Abstract
Introduction
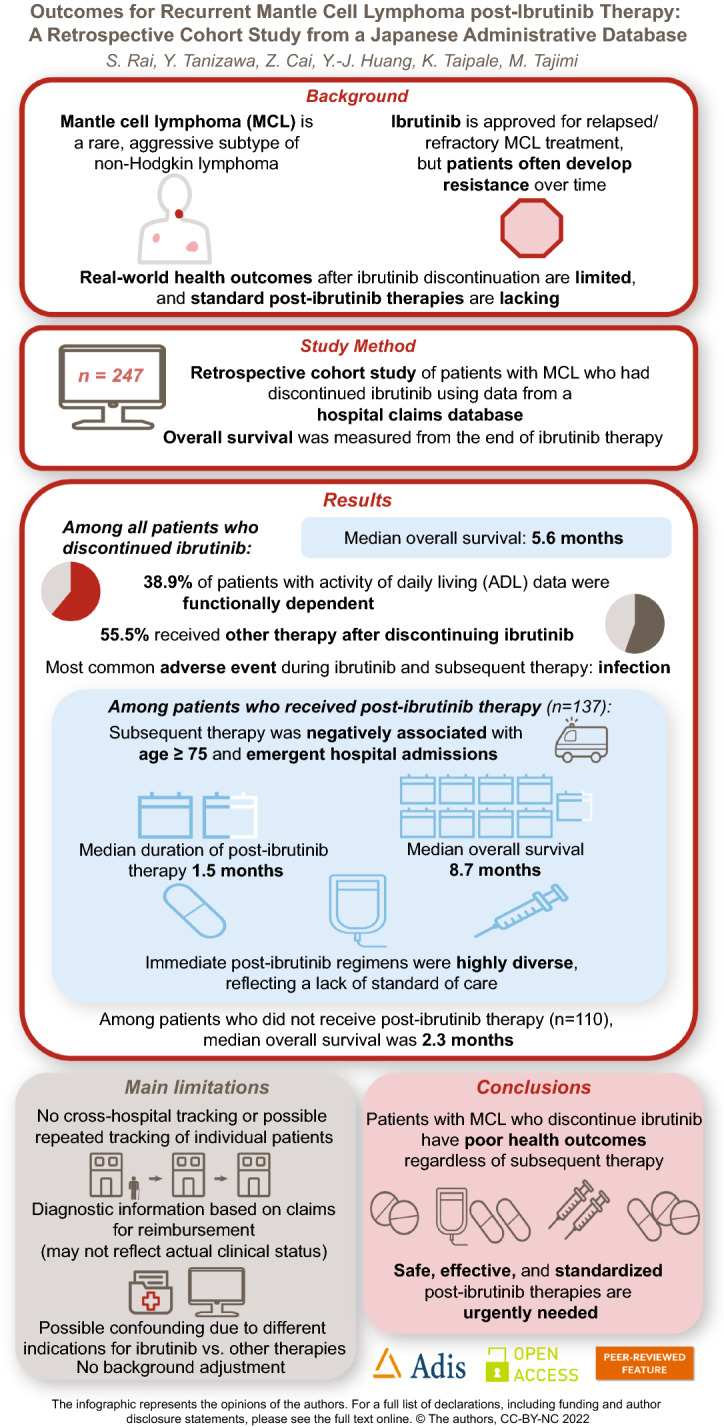
Treatment options in patients with mantle cell lymphoma (MCL) failing ibrutinib are limited, with no standard therapies defined. This study aimed to investigate real-world treatment patterns and outcomes for patients with MCL following ibrutinib.
Methods
This study utilized a de-identified hospital-based claims database (Medical Data Vision) in Japan. Eligible patients were adults who were diagnosed with MCL and had received antitumor drugs between December 2010 and July 2020. Patients were followed from the first antitumor drug treatment until the end of available data up to July 2021. Time-to-event analyses utilized the Kaplan–Meier method. Factors for receiving post-ibrutinib therapy were explored with logistic regression analysis.
Results
Of the 1386 patients who started antitumor drug therapy, 247 patients received and discontinued ibrutinib at any line of therapy. Among them, 137 patients (55.5%) received subsequent therapy. The median age at the end of ibrutinib therapy was 77 (range 42–95), and 44 patients had a dependent activity of daily living (ADL). Factors negatively associated with receiving post-ibrutinib therapy after discontinuation of ibrutinib were age ≥ 75 years (odds ratio [95% CI] 0.46 [0.26–0.80]) and emergency hospital admissions (0.37 [0.17–0.84]). Immediate post-ibrutinib therapy regimens were highly diverse, with BR (bendamustine, rituximab) only prescribed in more than 10% of patients. The median duration of post-ibrutinib therapy was 1.5 months (95% CI 1.07–2.07). The median overall survival from the end of ibrutinib therapy in patients regardless of the receipt of post-ibrutinib therapy (n = 247), in those who did not receive post-ibrutinib therapy (n = 110), and in those who received post-ibrutinib therapy (n = 137) was 5.6 months (95% CI 3.8–8.7), 2.3 months (95% CI 1.2–3.9), and 8.7 months (95% CI 5.6–13.8), respectively. The most common adverse event during post-ibrutinib therapy was infection, with the use of anti-infectives (17%).
Conclusions
Patients with MCL previously treated with ibrutinib have poor ability to carry out ADL and experience very poor outcomes. New safe, effective therapies are needed.
Graphical Abstract
Supplementary Information
The online version contains supplementary material available at 10.1007/s12325-022-02258-3.
Keywords: Ibrutinib, Japan, Mantle cell lymphoma, Real world, Treatment patterns
Key Summary Points
| Why carry out this study? |
| Mantle cell lymphoma (MCL) is an aggressive rare subtype of B-cell non-Hodgkin lymphomas. |
| Ibrutinib is a first-in-class Bruton tyrosine kinase (BTK) inhibitor approved for the treatment of relapsed/refractory MCL; however, its efficacy is limited, and no standard therapies are defined for patients failing ibrutinib therapy. |
| Real-world evidence on treatment patterns and outcomes of patients with relapsed/refractory MCL failing ibrutinib therapy are limited, including data characterizing overall survival (OS) and time to discontinuation. |
| What was learned from the study? |
| Following discontinuation of ibrutinib in patients with MCL, therapies were highly diverse, indicating a lack of defined standard regimens for this patient population. |
| Patients discontinuing ibrutinib therapy experienced poor outcomes, with a median OS of 5.6 months (95% CI 3.8–8.7). |
| For patients with post-ibrutinib therapy, the median time to discontinuation of immediate post-ibrutinib therapy was 1.5 months (95% CI 1.1–2.1). |
Digital Features
This article is published with digital features, including a graphical abstract, to facilitate understanding of the article. To view digital features for this article, go to https://doi.org/10.6084/m9.figshare.20211875
Introduction
Mantle cell lymphoma (MCL) is a rare subtype of B-cell non-Hodgkin lymphomas (NHL) that exhibits heterogeneous clinical behaviour which varies from indolent cases that may be managed with conservative measures, to aggressive cases requiring immediate treatment [1]. Current first-line combination chemotherapies followed by high-dose chemotherapy with autologous stem cell transplantation (ASCT) and introduction of rituximab maintenance have improved patient outcomes, particularly in transplant-eligible patients [2–6]; however, the disease is considered incurable, with frequent relapses, and chemotherapy resistance remains a significant burden [7].
