Abstract
Background:
Congenital adrenal hyperplasia (CAH) is a group of genetic disorders that affects the adrenal glands and is the most common cause of primary adrenal insufficiency in children. In the past few decades magnetic resonance imaging (MRI) has been implemented to investigate how the brain may be affected by CAH. A systematic review was conducted to evaluate and synthesize the reported evidence of brain findings related to CAH using structural, functional, and diffusion MRI.
Methods:
We searched bibliographical databases through July 2021 for brain MRI studies in individuals with CAH.
Results:
Twenty-eight studies were identified, including 13 case reports or series, 10 studies that recruited and studied CAH patients versus unaffected controls, and five studies without a matched control group. Eleven studies used structural MRI to identify structural abnormalities or quantify brain volumes, whereas three studies implemented functional MRI to investigate brain activity and three reported diffusion MRI findings to assess white matter microstructure. Some commonly reported findings across studies included cortical atrophy and differences in gray matter volumes, as well as white matter hyperintensities, altered white matter microstructure, and distinct patterns of emotion and reward-related brain activity.
Conclusions:
These findings suggest differences in brain structure and function in patients with CAH. Limitations of these studies highlight the need for CAH neuroimaging studies to incorporate larger sample sizes and follow best study design and MRI analytic practices, as well as clarify potential neurological effects seen across the lifespan and in relation to clinical and behavioral CAH phenotypes.
Keywords: congenital adrenal hyperplasia, adrenal insufficiency, 21-hydroxylase deficiency, magnetic resonance imaging (MRI), brain, neuroimaging, functional MRI, diffusion MRI
1. Introduction
Congenital adrenal hyperplasia (CAH) is the broad term used to describe a group of recessive genetic disorders that affect the adrenal glands.1,2 Most cases are caused by 21-hydroxylase (21-OH) deficiency, derived from a variant in the CYP21A2 gene, and involves deficiencies of cortisol and aldosterone with resultant increased adrenocorticotropic hormone (ACTH) signaling. This leads to overproduction of androgens by the adrenal glands, which may lead to pre- and/or postnatal virilization.3 CAH due to 21-OH deficiency widely ranges in prevalence across populations with an international incidence of approximately 1:15,000 births. Its classical form includes the onset of either the salt-wasting (SW) or simple virilizing (SV) forms at birth, while its nonclassical (NCAH) variations have a later age of onset. Salt-wasting is the most common manifestation of CAH due to 21-OH deficiency making up roughly 2/3 of overall cases and tending to be paired with the more extreme symptoms. The SV form may also pose a significant health risk to individuals, while those with nonclassical forms of CAH often see the least drastic of symptoms. Common symptoms of classical CAH include dehydration, low blood sodium levels (hyponatremia), high blood potassium levels (hyperkalemia), low blood glucose (hypoglycemia), metabolic acidosis, and like many androgen-linked disorders, ambiguous genitalia at birth in girls and infertility later in life in both sexes.4 All forms of CAH are best treated early through the administration of exogenous glucocorticoids to compensate for a lack of endogenous cortisol and exogenous mineralocorticoids in most cases to replace aldosterone.
Additionally, hormone imbalance and its unintentional overcorrection through glucocorticoid replacement therapy have called into question the impact of factors inherent to CAH and its therapy on the developing brain, considering the abundance of both glucocorticoid and androgen receptors found within both cortical and subcortical regions.5,6 Thus, in the past few decades, studies have begun to focus on the cognitive and behavioral phenotypes of CAH. Compared to age- and sex-matched controls, findings on general cognitive ability are mixed. Earlier studies found lower general intelligence (IQ) in patients with CAH, especially the SW form.7 Additionally, SV females had a higher rate of learning disabilities when compared to same-sex control siblings.8 On the contrary, some recent studies have found no IQ differences between CAH and controls, though SW patients still performed worse than SV patients.9,10 Beyond general cognitive ability, reduced working memory capacity has been noted in several CAH studies.11,12 Additionally, sex differences have been observed in verbal and spatial reasoning abilities. A 2008 meta-analysis by Puts et al.13 found better spatial ability in CAH females compared to control females, and worse spatial ability in CAH males compared to control males. More recent studies have replicated these findings, though some suggest superior spatial ability is only found in SW CAH females.14,15 In behavioral studies, CAH children likewise show reversal of gender-typical behavior. For instance, girls with CAH exhibit more masculine toy preference compared to control girls, independent of (but partially mediated by) parental socialization.16 Additionally, boys with CAH show reduced rough-and-tumble play compared to control boys, and girls with CAH show increased preference for playing with boys compared to unaffected girls.17 Moreover, higher recorded aggressive behavior in CAH female youth suggests psychological and potentially neurological changes due to increased androgen levels.18,19 Together, these findings suggest that CAH may impact cognitive functioning and behavior, yet questions remain about the underlying neurobiological phenotypes that may be present in individuals with CAH.
Advancements in non-invasive human magnetic resonance imaging (MRI) techniques and the field of pediatric psychoneuroendocrinology have provided the opportunity to begin to examine how specific regions of the brain may be affected by endocrine disorders such as CAH, resulting in cognitive, emotional, and behavioral differences.20 Specifically, structural MRI (sMRI) and functional MRI (fMRI), as their names imply, examine brain tissue and brain activation, respectively. MRI allows for pictures of anatomical and physiological processes within the body with an emphasis on soft tissue and hemodynamic blood responses that occur with brain activity. Depending on data preprocessing, structural MRI can assess gray matter (i.e., cell bodies) and white matter (i.e., myelination of axons) volume, surface area, density, and cortical thickness (i.e., distance between gray/white matter boundary and pia mater).21,22 Diffusion-weighted MRI (dMRI) provides additional insight on water diffusion to assess microstructural properties of tissue (e.g., diffusion coherency and directionality), enabling inferences about myelination, axonal organization, and/or axon caliber of white matter fiber bundles.23 FMRI measures the blood oxygen level-dependent (BOLD) signal as an estimate of neural activity.21,22 The BOLD signal capitalizes on the tight coupling of blood flow and oxygenation when local neurons are activated. Specifically, neuronal firing leads to an increase in blood flow carrying oxygenated blood, leading to displacement of paramagnetic, deoxygenated blood, and a higher MR signal. By mapping changes in the BOLD signal, we can indirectly measure neuronal activity across time while individuals rest, known as resting-state fMRI (rs-fMRI), and in relation to task demands, often referred to as task-based fMRI.21,22
In summary, MRI metrics hold great promise to probe brain structure and function affected in CAH. Thus, the objective of the current study is to conduct a systematic review of the current literature on brain health in individuals diagnosed with classical CAH, with a focus on studies implementing structural, functional, and/or diffusion MRI. In addition to reviewing the existing literature, we also provide suggestions on future directions that should be considered in examining neurobiological phenotypes of CAH using non-invasive MRI neuroimaging.
2. Materials and Methods
This systematic review is reported following the standard set in the Preferred Reporting Items for Systematic Reviews and Meta-analysis statement.24
2.1. Data Sources
We conducted a search of prior studies on CAH and the brain using the MEDLINE database through PubMed. A comprehensive strategy for combining key terms was developed individually by two authors (MH and DC) with two categories of search terms, including: (1) those pertaining to neuroimaging - brain imaging, structural and/or functional magnetic resonance imaging (MRI) - and (2) those related to CAH - Congenital Adrenal Hyperplasia, CAH, 21-hydroxylase deficiency, and adrenal deficiency. The search algorithm was as follows: [(“magnetic resonance image”[title/abstract] OR “magnetic resonance images”[title/abstract] OR “magnetic resonance imaging”[title/abstract] OR “MRI” [Title/Abstract] OR “white matter hyperintensity”[title/abstract] OR “white matter hyperintensities”[title/abstract] OR “white matter change*”[title/abstract] OR “neuroimage”[title/abstract] OR “neuroimages”[title/abstract] OR “neuroimaging”[title/abstract] OR “diffusion”[title/abstract] OR “DTI”[title/abstract] OR “diffuse tensor imaging”[title/abstract] OR “fractional anisotropy”[title/abstract] OR “mean diffusivity”[title/abstract] OR “neuroinflammation”[title/abstract] OR “white matter volume”[title/abstract] OR “white matter volumes”[title/abstract] OR “brain structure”[title/abstract] OR “brain volume”[title/abstract] OR “brain volumes”[title/abstract] OR “functional connectivity”[Title/Abstract] OR “Brain/pathology”[mesh] OR “Brain/physiopathology”[Majr] OR “Magnetic Resonance Imaging”[Mesh]) AND (“Congenital Adrenal Hyperplasia”[Title/Abstract] OR “CAH”[Title/Abstract] OR “Congenital Adrenocortical Hyperplasia”[Title/Abstract] OR “21-hydroxylase deficiency”[Title/Abstract] OR “21-OH deficiency”[Title/Abstract] OR “adrenal deficiency”[Title/Abstract])]. With the stored algorithms from the first search, a team member (NK) carried out the second round of searches by combining the medical subject headings (MeSH) and text keywords from the first round; this second round of searches included new key terms allowing for a more widespread identification of the presence of CAH (considering the multiple genetic factors in play). Only articles in the English language were collected. No limitations were placed on the year published or other content-related criteria at the time of these searches. The final PubMed search was completed on 7/21/2021. For resulting articles, we obtained their title, abstracts, plus full texts if needed, to determine each publication’s relevance to our review.
2.1.2. Study Selection and Data Extraction
The selection process to identify relevant papers for a full review consisted of two phases of screening, a title and abstract screening followed by a full-text screening. After the removal of duplicates from both rounds of searches, the titles and abstracts were individually screened by authors (MH, NK) to ascertain relevance to the study. The remainder of the articles was retrieved for full-text review. In the case of discrepancies, the two authors convened to determine a consensus of inclusion. Specific inclusionary criteria to be met required (1) full-length original research articles and (2) at least one of the brain MRI outcome measures be examined. Exclusionary criteria were articles with (1) animal subjects, (2) lack of brain MRI measures of interest (structural MRI, diffusion MRI, functional MRI), (3) no relevance to Congenital Adrenal Hyperplasia, (4) papers that were not original (i.e., review papers or identical replicates of previously published reports), and (5) not reported in English. Independent extraction of articles was performed by two authors using predefined data fields.
3. Results
Figure 1 outlines the study selection process. Using the comprehensive search strategy, 215 unique articles were identified from PubMed, with 37 of them meeting the inclusion and exclusion criteria based on title and abstract screening. Full-text review of these 37 articles led to the exclusion of nine additional articles given that the full-text review identified them as reported in a language other than English (n=2), non-original articles (n=3), and did not include one of the brain MRI outcomes of interest (n=4). Of note, two papers reported identical findings; thus, the second publication was excluded as one of the three non-original research reports.25 The remaining 28 articles were considered relevant original research articles and included in the final review. A division among the resultant articles was made to separate case reports or case series (n=13) from observational studies (n=16). The latter 16 studies were further divided by MRI modality. Two studies included both sMRI and dMRI, meaning 11 studies used sMRI, 3 studies used dMRI, and 3 studies used fMRI.
Figure 1.
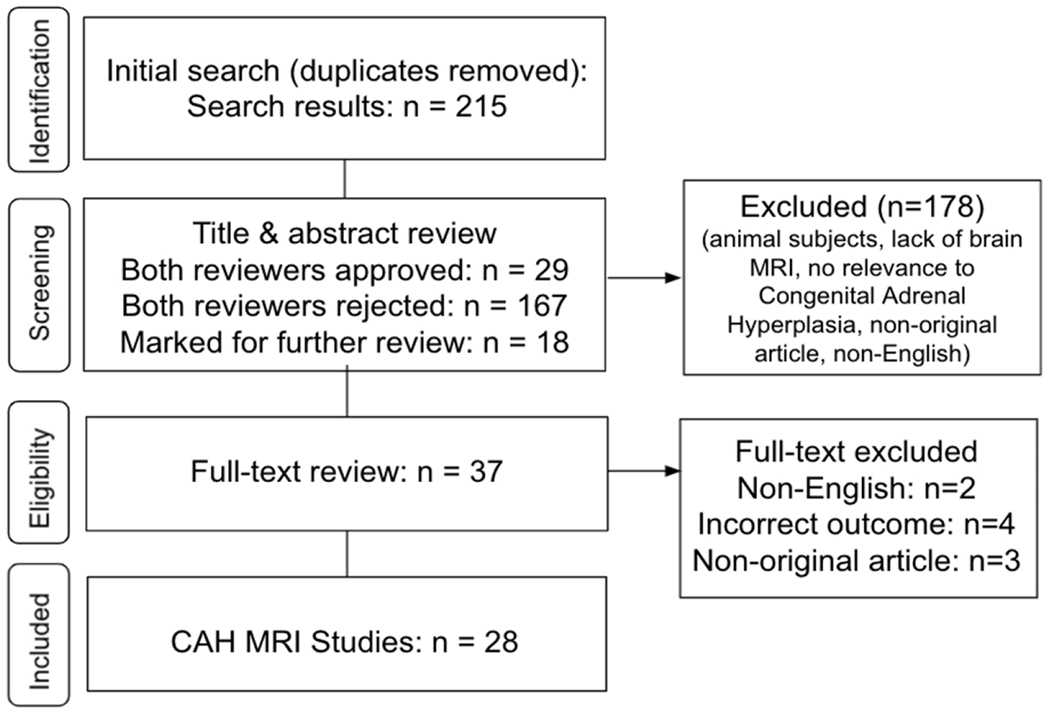
Systematic review flow diagram for included CAH MRI studies. Flow chart details the database search, the number of titles and abstracts screened, and the full texts retrieved.
