Abstract
Magnetic resonance imaging (MRI) has gained increasing importance in the management of rectal cancer over the last two decades. The role of MRI in patients with rectal cancer has expanded beyond the tumor-node-metastasis (TNM) system in both staging and restaging scenarios and has contributed to identifying “high” and “low” risk features that can be used to tailor and personalize patient treatment; for instance, selecting the patients for neoadjuvant chemoradiation (NCRT) before the total mesorectal excision (TME) surgery based on risk of recurrence. Among those features, the status of the circumferential resection margin (CRM), extramural vascular invasion (EMVI), and tumor deposits (TD) have stood out. Moreover, MRI also has played a role in surgical planning, especially when the tumor is located in the low rectum, when the relationship between tumor and the anal canal is important to choose the best surgical approach, and in cases of locally advanced or recurrent tumors invading adjacent pelvic organs that may require more complex surgeries such as pelvic exenteration. As approaches using organ preservation emerge, including transanal local excision and “watch-and-wait”, MRI may help in the patient selection for those treatments, follow up, and detection of tumor regrowth. Additionally, potential MRI-based prognostic and predictive biomarkers, such as quantitative and semi-quantitative metrics derived from functional sequences like DWI and DCE and radiomics, are under investigation. This review provides an overview of the current role of MRI in rectal cancer in staging and restaging and highlights the main areas under investigation and future perspectives.
Keywords: Magnetic resonance imaging, Rectal cancer
1. Introduction
Colorectal cancer is the third most common cancer and the second leading cause of death due to cancer in the world. Rectal cancer accounts for approximately one-third of these cases [1]. Magnetic resonance imaging (MRI) is the modality of choice for local staging and restaging due to its superb soft-tissue resolution. It also plays an essential role in evaluation of treatment response, surveillance and in detection of local recurrence after surgery.
Clinical assessment by digital rectal examination (DRE), endorectal ultrasound (ERUS), and computerized tomography (CT) were the first methods used for preoperative assessment in rectal cancer. However, DRE is limited by its subjective nature and inevitably understages extramural disease and mesorectal fascia involvement. ERUS is also highly operator-dependent and limited for proximal and stenotic rectal tumors, disease beyond the immediate lumen and for evaluation of the mesorectal fascia [2, 3]. CT lacks the inherent soft tissue resolution for discriminating rectal wall layers and involvement of the pelvic floor structures and its use is confined to determining the presence of distant metastases [4–6].
MRI technological improvements during the last two decades have enhanced our understanding of the detailed anatomy of the rectum and surrounding structures to allow accurate local staging essential to patient care. MRI has also advanced our knowledge of new prognostic imaging features such as extramural vascular invasion (EMVI), tumor deposits (TD) and tumor mucinous content [7–9]. Advances in functional MRI techniques which interrogate the tumor environment (e.g., diffusion weighted images (DWI)) may in the future play a role however, more research is needed to validate their prognostic utility.
In recognition of these rapid advances in our knowledge base, training sessions and hands-on workshops (e.g. offered by the American College of Radiology (ACR) [10] and the UK Professor Gina Brown [11]) are more frequently held and are essential tools in knowledge propagation given the proven learning curves among radiologists and trainees.
2. A Primer on Rectal Cancer Treatment
The goals of modern management of rectal cancer are to reduce perioperative and long-term morbidity, preserve organ function and quality of life, and minimize the risk of local recurrence and distant metastases.
Rectal cancer management has changed rapidly over the past decades and is more driven by imaging today. Until the early 1980s, patients were primarily treated with non-anatomic plane surgery alone, which resulted in a high risk of pelvic recurrence, morbidity, and mortality. Surgery was then supplemented with adjuvant therapy soon after numerous studies showed that postoperative chemoradiotherapy reduced pelvic recurrence rates and enhanced survival [12, 13]. In parallel, the importance of the surgical technique as demonstrated by Heald in the UK [14] and Enker in the USA [15] completely changed the landscape and demonstrated the superiority of total mesorectal excision (TME) (radical excision of the rectum and mesorectal envelope en bloc) over the non-anatomic perivisceral procedure being done at the time [14]. Later, the German Rectal Cancer Study found that preoperative chemoradiotherapy, as opposed to postoperative chemoradiotherapy, resulted in better pelvic control and sphincter preservation, and decreased morbidity [16], establishing yet a new standard of care.
MRI in parallel with histopathology has contributed to our knowledge of the prognostic inhomogeneity of T3 rectal cancers and has challenged the empiric neoadjuvant chemoradiation treatment paradigm often used irrespective of T3 depth [17]. Tumors extending ≤ 5mm beyond the muscularis propria have local recurrence and survival rates similar to T2 tumors. As such, European guidelines advise treatment of early rectal tumors ≤ T3b without clear involvement of the MRF with TME surgery alone, while locally advanced tumors ≥ T3c, with involved CRM on MRI (≤ 1mm), or presence of EMVI, are treated with neoadjuvant chemoradiotherapy (NCRT) followed TME surgery [18]. The empiric administration of preoperative radiotherapy (RT) to all patients with imaging suspicion for lymph node metastases is also still debatable [19], and the results of the PROSPECT trial testing selective use of RT are eagerly awaited [20].
As with other cancers, there is a growing interest in developing more patient-tailored approaches in recognition of the multiple (and increasing) risk factors/biomarkers that identify patients with “good prognosis” versus “bad prognosis” rectal tumors. The Quicksilver study, a prospective multicenter study whose primary endpoint was evaluating the safety and feasibility of MRI selected “good prognosis” patients (≤ T3b, distance to the mesorectal fascia greater than 1 mm from the primary tumor, discontinuous tumor nodule, or suspicious lymph node, and absent or equivocal EMVI) to undergo primary surgery, found low rates of positive circumferential resection margins (CRM), the known strongest predictor of local recurrence [21]
Another treatment paradigm gaining traction is avoidance of radical surgery altogether in selected candidates with excellent responses. It is well established that pathologic complete response (pCR) rates of up to 20% or higher have been observed after neoadjuvant chemoradiation [22]. A nonoperative management approach (also called “watch-and-wait” or organ-sparing) or perhaps local excision may be proven acceptable in this scenario, but because of limited long-term results and the lack of a reliable way to accurately identify these patients preoperatively, it is premature to make conclusions. The only prospective trial testing the non-operative management paradigm, the OPRA study, has finished accrual and long-term outcome results are eagerly awaited [23].
Among the rapidly evolving concepts of risk factors impacting treatment, lateral pelvic lymph nodes [primarily obturator and internal iliac] are approached very differently in Japan than in most western treatment centers. Routine dissection of these nodes has been historically recommended by Japanese societies for tumors located below the peritoneal reflection (generally 0–8 cm) to lower the risk of pelvic recurrence. This is not routinely recommended by European and American societies [17, 19]. Although it has been shown that selective lateral lymph node dissection may reduce local recurrence, no significant impact on overall survival can be demonstrated [24–27].
3. What We’ve Learned Through the Implementation of MRI into National Clinical Trials and the Impact of MRI on the Health Care System
The use of pelvic MRI for rectal cancer evaluation and management, in part, derives from its incorporation into clinical trials testing new treatments. This has been either in the form of (i) MRI-based risk categories determining treatment pathways (in concert with clinical findings) or more commonly as (ii) MRI serving an integral role in clinical staging and response evaluation allowing quality-controlled uniform retrospective assessment of MRI accuracy and efficacy. In the first category, and the first prospective study (although observational) was the MERCURY study [33,34] which determined that MRI measurement of tumor penetration into the fat is equivalent to findings at histopathology (to within 0.5mm) and confirmed that a 1mm distance of tumor from the mesorectal fascia (CRM) conferred poorer outcomes. Specifically, MRI was accurate in predicting CRM involvement with an accuracy of 91% and a NPV of 93% in patients that did not receive neoadjuvant therapy. The MERCURY II study [28, 29] revealed a safe low classification system in low rectal tumors. Tailored management based on “safe” low rectal cancer surgical resection plane on baseline MRI enabled optimal clinical management, reducing pathological CRM involvement to 9.0%. In the second category of MRI clinical trial incorporation, Pan-Ex (a pooled analysis of Expert and Expert-C trials testing Cetuximab in KRAS wild type patients) [30] found that mrTRG correlated with the long-term outcome and appeared to stratify patients based on the incremental benefit from sequential CRT. The Quicksilver [23] and RAPIDO trials [40] successfully categorized high- and low-risk patients using MRI-defined criteria to establish that low-risk groups had lower pathologic CRM; lower disease related treatment failures; and lower rates of distant metastases. Although results are not yet published, preliminary data from the TNT [31], OPRA [23] and PROSPECT [20] trials indicate that combining mrTRG with DWI [32], incorporating DWI, T2WI and endoscopy using an expert combined reference standard [33] and arbitrating response to therapy with a 20% reduction in tumor size [Phase II component of PROSPECT passed DSMB safety rules to proceed to Phase III] respectively are probably safe and successful uses of MRI in clinical trials (personal communication Marc Gollub, Radiologic PI or co-PI, all 3 studies). Finally, the phase III TRIGGER trial aims to determine whether patients with locally advanced rectal cancer can be recruited and subsequently randomized into a control trial that offers MRI-directed watch and wait for patients with a good response to CRT (mrTRG1–2) [34].
Fryback and Thornbury [35] created a useful hierarchical model to assess the role and usefulness of new medical technology. If applied to the use of MRI for rectal cancer, of 6 levels ranging from simple Technical Efficacy (Level 1) to Societal Efficacy (Level 6), the above studies firmly place MRI in the “Patient Outcome Efficacy” level (Level 5). As such, MRI is a very well-suited and validated medical test for rectal cancer evaluation [36].
4. European and American Guidelines on Rectal MRI
Rectal cancer management varies across the globe and between professional societies. Table 1 summarizes the main similarities and differences between the most recent guidelines published by the European Society of Gastrointestinal and Abdominal Radiology (ESGAR) [18] and the North American Society of Abdominal Radiology (SAR) [37].
Table 1.
Main similarities and differences between the most recent guidelines published by the European Society of Gastrointestinal and Abdominal Radiology (ESGAR) and the North American Society of Abdominal Radiology (SAR).
| Similarities | |
|
| |
| • MRI should be used for rectal cancer primary staging and restaging. | |
| • Endorectal ultrasound is recommended to differentiate between T1 and T2 tumors. | |
| • Minimal field strength requirement for the MRI system is 1.5 T. | |
| • 3 orthogonal planes T2W (sagittal, plus axial and coronal angulated to the tumor axis). | |
| • Coronal T2W parallel to anal canal in distal tumors. | |
| • Optimal slice thickness for T2W is 3 mm (ESGAR) and up to 3–4 mm (SAR) | |
| • DWI is recommended for restaging. | |
| • T1 and contrast images are not recommended. | |
| • MRF involved when distance between the tumor and MRF is < 1 mm | |
| • CR can be diagnosed when two-layered rectal wall is normalized on restaging. | |
| • Using only size threshold for LN staging is not universally accepted | |
|
| |
| Differences | |
|
| |
| SAR | ESGAR |
| • Measurement (mm) of the extent of tumor invasion beyond the bowel wall should be reported. • No consensus on the use of size criteria in primary staging of lymph nodes. • After CRT, nodal downsizing is considered a sign of sterilization. |
• Only the discrimination between T3ab (<5 mm) and T3cd (> 5mm) is required regarding the extramural extension depth. • Nodal size criteria, depending on the morphological features. • After CRT, nodes with a short axis < 5 mm are considered sterilized. |
5. MRI Technique
A good quality MRI more often results when a patient already has histopathologic confirmation of rectal cancer, and the tumor position is known from endoscopy. A distance estimation on MRI usually correlates quite well with endoscopy [38, 39] and can help avoid errors in tumor identification. We have also found (MJG, MCF) that high quality images may more often be achieved with the use of spasmolytics to reduce bowel peristalsis and with a 5-mL micro-enema administered just before the acquisition of MRI to reduce gas-related artifacts on DWI [40]. However, there is little consensus on their use. Some experts also recommend endorectal filling to help localize and depict the tumor [41], but this is not recommended by ESGAR or SAR guidelines [18, 37] and was shown not to improve tumor staging. Additionally, it can actually alter the distance of the tumor from the anorectal junction and mesorectal fascia [42].
The workhorse and obligatory MRI sequence is high-resolution fast spin-echo (FSE) T2-weighted (T2W) imaging. This depicts tumor and surrounding fat and organs with the highest intrinsic signal differences (contrast) allowing accurate differentiation. These sequences should be performed in sagittal, coronal, and axial planes perpendicular and parallel to the tumor axis using 3mm-slices and a field of view of 16cm (using 256 matrix) or 20 cm (using 384 matrix) and requires a minimum of 4 signal averages per acquisition. This gives an in-plane resolution of 0.6mm × 0.6mm and a voxel size of 1.1mm3. This allows an accurate assessment of the depth of invasion and involvement of adjacent structures and the correct distance from the mesorectal fascia [18, 37]. In low rectal tumors near or involving the anal sphincter complex, an additional high-resolution T2W oblique coronal parallel to the anal canal should be performed to assess the tumor relationship with the sphincter complex. For all tumors there should be coverage of the mesorectum for a minimum of 5cm above the upper border of the tumor to ensure that upward spread is also imaged at high resolution. In addition, a whole pelvis, larger field of view T2W sequence must be added to assess the nodes from the inferior mesenteric artery origin and caudad (e.g., common iliac nodes at the aortic bifurcation).
Gadolinium based contrast agent (GBCA) during MRI is optional and not recommended by ESGAR or SAR. Its use did not improve the diagnostic accuracy of T-staging, mesorectal fascia involvement, or invasion of adjacent organs in several independent, retrospective staging studies [43–45]. In the research arena, some studies showed that quantitative and qualitative features using GBCA-based DCE-MRI could help in detecting complete tumor response at restaging MRI [46–48].
There is an increasing recognition and validation of the importance of DWI in rectal cancer predominantly at restaging MRI by some researchers [49]. Other researchers would argue that in the absence of long-term outcome data, DWI remains a research tool given that it does not provide superior accuracy in identification of known and validated prognostic factors assessed using high resolution T2W sequences i.e. T depth >5mm, mrEMVI status pre and post treatment, mrTRG, mrCRM and mr Low Rectal stage [50–54]. Although the European consensus guidelines (ESGAR) only recommend DWI at restaging, the American guidelines recommend DWI at baseline also since it may increase the conspicuity of small primary tumors and nodes [18, 37]. Reduced field-of-view (FOV) DWI may provide better image quality of the primary tumor, and some authors have suggested that it may provide better diagnostic accuracy than full FOV DWI in the evaluation of CR at restaging [55–57].
5.1. Anatomy
Familiarity with rectal and pelvic anatomy is crucial for proper interpretation of rectal MRI. The definition of the rectum can be vague and confusing, and only more recently have there been efforts to attempt to clarify and standardize its definition in order to enhance patient management. One of the authors (MJG) can attest to upcoming proposed changes, based on ongoing discussions in the AJCC 9th edition committee, where instead of the arbitrary tripartite division based on millimetric measurement from the anal verge, a more physiologic and treatment-relevant anatomic division might be used with such landmarks as the anorectal junction [FIGURE 1], the anterior peritoneal reflection [FIGURE 2], and the “sigmoid takeoff” [FIGURE 3].
