Abstract
Necrotic enteritis causes economic losses estimated to be up to 6 billion US dollars per year. Clinical and subclinical infections in poultry are also both correlated with decreased growth and feed efficiency. Moreover, in a context of increased antibiotic resistance, feed additives with enhanced antimicrobial properties are a useful and increasingly needed strategy. In this study, the protective effects of a blend of thymol and organic acids against the effects of Clostridium perfringens type A (CP) on chicken intestinal epithelial cells were investigated and compared to bacitracin, a widely used antibiotic in poultry production. Primary chicken intestinal epithelial cells were challenged with CP for a total time of 3 h to assess the beneficial effect of 2 doses of citric acid, dodecanoic acid, and thymol-containing blend, and compare them with bacitracin. During the challenge, different parameters were recorded, such as transepithelial electrical resistance, cell viability, mRNA expression, and reactive oxygen species production. CP induced inflammation with cytokine production and loss of epithelial barrier integrity. It was also able to induce reactive oxygen species production and increase the caspase expression leading to cellular death. The high dose of the blend acted similarly to bacitracin, preventing the disruptive effects of CP and inducing also an increase in zonula occludens-1 mRNA expression. The low dose only partially prevented the disruptive effects of CP but successfully reduced the associated inflammation. This study shows that the usage of thymol combined with 2 organic acids can protect primary chicken intestinal epithelial cells from CP-induced damages creating a valid candidate to substitute or adjuvate the antibiotic treatment against necrotic enteritis.
Key words: chicken enterocytes, Clostridium perfringens, necrotic enteritis, organic acids, thymol
INTRODUCTION
The poultry industry is affected by a variety of pathogens such as Salmonella spp. (Vandelapidas et al., 2010), Eimeria spp. (Huang et al., 2018), Clostridium spp. (Baba et al., 1997), and many others, which are associated with high economic losses due to the increased morbidity/mortality rates, decreased growth and feed efficiency, and high costs for control strategies (Timbermont et al., 2011). Necrotic enteritis (NE) causes economic losses estimated to be up to 6 billion US dollars per year (Wade and Keyburn, 2015) and it is one of the most addressed diseases with high economic impact. The causative pathogen of NE is Clostridium perfringens (CP), a Gram-positive anaerobic spore-forming and rod-shaped bacterium (Baba et al., 1997; Paiva and McElroy, 2014). CP types A, C, and G are of particular interest to the poultry industry because they have been associated with diseases in poultry. Of the known 7 CP types, both A and G were the main prevalent causative agents of NE (Abd El-Hack et al., 2022). Moreover, a lot of different predisposing factors are needed to develop NE in poultry (Yang et al., 2019). Microscopic examination of the early stages of NE shows strong inflammatory reactions to CP in the intestine (Timbermont et al., 2011). Furthermore, CP and other pathogens can also be related to a subinflammatory status that can be difficult to diagnosticate and can contribute to decreased growth and feed efficiency (Homer et al., 1992; Abreu et al., 2016). In the past, chicken intestinal diseases were studied on alternative chicken cell models such as fibroblasts, liver, or cells of other kinds (Jagadish and Saxena, 2008; Felici et al., 2021). Few studies report the effects of pathogens or inflammation directly on chicken intestinal epithelial cells (cIECs) in vitro (Dimier-Poisson et al., 2004; Immerseel et al., 2004; Guo et al., 2015; Bar Shira and Friedman, 2018), but no one has reported a detailed investigation on different cellular parameters involved in intestinal barrier integrity and inflammation. Moreover, the improper use of antibiotics in food animals aggravates the issue of antibiotic resistance, contributing to the loss of efficacy of antibiotic treatments against NE and to the increased need for alternative solutions. Previously, Giovagnoni et al. (2019) investigated whether specific organic acids and natural identical compounds that are commonly found in feed additives could be used as alternative or adjuvant molecules along with conventional antibiotics against CP. Starting from there, a blend of thymol, citric acid, and dodecanoic acid was created to combine the antimicrobial power of organic acids with the antimicrobial and anti-inflammatory capabilities of thymol. Thymol is a well-studied anti-inflammatory that acts by inhibiting the nuclear factor kappa-light-chain-enhancer of activated B cells (Nf-kB) pathway, MAPKs, and iNOS (Liang et al., 2014; Chen et al., 2020; Rossi et al., 2020; Al-Khrashi et al., 2022). Thymol is also an effective antioxidant, which could scavenge free radicals and it can prevent oxidative stress increasing glutathione and superoxide dismutase levels through the Nrf2 pathway (Saleh et al., 2021). Moreover, thymol has also a good antimicrobial activity thanks to its pore-forming capabilities on the bacterial cell membrane also against CP (Du et al., 2015, 2016a,b). Together with thymol, medium-chain fatty acids exhibit a potent antibacterial pore-forming activity. Furthermore, dodecanoic and citric acids are particularly effective against CP, C. difficile, and other Gram-positive bacteria (Jackman et al., 2020). Unfortunately, the potential beneficial effects on epithelial cells of medium-chain fatty acids are not fully understood yet. Toschi et al. (2020) reported also beneficial effects of citric acid on the epithelial barrier function and tight junction gene expression in vitro. Bacitracin is an antimicrobial agent, used to control NE in poultry. Bacitracin is still used as antimicrobial in many countries including the United States, contributing to spread antimicrobial resistance.
This study aimed to develop and optimize an in vitro challenge model to study the effects of CP on cIECs and to help to characterize the beneficial and protective effects of high and low doses of a blend of thymol, citric acid, and dodecanoic acid that could be used as an alternative or adjuvant to bacitracin, in the future.
MATERIAL AND METHODS
Chemicals and Reagents
All chemicals were analytical grade: citric acid (≥99%), dodecanoic acid (≥99%), and thymol (≥98.5%), were obtained from Sigma-Aldrich (St. Louis, MO).
The blend of these compounds was tested at 2 different concentrations. The high dose (H-BLEND) contained thymol 470 µM, citric acid 6250 µM, and dodecanoic acid 116 µM. The low dose (L-BLEND) contained thymol 235 µM, citric acid 3125 µM, and dodecanoic acid 58 µM. In the supplementary materials, the minimal inhibitory concentration assay on CP is reported. The minimal inhibitory concentration assay was used to decide the dosages used in the study.
