Abstract
Objectives
To describe how a standardized pediatric medication therapy management (MTM) model identifies potential interventions and their impact on Medication Regimen Complexity Index (MRCI) scores in children with medical complexity (CMC) and polypharmacy.
Methods
This retrospective proof-of-concept study included pediatric patients receiving primary care in a large outpatient primary care medical home for CMC within a tertiary freestanding children’s hospital from August 2020 through July 2021. Medication profiles of established patients aged 0 to 18 years with ≥5 active medications at the time of the index visit were assessed for medication-related concerns, potential interventions, and potential impact of proposed interventions on MRCI scores.
Results
Among 100 patients, an average of 3.4 ± 0.3 medication-related concerns was identified using the pediatric MTM model. Common medication-related concerns (>25% of patients) included inappropriate/unnecessary therapy, suboptimal therapy, undertreated symptom, adverse effect, clinically significant drug-drug interaction, or duplication of therapy. Ninety-seven patients (97%) had opportunities for 5.0 ± 2.9 potential interventions. Most common proposed interventions included drug discontinuation trial (69%), patient/caregiver education (55%), dosage form modification (51%), dose modification (49%), and frequency modification (46%). The mean baseline MRCI score was 32.6 (95% CI 29.3–35.8) among all patients. MRCI scores decreased by a mean of 4.9 (95% CI 3.8–5.9) following application of the theoretical interventions (p<0.001). Mean potential score reduction was not significantly affected by patient age or number of complex chronic conditions. Potential impact of the proposed interventions on MRCI score was significantly greater in patients with higher baseline medication counts (p<0.001).
Conclusion
Most children with medical complexity would likely benefit from a pharmacist-guided pediatric MTM service. A standardized review of active medication regimens identified multiple medication-related concerns and potential interventions for nearly all patients. Proposed medication interventions would significantly reduce medication regimen complexity as measured by MRCI. Further prospective evaluation of a pharmacist-guided pediatric MTM service is warranted.
Keywords: medication therapy management, pharmacotherapy, polypharmacy, deprescribing, pediatric pharmacy, ambulatory care
INTRODUCTION
Children with medical complexity (CMC) make up a vulnerable subset of the pediatric population, defined by the presence of pediatric complex chronic condition (CCC) diagnoses (e.g., cerebral palsy, intractable epilepsy, trisomy 21) that are expected to last ≥12 months and require subspecialty care and/or tertiary care hospitalizations.1–3 CMC often have substantial functional limitations, high health care utilization, and frequently use supportive medical technology.1 To sustain quality of life and control debilitating symptoms, CMC often require treatment with complex polypharmacy.4,5 The Medication Regimen Complexity Index (MRCI), a tool developed to measure medication complexity in adult and geriatric populations with polypharmacy, has demonstrated potential for application in pediatric populations.6–10 In a recent pediatric study using the MRCI, medication regimen complexity was particularly high in CMC with severe neurological conditions; these children’s medication regimens consisted of a median of 31 doses per day overlaid with complicated dosing schedules and specialized medication instructions, and higher MRCI scores were associated with increased acute healthcare utilization.7
Indeed, CMC are more vulnerable to medication-related problems; for example, adverse drug event (ADE)-related emergency department utilization is nearly 5 times more likely in CMC.11 In the outpatient setting, CMC with polypharmacy may also have unrecognized or undertreated symptoms, receive suboptimal drug therapy, or experience preventable errors or effects.7,12–14 Factors including the necessary involvement of numerous specialty medical providers, potential need for numerous dispensing pharmacies (e.g., traditional community, specialty, compounding), and potential involvement of numerous parents, caregivers, or guardians in the medication use process all introduce risk for further complexity which has not been defined.15 Nonetheless, few pediatric-specific strategies exist to manage polypharmacy among CMC and to potentially mitigate downstream risks, including avoidable medication regimen complexity, caregiver burden, or ADEs.16,17
Medication therapy management (MTM) services have been shown to be widely effective among a wide variety of adult populations and disease states for improving health, reducing health care costs, reducing polypharmacy, and increasing patient knowledge.18 Such services consist of standardized, pharmacist-led efforts to provide education on medications, improve adherence, detect adverse drug reactions or drug-drug interactions, and address patterns of suboptimal or unsuitable medication use; thus, improving therapeutic outcomes.19,20 In addition to these clinical aims, studies evaluating the effects of MTM services in adults have demonstrated both improvements in patients’ perceptions of care and significant reductions in total health care costs, at times exceeding return on investment upwards of 12:1 per dollar spent on MTM services.18,19 Despite the numerous potential benefits of MTM-like services for the analogous pediatric population of CMC with polypharmacy, literature describing the use of a uniform MTM model in ambulatory pediatric settings is exceedingly limited.
Thus, we designed a study to determine whether applying a standardized MTM approach in a pediatric population of CMC might identify potential opportunities for pharmacists to manage polypharmacy and reduce medication regimen complexity as measured by MRCI scores.
OBJECTIVES
The aims of this study were to (1) identify the frequency by which a standardized, retrospective approach to pediatric MTM identifies potential medication-related concerns and subsequent interventions for CMC; (2) categorize the types of identified concerns and potential interventions for medication optimization resulting from the retrospective pediatric MTM process; and (3) evaluate the impact of the potential interventions on medication regimen complexity as measured by MRCI scores.
METHODS
Design and Setting
This single-center retrospective study evaluated the potential role of pediatric MTM services at the Special Care Clinic (SCC) at Children’s Hospital Colorado, a large multidisciplinary primary care medical home for CMC within a large, tertiary, freestanding children’s hospital, between August 1, 2020 and July 31, 2021. CMC are eligible to receive care in the SCC based upon the diagnosis of one or more CCC ICD-10 diagnosis and the requirement for subspecialty care and/or expected hospitalization.3 The study was deemed to be exempt from formal review by the Colorado Multiple Institutional Review Board, the institutional review board for the University of Colorado and its affiliates. Pharmacist support within the SCC is provided by a full-time embedded clinical pharmacist with pediatric board certification. The pharmacist works collaboratively with attending providers and multidisciplinary medical staff to provide recommendations in the management and monitoring of medication therapy, to educate patients and caregivers, and to otherwise assist in patient care within a reactive, consultation-based service model.
