Abstract
Purpose
To investigate if molecular subtype is associated with outcome in stage 1 breast cancer (BC).
Methods
Tissue samples from 445 women with node-negative BC ≤ 15 mm, treated in 1986–2004, were classified into surrogate molecular subtypes [Luminal A-like, Luminal B-like (HER2−), HER2-positive, and triple negative breast cancer (TNBC)]. Information on treatment, recurrences, and survival were gathered from medical records.
Results
Tumour subtype was not associated with overall survival (OS). Luminal B-like (HER2−) and TNBC were associated with higher incidence of distant metastasis at 20 years (Hazard ratio (HR) 2.26; 95% CI 1.08–4.75 and HR 3.24; 95% CI 1.17–9.00, respectively). Luminal B-like (HER2−) and TNBC patients also had worse breast cancer-specific survival (BCSS), although not statistically significant (HR 1.53; 95% CI 0.70–3.33 and HR 1.89; 95% CI 0.60–5.93, respectively). HER2-positive BC was not associated with poor outcome despite no patient receiving HER2-targeted therapy, with most of these tumours being ER+.
Conclusions
Stage 1 TNBC or Luminal B-like (HER2−) tumours behave more aggressively. Women with HER2+/ER+ tumours do not have an increased risk of distant metastasis or death, absent targeted treatment.
Supplementary Information
The online version contains supplementary material available at 10.1007/s10549-022-06691-4.
Keywords: Breast cancer, Molecular subtypes, TMA, Long-term outcome
Introduction
Most women with breast cancer (BC) are diagnosed with stage 1 disease in countries with generally available mammography screening and programmes for early detection [1]. As a result, focus has shifted from clinical stage to tumour biology or molecular subtype of breast cancer when deciding on adjuvant systemic therapy. The surrogate molecular subtypes used in clinical practice are based on those originally described by Sørlie [2] and include Luminal A-like, Luminal B-like (HER2−), HER2-positive (HER2+) and triple negative breast cancer (TNBC). All subtypes besides Luminal A implicate the patient is considered for adjuvant chemotherapy, with addition of targeted anti-HER2-therapy for the HER2+ tumours. However, most studies showing worse outcomes for these tumours and/or benefit of adjuvant chemotherapy and targeted anti-HER2 therapy included women with more advanced clinical stages of BC [3–5]. There are few studies on the potential independent prognostic value of tumour subtypes in women with small lymph node negative BC [6–10]. In these patients, the treatment benefit needs to be put into perspective of treatment induced morbidity since most women are long-term survivors. The prognostic value of different subtypes is also dependent on the length of follow-up, because the natural course of BC varies depending on subtype.
This study investigates the association of surrogate molecular subtypes with survival outcomes and recurrence in a cohort of Swedish women treated for small, lymph node negative BC between 1986–2004, a time period before multimodal treatment protocols were routine and before HER2-targeted therapy was approved for adjuvant BC treatment in Sweden.
Materials and methods
Study cohort and generation of tissue microarray (TMA)
The study cohort includes all women identified through the regional breast cancer quality of care registry and operated for unifocal BC with a radiological tumour size ≤ 15 mm at Uppsala university hospital or Västerås hospital between 1986 and 2004. The diagnosis and tumour size were verified through medical records. Additionally, the cohort includes women with breast carcinoma of any size operated at Uppsala university hospital 1986–2004 where the pathology report stated that there was an in situ-component as well as an invasive tumour. To generate a tissue microarray (TMA), we took two 1 mm core biopsies from the formalin fixed, paraffin embedded surgical resection specimens from each patient and embedded them in a recipient tissue block. A fully annotated pseudonymized clinical database included information on baseline characteristics, treatments, relapses, and causes of death with data collected from a review of medical records every other year until March 2015. For the present study, we excluded tumours where the final size on histology was > 15 mm, or there were metastases to axillary lymph nodes. The Regional Ethics Committee of Uppsala approved the study (Record No. 99 422, 2005:118, and 2005:118/2).
Histology
The TMAs were sectioned and stained for haematoxylin–eosin, oestrogen receptor (ER), progesterone receptor (PR), Ki-67 and HER2 at the Department of Clinical pathology of Umeå University Hospital, using externally validated protocols according to clinical routine. In each run, appropriate external controls were included [tonsil, prostate, endometrium, cervical tissue as well as breast tumor samples with known expression of HER2 (0, 1+, 2+, and 3+)]. For HER2 both immunohistochemistry and silver in situ hybridization (SISH) were performed. Two subspecialized breast pathologists at Umeå University Hospital (a tertiary care centre with approximately 1000 breast cancer cases/year), reviewed the slides. Expression of ER, PR, Ki67, and HER2 were scored separately by either pathologist, but the haematoxylin–eosin slides first separately and then together to reach consensus on the nuclear grade. For ER and PR, tumours were scored as positive (≥ 10%) or negative (< 10%). ER+ tumours with nuclear grade 2 were divided into low (0–13%), intermediate (14–19%) and high (> 20%) Ki67. If a tumour had positive PR but missing an ER value because of technical reasons, the tumour was considered ER positive as well. HER2 IHC was scored as 0–3+ and HER2 SISH as amplified/non-amplified respectively, both according to College of American Pathologists (CAP) guidelines 2018 [11]. Nuclear grade was scored 1–3 according to Elston and Ellis [12]
Surrogate molecular subtypes
Based on the surrogate classification suggested by St Gallen and revised by Maissonneuve and Ehinger [13, 14] we divided the tumours into four subtypes as defined below. We used nuclear grade instead of histologic grade since neither tubule formation nor mitotic activity can be adequately assessed in TMA cores of this size.
Luminal A-like (LumA): ER+ or PR+ with nuclear grade 1 or nuclear grade 2 with low Ki67 or nuclear grade 2 with intermediate Ki67 and PR+
Luminal B-like (HER2−) (LumB): ER+ or PR+ with nuclear grade 3 or nuclear grade 2 with high Ki67 or nuclear grade 2 with intermediate Ki67 and PR−
HER2-positive (HER2+): HER2-staining 3+ by IHC and/or amplified by SISH.
Triple negative (TNBC): ER−, PR−, and HER2−.
Statistics
To compare baseline characteristics between the four groups, we used chi-square test or one-way analysis of variance (ANOVA). Primary outcomes were overall survival (OS), breast cancer specific survival (BCSS) and recurrence-free survival (RFS). Secondary outcomes were cumulative incidences of locoregional recurrence and distant metastasis. OS was defined as time from surgery to death from any cause. BCSS was defined as time from surgery to death primarily caused by BC as judged by the researcher reviewing the patients’ medical records. Recurrence was defined as locoregional recurrence or distant metastasis as recorded in the medical records. RFS was defined as time from surgery to recurrence, and patients who died before recurrence were censored at the time of death. The reviewer of the medical records had no information on the subtype classification used in this study. The analyses of RFS and BCSS censored patients at the time of contralateral BC. The analysis of locoregional recurrence censored patients at the time of distant metastasis before locoregional recurrence. The analysis of distant metastasis censored patients at the time of contralateral BC before distant metastasis. To analyse survival outcomes and cumulative incidences, we used Kaplan–Meier curve statistics and compared differences in survival between tumour subtypes with the log rank test. p-values ≤ 0.05 were considered statistically significant.