Care and Use of Animals
Specific pathogen-free eggs were purchased from ValoBiomedia (Osterholz-Scharmbeck, Germany) and incubated at 37.7°C, 48% relative humidity in a semi-automated incubator. On the 19th day of incubation, according to the AVMA guidelines and animal welfare, chick embryos were sacrificed by decapitation. As chick embryos older than 14 d can experience pain, decapitation was recommended as a humane method of euthanasia. According to the Italian legislation (D.lgs. 26/2014, the act on the protection of animals used for scientific and educational purposes, which was passed in March 2014 and transposed Directive 2010/63/EU into current Italian legislation), avian embryos are not considered as “live vertebrate animals,” so the approval of Animal Ethics Commission was not required.
Bacterial Strain and Culture Conditions
The strain used in this study was ATCC 13124 CP type A. It was cultured in reinforced clostridial medium at 37°C under anaerobic conditions. For all the assays where CP was involved 5 × 105 CFU/mL was used as the initial standard inoculum, as recommended by the Clinical and Laboratory Standards Institute. Moreover, before this study, a set-up experiment was performed to find the optimal CP concentration to use on cells. We compared the effects on TEER of two concentrations of CP (104 and 105 CFU/mL). We decided to use 105 CFU/mL due to its more evident effects after 1.5 h and to be coherent with the CLSI reccomendations.
Growth Curve and α-Toxin Determination
The CP growth curve was determined through optical density (OD) measurement at 630 nm and represented using the Gompertz sigmoidal function (Zwietering et al., 1990).
The concentration of α-toxin (CPA) produced by the bacterium was assessed every hour from 0 h to 8 h after the passage of 105 CFU/mL in fresh reinforced clostridial medium, with the Bio-X α-toxin ELISA kit (Bio-X Diagnostics SPRL, Jemelle, Belgium) following the manufacturer instructions and using the quadratic regression curve that relates the OD values and CPA concentrations reported by Zang et al., (2006) for the kit.
Cell Cultures
cIECs were isolated with the method reported by Ghiselli et al. (2021). Briefly, starting from 19-day-old specific pathogen-free chicken embryos, the duodenal tract was recovered and placed in ice-cold Hank's balanced salt solution without calcium and magnesium (Cat. # 55021C—Sigma-Aldrich, St. Louis, MO), then it was cleaned from mesenteric fat and external mucus. Tissues were digested for 50 min at 37°C with a digestion medium containing collagenase and hyaluronidase. The recovered aggregates were ultimately seeded on cell supports coated with Matrigel matrix (Cat. # 356234—Corning Incorporated, Corning, NY) diluted at the final concentration of 0.8 mg/mL. Cells were cultured at 37°C and 5% CO2 onto different supports.
CP Challenge
To challenge cIECs, 4 different cell culture mediums were used:
-
(1)
Growth medium without penicillin/streptomycin as negative control (CTR- group);
-
(2)
Growth medium without penicillin/streptomycin containing CP in exponential phase at a concentration of 5 × 105 CFU/mL (CP group);
-
(3)
Growth medium without penicillin/streptomycin containing 5 × 105 CFU/mL CP in exponential phase and bacitracin 2ppm (Cat. # B0125—Sigma-Aldrich, St. Louis, MO—BAC group), used as bactericidal control;
-
(4)
Growth medium without penicillin/streptomycin containing 5 × 105 CFU/mL CP in exponential phase and H-BLEND (H-BLEND group);
-
(5)
Growth medium without penicillin/streptomycin containing 5 × 105 CFU/mL CP in exponential phase and L-BLEND (L-BLEND group);
Freshly isolated cIECs were then used to perform the different analyses listed below.
-
A.
Monolayer integrity: cIECs were cultured for 7 d before the challenge at 37°C and 5% CO2 onto coated Transwell inserts. Then they were adapted overnight to anaerobic conditions (100% CO2) at 37°C the day before the challenge. The anaerobiosis was generated in jars with an AnaeroGen gas pack (Thermo Fisher Scientific, Waltham, MA). On day 8 after seeding, transepithelial electrical resistance (TEER) was measured to check the monolayer integrity after the adaptation in anaerobiosis, and then cells were washed 3 times with Hank's balanced salt solution to remove penicillin/streptomycin contained in the normal growth medium. After the basal TEER measurement and washings, a medium change was performed to challenge the cells, accordingly to the 5 medium groups (n = 6 for each medium) listed at the beginning of the current paragraph. During the challenge, cells were maintained at 37°C and 100% CO2 in a jar with an AnaeroGen gas pack. TEER was measured 1.5 h and 3 h after the challenge started. At the end of the challenge, cells were harvested from filters for qPCR analyses.
-
B.
Viability and reactive oxygen species (ROS) production assays: cIECs were cultured for 3 d before the challenge at 37°C and 5% CO2 onto a coated 96-well plate. Then they were adapted overnight to anaerobic conditions (100% CO2) at 37°C, and on day 4 after seeding, they were challenged as described for the monolayer integrity assessment (n = 6 for each medium). During the challenge, cells were maintained at 37°C and 100% CO2 in a jar with an AnaeroGen gas pack. Viability was assessed after 1.5 h and 3 h from the beginning of the challenge, and the ROS production assay was performed only after 1.5 h due to the strong cytotoxic effect of CP that interferes with the proper ROS detection.
-
C.
Immunofluorescence (IF): cIECs were cultured for 3 d before the challenge at 37°C and 5% CO2 onto Lab-Tek II Chamber Slide System (Cat. # 154534—Thermo Scientific, Waltham, MA). Then they were adapted overnight to anaerobic conditions (100% CO2) at 37°C. Then on day 4 after seeding, they were challenged as described for the monolayer integrity assessment. During the challenge, cells were maintained at 37°C and 100% CO2 in a jar with an AnaeroGen gas pack. After 1.5 h, cells were fixed in 4% paraformaldehyde and used for the IF assay as described before.
