OVERVIEW
Early concepts of transport suggested that enzymes and transporters are evolutionarily related. In this brief minireview, evidence is presented suggesting that, contrary to this view, transport proteins and enzymes evolved independently of each other as two distinct classes of proteins from different precursor peptides. Transport systems probably evolved increasing degrees of complexity via the following pathway: channels → secondary carriers → primary carriers and group translocators.
THE PROBLEM IN PERSPECTIVE
All students of biology will agree to one essential point: life involves both chemical interconversions that we term metabolism and fluxes across the various membranes of a biological cell that we refer to as transport (3, 24, 25). No description of life is complete without the comprehensive inclusion of both phenomena. In order to be efficient, physiology integrates both processes into sets of coordinated networks. This fact was realized over 40 years ago by Peter Mitchell, who coined the term “vectorial metabolism.” Moreover, one form of transport that Mitchell and Moyle called group translocation, embodies both concepts, integrating them into a single mechanism (27, 28).
The metabolism of an exogenous substrate by a cell usually requires the participation of a transmembrane transport system that catalyzes entry of the substrate into the cell cytoplasm, where it is acted upon and altered by enzymes. Coordination of transport (the vectorial process) with metabolism (the chemical interconversion process) can be achieved by employing one or more of three potential mechanisms. First, metabolism can be directly coupled to transport, as in the case of the bacterial group translocating phosphoenolpyruvate-dependent phosphotransferase system (PTS) that phosphorylates its sugar substrates during transport (37, 38, 42, 43). Second, regulatory mechanisms can be superimposed upon both the transporter and the metabolic enzymes so that they are coordinately stimulated or inhibited (56). For example, the mammalian phagocytic NADPH oxidase which releases cytoplasmic protons during catalysis is regulated by arachidonate coordinately with a covalently linked proton channel that releases the oxidase-generated protons to the extracellular medium (13). Finally, the transport proteins and metabolic enzymes can form a higher-level, multiprotein complex referred to as a metabolon. Considerable evidence suggests, for example, that the PTS and the metabolic enzymes of glycolysis comprise such a metabolon in Escherichia coli and that the glycolytic enzymes in the human red blood cell, possibly together with the hexose transporter, comprise an equivalent mammalian metabolon (31). In such a complex, the product of one reaction (whether vectorial or chemical) can be passed directly from one protein active site to another. The product of the former serves as the substrate of the latter. Metabolon construction obviates the need for molecular diffusion through the cytoplasm. Metabolons may conceivably be either static or dynamic; in the latter case, protein association may be induced by the presence of appropriate metabolites and responsive to metabolite ratios that influence the conformations of the metabolon constituents, thereby controlling their activities (31).
VECTORIAL METABOLISM AND GROUP TRANSLOCATION
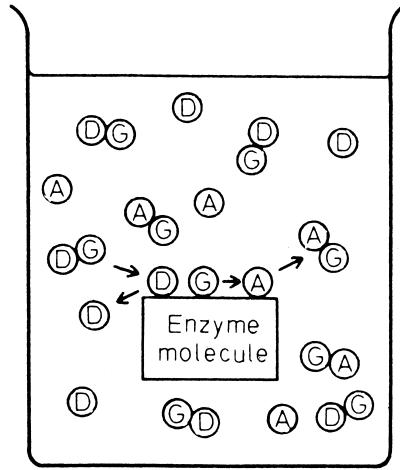
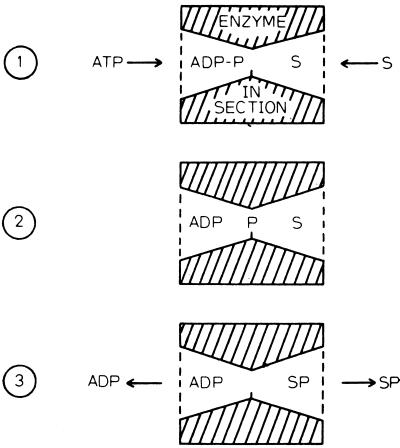
Vectorial metabolism and group translocation were extensively discussed by Peter Mitchell and Jennifer Moyle in the late 1950s (27, 28). In a later publication, Mitchell noted that most enzymes catalyze chemical reactions in an isotopic medium, the aqueous environment of the cell cytoplasm (26). Such a process does not give rise to a vectorial reaction. Thus, as illustrated in Fig. 1 (taken from reference 26), a simple chemical transformation involving a donor molecule, D-G, that transfers a reactive chemical group, G, to an acceptor, A, gives rise to the products D and AG. These are released into the same aqueous solution from which the reactants were derived. However, Mitchell and Moyle rationalized quite correctly that transport would occur if an enzyme catalyst were arranged anisotropically within a membrane, so that the enzyme active site spanned the membrane. This concept of enzyme-catalyzed group translocation is presented in Fig. 2. In this depiction, also taken from reference 26, ATP (the donor) approaches the transmembrane enzyme from one side of the membrane while the substrate, S, possibly a sugar, approaches from the other side (Fig. 2, part 1). The γ-phosphoryl group of ATP, P, is transferred first to an enzyme nucleophilic residue (Fig. 2, part 2) and then to the substrate, S. Finally, ADP and S-P dissociate from the enzyme active site into the two respective aqueous compartments separated by the membrane (Fig. 2, part 3). The phosphate moiety is thus transported across the membrane. According to Mitchell's definition of group translocation, transmembrane transport of a chemical moiety or group results from the anisotropic arrangement of an enzyme active site across a membrane. The enzyme is the transporter, and consequently, metabolism and transport are mechanistically inseparable.
FIG. 1.
Illustration of a chemical transformation reaction involving a group transfer process. D, donor; A, acceptor; G, group transferred. (Reproduced from reference 26 with permission.)
FIG. 2.
