Abstract
Purpose:
Programmed death or ligand-1 (PD-(L)1) pathway inhibitors confer improved survival as first-line treatment for advanced non-small cell lung cancer (aNSCLC) in patients with PD-L1 expression (PD-L1+e ≥50%) compared to platinum-doublet chemotherapy and have become a standard therapy. Some recent evidence suggests that among aNSCLC patients with PD-L1+e of ≥50% receiving pembrolizumab monotherapy, very high levels of PD-L1+e (≥90%) may be associated with better outcomes. We sought to assess whether very high PD-L1+e (≥90%) compared to high PD-L1+e (≥50–89%) is associated with an overall survival benefit in aNSCLC patients receiving anti PD-(L)1 monotherapies.
Methods:
We conducted a single-site retrospective cohort study of aNSCLC patients who initiated PD-(L)1 inhibitor monotherapy as first-line treatment from October 24, 2016, to August 25, 2021, and had a PD-L1+e ≥50%. The primary outcome was overall survival, measured from the start of first-line PD-(L)1 inhibitor monotherapy (index date) to date of death or last confirmed activity prior to the cohort exit date. Propensity score-based inverse probability weighting (IPW) was used to control for confounding in Kaplan-Meier curves and Cox proportional hazard regression analysis.
Results:
166 patients with aNSCLC receiving PD-(L)1 inhibitor monotherapy met inclusion criteria. Fifty four percent were female, 90% received pembrolizumab, median age was 68 years, 70% had non-squamous cell carcinoma, 94% had a history of smoking, 29% had a KRAS mutation, and 37% had very high PD-L1+e. Unweighted covariates at cohort entry were similar between groups (absolute standardized mean differences (SMDs) <0.1) except for race (SMD=0.2); age at therapy initiation (SMD=0.13); smoking status (SMD=0.13), and BRAF mutation status (SMD=0.11). After weighting, baseline covariates were well balanced (all absolute SMDs <0.1). In the weighted analysis, having a very high PD-L1+e was associated with lower mortality (weighted hazard ratio 0.57, 95% CI 0.36–0.90) and longer median survival: 3.85 vs 1.49 years.
Conclusions:
Very high PD-L1+e (≥90%) was associated with an overall survival benefit over high PD-L1+e (50–89%) in patients receiving first-line PD-(L)1 inhibitor monotherapy in a model controlling for potential confounders. These findings should be confirmed in a larger real-world data set.
Background
Lung cancer is the second most common cancer, and accounts for a quarter of all cancer deaths in the US.1 Non-small cell lung cancer accounts for approximately 85–90% of all lung cancer cases, with most patients presenting in advanced stages, where systemic treatment is required, and complete surgical resection is no longer an option.2 First-line treatment for advanced non-small cell lung cancer (aNSCLC) with programmed death ligand-1 (PD-(L)1) pathway inhibitors, a type of immunotherapy, confers improved survival compared to platinum-doublet chemotherapy, and has become a standard therapy in patients with high tumor expression of programmed death or ligand-1 (PD-L1+e).3
Factors affecting treatment decisions in aNSCLC patients include tumor histology, disease burden, symptoms, and tumor PD-L1+e. 4 Tumor PD-L1+e is a clinical biomarker, expressed as a percentage of tumor cells that exhibit membranous staining at any intensity. PD-L1+e ≥50% (which is present in 30% of NSCLC cases2) predicts a high likelihood of disease response and a longer overall survival in patients treated with first-line immunotherapy compared to lower PD-L1+e.3 As a result, newly diagnosed NSCLC patients are now routinely tested for PD-L1+e. There is some evidence that among aNSCLC patients with PD-L1+e ≥50% receiving pembrolizumab monotherapy first line, very high PD-L1+e (≥90%) is associated with improved survival.5 However, this previous study had limited follow up time during which median overall survival was not reached in the very high PD-L1+e group, and adjusted only for Eastern Cooperative Oncology Group (ECOG) performance status (PS). We hypothesized that compared with PD-L1+e of 50–89%, very high PD-L1+e (≥90%) is associated with prolonged overall survival, confirming the prior observation in a dataset with longer follow up time and the ability to control for more potentially important confounders.
