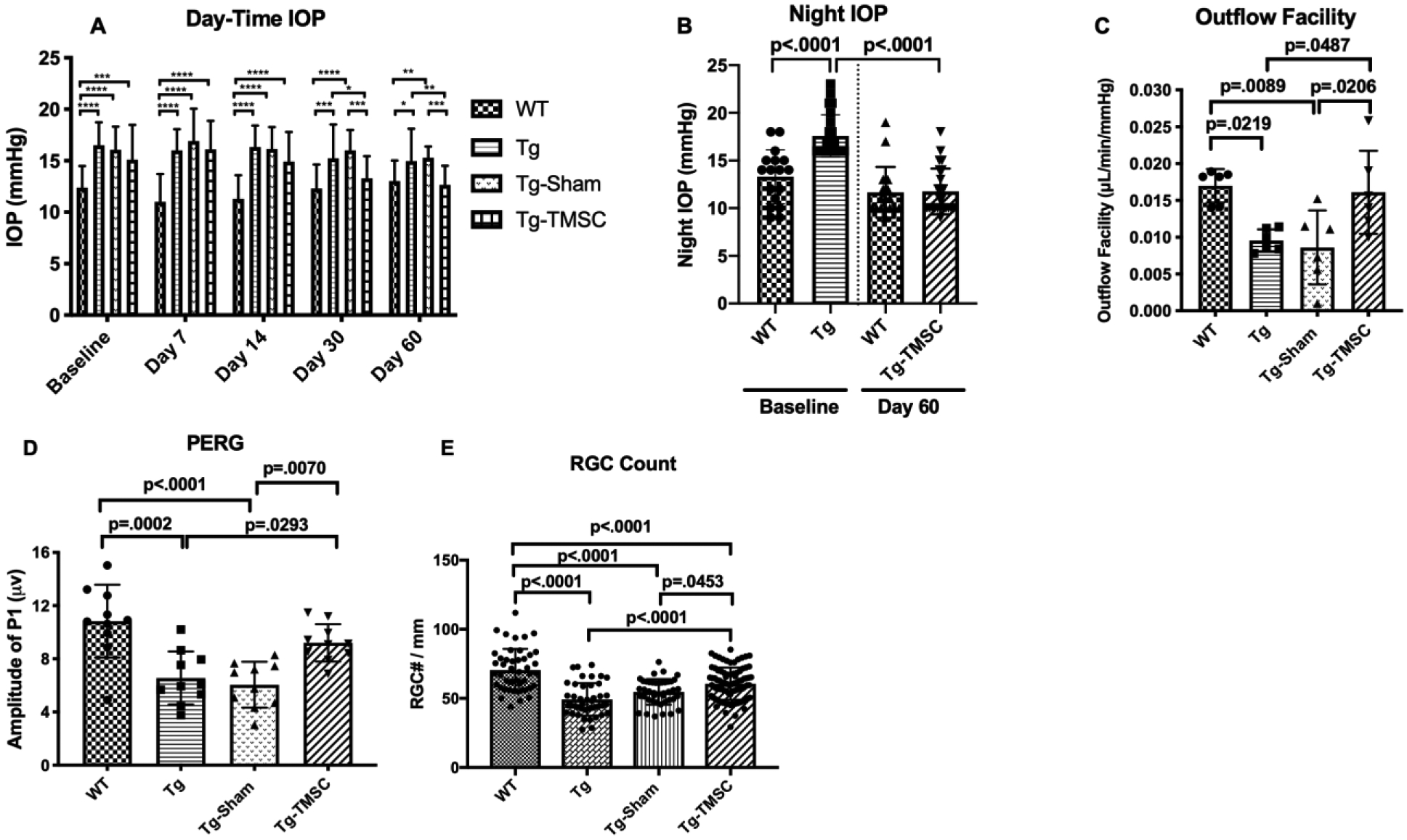
(A) Day-time IOP was measured in wildtype mice (WT, n=26), Tg-MyocY437H mice (Tg, n=26), Tg mice treated with basal medium (Tg-Sham, n=26) and Tg mice with TMSC transplantation (Tg-TMSC, n=26). (B) Night time IOP was measured in WT mice (n=17) and Tg-MyocY437H mice (n=24) before treatment and 2 months post treatment. Data are presented as mean ± SD. (C) Outflow facility was evaluated 2 months after TMSC transplantation (n=6 eyes/group). (D) Averaged P1 amplitude in pattern electroretinogram (PERG) (n = 10 eyes/group). (E) RGC numbers counted on the retinal sections in each group (n = 12–16 sections/eyes, and 4–6 eyes/group). Data are presented as mean ± SD. Two-way ANOVA (A) or one-way ANOVA (B,C,E) followed by Tukey’s multiple comparisons test. *p<0.05, **p<0.01, ***p<0.001, **** p<0.0001. Modified from Xiong et al. eLife, 2021, 10:e63677 under the Creative Commons Attribution License.
We have explored why TMSCs integrate into healthy TM. TMSCs have higher expression of CXCR4 while TM cells express more of SDF1. Chemokine pair CXCR4/SDF1 plays an important role for TMSCs to home to the TM cells. Further, we explored that a5b1 integrin plays an important role for TMSCs to integrate into the TM tissue (Xiong et al., 2020). Data from these studies suggests that cell-based therapy targeting the conventional outflow pathway may reduce IOP and possibly prevent optic nerve damage and vision loss.
1.4. Types of Stem Cells
Stem cells are characteristically capable of self-renewal, multilineage differentiation, and functional restoration of damaged tissue in vivo. They have also been proposed as mediators against oxidative stress, vascular insufficiency, and excitotoxicity (Pearson and Martin, 2015). Ocular stem cell research is particularly attractive in that the eye has features that can rapidly advance cell-based therapy in general: the eye is accessible for injection, allows direct visualization and monitoring, is immune privileged, and injection would theoretically have minimal systemic side effects (Roubeix et al., 2015; Streilein, 1990). Endogenous stem cells can be transplanted autologously and are less likely to face immune rejection (Pearson and Martin, 2015). Over the years, many have experimented with different types of stem cells for use in ocular regenerative medicine specifically for the treatment of glaucoma (Table 1).
Table 1:
Pros and Cons of Different Stem Cell Types
| Type of Stem Cell | Pros | Cons |
|---|---|---|
| Embryonic (ESC) |
|
|
| Induced pluripotent (iPSC) |
|
|
| Tissue specific/resident |
|
|
| Adult mesenchymal (MSC) |
|
Embryonic stem cells (ESC) were the first cell types to be identified as sources of stem cell therapy and are derived from the inner cell mass of blastocyst-stage embryos. These cells are pluripotent, have proliferative capacity, and can differentiate into all three embryonic germ layers. While these cells have great differentiation plasticity, they also have the most potential for tumor formation (Kelley et al., 2009; Prelle et al., 2002; Sun et al., 2015). This, in addition to the ethical issues associated with culturing cells from embryos and the concern for immune rejection of allogeneic transplants, currently limits clinical application of this cell type.
Induced pluripotent stem cells (iPSC) are obtained by reprogramming adult fibroblasts. This causes the cells to exhibit the morphology and properties of ESCs, while successfully avoiding the ethical and allogeneic issues of true ESC (Merkl et al., 2013; Yu et al., 2007). There are safety concerns that this cell type’s pluripotent reactivation could lead to tumorigenesis or teratoma formation (Sun et al., 2015). However, researchers have discovered ways to reduce this risk. For example, one group differentiated iPSC to TM cells then removed those still expressing markers of pluripotency prior to intracameral injection (Zhu et al., 2016). Others described a two-step induction of iPSC to neural crest cells to TM cells prior to use (Kumar et al., 2020a). It is also important to note that individual differences among donors have been found to be a major cause of transcriptional variation among heterogenous iPSC populations(Zhu et al., 2020).
Tissue-specific stem cells differentiate into the cells of that tissue and are less likely to trigger immune activation. As already discussed, much research has been done with native TMSCs. Because of their delicate location, harvesting enough of these cells from a living patient would be difficult. Furthermore, since patients with glaucoma have diseased TMs, it does not seem ideal to do autologous transplantation of their (presumably diseased or genetically mutated) TMSCs.
Adult mesenchymal stem cells (MSC) derived from bone marrow, umbilical cord, adipose tissue, skin, and amniotic fluid are multipotent, can develop into several cell types, and have a relatively large capacity for self-renewal (Kelley et al., 2009; Prelle et al., 2002; Zhou et al., 2020). These cells have also been shown to exert a neuroprotective effect on the central nervous system because they secrete neurotrophic factors and anti-inflammatory cytokines (Sun et al., 2015; Torrente and Polli, 2008). Recent studies showed that MSC transplantation protected against RGC degeneration in two rat models of ocular hypertension via their secretome (Harper et al., 2011; Johnson et al., 2010). We think that this type of stem cell shows great promise for the regeneration of the TM in glaucoma because of its secretome, multipotency, and decreased risk of immune rejection with autologous transplantation. However, the potential of MSCs for differentiation and proliferation may vary between different MSC sources due to the microenvironment in which they reside.
1.5. Trabecular Meshwork Regeneration by Stem Cells: Previous Models
Several studies in the last decade have shown that regeneration of the TM via stem cell therapy is a promising new method of reducing IOP, restoring damaged TM, and potentially preventing glaucomatous vision loss (Table 3) (Abu-Hassan et al., 2015; Du et al., 2013; Manuguerra-GagnÉ et al., 2013; Roubeix et al., 2015; Xiong et al., 2021; Yun et al., 2018, 2016; Zhou et al., 2020; Zhu et al., 2020, 2017, 2016).
Table 3:
Studies involving the use of different stem cells for glaucoma treatment
| Study | Cell Type | Origin | Model | Duration of Action | Pros | Cons | Reference |
|---|---|---|---|---|---|---|---|
| Use stem cells to treat Tg-MYOC Y437H POAG mouse model | TMSC | Human | Mouse Transgenic Age 4–6 months | 2 months | Precursor cell to cells being replaced or supported |
|
(Xiong et al., 2021) |
| Use stem cells in C57BL/6 WT mice | TMSC | Human | Mouse C57BL/6 WT (adult 10 weeks) | 4 months | Precursor cell to cells being replaced or supported |
|
(Du et al., 2013) |
| Use stem cells in laser photocoagulati on damaged mice | TMSC | Human | Mouse C57BL/6 WT (adult 10 weeks) | 4 weeks | Precursor cell to cells being replaced or supported |
|
(Yun et al., 2018) |
| Use stem cells to treat Tg-MYOC Y437H POAG mouse model | iPSC | Mouse | Mouse Transgenic Age 4 months | 9 weeks |
|
|
(Zhu et al., 2016) |
| Use stem cells to treat Tg-MYOC Y437H POAG mouse model | iPSC | Mouse | Mouse Transgenic Age 6 months | 3 months |
|
|
(Zhu et al., 2017) |
| Use stem cells in ex vivo human ocular perfusion organ culture system | iPSC | Human | Ex vivo human eyes Age 79.2 yrs +/− 14.6 | 2 weeks |
|
|
(Zhu et al., 2020) |
| Use stem cells in ex vivo human ocular perfusion organ culture system | iPSC | Human | Ex vivo human eyes using saponine to damage the TM cells | N/A |
|
|
(Abu-Hassan et al., 2015) |
| Use stem cells in C57BL/6 WT mice | adMSC | Human | Mouse C57BL/6 WT (adult 10 weeks) | 1 month |
|
Necessary to expand stem cells to achieve adequate number to transplant | (Zhou et al., 2020) |
| Use stem cells in laser-damaged ocular hypertension rat model | MSC | Mouse | Rat with laser damage to half circumferen ce of anterior chamber angle | 1 month |
|
Necessary to expand stem cells to achieve adequate number to transplant | (Manuguerr a-GagnÉ et al., 2013) |
| Use stem cells in vessel cauterized ocular hypertension rat model | MSC | Rat | Rat Long Evans WT with 3 cauterized episcleral veins | 1 month |
|
Necessary to expand stem cells to achieve adequate number to transplant | (Roubeix et al., 2015) |
Adapted and modified from (Mallick et al., 2021) under an open access Creative Commons CC BY 4.0 license.