Illustration of enzyme-catalyzed group translocation as proposed by Peter Mitchell. ATP and ADP, adenosine tri- and diphosphates; P, phosphoryl group; S, substrate. (Reproduced from reference 26 with permission.)
The question that I wish to pose is: has Mitchell's depiction of group translocation (Fig. 2) been documented in the scientific literature over the past 40 years since these concepts were proposed? Although it is hard to believe that such a mechanism does not exist somewhere in the vast diversity of living organisms found on Earth, my attempts to find convincing published evidence for such a mechanism have not been fruitful. Instead, and most surprisingly, it appears that transporters and enzymes evolved independently of each other, as two distinct classes of proteins, from different precursor peptides. In this article, we shall trace the evidence that led to this conclusion and propose a pathway for the evolution of transport systems.
THE EVOLUTIONARY ARGUMENT AS APPLIED TO TRANSPORTERS
“Nothing in biology makes sense except in the light of evolution” (6). Thus, any systematic analysis of biological entities must take cognizance of evolution. Since molecular phylogeny reflects the evolutionary process, it provides the most reliable guide to the structure, function, and mechanism of biological macromolecules. For this reason, it is imperative that we understand the evolutionary origins of the various protein types. We must know whether, for example, enzymes, structural proteins, regulatory proteins, and transporters are related in an evolutionary sense or if at least some of these classes evolved independently of each other from different precursor proteins.
TYPES OF TRANSPORTERS FOUND IN NATURE
Figure 3 shows five well-documented modes of transport, all found in bacteria. These processes include free diffusion of molecules through transmembrane channels (panel a), secondary active transport involving the coupling of solute transport to ion flux with or without an extracytoplasmic receptor (panel c or b, respectively), primary active transport involving the expenditure of a primary source of energy such as ATP (panel d), and group translocation in which the substrate is modified during transport (panel e). In panel a, a simple proteinaceous channel protein is depicted. Such systems allow the passive diffusion of solutes from one side of the membrane to the other without stereospecific recognition or energy coupling. The glycerol facilitator of E. coli, a member of the major intrinsic protein (MIP) family (transporter classification [TC] no. 1.A.8), provides an example (33). In panel b, a solute-H+ symporter is depicted. Such a carrier couples solute uptake with proton influx so that the flow of H+ down its electrochemical gradient drives the active uptake of the solute against a concentration gradient. The E. coli lactose permease of the major facilitator superfamily (MFS; TC no. 2.A.1; see references 48 to 50 and our website [http://www-biology.ucsd.edu/∼msaier/transport]) provides an example (17). In panel c, a symporter that uses a periplasmic solute binding protein to confer substrate specificity and high-affinity binding is shown. The three-component dicarboxylate transporter of Rhodobacter capsulatus, DctMPQ of the TRAP-T family (TC no. 2.A.56), provides an example (9, 39). In panel d, ATP-hydrolyzing subunits on the cytoplasmic side of the membrane and a periplasmic binding receptor confer a chemical energy-coupling mechanism and high-affinity solute recognition, respectively, on this primary active transporter. The E. coli maltose permease of the ABC superfamily (TC no. 3.A.1) provides an example (1). Finally, in panel e, the constituents of the PTS are depicted. Because this system modifies its substrate by phosphorylation during transport, it is considered to be a group-translocating system. The sugar-transporting constituent is IIC, while the energy-coupling phosphotransfer proteins are Enzyme I, HPr, IIA, and IIB. These last-mentioned proteins are sequentially phosphorylated in that order prior to transfer of the phosphoryl group to the incoming sugar (37, 38, 42, 43). The glucose permease of E. coli, a member of the glucose PTS (glc) family (TC no. 4.A.1) provides an example (41, 54).
FIG. 3.
Models of recognized classes of transporters. (a) Proteinaceous channel-mediated passive diffusion (e.g., the glycerol facilitator of E. coli, GlpF) of a substrate (●) across the cytoplasmic membrane of a gram-negative bacterial cell. (b) Carrier-mediated solute-H+ symport (e.g., the lactose permease of E. coli). (c) Carrier-mediated symport using an extracytoplasmic receptor (a solute binding protein [BP]) to confer high-affinity solute recognition (e.g., the DctMPQ dicarboxylate TRAP transporter of R. capsulatus. (d) ATP hydrolysis-driven primary active uptake via an ABC transporter (e.g., the maltose permease of E. coli). (e) A group-translocating permease of the PTS in which sugar is concomitantly transported and phosphorylated in a coupled process in which Enzyme I (EI), HPr, IIA, and IIB are sequentially phosphorylated in preparation for sugar transport and phosphorylation (e.g., the glucose permease of E. coli). PEP, phosphoenolpyruvate; P, phosphoryl group. (Reproduced from reference 39 with permission.)
PROPOSED PATHWAY FOR THE EVOLUTION OF SECONDARY ACTIVE TRANSPORT SYSTEMS
We have summarized extensive evidence showing that the proteins that comprise the majority of α-helical channel-type transporter families (TC classes 1.A and 1.C) consist of integral membrane oligomeric protein complexes in which each constituent polypeptide chain has just one or two transmembrane α-helical spanners (TMSs), although some have more (52). In some of the cases in which simple channel proteins have multiple TMSs, the channel consists of just one or two of these spanners and the other TMSs have biogenic and/or regulatory functions (29). We have also summarized evidence that the vast majority of primary and secondary active transporters, as well as group translocators, consist of polypeptide chains exhibiting between 10 and 14 TMSs, although some have about half of this number (50). Those with fewer than 10 TMSs are probably present in the membrane as dimers (42, 46, 47). How did these transporters evolve their differing degrees of complexity? I believe that channels were the primordial transporters, giving rise to secondary carriers and evolving into primary active transporters and group translocators via the following pathway: α-helical channels → secondary carriers → primary carriers and group translocators. The evidence for this postulate derives largely from (i) the observation noted above regarding channel versus carrier protein topology, (ii) sequence analyses that reveal the occurrence of repeat sequences in the proteins that comprise families of secondary and primary active transporters, and (iii) the fact that very few families of transporters include homologues that function in a capacity other than transport (55).