A greater understanding of the molecular pathogenesis of MCL has influenced the development of targeted therapies. The constitutive activation of B-cell receptor (BCR) signalling plays an important role in the development of MCL [8–10], and Bruton tyrosine kinase (BTK), an essential component of BCR signalling [11], has emerged as a therapeutic target. Ibrutinib, a first-in-class BTK inhibitor (BTKi), has exhibited efficacy in patents with relapsed/refractory (r/r) MCL in a large international phase 2 study, with an overall response rate of 68% [12]. Furthermore, a pooled analysis using 370 patients with r/r MCL treated with ibrutinib from three different studies reported that a subset of patients remained on therapy for ≥ 4 years [13, 14]. However, the sustained response to ibrutinib is found to be inadequate in most patients, which is likely attributable to discontinuation of ibrutinib therapy resulting from drug-dependent toxicities, or disease progression mediated by ibrutinib resistance [15–18].
Primary resistance to ibrutinib is observed in approximately one third of patients, while nearly all patients will progress to secondary resistance [15, 19, 20]. Numerous mechanisms of ibrutinib resistance have been put forward including mutations in BTK and upregulation of alternative survival pathways, while other non-BTK mechanisms that are not targeted by ibrutinib therapy are emerging [21]. It has been reported that patients who fail ibrutinib therapy experience poor outcomes, with a median overall survival (OS) less than 10 months [15–17, 22]. However, as the incidence rate of MCL is relatively low, there are limited data on the treatment patterns and clinical outcomes of post-ibrutinib therapy using a large cohort of patients with MCL failing ibrutinib.
Administrative databases generally contain data that are routinely collected in healthcare settings for a variety of purposes; these databases possess a readily available source of real-world data on a large population of unselected patients. Thus, they are valuable for capturing real-world data such as treatment patterns and outcomes, thereby complementing the evidence obtained from clinical trials. Recently, several studies have investigated the treatment patterns and clinical outcomes in patients with MCL using administrative databases [23–26]; however, data specifically in the post-ibrutinib setting are still lacking.
The aim of this study is to examine real-world treatment patterns and outcomes in patients with MCL failing ibrutinib therapy. To the best of our knowledge, this is the first published study using a large-scale administrative claims database, which will increase the understanding of this patient population and provide insights into the development of new safe and effective post-BTKi therapies.
Methods
Database
This was a real-world retrospective, observational analysis using administrative data from the Medical Data Vision Co., Ltd. (MDV) database (Tokyo, Japan) from 1 April 2008 to 31 July 2021. MDV provided de-identified inpatient and outpatient administrative data (claims and Diagnosis Procedure Combination [DPC] data) from acute care hospitals in Japan. Approximately 37.4 million patients from 451 acute-phase hospitals (representing approximately 26% of acute hospitals in Japan) were covered in the MDV database as of October 2021. The MDV provided claims information about patient characteristics including age, sex, treating hospital, diagnosis, prescribed medications, and medical procedures. Some additional clinical information was available in discharge summaries from hospitalizations including height, weight, the 10-item Barthel activities of daily living (ADL) index [27], and records of death events. This study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and are consistent with Good Pharmacoepidemiology Practices (GPPs). As this is a retrospective analysis with use of de-identified data, ethical review and informed consent were not required as per the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects.
Cohort Selection
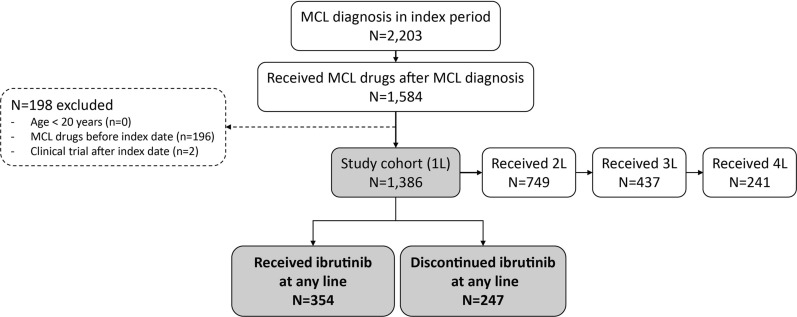
Antitumor drugs that are approved for use in Japan or recommended in the American Society of Hematology (ASH) guidelines or in the National Comprehensive Cancer Network (NCCN) guidelines for treatment of MCL [28, 29] are listed in Supplementary Table 1. MCL patients receiving these antitumor drugs were first identified, and their treatment pattern was then described. Patients were considered eligible if they (1) had ≥ 1 confirmed diagnosis of MCL (International Classification of Diseases, Tenth Revision [ICD-10] code of C83.1) between December 2010 and July 2020 (“index period”), (2) had ≥ 1 prescription for approved or recommended antitumor agents for the treatment of MCL (hereinafter referred to as “MCL drugs”; see details in Supplementary Table 1) in the same month or after the month of first MCL diagnosis identified during the index period (the first prescription date for the MCL agents during the index period was defined as the index date), and (3) were ≥ 20 years of age on the index date. Patients were further excluded if they received any antitumor therapy prior to the index date or if they had records of hospital admission as part of clinical trials on or after the index date. Patients were followed from the index date until the end of available data up to July 2021 (Supplementary Fig. 1). To describe patient characteristics, treatment patterns and outcomes of patients during and after ibrutinib therapy, subgroup cohorts of patients who received and discontinued ibrutinib therapy were defined (Fig. 1).
Fig. 1.
Flow chart of patient selection
Patient Characteristics
Patient characteristics were evaluated during the baseline periods (defined as 90 days prior to the index date) or during ibrutinib therapy. The line of therapy (LOT) was derived based on rules specified in the “Treatment sequence and assignment” section of these methods. Demographic variables included age and sex. Clinical characteristics of interest included the presence of bone marrow involvement and the Charlson Comorbidity Index (CCI) [30] as the total number of predefined comorbid conditions identifiable in the claims based on ICD codes. Discharge summaries from the last hospitalization within the baseline period were used to evaluate the ADL (determined using the 10-item Barthel index [27] to measure functional independence) and body mass index (BMI; based on weight and height data). All 10 items recorded as independent were reported as ADL-independent and any items recorded as not independent were reported as ADL-dependent. ADL was defined as “missing” where any ADL items were missing or unknown.
Treatment Sequence and Assignment
Treatment sequence with MCL drugs was described by LOT (first-line [1L], second-line [2L], third-line [3L], fourth-line and beyond [4L+]). The 1L was defined as the LOT that commenced on the index date. All therapies for MCL prescribed within 30 days from the start of the line (inclusive) constitute the regimen, and each subsequent LOT was defined when the first MCL drug(s) that had not been administered in the prior therapy were prescribed. Each LOT was considered as having ended when the patient terminated all the MCL drugs in the regimen or started a new MCL drug that was not included in the regimen. The start of ibrutinib therapy was separately defined as the first prescription of ibrutinib after starting the 1L therapy, regardless of which LOT it was included in. Post-ibrutinib regimens were categorized based on whether they included bendamustine, cytarabine or bortezomib.
