Abstract
Introduction
The cyclical changes of hormones during the menstrual cycle are responsible not only for reproductive function but also have other effects on dietary intake and appetite. The current study aimed to investigate the variations of appetite-related hormones (ghrelin and obestatin) during the menstrual cycle and their association with adipokines, estrogen, and BMI.
Methods
Fifty-six regularly menstruating female students were grouped into normal weight (BMI ≤24.9; n = 26), and overweight/obese subjects (BMI ≥25; n = 30). Serum ghrelin, obestatin, leptin, adiponectin, and estrogen levels were measured during the early follicular, preovulatory, and luteal phases of the menstrual cycle using the ELISA technique.
Results
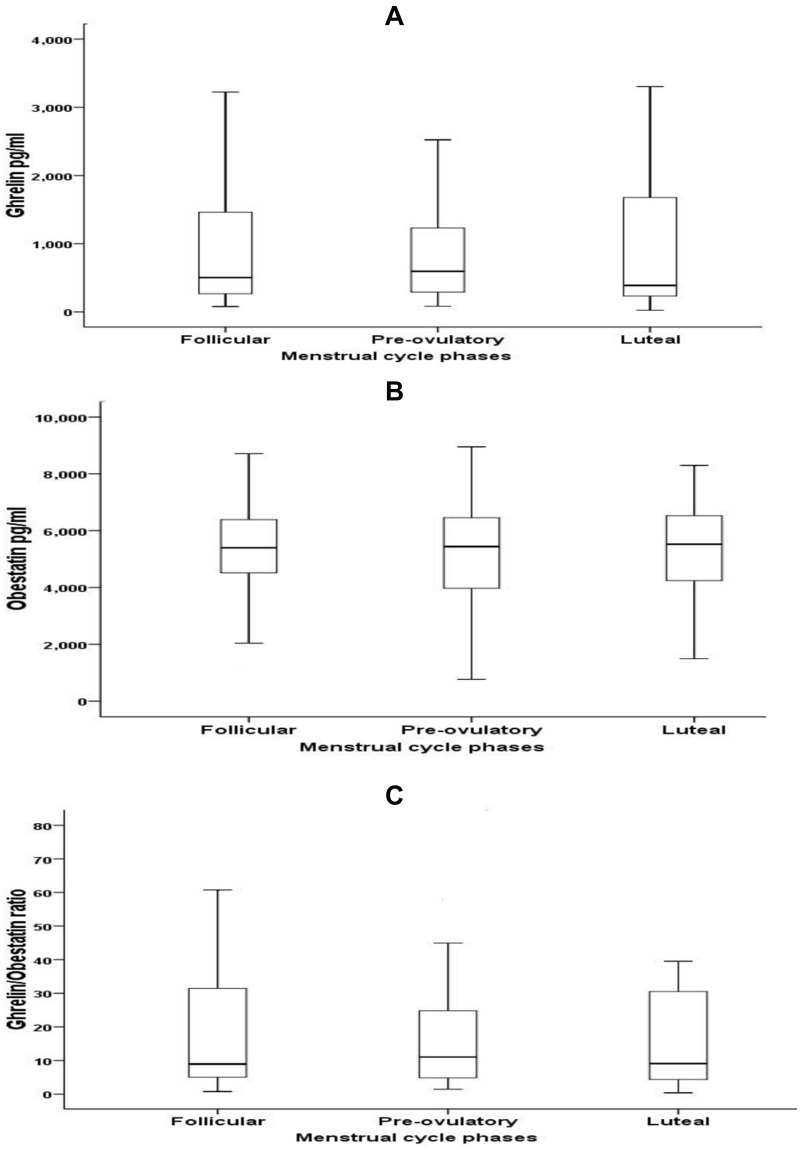
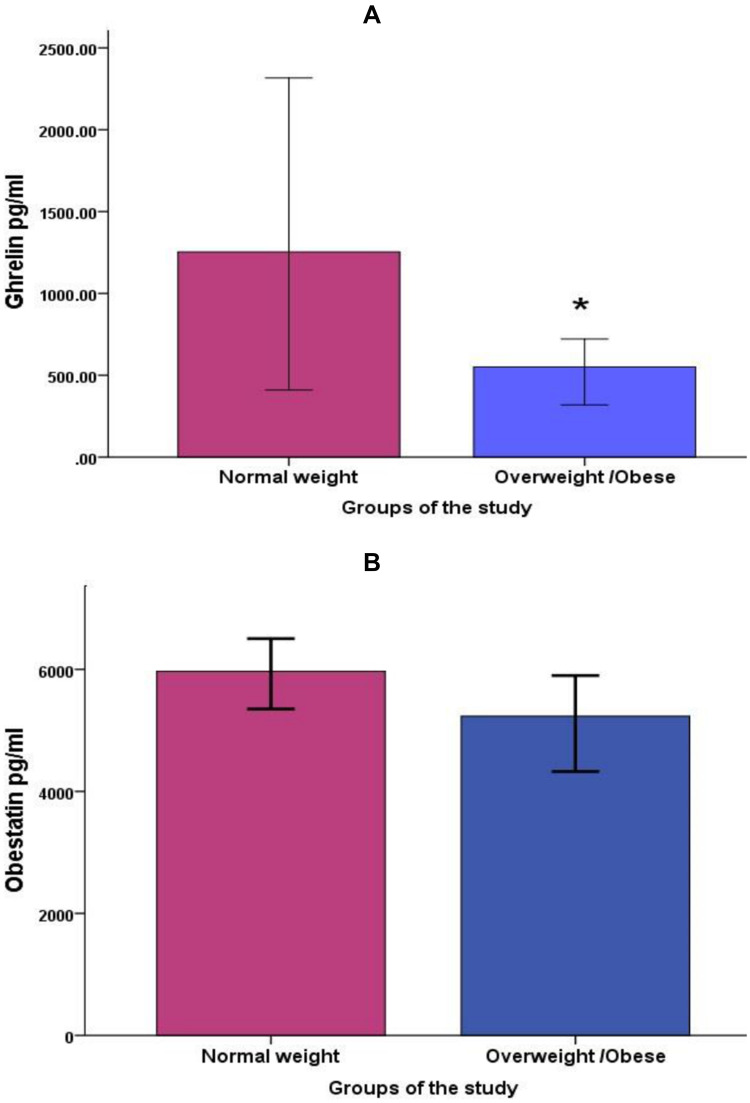
There were insignificant differences in the levels of serum ghrelin, obestatin, and ghrelin/obestatin ratio across menstrual cycle phases in the whole cohort as well as in each group separately (p > 0.05). Serum ghrelin was significantly less in OW-OB as compared to the NW group (p = 0.005), whereas the average serum obestatin did not show any significant differences between the two groups. No significant correlation was seen between ghrelin and obestatin with the adipokines and estradiol.
Conclusion
Significant low level of ghrelin was observed in obese group during the follicular phase. This finding may provide new insights into the altered ghrelin patterns in OW-OB individuals, as a cause or a consequence of obesity and related menstrual disorders.
Keywords: ghrelin, obestatin, adipokines, menstrual cycle, obesity
Introduction
There is evidence suggesting that women’s energy intake is not uniform throughout the menstrual cycle.1–3 In a study conducted in China, mean daily energy intake was significantly more in the luteal phase (6978 ± 1847 kJ) as compared to the follicular phase (6095 ± 1174 kJ), suggesting an increase of 14.5%.4 Both fat and carbohydrate intake increased in the luteal phase. Another study reported higher protein intake, and more food cravings in the luteal phase compared to the follicular phase.5 In addition, many adipokines were found to be related to human reproductive function.6 Leptin – a major adipokine that regulate the body energy store and inhibit hunger – showed fluctuation during different phases of the menstrual cycle.7 These variations in energy intake and energy-regulating hormones correspond to changes in ovarian hormones.8,9 The decrease in energy intake may be due to anorexigenic/appetite-suppressing effects of estradiol,10,11 and increased energy intake might be due to orexigenic/appetite stimulatory effects of progesterone.1,12