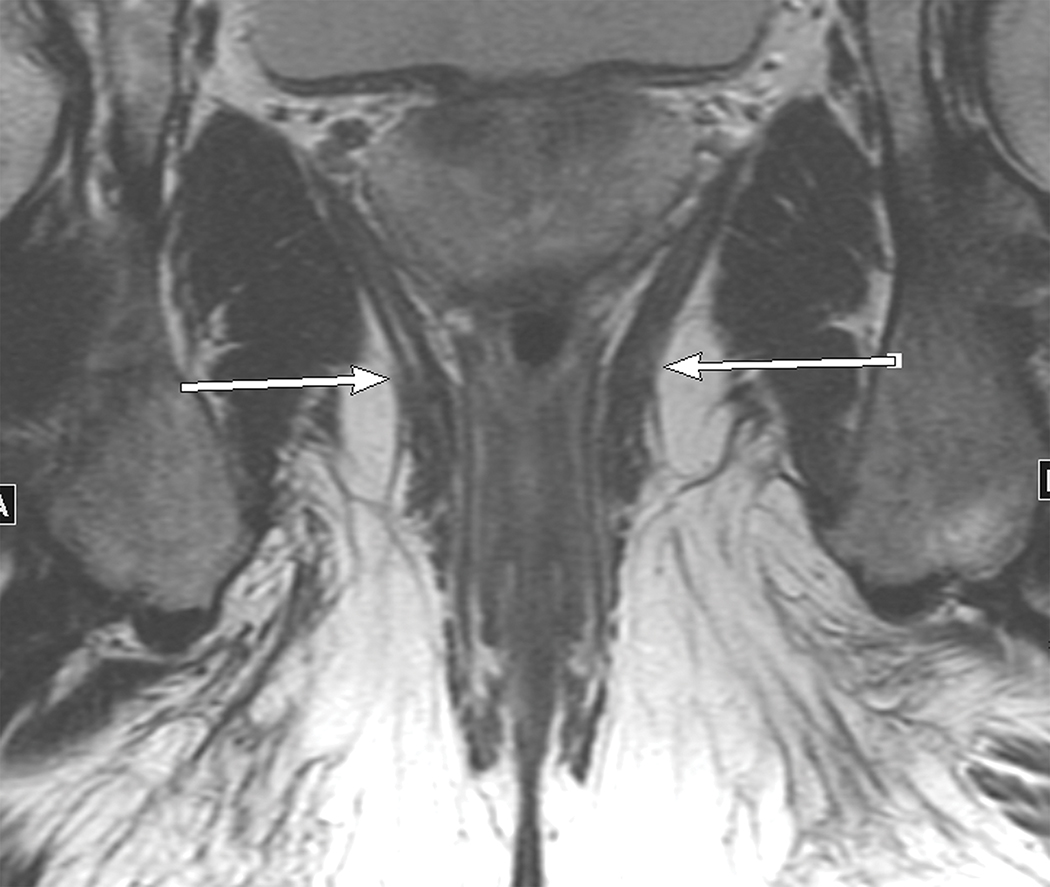
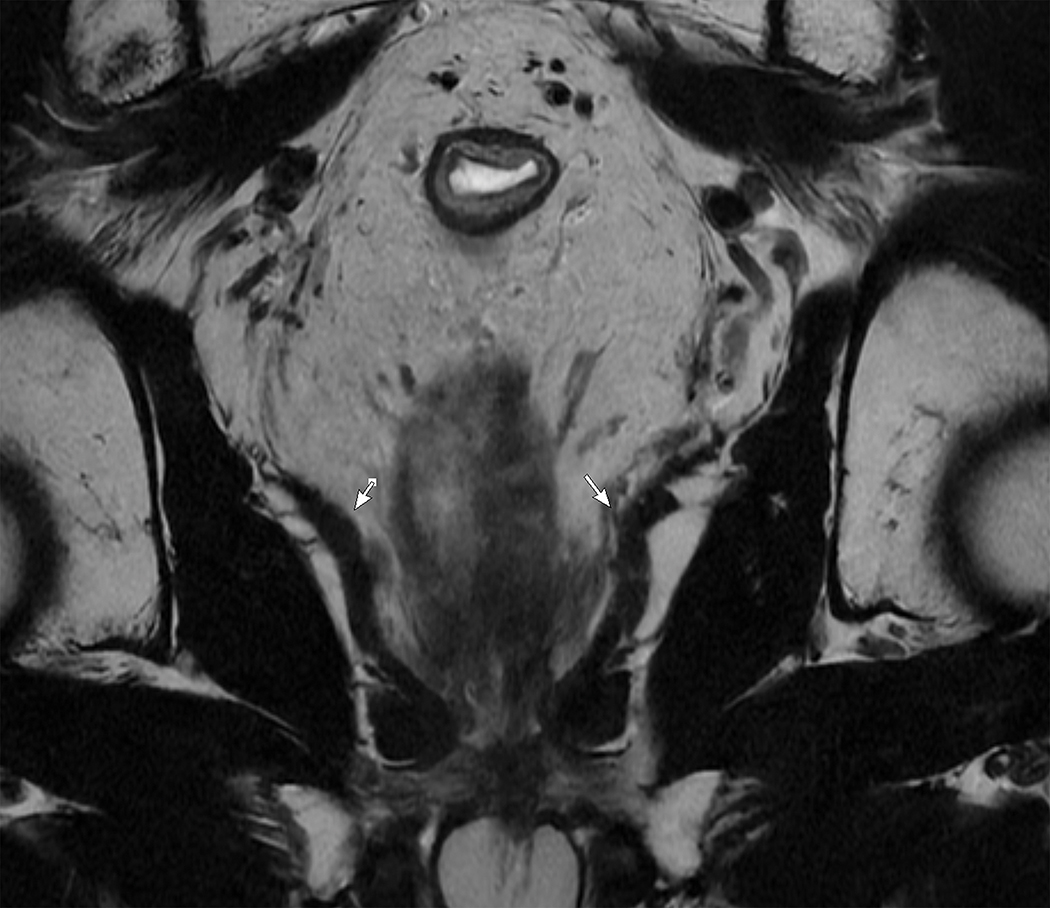
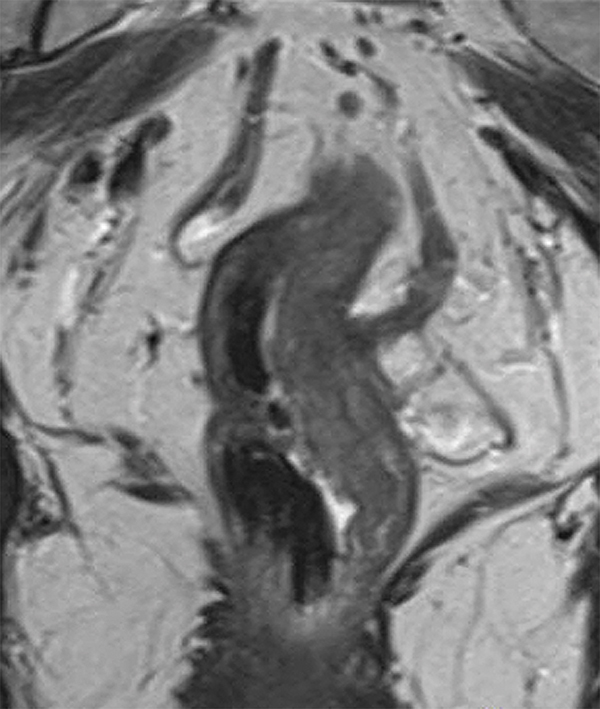
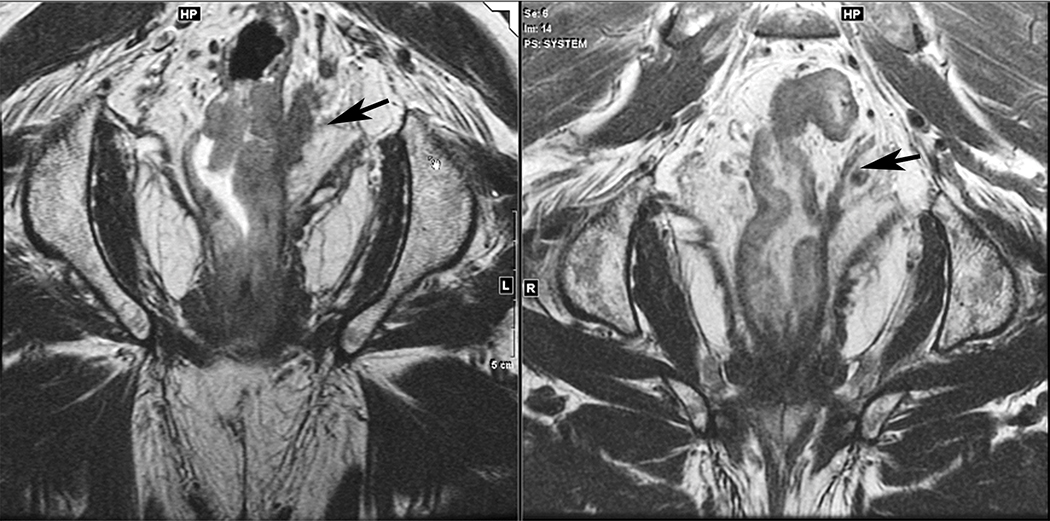
Figure 1.
Sagittal and coronal T2W MR pelvis images through the pelvic floor in a 52-year-old male with rectal cancer. Arrows indicate top of puborectalis/pubococcygeus muscles denoting the anorectal junction or “ring”.
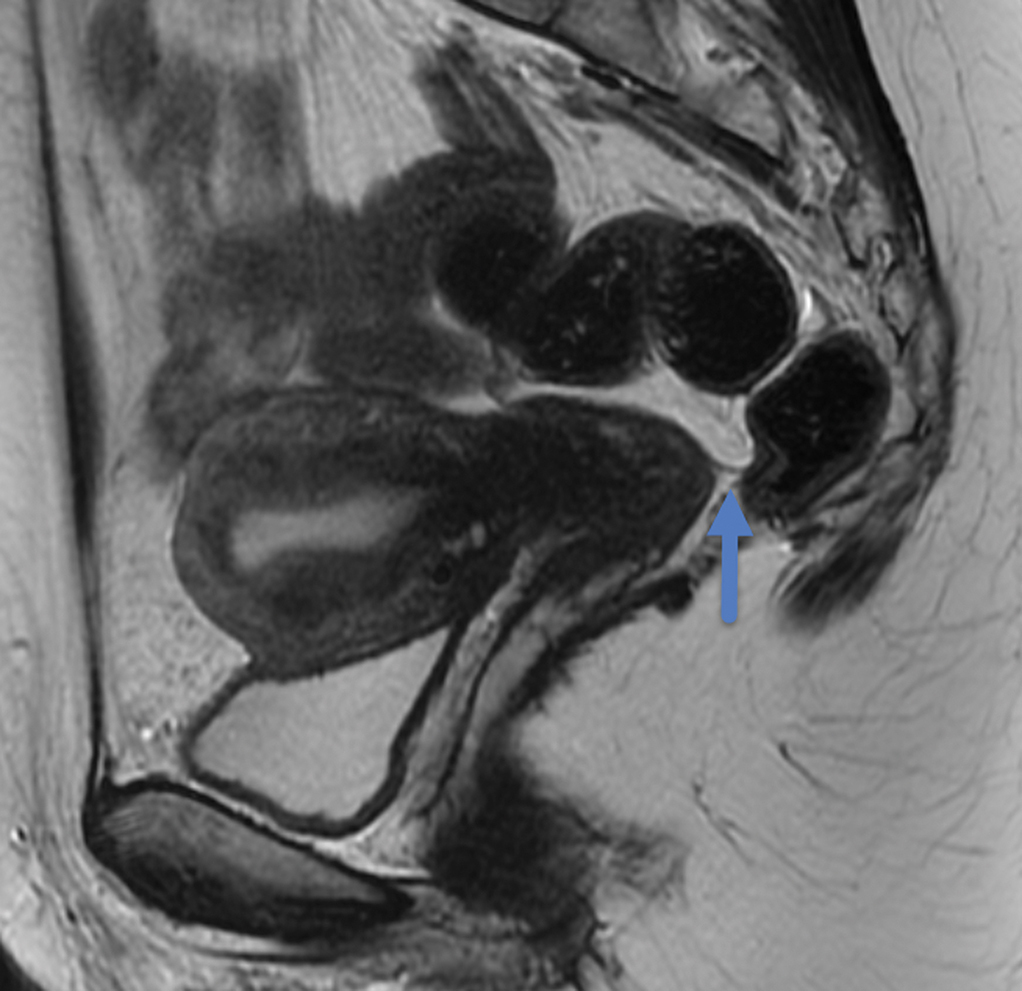
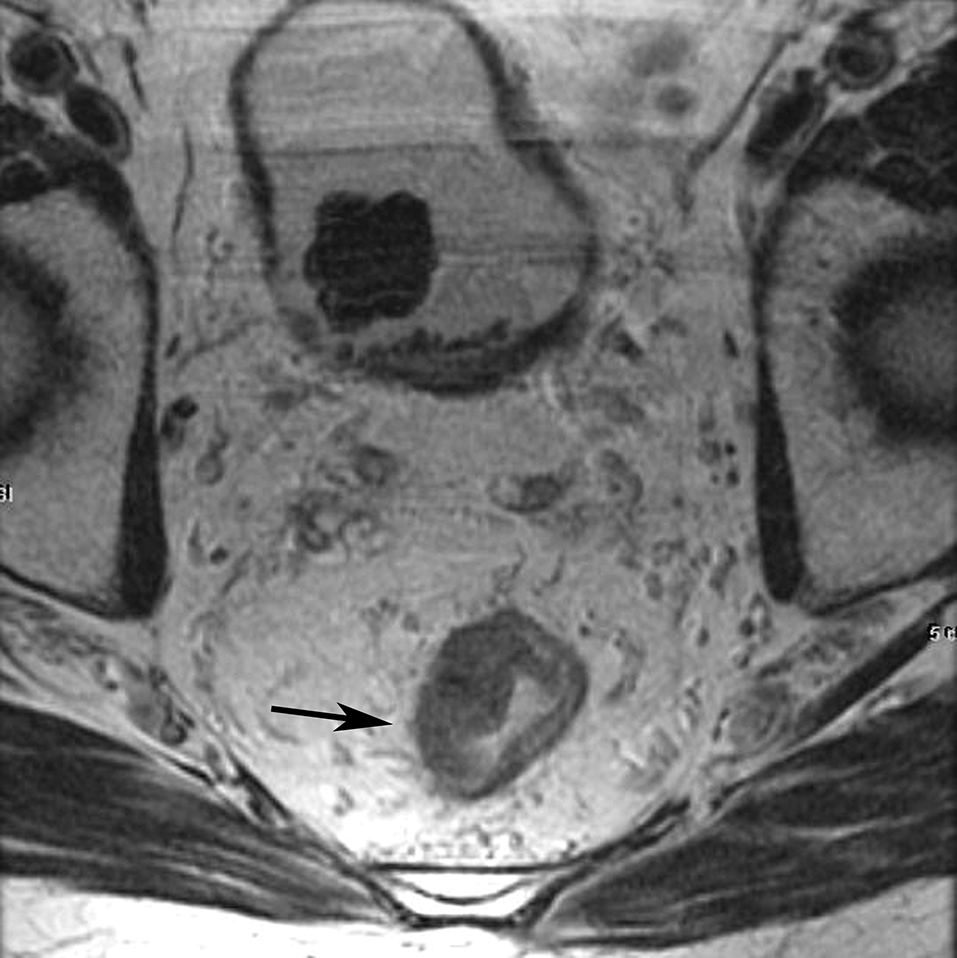
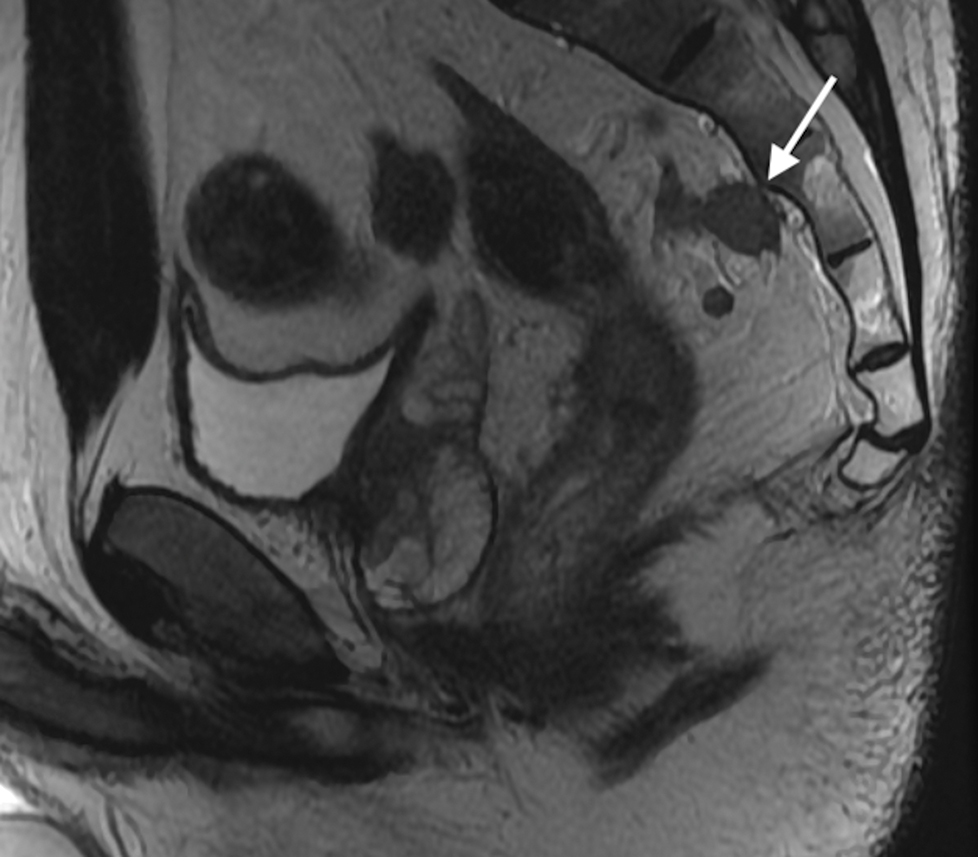
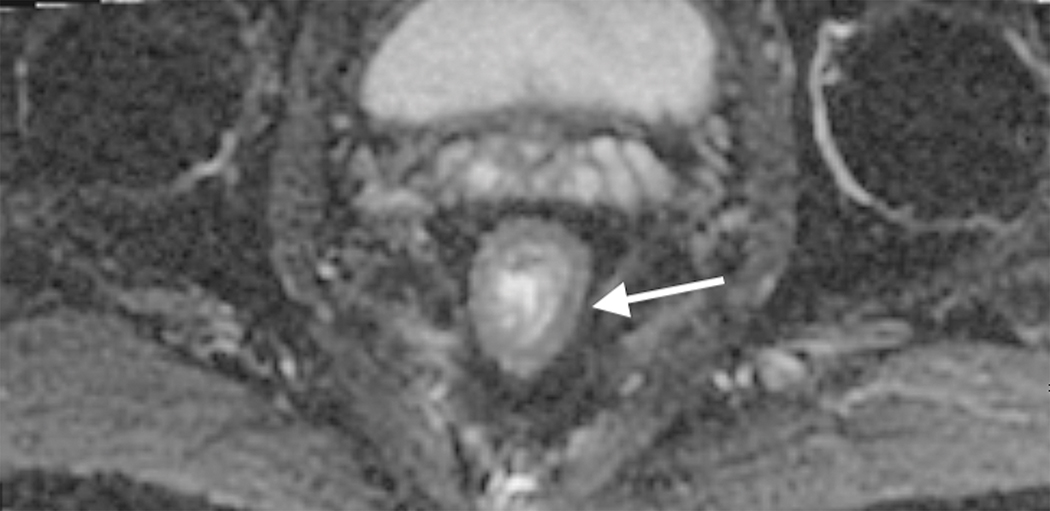
Figure 2.
Sagittal T2W MR of the pelvis in a 33-year-old female with rectal cancer. Arrow indicates the thin black line, the Anterior peritoneal reflection, inserting onto the rectum at the cul-de-sac.
