Abstract
Non-alcoholic fatty liver disease (NAFLD) is considered to be the hepatic manifestation of the metabolic syndrome and is characterized by ectopic accumulation of triglycerides in the cytoplasm of hepatocytes, i.e., steatosis. NAFLD has become the most common chronic liver disease, with an estimated global prevalence of 25%. Although the majority of NAFLD patients will never experience liver-related complications, the progressive potential of NAFLD is indisputable, with 5–10% of subjects progressing to cirrhosis, end-stage liver disease, or hepatocellular carcinoma. NAFLD patients with advanced fibrosis are at the highest risk of developing cardiovascular and cirrhosis-related complications. Liver biopsy has hitherto been considered the reference method for evaluation of hepatic steatosis and fibrosis stage. Given the limitations of biopsy for widescale screening, non-invasive tests (NITs) for assessment of steatosis and fibrosis stage, including serum-based algorithms and ultrasound- and magnetic resonance-based methods, will play an increasing role in the management of NAFLD patients. This comprehensive review presents the advantages and limitations of NITs for identification of steatosis and advanced fibrosis in NAFLD. The clinical implications of using NITs to identify and manage NAFLD patients are also discussed.
Keywords: Fibrosis, Non-alcoholic fatty liver disease, Non-invasive tests, Steatosis
Introduction
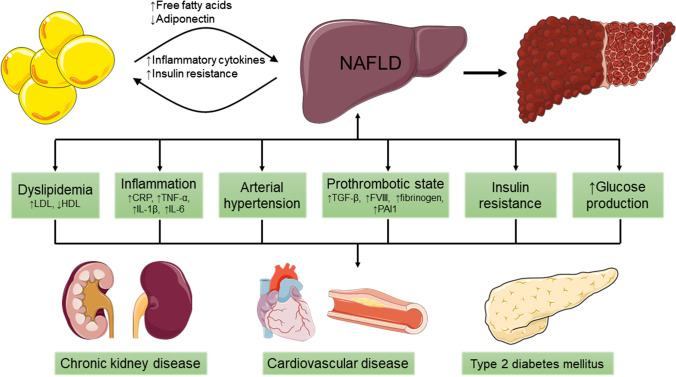
Non-alcoholic fatty liver disease (NAFLD) affects approximately 25% of the global population [1] and has in recent years surpassed viral hepatitis as a major cause of chronic liver disease [2, 3]. NAFLD is the hepatic manifestation of the metabolic syndrome and encompasses a spectrum of histopathological features that range from ectopic accumulation of triglycerides in the cytoplasm of hepatocytes (i.e., steatosis) via establishment of inflammation and hepatocellular injury (i.e., non-alcoholic steatohepatitis (NASH)), to progressive fibrosis and risk of progression to cirrhosis and development of end-stage liver disease (ESLD) or hepatocellular carcinoma (HCC) [4, 5]. The presence of NAFLD is associated with type 2 diabetes mellitus (T2DM), as well as with a greater prevalence and incidence of cardiovascular disease and chronic kidney disease [6] (Fig. 1). In patients with T2DM, NAFLD prevalence ranges from 70 to 95%, while the rate is even higher in morbid obesity at up to 98% [1]. Subjects with T2DM are particularly susceptible to more severe forms of NAFLD and its associated consequences [7, 8] as they have a higher prevalence of advanced fibrosis compared to the general population [9]. The swift increase in the prevalence of NAFLD is also mirrored in the incidence of ESLD. In the USA, NAFLD is currently the second leading indication for liver transplantation [10] and the most rapidly growing cause of HCC among subjects listed for liver transplantation [11]. A modeling study suggests that by 2030, the prevalence of NASH will have risen by as much as 56%, with ESLD and mortality expected to more than double [12].
Fig. 1.
NAFLD is associated with an increased risk of developing chronic kidney disease, cardiovascular disease, and type 2 diabetes mellitus. There are several putative mechanisms underlying this association. NAFLD contributes to an atherogenic milieu (dyslipidemia with decreased HDL and increased LDL) and a prothrombotic state (increased levels of TGF-β, fibrinogen, factor VIII, and PAI-1), as well as arterial hypertension, hepatic and systemic insulin resistance, increased glucose production, and increased secretion of proinflammatory biomarkers (such as CRP, IL6, IL-1β, and TNF-α). Furthermore, several of these pathological mechanisms seem to have a bidirectional effect, both increasing the presence of NAFLD and consequently increasing the risk of NAFLD progression to NASH and cirrhosis. Images from Servier Medical Art (https://smart.servier.com/). Abbreviations: CRP, C-reactive protein; FVIII, factor VIII; HDL, high-density lipoprotein; IL-1β, interleukin-1β; IL-6, interleukin-6; LDL, low-density lipoprotein; NAFLD, non-alcoholic fatty liver disease; NASH, non-alcoholic steatohepatitis; PAI-1, plasminogen activator inhibitor-1; TGF-β, transforming growth factor-β; TNF-α, tumor necrosis factor-α
Liver biopsy is considered the reference method for diagnosing hepatic steatosis. Moreover, histologic evaluation of liver tissue enables assessment of the presence of NASH with or without advanced fibrosis. However, liver biopsy sampling represents only approximately 1/50,000 of the organ volume and has its own inherent limitations, such as cost, sampling errors due to inadequate sample acquisition, incorrect sample representation, observer variability, and risk of adverse events, making it unsuitable for large-scale screening [13]. Therefore, simple, easily accessible, and validated non-invasive tests (NITs) are of utmost importance and much needed in the management of NAFLD patients.
Presence of hepatic steatosis is the prerequisite for diagnosing NAFLD [14]. Therefore, this review will first focus on the non-invasive diagnosis of steatosis. Although the severity of steatosis is associated with development of T2DM and mortality [15], hepatic fibrosis has been shown to be the key independent predictor that is associated with all-cause, cardiovascular, and liver-related mortality in NAFLD patients [16–19]. Lobular inflammation and hepatocellular ballooning, included in the histopathological NAFLD activity score (NAS), have not been shown to predict progression of fibrosis or mortality [17, 18]. After the diagnosis of steatosis, the stage of fibrosis should be assessed appropriately. In clinical practice, identification of NAFLD patients with advanced fibrosis (F3-F4) is a priority as these patients are at higher risk of mortality and development of liver-related complications [20]. It is widely accepted that NAFLD patients with advanced fibrosis should be followed up in secondary care and should be prioritized for treatment once medications for NAFLD are recommended. Thus, this review will also focus on NITs to diagnose advanced fibrosis in NAFLD.
The exclusion of other liver diseases, including “significant” alcohol consumption, is necessary to establish a diagnosis of NAFLD. A panel of international experts recently suggested an alternative approach, i.e., “positive criteria” to diagnose the liver disease associated with the metabolic syndrome, while they also proposed the name metabolic dysfunction-associated fatty liver disease (MAFLD) [21]. The criteria are based on evidence of hepatic steatosis (detected either by imaging techniques, blood biomarkers/scores, or liver histology), in addition to one of the following three criteria, namely, overweight/obesity, presence of T2DM, or evidence of metabolic dysregulation. In contrast to NAFLD, MAFLD can coexist with other chronic liver diseases, for example, viral hepatitis or alcohol-related liver disease. To our knowledge, there are to date no large studies that have evaluated the performance of NITs in MAFLD using the proposed definition. Thus, it is currently unknown if the data presented in this review can be extrapolated to MAFLD.
Steatosis
Hepatic triglyceride content (HTGC) can be assessed using various methods that measure fundamentally different tissue properties. The conventional histopathological methodology used to quantify liver fat content consists of a visual semiquantitative approach in which the histopathologist uses a four-graded scale (0–3). Grades 0–3 are considered to correspond to fat deposition in < 5%, 5–33%, 34–66%, and > 66% of the hepatocytes, respectively [22, 23]. The diagnosis of NAFLD requires that ≥ 5% of the hepatocytes contain fat globules in the absence of overconsumption of alcohol and other (secondary) causes of steatosis. It has previously been demonstrated that semiquantitative assessment of steatosis by a histopathologist frequently overestimates hepatic fat content when measured quantitatively [24]. An alternative approach is to assess steatosis quantitatively with stereological point counting, a method with higher reproducibility suggested as being preferable when accurate histopathological measurements of hepatic steatosis are required [24]. Both the semiquantitative histological method and stereological point counting rely on biopsies and, as such, are invasive tests, which issue will not be further discussed in this review.
Serum-based steatosis biomarkers
Although levels of alanine aminotransferase (ALT) are the best single biochemical correlate of hepatic steatosis, serum levels of hepatic enzymes can be within reference limits in 50–79% of NAFLD patients, fluctuating or slightly elevated [25, 26]. There have been suggestions that the current upper limit of normal (ULN) for ALT may be too high and should be reduced significantly [27, 28]. Slight to moderate elevation of serum ferritin can be caused by dysmetabolic iron overload associated with steatosis. However, in many NAFLD patients, elevated ferritin may reflect a subclinical inflammatory state rather than iron overload [29]. In general, a single biomarker cannot be used to identify NAFLD patients.
Several panels in which serum-based biomarkers are included have been evaluated as predictors of hepatic steatosis. Among them are fatty liver index (FLI) [30], hepatic steatosis index (HSI) [31], NAFLD liver fat score (NAFLD-LFS) [32], SteatoTest [33], visceral adiposity index (VAI) [34], triglyceride × glucose (TyG) index [35], and lipid accumulation product (LAP) [36, 37]. The diagnostic performance of these tests is summarized in Table 1. It should be noted that several of these tests have been evaluated using ultrasound as the gold standard to diagnose steatosis. However, ultrasound lacks sensitivity for detection of low grades of steatosis (see below) and, thus, the diagnostic performance of HSI, FLI, and LAP has probably been overestimated. In a study in which ultrasonography, SteatoTest, and liver biopsy were performed in 304 patients, it was shown that concordance between steatosis diagnosed both with ultrasonography and histopathologically was lower (kappa coefficient = 0.32 ± 0.05) than the concordance between SteatoTest and biopsy (kappa coefficient = 0.44 ± 0.06; P = 0.02) [33], indicating that both ultrasonography and serum-based tests have suboptimal sensitivity in detecting steatosis.
Table 1.
Serum-based steatosis markers
| Blood markers/algorithms | Components or formulas | AUROC | Study population (no. of participants)/diagnostic tools |
|---|---|---|---|
| Hepatic steatosis index[31] | 8 × ALT/AST + BMI (+ 2, if type 2 diabetes; + 2, if female) | 0.72–0.82 | Korean (n = 10,724)/ultrasound |
| Fatty liver index[30] |
(e0.953 × ln (TG) + 0.139*BMI + 0.718 × ln (GGT) + 0.053 × WC − 15.745)/(1 + e 0.953 × ln (TG) + 0.139 × BMI + 0.718 × ln (GGT) + 0.053 × WC − 15.745) × 100 |
0.79–0.85 | Italian (n = 496)/ultrasound |
| NAFLD liver fat score[32] | − 2.89 + 1.18 × metabolic syndrome (yes = 1/no = 0) + 0.45 × type 2 diabetes (yes = 2/no = 0) + 0.15 × insulin (mU/L) + 0.04 × AST − 0.94 × AST/ALT | 0.78–0.87 | Finnish (n = 470)/1H-MRS |
| SteatoTest[33] | ALT, α2-macroglobulin, apolipoprotein A1, haptoglobin, total bilirubin, GGT, total cholesterol, TG, glucose, age, gender, BMI | 0.72–0.86 |
Caucasians (n = 2272)/liver biopsy Patented test. The model equation was not presented |
| Lipid accumulation product (LAP)[36, 37] | [WC (cm)–65 (male) or –58 (female)] × [TG (mmol/L)] | 0.72–0.83 |
NHANES III (development, n = 9180), Italian (validation, n = 588)/ultrasound (in evaluation study) Originally developed as an index of cardiometabolic risk |
| Visceral adiposity index[34] |
Male: [WC/39.68 + (1.88 × BMI)] × (TG/1.03) × (1.31/HDL) Female: [WC/36.58 + (1.89 × BMI) × (TG/0.81) × (1.52/HDL) |
0.92 |
Validated in French NAFLD patients (n = 324)/liver biopsy Originally developed in patients with hepatitis C |
| Triglyceride/glucose index[35] | Log (TG × glucose/2) | 0.90 |
Validated in French NAFLD patients (n = 324)/liver biopsy Originally developed as a measure of insulin sensitivity |
Abbreviations: ALT, alanine aminotransferase; AST, aspartate aminotransferase; AUROC, area under the receiver operating characteristic; BMI, body mass index; GGT, gamma glutamyltransferase; HDL, high-density lipoprotein; NAFLD, non-alcoholic fatty liver disease; TG, triglycerides; WC, waist circumference
In a head-to-head comparison using liver biopsy as the reference standard, VAI outperformed four other algorithms with an area under the receiver operating characteristic (AUROC) of 0.92, a sensitivity of 79%, specificity of 92%, negative predictive value (NPV) of 16%, and positive predictive value (PPV) of 99% [38].
Given their diagnostic performance, the algorithms may have a role in identifying hepatic steatosis or cardiometabolic risk factors in population-based studies [36], but currently, they cannot be used in clinical practice for the diagnosis of NAFLD or in the decision-making process in the management of NAFLD patients. Further studies are needed to determine which algorithm(s) can be recommended as an initial tool for NAFLD screening. Moreover, it has not yet been clarified if these algorithms can be used for monitoring patients during interventions aiming to reduce liver fat.