Multivariable analysis with Cox regression for OS, RFS, and BCSS included age, tumour size, and mode of detection. We used SPSS statistics v.26 and STATA IC v 15 for the statistical analyses.
Results
Clinical characteristics of the study cohort and surrogate molecular subtypes
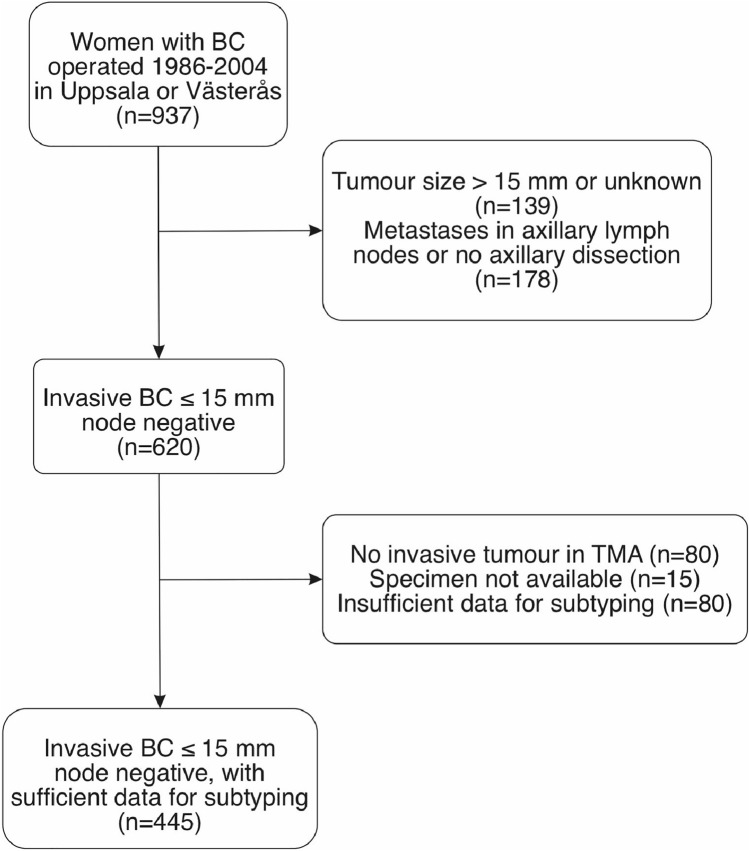
A total of 937 women diagnosed with BC were identified through a search of the regional breast cancer quality of care registry together with the hospital records during the study period. Out of these, 620 women had an invasive tumour ≤ 15 mm without lymph node metastases on final histology, and 445 of these tumours had sufficient material in the TMA for subtyping (Fig. 1). Dropout analysis showed that tumours without sufficient material in the TMA were smaller (mean 9 mm vs. 10 mm, p < 0.001), slightly more often lobular (12% vs. 6.7%, p = 0.02) and patients received less endocrine therapy (13.7% vs. 22.5%), compared to the tumours with material available. There were no statistically significant differences in age, mode of detection, locoregional treatment or chemotherapy (Supplementary Table S1).
Fig. 1.
Selection of patients for the study cohort. BC breast cancer, TMA tissue microarray
The follow-up time ranged from 0.2 to 29.6 years, with a median of 13.3 years. Median follow-up for women alive was 19.8 years. A high proportion of the tumours were Luminal A-like (59%) or Luminal B-like (HER2−) (28%). Within the group of luminal tumours with nuclear grade 2 (n = 267) 195 (73%) had low Ki-67, 36 (13.5%) had intermediate Ki-67 and 36 (13.5%) had high Ki-67. Most patients were diagnosed within the screening programme (71%), but women with Luminal B-like (HER2−), HER2-positive or TNBC more often had clinically detected tumours, compared to those with Luminal A-like tumours (34%, 42% and 37% vs. 25%, respectively) although this was not statistically significant (p = 0.08). Women with Luminal B-like (HER2−), HER2+, or TNBC tumours were younger than women with Luminal A-like tumours (mean age 59 years, 57 years, and 57 years vs. 62 years, respectively). Locoregional treatment did not differ between the groups. Most of the women (79%) received breast conserving surgery (BCS) in combination with radiotherapy (RT). A substantial minority (13%) were treated with BCS without RT, while the remainder received mastectomy with or without subsequent RT. Administration of systemic adjuvant therapy differed between groups, with endocrine therapy more often given to women with Luminal B-like (HER2−) BC, when compared to patients with Luminal A-like BC (27% vs. 23%). Very few patients (n = 9) received chemotherapy, most of those had HER2-positive BC (n = 3) or TNBC (n = 4) (Table 1).
Table 1.