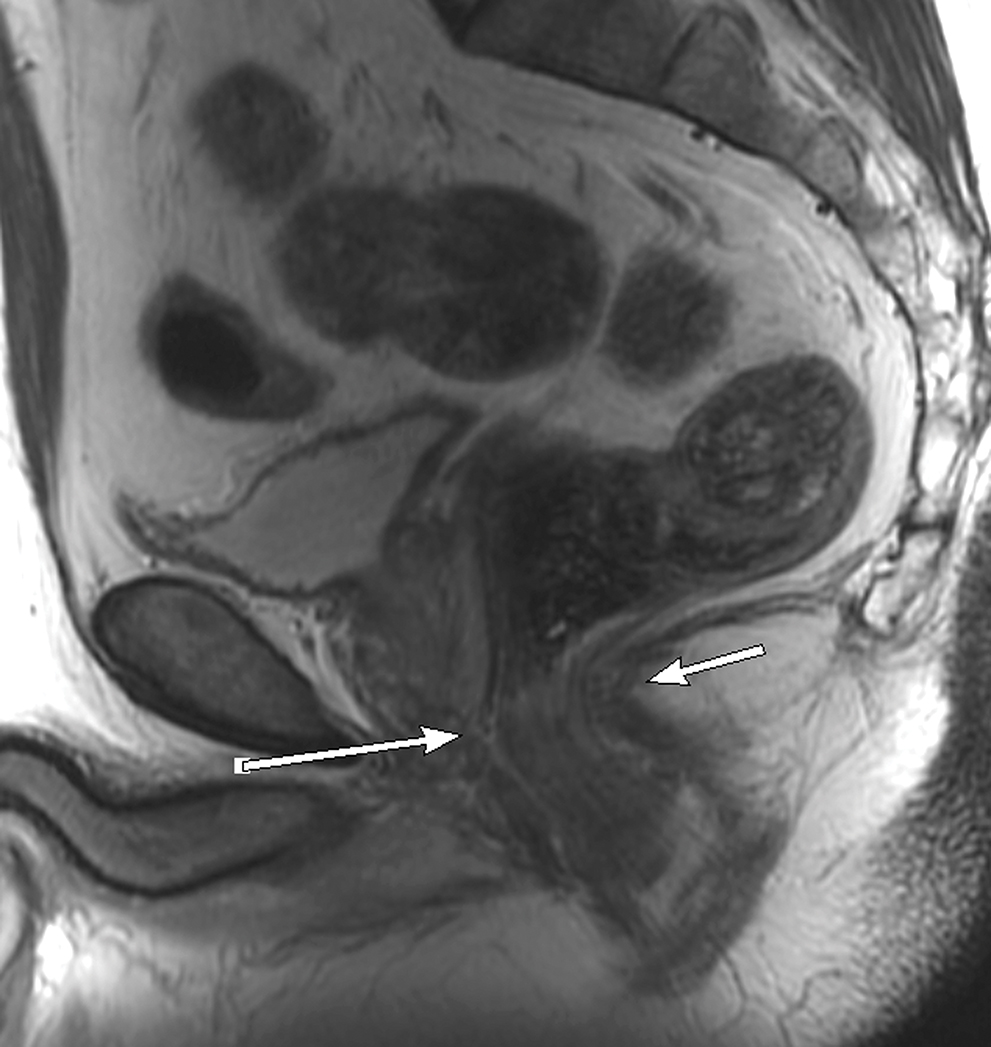
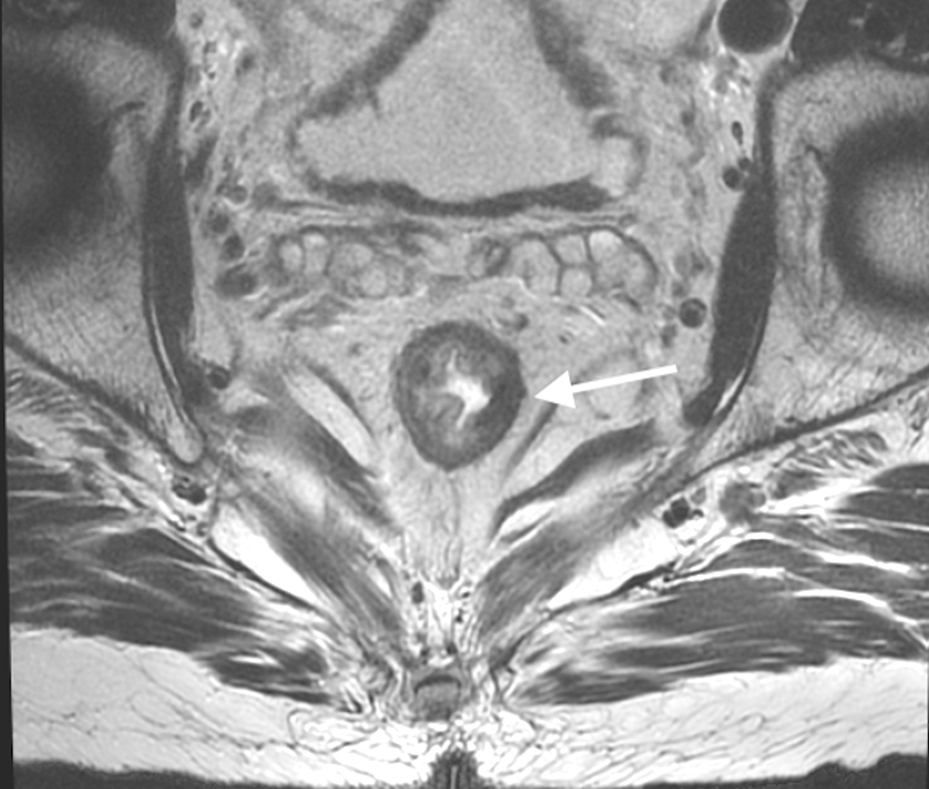
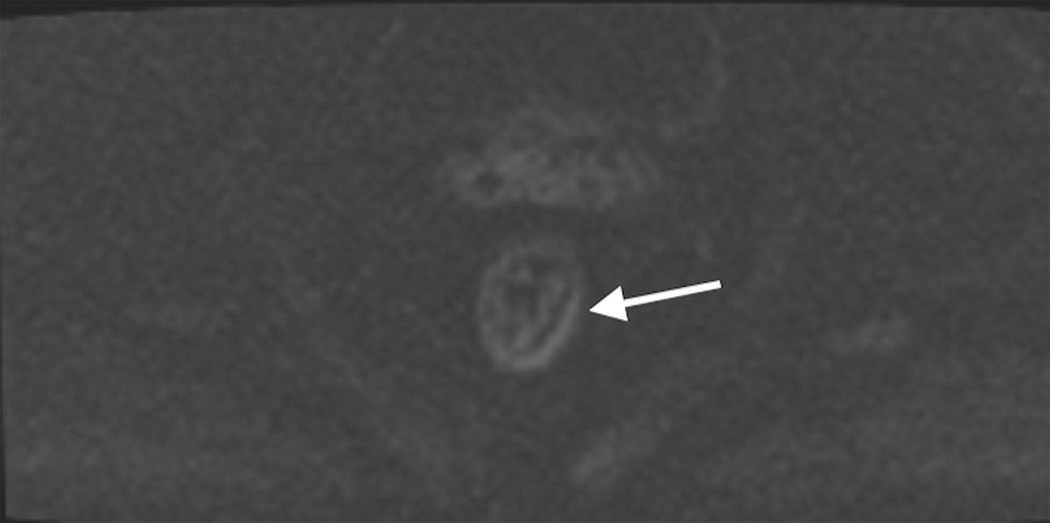
Figure 3.
Sagittal T2W MRI of pelvis in a 73-year-old old female with rectal cancer. Arrows indicate the acute forward angulation of the rectum (sigmoid takeoff) to become the horizontally oriented sigmoid colon.
The sigmoid take-off has been proposed as a useful radiological landmark to define the transition between the rectum and colon [58, 59]. It marks the confluence of the sigmoid mesocolon and mesorectum and is located where the sigmoid colon diverges from the sacrum in a ventral direction in the axial plane and takes a horizontal course in the sagittal plane. Currently, the most common definition is that the rectum is divided into three segments: upper, mid, and lower; arbitrarily set by the distance from the anal verge (0–5 cm, >5–10 cm, and >10–15 cm, respectively) [60]. The mesorectum is a compartment that surrounds the rectum composed mainly of fat, contains lymphatic structures and neurovascular bundles, and is enveloped by the mesorectal fascia. The mesorectal fascia is seen on MRI as a thin hypointense line on T2WI [60] and is caudally contiguous with the intersphincteric space and cranially with the peritoneal reflections [61]. Tumor involvement of the anal canal usually via growth of adenocarcinoma caudally from the dentate line is a common scenario in rectal cancer, and staging is slightly different. A working knowledge of anal canal anatomy is essential, including the internal anal sphincter, external anal sphincter, intersphincteric space and the levator ani muscles [FIGURE 4]. Staging challenges for such tumors will be discussed below.
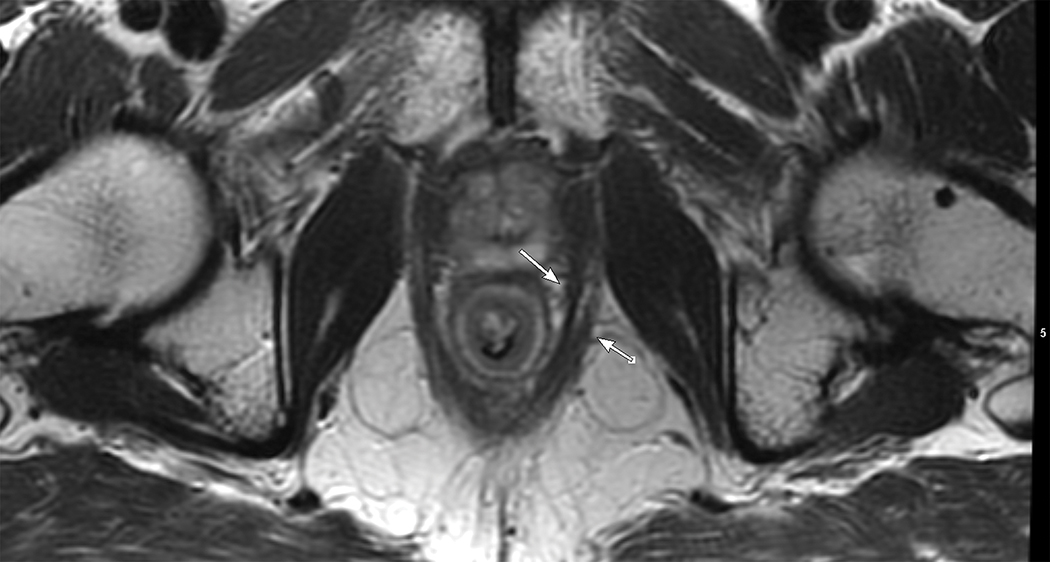
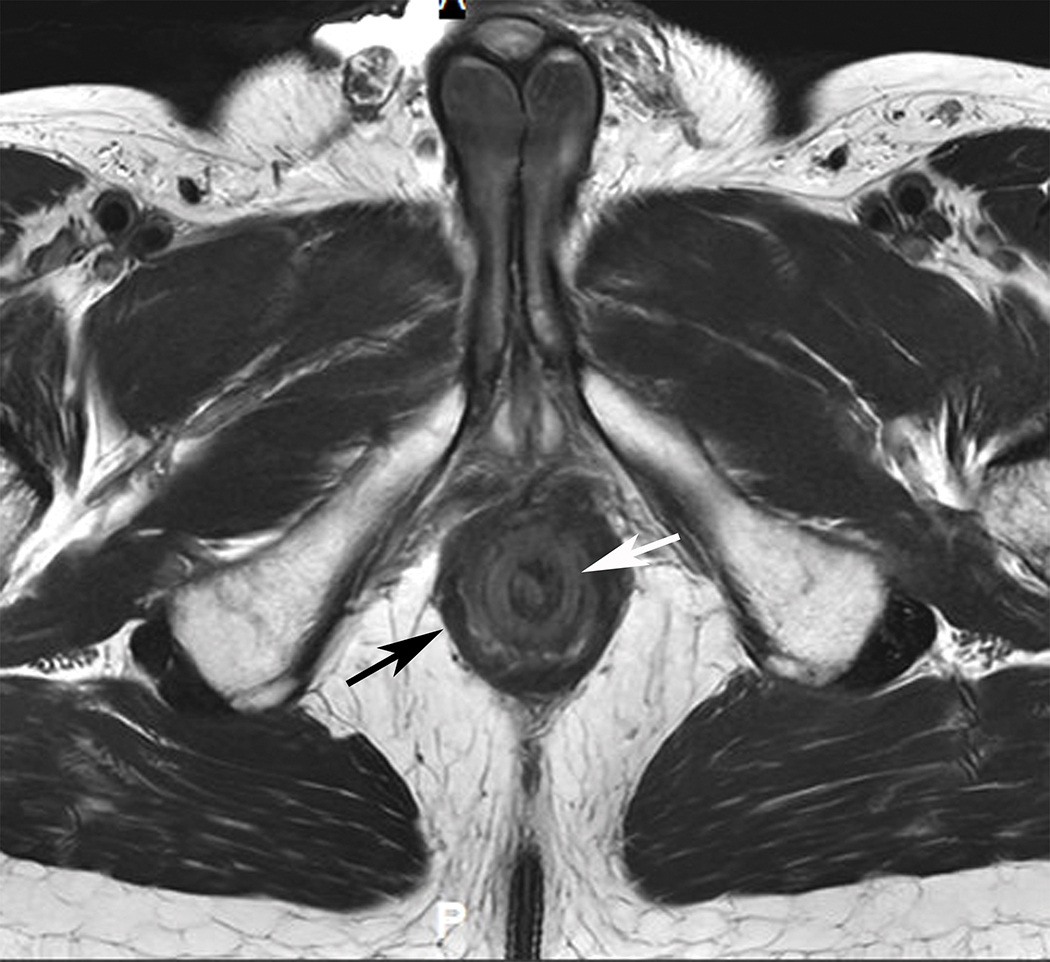
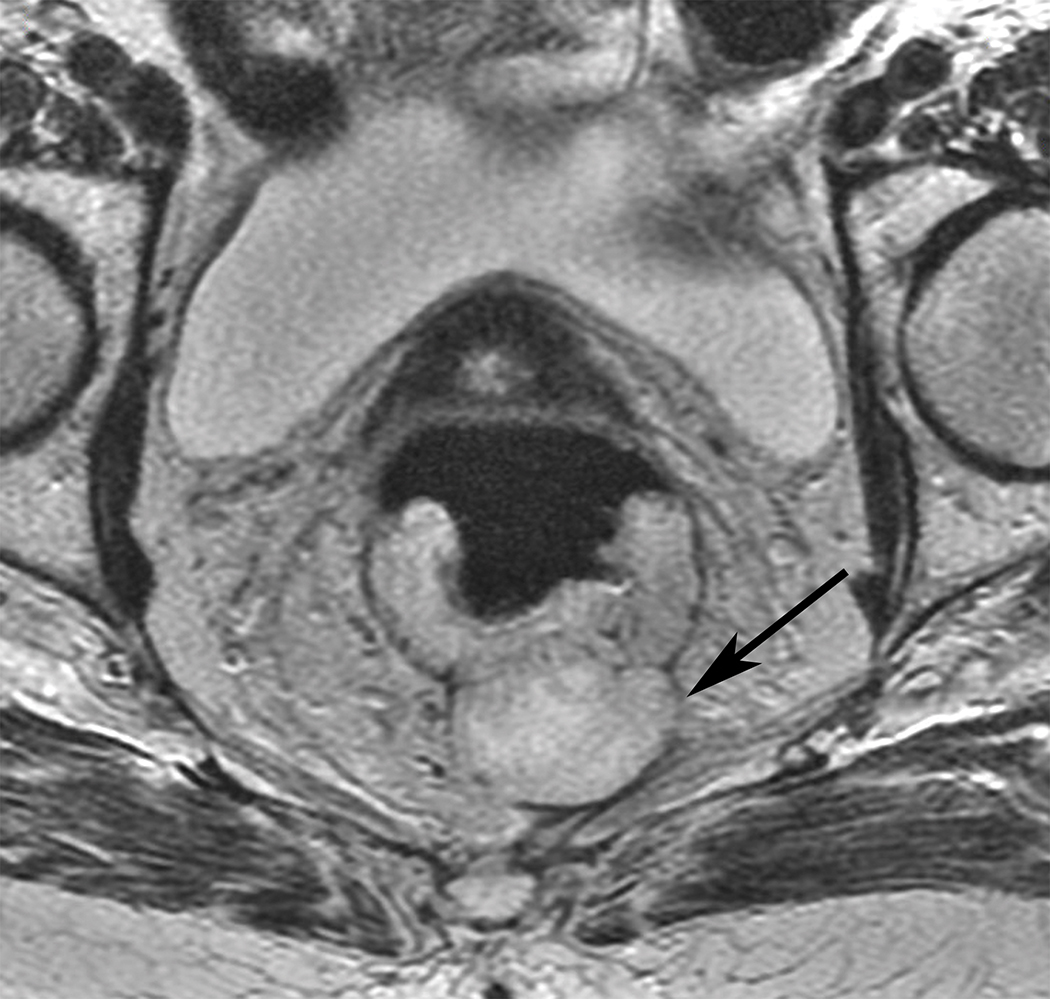
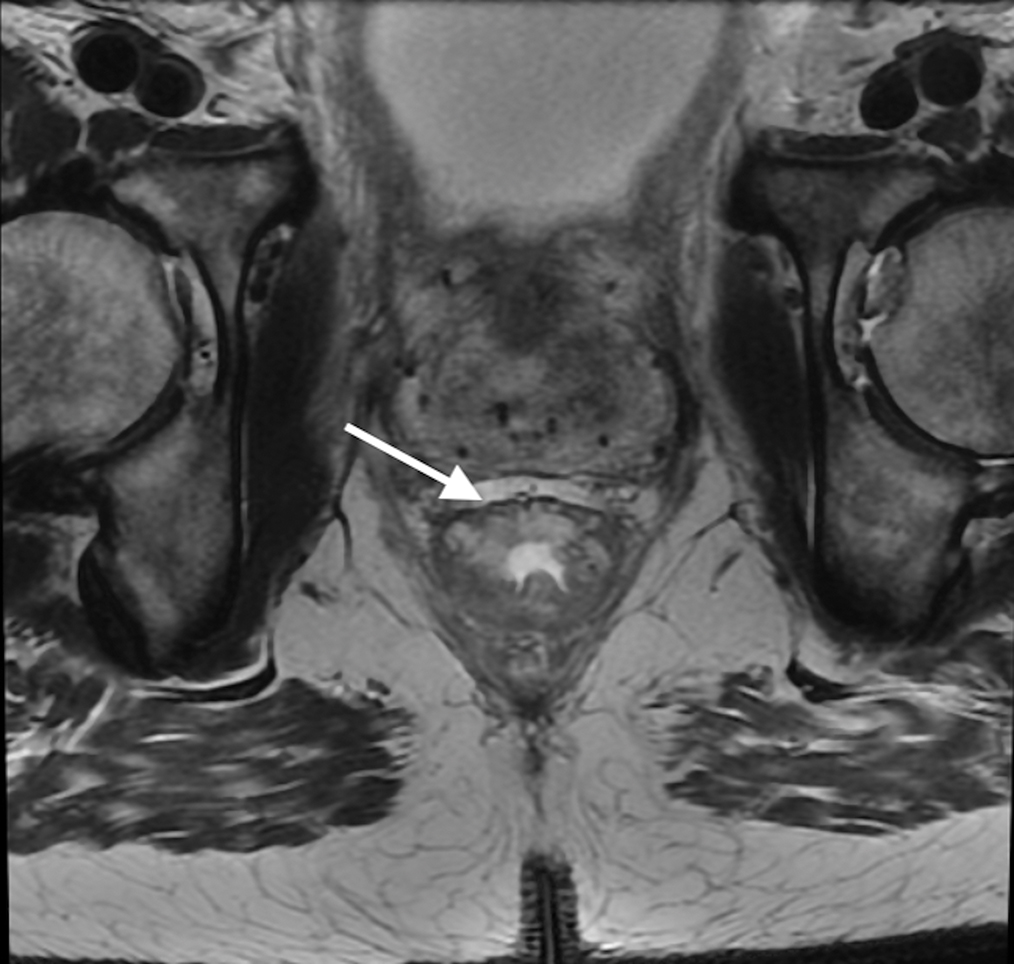
Figure 4.
Axial (A) and (B) and Coronal (B) T2W pelvic MRI in 59-year-old male with rectal cancer after treatment. The levator ani muscles comprise the puborectalis (inner thin arrow in (A), the pubococcygeus muscle (outer thin arrow in (A), and the iliococcygeus muscle (small arrows in (C). The anal sphincter muscles include external (black arrow (B)) and the internal (white arrow in (B)). Note the scar in the tumor bed at 6 pm in (A).
6. Baseline Staging MRI
6.1. Location, size, and morphology
Tumor location should be described as the distance from the inferior most aspect of the anal canal (“anal verge”) and from the upper most aspect of the anal canal (“anorectal junction”) to the most caudal aspect of the tumor and also may be specified with respect to its circumferential position (e.g., 12 o’clock to 7 o’clock). Although there is no consensus for which and how many dimensions should be reported, most commonly, the craniocaudal dimension and maximal wall thickness are described. Tumor morphology may be characterized as polypoid, circumferential, or partly circumferential or equivalent terms. Although tumor morphology does not determine the T stage, one study has shown that polypoid tumors are often associated with lower pathologic T and N stages [62]. In addition, the invasive border is the portion of the tumor with the deepest invasion and is usually located in the center of the lesion.