Ultrasonography
B-mode ultrasonography is widely used as the first-line imaging modality to detect hepatic steatosis due to its ease of use and low cost. This modality is the recommended screening method to detect steatosis in patients with T2DM by the European NAFLD guidelines [39]. Assessment of liver-to-kidney contrast, parenchymal brightness, deep beam attenuation, brightness of vessel walls, and gallbladder wall definition are used to diagnose excessive liver fat [40]. However, ultrasonography is hampered by limited sensitivity for detection of mild (< 20%) steatosis [40]. Detection of steatosis may be improved by the combination of various echographic parameters during the examination and technical improvements in equipment, reaching a sensitivity of 80–85% at a liver fat content of ≥ 12.5% [41, 42]. However, in hepatic fat content of 5 to 9%, sensitivity can be as low as 12% [43]. Since the definition of NAFLD includes the presence of ≥ 5% hepatic steatosis on liver biopsy, this means that a considerable number of NAFLD patients with steatosis grade 1 on liver histology will not be identified by ultrasonography. Other limitations of ultrasonography include its inability to quantify liver fat and its subjectivity and examiner-dependent characteristics, which limit inter- and intraobserver reproducibility [44].
Controlled attenuation parameter
During the last few years, several techniques based on ultrasonography have been developed, which provide an improved assessment of liver fat, as compared to conventional B-mode ultrasonography, by implementing quantitative approaches. Among them are ultrasound-guided attenuation parameter (UGAP) [45], attenuation imaging (ATI) [46], attenuation coefficient (ATT) [47], and controlled attenuation parameter (CAP), which quantify liver fat by measuring the attenuation of radiofrequency and are based on the principle that echo attenuation is larger in liver tissue with any grade of hepatic steatosis than in the normal liver. The number of studies evaluating UGAP, ATI, and ATT is limited. CAP can be measured simultaneously with liver stiffness measurement (LSM) by vibration-controlled transient elastography (VCTE) and is widely used to assess hepatic steatosis [48]. The diagnostic accuracy of CAP has been validated extensively. A meta-analysis that compared histologically graded steatosis with CAP and included 2375 patients from 19 studies reported AUROCs of 0.82 with a CAP threshold of 248 dB/m for steatosis of > 11%, 0.86 with 268 dB/m for steatosis of > 33%, and 0.89 with 280 dB/m for steatosis of > 66% [49]. However, the CAP value is affected by the presence of obesity and diabetes mellitus [48]. In NAFLD patients, the optimal thresholds for detecting magnetic resonance imaging proton density fat fraction (MRI-PDFF) of ≥ 5% and ≥ 10% were 288 dB/m and 306 dB/m, respectively, which were considerably higher than the optimal CAP thresholds obtained from a meta-analysis with multiple etiologies of liver disease [50]. In another meta-analysis including 2346 patients with chronic liver diseases, it was demonstrated that the accuracy of CAP in detecting histological liver fat > 5% was fair (AUROC of 0.819) [51]. CAP has a high interobserver reproducibility of 0.82 [52], this feature giving it a clear advantage over conventional B-mode ultrasonography. One disadvantage though with CAP is its high rate of measurement failure (0–24%), particularly in obese patients [53]. To overcome this shortcoming, an obesity-specific probe (known as the XL probe) has been developed [54]. However, in NAFLD patients, the threshold for detecting steatosis seems to be higher with the XL probe compared with the conventional probe (known as the M probe) [55]. Thus, the optimal thresholds for detecting steatosis with the different probes remain to be clarified.
Computed tomography
The radiodensity of different tissues can be assessed with computed tomography (CT). The radiodensity of water is 0 Hounsfield units (HUs) by definition, and air is defined as –1,000 HUs [56]. In non-contrast CT, normal liver parenchyma is approximately 50 to 60 HUs, while fat is –20 to − 100 HUs. Due to inconsistency in HU calibration by external factors, “fat-free spleen” can be used as an internal reference [56]. Hepatic HU < 40 has been suggested as a cut-off value for steatosis (> 30%) [57], while a liver HU–spleen HU value less than − 9 to − 10 can be used as a reference to detect steatosis [57–59]. However, as with ultrasonography, CT has limited sensitivity in detecting mild steatosis (< 30% liver fat) and is also limited by radiation exposure [56, 57]. Thus, CT cannot be recommended as a primary diagnostic tool to detect steatosis.
Magnetic resonance-based techniques
Proton magnetic resonance spectroscopy (1H-MRS) is widely considered the most accurate non-invasive method with which to measure HTGC [60]. The technique identifies signals from protons associated with triglycerides by their resonance frequencies. The fat fraction is given as the fat signal divided by the sum of the water and fat signals [57]. An excellent correlation has been shown between 1H-MRS and total lipid quantification in specimens of liver tissue, and it has been suggested that 1H-MRS can replace liver biopsy for the assessment of liver fat content [61]. Compared to liver biopsy, a much larger volume (2 × 2 × 2 or 3 × 3 × 3 cm) of liver tissue is assessed, minimizing the likelihood of sampling error. However, 1H-MRS remains primarily a research tool due to its low availability and limited clinical application [57, 62].
In contrast to MRS, magnetic resonance imaging (MRI) is available in many centers. MRI-PDFF is defined as the ratio of the mobile proton density from triglycerides and the total mobile proton density from triglycerides and water and reflects the concentration of triglycerides within liver tissue (Fig. 2). Although MRI-PDFF and histological assessment of fat content measure different properties, studies have demonstrated strong correlations between liver fat quantified by MRI-PDFF and steatosis grade as assessed by liver histology [43, 57]. Bannas et al. assessed the accuracy of MRI-PDFF through linear regression with 1H-MRS, triglyceride extraction, and histology using ex vivo human livers [63]. MRI-PDFF showed an excellent correlation with 1H-MRS (r = 0.984) and a strong correlation with histology (r = 0.850) and tissue triglyceride extraction (r = 0.871).
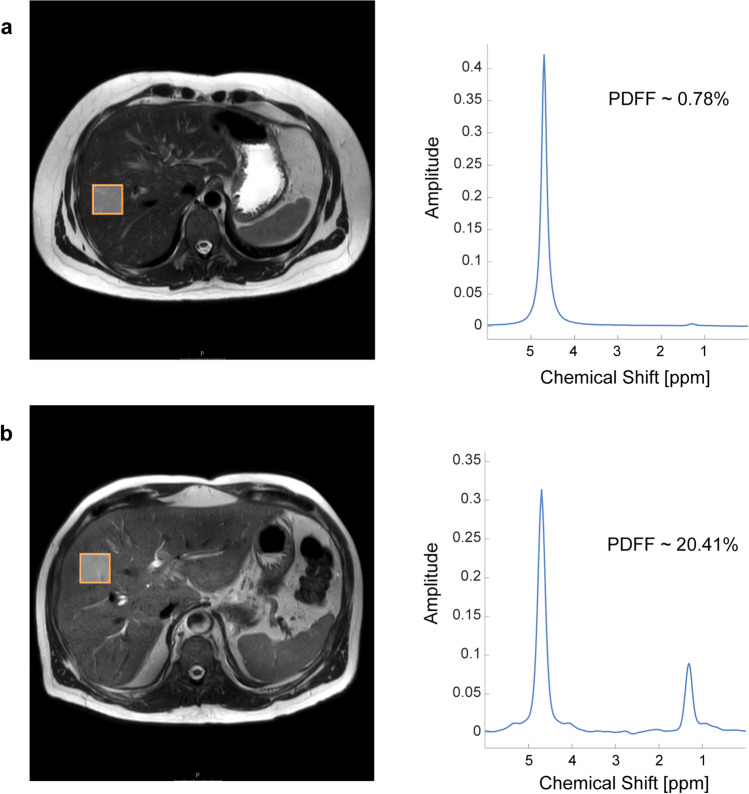
Fig. 2.
T2-weighted images (left) with schematic placement of 1H-MRS voxels (3 × 3 × 3 cm) placed in the right hepatic lobe and corresponding in vivo MRS spectra (right) for water (peak, 4.7 ppm) and triglycerides (peak, 1.3 ppm) for calculation of proton density fat fraction (PDFF) in a subject with a normal liver and in a patient with b NAFLD. Abbreviations: 1H-MRS, proton magnetic resonance spectroscopy; PDFF, proton density fat fraction; ppm, parts per million
In a recent meta-analysis based on six studies including 635 patients with biopsy-proven NAFLD [64], the AUROC value of MRI-PDFF for detecting steatosis was 0.98. Pooled sensitivity and specificity were 93 and 94%. The diagnostic accuracy of MRI-PDFF has been compared with that of CAP in NAFLD patients. MRI-PDFF was superior when a liver biopsy was used as the reference standard [65, 66]. Moreover, in contrast to CAP, the diagnostic performance of MRI-PDFF to diagnose steatosis is not lower in NAFLD patients [64]. An additional advantage of MRI-PDFF is that, unlike ultrasound-based techniques, it can reliably detect longitudinal changes in hepatic triglyceride content as small as 2% or less [67].
In studies and clinical practice using 1H-MRS or MRI-PDFF to quantify liver fat content, a cut-off of 5% [68] or 5.56% is usually used to define hepatic steatosis. The latter value is based on the results of Szczepaniak et al. who examined the distribution of HTGC with 1H-MRS in 345 subjects with a low risk for hepatic steatosis, i.e., BMI < 25 kg/m2, no glucose intolerance or excessive alcohol consumption, and normal serum ALT [62]. However, hepatic steatosis can be present in a significant proportion of lean subjects [69], as well as in individuals with normal serum ALT [70]. Nasr et al. [71] used histopathology as the reference standard to define the optimal cut-off for the definition of hepatic steatosis with 1H-MRS and reported that a cut-off of 3.02% could reliably identify patients with steatosis (specificity of 100%). A cut-off of 2% yielded the most reliable diagnostic accuracy, albeit with a few false positives (sensitivity of 87% and specificity of 94%). Thus, the cut-off for identifying NAFLD patients with MRI/MRS-based techniques can reliably be reduced to at least 3%. Other evidence also suggests that 5% as a limit for normal HTGC is too high. For example, it has been reported that a decrease in the suppression of endogenous glucose production is evident with HTGC as low as > 1.5% and that multiple extrahepatic metabolic changes, such as muscle insulin resistance, hypertriglyceridemia, and decreased plasma high-density lipoprotein cholesterol, are fully established at HTGC 6% [25, 41, 72, 73].
An overview of imaging techniques to diagnose steatosis is given in Table 2.
Table 2.
Imaging techniques to diagnose steatosis
| Modality | Description | Diagnostic performance | Advantages | Limitations |
|---|---|---|---|---|
| Ultrasonography |
-Tissue echogenicity depends on the degree of beam scattering -Fat deposition in tissue accentuates scattering |
AUROC 0.93 (Sn 60–80%, Sp 80–100%) |
-Widely available -Low cost -No radiation -Easy to perform |
-Low sensitivity for mild steatosis -Operator-dependent -Reduction of Sn and Sp in obese patients |
| Controlled attenuation parameter (CAP) | -Measurement of the degree of ultrasound attenuation by hepatic fat | AUROC 0.82 (Sn 69%, Sp 82%) |
-Portable device -Immediate assessment of steatosis -Simultaneous liver stiffness measurement |
-High rate of measurement failure particularly in obese patients |
| MRI-PDFF | -Option that can be added to MRI scanners to quantitatively assess steatosis | AUROC 0.98 (Sn 93%, Sp 94%) |
-Not affected by obesity -Simultaneous MRI for liver architecture and focal lesions |
-Time-consuming -Requires MRI facility -Costly -Cannot be used in some patients with implantable devices |
| 1H-MRS | -Provides a collection of spectra for signal fat fraction estimation, which requires a proper acquisition technique to estimate hepatic triglyceride content | AUROC 0.98 (Sn 89%, Sp 92%) | -The absolute hepatic triglyceride concentration can be directly measured, and very small amounts (as low as 0.5%) can be detected and quantified |
-See MRI-PDFF -Complex and time-consuming data analysis |
Abbreviations: AUROC, area under the receiver operating characteristic; MRI-PDFF, magnetic resonance imaging-proton density fat fraction; 1H-MRS, proton magnetic resonance spectroscopy; Sn, sensitivity; Sp, specificity
Fibrosis
Detecting fibrosis in NAFLD patients is critical as advanced fibrosis (F3-F4) independently predicts the development of liver-related complications, the need for liver transplantation, and liver-related and overall mortality [16–19]. Advanced fibrosis is also associated with a higher incidence of chronic kidney disease and increased mortality from cardiovascular disease [74]. Thus, there is a need to identify patients with bridging fibrosis (F3) and cirrhosis (F4) so that they can be managed to delay further progression, particularly given a large number of patients with undiagnosed cirrhosis within the general population (6–7%) [75].
Given the limitations of biopsy for widescale screening, NITs for assessment of fibrosis stage will play an increasing role in the management of NAFLD patients. The two main types of NITs used are the following: predictive models, which use clinical and laboratory data and imaging techniques, which estimate liver stiffness as a potential surrogate of hepatic fibrosis.
Serum-based fibrosis biomarkers
The most common approach to assessing the stage of fibrosis by serological means consists of routine biochemical and/or hematological tests. These are indirect serum markers and are based on the evaluation of common functional alterations in the liver, alterations that do not necessarily reflect extracellular matrix turnover and/or fibrogenic cell changes. A better understanding of the pathophysiology of liver fibrosis has prompted investigators to use more refined markers to identify different fibrosis stages. These so-called direct serum markers are intended to detect extracellular matrix turnover and/or fibrogenic cell changes. Markers may be used alone or combined with patient and clinical characteristics or with other direct or indirect markers to form panels. An overview of the most common combinations of non-invasive serum-based biomarkers is presented in Table 3.
