Cardiovascular disease is the leading cause of morbidity and mortality in the world [1]. Many individuals are unaware they may have cardiovascular disease, and the disease may go undetected until the occurrence of serious adverse events such as a stroke or heart attack. Therefore, identification of occult cardiovascular disease and early institution of medical interventions and lifestyle modifications is of paramount importance. Individuals with cardiovascular disease are at higher risk of developing retinal artery and vein occlusions, and even in the absence of overt retinal vascular occlusions, these patients may exhibit signs of subclinical retinal ischemia [2]. We previously demonstrated that lesions indicative of previous episodes of focal retinal ischemia, which we termed retinal ischemic perivascular lesions (RIPLs), are prevalent in patients with cardiovascular disease [2]. We showed that RIPLs, which are detected on routinely obtained spectral domain optical coherence tomography (SD-OCT) images, are associated with increased odds of having cardiovascular disease, even after adjusting for confounding variables including common cardiovascular risk factors [2]. As a legacy of PAMM [3, 4], RIPLs represent inner nuclear layer (INL) infarcts and have been documented in various conditions with ischemic etiologies including hypertension (HTN) [5] and in fellow eyes of patients with unilateral retinal vein occlusion [6]. In this study, we sought to determine whether detection of RIPLs in a real-world clinical setting led to identification of previously undiagnosed cardiovascular disease. We present the results of cardiovascular workup from a consecutive cohort of patients, with no pre-existing cardiovascular disease, in whom RIPLs were noted during retinal examination.
This is a review of a retrospective case series. The study adhered to the tenets of Declaration of Helsinki. Institutional Review Board approval was obtained from the University of California San Diego Health System with a waiver of informed consent for retrospective chart review. We identified consecutive patients from our clinical retinal practice in whom RIPLs were identified on SD-OCT taken for various clinical indications, and who did not have any evidence of underlying retinopathy. A RIPL was defined by the SD-OCT presence of focal atrophy of the INL associated with secondary expansion of the outer nuclear/Henle’s fiber layer, leading to a wavy appearance of the middle retinal layers as described by Long et al [2]. Inclusion criteria included a high-quality 6 x 6 mm SD-OCT volume macular scan consisting of 49 B-scans (cross sections). The SD-OCT scan part of the routine workflow at our institution for patients referred for retinal examination. Exclusion criteria were lack of follow-up at our institution, presence of macular edema, or any evidence of central retinal artery or vein occlusion, posterior uveitis, pars plana vitrectomy, intravitreal injection, or panretinal photocoagulation. At the time of examination, individuals with RIPLs but without a prior history of cardiovascular disease, other than essential hypertension (HTN), were advised to follow up with their primary care physician or cardiologist for an age-appropriate cardiovascular workup, at their physician’s discretion. We excluded those who did not seek a medical follow up.
We identified thirty-six patients with incidental RIPL identification on SD-OCT (Figure 1). Indications for retinal examination included floaters, posterior vitreous detachment, visual disturbance, screening for diabetic retinopathy, and referral for retinal examination following cataract surgery. A total of 25 patients were excluded. Ten were excluded due to presence of pre-existing cardiovascular disease, including coronary artery disease (CAD), congestive heart failure (CHF) and atrial fibrillation, as well as prior adverse cardiovascular events like pulmonary embolism, deep venous thrombosis, and stroke. Fifteen individuals chose not to pursue further cardiovascular workup or pursued it outside our institution. Of the 11 subjects that were included, 5 were males and 6 were females. Ages ranged from 44 to 80 years. The eleven subjects with no prior history of cardiovascular disease, other than essential HTN, followed up with their primary care physician or cardiologist for age-appropriate cardiovascular work-up (Table S1). Of these, newly diagnosed cardiovascular disease was identified in 8 individuals (72.7%), including multi-vessel CAD, significant carotid artery stenosis, soft carotid plaque, reduced cardiac ejection fraction, patent foramen ovale, cerebral infarction, subclavian steal syndrome and undiagnosed or poorly-controlled HTN. Two patients subsequently underwent invasive procedures – in Case #1, the patient required coronary artery bypass grafting (CABG) after being found to have three-vessel disease on coronary angiography (Figure S1) which was prompted by an abnormal echocardiogram, and in Case #2, the patient underwent carotid artery stent placement after observation of multifocal atherosclerotic disease on head and neck computed tomography angiography (Figure S2). Three patients initiated new medical therapy for their conditions, including antihypertensives and anticoagulants. Two patients were advised to undergo periodic monitoring.