6.2. TNM staging
The American Joint Committee on Cancer/Union for International Cancer Control (AJCC/UICC) tumor-node-metastasis (TNM) system has been an accepted standard around the world for rectal and other cancers. However, in rectal cancer, it has recently been suggested to place too much emphasis on lymph nodes while overlooking other routes available for metastases, such as via veins, lymphatics, and nerves [63]. Overlap between prognostic subgroups based on T and N stages alone exist (i.e., patients with stage IIc disease perform worse than patients with stage IIIa disease) underlining this limitation [64]. New iterations are in the works at AJCC and elsewhere which -it is hoped- may recognize these under-appreciated pathways of tumor spread.
6.2.1. T staging
The depth of the tumor invasion through the rectal wall into the mesorectal fat and/or into adjacent organs defines the T-category. A meta-analysis showed that the pooled sensitivity and specificity of MRI were 87% and 75% respectively, for T staging [65].
The differentiation between T1 (tumor limited to submucosa) and T2 tumors (tumor invading muscularis propria) [FIGURE 5] may be challenging on MRI due to an inherent soft tissue resolution limitation between tumor signal and muscularis propria signal.
Figure 5.
Axial T2W pelvic MRI in a 79-year-old female who underwent rectal filling. A sessile tumor between 12–2 PM is either T1 or T2 – but careful scrutiny of the muscularis propria shows its full preservation at the epicenter of the lesion indicating greater certainty of mrT1 stage and potentially amenable to local excision by TEM or TAMIS.
For those early-stage cases, ERUS is recommended since it has shown better specificity with similar sensitivity [66]. The muscularis propria is seen as a T2W hypointense layer on MRI, and its infiltration by intermediate signal intensity tumor, when special attention is given to its inner border, may suggest invasion and thereby a T2 tumor, with an accuracy of 67% [50]. A recent study suggests that with the use of GBCA, there is a “submucosal-enhancing-stripe-sign”, which has an accuracy of 87%, in differentiating T0-T1 from T2 tumors[67]. The high signal intensity of the submucosal layer stripe is also readily appreciated on high resolution T2 and has also been shown to distinguish early T1 from T1sm3/early T2; an ongoing trial (PRESERVE trial) is investigating the role of MRI, based on measuring the degree of preservation of the muscularis propria and submucosa in guiding the approach of early rectal tumor and therefore directing favorable patients towards local excision [68].
T3 tumor exists when tumor extends beyond the muscularis propria into the mesorectal fat [FIGURE 6]. Some authors have described inaccuracies differentiating between deep T2 and early T3 lesions. It can be hard to distinguish true mesorectal tumor invasion from desmoplastic reaction [66]. Desmoplastic reaction is typically seen as thin hypointense spiculations, while true tumor extension shows a more nodular/irregular character with an intermediate signal [60]. However, this differentiation may be more relevant when considering the USA guidelines since T2 vs. T3 can be a pivotal cut-off (in the absence of suspicious lymph nodes) as an indication for neoadjuvant treatment. Whereas the European guidelines more commonly use T3 with the extramural extension of <5mm vs. ≥5mm to tailor the treatment [FIGURE 7]. Nevertheless, the MERCURY group found that MRI was feasible and reproducible in measuring the depth of extramural tumor spread and that MRI and histopathologic assessments were considered equivalent to within 0.5 mm; in this trial radiologists ignored fine spiculations as simply representing expected desmoplastic reaction at the base of ulcerating tumors [50].
Figure 6.
Axial T2W pelvic MR in an 87-year-old female with T3 rectal cancer deeply penetrating wall at 5 pm position and mostly consisting of mucin.
Figure 7.
Axial T2W pelvic MR in middle aged male patient with T3 rectal cancer. On the left with < than 5mm of penetration from 6–9 pm and on the right, in a 48-year-old female with > 5mm at 10pm.
T4 is defined as involvement of adjacent organs and “other structures”- T4a when involving only the visceral peritoneum and T4b when involving organs and “adjacent structures”. Anterior peritoneal reflection (APR) involvement may manifest with direct tumor extension, thickening, and irregularity. Upper, anterior rectal tumors have a close relationship to the APR. Criteria to determine involvement at MRI are currently undefined. Even among pathologists, agreement is limited.
Although the AJCC 8th edition manual does not clearly define what ‘adjacent structures’ means in the T4b stage, there is a consensus among the members of the AJCC 9th edition committee (personal communication, Marc Gollub, 12/3/21) that the following structures should be included: pelvic organs (such as the bladder, prostate and uterus), bone (direct invasion not hematogenous spread), pelvic floor muscles (including levator ani, ischiococcygeus, puborectalis and external sphincter), urethra and ureters, sciatic and sacral nerves, vessels outside the mesorectum, small bowel loops in the pelvis, and any fat outside the mesorectum. Therefore it is possible to have tumor involving the mesorectal fascia but still be classified as T3
6.2.2. N staging
Accurate nodal staging remains one of the most challenging components of the preoperative evaluation. Multiple different criteria for lymph node staging have been proposed with varying, but consistently limited sensitivities and specificities. Traditional MRI methods have only moderate accuracy for predicting lymph node metastases if size is used to define involvement since there is considerable size overlap between benign and malignant lymph nodes [69]. High-resolution MRI-defined morphological criteria improve the accuracy of nodal staging. Studies have shown that size alone has low accuracy, but using morphological criteria based on the shape, border, and signal intensity features increases accuracy [69, 70]. It should be noted however, that lymph nodes <3mm are particularly difficult to characterize on current MRI, if at all, and up to 15% of these will be malignant [71]. Benign lymph nodes are homogenous with a regular contour, and malignant lymph nodes demonstrate heterogeneity and irregular borders [69]. A hybrid system combining size and morphology has been instituted for many years in Holland and, termed the “Dutch Consensus Criteria,” resulted in a reduction in over-staging [18].
Nevertheless, studies have demonstrated a reduction in the significance of mesorectal nodal status if a good quality TME with negative CRM is performed, and nodal status predicted local recurrence only when the patient underwent a non-TME or a TME surgery with incomplete specimens [53, 72, 73]. This finding and other similar ones call into question the mechanistic view that tumor spreads to LN and then to distant sites. In fact, a recent study showed that there was a 65% discordance in the sub-clonal origin of LN metastases compared with distant metastases [74]. Some even feel that a LN “traps” tumor and induces an immunological response, possibly even benefitting the patient [75]. While venous exit of tumor cells from a LN may occur, it may be more likely that primary tumor spreads via veins, nerves or lymphatic channels and are more common routes to distant metastases. So far, compared with lymph nodes, the MRI assessment of EMVI both as contiguous and discontinuous spread indicates that the vascular pathways of spread as depicted by MRI are prognostically more meaningful and more accurate. Brown et al. recommend that since tumor deposits or EMVI are the only features that are prognostically relevant for recurrent disease, radiologists should stage tumor based on the presence or absence of these features rather than attempting the prognostically inadequate MRI staging of lymph nodes [8]. Furthermore, since 40% of rectal cancer patients exhibit this feature, it would be logical to ensure that such patients receive preoperative therapy and also undergo close surveillance. Of necessity, more clinical trials are underway to prospectively gain a better understanding of the pathways of spread on imaging and how they can be disrupted to improve cancer survival [76].
On the other hand, extra-mesorectal lymph nodes (also called lateral lymph nodes, LLN) are not routinely resected and should be carefully assessed since, if suspicious, they may require additional morbid surgery risking bladder and sexual impairment. The nodes most often involved are internal iliac and obturator. These are still considered locoregional, whereas external iliac lymph nodes, inguinal and paraaortic lymph nodes are considered metastatic disease (M1). Recently the Lateral Lymph Node Consortium Study has derived some potentially useful criteria for suspicious nodes on MRI. This large multicenter study showed that nodes with a short axis diameter ≥ 7mm on pretreatment MRI in patients with low LARC (0–8 cm form the anal verge) were associated with an unacceptably high risk of lateral local recurrence (LLR) of 19.5% and thus, this was suggested as a reasonable cutoff size [25]. However, the choice of what an acceptable LLR is purely arbitrary, and the study did find a direct relationship between an increasing size of nodes and an increasing LLR if the nodes were not removed by LLND [25]. Based on the immunological protective theory of nodes, it may be argued that perhaps these nodes are not responsible for cancer death, and therefore a reduction in LLR does not equate to an improvement in survival.
6.2.3. M staging
Similar to colon cancer, rectal cancer most commonly metastasizes to the liver, lung and peritoneum. The vast majority of metastases will be detected using whole-body imaging with CT, MRI or combined PET/CT or PET/MRI. During locoregional staging with pelvic MRI, one may detect nodal metastases considered M1 (inguinal, external iliac and paraaortic lymph nodes). In cases of peritoneal carcinomatosis, implants can be detected in the pelvic cul-de-sac or elsewhere. Bone metastases are distinctly uncommon in rectal cancer, and if present are rarely seen in the absence of non-pelvic, often widespread M1 [77].
7. Beyond TNM
TNM staging, while essential, fails to convey all the necessary information about rectal cancer required by the multidisciplinary team to make management decisions because other evidence-based high and low risk factors have been found to be of utmost importance including; the circumferential resection margin (CRM), involvement of the anal canal, EMVI, tumor deposits (TD) (currently not captured well by AJCC staging), the presence of significant tumor mucin and the relationship of the tumor to the peritoneal reflection. We elaborate on these risk factors below.
7.1. CRM
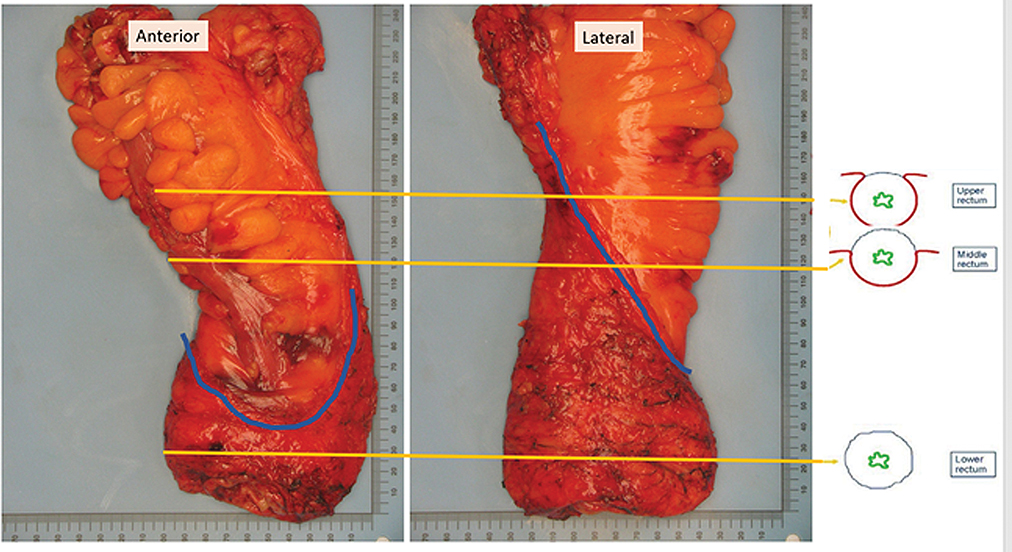
The mesorectal fascia (MRF), which encircles the fatty mesorectum surrounding the rectum, forms the plane of dissection in TME surgery and is the intended safe anatomic surgical margin CRM. Achievement of this radial plane is challenging, and gross specimens are carefully observed to ensure that a glistening serosal surface is preserved [FIGURE 8] and that at the level of the pelvic floor, no “waist” is present to suggest incursion into the mesorectal fat [72].
Figure 8.
Gross pathologic specimen from TME surgery; anterior and lateral. The blue line demarcates the peritoneal attachment which runs obliquely cephalad on the lateral Rectum. The lower, redder rectum is surrounded by the thin shiny intact mesorectal fascia. Superiorly, the fat of the sigmoid mesocolon is noted. Courtesy of Dr Jinru Shia Attending Pathologist Memorial Sloan Kettering Cancer Center.
Tumor within 1 mm of the mesorecta fascia strongly predicts poor oncological outcomes and is one of the main determinants of local recurrence [78]. Therefore, it is essential at MRI to preoperatively evaluate the relationship between tumor and the MRF to risk-stratify a patient who may require treatment. Fortunately, MRI has been shown to be highly accurate in predicting CRM involvement [66, 79]. The tumor-MRF distance should be measured as the shortest distance between the advancing edge of the primary tumor and the closest adjacent mesorectal fascia. A distance of ≤ 1mm is considered involved.
Some clarifications should be made regarding the CRM evaluation. Expressing a distance to the MRF can only be done where MRF exists. At a certain point anteriorly and above the anterior peritoneal reflection, such a measurement is no longer possible, because there is no longer any mesorectal fat to provide a margin. Similarly, at about the sacral promontory the posterior peritoneal reflection exists and laterally there is an oblique course of its attachment to the rectum. These landmarks are not well delineated compared with the anterior peritoneal reflection (APR) [18, 80]. For any component of a high tumor that is lateral and/or posterior, it is valid to express this distance, but anteriorly, we opt to say N/A (not applicable) since tumor is already visibly above the level of the APR.
Moreover, although the prognostic importance of the MRF involvement by the primary tumor is well established, the risk of its involvement by metastatic lymph nodes is of questionable significance. Nagtegaal found that metastatic lymph nodes involving the CRM did not increase the risk for local recurrence compared to those with negative margins [81]. In the MERCURY II study EMVI was an independent risk factor for pathologic CRM involvement and may relate to the fact that unlike lymph nodes which are bounded within the mesorectal compartment, vascular pathways of spread and discontinuous vascular tumor deposits do not respect the mesorectal fascia as a boundary [28].
7.2. Anal sphincter status
One of the surgeon’s primary goals (and challenges) for low rectal tumors is to preserve sphincter function without compromising oncological outcomes. Abdominopelvic resection is the conventional operation for low rectal tumors involving more than just a small upper portion of the anal sphincter, but it is associated with high local recurrence rates and morbidity [82]. Neoadjuvant chemoradiation has been shown to be helpful in downstaging tumors that initially involve the sphincter complex and at times may allow for sphincter preservation surgery; however, radiation to the anal sphincter itself compromises sphincter functionality and increases anastomotic leak rates [83]. Moreover, additional dissection planes have emerged depending on which component of the anal sphincter is involved and at times a wider excision will also remove the levator ani muscles (extra-levator abdominoperineal excision (AKA ELAPE)) [83].
MRI is particularly well suited as a non-invasive way to determine the safety of the distal TME plane – where the mesorectum naturally tapers and where the MRF is no longer present. In staging of low rectal cancers, the standard T1-T4 system used above the anorectal ring is not adequate and instead, an assessment based on the radial extent of tumor and the safety of the intersphincteric plane is used to guide the surgical plan. The “Level 1–4” staging system investigated in the MERCURY II study as well as another depth of anal canal invasion study [84] correlates invasion and >1mm clearance of tumor to the intersphincteric plane with surgical outcomes [28, 54, 84] (TABLE 2). As of now, there is no consensus on which system to implement. At our institution, and among USA surgeons, there is a general agreement to use a prose description of the relationship of tumor to the internal sphincter, the intersphincteric space, and the external sphincter so as to convey a clear message at a time when no specific staging system is universally accepted.
Table 2.
T2W coronal plane to anal canal and different levels of anal involvement.
| T2W coronal plane to anal canal | Level of anal involvement |
|---|---|
|
| |
| Tumor extends to the level of the internal sphincter but the muscularis propria of the rectal wall is intact with a fully preserved hypointense outer muscle coat. (MERCURY II; LEVEL 1) |
|
| Tumor also invades internal anal sphincter but interrupts the hypointense muscle coat without extension into the intersphincteric plane. (MERCURY II; LEVEL 2) |
|
| Tumor invades the intersphincteric space without involvement of the external anal sphincter/levator/puborectalis. (MERCURY II; LEVEL 3) |
|
| Tumor also invades the external anal sphincter and levator ani muscles. (MERCURY II; LEVEL 4) | |
7.3. EMVI, TD, and PNI
There is an increasing awareness that not every nodule within the mesorectum corresponds to a lymph node, and other entities of extra-nodal mesorectal spread may be at play – TD, EMVI, and perineural invasion (PNI). Studies have shown poorer prognosis associated with those entities [8, 63] with even greater prognostic significance than T and N stage [85].
Histopathologic EMVI is already well recognized as an independent predictor of local and distant recurrence and poor overall survival [86, 87]. More recently, studies have also shown that EMVI detected by MRI, in the pre [7] or post neoadjuvant CRT [52] settings, is also a poor prognostic factor with increased rate of synchronous metastases and of developing metastases after surgery [7]. MRI is now considered a gold standard in the detection of EMVI and can be used to help histopathological awareness [51, 88]. A study performed by Smith et al [51] proposed an MRI-EMVI score (raging 0 to 4) grading the suspicion of EMVI based on MRI. They showed that high scores (3 and 4) were associated with worse relapse-free survival. On MRI, EMVI manifests as tubular or serpiginous structures corresponding to the vessels with signal similar to the tumor (intermediate signal on T2) [FIGURE 9]. In addition, the vessels may show irregular or nodular borders. Although EMVI may or may not occur continuously with the primary tumor, whenever a tumor is near a vessel, EMVI should be considered – and confirmed as such if tumor is expanding and disrupting a vessel on two orthogonal views[89].
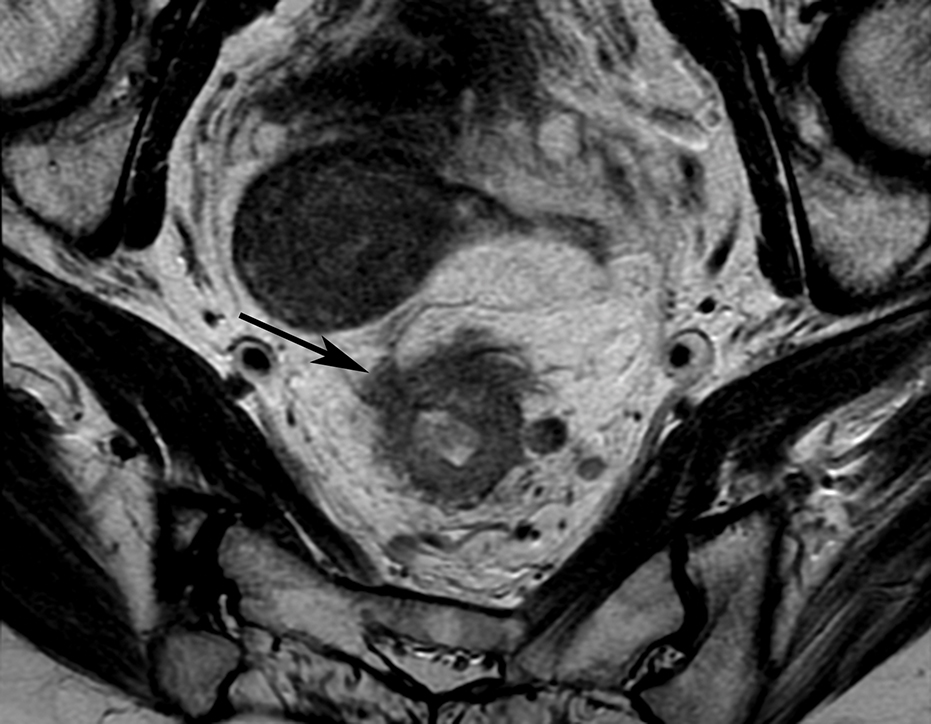
Figure 9.