Estradiol-induced anorexia might result from its direct effects or indirect effects through fluctuations of appetite-regulating gut hormones. A study reported variations in peptide YY and gastric emptying time in various phases of the menstrual cycle despite eating the same breakfast.3 Therefore, it seems logical to hypothesize those appetite-regulating gut hormone levels may fluctuate during each menstrual phase. For instance, ghrelin, an appetite stimulator known as the “hunger hormone”,13 may decrease in the follicular phase, thus diminishing hunger and consequent energy intake in this phase. Similarly, obestatin, an appetite suppressor,14 may increase in the follicular phase, resulting in a decrease in appetite. In fact, when exogenous estradiol was administered in ovariectomized rats, a decrease in the number of ghrelin-producing cells, the concentrations of ghrelin mRNA in the stomach, and ghrelin concentrations in plasma were seen.15 Moreover, ghrelin did not increase food consumption in ovariectomized estradiol-treated rats, suggesting a decrease in ghrelin-induced hunger by estradiol.16 The presence of estradiol receptors on ghrelin-producing cells in stomach indicates an explicit mechanism for the effect of estradiol on ghrelin production.15
Gut hormones have a significant role in the regulation of energy intake and appetite. Basal and postprandial responses of gut hormones may vary according to nutritional status. Studies involving obese versus lean/normal-weight young adults reported significantly impaired suppression of post-prandial ghrelin levels in obese subjects.17,18 The level of ghrelin in pre-prandial state was significantly lowered in obese (OB) compared to normal-weight (NW) subjects.19 It is yet to be confirmed whether an altered ghrelin pattern is a cause or a consequence of obesity.20 The ghrelin/obestatin balance is important for regulating nutritional homeostasis, and ghrelin/obestatin ratio acts as a marker reflecting the energy status. Nutritional pathologies such as anorexia nervosa and obesity showed disturbed ghrelin/obestatin ratio.21
Keeping in view the effects of estradiol on ghrelin and variations in gut hormones’ patterns according to nutritional status, it is vital to explore the fluctuations in selected appetite-regulating gut hormones (ghrelin and obestatin) throughout the menstrual cycle, and in subjects with variable BMI status. In this regard, there is a complete lack of studies on obestatin. However, few studies are available regarding ghrelin that reported no significant changes in its levels during menstrual cycle phases.22–24 However, none of these studies compared ghrelin levels in women of varying BMI status during various menstrual cycle phases nor did they explore the correlation with estradiol. Therefore, the primary aim of the present study was to explore the variation of ghrelin and obestatin throughout the menstrual cycle in females with different BMIs. As a secondary outcome, the correlation of these appetite-regulating hormones with estrogen and adipokines was also investigated.