Monolayer Integrity
To evaluate the monolayer's integrity and the effect of the CP challenge on the intestinal epithelium, the measurement of TEER was performed. cIECs were seeded at a density of 5 × 103 aggregates/filter onto 24-well Transwell polyethylene terephthalate inserts (0.4 µm pore) coated with Matrigel matrix (0.8 mg/mL). TEER was measured with a volt-ohm meter (Millicell ERS-2, Cat. # MERS00002—Millipore, Burlington, MA), then values were calculated as Cells reached confluence on Transwell inserts 3 d after seeding, but they were cultured until day 7 after seeding (before inducing the challenge) to allow the TEER stabilization. On day 8 after seeding, they were challenged.
qPCR
Total RNA was isolated from cells recovered from the Transwell supports after the challenge ended, using the NucleoSpin RNA Kit (Cat. # 750955—MACHEREY-NAGEL Inc., Bethlehem, PA), according to the manufacturer's protocol. RNA yield and quality were determined spectrophotometrically by detecting 260 and 280 nm absorbance by Varioskan LUX multimode microplate reader (Thermo Fisher Scientific, Waltham, MA). RNA was reverse transcribed with iScript cDNA Synthesis Kit (Cat. # 1708890—Bio-Rad Laboratories, Hercules, CA) according to the manufacturer's instructions. Lastly, real-time PCR reactions were performed in duplicate using CFX Connect Real-Time PCR System and iTaq Universal SYBR Green Supermix (Cat. # 1725120—Bio-Rad Laboratories, Hercules, CA).
Gene expression was reported as fold of change using the ΔΔCt method (Livak and Schmittgen, 2001), using as reference the 60S acidic ribosomal protein P0 (RPLP0). To analyze the effects of the 2 different challenges, specific markers of chicken cytokines, tight junction, and markers involved in the apoptotic pathway were chosen: Interleukin (IL)1β, IL6, IL8, IL10, Interferon-gamma (INFγ), mitogen-activated protein kinase 1 (MAPK), zonula occludens 1 (ZO1), occludin (OCCL), claudin-1 (CLDN1), caspase 1 (CASP1), caspase 3 (CASP3), inducible nitric oxide synthase (iNOS) and toll-like receptor 4 (TLR4).
All the primers (Sigma-Aldrich, St. Louis, MO) were designed using the PrimerBLAST tool (https://www.ncbi.nlm.nih.gov/tools/primer-blast/) and they are listed in Table 1.
Table 1.
Primer list used for gene expression.
| Gene | Primer sequence (5’-> 3’) | Product length (bp) | Accession N. | |
|---|---|---|---|---|
| Tight Junctions | Zonula occludens-1 | F: TCTGCACAGTGAGGTTGGCT R: GGCTGTCCTGCATCGGTGT |
145 | XM_004934975 |
| Occludin-1 | F: TGCTTTTGCCCAAGCAGGAA R: TGTGGGAGAGGCACCAGTTG |
153 | NM_204417 | |
| Claudin-1 | F: TCGGTGGTGGTCACTTCGTC R: CGCTGATTTACGGGCCGAAC |
113 | NM_001004768 | |
| Cytokines | IL1β | F: TGCCTGCAGAAGAAGCCTCG R: CTCCGCAGCAGTTTGGTCAT |
137 | NM_204524.1 |
| IL6 | F: GCAGGACGAGATGTGCAAGA R: ACCTTGGGCAGGTTGAGGTT |
84 | NM_204628.1 | |
| IL8 | F: AGCTGCTCTGTCGCAAGGTA R: GCTTGGCGTCAGCTTCACATC |
124 | NM_205498.1 | |
| INFγ | F: ACAACCTTCCTGATGGCGTG R: AGTTCATTCGCGGCTTTGCG |
100 | NM_205149.1 | |
| Inflammation markers | iNOS | F: CCCTCCAGCTGATCAGACTATC R: GTGTGCAAGCCGGAATCTTTT |
86 | NM_204961.1 |
| Toll-like receptor 4 | F: CCTGGGTCTAGCAGCCTTCC R: TGGCCCAGATTCAGCTCCTG |
129 | NM_001030693 | |
| Caspase 1 | F: CTGTGGGATTCTCCGACCCC R: GCCCTACGGGTTCGTCTCTC |
148 | XM_040650618 | |
| Caspase 3 | F: TACTCCTGGAGGAACGCAGC R: TGCCACTCTGCGATTTACACG |
123 | NM_204725.1 | |
| MAPK-1 | F: TGTGACTTCGGACTGGCTCG R: AGGAGCCCTGTACCAACGTG |
93 | XM_015275131 | |
| Ref. | RPLP0 | F: TCACGGTAAAGAGGGGAGGTG R: CTTGCTCAGTCCCCAGCCTT |
143 | NM_205179 |
Designed with PrimerBLAST.
Abbreviation: Ref., Reference gene.
Immunofluorescence Assay
IF staining was performed for ZO1, OCCL, claudin-3 (CLDN3), and MAPK. Freshly isolated cIECs were seeded at a density of 1 × 103 aggregates/well onto Matrigel-coated (0.8 mg/mL) Nunc Lab-Tek II Chamber Slide System cultured for 3 d, then adapted to anaerobic condition overnight, and challenged at day 4 after seeding. Then they were fixed for 20 min with 4% paraformaldehyde (Cat.# 158127—Sigma-Aldrich, St. Louis, MO) in Dulbecco's phosphate-buffered saline (DPBS—Cat.# D8537—Sigma-Aldrich St. Louis, MO). Cells were permeabilized with 0.5% Triton X-100 (Cat.# VARICP3418—VWR, Radnor, PA) for 15 min and then blocked in 10% goat serum (Cat.# G9023—Sigma-Aldrich St. Louis, MO) for 1 h. Primary monoclonal antibodies reported in Table 2 were diluted in 2% bovine serum albumin (BSA—Cat.# P6154—VWR, Radnor, PA) + 0.05% saponins (Cat.# A18820—Alfa Aesar, Haverhill, MA) in DPBS.
Table 2.