TMSCs
Human TMSCs can be expanded in vitro and preferentially integrate into the TM region and become functional TM cells after transplantation into normal, non-diseased mouse anterior chambers in vivo. Du showed that all cells maintained viability, as demonstrated by no apoptotic cell staining by TUNEL assay at four months (Du et al., 2013, 2012). Researchers also discovered that TMSCs were able to reconstruct the TM, reduce IOP, and restore outflow facility after laser photocoagulation (Yun et al., 2018) and in a mouse glaucoma model based on overexpression of mutant myocilin (Y437H) (Xiong et al., 2021). After laser-induced damage, TM cell death was evident on transmission electron microscopy (TEM) as loss of nuclei and mitochondria, ruptured cell membranes, and disorganized ECM. After TMSC transplantation, cells were normal size and morphology compared to controls, and the ECM was reorganized. TMSCs are multipotent and appear able to differentiate into cells with phenotypes similar to native TM cells.
MSCs
To investigate whether bone marrow-derived mesenchymal stem cells could promote TM regeneration in an animal glaucoma model, rats were treated with 180 degrees of laser photocoagulation to their anterior chamber angle to induce ocular hypertension (laser treated mice vs. healthy control mice) (Manuguerra-GagnÉ et al., 2013). Injection of MSCs into the AC resulted in angle structure and histology that approximated a normal eye; in contrast, eyes that did not receive MSCs had scarring in the TM area (Figure 5). MSCs were found to specifically migrate to the area of laser damage, but also did not remain in the eye for long. These investigators hypothesized that tissue repair from MSCs was induced by secreted factors and activation of an endogenous repair mechanism rather than direct integration into host tissue, which only lasted four days (Manuguerra-GagnÉ et al., 2013).
FIGURE 5. Mesenchymal stem cells (MSCs) induce a rapid return to normal intraocular pressure (IOP) levels in experimental glaucoma.
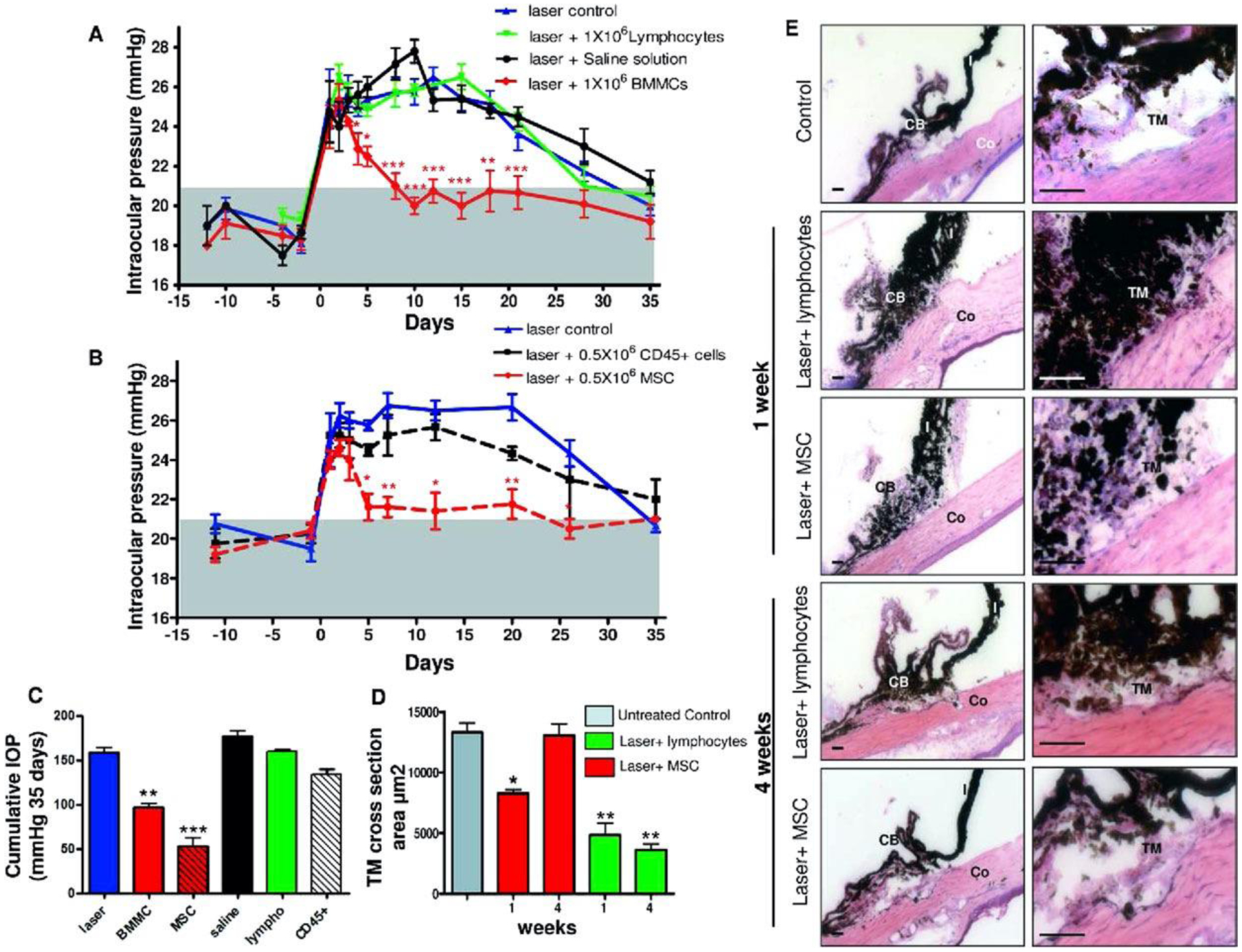
(A) Anterior chambers were injected either with 1 × 106 bone marrow-derived mesenchymal cells (BMMC, red), 1 × 106 lymphocytes (black), saline (green), or received no additional treatment (blue). The gray area represents IOP normal range. IOP was reported as mean ± SEM of four experiments (12 animals per group). (B) 0.5 × 106 CD45+ cells (black) or 0.5 × 106 MSC (red line) were injected intraocularly after laser exposure and IOP was evaluated as described above. We plot mean ± SEM of three experiments (9 animals per group). (C) Cumulative IOP exposure in eyes that received laser damage and injection of different cellular populations and controls. Cumulative IOP exposure is calculated as the temporal integral of IOP over the 4-week experimental period. (D) TM cross-sectional area from histologic sections for each experimental group (red, laser þ MSC group; green, laser þ lymphocyte group) and for a naïve TM (gray) (E) Representative images of hematoxylin-eosin (H&E) stained rat ocular anterior segments before (C: control) and 1 week after laser exposure alone or followed by MSC injection, as well as 4 weeks after laser damage alone or followed by MSC injection. Significance compared to laser control group: *, p<0.05; **, p<0.01; ***, p<0.001. Scale bar: 450μm. Abbreviations: CB, ciliary body; Co, cornea; I, iris. Reproduced with permission from Manuguerra-GagnÉ et al. Stem Cells. 2013;31:1136–1148. (license 1139632-2)
This work was furthered by Roubeix et al., who examined the molecular mechanisms of bone marrow-derived mesenchymal stem cells’ effect on TM protection and RGC loss. A model of ocular hypertension was induced in the right eye of rats by cauterization of three episcleral veins. As a control, the left eye of these rats underwent conjunctival dissection without episcleral vein cauterization. Several important findings came from this study: (1) Cauterization of three episcleral veins resulted in a significant increase in IOP as well as aqueous humor TGF-β2 levels (Figure 6). (2) Intracameral injection of MSCs into the episcleral vein cauterized (EVC) glaucoma model rats resulted in a significant decrease in IOP compared to controls (Figure 7). (3) Weeks after implantation, MSCs were located near the iridocorneal angle, on the corneal endothelium, and in the TM. (4) Peripheral RGC density in the ocular hypertensive eyes was higher in those treated with MSCs than in eyes not treated with MSCs. These findings confirmed that MSCs help reduce IOP in damaged TM eyes, can be found in the iridocorneal angle weeks after implantation, and may protect from peripheral RGC degeneration (Roubeix et al., 2015).
FIGURE 6. Effect of episcleral vein cautery on intraocular pressure (IOP) and levels of transforming growth factor beta 2 (TGF-β2) in aqueous humor.
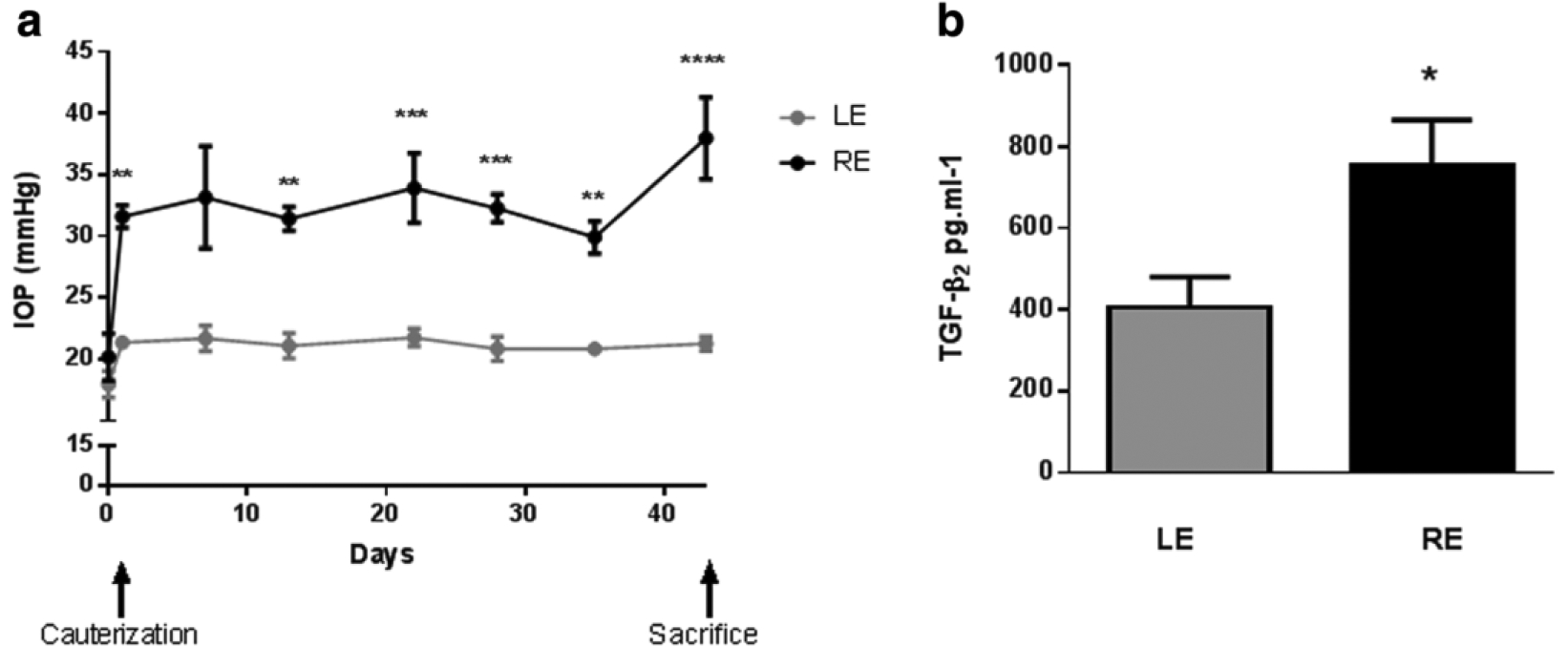
(a) IOP vs. time of right eyes (REs) having three episcleral veins cauterized (black symbols) and of untreated control left eyes (Les; grey symbols) over 44 days. (b) Aqueous humor levels of TGF-β2 from both cauterized (RE) and control (LE) eyes sampled 21 days post-cautery. *p <0.05, **p <0.01, ***p <0.001, ****p <0.0001 vs. control. . Reproduced under the Creative Commons CC BY license from Roubeix C, Godefroy D, Mias C, et al. Intraocular pressure reduction and neuroprotection conferred by bone marrow-derived mesenchymal stem cells in an animal model of glaucoma. Stem Cell Res Ther. 2015;6(1):177. doi:10.1186/s13287-015-0168-0.
FIGURE 7. Mesenchymal stem cell (MSC) transplantation decreases intraocular pressure (IOP) in a rat model of ocular hypertension.