Materials and Methods
This work is a part of a prospective cohort study conducted at the Physiology Department of Imam Abdulrahman Bin Faisal University (IAU), Dammam, KSA. One hundred and seven female students (18–25 years) were recruited by convenient sampling from various colleges of IAU. The sample size was determined based on previous studies that investigated the cyclical changes of different hormones during the menstrual cycle, where the number of participants ranged from 8 to 259.7,23,24 All participants were informed about the purpose of the study, in accordance with the Declaration of Helsinki, and an ethical approval was granted by the Institutional Review Board (2014-01-173) of IAU. Written consent was taken from all study participants.
Females with regular menstrual cycle (length between 25 and 35 days, with the variability of fewer than five days) were included. We excluded females who were pregnant or breastfeeding in the last six months, presence of menstrual or ovulatory disorders, chronic illness, or using hormonal contraceptives for the last six months. Finally, 23 subjects were excluded and 84 were selected, out of these only 56 (NW = 26 and OW/OB = 30) followed up for giving blood samples during all the three phases of the menstrual cycle.
Briefing/familiarization sessions were arranged for the selected subjects. During these sessions, measurement of anthropometric parameters was done by using standard procedures (light clothes, barefooted, empty rectum and bladder, and fasting of at least 4-hours). Weight was measured in kilograms by using digital scales. Height was measured via mounted vertical metal centimeter ruler. Waist and hip circumference were measured using a measuring tape. Calculation of waist–stature ratio and waist–hip ratio was done by waist circumference/height, waist circumference/hip circumference, respectively. Calculation of body mass index was done by the formula = weight in kg/height in m2. Two groups were created based on participants’ BMI: normal and underweight (BMI ≤24.9), and overweight and obese (BMI ≥25).
During these familiarization sessions, students were also guided about making their basal body temperature (BBT) chart. Moreover, to plan the dates of blood sampling, we kept a record of the expected date of the first day of the menstrual cycle of each subject. Based on this information, each subject has to come for the blood sampling at least three following times during her menstrual cycle, as per Latif and Rafique method:25 1) Early follicular phase: From day 2 to 5, after the beginning of menstruation. 2) Preovulatory phase: From days 11 to 16, prior to the beginning of the upcoming menstruation. 3) Luteal phase: 3 to 5 days prior to the beginning of the upcoming menstruation.
Menstrual cycle phases were confirmed based on basal body temperature (BBT),26 and fluctuation of serum estradiol levels.27
To minimize the effects of diet and circadian rhythm on hormones, all blood samples were obtained between 8 and 10 am by venipuncture after an overnight fast of 10–12 hours. Five milliliters of blood were drawn and allowed to clot at room temperature, centrifugation was done for 20 minutes at 4 °C at 3000 rpm and serum was frozen at −80 °C.
Measurement of serum ghrelin was done by “Human Ghrelin, EMD Millipore Kits EZGRT-89K” with inter-and intra-assay coefficient of variation of 5.1–7.8% and 0.90–1.91%. Serum obestatin levels were identified by “Obestatin Peninsula Laboratories, LLC” with inter and intra-assay coefficient of variation of <10%. Serum Leptin levels were determined by “Human Leptin, EMD Millipore kits (EZHL-80SK)” with inter and intra-assay coefficient of variation of 2.6–6.2% and 2.6–4.6%. Serum Adiponectin was quantified using “Human Adiponectin ELISA Kit, B-Bridge International (K1001-1)”, with inter and intra-assay coefficient of variation of 3.1–7.3% and 4.6–5.7%. Serum estradiol levels were measured with “Human estradiol Elisa kits Sigma Aldrich”, with inter-assay and intra-assay coefficient of variation <10%.