Antibodies used for immunofluorescence assay.
| Reagent | Dilution | Supplier | Product catalog number |
|---|---|---|---|
| Rabbit anti-chicken Claudin-3 | 10 µg/mL | Abcam plc | MA1-06326 |
| Rabbit anti-chicken zonula occludens 1 | 10 µg/mL | Thermo Fisher Scientific | 61-7300 |
| Rabbit anti-chicken pan-occludin | 2 µg/mL | Thermo Fisher Scientific | 71-1500 |
| Rabbit anti-chicken MAPK1/ERK2 | 1:100 | Thermo Fisher Scientific | MA5-15134 |
| Goat anti-rabbit secondary antibody, FITC conjugated | 4 µg/mL | Thermo Fisher Scientific | A27034 |
Cells were incubated with primary antibodies for 3 h at room temperature in a humidified chamber. Secondary antibodies conjugated to fluorescein isothiocyanate (FITC) were used to probe the bounded primary antibodies for 1 h (Table 2), followed by 2 successive washes with 0.2% BSA + 0.05% saponins in DPBS. The slides were then mounted with Fluoroshield containing 4’,6-diamidine-2’-phenylindole dihydrochloride (DAPI) (Cat.# F6057—Sigma-Aldrich, St. Louis, MO). Images were acquired with Nikon Eclipse Ci fluorescence upright microscope (Nikon corporation—www.nikon.com) and processed with NIS-Elements software (Nikon corporation—www.nikon.com).
Viability and ROS Production Assay
To assess the effect of the challenge on cIECs viability and ROS production, cIECs were seeded at a density of 1 × 103 aggregates/well onto a 96-well plate coated with Matrigel matrix (0.8 mg/mL) and were maintained at 37°C and 5% CO2 for 3 d after seeding, then adapted to anaerobic condition overnight and challenged 4 d after seeding. To determine the ROS production CellROX Deep Red Reagent kit was used (Thermo Fisher Scientific, Waltham, MA) and the effect on cell viability was assessed with the PrestoBlue Cell Viability Reagent (Thermo Fisher Scientific, Waltham, MA). Both assays were performed according to the manufacturer's protocols. Briefly, for the ROS production assay, 1.5 h after the challenge, cells were incubated for 30 min with the CellROX Deep Red Reagent and after 3 washes with DPBS the fluorescence (640 nm excitation and 695 nm emission) was read by Varioskan LUX multimode microplate reader. Instead, for the viability assay 10 µL of PrestoBlue Cell Viability Reagent were added to each well after 1.5 h and 3 h from the challenge start. The fluorescence (560 nm excitation and 590nm emission) was read by Varioskan LUX multimode microplate reader.
Statistical Analysis
For TEER measurement (n = 6), gene expression (n = 6), ROS production (n = 6), and cell viability (n = 6), data were represented as mean ± standard error (SEM). CP growth curve (n = 6) and α-toxin production (n = 6) were represented as mean ± standard deviation (SD). A 2-way ANOVA with Tukey's multiple comparisons was performed to analyze the effect of time and treatments on TEER and gene expression; ROS production data were analyzed with the Kruskal–Wallis's test. A 2-way ANOVA Šídák's multiple comparisons test was used to analyze the effect of time and treatments on cell viability. The level of significance was set at 0.05.
RESULTS
Bacterial Culture and α-Toxin Determination
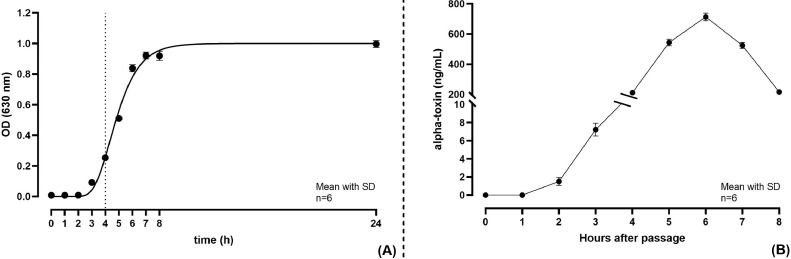
Figure 1 represents the bacterial growth curve (Figure 1A) and the CPA production by CP over time (Figure 1B). Using the ELISA kit, the CPA production was assessed to be 8 ng/mL 3 h after the bacterial passage.
Figure 1.
(A) Growth curve of C. perfringens ATCC 13124 determined through OD measurement at 630 nm and represented using the Gompertz sigmoidal function; (B) α-toxin production (ng/mL) overtime by C. perfringens determined through alpha-toxin ELISA kit. Mean with SD (n = 6). Abbreviation: OD, optical density.
CP Challenge
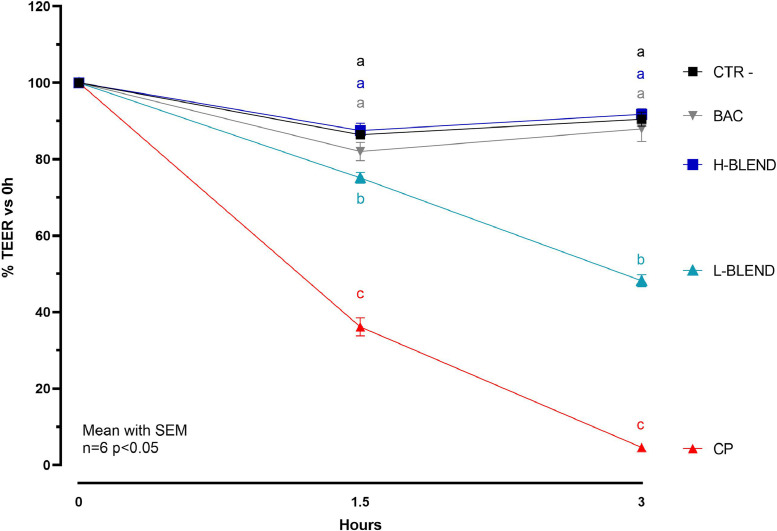
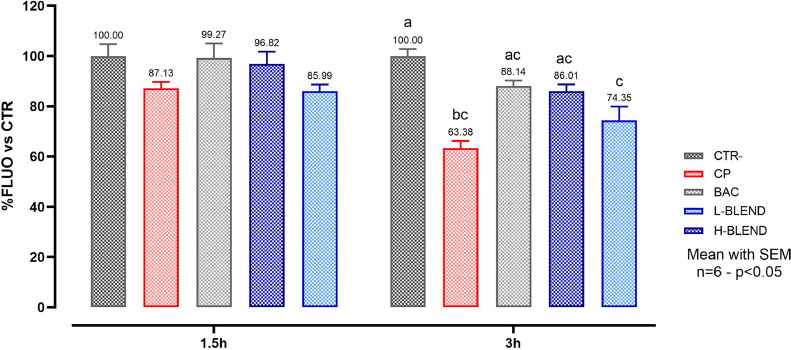
Figure 2 reports the TEER values measured at 1.5 and 3 h after infection. The 2-way ANOVA analysis on TEER measurements revealed that there was a statically significant interaction between time and treatments (P < 0.0001). CP showed a strong effect on the cIECs barrier, considering that it was able to completely disrupt the cell monolayer after 3 h. BAC used as bactericidal control, prevented the TEER drop which remained similar to the control in both time points. The H-BLEND protected the cells from the action of CP in a similar way to the antibiotic, while the L-BLEND partially failed with a 50% TEER drop after 3h. This result was also confirmed by the viability assay, reported in Figure 3, which showed a cell viability reduction, in the CP-challenged group, of 13% after 1.5 h and 37% after 3 h. BAC and the H-BLEND prevented cell death maintaining higher cell viability values. The L-BLEND recorded a viability reduction near 26% after 3 h. The 2-way ANOVA analysis on viability assay revealed that there was a statically significant interaction between time and treatments (P = 0.0359).