Participants
Participants were eligible for study inclusion if they received primary care in SCC; had ≥1 SCC routine, non-acute office or telehealth visit between August 1, 2000 to July 31, 2021; and had ≥5 active home medications at the time of the encounter. Eligible patients were identified via electronic health record (EHR) reporting tools. Criteria for exclusion included age >18 years, receipt of primary care services from a primary care provider (PCP) external to the SCC, or subsequent determination that the patient’s true home medication list consisted of <5 active medications at the time of the index visit. Comprehensive MTM visits in the adult population often require 15–60 minutes in duration of direct patient contact19,20; in anticipation of a similar requirement in the CMC population, approximately 30 minutes were allotted to the review of each patient chart. Consequently, a target sample size of 100 patients was selected a priori and was predicted to be sufficient for determination of pediatric MTM feasibility and proof-of-concept. After exclusion of participants on the basis of age and outpatient medication count using EHR reporting tools, patients were randomized for selection to study enrollment. Following randomization, additional manual EHR evaluation was performed in the sequence determined by automated randomization. Study eligibility was confirmed using the previously defined exclusion criteria until a sample size of 100 patients was attained. All remaining patients were excluded from the study analysis (Figure 1).
Figure 1.
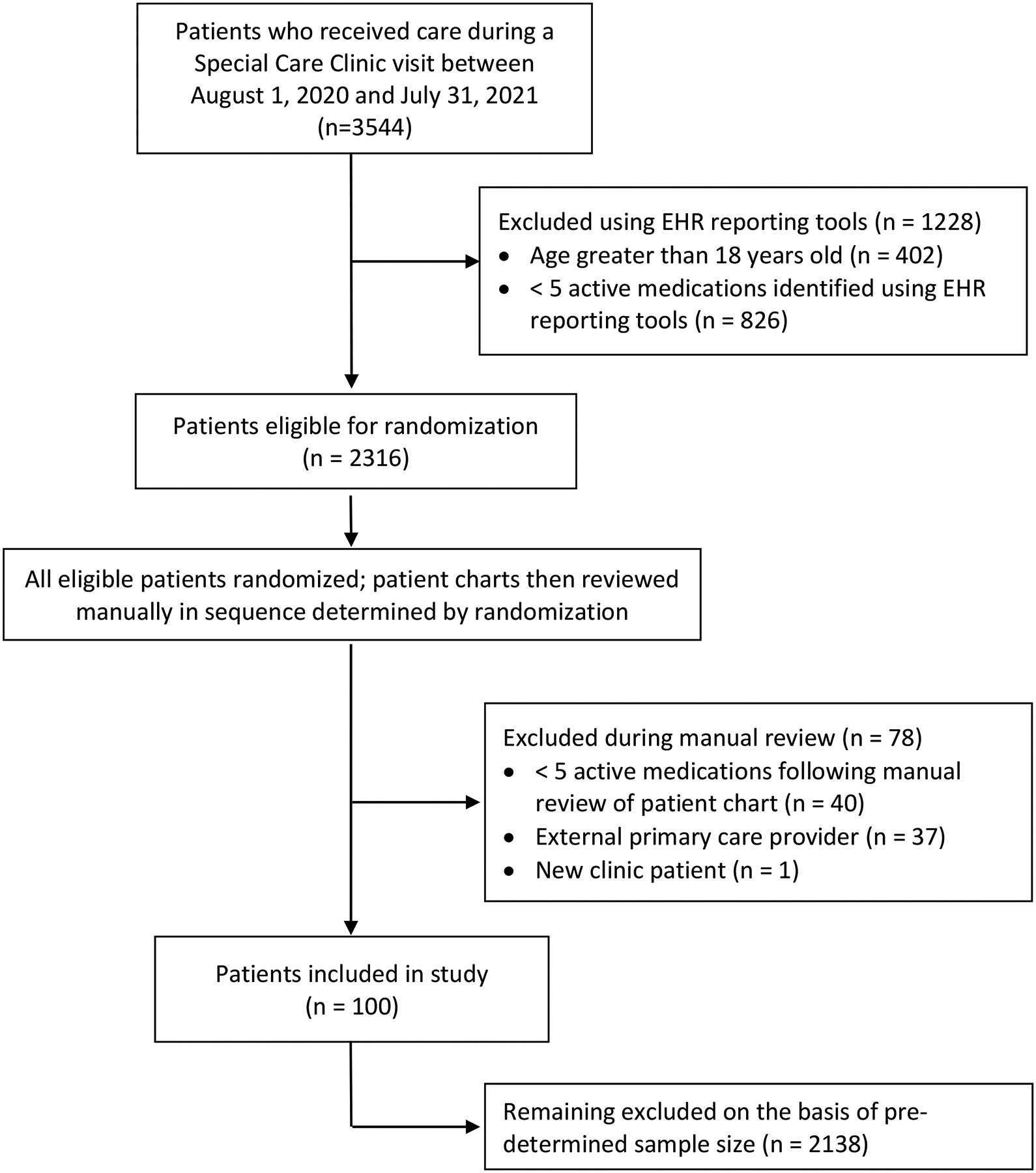
Patient Screening and Enrollment
Data Collection
Following patient identification, data were retrospectively collected via manual extraction from the EHR. Pertinent study data were collected for the 12-month period preceding each patient’s most recent SCC visit within the study window (henceforth referred to as the index visit). Demographic variables included age, sex, race, ethnicity, and insurer type. Counts of CCCs were determined using published classification systems based on International Classification of Diseases, Tenth Revision (ICD-10) diagnosis codes.21 Active medication lists, including all data elements necessary to calculate MRCI scores, were obtained from the EHR as they existed on the date of the index visit. High risk medications (those with potential to cause substantial patient harm if used in error) were identified using published guidance from the Institute for Safe Medication Practices.22 All study data were managed using REDCap™ electronic data collection tools.23
Medication-Related Concerns and Interventions
A standardized pediatric MTM assessment of medication therapy (Supplemental Appendix A) was performed for each patient to identify potential medication-related concerns and corresponding interventions, if appropriate. Each medication-related concern was classified within one of eight categories as described in Table 1. Following pediatric MTM assessment, each proposed intervention was categorically stratified into one of the following intervention categories: dose modification, drug discontinuation, dosage form modification, dosing frequency modification, initiation of new drug therapy, patient/caregiver education, change to alternative medication, laboratory monitoring for safety or efficacy, or none; qualifying examples of such interventions are also provided in Table 1. This review and classification process was first performed for each patient by one of two Doctor of Pharmacy candidates (C.M. and R.T.); all identified concerns or interventions were subsequently reviewed by the clinic’s embedded pediatric pharmacist (L.O.), at which time potential discrepancies were resolved through group discussion and consensus with the pharmacist. Finally, the pharmacist independently reviewed each patient’s active medication and problem lists for further optimization opportunities that would be reliant upon clinical judgement or pediatric evidence unlikely to be included within the current PharmD curriculum (Supplemental Appendix A, Step 10). All review and classification steps were performed prior to calculation of MRCI scores (described below) to avoid potential bias.