Methods
This study was approved by the University of Pennsylvania IRB and was conducted in accordance with all applicable University of Pennsylvania human subjects research requirements and federal regulations. We identified patients treated within the University of Pennsylvania Health System (UPHS) who 1) were 18 years of age or older, 2) had been diagnosed with advanced stage or metastatic stage IV or recurrent NSCLC (ICD‐9 codes 162.x or ICD‐10 codes C34.x or C39.9) according to the 2009 American Joint Committee on Cancer classification criteria – between October 24, 2016 (based on FDA approval of first- line pembrolizumab in those with PDL1 expression of at least 50%),6 and August 25, 2021 (the date of data abstraction), 3) had at least two clinical visits on or after October 24, 2016 4) initiated first line treatment with an anti PD-(L)1 monotherapy (i.e., pembrolizumab, durvalumab or atezolizumab) after diagnosis of advanced stage or metastasis, and 5) had a recorded value of percentage PD-L1 expression of at least 50% of tumor cells 6) had a recorded value of ECOG PS in the 100 days leading up to and including the index date. Patients with incomplete immunotherapy treatment data, those receiving first line immunotherapy as a part of clinical trial, those receiving chemo-immunotherapy combination, or patients harboring sensitizing alterations in EGFR, ALK or ROS1 genes were excluded because of harms associated with immunotherapy in patients with such alterations.7 We also excluded patients with a gap >90 days between diagnosis and first visit or medication order.
All prior baseline data up to and including the index date were used to ascertain baseline covariates. Baseline data and covariates were derived from prior literature,5, 8 and included (a) demographic factors age, sex, and race/ethnicity (b) pathological cancer diagnosis, date of diagnosis, and clinical and pathological stage of malignancy (c) smoking status; and (d) genetic mutation status e.g., KRAS, BRAF.
The prognostic variable of interest was very high PD-L1+e, which we defined as ≥90%. The cut off (≥ 90%) was formulated by Aguilar et al, where they performed a recursive partitioning algorithm to investigate an optimal grouping of PDL1+e with respect to overall survival among aNSCLC patients receiving immunotherapy.5
PD-L1+e is reported as a percentage of tumor cells with membranous staining using a variety of immunohistochemical antibodies and platforms but primarily assessed using the Dako PD-L1 22C3 assay (∼70% of the cohort).9 The outcome of interest was overall survival measured from the initiation of first-line anti-PD1 monotherapy, 10 which was considered the index date. Patients were followed until death, last structured electronic health record (EHR) activity (including visit or medication administration), or August 25, 2021.
Statistical Analysis
Baseline covariates were compared between the very high PD-L1+e and high PD-L1+e groups using absolute standardized mean differences to identify imbalances in continuous variables, and absolute standardized differences in proportion to assess imbalance for categorical variables, using a threshold of >0.1 to suggest factors with potentially meaningful imbalance between groups.11
To control for confounding, we used inverse probability weighting (IPW) by a function of the propensity score, which we defined as the probability of a patient having very high PD-L1+e conditional on baseline variables. The propensity score model was estimated using logistic regression and included all baseline covariates listed above. Patients with very high PD-L1+e were weighted by the inverse of the propensity score, and patients with high PD-L1+e were weighted by the inverse of the complement of the propensity score. The positivity assumption was assessed by examining overlap of propensity score distributions between the groups based on density plots. Absolute standardized differences were computed after weighting to assess whether any imbalances remained. Inverse probability weighted Kaplan-Meier curves comparing overall survival in the groups were plotted. Cox proportional hazards regression analysis with IPW, using a robust variance estimator, was used to estimate weighted hazard ratios (HRs), and 95% confidence intervals (CIs). We assessed the proportional hazards assumption using Schoenfeld residuals.12 Statistical analyses were performed using STATA V16.0/IC (College station, TX).