Figure 4 depicts the “established” pathways for the evolution of the protein constituents of three families of transporters. The mitochondrial carrier (MC) family (Fig. 4A; TC no. 2.A.29) arose from a primordial two-TMS-encoding genetic element by intragenic triplication to give the six-TMS protein topology found in every recognized member of this family (21). Although hundreds of these permeases have been sequenced, not a single MC family homologue has been identified in a bacterium or an archaeon. In fact, no member of this large family has yet been found in the cytoplasmic membrane of a eukaryotic cell! These proteins evidently arose in eukaryotes in order to provide intraorganellar-cytoplasmic communication.
FIG. 4.
Depiction of three independent pathways for the evolution of six- and seven-TMS transporter modules. A, MC family of exchange transporters; B, MIP family of aquaporins and glycerol facilitators; C, LCT family of eukaryotic organellar proteins. Arrows indicate the direction of passage through the membrane, while numbers refer to the TMSs in the repeat unit of the polypeptide chain.
The MIP family of aquaporins and glycerol facilitators (Fig. 4B; TC no. 1.A.8) arose from a three-TMS precursor by duplication, and consequently, the second halves of these proteins have the opposite orientation of the homologous first halves (33). Because these proteins are found universally in prokaryotes and eukaryotes, we presume they arose in prokaryotes before these organisms diverged from each other about 3 billion years ago (7, 8).
The lysosomal cystine transporter (LCT) family (59, 62) (Fig. 4C; TC no. 2.A.43) similarly arose from a three-TMS precursor polypeptide chain. However, in this case, duplication of the three-TMS-encoding genetic element gave rise to a seven-TMS protein because a novel central TMS was generated in the process (Y. Zhai, W. H. M. Heijne, D. W. Smith, and M. H. Saier, Jr. submitted for publication). This allowed the membrane orientation of the three-TMS precursor to be retained in both halves of the seven-TMS product. Although LCT family proteins are found only in eukaryotes (animals, plants, and fungi), they are distantly related to the fungal-archaeal rhodopsin family (TC no. 3.E.1) (Zhai et al., submitted). Although limited sequence similarity between the two halves of bacteriorhodopsin has been noted (60), it is insufficient to prove a common evolutionary origin (21). Nevertheless, recognition of the duplication event in the distantly related LCT family causes us to suggest that bacteriorhodopsin and its homologues similarly arose as a result of an internal gene duplication event (Zhai et al., submitted).
The proteins of the MC and MIP families exhibit half as many TMSs per polypeptide chain as most secondary carriers, but they are known to be present in the membrane as dimers or tetramers. The oligomeric structures of members of the LCT family are not known. However, these polypeptide chains are about half the size of most secondary carriers and they have half as many TMSs. As shown in Fig. 5, many secondary carrier proteins can be shown to have arisen from six-TMS polypeptide precursors. For example, all recognized permeases of the MFS (TC no. 2.A.1) exhibit either 12 or 14 TMSs and the 12-TMS proteins evidently arose by duplication of a 6-TMS element, giving rise to 12 with a large central loop (10, 14). In three of the 29 currently recognized MFS families (32, 57), the central loop apparently gained hydrophobic character and inserted itself into the membrane to yield proteins with 14 TMSs (34–36). In substantiation of these suggestions, we have recently identified an MFS homologue encoded within the genome of Bacillus subtilis that is about half as large as most prokaryotic MFS permeases and exhibits six putative TMSs (S. Goldman and M. H. Saier, Jr., unpublished observation). This gene product is presumably similar to the MFS precursor, and if active, it would be expected to be functional as a dimer.
FIG. 5.
Illustration of three independent duplication events that gave rise to current 12-TMS (or 14-TMS) transporter polypeptide chains. A, MFS; B, RND superfamily; C, PET family.
Recognized proteins of the ubiquitous RND superfamily (TC no. 2.A.6) have either 6 or 12 TMSs, but all exhibit a large extracytoplasmic domain between TMSs 1 and 2, as well as between TMSs 7 and 8 (1′ and 2′ in Fig. 5) (63). Analyses suggest that this duplication event did not occur just once but happened multiple times during the evolution of this family (55, 63). Finally, the PET family proteins (Fig. 5C; TC no. 9.B.4) apparently arose by a distinct and independent duplication event, even though this event similarly gave rise to a 12-TMS protein (12).
PROPOSED PATHWAY FOR THE EVOLUTION OF PRIMARY ACTIVE TRANSPORTERS
The origin of more complex, multidomain, multicomponent transporters, including primary active transporters and group translocators, can also be deduced. We have summarized evidence for another superfamily that we have termed the ion transporter (IT) superfamily because all functionally characterized members of the superfamily transport ionic, charged molecules (39). Most of the families within this superfamily consist of single-polypeptide secondary carriers consisting of 12 TMSs per polypeptide chain. However, two IT superfamily families consist of more complex structures. The TRAP-T family (Fig. 1 and 6; TC no. 2.A.56) arose by addition of two proteins, an essential small integral membrane protein of unknown biochemical function and a periplasmic solute binding protein (Fig. 6, top; 9, 39). The ArsAB family of arsenite-antimonite efflux pumps, on the other hand, arose by association of an ATP-hydrolyzing subunit (ArsA) with an IT superfamily homologue (ArsB) (39, 44, 45). If the former subunit is eliminated, for example, by deletion of the encoding gene, then the ArsB protein can still function in arsenite expulsion, but it does so by employing a secondary active transport mechanism, probably via uniport in response to the membrane potential. While ArsB exhibits sequence similarity to secondary permeases of the IT superfamily, ArsA is homologous to ATPases, many of which have nothing to do with transport (44, 45).