Statistical Analysis
Data were checked for normality using the Shapiro–Walk test, which revealed a non-normal distribution of the tested variables. Hence, the data were expressed as median with interquartile range (IQR). General linear model (one-way repeated measure ANOVA) was used to compare the levels of ghrelin, obestatin, and ghrelin/obestatin ratio between the menstrual cycle phases in the whole cohort as well as the individual groups, whereas post hoc multiple comparisons were performed using Bonferroni correction. Mann–Whitney test compared study variables between NW and OW/OB groups. The correlations between the gut hormones (ghrelin and obestatin) with estradiol and adipokines (leptin and adiponectin) were tested using Spearman correlation coefficients. Statistical Package of Social Science (SPSS) version 21 was used to analyze the data. P-value of <0.05 was considered significant.
Results
The general characteristics of (NW) and (OW/OB) women are listed in Table 1. The menstrual cycle duration ranged from 27 to 31 days with an average of 29 ±1 (mean ±SD) days. Bodyweight, BMI, waist circumference, and hip circumference were significantly higher in OW/OB group as expected. Leptin hormone was significantly higher (p = 0.022), and adiponectin was significantly less (p = 0.026) in the OW/OB group in comparison to normal weight.
Table 1.
Anthropometric and Hormonal Measurements of the Participants
| Variables (Mean ± SD) | Normal Weight Group (n = 26) | Overweight–Obese Group (n = 30) | P value |
|---|---|---|---|
| Body mass index (BMI) (kg/m2) | 21.97 ± 1.89 | 31.39 ± 3.57 | < 0.001* |
| Waist circumference (cm) | 75.69 ± 5.88 | 87.25 ±14.70 | < 0.001* |
| Hip circumference (cm) | 98.58 ± 7.05 | 111.43 ±16.53 | 0.001* |
| Waist/hip (W/H) ratio | 0.77± 0.06 | 0.78 ± 0.07 | 0.711 |
| Serum leptin (pg/mL) | 9482.9 ± 4385.7 | 12,986.9 ± 6386.7 | 0.022* |
| Serum adiponectin (µg/mL) | 7.35± 3.28 | 5.47 ± 2.84 | 0.026* |
| Serum estrogen (pg/mL) | 65.6 ±19.8 | 60.6 ± 24.9 | 0.437 |
Note: *Significance level at P < 0.05.
Ghrelin, obestatin, and ghrelin/obestatin ratio did not show any significant differences across menstrual cycle phases either in the whole cohort – Figure 1 or in each group individually Table 2.
Figure 1.
Variation of ghrelin (A), obestatin (B), and ghrelin/obestatin ratio (C) during different phases of the menstrual cycle, data was represented as median (IQR).
Table 2.
Serum Ghrelin, Obestatin, and Ghrelin/Obestatin Ratio Across Different Phases of the Menstrual Cycle in Normal Weight and Overweight/Obese Groups
| Hormones Median (IQR) | Phase of the Menstrual Cycle | Normal Weight Group | Overweight Obese Group | P value (Between Groups) |
|---|---|---|---|---|
| Ghrelin (pg/mL) | Follicular phase (F) | 534.0 (2510) | 345 (499.3) | 0.005* |
| N | 17 | 25 | ||
| Preovulatory phase (PO) | 596.3 (1470) | 575 (620.5) | 0.116 | |
| N | 19 | 26 | ||
| Luteal phase (L) | 1060 (2440) | 316 (965) | 0.363 | |
| N | 19 | 26 | ||
| P value (within group) | 0.250 | 0.225 | ||
| Obestatin (pg/ mL) | Follicular phase (F) | 6024.7 (1869.9) | 5188.8 (2170.3) | 0.797 |
| N | 25 | 29 | ||
| Preovulatory phase (PO) | 6059.4 (2460.1) | 5058.3 (2433.8) | 0.720 | |
| N | 25 | 29 | ||
| Luteal phase (L) | 5591.6 (2590.6) | 5210.6 (2149.1) | 0.977 | |
| N | 25 | 29 | ||
| P value (within group) | 0.911 | 0 0.823 | ||
| Ghrelin/obestatin ratio | Follicular phase (F) | 0.22 (0.43) | 0.07 (0.08) | 0.134 |
| N | 16 | 24 | ||
| Preovulatory phase (PO) | 0.22 (0.33) | 0.10 (0.20) | 0.181 | |
| N | 18 | 25 | ||
| Luteal phase (L) | 0.25 (0.78) | 0.08 (0.21) | 0.403 | |
| N | 18 | 25 | ||
| P value (within group) | 0.181 | 0.383 |
Note: *p value < 0.05.
The ghrelin level showed a significantly lower level in overweight/obese subjects compared to control subjects with normal weight (p-value = 0.019), while serum obestatin concentration did not show any significant differences between groups (p value = 0.819) Figure 2. When comparing both groups during different phases of the menstrual cycle, ghrelin was significantly less in the follicular phase in obese/overweight group compared to the normal weight group Table 2.
Figure 2.
Comparisons of average serum ghrelin (A), and obestatin (B) between normal weight and overweight/obese groups, data was shown as median (95% CI), *p value < 0.05.
