Highlights
-
•
Beam on MR acquisition on the MR-Linac can be used to compute DDOTD.
-
•
Intrafraction motion via volumetric variability of OARs can impact dosimetry.
-
•
Computation of the DDOTD may help inform prospective fractions for SBRT prostate.
Keywords: Intra-fraction motion, Adaptive radiotherapy, DDOTD, MR-Linac, Prostate SBRT
Abstract
Purpose
The purpose of this study is to evaluate the impact of intrafraction pelvic motion by comparing the adapted plan dose (APD) and the computed delivered dose of the day (DDOTD) for patients with prostate cancer (PCa) treated with SBRT on the MR-Linac.
Methods
Twenty patients with PCa treated with MR-guided adaptive SBRT were included. A 9-field IMRT distribution was adapted based on the anatomy of the day to deliver a total prescription dose of 3000 cGy in 5 fractions to the prostate plus a 5 mm isotropic margin. Prostate, bladder, and rectum were re-contoured on the MR-image acquired during treatment delivery (MRBO). DDOTD was computed by propagating the dose from the daily adapted plan generated during treatment onto the MRBO.
Results
Target coverage was met for all fractions, however, computed DDOTD was significantly less than the APD (p < 0.05). During an average treatment of 53 min, mean bladder volume increased by 116%, which led to a significant decrease in the DDOTD bladder D40% (p < 0.001). However, DDOTD to bladder 5 cc was significantly higher (p < 0.001) than APD. Rectum intrafraction changes were observed based on a volume change of −20% to 83% and presence of significant dose changes from APD to DDOTD for rectum D20% (p < 0.05) and D1cc (p < 0.0001).
Conclusions
Intrafraction motion observed during prostate SBRT treatment on the MR-Linac have dosimetric impacts on both the target and organs at risk. Post-treatment computation using DDOTD may inform adaptation beyond anatomic changes in subsequent treatment fractions to best capitalize on MR-Linac technology and widen the therapeutic index of SBRT for PCa.
Introduction
The adoption of stereotactic body radiotherapy (SBRT) for prostate cancer treatments has been enabled by the technical advancements in treatment planning and delivery. However, with SBRT, a larger dose per fraction is delivered, furthering the importance of precise and accurate treatment delivery. Inter-fraction changes noted over the course of treatment have substantiated the need for adaptive online re-planning [19]. The current use of an integrated MRI and linear accelerator (MR-Linac) allows the convergence of improved soft tissue visualization and adaptation to anatomical changes, allowing the generation and delivery of more accurate and individualized treatment [4]. There are two workflow options on the Unity MR-Linac (Elekta Unity, Stockholm, Sweden); adapt-to-position (ATP) workflow (MR image acquisition for registration, multi-leaf collimator translation based on the image registration) and adapt-to-shape (ATS) workflow (MR image acquisition for registration, contour propagation/re-contouring and plan optimization) [8] both of which result in much longer treatment times than on a conventional linear accelerator (linac). This increases the likelihood of intrafraction motion and associated potential dosimetric impact.
Intrafraction motion in the pelvis may be attributed to bladder and rectal changes [1]. Bladder filling, and unexpected rectal changes over a treatment session have the potential to deform the target and influence target coverage [2], impacting delivered dose, and hence the need for bladder and bowel preparation [28]. To spare these organs at risk (OARs), a comfortably full bladder is often recommended [20]. Considering the increased treatment time on the MR-Linac, conventional bladder requirements may not be necessary since bladder filling will continue to occur during the treatment session, thus, patients who start with a smaller bladder may still have their dosimetric constraints met [22].
In conventional dose reconstruction, dose of the day provides insight into the impact of inter-fraction changes [25] and following a similar framework it is possible to assess intrafraction dosimetric impact on accumulated treatment dose. Accumulated treatment dose provides a more accurate insight into the characterization of normal-tissue and target response [12]. The combination of adaptive radiotherapy and MR imaging enables the treatment team to adjust the daily planned dose based on the patient’s anatomy [21]. Previous studies have shown that intrafraction motion from both bladder filling and rectal changes exist [8] and may lead to a decrease in target coverage on the MR-Linac from the localization to verification imaging [16]. Work has also been done to determine the influence of intrafraction motion on near max-point dose to OARs of PCa patients treated with 60 Gy in 20 fractions via 2D cine imaging on the MR-Linac [18]. However, these studies do not address the implications of intrafraction motion on volumetric dosimetry of OARs. The purpose of this study is to quantify the volumetric dosimetric implications of such intrafraction motion by computing the delivered dose of the day (DDOTD) using a 3D MRI image acquired during beam on (MRBO) for PCa patients treated with SBRT on the MR-Linac.