FIG. 6.
Proposed pathway for the construction of present-day TRAP-T and ArsAB family permeases. The presumed precursor transporter was a single 12-TMS secondary transporter in both cases, but auxiliary proteins were superimposed upon them during evolution to confer either high-affinity substrate recognition (TRAP-T) or ATP hydrolysis-driven energy coupling (ARS).
Figure 7 provides another example of a hybrid primary active transporter that evidently evolved by bringing together two (or more) catalytic proteins that function in transport and metabolism. The protein complex depicted is the Na+-pumping oxaloacetate decarboxylase of Klebsiella pneumoniae (16). This transporter consists of (i) the biotin-containing oxaloacetate decarboxylase α subunit, (ii) the H+-Na+ antiporter β subunit, and (iii) an additional γ subunit which may help to hold the complex together (16). This protein complex is the best-characterized member of the Na+-transporting carboxylic acid decarboxylase family (TC no. 3.B.1). Limited evidence suggests that the β subunits of the Na+-transporting carboxylic acid decarboxylase family are distantly related to IT superfamily carriers (unpublished observation), although the former proteins exhibit a dissimilar 11-TMS topology (16). Moreover, the α subunits are homologous to a large number of biotin-containing enzymes that do not play a role in transport. A hybrid origin for these primary active transporters is apparent.
FIG. 7.
Illustration of the overall geometry and features of the catalytic events for the Na+-transporting oxaloacetate decarboxylase. B-H, biotin; B-CO2−, carboxybiotin; Lys, biotin-binding residue; 1, carboxyltransferase reaction; 2, decarboxylase reaction. The α subunit catalyzes decarboxylation, the β subunit catalyzes transport, and the γ subunit is proposed to facilitate linkage of these two subunits into a single membrane-embedded protein complex. (Reproduced from reference 5 with permission.)
MOSAIC ORIGIN OF THE GROUP-TRANSLOCATING PTS
As noted above, the type of group translocation described by Peter Mitchell and Jennifer Moyle in the 1950s (27, 28; Fig. 2) has not been convincingly documented in the scientific literature. However, the bacterial PTS catalyzes a related process and Mitchell's term “group translocation” has been adopted for this process. In the PTS-mediated vectorial reaction, transmembrane movement of a sugar is driven by its chemical modification which, however, occurs on just one side of the membrane. Thus, the enzymatic machinery appears to be superimposed on the transporter domain in a fashion reminiscent of the primary active transporters noted above. Metabolism and transport are therefore separable (42, 43).
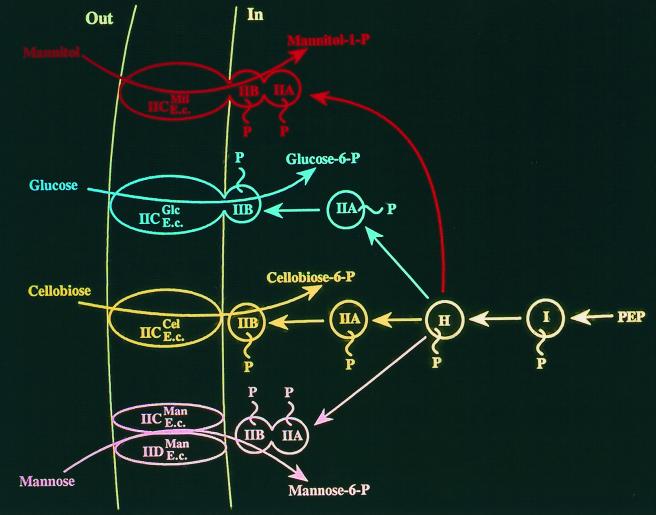
Figure 8 depicts four PTS permeases specific for mannitol, glucose, cellobiose, and mannose. In all four cases, the general energy-coupling proteins of the PTS, Enzyme I and HPr, function to phosphorylate the sugar-specific PTS proteins. These sugar-specific proteins are first the IIA proteins and then the IIB proteins. A IIB protein must be phosphorylated as a prelude to sugar phosphorylation (37, 42, 43).
FIG. 8.
Schematic depiction of four E. coli (E.c.) PTS permeases, those specific for mannitol (Mtl, top), glucose (Glc), cellobiose (Cel), and mannose (Man, bottom). Abbreviations of the proteins are as follows: I, Enzyme I; H, HPr; IIA, IIB, and IIC, the three functionally distinct domains of the Enzyme II complexes; IID, an extra domain found only in the mannose permease. The scheme illustrates the coupling of phosphoryl transfer from phosphoenolpyruvate (PEP) through various protein intermediates to give cytoplasmic sugar-phosphate (P) from exogenous sugars.
Sequence and motif analyses of the actual transporting protein constituents of the PTS, the IIC proteins or protein domains, suggest that those specific for mannitol, glucose, and cellobiose are distantly related, but the mannose IIC protein and its homologues (TC family 4.A.6) appear to have evolved independently (41, 54). The mannose IIC protein is apparently related to sugar transporters of the CUT2 family within the ABC superfamily (TC no. 3.A.1.2) (unpublished results).
High-resolution X-ray crystallographic data for the integral membrane proteins of the PTS are not yet available (20). However, X-ray crystallographic data for the IIA proteins of the mannitol-, glucose-, and mannose-specific PTSs have revealed that these three proteins exhibit completely different three-dimensional folds (41, 54). Moreover, the same observation has been made for the glucose-, cellobiose-, and mannose-specific IIB proteins (41, 58, 64). It seems clear that these PTS permeases arose as mosaic systems from a large number of evolutionary sources.