3.1. Case Reports and Case Series
Thirteen case reports and series were identified as part of the current systematic review using brain MRI to assess CAH (Table 1). While case reports can be helpful to identify new trends or important variation in CAH, or even highlight unexpected events and concomitant health conditions, these reports provide the lowest level of evidence as they are likely not generalizable or based on systematic study, nor do they explore or adjust for other explanations of findings. However, we note some of the common comorbid health conditions and findings seen in more than one case report below.
Table 1.
CAH MRI Case Reports and Case Series
| Study | Participant(s) | Phenotype, Age at Diagnosis | Treatment | MRI Type(s) | MRI Findings |
|---|---|---|---|---|---|
| Bergamaschi et al. 200426 | 22 yr, female (longitudinal) | SW CAH; Sudden visual loss; cranial nerve impairment after 4 mo; Multiple Sclerosis | Hydrocortisone and fludrocortisone; Methylprednisolone 1 g/d (3d) | Spin echo proton density–weighted image; T1w and T2w sagittal/coronal images, 0.5T Philips (Gyroscan NT) |
Baseline: WM hyperintensities on T2w in periventricular areas (diffuse); corpus callosum, pons, and cerebellum (focal); Follow-up (7 yrs): T1w showed enhancing WM hyperintensities |
| Bhangoo et al. 200527 | N=3; A: 19 mo, phenotypic female (XY) B: Age during study NS, female (XX) C: Age during study NS, female (XX) *B and C are siblings |
A: Lipoid CAH, diagnosed at 5 months; Developmental delay; Homozygous missense mutation in exon 5 at nucleotide 670 CGT3TGT B: Lipoid CAH, diagnosed at 2 weeks; ADHD; IQ 80; Deletion from nucleotide 327 to 328 (327_328delCT) in exon 3 C: Lipoid CAH, diagnosed at 2 days; Tic disorder; Deletion from nucleotide 327 to 328 |
A: NS B: Dex (0.5 mg qID x 2d at age 6 yr when ACTH high); hydrocortisone and fludrocortisone replacement C: Glucocorticoids, mineralocorticoid; Dex (0.5 mg QID x 2d when ACTH high) |
A: T1w axial results provided (NS) B: T2w axial results provided (NS) C: T1w sagittal results provided (NS) |
A: Enlarged subarachnoid spaces consistent with frontal and temporal atrophy; static encephalopathy B: Focal supratentorial WM hyperintensities, perhaps due to demyelination, ischemic damage, or gliosis C: Chiari-I malformation |
| Okamoto et al. 200628 | 6 yr, female (longitudinal) | NS; Gastroenteritis; seizures; hypotension; acidosis; brain edema; nuchal and truncal hypotonia; pathological reflexes; forced mouth opening | Hydrocortisone and fludrocortisone | T2w, FLAIR, DWI (NS) | Baseline: Widespread hyperintensities in frontal cortex on DWI; Follow-up (2 mo): widespread hyperintensities in bilateral frontal lobes, smaller hyperintensities in parietal and temporal lobes, involving both GM and WM, on T2w and FLAIR |
| Saito et al. 200829 | 6 yr, female | Hypoglycemia and hypoxia/ischemia in the setting of adrenal insufficiency; Seizures; cyanosis; irregular respiration; hypotension | NS | T1w, T2w, FLAIR, DWI (NS) | Brain edema; laminar cortical atrophy; fronto-parietal cerebral cortex hyperintensities on T2w and DWI (with reduced ADC); cerebral hyperintensities on T1w and FLAIR; multi-layer cortical WM hyperintensities on T2w and FLAIR; Widespread hypointensities on T1w and hyperintensities on T2w and FLAIR; evidence of laminar cortical necrosis (T2w isointensity/FLAIR hyperintensity) |
| Gaudiano et al. 2010 30 | N=3; A: 27-yr-old CAH daughter; B: 54-yr-old CAH father; C: 50-yr-old CAH mother |
A: Daughter: 21-OHD, diagnosed at birth; Ataxia; frequent tremors; positive Babinski sign; leukoencephalopathy B: Leukoencephalopathy C: Leukoencephalopathy |
A: Prednisolone 5 mg/d birth to 14 yr; then Dex (0.75 mg/d) | NS | A: Periventricular white matter hyperintensities B: Bilateral, periventricular leukoencephalopathy C: Diffuse periventricular and subcortical leukoencephalopathy |
| Samia et al. 201031 | 15 yr, male | SW; Two adrenal crises occurred following viral illness (ages 1 and 9. yr); 1-yr history of worsening tremors | Hydrocortisone (60 mg/m2/day); Fludrocortisone (100 μg BID) | T1w, T2w, FLAIR (NS) | Bilateral periventricular hyperintensities on T2w and FLAIR (mostly posterior); Cortico-subcortical atrophy and agenesis of the corpus callosum evident on T1w |
| Lee et al. 201132 | N=3; A: 9 yr, female B: 1 yr 5 mo, male C: 2 yr 11 mo, male (longitudinal) |
A: SV, 21-OHD diagnosed neonatally; presented with altered state, fever, vomiting B: SW, 21-OHD diagnosed neonatally; presented with altered state, fever, diarrhea C: SW, 21-OHD diagnosed neonatally; Mild developmental delay; presented with seizures, fever, vomiting, diarrhea, exhibited mild left hemiparesis and epilepsy |
A: Hydrocortisone B: Hydrocortisone and fluorocortisol C: Hydrocortisone and fluorocortisol |
A: T1w, T2w, FLAIR, DWI (NS) B: FLAIR (NS) C: DWI (NS) |
A: WM hyperintensities (diffuse) and in corpus callosum (focal) apparent on DWI (with reduced ADC); no T1w, T2w, or FLAIR abnormalities B: Multiple hyperintensities in cerebral white matter on FLAIR C: Baseline: Hyperintensities in the R frontal subcortical white matter on DWI (with reduced ADC); Follow-up (11 days): Hyperintensities in the R cortical hemisphere on DWI (with reduced ADC) |
| Kaga et al. 201333 | 12 d, male (longitudinal) | SW CAH; Tonic seizures | Hydrocortisone (200 mg/m2/day); Fludrocortisone and NaCl administered after first scan | T1w, T2w (NS) |
Baseline: Bilateral diffuse hyperintensities on T1w, hypointensities in watershed area on T2w; Follow-up (1 yr): decreased hyperintensities on T1w compared to baseline |
| Grunt et al. 201334 | N=2; A: 5 yr 3 mo, female (longitudinal) B: 1 yr 10 mo, male (longitudinal) |
A: SW, 21-OHD, diagnosed neonatally; 2 focal seizures 7 mos after hospital admission; at the time of study, she was 13 yr and seizure-free without anti-epileptics B: SW, 21-OHD, diagnosed neonatally; irritability, vomiting, fever, diarrhea, and hypoglycemia (0.9 mmol/l) developed within 24 hr prior to admission |
A: Prenatal Dex; hydrocortisone and fludrocortisone B: Prenatal Dex; hydrocortisone and fludrocortisone |
DWI, T2w (NS) DWI, T2w (NS) |
A: Baseline: Focal hyperintensities in the left parietal cortex on DWI and Tw2; Follow-up (3 mo): Thinning of cortex and hyperintensities on T2w B: Baseline: Extensive hyperintensities on DWI and focal hyperintense lesions on T2w in L parieto-occipital lobe, medulla, L middle and inferior frontal gyrus and R occipital lobe; Follow-up (5 yrs): L-sided cortical and subcortical atrophy in the temporo-occipital lobe and smaller defects in the L frontal and R occipital lobe than on initial scan |
| Winfeld et al. 201335 | 11 d, male (longitudinal) | SW, 21-OHD (Q318X and intron I2splice mutations); Elongated and thickened adrenal glands; seizures; hypoxia and hypocalcemia | Prenatal Dex; hydrocortisone (15 mg Q6h, reduced to 10 mg q6h on day 3); calcium supplementation; phenobarbital; mineralocorticoid; NaCl 13 months: hydrocortisone 13.6 mg/m2 per day; fludrocortisone 0.1 mg BID; NaCl 500 mg TID |
T1w, T2w, DWI (NS) |
Baseline: T2w hyperintensity in the genu and splenium of the corpus callosum; T1w hyperintensity and reduced diffusion in periventricular white matter regions; Follow-up (1 yr): T1w abnormalities and reduced diffusion resolved; T2w abnormalities persisted; mild thinning of corpus callosum; focal prominence of atria of lateral ventricles; adjacent abnormal periventricular T2w hyperintensity |
| Serter et al. 201536 | 3 yr, male (longitudinal) | 21-OHD, diagnosed at birth; unconscious with seizures and hypoglycemic after stopping hydrocortisone treatment; acute encephalopathy | Hydrocortisone | DWI, SWI, T2w FLAIR (NS) |
Baseline: Effacement of sulcal spaces; Hyperintense T2w and FLAIR signals in the bilateral frontal and parieto-occipital cortical-subcortical regions; T2w hyperintensities in the splenium of the corpus callosum and cortical-subcortical regions; Follow-up (20 days): Partial resolution of T2w hyperintensities; ventricular dilatation; mild cerebral cortical atrophy; hyperintense T2w, FLAIR, and DWI signal changes in bilateral occipital lobes |
| O’shea et al. 201637 | 45 yr, female | SV, 21-OHD, diagnosed in childhood with premature adrenarche; hyperandrogenemia; grade 1 meningioma stained strongly for progesterone receptors, negative for estrogen receptors | Glucocorticoid therapy | NS | Meningioma centered in the greater R wing of the sphenoid |
| Abe et al. 201638 | Retrospective case report review study: 15 CAH (4 female, 11 male), mean age: 3.3 yr (range: 1-9 years) *Includes patient from Saito et al. 2008 & Lee et al. 2011 |
13 with 21-OHD (87%); Encephalopathy 2 with Lipoid CAH (13%); Encephalopathy |
All treated with glucocorticoids and/or mineralocorticoids | T1w, T2w, FLAIR, DWI | All patients exhibited appropriate neurological development prior to encephalopathy; 8 MRI findings were heterogeneous; acute period: 8 bilateral diffuse or multifocal brain lesions using DWI; chronic period: 3 normal, 5 WM atrophy, 2 cortical necrosis |
Abbreviations: 3T: 3 Tesla, ACTH: adrenocorticotropic hormone, ADC: apparent diffusion coefficient, ADHD: attention-deficit/hyperactivity disorder, BID: twice a day, CAH: congenital adrenal hyperplasia, d: day, Dex: dexamethasone, DWI: diffusion-weighted imaging, FA: fractional anisotropy, GC: glucocorticoid, GM: Gray Matter, IQ: intelligence quotient, L: left hemisphere, MD: mean diffusivity, mo: month, NaCl: sodium chloride, NCAH: non-classic, NS: not specified, OHD: hydroxylase deficiency, q6h: once every six hours, qID: four times a day, R: right hemisphere, SV: simple-virilizing, SW: salt-wasting, T1w: T1-weighted imaging, T2w: T2-weighted imaging, wk: week, WM: white matter, yr: year
CAH case reports or series involving MRI scanning included patients ranging from 11 days to 54 years old. Reasons for brain scanning varied, but included comorbidity of seizures, worsening tremors, multiple sclerosis, or severe illness that was, or was not, related to adrenal crisis. Common notable brain findings included focal and diffuse hyperintensities on scans of T1-, T2- and diffusion-weighted sequences, the pathologies of which can vary significantly, including ischemia, gliosis, damage to small blood vessels, or loss and deformation of myelinated axons.39,40 Other less common findings included edema, cortical atrophy, meningioma, and Chiari-I malformation. More recently, a retrospective review of case reports in Japan surveyed 1,061 child neurology patients who presented with CAH-associated encephalopathy (CAHE).38 Twenty-five patients with suspected CAHE were identified, and clinical data for 15 patients with CAHE, ranging from 1 to 9 years of age, with an average CAHE onset of 3.3 years of age, were provided for further review. Eight completed MRI scanning with heterogeneous findings. Diffusion-weighted images acquired during the acute period showed bilateral diffuse or multifocal brain lesions. In follow-up scans to assess chronic effect, DWI revealed that three patients no longer showed lesions, while five displayed white matter atrophy, two of which presented with cortical necrosis.
While the findings in CAH case reports or case series have largely been variable, they point to observable neurological differences on MRI scans that warrant further investigations to determine if these brain findings are due to CAH specifically or arise due to comorbid health conditions in individuals affected by CAH. As such, we next discuss observational studies that have been performed in study samples using distinct MRI sequences to determine how brain structure and brain activity may be affected in patients with CAH, as well as to what degree these patterns differ from control individuals.
3.2. Structural MRI (sMRI)
SMRI is based on tissue relaxation properties, which allows for quantification of brain morphology in terms of size and shape of white matter (WM) and gray matter (GM). GM is composed of neuropil (i.e., cell bodies and their processes), whereas WM consists primarily of myelinated axons connecting distal cortical and subcortical brain regions.41 Thus, sMRI allows for quantification of GM and WM regions of interest within the brain, as well as visualizes hyperintensities, edema, demyelination, atrophy, and other brain abnormalities.