Methods
Patient population
This retrospective planning study included twenty patients treated with 3000 cGy in five fractions on the MR-Linac. These patients were enrolled on a prospective study (REB 09-0026) where they also received a single fraction 1500 cGy focal MR-guided high dose rate (HDR) brachytherapy boost to the gross disease.
Patient preparation
Bladder and bowel preparation were required for prostate patients treated using MR-Linac. Patients were asked to have, where possible, a bowel movement either in the morning or prior to simulation and treatment. Select patients also had a rectal spacer (SpaceOAR hydrogel, Boston Scientific, MA, US) inserted at the discretion of their Radiation Oncologist (RO) at minimum 3 days prior to simulation. For bladder preparation, patients were instructed to drink 300 mL of water at the beginning of the treatment session. This is to ensure that the desired filling is achieved at time of treatment delivery and minimize discomfort for patients during treatment.
Reference planning
Patients underwent both CT and MRI simulation. The CT and additional MR images were fused to the MR-Linac acquired high-resolution 3D T2-weighted (T2w) image for reference planning. The prostate - clinical target volume (CTV), which may have included 1 cm of the seminal vesicles as per the RO’s discretion, and all OARs were contoured by the RO. A planning target volume (PTV) was created with a 5 mm uniform expansion around the CTV. The intensity modulated radiotherapy (IMRT) plans were generated with nine beams at gantry angles 215, 265, 295, 325, 0, 35, 65, 95 and 145, and a total of 70 segments, with the minimum segment area of 4 cm2 and a minimum of 3MU per segment using the MR-Linac treatment planning system (Monaco v5.4, Elekta AB, Stockholm, Sweden) to achieve the dosimetric evaluation criteria (Table 1). Reference plans were either generated on the CT or MR-Linac acquired image.
Table 1.
Mean doses to targets and OARs based on 100 treated fractions. (APD: Adapted Plan Dose; DDOTD: Delivered Dose of The Day).
| Structure of Interest | Parameters | Acceptance criteria per fraction (cGy) | Mean (IQR) APD per fraction(cGy) | Mean (IQR) DDOTD (cGy) |
|---|---|---|---|---|
| CTV | D95% | 600* | 661 (659–664) | 652 (644–661) |
| Rectum | D1cc | 600 | 488 (239–622) | 453 (206–679) |
| D20% | 400 | 276 (188–335) | 263 (180–321) | |
| D50% | 200 | 144 (111–175) | 142 (114–169) | |
| Bladder | D5cc | 600 | 570 (546–599) | 589 (563–625) |
| D40% | 300 | 262 (166–365) | 160 (54–234) | |
As per study planning protocol the clinical target volume (CTV) can be boosted to 110% of prescription dose.
Online treatment workflow
The adapt to shape (ATS) workflow was performed for each MR-Linac treatment session, beginning with the acquisition of a localization T2w MR image (MRLoc). The image was rigidly registered with the image dataset used for reference planning. Once rigid registration was complete, the contours were either rigidly or deformably propagated onto the MRLoc for RO assessment and modification as required. Contours were reviewed by the treating team consisting of the RO, Radiation Therapists (RTs) and the Medical Physicist. An adapted plan was optimized based on the patient’s contours of the day. The optimized plan was reviewed and then exported for quality control (QC) including second dose calculation, and then published using an in-house software for documentation purposes.
During the QC process, a verification image was acquired (MRVer) to ensure the CTV was within the PTV contour and the presence of gross anatomical changes were assessed. If no gross changes were observed, motion monitoring (MM) was initiated through 2D cine imaging in all three planes. If gross changes were noted, and the CTV moved outside of the PTV contour, an ATP planning process was initiated followed by an additional MRVer and plan QC. MM was initiated following the assessment of the additional MRVer. Once plan QC was completed, and the plan was approved by all three professionals, MM would conclude and IMRT treatment commenced along with the beam-on MR (MRBO) acquisition. The MRBO is a 6-minute, high resolution T2w image acquired during beam on and used for the DDOTD calculation.