Time-to-Event Outcomes
Time to treatment discontinuation (TTD) was defined as the time between the start and end dates for each of 1L, 2L, and 3L (each LOT for MCL drugs during the index period), treatment line with ibrutinib, and treatment line post-ibrutinib. Patients were considered to have discontinued the LOT if the interval between the end date of the line and the end of hospital data was 90 days or longer, or the patient received the subsequent LOT. Patients without discontinuation based on this definition were censored at the end date of the line. The proportion of patients who received a subsequent LOT was calculated among those who had discontinued (not censored). The date of discharge from the last hospitalization with death outcome was defined as the date of death event, and time from the index date was evaluated. Time to death was censored at the end date of the last hospital visit if the patient did not have a recorded death event.
Supportive Care and Adverse Events
Supportive care utilization and the incidence of adverse events (AEs), defined as the number of new cases (being absent in the baseline period) during the specified time interval, were evaluated for each treatment line and included only patients who discontinued the LOT. Patients were considered to have supportive care if they had ≥ 1 claim associated with a supportive care event during each treatment line. Supportive care of interest included emergency hospital admission, blood transfusion, radiotherapy, procedures for treating arrhythmia, anti-infectives, oral anticoagulants, and drugs for arrhythmia treatment. Patients were considered to have an AE if they had ≥ 1 claim that fulfilled the definition of an AE during each treatment line. AEs of interest were selected based on those observed in clinical trials with ibrutinib treatment [31]. A full list of AEs evaluated and their definitions are provided in Supplementary Table 2.
Statistical Analysis
Descriptive statistics were used for patient, hospital, and treatment characteristics, with n (%) for categorical variables, and mean and standard deviation (SD) for continuous variables. The Kaplan–Meier method was used to determine the median (95% confidence interval [CI]) for time to discontinuation and OS. Multivariable logistic regression analysis was used to identify factors associated with receiving post-ibrutinib therapy among the patients who discontinued ibrutinib therapy. Factors included in the multivariable analysis were age, sex, bone marrow involvement, hospital admission, blood transfusion, radiotherapies, oral anticoagulants, atrial fibrillation/flutter, infection with anti-infectives, gastrointestinal haemorrhage, and duration of ibrutinib therapy. Subgroup analysis on identifying factors associated with receiving post-ibrutinib therapy was conducted on patients with available ADL data. Analyses were performed using Instant Health Data (IHD) software (Panalgo, Boston MA, USA) and R version 3.2.1 (R Foundation for Statistical Computing, Vienna, Austria).
Results
Study Cohort and Patient Characteristics
Of the 2203 patients who had ≥ 1 confirmed claims recorded for MCL diagnosis in the index period (Dec 2010–July 2020) in the MDV database, 1584 patients received therapies for MCL in the same month or after the month of their MCL diagnosis. Of these, 1386 met all eligibility criteria and were included in this study. Of these patients who started the 1L therapy, 749 received a 2L therapy, 437 received a 3L therapy and 241 received a 4L therapy (Fig. 1). The baseline characteristics of these 1386 patients are shown in Table 1. The median age was 72 (range 23–96) and 1033 patients (74.5%) were male. The proportion of patients with bone marrow involvement was 5.7%. The mean CCI for 1L patients was 2.2. Among patients whose ADL data were available from hospitalization records, 13.0% (n = 152/1167) of 1L patients had a dependent ADL (Table 1).
Table 1.
Patient characteristics
| Characteristic | Overall population (N = 1386) | Patients who discontinued ibrutinib therapy (N = 247) |
|---|---|---|
| Age, median years (min–max) | 72 (23–96)c | 77 (42–95)d |
| Age ≥ 65, n (%) | 1082 (78.1)c | 221 (89.5)d |
| Age ≥ 75, n (%) | 560 (40.4)c | 145 (58.7)d |
| Sex, n (%) | ||
| Male | 1033 (74.5) | 183 (74.1) |
| Female | 353 (25.5) | 64 (25.9) |
| Bone marrow involvement, n (%) | 79 (5.7)e | 15 (6.1)f |
| CCI, mean (SD) | 2.2 (1.4)e | 2.8 (1.6)f |
| BMI, mean (SD)a | 22.6 (3.4)e | 22.3 (3.7)f |
| Total ADL independence, n (%)b | ||
| Independent | 1015 (87.0)e | 69 (61.1)f |
| Dependent | 152 (13.0)e | 44 (38.9)f |
aResults were available only from discharge summaries for 1211 patients in the overall population and 119 post-ibrutinib therapy patients
bResults were available only from discharge summaries for 1167 patients in the overall population and 113 post-ibrutinib therapy patients
cEvaluated at index date
dEvaluated at the end of ibrutinib therapy
eEvaluated at baseline before 1L
fEvaluated during ibrutinib therapy
ADL activities of daily living, BMI body mass index, CCI Charlson Comorbidity Index, SD standard deviation
Treatment Patterns
As a 1L therapy, bendamustine and rituximab ([BR]; n = 432, 31.2%) was the most common regimen, followed by R-CHOP ([rituximab, cyclophosphamide, doxorubicin, and prednisolone]; n = 210, 15.2%) (Supplementary Fig. 2). Among patients who discontinued 1L (n = 1326), 2L (n = 687), and 3L (n = 399), the proportion of patients who transitioned to a subsequent LOT was 56.5% (n = 749), 63.6% (n = 437), and 60.4% (n = 241), respectively. Overall, 134 of 1386 patients received hematopoietic cell transplantation after starting the 1L therapy, which included 128 (9.2%) with ASCT and 6 (0.4%) who received cord blood transplantation. The most common regimen among patients who received 2L (N = 749) and 3L (N = 437) therapy was ibrutinib (2L: n = 142 [19.0%], 3L: n = 78 [17.9%]) (Supplementary Fig. 2). Overall, ibrutinib was received by 354 patients at a median dose of 430 mg/day at any line, and the median TTD and OS from the start of ibrutinib therapy at any line were 7.3 months (95% CI 6.3–9.8) and 30.0 months (95% CI 22.0–38.3), respectively.
Patient Characteristics in Patients Failing Ibrutinib Therapy
Among these 354 patients who received ibrutinib at any line, 247 (69.8%) patients discontinued ibrutinib therapy (Fig. 1). The median age at the end of ibrutinib therapy was 77 (range 42–95), and the mean CCI was 2.8. During ibrutinib therapy, the proportion of patients with bone marrow involvement who discontinued ibrutinib was 6.1%. Among patients whose ADL data were available from hospitalization records, 38.9% (n = 44/113) patients who discontinued ibrutinib had a dependent ADL (Table 1).
Treatment Patterns in Patients Failing Ibrutinib Therapy
Of these patients who discontinued ibrutinib (n = 247), 137 patients (55.5%) received subsequent therapy, and the median time from the end of ibrutinib therapy to the start of post-ibrutinib therapy was 5 days (interquartile range [IQR] 1–19). Multivariable logistic regression analysis revealed that factors negatively associated with receiving the post-ibrutinib therapy were age ≥ 75 years at end of ibrutinib therapy (odds ratio [95% CI] 0.46 [0.26–0.80], p = 0.01) and emergency hospital admissions during ibrutinib therapy (odds ratio [95% CI] 0.37 [0.17–0.84, p = 0.02]) (Table 2). Subgroup analysis on patients with ADL data available during ibrutinib therapy (n = 113) revealed that an independent ADL (odds ratio [95% CI] 2.90 [1.09–7.73], p = 0.03) was positively associated with receiving post-ibrutinib therapy (Supplementary Table 3).
Table 2.