The PTS proves to be bacterium specific, as PTS permease homologues have not been identified in archaea or eukaryotes. Amazingly, although hundreds of genes encoding PTS protein homologues have been identified in the genomes of very diverse bacteria, not a single such homologue has yet been found in an archaeon or a eukaryote. This is particularly surprising because some PTS proteins function in the regulation of bacterial metabolism, as well as or instead of energy coupling to transport. We suggest that the PTS arose in the bacterial domain after archaea, bacteria, and eukaryotes separated from each other some 3 billion years ago (7, 8). This presupposition implies that little or no horizontal transfer of pts genes has occurred across domain lines.
PERSPECTIVES AND CONCLUSIONS
In this minireview, I have tried to summarize the available evidence concerning the evolution of integral membrane transport systems. This evidence follows from an earlier report in which this topic was considered from a different standpoint (55). The interested reader is referred to reference 55 for discussions regarding several key observations related to the evolutionary appearance of different transporter types.
All major evolutionarily well-characterized transporter families appear to have arisen via a pathway that started with a simple one-, two-, or three-TMS polypeptide chain that duplicated one or more times to give rise to larger proteins. In some cases, such as the large voltage-gated ion channel family (TC no. 1.A.1), gene fusion events apparently gave rise to single polypeptide chains with different parts of the polypeptide chain derived from different precursor sources (29). The basic channel-forming element in these proteins is therefore the same as in the smaller primordial polypeptides.
We have found no concrete evidence that transporters arose from enzymes, one of Peter Mitchell's key concepts for the origin of group translocation. However, it should be pointed out that negative results never prove a point. In fact, several investigators have proposed that enzymes mediate transmembrane transport. For example, the notion that fatty acyl synthases or their catalytically altered homologues catalyze transmembrane fatty acid transport has been argued (15) and countered (53). Bacterial cytoplasmic siderophore synthases that are at least loosely membrane associated and, mysteriously, can be released from the bacterial cell by osmotic shock have been suggested to mediate siderophore export (11). Moreover, chitin synthases (2), hyaluronate synthase of Streptococcus pyogenes (4, 40), and the WbbF lipopolysaccharide glycosyl transferase of Salmonella serovar Borreze (19) have all been proposed to provide a transmembrane transport pathway for carbohydrate export. Some of these enzymes comprise the putative vectorial glycosyl polymerization family (TC no. 9.B.32). Thus, the possibility that enzymes can sometimes catalyze transport should not be excluded.
As search tools become more refined and three-dimensional structures of integral membrane transport proteins become available, we should be able to come to more reliable and general conclusions regarding the breadth of superfamilies and the interrelationships of distantly related protein families. This information should allow us to delve more deeply into the evolutionary histories of transport proteins. However, the reluctance with which integral membrane secondary and primary active transporters, as well as group translocators, have yielded their three-dimensional structures may have a bright side. Because of the requirement for these proteins to form transmembrane helical bundles, their structures may be subject to topological restrictions which will allow the application of programs and derivation of novel algorithms that will allow reliable structural predictions long before the same is possible for water-soluble proteins, to which no such restrictions apply. Work in our laboratory is currently under way to explore such possibilities (Zhai et al., submitted).
The classification scheme we have devised for transporters (50), based on both phylogeny and function (48), has yielded a wealth of information of a most unexpected nature (46, 47, 49, 51, 52). However, of greatest significance is information related to the evolutionary pathways taken for the appearance and elaboration of these proteins. Evolutionary ancestry provides a reliable guide to structure, function, and mechanism, and therefore, by knowing the familial relationships of various groups of proteins, it is immediately apparent when extrapolation of structural, functional, or mechanistic information is likely to be possible. Further, knowledge of the phylogenetic relationships of the various proteins within a family also reveals the relative degrees to which such extrapolations will prove to be reliable. The time will come when laboratory experimental work always relies on phylogenetic predictions. The age of theoretical biology is just around the corner.
The conclusion that transporters and enzymes in general evolved independently raises the question of whether other classes of proteins evolved independently. Answers to this interesting question are at least partially available. Thus, one class of bacterial transcription factors (regulatory proteins) are homologous to sugar kinases (enzymes) with fused DNA binding domains (61), and another family of these factors is phylogenetically related to periplasmic binding proteins (receptors) (30). Many enzymes are known to interact with the genetic apparatus that encodes them in order to autoregulate their own syntheses (23), and ribosomal proteins are known to bind to both the relevant DNAs and mRNAs in order to autoregulate their own gene expression at both the transcriptional and translational levels (18). Thus, it seems clear that regulatory proteins, unlike transporters, have multiple origins grounded in a variety of other functional types. However, the extent to which these isolated cases are relevant to a generalized view of regulatory protein evolution will not be known until these proteins are systematically studied, classified, and analyzed in a quantitative fashion. We are only now emerging from the darkness of a strictly empirical science. The promises of a future with an entirely new discipline of theoretical, predictive biology are already in view. We should embrace and encourage the development of this new discipline. Challenges in bioinformatics and biosystematics, rendered essential by the ever-expanding genomics revolution, are likely to provide tremendous scientific excitement in the immediate years to come.
ACKNOWLEDGMENTS
I thank Mary Beth Hiller and Milda Simonaitis for assistance in the preparation of the manuscript.
Work in my laboratory was supported by NIH grants 2R01 AI14176 from The National Institute of Allergy and Infectious Diseases and 9RO1 GM55434 from the National Institute of General Medical Sciences, as well as by the M. H. Saier, Sr., Memorial Research Fund.
Footnotes
This minireview is dedicated to Fred C. Neidhardt to honor his distinguished career of teaching and research.