Figure 2.
Relative transepithelial electrical resistance percentage after 1.5h and 3h of C. perfringens challenge—CTR = Control; CP = C. perfringens 5 × 105 CFU/mL; BAC = Bacitracin 2 ppm; H-BLEND = thymol 470 µM, citric acid 6250 µM, and dodecanoic acid 115 µM; L-BLEND = thymol 235 µM, citric acid 3125 µM, and do-decanoic acid 58 µM. Mean with SEM (n = 6). Letters represent significant differences (P < 0.05); (2-way ANOVA Tukey's multiple comparisons).
Figure 3.
Cell viability after 1.5h and 3h of C. perfringens challenge—CTR = Control; CP = C. perfringens 5 × 105 CFU/mL; BAC = Bacitracin 2 ppm; H-BLEND = thymol 470 µM, citric acid 6250 µM, and dodecanoic acid 115 µM; L-BLEND = thymol 235 µM, citric acid 3125 µM, and dodecanoic acid 58 µM. Mean with SEM (n = 6). Letters represent significant differences (P < 0.05); (2-way ANOVA Tukey's multiple comparisons).
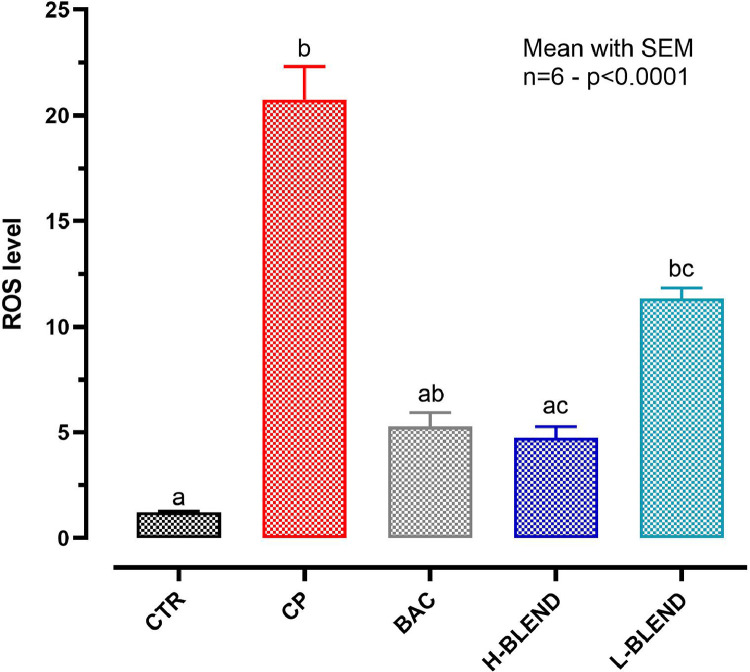
Figure 4 reports the effects of CP on ROS production showing a 20-fold increase of ROS production in the challenged group, compared to control, already after 1.5 h. ROS levels were 4 times reduced by H-BLEND and BAC, and only 2-fold by L-BLEND.
Figure 4.
ROS production after 1.5 h of C. perfringens challenge. The reactive oxygen species production assay was performed only after 1.5 h due to the strong cytotoxic effect of CP that interferes with the proper ROS detection – CTR = Control; CP = C. perfringens 5 × 105 CFU/mL; BAC = Bacitracin 2 ppm; H-BLEND = thymol 470 µM, citric acid 6250 µM, and dodecanoic acid 115 µM; L-BLEND = thymol 235 µM, citric acid 3125 µM, and dodecanoic acid 58 µM - Mean with SEM (n = 6). Letters represent significant differences (P < 0.0001); (Kruskal–Wallis test multiple comparisons). Abbreviation: ROS, reactive oxygen species.
The ROS production assay was performed only after 1.5 h due to the strong cytotoxic effect of CP that interferes with the proper ROS detection.
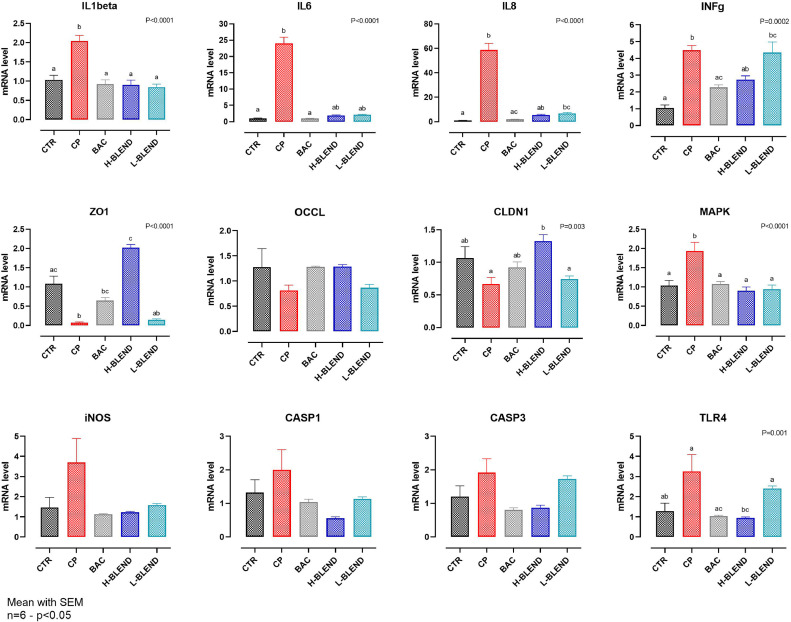
In Figure 5, the qPCR results are reported. CP reduced the expression of ZO1 and CLDN1 and significantly increased the expression of pro-inflammatory cytokines: IL1β (2 times higher), IL6 (25 times higher), IL8 (60 times higher), and INFγ (5 times higher). The blend at both doses significantly reduced the expression of IL1β, IL6, and IL8. The H-BLEND managed to reduce INFγ and increase the expression of ZO1 compared to the control and BAC. The L-BLEND was ineffective against INFγ expression increase and ZO1 and CLND1 expression reduction.
