Abstract
Infective endocarditis is a life-threatening disease that is associated with a significant risk of morbidity and mortality. One of the most serious complications of infective endocarditis is perivalvular and aortic root abscess formation. Due to the high propensity for rupture and continued spread within the aorta and surrounding organs, surgical management is recommended and can improve long-term survival. Imaging plays a critical role in diagnosis of infective endocarditis and its sequalae. Initial workup includes transthoracic and/or transesophageal echocardiography, as part of the modified Duke criteria for diagnosing infective endocarditis. If paravalvular abscesses are suspected, CTA chest can characterize invasion and spread of the abscess. Here, we present a 55-year-old male with recurrent infective endocarditis with an aortic root abscess. The abscess was first identified through transesophageal echocardiography and subsequently confirmed using CTA chest. Surgically, the patient required pulmonic and aortic valve replacement along with aortic root reconstruction.
Keywords: Aortic root abscess, Paravalvular abscess, Infective endocarditis, Modified Duke criteria, Bentall procedure
Abbreviations: CTA, computed tomography angiography; MRI, magnetic resonance imaging; SPECT, single photon emission computed tomography; TEE, transesophageal echocardiogram; TTE, transthoracic echocardiogram; IE, infective endocarditis; GMS, Grocott's Methenamine Silver
Background
An abscess involving the aortic root almost exclusively occurs in the setting of endocarditis [1]. Incidence of infective endocarditis is generally between 1.5 and 11.6 cases per 100,000 person-years [2], [3], [4], with incidence of death at 1.42 per 100,000 person-years [5]. Overall, mortality of infective endocarditis is approximately 25% [6], including in-hospital mortality (28.9%) and mortality at 1 year (11.2%) [7]. Risk factors for infective endocarditis include structural heart disease, prosthetic valves, implanted cardiac devices, congenital heart disease, immunosuppressive therapy or diseases, and injection drug use [6].
The microbiological origin of infective endocarditis includes staphylococcal and streptococcal species in 80% of diagnoses made in children and adults [6,8]. Important streptococcal species include Viridans Streptococci and Streptococcus gallolyticus, with other causes of infective endocarditis including Enterococci, such as Enterococcus faecalis, and Gram-negative bacilli [9]. Healthcare-associated infective endocarditis is also a concern, leading to rising rates of staphylococcal infections [10], [11], [12]. Outside of bacterial origins, infective endocarditis can be the result of Candida and Aspergillus species [13], which typically present in a subacute manner.
Aortic root abscesses generally require surgical intervention, which has been associated with significantly reduced morbidity and mortality [3,7]. The most common interventions include complete resection/reconstruction of the aortic root abscess through graft placement with a new mechanical valve [1,14]. Alternatively, in patients where the mechanical valve is still functional, it is possible to perform a valve-sparing technique that only involves resection or reconstruction of the aortic root [15]. While postsurgical complications should always be considered, the risk of recurrent infective endocarditis, as well as complications to the distal aorta, should be monitored [16].
The patient presented is a 55-year-old male with recurrent infective endocarditis with an aortic root abscess. Surgically, the patient required pulmonic and aortic valve replacement along with aortic root reconstruction through the Bentall procedure (ie, utilizing a mechanical valved conduit to replace the aortic root [17]).
Case report
We present a 55-year-old male who was admitted to the emergency department for tremors, rigors, and night sweats for a 2–3-month duration. Patient had 60 lbs. weight loss in the prior year. He denied chest pain, but had worsening dyspnea with exertion. Past medical history was significant for hypertension, mixed hyperlipidemia, and endocarditis and a past surgical history of pulmonic (cadaveric, 28 years prior) and aortic (Ross procedure, 28 years prior with mechanical revision 5 years prior) valve replacements.