Coronal T2W pelvic MR in 37-year-old female with rectal cancer and left-sided EMVI
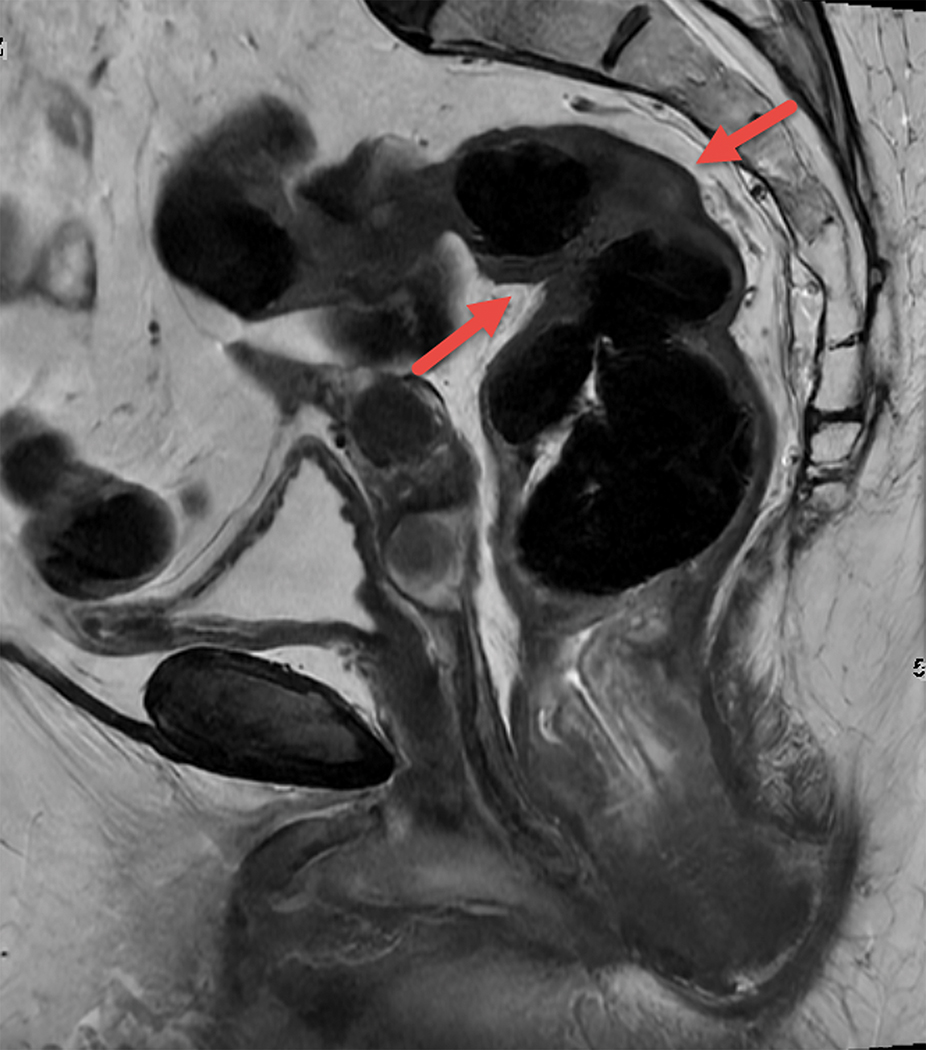
Tumor deposits (TD) are nodules of tumor cells within the mesorectum discontinuous from the primary tumor [FIGURE 10], still of uncertain origin, and may occur in association with EMVI [8]. TD was first introduced within the AJCC staging system in the 5th edition in 1977 and, since the 7th edition, has been recognized as the separate category N1c [8]. On MRI, they are seen as nodules with irregular margins, often located along the vessels, but, differently from the lymph nodes, they interrupt the vessel course [85]. Controversy arises however in that the current TNM places pN1c as a lower category than pN2 for example, and yet outcomes for pN1c are worse [63]. It is thus felt that the current manner of incorporating TD into the TNM system does not properly convey the patient’s risk stratum. The current AJCC 9th edition committee is discussing these 2 emerging risk factors and how best to incorporate them into a modern staging system that properly reflects patient risk (personal communication Marc Gollub 12/7/21).
Figure 10.
Sagittal T2W shows a posterior mesorectal irregular nodule arising from and interrupting the superior rectal vessel consistent with tumor deposit.
PNI occurs when tumor cells travel along nerve sheaths and is a known poor prognosticator [90]. Its role in causing distant metastases is not yet clear, but it would seem likely to be a risk for local invasion and local recurrence. Here too, more studies are needed.
7.4. Peritoneal reflection
The APR is a landmark that divides the intra- and extra-peritoneal portions of the rectum (anteriorly) and can often be identified on MRI. This landmark has oncological implications since (a) tumors below APR have lymphatic drainage to the pelvic basin whereas tumors above the APR mainly drain to the sigmoid mesentery, (b) below the APR tumors have a worse prognosis with higher risk of local recurrence, (c) APR involvement by the tumor also increases the recurrence risk [91] and is staged as T4a, and finally; (d) the APR approximates the 10-cm distance from the anal verge at which, historically it was determined (in the Dutch TME trial) that there was no survival advantage of administering radiation [92]. Hence, the tumor relationship to the APR could potentially be used to tailor optimal treatment strategies.
On T2W images, APR can be recognized as a hypointense thin line well identified on axial and sagittal planes with its attachment on the anterior rectum with V-shaped configuration on axial plan. The junction of the seminal vesicles in men and the uterocervical angle in women are useful anatomical references to find the APR [93].
7.5. Mucin
Mucin may occur in two different scenarios in rectal tumors: primary mucinous tumors and mucinous response to chemoradiation of an originally non-mucinous tumor (sometimes referred to as “colloid degeneration”). Primary mucinous adenocarcinomas are poorly differentiated tumors with poor prognosis and poor response to neoadjuvant chemotherapy [9, 94]. On the other hand, mucinous response is usually a sign of successful treatment and does not carry the poor prognosis primary mucinous tumors do, however its prognostic significance compared to other types of responses is still debatable [95].
MRI has shown good accuracy in differentiating mucinous and non-mucinous tumors [96] and has demonstrated even higher accuracy than presurgical biopsy in detecting mucin due to the randomness of biopsy [97]. Moreover, mucinous tumor defined by baseline MRI have been shown to be an independent imaging biomarker for poor prognosis and poor response to preoperative chemoradiotherapy [97]. On MRI, mucin is identified as very high signal on T2W images [FIGURE 11] and predominantly hypo-enhancing in contrast images. DWI is of limited value due the T2 shine-through effect [98]. Mucinous response may be suggested when there is an increase in tumor signal intensity on T2W relative to pretreatment MRI [99].
Figure 11.
T2W axial imaging. Note the markedly high signal of the anterior rectal tumor.
Evaluation of response after neoadjuvant therapy in mucinous tumors is challenging since MRI is limited in differentiating cellular from acellular mucin pools and its interpretation remains uncertain. Residual tumor cells within the mucin pools may be suggested by heterogeneity with intermediate signal foci and restricted diffusion [100].
8. Restaging MRI
Assessment of response to neoadjuvant therapy was historically accomplished with endoscopy, clinical assessment, and routine imaging with CT scan to exclude progression or development of metastases. Although baseline tumor evaluation using MRI rapidly established itself as a useful tool and was widely proliferated, the use of MRI for re-staging has been adopted more sluggishly, in part because of confusing appearances of tumor versus the effects of treatment such as fibrosis, inflammation and edema. A meta-analysis of restaging MRI determined just how limited this application of MRI was using conventional T2W sequences [101]. Nonetheless, the rapid proliferation of magnets, radiologist’s comfort with MRI and its availability were such that research determined that there were advantages to its use in spite of its limitations. In 2022, it is the preferred method for restaging and is routinely performed in conjunction with clinical and endoscopic evaluation. The modern goals of rectal cancer restaging are: a) to differentiate “good responders” versus “poor responders” in order to personalize therapy b) to differentiate complete response (CR) (yT0N0) from residual tumor (yT1–4), and therein contemplate possible non-operative management (“watch and wait” policy), c) to identify tumors limited to rectal wall (yT0–2N0 versus T3–4 or N+), which might lend themselves to limited surgeries such as transanal excision, and d) to reassess CRM, anal sphincter, and adjacent organ involvement to ensure a safe surgical plane.
8.1. Morphologic and size changes
Scar/fibrosis formation is one of the main responses seen after neoadjuvant therapy. On MRI, it is characterized as very low T2 signal [FIGURE 12], whereas residual tumor manifests as intermediate signal areas. Moreover, fibrosis usually has a low cellular density, resulting in low signal intensity on high b-value DWI [FIGURE 12], while residual tumor has a high cellularity and therefore a high signal intensity on DWI. The extent of fibrosis should be recorded separately from the extent of tumor signal [102]. Nevertheless, MRI is limited in detecting microscopic tumoral cells within the fibrosis. In addition, after radiation, normal rectal wall adjacent to the tumor may show edema and become prominent, which may simulate a tumor and cause confusion in less experienced readers.
Figure 12.
Restaging MRI in a patient with complete response after chemoradiation. (A) Axial T2W, (B) DWI, and (C) ADC map shows a T2W dark scar in the left lateral rectal wall with no focal restriction diffusion.
Responding tumors also decrease in size after neoadjuvant therapy. Reduction of the tumor volume in > 60–80% correlates with good response [103] and tumor volume reduction rate has been shown to be an independent prognostic factor [104]. However, volumetry is time-consuming and therefore many societies recommend the measurement of the tumor length as a proxy [18, 37].
In a small percentage of cases, the tumor completely disappears with normalization of the rectal wall. In a study, the normalization of rectal wall corresponded to complete response in all cases [105].
8.2. MRI tumor regression grade (mrTRG)
mrTRG is an imaging adaptation from the TRG grading system used in histopathology. It is a 1–5 scale to grade the degree of tumor response after neoadjuvant therapy based on the proportions of fibrosis and residual tumor seen on T2W. It is well established that the pathologic TRG (pTRG) is a significant prognostic factor and predicts overall survival [73]. The MERCURY study showed that the mrTRG also correlated with survival outcomes (disease-free survival and overall survival), and with a greater statistical significance than ymrT [53]. However, other studies showed low agreement between pTRG and mrTRG [106, 107]. Brown et al. hypothesize that mrTRG1–2 represents non-viable tumor within fibrotic stroma and therefore would not correlate with pathology but would correlate with long term lack of tumor regrowth in a watch and wait setting. This hypothesis is being tested in the TRIGGER trial [34].
8.3. ymrTNM (including lateral LN)
8.3.1. ymrT staging
Posttreatment T staging criteria on MRI (ymrTNM) are identical to the baseline staging parameters (mrTNM). However, accuracy of MRI in the posttreatment setting is lower than in pretreatment setting [101]. A meta-analysis has shown an overall low sensitivity of 50.4% with a good sensitivity of 91.2% [101]. DWI increased the sensitivity to 83.6% without significant decrease in specificity (84.8%) [101]. DWI has continued to emerge as a powerful tool in rectal cancer re-evaluation [49].
8.3.2. ymrN staging
As organ preservation therapy has been increasingly considered for good responders to CRT, accurate nodal restaging has become more important. Chemoradiation often decreases the size and quantity of both benign and malignant lymph nodes. MRI restaging using size criteria has been shown to be more accurate for lymph node staging when compared to the baseline scan probably due to the decrease in the number and size of all lymph nodes whereby residual enlarged nodes are statistically more likely to be metastatic lymph nodes [108].
In the posttreatment setting, chemoradiation induced fibrosis and inflammatory changes may be responsible for irregular contour and signal intensity heterogeneity in the lymph nodes, and also the lymph nodes get smaller making morphologic features more difficult to evaluate [109]. Therefore, size becomes an important feature. Optimal size cut-offs for short axis have found to be 2.5 mm [108] - 3.3 mm [109] with sensitivity and specificity 85% and 78% and 75% and 64%, respectively. However, the use of a 5mm cut-off has become common and pragmatic with a chance varying only between 11–14% that such a node will contain tumor. Furthermore, one study showed that the absence of nodes at DWI, although not a common finding, was a reliable predictor of yN0 [110].
On the other hand, criteria for tumor risk in lateral lymph nodes (“extra-mesorectal” lymph nodes) have been the topic of a recent, large, multicenter cohort study performed by the Lateral Lymph Node Consortium. This showed a progressive increased risk of local recurrence (LR) with size of the internal iliac and obturator nodes. While no absolute size cutoff existed to confer no risk, the Consortium suggested an acceptable risk of LR using a short-axis lateral node size < 7mm on pretreatment MRI. Furthermore, if that criterion is used, the group noted that shrinkage to ≤ 4mm on post CRT MRI resulted in no local recurrences at 3 years of follow up, suggesting this as a possible cutoff in the decisions about the need to perform lateral lymph node dissection. While the baseline criteria are slowly being adopted in the USA, the post-treatment criteria are felt to be less reliable based on the high variability in treatment types in the study population [24].
8.4. Posttreatment CRM (ymrCRM)
Evaluation of CRM involvement after chemoradiation is more challenging due to the limitation of MRI in identifying residual tumor within fibrosis, and consequently leading to overestimation of local tumor invasion. A study showed that the positive predictive value for pCRM decreased from 80% in the pretreatment setting to 42% in the posttreatment setting [111]. A meta-analysis showed moderate results for CRM restaging with sensitivity of 76% and specificity of 85.9% [101].
8.5. Posttreatment EMVI/TD
EMVI and TD have increasingly been studied on pretreatment MRI; however, only a few studies have investigated their characteristics on MRI after neoadjuvant chemoradiation. Some studies have shown that mrEMVI regression or the replacement of tumor signal within vessels by fibrosis after CRT was associated with improvements in survival outcomes [FIGURE13] [112–114]. Therefore, patients with minimal or no regression of mrEMVI might be considered for intensification of treatment and/or closer follow-up. Validation of existing studies is needed to further understand the implications of this important tumor biomarker, and the ongoing MARVEL study [76] (NCT01995942) is a European multicenter observational study undertaken to accomplish this task.
Figure 13.
Coronal T2W pelvic MRI (A) pre and (B) post treatment in a 45 yr. old male with rectal cancer. The left sided EMVI is smaller and T2 darker indicating at least partial regression.
8.6. Complete response and watch-and-wait policy
Approximately 15–20% of patients with rectal cancer that receive neoadjuvant chemoradiation achieve complete response [115]. In 2004, Habr-Gama et al [116] suggested that surgery could be deferred in a selective group of patients who had clinical complete response after neoadjuvant therapy and that those patients could be closely followed clinically (a strategy known as “wait-and-see” policy). Since then, there has been a rapidly growing interest in organ preservation strategies including local excision and non-operative management. More recent studies have shown promising long-term oncological and functional outcomes [115, 117]. A remaining challenge is how to preoperatively diagnose complete response and safely select patients for organ preservation strategies. The outcomes of the first prospective study, the OPRA trial [23], including an expert-panel created a priori MRI system are eagerly awaited and have been presented at ASCO 2021 [118].
Studies have shown that clinical assessment (DRE and endoscopy) are inadequate to predict complete response. For instance, the presence of ulcer on endoscopy, although significantly associated with pathological incomplete response, occurred in 66% of cases with complete response on pathology. On the other hand, even though absence of mucosal abnormalities or presence of mucosal scarring alone on endoscopy were significantly associated with pathological complete response, 27% of those cases had residual tumor on surgical pathology [119]. Moreover, endoscopy provides a good assessment of the mucosa, however, it is limited in evaluating possible residual tumor deeper in the rectal wall and mesorectum [120, 121].
The combination of DRE, endoscopy, and MRI (T2W with DWI) seems to give the highest accuracy to predict CR. A seminal study showed that when all three assessments indicate a CR, it was correct in 98% of the cases [122]. Complete response on MRI is suggested when there is a normalization of the rectal wall or when there is only the T2 hypointense scar with no restricted diffusion. DWI has improved the performance of MRI in predicting complete response [123, 124] and in detecting tumor regrowth when an organ preservation strategy was chosen. A recent small study found that while most DWI positive cases corresponded to endoscopy positive cases, false positive DWI was also common. However, in approximately one fifth of cases where there was a positive DWI with a negative endoscopy, endoscopy turned positive later, while DWI remained positive, or increased in the identical location, suggesting that DWI may detect tumor regrowth before endoscopy [121]. However, this remains to be validated. DWI, admittedly, is a fastidious sequence to perform and interpret with myriad artifacts and interpretation nuances including T2 shine through and T2-dark-through, among others [125].
On the flip side, radiologists and surgeons are still missing some cases of CR. Some studies have found that when MRI or endoscopy indicated residual tumors approximately 15% of the cases were in fact CR on surgical pathology [122, 126]. The most common pitfalls on those false positive cases were mucosal abnormalities on endoscopy, mixed signal, or irregular appearance on T2W, residual high signal on DWI, and over-staging nodes [127].
As discussed previously, one of the main limitations of MRI is the nodal assessment. It is accepted that the response on lymph nodes tends to follow the response on the primary tumor. However, the risk of residual nodal disease when a complete response is reached in the primary tumor is variable in the literature, from as low as 1.8–5% [115, 128] to as high as 17% [129].
9. Beyond TME-Surgery and the Role of MRI
Recurrent rectal tumors and locally advanced primary rectal cancers that have spread beyond the TME planes are challenging due to necessity of more complex surgeries to achieve complete resection with negative surgical margins (R0). Those surgeries are composed of pelvic exenterations or multivisceral/multicompartmental resections of adjacent organs or of the pelvic sidewall and they may require surgeons from different specialties with high expertise. An R0 surgery improves the oncological outcomes, but at the expense of increased morbidity and postoperative mortality [130, 131]. Therefore, patient selection should be done carefully.
MRI has been shown to have a high accuracy to determine invasion of adjacent organs and may be used to select eligible patients for surgery and as a road map for surgical planning [132, 133]. A classification into seven anatomical compartments according to the fascial limits and the anatomical planes of dissection has been suggested [134]. The same study investigated the performance of MRI detecting invasion of each pelvic compartment [134] and showed that MRI has a sensitivity superior to 93.3% in all except for the lateral compartment where it was 89.3%. Another simplified classification recently proposed has shown that a peri-anastomotic recurrence is associate with better DFS after salvage surgery [135]. The Beyond TME trial [136] is an ongoing multicenter prospective trial that has been studying the role of MRI in selecting and guiding surgery.