Table 1.
Medication-Related Concerns and Interventions: Definitions and Examples
| Categories | Definition or criteria | Examples |
|---|---|---|
| Medication-related concerns | ||
| Inappropriate or unnecessary therapy |
|
|
| Suboptimal therapy |
|
|
| Undertreated symptoms |
|
|
| Adverse drug event |
|
|
| Drug-drug interaction |
|
|
| Duplication of therapy |
|
|
| Unclear prescription instructions |
|
|
| Interventions | ||
| Dose modification |
|
|
| Drug discontinuation |
|
|
| Dosage form modification |
|
|
| Dosing frequency modification |
|
|
| Initiation of new drug therapy |
|
|
| Patient/caregiver education |
|
|
| Change to alternative medication |
|
|
| Laboratory monitoring for safety or efficacy |
|
|
GDMT: guideline-directed medical therapy; PPI: Proton pump inhibitor; H2RA: histamine-2 receptor antagonist; ER: extended release; SABA: short-acting beta agonist
MRCI Score and Calculations
A pre-intervention MRCI score was calculated for each patient using the index visit medication regimen (prior to any proposed pediatric MTM intervention), and a subsequent MRCI score was calculated using the potential regimen following application of all proposed interventions identified during the pediatric MTM process. The MRCI score is composed of weighted subscores for complexity in the areas of dosage form, dosing frequency, and specialized instructions.6 The dosage form subscore ascribes a complexity score (e.g., tablet = 1, liquid = 2) to each unique dosage form within the regimen. The dosage frequency subscore weights complexity according to how often a specific medication’s administration occurs within the regimen (e.g., once daily = 1, twice daily = 2, every 8 hours = 3.5). Lastly, the specialized instructions subscore assigns weight to the number of unique instructions prescribed for each medication in the regimen. As an example, a tablet taken once daily before breakfast has a dosage form score of 1 (tablet), a dosage frequency score of 1 (once daily), and a specialized instructions score of 1 (before breakfast), contributing a combined score of 3 to a regimen’s total MRCI score. The total MRCI score has no upper limit, as the score is a function of the number of medications in a regimen; higher scores indicate a more complex regimen, and lower scores indicate a less complex regimen.
Statistical Analysis
Patient demographics and clinical characteristics, categorization of medication-related concerns and corresponding interventions, and components of MRCI score analysis were analyzed using descriptive statistics. Reported statistics include mean and 95% confidence intervals for all normally distributed data. A paired samples t-test was used for analyzing differences between pre-MTM and potential post-MTM MRCI scores, and analysis of variance (ANOVA) was used to analyze differences between defined subgroups. Simple linear regression was used to analyze the relationship between medication counts and potential changes in MRCI scores. Data were analyzed using Stata 17.0, and significance was set at a 2-tailed P < 0.05.
RESULTS
Patient Demographics and Clinical Characteristics
A total of 3,544 patients receiving care at the SCC during the study window were screened for inclusion. One-hundred patients were included for analysis following application of enrollment and exclusion criteria, as outlined in Figure 1. The mean age of patients during the study period was 9.5 ± 0.6 years; the population was predominantly male (58%); white (53%); Hispanic or Latino (53%); and used public insurance (83%) (Table 2). The average number of CCC’s per patient was 2.4 ± 1.6. Patients were prescribed an average of 10.3 ± 5.3 active medications at the time of the index visit; the majority used ≥2 high risk medications (61%), and an additional 14% received non-commercially available compounded medications. Forty-five percent of patients had 10 or more outpatient clinic encounters within the health-system in the preceding 12 months.
Table 2.