Cohort characteristics
| Whole cohort | Luminal A-like | Luminal B-like (HER2−) | HER2-positive | Triple negative | p-value | ||
|---|---|---|---|---|---|---|---|
| n = 445 | n = 264 (59%) | n = 125 (28%) | n = 26 (6%) | n = 30 (7%) | |||
| Age years, mean (IQR) | 61 (52–68) | 62 (54–69) | 59 (51–68) | 57 (46–67) | 57 (48–67) | 0.009 | |
| < 50 years | 85 (19%) | 38 (14.4%) | 28 (22.4%) | 11 (42.3%) | 8 (26.7%) | ||
| ≥ 50 years | 360 (81%) | 226 (85.6%) | 97 (77.6%) | 15 (57.7%) | 22 (73.3%) | ||
| Size, mm, mean, (IQR) | 10 (8–13) | 10 (8–12) | 11 (8–14) | 10 (7–11) | 12 (10–15) | 0.006 | |
| pT1a, n (%) | 22 (5%) | 15 (5.7%) | 4 (3.2%) | 3 (11.5%) | 0 | ||
| pT1b | 219 (49%) | 139 (52.7%) | 53 (42.4%) | 14 (53.8%) | 13 (43.3%) | ||
| pT1c | 204 (46%) | 110 (41.7%) | 68 (54.4%) | 9 (34.6%) | 17 (56.7%) | ||
| Mode of detection | 0.08 | ||||||
| Screening | 319 (71%) | 199 (75.4%) | 83 (66.4%) | 15 (57.7%) | 19 (63.3%) | ||
| Clinical | 129 (29%) | 65 (24.6%) | 42 (33.6%) | 11 (42.3%) | 11 (36.7%) | ||
| Histological subtypea, n (%) | 0.28 | ||||||
| Ductal | 393 (88%) | 224 (84.8%) | 118 (94.4%) | 26 (100%) | 25 (83.3%) | ||
| Papillary/EPC | 2 (0.4%) | 1 (0.4%) | 1 (0.8%) | 0 | 0 | ||
| Lobular | 30 (7%) | 23 (8.7%) | 4 (3.2%) | 0 | 3 (10%) | ||
| Mucinous | 14 (3%) | 12 (4.5%) | 1 (0.8%) | 0 | 1 (3.3%) | ||
| Other | 6 (1%) | 4 (1.5%) | 1 (0.8%) | 0 | 1 (3.3%) | ||
| Oestrogen receptors (ER) | < 0.001 | ||||||
| ER+ (≥ 10%) | 395 (88.8%) | 254 (96.2%) | 124 (99.2%) | 17 (65.4%) | 0 | ||
| ER− (< 10%) | 37 (8.3%) | 0 | 1 (0.8%) | 8 (30.8%) | 28 (93.3%) | ||
| Missing | 13 (2.9%) | 10 (3.8%) | 0 | 1 (3.8%) | 2 (6.7%) | ||
| Progesterone receptors (PR) | < 0.001 | ||||||
| PR+ (≥ 10%) | 326 (73.3%) | 222 (84.1%) | 93 (74.4%) | 11 (42.3%) | 0 | ||
| PR− (< 10%) | 108 (24.3%) | 36 (13.6%) | 28 (22.4%) | 14 (53.8%) | 30 (100%) | ||
| Missing | 11 (2.5%) | 6 (2.3%) | 4 (3.2%) | 1 (3.8%) | 0 | ||
| Nuclear grade | < 0.001 | ||||||
| 1 | 41 (9.2%) | 39 (14.8%) | 0 | 2 (7.7%) | 0 | ||
| 2 | 292 (65.6%) | 225 (85.2%) | 42 (33.6%) | 13 (50%) | 12 (40%) | ||
| 3 | 112 (25.2%) | 0 | 83 (66.4%) | 11 (42.3%) | 18 (60%) | ||
| Locoregional treatment | 0.56 | ||||||
| BCS and RT | 352 (79.1%) | 202 (76.5%) | 104 (83.2%) | 21 (80.8%) | 25 (83.3%) | ||
| Mastectomy and RT | 7 (1.6%) | 5 (1.9%) | 2 (1.6%) | 0 | 0 | ||
| Mastectomy w/o RT | 30 (6.7%) | 16 (6.1%) | 8 (6.4%) | 3 (11.5%) | 3 (10%) | ||
| BCS w/o RT | 56 (12.6%) | 41 (15.5%) | 11 (8.8%) | 2 (7.7%) | 2 (6.7%) | ||
| Endocrine therapy | 0.04 | ||||||
| Yes | 100 (22.5%) | 60 (22.7%) | 34 (27.2%) | 5 (19.2%) | 1 (3.3%) | ||
| No | 345 (77.5%) | 204 (77.3%) | 91 (72.8%) | 21 (80.8%) | 29 (96.7%) | ||
| Chemotherapy | < 0.001 | ||||||
| Yes | 9 (2%) | 0 | 2 (1.6%) | 3 (11.5%) | 4 (13.3%) | ||
| No | 436 (98%) | 264 (100%) | 123 (98.4%) | 23 (88.5%) | 26 (86.7%) |
p-values indicate level of significance for overall difference between subtype groups, using chi-square test for categorical variables and one-way ANOVA for continuous variables
BC breast cancer, IQR interquartile range, EPC encapsulated papillary carcinoma, BCS breast conserving surgery, RT radiotherapy
aHistologic subtype based on medical records
Overall survival
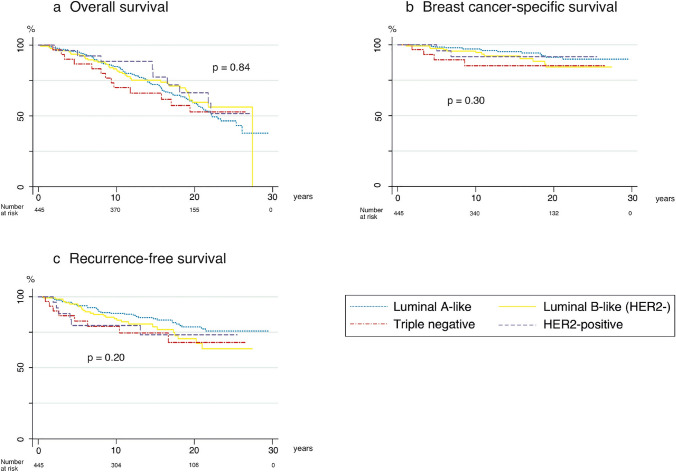
A total of 168 women died, whereof 33 from breast cancer. The univariate analysis showed no statistically significant differences in OS between patients based on surrogate tumour subtypes (Fig. 2a). Multivariable analysis showed numerically increased hazard ratios (HR) for the non-Luminal A-like subtypes; 1.08 [Luminal B-like (HER2−); 95% confidence interval (CI) 0.75–1.55], 1.41 (TNBC; 95% CI 0.78–2.54), and 1.01 (HER2+; 95% CI 0.51–2.02) but the differences were not statistically significant. Clinical detection (HR 1.58; CI 1.12–2.23) and high age (HR 1.10; CI 1.08–1.12) increased the risk of dying. Table 2 summarizes the results of the multivariable analysis.
Fig. 2.
Survival outcomes (Kaplan Meier), a overall survival, b breast cancer-specific survival, c recurrence-free survival
Table 2.
Multivariable analysis of risk factors for overall survival
| n | HR (95% CI) | p-value | |
|---|---|---|---|
| Multivariable analysis of overall survival | |||
| Tumour subtype | |||
| Luminal A-like | 264 | Ref. | |
| Luminal B-like (HER2−) | 125 | 1.08 (0.75–1.55) | 0.70 |
| Triple negative | 30 | 1.41 (0.78–2.54) | 0.25 |
| HER2-positive | 26 | 1.01 (0.51–2.02) | 0.97 |
| Clinical detectiona | 129 | 1.58 (1.12–2.23) | 0.01 |
| Tumour size (mm) | 1.01 (0.96–1.07) | 0.71 | |
| Age (years) | 1.10 (1.08–1.12) | < 0.001 | |
n number of patients with risk factor, HR hazard ratio, Ref. reference, RT radiotherapy, CI confidence interval
aCompared to detection by screening
Breast cancer-specific survival
Univariate analysis showed no statistically significant difference in BCSS between subtypes. (Fig. 2b). The 5-, 10-, and 20-year BCSS for the whole cohort were 98%, 96%, and 89%, respectively. Table 3 shows the survival rates for the respective subtypes. Of the 33 women who died from breast cancer, 15 had Luminal A-like tumours, 12 had Luminal B-like (HER2−) tumours, four had TNBC and two had HER2-positive tumours. In multivariable analysis, the non-Luminal A-like subtypes all had higher HRs than the Luminal A-like [Luminal B-like (HER2−) 1.53 (95% CI 0.70–3.33), TNBC 1.89 (95% CI 0.60–5.93), and HER2 + 1.23 (95% CI 0.28–5.40)] but the differences did not reach statistical significance (Table 4).