Table 3.
Serum-based biomarkers of liver fibrosis in NAFLD
| Algorithm | Formula/parameters | Number of NAFLD patients | AUROC/Sn/Sp/NPV/PPV |
|---|---|---|---|
| APRI[88] | (AST (U/L)/(AST upper limit of normal))/(platelet count (× 109/L) × 100) | 145 | 0.67/27/89/84/37 |
| FIB-4[88–90] | (age (years) × AST (U/L)) / ((platelet count (× 109/L)) × (ALT(U/L))1/2) |
145 541 1038 |
0.86/85/65/95/36 0.80/52/90/–/– 0.85/84/69/–/– |
| NFS[88–90] | − 1.675 + 0.037 × age (years) + 0.094 × BMI (kg/m2) + 1.13 × impaired fasting glucose/diabetes (yes = 1, no = 0) + 0.99 × AST/ALT ratio – 0.013 × platelet count (× 109/L) – 0.66 × albumin (g/dL) |
733 145 1038 |
0.82–0.88/77–82/71–77/88–93/52–56 |
| BARD[89, 91, 92] | BMI ≥ 28: No = 0, Yes = 1; AST/ALT ratio ≥ 0.8: No = 0, Yes = 2; and diabetes: No = 0, Yes = 1 |
827 145 138 1038 |
0.81/–/–/–/– 0.77/89/44/95/27 0.67/51/77/81/45 0.76/74/66/–/– |
| ELF[93, 94] | 2.2781 + 0.851 × ln [HA] (µg/L) + 10.751 × ln [P3NP] (µg/L) + 10.934 × ln [TIMP 1] (µg/L) |
61 192 |
0.87/89/96/96/80 0.90/80/90/94/71 |
| FibroMeter[95] | Platelets, prothrombin index, AST, α2-macroglobulin, hyaluronic acid, urea, age | 383 | 0.89*/81*/84*/77*/86* |
| FibroTest[96] | Haptoglobin, α2-macroglobulin, apolipoprotein A1, GGT, bilirubin, age, gender | 267 | 0.81/92/71/98/33 |
*Values are for prediction of significant fibrosis. ALT, alanine aminotransferase; APRI, AST-to-platelet ratio index; AST, aspartate aminotransferase; AUROC, area under the receiver operating characteristic; BMI, body mass index; DM, diabetes mellitus; ELF, enhanced liver fibrosis; FIB-4, fibrosis-4 score; GGT, gamma glutamyltransferase; IFG, impaired fasting glycemia; MMP, matrix metalloproteinases; NAFLD, non-alcoholic fatty liver disease; NFS, non-alcoholic fatty liver disease fibrosis score; NPV, negative predictive value; P3NP, N-terminal peptide of procollagen III; PPV, positive predictive value; TIMP, tissue inhibitors of metalloproteinases
Fibrosis-4 (FIB-4) and NAFLD fibrosis score (NFS) are the most widely used algorithms and can be easily calculated using freely available online calculators [76, 77]. Both algorithms have high negative predictive values (approximately 90%) and can reliably exclude advanced fibrosis, thus identifying lower-risk patients who do not need secondary care referral. However, although FIB-4 and NFS are effective at excluding advanced fibrosis, as well as predictive of liver outcomes over time [78], they have limited ability to identify NAFLD patients with advanced fibrosis. Approximately 30% of NAFLD patients will be classified as “indeterminate” when using FIB-4 and NFS, and a significant number are falsely classified as having advanced fibrosis. Since there is abundant evidence that NFS and FIB-4 provide higher rates of false-positive results in older populations, it has been proposed that different diagnostic cut-offs should be used in patients ≥ 65 years old [79]. However, the latter proposal has been criticized since a recent study suggested that these adapted cut-offs significantly decreased the sensitivity to only 60% in patients over 65 years of age [80]. It should be noted that FIB-4 and NFS have been developed and, therefore, their coefficients for age have been calibrated in cohorts of patients aged around 45–50 years. Consequently, they are less sensitive in younger people and should not be used in patients < 35 years old [81]. This could become a matter of importance if cases of advanced liver fibrosis in young adults increase as expected due to the dramatic increase in the prevalence of obesity and insulin resistance in children [82].
Commercial biomarker panels such as the enhanced liver fibrosis (ELF) test, FibroTest (FibroSure), and FibroMeter have been developed, though they are more expensive and less available than FIB-4 and NFS. ELF is a simplified algorithm comprising three direct fibrosis markers which, aside from distinguishing patients with advanced fibrosis, may also be a good predictor of liver-related morbidity and mortality [82]. Therefore, this test may be considered in patients where FIB-4 and NFS indicate advanced fibrosis.
Several new scoring systems have also reported good accuracy in detecting advanced fibrosis in patients with NASH. Among them, an algorithm based on the measurement of serum PRO-C3 (a marker of type III collagen formation), age, presence of diabetes, and platelet count (ADAPT) [83], and the LINKI algorithms, which are based on hyaluronic acid, fasting glucose, AST, age, and platelet count [84]. In addition, the Hepamet fibrosis score has recently demonstrated superior diagnostic accuracy, compared to FIB-4 and NFS, in a multinational cohort of 1500 patients [85, 86]. Recently, a Cox regression model based on age, AST/ALT-ratio, and ALT-level (dAAR) was reported to predict the risk of incident severe liver outcomes in the general population and may be of value for detecting advanced liver fibrosis [87].
Ultrasound-based methods
Techniques based on elastography measure tissue stiffness as a physical property termed Young’s modulus [97]. VCTE measures the speed of a mechanically induced shear wave through the hepatic parenchyma using pulse-echo ultrasonic acquisitions to obtain a liver stiffness measurement (LSM) as a marker of hepatic fibrosis. The velocity of the shear wave, which is perpendicular to the direction of pulse wave propagation, is proportional to liver stiffness, with quantitative results available as the algebraically derived Young’s modulus in kilopascals [97]. The result of VCTE is obtained as a median of at least 10 measurements and measures approximately a 1-cm diameter by 4-cm length region of liver tissue, which is approximately 100 times larger than that evaluated with liver biopsy [97]. VCTE is an easy-to-perform tool using a portable ultrasound probe, either an M-probe (3.5 MHz, at 2.5 to 6.5 cm depth) or, in the case of morbid obesity, an XL-probe (2.5 MHz, at 3.5 to 7.5 cm depth). Values of LSM < 8 kPa exclude advanced fibrosis with a very high probability [98]. However, the ability to rule in advanced fibrosis is considerably lower. Cut-offs ranging from 8 to 12 kPa have been proposed, with sensitivities ranging from 84 to 100% and specificities from 83 to 97% [48].
Obesity increases the probability of invalid results (by up to 30%) and may also lead to an overestimation of LSM [99, 100]. With the availability of the XL probe, which has been approved for use in patients with morbid obesity, the failure rate of VCTE was reported to be < 5%. VCTE data are not reliable under conditions in which a rapidly developing mass effect inside the liver increases intrahepatic pressure and, thereby, reduces liver elasticity. This phenomenon is observed in right-sided congestive heart failure, acute inflammation and/or edema of the liver, and extrahepatic cholestasis [48]. In addition, regarding food intake, a minimum 2-h fast is currently recommended prior to the examination [48].
Shear wave elastography (SWE) and acoustic radiation force impulse (ARFI) are integrated into conventional ultrasonography devices to measure LSM [101]. Although SWE has been reported to perform better than ARFI in differentiating moderate fibrosis in NAFLD patients, VCTE, SWE, and ARFI showed similar performances in differentiating advanced fibrosis and suffered from a significant rate of failures or unreliable results [102].
Magnetic resonance-based methods
With magnetic resonance elastography (MRE), low-frequency vibrations are applied to the abdominal wall, which are tracked as propagating hepatic shear waves by acquiring images with wave motion-sensitized phase-contrast sequences and processing raw images (i.e., magnitude and phase images) to “wave images” and then to “elastogram” (Fig. 3). The cross-sectional elastogram images reflect the stiffness generated from the wave propagation information [103]. An MRE protocol can be performed with most MR scanners but requires adding hardware to generate mechanical waves and software for acquisition and processing. In theory, the whole liver can be examined, but typically four axial (or transverse) slices with a thickness of 5–10 mm are placed in the widest part of the liver, which generally corresponds to 5–35% of the total liver volume. Liver stiffness measurement is performed by drawing a region of interest on the generated elastograms, avoiding edge effects, large vessels, the gallbladder fossa, and any areas affected by cardiac and vascular artifacts. The mean stiffness is calculated using the mean from all regions of interest. Thus, MRE evaluates much larger volumes of the total liver than liver biopsy and can be performed in conjunction with conventional MRI.
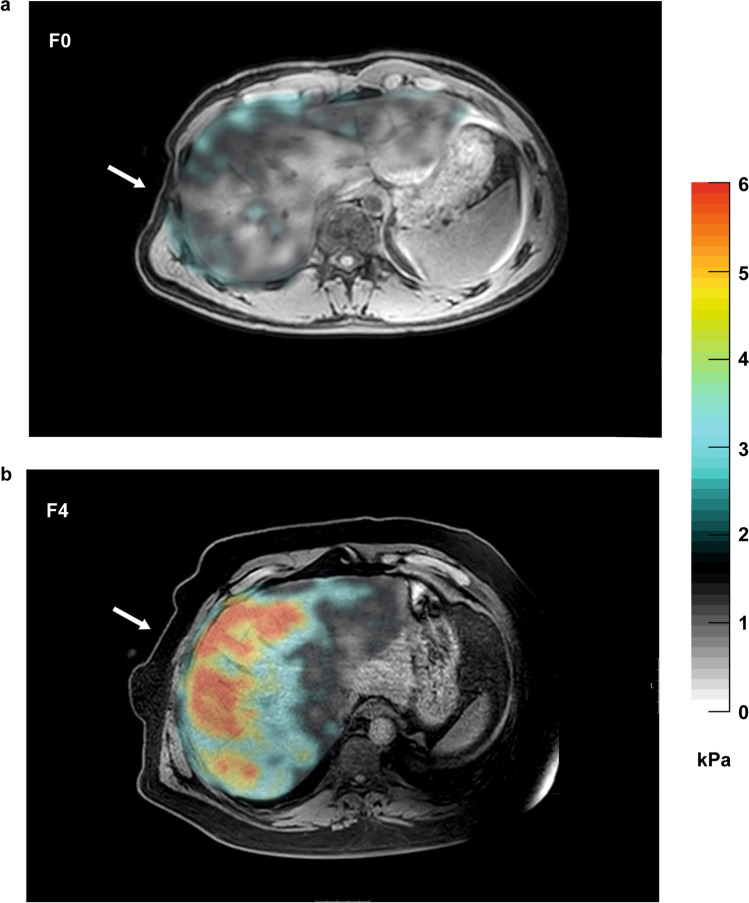
Fig. 3.
Hepatic magnetic resonance elastography (MRE) images captured from two NAFLD patients using an active electrodynamic transducer transmitting mechanical waves at 54 Hz. Arrow indicates placement of transducer when strapped to the patient. Tissue stiffness is illustrated in a corresponding elastogram, shown as a color gradient with higher degrees of tissue stiffness indicated in red. Liver biopsies obtained immediately after the MRE-examination showed a absence of fibrosis (F0) and b cirrhosis (F4), respectively. Abbreviations: kPa, kilopascal; NAFLD, non-alcoholic fatty liver disease
Shear stiffness values in the normal liver are < 3 kPa [103]. Studies have demonstrated that MRE has high diagnostic accuracy, with an AUROC of ≥ 0.90 for identifying advanced fibrosis in NAFLD with MRE cut-off values ranging from 3.64 to 4.15 kPa [67, 104–106]. As with VCTE, MRE needs to be performed in the fasting state, at least for 2 h, because increased postprandial portal blood flow may cause a dynamic increase in liver stiffness, potentially leading to an overestimation of the extent of fibrosis [103]. In contrast to VCTE, diagnostic accuracy is not affected by patient body factors. In addition, the inter-observer agreement for staging fibrosis is nearly perfect and higher than that seen with histopathology [67, 105, 106].
The performance of MRE is better than that of VCTE-LSM in diagnosing advanced fibrosis in NAFLD patients [66]. However, unlike VCTE, it is difficult to implement MRE at a large scale since it requires an MRI facility, is associated with a high cost, and is time-consuming.
Evolving non-invasive tests
Genetic markers
Genome-wide association studies have revealed several genetic risk factors associated with NAFLD prevalence and progression. Moreover, functional studies on those genes have added much knowledge on the pathogenesis of NAFLD and the complex pathophysiologic pathways related to the disease [107]. The risk variant with the most pronounced effect on the development and progression of NAFLD is the rs738409 C > G single nucleotide polymorphism of patatin-like phospholipase domain-containing 3 (PNPLA3), which encodes the I148M protein variant of PNPLA3, also known as adiponutrin, a protein involved in lipid remodeling [108]. The primary hepatic effects of the I148M protein variant are most likely attributable to its repression of lipase activity [109], resulting in increased intracellular accumulation of triglycerides with the number of risk alleles (the G allele). In a recent study in which subjects were phenotyped by MRI-PDFF, each copy of the PNPLA3 risk allele was associated with a 2.56% increase in hepatic steatosis [110]. In addition, cumulative evidence also suggests that PNPLA3 is associated with fibrosis progression, development of HCC, ESLD, and all-cause mortality [111].