Patient demographics and clinical characteristics
| All Patients (N=100) | Baseline MRCI score, mean (95% CI) | |
|---|---|---|
| Age, mean ± SD, y | 9.5 ± 0.6 | 32.6 (29.3–35.8) |
| Sex, n (%), male | 58 (58) | 32.6 (28.3–36.9) |
| Race, n (%) | ||
| White | 53 (53) | 33.7 (29.3–38.0) |
| Other | 20 (20) | 30.5 (23.4–37.5) |
| Black or African American | 12 (12) | 35.5 (21.3–49.7) |
| >1 Race | 7 (7) | 25.7 (22.2–29.2) |
| Asian | 6 (6) | 36.4 (27.2–45.7) |
| American Indian or Alaskan Native | 1 (1) | 26.5 |
| Unknown | 1 (1) | 12.5 |
| Ethnicity, n (%) | ||
| Hispanic or Latino | 53 (53) | 30.4 (26.8–33.9) |
| Not Hispanic or Latino | 46 (46) | 35.5 (29.9–41.2) |
| Unknown | 1 (1) | 12.5 |
| Insurance type, n (%) | ||
| Public | 83 (83) | 31.6 (28.0–35.2) |
| Private | 17 (17) | 37.2 (30.0–44.4) |
| High-risk medications, n (%) | ||
| 0–1 | 39 (39) | 27.4 (23.6–31.3) |
| 2–3 | 30 (30) | 28.6 (24.4–32.8) |
| ≥4 | 31 (31) | 42.9 (35.6–50.2) |
| Any compounded medications, n (%) | ||
| Yes | 14 (14) | 32.7 (23.2–42.2) |
| No | 86 (86) | 32.6 (29.1–36.0) |
| Complex chronic conditions, n (%) | ||
| 0 | 11 (11) | 26.5 (15.6–37.4) |
| 1–2 | 47 (47) | 29.6 (25.1–34.0) |
| ≥3 | 42 (42) | 37.6 (32.8–42.3) |
| Outpatient visits in past year, n (%) | ||
| 0–9 | 55 (55) | 27.5 (23.9–31.2) |
| 10–19 | 27 (27) | 33.2 (27.3–39.1) |
| 20–29 | 11 (11) | 51.3 (41.1–61.5) |
| ≥30 | 7 (7) | 40.6 (27.2–53.9) |
Medication-Related Concerns and Potential Interventions by Type
Among all patients, an average of 3.4 ± 0.3 medication-related concerns were identified. Ninety-one patients had ≥1 medication-related concern which included: inappropriate/unnecessary therapy (41%), suboptimal therapy (40%), under-treated symptoms (34%), likely ADE (33%), pertinent drug-drug interaction (32%), duplication of therapy (29%), and unclear prescription instructions (11%) (Table 3). Among all patients, an average of 5.0 ± 2.9 potential interventions were identified. Mean intervention count was not significantly different when stratified by patient age (i.e., 0–4 years, 5–8 years, 9–12 years, 13–18 years) (p=0.074) or CCC count (i.e., no CCC, 1–2 CCCs, ≥3 CCCs) (p=0.570). The number of identified interventions was significantly higher in patients with higher baseline medication counts; mean intervention count was 3.5 (95% CI: 3.0–4.0), 6.5 (95% CI: 5.5–7.5), and 8.0 (95% CI: 6.7–9.3) in patients using 5–9 medications, 10–14 medication, and ≥15 medications at the time of the index visit, respectively (p<0.001) (Figure 2). A total of 97 patients were identified as potentially benefiting from ≥1 intervention, which included: drug discontinuation (69%), dose modification (49%), drug therapy initiation (23%), change to alternative medication (21%), modification of frequency or dosage form (46% and 51%, respectively), and medication education (55%) (Table 3).
Table 3.
Identified medication-related concerns and interventions by type
| Patients, n (%) (N = 100) |
|
|---|---|
| Identified medication-related concem(s)* | |
| Inappropriate or unnecessary therapy | 41 (41) |
| Suboptimal therapy | 40 (40) |
| Undertreated symptoms | 34 (34) |
| Adverse drug event | 33 (33) |
| Drug-drug interaction | 32 (32) |
| Duplication of therapy | 29 (29) |
| Unclear prescription instructions | 11 (11) |
| None | 9 (9) |
| Identified intervention(s)* | |
| Drug discontinuation | 69 (69) |
| Patient/caregiver education† | 55 (55) |
| Dosage form modification | 51 (51) |
| Dose modification | 49 (49) |
| Dosing frequency modification | 46 (46) |
| Initiation of drug therapy | 23 (23) |
| Change to alternative medication | 21 (21) |
| Laboratory monitoring for safety/efficacy | 4 (4) |
| None | 3 (3) |
Neither concern nor intervention subcategories were mutually exclusive.
Education interventions referred to those intended to prevent administration errors on a recurring basis (e.g., compounded liquid medication volume/concentration, appropriate injection technique for parenteral medications).
Figure 2.
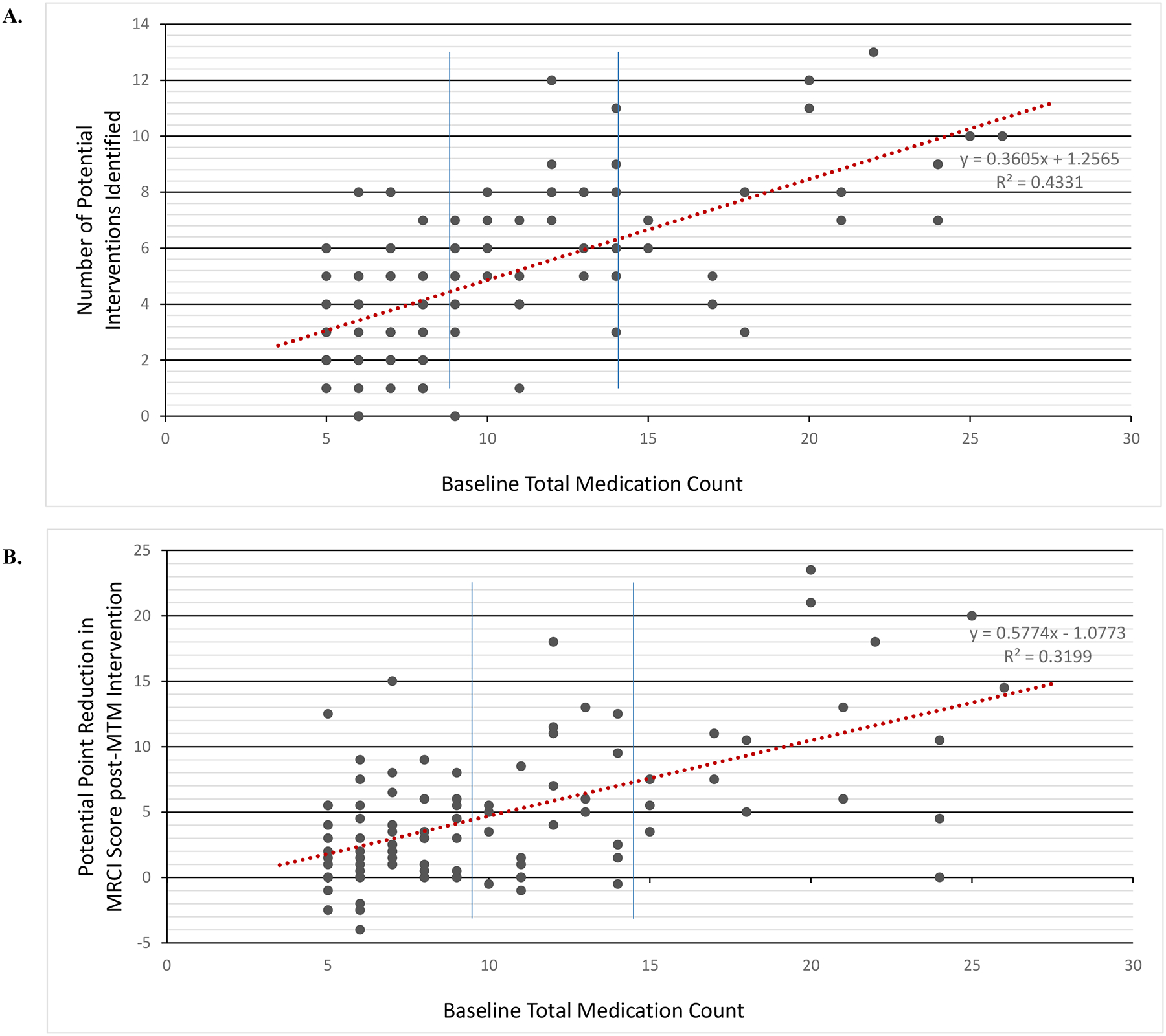
Association between Baseline Medication Count and Potential Impact of Pediatric MTM Approach
Panel A illustrates the association between baseline medication count and the number of interventions identified through the standardized pediatric MTM approach. The number of identified interventions was significantly higher in patients with higher medication counts; mean intervention count was 3.5 (95% CI: 3.0–4.0), 6.5 (95% CI: 5.5–7.5), and 8.0 (95% CI: 6.7–9.3) in patients using 5–9 medications, 10–14 medication, and ≥15 medications at the time of the index visit, respectively (all p<0.001). Panel B illustrates the association between baseline medication count and the magnitude of the pediatric MTM’s potential effect on MRCI score. Impact of the proposed pediatric MTM intervention on MRCI score was also significantly greater in patients with higher counts; mean MRCI reduction was 2.9 points (95% CI: 2.0–3.7) in patients using 5–9 medications, 5.7 (95% CI: 3.6–7.8) in patients using 10–14 medications, and 10.7 (95% CI: 7.5–13.8 in patients using ≥15 medications at the time of the index visit (all p<0.001), Each data point represents an individual patient from the study cohort. Solid blue lines indicate the thresholds used for stratification and analysis.