Offline dose computation and analysis
To quantify the dosimetric changes induced by intrafraction motion exhibited between the time of planning and time of delivery, the MRLOC, MRBO, their associated contours and the adapted dose distribution of each treatment fraction were exported to Raystation (V8.1.2.5, Raysearch, Sweden). The CTV, rectum and bladder were re-contoured on the MRBO by either a RT or RO and independently reviewed by a second RO. The dose from the adapted plans of all treated fractions were transferred onto their respective MRBO for the estimation of the DDOTD to the structures of interest. Two tailed paired t-test was used to compare the doses computed based on the MRLoc and MRBO, where p ≤ 0.05 was used as the threshold for statistical significance.
Results
Dosimetric data from twenty patients who completed their treatment on the MR-Linac with a prescription of 3000 cGy in 5 fractions were analyzed for a total of 100 fractions. Of the 20 patients, 13 had a rectal spacer (SpaceOAR hydrogel, Boston Scientific, MA, US). Median treatment time (determined by when the patient entered and exited the room) was 50 min (IQR = 44–61 min). ATP was performed for five fractions (3 patients) due to target displacement noted during the QC phase of the treatment session.
Rectum
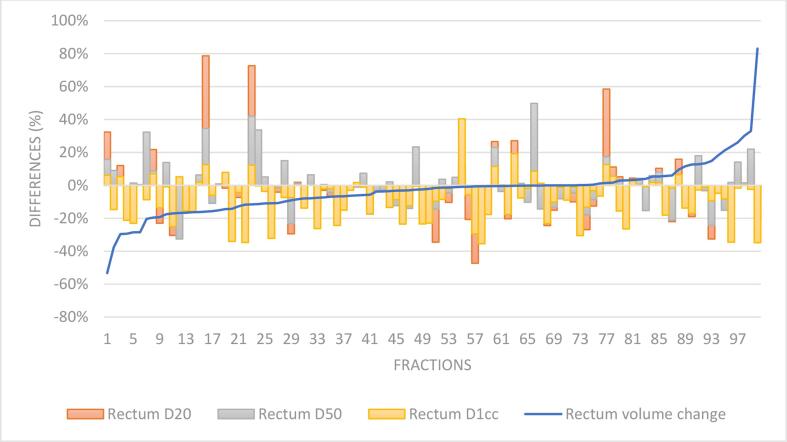
Median rectal volume difference based on the contours from the MRLoc to the MRBO was −1.9% (IQR: −10.9% to 0.6%) (Fig. 1). There were no significant differences in volume changes for patients with and without a hydrogel spacer (spacer mean = -4.2%, SD 14.1%, no spacer mean = −1.1%, SD 18.4%, p > 0.05). Median dosimetric differences for rectum D20% was −4.5% (IQR: −15% to 3%), D50% was −1.7% (IQR: −10% to 5%) and D1cc was −4% (IQR: −16% to 0%). Significant differences were identified between the adapted plan dose (APD) and the delivered dose of the day (DDOTD) for rectum D20% (Mean (cGy) = 276 (SD: 86) for APD and 263 (SD: 88) for DDOTD, p < 0.05) and rectum D1cc (Mean (cGy) = 488 (SD: 124) for APD and 453 (SD: 136) for DDOTD, p < 0.0001) (Fig. 1). However, no significant differences were identified between the APD and DDOTD for the rectum D50% (APD mean D50% = 145 cGy, SD = 53 cGy; DDOTD mean D50% = 142 cGy, SD = 52 cGy).
Fig. 1.
Rectal volume and dose differences between APD and DDOTD across 100 fractions ordered by rectal volume changes.