Results
We identified 196 aNSCLC patients who received anti PD-(L)1 monotherapy, of whom 166 met inclusion criteria. The median (IQR) age was 68 (61–75) years; 116 (70%) were White, and 89 (54%) were female. A total of 117 patients (70%) had non squamous cell carcinoma and 156 (94%) had a history of smoking. In the cohort, 150 patients (90%) received pembrolizumab monotherapy, 48 (29%) had a KRAS mutation, and 62 (37%) patients had very high PDL1+e. Unweighted baseline characteristics were generally similar between exposure groups (absolute standardized mean differences (SMDs) <0.1) except for race (SMD=0.2); age at therapy initiation (SMD=0.13); smoking status (SMD=0.13), and BRAF mutation status (SMD=0.11). After IPW, baseline covariates were well balanced between groups (all absolute standardized differences<0.1) (Table 1). Thirty five percent of patients went on to receive a second-line therapy (n=19 (31%) in the ≥90% group and n=40 (38%) in the 50–89% group). Median time on first line treatment was higher for the time PD-L1+e (≥90%) group [median 229.5 days IQR 75–518] versus PD-L1+e (50–89%) group [median 139 days IQR 41.5–448.5].
Table 1.
Unweighted and weighted baseline Characteristics of the Cohort by PD-L1 Expression
| Unweighted population | Weighted population (scaled) | |||||
|---|---|---|---|---|---|---|
| Very High PDL1+e | High PDL1+e | Absolute Standardized Difference Before IPW | Very High PDL1+e | High PDL1+e | Absolute Weighted Standardized Difference | |
| N | N=62 | N=104 | N=166 | N=166 | ||
| Age at therapy initiation, median (IQR) | 68(62–75) | 67(61–76) | 0.13 | 68 (60–74) | 68 (61–76) | 0.01 |
| Gender n (%) | 0.06 | 0.02 | ||||
| Female | 32(52) | 57(55) | 91 (55) | 89 (54) | ||
| Male | 30(48) | 47(45) | 75 (45) | 77 (46) | ||
| Race n (%) | 0.2 | 0.02 | ||||