Potential Impact of Pediatric MTM Interventions on MRCI Scores
Among all study patients, the mean pre-intervention MRCI score was 32.6 (95% CI: 29.3–35.8). The average potential reduction in MRCI score was 4.9 points (95% CI 3.8–5.9; p<0.001) following application of proposed interventions resulting from the pediatric MTM process. Mean potential reduction in MRCI scores was not significantly different when stratified by patient age (i.e., 0–4 years, 5–8 years, 9–12 years, 13–18 years) (p=0.219) or by CCC count (i.e., no CCC, 1–2 CCCs, ≥3 CCCs) (p=0.128) (Supplemental Table 1). Potential impact of the proposed interventions on MRCI score was significantly greater in patients with higher baseline medication counts; mean potential MRCI reduction was 2.9 points (95% CI: 2.0–3.7) in patients using 5–9 medications, 5.7 (95% CI: 3.6–7.8) in patients using 10–14 medications, and 10.7 (95% CI: 7.5–13.8 in patients using ≥15 medications at the time of the index visit (all p<0.001) (Figure 2).
DISCUSSION
In this retrospective proof-of-concept study, the potential benefit of a pharmacist-guided strategy for pharmacotherapy optimization was explored through a systematic pediatric MTM model. Using a standardized approach aimed at the identification of potential concerns and which targets medication deprescribing or simplification where appropriate, our findings suggest that ≥ 90% of CMC could benefit from one or more medication-related intervention. Specifically, CMC with polypharmacy experienced, on average, the potential for a 5.4 point reduction in MRCI scores following application of the pediatric MTM process, and overall regimen complexity would be reduced significantly if proposed interventions were adopted, regardless of patient age, medication count, or number of CCCs. Most commonly, 69% of patients were appropriate candidates for a drug discontinuation trial on the basis of unnecessary treatment, likely contribution to ADE, or duplication of therapy. Such findings suggest an unmet need to improve medication safety, efficacy, and coordination of medication decisions within the current ambulatory care model for CMC in the United States. Pharmacists are well positioned to systematically identify and deliver medication-related interventions that might not otherwise be identified or addressed by clinicians during routine care. As such, several of our specific findings warrant further discussion.
First, our findings provide theoretical evidence that the standardized pediatric MTM intervention produces a significant reduction in medication regimen complexity regardless of patient age, number of CCCs, or number of medications. Due to the challenging nature of the clinical management of CMC, polypharmacy in this population is common. At baseline, the mean pre-intervention MRCI score of 32.6 (95% CI: 29.3–35.8) indicates that CMC with polypharmacy display a degree of medication regimen complexity equal to or greater than comparable adult and geriatric populations.10,24–30 Through application the pediatric MTM intervention, at least one medication-related concern was identified for 91% of patients. All identified concerns had the potential to negatively impact patient outcomes, and many could be addressed in a manner that subsequently reduced medication regimen complexity. Meaningful interventions, including drug de-escalation, dose modification, simplification of dosage form or frequency, and high-risk medication education were identified frequently. Because the current study was unable to assess parental opinion of proposed interventions, it is possible that the impact of such interventions may be under- or overestimated. While a parent or patient may be reluctant to discontinue use of a medication deemed to be unnecessary31–33, a previous study by Blackmer and colleagues demonstrated that, among caregivers of CMC with polypharmacy, 87% express willingness to discontinue use of ≥1 medication if recommended by their child’s PCP.34 We are therefore encouraged to believe that parental agreement with the pharmacist-proposed interventions would be favorable in a prospective pediatric MTM model.