The gene encoding transmembrane 6 superfamily member 2 (TM6SF2) is also associated with NAFLD progression. The gene product of TM6SF2 is involved in VLDL secretion from hepatocytes, and the TM6SF2 E167K variant results in a loss of this function, resulting in the hepatic accumulation of lipids [112]. In NAFLD patients, TM6SF2 E167K is associated with significant fibrosis (stages 2–4), with an odds ratio (OR) of 1.88, independent of the PNPLA3 genotype [113].
Glucokinase regulatory protein (GCKR) is an inhibitor of glucokinase and regulates de novo lipogenesis [114]. The GCKRP446L variant disrupts negative regulation of glucokinase, which leads to glucose uptake and increased de novo lipogenesis. In a study comparing patients with NAFLD with healthy control individuals, significant associations were found between GCKR rs780094 and susceptibility to NAFLD (OR 1.49), NASH (OR, 1.55), and NASH with significant fibrosis (OR, 1.50) [115]. High levels of liver fat caused by these genetic mutations seem to be associated with disease progression in NAFLD. Thus, due to their influence on hepatic lipid accumulation, these genetic risk variants further support the hypothesis that high levels of liver fat are associated with the progression of NAFLD and probably other chronic liver diseases as well.
Interestingly, several genes protective of hepatic steatosis and fibrosis have recently been reported, namely, mitochondrial amidoxime-reducing component 1 (MARC1), 17β-hydroxysteroid dehydrogenase 13 (HSD17B13), LPIN1, uncoupling protein 2 (UCP2), interleukin-28B (IL-28B), Kruppel-like factor 6 (KLF6), and the MER protocol-oncogene tyrosine kinase (MERTK) [111].
Omics-based markers
Through their ability to detect thousands of different molecules, “omics technologies” represent a new diagnostic approach that could be useful for the diagnosis of hepatic steatosis. A proteomics study conducted on 70 patients (35 controls and 35 NAFLD) identified 20 protein peaks in NAFLD versus controls (sensitivity of 89% and specificity of 83%). Moreover, it was reported that NAFLD patients had a higher basal hemoglobin level [116]. Metabolomics has been applied to identify the metabolic profile specific for NAFLD, such as glutathione and bile acids, whose levels are altered during NAFLD onset [117]. Lipidomic-based studies identify the alteration of lipid species levels, for instance, eicosanoids and short-chain fatty acids. It has been reported that three specific lipids (defined as “lipid triplet”) can identify NAFLD. However, the diagnostic performance was modest (AUROC of 0.71–0.74) [118]. Omics-based biomarkers may also be of value to evaluate disease severity. It was recently reported that a signature of triglycerides was able to differentiate steatosis from NASH, however, with limited diagnostic performance (AUROC of 0.79) [119].
RNA biomarkers
In biological fluids, there are RNA molecules belonging to different classes. A key feature making these RNAs potentially valuable biomarkers is their high stability since they are not present in free form in the circulation. Instead, they are encapsulated in membranous vesicles, complexed to RNA-binding proteins, or associated with lipoproteins, features that protect these RNAs from degradation [120]. Moreover, the techniques used for their detection are extremely sensitive. In theory, even a single RNA molecule could be detected through quantitative PCR [121].
Most of the studies concerning circulating RNAs as NAFLD biomarkers are limited to microRNAs (miRNAs). It has been reported that a miRNA panel (miR-122, miR-1290, miR-27b, miR-192) showed a high NAFLD diagnostic accuracy (AUROC of 0.86) [122]. Moreover, in a recent study, 2083 serum miRNAs were profiled in NAFLD patients representing the complete NAFLD spectrum and compared with population controls. The most robust finding was that serum miR-193a-5p levels correlated strongly with NAFLD activity grade and fibrosis stage. It was concluded that miR-193a-5p is a potential clinically tractable circulating biomarker for progressive NAFLD [123].
Gut microbiota
Several hypotheses have provided mechanistic insights into the pathways of how the gut microbiota might contribute to NAFLD development and progression [124]. Some studies have focused on microbiome signatures in NAFLD with fibrosis of different stages. When compared with NAFLD patients with severe fibrosis, individuals with absence of or mild fibrosis as well as healthy controls display a decreased abundance of Gram-negative bacteria, decreased Fusobacteria phylum, increased Enterobacteriaceae family (Bacteroides, Ruminococcus, and Shigella genera [125, 126]), and, by contrast, increased Gram-positive bacteria, Firmicutes phylum, Prevotellaceae family, and Prevotella genus [127].
In a recent study, Lang et al. used gut microbiota-based approaches, VCTE, NFS, and FIB-4, to predict advanced fibrosis in 83 biopsy-proven NAFLD patients. A random forest model composed of clinical features and bacterial taxa achieved an AUROC of 0.87, which was similar to the AUROCs of NFS and FIB-4 (0.86 and 0.85, respectively). VCTE had the best diagnostic performance with an AUROC of 0.93 [128].
Clinical implications
Which patient populations should be screened for NAFLD?
Current guidelines do not recommend widespread or community screening, mainly due to the perceived associated direct and indirect medical costs [20, 39]. However, some national and international initiatives (ETHON project, Spain [129]; international LiverScreen project [NCT03789825]) are currently investigating the effectiveness of screening the general population for significant liver disease. Screening at-risk patients has been shown to be cost-effective in several studies across different countries [130–132]. To define at-risk populations, important information can be obtained from population-based studies evaluating the screening for liver fibrosis using VCTE [81, 133–135]. Using the 8.0 kPa threshold, the prevalence of patients at risk of significant liver fibrosis among adults in the general population was similar across studies (6–7%). However, in some subgroups, the prevalence was significantly higher. T2DM has been shown to be the condition associated with the highest risk of increased LSM [133]. The prevalence of liver stiffness ≥ 8.0 kPa was approximately 9% in patients with diabetes but no liver steatosis, and it reached 17.2% in patients with both diabetes and liver steatosis [134].
Other factors associated with elevated liver stiffness are closely related to metabolic conditions such as obesity, impaired fasting glucose, low HDL-cholesterol, and high triglyceride levels. [8, 136–138]. The available evidence, therefore, suggests that patients with BMI > 25 kg/m2, particularly those with metabolic risk factors, and especially T2DM, might be a relevant population for the identification of NAFLD patients with advanced fibrosis. However, it is unclear at which age at-risk patients should be screened. Most studies have been performed in adults aged > 40 years, although obesity and T2DM have become more prevalent in children. Moreover, T2DM tends to be more aggressive in young people with poorer responses to glucose-lowering medication and greater insulin resistance [139–141]. As the length of exposure to insulin resistance is probably a key factor in the development of NAFLD with advanced fibrosis, it is reasonable to assume that more cases of advanced NAFLD will be identified in young adults in the near future. In this context, it was recently reported that the prevalence of elevated LSM (≥ 7.9 kPa) in young adults aged 22–25 years was 2.7% [81].
Which method should be used to identify steatosis?
As stated above, 1H-MRS and MRI-PDFF can be regarded as reference methods to diagnose steatosis. These methods provide a more accurate assessment of steatosis than conventional histopathology, which often overestimates hepatic triglyceride content [24, 71]. However, despite the high accuracy of MRI-PDFF for detecting and quantifying steatosis, cost and limited availability restrict its use on a larger scale.
Serum-based algorithms to identify steatosis have been proposed for screening purposes, but, in general, their diagnostic performance is suboptimal compared to other methods, and currently, they do not add much to the information provided by the clinical, laboratory, and imaging examinations that are routinely performed in patients with suspected NAFLD.
Conventional ultrasonography is currently recommended as the first-line tool for the diagnosis of steatosis in clinical practice [39]. It is widely available, cheap, and well-established. However, ultrasonography has poor sensitivity for detecting steatosis < 20% and many NAFLD patients may thus be missed when using this modality. CAP is a promising technique for the detection of steatosis, but it is unknown whether its diagnostic accuracy is better than that of ultrasonography in patients with steatosis < 20%. If NAFLD patients with steatosis missed by ultrasound-based techniques have a low risk of developing advanced fibrosis, the inability of conventional ultrasonography and CAP to identify steatosis < 20% would be of limited clinical importance. However, a phenomenon exists in NAFLD known as burned-out NASH, in which steatosis decreases with the progression of fibrosis [142]. In a study including 458 NAFLD patients with advanced fibrosis, overall mortality was higher in patients with histological steatosis < 33% than in those with steatosis ≥ 33% [143]. Thus, some NAFLD patients with a low steatosis grade not identified with ultrasound-based methods might have advanced fibrosis and an increased risk of liver-related events and mortality. The characteristics of NAFLD patients diagnosed with MRI-PDFF but missed by ultrasound-based techniques will be elucidated in the currently ongoing prospective Swedish EPSONIP study [144] in which patients with T2DM consecutively enrolled from primary care health centers will undergo serological, ultrasound-based, and magnetic resonance-based repeated measurements of steatosis and fibrosis.
Which method should be used to identify advanced fibrosis in NAFLD?
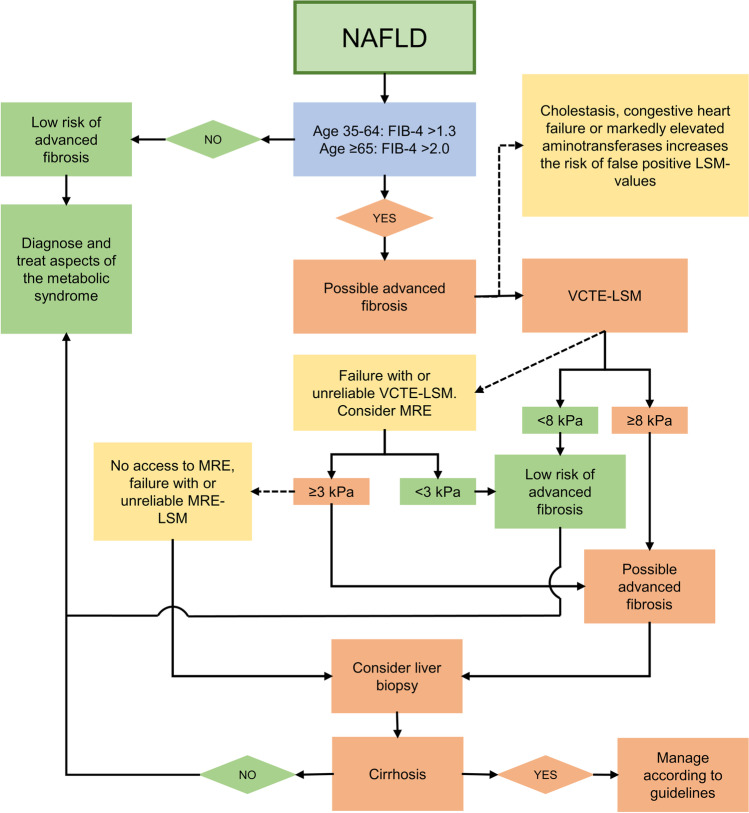
The most validated NITs for assessment of fibrosis stage in NAFLD are NFS and FIB-4, which are non-patented and widely available tests. FIB-4 is easier to calculate since it is composed of only three serum-based parameters and the subject’s age, and thus may be most suitable for screening purposes. Both algorithms can exclude the presence of advanced fibrosis with a high NPV (> 90%) [145]. However, their PPV for confirming advanced fibrosis is modest (< 70%), with a significant risk of false-positive results [145]. Moreover, approximately one-third of patients fall in between the lower and upper cut-offs, resulting in undetermined results [145]. The insufficient PPV of these tests has led to the development of strategies using FIB-4 or NFS as a first-line procedure, followed, if positive, by a second-line confirmatory test (elastography or specialized blood test). A proposed pathway to assess the fibrosis stage in NAFLD patients is shown in Fig. 4.
Fig. 4.
Suggested algorithm for non-invasive risk stratification of NAFLD patients without signs of end-stage liver disease. Abbreviations: FIB-4, fibrosis-4; kPa, kilopascal; LFT, liver function test; LSM, liver stiffness measurement; MRE, magnetic resonance elastography; NAFLD, non-alcoholic fatty liver disease; VCTE, vibration-controlled transient elastography
The two most validated patented serum fibrosis biomarkers are FibroMeter and ELF. Overall, the diagnostic accuracy of patented serum fibrosis tests for staging fibrosis is similar [146] to that of FIB-4 and NFS or only slightly higher [147]. However, high costs and limited availability limit the widespread application of these tests in clinical practice.
VCTE is the most widely available device for LSM with the largest amount of data in the NAFLD setting. It has significantly better diagnostic accuracy compared to FIB-4 and NFS [145] and is, therefore, better suited as a second-line test. There is no agreement in clinical practice on LSM cut-offs for ruling out advanced fibrosis, even though 8 kPa is the most validated threshold, with an NPV > 90%. However, the PPV to confirm advanced fibrosis is not sufficiently high to avoid liver biopsies in many NAFLD patients. According to the results of a recent meta-analysis [98], values of LSM by VCTE > 12 kPa could be used to rule in advanced fibrosis, thus limiting the need for confirmatory liver biopsy in NAFLD patients with LSM values by VCTE between 8 and 12 kPa, albeit this must be confirmed in further studies. Regarding SWE, meta-analyses [148, 149] suggest performance in detecting advanced fibrosis similar to that reported for VCTE [102]. However, SWE is less available and relevant data in NAFLD patients remain limited.
MRE has superior diagnostic accuracy compared to ultrasound-based elastography techniques. However, the use of MRE is limited to tertiary specialized centers because of its high cost and the need for advanced equipment.