Of the 11 sMRI studies identified, two focused on CAH patients only (i.e., no control group) and nine included both CAH patients as well as a ‘control’ comparison dataset or matched control sample (Table 2). The age of participants ranged from 3 months to 48 years, with relatively small sample sizes (7-39 CAH participants per study). Moreover, the exact sMRI scanner strength and image acquisition type (i.e., T1- vs. T2-weighted) as well as brain outcome, such as the brain regions of interest studied and/or phenotype (i.e., hyperintensities, volumes, etc.), varied widely across studies. Below, we describe each of these studies in greater detail.
Table 2.
CAH structural MRI studies
| Study | Participants | Age (Mean ± SD or range) | Sex | Phenotype & Age at Diagnosis | Treatment | MRI Type(s) | MRI Findings | Behavioral or Clinical Correlates |
|---|---|---|---|---|---|---|---|---|
| Sinforiani et al. 19948 | 19 CAH, 19 Control | 19.2 ± 2.6 yr (range: 16-24 yr) | CAH: 7 females (37%) Control: 7 females (37%) |
10 SW (53%) 9 SV (47%) Mean age at diagnosis: SV: 39.6 +/− 23.7 mo.; SW: 2.4 ± 5.8 mo. |
All treated with Dex (0.25-0.5 mg qHS); N=15 took hydrocortisone (10-25 qAM); All SW treated with fludrocortisone 0.1-0.15 mg/d | 0.5T Philips; T1w & T2w: 7-mm slice; | L peritrigonal hyperintensities (N=2); Cortical atrophy (N=2); R corona radiata hyperintensities (N=2); L corona radiata hyperintensities (N=2); R frontal hyperintensities (N=1) | None |
| Speiser et al. 19953 | 7 CAH, No Control Group | 27.5 years (range 14-33 yr) | CAH: 3 female (43%) | 4 SW (57%) 3 SV (43%) Age at diagnosis: NS |
Glucocorticoid (12.5-40 mg/day or 10.5-27 mg/m2/d hydrocortisone equivalent) | SW: 1.5T (NS); T1w: 3-mm slice, 1mm gap; T2w: 5-mm slice, 2.5-mm gap SV: 0.6T (NS); T1w: 3.7-mm thick slice, 2-mm gap; T2w: 7.5-mm slice, 0.5-mm gap |
Anterior pituitary hyperintensities (N=3); Microadenoma (N=3); Empty sella (N=1) | None |
| Plante et al. 199642 | 7 CAH, 3 unaffected siblings ‘Control’ MRI dataset |
Range: 5-16 yr | CAH: 6 female (86%) Unaffected siblings: NS | 6 SW (86%) 1 SV (14%) Age at diagnosis: NS | NS | 1.5T (NS) or 0.5T (NS); T2w: 5-mm slice | Atypical perisylvian asymmetries (N=5); No WM abnormalities noted; Focal migrational abnormality (N=1) | Brain findings paralleled poor language skills in four of the seven subjects with CAH; not all CAH patients with atypical asymmetry had poor language skills - some with asymmetry were also noted to be intellectually gifted. |
| Nass et al. 199743 | 39 CAH, No Control Group ‘Control’ MRI dataset |
19.2 ± 2.6 yr (range: 3 mo-36 yr) | CAH: 19 female (49%) | 27 SW (69%) 12 SV (31%) Age at diagnosis: NS |
NS | 1.5T Siemens or 0.5T (NS); T1w & T2w; 7.5- or 5-mm slice | WM abnormalities (N=14); Temporal lobe atrophy (N=11); Pituitary abnormalities (N=9) | Results did not differ by CAH treatment status or type of magnet (0.6 or 1.5 Tesla) |
| Merke et al. 200344 | 27 CAH, 47 Control | Range: 4-17 yr | CAH: 11 female (41%) Control: 13 female (48%) |
SW 18 (67%) SV 9 (33%) Age during treatment: SW: Birth-7 wk SV: Birth-5 yr |
Average hydrocortisone dose 14.6 +/− 6.4 mg/m2/d | 1.5T GE; T1w: 1.5x2 mm voxels | Temporal lobe, ventricle, and hippocampal volumes not different between groups; Decrease in L amygdala volume in males; Decrease in L and R amygdala volume in females; Cerebral volumes tended to be smaller in CAH females, but not significant | Prenatal glucocorticoid deficiency, excess adrenal sex steroids, or a combination of the two may stunt growth and development of the amygdala |
| Rose et al. 200445 | 16 CAH, 34 Control | 10.5 ± 2.9 yr (range: 5-17 yr) | CAH: No females | Type NS Age at diagnosis: NS | NS | 1.5T GE; T1w: 1.5x2 mm voxels | Decrease in total amygdala volume; Decrease in L amygdala volume | None |
| Bergamaschi et al. 200646 | 22 CAH, No Control Group 12 CAH studied ~11 yr prior as part of Sinforiani et al. 1994 |
19.9 yr (range: 16-25 yr) | CAH: 13 females (59%) | 12 SW (55%) 7 SV (32%) 3 NCAH (14%) Age during Treatment: Birth-6 wk | All treated with hydrocortisone and fludrocortisone | 0.5T Philips; T1w & T2w; no sequence specification | WM abnormalities (N=10) | No significant relationship was detected between brain abnormalities and clinical features |
| Mnif et al. 201247 (same results reported in Mnif et al. 201325) | 26 CAH, No Control Group | 27.5 ± 8.2 yr (range: 6.5-48 yr) | CAH: 15 female (58%) | 10 SW (38%) 8 SV (31%) 8 NCAH (31%); Age during treatment: SW: Birth-1 yr SV: Birth-6 yr | Hydrocortisone (N=21, first 2 yr: 27.9 mg/m2/d, childhood: 17.6 mg/m2/d, adulthood classical: 17.3 mg/m2/d, adulthood NCAH: 16.04 mg/m2/d); Dex (N=5, 0.25-0.75 mg/d) | 1.5T Philips; T1w & T2w (proton density & FLAIR) | Cerebellar WM hyperintensities (N=1) Periventricular WM hyperintensities (N=7); Partially empty sella (N=1); Cortico-subcortical atrophy (N=1); Complete agenesis of corpus callosum (N=1); R hippocampal dysgenesis (N=2, only NC); No cases of amygdala atrophy noted | Of 11 CAH patients with brain abnormalities, one was overtreated with glucocorticoids, two were adequately treated with wellcontrolled hormone levels, eight were non-compliant and thus undertreated |
| Webb et al. 201848 | 19 CAH, 19 Control | CAH: 30.6 ± 8.9 yr (range: 18-49 yr) Control: 32.8 ± 8.5 yr (range: 21-50 yr) |
CAH: 19 female (100%) Control: 19 female (100%) |
18 SW (95%) 1 NCAH (5%) Age at diagnosis: Birth-17 yr | All treated with glucocorticoids (11 Hydrocortisone, 8 Prednisolone) Hydrocortisone (N=11/19; median dose 10-13.8 mg/m2/d); Fludrocortisone (N=16/19, Median dose 150 μg/d) | 3T Philips; T1w | Smaller R hippocampus; Smaller R & L Thalamus; Smaller Cerebellum Smaller Brain Stem | Brain volumes did not correlate with glucocorticoid dose or androgen levels; Cerebellum volume associated with matrix reasoning scores; Brainstem volumes associated with working memory as well as digit span scores |
| Herting et al. 20209 | 27 CAH, 35 Control | CAH: 12.63 ± 3.35 yr (range: 8-18 yr) Control: 13.0 ± 2.8 yr (range: 8-18 yr) |
CAH: 16 female (59%) Control: 20 female (57%) |
25 SW (93%) 2 SV (7%) Age at diagnosis: diagnosed by positive newborn screen (n = 12) or biochemically and/or by genotype (n = 15; age of testing 11.2 ± 27.4 mo) |
All treated with glucocorticoids (16.5 ± 4.7 mg/m2/d); Fludrocortisone (N = 26/27, 0.11 ± 0.04 mg/m2/d) | 3T Siemens; T1w & T2w; 0.8-mm3 voxels | Smaller ICV Larger CSF volumes; Smaller L hippocampus volume; Smaller lateral nucleus of the amygdala; Smaller presubiculum and subiculum and CA1 subfields; Larger CA3 region of hippocampus | No associations were seen between brain volumes and CAH clinical features (e.g., BA SD, total testosterone and androstenedione, 17-hydroxyprogesterone, or glucocorticoid daily dose) |
| Van’t Westeinde et al. 202049 | 37 CAH unexposed to Dex (CAH noDex) 8 CAH exposed prenatally to Dex (CAH Dex) 43 Control |
21.7 ± 4.0 years (range: 16-33 yr) | CAH noDex: 21 female (57%) CAH Dex: 2 female (25%) Control: 26 female (60%) |
3 NCAH (8.1%) 16 SV (43.2%) 18 SW (48.6%) Age at diagnosis: NS |
Hydrocortisone: CAH female noDex: 12.5 ± 4.15 mg/m2 CAH male noDex: 14.89 ± 4.65 mg/m2 CAH female Dex: 12.07 ± 0.27 mg/m2 CAH male Dex: 13.68 ± 3.93 mg/m2 |
3T GE; T1w; 1 mm3 | CAH female noDex: Reduced whole brain volume CAH male noDex: Larger surface area in the L parietal and occipital lobes, including the pericalcarine and cuneus cortex CAH female Dex: Smaller bilateral rostral middle frontal gyrus, L superior and L inferior parietal cortex thickness CAH male Dex: Smaller precuneus volumes using VBM |
CAH patients performed worse on visuospatial working memory performance; After multiple comparison correction, volume of L precuneus and visuospatial memory were significant in controls; No associations were seen between gray matter outcomes and glucocorticoid dose, phenotype, CYP21A2 genotype; FAIM2 methylation was associated with surface area of the lateral occipital gyrus |
Abbreviations: 3T: 3 Tesla, BA: Bone Age, CAH: Congenital adrenal hyperplasia, CSF: cerebral spinal fluid, d: day, Dex: dexamethasone, FLAIR: fluid-attenuated inversion recovery, GC: glucocorticoid, GE: General Electric, L: left hemisphere; NS: not specified, mo: month, NCAH: non-classic, qAM: once every morning, qHS: once every night, R: right hemisphere, SD: Standard Deviation, SV: simple-virilizing, SW: salt-wasting, T1w: T1-weighted imaging, T2w: T2-weighted imaging, VBM: voxel-based morphometry, wk: week, WM: white matter, yr: year
3.2.1. CAH versus control sMRI findings
The first study to use MRI in patients with CAH was conducted in 1994.8 Of 19 CAH patients who also underwent neuropsychological evaluation, 15 had brain imaging on a 0.5T Philips scanner to examine the presence and grading of cortical or ventricular atrophy and/or white and gray matter abnormalities. The ‘control’ MRI dataset (N=50) included individuals referred for a scan due to either headaches or psychological disturbances without any notable structural central lesions; however, no other demographic information or MRI acquisition was provided about these individuals. Within the CAH group, 4/15 (27%) were found to have increased signal intensities in WM, including in the left peritrigonal region, the corona radiata and external capsule, and frontal WM. Mild ventricular dilation and cortical atrophy were also noted in these patients. As expected, none of the scan abnormalities were noted in the ‘control’ MRI dataset.8
In 1995, Speiser et al.3 studied 7 individuals with CAH (ages 14-33 years), and included imaging of the pituitary gland to examine glucocorticoid replacement therapy and the hypothalamic-pituitary-adrenal axis. Four SW CAH patients (57%) were found to have anterior pituitary abnormalities, including hypointensities congruent with microadenoma and empty sella turcica (n=1). Key limitations of this study include no specification of a control group for MRI comparison, and differing magnet strength for patients with SW (1.5T) vs. SV phenotypes (0.6T).3
In a two-part study, Plante and colleagues42 examined behavior and asymmetries in the perisylvian regions of the brain, known to be responsible for language, in children with CAH, unaffected siblings of the patients (“non-CAH siblings”), and controls. Seven children with CAH and three non-CAH siblings completed the imaging protocol. The CAH youth were compared to control scans matched for sex and ethnic background, albeit not age, from a database of healthy volunteers. Three CAH participants were also compared to their non-CAH siblings, with blind raters measuring bilateral perisylvian gray matter regions. Left-greater-than-right asymmetries are known to be reported in typically developing individuals, as the left perisylvian region is specifically related to language. However, six out of seven children affected by CAH (85%), and all three non-CAH siblings, were found to have atypical perisylvian asymmetries as they either showed left-equal-to-right or right-greater-than-left asymmetries. In contrast, only one control individual showed this atypical asymmetry. Diverging from the prior studies in older individuals, no WM abnormalities were noted in this sample of children affected by CAH, although one child had a focal migrational abnormality in the left hemisphere. Although this study did not correlate brain with behavior, poor language skills were noted in four of the eleven subjects with CAH; albeit not all CAH patients with poor language skills had atypical perisylvian asymmetry.42 Similar to previous studies, not all study participants were scanned on the same MRI scanner (most at 1.5T and two individuals at 0.5T) and the ‘control’ data were not matched on key sociodemographic variables, such as age.42
Nass et al.43 examined WM changes and temporal lobe atrophy in 39 young CAH patients, with ages ranging from 3 months to 36 years. T1- and T2-weighted images were taken on a 0.6T machine for approximately half of patients, while the other patients were scanned on a 1.5T Siemens scanner. Thirty-six percent of the CAH patients (N=14: 10 SW, 4 SV) were found to have WM abnormalities. The extent of these abnormalities ranged from a single lesion to diffuse patterns noted in periventricular and cerebellar regions. In terms of temporal lobe atrophy, twenty-eight percent of patients (N=11:7 SW, 4SV) were affected. Additional notable abnormalities included bifrontal atrophy (N=1), mild ventricular enlargement (N=4), pituitary abnormalities such as empty sella or microadenoma (N=9), and Chiari malformations (N=8). However, these abnormalities in CAH patients did not vary by age, phenotype (SW vs SV), treatment status (17-hydroxyprogesterone 500-2,000 ng/dL), or magnet strength (0.6T and 1.5T).43 While these abnormalities appeared in the two existing MRI data sources that Nass et al. 43 utilized as a ‘control’ group proxy, they were rare (e.g., one out of 301 scans (0.33%) in one sample). However, no demographic information, such as age or sex, was provided about these historic control samples extracted from existing databases.