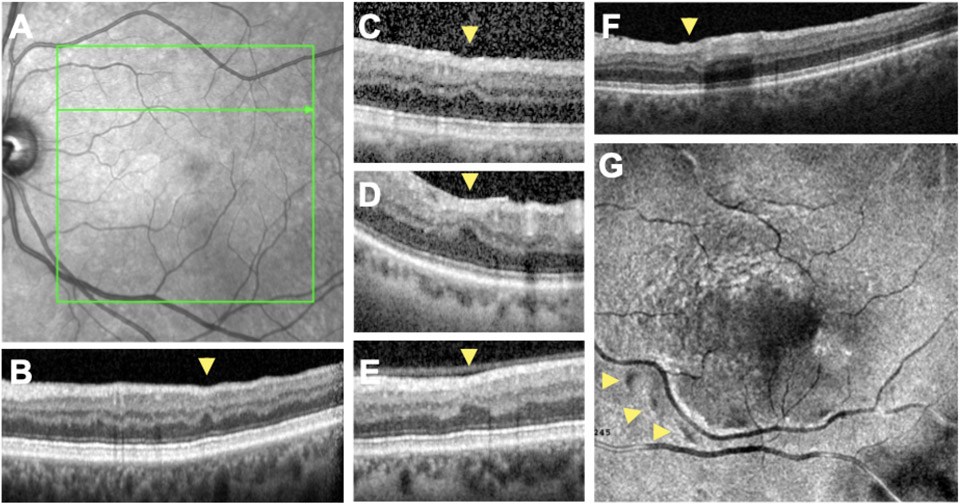
Figure 1. RIPLs detected on spectral domain optical coherence tomography (SD-OCT) scans of study subjects.
A) Infrared image of retina of the left eye of Case #1 with no appreciable abnormalities. B) SD-OCT B-scan of the same individual corresponding to the green arrow in A demonstrating a RIPL (yellow arrowhead). C-F) SD-OCT B-scans of Cases 3 (C), 5 (D), 4 (E) and 11 (F). RIPLs are denoted with yellow arrowheads. G) En face 3D reconstruction, at the level of the outer plexiform layer demonstrating three RIPLs appearing as dark spots (yellow arrowheads) with a perivascular predilection.
Our report highlights the potential of using RIPLs to uncover underlying cardiovascular disease in a real-world clinical setting. We had previously demonstrated, in a retrospective series of 160 individuals, that the presence of one, two or three RIPLs was associated with an odds ratio of 2.34, 4.17, and 5.34 of having cardiovascular disease, respectively, after adjusting for age, sex and smoking status [2]. RIPLs, which are anatomical biomarkers of retinal ischemia [2-4], have also been documented in retrospective studies in individuals with HTN [5] and in fellow eyes of patients with unilateral retinal vein occlusion [6]. As a legacy of PAMM [3, 4], these lesions represent INL infarcts and may represent the earliest evidence of macular ischemia [4]. In this series, we present real-world data which further supports the role of RIPL identification in detecting co-existing cardiovascular disease, with the goal of preventing major systemic cardiovascular events. Besides the obvious clinical benefit in reducing morbidity and mortality, early intervention could also reduce the financial burden associated with managing complications such as stroke and heart attack 2. RIPL detection with SD-OCT is a simple, fast technique that is inexpensive, non-invasive, already part of the routine ocular examination, and widely used in ophthalmology and optometry clinics.
In conclusion, our real-world findings strongly suggest that RIPLs may provide an additional biomarker to predict the existence of a subclinical cardiovascular disease. Major limitations to our study include the relatively small number and nonconsecutive ascertainment of patients and lack of standardized workup. It also remains to be determined whether significance of RIPLs may vary in different age groups. For example, retinal vein occlusions are associated with stroke independent of underlying cardiovascular co-morbidities [7]. This association was highly significant in younger individuals but not in those older than 85 years of age. Future prospective studies in which subjects with and without RIPLs are offered a standardized cardiovascular evaluation are needed to assess the utility of RIPLs in detecting underlying cardiovascular disease.
Supplementary Material
Acknowledgements.
CYB is funded by The American Heart Association Award 857722 and NIDDK K23 DK129836. MFB is supported by NIH Research Grant P30CA016359 from the National Cancer Institute.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest: DS is a consultant for Heidelberg, Topcon, Amgen, Bayer, Iveric Bio, Novartis and Optovue, and receives equipment for research from Heidelberg, Optovue and Topcon. No conflicting relationship exists for any other author.
References
- 1.Benjamin EJ, et al. , Heart Disease and Stroke Statistics-2019 Update: A Report From the American Heart Association. Circulation, 2019. 139(10): p. e56–e528. [DOI] [PubMed] [Google Scholar]
- 2.Long CP, et al. , Prevalence of subclinical retinal ischemia in patients with cardiovascular disease - a hypothesis driven study. EClinicalMedicine, 2021. 33: p. 100775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Yu S, et al. , Multimodal imaging findings in retinal deep capillary ischemia. Retina, 2014. 34(4): p. 636–46. [DOI] [PubMed] [Google Scholar]
- 4.Bakhoum MF, et al. , Paracentral Acute Middle Maculopathy and the Ischemic Cascade Associated With Retinal Vascular Occlusion. Am J Ophthalmol, 2018. 195: p. 143–153. [DOI] [PubMed] [Google Scholar]
- 5.Burnasheva MA, et al. , Association of Chronic Paracentral Acute Middle Maculopathy Lesions with Hypertension. Ophthalmol Retina, 2020. 4(5): p. 504–509. [DOI] [PubMed] [Google Scholar]
- 6.Maltsev DS, et al. , Prevalence of resolved paracentral acute middle maculopathy lesions in fellow eyes of patients with unilateral retinal vein occlusion. Acta Ophthalmol, 2020. 98(1): p. e22–e28. [DOI] [PubMed] [Google Scholar]
- 7.Bakhoum CY, et al. , Retinal vein occlusion is associated with stroke independent of underlying cardiovascular disease. Eye (Lond), 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.