Should screening aim to identify steatosis, advanced fibrosis, or earlier stages of fibrosis?
In light of the pathophysiology of NAFLD, which implies the initial accumulation of triglycerides leading to steatosis and, in some individuals, subsequent progressive fibrosis, it is reasonable to screen for steatosis in patients with metabolic risk factors such as T2DM and hyperlipidemia, or with BMI > 25 kg/m2. Considering that high levels of liver fat appear to be associated with the progression of NAFLD, ultrasonography, which is widely available and has sufficient sensitivity to identify pronounced steatosis, is currently the screening method of choice. Further investigations can be avoided in subjects without steatosis, while in those with steatosis, an NIT, preferably FIB-4, should be used to assess the probability of advanced fibrosis (F3-F4). If positive, a second-line confirmatory test (elastography or specialized blood test) should be conducted.
However, applying the strategy above theoretically entails a risk of missing advanced fibrosis in burned-out NASH or in NAFLD patients with low steatosis grade not detectable with ultrasonography. Therefore, an alternative screening approach may be to disregard steatosis and focus on screening for advanced fibrosis with serum-based NITs and/or elastography in populations at risk for NAFLD. Future studies in subjects with metabolic risk factors assessing the prevalence of advanced fibrosis with normal HTGC or with low steatosis grade undetectable with ultrasonography, as well as the diagnostic performance of NITs in these patients, will elucidate whether this strategy may be preferable.
Identifying earlier stages of fibrosis in NAFLD is appealing since this would give physicians opportunities to intervene at an earlier stage, minimizing the risk of future liver-related complications. However, considering the inferior diagnostic performance of NITs to distinguish fibrosis stages 1–2 and in light of the absence of approved pharmacological treatments to halt or retard disease progression, it is currently advisable to focus on identifying NAFLD patients with advanced fibrosis. The presence of advanced fibrosis, particularly cirrhosis, alters clinical management, especially the initiation of surveillance for gastroesophageal varices and HCC.
Conclusions
The increasing prevalence of obesity has changed the landscape of chronic liver disease, with increasing numbers of NAFLD patients presenting with complications of ESLD. In many of these patients, NAFLD and its severity have previously been undiagnosed or neglected, and opportunities for early interventions and surveillance have been missed. Accumulating evidence supports the use of NITs for diagnosing steatosis and assessing the presence of advanced fibrosis in NAFLD. The diagnostic performance of serum-based algorithms is currently insufficient for clinical use to identify patients with steatosis. CAP and other ultrasound-based methods are promising, but diagnostic cut-offs for steatosis are yet to be defined. 1H-MRS or MRI-PDFF can reliably diagnose steatosis and replace liver biopsy as the reference method in this aspect. On the other hand, their limited availability limits the implementation of these methods on a large scale. Serum-based algorithms are recommended as the first step to assess the fibrosis stage in NAFLD. If advanced fibrosis cannot be excluded with these algorithms, VCTE offers higher diagnostic accuracy with excellent NPV but with considerably lower PPV. Thus, liver biopsy may still be needed to assess the fibrosis stage in a significant number of NAFLD patients. MRE may reduce the need for liver biopsy, although further studies are needed to clarify its usefulness in the evaluation and follow-up of NAFLD patients. Genetic, omics-based, and RNA markers, as well as gut microbiota-based approaches are promising methods, but their role in the management of NAFLD patients remains to be clarified.
Abbreviations
- ALT
Alanine aminotransferase
- ARFI
Acoustic radiation force impulse
- AST
Aspartate aminotransferase
- ATI
Attenuation imaging
- ATT
Attenuation coefficient
- AUROC
Area under the receiver operating characteristic
- BMI
Body mass index
- CAP
Controlled attenuation parameter
- CT
Computed tomography
- ELF
Enhanced Liver Fibrosis
- ESLD
End-stage liver disease
- FLI
Fatty liver index
- FIB-4
Fibrosis-4
- GCKR
Glucokinase regulatory protein
- GGT
Gamma glutamyltransferase
- HCC
Hepatocellular carcinoma
- HDL
High-density lipoprotein
- 1H-MRS
Proton magnetic resonance spectroscopy
- HSD17B13
17β-Hydroxysteroid dehydrogenase 13
- HSI
Hepatic steatosis index
- HTGC
Hepatic triglyceride content
- HU
Hounsfield unit
- IL-28B
Interleukin-28B
- KLF6
Kruppel-like factor 6
- LAP
Lipid accumulation product
- LSM
Liver stiffness measurement
- MARC1
Mitochondrial amidoxime-reducing component 1
- MAFLD
Metabolic dysfunction-associated fatty liver disease
- MERTK
MER protocol-oncogene tyrosine kinase
- MRE
Magnetic resonance elastography
- MRI
Magnetic resonance imaging
- MRI-PDFF
Magnetic resonance imaging proton density fat fraction
- NAFLD
Non-alcoholic fatty liver disease
- NAFLD-LFS
NAFLD liver fat score
- NAS
NAFLD activity score
- NASH
Non-alcoholic steatohepatitis
- NFS
NAFLD fibrosis score
- NIT
Non-invasive test
- NPV
Negative predictive value
- OR
Odds ratio
- PNPLA3
Patatin-like phospholipase domain-containing 3
- PPV
Positive predictive value
- Sn
Sensitivity
- Sp
Specificity
- SPC
Stereological point counting
- SWE
Shear wave elastography
- T2DM
Type 2 diabetes mellitus
- TG
Triglycerides
- TyG
Triglyceride x glucose
- TM6SF2
Transmembrane 6 superfamily member 2
- UCP2
Uncoupling protein 2
- UGAP
Ultrasound-guided attenuation parameter
- ULN
Upper limit of normal
- VAI
Visceral adiposity index
- VCTE
Vibration controlled transient elastography
- VLDL
Very low-density lipoprotein
- WC
Waist circumference
Funding
Open access funding provided by Linköping University.
Declarations
Competing interests
The authors declare no competing interests.
Footnotes
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Younossi ZM, Koenig AB, Abdelatif D, Fazel Y, Henry L, Wymer M. Global epidemiology of nonalcoholic fatty liver disease - meta-analytic assessment of prevalence, incidence, and outcomes. Hepatology. 2016;64:73–84. doi: 10.1002/hep.28431. [DOI] [PubMed] [Google Scholar]
- 2.Marcellin P, Kutala BK. Liver diseases: a major, neglected global public health problem requiring urgent actions and large-scale screening. Liver Int. 2018;38(Suppl 1):2–6. doi: 10.1111/liv.13682. [DOI] [PubMed] [Google Scholar]
- 3.Younossi ZM. Non-alcoholic fatty liver disease - a global public health perspective. J Hepatol. 2019;70:531–544. doi: 10.1016/j.jhep.2018.10.033. [DOI] [PubMed] [Google Scholar]
- 4.Ekstedt M, Franzen LE, Mathiesen UL, Thorelius L, Holmqvist M, Bodemar G, et al. Long-term follow-up of patients with NAFLD and elevated liver enzymes. Hepatology. 2006;44:865–873. doi: 10.1002/hep.21327. [DOI] [PubMed] [Google Scholar]
- 5.Nasr P, Ignatova S, Kechagias S, Ekstedt M. Natural history of nonalcoholic fatty liver disease: a prospective follow-up study with serial biopsies. Hepatol Commun. 2017;2:199–210. doi: 10.1002/hep4.1134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Targher G, Tilg H, Byrne CD. Non-alcoholic fatty liver disease: a multisystem disease requiring a multidisciplinary and holistic approach. Lancet Gastroenterol Hepatol. 2021;6:578–588. doi: 10.1016/S2468-1253(21)00020-0. [DOI] [PubMed] [Google Scholar]
- 7.Targher G, Bertolini L, Padovani R, Rodella S, Tessari R, Zenari L, et al. Prevalence of nonalcoholic fatty liver disease and its association with cardiovascular disease among type 2 diabetic patients. Diabetes Care. 2007;30:1212–1218. doi: 10.2337/dc06-2247. [DOI] [PubMed] [Google Scholar]
- 8.Kwok R, Choi KC, Wong GL, Zhang Y, Lik-Yuen Chan H, On-Yan Luk A, et al. Screening diabetic patients for non-alcoholic fatty liver disease with controlled attenuation parameter and liver stiffness measurements: a prospective cohort study. Gut. 2016;65:1359–1368. doi: 10.1136/gutjnl-2015-309265. [DOI] [PubMed] [Google Scholar]
- 9.Bril F, Cusi K. Management of nonalcoholic fatty liver disease in patients with type 2 diabetes: a call to action. Diabetes Care. 2017;40:419–430. doi: 10.2337/dc16-1787. [DOI] [PubMed] [Google Scholar]
- 10.Noureddin M, Vipani A, Bresee C, Todo T, Kim IK, Alkhouri N, et al. NASH leading cause of liver transplant in women: updated analysis of indications for liver transplant and ethnic and gender variances. Am J Gastroenterol. 2018;113:1649–1659. doi: 10.1038/s41395-018-0088-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Younossi Z, Stepanova M, Ong JP, Jacobson IM, Bugianesi E, Duseja A, et al. Nonalcoholic steatohepatitis is the fastest growing cause of hepatocellular carcinoma in liver transplant candidates. Clin Gastroenterol Hepatol. 2019;17:748–755. doi: 10.1016/j.cgh.2018.05.057. [DOI] [PubMed] [Google Scholar]
- 12.Estes C, Anstee QM, Arias-Loste MT, Bantel H, Bellentani S, Caballeria J, et al. Modeling NAFLD disease burden in China, France, Germany, Italy, Japan, Spain, United Kingdom, and United States for the period 2016–2030. J Hepatol. 2018;69:896–904. doi: 10.1016/j.jhep.2018.05.036. [DOI] [PubMed] [Google Scholar]
- 13.Sumida Y, Nakajima A, Itoh Y. Limitations of liver biopsy and non-invasive diagnostic tests for the diagnosis of nonalcoholic fatty liver disease/nonalcoholic steatohepatitis. World J Gastroenterol. 2014;20:475–485. doi: 10.3748/wjg.v20.i2.475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Adams LA, Angulo P, Lindor KD. Nonalcoholic fatty liver disease. CMAJ. 2005;172:899–905. doi: 10.1503/cmaj.045232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Nasr P, Fredrikson M, Ekstedt M, Kechagias S. The amount of liver fat predicts mortality and development of type 2 diabetes mellitus in non-alcoholic fatty liver disease. Liver Int. 2020;40:1069–1078. doi: 10.1111/liv.14414. [DOI] [PubMed] [Google Scholar]
- 16.Taylor RS, Taylor RJ, Bayliss S, Hagström H, Nasr P, Schattenberg JM, et al. Association between fibrosis stage and outcomes of patients with nonalcoholic fatty liver disease: a systematic review and meta-analysis. Gastroenterology. 2020;158:1611–1625. doi: 10.1053/j.gastro.2020.01.043. [DOI] [PubMed] [Google Scholar]
- 17.Ekstedt M, Hagström H, Nasr P, Fredrikson M, Stål P, Kechagias S, et al. Fibrosis stage is the strongest predictor for disease-specific mortality in NAFLD after up to 33 years of follow-up. Hepatology. 2015;61:1547–1554. doi: 10.1002/hep.27368. [DOI] [PubMed] [Google Scholar]
- 18.Angulo P, Kleiner DE, Dam-Larsen S, Adams LA, Björnsson ES, Charatcharoenwitthaya P, et al. Liver fibrosis, but no other histologic features, is associated with long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2015;149:389–397. doi: 10.1053/j.gastro.2015.04.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, et al. Fibrosis stage but not NASH predicts mortality and time to development of severe liver disease in biopsy-proven NAFLD. J Hepatol. 2017;67:1265–1273. doi: 10.1016/j.jhep.2017.07.027. [DOI] [PubMed] [Google Scholar]
- 20.Chalasani N, Younossi Z, Lavine JE, Charlton M, Cusi C, Rinella M, et al. The diagnosis and management of nonalcoholic fatty liver disease: practice guidance from the American Association for the Study of Liver Diseases. Hepatology. 2018;67:328–357. doi: 10.1002/hep.29367. [DOI] [PubMed] [Google Scholar]
- 21.Eslam M, Newsome PN, Sarin SK, Anstee QM, Targher G, Romero-Gomez M, et al. A new definition for metabolic dysfunction-associated fatty liver disease: an international expert consensus statement. J Hepatol. 2020;73:202–209. doi: 10.1016/j.jhep.2020.03.039. [DOI] [PubMed] [Google Scholar]
- 22.Brunt EM, Janney CG, Di Bisceglie AM, Neuschwander-Tetri BA, Bacon BR. Nonalcoholic steatohepatitis: a proposal for grading and staging the histological lesions. Am J Gastroenterol. 1999;94:2467–2474. doi: 10.1111/j.1572-0241.1999.01377.x. [DOI] [PubMed] [Google Scholar]