Merke et al.44 performed the first study to recruit and scan CAH patients with an age- and sex-matched control group. MRI images were acquired for 27 CAH youth and 47 age- and sex-matched healthy controls using a 1.5T MRI scanner. The two groups were also similar in height, weight, pubertal stage, handedness, and IQ. Total cerebral volume as well as the ventricles, temporal lobe, hippocampal, and amygdala volumes were quantified by manual tracing performed by raters blind to subject characteristics. Total cerebral and temporal lobe volumes were not statistically different between CAH and control groups, but CAH females tended to have smaller volumes. A decrease of approximately 20% was also noted in the left amygdala volumes in CAH males and bilateral amygdala volumes in females, and these values remained significant after adjustments were made for total brain volume and age. Where healthy controls showed age-related increases in amygdala volumes, these patterns were diminished in children with CAH. In contrast to the amygdala, no significant differences were seen in hippocampal volume or the ventricles in CAH and control youth. A study conducted by Rose et al.45 on 16 CAH male participants and 34 age- and sex-matched control youth reported decreased total amygdala volume in both groups, likely driven by decreases in the left amygdala. It is, however, unclear if the 16 male participants from this study were independent or the same sample of 16 CAH males reported on from the original study published by Merke and colleagues in 2003.
Smaller brain volumes were also seen in a cross-sectional study of both children and adults conducted by Webb et al.48 In this study, 19 female CAH subjects and 19 healthy controls matched for age, sex, and educational status were scanned on a 3T scanner. CAH patients were either taking hydrocortisone (11 patients with a median dose of 11.1 mg/m2/d) or prednisolone (¼ the equivalent dose of hydrocortisone for each individual). Radiological review of the scans also revealed Type I Chiari malformations in 21% of participants (N=4). No other abnormalities were noted. Total brain volumes as well as cerebral spinal fluid (CSF) volume were quantified using a semi-automatic segmentation software along with a few a priori regions, namely the amygdala, hippocampus, thalamus, brainstem, and cerebellum, due to high concentrations of androgen, mineralocorticoid, and/or glucocorticoid receptors. After adjusting for total brain volumes and correcting for multiple comparisons, localized reductions in neural volumes were seen for the right hippocampus, thalamus, cerebellum, and brainstem. Interestingly, correlations were seen between cerebellar volume and matrix reasoning scores, as well as brainstem volumes and working memory and digit span scores. There were no associations between brain volumes and glucocorticoid dose or androgen levels.
Similarly, our group examined brain structure using 3T MRI for 27 CAH and 35 typically developing healthy control youth.9 The control group was matched for age, sex, and income, with no significant group differences in handedness, ethnicity/race, maternal education, IQ, and pubertal development; however, the CAH group was reported as having a higher body mass index on average. Brain volumes were quantified using a semi-automatic segmentation software and regions examined included whole brain (i.e., total brain volume, intracranial volumes, and total gray matter) as well as prefrontal and limbic regions of interest, including total amygdala and hippocampal volumes and their subregions. We found that CAH youth had smaller intracranial volumes, but more CSF on average when compared to controls. Moreover, after adjusting for intracranial volume, the superior frontal, caudal middle frontal, and left rostral middle frontal regions were significantly smaller in CAH youth compared to controls, with no group differences in amygdala volumes. However, decreases in left hippocampal volumes, with differences in hippocampal subregion composition noted, as well as a smaller lateral nucleus of the amygdala were seen in CAH as compared to controls. All CAH youth received glucocorticoid treatment in addition to fludrocortisone treatment, albeit brain differences in the CAH group did not relate to treatment doses or other clinical features.
Lastly, Van’t Westeinde and colleagues49 examined cortical thickness, surface area, and subcortical volumes as well as conducted voxel-based morphometry (VBM) using a 3T scanner to examine gray matter in 37 CAH individuals who were not prenatally exposed to dexamethasone (NoDex group) and 43 controls (ages 16-33 years). The authors also reported separately on a small sample of 8 CAH patients who were treated prenatally with dexamethasone (Dex group). No structural abnormalities were noted in the CAH group, and analyses were adjusted for age, sex, and total brain volumes as covariates given that CAH patients showed 4.23% smaller total brain volumes. Results showed a reduced bilateral rostral middle frontal gyrus, and left superior and left inferior parietal cortex, but increased surface area in the left parietal and occipital lobes, including the pericalcarine and cuneus cortex in CAH patients versus controls. A similar finding of smaller precuneus volumes in CAH and controls was also seen using VBM. No effects were reported for subcortical volumes, and no significant CAH by sex interactions were seen. Follow-up analyses corrected for multiple comparisons showed the smaller left precuneus result was associated with worse visuospatial working memory in control participants albeit the CAH group performed worse than controls on this task. Moreover, no associations with gray matter were seen with either glucocorticoid dose, phenotype, or CYP21A2 genotype. However, associations of brain metrics with FAIM2 methylation were also studied, with a negative association detected between FAIM2 methylation and surface area of the occipital gyrus. The FAIM2 gene is involved in protection against neuronal apoptosis and was previously reported by the same group to be hypermethylated in subjects with CAH. Interestingly, the small sample of prenatal dexamethasone-treated CAH patients had reduced surface area of the bilateral pericalcarine, as well as smaller gray matter volumes in the left pericalcarine and right superior parietal cortex as compared to the larger group of CAH patients not treated prenatally with dexamethasone.
3.2.2. Single group CAH sMRI findings
Two studies focus on a single sample of participants with varying CAH phenotypes. In the first, 22 CAH patients (ages 16-25 years) were scanned using a 0.5T Philips scanner.46 Focal WM hyperintensities were rated by two neuroradiologists using a visual rating scale and were seen in 45% (N=10) of CAH patients, with 3 patients showing focal hyperintensities (i.e., in a distinct brain location), 4 with diffuse hyperintensities (i.e., widespread throughout the brain), and 3 patients displayed both focal and diffuse abnormalities. Within the affected patients, focal hyperintensities were primarily localized in the corpus callosum or the periventricular region. Interestingly, of the 22 CAH patients, 12 had been previously scanned in a study done by Sinforiani 11 years prior and the patterns were unchanged.8 Albeit limited, these findings may suggest that, while WM abnormalities may be common in CAH patients, they may not be a sign of pathological progression.46
In the second study, 26 CAH patients (ages 16.5-48 years) were scanned using a 1.5T Philips scanner to examine WM hyperintensities and morphological abnormalities in the temporal lobe (i.e., hippocampus and amygdala).47 Of the 26 participants, 42.3% (N=11) had notable MRI abnormalities, including right hippocampal dysgenesis (N=2), cerebellar WM hyperintensities (N=1), periventricular WM hyperintensities (N=7), partially empty sella (N=1), cortico-subcortical atrophy (N=1), and agenesis of the corpus callosum (N=1). No cases of amygdala atrophy were noted, which contrasts with results from previously mentioned sMRI studies. Treatment conditions and CAH phenotypes were not explicitly specified in this study, limiting our understanding of observed neuropathophysiology.
3.2.3. Summary of sMRI
In the sMRI studies discussed above, sample sizes were relatively small and methods varied widely. Visual review of scans from CAH patients have noted similarities such as WM hyperintensities, cortical or subcortical atrophy, pituitary abnormalities, and Chiari malformations. Fifty-four percent of the studies to date have lacked a formal control group, rather relying on existing MRI datasets without clear sociodemographic or health information, or only studying a sample of individuals affected by CAH. A number of studies also used low-field scanners (i.e., 0.5T or 0.6T). Although the three studies on youth with control groups noted quantitative differences in limbic regions, such as the amygdala and hippocampus, the exact findings regarding significantly smaller volumes of these regions have been mixed. Moreover, only one study to date has investigated differences in typically noted brain asymmetries, such as the perisylvian region. Larger studies including both CAH patients and controls recruited and scanned on similar high-field scanners (i.e., 3T and 7T) are necessary. Lastly, existing studies to date suggest that overall brain volumes may be smaller in individuals affected by CAH and these differences may account for regional differences in gray or white matter.9,49 Thus, future structural MRI studies examining regional patterns of volumetric differences in CAH should examine and report group effects controlling for total whole brain or intracranial volumes in order to understand if regional differences are primarily driven by overall differences in brain sizes. Best practices may seek to publish both corrected and uncorrected findings, as has been done by Khorashad et al.50, in order to allow future studies to compare these distinct results.
3.3. Diffusion-weighted MRI (dMRI)
Diffusion-weighted MRI (dMRI), also known as diffusion-weighted imaging (DWI), utilizes Brownian motion of water molecules to indirectly determine tissue microstructure. DMRI looks at both isotropic diffusion, or free flow of water molecules equally in all directions, and anisotropic diffusion, in which diffusion may be restricted due to various cellular boundaries.51 Water diffusion is anisotropic in brain white matter because axon membranes limit molecular movement perpendicular to the fibers. Diffusion tensor imaging (DTI) and other mathematical models of diffusion can be used to exploit these physical properties to estimate micro-architectural detail of white matter tracts and provide information about white matter integrity.
Only three dMRI studies were identified, all of which included both CAH patients as well as a recruited and scanned control group (Table 3). Participant ages across the three studies ranged from 8-49 years, with sample sizes ranging from 15-55 subjects. Below, we discuss these three studies in further detail.
Table 3.
CAH diffusion MRI studies
| Study | Participants | Age (Mean ± SD) | Sex | Phenotype & Age at Diagnosis | Treatment | MRI Type | MRI Findings | Behavioral or Clinical Correlates |
|---|---|---|---|---|---|---|---|---|
| Webb et al. 201848 | 19 CAH 19 Control | CAH: 30.6 ± 8.9 yr Control: 32.8 ± 8.5 yr |
CAH: 19 Female Control: 19 Female |
18 SW (95%) 1 NCAH (5%) Mean age at diagnosis at 2 wk |
16 CAH treated with fludrocortisone (median: 150 μg/d) 8 CAH treated with prednisolone 11 CAH treated with hydrocortisone (median: 11.1 mg/m2/d) |
DWI; 3T Philips Achieva; 32-channel head coil; 2-mm3 voxel size; 60 directions b=1500s/m m2, 1 direction b=0 s/mm2 | Reduced FA and increased MD in fronto-occipital fasciculus, cingulate gyrus, and corticospinal tract | Higher GC dose correlated with bilaterally reduced mean diffusivity (r = 20.5, P = 0.03) No association seen between WM findings and androgen exposure or treatment type |
| Van’t Westeinde et al. 202049 | 35 CAH unexposed to Dex (CAH noDex) 8 CAH exposed prenatally to Dex (CAH Dex) 42 Control |
21.7 ± 4.0 yr | CAH NoDex: 20 Female CAH Dex: 2 Female Control: 25 Female |
Age at diagnosis NS CAH NoDex: 3 NC, 16, SV, 18 SW CAH Dex: 1 NC, 1 SV, 6 SW | CAH NoDex: female 12.5 ± 4.15 mg/m2, male 14.89 ± 4.65 mg/m2 CAH Dex: Female 12.07 ± 0.27 mg/m2, male 13.68 ± 3.93 mg/m2 | DWI; 3T GE; 8-channel head coil; 2.3-mm3 voxel size; 60 directions b=1500 s/mm2, 8 directions b=0 s/mm2 | CAH NoDex: Reduced FA in corpus callosum (forceps minor), R inferior longitudinal fasciculus, and cingulum Increased MD in bilateral inferior fronto-occipital fasciculus, inferior and superior longitudinal fasciculus Increased RD in R inferior fronto-occipital fasciculus and R anterior thalamic Age-by-sex interaction for bilateral corticospinal tract and R superior longitudinal fasciculus No findings significant after correcting for whole brain volumes CAH Dex: No white matter alterations compared with CAH NoDex |
No associations between WM findings and visuospatial memory, phenotype, CYP21A2 genotype, or FAIM2 methylation Higher GC dose was associated with increased mean FA and reduced mean RD, both with and without correcting for total brain volumes |
| Cotter et al. 202152 | 23 CAH 33 Control | CAH: 12.95 ± 3.49 yr Control: 13.13 ± 2.77 yr |
CAH: 14 (60.9%) Control: 20 (60.6%) |
Diagnosed via positive newborn screen and confirmatory serum analytes (n = 10) or biochemically with +/− genotype (n = 13) | Daily GC dosing (16.6 ± 5.0 mg/m2/d) | DWI; 3T Siemens; 32-channel head coil; 2-mm3 voxel size; 33 directions b=1000 s/mm2, 67 directions b=2500 s/mm2 | CAH youth has lower FA and higher MD in the fornix and stria terminalis Higher ODI in the stria terminalis Decreases in white matter integrity in the fornix associated with decreases in hippocampal volume Decreases in white matter integrity in the stria terminalis associated with decreases in amygdala volume | None |
Abbreviations: 3T: 3-Tesla, AD: axial diffusivity, CAH: Congenital adrenal hyperplasia, Dex: dexamethasone, DWI: diffusion weighted imaging, FA: fractional anisotropy, GC: glucocorticoid, L: left hemisphere, MD: mean diffusivity, NC: non-classic, NS: not specified, ODI: Orientation Dispersion Index, R: right hemisphere, RD: radial diffusivity, SV: simple-virilizing, SW: salt-wasting, WM: White Matter, yr: year
3.3.1. CAH vs control dMRI studies
Only three studies to date have examined dMRI outcomes in both a CAH sample and a control group. The first to do so was part of the previously mentioned study conducted by Webb and colleagues.48 Of the 19 female CAH adult subjects (SW: 18, NC: 1), dMRI on a 3T scanner were acquired for 18 CAH participants and 19 age- and sex-matched healthy controls. Again, all CAH patients from this study were either taking hydrocortisone or prednisolone. DTI modeling was then used to examine voxel-wise white matter microstructure across a white matter skeleton common to all participants. Results showed reductions in fractional anisotropy (FA) throughout the inferior fronto-occipital fasciculus, superior longitudinal fasciculus, uncinate fasciculus, corpus callosum, hippocampus, cingulate gyrus, and corticospinal tract. Bilateral mean diffusivity (MD), which is inversely related to FA, was also found to be increased in these aforementioned regions. Moreover, higher glucocorticoid-equivalent doses were associated with reduced MD. Diffusion findings did not correlate, however, with androgen exposure or treatment type (prednisolone vs. hydrocortisone).