Physical exam revealed an afebrile, acutely ill-appearing man. Because of suspected infective endocarditis, blood cultures were obtained and transthoracic echocardiogram (TTE) and computed tomography angiography (CTA) chest/abdomen/pelvis performed. Troponin initially 40 ng/mL and BNP 500 pg/mL. Vancomycin and ceftriaxone empirically started, and blood cultures grew Enterococcus faecalis.
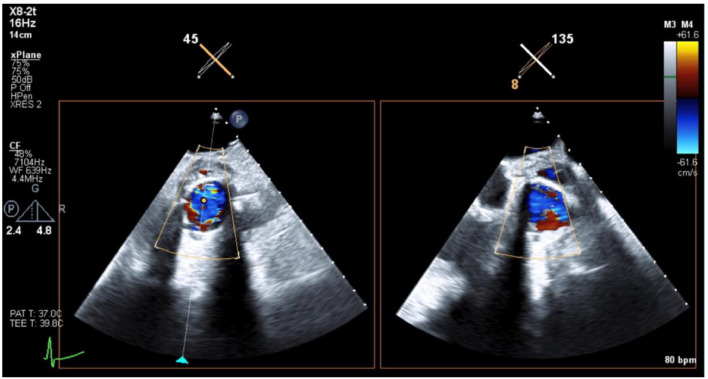
TTE revealed trace aortic regurgitation. The aortic prosthesis demonstrated a normal transvalvular gradient for valve type and size, with no evidence of paravalvular aortic regurgitation (Fig. 1). The pulmonic valve revealed evidence of pulmonic regurgitation. A mobile density was observed on the pulmonic valve compatible with a vegetation (Fig. 2). Increased gradient across the pulmonic valve suggested severe stenosis. Transesophageal echocardiogram (TEE) revealed tissue changes in the aortic paravalvular area, which were concerning for abscess, but could not be clearly delineated. CTA chest was subsequently performed and revealed an oval thrombus (8 × 16 mm) at the level of the pulmonary valve (Figs. 3A and B). CTA chest also revealed near circumferential extraluminal contrast of the aortic root suggesting aortic root abscess, 10 mm thickness × 29 mm craniocaudal (Figs. 4A and B). No extraluminal gas or pneumomediastinum was appreciated.
Fig. 1.
Transthoracic echocardiogram (TTE) images centered at the aortic valve showed mild nonspecific peri-valvular thickening around the aortic valve, without evidence of extraluminal flow or focal fluid collection.
Fig. 2.
Transthoracic echocardiogram (TTE) centered at the pulmonic valve showed a thrombus originating from valve surface.
Fig. 3.
CTA heart morphology axial (A) and coronal (B) images showed an 8 mm × 16 mm soft oval tissue originating from the pulmonic valve. Considering the patients presentation this was concerning for infective pulmonic valve endocarditis, bland thrombus was another consideration.
Fig. 4.
CTA heart morphology axial (A-C) shows bileaflet prosthetic aortic valve with circumferential extravascular contrast around the aortic root measuring 10 mm in thickness and 29 mm in craniocaudal dimension (yellow arrow). In addition to circumferential aortic root thickening (white arrows) measuring 10 mm, suggestive of aortic root abscess with contained leak. Filling defect around the prosthetic valve was also present suggesting potential endocarditis (red arrow). No evidence of intramural gas.