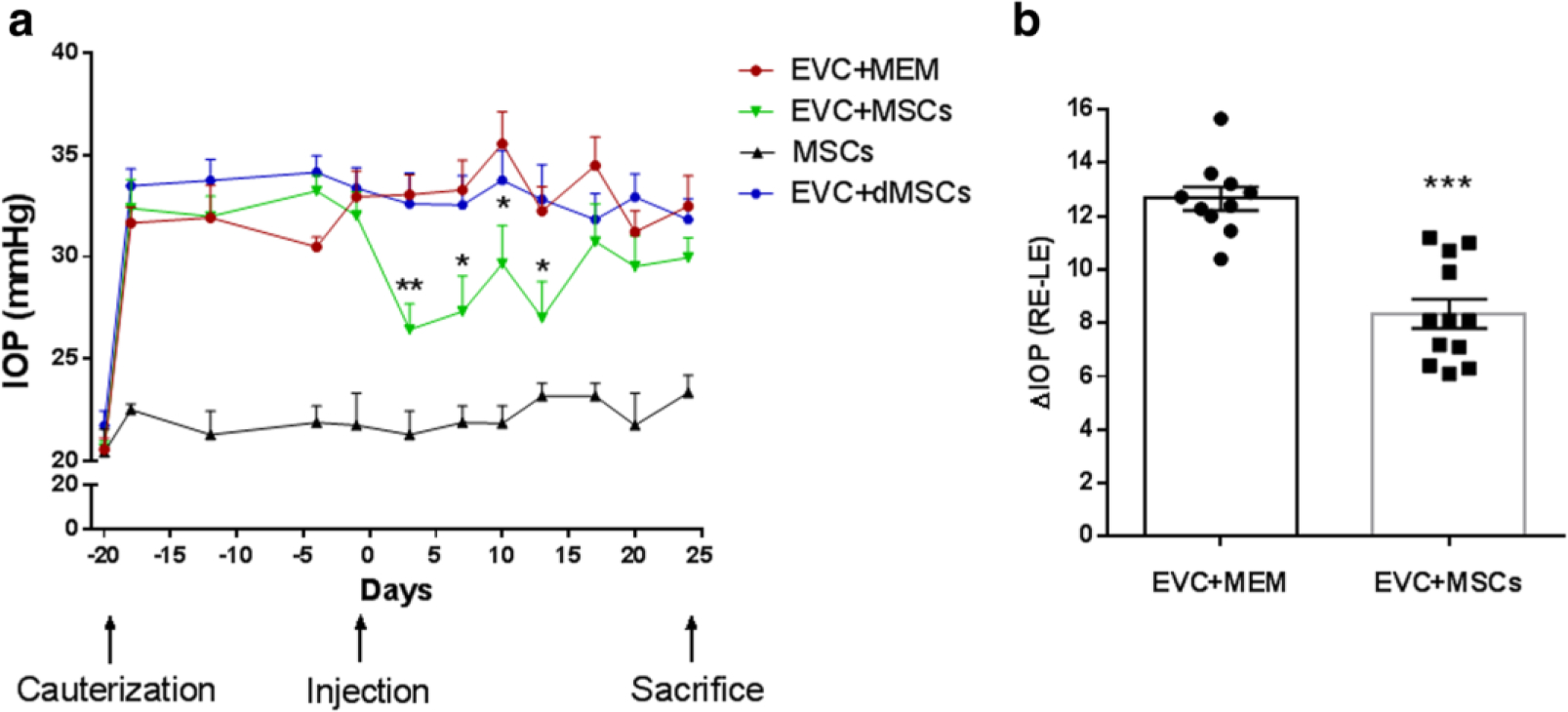
(a) IOP was monitored over time in both eyes of rats that received episcleral vein cauterization (EVC) (right eye (RE)) or not (left eye (LE)). IOP measurements in REs are reported for the following groups: eyes injected with 5 × 105 MSCs (EVC + MSCs, green symbols; n = 12), eyes injected with culture medium alone (EVC + MEM, red symbols; n = 10), or normotensive eyes injected with 5 × 105 MSCs (MSCs, black symbols; n = 5). (b) Individual average change in IOP (ΔIOP = RE IOP − LE IOP) from injection to sacrifice times for both EVC + MSCs and EVC + MEM groups. *p <0.05, **p <0.05, ***p <0.001 vs. EVC + MEM. dMSCs differentiated mesenchymal stem cells, MEM minimum essential medium. Reproduced under the Creative Commons CC BY license from Roubeix C, Godefroy D, Mias C, et al. Intraocular pressure reduction and neuroprotection conferred by bone marrow-derived mesenchymal stem cells in an animal model of glaucoma. Stem Cell Res Ther. 2015;6(1):177. doi:10.1186/s13287-015-0168-0.
Another arm of Roubeix et al.’s study investigated how MSCs affect IOP regulation through TM cell viability, contractility, and TM cell phenotype transition. Human TM cells (hTM) were exposed in vitro to cytotoxic levels of benzalkonium chloride (BAC), which induced TM cell death in a dose dependent manner. When co-applying MSC-cultured medium (MSC-CM) to hTM, there was a significant shift in the lethal concentration 50 (LC50) of BAC, suggesting a positive effect of MSC-CM on hTM cell viability. TM cell contractility in glaucoma involves TGF-β2 triggering phosphorylation of myosin fibers, inducing TM cell contraction, reduction of outflow, and IOP increase. hTM cells exposed to TGF-β2 and MSC-CM showed lower levels of phosphorylated myosin fibers than cells exposed to TGF-β2 alone, suggesting a positive effect of MSC-CM on reducing hTM cell contractility. It is thought that fibrosis in the TM in glaucoma may result from TM cell phenotype transition. hTM cells were exposed to TGF-β2, causing increased mRNA expression of specific ECM compounds. This expression was reduced when cells were exposed to TGF-β2 and MSC-CM together (Roubeix et al., 2015). These findings confirm that MSCs have valuable properties that allow for IOP regulation through the TM.
iPSCs
The ability of iPSCs to restore TM function has also been investigated. In 2015, it was shown that outflow pathway cell loss leads to elevated IOP and that replacement of lost cells with iPSCs restores IOP homeostasis (Figure 8) (Abu-Hassan et al., 2015). In this study, primary TM cells and anterior segments were isolated from porcine and human eyes. Human iPSCs were derived from dermal foreskin fibroblasts. To simulate TM cell loss, anterior segments were treated with saponin, a detergent that caused cell death with minimal outflow pathway disruption, simulating certain aspects of glaucoma. Partial repopulation of the damaged TM with cultured TM cells restored outflow pathway structure, providing more evidence that cellularity of the TM is key in maintaining IOP. Further, transplantation of differentiated TM-like iPSCs was able to restore IOP homeostasis. This work was important in its proof of the conceptual basis of stem cell therapy in TM regeneration. The ability of stem cells to restore TM homeostasis was confirmed by another study, whereby MSCs were delivered into a similar anterior segment glaucoma model with restoration of the TM’s capacity to adapt to an imposed pressure transient (Snider et al., 2021). It is important to observe that saponin treatment would be expected to damage both resident TM cells and any TM progenitor cells, which may complicate interpretation of the results of this model as compared to human glaucoma.
FIGURE 8. Replacement of endogenous saponin‐depleted human trabecular meshwork (HTM) cells with cultured HTM cells.
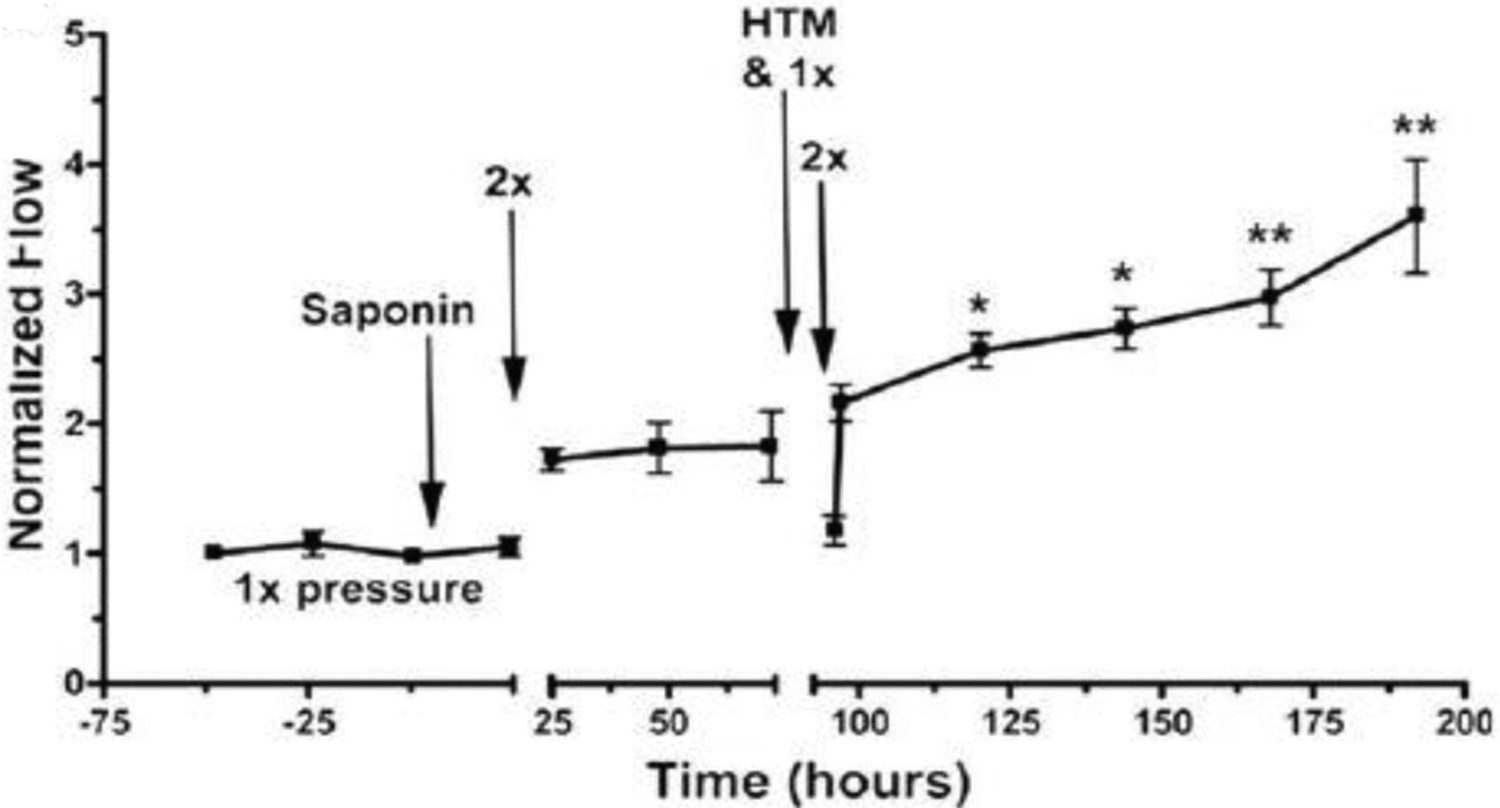
Transplanted replacement HTM cells (added at the time indicated) restored the intraocular pressure homeostatic response to 2x pressure elevation, which had been compromised by saponin-induced depletion of endogenous HTM cells. “1x” refers to imposition of normal perfusion pressure, while “2x” refers to a doubling of pressure. Flow has been normalized to baseline values. Data shown are mean for six experiments using separate anterior segments and error bars represent the SEM with significance by one-way ANOVA where * indicates p < .05 and ** indicates p < .001. Abu-Hassan et al. Stem Cells. 2015;33:751–761 with permission license # 1139632-3
iPSCs can be differentiated into a cell type that resembles primary TM cells (iPSC-TM) (Ding et al., 2014). These iPSC-TM cells were found to be morphologically similar to TM cells, to express many of the same proteins, and to functionally respond to various stimuli in a manner similar to TM cells. This work was furthered by transplanting iPSC-TM into mouse models of glaucoma to determine whether IOP reduction and prevention of RGC degeneration were possible. The study relied on the use of a transgenic mouse model of glaucoma whereby animals expressed human myocilin harboring a pathogenic mutation (Tg-MYOCY437H), causing endoplasmic reticulum stress, TM cellular dysfunction, and cell loss. However, importantly, the TM develops normally in these mice and disturbances in outflow dynamics only occur as animals age. Data demonstrated that transplantation of iPSC-TM into the eyes of 4-month-old Tg-MYOCY437H mice prevented the development of elevated IOP, preserved normal aqueous outflow for at least nine weeks, and prevented RGC loss. iPSC-TM cells also appeared to result in stimulation or proliferation of primary TM cells (Zhu et al., 2016). This suggested that functional renewal of aqueous outflow after degeneration was possible with iPSCs. This group then tested the same hypothesis in older mice (6-months-old) with more clearly reduced TM cellularity or later stage of disease. Results were positive - with improved outflow facility, lower IOP, and increased TM cellularity (Figure 9) (Zhu et al., 2017).