Table 3.
Survival rates depending on molecular subtype
| n | 5 years | 10 years | 20 years | ||||
|---|---|---|---|---|---|---|---|
| OS (%) | BCSS (%) | OS (%) | BCSS (%) | OS (%) | BCSS (%) | ||
| Cumulative survival rates depending on molecular subtype | |||||||
| All | 445 | 94 | 98 | 84 | 96 | 60 | 89 |
| LumA | 209 | 95 | 98 | 85 | 97 | 60 | 91 |
| LumB | 180 | 94 | 97 | 83 | 96 | 59 | 83 |
| TNBC | 30 | 87 | 89 | 70 | 85 | 53 | 85 |
| HER2+ | 26 | 96 | 100 | 88 | 91 | 68 | 91 |
OS overall survival, BCSS breast cancer specific survival, LumA Luminal A-like, LumB Luminal B-like (HER2−), TNBC triple negative breast cancer, HER2+ HER2-positive
Table 4.
Multivariable analysis of breast cancer-specific survival
| n | HR (95% CI) | p-value | |
|---|---|---|---|
| Multivariable analysis of breast cancer specific survival | |||
| Tumour subtype | |||
| Luminal A-like | 264 | Ref. | |
| Luminal B-like (HER2−) | 125 | 1.53 (0.70–3.33) | 0.28 |
| Triple negative | 30 | 1.89 (0.60–5.93) | 0.27 |
| HER2-positive | 26 | 1.23 (0.28–5.40) | 0.79 |
| Clinical detectiona | 129 | 1.46 (0.69–3.06) | 0.32 |
| Tumour size (mm) | 1.08 (0.96–1.21) | 0.21 | |
| Age (years) | 0.99 (0.96–1.02) | 0.47 | |
n number of patients with risk factor, HR hazard ratio, Ref. reference, CI confidence interval
aCompared to detection by screening
Recurrence-free survival
We found no statistically significant difference in RFS between subgroups in the present cohort either in univariate (Fig. 2c) or multivariable analysis (Table 5). The non-Luminal A-like subtypes, however all had higher estimated HRs compared to the Luminal A-like subtype [Luminal B-like (HER2−) 1.41 (95% CI 0.86–2.32), TNBC 1.66 (95% CI 0.76–3.63), and HER2 + 1.48 (95% CI 0.63–3.51)]. More extensive models for multivariable analysis of OS, BCSS, and RFS including treatment variables (radiotherapy, type of surgery, chemotherapy, and endocrine therapy) did not substantially change the estimated hazard ratios for the different subtypes (data not shown).
Table 5.
Multivariable analysis of recurrence-free survival
| n | HR (95% CI) | p-value | |
|---|---|---|---|
| Multivariable analysis of recurrence-free survival | |||
| Tumour subtype | |||
| Luminal A-like | 264 | Ref. | |
| Luminal B-like (HER2−) | 125 | 1.41 (0.86–2.32) | 0.17 |
| Triple negative | 30 | 1.66 (0.76–3.63) | 0.21 |
| HER2-positive | 26 | 1.48 (0.63–3.51) | 0.37 |
| Clinical detectiona | 129 | 1.49 (0.92–2.40) | 0.10 |
| Tumour size (mm) | 1.01 (0.94–1.09) | 0.78 | |
| Age (years) | 0.99 (0.97–1.01) | 0.39 | |
n number of patients with risk factor, HR hazard ratio, Ref. reference, CI confidence interval
aCompared to detection by screening
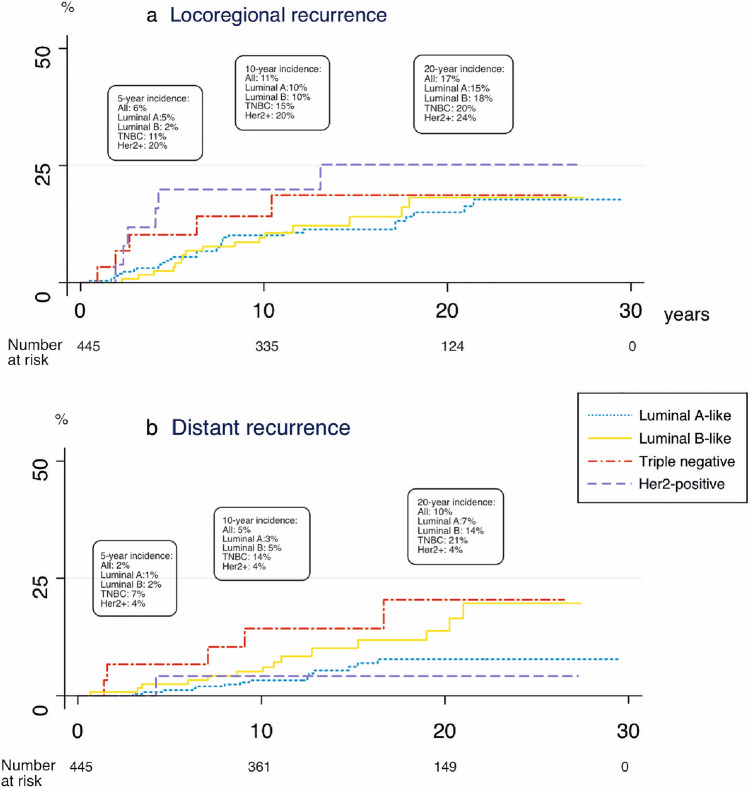
Locoregional and distant recurrence
In total 60 women (13%) had locoregional recurrence and 34 women (8%) had distant metastasis. We found no statistically significant difference in locoregional recurrence across subtypes (Fig. 3a). By contrast, there was a difference in the incidence of distant metastasis (p = 0.03) where the TNBC (HR 3.24; 95% CI 1.17–9.00) and Luminal B-like (HER2−) (HR 2.26; CI 1.08–4.75) had more distant metastases as compared with the Luminal A-like subtype. Distant recurrence occurred early in TNBC, and later in the Luminal B-like (HER2−) BC, hence the difference between these two groups gradually diminished with longer follow-up (Fig. 3b).
Fig. 3.