Figure 5.
Gene expression after 3 h of C. perfringens challenge—CTR = Control; CP = C. perfringens 5 × 105 CFU/mL; BAC = Bacitracin 2 ppm; H-BLEND = thymol 470 µM, citric acid 6250 µM, and do-decanoic acid 115 µM; L-BLEND = thymol 235 µM, citric acid 3125 µM, and dodecanoic acid 58 µM; INFg = Interferon γ; ZO1 = Zonula occludens 1; OCCL = Occludin; CLDN1 = Claudin1; MAPK = Mitogen-activated protein kinase; iNOS = inducible nitric oxide synthase; TLR4 = toll-like receptor 4; CASP1 = caspase 1; CASP3 = caspase 3; Mean with SEM (n = 6). Letters represent significant differences (P < 0.05); (1-way ANOVA Tukey's multiple comparisons).
Moreover, iNOS, TLR4, and CASP1 levels were numerically increased in cells challenged with CP, compared to the control ones (negative control and BAC group). The H-BLEND managed to reduce the levels of these molecular markers near the control. Lastly, both H-BLEND and BAC managed to significantly reduce the expression levels of CASP3 compared to the challenged cells.
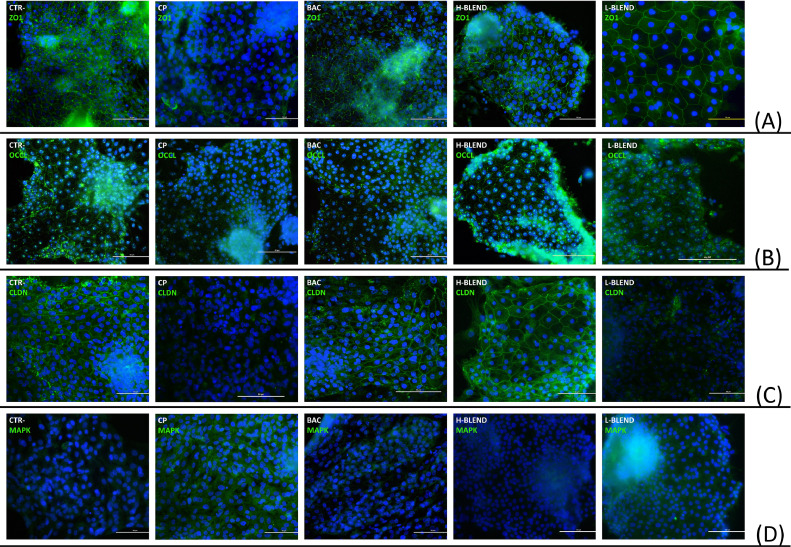
This result was also confirmed by the IF assay reported in Figure 6, where it was possible to observe a loss of tight junctions and activation of the MAPK pathway in the CP group. Interestingly, the H-BLEND completely inhibited the effect of CP on ZO1, OCCL, CLDN3, and MPAK.
Figure 6.
Immunofluorescence assay after 1.5 h of C. perfringens challenge—CTR = Control; CP = C. perfringens 5 × 105 CFU/mL; BAC = Bacitracin 2 ppm; H-BLEND = thymol 470 µM, citric acid 6250 µM, and dodecanoic acid 115 µM; L-BLEND = thymol 235 µM, citric acid 3125 µM, and dodecanoic acid 58 µM; (A) ZO1=Zonula occludens 1; (B) OCCL = Occludin; (C) CLDN = Claudin-3; (D) MAPK = Mitogen-activated protein kinase.
DISCUSSION
The poultry industry is affected by different pathogens and problems that cause losses of billions of US dollars every year (Espinosa et al., 2020). Farmers are constantly looking for ways to improve productivity and reduce the impact of various diseases. CP is the causative pathogen of NE and the early stages of NE are associated with strong inflammatory reactions at the intestinal level (Paiva and McElroy, 2014). To find feed additives that can act against these pathologies, it is necessary to develop an in vitro standardized model that allows an in-depth study of the pathological mechanisms related to CP pathogenesis. This study aimed to develop and optimize an in vitro challenge model to study the effects on cIECs of CP and to test the protective effects of a blend composed of thymol, citric acid, and dodecanoic acid as an alternative or adjuvant to a commonly used antibiotic in poultry production. In particular, the H-BLEND contained an inhibitory concentration of citric acid and dodecanoic acid to compare the blend to BAC. Instead, L-BLEND contained a sub-inhibitory concentration of all 3 compounds to test a possible synergistic effect against the CP-induced effects on cells.
To study the effects of a challenge induced by CP on the epithelial cells cIECs were cultured on Transwell inserts to mimic the intestinal barrier. CP reduced the TEER values up to 50–80% compared to control, indicating a disruptive effect on the epithelial barrier. CP strongly affected ZO1, OCCL, and CLDN1 mRNA expression. The tested blend of citric acid, dodecanoic acid, and thymol at the higher dose managed to protect the intestinal barrier preventing the TEER drop as well as BAC. Interestingly, the H-BLEND also increased the mRNA expression of ZO1 acting as a protecting and reinforcing agent. BAC used at his minimal inhibitory concentration, instead prevented the TEER drop but without inducing an increase in ZO1 mRNA expression. L-BLEND protected only partially from the TEER drop without being able to increase the mRNA expression of ZO1.
In this study, the blend containing thymol reduced the activation of the inflammatory pathway induced by CP, reducing the expression of cytokines and enzymes related to the inflammation and it helped to maintain normal cellular functions and low ROS levels during the CP challenge. Moreover, thymol has also a good antimicrobial activity thanks to its pore-forming capabilities on the bacterial cell membrane.