REFERENCES
- 1.Boos W, Shuman H. Maltose/maltodextrin system of Escherichia coli: Transport, metabolism, and regulation. Microbiol Mol Biol Rev. 1998;62:204–229. doi: 10.1128/mmbr.62.1.204-229.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cabib E, Bowers B, Roberts R L. Vectorial synthesis of a polysaccharide by isolated plasma membranes. Proc Natl Acad Sci USA. 1983;80:3318–3321. doi: 10.1073/pnas.80.11.3318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cohen G N, Monod J. Bacterial permeases. Bacteriol Rev. 1957;21:169–194. doi: 10.1128/br.21.3.169-194.1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.DeAngelis P L, Papaconstantinou J, Weigel P H. Molecular cloning, identification, and sequence of the hyaluronan synthase gene from group A Streptococcus pyogenes. J Biol Chem. 1993;268:19181–19184. [PubMed] [Google Scholar]
- 5.Dimroth P, Schink B. Energy conservation in the decarboxylation of dicarboxylic acids by fermenting bacteria. Arch Microbiol. 1998;170:69–77. doi: 10.1007/s002030050616. [DOI] [PubMed] [Google Scholar]
- 6.Dobzhansky T. Nothing makes sense in biology except in the light of evolution. Am Biol Teach. 1973;35:125–129. [Google Scholar]
- 7.Doolittle W F. Fun with genealogy. Proc Natl Acad Sci USA. 1997;94:12751–12753. doi: 10.1073/pnas.94.24.12751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Feng D-F, Cho G, Doolittle R F. Determining divergence times with a protein clock: update and reevaluation. Proc Natl Acad Sci USA. 1997;94:13028–13033. doi: 10.1073/pnas.94.24.13028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Forward J A, Behrendt M C, Wyborn N R, Cross R, Kelly D J. TRAP transporters: a new family of periplasmic solute transport systems encoded by the dctPQM genes of Rhodobacter capsulatus and by homologs in diverse gram-negative bacteria. J Bacteriol. 1997;179:5482–5493. doi: 10.1128/jb.179.17.5482-5493.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Griffith J K, Baker M E, Rouch D A, Page M G P, Skurray R A, Paulsen I T, Chater K F, Baldwin S A, Henderson P J F. Membrane transport proteins: implications of sequence comparisons. Curr Opin Cell Biol. 1992;4:684–695. doi: 10.1016/0955-0674(92)90090-y. [DOI] [PubMed] [Google Scholar]
- 11.Hantash F M, Earhart C F. Membrane association of the Escherichia coli enterobactin synthase proteins EntB/G, EntE, and EntF. J Bacteriol. 2000;182:1768–1773. doi: 10.1128/jb.182.6.1768-1773.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Harley K T, Saier M H., Jr A novel ubiquitous family of putative efflux transporters. J Mol Microbiol Biotechnol. 2000;2:195–198. [PubMed] [Google Scholar]
- 13.Henderson L M, Thomas S, Banting G, Chappel J B. The arachidonate-activatable, NADPH oxidase-associated H+ channel is contained within the multi-membrane-spanning N-terminal region of gp91 phox. Biochem J. 1997;325:701–705. doi: 10.1042/bj3250701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Henderson P J F, Maiden M C J. Homologous sugar transport proteins in Escherichia coli and their relatives in both prokaryotes and eukaryotes. Philos Trans R Soc Lond B. 1990;326:391–410. doi: 10.1098/rstb.1990.0020. [DOI] [PubMed] [Google Scholar]
- 15.Hirsch D, Stahl A, Lodish H F. A family of fatty acid transporters conserved from mycobacterium to man. Proc Natl Acad Sci USA. 1998;95:8625–8629. doi: 10.1073/pnas.95.15.8625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jockel P, Di Berardino M, Dimroth P. Membrane topology of the β-subunit of the oxaloacetate decarboxylase Na+ pump from Klebsiella pneumoniae. Biochemistry. 1999;38:13461–13472. doi: 10.1021/bi990303+. [DOI] [PubMed] [Google Scholar]
- 17.Kaback H R, Voss J, Wu J. Helix packing in polytopic membrane proteins: the lactose permease of Escherichia coli. Curr Opin Struct Biol. 1997;7:537–542. doi: 10.1016/s0959-440x(97)80119-4. [DOI] [PubMed] [Google Scholar]
- 18.Keener J, Nomura M. Regulation of ribosome synthesis. In: Neidhardt F C, editor. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C.: ASM Press; 1996. pp. 1417–1431. [Google Scholar]
- 19.Keenleyside W J, Whitfield C. A novel pathway for O-polysaccharide biosynthesis in Salmonella enterica serovar Borreze. J Biol Chem. 1996;271:28581–28592. doi: 10.1074/jbc.271.45.28581. [DOI] [PubMed] [Google Scholar]
- 20.Koning R I, Keegstra W, Oostergetel G T, Schuurman-Wolters G, Robillard G T, Brisson A. The 5 Å projection structure of the transmembrane domain of the mannitol transporter Enzyme II. J Mol Biol. 1999;287:845–851. doi: 10.1006/jmbi.1999.2650. [DOI] [PubMed] [Google Scholar]
- 21.Kuan J, Saier M H., Jr The mitochondrial carrier family of transport proteins: structural, functional and evolutionary relationships. Crit Rev Biochem Mol Biol. 1993;28:209–233. doi: 10.3109/10409239309086795. [DOI] [PubMed] [Google Scholar]
- 22.Kuan G, Saier M H., Jr Phylogenetic relationships among bacteriorhodopsins. Res Microbiol. 1994;145:273–285. doi: 10.1016/0923-2508(94)90183-x. [DOI] [PubMed] [Google Scholar]
- 23.McFall E, Newman E B. Amino acids as carbon sources. In: Neidhardt F C, editor. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C.: ASM Press; 1996. pp. 358–379. [Google Scholar]
- 24.Mitchell P. Transport of phosphate across the osmotic barrier of Micrococcus pyogenes: specificity and kinetics. J Gen Microbiol. 1954;11:73–82. doi: 10.1099/00221287-11-1-73. [DOI] [PubMed] [Google Scholar]
- 25.Mitchell P. Symp. Soc. Exp. Biol. 8:254–261. 1954. Transport of phosphate through an osmotic barrier. [Google Scholar]
- 26.Mitchell P. The ninth Sir Hans Krebs lecture. Compartmentation and communication in living systems. Ligand conduction: a general catalytic principle in chemical, osmotic and chemiosmotic reaction systems. Eur J Biochem. 1979;95:1–20. doi: 10.1111/j.1432-1033.1979.tb12934.x. [DOI] [PubMed] [Google Scholar]