DMRI via a 3T scanner was also assessed in the previously mentioned adult study by van’t Westeinde and colleagues.49 The final sample for these analyses included 35 CAH patients and 42 controls. A similar dMRI analysis approach was utilized as Webb and colleagues, with traditional DTI modeling performed and analyses examined in a common white matter skeleton. Reduced FA was seen in portions of the corpus callosum, right inferior longitudinal fasciculus, and cingulum, whereas increases in MD were noted in the bilateral inferior fronto-occipital fasciculus and bilateral inferior and superior longitudinal fasciculus. Increased radial diffusivity (RD) was also noted in the right inferior fronto-occipital fasciculus and the anterior thalamic radiation. An interaction was also noted between group status (CAH vs. control) and sex, for axial diffusivity (AD) in the bilateral corticospinal tract and the right superior longitudinal fasciculus. Interestingly, however, after adjusting for overall smaller total brain volumes, these associations were no longer significant. There was an association between higher levels of glucocorticoid treatment dose and increased mean FA as well as decreased mean MD, whereas phenotype, CYP21A2 genotype, FAIM2 methylation, and prenatal dexamethasone exposure were not found to relate to white matter microstructure in this sample.
The third and most recent dMRI study was conducted by our group, where we implemented a traditional DTI modeling approach as well as the more novel neurite orientation dispersion and density imaging (NODDI) modeling approach to investigate white matter microstructural integrity in a group of 23 CAH and 33 age- and sex-matched control youth.52 We were interested in key limbic and prefrontal cortex tracts, due to previous research indicating the involvement of these regions in association with CAH status. Results indicated that measures of white matter microstructural integrity were altered in the fornix and stria terminalis in CAH youth compared to controls. Specifically, CAH youth were found to have lower FA in both the fornix and stria terminalis as well as higher MD and RD in the fornix, using DTI modeling. Utilizing the NODDI approach, CAH youth were found to have a higher orientation dispersion index (ODI). Employing tractography, we additionally found significant group differences in along-tract microstructural properties within the fornix and the stria terminalis. There were group differences in FA, MD, and RD along the upper surface of the thalamus, near the commissure of the fornix, in both hemispheres. Along-tract group differences were found only within the FA of the right stria terminalis, similarly located along the upper surface of the thalamus; lower FA in the left hemisphere as well as higher bilateral ODI were homogeneous throughout the stria terminalis. Lastly, we investigated the association between gray matter volume and white matter integrity in corresponding ROIs, namely the fornix and hippocampus as well as the stria terminalis and the amygdala. Results indicated that, across the entire sample, smaller hippocampal volumes were associated with lower fornix FA, and smaller amygdala volumes were associated with both lower FA and higher ODI in the stria terminalis. However, there were no significant group differences in these associations.
3.3.3. Summary of dMRI
While studies utilizing dMRI in CAH populations are sparse (N=3) with relatively modest sample sizes (19-35 CAH participants), all demonstrate reduced FA and increased MD in CAH patients compared to controls, indicating that compromised white matter microstructural integrity is associated with CAH status. Regions identified include tracts within the limbic system (cingulum, fornix, stria terminalis) as well as tracts involved in connecting various brain regions with the frontal lobe (longitudinal fasciculus, fronto-occipital fasciculus), which are important for emotional processing and executive function, respectively.53
In addition to characterizing the regionality of white matter microstructural abnormalities, these studies investigated clinical correlates, such as glucocorticoid dose. Here, results were mixed: two studies found that higher glucocorticoid doses were associated with decreased white matter microstructural integrity (decreased FA, increased MD),48,49 while the third found no associations between glucocorticoid dosage and measures of white matter microstructure.52 Methodological differences may contribute to differences in findings: two studies (Webb et al.48 and Van’t Westeinde et al.49) utilized an adult population with a voxel-wise white matter skeleton approach, while the study by Cotter et al.52 consisted of a much younger adolescents and implemented subject-specific tractography to examine specific white matter tracts of interest. As such, the noted differences in glucocorticoid dosage associations with white matter microstructure could be due to length of exposure time. Additionally, while all three studies used a 3T scanner, each used a different manufacturer (Phillips, GE, Siemens) as well as varying numbers of head coil channels (i.e. 8-32). The consistency in the direction of the relationship between CAH and measures of white matter microstructure is encouraging, but more studies with larger sample sizes are needed to increase confidence in these results.
3.4. Functional MRI (fMRI)
Functional MRI (fMRI), in contrast to sMRI and dMRI, does not emphasize structural anatomy and pathology, but detects brain activity by measuring changes associated with blood flow and relative oxygen concentration in the blood supply.22 Due to the higher magnetic susceptibility of oxygenated blood compared to its deoxygenated counterpart, the powerful magnetic fields, field gradients, and radio waves produced by MRI machines offer insight into brain activity by following the ratio of oxygenated/deoxygenated blood over time.21 The brain’s relative oxygen concentration in its regional blood supply is closely tied to neural activity, and the measured hemodynamic response can be followed to determine how certain ailments affect brain activity in a given task relative to how the brain normally responds to said task.54 By measuring brain activity of individuals with CAH during behavioral tasks, impairments in normal functionality can be tied to regional deficiencies in the brain.
Only three fMRI studies focused on CAH were identified in our search of the extant literature (Table 4). All studies included CAH patients as well as a control group, with sample sizes ranging from 20-36 subjects. Below, we describe these studies and their findings.
Table 4.
CAH Functional MRI studies
| Study | Participants | Age (Mean ± SD) | Sex | Phenotype & Age of Diagnosis | Treatment | MRI Type(s) | fMRI Stimuli | MRI Findings | Behavioral & Clinical Correlates |
|---|---|---|---|---|---|---|---|---|---|
| Ernst et al., 200718 | 14 CAH, 14 control | 13.5 ± 2.10 yr Range: 9-18 yr |
CAH: 7 females (50%) Control: 7 females (50%) |
12 SW (86%) Females: 7 at birth (100%) Males: 6 in first year of life (86%), 1 at age 4 yr (14%) |
14 CAH (100%) treated with hydrocortisone (12.1 ± 5.3 mg/m2/day) and fludrocortisone (120 ± 48 mcg/day) | 3T GE; T2w EPI; voxel size = 3.75 x 3.75 x 5 mm | Emotional rating Task; Emotive faces, 4 emotion conditions, 4 rating conditions |
Angry vs neutral faces: L (p = 0.027) and R (p = 0.031) amygdala activation: CAH females > control females BL activation in the fusiform gyrus and occipital cortex: CAH > controls (p < 0.001) |
Negative faces rated more negative in CAH |
| Mazzone et al., 201155 | 14 CAH, 22 control | 13.98 ± 2.76 yr Range: 9-18 yr |
CAH: 7 females (50%) Control: 11 females (50%) |
NS | NS | 3T GE; T2w EPI; voxel size = 3.75 x 3.75 x 5 mm | Viewing emotive faces; 4 emotion conditions |
Remembered fear faces vs baseline: Sex by group interaction effects in hippocampal regions (BL posterior and anterior, p < 0.05) and R amygdala (p < 0.05); nonsignificant trend in L amygdala (p = 0.085); Activation patterns: CAH males > control males; CAH females < control females Remembered vs forgotten fear faces: L fusiform gyrus and L superior parietal lobule: Controls > CAH; BL pregenual cingulate cortices: CAH males > control males; BL pregenual cingulate cortices: Control females > CAH females |
Worse memory encoding of fearful faces in CAH BL posterior, R anterior hippocampal, and R amygdala activity negatively correlated with memory performance for fearful faces in control females and CAH males |
| Beltz et al., 202156 | 13 CAH, 7 Control | NS; all over 18 yr | CAH: 13 females (100%) Control: 7 females (100%) |
13 Classical (100%) | NS | 3T Siemens Trio or Prisma Fit; T2w EPI; voxel size = 3 mm3 | Card-guessing game reward task with monetary incentive; 3 runs each with 10 win and 10 loss trials | Approach network density: CAH > control (ventral striatum, orbitofrontal cortex, and ventromedial prefrontal cortex connectivity) No group differences in salience or regulatory network density |
No significant group differences in sensation seeking on outside of scanner survey |
Abbreviations: 3T: 3-Tesla, BL: bilateral, CAH: Congenital adrenal hyperplasia, EPI: Echo Planar Imaging, GC: glucocorticoid, GE: General Electric, L: left hemisphere, NC: non-classic, NS: not specified, R: right hemisphere, SV: simple-virilizing, SW: salt-wasting, T2w: T2-weighted imaging, yr: year
3.4.1. CAH vs control fMRI studies
The first of the two fMRI studies of CAH youth focused on amygdala function during a face-viewing task in 14 youth aged 9-18 with Classical CAH due to 21-hydroxylase deficiency and 14 age- and sex-matched controls.18 The groups did not differ by age, sex, or IQ. All 7 CAH girls were diagnosed at birth, and all but one of the boys were diagnosed in the first year of life. All CAH patients were treated with hydrocortisone and fludrocortisone and entered the study with normal cortisol and testosterone levels (save one female with high testosterone, whose exclusion did not change results and was thus retained). Whole-brain blood oxygen level dependent (BOLD) fMRI data was gathered in a single 14-minute run on a 3T scanner, during which each participant was exposed to 160 total images featuring faces with happy, angry, fearful, and neutral expressions. When viewing angry as compared to neutral facial expressions, CAH females displayed significantly higher left and right amygdala activation compared to control females, but no significant differences were observed between CAH and control males. Whole-brain exploratory analyses found significantly higher bilateral activation in the fusiform gyrus and occipital cortex in response to angry and fearful (as compared to neutral) faces in all CAH youth compared to controls.
The second fMRI study focused on emotional memory encoding and consisted of the same 14 CAH and 22 control youth.55 CAH phenotypes, age of diagnosis, and treatments were not specified. Whole-brain BOLD fMRI data were gathered in a single 14.2-minute run in a General Electric Signa 3T scanner, during which each participant was exposed to a total of 160 images: 10 images per facial expression condition (happy, angry, fearful, neural) and rating condition (rating anger, fear, width of nose, and looking without rating). Fixation trials served as a baseline for fMRI contrasts. After the scans and ratings, participants were shown 24 previously seen and 24 new faces and tested for memory outside of the scanner. Relative to controls, CAH had overall lower memory scores, with a significant group-by-emotion interaction effect, performing significantly worse for encoding of fearful faces. There were significant group differences in brain activation in response to remembered fear faces compared to baseline, with significant sex-by-diagnosis interaction effects in hippocampal regions and the right amygdala as well as a non-significant trend in the left amygdala. Specifically, CAH males had significantly higher BOLD activation in these regions compared to control males, while CAH females had significantly lower BOLD activation compared to control females. Activation patterns in CAH males, but not females, more closely resembled those of healthy females, with bilateral posterior and right anterior hippocampal and right amygdala activation being negatively correlated with memory performance for fearful faces. Additionally, whole-brain exploratory analyses showed that in response to fearful faces which were remembered as opposed to those which were forgotten, controls had higher BOLD activation in the left fusiform gyrus and left superior parietal lobule compared to CAH; CAH males had higher activation in the left and right pregenual cingulate cortices compared to control males; and CAH females had lower activation in the left and right pregenual cingulate cortices compared to control females.
The final fMRI study on CAH presented pilot data illustrating new methodologies in the context of an earlier CAH review paper and featured 13 adult females with classical CAH, compared to 7 non-affected same-sex siblings.56 Age of participants, age at diagnosis, and treatments were not specified. Whole-brain BOLD fMRI data were acquired using an echo planar imaging sequence on a 3T Siemens Trio (upgraded to a Magnetom Prisma Fit) scanner. Participants played three rounds of a card-guessing game with monetary incentive in the scanner, and had sensation-seeking measured via the Zuckerman Sensation Seeking Scale-V outside the scanner.57,58 Group differences in reward processing in approach, salience, and regulatory systems of the brain were examined by functional connectivity of 12 a priori regions of interest, as well their relation to sensation-seeking. CAH females had greater network density in the approach system in the brain (i.e., the ventral striatum, orbitofrontal cortex, and ventromedial prefrontal cortex) than control females. No significant group differences were observed in functional connectivity of the salience system (i.e., the amygdala and insula), the regulatory system (i.e., the dorsolateral prefrontal cortex and anterior cingulate cortex), or in sensation-seeking behaviors during the task.