| White | 47(76) | 69(66) | 116 (70) | 116 (70) | ||
| Non-White | 15(24) | 35(34) | 50 (30) | 50 (30) | ||
| Smoking Status n (%) | 0.13 | <0.01 | ||||
| Current/Former | 57(92) | 99(95) | 156 (94) | 156 (94) | ||
| Never | 5(8) | 5(5) | 10(6) | 10(6) | ||
| ECOG PS n (%) | 0.01 | 0.01 | ||||
| 0–1 | 45(73) | 75(72) | 121(72) | 120(72) | ||
| ≥2 | 17(27) | 29(28) | 45(27) | 46(28) | ||
| Tumor histology n (%) | 0.07 | <0.01 | ||||
| Non squamous cell carcinoma | 45(73) | 72(69) | 117 (70) | 117(70) | ||
| Squamous cell carcinoma | 13(21) | 21(20) | 38(23) | 31(19) | ||
| NSCLC NOS | 4(6) | 11(11) | 11 (7) | 18(11) | ||
| KRAS mutation n (%) | 0.05 | <0.01 | ||||
| Yes | 17(27) | 31(30) | 48 (29) | 48 (29) | ||
| No | 45(73) | 73(70) | 118 (71) | 118 (71) | ||
| BRAF mutation n (%) | 0.11 | <0.01 | ||||
| Yes | 4(6) | 4(4) | 7 (4) | 8 (5) | ||
| No | 58(94) | 100(96) | 159 (96) | 158 (95) | ||
IQR, Interquartile range
ECOG, Eastern Cooperative Oncology Group
NSCLC NOS, non-small cell lung cancer not otherwise specified
IPW, inverse probability weighting
Overall survival
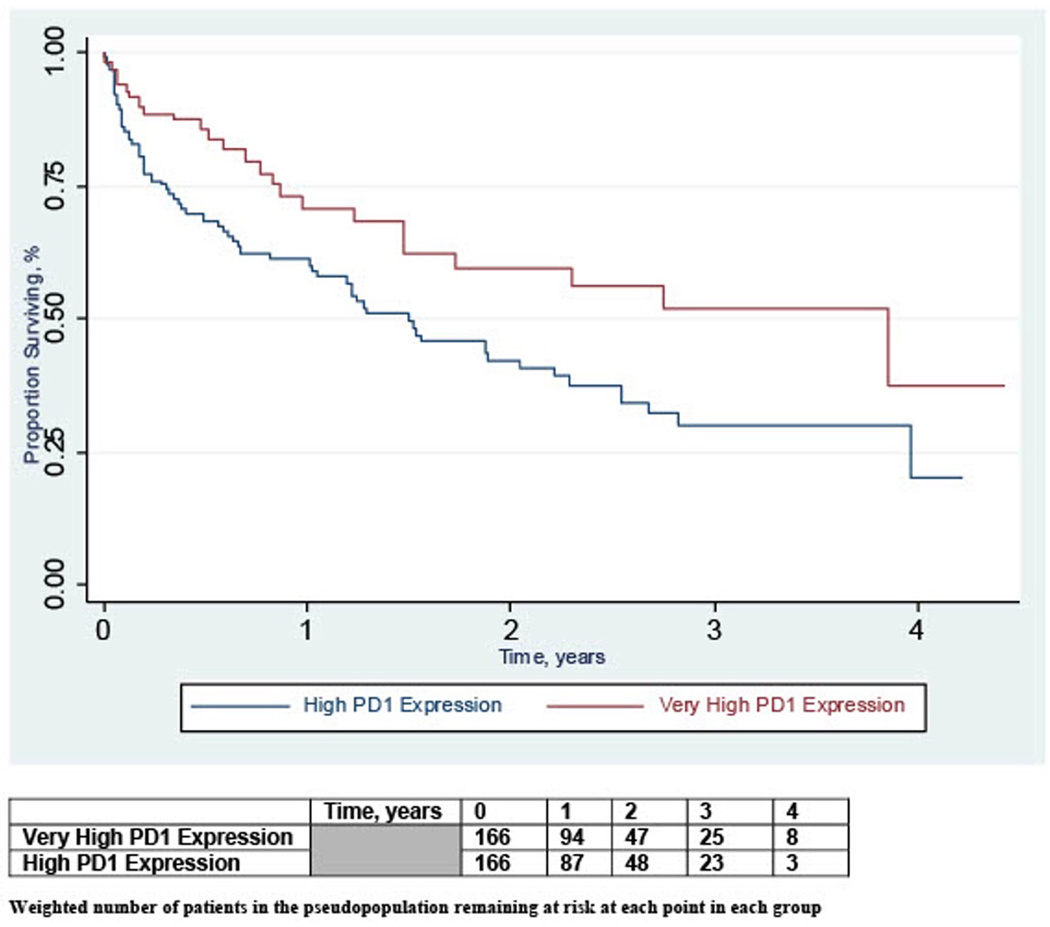
Median follow-up time from initiation of first-line anti PD-(L)1 monotherapy for the entire cohort in the unweighted sample was 1.20 years (interquartile range 0.40–2.36). During follow-up, there were 87 deaths, 25 (40%) in the very high PD-L1+e and 62 (60%) in the high PD-L1+e group. Inverse probability weighted Kaplan-Meier curves are presented in Figure 1. In the weighted analysis, PD-L1+e ≥90% was associated with lower mortality compared to PD-L1+e of 50–89% (weighted HR 0.57, 95% CI 0.36–0.90, Table 2). Median overall survival from the index date in the inverse probability weighted sample was 3.85 years in patients with PD-L1+e ≥90% and 1.49 years in patients with PD-L1+e of 50–89% (p=0.01).
Figure 1.