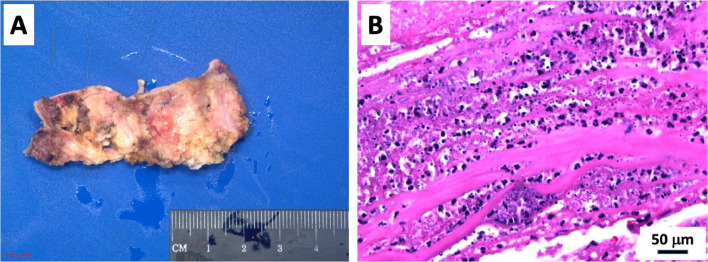
Following imaging, surgical revision of the pulmonic and aortic valves was completed. A sternotomy was performed, aortic root abscess/phlegmon debrided, and double root replacement (ie, aortic root replacement with St. Jude valved graft 23 mm (Bentall procedure) and right ventricular outflow tract reconstruction with pulmonary homograft 29 mm). The aortic and pulmonic valves were sent to Pathology, revealing marked fibrosis, calcification and vegetations, consistent with infective endocarditis on the pulmonic valve (Figs. 5A and B). Gram and Grocott's Methenamine Silver (GMS) stain showed numerous colonies of Gram variable coccal organisms in the vegetation (Figs. 6A and B). The mechanical aortic valve revealed adherent connective tissue containing fibrin and vegetations consistent with history of abscess of the aortic root.
Fig. 5.
Excised pulmonic valve homograft showing adherent vegetations. Hematoxylin and eosin stain showing the necro-inflammatory vegetations on the pulmonary homograft.
Fig. 6.
Gram stain highlighting numerous colonies of Gram variable coccal organisms in the vegetation. GMS stain highlighting numerous colonies of nonviable coccal organisms in the vegetation.
Currently, the patient continues to follow with cardiology and cardiothoracic surgery and is undergoing cardiac rehabilitation. He has remained afebrile, without rigors or night sweats and was discharged from the hospital.
Discussion
Imaging is pivotal in the diagnosis and management of infective endocarditis and its complications. The major components of the modified Duke criteria include 1) bacteremia by suspected organisms and 2) echocardiographic findings of valvular vegetations or new regurgitant murmur [18]. In comparing our patient's presentation, vegetations on the pulmonic valve were able to be visualized by TTE, while TEE was needed to appreciate the aortic root abscess. On echocardiography, paravalvular abscesses are likely to appear as areas of nonhomogeneous perivalvular thickening with hyperechoic features [19,20].
The use of CTA can more conclusively define aortic root and perivalvular abscesses. As in the presented patient, extraluminal contrast provides a marker for abscess formation, with concurrent inflammatory processes represented as hypodense thickening, which was difficult to appreciate using TEE. Other complications from aortic root abscesses can include phlegmon accumulation and gas locules within surrounding tissue [21]. Other imaging techniques useful in aiding management of aortic root abscesses include CT, MRI, and Gallium-67 SPECT [22].
The formation of a paravalvular abscess in the setting of infective endocarditis ranges from 10% to 30% [23]. While congenital, structural, and valvular changes to the heart are considered traditional risk factors for acquiring infective endocarditis, these same indices can also predict progression toward abscess formation. For example, aortic paravalvular leak, mechanical prosthesis, aortic valve vegetations, and an undetected organism on blood culture have been associated with higher likelihood of aortic root abscess formation [24]. Likewise, prosthetic valve involvement, increasing age, pulmonary edema, mitral valve vegetation, and paravalvular complications have been linked to increased in-hospital mortality [12].
Our patient presented with Enterococcus faecalis blood culture positive infective endocarditis. Enterococcus endocarditis is the third leading cause of endocarditis and the source of infection remains undetermined in the majority of cases [25]. While genitourinary sources were evaluated in the current case, through both CT abdomen/pelvis and cystoscopy, the source was never determined. Understanding the microbial origin of infective endocarditis is important in determining treatment, but may also contribute to understanding prognosis, with Staphylococcus aureus infection and coagulase-negative staphylococcal infection increasing risk of in-hospital death, while Viridans Streptococci infection decreases risk [12].
Patient consent
Consent was obtained for the publication of current case. No patient identifiers disclosed.
Footnotes
Competing Interests: None.