10. Non-Standard MRI Biomarkers
Quantitation of diffusion weighted imaging using pharmacokinetic modeling results in the apparent diffusion coefficient (ADC). The ADC-value has also been investigated as a biomarker in LARC. Studies have shown that ADC-value of the primary tumor can help predict tumor aggressiveness [137], presence of nodal metastases [138–140] and of distant metastases [139]. Moreover, it can also be helpful in predicting CR on restaging MRI, with an accuracy of 87% but a low PPV of 52%, probably due to significant overlap of ADC values between CR and near-CR [141]. These promising small retrospective studies also await large-scale prospective validation.
DCE-MRI is a technique that can assess the tumor vascularity and therefore provide information regarding aggressiveness and degree of angiogenesis and by extension hypoxia, all important features of the microenvironment. The value of quantitative and semiquantitative features extracted from DCE-MRI in rectal cancer has been investigated. A systematic review showed that high Ktrans before CRT and a reduction of Ktrans of 32%−36% was associated with good response [48]. The future role of DCE-MRI is unclear given the agreement among guidelines that GBCA are not required for the evaluation of rectal cancer during MRI. As such, similar to quantitative DWI (ADC and IVIM) these techniques remain largely in the research arena.
11. Structured Reports
The introduction of proformas, or structured (sometimes synoptic) reporting improves the completeness of rectal cancer assessment reports significantly [142, 143]. A prospective study showed that crucial information for therapeutic decisions on oncologic staging imaging were missing in 52% of radiology reports with an improvement of 78% in report completeness after the implementation of structured reports [144].
12. New Frontiers: MR-Based Radiomics
Radiomics and radiogenomics have been targets of investigation in the last several years for several types of cancers, including rectal cancer. Radiomics refers to the extraction of a vast number of qualitative and quantitative features from routine images using a computer that are effectively invisible to the human eye [145]. Several studies have been investigating the association between radiomics and different endpoints, including prognosis and survival [146], prediction of pathological complete response on baseline MRI [147] and on restaging MRI [148], genetic profiles of tumors (radiogenomics) [149], and prediction of distant metastases [150]. Although promising, the generalizability and therefore applicability into clinical practice is still limited and further prospective, multicenter, and standardized studies are needed.
Acknowledgments
This research was funded in part through the NIH/NCI Cancer Center Support Grant P30 CA008748.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- [1].Sung H, Ferlay J, Siegel RL, Laversanne M, Soerjomataram I, Jemal A, Bray F, Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries, CA Cancer J Clin 71(3) (2021) 209–249. [DOI] [PubMed] [Google Scholar]
- [2].Brown G, Davies S, Williams GT, Bourne MW, Newcombe RG, Radcliffe AG, Blethyn J, Dallimore NS, Rees BI, Phillips CJ, Maughan TS, Effectiveness of preoperative staging in rectal cancer: digital rectal examination, endoluminal ultrasound or magnetic resonance imaging?, Br J Cancer 91(1) (2004) 23–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Akasu T, Kondo H, Moriya Y, Sugihara K, Gotoda T, Fujita S, Muto T, Kakizoe T, Endorectal ultrasonography and treatment of early stage rectal cancer, World J Surg 24(9) (2000) 1061–8. [DOI] [PubMed] [Google Scholar]
- [4].Vliegen R, Dresen R, Beets G, Daniels-Gooszen A, Kessels A, van Engelshoven J, Beets-Tan R, The accuracy of Multi-detector row CT for the assessment of tumor invasion of the mesorectal fascia in primary rectal cancer, Abdom Imaging 33(5) (2008) 604–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [5].Maizlin ZV, Brown JA, So G, Brown C, Phang TP, Walker ML, Kirby JM, Vora P, Tiwari P, Can CT replace MRI in preoperative assessment of the circumferential resection margin in rectal cancer?, Dis Colon Rectum 53(3) (2010) 308–14. [DOI] [PubMed] [Google Scholar]
- [6].Mathur P, Smith JJ, Ramsey C, Owen M, Thorpe A, Karim S, Burke C, Ramesh S, Dawson PM, Comparison of CT and MRI in the pre-operative staging of rectal adenocarcinoma and prediction of circumferential resection margin involvement by MRI, Colorectal Dis 5(5) (2003) 396–401. [DOI] [PubMed] [Google Scholar]
- [7].Siddiqui MRS, Simillis C, Hunter C, Chand M, Bhoday J, Garant A, Vuong T, Artho G, Rasheed S, Tekkis P, Abulafi AM, Brown G, A meta-analysis comparing the risk of metastases in patients with rectal cancer and MRI-detected extramural vascular invasion (mrEMVI) vs mrEMVI-negative cases, Br J Cancer 116(12) (2017) 1513–1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Lord AC, D’Souza N, Pucher PH, Moran BJ, Abulafi AM, Wotherspoon A, Rasheed S, Brown G, Significance of extranodal tumour deposits in colorectal cancer: A systematic review and meta-analysis, Eur J Cancer 82 (2017) 92–102. [DOI] [PubMed] [Google Scholar]
- [9].McCawley N, Clancy C, O’Neill BD, Deasy J, McNamara DA, Burke JP, Mucinous Rectal Adenocarcinoma Is Associated with a Poor Response to Neoadjuvant Chemoradiotherapy: A Systematic Review and Meta-analysis, Dis Colon Rectum 59(12) (2016) 1200–1208. [DOI] [PubMed] [Google Scholar]
- [10].A.c.o. Radiology. https://www.acr.org/Lifelong-Learning-and-CME/Learning-Activities/Rectal-Cancer-Staging. (Accessed 12/12/2021.
- [11].Brown PG https://profginabrown.com/virtual-handson-workshops/. (Accessed 12/12/2021.
- [12].Gastrointestinal Tumor Study G, Prolongation of the disease-free interval in surgically treated rectal carcinoma, N Engl J Med 312(23) (1985) 1465–72. [DOI] [PubMed] [Google Scholar]
- [13].Krook JE, Moertel CG, Gunderson LL, Wieand HS, Collins RT, Beart RW, Kubista TP, Poon MA, Meyers WC, Mailliard JA, et al. , Effective surgical adjuvant therapy for high-risk rectal carcinoma, N Engl J Med 324(11) (1991) 709–15. [DOI] [PubMed] [Google Scholar]
- [14].Heald RJ, Husband EM, Ryall RD, The mesorectum in rectal cancer surgery--the clue to pelvic recurrence?, Br J Surg 69(10) (1982) 613–6. [DOI] [PubMed] [Google Scholar]
- [15].Enker WE, Total mesorectal excision--the new golden standard of surgery for rectal cancer, Ann Med 29(2) (1997) 127–33. [DOI] [PubMed] [Google Scholar]
- [16].Sauer R, Becker H, Hohenberger W, Rodel C, Wittekind C, Fietkau R, Martus P, Tschmelitsch J, Hager E, Hess CF, Karstens JH, Liersch T, Schmidberger H, Raab R, German G Rectal Cancer Study, Preoperative versus postoperative chemoradiotherapy for rectal cancer, N Engl J Med 351(17) (2004) 1731–40. [DOI] [PubMed] [Google Scholar]
- [17].You YN, Hardiman KM, Bafford A, Poylin V, Francone TD, Davis K, Paquette IM, Steele SR, Feingold DL, On C Behalf of the Clinical Practice Guidelines Committee of the American Society of, S. Rectal, The American Society of Colon and Rectal Surgeons Clinical Practice Guidelines for the Management of Rectal Cancer, Dis Colon Rectum 63(9) (2020) 1191–1222. [DOI] [PubMed] [Google Scholar]
- [18].Beets-Tan RGH, Lambregts DMJ, Maas M, Bipat S, Barbaro B, Curvo-Semedo L, Fenlon HM, Gollub MJ, Gourtsoyianni S, Halligan S, Hoeffel C, Kim SH, Laghi A, Maier A, Rafaelsen SR, Stoker J, Taylor SA, Torkzad MR, Blomqvist L, Magnetic resonance imaging for clinical management of rectal cancer: Updated recommendations from the 2016 European Society of Gastrointestinal and Abdominal Radiology (ESGAR) consensus meeting, Eur Radiol 28(4) (2018) 1465–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [19].Glynne-Jones R, Wyrwicz L, Tiret E, Brown G, Rodel C, Cervantes A, Arnold D, Committee EG, Rectal cancer: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up, Ann Oncol 29(Suppl 4) (2018) iv263. [DOI] [PubMed] [Google Scholar]
- [20].A.f.C.T.i. Oncology, N.C. Institute, C.C.T. Group, PROSPECT: Chemotherapy Alone or Chemotherapy Plus Radiation Therapy in Treating Patients With Locally Advanced Rectal Cancer Undergoing Surgery, https://ClinicalTrials.gov/show/NCT01515787, 2012.
- [21].Kennedy ED, Simunovic M, Jhaveri K, Kirsch R, Brierley J, Drolet S, Brown C, Vos PM, Xiong W, MacLean T, Kanthan S, Stotland P, Raphael S, Chow G, O’Brien CA, Cho C, Streutker C, Wong R, Schmocker S, Liberman S, Reinhold C, Kopek N, Marcus V, Bouchard A, Lavoie C, Morin S, Perigny M, Wright A, Neumann K, Clarke S, Patil NG, Arnason T, Williams L, McLeod R, Brown G, Mathieson A, Pooni A, Baxter NN, Safety and Feasibility of Using Magnetic Resonance Imaging Criteria to Identify Patients With “Good Prognosis” Rectal Cancer Eligible for Primary Surgery: The Phase 2 Nonrandomized QuickSilver Clinical Trial, JAMA Oncol 5(7) (2019) 961–966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Ryan JE, Warrier SK, Lynch AC, Ramsay RG, Phillips WA, Heriot AG , Predicting pathological complete response to neoadjuvant chemoradiotherapy in locally advanced rectal cancer: a systematic review, Colorectal Dis 18(3) (2016) 234–46. [DOI] [PubMed] [Google Scholar]
- [23].M.S.K.C. Center, Colon, R.S. Inc., T.C. Clinic, J.M. Health, O. Health, S. University, S.J.H.o. Orange, I. University of California, S.F. University of California, U.o. Chicago, U.o.S. Florida, U.o. Vermont, U.o. Washington, M.H.R. Institute, W.U.S.o. Medicine, C.U.M. Center, T.M.H.R. Institute, U.o. Rochester, U.o. Virginia, S.P.s. Hospital, F. St. Joseph, C.C. Florida, D. University of Colorado, U.o. Michigan, Trial Evaluating 3-year Disease Free Survival in Patients With Locally Advanced Rectal Cancer Treated With Chemoradiation Plus Induction or Consolidation Chemotherapy and Total Mesorectal Excision or Non-operative Management, https://ClinicalTrials.gov/show/NCT02008656, 2013.
- [24].Ogura A, Konishi T, Beets GL, Cunningham C, Garcia-Aguilar J, Iversen H, Toda S, Lee IK, Lee HX, Uehara K, Lee P, Putter H, van de Velde CJH, Rutten HJT, Tuynman JB, Kusters M, Lateral Node Study C, Lateral Nodal Features on Restaging Magnetic Resonance Imaging Associated With Lateral Local Recurrence in Low Rectal Cancer After Neoadjuvant Chemoradiotherapy or Radiotherapy, JAMA Surg 154(9) (2019) e192172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Ogura A, Konishi T, Cunningham C, Garcia-Aguilar J, Iversen H, Toda S, Lee IK, Lee HX, Uehara K, Lee P, Putter H, van de Velde CJH, Beets GL, Rutten HJT, Kusters M, Lateral Node Study C, Neoadjuvant (Chemo)radiotherapy With Total Mesorectal Excision Only Is Not Sufficient to Prevent Lateral Local Recurrence in Enlarged Nodes: Results of the Multicenter Lateral Node Study of Patients With Low cT3/4 Rectal Cancer, J Clin Oncol 37(1) (2019) 33–43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [26].Schaap DP, Boogerd LSF, Konishi T, Cunningham C, Ogura A, Garcia-Aguilar J, Beets GL, Suzuki C, Toda S, Lee IK, Sammour T, Uehara K, Lee P, Tuynman JB, van de Velde CJH, Rutten HJT, Kusters M, Lateral Node Study C, Rectal cancer lateral lymph nodes: multicentre study of the impact of obturator and internal iliac nodes on oncological outcomes, Br J Surg 108(2) (2021) 205–213. [DOI] [PubMed] [Google Scholar]
- [27].Fujita S, Mizusawa J, Kanemitsu Y, Ito M, Kinugasa Y, Komori K, Ohue M, Ota M, Akazai Y, Shiozawa M, Yamaguchi T, Bandou H, Katsumata K, Murata K, Akagi Y, Takiguchi N, Saida Y, Nakamura K, Fukuda H, Akasu T, Moriya Y, Colorectal G Cancer Study Group of Japan Clinical Oncology, Mesorectal Excision With or Without Lateral Lymph Node Dissection for Clinical Stage II/III Lower Rectal Cancer (JCOG0212): A Multicenter, Randomized Controlled, Noninferiority Trial, Ann Surg 266(2) (2017) 201–207. [DOI] [PubMed] [Google Scholar]
- [28].Battersby NJ, How P, Moran B, Stelzner S, West NP, Branagan G, Strassburg J, Quirke P, Tekkis P, Pedersen BG, Gudgeon M, Heald B, Brown G, Group MIS, Prospective Validation of a Low Rectal Cancer Magnetic Resonance Imaging Staging System and Development of a Local Recurrence Risk Stratification Model: The MERCURY II Study, Ann Surg 263(4) (2016) 751–60. [DOI] [PubMed] [Google Scholar]
- [29].R.M.N.F. Trust, P.C. Foundation, Low Rectal Cancer Study (MERCURY II), https://ClinicalTrials.gov/show/NCT02005965, 2007.
- [30].Sclafani F, Brown G, Cunningham D, Wotherspoon A, Tait D, Peckitt C, Evans J, Yu S, Sena Teixeira Mendes L, Tabernero J, Glimelius B, Cervantes A, Thomas J, Begum R, Oates J, Chau I, PAN-EX: a pooled analysis of two trials of neoadjuvant chemotherapy followed by chemoradiotherapy in MRI-defined, locally advanced rectal cancer, Ann Oncol 27(8) (2016) 1557–65. [DOI] [PubMed] [Google Scholar]
- [31].N.C. Institute, N. Oncology, Veliparib, Pembrolizumab, and Combination Chemotherapy in Treating Patient With Locally Advanced Rectal Cancer, https://ClinicalTrials.gov/show/NCT02921256, 2016.