Bladder
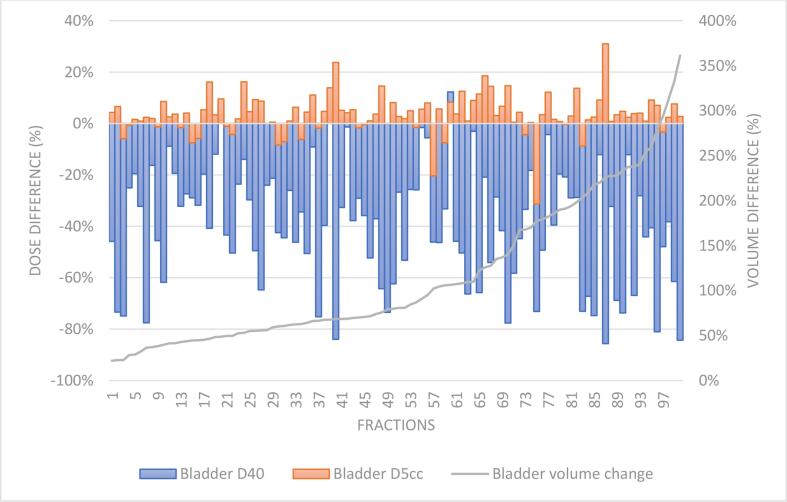
Bladder volume difference between the MRLoc and MRBO ranged from 22% to 361%, with 44 fractions showing at least a doubling in bladder volume (Fig. 2). The in-house volumetric dose criterion for bladder D40% (Table 1) was exceeded on 45 fractions based on the APD on the MRLoc and was exceeded on 18 fractions for the DDOTD. With bladder filling throughout the MR-Linac session, there was a significant reduction to the bladder D40% (mean APD 262 cGy; SD = 129 cGy vs mean DDOTD = 160 cGy; SD = 116 cGy, p < 0.001). However, the DDOTD for bladder D5cc was significantly higher than the APD (Mean APD = 570 cGy, SD: 41 cGy vs mean DDOTD = 589 cGy, SD: 54 cGy, p < 0.001).
Fig. 2.
Changes to bladder volume and dose differences between APD and DDOTD across 100 fractions ordered by bladder volume differences.
CTV
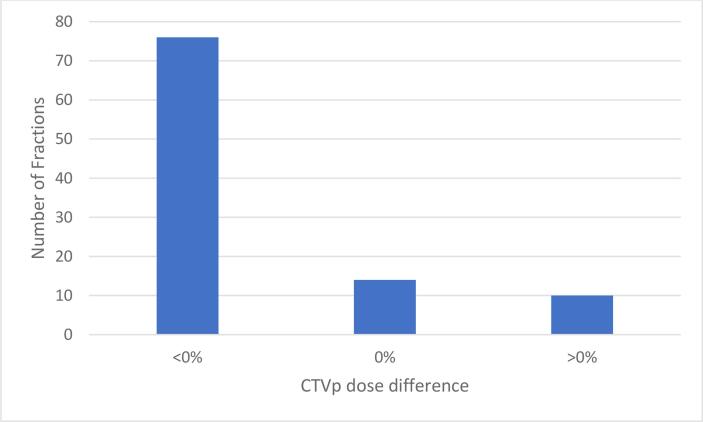
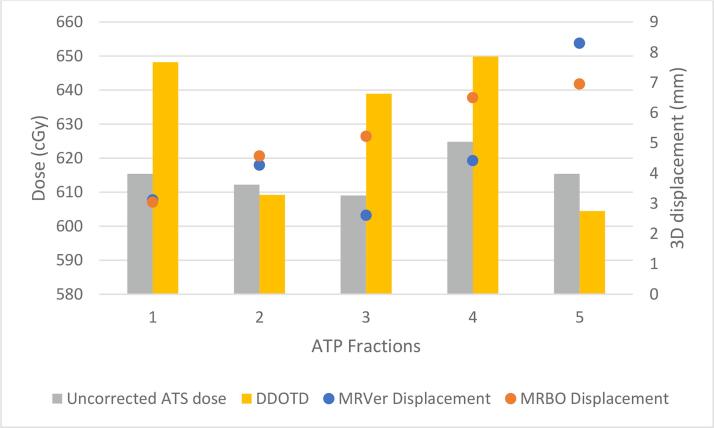
CTV coverage dose objective (Table 1) was achieved in all delivered fractions. However, CTV dose did decrease from ADP to DDOTD for most patients (Fig. 3). A significant decrease between the mean CTV D95% from the APD and the DDOTD was observed (APD = 660.8 cGy, SD = 7.4 cGy, DDOTD = 651.6 cGy, SD = 14.7 cGy, p < 0.01). Overall, no significant differences in coverage based on the DDOTD for patients with and without a hydrogel spacer were observed (p > 0.05). There were also no correlations between the difference in CTV coverage and bladder or rectal volumetric changes based on the computed coefficient of determination (r2 = 0.0002 and 0.0014, respectively). For the five treatment sessions requiring ATP, CTV displacement determined from the MRVer was greatest in the anterior-posterior (AP) direction (mean = 3.9 mm), followed by the superior-inferior (SI) direction (mean = 1.9 mm) and smallest in the right-left (RL) direction (mean = 0.2 mm). Of the five ATP sessions, only three demonstrated improved coverage (Fig. 4).