Cumulative incidence of locoregional (a) and distant recurrence (b)
Discussion
No certain association of surrogate molecular subtypes with overall survival was found in this population-based cohort of women with small node negative breast cancers (BC) and a very long follow-up time. Women with small TNBC or Luminal B-like (HER2−) tumours were however three times (TNBC) or twice [Luminal B-like (HER2−)] as likely to have had a distant recurrence compared to woman with a Luminal A-like tumour after 20 years. These women also had worse BCSS compared to women with Luminal A-like tumours, although the difference was not statistically significant. Women with HER2+ tumours had neither worse BCSS, nor higher incidence of distant recurrence than the women with Luminal A-like tumours, despite not receiving any targeted anti-HER2 therapy.
TNBCs are more aggressive and may merit systemic therapy even when they are small
Women with TNBC fared worse than women with Luminal A-like tumours in terms of OS, BCSS and RFS although differences were statistically significant only for distant recurrences. Our findings are supported by a SEER database study showing that women with pT1abN0 ER− tumours more often died from BC and those with ER+ more often of other causes [15], as well as other studies showing that TNBC is associated with worse RFS and distant RFS in pT1abN0 [16] or pT1bN0 tumours [9]. Thus, it would seem prudent to consider chemotherapy even for small node negative TNBC. Indeed, results from older prospective trials show a benefit of chemotherapy for ER− pT1abN0 tumours [17]. Several newer retrospective studies, however, failed to show any association between chemotherapy and outcome in pT1abN0 TNBC tumours [16, 18, 19], emphasizing the need for prospective trials on this group of patients, receiving modern locoregional therapy and using relevant definitions of hormone receptor status and HER2-status.
Small HER2-positive tumours have a favourable prognosis even in the absence of HER2-targeted therapy
The most surprising finding in our study was that HER2-positive tumours did not have a significantly worse long-term outcome compared to Luminal A-like tumours, even though no patient received targeted HER2 therapy. The numbers are small and warrant a cautious interpretation but are nonetheless interesting. HER2-positivity had no association with OS, possibly because women with HER2+ tumours were younger on average, a finding consistent with other studies [10, 20]. The BCSS was worse compared to Luminal A-like tumours after 10 years (91% vs. 97%) but after this the difference evened out. The only analysis where the HER2+ tumours stood out was in the locoregional recurrences where the cumulative incidence was fourfold higher after five years and still almost twice as high compared to Luminal A-like tumours after 20 years. Other retrospective studies have also failed to show HER2-positivity in pT1abN0 BC being an independent factor for worse DFS [20] or distant RFS [8]. In contrast, two retrospective studies have shown worse RFS and distant RFS for HER2 + pT1abN0 tumours [7] or pT1bN0 tumours [9], respectively. The second study did not, however, find any worse outcome for HER2 + pT1aN0 tumours. Another retrospective study described worse RFS and BCSS for HER2 + pT1mic/ab N0 tumours [6].
One of the reasons for the conflicting results in the literature may be how the molecular surrogate HER2-positive group is defined. By the original definition, “HER2-enriched” is ER-negative [2] but many authors define it as HER2 + regardless of hormone receptor status, as was also the case for the present cohort. The rationale behind this is that treatment recommendations generally do not consider ER-status in HER2 + BC [21, 22]. In our cohort, most (17/26) HER2 + tumours were ER+, but the BC deaths and the distant recurrences all occurred in the HER2+/ER− subgroup, while locoregional recurrences occurred equally in the HER2+/ER− and HER2+/ER+ groups (data not shown). Furthermore, there are retrospective studies showing a benefit of endocrine therapy alone (for HER2+/ER+ tumours) but not chemotherapy with/without trastuzumab, for women with HER2-positive pT1abN0 tumours [19, 20]. A retrospective study describing a better OS for patients with HER2-positive pT1abN0 tumours receiving trastuzumab and chemotherapy [23] included women who received only chemotherapy, only endocrine therapy or no adjuvant treatment at all in the same comparison group making the results somewhat hard to disentangle. Together with our findings, this suggests that small node negative HER2+ tumours have a good prognosis if they are ER+, and hence that the benefits of adding chemotherapy and HER2-targeted therapy on top of endocrine therapy may not be substantial.
Outcome of luminal B-like (HER2−) tumours depends strongly on the length of follow-up
The natural course of BC varies depending on subtype, where the Luminal-like tumours have a slow but steady rate of recurrence and death over the years, while the HER2+ and TNBC have a higher mortality rate initially, which declines after the first 5 years [24, 25]. The rate of recurrence and death for Luminal B-like (HER2−) tumours is only slightly higher than for the Luminal A-like, with almost no difference at five years of follow-up, but after 20 years actually being on the same level as TNBC. In our cohort the 5-year BCSS were similar 98% (Luminal A-like) and 97% [Luminal B-like (HER2−)], but after 20 years had diverged to 91% (Luminal A-like) and 83% [Luminal B-like (HER2−)]. The same held true for both locoregional and distant recurrences. Our findings are consistent with a recent registry-based study with long-term follow-up in which Luminal B-like (HER2−) and TNBC eventually had the same risk of BC events [26]. Only a third of the women with Luminal B-like (HER2−) tumours in the present cohort received endocrine therapy and a tenth received lumpectomy without subsequent RT, which certainly influenced the long-term outcome [27]. Today, endocrine therapy would have been recommended for all of them, and likely none would have had BCS without RT. These results suggest that the possible benefit from addition of chemotherapy is highly dependent on the expected life span of the woman.
Limitations
The strength in having a cohort with very long follow-up confers a limitation in that old specimens are used. It is known that the levels of detectable protein in archival FFPE tissue decrease over time but also that many proteins are still detectable after 70 years [28–31]. For each biomarker the effect of analysing TMA rather than whole slides also has to be considered, although since the tumours in our cohort are small, this risk is probably lower than average. For ER the effect of aging should be negligible due to its bimodal distribution [33, 34] and even with a 10% decrease per decade [32] the vast majority of ER+ tumours would still be scored as ER+. For the same reason, concordance between TMA and whole slide evaluation is assumably excellent [35, 36]. This is confirmed by comparison with the available original pathology reports, showing that 95% of the ER+ tumours were classified as ER+ by TMA-scoring (data not shown). For PR the distribution is somewhat different [37], leading to discrepancy between cores in 7% of cases in one study [35], possibly leading to misclassification of a small number of Luminal tumours with intermediate proliferation.