3.4.2. Summary of fMRI
The extant fMRI literature on CAH is limited to three studies examining emotion perception,18 emotional memory,55 and reward processing.56 Though limited to only three small-scale studies, fMRI investigations of CAH suggest that activation patterns and functional connectivity of the brain may be altered, particularly in regions related to emotional appraisal and approach/avoidance activity. In both studies of emotion in CAH youth, significant group differences were observed in the amygdala.18,55 However, no group differences in task-based functional connectivity were observed in the amygdala in a later study of adult CAH females, suggesting that these group differences in amygdala activation are not a result of altered functional connectivity, or do not generalize to older populations.56 Other significant group differences which were not replicated across studies included significantly higher bilateral activation in the fusiform gyrus and occipital cortex in response to negative (i.e., angry and fearful) stimuli in CAH youth as compared to controls.18 Additionally, hyperactivation in CAH males and hypoactivation in CAH females, as compared to same-sex controls, was observed in the bilateral posterior and anterior hippocampi in response to remembered fear faces.55 Lastly, the single study examining functional connectivity found greater network density in the approach system in the brain (i.e., the ventral striatum, orbitofrontal cortex, and ventromedial prefrontal cortex) in CAH females as compared to control females.56
These three studies are limited by small sample sizes ranging from 13-14 CAH and 7-22 control participants. Methodologically, these studies each feature participants involved in active tasks inside a 3T MRI machine examining whole brain structure, function, and, in the case of the latter study by Beltz and colleagues56, task-based functional connectivity. Future research may expand on these methodologies by recruiting larger samples, and continuing to use high-field (i.e., 3T and 7T) MRI scanners, and implementing novel fMRI-based tasks to more completely understand the mechanisms by which differences in cognitive and emotional behavioral differences may emerge in CAH.
4. Risk of Bias Assessment
Risk of bias was assessed using JBI critical appraisal checklists for Case/Control, Case Series, and Case Report studies (Table 5).59,60 These checklists help to systematically review the quality of methodology implemented as well as address potential bias in design, conduct, and analysis of each study. For each set of questions, a score of 0 was given in the case that the criterion was met, whereas a score of 0.5 was given if the criteria was partially met, and a score of 1 was given if criteria was not at all met.
Table 5.
Risk of Bias Assessment
| CASE/CONTROL | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MRI Modality | Study | Were the groups comparable other than the presence of disease in cases or the absence of disease in controls? | Were cases and controls matched appropriately? | Were the same criteria used for identificatio n of cases and controls? | Was exposure measured in a standard, valid and reliable way? | Was exposure measured in the same way for cases and controls? | Were confoundin g factors identified? | Were strategies to deal with confoundin g factors stated? | Were outcomes assessed in a standard, valid and reliable way for cases and controls? | Was the exposure period of interest long enough to be meaningful? | Was appropriate statistical analysis used? | Overall Bias Score |
| Structural | Sinforiani et al. 1994 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | NA | 2 |
| Speiser et al. 1995 | 0 | 1 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 5 | |
| Plante et al. 1996 | 0 | 0.5 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 1 | 4.5 | |
| Nass et al. 1997 | 0 | 1 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 2 | |
| Merke et al. 2003 | 0 | 0.5 | 1 | 0 | 0 | 0.5 | 0.5 | 0 | 0 | 0 | 2.5 | |
| Rose et al. 2004 | 0 | 0.5 | 1 | 0 | 0 | 0.5 | 1 | 0 | 0 | 1 | 4 | |
| Herting et al. 2020 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |
| Structural + Diffusion | Webb et al. 2018 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Van’t Westeinde et al. 2020 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | |
| Diffusion | Cotter et al. 2021 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
| Functional | Ernst et al., 2007 | 0 | 0 | 1 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 |
| Mazzone et al., 2011 | 0 | 0 | 1 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 3 | |
| Beltz et al., 2021 | 0 | 0 | 0 | 0 | 0 | 1 | 1 | 0 | 0 | 0 | 2 | |
| CASE SERIES | ||||||||||||
| MRI Modality | Study | Were there clear criteria for inclusion in the case series? | Was the condition measured in a standard, reliable way for all participants included in the case series? | Were valid methods used for identificatio n of the condition for all participants included in the case series? | Did the case series have consecutive inclusion of participants? | Did the case series have complete inclusion of participants? | Was there clear reporting of the demographi cs of the participants in the study? | Was there clear reporting of clinical information of the participants? | Were the outcomes or follow up results of cases clearly reported? | Was there clear reporting of the presenting site(s)/clinic(s) demographic information? | Was statistical analysis appropriate? | Overall score |
| Structural | Bergamaschi et al. 2006 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 1 | 0 | 1 |
| Mnif et al. 2012 (same results reported in Mnif et al. 2013) | 0 | 0 | 0 | 0 | 0.5 | 0 | 0 | 0 | 1 | 0 | 1.5 | |
| Diffusion | Abe et al. 2016 | 0 | 0 | 0 | 1 | 1 | 1 | 0 | 0 | 1 | 0 | 4 |
| CASE REPORTS | ||||||||||||
| MRI Modality | Study | Were patient’s demographic characteristics clearly described? | Was the patient’s history clearly described and presented as a timeline? | Was the current clinical condition of the patient on presentation clearly described? | Were diagnostic tests or assessment methods and the results clearly described? | Was the intervention(s) or treatment procedure(s) clearly described? | Was the post-intervention clinical condition clearly described? | Were adverse events (harms) or unanticipated events identified and described? | Does the case report provide takeaway lessons? | Overall score | ||
| Structural | Bergamaschi et al. 2004 | 0.5 | 0 | 0 | 0 | 0 | 0 | NA | 0 | 0.5 | ||
| Bhangoo et al. 2005 | 0 | 0 | 0 | 0 | 0 | 0 | NA | 0 | 0 | |||
| Okamoto et al. 2006 | 0.5 | 0 | 0 | 0 | 0 | 0 | NA | 0 | 0.5 | |||
| O’shea et al. 2016 | 0.5 | 0 | 0 | 0 | 0 | 0 | NA | 0 | 0.5 | |||
| Gaudiano et al. 2010 | 0 | 0 | 0 | 0 | 0 | NA | NA | 0 | 0 | |||
| Samia et al. 2010 | 0.5 | 0 | 0 | 0 | 0 | 0.5 | NA | 0 | 1 | |||
| Kaga et al. 2013 | 0 | 0 | 0 | 0 | 0 | 0 | NA | 0 | 0 | |||
| Diffusion | Winfeld et al. 2013 | 0.5 | 0 | 0 | 0 | 0 | 0 | NA | 0 | 0.5 | ||
| Grunt et al. 2013 | 0.5 | 0 | 0 | 0 | 0 | 0 | NA | 0 | 0.5 | |||
| Saito et al. 2008 | 0.5 | 0 | 0 | 0 | 0 | 0 | NA | 0 | 0.5 | |||
| Lee et al. 2011 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | |||
| Serter et al. 2015 | 0.5 | 0 | 0 | 0 | 0 | 0 | NA | 0 | 0.5 | |||
Higher scores reflect greater bias. Individual criterion answer key: 0: Criteria Met, 0.5: Criteria Partially Met, 1: Criteria Not Met, NA: Criteria Not applicable.
With the low prevalence of CAH, it becomes difficult to recruit a significant number of CAH participants per study. This leads to uncertainty over whether CAH is the cause of the observed brain abnormalities within these studies. Moreover, some of the earlier dated sMRI studies also either failed to correct for confounding variables or omitted them entirely, and often failed to properly match the CAH and control groups. Specifically, in many of these earlier studies, the age range of the control groups was too broad, the controls were not sex-matched, and/or they underwent a completely different selection process than the CAH group. While this limitation accounts for a large portion of internal validity concerns in the CAH versus control studies, it should be noted that several of the newer studies conducted did implement proper statistical analysis, factored in confounding variables, and, where applicable, closely matched controls to the CAH group. Thus, these more recent studies have implemented more robust methods to alleviate potential errors in internal validity.
Risk of bias was also assessed separately for case series and case reports (Table 5). All case series studies failed to provide site/clinic demographic information. Additionally, the case series conducted by Abe et al.38 failed to meet several criteria due to lack of cooperability. Specifically, it was a retroactive study that relied on requested data from clinicians; several clinicians did not respond, and others failed to provide sufficient demographic information.38 Similarly, across the case reports, a consistent limitation was the lack of clear demographic descriptions of the patients (i.e., not reporting race, ethnicity, and socioeconomic status).
In addition to the more objective risk of bias assessment presented above, we also considered performance and detection bias (i.e., blinding of personnel and outcome assessment) as well as reporting bias in the reviewed papers. Case reports and case series have high risk of both performance and detection bias given they focus solely on patients diagnosed with CAH. All other studies did not include enough detail to assess whether personnel working with the participants or conducting the brain imaging scan were blinded to participants’ diagnosis, making it unclear as to the risk for performance bias. In terms of detection bias, a handful of the observational sMRI studies included details of multiple raters and/or blind raters to the group membership of each MRI scan observation. Moreover, many of the observational studies with control groups that were also recruited and scanned utilized computer programs and algorithms widely accepted in the neuroimaging community; thus, reducing the risk that could bias estimating morphometry, microstructure, or functional results for each subject. Lastly, selective reporting bias was also unclear from the current literature. Most studies examined a certain set of a priori brain regions of interest and did not report all possible data collected in addition to MRI, or if they examined potential correlations (or lack thereof) with clinical features of CAH.
5. Future Directions
Structural, diffusion, and functional MRI have begun to provide a glimpse into the neurological phenotypes in patients affected by CAH. While common findings include notable WM hyperintensities and mention of cortical atrophy, few well-designed MRI studies have been conducted to examine gray and white matter volumetrics, white matter microstructure, and brain activity in patients affected by CAH as compared to a well-matched control sample. In the observational CAH versus matched control studies that have been conducted, findings suggest structural and functional brain differences in individuals with CAH. Smaller overall brain volumes and smaller subcortical volumes in regions with high expression of sex steroids, along with altered white matter microstructure in tracts connecting brain regions involved in both cognitive and emotional processes, have been noted in CAH patients as compared to controls. Subcortical regions, including the amygdala, hippocampus, and portions of the striatum, may also activate differently in patients with CAH when attending to tasks of emotion or reward processing. With that said, however, the directionality and regional specificity of the results are mixed with no strong consensus to date. Together, the existing literature suggests non-invasive MRI methodology holds great promise in detecting neurological differences in patients affected by CAH, but also emphasizes the need for additional larger and well-designed studies to begin to characterize when and how brain structure and function may be affected by this endocrine disorder. Below, we outline ways to improve methodological rigor, as well as important challenges and key questions to be addressed by this emerging field of brain MRI research in CAH.
Probing CAH patient variability with big data
As with many clinical disorders, age at diagnosis, genotype and phenotype variation, treatment choices and adherence, and potential comorbid health conditions contribute to wide variations in a single patient population.61 While these clinical features pose a challenge, they also are likely to have widespread implications in moderating neurological effects noted on MRI in patients with CAH. As such, future longitudinal MRI studies will allow for a better appreciation of the potential individual differences in disease progression, maintenance, treatment adherence, and brain changes over time in those diagnosed with CAH. As previously mentioned, with the relatively rare prevalence of CAH, it can be challenging to obtain large CAH study samples. Thus, to aid in achieving these goals, consortium-based efforts, such as ENIGMA62 should be pursued that utilize previously collected MRI data to analyze key questions in unison. Specifically, the ENIGMA Consortium brings together researchers worldwide to “investigate structure, function, and disease using neuroimaging and genetic datasets”. Such large-scale consortiums may provide the ability to utilize small samples to study important empirical questions in patient populations, such as allowing for researchers to answer key questions as to how clinical features of CAH may uniquely map onto brain structure and function in both national and international patient samples. Two approaches widely used by ENIGMA working groups are meta-analysis and mega-analysis. Meta-analysis includes synthesizing summary statistics of individual MRI studies, whereas mega-analysis pools raw data across existing MRI studies. Thus, similar to recent efforts to utilize mega-analytic methods to better study brain variability in transgender persons63 statistical modeling while adjusting for site-effects could be employed to individual MRI data of CAH and control participants collected across various sites and MRI scanners to overcome these challenges. This type of mega-analytic framework would ultimately allow for examining otherwise underpowered testing of CAH-related brain differences between SW versus SV phenotypes, potential effects of various types and dosages of glucocorticoid replacement treatments, as well as variation in neuroanatomy as it pertains to the several genetic mutations that cause CAH (e.g., CYP21A2, HSD3B2). In other words, pooled analyses across various cohorts of CAH will be essential for us to determine how age at diagnosis, as well as genotype and phenotype variation map on to neuroanatomical differences and subsequent brain function.