- [32].Hall W, Li J, You YN, Gollub M, Grajo JR, Rosen M, dePrisco G, Yothers G, Dorth J, Gross HM, Peterson RA, Faller B, Moxley K, Jacobs S, Stella P, Haddock M, Hong T, George TJ, Prospective Validation of the Magnetic Resonance Tumor Regression Grade (MR-TRG) and Correlation With Pathologic Endpoints Score in NRG Oncology GI002, International Journal of Radiation Oncology*Biology*Physics 111 (2021) S37. [Google Scholar]
- [33].Garcia-Aguilar J, Patil S, Kim JK, Yuval JB, Thompson H, Verheij F, Lee M, Saltz LB, o.b.o.t.O. Consortium, Preliminary results of the organ preservation of rectal adenocarcinoma (OPRA) trial, Journal of Clinical Oncology 38(15_suppl) (2020) 4008–4008. [Google Scholar]
- [34].Battersby NJ, Dattani M, Rao S, Cunningham D, Tait D, Adams R, Moran BJ, Khakoo S, Tekkis P, Rasheed S, Mirnezami A, Quirke P, West NP, Nagtegaal I, Chong I, Sadanandam A, Valeri N, Thomas K, Frost M, Brown G, A rectal cancer feasibility study with an embedded phase III trial design assessing magnetic resonance tumour regression grade (mrTRG) as a novel biomarker to stratify management by good and poor response to chemoradiotherapy (TRIGGER): study protocol for a randomised controlled trial, Trials 18(1) (2017) 394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [35].Fryback DG, Thornbury JR, The efficacy of diagnostic imaging, Med Decis Making 11(2) (1991) 88–94. [DOI] [PubMed] [Google Scholar]
- [36].Taylor FG, Quirke P, Heald RJ, Moran B, Blomqvist L, Swift I, Sebag-Montefiore DJ, Tekkis P, Brown G, M.s. group, Preoperative high-resolution magnetic resonance imaging can identify good prognosis stage I, II, and III rectal cancer best managed by surgery alone: a prospective, multicenter, European study, Ann Surg 253(4) (2011) 711–9. [DOI] [PubMed] [Google Scholar]
- [37].Gollub MJ, Arya S, Beets-Tan RG, dePrisco G, Gonen M, Jhaveri K, Kassam Z, Kaur H, Kim D, Knezevic A, Korngold E, Lall C, Lalwani N, Blair Macdonald D, Moreno C, Nougaret S, Pickhardt P, Sheedy S, Harisinghani M, Use of magnetic resonance imaging in rectal cancer patients: Society of Abdominal Radiology (SAR) rectal cancer disease-focused panel (DFP) recommendations 2017, Abdom Radiol (NY) 43(11) (2018) 2893–2902. [DOI] [PubMed] [Google Scholar]
- [38].Bates DDB, Fuqua JL 3rd, Zheng J, Capanu M, Golia Pernicka JS, Javed-Tayyab S, Paroder V, Petkovska I, Gollub MJ, Measurement of rectal tumor height from the anal verge on MRI: a comparison of internal versus external anal sphincter, Abdom Radiol (NY) 46(3) (2021) 867–872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Johnson W, Taylor MB, Carrington BM, Bonington SC, Swindell R, The value of hyoscine butylbromide in pelvic MRI, Clin Radiol 62(11) (2007) 1087–93. [DOI] [PubMed] [Google Scholar]
- [40].Jayaprakasam VS, Javed-Tayyab S, Gangai N, Zheng J, Capanu M, Bates DDB, Fuqua JL 3rd, Paroder V, Golia-Pernicka J, Gollub MJ, Petkovska I, Does microenema administration improve the quality of DWI sequences in rectal MRI?, Abdom Radiol (NY) 46(3) (2021) 858–866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [41].Kim SH, Lee JM, Lee MW, Kim GH, Han JK, Choi BI, Sonography transmission gel as endorectal contrast agent for tumor visualization in rectal cancer, AJR Am J Roentgenol 191(1) (2008) 186–9. [DOI] [PubMed] [Google Scholar]
- [42].Stijns RC, Scheenen TW, de Wilt JH, Futterer JJ, Beets-Tan RG, The influence of endorectal filling on rectal cancer staging with MRI, Br J Radiol 91(1089) (2018) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [43].Gollub MJ, Lakhman Y, McGinty K, Weiser MR, Sohn M, Zheng J, Shia J, Does gadolinium-based contrast material improve diagnostic accuracy of local invasion in rectal cancer MRI? A multireader study, AJR Am J Roentgenol 204(2) (2015) W160–7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Vliegen RF, Beets GL, von Meyenfeldt MF, Kessels AG, Lemaire EE, van Engelshoven JM, Beets-Tan RG, Rectal cancer: MR imaging in local staging--is gadolinium-based contrast material helpful?, Radiology 234(1) (2005) 179–88. [DOI] [PubMed] [Google Scholar]
- [45].Jao SY, Yang BY, Weng HH, Yeh CH, Lee LW, Evaluation of gadolinium-enhanced T1-weighted magnetic resonance imaging in the preoperative assessment of local staging in rectal cancer, Colorectal Dis 12(11) (2010) 1139–48. [DOI] [PubMed] [Google Scholar]
- [46].Gollub MJ, Gultekin DH, Akin O, Do RK, Fuqua JL 3rd, Gonen M, Kuk D, Weiser M, Saltz L, Schrag D, Goodman K, Paty P, Guillem J, Nash GM, Temple L, Shia J, Schwartz LH, Dynamic contrast enhanced-MRI for the detection of pathological complete response to neoadjuvant chemotherapy for locally advanced rectal cancer, Eur Radiol 22(4) (2012) 821–31. [DOI] [PubMed] [Google Scholar]
- [47].Tong T, Sun Y, Gollub MJ, Peng W, Cai S, Zhang Z, Gu Y, Dynamic contrast-enhanced MRI: Use in predicting pathological complete response to neoadjuvant chemoradiation in locally advanced rectal cancer, J Magn Reson Imaging 42(3) (2015) 673–80. [DOI] [PubMed] [Google Scholar]
- [48].Dijkhoff RAP, Beets-Tan RGH, Lambregts DMJ, Beets GL, Maas M, Value of DCE-MRI for staging and response evaluation in rectal cancer: A systematic review, Eur J Radiol 95 (2017) 155–168. [DOI] [PubMed] [Google Scholar]
- [49].Schurink NW, Lambregts DMJ, Beets-Tan RGH, Diffusion-weighted imaging in rectal cancer: current applications and future perspectives, Br J Radiol 92(1096) (2019) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [50].Group MS, Extramural depth of tumor invasion at thin-section MR in patients with rectal cancer: results of the MERCURY study, Radiology 243(1) (2007) 132–9. [DOI] [PubMed] [Google Scholar]
- [51].Smith NJ, Barbachano Y, Norman AR, Swift RI, Abulafi AM, Brown G, Prognostic significance of magnetic resonance imaging-detected extramural vascular invasion in rectal cancer, Br J Surg 95(2) (2008) 229–36. [DOI] [PubMed] [Google Scholar]
- [52].Chand M, Evans J, Swift RI, Tekkis PP, West NP, Stamp G, Heald RJ, Brown G, The prognostic significance of postchemoradiotherapy high-resolution MRI and histopathology detected extramural venous invasion in rectal cancer, Ann Surg 261(3) (2015) 473–9. [DOI] [PubMed] [Google Scholar]
- [53].Patel UB, Taylor F, Blomqvist L, George C, Evans H, Tekkis P, Quirke P, Sebag-Montefiore D, Moran B, Heald R, Guthrie A, Bees N, Swift I, Pennert K, Brown G, Magnetic resonance imaging-detected tumor response for locally advanced rectal cancer predicts survival outcomes: MERCURY experience, J Clin Oncol 29(28) (2011) 3753–60. [DOI] [PubMed] [Google Scholar]
- [54].Shihab OC, How P, West N, George C, Patel U, Quirke P, Heald RJ, Moran BJ, Brown G, Can a novel MRI staging system for low rectal cancer aid surgical planning?, Dis Colon Rectum 54(10) (2011) 1260–4. [DOI] [PubMed] [Google Scholar]
- [55].Jang S, Lee JM, Yoon JH, Bae JS, Reduced field-of-view versus full field-of-view diffusion-weighted imaging for the evaluation of complete response to neoadjuvant chemoradiotherapy in patients with locally advanced rectal cancer, Abdom Radiol (NY) 46(4) (2021) 1468–1477. [DOI] [PubMed] [Google Scholar]
- [56].Cai JS, Chen HY, Chen JY, Lu YF, Sun JZ, Zhou Y, Yu RS, Reduced field-of-view diffusion-weighted imaging (DWI) in patients with gastric cancer: Comparison with conventional DWI techniques at 3.0T: A preliminary study, Medicine (Baltimore) 99(1) (2020) e18616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Hwang J, Hong SS, Kim HJ, Chang YW, Nam BD, Oh E, Lee E, Cha H, Reduced field-of-view diffusion-weighted MRI in patients with cervical cancer, Br J Radiol 91(1087) (2018) . [DOI] [PMC free article] [PubMed] [Google Scholar]
- [58].D’Souza N, Lord A, Shaw A, Patel A, Balyasnikova S, Tudyka V, Abulafi M, Moran B, Rasheed S, Tekkis P, Terlizzo M, West N, Quirke P, Brown G, The sigmoid take-off: An anatomical imaging definition of the rectum validated on specimen analysis, Eur J Surg Oncol 46(9) (2020) 1668–1672. [DOI] [PubMed] [Google Scholar]
- [59].D’Souza N, de Neree Tot Babberich MPM, d’Hoore A, Tiret E, Xynos E, Beets-Tan RGH, Nagtegaal ID, Blomqvist L, Holm T, Glimelius B, Lacy A, Cervantes A, Glynne-Jones R, West NP, Perez RO, Quadros C, Lee KY, Madiba TE, Wexner SD, Garcia-Aguilar J, Sahani D, Moran B, Tekkis P, Rutten HJ, Tanis PJ, Wiggers T, Brown G, Definition of the Rectum: An International, Expert-based Delphi Consensus, Ann Surg 270(6) (2019) 955–959. [DOI] [PubMed] [Google Scholar]
- [60].Nougaret S, Reinhold C, Mikhael HW, Rouanet P, Bibeau F, Brown G, The use of MR imaging in treatment planning for patients with rectal carcinoma: have you checked the “DISTANCE”?, Radiology 268(2) (2013) 330–44. [DOI] [PubMed] [Google Scholar]
- [61].Santiago I, Figueiredo N, Pares O, Matos C, MRI of rectal cancer-relevant anatomy and staging key points, Insights Imaging 11(1) (2020) 100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Golia Pernicka JS, Bates DDB, Fuqua JL 3rd, Knezevic A, Yoon J, Nardo L, Petkovska I, Paroder V, Nash GM, Markowitz AJ, Gollub MJ, Meaningful words in rectal MRI synoptic reports: How “polypoid” may be prognostic, Clin Imaging 80 (2021) 371–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Nagtegaal ID, Knijn N, Hugen N, Marshall HC, Sugihara K, Tot T, Ueno H, Quirke P, Tumor Deposits in Colorectal Cancer: Improving the Value of Modern Staging-A Systematic Review and Meta-Analysis, J Clin Oncol 35(10) (2017) 1119–1127. [DOI] [PubMed] [Google Scholar]
- [64].Nitsche U, Maak M, Schuster T, Kunzli B, Langer R, Slotta-Huspenina J, Janssen KP, Friess H, Rosenberg R, Prediction of prognosis is not improved by the seventh and latest edition of the TNM classification for colorectal cancer in a single-center collective, Ann Surg 254(5) (2011) 793–800; discussion 800–1. [DOI] [PubMed] [Google Scholar]
- [65].Al-Sukhni E, Milot L, Fruitman M, Beyene J, Victor JC, Schmocker S, Brown G, McLeod R, Kennedy E, Diagnostic accuracy of MRI for assessment of T category, lymph node metastases, and circumferential resection margin involvement in patients with rectal cancer: a systematic review and meta-analysis, Ann Surg Oncol 19(7) (2012) 2212–23. [DOI] [PubMed] [Google Scholar]
- [66].Beets-Tan RG, Beets GL, Vliegen RF, Kessels AG, Van Boven H, De Bruine A, von Meyenfeldt MF, Baeten CG, van Engelshoven JM, Accuracy of magnetic resonance imaging in prediction of tumour-free resection margin in rectal cancer surgery, Lancet 357(9255) (2001) 497–504. [DOI] [PubMed] [Google Scholar]
- [67].Wan LJ, Liu Y, Peng WJ, Zou SM, Ye F, Ouyang H, Zhao XM, Zhou CW, Zhang HM, Submucosal Enhancing Stripe as a Contrast Material-enhanced MRI-based Imaging Feature for the Differentiation of Stage T0-T1 from Early T2 Rectal Cancers, Radiology 298(1) (2021) 93–101. [DOI] [PubMed] [Google Scholar]
- [68].R.M.N.F. Trust, P.C. Foundation, Pre-therapeutic MRI Assessment of Early Stage Rectal Cancer and Significant Rectal Polyps to Avoid Major Resectional Surgery: A New Approach to the Management of Early Stage Rectal Cancer, https://ClinicalTrials.gov/show/NCT04103372, 2019.
- [69].Brown G, Richards CJ, Bourne MW, Newcombe RG, Radcliffe AG, Dallimore NS, Williams GT, Morphologic predictors of lymph node status in rectal cancer with use of high-spatial-resolution MR imaging with histopathologic comparison, Radiology 227(2) (2003) 371–7. [DOI] [PubMed] [Google Scholar]
- [70].Kim JH, Beets GL, Kim MJ, Kessels AG, Beets-Tan RG, High-resolution MR imaging for nodal staging in rectal cancer: are there any criteria in addition to the size?, Eur J Radiol 52(1) (2004) 78–83. [DOI] [PubMed] [Google Scholar]
- [71].Park JS, Jang YJ, Choi GS, Park SY, Kim HJ, Kang H, Cho SH, Accuracy of preoperative MRI in predicting pathology stage in rectal cancers: node-for-node matched histopathology validation of MRI features, Dis Colon Rectum 57(1) (2014) 32–8. [DOI] [PubMed] [Google Scholar]
- [72].Quirke P, Steele R, Monson J, Grieve R, Khanna S, Couture J, O’Callaghan C, Myint AS, Bessell E, Thompson LC, Parmar M, Stephens RJ, Sebag-Montefiore D, Investigators MCN-CCT, Group NCCS, Effect of the plane of surgery achieved on local recurrence in patients with operable rectal cancer: a prospective study using data from the MRC CR07 and NCIC-CTG CO16 randomised clinical trial, Lancet 373(9666) (2009) 821–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Vecchio FM, Valentini V, Minsky BD, Padula GD, Venkatraman ES, Balducci M, Micciche F, Ricci R, Morganti AG, Gambacorta MA, Maurizi F, Coco C, The relationship of pathologic tumor regression grade (TRG) and outcomes after preoperative therapy in rectal cancer, Int J Radiat Oncol Biol Phys 62(3) (2005) 752–60. [DOI] [PubMed] [Google Scholar]
- [74].Naxerova K, Reiter JG, Brachtel E, Lennerz JK, van de Wetering M, Rowan A, Cai T, Clevers H, Swanton C, Nowak MA, Elledge SJ, Jain RK, Origins of lymphatic and distant metastases in human colorectal cancer, Science 357(6346) (2017) 55–60. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [75].Markl B, Stage migration vs immunology: The lymph node count story in colon cancer, World J Gastroenterol 21(43) (2015) 12218–33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [76].R.M.N.F. Trust, P.C. Foundation, Molecular, Pathologic and MRI Investigation of the Prognostic and Redictive Importance of Extramural Venous Invasion in Rectal Cancer (MARVEL) Trial, https://ClinicalTrials.gov/show/NCT01995942, 2013.
- [77].Levine J, Petkovska I, Landa J, Bates DDB, Capanu M, Fuqua JL 3rd, Paroder V, Zheng J, Gollub MJ, Pernicka JSG, Bone lesions on baseline staging rectal MRI: prevalence and significance in patients with rectal adenocarcinoma, Abdom Radiol (NY) 46(6) (2021) 2423–2431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [78].Birbeck KF, Macklin CP, Tiffin NJ, Parsons W, Dixon MF, Mapstone NP, Abbott CR, Scott N, Finan PJ, Johnston D, Quirke P, Rates of circumferential resection margin involvement vary between surgeons and predict outcomes in rectal cancer surgery, Ann Surg 235(4) (2002) 449–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Kim SH, Lee JM, Park HS, Eun HW, Han JK, Choi BI, Accuracy of MRI for predicting the circumferential resection margin, mesorectal fascia invasion, and tumor response to neoadjuvant chemoradiotherapy for locally advanced rectal cancer, J Magn Reson Imaging 29(5) (2009) 1093–101. [DOI] [PubMed] [Google Scholar]
- [80].Salerno G, Daniels IR, Moran BJ, Wotherspoon A, Brown G, Clarifying margins in the multidisciplinary management of rectal cancer: the MERCURY experience, Clin Radiol 61(11) (2006) 916–23. [DOI] [PubMed] [Google Scholar]
- [81].Nagtegaal ID, Marijnen CA, Kranenbarg EK, van de Velde CJ, van Krieken JH, Pathology Review C, Cooperative Clinical I, Circumferential margin involvement is still an important predictor of local recurrence in rectal carcinoma: not one millimeter but two millimeters is the limit, Am J Surg Pathol 26(3) (2002) 350–7. [DOI] [PubMed] [Google Scholar]
- [82].Nagtegaal ID, van de Velde CJ, Marijnen CA, van Krieken JH, Quirke P, Dutch Colorectal Cancer G, Pathology Review C, Low rectal cancer: a call for a change of approach in abdominoperineal resection, J Clin Oncol 23(36) (2005) 9257–64. [DOI] [PubMed] [Google Scholar]
- [83].Weiser MR, Quah HM, Shia J, Guillem JG, Paty PB, Temple LK, Goodman KA, Minsky BD, Wong WD, Sphincter preservation in low rectal cancer is facilitated by preoperative chemoradiation and intersphincteric dissection, Ann Surg 249(2) (2009) 236–42. [DOI] [PubMed] [Google Scholar]
- [84].Bamba Y, Itabashi M, Kameoka S, Preoperative evaluation of the depth of anal canal invasion in very low rectal cancer by magnetic resonance imaging and surgical indications for intersphincteric resection, Surg Today 42(4) (2012) 328–33. [DOI] [PubMed] [Google Scholar]
- [85].Lord AC, D’Souza N, Shaw A, Rokan Z, Moran B, Abulafi M, Rasheed S, Chandramohan A, Corr A, Chau I, Brown G, MRI-Diagnosed Tumour Deposits and EMVI Status Have Superior Prognostic Accuracy to Current Clinical TNM Staging in Rectal Cancer, Ann Surg (2020). [DOI] [PubMed] [Google Scholar]
- [86].Krasna MJ, Flancbaum L, Cody RP, Shneibaum S, Ben Ari G, Vascular and neural invasion in colorectal carcinoma. Incidence and prognostic significance, Cancer 61(5) (1988) 1018–23. [DOI] [PubMed] [Google Scholar]
- [87].Shirouzu K, Isomoto H, Kakegawa T, Morimatsu M, A prospective clinicopathologic study of venous invasion in colorectal cancer, Am J Surg 162(3) (1991) 216–22. [DOI] [PubMed] [Google Scholar]
- [88].Jhaveri KS, Hosseini-Nik H, Thipphavong S, Assarzadegan N, Menezes RJ, Kennedy ED, Kirsch R, MRI Detection of Extramural Venous Invasion in Rectal Cancer: Correlation With Histopathology Using Elastin Stain, AJR Am J Roentgenol 206(4) (2016) 747–55. [DOI] [PubMed] [Google Scholar]
- [89].Smith NJ, Shihab O, Arnaout A, Swift RI, Brown G, MRI for detection of extramural vascular invasion in rectal cancer, AJR Am J Roentgenol 191(5) (2008) 1517–22. [DOI] [PubMed] [Google Scholar]
- [90].Knijn N, Mogk SC, Teerenstra S, Simmer F, Nagtegaal ID, Perineural Invasion is a Strong Prognostic Factor in Colorectal Cancer: A Systematic Review, Am J Surg Pathol 40(1) (2016) 103–12. [DOI] [PubMed] [Google Scholar]
- [91].Shepherd NA, Baxter KJ, Love SB, Influence of local peritoneal involvement on pelvic recurrence and prognosis in rectal cancer, J Clin Pathol 48(9) (1995) 849–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [92].van Gijn W, Marijnen CA, Nagtegaal ID, Kranenbarg EM, Putter H, Wiggers T, Rutten HJ, Pahlman L, Glimelius B, van de Velde CJ, Dutch Colorectal Cancer G, Preoperative radiotherapy combined with total mesorectal excision for resectable rectal cancer: 12-year follow-up of the multicentre, randomised controlled TME trial, Lancet Oncol 12(6) (2011) 575–82. [DOI] [PubMed] [Google Scholar]
- [93].Gollub MJ, Maas M, Weiser M, Beets GL, Goodman K, Berkers L, Beets-Tan RG, Recognition of the anterior peritoneal reflection at rectal MRI, AJR Am J Roentgenol 200(1) (2013) 97–101. [DOI] [PubMed] [Google Scholar]