For Ki67, the decrease is perhaps more problematic and could also affect the subdivision between Luminal A-like versus Luminal B-like (HER2−). In our material there were indeed slightly more Luminal B-like (HER2−) tumours in the samples from more recent years, (data not shown) The proportion of HER2-positive tumours in our cohort was as expected in a sample of small tumours [6, 20] even though this is the most intratumourally heterogenous stain [28, 35]. Since we performed SISH on all samples and DNA is thought to be more stable than membrane proteins [32], as well as less heterogenous [38, 39] we consider the risk that we missed any HER2-positive cases very low. Still, the small number of HER2-positive tumours prohibited further subdivision of these patients into ER+ and ER− groups. Likewise, the number of TNBC were low, as was the number of events in each group. While this underscores the generally good prognosis for these women, it makes it difficult to reach statistical significance. Using surrogate molecular subtypes, rather than the actual molecular subtypes based on gene expression analysis may also be considered a weakness, since these do not correlate perfectly [40]. On the other hand, surrogate molecular subtypes are currently used in clinical practice in many settings and the molecular subtypes can also vary depending on signature and gene expression test used [41]. In summary, our limitations all mainly affect the subdivision between Luminal A-like and Luminal B-like (HER2−) breast cancer. However, the resulting groups still had measurably different outcomes. The clinicopathological factors used to guide treatment during the recruitment period of the cohort correlates with breast cancer subtypes and may have influenced analysis of the association between prognosis and subtype. At the time of treatment, modern protocols were however not in practice and few women were given adjuvant systemic treatment.
Strenghts
The strengths of this study include a very long follow-up with no patients lost to follow-up. This is in contrast with most of the currently available studies for this patient group, and essential for analysing the outcome of the Luminal B-tumours. This means that the accuracy of the findings is high, compared to the data that may be extracted from a registry. The cohort is population based, and the analyses of TMA data include more than 70% of the eligible women, making it representative for women with small lymph node negative BC at that time. There was no difference in primary surgery or RT between the groups, indicating that they were probably comparable with respect to co-morbidity. None of the women with HER2-positive tumours were treated with targeted therapy, making it possible to observe the natural course of these tumours. Finally, the histological evaluation was done by the same two subspecialised breast pathologists, reducing interobserver variation.
Conclusion
Small TNBC and Luminal B-like (HER2−) tumours behave more aggressively than Luminal A-like tumours. These subtypes follow different courses, where the TNBC recur mostly early on or not at all, while the Luminal B-like (HER2−) tumours recur at a slow but consistent rate over the years. This means that a young and otherwise healthy woman with a stage 1 Luminal B tumour might benefit substantially from systemic adjuvant therapy, while for an older woman the risks may outweigh the benefits. For early stage TNBC, our study confirms a high 10-year risk of recurrence and death, and thus patients should stand to gain from systemic adjuvant therapy. For the women with HER2+ tumours however, neither our findings, nor the available literature unequivocally support an increased risk of distant metastasis or death for pT1abN0-tumours in absence of HER2-targeted treatment. It is possible that treatment recommendations for HER2+ tumours need to take ER-status into account, and that women with ER+/HER2+ tumours have no need for adjuvant therapy in addition to locoregional radiotherapy and endocrine treatment. Despite the different risks associated with the subtypes described above, no association of tumour subtype with OS was observed. Prospective trials using modern locoregional therapy and endocrine therapy are thus needed to evaluate whether the more aggressive behaviour of non-Luminal A-like subtypes translate into a benefit of systemic adjuvant chemotherapy and HER2-targeted therapy for women with small lymph node negative BC.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Johan Svensson at Umeå school of Business, Economics, and Statistics for kind and patient help with statistical analyses.
Authors contributions
GR—histologic examination of the material, data analysis and interpretation, draft writing. AN—histologic examination of the material, critical review of the final draft. MJ—data analysis, critical review of the final draft. CW—data interpretation, critical review of the final draft. FW–acquisition of surgical specimens, construction of TMA and acquisition of patient data. Critical review of the final draft. LH—conception of study and critical review of the final draft. GN—conception of study and critical review of the final draft. CB—conception of study and critical review of the final draft. MS—conception and design of study, data interpretation, and manuscript drafting.
Funding
Open access funding provided by Umeå University. This study was supported by grants from the Lions Cancer Research Foundation in Uppsala, Swedish Breast Cancer Association, the Percy Falk Foundation, VISARE NORR funding of the Northern County Councils Regional Federation (Grant Nos. VISARENORR750491 and VISARENORR931408) and ALF funding from Region Västerbotten.
Availability of data and materials
Data is not uploaded to a publicly available platform. Researchers have access to data through application to the study PI (malin.sund@umu.se) or the corresponding author under standard rules of protecting data integrity and existing ethics permissions.
Code availability
Not applicable.
Declarations
Conflict of interest
All author declares that they have no conflict of interest.
Consent for publication
Not applicable.
Consent to participate
After approval of the regional Ethics Committee, no patients were informed of the study, since the material and information was gathered after such time that any result of the study could not affect treatment decision for the women studied.
Ethics approval
Record number 99 422, 2005:118, and 2005:118/2; The Regional Ethics Committee of Uppsala.