New CAH studies to study brain networks and connectomics
For researchers that aim to launch a new CAH neuroimaging study, exciting possibilities continue to emerge with advancements in both multimodal MRI data acquisition and analytic approaches. For example, 3T and 7T scanners combined with a 32- or 64-channel radiofrequency head-coil allow for improved MRI signal-to-noise ratios, whereas recent sequences that couple contrasting weighted images, such as collecting 3D T1-weighted and T2-weighted structural scans, with higher spatial resolution (< 1mm3 voxel sizes) vastly improves accuracy in sMRI outcome estimations. Similarly, novel dMRI sequences now exist that can be used for biophysical modeling of water diffusion in intracellular and extracellular spaces, such as the NODDI method implemented in Cotter et al.52 These new methods reduce issues with crossing fibers in studying white matter microstructure and aid in characterizing biological factors that may be contributing to altered signals in more common DTI outcomes. Similar to Webb et al.48 and van’t Westeinde et al.49, studies can perform various imaging modalities (sMRI and dMRI) within the same populations to allow for a more complete quantification of altered brain structure. In looking at size, shape, and microstructural properties as the primary outcome, the field of CAH brain imaging has focused primarily on quantifying group differences using a priori brain regions of interest including the prefrontal cortex and subcortical volumes based on the high concentration of both androgen and glucocorticoid receptors.9,44,52 Moving forward, future multimodal studies have the opportunity to expand upon these findings and to examine differences in larger-scaled connectome properties that may shed light on alterations to larger brain systems involved in emotion and cognitive processes (e.g., Markett et al.64). For example, despite functional connectivity studies showing robust and stable neural networks that map onto various brain functions65, functional organization and connectivity of brain networks in patients with CAH remains unknown. More importantly, several studies have shown resting-state fMRI to be useful in identifying patient populations as well as potential sub-populations within various clinical conditions (e.g., Fair et al.66). Thus, by examining various brain outcomes using multi-modal imaging techniques, localization of macro- and microstructural as well as functional differences in specific brain regions and/or large scaled networks may offer potential insight as to what may be ailing CAH children. Similarly, despite behavioral reports of potential CAH differences in spatial ability and working memory, few studies have implemented fMRI task paradigms to study the neurobiological underpinnings contributing to these differences.11–13 Of course, in implementing these advanced neuroimaging approaches, new studies should follow well-known best practices for collecting and reporting acquisition and analyses for MRI neuroimaging67 as doing so will help to improve study rigor and reproducibility, and strengthen our understanding of how the brain may be impacted by CAH.
Understanding of hormone and brain changes across development
Understanding how various clinical features of CAH relate to MRI outcomes holds relevance not only for improved precision-medicine in treating individuals affected by CAH, but as previously outlined by Mueller20, may also provide information as to how prenatal and childhood hormonal factors influence neurodevelopment as well as cognitive and emotional outcomes. For example, given that the brain undergoes dynamic changes in development both pre- and postnatally, with many larger-scaled cognitive and emotional systems continuing to refine themselves across adolescence and into young adulthood, pertinent questions about neurological CAH phenotypes at various ages may also shed light on what roles hormones have in neuromaturation.68,69 Specifically, animal models suggest testosterone exposure in utero contributes to specific brain and behavioral phenotypes based on the animal’s biological sex chromosome (XX or XY).70 Taken together, these studies have led to the organizational versus activational effects of sex steroids, which postulates sex steroids prenatally contribute to the wiring of the brain prior to birth, whereas sex steroids postnatally (i.e. puberty, adulthood) contribute to activational or behavioral phenotypes.71 In CAH, the disruption to glucocorticoid synthesis also results in an overproduction of androgens by the adrenal glands beginning in utero. Thus, a firm understanding of potential differing effects on brain maturation in male or female patients with CAH as compared to unaffected controls may help shed light as to ways androgen levels do (or do not) influence brain structure and function. If various differences in brain structure are apparent at birth in patients diagnosed with CAH as compared to unaffected controls, these findings may support the organizational hypothesis as to the importance of prenatal hormones. On the contrary, if these patterns emerge with development as a function of either age or pubertal maturation, and/or if differences are exacerbated in adulthood, it may suggest the organizational effects of hormones continue into various sensitive periods of postnatal development, such as adolescence. In conducting CAH research to help study these larger neurodevelopmental questions, researchers may also consider leveraging existing big datasets as well as collecting new CAH MRI data. For instance, large developmental MRI studies such as the developmental Human Connectome Project (HCP)72 as well as the Adolescent Brain Cognitive Development (ABCD)73 studies, provide researchers with large public datasets as well as established MRI acquisition and preprocessing pipelines that may be used in CAH neuroimaging studies to provide a well-matched sociodemographic sample (i.e., age, sex, race/ethnicity, socioeconomic status, pubertal stage, etc.) or help to interpret specific cross-sectional and longitudinal CAH findings in the wider context of age- and sex-related patterns of neurodevelopment.
6. Conclusion
In summary, the existing literature on brain structure and function in individuals affected by CAH is an emerging field. More than half of the existing literature consists of case studies that utilized MRI to visually assess white matter hyperintensities, cortical atrophy, or incidental findings. While these studies have been useful for generating potential hypotheses and/or identifying common comorbidities noted in patients with CAH, few observational studies have been designed and implemented to examine brain structure and function in those affected by CAH as compared to a control sample. While some common findings are seen across these observational CAH versus control MRI studies, the small sample sizes and mixed patterns of results highlight the need to leverage pooled data strategies and cutting-edge MRI methods as well as follow best practices to improve rigor and reproducibility for MRI neuroimaging CAH studies. Future studies also need to address potential neurological influences of CAH across the lifespan and in relation to known clinical and behavioral phenotypes seen across individuals impacted by CAH. Examining the potential heterogeneity in CAH phenotypes that may influence regional and large-scaled neural networks will greatly advance our understanding of CAH, as well as contribute to the critical knowledge base that may help develop prevention and treatment strategies available to patients with CAH.
Role of the funding source
Grants supporting the writing of the paper include the National Institutes of Health K01 MH1087610 (MMH), K23HD084735 and R03 HD101718-01 (MSK). The funding sources had no involvement in the preparation of the article nor in the analysis or interpretation of the work presented in this article.
Declaration of interest
MSK receives research support from Neurocrine Biosciences. MEG receives grant support from Novo Nordisk; consultant fees from Daiichi Sankyo, Ferring, Novo Nordisk, Nutrition & Growth Solutions, Pfizer, Sandoz, and Spruce Biosciences; serves on data safety monitoring boards for Ascendis, Millendo, and Tolmar; and receives royalties from McGraw-Hill and UpToDate.
References:
- 1.White PC, Speiser PW. Congenital Adrenal Hyperplasia due to 21-Hydroxylase Deficiency*. Endocr Rev. 2000;21(3):245–291. doi: 10.1210/edrv.21.3.0398 [DOI] [PubMed] [Google Scholar]
- 2.Witchel SF. Congenital Adrenal Hyperplasia. J Pediatr Adolesc Gynecol. 2017;30(5):520–534. doi: 10.1016/j.jpag.2017.04.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Speiser PW, Heier L, Serrat J, New MI, Nass R. Failure of Steroid Replacement to Consistently Normalize Pituitary Function in Congenital Adrenal Hyperplasia: Hormonal and MRI Data. Horm Res. 1995;44(6):241–246. doi: 10.1159/000184635 [DOI] [PubMed] [Google Scholar]
- 4.Almasri J, Zaiem F, Rodriguez-Gutierrez R, et al. Genital Reconstructive Surgery in Females With Congenital Adrenal Hyperplasia: A Systematic Review and Meta-Analysis. J Clin Endocrinol Metab. 2018;103(11):4089–4096. doi: 10.1210/jc.2018-01863 [DOI] [PubMed] [Google Scholar]
- 5.Mueller SC, Grissom EM, Dohanich GP. Assessing gonadal hormone contributions to affective psychopathologies across humans and animal models. Psychoneuroendocrinology. 2014;46:114–128. doi: 10.1016/j.psyneuen.2014.04.015 [DOI] [PubMed] [Google Scholar]
- 6.McEwen BS, Bowles NP, Gray JD, et al. Mechanisms of stress in the brain. Nat Neurosci. 2015;18(10):1353–1363. doi: 10.1038/nn.4086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Helleday J, Bartfai A, Martin Ritzén E, Forsman M. General intelligence and cognitive profile in women with congenital adrenal hyperplasia (CAH). Psychoneuroendocrinology. 1994;19(4):343–356. doi: 10.1016/0306-4530(94)90015-9 [DOI] [PubMed] [Google Scholar]
- 8.Sinforiani E, Livieri C, Mauri M, et al. Cognitive and neuroradiological findings in congenital adrenal hyperplasia. Psychoneuroendocrinology. 1994;19(1):55–64. doi: 10.1016/0306-4530(94)90059-0 [DOI] [PubMed] [Google Scholar]
- 9.Herting MM, Azad A, Kim R, Tyszka JM, Geffner ME, Kim MS. Brain Differences in the Prefrontal Cortex, Amygdala, and Hippocampus in Youth with Congenital Adrenal Hyperplasia. J Clin Endocrinol Metab. 2020;105(4):1098–1111. doi: 10.1210/clinem/dgaa023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Messina V, Karlsson L, Hirvikoski T, Nordenström A, Lajic S. Cognitive Function of Children and Adolescents With Congenital Adrenal Hyperplasia: Importance of Early Diagnosis. J Clin Endocrinol Metab. 2020;105(3):e683–e691. doi: 10.1210/clinem/dgaa016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Browne WV, Hindmarsh PC, Pasterski V, et al. Working memory performance is reduced in children with congenital adrenal hyperplasia. Horm Behav. 2015;67:83–88. doi: 10.1016/j.yhbeh.2014.11.014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Karlsson L, Gezelius A, Nordenström A, Hirvikoski T, Lajic S. Cognitive impairment in adolescents and adults with congenital adrenal hyperplasia. Clin Endocrinol (Oxf). 2017;87(6):651–659. doi: 10.1111/cen.13441 [DOI] [PubMed] [Google Scholar]
- 13.Puts DA, McDaniel MA, Jordan CL, Breedlove SM. Spatial Ability and Prenatal Androgens: Meta-Analyses of Congenital Adrenal Hyperplasia and Digit Ratio (2D:4D) Studies. Arch Sex Behav. 2008;37(1):100–111. doi: 10.1007/s10508-007-9271-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Berenbaum SA, Bryk KLK, Beltz AM. Early androgen effects on spatial and mechanical abilities: Evidence from congenital adrenal hyperplasia. Behav Neurosci. 2012;126(1):86–96. doi: 10.1037/a0026652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hampson E, Rovet JF. Spatial function in adolescents and young adults with congenital adrenal hyperplasia: Clinical phenotype and implications for the androgen hypothesis. Psychoneuroendocrinology. 2015;54:60–70. doi: 10.1016/j.psyneuen.2015.01.022 [DOI] [PubMed] [Google Scholar]
- 16.Wong WI, Pasterski V, Hindmarsh PC, Geffner ME, Hines M. Are There Parental Socialization Effects on the Sex-Typed Behavior of Individuals with Congenital Adrenal Hyperplasia? Arch Sex Behav. 2013;42(3):381–391. doi: 10.1007/s10508-012-9997-4 [DOI] [PubMed] [Google Scholar]
- 17.Hines M, Kaufman FR. Androgen and the development of human sex-typical behavior: rough-and-tumble play and sex of preferred playmates in children with congenital adrenal hyperplasia (CAH). Child Dev. 1994;65(4):1042–1053. [PubMed] [Google Scholar]
- 18.Ernst M, Maheu F, Schroth E, et al. Amygdala function in adolescents with congenital adrenal hyperplasia: A model for the study of early steroid abnormalities. Neuropsychologia. 2007;45(9):2104–2113. doi: 10.1016/j.neuropsychologia.2007.01.019 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Berenbaum SA, Resnick SM. Early androgen effects on aggression in children and adults with congenital adrenal hyperplasia. Psychoneuroendocrinology. 1997;22(7):505–515. doi: 10.1016/S0306-4530(97)00049-8 [DOI] [PubMed] [Google Scholar]