- [94].Hyngstrom JR, Hu CY, Xing Y, You YN, Feig BW, Skibber JM, Rodriguez-Bigas MA, Cormier JN, Chang GJ, Clinicopathology and outcomes for mucinous and signet ring colorectal adenocarcinoma: analysis from the National Cancer Data Base, Ann Surg Oncol 19(9) (2012) 2814–21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Rullier A, Laurent C, Vendrely V, Le Bail B, Bioulac-Sage P, Rullier E, Impact of colloid response on survival after preoperative radiotherapy in locally advanced rectal carcinoma, Am J Surg Pathol 29(5) (2005) 602–6. [DOI] [PubMed] [Google Scholar]
- [96].Hussain SM, Outwater EK, Siegelman ES, Mucinous versus nonmucinous rectal carcinomas: differentiation with MR imaging, Radiology 213(1) (1999) 79–85. [DOI] [PubMed] [Google Scholar]
- [97].Yu SK, Chand M, Tait DM, Brown G, Magnetic resonance imaging defined mucinous rectal carcinoma is an independent imaging biomarker for poor prognosis and poor response to preoperative chemoradiotherapy, Eur J Cancer 50(5) (2014) 920–7. [DOI] [PubMed] [Google Scholar]
- [98].Horvat N, Hope TA, Pickhardt PJ, Petkovska I, Mucinous rectal cancer: concepts and imaging challenges, Abdom Radiol (NY) 44(11) (2019) 3569–3580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [99].Taylor FG, Swift RI, Blomqvist L, Brown G, A systematic approach to the interpretation of preoperative staging MRI for rectal cancer, AJR Am J Roentgenol 191(6) (2008) 1827–35. [DOI] [PubMed] [Google Scholar]
- [100].Park SH, Lim JS, Lee J, Kim HY, Koom WS, Hur H, Park MS, Kim MJ, Kim H, Rectal Mucinous Adenocarcinoma: MR Imaging Assessment of Response to Concurrent Chemotherapy and Radiation Therapy-A Hypothesis-generating Study, Radiology 285(1) (2017) 124–133. [DOI] [PubMed] [Google Scholar]
- [101].van der Paardt MP, Zagers MB, Beets-Tan RG, Stoker J, Bipat S, Patients who undergo preoperative chemoradiotherapy for locally advanced rectal cancer restaged by using diagnostic MR imaging: a systematic review and meta-analysis, Radiology 269(1) (2013) 101–12. [DOI] [PubMed] [Google Scholar]
- [102].Patel UB, Blomqvist LK, Taylor F, George C, Guthrie A, Bees N, Brown G, MRI after treatment of locally advanced rectal cancer: how to report tumor response--the MERCURY experience, AJR Am J Roentgenol 199(4) (2012) W486–95. [DOI] [PubMed] [Google Scholar]
- [103].Martens MH, van Heeswijk MM, van den Broek JJ, Rao SX, Vandecaveye V, Vliegen RA, Schreurs WH, Beets GL, Lambregts DM, Beets-Tan RG, Prospective, Multicenter Validation Study of Magnetic Resonance Volumetry for Response Assessment After Preoperative Chemoradiation in Rectal Cancer: Can the Results in the Literature be Reproduced?, Int J Radiat Oncol Biol Phys 93(5) (2015) 1005–14. [DOI] [PubMed] [Google Scholar]
- [104].Yeo SG, Kim DY, Park JW, Oh JH, Kim SY, Chang HJ, Kim TH, Kim BC, Sohn DK, Kim MJ, Tumor volume reduction rate after preoperative chemoradiotherapy as a prognostic factor in locally advanced rectal cancer, Int J Radiat Oncol Biol Phys 82(2) (2012) e193–9. [DOI] [PubMed] [Google Scholar]
- [105].Dresen RC, Beets GL, Rutten HJ, Engelen SM, Lahaye MJ, Vliegen RF, de Bruine AP, Kessels AG, Lammering G, Beets-Tan RG, Locally advanced rectal cancer: MR imaging for restaging after neoadjuvant radiation therapy with concomitant chemotherapy. Part I. Are we able to predict tumor confined to the rectal wall?, Radiology 252(1) (2009) 71–80. [DOI] [PubMed] [Google Scholar]
- [106].Sclafani F, Brown G, Cunningham D, Wotherspoon A, Mendes LST, Balyasnikova S, Evans J, Peckitt C, Begum R, Tait D, Tabernero J, Glimelius B, Rosello S, Thomas J, Oates J, Chau I, Comparison between MRI and pathology in the assessment of tumour regression grade in rectal cancer, Br J Cancer 117(10) (2017) 1478–1485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [107].Nahas SC, Nahas CSR, Cama GM, de Azambuja RL, Horvat N, Marques CFS, Menezes MR, Junior UR, Cecconello I, Diagnostic performance of magnetic resonance to assess treatment response after neoadjuvant therapy in patients with locally advanced rectal cancer, Abdom Radiol (NY) 44(11) (2019) 3632–3640. [DOI] [PubMed] [Google Scholar]
- [108].Heijnen LA, Maas M, Beets-Tan RG, Berkhof M, Lambregts DM, Nelemans PJ, Riedl R, Beets GL, Nodal staging in rectal cancer: why is restaging after chemoradiation more accurate than primary nodal staging?, Int J Colorectal Dis 31(6) (2016) 1157–62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [109].Lahaye MJ, Beets GL, Engelen SM, Kessels AG, de Bruine AP, Kwee HW, van Engelshoven JM, van de Velde CJ, Beets-Tan RG, Locally advanced rectal cancer: MR imaging for restaging after neoadjuvant radiation therapy with concomitant chemotherapy. Part II. What are the criteria to predict involved lymph nodes?, Radiology 252(1) (2009) 81–91. [DOI] [PubMed] [Google Scholar]
- [110].van Heeswijk MM, Lambregts DM, Palm WM, Hendriks BM, Maas M, Beets GL, Beets-Tan RG, DWI for Assessment of Rectal Cancer Nodes After Chemoradiotherapy: Is the Absence of Nodes at DWI Proof of a Negative Nodal Status?, AJR Am J Roentgenol 208(3) (2017) W79–W84. [DOI] [PubMed] [Google Scholar]
- [111].Oberholzer K, Junginger T, Heintz A, Kreft A, Hansen T, Lollert A, Ebert M, Duber C, Rectal Cancer: MR imaging of the mesorectal fascia and effect of chemoradiation on assessment of tumor involvement, J Magn Reson Imaging 36(3) (2012) 658–63. [DOI] [PubMed] [Google Scholar]
- [112].Chand M, Swift RI, Tekkis PP, Chau I, Brown G, Extramural venous invasion is a potential imaging predictive biomarker of neoadjuvant treatment in rectal cancer, Br J Cancer 110(1) (2014) 19–25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [113].Lee ES, Kim MJ, Park SC, Hur BY, Hyun JH, Chang HJ, Baek JY, Kim SY, Kim DY, Oh JH, Magnetic Resonance Imaging-Detected Extramural Venous Invasion in Rectal Cancer before and after Preoperative Chemoradiotherapy: Diagnostic Performance and Prognostic Significance, Eur Radiol 28(2) (2018) 496–505. [DOI] [PubMed] [Google Scholar]
- [114].Prampolini F, Taschini S, Pecchi A, Sani F, Spallanzani A, Gelsomino F, Kaleci S, Torricelli P, Magnetic resonance imaging performed before and after preoperative chemoradiotherapy in rectal cancer: predictive factors of recurrence and prognostic significance of MR-detected extramural venous invasion, Abdom Radiol (NY) 45(10) (2020) 2941–2949. [DOI] [PubMed] [Google Scholar]
- [115].Maas M, Nelemans PJ, Valentini V, Das P, Rodel C, Kuo LJ, Calvo FA, Garcia-Aguilar J, Glynne-Jones R, Haustermans K, Mohiuddin M, Pucciarelli S, Small W Jr., Suarez J, Theodoropoulos G, Biondo S, Beets-Tan RG, Beets GL, Long-term outcome in patients with a pathological complete response after chemoradiation for rectal cancer: a pooled analysis of individual patient data, Lancet Oncol 11(9) (2010) 835–44. [DOI] [PubMed] [Google Scholar]
- [116].Habr-Gama A, Perez RO, Nadalin W, Sabbaga J, Ribeiro U Jr., Silva e Sousa AH Jr., Campos FG, Kiss DR, Gama-Rodrigues J, Operative versus nonoperative treatment for stage 0 distal rectal cancer following chemoradiation therapy: long-term results, Ann Surg 240(4) (2004) 711–7; discussion 717–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [117].Martens MH, Maas M, Heijnen LA, Lambregts DM, Leijtens JW, Stassen LP, Breukink SO, Hoff C, Belgers EJ, Melenhorst J, Jansen R, Buijsen J, Hoofwijk TG, Beets-Tan RG, Beets GL, Long-term Outcome of an Organ Preservation Program After Neoadjuvant Treatment for Rectal Cancer, J Natl Cancer Inst 108(12) (2016). [DOI] [PubMed] [Google Scholar]
- [118].Thompson H, Kim JK, Yuval JB, Verheij F, Patil S, Gollub MJ, Wu AJ-C, Lee M, Hezel AF, Marcet J, Cataldo P, Polite BN, Herzig D, Liska D, Oommen S, Friel C, Ternent CA, Coveler AL, Hunt SR, Garcia-Aguilar J, Survival and organ preservation according to clinical response after total neoadjuvant therapy in locally advanced rectal cancer patients: A secondary analysis from the organ preservation in rectal adenocarcinoma (OPRA) trial, Journal of Clinical Oncology 39(15_suppl) (2021) 3509–3509. [Google Scholar]
- [119].Smith FM, Wiland H, Mace A, Pai RK, Kalady MF, Clinical criteria underestimate complete pathological response in rectal cancer treated with neoadjuvant chemoradiotherapy, Dis Colon Rectum 57(3) (2014) 311–5. [DOI] [PubMed] [Google Scholar]
- [120].Duldulao MP, Lee W, Streja L, Chu P, Li W, Chen Z, Kim J, Garcia-Aguilar J, Distribution of residual cancer cells in the bowel wall after neoadjuvant chemoradiation in patients with rectal cancer, Dis Colon Rectum 56(2) (2013) 142–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [121].Gollub MJ, Das JP, Bates DDB, Fuqua JL 3rd, Golia Pernicka JS, Javed-Tayyab S, Paroder V, Petkovska I, Garcia-Aguilar J, Rectal cancer with complete endoscopic response after neoadjuvant therapy: what is the meaning of a positive MRI?, Eur Radiol 31(7) (2021) 4731–4738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [122].Maas M, Lambregts DM, Nelemans PJ, Heijnen LA, Martens MH, Leijtens JW, Sosef M, Hulsewe KW, Hoff C, Breukink SO, Stassen L, Beets-Tan RG, Beets GL, Assessment of Clinical Complete Response After Chemoradiation for Rectal Cancer with Digital Rectal Examination, Endoscopy, and MRI: Selection for Organ-Saving Treatment, Ann Surg Oncol 22(12) (2015) 3873–80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [123].Lambregts DM, Vandecaveye V, Barbaro B, Bakers FC, Lambrecht M, Maas M, Haustermans K, Valentini V, Beets GL, Beets-Tan RG, Diffusion-weighted MRI for selection of complete responders after chemoradiation for locally advanced rectal cancer: a multicenter study, Ann Surg Oncol 18(8) (2011) 2224–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [124].Park MJ, Kim SH, Lee SJ, Jang KM, Rhim H, Locally advanced rectal cancer: added value of diffusion-weighted MR imaging for predicting tumor clearance of the mesorectal fascia after neoadjuvant chemotherapy and radiation therapy, Radiology 260(3) (2011) 771–80. [DOI] [PubMed] [Google Scholar]
- [125].Lambregts DMJ, van Heeswijk MM, Delli Pizzi A, van Elderen SGC, Andrade L, Peters N, Kint PAM, Osinga-de Jong M, Bipat S, Ooms R, Lahaye MJ, Maas M, Beets GL, Bakers FCH, Beets-Tan RGH, Diffusion-weighted MRI to assess response to chemoradiotherapy in rectal cancer: main interpretation pitfalls and their use for teaching, Eur Radiol 27(10) (2017) 4445–4454. [DOI] [PubMed] [Google Scholar]
- [126].Nahas SC, Rizkallah Nahas CS, Sparapan Marques CF, Ribeiro U Jr., Cotti GC, Imperiale AR, Capareli FC, Chih Chen AT, Hoff PM, Cecconello I, Pathologic Complete Response in Rectal Cancer: Can We Detect It? Lessons Learned From a Proposed Randomized Trial of Watch-and-Wait Treatment of Rectal Cancer, Dis Colon Rectum 59(4) (2016) 255–63. [DOI] [PubMed] [Google Scholar]
- [127].van der Sande ME, Beets GL, Hupkens BJ, Breukink SO, Melenhorst J, Bakers FC, Lambregts DM, Grabsch HI, Beets-Tan RG, Maas M, Response assessment after (chemo)radiotherapy for rectal cancer: Why are we missing complete responses with MRI and endoscopy?, Eur J Surg Oncol 45(6) (2019) 1011–1017. [DOI] [PubMed] [Google Scholar]
- [128].Coco C, Manno A, Mattana C, Verbo A, Rizzo G, Valentini V, Gambacorta MA, Vecchio FM, D’Ugo D, The role of local excision in rectal cancer after complete response to neoadjuvant treatment, Surg Oncol 16 Suppl 1 (2007) S101–4. [DOI] [PubMed] [Google Scholar]
- [129].Hughes R, Glynne-Jones R, Grainger J, Richman P, Makris A, Harrison M, Ashford R, Harrison RA, Livingstone JI, McDonald PJ, Meyrick Thomas J, Mitchell IC, Northover JM, Phillips R, Wallace M, Windsor A, Novell JR, Can pathological complete response in the primary tumour following pre-operative pelvic chemoradiotherapy for T3-T4 rectal cancer predict for sterilisation of pelvic lymph nodes, a low risk of local recurrence and the appropriateness of local excision?, Int J Colorectal Dis 21(1) (2006) 11–7. [DOI] [PubMed] [Google Scholar]
- [130].Radwan RW, Jones HG, Rawat N, Davies M, Evans MD, Harris DA, Beynon J, Swansea Pelvic Oncology G, Determinants of survival following pelvic exenteration for primary rectal cancer, Br J Surg 102(10) (2015) 1278–84. [DOI] [PubMed] [Google Scholar]
- [131].Kusters M, Austin KK, Solomon MJ, Lee PJ, Nieuwenhuijzen GA, Rutten HJ, Survival after pelvic exenteration for T4 rectal cancer, Br J Surg 102(1) (2015) 125–31. [DOI] [PubMed] [Google Scholar]
- [132].Robinson P, Carrington BM, Swindell R, Shanks JH, O’Dwyer S T, Recurrent or residual pelvic bowel cancer: accuracy of MRI local extent before salvage surgery, Clin Radiol 57(6) (2002) 514–22. [DOI] [PubMed] [Google Scholar]
- [133].Dresen RC, Kusters M, Daniels-Gooszen AW, Cappendijk VC, Nieuwenhuijzen GA, Kessels AG, de Bruine AP, Beets GL, Rutten HJ, Beets-Tan RG, Absence of tumor invasion into pelvic structures in locally recurrent rectal cancer: prediction with preoperative MR imaging, Radiology 256(1) (2010) 143–50. [DOI] [PubMed] [Google Scholar]
- [134].Georgiou PA, Tekkis PP, Constantinides VA, Patel U, Goldin RD, Darzi AW, John Nicholls R, Brown G, Diagnostic accuracy and value of magnetic resonance imaging (MRI) in planning exenterative pelvic surgery for advanced colorectal cancer, Eur J Cancer 49(1) (2013) 72–81. [DOI] [PubMed] [Google Scholar]
- [135].Jimenez-Rodriguez RM, Yuval JB, Sauve CG, Wasserman I, Aggarwal P, Romesser PB, Crane CH, Yaeger R, Cercek A, Guillem JG, Weiser MR, Wei IH, Widmar M, Nash GM, Pappou EP, Garcia-Aguilar J, Gollub MJ, Paty PB, Smith JJ, Type of recurrence is associated with disease-free survival after salvage surgery for locally recurrent rectal cancer, Int J Colorectal Dis 36(12) (2021) 2603–2611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [136].R.M.N.F. Trust, P.C. Foundation, The Beyond TME Trial, https://ClinicalTrials.gov/show/NCT02292641, 2014.
- [137].Curvo-Semedo L, Lambregts DM, Maas M, Beets GL, Caseiro-Alves F, Beets-Tan RG, Diffusion-weighted MRI in rectal cancer: apparent diffusion coefficient as a potential noninvasive marker of tumor aggressiveness, J Magn Reson Imaging 35(6) (2012) 1365–71. [DOI] [PubMed] [Google Scholar]
- [138].Li H, Chen GW, Liu YS, Pu H, Yin LL, Hou NY, Chen XL, Assessment of histologic prognostic factors of resectable rectal cancer: comparison of diagnostic performance using various apparent diffusion coefficient parameters, Sci Rep 10(1) (2020) 11554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [139].Nerad E, Delli Pizzi A, Lambregts DMJ, Maas M, Wadhwani S, Bakers FCH, van den Bosch HCM, Beets-Tan RGH, Lahaye MJ, The Apparent Diffusion Coefficient (ADC) is a useful biomarker in predicting metastatic colon cancer using the ADC-value of the primary tumor, PLoS One 14(2) (2019) e0211830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [140].Sun Y, Tong T, Cai S, Bi R, Xin C, Gu Y, Apparent Diffusion Coefficient (ADC) value: a potential imaging biomarker that reflects the biological features of rectal cancer, PLoS One 9(10) (2014) e109371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [141].Kim SH, Lee JY, Lee JM, Han JK, Choi BI, Apparent diffusion coefficient for evaluating tumour response to neoadjuvant chemoradiation therapy for locally advanced rectal cancer, Eur Radiol 21(5) (2011) 987–95. [DOI] [PubMed] [Google Scholar]
- [142].Sahni VA, Silveira PC, Sainani NI, Khorasani R, Impact of a Structured Report Template on the Quality of MRI Reports for Rectal Cancer Staging, AJR Am J Roentgenol 205(3) (2015) 584–8. [DOI] [PubMed] [Google Scholar]
- [143].Norenberg D, Sommer WH, Thasler W, D’Haese J, Rentsch M, Kolben T, Schreyer A, Rist C, Reiser M, Armbruster M, Structured Reporting of Rectal Magnetic Resonance Imaging in Suspected Primary Rectal Cancer: Potential Benefits for Surgical Planning and Interdisciplinary Communication, Invest Radiol 52(4) (2017) 232–239. [DOI] [PubMed] [Google Scholar]
- [144].Patel A, Rockall A, Guthrie A, Gleeson F, Worthy S, Grubnic S, Burling D, Allen C, Padhani A, Carey B, Cavanagh P, Peake MD, Brown G, Can the completeness of radiological cancer staging reports be improved using proforma reporting? A prospective multicentre non-blinded interventional study across 21 centres in the UK, BMJ Open 8(10) (2018) e018499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [145].Horvat N, Bates DDB, Petkovska I, Novel imaging techniques of rectal cancer: what do radiomics and radiogenomics have to offer? A literature review, Abdom Radiol (NY) 44(11) (2019) 3764–3774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [146].Staal FCR, van der Reijd DJ, Taghavi M, Lambregts DMJ, Beets-Tan RGH, Maas M, Radiomics for the Prediction of Treatment Outcome and Survival in Patients With Colorectal Cancer: A Systematic Review, Clin Colorectal Cancer 20(1) (2021) 52–71. [DOI] [PubMed] [Google Scholar]
- [147].Petkovska I, Tixier F, Ortiz EJ, Golia Pernicka JS, Paroder V, Bates DD, Horvat N, Fuqua J, Schilsky J, Gollub MJ, Garcia-Aguilar J, Veeraraghavan H, Clinical utility of radiomics at baseline rectal MRI to predict complete response of rectal cancer after chemoradiation therapy, Abdom Radiol (NY) 45(11) (2020) 3608–3617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [148].Horvat N, Veeraraghavan H, Khan M, Blazic I, Zheng J, Capanu M, Sala E, Garcia-Aguilar J, Gollub MJ, Petkovska I, MR Imaging of Rectal Cancer: Radiomics Analysis to Assess Treatment Response after Neoadjuvant Therapy, Radiology 287(3) (2018) 833–843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [149].Horvat N, Veeraraghavan H, Pelossof RA, Fernandes MC, Arora A, Khan M, Marco M, Cheng CT, Gonen M, Golia Pernicka JS, Gollub MJ, Garcia-Aguillar J, Petkovska I, Radiogenomics of rectal adenocarcinoma in the era of precision medicine: A pilot study of associations between qualitative and quantitative MRI imaging features and genetic mutations, Eur J Radiol 113 (2019) 174–181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [150].Liu X, Zhang D, Liu Z, Li Z, Xie P, Sun K, Wei W, Dai W, Tang Z, Ding Y, Cai G, Tong T, Meng X, Tian J, Deep learning radiomics-based prediction of distant metastasis in patients with locally advanced rectal cancer after neoadjuvant chemoradiotherapy: A multicentre study, EBioMedicine 69 (2021) 103442. [DOI] [PMC free article] [PubMed] [Google Scholar]