The ATCC 13124 C. perfringens type A used in this study produces CPA and perfringolysin-o (PFOA). PFOA and CPA are 2 toxins that have strong pore-forming activity. PFOA acts on cholesterol creating holes in cholesterol-containing membranes (Yamamura et al., 2019).
CPA acts mostly on phospholipids such as phosphatidylcholine and it can activate the CASP3, causing cell death (Porter and Jänicke, 1999), and MEK1/2, ERK1/2 system related to NF-κB activation, (Oda et al., 2006; Monturiol-Gross et al., 2014) actively fought by thymol.
Monturiol-Gross et al. (2014) report that CPA causes oxidative stress and MEK/ERK pathway activation at a concentration of 4 ng/mL, which is half of 8 ng/mL recorded in this study after 3 h. Moreover, MEK1/2 and ERK1/2 pathways are antagonized by thymol and they recover an important role in the generation of IL8 as reported by Wang et al. (2006). In addition, in this study, the activation of MAPK (ERK2) was observed, which is believed to stabilize IL8-mRNA and is associated with the generation of ROS (Oda et al., 2012). The blend used strongly reduced ROS generation thanks to the antioxidant capabilities of thymol and the activation of the Nrf2 pathway by citric acid (Ashrafian et al., 2012) and thymol (Saleh et al., 2021).
Interestingly, TLR4 mRNA expression was increased by CP. CP is a Gram-positive bacterium and its main cell wall component is peptidoglycan (PGN) (Kim et al., 2015). In human and mouse studies, PGN has been reported to activate the innate immune response via TLR2 (Wu et al., 2007). Guo et al. (2015) already observed that PGN treatment did not significantly affect TLR2 expression in chicken intestinal epithelial cells. Recently, Takehara et al. (2021) speculated that CPA and PFOA could activate the TLR4 on intestinal cells. This result was observed also in this study by the qPCR expression of TLR4. The TLR4 activation should be also related to an increase in IL6, IL8, and IL1β expression through the Nf-kB pathway (Nyati et al., 2017 pp. 2687–2703). The blend used in this study managed to strongly reduce the effect of CP on those 3 cytokines at both dosages. Interestingly, also the L-BLEND that contains sub-inhibitory concentrations of the compounds managed to reduce the pro-inflammatory cytokines mRNA expression. This indicates an anti-inflammatory property of the blend also at subinhibitory concentrations.
Lastly, after an acute mucosal injury, epithelial cells also start to produce nitric oxide through the iNOS (Chokshi et al., 2008) activated by Nf-kB. Indeed, qPCR showed a 4-fold numerical increase in iNOS expression on cIECs challenged with CP confirming again the Nf-kB involvement.
All the reported effects of CPA and PFOA on cells seem to be mainly connected to the NF-κB activation which is reported to be actively inhibited by thymol (Liang et al., 2014; Chen et al., 2020; Rossi et al., 2020; Al-Khrashi et al., 2022). Together with the 2 organic acids used, thymol forms a blend that has strong antimicrobial power against CP, reducing the bacterial load, and thus protecting against the inflammation and oxidative stress caused by the challenge. Moreover, the tested blend showed anti-inflammatory effects at subinhibitory concentration, but without fully protecting the epithelial barrier. These results could be an interesting starting point for a future test of the effects of this blend in-vivo and candidate it as a possible alternative to bacitracin in poultry production.
ACKNOWLEDGMENTS
This work was supported by a grant from Vetagro S.p.A. (Reggio Emilia, Italy). Funders participated in the study design, collection, analysis, interpretation of data, and writing of the manuscript.
DISCLOSURES
Ester Grilli reports financial support was provided by Vetagro S.p.A. Andrea Piva reports a relationship with Vetagro S.p.A. that includes: board membership. Andrea Piva reports a relationship with University of Bologna that includes: employment. Ester Grilli reports a relationship with Vetagro Inc. that includes: board membership. Ester Grilli reports a relationship with University of Bologna that includes: employment.
REFERENCES
- Abd El-Hack M.E., El-Saadony M.T., Elbestawy A.R., El-Shall N.A., Saad A.M., Salem H.M., El-Tahan A.M., Khafaga A.F., Taha A.E., AbuQamar S.F., El-Tarabily K.A. Necrotic enteritis in broiler chickens: disease characteristics and prevention using organic antibiotic alternatives—a comprehensive review. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abreu D.L.C., Santos F.F., José D.S., Tortelly R., Nascimento E.R., Pereira V.L.A. Pathological aspects of a subclinical Marek's disease case in free-range chickens. Braz. J. Poult. Sci. 2016;18:197–200. [Google Scholar]
- Al-Khrashi L.A., Badr A.M., AL-Amin M.A., Mahran Y.F. Thymol ameliorates 5-fluorouracil-induced intestinal mucositis: evidence of down-regulatory effect on TGF-β/MAPK pathways through NF-κB. J. Biochem. Mol. Toxicol. 2022;36:e22932. doi: 10.1002/jbt.22932. [DOI] [PubMed] [Google Scholar]
- Ashrafian H., Czibik G., Bellahcene M., Aksentijević D., Smith A.C., Mitchell S.J., Dodd M.S., Kirwan J., Byrne J.J., Ludwig C., Isackson H., Yavari A., Støttrup N.B., Contractor H., Cahill T.J., Sahgal N., Ball D.R., Birkler R.I.D., Hargreaves I., Tennant D.A., Land J., Lygate C.A., Johannsen M., Kharbanda R.K., Neubauer S., Redwood C., de Cabo R., Ahmet I., Talan M., Günther U.L., Robinson A.J., Viant M.R., Pollard P.J., Tyler D.J., Watkins H. Fumarate is cardioprotective via activation of the Nrf2 antioxidant pathway. Cell Metab. 2012;15:361–371. doi: 10.1016/j.cmet.2012.01.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baba E., Ikemoto T., Fukata T., Sasai K., Arakawa A., McDougald L.R. Clostridial population and the intestinal lesions in chickens infected with Clostridium perfringens and Eimeria necatrix. Vet. Microbiol. 1997;54:301–308. doi: 10.1016/s0378-1135(96)01289-8. [DOI] [PubMed] [Google Scholar]