- 27.Mitchell P, Moyle J. Group-translocation: a consequence of enzyme-catalysed group-transfer. Nature. 1958;182:372–373. doi: 10.1038/182372a0. [DOI] [PubMed] [Google Scholar]
- 28.Mitchell P, Moyle J. Coupling of metabolism and transport by enzymic translocation of substrates through membranes. Proc R Phys Soc Edinburgh. 1959;28:19–27. [Google Scholar]
- 29.Nelson R D, Kuan G, Saier M H, Jr, Montal M. Modular assembly of voltage-gated channel proteins: a sequence analysis and phylogenetic study. J Mol Microbiol Biotechnol. 1999;1:281–287. [PubMed] [Google Scholar]
- 30.Nguyen C C, Saier M H., Jr Phylogenetic, structural and functional analyses of the LacI-GalR family of bacterial transcription factors. FEBS Lett. 1995;377:98–102. doi: 10.1016/0014-5793(95)01344-x. [DOI] [PubMed] [Google Scholar]
- 31.Norris V, Gascuel P, Guespin-Michel J, Ripoll C, Saier M H., Jr Metabolite-induced metabolons: the activation of transporter-enzyme complexes by substrate binding. Mol Microbiol. 1999;31:1591–1595. doi: 10.1046/j.1365-2958.1999.01275.x. [DOI] [PubMed] [Google Scholar]
- 32.Pao S S, Paulsen I T, Saier M H., Jr The major facilitator superfamily. Microbiol Mol Biol Rev. 1998;62:1–32. doi: 10.1128/mmbr.62.1.1-34.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Park J H, Saier M H., Jr Phylogenetic characterization of the MIP family of transmembrane channel proteins. J Membr Biol. 1996;153:171–180. doi: 10.1007/s002329900120. [DOI] [PubMed] [Google Scholar]
- 34.Paulsen I T, Skurray R A. Topology, structure and evolution of two families of proteins involved in antibiotic and antiseptic resistance in eukaryotes and prokaryotes—an analysis. Gene. 1993;124:1–11. doi: 10.1016/0378-1119(93)90755-r. [DOI] [PubMed] [Google Scholar]
- 35.Paulsen I T, Brown M H, Littlejohn T G, Mitchell B A, Skurray R A. Multidrug resistance proteins QacA and QacB from Staphylococcus aureus: membrane topology and identification of residues involved in substrate specificity. Proc Natl Acad Sci USA. 1996a;93:3630–3635. doi: 10.1073/pnas.93.8.3630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Paulsen I T, Brown M H, Skurray R A. Proton-dependent multidrug efflux systems. Microbiol Rev. 1996b;60:575–608. doi: 10.1128/mr.60.4.575-608.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Postma P W, Lengeler J W, Jacobson G R. Phosphoenolpyruvate:carbohydrate phosphotransferase systems of bacteria. Microbiol Rev. 1993;57:543–594. doi: 10.1128/mr.57.3.543-594.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Postma P W, Lengeler J W, Jacobson G R. Phosphoenolpyruvate:carbohydrate phosphotransferase systems. In: Neidhardt F C, editor. Escherichia coli and Salmonella: cellular and molecular biology. Washington, D.C.: ASM Press; 1996. pp. 1149–1174. [Google Scholar]
- 39.Rabus R, Jack D L, Kelly D J, Saier M H., Jr TRAP transporters: an ancient family of extracytoplasmic solute-receptor-dependent secondary active transporters. Microbiology. 1999;145:3431–3445. doi: 10.1099/00221287-145-12-3431. [DOI] [PubMed] [Google Scholar]
- 40.Reeves P R, Hobbs M, Valvano M A, Skurnik M, Whitfield C, Coplin D, Kido N, Klena J, Maskell D, Raetz C R, Rick P D. Bacterial polysaccharide synthesis and gene nomenclature. Trends Microbiol. 1996;4:495–503. doi: 10.1016/s0966-842x(97)82912-5. [DOI] [PubMed] [Google Scholar]
- 41.Reizer J, Saier M H., Jr Modular multidomain phosphoryl transfer proteins of bacteria. Curr Opin Struct Biol. 1997;7:407–415. doi: 10.1016/s0959-440x(97)80059-0. [DOI] [PubMed] [Google Scholar]
- 42.Robillard G T, Broos J. Structure/function studies on the bacterial carbohydrate transporters, Enzymes II of the phosphoenolpyruvate-dependent phosphotransferase system. Biochim Biophys Acta. 1999;1422:73–104. doi: 10.1016/s0304-4157(99)00002-7. [DOI] [PubMed] [Google Scholar]
- 43.Robillard G T, Lolkema J S. Enzymes II of the phosphoenolpyruvate-dependent sugar transport systems: a review of their structure and mechanism of sugar transport. Biochim Biophys Acta. 1988;947:493–519. doi: 10.1016/0304-4157(88)90005-6. [DOI] [PubMed] [Google Scholar]
- 44.Rosen B P. Families of arsenic transporters. Trends Microbiol. 1999;7:207–212. doi: 10.1016/s0966-842x(99)01494-8. [DOI] [PubMed] [Google Scholar]
- 45.Rosen B P, Bhattacharjee H, Zhou T, Walmsley A R. Mechanism of the ArsA ATPase. Biochim Biophys Acta. 1999;1461:207–215. doi: 10.1016/s0005-2736(99)00159-5. [DOI] [PubMed] [Google Scholar]
- 46.Saier M H., Jr Computer-aided analyses of transport protein sequences: gleaning evidence concerning function, structure, biogenesis, and evolution. Microbiol Rev. 1994;58:71–93. doi: 10.1128/mr.58.1.71-93.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Saier M H., Jr Phylogenetic approaches to the identification and characterization of protein families and superfamilies. Microb Comp Genomics. 1996;1:129–150. doi: 10.1089/mcg.1996.1.129. [DOI] [PubMed] [Google Scholar]
- 48.Saier M H., Jr Molecular phylogeny as a basis for the classification of transport proteins from bacteria, archaea and eukarya. Adv Microb Physiol. 1998;40:81–136. doi: 10.1016/s0065-2911(08)60130-7. [DOI] [PubMed] [Google Scholar]
- 49.Saier M H., Jr Genome archeology leading to the characterization and classification of transport proteins. Curr Opin Microbiol. 1999;2:555–561. doi: 10.1016/s1369-5274(99)00016-8. [DOI] [PubMed] [Google Scholar]
- 50.Saier M H., Jr A functional phylogenetic classification system for transmembrane solute transporters. Microbiol Mol Biol Rev. 2000;64:354–411. doi: 10.1128/mmbr.64.2.354-411.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Saier, M. H., Jr. Families of transporters specific for amino acids and their derivatives. Microbiology, in press.