Second, the proposed pediatric MTM structure includes additional opportunities for interventions which do not directly reduce medication regimen complexity but may improve outcomes. Parents of CMC have reported concerns about their own understanding of medication administration and safety and have additionally demonstrated inaccuracies in the measurement and administration of doses.34–38 Furthermore, parents of CMC may also identify additional symptom targets for medication-related interventions or vocalize concerns for unnecessary prescription use that would not be identifiable through chart review alone.13,34 In the prospective implementation of this pediatric MTM model, customized support components, such as discussion of ADE likelihood and in-the-moment caregiver education related to medication administration, could increase confidence and understanding amongst caregivers. Incorporation of strategies targeted towards medication adherence or caregiver understanding, such as pictogram-based medication instruction sheets, electronic adherence tools, or “see one, do one” visual instruction sessions should be tailored towards individual family needs and preferences and may lead to subsequent benefits in medication effectiveness and safety in this vulnerable population.34,39–43
Third, even within the focused population of CMC with polypharmacy, limited availability of pediatric pharmacist resources within the ambulatory setting necessitates proper identification of patients most likely to benefit from pediatric MTM to maximize the intervention’s effectiveness. When this study’s population was stratified by total medication count, patients requiring a higher number of medications at baseline had the potential to receive increased benefit in MRCI score reduction. The greatest potential reduction in MRCI scores was found in the 17% of children receiving 15 or more medications, with an average maximal reduction of 10.7 points from their pre-MTM MRCI score of 62.1 (95% CI: 56.4–67.7). Interestingly, stratification by age and CCC count showed no significant differences in magnitude of potential MRCI score reduction between subgroups. While the CCC classification system is considered the gold standard for classification of children with life-limiting illness, this system does not necessarily reflect anticipated medication use; some CCCs are less reliant on medication management. Therefore, there may be benefit to further identification of specific CCCs in which medication use is common. Nevertheless, these findings suggest that, in general, pharmacists should focus initially on CMC with the highest medication counts, but that this strategy may be refined and updated based on further testing of pediatric MTM among CMC.
Finally, it will be necessary to longitudinally collect and study relevant patient outcomes before and after pediatric MTM to fully assess the true benefits of the proposed interventions. To support widespread adoption, pragmatic data demonstrating effectiveness, value, and feasibility of the described model or a similar service are needed. Meaningful metrics for evaluation of the prospective implementation of pediatric MTM may include changes in medication regimen complexity (e.g., MRCI score) or validated parent-reported symptom scoring tools as direct measure of impact at the patient and caregiver level. Further, improvement in medication adherence and reduction in both healthcare utilization and total medication costs have been demonstrated as benefits of MTM services in comparable adult populations and should be evaluated within the pediatric population.44 Assessment of parental and provider acceptance of proposed interventions, the potential for maintenance of the pediatric MTM intervention over an extended period, and the direct and indirect costs of pediatric MTM implementation may also encourage or guide the adoption and employment of this model on a wider scale.
Our study’s findings must be considered in the context of several limitations. First, the retrospective design necessitates assumption of accuracy of the EHR medication list, as well as that documented medications were used as prescribed; variability in adherence may cause error in the assessment of an intervention’s impact on regimen complexity. Prospective use of the standardized pediatric MTM intervention should ensure thorough medication reconciliation has occurred prior to recommendation of any medication changes. Second, the MRCI tool is not designed to capture all relevant pediatric-specific aspects of medication administration that may increase complexity, including the use of enteral feeding tubes, compounded medications, or medication selection based on an algorithmic approach (e.g., escalating medication use for migraine or seizure management). Third, the impact of pediatric MTM services may vary according to the experience of a pharmacist performing the intervention; a pharmacist with pediatric residency training, board certification, or experience working directly with CMC may, for example, be more likely to identify an unnecessary medication than a pharmacist without such training. This potential variance may be mitigated through use of a standardized decision-making tool, as described in the methods section and included in Supplemental Appendix A. Lastly, the impact of pharmacist recommendations resulting from this standardized pediatric MTM model are reliant on adoption of the proposed interventions by both caregivers and providers. It is expected that initial adoption would be highest at practice sites in which pharmacists have developed strong interdisciplinary relationships and caregiver trust, though recognition of the benefits of such a model may increase over time through demonstration of effectiveness and value of the pediatric MTM model for CMC.
CONCLUSION
In this retrospective study, a standardized systematic approach to pediatric MTM identified medication-related concerns and potential interventions for nearly all patients with CMC. Pediatric patients receiving 5 or more medications had the potential to experience significant reductions in medication regimen complexity following the pediatric MTM intervention, suggesting opportunities for targeted services to improve medication-related outcomes. Our findings support further prospective evaluation of the effectiveness of a pharmacist-guided pediatric MTM service for CMC with polypharmacy.
Supplementary Material
Key points.
What was already known:
Medication therapy management services have been shown to be effective at improving both patient outcomes and economic outcomes in adult patients.
Current pediatric medication management strategies are primarily reactive, rather than proactive; literature describing a systematic MTM model for children is scarce.
Increased medication regimen complexity, as measured by the Medication Regimen Complexity Index, has been associated with increased acute healthcare utilization in adult and pediatric populations.
What this study adds:
A systematic, retrospective, pharmacist-performed pediatric MTM intervention led to a significant decrease in Medication Regimen Complexity Index scores in children with medical complexity and polypharmacy.
A standardized approach to pediatric MTM services may provide benefit to CMC, particularly those with high baseline total medication counts.
Funding support:
Dr. Feinstein was supported by the Eunice Kennedy Shriver National Institute of Child Health & Human Development of the National Institutes of Health under award number K23HD091295.
Footnotes
Disclosures of conflicts of interest: All authors have no conflicts of interest to report.
Previous presentations of the work: Portions of this work were presented at the 2021 ASHP Midyear Clinical Meeting and Exhibition Student Poster Session (virtual).
Data statement:
De-identified data set is available upon request.