Considering whole cohort, there was no significant association of gastric hormones (ghrelin, obestatin) with adipose tissue hormones (leptin, adiponectin), and estradiol Table 3. The same pattern of no correlation was also found in normal-weight and overweight/obese groups separately (data is not shown).
Table 3.
Correlation Coefficient Between Gastric Hormones (Ghrelin, Obestatin) and Adipose Tissue Hormones (Leptin, Adiponectin), and Estradiol
| Gastric Hormones | Leptin | Adiponectin | Estradiol | |
|---|---|---|---|---|
| Ghrelin | r | −0.200 | 0.221 | 0.232 |
| p | 0.204 | 0.159 | 0.150 | |
| Obestatin | r | 0.030 | 0.243 | −0.029 |
| p | 0.830 | 0.076 | 0.845 | |
Note: r: correlation coefficient using Spearman test.
Discussion
Our results showed that variations of ghrelin, obestatin, and ghrelin/obestatin ratio during the menstrual cycle and its relation to estradiol and adipokines are not substantial. However, serum ghrelin showed a significantly lower level in overweight/obese compared to normal-weight subjects, while serum obestatin did not show any significant differences.
Ghrelin is an endocrine hormone mainly secreted by the stomach, serving as a key regulatory metabolic chemical to maintain energy hemostasis.28 Data regarding ghrelin variation during the menstrual cycle are still limited. A study that examined acylated and unacylated ghrelin levels across the menstrual cycle reported similar non-significant changes of ghrelin levels across menstrual cycle phases with no relationship between estradiol and ghrelin levels in the menstrual cycle with ghrelin.23 Furthermore, Šrámková et al showed a relatively stable level of ghrelin across different stages of the menstrual cycle.24 In addition, regularly exercising women with increased reproductive hormones in the luteal phase were not found to show an associated elevation in levels of ghrelin.29 Interestingly, ghrelin values were not affected by estrogen replacement therapy in pre- and post-menopausal women.30 This is also further supported by an animal study where no cyclic changes in serum ghrelin or in stomach expression were observed in the rat ovary.31 However, the absence of ghrelin variation does not eliminate its role in the hypothalamus-pituitary-gonadal axis in females. Many studies have reported variations in food consumption across the menstrual cycle with high caloric consumption observed in the luteal phase compared to the follicular phase, and this variation corresponded to the cyclic changes in ovarian hormones.8,9 High estradiol levels during the follicular phase could produce anorexigenic/appetite-suppressing effects,10,11 while high luteal progesterone might be responsible for the increased energy intake due to its orexigenic/appetite stimulatory effects.1,12 Furthermore, other studies demonstrated a relationship between menstrual disturbances with energy intake, nutritional status, and lifestyle.28,32 Young women with exercise-associated amenorrhea were found to have significantly elevated levels of ghrelin, which indicate that chronic energy deficit status with high ghrelin observed in athletic exercising women could affect the menstrual hormonal changes leading to menstrual disturbances such as amenorrhea.28 These contradictory shreds of evidence may reflect a complicated interrelationship between ovarian hormones and ghrelin that could be mediated at the level of ghrelin gene expression and not necessarily be reflected on the serum level of ghrelin. The clue to this suggestion comes from a previous animal study, which showed cyclic ghrelin gene expression during the estrous cycle of rats. Ghrelin-related mRNA levels varied based on the estrous cycle phases, with peak expression in the luteal phase. Noteworthy, this cyclic variation in ghrelin expression was noticeable in ovarian tissue only, and was not observed in the ghrelin-producing gastric cell nor in the serum ghrelin levels.31
We noted lower serum concentrations of ghrelin in OW/OB subjects compared to NW controls. In agreement with our results, ghrelin levels tend to drop in OW/OB subjects, and increasing body mass index was found to be inversely correlated with serum ghrelin.33,34 One possible explanation of the lower serum ghrelin levels in obesity is due to abundant energy reserve. In contrast, during circumstances where energy deficiency arises such as in anorexic patients, ghrelin remains high to stimulate appetite and replenish energy stores.35,36 However, there is no consensus in the literature in this regard, a recent study showed no significant differences in ghrelin serum concentration between obese and normal-weight people.37,38
Obestatin, a peptide hormone secreted mainly by the gastric cells, is one of the appetite-regulating hormones. In contrast to ghrelin, which triggers increased dietary intake, obestatin decreases food consumption and body weight through an anorectic effect.14,39 There is an obvious lack of studies that evaluate the obestatin level at different phases of the menstrual cycle. To our knowledge, the present study is the first one to report obestatin levels through various menstrual cycle phases. Additionally, studies that compared obestatin levels in different nutritional statuses showed contradictory results. Some studies showed higher obestatin levels in obese individuals,29,40 while others reported a reduction in obestatin levels associated with increasing BMI.41–44 However, another study did not find any significant reduction in obestatin level after weight reduction surgery.45 The difference in the results between studies was attributed to the differences in the obesity status; Vicennati et al40 and Haider et al42 investigated massively obese subjects, while the subjects of Guo et al41 were Chinese individuals who were known to have values of BMI and waist circumference specific for their ethnicity.