- 20.Mueller SC. Magnetic Resonance Imaging in Paediatric Psychoneuroendocrinology: A New Frontier for Understanding the Impact of Hormones on Emotion and Cognition. J Neuroendocrinol. 2013;25(8):762–770. doi: 10.1111/jne.12048 [DOI] [PubMed] [Google Scholar]
- 21.Buxton RB. Introduction to Functional Magnetic Resonance Imaging: Principles and Techniques. 2nd ed. Cambridge University Press; 2009. [Google Scholar]
- 22.Huettel SA, Song AW, McCarthy G. Functional Magnetic Resonance Imaging. 2nd ed. Sinauer Associates; 2008. [Google Scholar]
- 23.Lebel C, Deoni S. The development of brain white matter microstructure. NeuroImage. 2018;182:207–218. doi: 10.1016/j.neuroimage.2017.12.097 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Page MJ, Moher D, Bossuyt PM, et al. PRISMA 2020 explanation and elaboration: updated guidance and exemplars for reporting systematic reviews. BMJ. Published online March 29, 2021:n160. doi: 10.1136/bmj.n160 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mnif M, Kamoun M, Mnif F, et al. Brain magnetic resonance imaging findings in adult patients with congenital adrenal hyperplasia: Increased frequency of white matter impairment and temporal lobe structures dysgenesis. Indian J Endocrinol Metab. 2013;17(1):121. doi: 10.4103/2230-8210.107833 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bergamaschi R, Livieri C, Candeloro E, Uggetti C, Franciotta D, Cosi V. Congenital Adrenal Hyperplasia and Multiple Sclerosis: Is There an Increased Risk of Multiple Sclerosis in Individuals With Congenital Adrenal Hyperplasia? Arch Neurol. 2004;61(12). doi: 10.1001/archneur.61.12.1953 [DOI] [PubMed] [Google Scholar]
- 27.Bhangoo A, Gu WX, Pavlakis S, et al. Phenotypic Features Associated with Mutations in Steroidogenic Acute Regulatory Protein. J Clin Endocrinol Metab. 2005;90(11):6303–6309. doi: 10.1210/jc.2005-0434 [DOI] [PubMed] [Google Scholar]
- 28.Okamoto R, Saito Y, Inoue T, Maegaki Y, Nagaishi J ichi, Ohno K. Forced mouth opening reaction: A primitive reflex released from cortical inhibition. Brain Dev. 2006;28(4):272–274. doi: 10.1016/j.braindev.2005.09.001 [DOI] [PubMed] [Google Scholar]
- 29.Saito Y, Ogawa T, Nagaishi J ichi, Inoue T, Maegaki Y, Ohno K. Laminar cortical necrosis in adrenal crisis: Sequential changes on MRI. Brain Dev. 2008;30(1):77–81. doi: 10.1016/j.braindev.2007.05.007 [DOI] [PubMed] [Google Scholar]
- 30.Gaudiano C, Malandrini A, Pollazzon M, et al. Leukoencephalopathy in 21-beta hydroxylase deficiency: report of a family. Brain Dev. 2010;32(5):421–424. doi: 10.1016/j.braindev.2009.04.004 [DOI] [PubMed] [Google Scholar]
- 31.Samia YM, Mahdi K, Baha Z, Saida JO, Tahar SM, Habib SM. Congenital Adrenal Hyperplasia and Brain Magnetic Resonance Imaging Abnormalities. Clin Pediatr Endocrinol. 2010;19(4):109–113. doi: 10.1297/cpe.19.109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lee S, Sanefuji M, Watanabe K, et al. Clinical and MRI characteristics of acute encephalopathy in congenital adrenal hyperplasia. J Neurol Sci. 2011;306(1-2):91–93. doi: 10.1016/j.jns.2011.03.037 [DOI] [PubMed] [Google Scholar]
- 33.Kaga A, Saito-Hakoda A, Uematsu M, et al. Brain white matter abnormality in a newborn infant with congenital adrenal hyperplasia. Clin Pediatr Endocrinol Case Rep Clin Investig Off J Jpn Soc Pediatr Endocrinol. 2013;22(4):77–81. doi: 10.1292/cpe.22.77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Grunt S, Steinlin M, Weisstanner C, Schöning M, Mullis PE, Flück CE. Acute Encephalopathy with Unilateral Cortical-Subcortical Lesions in Two Unrelated Kindreds Treated with Glucocorticoids Prenatally for Congenital Adrenal Hyperplasia due to 21-Hydroxylase Deficiency: Established Facts and Novel Insight. Horm Res Paediatr. 2013;80(1):57–63. doi: 10.1159/000348515 [DOI] [PubMed] [Google Scholar]
- 35.Winfeld M, Patel P, Shah B, Nass R, Milla S. Early occurrence of cerebral white matter abnormality detected in a neonate with salt-wasting congenital adrenal hyperplasia. J Pediatr Endocrinol Metab. 2013;26(1-2). doi: 10.1515/jpem-2012-0154 [DOI] [PubMed] [Google Scholar]
- 36.Serter A, Alkan A, Demirkol D. Diffusion MRI features of acute encephalopathy due to stopping steroid medication abruptly in congenital adrenal hyperplasia. Ann Indian Acad Neurol. 2015;18(3):342. doi: 10.4103/0972-2327.152086 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.O’Shea T, Crowley RK, Farrell M, et al. Growth of a progesterone receptor-positive meningioma in a female patient with congenital adrenal hyperplasia. Endocrinol Diabetes Metab Case Rep. 2016;2016. doi: 10.1530/EDM-16-0054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Abe Y, Sakai T, Okumura A, et al. Manifestations and characteristics of congenital adrenal hyperplasia-associated encephalopathy. Brain Dev. 2016;38(7):638–647. doi: 10.1016/j.braindev.2016.01.007 [DOI] [PubMed] [Google Scholar]
- 39.Trip SA, Miller DH. Imaging in multiple sclerosis. J Neurol Neurosurg Psychiatry. 2005;76(suppl 3):iii11–iii18. doi: 10.1136/jnnp.2005.073213 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Gouw AA, Seewann A, van der Flier WM, et al. Heterogeneity of small vessel disease: a systematic review of MRI and histopathology correlations. J Neurol Neurosurg Psychiatry. 2011;82(2):126–135. doi: 10.1136/jnnp.2009.204685 [DOI] [PubMed] [Google Scholar]
- 41.Symms M A review of structural magnetic resonance neuroimaging. JNeurol Neurosurg Psychiatry. 2004;75(9): 1235–1244. doi: 10.1136/jnnp.2003.032714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Plante E, Boliek C, Binkiewicz A, Erly WK. Elevated androgen, brain development and language/leaming disabilities in children with congenital adrenal hyperplasia. Dev Med Child Neurol. 1996;38(5):423–437. doi: 10.1111/j.l469-8749.1996.tbl5100.x [DOI] [PubMed] [Google Scholar]
- 43.Nass R, Heier L, Moshang T, et al. Magnetic Resonance Imaging in the Congenital Adrenal Hyperplasia Population: Increased Frequency of White-Matter Abnormalities and Temporal Lobe Atrophy. J Child Neurol. 1997; 12(3): 181–186. doi: 10.1177/088307389701200306 [DOI] [PubMed] [Google Scholar]
- 44.Merke DP, Fields JD, Keil MF, Vaituzis AC, Chrousos GP, Giedd JN. Children with Classic Congenital Adrenal Hyperplasia Have Decreased Amygdala Volume: Potential Prenatal and Postnatal Hormonal Effects. J Clin Endocrinol Metab. 2003;88(4): 1760–1765. doi: 10.1210/jc.2002-021730 [DOI] [PubMed] [Google Scholar]
- 45.Rose AB, Merke DP, Clasen LS, et al. Effects of Hormones and Sex Chromosomes on Stress-Influenced Regions of the Developing Pediatric Brain. Ann N Y Acad Sci. 2004; 1032(1):231–233. doi: 10.1196/annals.1314.027 [DOI] [PubMed] [Google Scholar]
- 46.Bergamaschi R, Livieri C, Uggetti C, et al. Brain white matter impairment in congenital adrenal hyperplasia. Arch Neurol. 2006;63(3):413–416. doi: 10.1001/archneur.63.3.413 [DOI] [PubMed] [Google Scholar]
- 47.Mnif MF, Kamoun M, Mnif F, et al. Long-Term Outcome of Patients With Congenital Adrenal Hyperplasia Due to 21-hydroxylase Deficiency. Am J Med Sci. 2012;344(5):363–373. doi: 10.1097/MAJ.ObO13e31824369e4 [DOI] [PubMed] [Google Scholar]
- 48.Webb EA, Elliott L, Carlin D, et al. Quantitative Brain MRI in Congenital Adrenal Hyperplasia: In Vivo Assessment of the Cognitive and Structural Impact of Steroid Hormones. J Clin Endocrinol Metab. 2018; 103(4): 1330–1341. doi: 10.1210/jc.2017-01481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Van’t Westeinde A, Karlsson L, Thomsen Sandberg M, Nordenström A, Padilla N, Lajic S. Altered Gray Matter Structure and White Matter Microstructure in Patients with Congenital Adrenal Hyperplasia: Relevance for Working Memory Performance. Cereb Cortex N Y N 1991. 2020;30(5):2777–2788. doi: 10.1093/cercor/bhz274 [DOI] [PubMed] [Google Scholar]
- 50.Sorouri Khorashad B, Khazai B, Talaei A, et al. Neuroanatomy of transgender persons in a Non-Western population and improving reliability in clinical neuroimaging. J Neurosci Res. 2020;98(11):2166–2177. doi: 10.1002/jnr.24702 [DOI] [PubMed] [Google Scholar]
- 51.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–329. doi: 10.1016/j.nurt.2007.05.011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cotter DL, Azad A, Cabeen RP, et al. White Matter Microstructural Differences in Youth With Classical Congenital Adrenal Hyperplasia. J Clin Endocrinol Metab. 2021; 106(11):3196–3212. doi: 10.1210/clinem/dgab520 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.RajMohan V, Mohandas E. The limbic system. Indian J Psychiatry. 2007;49(2): 132. doi: 10.4103/0019-5545.33264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Glover GH. Overview of Functional Magnetic Resonance Imaging. Neurosurg Clin N Am. 2011;22(2): 133–139. doi: 10.1016/j.nec.2010.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazzone L, Mueller SC, Maheu F, VanRyzin C, Merke DP, Ernst M. Emotional Memory in Early Steroid Abnormalities: An fMRI Study of Adolescents With Congenital Adrenal Hyperplasia. Dev Neuropsychol. 2011;36(4):473–492. doi: 10.1080/87565641.2010.549866 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Beltz AM, Demidenko MI, Wilson SJ, Berenbaum SA. Prenatal androgen influences on the brain: A review, critique, and illustration of research on congenital adrenal hyperplasia. J Neurosci Res. Published online June 17, 2021:jnr.24900. doi: 10.1002/jnr.24900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Roberti JW, Storch EA, Bravata E. Further Psychometric Support for the Sensation Seeking Scale-Form V. J Pers Assess. 2003;81(3):291–292. doi: 10.1207/S15327752JPA8103_12 [DOI] [PubMed] [Google Scholar]
- 58.Zuckerman M, Eysenck SB, Eysenck HJ. Sensation seeking in England and America: Cross-cultural, age, and sex comparisons. J Consult Clin Psychol. 1978;46(1):139–149. doi: 10.1037/0022-006X.46.1.139 [DOI] [PubMed] [Google Scholar]
- 59.Munn Z, Barker TH, Moola S, et al. Methodological quality of case series studies: an introduction to the JBI critical appraisal tool. JBI Evid Synth. 2020;18(10):2127–2133. doi: 10.11124/JBISRIR-D-19-00099 [DOI] [PubMed] [Google Scholar]
- 60.Aromataris E, Munn Z, Joanna Briggs Institute. JBI Manual for Evidence Synthesis. Joanna Briggs Institute; 2020. [Google Scholar]
- 61.Fuqua JS, Rotenstein D, Lee PA. Duration of Suppression of Adrenal Steroids after Glucocorticoid Administration. Int J Pediatr Endocrinol. 2010;2010:1–8. doi: 10.1155/2010/712549 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.for the ENIGMA Consortium, Thompson PM, Jahanshad N, et al. ENIGMA and global neuroscience: A decade of large-scale studies of the brain in health and disease across more than 40 countries. Transl Psychiatry. 2020;10(1):100. doi: 10.1038/s41398-020-0705-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Mueller SC, Guillamon A, Zubiaurre-Elorza L, et al. The Neuroanatomy of Transgender Identity: Mega-Analytic Findings From the ENIGMA Transgender Persons Working Group. J Sex Med. 2021;18(6):1122–1129. doi: 10.1016/j.jsxm.2021.03.079 [DOI] [PubMed] [Google Scholar]
- 64.Markett S, Jawinski P, Kirsch P, Gerchen MF. Specific and segregated changes to the functional connectome evoked by the processing of emotional faces: A task-based connectome study. Sci Rep. 2020;10(1):4822. doi: 10.1038/s41598-020-61522-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Lee MH, Smyser CD, Shimony JS. Resting-State fMRI: A Review of Methods and Clinical Applications. Am J Neuroradiol. 2013;34(10):1866–1872. doi: 10.3174/ajnr.A3263 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Fair DA, Bathula D, Nikolas MA, Nigg JT. Distinct neuropsychological subgroups in typically developing youth inform heterogeneity in children with ADHD. Proc Natl Acad Sci. 2012;109(17):6769–6774. doi: 10.1073/pnas.1115365109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Nichols TE, Das S, Eickhoff SB, et al. Best practices in data analysis and sharing in neuroimaging using MRI. Nat Neurosci. 2017;20(3):299–303. doi: 10.1038/nn.4500 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Lagercrantz H Infant Brain Development: Formation of the Mind and the Emergence of Consciousness. Springer Science+Business Media; 2016. [Google Scholar]
- 69.Gibb R, Kolb B. The Neurobiology of Brain and Behavioral Development. Academic Press, an imprint of Elsevier; 2018. Accessed November 28, 2021. https://www.sciencedirect.com/science/book/9780128040362 [Google Scholar]
- 70.Armoskus C, Mota T, Moreira D, Tsai HW. Effects of Prenatal Testosterone Exposure on Sexually Dimorphic Gene Expression in the Neonatal Mouse Cortex and Hippocampus. J Steroids Horm Sci. 2014;5(3):1000139. [PMC free article] [PubMed] [Google Scholar]
- 71.Henley CL, Nunez AA, Clemens LG. Exogenous androgen during development alters adult partner preference and mating behavior in gonadally intact male rats. Horm Behav. 2010;57(4-5):488–495. doi: 10.1016/j.yhbeh.2010.02.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Setsompop K, Kimmlingen R, Eberlein E, et al. Pushing the limits of in vivo diffusion MRI for the Human Connectome Project. NeuroImage. 2013;80:220–233. doi: 10.1016/j.neuroimage.2013.05.078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Jernigan TL, Brown SA, Dowling GJ. The Adolescent Brain Cognitive Development Study. J Res Adolesc Off J Soc Res Adolesc. 2018;28(1):154–156. doi: 10.11n/jora.12374 [DOI] [PMC free article] [PubMed] [Google Scholar]