FIGURE 9. Effects of trabecular meshwork (TM) induced pluripotent stem cells (iPSC-TM) on intraocular pressure (IOP) and aqueous humor outflow in aged Tg-MYOC mice.
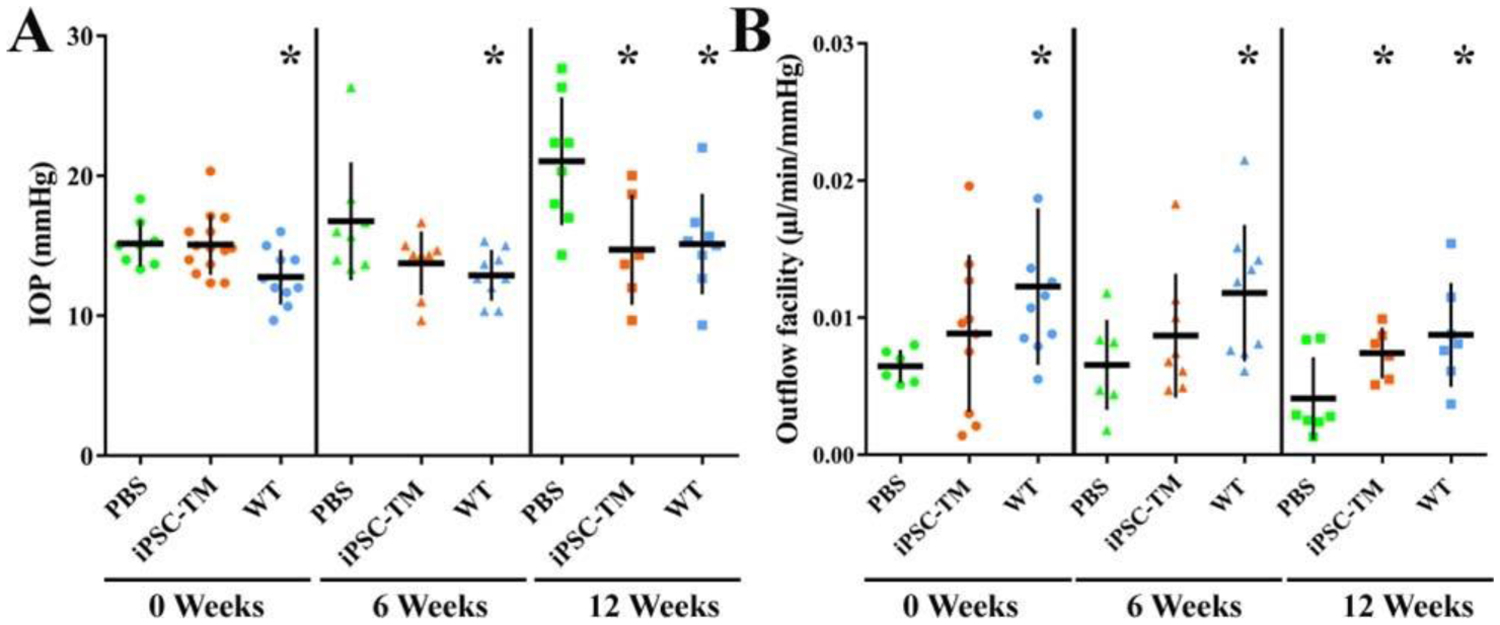
IOP (A) and outflow facility (B) were determined in PBS recipient controls, iPSC-TM recipients, and wild-type (WT) mice. Measurements were taken before transplantation (0 weeks), and 6 and 12 weeks after transplantation. *P < 0.05. Reprinted from Zhu et al. Invest Ophthalmol Vis Sci. 2017;58:2054–2062 under a CC BY license.
Zhu et al evaluated if transplantation of human donor derived iPSC-TM restores TM cellularity and function in human eyes. In this study, human iPSC were differentiated into Ipsc-TM, then injected into the anterior segments of human eyes maintained in perfusion culture. A Perfusion Organ Culture (POC) system allows for investigation of cellular responses as well as estimation of functional parameters like outflow facility. An important observation was that transplantation of iPSC-TM stimulated proliferation of endogenous TM cells, with fewer than 1% of all cells being iPSC-TM at the end of the experiment. The restored cellularity did not increase outflow facility in this study; however, the eyes used were without glaucomatous damage or elevated IOP (Zhu et al., 2020).
adMSCs
Investigators have recently investigated restoration of the TM with adipose derived mesenchymal stem cells (adMSCs). There are multiple approaches to quantify differentiation of adMSCs into TM-like cells. and they can be specifically delivered to TM tissue using magnetic nanoparticles (E. J. Snider et al., 2018; Eric J. Snider et al., 2018). Human adMSCs can differentiate into morphologic and functional TM-like cells with the ability to maintain aqueous humor dynamics in mouse models in vivo (Zhou et al., 2020). Since adMSCs are autologous and can be obtained in large quantities with minimally invasive procedures, these stem cells may be ideal candidates for future clinical studies investigating restoration of the TM in glaucoma.
1.6. Future Directions
While the results of current animal studies on the effectiveness of stem cell therapy for glaucoma have been very promising in rodent and ex vivo models, testing stem cell therapies in larger animals that are physiologically closer to humans (e.g. non-human primates) would be ideal. Unfortunately, such testing is severely limited by a lack of suitable non-human primate models, where the most common model uses laser photocoagulation of the monkey TM to elevate IOP. Although this model is appropriate for studying the effects of ocular hypertension on retinal ganglion cells, it is a poor analogue of the TM changes that occur in human POAG, thus limiting the relevant pre-clinical functional information that it can provide. Of course, there exists other non-rodent POAG models, including steroid-induced glaucoma in dogs, cows, and sheep (Candia et al., 2010; Gelatt and Mackay, 1998; Gerometta et al., 2004); laser photocoagulation, iridocorneal angle obstruction using autologous red blood cells or microbeads in rabbits and monkeys (Ngumah et al., 2006; Ollivier et al., 2004; Quigley and Addicks, 1980; Toris et al., 2000; Weber and Zelenak, 2001); and a mutation in LTBP2 in cats (Kuehn et al., 2016); however, these models all suffer drawbacks, primarily poor fidelity to human POAG pathophysiological changes in the TM and/or incomplete characterization. Further, it is not known whether other forms of glaucoma aside from POAG are associated with a loss of TM cells, and thus whether restoration of normal TM cellularity would be beneficial in these conditions. Thus, the myocilin mutant mouse model is likely the best current model for testing stem cell therapy for the TM, since it is relatively well-characterized and replicates important aspects of human POAG: IOP elevation, TM cell loss, ECM changes in the TM, as well as retinal ganglion cell loss. In summary, insights from non-rodent models into the therapeutic benefits of stem cell treatments are expected to be limited, although the laser TM photocoagulation monkey model can be beneficial for providing further safety data.
This scarcity of animal models is not limited to glaucoma. Diseases such as Alzheimer’s disease, Parkinson’s disease, ischemic stroke, amyotrophic lateral sclerosis and cancer lack an animal model that captures all the aspects of the disease, in part due to the complexity of the pathology, comorbidities, and age-related co-factors (Dawson et al., 2018; Mak et al., 2014; Pound and Ritskes-Hoitinga, 2018; van der Worp et al., 2010). Therefore, transgenic mouse models that better represent the complexity of the disease are heavily relied on for preclinical assessment of safety and effectiveness of the treatments (Cook et al., 2012; Dawson et al., 2018; Epstein et al., 2017).
Stem cells have introduced exciting new possibilities for the potential treatment of glaucoma. Research over the last few decades has investigated various types of stem cells and their ability to restore aqueous humor outflow facility through TM cell regeneration. It has been demonstrated that stem cells (i.e., native TMSCs, adipose derived MSCs, bone marrow derived MSCs, iPSCs) can be differentiated into TM-like cells, can be safely implanted into/home to the TM in mouse models in vivo, and can confer functional benefit by restoring IOP homeostasis. It has also been shown that these cells remain stable for a significant period without serious side effects. We propose a human clinical study as the next step to further the progress of this novel treatment paradigm.
1.6.1. Proposed Phase I Clinical Study
1.6.1.1. Type of Stem Cells
After review of the current evidence, we suggest that further investigation of adMSCs in human clinical studies of trabecular meshwork regeneration would yield promising results. Although the eye is thought to be an immune-privileged site, using autologous stem cells is preferred and seems optimal to decrease risk of immune rejection. Adipose stem cells are readily available and can be obtained easily with minimally invasive procedures. Further, and importantly, there is less risk of tumorigenesis with MSC use (Musiał-Wysocka et al., 2019).
Use of iPSCs would be attractive from a clinical convenience and commercial standpoint, in that such cells could be readily available from stem cell facilities. While not autologous, and therefore carrying the associated risks, iPSCs obtained in this way would be less expensive to produce and use than adMSCs or other stem cells requiring extraction from the patient, purification and expansion. Such iPSCs could be the basis of a second arm in a clinical study.
There is also a possibility of cell-free treatment, which could further reduce patient risk. In recent work, investigators discovered that secretome derived from human TM stem cells reduces intraocular pressure as well as restores TM homeostasis and RGC function in both steroid-induced and genetic myocilin mutant mouse models of glaucoma. This could be the basis of a third arm in a clinical study (Kumar et al., 2021).
1.6.1.2. Potential Side Effects
The incidence of tumor or teratoma formation in clinical trials of stem cell therapy are rare. The safety of mesenchymal stem cells (MSC) is evident by their use in more than 1000 clinical trials, where systematic reviews of the published results from these clinical trials showed no evidence of tumorigenicity (Lalu et al., 2012; Rodriguez-Fuentes et al., 2021). In rare instances where malignant tumor formation of MSCs in culture has been reported, the studies have later been retracted due to cancer cell cross-contamination (Rosland et al., 2009; Rubio et al., 2005). One study reported a 4% possibility for genetic aberrations in adult human mesenchymal cells in culture, but these results are controversial (Ben-David et al., 2011; Sensebe et al., 2012). As a result, MSCs are considered to be safe for clinical applications and extremely unlikely to form tumors (Lukomska et al., 2019; Neri, 2019; Vitale et al., 2017).
Such intrinsic tumor-resistance does not necessarily apply to iPSCs, where concerns about tumorigenicity were more common in the past (Lamm et al., 2016; Neri, 2019; Vitale et al., 2017). Such tumorigenicity was largely attributed to the oncogenic factors used for induction of pluripotency, remnant undifferentiated stem cells in the colony, and genetic aberrations caused by prolonged in vitro expansion. To overcome these problems, methods such as non-integrating vectors are used to minimize genomic disruption during induction. Proper purification using methods such as fluorescent activated cell sorting (FACS) and magnetic activated cell sorting (MACS) have also been shown to significantly increase the quality of transplants (Lee et al., 2013; Yamanaka, 2020). Thus, the first clinical trial for transplantation of iPSCs-derivatives has reported for a patient with age-related macular degeneration (Cyranoski, 2014) and iPSC-derived adult cells have subsequently been used in clinical trials targeting ocular diseases, cardiomyopathy, spinal cord injury (Cyranoski, 2019; Mitton, 2019). Encouragingly, a recent systematic review of clinical trials that have used iPSC-derivatives has reported no sign of tumorigenicity in humans (Deinsberger et al., 2020); however, the published results are scarce and recent, and more evidence is needed to verify safety.
1.6.1.3. Characterization of Cells
Production and manufacturing of cell-based therapies is subject to standardized quality systems regulated by good manufacturing practice (GMP) rules. In 2006, the International Society for Cellular Therapy (ISCT) proposed a set of standards to define MSCs for both laboratory-based scientific investigations and pre-clinical studies. The three criteria to define MSC included: adherence to plastic, specific surface antigen (Ag) expression, and multi-potent differentiation potential. Standards for surface Ag expression were that >95% of the MSC population express CD105, CD73, and CD90 and <2% express CD45, CD34, CD14 or CD11b, CD79a or CD19. The cells must also be able to differentiate to osteoblasts, adipocytes, and chondroblasts (Dominici et al., 2006) Other than appropriate identification of these cells, critical quality attributes of the product such as safety, identity, and purity must be ensured. Safety comes from the demonstration that the product does not contain bacteria, fungi, and/or viruses; identity refers to the presence of a substance that consists of a specific phenotypic and genotypic definition; purity demonstrates that the product contains a high concentration of the desired substance, free from unwanted cell populations (Viganò et al., 2018). It would be critical to ensure that stem cells taken from patients meet these GMP standards of characterization.