We observed no significant relation between obestatin and adipokines (leptin and adiponectin). In concordance with our finding, some studies showed an absence of correlation between obestatin, ghrelin/obestatin ratio, and adiponectin.40 In contrast, a negative correlation pattern between obestatin and leptin was reported in obese subjects.46 In light of this controversy, further research is recommended about the mechanism of the interaction between adipose tissue hormones and gut hormones in different nutritional statuses.
Conclusions
Average serum ghrelin levels are significantly less in OW/OB in comparison to NW subjects. Ghrelin levels are also significantly lower in OW/OB as compared to the NW group in the follicular phase. These findings may provide new insights into the altered Ghrelin patterns in OW-OB individuals, as a cause or a consequence of obesity and menstrual disorders.
Funding Statement
Funded by the deanship of scientific research at IAU [grant number 2014293].
Disclosure
The authors declare no conflicts of interest.
References
- 1.Asarian L, Geary N. Sex differences in the physiology of eating. Am J Physiol Regul Integr Comp Physiol. 2013;305(11):31. doi: 10.1152/ajpregu.00446.2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barr SI, Janelle KC, Prior JC. Energy intakes are higher during the luteal phase of ovulatory menstrual cycles. Am J Clin Nutr. 1995;61(1):39–43. doi: 10.1093/ajcn/61.1.39 [DOI] [PubMed] [Google Scholar]
- 3.Campolier M, Thondre SP, Clegg M, Shafat A, McIntosh A, Lightowler H. Changes in PYY and gastric emptying across the phases of the menstrual cycle and the influence of the ovarian hormones. Appetite. 2016;107:106–115. doi: 10.1016/j.appet.2016.07.027 [DOI] [PubMed] [Google Scholar]
- 4.Li ET, Tsang LB, Lui SS. Menstrual cycle and voluntary food intake in young Chinese women. Appetite. 1999;33(1):109–118. doi: 10.1006/appe.1999.0235 [DOI] [PubMed] [Google Scholar]
- 5.Gorczyca AM, Sjaarda LA, Mitchell EM, et al. Changes in macronutrient, micronutrient, and food group intakes throughout the menstrual cycle in healthy, premenopausal women. Eur J Nutr. 2016;55(3):1181–1188. doi: 10.1007/s00394-015-0931-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Comninos AN, Jayasena CN, Dhillo WS. The relationship between gut and adipose hormones, and reproduction. Hum Reprod Update. 2013;20(2):153–174. doi: 10.1093/humupd/dmt033 [DOI] [PubMed] [Google Scholar]
- 7.Salem AM. Variation of leptin during menstrual cycle and its relation to the Hypothalamic-Pituitary-Gonadal (HPG) axis: a systematic review. Int J Womens Health. 2021;13:445–458. doi: 10.2147/IJWH.S309299 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shi H, Clegg DJ. Sex differences in the regulation of body weight. Physiol Behav. 2009;97(2):199–204. doi: 10.1016/j.physbeh.2009.02.017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sinchak K, Wagner EJ. Estradiol signaling in the regulation of reproduction and energy balance. Front Neuroendocrinol. 2012;33(4):342–363. doi: 10.1016/j.yfrne.2012.08.004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Santollo J, Daniels D. Anorexigenic effects of estradiol in the medial preoptic area occur through membrane-associated estrogen receptors and metabotropic glutamate receptors. Horm Behav. 2019;107:20–25. doi: 10.1016/j.yhbeh.2018.11.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Stincic TL, Grachev P, Bosch MA, Rønnekleiv OK, Kelly MJ. Estradiol drives the anorexigenic activity of proopiomelanocortin neurons in female mice. eNeuro. 2018;5(4):0103–0118. doi: 10.1523/ENEURO.0103-18.2018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stelmańska E, Sucajtys-Szulc E. Enhanced food intake by progesterone-treated female rats is related to changes in neuropeptide genes expression in hypothalamus. Endokrynol Pol. 2014;65(1):46–56. doi: 10.5603/EP.2014.0007 [DOI] [PubMed] [Google Scholar]
- 13.Levin F, Edholm T, Schmidt PT, et al. Ghrelin stimulates gastric emptying and hunger in normal-weight humans. J Clin Endocrinol Metab. 2006;91(9):3296–3302. doi: 10.1210/jc.2005-2638 [DOI] [PubMed] [Google Scholar]