Inverse Probability Weighted Kaplan Meier Curves of Overall Survival by PD-L1 Expression
Table 2:
Overall Survival in patients with Very High PD-L1 expression
| Study groups | No. of subjects | Median duration of follow up, years [IQR] | No. of deaths in the follow up period | Weighted median survival, years [IQR] | Weighted Hazard Ratio [95% CI] | P-value* |
|---|---|---|---|---|---|---|
| Very high PD-L1 expression (90–100%) | 62 | 1.38 [0.60–2.37] | 25 | 3.85 [0.86-NR] | 0.57 [0.36–0.90] | 0.01 |
| High PD-L1 expression (50–89%) | 104 | 1.03 [0.32–2.32] | 62 | 1.49 [0.31–3.96] | 1.00 [Reference] |
IQR, interquartile range; NR, Not reached;
Based on test for two survival curves using cox proportional hazards regression analysis adjusted with IPW
Discussion
We found that among aNSCLC patients with PD-L1+e ≥50%, treated first-line with anti PD-(L)1 inhibitors, 37% had PDL1+e ≥90%, and that having a PDL1+e ≥90% was associated with better overall survival [weighted HR 0.57 (0.36–0.90)], and longer median survival (3.85 vs. 1.49 years).
Tumor PD-L1 expression has been used to predict immunotherapy benefit compared to conventional chemotherapy and is routinely used in clinical practice to select first-line therapy among aNSCLC patients. Our finding that, among patients with PD-L1+e ≥50%, 37% have PDL1+e ≥90% is similar to analogous findings in Japan (34%)13 and the US (44%).5 As PD-L1+e ≥50% is found in nearly one third of all real-world non-small cell lung cancer patients,2 this implies that very high PD-L1+e is present in 10–13% of real-world aNSCLC cases.
Our study builds on this prior work by including longer follow up, and accounting for a wider range of potential confounders. Our finding that PD-L1+e ≥90% was associated with a lower mortality after IPW is in line with a previous study of very high PD-L1+e in patients treated with pembrolizumab monotherapy [median overall survival not reached for the very high PD-L1+e group vs 1.32 years, HR 0.39 (0.21–0.70)].5
This study’s strengths include a clearly defined cohort, and similar distributions of most baseline covariates even before weighting. Potential limitations include its modest sample size, potentially limited generalizability, and the potential for confounding from unmeasured baseline factors such as site of metastasis and body mass index.
In addition to the prognostic information provided by this study, its findings have potential implications in design of future clinical trials. Ongoing aNSCLC clinical trials collectively group baseline PD-L1+e ≥50% and report their outcomes in aggregate. This leads to patients with very high PD-L1+e and high PD-L1+e being placed in the same group (PD-L1+e ≥50%), and treatment arms that, especially for small trials, may not be evenly balanced on PD-L1+e, which could lead to treatment effect estimates that may not be reliable and valid. Building on recommendations by Kernan et al, 14 we believe that anti PD-(L)1 immunotherapy trials with fewer than 100 patients per treatment arm should consider utilizing PD-L1+e stratified randomization (50–89% and ≥90%), and report outcomes accordingly. This would ensure that randomized groups are evenly balanced with respect to prognostic indicators like very high PD-L1+e levels. For larger anti PD-(L)1 monotherapy clinical trials (>200 patients per treatment arm), post-randomization stratification based on a 50–89% and ≥90% scale may be equivalent to stratified randomization.14
Conclusions
Among aNSCLC patients treated with anti PD-(L)1 monotherapies and PD-L1+e levels of ≥50%, having very high PD-L1+e is associated with improved survival. Future studies are needed to confirm these findings in larger real world data sets.
Key points.
Programmed death or ligand-1 (PD-(L)1) pathway inhibitors confer improved survival as first-line treatment for advanced non-small cell lung cancer (aNSCLC) in patients with PD-L1 expression (PD-L1+e ≥50%) compared to platinum-doublet chemotherapy and have become a standard therapy. Some recent evidence suggests that among aNSCLC patients with PD-L1+e of ≥50% receiving pembrolizumab monotherapy, very high levels of PD-L1+e (≥90%) may be associated with better outcomes.