In 2016, a protocol was developed for identifying clinical-grade adMSCs and validating new biomarkers for adMSC characterization that can guide release criteria (Table 2). In brief, the results were as follows: (1) adMSCs expressed classical markers including CD73, CD90, CD105, CD44, and HLA-ABC, and lacked CD14, CD45, and HLA-DR. (2) Nine other non-classical markers (positive for CD248, CD36, CD140B, CD271, CD273; negative for CD163, CD146, CD200, CD 274) were able to distinguish adMSCs from other cell types and provide insight into the biological function of these cells. (3) Markers are expressed irrespective of manufacturing conditions (i.e., fresh expansion; cryopreserved, thawed, and immediately administered; cryopreserved and allowed to recover in culture for up to 4 days). However, recovery in culture may allow optimal surface antigen expression. (4) Expansion in human platelet lysate (hPL), which supports proliferation and genomic stability, is achievable and optimal (Camilleri et al., 2016). Based on these results, we would feel comfortable cryopreserving our patients’ stem cells (if needed) but would allow recovery for up to 4 days prior to use. We would also expand the cells in hPL rather than fetal bovine serum (FBS) culture media to avoid potential zoonotic pathogens.
Table 2:
Protocol for identifying clinical grade adipose derived mesenchymal stem cells based on surface antigen expression
| Classical markers | Non-classical markers |
|---|---|
| CD73, CD90, CD105, CD44, HLA-ABC positive | CD248, CD36, CD140B, CD271, CD273 positive |
| CD14, CD45, HLA-DR negative | CD163, CD146, CD200, CD 274 negative |
In recent work, adMSCs could be induced to differentiate into TM-like cells with characteristics of TM cells, including response to dexamethasone (DEX) stimulation, phagocytic activity, and ability to maintain aqueous outflow (Zhou et al., 2020). adMSCs were induced towards TM phenotypes using three different conditions: coculture with TM cells, exposure to ECM and conditioned media from TM cells (ECM+CM), and exposure to ECM from TM cells and culture in advanced MEM (ECM+AdvM). After 10 days, decreased expression of the stem cell marker OCT4 and increased expression of TM cell markers CHI3L1 and AQP1 were noted, similar to levels in primary TM cells. Another important characteristic of TM cells is their response to DEX exposure, including formation of CLANs and upregulation of myocilin. Cells cultured in ECM+CM had comparable percentages of CLANs and appropriate upregulation of myocilin compared with primary TM cells. This study also demonstrated that human adMSCs homed to wildtype mice TM tissue after intracameral injection and did not adversely affect IOP for up to 30 days after xenotransplantation. Based on these results, our study would differentiate adMSCs using ECM+CM media (Zhou et al., 2020).
1.6.1.4. Delivery
In studying stem cells for TM regeneration, it would seem best to deliver the SCs intracamerally, which is facilitated by natural flow patterns, as well as past work that has suggested an innate chemotaxis mechanism (CXCR4/SDF1) for homing of stem cells to the TM.
The main outcome parameter will be IOP and outflow facility. Inflammation and visual acuity will be monitored, and optical coherence tomography (OCT) will be used to monitor TM, SC and outflow channel structures. It would be possible to use GFP to assess integration anatomically, but that risks unintended effects on Schlemm’s canal and therefore is less desirable. A consortium would be well suited in order to provide the many necessary skill sets necessary for such a study. Expertise will be required in stem cell biology, acquisition, expansion and transplantation, aqueous outflow physiology and pathophysiology, ocular perfusion for assessment of outflow function/facility, Good Manufacturing Practice and other regulatory matters, clinical study design and conduct and clinical ophthalmology, among other areas. Difficulties can present in each of the areas above and skills and experience in each of these domains will be necessary for a successful clinical trial.
For the transplanted cell numbers in mice, we did optimization before our animal experiments. For the purpose of clinical trials, more detailed optimization of transplanted cells will be needed. Different cell numbers will be injected and long-term effects and side effects in mice (6 months to 1 year) will be discovered. Further, an animal model with similar size to humans, such as rabbit or monkey, will be needed to optimize the transplanted cell number based on the mouse results.
1.6.1.5. Potential Complications Related to Delivery
Stem cell therapies for TM restoration and glaucoma will use clinical outputs specific to the TM. However, a brief review about the recent advances in adMSC and iPSC-derived cell therapies for other ocular pathology remains useful to better understand the safety of such treatments in clinical trials of other ocular diseases.
Suprachoroidal injection of a mixture of adMSCs, stromal vascular fractions and platelet-rich plasma in 12 eyes in patients with dry AMD showed a significant increase in the scotopic but not in the photopic ERG thirty days after transplantation – indicating no change in foveal activity (Limoli et al., 2014). A follow-up study of 36 eyes showed a statistically significant improvement in best corrected visual acuity (BCVA) and improvement of visual performance in 53% of the eyes and no change in 39%. However, details of visual performance assessment are not well documented in the paper (Limoli et al., 2016). This group applied the same treatment to 34 eyes of patients with retinitis pigmentosa (RP) and observed no significant improvement in BCVA (Limoli et al., 2020, 2019). No adverse event was reported in any of these four studies.
Oner et al. have reported subretinal implantation of adMSCs in 11 patients with RP, resulting in the improvement of visual acuity from 20/2000 to 20/200 in just one case. Five patients experienced epiretinal membrane formation at the site of transplantation and choroidal neovascular membrane was observed in one patient. The authors report no evidence of tumorigenicity, graft rejection or systemic symptoms associated with the implant (Oner et al., 2016). By changing their approach from subretinal to suprachoroidal delivery of adMSCs, this group subsequently achieved improved visual acuity and visual fields in all patients with optic atrophy, dry AMD and Stargardt’s macular dystrophy within 6 months post treatment (4 patients in each disease group) with no systemic or ocular complications (Oner et al., 2019, 2018).
In 2014, Takahashi et al. transplanted autologous induced pluripotent stem cell-derived retinal pigment epithelium (RPE) in a patient with wet AMD. Four years later, disease progression was stopped and visual acuity was stabilized without additional anti-VEGF treatment but did not improve. The study reported no sign of graft rejection or adverse events (Mandai et al., 2017; Takagi et al., 2019). In another clinical trial this group used HLA-matched allogenic iPSC-derived RPE cells as a more clinically practical approach in five patients with wet AMD (Sugita et al., 2020). Only one of the patients showed mild immune rejection which was controlled by local steroid administration. Additional adverse events included ERM formation, retinal edema, and IOP elevation, all of which were managed by standard interventions. No abnormal growth of transplanted cells is reported and graft survival was confirmed one year after surgery. The study does not provide evidence of improvement in disease progression or visual acuity.
The most significant adverse event reported for adMSC transplantation in the eye occurred in a study on three patients who received intravitreal adMSC injection (Kuriyan et al., 2017). Sadly, this treatment resulted in vision loss in all patients, as well as ocular hypertension, retinal hemorrhage, vitreous hemorrhage, and retinal detachment. We note that these patients were treated with no regulatory input from the FDA which makes clinical interpretation of these results more complex.
While the studies discussed above provide examples of the effectiveness and potential complications associated with using adMSCs and iPSC-derived cells in ophthalmologic diseases, it should be noted that the effectiveness of the discussed methods seem to depend substantially on the surgical method used, and complications are specific to retinal diseases. There is no evidence of adverse effects associated with the general use of stem cell transplants.
The current literature we have reviewed proposes two methods of delivery for stem cells. While most of the studies rely on the hydrodynamic forces of aqueous humor for “passive” delivery of cells to the TM or “active” homing by stem cell themselves involving in a specific chemokine pair CXCR4/SDF1 (Yun et al., 2018; Zhou et al., 2020), Snider et al. used magnetically labeled stem cells for delivery under an external magnetic field (E. J. Snider et al., 2018). This approach is intended to avoid non-uniform delivery of cells caused by increased segmental flow in glaucomatous eyes and to reduce the off-target delivery to non-TM tissues in the anterior segment. The most widely used nanoparticles for stem cell labeling are superparamagnetic iron oxide nanoparticles (SPIONs), which have been used to deliver and label stem cells in multiple clinical trials (Bulte and Daldrup-Link, 2018; Karussis et al., 2010; Ma et al., 2020; Mathiasen et al., 2019). While originally several SPIONs were approved by FDA, most of these products were later discontinued. Currently, ferumoxytol is the only FDA-approved SPION. Unfortunately, we could not find a clinical trial with published results which utilized ferumoxytol-labeled cells and, therefore, the inclusion of magnetic nanoparticles in a clinical trial would require additional data on safety and effectiveness.
1.6.1.6. Structure of Study
To reduce risk to the subjects, we propose that the study population for an initial stem cell-based TM regeneration trial include blind (no light perception) eyes from people with open-angle glaucoma. It would be most attractive to include subjects with eyes that are planned to be enucleated due to their underlying pathology in 1–2 months. This would allow a short term follow up to evaluate the effects of the stem cells on TM and ocular anatomy and physiology, specifically with regard to TM regeneration, aqueous outflow and extraneous migration and implantation of the injected stem cells. This approach would also permit investigators to follow long term effects in subjects post stem cell injection with a lower risk of non-ocular effects for the patient. Additionally, impending enucleation would reduce the consequences of an unanticipated complication such as tumorigenesis, infection, or rejection. Unfortunately, it is likely that such an approach of using only eyes shortly to undergo enucleation would severely hamper enrollment in such a study and would create an unworkably long enrollment period. For this reason, we recommend including such subjects opportunistically to whatever extent possible, but not limiting enrollment to subjects for whom enucleation is planned. It would also be beneficial to evaluate TM function after transplantation of stem cells; clinically, this can be done using tonography and possibly also by extensions to OCT-based imaging methods to determine TM biomechanical properties, related to TM function (Li et al., 2021, 2019; Wang et al., 2017a). Such translation of these OCT-based technologies is currently under development.
1.7. Cell-Based Regeneration of the Trabecular Meshwork versus Other Therapeutic Methods
Clinically approved methods of lowering intraocular pressure through the conventional outflow pathway include some topical medications, laser trabeculoplasty, and surgical procedures that directly target the TM and Schlemm’s canal. Other experimental methods such as small interfering RNA (siRNA) therapies that target the ß2-adrenergic receptors at the ciliary body and viral vectors that target diseased genes within the TM are being investigated. New classes of medication with different mechanisms of action are likely soon to come (Kaufman and Rasmussen, 2012; O’Callaghan et al., 2017). However, stem cell regeneration of the trabecular meshwork is unique in the direct target of diseased tissue.
1.8. Conclusion
Glaucoma patients have reduced trabecular meshwork cellularity, which associates with elevated intraocular pressure. Research over the years has demonstrated that stem cell therapy has the potential to regenerate damaged trabecular meshwork. Adipose-derived mesenchymal stem cells have a favorable safety profile with minimal risk of tumorigenesis, are multipotent, and readily available. Induced pluripotential stem cells are readily available and relatively inexpensive but are not autologous. Both cell types have been shown to differentiate into TM-like cells, integrate into TM tissue, and maintain normal IOP. Transplantation of human stem cells to restore TM function in glaucomatous eyes is a potentially vision saving, revolutionary treatment that could impact the lives of millions.
Article Highlights.
Cellularity of the TM is decreased in eyes with glaucoma.
Decreased cellularity of the TM correlates with elevated intraocular pressure.
Human stem cell transplantation in glaucomatous eyes can restore IOP homeostasis.
We propose investigating transplantation of adipose-derived mesenchymal stem cells (adMSCs) and induced pluripotent stem cells (iPSCs) into eyes with glaucoma.