- 14.Lacquaniti A, Donato V, Chirico V, Buemi A, Buemi M. Obestatin: an interesting but controversial gut hormone. Ann Nutr Metab. 2011;59(2–4):193–199. doi: 10.1159/000334106 [DOI] [PubMed] [Google Scholar]
- 15.Matsubara M, Sakata I, Wada R, Yamazaki M, Inoue K, Sakai T. Estrogen modulates ghrelin expression in the female rat stomach. Peptides. 2004;25(2):289–297. doi: 10.1016/j.peptides.2003.12.020 [DOI] [PubMed] [Google Scholar]
- 16.Clegg DJ, Brown LM, Zigman JM, et al. Estradiol-dependent decrease in the orexigenic potency of ghrelin in female rats. Diabetes. 2007;56(4):1051–1058. doi: 10.2337/db06-0015 [DOI] [PubMed] [Google Scholar]
- 17.le Roux CW, Patterson M, Vincent RP, Hunt C, Ghatei MA, Bloom SR. Postprandial plasma ghrelin is suppressed proportional to meal calorie content in normal-weight but not obese subjects. J Clin Endocrinol Metab. 2005;90(2):1068–1071. doi: 10.1210/jc.2004-1216 [DOI] [PubMed] [Google Scholar]
- 18.Yang N, Liu X, Ding EL, et al. Impaired ghrelin response after high-fat meals is associated with decreased satiety in obese and lean Chinese young adults. J Nutr. 2009;139(7):1286–1291. doi: 10.3945/jn.109.104406 [DOI] [PubMed] [Google Scholar]
- 19.Korek E, Krauss H, Gibas-Dorna M, Kupsz J, Piątek M, Piątek J. Fasting and postprandial levels of ghrelin, leptin and insulin in lean, obese and anorexic subjects. Prz Gastroenterol. 2013;8(6):383–389. doi: 10.5114/pg.2013.39922 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makris MC, Alexandrou A, Papatsoutsos EG, et al. Ghrelin and Obesity: identifying Gaps and Dispelling Myths. A Reappraisal in Vivo. 2017;31(6):1047–1050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hassouna R, Zizzari P, Tolle V. The ghrelin/obestatin balance in the physiological and pathological control of growth hormone secretion, body composition and food intake. J Neuroendocrinol. 2010;22(7):793–804. doi: 10.1111/j.1365-2826.2010.02019.x [DOI] [PubMed] [Google Scholar]
- 22.Ihalainen JK, Löfberg I, Kotkajuuri A, Kyröläinen H, Hackney AC, Taipale-Mikkonen RS. Influence of menstrual cycle or hormonal contraceptive phase on energy intake and metabolic hormones-a pilot study. Endocrines. 2021;2(2):79–90. doi: 10.3390/endocrines2020008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Dafopoulos K, Sourlas D, Kallitsaris A, Pournaras S, Messinis IE. Blood ghrelin, resistin, and adiponectin concentrations during the normal menstrual cycle. Fertil Steril. 2009;92(4):1389–1394. doi: 10.1016/j.fertnstert.2008.07.1773 [DOI] [PubMed] [Google Scholar]
- 24.Šrámková M, Dušková M, Vítků J, et al. Levels of adipokines and some steroids during the menstrual cycle. Physiol Res. 2015;64(Suppl 2):933116. [DOI] [PubMed] [Google Scholar]
- 25.Latif R, Rafique N. Serum kisspeptin levels across different phases of the menstrual cycle and their correlation with serum oestradiol. Neth J Med. 2015;73(4):175–178. [PubMed] [Google Scholar]
- 26.Freedman RR, Girgis R. Effects of menstrual cycle and race on peripheral vascular alpha-adrenergic responsiveness. Hypertension. 2000;35(3):795–799. doi: 10.1161/01.HYP.35.3.795 [DOI] [PubMed] [Google Scholar]
- 27.Bell HK, Bloomer RJ. Impact of serum estradiol on postprandial lipemia, oxidative stress, and inflammation across a single menstrual cycle. Gend Med. 2010;7(2):166–178. doi: 10.1016/j.genm.2010.03.001 [DOI] [PubMed] [Google Scholar]
- 28.De Souza MJ, Leidy HJ, O’Donnell E, Lasley B, Williams NI. Fasting ghrelin levels in physically active women: relationship with menstrual disturbances and metabolic hormones. J Clin Endocrinol Metab. 2004;89(7):3536–3542. doi: 10.1210/jc.2003-032007 [DOI] [PubMed] [Google Scholar]
- 29.Kamemoto K, Yamada M, Matsuda T, et al. Effects of menstrual cycle on appetite-regulating hormones and energy intake in response to cycling exercise in physically active women. J Appl Physiol. 1985;132(1):224–235. doi: 10.1152/japplphysiol.01117.2020 [DOI] [PubMed] [Google Scholar]
- 30.Dafopoulos K, Chalvatzas N, Kosmas G, Kallitsaris A, Pournaras S, Messinis IE. The effect of estrogens on plasma ghrelin concentrations in women. J Endocrinol Invest. 2010;33(2):109–112. doi: 10.1007/BF03346563 [DOI] [PubMed] [Google Scholar]