- 52.Saier M H., Jr Families of proteins forming transmembrane channels. J Membr Biol. 2000;175:165–180. doi: 10.1007/s00232001065. [DOI] [PubMed] [Google Scholar]
- 53.Saier M H, Jr, Kollman J M. Is FatP a long-chain fatty acid transporter? Mol Microbiol. 1999;33:670–672. doi: 10.1046/j.1365-2958.1999.01512.x. [DOI] [PubMed] [Google Scholar]
- 54.Saier M H, Jr, Reizer J. The bacterial phosphotransferase system: new frontiers 30 years later. Mol Microbiol. 1994;13:755–764. doi: 10.1111/j.1365-2958.1994.tb00468.x. [DOI] [PubMed] [Google Scholar]
- 55.Saier M H, Jr, Tseng T-T. Evolutionary origins of transmembrane transport systems. In: Broome-Smith J K, Baumberg S, Stirling C J, Ward F B, editors. Transport of Molecules Across Microbial Membranes, Symposium 58, Society for General Microbiology. Cambridge, United Kingdom: Cambridge University Press; 1999. pp. 252–274. [Google Scholar]
- 56.Saier M H, Jr, Chauvaux S, Deutscher J, Reizer J, Ye J-J. Protein phosphorylation and the regulation of carbon metabolism: comparisons in gram-negative versus gram-positive bacteria. Trends Biochem Sci. 1995;20:267–271. doi: 10.1016/s0968-0004(00)89041-6. [DOI] [PubMed] [Google Scholar]
- 57.Saier M H, Jr, Beatty J T, Goffeau A, Harley K T, Heijne W H M, Huang S-C, Jack D L, Jahn P S, Lew K, Liu J, Pao S S, Paulsen I T, Tseng T-T, Virk P S. The major facilitator superfamily. J Mol Microbiol Biotechnol. 1999;1:257–279. [PubMed] [Google Scholar]
- 58.Schauder S, Nunn R S, Lanz R, Erni B, Schirmer T. Crystal structure of the IIB subunit of a fructose permease (IIBLev) from Bacillus subtilis. J Mol Biol. 1998;276:591–602. doi: 10.1006/jmbi.1997.1544. [DOI] [PubMed] [Google Scholar]
- 59.Smith M L, Greene A A, Potashnik R, Mendoza S A, Schneider J A. Lysosomal cystine transport. Effect of intralysosomal pH and membrane potential. J Biol Chem. 1987;262:1244–1253. [PubMed] [Google Scholar]
- 60.Taylor E W, Agarwal A. Sequence homology between bacteriorhodopsin and G-protein coupled receptors: exon shuffling or evolution by duplication? FEBS Lett. 1993;325:161–166. doi: 10.1016/0014-5793(93)81065-8. [DOI] [PubMed] [Google Scholar]
- 61.Titgemeyer F, Reizer J, Reizer A, Saier M H., Jr Evolutionary relationships between sugar kinases and transcriptional repressors in bacteria. Microbiology. 1995;140:2349–2354. doi: 10.1099/13500872-140-9-2349. [DOI] [PubMed] [Google Scholar]
- 62.Town M, Jean G, Cherqui S, Attard M, Forestier L, Whitmore S A, Callen D F, Gribouval O, Broyer M, Bates G P, van't Hoff W, Antignac C. A novel gene encoding an integral membrane protein is mutated in nephropathic cystinosis. Nat Genet. 1998;18:319–324. doi: 10.1038/ng0498-319. [DOI] [PubMed] [Google Scholar]
- 63.Tseng T-T, Gratwick K S, Kollman J, Park D, Nies D H, Goffeau A, Saier M H., Jr The RND permease superfamily: an ancient, ubiquitous and diverse family that includes human disease and development proteins. J Mol Microbiol Biotechnol. 1999;1:107–125. [PubMed] [Google Scholar]
- 64.van Montfort R L M, Pijning T, Kalk K H, Reizer J, Saier M H, Jr, Thunnissen M M G M, Robillard G T, Dijkstra B W. The structure of an energy-coupling protein from bacteria, IIBcellobiose, reveals similarity to eukaryotic protein tyrosine phosphatases. Structure. 1997;5:217–225. doi: 10.1016/s0969-2126(97)00180-9. [DOI] [PubMed] [Google Scholar]