Acknowledgments
Supported in part by an unrestricted grants to NYU Langone Health Department of Ophthalmology from Suzie Welch (New York, NY) and Research to Prevent Blindness (RPB, New York, NY), NIH grants R01 EY025643 (YD), EY030071 (CRE) and the Georgia Research Alliance (CRE).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
CRediT author statement
Sara J. Coulon, MD: Conceptualization; Investigation; Methodology; Visualization; Roles/Writing - original draft; Writing - review & editing
Joel S. Schuman, MD: Conceptualization; Investigation; Methodology; Project administration; Resources; Supervision; Validation; Visualization; Roles/Writing - original draft; Writing - review & editing
Yiqin Du, MD, PhD: Conceptualization; Investigation; Methodology; Visualization; Roles/Writing - original draft; Writing - review & editing
Mohammad Reza Bahrani Fard, PhD: Writing - review & editing
C. Ross Ethier, PhD: Conceptualization; Investigation; Methodology; Visualization; Roles/Writing - original draft; Writing - review & editing
W. Daniel Stamer, PhD: Conceptualization; Investigation; Methodology; Visualization; Roles/Writing - original draft; Writing - review & editing
Disclosures:
Authors report no relevant conflicts of interest
References
- Abu-Hassan DW, Li X, Ryan EI, Acott TS, Kelley MJ, 2015. Induced Pluripotent Stem Cells Restore Function in a Human Cell Loss Model of Open-Angle Glaucoma: iPS Cells for Glaucoma. Stem Cells 33, 751–761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Acott TS, Bacon DR, 1989. Trabecular Repopulation by Anterior Trabecular Meshwork Cells After Laser Trabeculoplasty. Am. J. Ophthalmol 6. [DOI] [PubMed] [Google Scholar]
- Acott TS, Kelley MJ, Keller KE, Vranka JA, Abu-Hassan DW, Li X, Aga M, Bradley JM, 2014. Intraocular Pressure Homeostasis: Maintaining Balance in a High-Pressure Environment. J. Ocul. Pharmacol. Ther 30, 94–101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alvarado J, Murphy C, Juster R, 1984. Trabecular Meshwork Cellularity in Primary Open-angle Glaucoma and Nonglaucomatous Normals. Ophthalmology 91, 564–579. [DOI] [PubMed] [Google Scholar]
- Alvarado J, Murphy C, Polansky J, Juster R, 1981. Age-related changes in trabecular meshwork cellularity 14. [PubMed] [Google Scholar]
- Baleriola J, García-Feijoo J, Martínez-de-la-Casa JM, Fernández-Cruz A, de la Rosa EJ, Fernández-Durango R, 2008. Apoptosis in the trabecular meshwork of glaucomatous patients. Mol. Vis 14, 1513–1516. [PMC free article] [PubMed] [Google Scholar]
- Barnebey HS, Robin AL, 2017. Adherence to Fixed-Combination Versus Unfixed Travoprost 0.004%/Timolol 0.5% for Glaucoma or Ocular Hypertension: A Randomized Trial. Am. J. Ophthalmol 176, 61–69. [DOI] [PubMed] [Google Scholar]
- Ben-David U, Mayshar Y, Benvenisty N, 2011. Large-scale analysis reveals acquisition of lineage-specific chromosomal aberrations in human adult stem cells. Cell Stem Cell 9, 97–102. 10.1016/j.stem.2011.06.013 [DOI] [PubMed] [Google Scholar]
- Braunger BM, Ademoglu B, Koschade SE, Fuchshofer R, Gabelt BT, Kiland JA, Hennes-Beann EA, Brunner KG, Kaufman PL, Tamm ER, 2014. Identification of Adult Stem Cells in Schwalbe’s Line Region of the Primate Eye. Invest. Ophthalmol. Vis. Sci 55, 7499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bull ND, Irvine K-A, Franklin RJM, Martin KR, 2009. Transplanted Oligodendrocyte Precursor Cells Reduce Neurodegeneration in a Model of Glaucoma. Invest. Ophthalmol. Vis. Sci 50, 4244. [DOI] [PubMed] [Google Scholar]
- Bulte JWM, Daldrup-Link HE, 2018. Clinical Tracking of Cell Transfer and Cell Transplantation: Trials and Tribulations. Radiology 289, 604–615. 10.1148/radiol.2018180449 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Camilleri ET, Gustafson MP, Dudakovic A, Riester SM, Garces CG, Paradise CR, Takai H, Karperien M, Cool S, Sampen H-JI, Larson AN, Qu W, Smith J, Dietz AB, van Wijnen AJ, 2016. Identification and validation of multiple cell surface markers of clinical-grade adipose-derived mesenchymal stromal cells as novel release criteria for good manufacturing practice-compliant production. Stem Cell Res. Ther 7, 107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Candia OA, Gerometta R, Millar JC, Podos SM, 2010. Suppression of corticosteroid-induced ocular hypertension in sheep by anecortave. Arch. Ophthalmol. Chic. Ill 1960 128, 338–343. 10.1001/archophthalmol.2009.387 [DOI] [PubMed] [Google Scholar]
- Castro A, Du Y, 2019. Trabecular Meshwork Regeneration—a Potential Treatment for Glaucoma. Curr. Ophthalmol. Rep 7, 80–88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang EE, Goldberg JL, 2012. Glaucoma 2.0: Neuroprotection, Neuroregeneration, Neuroenhancement. Ophthalmology 119, 979–986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook N, Jodrell DI, Tuveson DA, 2012. Predictive in vivo animal models and translation to clinical trials. Drug Discov. Today 17, 253–260. 10.1016/j.drudis.2012.02.003 [DOI] [PubMed] [Google Scholar]
- Cyranoski D, 2019. ‘Reprogrammed’ stem cells to treat spinal-cord injuries for the first time. Nature d41586-019-00656-2. 10.1038/d41586-019-00656-2 [DOI] [Google Scholar]
- Cyranoski D, 2014. Japanese woman is first recipient of next-generation stem cells. Nature nature.2014.15915. 10.1038/nature.2014.15915 [DOI] [Google Scholar]
- Dawson TM, Golde TE, Lagier-Tourenne C, 2018. Animal models of neurodegenerative diseases. Nat. Neurosci 21, 1370–1379. 10.1038/s41593-018-0236-8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deinsberger J, Reisinger D, Weber B, 2020. Global trends in clinical trials involving pluripotent stem cells: a systematic multi-database analysis. NPJ Regen. Med 5, 15. 10.1038/s41536-020-00100-4 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding QJ, Zhu W, Cook AC, Anfinson KR, Tucker BA, Kuehn MH, 2014. Induction of Trabecular Meshwork Cells From Induced Pluripotent Stem Cells. Invest. Ophthalmol. Vis. Sci 55, 7065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dominici M, Le Blanc K, Mueller I, Slaper-Cortenbach I, Marini FC, Krause DS, Deans RJ, Keating A, Prockop DJ, Horwitz EM, 2006. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 8, 315–317. [DOI] [PubMed] [Google Scholar]
- Du Y, Carlson EC, Funderburgh ML, Birk DE, Pearlman E, Guo N, Kao WW-Y, Funderburgh JL, 2009. Stem cell therapy restores transparency to defective murine corneas. Stem Cells Dayt. Ohio 27, 1635–1642. 10.1002/stem.91 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Roh DS, Mann MM, Funderburgh ML, Funderburgh JL, Schuman JS, 2012. Multipotent Stem Cells from Trabecular Meshwork Become Phagocytic TM Cells. Invest. Ophthalmol. Vis. Sci 53, 1566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du Y, Yun H, Yang E, Schuman JS, 2013. Stem Cells from Trabecular Meshwork Home to TM Tissue In Vivo. Invest. Ophthalmol. Vis. Sci 54, 1450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein DL, 2012. Framing Glaucoma Questions: What are the Opportunities for Glaucoma Treatment? A Personal Perspective. Investig. Opthalmology Vis. Sci 53, 2462. 10.1167/iovs.12-9483c [DOI] [PubMed] [Google Scholar]
- Epstein SE, Luger D, Lipinski MJ, 2017. Large Animal Model Efficacy Testing Is Needed Prior to Launch of a Stem Cell Clinical Trial: An Evidence-Lacking Conclusion Based on Conjecture. Circ. Res 121, 496–498. 10.1161/CIRCRESAHA.117.311562 [DOI] [PubMed] [Google Scholar]
- Gelatt KN, Mackay EO, 1998. The ocular hypertensive effects of topical 0.1% dexamethasone in beagles with inherited glaucoma. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther 14, 57–66. 10.1089/jop.1998.14.57 [DOI] [PubMed] [Google Scholar]
- Gerometta R, Podos SM, Candia OA, Wu B, Malgor LA, Mittag T, Danias J, 2004. Steroid-induced ocular hypertension in normal cattle. Arch. Ophthalmol. Chic. Ill 1960 122, 1492–1497. 10.1001/archopht.122.10.1492 [DOI] [PubMed] [Google Scholar]
- Gong H, Swain D, 2016. The histopathological changes in the trabecular outflow pathway and their possible effects on aqueous outflow in eyes with primary open-angle glaucoma. pp. 17–40. [Google Scholar]
- Gonzalez P, Epstein DL, Luna C, Liton PB, 2006. Characterization of free-floating spheres from human trabecular meshwork (HTM) cell culture in vitro. Exp. Eye Res 82, 959–967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harper MM, Grozdanic SD, Blits B, Kuehn MH, Zamzow D, Buss JE, Kardon RH, Sakaguchi DS, 2011. Transplantation of BDNF-Secreting Mesenchymal Stem Cells Provides Neuroprotection in Chronically Hypertensive Rat Eyes. Invest. Ophthalmol. Vis. Sci 52, 4506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He Y, Leung KW, Zhang Y-H, Duan S, Zhong X-F, Jiang R-Z, Peng Z, Tombran-Tink J, Ge J, 2008. Mitochondrial complex I defect induces ROS release and degeneration in trabecular meshwork cells of POAG patients: protection by antioxidants. Invest. Ophthalmol. Vis. Sci 49, 1447–1458. 10.1167/iovs.07-1361 [DOI] [PubMed] [Google Scholar]
- Johnson M, 2006. ‘What controls aqueous humour outflow resistance?’ Exp. Eye Res 82, 545–557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson TV, Bull ND, Hunt DP, Marina N, Tomarev SI, Martin KR, 2010. Neuroprotective Effects of Intravitreal Mesenchymal Stem Cell Transplantation in Experimental Glaucoma. Invest. Ophthalmol. Vis. Sci 51, 2051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnstone MA, Grant WM, 1973. Pressure-Dependent Changes in Structures of the Aqueous Outflow System of Human and Monkey Eyes. Am. J. Ophthalmol 75, 365–383. [DOI] [PubMed] [Google Scholar]
- Kahook M, Schuman J, Schuman JS, Kahook MY, 2012. Chandler and Grant’s Glaucoma. SLACK Incorporated, Thorofare, UNITED STATES. [Google Scholar]