- 31.Caminos JE, Tena-Sempere M, Gaytán F, et al. Expression of ghrelin in the cyclic and pregnant rat ovary. Endocrinology. 2003;144(4):1594–1602. doi: 10.1210/en.2002-221058 [DOI] [PubMed] [Google Scholar]
- 32.Doğan H, Eroğlu S, Akbayrak T. The effect of kinesio taping and lifestyle changes on pain, body awareness and quality of life in primary dysmenorrhea. Complement Ther Clin Pract. 2020;39:101120. doi: 10.1016/j.ctcp.2020.101120 [DOI] [PubMed] [Google Scholar]
- 33.Adamska-Patruno E, Ostrowska L, Goscik J, Pietraszewska B, Kretowski A, Gorska M. The relationship between the leptin/ghrelin ratio and meals with various macronutrient contents in men with different nutritional status: a randomized crossover study. Nutr J. 2018;17(1):018–0427. doi: 10.1186/s12937-018-0427-x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Alamri BN, Shin K, Chappe V, Anini Y. The role of ghrelin in the regulation of glucose homeostasis. Horm Mol Biol Clin Investig. 2016;26(1):3–11. doi: 10.1515/hmbci-2016-0018 [DOI] [PubMed] [Google Scholar]
- 35.Otto B, Cuntz U, Fruehauf E, et al. Weight gain decreases elevated plasma ghrelin concentrations of patients with anorexia nervosa. Eur J Endocrinol. 2001;145(5):669–673. doi: 10.1530/EJE-1450669 [DOI] [PubMed] [Google Scholar]
- 36.Nedvídková J, Krykorková I, Barták V, et al. Loss of meal-induced decrease in plasma ghrelin levels in patients with anorexia nervosa. J Clin Endocrinol Metab. 2003;88(4):1678–1682. doi: 10.1210/jc.2002-021669 [DOI] [PubMed] [Google Scholar]
- 37.Sitar-Tǎut AV, Cozma A, Fodor A, et al. New insights on the relationship between leptin, ghrelin, and leptin/ghrelin ratio enforced by body mass index in obesity and diabetes. Biomedicines. 2021;9(11). doi: 10.3390/biomedicines9111657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Atas U, Erin N, Tazegul G, Elpek GO, Yildirim B. Changes in ghrelin, substance P and vasoactive intestinal peptide levels in the gastroduodenal mucosa of patients with morbid obesity. Neuropeptides. 2021;89(102164):3. doi: 10.1016/j.npep.2021.102164 [DOI] [PubMed] [Google Scholar]
- 39.Li JB, Asakawa A, Cheng K, et al. Biological effects of obestatin. Endocrine. 2011;39(3):205–211. doi: 10.1007/s12020-011-9453-6 [DOI] [PubMed] [Google Scholar]
- 40.Vicennati V, Genghini S, De Iasio R, Pasqui F, Pagotto U, Pasquali R. Circulating obestatin levels and the ghrelin/obestatin ratio in obese women. Eur J Endocrinol. 2007;157(3):295–301. doi: 10.1530/EJE-07-0059 [DOI] [PubMed] [Google Scholar]
- 41.Guo ZF, Zheng X, Qin YW, Hu JQ, Chen SP, Zhang Z. Circulating preprandial ghrelin to obestatin ratio is increased in human obesity. J Clin Endocrinol Metab. 2007;92(5):1875–1880. doi: 10.1210/jc.2006-2306 [DOI] [PubMed] [Google Scholar]
- 42.Haider DG, Schindler K, Prager G, et al. Serum retinol-binding protein 4 is reduced after weight loss in morbidly obese subjects. J Clin Endocrinol Metab. 2007;92(3):1168–1171. doi: 10.1210/jc.2006-1839 [DOI] [PubMed] [Google Scholar]
- 43.Lippl F, Erdmann J, Lichter N, et al. Relation of plasma obestatin levels to BMI, gender, age and insulin. Horm Metab Res. 2008;40(11):806–812. doi: 10.1055/s-2008-1081503 [DOI] [PubMed] [Google Scholar]
- 44.Zamrazilová H, Hainer V, Sedláčková D, et al. Plasma obestatin levels in normal weight, obese and anorectic women. Physiol Res. 2008;57(1):S49–S55. doi: 10.33549/physiolres.931489 [DOI] [PubMed] [Google Scholar]
- 45.Roth CL, Reinehr T, Schernthaner GH, Kopp HP, Kriwanek S, Schernthaner G. Ghrelin and obestatin levels in severely obese women before and after weight loss after Roux-en-Y gastric bypass surgery. Obes Surg. 2009;19(1):29–35. doi: 10.1007/s11695-008-9568-x [DOI] [PubMed] [Google Scholar]
- 46.Nakahara T, Harada T, Yasuhara D, et al. Plasma obestatin concentrations are negatively correlated with body mass index, insulin resistance index, and plasma leptin concentrations in obesity and anorexia nervosa. Biol Psychiatry. 2008;64(3):252–255. doi: 10.1016/j.biopsych.2007.08.005 [DOI] [PubMed] [Google Scholar]