- 23.Kleiner DE, Brunt EM, Van Natta M, Behling C, Contos MJ, Cummings OW, et al. Design and validation of a histological scoring system for nonalcoholic fatty liver disease. Hepatology. 2005;41:1313–1321. doi: 10.1002/hep.20701. [DOI] [PubMed] [Google Scholar]
- 24.Franzén LE, Ekstedt M, Kechagias S, Bodin L. Semiquantitative evaluation overestimates the degree of steatosis in liver biopsies: a comparison to stereological point counting. Mod Pathol. 2005;18:912–916. doi: 10.1038/modpathol.3800370. [DOI] [PubMed] [Google Scholar]
- 25.Browning JD, Szczepaniak LS, Dobbins R, Nuremberg P, Horton JD, Cohen JC, et al. Prevalence of hepatic steatosis in an urban population in the United States: impact of ethnicity. Hepatology. 2004;40:1387–1395. doi: 10.1002/hep.20466. [DOI] [PubMed] [Google Scholar]
- 26.Bhatia LS, Curzen NP, Calder PC, Byrne CD. Non-alcoholic fatty liver disease: a new and important cardiovascular risk factor? Eur Heart J. 2012;33:1190–1200. doi: 10.1093/eurheartj/ehr453. [DOI] [PubMed] [Google Scholar]
- 27.Kunde SS, Lazenby AJ, Clements RH, Abrams GA. Spectrum of NAFLD and diagnostic implications of the proposed new normal range for serum ALT in obese women. Hepatology. 2005;42:650–656. doi: 10.1002/hep.20818. [DOI] [PubMed] [Google Scholar]
- 28.Prati D, Colli A, Conte D, Colombo M. Spectrum of NAFLD and diagnostic implications of the proposed new normal range for serum ALT in obese women. Hepatology. 2005;42:1460–1461. doi: 10.1002/hep.20964. [DOI] [PubMed] [Google Scholar]
- 29.Kechagias S, Nasr P, Blomdahl J, Ekstedt M. Established and emerging factors affecting the progression of nonalcoholic fatty liver disease. Metabolism. 2020;111S:154183. doi: 10.1016/j.metabol.2020.154183. [DOI] [PubMed] [Google Scholar]
- 30.Bedogni G, Bellentani S, Miglioli L, Masutti F, Passalacqua M, Castiglione A, et al. The fatty liver index: a simple and accurate predictor of hepatic steatosis in the general population. BMC Gastroenterol. 2006;6:33. doi: 10.1186/1471-230X-6-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lee JH, Kim D, Kim HJ, Lee CH, Yang JI, Kim W, et al. Hepatic steatosis index: a simple screening tool reflecting nonalcoholic fatty liver disease. Dig Liver Dis. 2010;42:503–508. doi: 10.1016/j.dld.2009.08.002. [DOI] [PubMed] [Google Scholar]
- 32.Kotronen A, Peltonen M, Hakkarainen A, Sevastianova K, Bergholm R, Johansson LM, et al. Prediction of non-alcoholic fatty liver disease and liver fat using metabolic and genetic factors. Gastroenterology. 2009;137:865–872. doi: 10.1053/j.gastro.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 33.Poynard T, Ratziu V, Naveau S, Thabut D, Charlotte F, Messous D, et al. The diagnostic value of biomarkers (SteatoTest) for the prediction of liver steatosis. Comp Hepatol. 2005;4:10. doi: 10.1186/1476-5926-4-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petta S, Amato M, Cabibi D, Camma C, Di Marco V, Giordano C, et al. Visceral adiposity index is associated with histological findings and high viral load in patients with chronic hepatitis C due to genotype 1. Hepatology. 2010;52:1543–1552. doi: 10.1002/hep.23859. [DOI] [PubMed] [Google Scholar]
- 35.Guerrero-Romero F, Simental-Mendía LE, González-Ortiz M, Martinez-Abundis E, Ramos-Zavala MG, Hernandez-Gonzales SO, et al. The product of triglycerides and glucose, a simple measure of insulin sensitivity. Comparison with the euglycemic-hyperinsulinemic clamp. J Clin Endocrinol Metab. 2010;95:3347–3351. doi: 10.1210/jc.2010-0288. [DOI] [PubMed] [Google Scholar]
- 36.Bedogni G, Kahn HS, Bellentani S, Tiribelli C. A simple index of lipid overaccumulation is a good marker of liver steatosis. BMC Gastroenterol. 2010;10:98. doi: 10.1186/1471-230X-10-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kahn HS. The “lipid accumulation product” performs better than the body mass index for recognizing cardiovascular risk: a population-based comparison. BMC Cardiovasc Disord. 2005;5:26. doi: 10.1186/1471-2261-5-26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fedchuk L, Nascimbeni F, Pais R, Charlotte F, Housset C, Ratziu V. Performance and limitations of steatosis biomarkers in patients with nonalcoholic fatty liver disease. Aliment Pharmacol Ther. 2014;40:1209–1222. doi: 10.1111/apt.12963. [DOI] [PubMed] [Google Scholar]
- 39.European Association for the Study of the Liver (EASL); European Association for the Study of Diabetes (EASD); European Association for the Study of Obesity (EASO). EASL-EASD-EASO clinical practice guidelines for the management of non-alcoholic fatty liver disease (2016) Diabetologia 59:1121–1140. 10.1007/s00125-016-3902-y [DOI] [PubMed]
- 40.Hernaez R, Lazo M, Bonekamp S, Kamel I, Brancati FL, Guallar E, et al. Diagnostic accuracy and reliability of ultrasonography for the detection of fatty liver: a meta-analysis. Hepatology. 2011;54:1082–1090. doi: 10.1002/hep.24452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Stefan N, Haring HU, Cusi K. Non-alcoholic fatty liver disease: causes, diagnosis, cardiometabolic consequences, and treatment strategies. Lancet Diabetes Endocrinol. 2019;7:313–324. doi: 10.1016/S2213-8587(18)30154-2. [DOI] [PubMed] [Google Scholar]
- 42.Bril F, Ortiz-Lopez C, Lomonaco R, Orsak B, Freckleton M, Chintapalli K, et al. Clinical value of liver ultrasound for the diagnosis of nonalcoholic fatty liver disease in overweight and obese patients. Liver Int. 2015;35:2139–2146. doi: 10.1111/liv.12840. [DOI] [PubMed] [Google Scholar]
- 43.Ryan CK, Johnson LA, Germin BI, Marcos A. One hundred consecutive hepatic biopsies in the workup of living donors for right lobe liver transplantation. Liver Transpl. 2002;8:1114–1122. doi: 10.1053/jlts.2002.36740. [DOI] [PubMed] [Google Scholar]
- 44.Strauss S, Gavish E, Gottlieb P, Katsnelson L. Interobserver and intraobserver variability in the sonographic assessment of fatty liver. Am J Roentgenol. 2007;189:W320–W323. doi: 10.2214/AJR.07.2123. [DOI] [PubMed] [Google Scholar]
- 45.Fujiwara Y, Kuroda H, Abe T, Ishida K, Oguri T, Noguchi S, et al. The B-mode image-guided ultrasound attenuation parameter accurately detects hepatic steatosis in chronic liver disease. Ultrasound Med Biol. 2018;44:2223–2232. doi: 10.1016/j.ultrasmedbio.2018.06.017. [DOI] [PubMed] [Google Scholar]
- 46.Tada T, Iijima H, Kobayashi N, Yoshida M, Nishimura T, Kumada T, et al. Usefulness of attenuation imaging with an ultrasound scanner for the evaluation of hepatic steatosis. Ultrasound Med Biol. 2019;45:2679–2687. doi: 10.1016/j.ultrasmedbio.2019.05.033. [DOI] [PubMed] [Google Scholar]
- 47.Tamaki N, Koizumi Y, Hirooka M, Yada N, Takada H, Nakashima O, et al. Novel quantitative assessment system of liver steatosis using a newly developed attenuation measurement method. Hepatol Res. 2018;48:821–828. doi: 10.1111/hepr.13179. [DOI] [PubMed] [Google Scholar]
- 48.Mikolasevic I, Orlic L, Franjic N, Hauser G, Stimac D, Milic S. Transient elastography (FibroScan®) with controlled attenuation parameter in the assessment of liver steatosis and fibrosis in patients with nonalcoholic fatty liver disease: where do we stand? World J Gastroenterol. 2016;22:7236–7251. doi: 10.3748/wjg.v22.i32.7236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Karlas T, Petroff D, Sasso M, Fan JG, Mi YQ, de Lédinghen V, et al. Individual patient data meta-analysis of controlled attenuation parameter (CAP) technology for assessing steatosis. J Hepatol. 2017;66:1022–1030. doi: 10.1016/j.jhep.2016.12.022. [DOI] [PubMed] [Google Scholar]
- 50.Caussy C, Alquiraish MH, Nguyen P, Hernandez C, Cepin S, Fortney LE, et al. Optimal threshold of controlled attenuation parameter with MRI-PDFF as the gold standard for the detection of hepatic steatosis. Hepatology. 2018;67:1348–1359. doi: 10.1002/hep.29639. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Petroff D, Blank V, Newsome PN, Shalimar VCS, Thiele M, et al. Assessment of hepatic steatosis by controlled attenuation parameter using the M and XL probes: an individual patient data meta-analysis. Lancet Gastroenterol Hepatol. 2021;6:185–198. doi: 10.1002/hep.29639. [DOI] [PubMed] [Google Scholar]
- 52.Ferraioli G, Tinelli C, Lissandrin R, Zicchetti M, Rondanelli M, Perani G, et al. Interobserver reproducibility of the controlled attenuation parameter (CAP) for quantifying liver steatosis. Hepatol Int. 2014;8:576–581. doi: 10.1007/s12072-014-9573-1. [DOI] [PubMed] [Google Scholar]
- 53.de Lédinghen V, Vergniol J, Capdepont M, Chermak F, Hiriart JB, Cassinotto C, et al. Controlled attenuation parameter (CAP) for the diagnosis of steatosis: a prospective study of 5323 examinations. J Hepatol. 2014;60:1026–1031. doi: 10.1016/j.jhep.2013.12.018. [DOI] [PubMed] [Google Scholar]
- 54.Sasso M, Audière S, Kemgang A, Gaouar F, Corpechot C, Chazouillères O, et al. Liver steatosis assessed by controlled attenuation parameter (CAP) measured with the XL probe of the FibroScan: a pilot study assessing diagnostic accuracy. Ultrasound Med Biol. 2016;42:92–103. doi: 10.1016/j.ultrasmedbio.2015.08.008. [DOI] [PubMed] [Google Scholar]
- 55.Caussy C, Brissot J, Singh S, Bassirian S, Hernandez C, Bettencourt R, et al. Prospective, same-day, direct comparison of controlled attenuation parameter with the M vs the XL Probe in patients with nonalcoholic fatty liver disease, using magnetic resonance imaging-proton density fat fraction as the standard. Clin Gastroenterol Hepatol. 2020;18:1842–1850.e6. doi: 10.1016/j.cgh.2019.11.060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Lee YH, Cho Y, Lee BW, Park CY, Lee DH, Cha BS, et al. Nonalcoholic fatty liver disease in diabetes. Part I: epidemiology and diagnosis. Diabetes Metab J. 2019;43:31–45. doi: 10.4093/dmj.2019.0011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chartampilas E. Imaging of nonalcoholic fatty liver disease and its clinical utility. Hormones (Athens) 2018;17:69–81. doi: 10.1007/s42000-018-0012-x. [DOI] [PubMed] [Google Scholar]
- 58.Piekarski J, Goldberg HI, Royal SA, Axel L, Moss AA. Difference between liver and spleen CT numbers in the normal adult: its usefulness in predicting the presence of diffuse liver disease. Radiology. 1980;137:727–729. doi: 10.1148/radiology.137.3.6934563. [DOI] [PubMed] [Google Scholar]
- 59.Park SH, Kim PN, Kim KW, Lee SW, Yoon SE, Park SW, et al. Macrovesicular hepatic steatosis in living liver donors: use of CT for quantitative and qualitative assessment. Radiology. 2006;239:105–112. doi: 10.1148/radiol.2391050361. [DOI] [PubMed] [Google Scholar]
- 60.Charatcharoenwitthaya P, Lindor KD. Role of radiologic modalities in the management of non-alcoholic steatohepatitis. Clin Liver Dis. 2007;11:37–54. doi: 10.1016/j.cld.2007.02.014. [DOI] [PubMed] [Google Scholar]
- 61.Roldan-Valadez E, Favila R, Martínez-López M, Uribe M, Ríos C, Méndez-Sánchez N. In vivo 3T spectroscopic quantification of liver fat content in nonalcoholic fatty liver disease: correlation with biochemical method and morphometry. J Hepatol. 2010;53:732–737. doi: 10.1016/j.jhep.2010.04.018. [DOI] [PubMed] [Google Scholar]
- 62.Szczepaniak LS, Nurenberg P, Leonard D, Browning JD, Reingold JS, Grundy S, et al. Magnetic resonance spectroscopy to measure hepatic triglyceride content: prevalence of hepatic steatosis in the general population. Am J Physiol Endocrinol Metab. 2005;288:E462–E468. doi: 10.1152/ajpendo.00064.2004. [DOI] [PubMed] [Google Scholar]
- 63.Bannas P, Kramer H, Hernando D, Agni R, Cunningham AM, Mandal R, et al. Quantitative magnetic resonance imaging of hepatic steatosis: validation in ex vivo human livers. Hepatology. 2015;62:1444–1455. doi: 10.1002/hep.28012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Gu J, Liu S, Du S, Zhang Q, Xiao J, Dong Q, et al. Diagnostic value of MRI-PDFF for hepatic steatosis in patients with non-alcoholic fatty liver disease: a meta-analysis. Eur Radiol. 2019;29:3564–3573. doi: 10.1007/s00330-019-06072-4. [DOI] [PubMed] [Google Scholar]
- 65.Park CC, Nguyen P, Hernandez C, Bettencourt R, Ramirez K, Fortney L, et al. Magnetic resonance elastography vs transient elastography in detection of fibrosis and noninvasive measurement of steatosis in patients with biopsy-proven nonalcoholic fatty liver disease. Gastroenterology. 2017;152:598–607.e2. doi: 10.1053/j.gastro.2016.10.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Imajo K, Kessoku T, Honda Y, Tomeno W, Ogawa Y, Mawatari H, et al. Magnetic resonance imaging more accurately classifies steatosis and fibrosis in patients with nonalcoholic fatty liver disease than transient elastography. Gastroenterology. 2016;150:626–637.e7. doi: 10.1053/j.gastro.2015.11.048. [DOI] [PubMed] [Google Scholar]