- Karussis D, Karageorgiou C, Vaknin-Dembinsky A, Gowda-Kurkalli B, Gomori JM, Kassis I, Bulte JWM, Petrou P, Ben-Hur T, Abramsky O, Slavin S, 2010. Safety and immunological effects of mesenchymal stem cell transplantation in patients with multiple sclerosis and amyotrophic lateral sclerosis. Arch. Neurol 67, 1187–1194. 10.1001/archneurol.2010.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaufman PL, Rasmussen CA, 2012. Advances in Glaucoma Treatment and Management: Outflow Drugs. Investig. Opthalmology Vis. Sci 53, 2495. 10.1167/iovs.12-9483m [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller KE, Bhattacharya SK, Borrás T, Brunner TM, Chansangpetch S, Clark AF, Dismuke WM, Du Y, Elliott MH, Ethier CR, Faralli JA, Freddo TF, Fuchshofer R, Giovingo M, Gong H, Gonzalez P, Huang A, Johnstone MA, Kaufman PL, Kelley MJ, Knepper PA, Kopczynski CC, Kuchtey JG, Kuchtey RW, Kuehn MH, Lieberman RL, Lin SC, Liton P, Liu Y, Lütjen-Drecoll E, Mao W, Masis-Solano M, McDonnell F, McDowell CM, Overby DR, Pattabiraman PP, Raghunathan VK, Rao PV, Rhee DJ, Chowdhury UR, Russell P, Samples JR, Schwartz D, Stubbs EB, Tamm ER, Tan JC, Toris CB, Torrejon KY, Vranka JA, Wirtz MK, Yorio T, Zhang J, Zode GS, Fautsch MP, Peters DM, Acott TS, Stamer WD, 2018. Consensus recommendations for trabecular meshwork cell isolation, characterization and culture. Exp. Eye Res 171, 164–173. 10.1016/j.exer.2018.03.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelley MJ, Rose AY, Keller KE, Hessle H, Samples JR, Acott TS, 2009. Stem cells in the trabecular meshwork: Present and future promises. Exp. Eye Res 88, 747–751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn MH, Lipsett KA, Menotti-Raymond M, Whitmore SS, Scheetz TE, David VA, O’Brien SJ, Zhao Z, Jens JK, Snella EM, Ellinwood NM, McLellan GJ, 2016. A Mutation in LTBP2 Causes Congenital Glaucoma in Domestic Cats (Felis catus). PloS One 11, e0154412. 10.1371/journal.pone.0154412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Cheng T, Song W, Cheuk B, Yang E, Yang L, Xie Y, Du Y, 2020a. Two-step induction of trabecular meshwork cells from induced pluripotent stem cells for glaucoma. Biochem. Biophys. Res. Commun 529, 411–417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Xu Y, Du Y, 2020b. Stem Cells from Human Trabecular Meshwork Hold the Potential to Develop into Ocular and Non-Ocular Lineages After Long-Term Storage. Stem Cells Dev. 29, 49–61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar Ajay, Siqi X, Zhou M, Chen W, Yang E, Price A, Le L, Zhang Y, Florens L, Washburn M, Kumar Akshay, Li Y, Xu Y, Lathrop K, Davoli K, Chen Y, Schuman JS, Xie T, Du Y, 2021. Stem cell-free therapy for glaucoma to preserve vision (preprint). Cell Biology. 10.1101/2021.06.18.449038 [DOI] [Google Scholar]
- Kuriyan AE, Albini TA, Townsend JH, Rodriguez M, Pandya HK, Leonard RE, Parrott MB, Rosenfeld PJ, Flynn HW, Goldberg JL, 2017. Vision Loss after Intravitreal Injection of Autologous “Stem Cells” for AMD. N. Engl. J. Med 376, 1047–1053. 10.1056/NEJMoa1609583 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lalu MM, McIntyre L, Pugliese C, Fergusson D, Winston BW, Marshall JC, Granton J, Stewart DJ, Canadian Critical Care Trials Group, 2012. Safety of cell therapy with mesenchymal stromal cells (SafeCell): a systematic review and meta-analysis of clinical trials. PloS One 7, e47559. 10.1371/journal.pone.0047559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamm N, Ben-David U, Golan-Lev T, Storchová Z, Benvenisty N, Kerem B, 2016. Genomic Instability in Human Pluripotent Stem Cells Arises from Replicative Stress and Chromosome Condensation Defects. Cell Stem Cell 18, 253–261. 10.1016/j.stem.2015.11.003 [DOI] [PubMed] [Google Scholar]
- Lee AS, Tang C, Rao MS, Weissman IL, Wu JC, 2013. Tumorigenicity as a clinical hurdle for pluripotent stem cell therapies. Nat. Med 19, 998–1004. 10.1038/nm.3267 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Lee C, Agrahari V, Wang K, Navarro I, Sherwood JM, Crews K, Farsiu S, Gonzalez P, Lin C-W, Mitra AK, Ethier CR, Stamer WD, 2019. In vivo measurement of trabecular meshwork stiffness in a corticosteroid-induced ocular hypertensive mouse model. Proc. Natl. Acad. Sci 116, 1714–1722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li G, Lee C, Read AT, Wang K, Ha J, Kuhn M, Navarro I, Cui J, Young K, Gorijavolu R, Sulchek T, Kopczynski C, Farsiu S, Samples J, Challa P, Ethier CR, Stamer WD, 2021. Anti-fibrotic activity of a rho-kinase inhibitor restores outflow function and intraocular pressure homeostasis. eLife 10, e60831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limoli PG, Limoli C, Vingolo EM, Scalinci SZ, Nebbioso M, 2016. Cell surgery and growth factors in dry age-related macular degeneration: visual prognosis and morphological study. Oncotarget 7, 46913–46923. 10.18632/oncotarget.10442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limoli PG, Limoli CSS, Morales MU, Vingolo EM, 2020. Mesenchymal stem cell surgery, rescue and regeneration in retinitis pigmentosa: clinical and rehabilitative prognostic aspects. Restor. Neurol. Neurosci 38, 223–237. 10.3233/RNN-190970 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limoli PG, Vingolo EM, Limoli C, Nebbioso M, 2019. Stem Cell Surgery and Growth Factors in Retinitis Pigmentosa Patients: Pilot Study after Literature Review. Biomedicines 7, E94. 10.3390/biomedicines7040094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Limoli PG, Vingolo EM, Morales MU, Nebbioso M, Limoli C, 2014. Preliminary study on electrophysiological changes after cellular autograft in age-related macular degeneration. Medicine (Baltimore) 93, e355. 10.1097/MD.0000000000000355 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liton PB, Challa P, Stinnett S, Luna C, Epstein DL, Gonzalez P, 2005. Cellular senescence in the glaucomatous outflow pathway. Exp. Gerontol 40, 745–748. 10.1016/j.exger.2005.06.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llobet A, Gasull X, Gual A, 2003. Understanding Trabecular Meshwork Physiology: A Key to the Control of Intraocular Pressure? Physiology 18, 205–209. [DOI] [PubMed] [Google Scholar]
- Lukomska B, Stanaszek L, Zuba-Surma E, Legosz P, Sarzynska S, Drela K, 2019. Challenges and Controversies in Human Mesenchymal Stem Cell Therapy. Stem Cells Int. 2019, 9628536. 10.1155/2019/9628536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ma M, Shu Y, Tang Y, Chen H, 2020. Multifaceted application of nanoparticle-based labeling strategies for stem cell therapy. Nano Today 34, 100897. 10.1016/j.nantod.2020.100897 [DOI] [Google Scholar]
- Mak IW, Evaniew N, Ghert M, 2014. Lost in translation: animal models and clinical trials in cancer treatment. Am. J. Transl. Res 6, 114–118. [PMC free article] [PubMed] [Google Scholar]
- Mallick S, Sharma M, Kumar A, Du Y, 2021. Cell-Based Therapies for Trabecular Meshwork Regeneration to Treat Glaucoma. Biomolecules 11, 1258. 10.3390/biom11091258 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mandai M, Watanabe A, Kurimoto Y, Hirami Y, Morinaga C, Daimon T, Fujihara M, Akimaru H, Sakai N, Shibata Y, Terada M, Nomiya Y, Tanishima S, Nakamura M, Kamao H, Sugita S, Onishi A, Ito T, Fujita K, Kawamata S, Go MJ, Shinohara C, Hata K-I, Sawada M, Yamamoto M, Ohta S, Ohara Y, Yoshida K, Kuwahara J, Kitano Y, Amano N, Umekage M, Kitaoka F, Tanaka A, Okada C, Takasu N, Ogawa S, Yamanaka S, Takahashi M, 2017. Autologous Induced Stem-Cell-Derived Retinal Cells for Macular Degeneration. N. Engl. J. Med 376, 1038–1046. 10.1056/NEJMoa1608368 [DOI] [PubMed] [Google Scholar]
- Manuguerra-GagnÉ R, Boulos PR, Ammar A, Leblond FA, Krosl G, Pichette V, Lesk MR, Roy D-C, 2013. Transplantation of Mesenchymal Stem Cells Promotes Tissue Regeneration in a Glaucoma Model Through Laser-Induced Paracrine Factor Secretion and Progenitor Cell Recruitment. Stem Cells 31, 1136–1148. [DOI] [PubMed] [Google Scholar]
- Mathiasen AB, Qayyum AA, Jørgensen E, Helqvist S, Ekblond A, Ng M, Bhakoo K, Kastrup J, 2019. In Vivo MRI Tracking of Mesenchymal Stromal Cells Labeled with Ultrasmall Paramagnetic Iron Oxide Particles after Intramyocardial Transplantation in Patients with Chronic Ischemic Heart Disease. Stem Cells Int. 2019, 2754927. 10.1155/2019/2754927 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkl C, Saalfrank A, Riesen N, Kühn R, Pertek A, Eser S, Hardt MS, Kind A, Saur D, Wurst W, Iglesias A, Schnieke A, 2013. Efficient Generation of Rat Induced Pluripotent Stem Cells Using a Non-Viral Inducible Vector. PLoS ONE 8, e55170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitton N, 2019. Woman is first to receive cornea made from ‘reprogrammed’ stem cells. mymed.com d41586-019-02597-2. 10.1038/d41586-019-02597-2 [DOI] [PubMed] [Google Scholar]
- Musiał-Wysocka A, Kot M, Majka M, 2019. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 28, 801–812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadri S, Yazdani S, Arefian E, Gohari Z, Eslaminejad MB, Kazemi B, Soleimani M, 2013. Mesenchymal stem cells from trabecular meshwork become photoreceptor-like cells on amniotic membrane. Neurosci. Lett 541, 43–48. [DOI] [PubMed] [Google Scholar]
- Neri S, 2019. Genetic Stability of Mesenchymal Stromal Cells for Regenerative Medicine Applications: A Fundamental Biosafety Aspect. Int. J. Mol. Sci 20, E2406. 10.3390/ijms20102406 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ngumah QC, Buchthal SD, Dacheux RF, 2006. Longitudinal non-invasive proton NMR spectroscopy measurement of vitreous lactate in a rabbit model of ocular hypertension. Exp. Eye Res 83, 390–400. 10.1016/j.exer.2006.01.015 [DOI] [PubMed] [Google Scholar]
- O’Callaghan J, Cassidy PS, Humphries P, 2017. Open-angle glaucoma: therapeutically targeting the extracellular matrix of the conventional outflow pathway. Expert Opin. Ther. Targets 21, 1037–1050. 10.1080/14728222.2017.1386174 [DOI] [PubMed] [Google Scholar]
- Ollivier FJ, Brooks DE, Kallberg ME, Sapp HL, Komáromy AM, Stevens GR, Dawson WW, Sherwood MB, Lambrou GN, 2004. Time-specific intraocular pressure curves in Rhesus macaques (Macaca mulatta) with laser-induced ocular hypertension. Vet. Ophthalmol 7, 23–27. 10.1111/j.14635224.2004.00316.x [DOI] [PubMed] [Google Scholar]
- Oner A, Gonen ZB, Sevim DG, Sinim Kahraman N, Unlu M, 2019. Six-month results of suprachoroidal adipose tissue-derived mesenchymal stem cell implantation in patients with optic atrophy: a phase 1/2 study. Int. Ophthalmol 39, 2913–2922. 10.1007/s10792-019-01141-5 [DOI] [PubMed] [Google Scholar]
- Oner A, Gonen ZB, Sevim DG, Smim Kahraman N, Unlu M, 2018. Suprachoroidal Adipose Tissue-Derived Mesenchymal Stem Cell Implantation in Patients with Dry-Type Age-Related Macular Degeneration and Stargardt’s Macular Dystrophy: 6-Month Follow-Up Results of a Phase 2 Study. Cell. Reprogramming 20, 329–336. 10.1089/cell.2018.0045 [DOI] [PubMed] [Google Scholar]
- Oner A, Gonen ZB, Sinim N, Cetin M, Ozkul Y, 2016. Subretinal adipose tissue-derived mesenchymal stem cell implantation in advanced stage retinitis pigmentosa: a phase I clinical safety study. Stem Cell Res. Ther 7, 178. 10.1186/s13287-016-0432-y [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel SA, Sherman L, Munoz J, Rameshwar P, 2008. Immunological properties of mesenchymal stem cells and clinical implications. Arch. Immunol. Ther. Exp. (Warsz.) 56, 1–8. 10.1007/s00005-008-0001-x [DOI] [PubMed] [Google Scholar]
- Pearson C, Martin K, 2015. Stem cell approaches to glaucoma, in: Progress in Brain Research. Elsevier, pp. 241–256. 10.1016/bs.pbr.2015.04.005 [DOI] [PubMed] [Google Scholar]
- Polansky JR, Weinreb RN, Baxter JD, Alvarado J, 1979. Human trabecular cells: Establishment in tissue culture and growth characteristics. Invest. Ophthalmol. Vis. Sci 10, 1043–9. [PubMed] [Google Scholar]
- Polansky JR, Wood IS, Maglio MT, Alvarado JA, 1984. Trabecular Meshwork Cell Culture in Glaucoma Research: Ophthalmology 91, 580–595. [DOI] [PubMed] [Google Scholar]
- Pound P, Ritskes-Hoitinga M, 2018. Is it possible to overcome issues of external validity in preclinical animal research? Why most animal models are bound to fail. J. Transl. Med 16, 304. 10.1186/s12967-018-1678-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prelle K, Zink N, Wolf E, 2002. Pluripotent Stem Cells - Model of Embryonic Development, Tool for Gene Targeting, and Basis of Cell Therapy. Anat. Histol. Embryol. J. Vet. Med. Ser C 31, 169–186. [DOI] [PubMed] [Google Scholar]