Fig. 3.
CTV dose differences between APD and DDOTD.
Fig. 4.
Impact to CTV dose due to ATP prompted by MRVer displacement. Computed 3D displacement is relative to MRLoc. Fractions 1 and 3 were from Patient 9, fraction 2 was from Patient 11, and fractions 4 and 5 were from Patient 7.
Discussion
In this study, intrafraction motion was identified through changes observed to CTV coverage for some patients and through both volumetric and dosimetric changes to OARs. However, clinically acceptable coverage was achieved for all delivered fractions for all patients even when intrafraction motion was observed, confirming the sufficiency of the current 5 mm PTV margin. The displacement results noted in session are similar to the intrafraction motion identified by Adamson et al. [2] using online kV fluoroscopy and post-treatment cone-beam CTs (CBCTs) showing the need for a minimum non-uniform margin of 2 mm in the RL direction, 3 mm in the SI direction and 4 mm in the AP direction. Additionally, prostate intrafraction motion was identified to be 2 mm LR, 3 mm SI, and 3 mm AP during SBRT delivery on a standard linac [15]. There is an opportunity to explore PTV margin reduction based on these results or personalized PTV margins considering the extent of target coverage change and displacement varied between patients. However, PTV reduction to <4 mm should be implemented with caution due to intrafraction motion over the course of treatment adaptation [7], [14]. It has been shown that over the course of a 10-minute cine imaging session reflective of treatment delivery time on a MR-Linac; using fiducial markers as a prostate tracking surrogate, translation trends of 1 mm posterior and 0.9 mm inferior occur [6].
The displacement data from the ATP sessions indicate that from the five fractions where ATP was performed, quantitatively, ATP was only required on one fraction as the 3D prostate soft tissue displacement on the MRVer was <5 mm, for the other fractions. However, a PTV margin reduction to <5 mm will likely result in an increased number of ATP plans after ATS on the MR-Linac, further increasing overall treatment time. The advantage of ATP after ATS is that it may help account for the additional motion, and this has been noted by [7], where ATP after every ATS fraction resulted in a reduction in prostate drift in the posterior and inferior direction and can help decrease the bladder volume in the high dose region. However, with longer treatment times, there would be an increased likelihood of intrafraction motion. Previous studies have suggested that the shorter the treatment time, the less the intrafraction motion observed, which yields a preference for volumetric arc therapy (VMAT) over IMRT [3].
Currently, implementation of VMAT is not clinically available on the Elekta MRL thus, alternative methods to decrease treatment time, or different methods of intrafraction motion mitigation need to be explored. Various studies have suggested using dietary interventions to help stabilize and reproduce rectum size over the course of treatment, mitigating inter-fractional changes. However, results from such studies showed that interventions such as the use of milk of magnesia to decrease flatulence [11] or advising regular fibre and fluid intake [17], or having regular bowel movements [28] may not always yield reproducible rectal geometry. Volumetric and dosimetric findings from this study suggests a similar finding; that rectal preparation may not necessarily lead to a reproducible rectal geometry daily, or even during a single treatment session, and these changes may be more patient dependent than intervention dependent. For prostate radiotherapy, the need for target positional consistency is generally associated with avoiding increased dose to the rectum and prostate motion. Dose reduction to the rectum has shown to decrease rectal toxicities [13].
Use of the hydrogel spacer has shown to help reduce dose to the rectum [5], however, it does not prevent rectal deformation during treatment as shown here. An alternative method to mitigate rectal changes is the use of the endorectal balloon, which have shown on planning studies to reduce posterior rectal wall dose and immobilize the prostate [26], however, has also been noted to increase rectal wall volume receiving higher doses [27]. Additionally, decreased rectum, bladder and penile bulb doses were found in patients treated with an endorectal balloon compared to patients treated without [9]. However, in a study comparing the use of the hydrogel spacer and the endorectal balloon with regards to the minimum PTV margin required, very small differences were found between the two groups [23]. Similar findings were established regarding intrafraction prostate motion for patients with a hydrogel spacer compared to those with an endorectal balloon during proton therapy [10]. Due to the position of the rectum relative to the prostate, changes to rectal filling via gas or feces can influence prostate motion, a characteristic that has been identified by both Adamson et al. [2] and Levin-Epstein et al. [15]. This contributes to the change in target coverage. Thus, the ability to image and define these inter- and intra-fractional changes and adapt is necessary when a reproducible method to mitigate rectal deformation has yet to be established [29].