Footnotes
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.NKBC (https://statistik.incanet.se/brostcancer/)
- 2.Sorlie T, Perou CM, Tibshirani R, Aas T, Geisler S, Johnsen H, Hastie T, Eisen MB, van de Rijn M, Jeffrey SS, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci USA. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Piccart-Gebhart MJ, Procter M, Leyland-Jones B, Goldhirsch A, Untch M, Smith I, Gianni L, Baselga J, Bell R, Jackisch C, et al. Trastuzumab after adjuvant chemotherapy in HER2-positive breast cancer. N Engl J Med. 2005;353(16):1659–1672. doi: 10.1056/NEJMoa052306. [DOI] [PubMed] [Google Scholar]
- 4.Gianni L, Pienkowski T, Im Y-H, Roman L, Tseng L-M, Liu M-C, Lluch A, Staroslawska E, de la Haba-Rodriguez J, Im S-A, et al. Efficacy and safety of neoadjuvant pertuzumab and trastuzumab in women with locally advanced, inflammatory, or early HER2-positive breast cancer (NeoSphere): a randomised multicentre, open-label, phase 2 trial. Lancet Oncol. 2012;13(1):25–32. doi: 10.1016/S1470-2045(11)70336-9. [DOI] [PubMed] [Google Scholar]
- 5.Hugh J, Hanson J, Cheang MCU, Nielsen TO, Perou CM, Dumontet C, Reed J, Krajewska M, Treilleux I, Rupin M, et al. Breast cancer subtypes and response to docetaxel in node-positive breast cancer: use of an immunohistochemical definition in the BCIRG 001 trial. J Clin Oncol. 2009;27(8):1168–1176. doi: 10.1200/JCO.2008.18.1024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cancello G, Maisonneuve P, Rotmensz N, Viale G, Mastropasqua MG, Pruneri G, Montagna E, Dellapasqua S, Iorfida M, Cardillo A, et al. Prognosis in women with small (T1mic, T1a, T1b) node-negative operable breast cancer by immunohistochemically selected subtypes. Breast Cancer Res Treat. 2011;127(3):713–720. doi: 10.1007/s10549-011-1465-7. [DOI] [PubMed] [Google Scholar]
- 7.Gonzalez-Angulo AM, Litton JK, Broglio KR, Meric-Bernstam F, Rakkhit R, Cardoso F, Peintinger F, Hanrahan EO, Sahin A, Guray M, et al. High risk of recurrence for patients with breast cancer who have human epidermal growth factor receptor 2-positive, node-negative tumors 1 cm or smaller. J Clin Oncol. 2009;27(34):5700–5706. doi: 10.1200/JCO.2009.23.2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Livi L, Meattini I, Saieva C, Franzese C, Di Cataldo V, Greto D, Franceschini D, Scotti V, Bonomo P, Nori J, et al. Prognostic value of positive human epidermal growth factor receptor 2 status and negative hormone status in patients with T1a/T1b, lymph node-negative breast cancer. Cancer. 2012;118(13):3236–3243. doi: 10.1002/cncr.26647. [DOI] [PubMed] [Google Scholar]
- 9.Park YH, Kim ST, Cho EY, Choi YL, Ok ON, Baek HJ, Lee JE, Nam SJ, Yang JH, Park W, et al. A risk stratification by hormonal receptors (ER, PgR) and HER-2 status in small (< or = 1 cm) invasive breast cancer: who might be possible candidates for adjuvant treatment? Breast Cancer Res Treat. 2010;119(3):653–661. doi: 10.1007/s10549-009-0665-x. [DOI] [PubMed] [Google Scholar]
- 10.Kolben T, Harbeck N, Wuerstlein R, Schubert-Fritschle G, Bauerfeind I, Schrodi S, Engel J. Endocrine sensitivity is decisive for patient outcome in small node-negative breast cancers (BC) (pT1a, b)—results from the Munich Cancer Registry. Breast. 2015;24(1):24–31. doi: 10.1016/j.breast.2014.10.007. [DOI] [PubMed] [Google Scholar]
- 11.Wolff AC, Hammond MEH, Allison KH, Harvey BE, Mangu PB, Bartlett JMS, Bilous M, Ellis IO, Fitzgibbons P, Hanna W, et al. Human epidermal growth factor receptor 2 testing in breast cancer: American Society of Clinical Oncology/College of American Pathologists Clinical Practice Guideline Focused Update. J Clin Oncol. 2018;36(20):2105–2122. doi: 10.1200/JCO.2018.77.8738. [DOI] [PubMed] [Google Scholar]
- 12.Elston CW, Ellis IO (1991) Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. In: Elston CW, Ellis IO (eds) Histopathology 19:403–410; Histopathology 2002, 41(3a):151–152, discussion 152–153 [PubMed]
- 13.Ehinger A, Malmström P, Bendahl PO, Elston CW, Falck AK, Forsare C, Grabau D, Rydén L, Stål O, Fernö M. Histological grade provides significant prognostic information in addition to breast cancer subtypes defined according to St Gallen 2013. Acta Oncol. 2017;56(1):68–74. doi: 10.1080/0284186X.2016.1237778. [DOI] [PubMed] [Google Scholar]
- 14.Maisonneuve P, Disalvatore D, Rotmensz N, Curigliano G, Colleoni M, Dellapasqua S, Pruneri G, Mastropasqua MG, Luini A, Bassi F, et al. Proposed new clinicopathological surrogate definitions of luminal A and luminal B (HER2-negative) intrinsic breast cancer subtypes. Breast Cancer Res. 2014;16(3):R65–R65. doi: 10.1186/bcr3679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Hanrahan EO, Gonzalez-Angulo AM, Giordano SH, Rouzier R, Broglio KR, Hortobagyi GN, Valero V. Overall survival and cause-specific mortality of patients with stage T1a, bN0M0 breast carcinoma. J Clin Oncol. 2007;25(31):4952–4960. doi: 10.1200/JCO.2006.08.0499. [DOI] [PubMed] [Google Scholar]
- 16.Kwon JH, Kim YJ, Lee KW, Oh DY, Park SY, Kim JH, Chie EK, Kim SW, Im SA, Kim IA, et al. Triple negativity and young age as prognostic factors in lymph node-negative invasive ductal carcinoma of 1 cm or less. BMC Cancer. 2010;10:557. doi: 10.1186/1471-2407-10-557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fisher B, Redmond C, Dimitrov NV, Bowman D, Legault-Poisson S, Wickerham DL, Wolmark N, Fisher ER, Margolese R, Sutherland C, et al. A randomized clinical trial evaluating sequential methotrexate and fluorouracil in the treatment of patients with node-negative breast cancer who have estrogen-receptor-negative tumors. N Engl J Med. 1989;320(8):473–478. doi: 10.1056/NEJM198902233200801. [DOI] [PubMed] [Google Scholar]
- 18.Ho AY, Gupta G, King TA, Perez CA, Patil SM, Rogers KH, Wen YH, Brogi E, Morrow M, Hudis CA, et al. Favorable prognosis in patients with T1a/T1bN0 triple-negative breast cancers treated with multimodality therapy. Cancer. 2012;118(20):4944–4952. doi: 10.1002/cncr.27480. [DOI] [PubMed] [Google Scholar]
- 19.Olszewski AJ, Migdady Y, Boolbol SK, Klein P, Boachie-Adjei K, Sakr BJ, Sikov W, Shao T. Effects of adjuvant chemotherapy in HER2-positive or triple-negative pT1ab breast cancers: a multi-institutional retrospective study. Breast Cancer Res Treat. 2013;138(1):215–223. doi: 10.1007/s10549-013-2423-3. [DOI] [PubMed] [Google Scholar]
- 20.Bao J, Donovan C, Amersi F, Zhang X, Giuliano AE, Chung A. Outcomes in patients with small node-negative invasive breast cancer. Breast J. 2019;25(4):638–643. doi: 10.1111/tbj.13288. [DOI] [PubMed] [Google Scholar]