- Quigley HA, Addicks EM, 1980. Chronic experimental glaucoma in primates. I. Production of elevated intraocular pressure by anterior chamber injection of autologous ghost red blood cells. Invest. Ophthalmol. Vis. Sci 19, 126–136. [PubMed] [Google Scholar]
- Raviola G, 1982. Schwalbe line’s cells: a new cell type in the trabecular meshwork of Macaca mulatta 22, 12. [PubMed] [Google Scholar]
- Reardon G, Kotak, Schwartz G, 2011. Objective assessment of compliance and persistence among patients treated for glaucoma and ocular hypertension: a systematic review. Patient Prefer. Adherence 441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez-Fuentes DE, Fernandez-Garza LE, Samia-Meza JA, Barrera-Barrera SA, Caplan AI, Barrera-Saldaña HA, 2021. Mesenchymal Stem Cells Current Clinical Applications: A Systematic Review. Arch. Med. Res 52, 93–101. 10.1016/j.arcmed.2020.08.006 [DOI] [PubMed] [Google Scholar]
- Rosland GV, Svendsen A, Torsvik A, Sobala E, McCormack E, Immervoll H, Mysliwietz J, Tonn J-C, Goldbrunner R, Lønning PE, Bjerkvig R, Schichor C, 2009. Long-term cultures of bone marrow-derived human mesenchymal stem cells frequently undergo spontaneous malignant transformation. Cancer Res. 69, 5331–5339. 10.1158/0008-5472.CAN-08-4630 [DOI] [PubMed] [Google Scholar]
- Roubeix C, Godefroy D, Mias C, Sapienza A, Riancho L, Degardin J, Fradot V, Ivkovic I, Picaud S, Sennlaub F, Denoyer A, Rostene W, Sahel JA, Parsadaniantz SM, Brignole-Baudouin F, Baudouin C, 2015. Intraocular pressure reduction and neuroprotection conferred by bone marrow-derived mesenchymal stem cells in an animal model of glaucoma. Stem Cell Res. Ther 6, 177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubio D, Garcia-Castro J, Martín MC, de la Fuente R, Cigudosa JC, Lloyd AC, Bernad A, 2005. Spontaneous human adult stem cell transformation. Cancer Res. 65, 3035–3039. 10.1158/0008-5472.CAN-04-4194 [DOI] [PubMed] [Google Scholar]
- Sensebe L, Tarte K, Galipeau J, Krampera M, Martin I, Phinney DG, Shi Y, MSC Committee of the International Society for Cellular Therapy, 2012. Limited acquisition of chromosomal aberrations in human adult mesenchymal stromal cells. Cell Stem Cell 10, 9–10; author reply 10–11. 10.1016/j.stem.2011.12.005 [DOI] [PubMed] [Google Scholar]
- Shepard AR, Jacobson N, Millar JC, Pang I-H, Steely HT, Searby CC, Sheffield VC, Stone EM, Clark AF, 2007. Glaucoma-causing myocilin mutants require the Peroxisomal targeting signal-1 receptor (PTS1R) to elevate intraocular pressure. Hum. Mol. Genet 16, 609–617. [DOI] [PubMed] [Google Scholar]
- Snider EJ, Hardie BA, Li Y, Gao K, Splaine F, Kim RK, Vannatta RT, Read AT, Ethier CR, 2021. A Porcine Organ-Culture Glaucoma Model Mimicking Trabecular Meshwork Damage Using Oxidative Stress. Invest. Ophthalmol. Vis. Sci 62, 18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider EJ, Kubelick KP, Tweed K, Kim RK, Li Y, Gao K, Read AT, Emelianov S, Ethier CR, 2018. Improving Stem Cell Delivery to the Trabecular Meshwork Using Magnetic Nanoparticles. Sci. Rep 8, 12251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snider Eric J., Vannatta RT, Schildmeyer L, Stamer WD, Ethier CR, 2018. Characterizing differences between MSCs and TM cells: Toward autologous stem cell therapies for the glaucomatous trabecular meshwork. J. Tissue Eng. Regen. Med 12, 695–704. [DOI] [PubMed] [Google Scholar]
- Streilein JW, 1990. Anterior chamber associated immune deviation: The privilege of immunity in the eye. Surv. Ophthalmol 35, 67–73. [DOI] [PubMed] [Google Scholar]
- Sugita S, Mandai M, Hirami Y, Takagi S, Maeda T, Fujihara M, Matsuzaki M, Yamamoto M, Iseki K, Hayashi N, Hono A, Fujino S, Koide N, Sakai N, Shibata Y, Terada M, Nishida M, Dohi H, Nomura M, Amano N, Sakaguchi H, Hara C, Maruyama K, Daimon T, Igeta M, Oda T, Shirono U, Tozaki M, Totani K, Sugiyama S, Nishida K, Kurimoto Y, Takahashi M, 2020. HLA-Matched Allogeneic iPS Cells-Derived RPE Transplantation for Macular Degeneration. J. Clin. Med 9, E2217. 10.3390/jcm9072217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Williams A, Waisbourd M, Iacovitti L, Katz LJ, 2015. Stem cell therapy for glaucoma: Science or snake oil? Surv. Ophthalmol 60, 93–105. [DOI] [PubMed] [Google Scholar]
- Sundaresan Y, Manivannan LP, Radhakrishnan S, Ramasamy KS, Veerappan M, Chidambaranathan GP, 2021. Reduction in trabecular meshwork stem cell content in donor eyes with primary open angle glaucoma. Sci. Rep 11, 24518. 10.1038/s41598-021-03345-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundaresan Y, Veerappan M, Ramasamy KS, Chidambaranathan GP, 2019. Identification, quantification and age-related changes of human trabecular meshwork stem cells. Eye Vis. Lond. Engl 6, 31. 10.1186/s40662-019-0156-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi S, Mandai M, Gocho K, Hirami Y, Yamamoto M, Fujihara M, Sugita S, Kurimoto Y, Takahashi M, 2019. Evaluation of Transplanted Autologous Induced Pluripotent Stem Cell-Derived Retinal Pigment Epithelium in Exudative Age-Related Macular Degeneration. Ophthalmol. Retina 3, 850–859. 10.1016/j.oret.2019.04.021 [DOI] [PubMed] [Google Scholar]
- Tham Y-C, Li X, Wong TY, Quigley HA, Aung T, Cheng C-Y, 2014. Global Prevalence of Glaucoma and Projections of Glaucoma Burden through 2040. Ophthalmology 121, 2081–2090. [DOI] [PubMed] [Google Scholar]
- Toris CB, Zhan GL, Wang YL, Zhao J, McLaughlin MA, Camras CB, Yablonski ME, 2000. Aqueous humor dynamics in monkeys with laser-induced glaucoma. J. Ocul. Pharmacol. Ther. Off. J. Assoc. Ocul. Pharmacol. Ther 16, 19–27. 10.1089/jop.2000.16.19 [DOI] [PubMed] [Google Scholar]
- Torrente Y, Polli E, 2008. Mesenchymal Stem Cell Transplantation for Neurodegenerative Diseases. Cell Transplant. 17, 1103–1113. [DOI] [PubMed] [Google Scholar]
- van der Worp HB, Howells DW, Sena ES, Porritt MJ, Rewell S, O’Collins V, Macleod MR, 2010. Can animal models of disease reliably inform human studies? PLoS Med. 7, e1000245. 10.1371/journal.pmed.1000245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verfaillie CM, 2002. Adult stem cells: assessing the case for pluripotency. Trends Cell Biol. 12, 502–508. [DOI] [PubMed] [Google Scholar]
- Viganò M, Budelli S, Lavazza C, Montemurro T, Montelatici E, de Cesare S, Lazzari L, Orlandi AR, Lunghi G, Giordano R, 2018. Tips and Tricks for Validation of Quality Control Analytical Methods in Good Manufacturing Practice Mesenchymal Stromal Cell Production. Stem Cells Int. 2018, 1–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vitale I, Manic G, De Maria R, Kroemer G, Galluzzi L, 2017. DNA Damage in Stem Cells. Mol. Cell 66, 306–319. 10.1016/j.molcel.2017.04.006 [DOI] [PubMed] [Google Scholar]
- Wang K, Johnstone MA, Xin C, Song S, Padilla S, Vranka JA, Acott TS, Zhou K, Schwaner SA, Wang RK, Sulchek T, Ethier CR, 2017a. Estimating Human Trabecular Meshwork Stiffness by Numerical Modeling and Advanced OCT Imaging. Invest. Ophthalmol. Vis. Sci 58, 4809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Li G, Read AT, Navarro I, Mitra AK, Stamer WD, Sulchek T, Ethier CR, 2018. The relationship between outflow resistance and trabecular meshwork stiffness in mice. Sci. Rep 8, 5848. 10.1038/s41598-018-24165-w [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang K, Read AT, Sulchek T, Ethier CR, 2017b. Trabecular meshwork stiffness in glaucoma. Exp. Eye Res 158, 3–12. 10.1016/j.exer.2016.07.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber AJ, Zelenak D, 2001. Experimental glaucoma in the primate induced by latex microspheres. J. Neurosci. Methods 111, 39–48. 10.1016/s0165-0270(01)00443-5 [DOI] [PubMed] [Google Scholar]
- Whikehart DR, Parikh CH, Vaughn AV, Mishler K, Edelhauser HF, 2005. Evidence suggesting the existence of stem cells for the human corneal endothelium. Mol. Vis 9. [PubMed] [Google Scholar]
- Xiong S, Kumar A, Tian S, Taher EE, Yang E, Kinchington PR, Xia X, Du Y, 2021. Stem cell transplantation rescued a primary open-angle glaucoma mouse model. eLife 10, e63677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong S, Xu Y, Wang Y, Kumar A, Peters DM, Du Y, 2020. α5β1 Integrin Promotes Anchoring and Integration of Transplanted Stem Cells to the Trabecular Meshwork in the Eye for Regeneration. Stem Cells Dev. 29, 290–300. 10.1089/scd.2019.0254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamanaka S, 2020. Pluripotent Stem Cell-Based Cell Therapy-Promise and Challenges. Cell Stem Cell 27, 523–531. 10.1016/j.stem.2020.09.014 [DOI] [PubMed] [Google Scholar]
- Yu J, Vodyanik MA, Smuga-Otto K, Antosiewicz-Bourget J, Frane JL, Tian S, Nie J, Jonsdottir GA, Ruotti V, Stewart R, Slukvin II, Thomson JA, 2007. Induced Pluripotent Stem Cell Lines Derived from Human Somatic Cells 318, 5. [DOI] [PubMed] [Google Scholar]
- Yun H, Wang Y, Zhou Y, Wang K, Sun M, Stolz DB, Xia X, Ethier CR, Du Y, 2018. Human stem cells home to and repair laser-damaged trabecular meshwork in a mouse model. Commun. Biol 1, 216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yun H, Zhou Y, Wills A, Du Y, 2016. Stem Cells in the Trabecular Meshwork for Regulating Intraocular Pressure. J. Ocul. Pharmacol. Ther 32, 253–260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y, Cai S, Tseng SCG, Zhu Y-T, 2018. Isolation and Expansion of Multipotent Progenitors from Human Trabecular Meshwork. Sci. Rep 8, 2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Z, Dhaliwal AS, Tseng H, Kim JD, Schuman JS, Weinreb RN, Loewen NA, 2014. Outflow tract ablation using a conditionally cytotoxic feline immunodeficiency viral vector. Invest. Ophthalmol. Vis. Sci 55, 935–940. 10.1167/iovs.13-12890 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou Y, Xia X, Yang E, Wang Y, Marra KG, Ethier CR, Schuman JS, Du Y, 2020. Adipose‐derived stem cells integrate into trabecular meshwork with glaucoma treatment potential. FASEB J. 34, 7160–7177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Godwin CR, Cheng L, Scheetz TE, Kuehn MH, 2020. Transplantation of iPSC-TM stimulates division of trabecular meshwork cells in human eyes. Sci. Rep 10, 2905. 10.1038/s41598-020-59941-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Gramlich OW, Laboissonniere L, Jain A, Sheffield VC, Trimarchi JM, Tucker BA, Kuehn MH, 2016. Transplantation of iPSC-derived TM cells rescues glaucoma phenotypes in vivo. Proc. Natl. Acad. Sci 113, E3492–E3500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu W, Jain A, Gramlich OW, Tucker BA, Sheffield VC, Kuehn MH, 2017. Restoration of Aqueous Humor Outflow Following Transplantation of iPSC-Derived Trabecular Meshwork Cells in a Transgenic Mouse Model of Glaucoma. Invest. Ophthalmol. Vis. Sci 58, 2054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zode GS, Kuehn MH, Nishimura DY, Searby CC, Mohan K, Grozdanic SD, Bugge K, Anderson MG, Clark AF, Stone EM, Sheffield VC, 2011. Reduction of ER stress via a chemical chaperone prevents disease phenotypes in a mouse model of primary open angle glaucoma. J. Clin. Invest 121, 3542–3553. [DOI] [PMC free article] [PubMed] [Google Scholar]