- 67.Dulai PS, Sirlin CB, Loomba R. MRI and MRE for non-invasive quantitative assessment of hepatic steatosis and fibrosis in NAFLD and NASH: clinical trials to clinical practice. J Hepatol. 2016;65:1006–1016. doi: 10.1016/j.jhep.2016.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Loomba R, Sirlin CB, Ang B, Bettencourt R, Jain R, Salotti J, et al. Ezetimibe for the treatment of nonalcoholic steatohepatitis: assessment by novel magnetic resonance imaging and magnetic resonance elastography in a randomized trial (MOZART trial) Hepatology. 2015;61:1239–1250. doi: 10.1002/hep.27647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Hagström H, Nasr P, Ekstedt M, Hammar U, Stål P, Hultcrantz R, et al. Risk for development of severe liver disease in lean patients with nonalcoholic fatty liver disease: a long-term follow-up study. Hepatol Commun. 2017;2:48–57. doi: 10.1002/hep4.1124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Grgurevic I, Podrug K, Mikolasevic I, Kukla M, Madir A, Tsochatzis EA. Natural history of nonalcoholic fatty liver disease: implications for clinical practice and an individualized approach. Can J Gastroenterol Hepatol. 2020;2020:9181368. doi: 10.1155/2020/9181368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nasr P, Forsgren MF, Ignatova S, Dahlström N, Cedersund G, Leinhard OD, et al. Using a 3% proton density fat fraction as a cut-off value increases sensitivity of detection of hepatic steatosis, based on results from histopathology analysis. Gastroenterology. 2017;153:53–55.e7. doi: 10.1053/j.gastro.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 72.Bril F, Barb D, Portillo-Sanchez P, Biernacki D, Lomonaco R, Suman A, et al. Metabolic and histological implications of intrahepatic triglyceride content in nonalcoholic fatty liver disease. Hepatology. 2017;65:1132–1144. doi: 10.1002/hep.28985. [DOI] [PubMed] [Google Scholar]
- 73.Wildman-Tobriner B, Middleton MM, Moylan CA, Rossi S, Flores O, Chang ZA, et al. Association between magnetic resonance imaging-proton density fat fraction and liver histology features in patients with nonalcoholic fatty liver disease or nonalcoholic steatohepatitis. Gastroenterology. 2018;155:1428–1435. doi: 10.1053/j.gastro.2018.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.VanWagner LB, Rinella ME. Extrahepatic manifestations of nonalcoholic fatty liver disease. Curr Hepatol Rep. 2016;15:75–85. doi: 10.1007/s11901-016-0295-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Ginès P, Graupera I, Lammert F, Angeli P, Caballeria L, Krag A, et al. Screening for liver fibrosis in the general population: a call for action. Lancet Gastroenterol Hepatol. 2016;1:256–260. doi: 10.1016/S2468-1253(16)30081-4. [DOI] [PubMed] [Google Scholar]
- 76.Angulo P, Hui JM, Marchesini G, Bugianesi E, George J, Farrell GC, et al. The NAFLD fibrosis score: a noninvasive system that identifies liver fibrosis in patients with NAFLD. Hepatology. 2007;45:846–854. doi: 10.1002/hep.21496. [DOI] [PubMed] [Google Scholar]
- 77.Sterling RK, Lissen E, Clumeck N, Sola R, Correa MC, Montaner J, et al. Development of a simple noninvasive index to predict significant fibrosis in patients with HIV/HCV coinfection. Hepatology. 2006;43:1317–1325. doi: 10.1002/hep.21178. [DOI] [PubMed] [Google Scholar]
- 78.Angulo P, Bugianesi E, Björnsson ES, Charatcharoenwitthaya P, Mills PR, Barrera F, et al. Simple noninvasive systems predict long-term outcomes of patients with nonalcoholic fatty liver disease. Gastroenterology. 2013;145:782–789.e4. doi: 10.1053/j.gastro.2013.06.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.McPherson S, Hardy T, Dufour JF, Petta S, Romero-Gomez M, Allison M, et al. Age as a confounding factor for the accurate non-invasive diagnosis of advanced NAFLD fibrosis. Am J Gastroenterol. 2017;112:740–751. doi: 10.1038/ajg.2016.453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Boursier J, Guillaume M, Leroy V, Irles M, Roux M, Lannes A, et al. New sequential combinations of non-invasive fibrosis tests provide an accurate diagnosis of advanced fibrosis in NAFLD. J Hepatol. 2019;71:389–396. doi: 10.1016/j.jhep.2019.04.020. [DOI] [PubMed] [Google Scholar]
- 81.Abeysekera KWM, Fernandes GS, Hammerton G, Portal AJ, Gordon FH, Heron J, et al. Prevalence of steatosis and fibrosis in young adults in the UK: a population-based study. Lancet Gastroenterol Hepatol. 2020;5:295–305. doi: 10.1016/S2468-1253(19)30419-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Parkes J, Roderick P, Harris S, Day C, Mutimer D, Collier J, et al. Enhanced liver fibrosis test can predict clinical outcomes in patients with chronic liver disease. Gut. 2010;59:1245–1251. doi: 10.1136/gut.2009.203166. [DOI] [PubMed] [Google Scholar]
- 83.Daniels SJ, Leeming DJ, Eslam M, Hashem AM, Nielsen MJ, Krag A, et al. ADAPT: An algorithm incorporating PRO-C3 accurately identifies patients with NAFLD and advanced fibrosis. Hepatology. 2019;69:1075–1086. doi: 10.1002/hep.30163. [DOI] [PubMed] [Google Scholar]
- 84.Lykiardopoulos B, Hagström H, Fredrikson M, Ignatova S, Stål P, Hultcrantz R, et al. Development of serum marker models to increase diagnostic accuracy of advanced fibrosis in nonalcoholic fatty liver disease: the new LINKI algorithm compared with established algorithms. PLoS One. 2016;11(12):e0167776. doi: 10.1371/journal.pone.0167776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Ampuero J, Pais R, Aller R, Gallego-Durán R, Crespo J, Garcia-Monzón C, et al. Development and validation of Hepamet fibrosis scoring system-a simple, noninvasive test to identify patients with nonalcoholic fatty liver disease with advanced fibrosis. Clin Gastroenterol Hepatol. 2020;18:216–225. doi: 10.1016/j.cgh.2019.05.051. [DOI] [PubMed] [Google Scholar]
- 86.Ampuero J, Aller R, Gallego-Durán R, Banales J, Crespo J, Mora-Cuadrado N, et al. Clinical outcomes in biopsy-proven NAFLD patients from the HEPAmet Spanish Registry. J Hepatol. 2018;68(Suppl 1):S833. doi: 10.1016/S0168-8278(18)31942-1. [DOI] [Google Scholar]
- 87.Åberg F, Danford CJ, Thiele M, Talbäck M, Rasmussen DN, Jiang ZG, et al. A dynamic aspartate-to-alanine aminotransferase ratio provides valid predictions of incident severe liver disease. Hepatol Commun. 2021;5:1021–1035. doi: 10.1002/hep4.1700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.McPherson S, Stewart SF, Henderson E, Burt AD, Day CP. Simple non-invasive fibrosis scoring systems can reliably exclude advanced fibrosis in patients with non-alcoholic fatty liver disease. Gut. 2010;59:1265–1269. doi: 10.1136/gut.2010.216077. [DOI] [PubMed] [Google Scholar]
- 89.Sun W, Cui H, Li N, Wei Y, Lai S, Yang Y, et al. Comparison of FIB-4 index, NAFLD fibrosis score and BARD score for prediction of advanced fibrosis in adult patients with non-alcoholic fatty liver disease: a meta-analysis study. Hepatol Res. 2016;46:862–870. doi: 10.1111/hepr.12647. [DOI] [PubMed] [Google Scholar]
- 90.Shah AG, Lydecker A, Murray K, Tetri BN, Contos MJ, Sanyal AJ. Comparison of noninvasive markers of fibrosis in patients with nonalcoholic fatty liver disease. Clin Gastroenterol Hepatol. 2009;7:1104–1112. doi: 10.1016/j.cgh.2009.05.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Harrison SA, Oliver D, Arnold HL, Gogia S, Neuschwander-Tetri BA. Development and validation of a simple NAFLD clinical scoring system for identifying patients without advanced disease. Gut. 2008;57:1441–1447. doi: 10.1136/gut.2007.146019. [DOI] [PubMed] [Google Scholar]
- 92.Ruffillo G, Fassio E, Alvarez E, Landeira G, Longo C, Dominguez N, et al. Comparison of NAFLD fibrosis score and BARD score in predicting fibrosis in nonalcoholic fatty liver disease. J Hepatol. 2011;54:160–163. doi: 10.1016/j.jhep.2010.06.028. [DOI] [PubMed] [Google Scholar]
- 93.Guha IN, Parkes J, Roderick P, Chattopadhyay D, Cross R, Harris S, et al. Noninvasive markers of fibrosis in nonalcoholic fatty liver disease: validating the European Liver Fibrosis Panel and exploring simple markers. Hepatology. 2008;47:455–460. doi: 10.1002/hep.21984. [DOI] [PubMed] [Google Scholar]
- 94.Rosenberg WM, Voelker M, Thiel R, Becka M, Burt A, Schuppan D, et al. Serum markers detect the presence of liver fibrosis: a cohort study. Gastroenterology. 2004;127:1704–1713. doi: 10.1053/j.gastro.2004.08.052. [DOI] [PubMed] [Google Scholar]
- 95.Calès P, Oberti F, Michalak S, Hubert-Fouchard I, Rousselet MC, Konaté A, et al. A novel panel of blood markers to assess the degree of liver fibrosis. Hepatology. 2005;42:1373–1381. doi: 10.1002/hep.20935. [DOI] [PubMed] [Google Scholar]
- 96.Ratziu V, Massard J, Charlotte F, Messous D, Imbert-Bismout F, Bonyhay L, et al. Diagnostic value of biochemical markers (FibroTest-FibroSURE) for the prediction of liver fibrosis in patients with non-alcoholic fatty liver disease. BMC Gastroenterol. 2006;6:6. doi: 10.1186/1471-230X-6-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ozturk A, Grajo JR, Dhyani M, Anthony BW, Samir AE. Principles of ultrasound elastography. Abdom Radiol (NY) 2018;43:773–785. doi: 10.1007/s00261-018-1475-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Papatheodoridi M, Hiriart JB, Lupsor-Platon M, Bronte F, Boursier J, Elshaarawy O, Marra F, et al. Refining the Baveno VI elastography criteria for the definition of compensated advanced chronic liver disease. J Hepatol. 2021;74:1109–1116. doi: 10.1016/j.jhep.2020.11.050. [DOI] [PubMed] [Google Scholar]
- 99.Shen F, Zheng RD, Shi JP, Mi YQ, Chen GF, Hu X, et al. Impact of skin capsular distance on the performance of controlled attenuation parameter in patients with chronic liver disease. Liver Int. 2015;35:2392–2400. doi: 10.1111/liv.12809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Vuppalanchi R, Siddiqui MS, Van Natta ML, Hallinan E, Brandman D, Kowdley K, et al. Performance characteristics of vibration-controlled transient elastography for evaluation of nonalcoholic fatty liver disease. Hepatology. 2018;67:134–144. doi: 10.1002/hep.29489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101.Zhou JH, Cai JJ, She ZG, Li HL. Noninvasive evaluation of nonalcoholic fatty liver disease: current evidence and practice. World J Gastroenterol. 2019;25:1307–1326. doi: 10.3748/wjg.v25.i11.1307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Cassinotto C, Boursier J, de Ledinghen V, Lebigot J, Lapuyade B, Cales P, et al. Liver stiffness in nonalcoholic fatty liver disease: a comparison of supersonic shear imaging, FibroScan, and ARFI with liver biopsy. Hepatology. 2016;63:1817–1827. doi: 10.1002/hep.28394. [DOI] [PubMed] [Google Scholar]
- 103.Venkatesh SK, Yin M, Ehman RL. Magnetic resonance elastography of liver: technique, analysis, and clinical applications. J Magn Reson Imaging. 2013;37:544–555. doi: 10.1002/jmri.23731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Singh S, Venkatesh SK, Loomba R, Wang Z, Sirlin C, Chen J, et al. Magnetic resonance elastography for staging liver fibrosis in non-alcoholic fatty liver disease: a diagnostic accuracy systematic review and individual participant data pooled analysis. Eur Radiol. 2016;26:1431–1440. doi: 10.1007/s00330-015-3949-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim D, Kim WR, Talwalkar JA, Kim HJ, Ehman RL. Advanced fibrosis in nonalcoholic fatty liver disease: noninvasive assessment with MR elastography. Radiology. 2013;268:411–419. doi: 10.1148/radiol.13121193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Loomba R, Wolfson T, Ang B, Hooker J, Behling C, Peterson M, et al. Magnetic resonance elastography predicts advanced fibrosis in patients with nonalcoholic fatty liver disease: a prospective study. Hepatology. 2014;60:1920–1928. doi: 10.1002/hep.27362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Krawczyk M, Liebe R, Lammert F. Toward genetic prediction of nonalcoholic fatty liver disease trajectories: PNPLA3 and beyond. Gastroenterology. 2020;158:1865–1880. doi: 10.1053/j.gastro.2020.01.053. [DOI] [PubMed] [Google Scholar]