As expected, the bladder D40% decreased significantly from the adapted plan dose due to bladder filling during the treatment session. This suggests that there should be flexibility during online treatment regarding the acceptance criteria for this dosimetric constraint. Additionally, there were no correlation found between the extent of bladder filling and the changes to target coverage. Similarly, it has been reported that changes in bladder filling does not play a significant role in impacting target position based on daily CBCTs [24]. Conversely, it has been noted that bladder filling influences prostate drift during treatment [14]. As no correlation was observed in this study, it is uncertain whether bladder filling itself contributes to prostate motion or it is just the effect of natural gravitational sag that was also noted by Kontaxis et al. [14].
Currently, computing the DDOTD is a resource intensive task. However, it can be used to assess the adequacy of treatment delivery in terms of dose to the target and OARs. This information can be used to inform following treatment fractions, to determine whether there is an opportunity to further spare the OARs or increase coverage to the target. Still, there are several limitations when interpreting the results of this study. First, the computed DDOTD is an estimation considering the image acquired for the computation is a 6-minute-long acquisition during beam delivery, however beam delivery often time is longer than 6 min. As the last few minutes of treatment delivery was not captured, it is possible that more motion could have been observed, impacting the delivered dose. There is a possibility that this can be remedied through ongoing imaging with cine or an additional 3D acquisition. However, treatment delivery times can easily vary depending on the number of monitor units and segments generated from the adapted plan and any treatment interruptions due to machine faults. The second limitation involves the human-generated contours and the associated interobserver variability during re-contouring since the RO who delineated the treatment contours was not the same person who contoured on the MRBO. Further exploration of machine learning could enable us to minimize such interobserver differences via auto-contouring and help decrease the number of resources that are required for this process.
Conclusion
Findings from this study demonstrated the dosimetric implications of intrafraction motion due to pelvic organ changes during prostate SBRT treatment on the MR-Linac. Volumetric variability of OARs does not necessarily influence CTV coverage but does impact their own dosimetry. The computation of the DDOTD may help inform prospective treatment fractions for SBRT prostate treatments by evaluating whether there is potential to further boost the target or spare OARs based on what was delivered.
Declaration of Competing Interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
References
- 1.Adamson J., Wu Q. Inferences about Prostate Intrafraction Motion from Pre- and Post-Treatment Volumetric Imaging. Int J Radiat Onocol Biol Phys. 2009;75(1):260–267. doi: 10.1016/j.ijrobp.2009.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Adamson J., Wu Q., Yan D. Dosimetric Effect of Intrafraction Motion and Residual Setup Error for Hypofractionated Prostate IMRT with CBCT Image Guidance. Int J Radiat Onocol Biol Phys. 2011:453–461. doi: 10.1016/j.ijrobp.2010.02.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ballhausen H., Li M., Ganswindt U., Belka C. Shorter treatment times reduce the impact of intra-fractional motionKürzere Behandlungszeit reduziert den Einfluss intrafraktioneller Bewegung: Eine Echtzeit-4DUS-Studie vergleicht VMAT vs. Step-and-shoot-IMRT beim Prostatakarzinom. Strahlenther Onkol. 2018;194(7):664–674. doi: 10.1007/s00066-018-1286-2. [DOI] [PubMed] [Google Scholar]