REFERENCES
- 1.Kuo DZ, Houtrow AJ, Council on Children with Disabilities. Recognition and management of medical complexity. Pediatrics. Dec 2016;138(6):e20163021. doi: 10.1542/peds.2016-3021 [DOI] [PubMed] [Google Scholar]
- 2.Feudtner C, Christakis DA, Connell FA. Pediatric deaths attributable to complex chronic conditions: a population-based study of Washington State, 1980–1997. Pediatrics. 2000;106(supplement 1): 205–9. doi: 10.1542/peds.106.S1.205 [DOI] [PubMed] [Google Scholar]
- 3.Feudtner C, Feinstein HA, Zhong W, Hall M, Dai D. Pediatric complex chronic conditions classification system version 2: updated for ICD-10 and complex medical technology dependence and transplantation. BMC Pediatr. 2014;14:199. doi: 10.1186/1471-2431-14-199 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Feudtner C, Dai D, Hexem KR, Luan X, Metjian TA. Prevalence of polypharmacy exposure among hospitalized children in the United States. Arch Pediatr Adolesc Med. Jan 2012;166(1):9–16. doi: 10.1001/archpediatrics.2011.161 [DOI] [PubMed] [Google Scholar]
- 5.Feinstein JA, Hall M, Antoon JW, et al. Chronic Medication Use in Children Insured by Medicaid: A Multistate Retrospective Cohort Study. Pediatrics. Apr 2019;143(4) doi: 10.1542/peds.2018-3397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.George J, Phun YT, Bailey MJ, Kong DC, Stewart K. Development and validation of the medication regimen complexity index. Ann Pharmacother. Sep 2004;38(9):1369–76. doi: 10.1345/aph.1D479 [DOI] [PubMed] [Google Scholar]
- 7.Feinstein JA, Friedman H, Orth LE, et al. Complexity of Medication Regimens for Children With Neurological Impairment. JAMA Netw Open. Aug 2 2021;4(8):e2122818. doi: 10.1001/jamanetworkopen.2021.22818 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hirsch JD, Metz KR, Hosokawa PW, Libby AM. Validation of a patient-level medication regimen complexity index as a possible tool to identify patients for medication therapy management intervention. Pharmacotherapy. Aug 2014;34(8):826–35. doi: 10.1002/phar.1452 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tjugum SL, Hansen BK, McKinzie CJ. Association of Medication Regimen Complexity With Clinical Endpoints in Pediatric Patients With Cystic Fibrosis. J Pediatr Pharmacol Ther. 2021;26(3):248–252. doi: 10.5863/1551-6776-26.3.248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Schoonover H, Corbett CF, Weeks DL, Willson MN, Setter SM. Predicting potential postdischarge adverse drug events and 30-day unplanned hospital readmissions from medication regimen complexity. J Patient Saf. Dec 2014;10(4):186–91. doi: 10.1097/PTS.0000000000000067 [DOI] [PubMed] [Google Scholar]
- 11.Feinstein JA, Feudtner C, Kempe A. Adverse drug event-related emergency department visists associated with complex chronic conditions. Pediatrics. Jun 2014;133(6):e1575–85. doi: 10.1542/peds.2013-3060 [DOI] [PubMed] [Google Scholar]
- 12.Antoon JW, Hall M, Herndon A, et al. Prevalence of Clinically Significant Drug-Drug Interactions Across US Children’s Hospitals. Pediatrics. Nov 2020;146(5)doi: 10.1542/peds.2020-0858 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Feinstein JA, Feudtner C, Blackmer AB, et al. Parent-Reported Symptoms and Medications Used Among Children With Severe Neurological Impairment. JAMA Netw Open. Dec 1 2020;3(12):e2029082. doi: 10.1001/jamanetworkopen.2020.29082 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Feinstein JA, Feudtner C, Valuck RJ, et al. Identifying Important Clinical Symptoms in Children With Severe Neurological Impairment Using Parent-Reported Outcomes of Symptoms. JAMA Pediatr. Nov 1 2020;174(11):1114–1117. doi: 10.1001/jamapediatrics.2020.2987 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Horace AE, Ahmed F. Polypharmacy in pediatric patients and opportunities for pharmacists’ involvement. Integr Pharm Res Pract. Aug 2015;4:113–26. doi: 10.2147/IPRP.S64535 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Benavides S, Madzhidova S, Hernandez A, Le T, Palma SM, Stephen S. Establishment of Pediatric Medication Therapy Management: A Proposed Model. Pharmacy (Basel). Jan 19 2016;4(1)doi: 10.3390/pharmacy4010005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sneed G, Kappeler K, Gilmore T, Kuhn C. Implementation of a medication therapy management collaborative within a pediatric health system. J Am Pharm Assoc (2003). Jul - Aug 2018;58(4S):S114–S119. doi: 10.1016/j.japh.2018.04.021 [DOI] [PubMed] [Google Scholar]
- 18.Centers for Disease Control and Prevention. Community Pharmacists and Medication Therapy Management. https://www.cdc.gov/dhdsp/pubs/guides/best-practices/pharmacist-mtm.htm. Accessed October 15, 2021.