Logistic regression analysis of factors associated with receiving immediate post-ibrutinib therapy after discontinuation of ibrutinib therapy (N = 247)
| Covariate | Odds ratio | 95% CI | P value | |
|---|---|---|---|---|
| Age at end of ibrutinib therapy | ≥ 75 vs < 75 | 0.46 | 0.26–0.80 | 0.01 |
| Sex | Male vs females | 1.06 | 0.58–1.96 | 0.84 |
| Bone marrow involvement | Yes vs no | 1.12 | 0.35–3.56 | 0.85 |
| Hospital admission | Yes vs no | 0.37 | 0.17–0.84 | 0.02 |
| Blood transfusions | Yes vs no | 0.50 | 0.24–1.04 | 0.06 |
| Radiotherapies | Yes vs no | 1.33 | 0.39–4.57 | 0.65 |
| Any oral anticoagulants | Yes vs no | 1.25 | 0.41–3.87 | 0.69 |
| Atrial fibrillation and flutter (newly emerged) | Yes vs no | 0.82 | 0.22–3.10 | 0.77 |
| Infection associated with prescription of anti-infectives (newly emerged) | Yes vs no | 0.72 | 0.34–1.53 | 0.40 |
| Gastrointestinal haemorrhage (newly emerged) | Yes vs no | 1.12 | 0.26–4.92 | 0.88 |
| Duration of ibrutinib therapy in months | By 1-month increments | 1.02 | 0.99–1.06 | 0.13 |
All covariates were evaluated during ibrutinib therapy except for age and sex
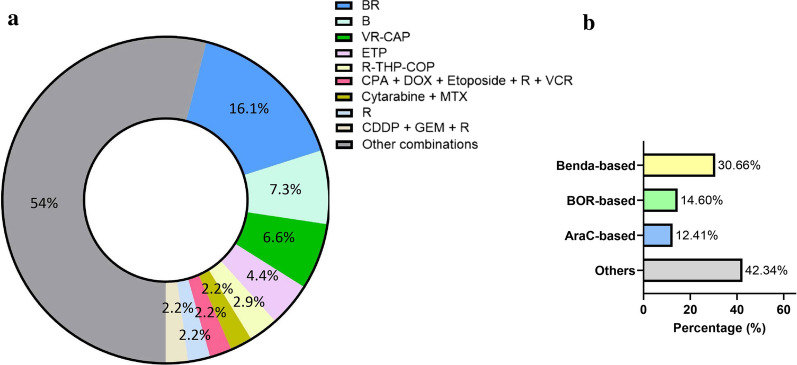
The most common regimens as immediate post-ibrutinib therapy were BR (16.1%), bendamustine monotherapy (7.3%), and VR-CAP (bortezomib, rituximab, cyclophosphamide, doxorubicin, and prednisolone) (6.6%) (Fig. 2a). Based on the backbone chemotherapeutics included in the post-ibrutinib therapies, 30.7% of the patients (n = 42) received bendamustine-based, 14.6% (n = 20) received bortezomib-based, and 12.4% (n = 17) received cytarabine-based therapies (Fig. 2b). Among patients whose ADL data were available from hospitalization records during ibrutinib therapy, the proportion of patients with dependent ADL was 5.6% (n = 1/18) in the patients who received bendamustine-based post-ibrutinib therapy, 33.3% (n = 4/12) among those with bortezomib-based therapies, and 36.4% (n = 4/11) among those with cytarabine-based therapy, while it was 55.3% (n = 26/47) in the patients without post-ibrutinib therapy.
Fig. 2.
Treatment pattern after Ibrutinib discontinuation (N = 137). Proportion of drugs used as post-ibrutinib therapy (a) and classification of post-ibrutinib regimens (b). B, bendamustine; VR-CAP, bortezomib/rituximab/cyclophosphamide/doxorubicin/prednisolone; CHOP, cyclophosphamide/doxorubicin/vincristine/prednisolone; ETP, etoposide; R-THP-COP, rituximab/pirarubicin/cyclophosphamide/vincristine/prednisolone; CPA, cyclophosphamide; DOX, doxorubicin; VCR, bortezomib/cladribine/rituximab; MTX, methotrexate; R, rituximab; CDDP, cisplatin; GEM, gemcitabine; BOR, bortezomib; Benda, bendamustine; AraC, cytarabine
Clinical Outcomes
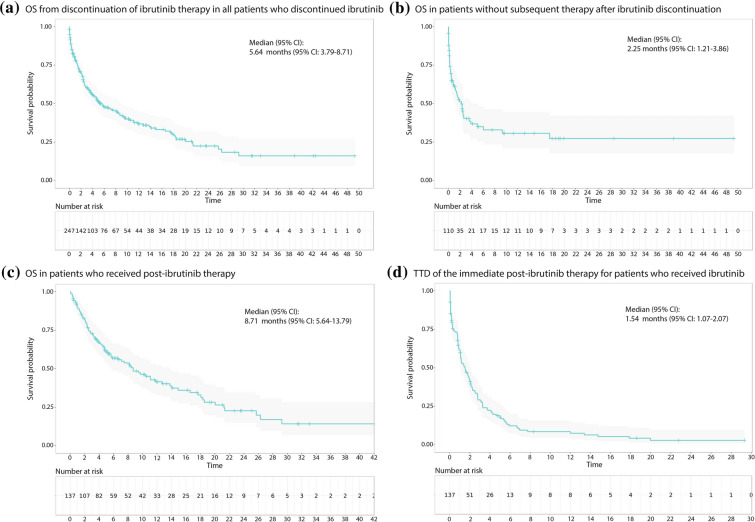
In patients who discontinued ibrutinib (n = 247) at any line, the median OS from the end of ibrutinib therapy regardless of presence of post-ibrutinib therapy was 5.6 months (95% CI 3.8–8.7) (Fig. 3a). When evaluated by the LOT in which ibrutinib was prescribed, the median OS from the end of ibrutinib therapy was 25.7 months (95% CI 11.1-not estimable [NA]), 8.2 months (95% CI 4.0–12.7), and 3.2 months (95% CI 1.8–5.6) for the patients who received ibrutinib as the 1L (N = 54), 2L (N = 93), and 3L or later (N = 100), respectively. Among those who discontinued ibrutinib at any line, the median OS in patients who did not receive post-ibrutinib therapy (n = 110) was 2.3 months (95% CI 1.2–3.9) (Fig. 3b), and in the patients who received post-ibrutinib therapy (n = 137) was 8.7 months (95% CI 5.6–13.8) (Fig. 3c). For patients with post-ibrutinib therapy, the median time to discontinuation of immediate post-ibrutinib therapy was 1.5 months (95% CI 1.1–2.1) (Fig. 3d). The median OS from the end of ibrutinib therapy of 20.0 months (95% CI 6.7-NR) and the median TTD of post-ibrutinib therapy of 2.1 months (95% CI 1.4–5.3) were numerically longest in patients who received bendamustine-based therapy among the major regimen groups based on the backbone chemotherapeutics (Supplementary Fig. 3a, b).
Fig. 3.