References
- 1.Chen GJ, Lo WC, Tseng HW, Pan SC, Chen YS, Chang SC. Outcome of surgical intervention for aortic root abscess: A meta-analysis. Eur J Cardiothorac Surg. 2018;53(4):807–814. doi: 10.1093/ejcts/ezx421. [DOI] [PubMed] [Google Scholar]
- 2.Cresti A, Chiavarelli M, Scalese M, Nencioni C, Valentini S, Guerrini F, et al. Epidemiological and mortality trends in infective endocarditis, a 17-year population-based prospective study. Cardiovasc Diagn Ther. 2017;7(1):27–35. doi: 10.21037/cdt.2016.08.09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sunder S, Grammatico-Guillon L, Lemaignen A, Lacasse M, Gaborit C, Boutoille D, et al. Incidence, characteristics, and mortality of infective endocarditis in France in 2011. PLoS One. 2019;14(10) doi: 10.1371/journal.pone.0223857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bin Abdulhak AA, Baddour LM, Erwin PJ, Hoen B, Chu VH, Mensah GA, et al. Global and regional burden of infective endocarditis, 1990-2010: A systematic review of the literature. Glob Heart. 2014;9(1):131–143. doi: 10.1016/j.gheart.2014.01.002. [DOI] [PubMed] [Google Scholar]
- 5.Ahtela E, Oksi J, Sipila J, Rautava P, Kyto V. Occurrence of fatal infective endocarditis: A population-based study in Finland. BMC Infect Dis. 2019;19(1):987. doi: 10.1186/s12879-019-4620-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Holland TL, Baddour LM, Bayer AS, Hoen B, Miro JM, Fowler VG., Jr. Infective endocarditis. Nat Rev Dis Primers. 2016;2:16059. doi: 10.1038/nrdp.2016.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Munoz P, Kestler M, De Alarcon A, Miro JM, Bermejo J, Rodriguez-Abella H, et al. Current epidemiology and outcome of infective endocarditis: A multicenter, prospective, cohort study. Medicine (Baltimore) 2015;94(43):e1816. doi: 10.1097/MD.0000000000001816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gupta S, Sakhuja A, McGrath E, Asmar B. Trends, microbiology, and outcomes of infective endocarditis in children during 2000-2010 in the United States. Congenit Heart Dis. 2017;12(2):196–201. doi: 10.1111/chd.12425. [DOI] [PubMed] [Google Scholar]
- 9.Liesman RM, Pritt BS, Maleszewski JJ, Patel R. Laboratory diagnosis of infective endocarditis. J Clin Microbiol. 2017;55(9):2599–2608. doi: 10.1128/JCM.00635-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Federspiel JJ, Stearns SC, Peppercorn AF, Chu VH, Fowler VG., Jr. Increasing US rates of endocarditis with Staphylococcus aureus: 1999-2008. Arch Intern Med. 2012;172(4):363–365. doi: 10.1001/archinternmed.2011.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Holland DJ, Simos PA, Yoon J, Sivabalan P, Ramnarain J, Runnegar NJ. Infective endocarditis: A contemporary study of microbiology, echocardiography and associated clinical outcomes at a major tertiary referral centre. Heart Lung Circ. 2020;29(6):840–850. doi: 10.1016/j.hlc.2019.07.006. [DOI] [PubMed] [Google Scholar]
- 12.Murdoch DR, Corey GR, Hoen B, Miro JM, Fowler VG, Jr., Bayer AS, et al. Clinical presentation, etiology, and outcome of infective endocarditis in the 21st century: the International Collaboration on Endocarditis-Prospective Cohort Study. Arch Intern Med. 2009;169(5):463–473. doi: 10.1001/archinternmed.2008.603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Yuan SM. Fungal endocarditis. Braz J Cardiovasc Surg. 2016;31(3):252–255. doi: 10.5935/1678-9741.20160026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kirali K, Sarikaya S, Ozen