- 19.Pellegrino AN, Martin MT, Tilton JJ, Touchette DR. Medication therapy management services: definitions and outcomes. Drugs. 2009;69(4):393–406. doi: 10.2165/00003495-200969040-00001 [DOI] [PubMed] [Google Scholar]
- 20.McGivney MS, Meyer SM, Duncan-Hewitt W, Hall DL, Goode JV, Smith RB. Medication therapy management: its relationship to patient counseling, disease management, and pharmaceutical care. J Am Pharm Assoc (2003). Sep-Oct 2007;47(5):620–8. doi: 10.1331/JAPhA.2007.06129 [DOI] [PubMed] [Google Scholar]
- 21.Feinstein JA, Russell S, DeWitt PE, Feudtner C, Dai D, Bennett TD. R package for pediatric complex chronic condition classification. JAMA Pediatr. 2018;172(6):596–598. doi: 10.1001/jamapediatrics.2018.0256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Institute for Safe Medication Practices. High-alert medications in community/ambulatory settings. Published January 31, 2011. Accessed November 3, 2021. https://www.ismp.org/recommendations/high-alert-medications-community-ambulatory-list
- 23.Harris PA, Taylor R, Thielke R, Payne J, Gonzalez N, Conde JG. Research electronic data capture (REDCap)--a metadata-driven methodology and workflow process for providing translational research informatics support. J Biomed Inform. 2009. Apr;42(2):377–81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Barnette DJ, Hanks C, Li W, Porter K. Patient-level medication regimen complexity in an adolescent and adult population with autism spectrum disorders. Pharmacotherapy. 2019;39(6):636–644. doi: 10.1002/phar.2202 [DOI] [PubMed] [Google Scholar]
- 25.Ferreira JM, Galato D, Melo AC. Medication regimen complexity in adults and the elderly in a primary healthcare setting: determination of high and low complexities. Pharm Pract (Granada). 2015;13(4):659. doi: 10.18549/PharmPract.2015.04.659 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Advinha AM, de Oliveira-Martins S, Mateus V, Pajote SG, Lopes MJ. Medication regimen complexity in institutionalized elderly people in an aging society. Int J Clin Pharm. 2014;36(4):750–756. doi: 10.1007/s11096-014-9963-4 [DOI] [PubMed] [Google Scholar]
- 27.Mansur N, Weiss A, Beloosesky Y. Looking beyond polypharmacy: quantification of medication regimen complexity in the elderly. Am J Geriatr Pharmacother. 2012;10(4):223–229. doi: 10.1016/j.amjopharm.2012.06.002 [DOI] [PubMed] [Google Scholar]
- 28.Libby AM, Fish DN, Hosokawa PW, et al. Patient-level medication regimen complexity across populations with chronic disease. Clin Ther. 2013;35(4):385–398.e1. doi: 10.1016/j.clinthera.2013.02.019 [DOI] [PubMed] [Google Scholar]
- 29.Patel CH, Zimmerman KM, Fonda JR, Linsky A. Medication complexity, medication number, and their relationships to medication discrepancies. Ann Pharmacother. 2016;50(7):534–540. doi: 10.1177/1060028016647067 [DOI] [PubMed] [Google Scholar]
- 30.Linnebur SA, Vande Griend JP, Metz KR, Hosokawa PW, Hirsch JD, Libby AM. Patient-level medication regimen complexity in older adults with depression. Clin Ther. 2014;36(11):1538–1546.e1. doi: 10.1016/j.clinthera.2014.10.004 [DOI] [PubMed] [Google Scholar]
- 31.Barnett ER, Trepman AZ, Fuson HA, et al. Deprescribing psychotropic medications in children: results of a national qualitative study. BMJ Qual Saf. Aug 2020;29(8):655–663. doi: 10.1136/bmjqs-2019-010033 [DOI] [PubMed] [Google Scholar]
- 32.Grudnikoff E, Bellonci C. Deprescribing in child and adolescent psychiatry–a sorely needed intervention. Am J Ther. Jan 2017;24(1):e1–e2. doi: 10.1097/MJT.0000000000000552 [DOI] [PubMed] [Google Scholar]
- 33.Buzancic I, Dragovic P, Pejakovic TI, Markulin L, Orthner-Hadziabdic M. Exploring patients’ attitudes toward deprescribing and their perception of pharmacist involvement in a European country: a cross-sectional study. Patient Prefer Adherence. Sep 2021;15:2197–2208. doi: 10.2147/PPA.S323846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Blackmer AB, Fox D, Arendt D, Phillips K, Feinstein JA. Perceived Versus Demonstrated Understanding of the Complex Medications of Medically Complex Children. J Pediatr Pharmacol Ther. 2021;26(1):62–72. doi: 10.5863/1551-6776-26.1.62 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edelstein H, Schippke J, Sheffe S, Kingsnorth S. Children with medical complexity: a scoping review of interventions to support caregiver stress. Child Care Health Dev. May 2017;43(3):323–333. doi: 10.1111/cch.12430 [DOI] [PubMed] [Google Scholar]
- 36.Philips K, Zhou R, Lee DS, et al. Caregiver Medication Management and Understanding After Pediatric Hospital Discharge. Hosp Pediatr. Nov 2019;9(11):844–850. doi: 10.1542/hpeds.2019-0036 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yin HS, Mendelsohn AL, Wolf MS, et al. Parents’ medication administration errors: role of dosing instruments and health literacy. Arch Pediatr Adolesc Med. Feb 2010;164(2):181–6. doi: 10.1001/archpediatrics.2009.269 [DOI] [PubMed] [Google Scholar]
- 38.You MA, Nam SM, Son YJ. Parental Experiences of Medication Administration to Children at Home and Understanding of Adverse Drug Events. J Nurs Res. Sep 2015;23(3):189–96. doi: 10.1097/jnr.0000000000000080 [DOI] [PubMed] [Google Scholar]
- 39.Yin HS, Dreyer BP, van Schaick L, Foltin GL, Dinglas C, Mendelsohn AL. Randomized controlled trial of a pictogram-based intervention to reduce liquid medication dosing errors and improve adherence among caregivers of young children. Arch Pediatr Adolesc Med. Sep 2008;162(9):814–822. doi: 10.1001/archpedi.162.9.814 [DOI] [PubMed] [Google Scholar]
- 40.Yin HS, Parker RM, Sanders LM, et al. Pictograms, units and dosing tools, and parent medication errors: a randomized study. Pediatr. Jul 2017;140(1):e20163237. doi: 10.1542/peds.2016-3237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Allshouse C, Comeau M, Rodgers R, Wells N. Families of children with medical complexity: a view from the front lines. Pediatr. Mar 2018;141(s3):S195–201. doi: 10.1542/peds.2017-1284D [DOI] [PubMed] [Google Scholar]
- 42.El-Rachidi S, LaRochelle JM, Morgan JA. Pharmacists and pediatric medication adherence: bridging the gap. Hosp Pharm. Feb 2017;52(2):124–131. doi: 10.1310/hpj5202-124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Morawski K, Ghazinouri R, Krumme A, et al. Association of a smartphone application with medication adherence and blood pressure control: the MediSAFE-BP randomized clinical trial. JAMA Intern Med. Jun 2018;178(6):802–809. doi: 10.1001/jamainternmed.2018.0447 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Center for Medicare and Medicaid Innovation. Part D Enhanced Medication Therapy Management Model. Updated Aug 2021. https://innovation.cms.gov/innovation-models/enhancedmtm. Accessed December 21, 2021.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
De-identified data set is available upon request.