Kaplan–Meier survival curves of overall survival (OS) and time to treatment discontinuation (TTD). Kaplan–Meier survival curves for OS from discontinuation of ibrutinib therapy in all patients who discontinued ibrutinib (a), in patients without subsequent therapy after ibrutinib discontinuation (b), and in patients who received post-ibrutinib therapy (c). TTD of the immediate post-ibrutinib therapy (d)
Adverse Events (AE) and Supportive Care
The most common AE of interest during both ibrutinib and post-ibrutinib therapy was infection with documented use of anti-infectives (ibrutinib therapy, 20.2%; post-ibrutinib therapy, 17.0%). Rates of emergency hospital admissions (18.6% vs 5.9%), atrial fibrillation (4.9% vs 1.7%), infection requiring hospitalization (4.9% vs 0.9%), and gastrointestinal haemorrhage (4.1% vs 1.7%) were higher during ibrutinib therapy than post-ibrutinib therapy, respectively (Supplementary Table 4). For supportive care, rates of prescription of anti-infectives (36.8% vs 50.0%), granulocyte colony stimulating factor (1.62% vs 11.9%), and blood transfusions (18.6% vs 37.3%) were higher during post-ibrutinib therapy than ibrutinib therapy, respectively (Supplementary Table 4). When stratified by regimen classification, patients receiving bendamustine-based therapy had lower rates of infection with documented use of anti-infectives (5.9%) and blood transfusions (26.5%) compared with cytarabine-based (31.3% and 43.8%) and bortezomib-based regimens (22.2% and 61.1%), respectively (Supplementary Table 5).
Discussion
Treatment options in patients with MCL failing ibrutinib therapy have been limited, with no standard regimens established in many countries. This study examined real-world treatment patterns and clinical outcomes of post-ibrutinib therapy using a hospital-based administrative database in Japan. Among patients who discontinued ibrutinib (n = 247), 137 patients (55.5%) received subsequent therapies, and the treatment patterns were highly diverse indicating no clear standard of care. Median OS in patients who received the post-ibrutinib therapy was 8.7 months. Thus, there remains an unmet need for safe and effective therapies to improve these dismal outcomes in patients with MCL failing ibrutinib therapy.
1L therapies for patients with MCL were generally in line with the Japanese and NCCN guidelines [29, 32]. In our study, BR and R-CHOP were the most common 1L regimens, which was consistent with a prior real-world analysis in Japan [26] as well as two retrospective US analyses [33, 34]. The most common regimen among patients who received 2L and 3L was ibrutinib. Ibrutinib was also found as 1L therapy in this study regardless of the indication of ibrutinib only for r/r MCL in Japan. This observation may be explained by off-label use or misclassification of LOT caused by limitations of the database that cannot track beyond a single hospital. The proportion of patients who underwent ASCT was 9.2%, which was similar to previous findings which utilized the MDV database in Japan (8.5%) and the Optum Clinformatics Data Mart database in the USA (6.9–7.3%) [26, 35]. However, other studies from the UK and Norway reported higher rates of ASCT of 24% [36, 37]. The differences observed between these studies may be influenced by a number of factors including patient characteristics such as age, differences in regional practices and study variation in calculating rates of ASCT. In the presented study, following discontinuation of treatment with ibrutinib, patients experienced extremely poor outcomes, regardless of the presence of post-ibrutinib therapy, with a median OS after ibrutinib therapy of 5.6 months, which is consistent with previous studies [15–17, 22]. In particular, outcomes in patients who did not receive post-ibrutinib therapy were extremely poor, with a median OS of 2.3 months. Although these results were not surprising, we speculate that failing to receive subsequent therapy likely contributes to this dismal prognosis. In this study, age (≥ 75 years) was a significant risk factor associated with failure of receiving subsequent therapy. Previous studies have reported similar findings [38–40], indicating a lack of effective therapy for this patient population. Other factors indicative of failure to receive post-ibrutinib therapy were emergency hospital admissions and poor ADL during ibrutinib therapy. These findings suggest that improving health status overall may contribute to the success of continuing active antitumor treatment, which may ultimately lead to improved patient outcomes.
Post-ibrutinib regimens were highly diverse, with BR only prescribed in more than 10% of patients. In addition, TTD of immediate post-ibrutinib therapy was 1.5 months (95% CI 1.1–2.1), further indicating a lack of effective standard of care therapy in this setting. It has been reported that specific regimens such as R-BAC (rituximab, bendamustine, cytarabine) have demonstrated a high overall response rate (83%) in the post-BTKi setting [41]. In the present study, only two patients received R-BAC (classified as part of the bendamustine group) as post-ibrutinib treatment. Patients who received bendamustine-based therapy demonstrated numerically longer OS from the end of ibrutinib therapy and TTD of post-ibrutinib therapy. Although this potentially indicates the high effectiveness of bendamustine-based therapy as the immediate post-ibrutinib therapy, it is likely that this result was confounded by the difference in patient background associated with the choice of post-ibrutinib therapy (confounding by indication [42]). For example, we observed that the proportion of the patients with fully dependent ADL was higher with bendamustine-based therapy than other therapies. Additionally, patients receiving bendamustine-based therapy had lower rates of infection with documented use of anti-infectives and blood transfusions. This result suggests that patients with bendamustine-based therapy were in better health condition than those with other therapies, which may have contributed to longer TTD and OS.
It is well known that ibrutinib therapy may be limited by AE [43]. In this study, the most common AE of interest during both ibrutinib and post-ibrutinib therapy was infection with documented use of anti-infectives. Indeed, a retrospective chart review study which included 254 patients on ibrutinib therapy reported an increased risk of infections in MCL relative to other hematologic malignancies [44]. Moreover, patients also experienced cardiac side effects such as atrial fibrillation, consistent with a warning/precaution for cardiac arrhythmias in the prescribing information for ibrutinib [24, 45]. Other frequent AE and supportive care observed in this study may be indicative of high disease and/or treatment burden with the current therapies available after ibrutinib use. Such burdens may have contributed to dose reductions or treatment gaps for patients in this study, resulting in the observed average dose (430 mg/day) which is lower than the standard dose for ibrutinib (560 mg/day).
Several novel targeted therapies have been developed as monotherapy for patients with r/r MCL, including second-generation BTKi (acalabrutinib [46], zanubrutinib [47], pirtobrutinib [48]), a BCL-2 inhibitor (venetoclax [49]), chimeric antigen receptor T-cell (CAR-T) therapy (KTE-X19; [50]), and bispecific antibodies (mosunetuzumab, glofitamab, and epcoritamab; [51]), while combined targeted therapies currently remain under evaluation [52]. Although second-generation BTKi provide greater selectivity and limit off-target toxicity, their efficacy beyond ibrutinib is limited, as they do not overcome mechanisms of resistance [53]. Recently, pirtobrutinib, a highly selective and non-covalent BTKi, showed favourable efficacy among patients with MCL who had prior covalent BTKi therapy in the phase 1/2 BRUIN study [54]. An interim analysis demonstrated that 57% of all patients with MCL remained on treatment, with no evidence of disease progression, and the overall response rate was 52% among patients with prior BTKi exposure [54]. Meanwhile, CAR-T therapy such as KTE-X19 has demonstrated induction of durable remission after failed BTKi therapy in r/r MCL; however, it led to serious and life-threatening toxic events [50]. Additionally, bispecific antibodies targeting CD3 on T-cells and CD20 on malignant B-cells (mosunetuzumab, glofitamab, and epcoritamab) have demonstrated promising efficacy, with favourable toxicity profiles, in r/r MCL [55–57]. These novel therapies have the potential to improve outcomes for r/r MCL, but whether they will have a significant impact for patients with this challenging disease remains unknown.