- 108.Romeo S, Kozlitina J, Xing C, Pertsemlidis A, Cox D, Pennachio LA, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2008;40:1461–1465. doi: 10.1038/ng.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wang Y, Kory N, BasuRay S, Cohen JC, Hobbs HH. PNPLA3, CGI-58, and inhibition of hepatic triglyceride hydrolysis in mice. Hepatology. 2019;69:2427–2441. doi: 10.1002/hep.30583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Ajmera V, Liu A, Bettencourt R, Dhar D, Richards L, Loomba R. The impact of genetic risk on liver fibrosis in non-alcoholic fatty liver disease as assessed by magnetic resonance elastography. Aliment Pharmacol Ther. 2021;54:68–77. doi: 10.1111/apt.16392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Eslam M, Valenti L, Romeo S. Genetics and epigenetics of NAFLD and NASH: clinical impact. J Hepatol. 2018;68:268–279. doi: 10.1016/j.jhep.2017.09.003. [DOI] [PubMed] [Google Scholar]
- 112.Kozlitina J, Smagris E, Stender S, Nordestgaard BG, Zhou HH, Tybjaerg-Hansen A, et al. Exome-wide association study identifies a TM6SF2 variant that confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. 2014;46:352–356. doi: 10.1038/ng.2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Liu YL, Reeves HL, Burt AD, Tiniakos D, McPherson S, Leathart JBS, et al. TM6SF2 rs58542926 influences hepatic fibrosis progression in patients with nonalcoholic fatty liver disease. Nat Commun. 2014;5:4309. doi: 10.1038/ncomms5309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Beer NL, Tribble ND, McCulloch LJ, Roos C, Johnson PRV, Orho-Melander M, et al. The P446L variant in GCKR associated with fasting plasma glucose and triglyceride levels exerts its effect through increased glucokinase activity in liver. Hum Mol Genet. 2009;18:4081–4088. doi: 10.1093/hmg/ddp357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tan HL, Zain SM, Mohamed R, Rampal S, Chin KH, Basu RC, et al. Association of glucokinase regulatory gene polymorphisms with risk and severity of nonalcoholic fatty liver disease: an interaction study with adiponutrin gene. J Gastroenterol. 2014;49:1056–1064. doi: 10.1007/s00535-013-0850-x. [DOI] [PubMed] [Google Scholar]
- 116.Yu C, Xu C, Xu L, Yu J, Miao M, Li Y. Serum proteomic analysis revealed diagnostic value of hemoglobin for nonalcoholic fatty liver disease. J Hepatol. 2012;56:241–247. doi: 10.1016/j.jhep.2011.05.027. [DOI] [PubMed] [Google Scholar]
- 117.Kalhan SC, Guo L, Edmison J, Dasarathy S, McCullough AJ, Hanson RW, et al. Plasma metabolomic profile in nonalcoholic fatty liver disease. Metabolism. 2011;60:404–413. doi: 10.1016/j.metabol.2010.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Oresic M, Hyotylainen T, Kotronen A, Gopalacharyulu P, Nygren H, Arola J, et al. Prediction of non-alcoholic fatty-liver disease and liver fat content by serum molecular lipids. Diabetologia. 2013;56:2266–2274. doi: 10.1007/s00125-013-2981-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mayo R, Crespo J, Martinez-Arranz I, Banales JM, Arias M, Minchole I, et al. Metabolomic-based noninvasive serum test to diagnose nonalcoholic steatohepatitis: Results from discovery and validation cohorts. Hepatol Commun. 2018;2:807–820. doi: 10.1002/hep4.1188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Pos O, Biro O, Szemes T, Nagy B. Circulating cell-free nucleic acids: Characteristics and applications. Eur J Hum Genet. 2018;26:937–945. doi: 10.1038/s41431-018-0132-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.Turchinovich A, Baranova A, Drapkina O, Tonevitsky A. Cell-free circulating nucleic acids as early biomarkers for NAFLD and NAFLD-associated disorders. Front Physiol. 2018;9:1256. doi: 10.3389/fphys.2018.01256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tan Y, Ge G, Pan T, Wen D, Gan J. A pilot study of serum microRNAs panel as potential biomarkers for diagnosis of nonalcoholic fatty liver disease. PLoS ONE. 2014;9:e105192. doi: 10.1371/journal.pone.0105192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Johnson K, Leary PJ, Govaere O, Barter MJ, Charlton SH, Cockell SJ, et al. Increased serum miR-193a-5p during non-alcoholic fatty liver disease progression: Diagnostic and mechanistic relevance. JHEP Rep. 2021;4:100409. doi: 10.1016/j.jhepr.2021.100409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Aron-Wisnewsky J, Gaborit B, Dutour A, Clement K. Gut microbiota and non-alcoholic fatty liver disease: new insights. Clin Microbiol Infect. 2013;19:338–348. doi: 10.1111/1469-0691.12140. [DOI] [PubMed] [Google Scholar]
- 125.Shen F, Zheng RD, Sun XQ, Ding WJ, Wang XY, Fan JG. Gut microbiota dysbiosis in patients with non-alcoholic fatty liver disease. Hepatobiliary Pancreat Dis Int. 2017;16:375–381. doi: 10.1016/S1499-3872(17)60019-5. [DOI] [PubMed] [Google Scholar]
- 126.Boursier J, Mueller O, Barret M, Machado M, Fizanne L, Araujo-Perez F, et al. The severity of nonalcoholic fatty liver disease is associated with gut dysbiosis and shift in the metabolic function of the gut microbiota. Hepatology. 2016;63:764–775. doi: 10.1002/hep.28356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Loomba R, Seguritan V, Li W, Long T, Klitgord N, Bhatt A, et al. Gut microbiome-based metagenomic signature for non-invasive detection of advanced fibrosis in human nonalcoholic fatty liver disease. Cell Metab. 2017;25:1054–1062. doi: 10.1016/j.cmet.2017.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Lang S, Farowski F, Martin A, Wisplinghoff H, Vehreschild MJGT, Krawczyk M, et al. Prediction of advanced fibrosis in non-alcoholic fatty liver disease using gut microbiota-based approaches compared with simple non-invasive tools. Sci Rep. 2020;10:9385. doi: 10.1038/s41598-020-66241-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Llop E, Iruzubieta P, Perelló C, Cabezas J, Escudero-Garcia D, Gonzáles M, et al. Transient elastography as a method of screening of chronic hepatic disease in apparently healthy population. Results from ETHON cohort J Hepatol. 2018;68(Suppl 1):S634. [Google Scholar]
- 130.Zhang E, Wartelle-Bladou C, Lepanto L, Lachaine J, Cloutier G, Tang A. Cost-utility analysis of nonalcoholic steatohepatitis screening. Eur Radiol. 2015;25:3282–3294. doi: 10.1007/s00330-015-3731-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Phisalprapa P, Supakankunti S, Charatcharoenwitthaya P, Apisarnthanarak P, Charoensak A, Washirasaksiri C, et al. Cost-effectiveness analysis of ultrasonography screening for nonalcoholic fatty liver disease in metabolic syndrome patients. Medicine (Baltimore) 2017;96:e6585. doi: 10.1097/MD.0000000000006585. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 132.Tanajewski L, Harris R, Harman DJ, Aithal GP, Card TR, Gkountouras G, et al. Economic evaluation of a community-based diagnostic pathway to stratify adults for nonalcoholic fatty liver disease: a Markov model informed by a feasibility study. BMJ Open. 2017;7:e015659. doi: 10.1136/bmjopen-2016-015659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Caballeria L, Pera G, Arteaga I, Rodriguez L, Aluma A, Morillas RM, et al. High prevalence of liver fibrosis among European adults with unknown liver disease: a population-based study. Clin Gastroenterol Hepatol. 2018;16(1138–1145):e1135. doi: 10.1016/j.cgh.2017.12.048. [DOI] [PubMed] [Google Scholar]
- 134.Koehler EM, Plompen EP, Schouten JN, Hansen BE, Darwish Murad S, Taimr P, et al. Presence of diabetes mellitus and steatosis is associated with liver stiffness in a general population: the Rotterdam study. Hepatology. 2016;63:138–147. doi: 10.1002/hep.27981. [DOI] [PubMed] [Google Scholar]
- 135.Roulot D, Costes JL, Buyck JF, Warzocha U, Gambier N, Czernichow S, et al. Transient elastography as a screening tool for liver fibrosis and cirrhosis in a community-based population aged over 45 years. Gut. 2011;60:977–984. doi: 10.1136/gut.2010.221382. [DOI] [PubMed] [Google Scholar]
- 136.Sporea I, Mare R, Popescu A, Nistorescu S, Baldea V, Sirli R, et al. Screening for liver fibrosis and steatosis in a large cohort of patients with type 2 diabetes using vibration controlled transient elastography and controlled attenuation parameter in a single-center real-life experience. J Clin Med. 2020;9:1032. doi: 10.3390/jcm9041032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Lai LL, Wan Yusoff WNI, Vethakkan SR, Nik Mustapha NR, Mahadeva S, Chan WK. Screening for non-alcoholic fatty liver disease in patients with type 2 diabetes mellitus using transient elastography. J Gastroenterol Hepatol. 2019;34:1396–1403. doi: 10.1111/jgh.14577. [DOI] [PubMed] [Google Scholar]
- 138.Roulot D, Roudot-Thoraval F, NKontchou G, Kouacou N, Costes JL, Elourimi G, et al. Concomitant screening for liver fibrosis and steatosis in French type 2 diabetic patients using Fibroscan. Liver Int. 2017;37:1897–1906. doi: 10.1111/liv.13481. [DOI] [PubMed] [Google Scholar]
- 139.Di Cesare M, Soric M, Bovet P, Miranda JJ, Bhutta Z, Stevens GA, et al. The epidemiological burden of obesity in childhood: a worldwide epidemic requiring urgent action. BMC Med. 2019;17:212. doi: 10.1186/s12916-019-1449-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Type 2 diabetes: the urgent need to protect young people (2018) Lancet 392:2325. 10.1016/S0140-6736(18)33015-0 [DOI] [PubMed]
- 141.Lascar N, Brown J, Pattison H, Barnett AH, Bailey CJ, Bellary S. Type 2 diabetes in adolescents and young adults. Lancet Diabetes Endocrinol. 2018;6:69–80. doi: 10.1016/S2213-8587(17)30186-9. [DOI] [PubMed] [Google Scholar]
- 142.Powell EE, Cooksley WG, Hanson R, Searle J, Halliday JW, Powell LW. The natural history of nonalcoholic steatohepatitis: a follow-up study of forty-two patients for up to 21 years. Hepatology. 1990;11:74–80. doi: 10.1002/hep.1840110114. [DOI] [PubMed] [Google Scholar]
- 143.Vilar-Gomez E, Calzadilla-Bertot L, Wai-Sun Wong V, Castellanos M, Aller-de la Fuente R, Metwally M, et al. Fibrosis severity as a determinant of cause-specific mortality in patients with advanced nonalcoholic fatty liver disease: a multi-national cohort study. Gastroenterology. 2018;155(443–57):e17. doi: 10.1053/j.gastro.2018.04.034. [DOI] [PubMed] [Google Scholar]
- 144.Nasr P, Iredahl F, Dahlström N, Rådholm K, Henriksson P, Cedersund G, et al. Evaluating the prevalence and severity of NAFLD in primary care: the EPSONIP study protocol. BMC Gastroenterol. 2021;21:180. doi: 10.1186/s12876-021-01763-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Xiao G, Zhu S, Xiao X, Yan L, Yang J, Wu G. Comparison of laboratory tests, ultrasound, or magnetic resonance elastography to detect fibrosis in patients with nonalcoholic fatty liver disease: a meta-analysis. Hepatology. 2017;66:1486–1501. doi: 10.1002/hep.2930. [DOI] [PubMed] [Google Scholar]
- 146.Anstee QM, Lawitz EJ, Alkhouri N, Wong VW, Romero-Gomez M, Okanoue T, et al. Noninvasive tests accurately identify advanced fibrosis due to NASH: baseline data from the STELLAR trials. Hepatology. 2019;70:1521–1530. doi: 10.1002/hep.30842. [DOI] [PubMed] [Google Scholar]
- 147.Guillaume M, Moal V, Delabaudiere C, Zuberbuhler F, Robic MA, Lannes A, et al. Direct comparison of the specialised blood fibrosis tests FibroMeter(V2G) and Enhanced Liver Fibrosis score in patients with non-alcoholic fatty liver disease from tertiary care centres. Aliment Pharmacol Ther. 2019;50:1214–1222. doi: 10.1111/apt.15529. [DOI] [PubMed] [Google Scholar]
- 148.Herrmann E, de Ledinghen V, Cassinotto C, Chu WC, Leung VY, Ferraioli G, et al. Assessment of biopsy-proven liver fibrosis by two-dimensional shear wave elastography: an individual patient data-based meta-analysis. Hepatology. 2018;67:260–272. doi: 10.1002/hep.29179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Jiang W, Huang S, Teng H, Wang P, Wu M, Zhou X, et al. Diagnostic accuracy of point shear wave elastography and transient elastography for staging hepatic fibrosis in patients with non-alcoholic fatty liver disease: a meta-analysis. BMJ Open. 2018;8:e021787. doi: 10.1136/bmjopen-2018-021787. [DOI] [PMC free article] [PubMed] [Google Scholar]