- 21.Nationellt Vårdprogram—Bröstcancer (https://www.cancercentrum.se/samverkan/cancerdiagnoser/brost/vardprogram/gallande-vardprogram)
- 22.Tryfonidis K, Zardavas D, Cardoso F. Small breast cancers: when and how to treat. Cancer Treat Rev. 2014;40(10):1129–1136. doi: 10.1016/j.ctrv.2014.09.004. [DOI] [PubMed] [Google Scholar]
- 23.Ignatov T, Eggemann H, Burger E, Costa SD, Ignatov A. Management of small T1a/b breast cancer by tumor subtype. Breast Cancer Res Treat. 2017;163(1):111–118. doi: 10.1007/s10549-017-4168-x. [DOI] [PubMed] [Google Scholar]
- 24.Chia SK, Speers CH, Bryce CJ, Hayes MM, Olivotto IA. Ten-year outcomes in a population-based cohort of node-negative, lymphatic, and vascular invasion-negative early breast cancers without adjuvant systemic therapies. J Clin Oncol. 2004;22(9):1630–1637. doi: 10.1200/JCO.2004.09.070. [DOI] [PubMed] [Google Scholar]
- 25.Blows FM, Driver KE, Schmidt MK, Broeks A, van Leeuwen FE, Wesseling J, Cheang MC, Gelmon K, Nielsen TO, Blomqvist C, et al. Subtyping of breast cancer by immunohistochemistry to investigate a relationship between subtype and short and long term survival: a collaborative analysis of data for 10,159 cases from 12 studies. PLoS Med. 2010;7(5):e1000279. doi: 10.1371/journal.pmed.1000279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jaraj D (2020) Clinical epidemiology division DoMSKISS, Department of Surgery CStGHSS, Höijer J, Unit of Biostatistics IoEMKISS, Widman L, Unit of Biostatistics IoEMKISS, Ahlgren J, Department of Oncology FoM, Health ÖUÖS et al: Long-term prognostication for 20,114 women with small and node-negative breast cancer (T1abN0). JNCI Cancer Spectrum [DOI] [PMC free article] [PubMed]
- 27.Davies C, Godwin J, Gray R, Clarke M, Cutter D, Darby S, McGale P, Pan HC, Taylor C, Wang YC, et al. Relevance of breast cancer hormone receptors and other factors to the efficacy of adjuvant tamoxifen: patient-level meta-analysis of randomised trials. Lancet. 2011;378(9793):771–784. doi: 10.1016/S0140-6736(11)60993-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Camp RL, Charette LA, Rimm DL. Validation of tissue microarray technology in breast carcinoma. Lab Invest. 2000;80(12):1943–1949. doi: 10.1038/labinvest.3780204. [DOI] [PubMed] [Google Scholar]
- 29.Fergenbaum JH, Garcia-Closas M, Hewitt SM, Lissowska J, Sakoda LC, Sherman ME. Loss of antigenicity in stored sections of breast cancer tissue microarrays. Cancer Epidemiol Biomarkers Prev. 2004;13(4):667–672. doi: 10.1158/1055-9965.667.13.4. [DOI] [PubMed] [Google Scholar]
- 30.Jacobs TW, Prioleau JE, Stillman IE, Schnitt SJ. Loss of tumor marker-immunostaining intensity on stored paraffin slides of breast cancer. J Natl Cancer Inst. 1996;88(15):1054–1059. doi: 10.1093/jnci/88.15.1054. [DOI] [PubMed] [Google Scholar]
- 31.Grillo F, Bruzzone M, Pigozzi S, Prosapio S, Migliora P, Fiocca R, Mastracci L. Immunohistochemistry on old archival paraffin blocks: Is there an expiry date? J Clin Pathol. 2017;70(11):988–993. doi: 10.1136/jclinpath-2017-204387. [DOI] [PubMed] [Google Scholar]
- 32.Combs SE, Han G, Mani N, Beruti S, Nerenberg M, Rimm DL. Loss of antigenicity with tissue age in breast cancer. Lab Invest. 2016;96(3):264–269. doi: 10.1038/labinvest.2015.138. [DOI] [PubMed] [Google Scholar]
- 33.Collins LC, Botero ML, Schnitt SJ. Bimodal frequency distribution of estrogen receptor immunohistochemical staining results in breast cancer: an analysis of 825 cases. Am J Clin Pathol. 2005;123(1):16–20. doi: 10.1309/HCF035N9WK40ETJ0. [DOI] [PubMed] [Google Scholar]
- 34.Nadji M, Gomez-Fernandez C, Ganjei-Azar P, Morales AR. Immunohistochemistry of estrogen and progesterone receptors reconsidered: experience with 5993 breast cancers. Am J Clin Pathol. 2005;123(1):21–27. doi: 10.1309/4WV79N2GHJ3X1841. [DOI] [PubMed] [Google Scholar]
- 35.Allott EH, Geradts J, Sun X, Cohen SM, Zirpoli GR, Khoury T, Bshara W, Chen M, Sherman ME, Palmer JR, et al. Intratumoral heterogeneity as a source of discordance in breast cancer biomarker classification. Breast Cancer Res. 2016;18(1):68. doi: 10.1186/s13058-016-0725-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zhang D, Salto-Tellez M, Putti TC, Do E, Koay ES. Reliability of tissue microarrays in detecting protein expression and gene amplification in breast cancer. Mod Pathol. 2003;16(1):79–84. doi: 10.1097/01.MP.0000047307.96344.93. [DOI] [PubMed] [Google Scholar]
- 37.Prat A, Cheang MC, Martín M, Parker JS, Carrasco E, Caballero R, Tyldesley S, Gelmon K, Bernard PS, Nielsen TO, et al. Prognostic significance of progesterone receptor-positive tumor cells within immunohistochemically defined luminal A breast cancer. J Clin Oncol. 2013;31(2):203–209. doi: 10.1200/JCO.2012.43.4134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Andersson J, Linderholm B, Bergh J, Elmberger G. HER-2/neu (c-erbB-2) evaluation in primary breast carcinoma by fluorescent in situ hybridization and immunohistochemistry with special focus on intratumor heterogeneity and comparison of invasive and in situ components. Appl Immunohistochem Mol Morphol. 2004;12(1):14–20. doi: 10.1097/00129039-200403000-00003. [DOI] [PubMed] [Google Scholar]
- 39.Glöckner S, Buurman H, Kleeberger W, Lehmann U, Kreipe H. Marked intratumoral heterogeneity of c-myc and cyclinD1 but not of c-erbB2 amplification in breast cancer. Lab Invest. 2002;82(10):1419–1426. doi: 10.1097/01.LAB.0000032371.16521.40. [DOI] [PubMed] [Google Scholar]
- 40.Lundgren C, Bendahl PO, Borg Å, Ehinger A, Hegardt C, Larsson C, Loman N, Malmberg M, Olofsson H, Saal LH, et al. Agreement between molecular subtyping and surrogate subtype classification: a contemporary population-based study of ER-positive/HER2-negative primary breast cancer. Breast Cancer Res Treat. 2019;178(2):459–467. doi: 10.1007/s10549-019-05378-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bartlett JMS, Bayani J, Marshall A, Dunn JA, Campbell A, Cunningham C, Sobol MS, Hall PS, Poole CJ, Cameron DA, et al. Comparing breast cancer multiparameter tests in the OPTIMA prelim trial: no test is more equal than the others. J Natl Cancer Inst. 2016;108(9):djw050. doi: 10.1093/jnci/djw050. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
Data is not uploaded to a publicly available platform. Researchers have access to data through application to the study PI (malin.sund@umu.se) or the corresponding author under standard rules of protecting data integrity and existing ethics permissions.
Not applicable.