This study has limitations that were inherent to hospital-based real-world database analysis and should be considered when interpreting the results. The MDV database cannot track patients beyond each participating hospital; therefore, it was possible that patients moved to another hospital in or not in the MDV database, preventing follow-up. Patients may have also been counted multiple times in the database; however, it can be reasonably assumed that patients with refractory MCL have aggressive disease and are elderly, requiring rigorous disease management. Although we were not able to quantify the number of patients who changed hospitals, we presume that they are not likely to change their treating hospitals often; therefore, this limitation may have minimal influence on our results. Some clinical information such as ADL, height and weight, and death were only available from hospitalization records. Diagnostic information was based on claims for reimbursement and may not correctly reflect the actual clinical status of the patients. Confounding by indication [58] was possible, as we observed a difference in patient age and ADL between those who received ibrutinib and those receiving other therapies in the 2L and 3L. Clinical and disease information in the database was poorly populated, and therefore background adjustment for comparison between different treatments was not conducted.
Conclusions
These data add to the body of evidence for patients with MCL receiving ibrutinib therapy, demonstrating that following discontinuation of ibrutinib treatment, subsequent therapy was very diverse, indicating a lack of standard of care. Outcomes of patients receiving and discontinuing ibrutinib, regardless of post-ibrutinib therapy, were poor, which may be due in part to the poor health status of these patients. Safe and effective therapy after progression on ibrutinib is urgently needed to improve the otherwise dismal outcomes of patients with MCL.
Supplementary Information
Below is the link to the electronic supplementary material.
Acknowledgements
Funding
This study, including the journal’s Rapid Service and Open Access fees, was sponsored by Eli Lilly & Company.
Medical Writing Assistance
Medical writing assistance was provided by Garreth Lawrence, Ph.D (Eli Lilly & Company).
Author Contributions
All authors contributed to the study conception and design. Data collection and analysis was performed by Yoshinori Tanizawa and Zhihong Cai. All authors drafted, reviewed, and edited the manuscript. All authors read and approved the final version of the manuscript.
Disclosures
Yoshinori Tanizawa, Zhihong Cai, Yu-Jing Huang, and Masaomi Tajimi are employees and shareholders of Eli Lilly Japan K.K; Kaisa Taipale is an employee and shareholder of Eli Lilly & Company; Shinya Rai has received payment for lectures from Chugai, Eisai, Ono, and Janssen Pharmaceuticals.
Compliance with Ethics Guidelines
This study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with Good Pharmacoepidemiology Practices (GPPs). As this was a retrospective analysis with use of de-identified data, ethical review and informed consent were not required as per the Japanese Ethical Guidelines for Medical and Health Research Involving Human Subjects.
Data Availability
The data in this study belong to Medical Data Vision Co., Ltd (http://www.mdv.co.jp/) and were used under licence, funded by Eli Lilly Japan K.K. As the data are not publicly available, researchers looking to access the data used in this study should contact Medical Data Vision Co., Ltd via their website (http://www.mdv.co.jp/ [Japanese] or https://en.mdv.co.jp/ [English]).
References
- 1.Swerdlow SH, Campo E, Pileri SA, Harris NL, Stein H, Siebert R, et al. The 2016 revision of the World Health Organization classification of lymphoid neoplasms. Blood. 2016;127(20):2375–2390. doi: 10.1182/blood-2016-01-643569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hermine O, Hoster E, Walewski J, Bosly A, Stilgenbauer S, Thieblemont C, et al. Addition of high-dose cytarabine to immunochemotherapy before autologous stem-cell transplantation in patients aged 65 years or younger with mantle cell lymphoma (MCL Younger): a randomised, open-label, phase 3 trial of the European Mantle Cell Lymphoma Network. Lancet. 2016;388(10044):565–575. doi: 10.1016/S0140-6736(16)00739-X. [DOI] [PubMed] [Google Scholar]
- 3.Le Gouill S, Thieblemont C, Oberic L, Moreau A, Bouabdallah K, Dartigeas C, et al. Rituximab after autologous stem-cell transplantation in mantle-cell lymphoma. N Engl J Med. 2017;377(13):1250–1260. doi: 10.1056/NEJMoa1701769. [DOI] [PubMed] [Google Scholar]
- 4.Zoellner AK, Unterhalt M, Stilgenbauer S, Hübel K, Thieblemont C, Metzner B, et al. Long-term survival of patients with mantle cell lymphoma after autologous haematopoietic stem-cell transplantation in first remission: a post-hoc analysis of an open-label, multicentre, randomised, phase 3 trial. Lancet Haematol. 2021;8(9):e648–e657. doi: 10.1016/S2352-3026(21)00195-2. [DOI] [PubMed] [Google Scholar]
- 5.Dreyling M, Lenz G, Hoster E, Van Hoof A, Gisselbrecht C, Schmits R, et al. Early consolidation by myeloablative radiochemotherapy followed by autologous stem cell transplantation in first remission significantly prolongs progression-free survival in mantle-cell lymphoma: results of a prospective randomized trial of the European MCL Network. Blood. 2005;105(7):2677–2684. doi: 10.1182/blood-2004-10-3883. [DOI] [PubMed] [Google Scholar]
- 6.Ogura M, Yamamoto K, Morishima Y, Wakabayashi M, Tobinai K, Ando K, et al. R-High-CHOP/CHASER/LEED with autologous stem cell transplantation in newly diagnosed mantle cell lymphoma: JCOG0406 STUDY. Cancer Sci. 2018;109(9):2830–2840. doi: 10.1111/cas.13719. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Eskelund CW, Kolstad A, Jerkeman M, Räty R, Laurell A, Eloranta S, et al. 15-year follow-up of the second nordic mantle cell lymphoma trial (MCL2): prolonged remissions without survival plateau. Br J Haematol. 2016;175(3):410–418. doi: 10.1111/bjh.14241. [DOI] [PubMed] [Google Scholar]