We hypothesized that compared with PD-L1+e of 50–89%, very high PD-L1+e (≥90%) is associated with prolonged overall survival, confirming the prior observation in a dataset with longer follow up time and the ability to control for more potentially important confounders.
We observed among aNSCLC patients with PD-L1+e ≥50% treated first-line with anti PD-(L)1 inhibitors in routine practice, 37% had PD-L1+e ≥90%.
Having a PD-L1+e ≥90% was associated with better overall survival [inverse probability weighted HR 0.57 (0.36–0.90)], and longer median survival (3.85 vs. 1.49 years).
In addition to the prognostic information provided by this study, its findings have potential implications in design of future clinical trials.
Funding
Shah was supported by a NIH NIGMS T32 pharmacoepidemiology fellowship award (T32GM-075766)
Figure abbreviations
- IP
Inverse Probability
References
- 1.Bray F, et al. , Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA: a cancer journal for clinicians, 2018. 68(6): p. 394–424. [DOI] [PubMed] [Google Scholar]
- 2.Holmes M, et al. , Real-world programmed death-ligand 1 prevalence rates in non-small cell lung cancer: correlation with clinicopathological features and tumour mutation status. Journal of Clinical Pathology, 2021. 74(2): p. 123–128. [DOI] [PubMed] [Google Scholar]
- 3.Reck M, et al. , Updated analysis of KEYNOTE-024: pembrolizumab versus platinum-based chemotherapy for advanced non-small-cell lung cancer with PD-L1 tumor proportion score of 50% or greater. 2019. [DOI] [PubMed] [Google Scholar]
- 4.Parikh RB, et al. , Uptake and Survival Outcomes Following Immune Checkpoint Inhibitor Therapy Among Trial-Ineligible Patients With Advanced Solid Cancers. JAMA oncology. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Aguilar EJ, et al. , Outcomes to first-line pembrolizumab in patients with non-small-cell lung cancer and very high PD-L1 expression. Annals of Oncology, 2019. 30(10): p. 1653–1659. [DOI] [PubMed] [Google Scholar]
- 6.Reck M, et al. , Pembrolizumab versus chemotherapy for PD-L1–positive non–small-cell lung cancer. N engl J med, 2016. 375: p. 1823–1833. [DOI] [PubMed] [Google Scholar]
- 7.Gainor JF, et al. , EGFR mutations and ALK rearrangements are associated with low response rates to PD-1 pathway blockade in non–small cell lung cancer: a retrospective analysis. Clinical cancer research, 2016. 22(18): p. 4585–4593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Moutafi MK, et al. , Comparison of programmed death-ligand 1 protein expression between primary and metastatic lesions in patients with lung cancer. Journal for immunotherapy of cancer, 2021. 9(4): p. e002230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lantuejoul S, et al. , PD-L1 testing for lung cancer in 2019: perspective from the IASLC Pathology Committee. Journal of Thoracic Oncology, 2020. 15(4): p. 499–519. [DOI] [PubMed] [Google Scholar]
- 10.Zhang Q, et al. , Validation analysis of a composite real-world mortality endpoint for US cancer patients. 2020, AACR. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang Z, et al. , Balance diagnostics after propensity score matching. Annals of translational medicine, 2019. 7(1). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schoenfeld D, Partial residuals for the proportional hazards regression model. Biometrika, 1982. 69(1): p. 239–241. [Google Scholar]
- 13.Edahiro R, et al. , Clinical outcomes in non-small cell lung cancer patients with an ultra-high expression of programmed death ligand-1 treated using pembrolizumab as a first-line therapy: A retrospective multicenter cohort study in Japan. PLoS One, 2019. 14(7): p. e0220570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kernan WN, et al. , Stratified randomization for clinical trials. Journal of clinical epidemiology, 1999. 52(1): p. 19–26. [DOI] [PubMed] [Google Scholar]