- 4.Corradini S., Alongi F., Andratschke N., Belka C., Boldrini L., Cellini F., et al. MR-guidance in clinical reality: current treatment challenges and future perspectives. Radiat Oncol. 2019;14(1) doi: 10.1186/s13014-019-1308-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cuccia F., Mazzola R., Nicosia L., Figlia V., Giaj-Levra N., Ricchetti F., et al. Impact of hydrogel peri-rectal spacer insertion on prostate gland intra-fraction motion during 1.5 T MR-guided stereotactic body radiotherapy. Radiat Oncol. 2020;15(1):1–9. doi: 10.1186/s13014-020-01622-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.de Muinck Keizer D.M., Pathmanathan A.U., Andreychenko A., Kerkmeijer L.G.W., van der Voort van Zyp J.R.N., Tree A.C., et al. Fiducial marker based intra-fraction motion assessment on cine-MR for MR-linac treatment of prostate cancer. Phys Med Biol. 2019;64(7):07NT02. doi: 10.1088/1361-6560/ab09a6. [DOI] [PubMed] [Google Scholar]
- 7.de Muinck Keizer D.M., van der Voort van Zyp J.R.N., de Groot-van Breugel E.N., Raaymakers B.W., Lagendijk J.J.W., de Boer H.C.J. On-line daily plan optimization combined with a virtual couch shift procedure to address intrafraction motion in prostate magnetic resonance guided radiotherapy. Phys Imaging Radiat Oncol. 2021;19:90–95. doi: 10.1016/j.phro.2021.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dunlop A., Mitchell A., Tree A., Barnes H., Bower L., Chick J., et al. Daily adaptive radiotherapy for patients with prostate cancer using a high field MR-linac: Initial clinical experiences and assessment of delivered doses compared to a C-arm linac. Clin Translat Radiat Oncol. 2020;23:35–42. doi: 10.1016/j.ctro.2020.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gündoğ M., Başaran H., Orhan O., Yaray K., Aksözen T., Ki̇raz E., Eroğlu C. The Use of High-Volume Endorectal Balloon is Effective to Reduce Doses of Rectum and Bladder in Prostate Cancer Patients Treated with Linac-Based Stereotactic Body Radiotherapy. Turk J Oncol. 2020;35(4) [Google Scholar]
- 10.Hedrick S.G., Fagundes M., Robison B., Blakey M., Renegar J., Artz M., et al. A comparison between hydrogel spacer and endorectal balloon: an analysis of intrafraction prostate motion during proton therapy. J Appl Clin Med Phys. 2017;18(2):106–112. doi: 10.1002/acm2.12051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hosni A., Rosewall T., Craig T., Kong V., Bayley A., Berlin A., et al. The effect of bowel preparation regime on interfraction rectal filling variation during image guided radiotherapy for prostate cancer. Radiat Oncol. 2017;12(1) doi: 10.1186/s13014-017-0787-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Jaffray D.A., Lindsay P.E., Brock K.K., Deasy J.O., Tomé W.A. Accurate accumulation of dose for improved understanding of radiation effects in normal tissue. Int J Radiat Oncol Biol Phys. 2010;76(3 Suppl):S135–S139. doi: 10.1016/j.ijrobp.2009.06.093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Karsh L.I., Gross E.T., Pieczonka C.M., Aliotta P.J., Skomra C.J., Ponsky L.E., et al. Absorbable hydrogel spacer use in prostate radiotherapy: a comprehensive review of phase 3 clinical trial published data. Urology. 2018;115:39–44. doi: 10.1016/j.urology.2017.11.016. [DOI] [PubMed] [Google Scholar]
- 14.Kontaxis C., de Muinck Keizer D.M., Kerkmeijer L.G., Willigenburg T., den Hartogh M.D., de Groot-van Breugel E.N., et al. Delivered dose quantification in prostate radiotherapy using online 3D cine imaging and treatment log files on a combined 1.5 T magnetic resonance imaging and linear accelerator system. Phys Imaging Radiat Oncol. 2020;15:23–29. doi: 10.1016/j.phro.2020.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Levin-Epstein R., Qiao-Guan G., Juarez J.E., Shen Z., Steinberg M.L., Ruan D., et al. Clinical assessment of prostate displacement and planning target volume margins for stereotactic body radiotherapy of prostate cancer. Front Oncol. 2020;10 doi: 10.3389/fonc.2020.00539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mannerberg A., Persson E., Jonsson J., Gustafsson C.J., Gunnlaugsson A., Olsson L.E., et al. Dosimetric effects of adaptive prostate cancer radiotherapy in an MR-linac workflow. Radiat Oncol. 2020;15(1):1–9. doi: 10.1186/s13014-020-01604-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.McNair H.A., Wedlake L., McVey G.P., Thomas K., Andreyev J., Dearnaley D.P. Can diet combined with treatment scheduling achieve consistency of rectal filling in patients receiving radiotherapy to the prostate? Radiother Oncol. 