- 8.Wiestner A. Targeting B-Cell receptor signaling for anticancer therapy: the Bruton's tyrosine kinase inhibitor ibrutinib induces impressive responses in B-cell malignancies. J Clin Oncol. 2013;31(1):128–130. doi: 10.1200/JCO.2012.44.4281. [DOI] [PubMed] [Google Scholar]
- 9.Jares P, Colomer D, Campo E. Molecular pathogenesis of mantle cell lymphoma. J Clin Invest. 2012;122(10):3416–3423. doi: 10.1172/JCI61272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Young RM, Staudt LM. Targeting pathological B cell receptor signalling in lymphoid malignancies. Nat Rev Drug Discov. 2013;12(3):229–243. doi: 10.1038/nrd3937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Buggy JJ, Elias L. Bruton tyrosine kinase (BTK) and its role in B-cell malignancy. Int Rev Immunol. 2012;31(2):119–132. doi: 10.3109/08830185.2012.664797. [DOI] [PubMed] [Google Scholar]
- 12.Wang ML, Rule S, Martin P, Goy A, Auer R, Kahl BS, et al. Targeting BTK with ibrutinib in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2013;369(6):507–516. doi: 10.1056/NEJMoa1306220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rule S, Dreyling M, Goy A, Hess G, Auer R, Kahl B, et al. Ibrutinib for the treatment of relapsed/refractory mantle cell lymphoma: extended 3.5-year follow up from a pooled analysis. Haematologica. 2019;104(5):e211-e4. [DOI] [PMC free article] [PubMed]
- 14.Rule S, Dreyling M, Goy A, Hess G, Auer R, Kahl B, et al. Outcomes in 370 patients with mantle cell lymphoma treated with ibrutinib: a pooled analysis from three open-label studies. Br J Haematol. 2017;179(3):430–438. doi: 10.1111/bjh.14870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Martin P, Maddocks K, Leonard JP, Ruan J, Goy A, Wagner-Johnston N, et al. Postibrutinib outcomes in patients with mantle cell lymphoma. Blood. 2016;127(12):1559–1563. doi: 10.1182/blood-2015-10-673145. [DOI] [PubMed] [Google Scholar]
- 16.Jain P, Kanagal-Shamanna R, Zhang S, Ahmed M, Ghorab A, Zhang L, et al. Long-term outcomes and mutation profiling of patients with mantle cell lymphoma (MCL) who discontinued ibrutinib. Br J Haematol. 2018;183(4):578–587. doi: 10.1111/bjh.15567. [DOI] [PubMed] [Google Scholar]
- 17.Epperla N, Hamadani M, Cashen AF, Ahn KW, Oak E, Kanate AS, et al. Predictive factors and outcomes for ibrutinib therapy in relapsed/refractory mantle cell lymphoma-a “real world” study. Hematol Oncol. 2017;35(4):528–535. doi: 10.1002/hon.2380. [DOI] [PubMed] [Google Scholar]
- 18.Shah B, Zhao X, Silva AS, Shain KH, Tao J. Resistance to Ibrutinib in B cell malignancies: one size does not fit all. Trends Cancer. 2018;4(3):197–206. doi: 10.1016/j.trecan.2018.01.004. [DOI] [PubMed] [Google Scholar]
- 19.Roué G, Sola B. Management of drug resistance in mantle cell lymphoma. Cancers (Basel) 2020;12(6):1565. doi: 10.3390/cancers12061565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hershkovitz-Rokah O, Pulver D, Lenz G, Shpilberg O. Ibrutinib resistance in mantle cell lymphoma: clinical, molecular and treatment aspects. Br J Haematol. 2018;181(3):306–319. doi: 10.1111/bjh.15108. [DOI] [PubMed] [Google Scholar]
- 21.George B, Chowdhury SM, Hart A, Sircar A, Singh SK, Nath UK, et al. Ibrutinib resistance mechanisms and treatment strategies for B-cell lymphomas. Cancers (Basel). 2020;12(5). [DOI] [PMC free article] [PubMed]
- 22.Cheah CY, Chihara D, Romaguera JE, Fowler NH, Seymour JF, Hagemeister FB, et al. Patients with mantle cell lymphoma failing ibrutinib are unlikely to respond to salvage chemotherapy and have poor outcomes. Ann Oncol. 2015;26(6):1175–1179. doi: 10.1093/annonc/mdv111. [DOI] [PubMed] [Google Scholar]
- 23.Weaver JA, Peng Y, Ji Y, Gilbertson D, Pease DF, Morrison VA. A medicare database analysis of practice patterns in patients with mantle cell lymphoma. J Geriatr Oncol. 2021;12(6):894–901. doi: 10.1016/j.jgo.2020.12.013. [DOI] [PubMed] [Google Scholar]
- 24.Goyal RK, Jain P, Nagar SP, Le H, Kabadi SM, Davis K, et al. Real-world evidence on survival, adverse events, and health care burden in Medicare patients with mantle cell lymphoma. Leuk Lymphoma. 2021;62(6):1325–1334. doi: 10.1080/10428194.2021.1919662. [DOI] [PubMed] [Google Scholar]
- 25.Sharman J, Kabadi SM, Clark J, Andorsky D. Treatment patterns and outcomes among mantle cell lymphoma patients treated with ibrutinib in the United States: a retrospective electronic medical record database and chart review study. Br J Haematol. 2021;192(4):737–746. doi: 10.1111/bjh.16922. [DOI] [PubMed] [Google Scholar]
- 26.Izutsu K, Suzumiya J, Takizawa J, Fukase K, Nakamura M, Jinushi M, et al. Real world treatment practices for mantle cell lymphoma in Japan: an observational database research study (CLIMBER-DBR) J Clin Exp Hematop. 2021;61(3):135–144. doi: 10.3960/jslrt.20056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Wade DT, Collin C. The Barthel ADL Index: a standard measure of physical disability? Int Disabil Stud. 1988;10(2):64–67. doi: 10.3109/09638288809164105. [DOI] [PubMed] [Google Scholar]
- 28.Okamoto M, Kusumoto S. JSH practical guidelines for hematological malignancies, 2018: II. Lymphoma-4. Mantle cell lymphoma (MCL). Int J Hematol. 2020;111(1):5–15. [DOI] [PubMed]
- 29.Zelenetz AD, Gordon LI, Chang JE, et al. NCCN Guidelines® Insights: B-Cell Lymphomas, Version 5.2021. Compr Canc Netw. 2021;19(11):1218–1230. doi: 10.6004/jnccn.2021.0054. [DOI] [PubMed] [Google Scholar]
- 30.Quan H, Sundararajan V, Halfon P, Fong A, Burnand B, Luthi JC, et al. Coding algorithms for defining comorbidities in ICD-9-CM and ICD-10 administrative data. Med Care. 2005;43(11):1130–1139. doi: 10.1097/01.mlr.0000182534.19832.83. [DOI] [PubMed] [Google Scholar]
- 31.Paydas S. Management of adverse effects/toxicity of ibrutinib. Crit Rev Oncol Hematol. 2019;136:56–63. doi: 10.1016/j.critrevonc.2019.02.001. [DOI] [PubMed] [Google Scholar]
- 32.National Comprehensive Cancer Network (NCCN) Guidelines for Patients. Mantle Cell Lymhoma. 2021. Available at https://www.nccn.org/patients/guidelines/content/PDF/Mantle-patient-guideline.pdf.