Y, Sacli H, Basaran E, Yerlikhan OA, et al. Surgery for aortic root abscess: A 15-year experience. Tex Heart Inst J. 2016;43(1):20–28. doi: 10.14503/THIJ-14-4747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ahmed A, Ammar A, Elnahas Y, Abd Al Jawad M. Mechanical prosthetic valve sparing for aortic root abscess complicated by infective endocarditis. Tex Heart Inst J. 2020;47(4):280–283. doi: 10.14503/THIJ-19-7059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gott VL, Gillinov AM, Pyeritz RE, Cameron DE, Reitz BA, Greene PS, et al. Aortic root replacement. Risk factor analysis of a seventeen-year experience with 270 patients. J Thorac Cardiovasc Surg. 1995;109(3):536–544. doi: 10.1016/S0022-5223(95)70286-5. discussion 544-535. [DOI] [PubMed] [Google Scholar]
- 17.Mookhoek A, Korteland NM, Arabkhani B, Di Centa I, Lansac E, Bekkers JA, et al. Bentall procedure: A systematic review and meta-analysis. Ann Thorac Surg. 2016;101(5):1684–1689. doi: 10.1016/j.athoracsur.2015.10.090. [DOI] [PubMed] [Google Scholar]
- 18.Habib G, Derumeaux G, Avierinos JF, Casalta JP, Jamal F, Volot F, et al. Value and limitations of the Duke criteria for the diagnosis of infective endocarditis. J Am Coll Cardiol. 1999;33(7):2023–2029. doi: 10.1016/s0735-1097(99)00116-3. [DOI] [PubMed] [Google Scholar]
- 19.Habib G, Lancellotti P, Antunes MJ, Bongiorni MG, Casalta JP, Del ZF, et al. 2015 ESC Guidelines for the management of infective endocarditis: The Task Force for the management of infective endocarditis of the European Society of Cardiology (ESC). Endorsed by: European Association for Cardio-Thoracic Surgery (EACTS), the European Association of Nuclear Medicine (EANM) Eur Heart J. 2015;36(44):3075–3128. doi: 10.1093/eurheartj/ehv319. [DOI] [PubMed] [Google Scholar]
- 20.Saeedan MB, Wang TKM, Cremer P, Wahadat AR, Budde RPJ, Unai S, et al. Role of cardiac CT in infective endocarditis: current evidence, opportunities, and challenges. Radiol Cardiothorac Imaging. 2021;3(1) doi: 10.1148/ryct.2021200378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Pham N, Zaitoun H, Mohammed TL, DeLaPena-Almaguer E, Martinez F, et al. Complications of aortic valve surgery: manifestations at CT and MR imaging. Radiographics. 2012;32(7):1873–1892. doi: 10.1148/rg.327115735. [DOI] [PubMed] [Google Scholar]
- 22.McWilliams ET, Yavari A, Raman V. Aortic root abscess: multimodality imaging with computed tomography and gallium-67 citrate single-photon emission computed tomography/computed tomography hybrid imaging. J Cardiovasc Comput Tomogr. 2011;5(2):122–124. doi: 10.1016/j.jcct.2010.10.004. [DOI] [PubMed] [Google Scholar]
- 23.Ye W, Ren G, Zhong X, Jian X, Chen O, Ma Q, et al. ECG-gated CT in aortic perivalvular abscess: comparison with transesophageal echocardiography and intraoperative findings. Radiology. 2020;297(2):334–341. doi: 10.1148/radiol.2020200685. [DOI] [PubMed] [Google Scholar]
- 24.Mahmoud K, Hammouda T, Kandil H, Mashaal M. Prevalence and predictors of aortic root abscess among patients with left-sided infective endocarditis: A cross-sectional comparative study. Egypt Heart J. 2020;72(1):62. doi: 10.1186/s43044-020-00098-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khan Z, Siddiqui N, Saif MW. Enterococcus faecalis infective endocarditis and colorectal carcinoma: case of new association gaining ground. Gastroenterol Res. 2018;11(3):238–240. doi: 10.14740/gr996w. [DOI] [PMC free article] [PubMed] [Google Scholar]