2011;101(3):471–478. doi: 10.1016/j.radonc.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 18.Menten M.J., Mohajer J.K., Nilawar R., Bertholet J., Dunlop A., Pathmanathan A.U., et al. Automatic reconstruction of the delivered dose of the day using MR-linac treatment log files and online MR imaging. Radiother Oncol. 2020;145:88–94. doi: 10.1016/j.radonc.2019.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Peng C., Ahunbay E., Chen G., Anderson S., Lawton C., Li X.A. Characterizing interfraction variations and their dosimetric effects in prostate cancer radiotherapy. Int J Radiat Oncol Biol Phys. 2011;79(3):909–914. doi: 10.1016/j.ijrobp.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 20.Pinkawa M., Asadpour B., Gagel B., Piroth M.D., Holy R., Eble M.J. Prostate position variability and dose-volume histograms in radiotherapy for prostate cancer with full and empty bladder. Int J Radiat Oncol Biol Phys. 2006;64(3):856–861. doi: 10.1016/j.ijrobp.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 21.Raaymakers B.W., Jurgenliemk-Schulz I.M., Bol G.H., Glitzner M., Kotte A.N.T.J., van Asselen B., et al. First patients treated with a 1.5 T MRI-Linac: clinical proof of concept of a high- precision, high-field MRI guided radiotherapy treatment. Phys Med Biol. 2017:L41–L50. doi: 10.1088/1361-6560/aa9517. [DOI] [PubMed] [Google Scholar]
- 22.Smith G.A., Dunlop A., Barnes H., Herbert T., Lawes R., Mohajer J., et al. Bladder filling in patients undergoing prostate radiotherapy on a MR-linac: The dosimetric impact. Tech Innov Patient Support Radiat Oncol. 2022;21:41–45. doi: 10.1016/j.tipsro.2022.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Su Z., Henderson R., Nichols R., Bryant C., Hoppe B., Mendenhall W., et al. A comparative study of prostate PTV margins for patients using hydrogel spacer or rectal balloon in proton therapy. Physica Med. 2021;81:47–51. doi: 10.1016/j.ejmp.2020.11.033. [DOI] [PubMed] [Google Scholar]
- 24.Tsai C.-L., Wu J.-K., Wang C.-W., Hsu F.-M., Lai M.-K., Chia-Hsien Cheng J. Using cone-beam computed tomography to evaluate the impact of bladder filling status on target position in prostate radiotherapyEinsatz der Cone-Beam-Computertomographie zur Beurteilung des Einflusses des Blasenfüllungszustands auf die Zielposition bei der Radiotherapie der Prostata. Strahlenther Onkol. 2009;185(9):588–595. doi: 10.1007/s00066-009-1987-7. [DOI] [PubMed] [Google Scholar]
- 25.Veiga C., McClelland J., Moinuddin S., Lourenço A., Ricketts K., Annkah J., et al. Toward adaptive radiotherapy for head and neck patients: feasibility study on using CT-to-CBCT deformable registration for “dose of the day” calculations. Med Phys. 2014;41(3):031703. doi: 10.1118/1.4864240. [DOI] [PubMed] [Google Scholar]
- 26.Wachter S., Gerstner N., Dorner D., Goldner G., Colotto A., Wambersie A., et al. The influence of a rectal balloon tube as internal immobilization device on variations of volumes and dose-volume histograms during treatment course of conformal radiotherapy for prostate cancer. Int J Radiat Oncol Biol Phys. 2002;52(1):91–100. doi: 10.1016/s0360-3016(01)01821-1. [DOI] [PubMed] [Google Scholar]
- 27.Wong A.T., Schreiber D., Agarwal M., Polubarov A., Schwartz D. Impact of the use of an endorectal balloon on rectal dosimetry during stereotactic body radiation therapy for localized prostate cancer. Practical Radiat Oncol. 2016;6(4):262–267. doi: 10.1016/j.prro.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 28.Yaver M., Foo A., Larsen T., Fineberg H., Zeng G., McGowan T., et al. Consistency of Organ Geometries during Prostate Radiotherapy with Two Different Bladder and Bowel Regimens. J Med Imaging Radiat Sci. 2015;46(4):380–387. doi: 10.1016/j.jmir.2015.09.001. [DOI] [PubMed] [Google Scholar]
- 29.Schaule J, Chamberlain M, Wilke L, Baumgartl M, Krayenbuhl J, Zamburlini M, et al. Intrafractional stability of MR-guided online adaptive SBRT for prostate cancer. Radiation Oncology. 2021;16:1–7. doi: 10.1186/s13014-021-01916-0. [DOI] [PMC free article] [PubMed] [Google Scholar]