- 33.Kabadi SM, Near A, Wada K, Burudpakdee C. Treatment patterns, adverse events, healthcare resource use and costs among commercially insured patients with mantle cell lymphoma in the United States. Cancer Med. 2019;8(17):7174–7185. doi: 10.1002/cam4.2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sharman J, Kabadi SM, Clark J, Andorsky D. Treatment patterns and outcomes among mantle cell lymphoma patients treated with ibrutinib in the United States: a retrospective electronic medical record database and chart review study. Br J Haematol. 2021;192(4):737–746. doi: 10.1111/bjh.16922. [DOI] [PubMed] [Google Scholar]
- 35.Ghosh N, Emond B, Lafeuille MH, Côté-Sergent A, Lefebvre P, Huang Q. Treatment patterns among patients with mantle cell lymphoma and comparison of healthcare resource utilization and costs among relapsed/refractory patients treated with ibrutinib or chemoimmunotherapy: a real-world retrospective study. Clin Ther. 2021;43(8):1285–1299. doi: 10.1016/j.clinthera.2021.06.012. [DOI] [PubMed] [Google Scholar]
- 36.McCulloch R, Lewis D, Crosbie N, Eyre TA, Bolam S, Arasaretnam A, et al. Ibrutinib for mantle cell lymphoma at first relapse: a United Kingdom real-world analysis of outcomes in 211 patients. Br J Haematol. 2021;193(2):290–298. doi: 10.1111/bjh.17363. [DOI] [PubMed] [Google Scholar]
- 37.Abrahamsson A, Albertsson-Lindblad A, Brown PN, Baumgartner-Wennerholm S, Pedersen LM, D’Amore F, et al. Real world data on primary treatment for mantle cell lymphoma: a nordic lymphoma group observational study. Blood. 2014;124(8):1288–1295. doi: 10.1182/blood-2014-03-559930. [DOI] [PubMed] [Google Scholar]
- 38.Pfreundschuh M. Age and sex in non-hodgkin lymphoma therapy: it's not all created equal, or is it? Am Soc Clin Oncol Educ Book. 2017;37:505–511. doi: 10.1200/EDBK_175447. [DOI] [PubMed] [Google Scholar]
- 39.Karmali R, Switchenko JM, Goyal S, Shanmugasundaram K, Churnetski MC, Kolla B, et al. Multi-center analysis of practice patterns and outcomes of younger and older patients with mantle cell lymphoma in the rituximab era. Am J Hematol. 2021;96(11):1374–1384. doi: 10.1002/ajh.26306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Rampotas A, Wilson MR, Lomas O, Denny N, Leary H, Ferguson G, et al. Treatment patterns and outcomes of unfit and elderly patients with Mantle cell lymphoma unfit for standard immunochemotherapy: a UK and Ireland analysis. Br J Haematol. 2021;194(2):365–377. doi: 10.1111/bjh.17513. [DOI] [PubMed] [Google Scholar]
- 41.McCulloch R, Visco C, Eyre TA, Frewin R, Phillips N, Tucker DL, et al. Efficacy of R-BAC in relapsed, refractory mantle cell lymphoma post BTK inhibitor therapy. Br J Haematol. 2020;189(4):684–688. doi: 10.1111/bjh.16416. [DOI] [PubMed] [Google Scholar]
- 42.McMahon AD, MacDonald TM. Design issues for drug epidemiology. Br J Clin Pharmacol. 2000;50(5):419–425. doi: 10.1046/j.1365-2125.2000.00289.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.O'Brien SM, Brown JR, Byrd JC, Furman RR, Ghia P, Sharman JP, et al. Monitoring and managing BTK inhibitor treatment-related adverse events in clinical practice. Front Oncol. 2021;11:720704 [DOI] [PMC free article] [PubMed]
- 44.Holowka T, Cheung H, Malinis MF, Perreault S, Isufi I, Azar MM. 1092. Increased risk of serious infections in mantle cell lymphoma versus other hematologic malignancies in patients on ibrutinib. Open Forum Infect Dis. 2020;7(Suppl 1):S575-S6.
- 45.Mato AR, Nabhan C, Thompson MC, Lamanna N, Brander DM, Hill B, et al. Toxicities and outcomes of 616 ibrutinib-treated patients in the United States: a real-world analysis. Haematologica. 2018;103(5):874–879. doi: 10.3324/haematol.2017.182907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Girard J, Reneau J, Devata S, Wilcox RA, Kaminski MS, Mercer J, et al. Evaluating acalabrutinib in the treatment of mantle cell lymphoma: design, development, and place in therapy. Onco Targets Ther. 2019;12:8003–8014. doi: 10.2147/OTT.S155778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Song Y, Zhou K, Zou D, Zhou J, Hu J, Yang H, et al. Treatment of patients with relapsed or refractory mantle-cell lymphoma with zanubrutinib, a selective inhibitor of Bruton's tyrosine kinase. Clin Cancer Res. 2020;26(16):4216–4224. doi: 10.1158/1078-0432.CCR-19-3703. [DOI] [PubMed] [Google Scholar]
- 48.Mato AR, Shah NN, Jurczak W, Cheah CY, Pagel JM, Woyach JA, et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. Lancet. 2021;397(10277):892–901. doi: 10.1016/S0140-6736(21)00224-5. [DOI] [PubMed] [Google Scholar]
- 49.Eyre TA, Walter HS, Iyengar S, Follows G, Cross M, Fox CP, et al. Efficacy of venetoclax monotherapy in patients with relapsed, refractory mantle cell lymphoma after Bruton tyrosine kinase inhibitor therapy. Haematologica. 2019;104(2):e68–e71. doi: 10.3324/haematol.2018.198812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wang M, Munoz J, Goy A, Locke FL, Jacobson CA, Hill BT, et al. KTE-X19 CAR T-cell therapy in relapsed or refractory mantle-cell lymphoma. N Engl J Med. 2020;382(14):1331–1342. doi: 10.1056/NEJMoa1914347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bock AM, Nowakowski GS, Wang Y. Bispecific antibodies for non-hodgkin lymphoma treatment. Curr Treat Options Oncol. 2022;23(2):155–170. doi: 10.1007/s11864-021-00925-1. [DOI] [PubMed] [Google Scholar]
- 52.Silkenstedt E, Linton K, Dreyling M. Mantle cell lymphoma - advances in molecular biology, prognostication and treatment approaches. Br J Haematol. 2021;195(2):162–173. doi: 10.1111/bjh.17419. [DOI] [PubMed] [Google Scholar]
- 53.Bond DA, Woyach JA. Targeting BTK in CLL: beyond ibrutinib. Curr Hematol Malig Rep. 2019;14(3):197–205. doi: 10.1007/s11899-019-00512-0. [DOI] [PubMed] [Google Scholar]
- 54.Mato AR, Shah NN, Jurczak W, Cheah CY, Pagel JM, Woyach JA, et al. Pirtobrutinib in relapsed or refractory B-cell malignancies (BRUIN): a phase 1/2 study. The Lancet. 2021;397(10277):892–901. doi: 10.1016/S0140-6736(21)00224-5. [DOI] [PubMed] [Google Scholar]
- 55.Budde LE, Assouline S, Sehn LH, Schuster SJ, Yoon SS, Yoon DH, et al. Single-agent mosunetuzumab shows durable complete responses in patients with relapsed or refractory B-Cell lymphomas: phase i dose-escalation study. J Clin Oncol. 2022;40(5):481–491. doi: 10.1200/JCO.21.00931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Hutchings M, Mous R, Clausen MR, Johnson P, Linton KM, Chamuleau MED, et al. Dose escalation of subcutaneous epcoritamab in patients with relapsed or refractory B-cell non-Hodgkin lymphoma: an open-label, phase 1/2 study. Lancet. 2021;398(10306):1157–1169. doi: 10.1016/S0140-6736(21)00889-8. [DOI] [PubMed] [Google Scholar]
- 57.Hutchings M, Morschhauser F, Iacoboni G, Carlo-Stella C, Offner FC, Sureda A, et al. Glofitamab, a novel, bivalent CD20-targeting T-cell-engaging bispecific antibody, induces durable complete remissions in relapsed or refractory B-cell lymphoma: a phase i trial. J Clin Oncol. 2021;39(18):1959–1970. doi: 10.1200/JCO.20.03175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Brookhart MA, Stürmer T, Glynn RJ, Rassen J, Schneeweiss S. Confounding control in healthcare database research: challenges and potential approaches. Med Care. 2010;48(6 Suppl):S114–S120. doi: 10.1097/MLR.0b013e3181dbebe3. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data in this study belong to Medical Data Vision Co., Ltd (http://www.mdv.co.jp/) and were used under licence, funded by Eli Lilly Japan K.K. As the data are not publicly available, researchers looking to access the data used in this study should contact Medical Data Vision Co., Ltd via their website (http://www.mdv.co.jp/ [Japanese] or https://en.mdv.co.jp/ [English]).