- Bar Shira E., Friedman A. Innate immune functions of avian intestinal epithelial cells: response to bacterial stimuli and localization of responding cells in the developing avian digestive tract (TA Kufer, Ed.) PLoS One. 2018;13 doi: 10.1371/journal.pone.0200393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Li D.L., Xie L.N., ran Ma Y., Wu P.P., Li C., Liu W.F., Zhang K., Zhou R.P., Xu X.T., Zheng X., Liu X. Synergistic anti-inflammatory effects of silibinin and thymol combination on LPS-induced RAW264.7 cells by inhibition of NF-κB and MAPK activation. Phytomedicine. 2020;78 doi: 10.1016/j.phymed.2020.153309. [DOI] [PubMed] [Google Scholar]
- Chokshi N.K., Hunter C.J., Guner Y.S., Grishin A., Ford H.R. The role of nitric oxide in intestinal epithelial injury and restitution in neonatal NEC. Semin. Perinatol. 2008;32:92–99. doi: 10.1053/j.semperi.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimier-Poisson I.H., Bout D.T., Quéré P. Chicken primary enterocytes: inhibition of Eimeria tenella replication after activation with crude interferon-γ supernatants. Avian Dis. 2004;48:617–624. doi: 10.1637/7180-031604R. [DOI] [PubMed] [Google Scholar]
- Du E., Gan L., Li Z., Wang W., Liu D., Guo Y. In vitro antibacterial activity of thymol and carvacrol and their effects on broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2015;6:58. doi: 10.1186/s40104-015-0055-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du E., Wang W., Gan L., Li Z., Guo S., Guo Y. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2016;7:19. doi: 10.1186/s40104-016-0079-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Du E., Wang W., Gan L., Li Z., Guo S., Guo Y. Effects of thymol and carvacrol supplementation on intestinal integrity and immune responses of broiler chickens challenged with Clostridium perfringens. J. Anim. Sci. Biotechnol. 2016;7:9. doi: 10.1186/s40104-016-0079-7. http://www.jasbsci.com/content/7/1/19 (Verified May 14, 2019) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa R., Tago D., Treich N. Infectious diseases and meat production. Environ. Resour. Econ. 2020;76:1019–1044. doi: 10.1007/s10640-020-00484-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felici M., Tugnoli B., Piva A., Grilli E. In Vitro assessment of anticoccidials: methods and molecules. Anim. Open Access J. MDPI. 2021;11:1962. doi: 10.3390/ani11071962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghiselli F., Rossi B., Felici M., Parigi M., Tosi G., Fiorentini L., Massi P., Piva A., Grilli E. Isolation, culture, and characterization of chicken intestinal epithelial cells. BMC Mol. Cell Biol. 2021;22:12. doi: 10.1186/s12860-021-00349-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giovagnoni G., Tugnoli B., Piva A., Grilli E. Organic acids and nature identical compounds can increase the activity of conventional antibiotics against Clostridium perfringens and enterococcus cecorum in vitro. J. Appl. Poult. Res. 2019;28:1398–1407. [Google Scholar]
- Guo S., Li C., Liu D., Guo Y. Inflammatory responses to a Clostridium perfringens type A strain and α-toxin in primary intestinal epithelial cells of chicken embryos. Avian Pathol. 2015;44:81–91. doi: 10.1080/03079457.2015.1005573. [DOI] [PubMed] [Google Scholar]
- Homer B.L., Butcher G.D., Miles R.D., Rossi A.F. Subclinical infectious bursal disease in an integrated broiler production operation. J. Vet. Diagn. Investig. 1992;4:406–411. doi: 10.1177/104063879200400406. [DOI] [PubMed] [Google Scholar]
- Huang G., Tang X., Bi F., Hao Z., Han Z., Suo J., Zhang S., Wang S., Duan C., Yu Z., Yu F., Yu Y., Lv Y., Suo X., Liu X. Eimeria tenella infection perturbs the chicken gut microbiota from the onset of oocyst shedding. Vet. Parasitol. 2018;258:30–37. doi: 10.1016/j.vetpar.2018.06.005. [DOI] [PubMed] [Google Scholar]
- Immerseel F.V., Buck J.D., Smet I.D., Pasmans F., Haesebrouck F., Ducatelle R. Interactions of butyric acid- and acetic acid-treated salmonella with chicken primary cecal epithelial cells in vitro. Avian Dis. 2004;48:384–391. doi: 10.1637/7094. [DOI] [PubMed] [Google Scholar]
- Jackman J.A., Boyd R.D., Elrod C.C. Medium-chain fatty acids and monoglycerides as feed additives for pig production: towards gut health improvement and feed pathogen mitigation. J. Anim. Sci. Biotechnol. 2020;11:44. doi: 10.1186/s40104-020-00446-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagadish B., Saxena M. Salmonella Typhimurium invasion induces apoptosis in chicken embryo fibroblast. Curr. Sci. 2008;95:512–514. [Google Scholar]
- Kim S.J., Chang J., Singh M. Peptidoglycan architecture of gram-positive bacteria by solid-state NMR. Biochim. Biophys. Acta. 2015;1848:350–362. doi: 10.1016/j.bbamem.2014.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang D., Li F., Fu Y., Cao Y., Song X., Wang T., Wang W., Guo M., Zhou E., Li D., Yang Z., Zhang N. Thymol inhibits LPS-stimulated inflammatory response via down-regulation of NF-κB and MAPK signaling pathways in mouse mammary epithelial cells. Inflammation. 2014;37:214–222. doi: 10.1007/s10753-013-9732-x. [DOI] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods San Diego Calif. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Monturiol-Gross L., Flores-Díaz M., Campos-Rodríguez D., Mora R., Rodríguez-Vega M., Marks D.L., Alape-Girón A. Internalization of Clostridium perfringens α-toxin leads to ERK activation and is involved on its cytotoxic effect. Cell. Microbiol. 2014;16:535–547. doi: 10.1111/cmi.12237. [DOI] [PubMed] [Google Scholar]
- Nyati K.K., Masuda K., Zaman M.M.-U., Dubey P.K., Millrine D., Chalise J.P., Higa M., Li S., Standley D.M., Saito K., Hanieh H., Kishimoto T. TLR4-induced NF-κB and MAPK signaling regulate the IL-6 mRNA stabilizing protein Arid5a. Nucleic Acids Res. 2017;45:2687–2703. doi: 10.1093/nar/gkx064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda M., Ikari S., Matsuno T., Morimune Y., Nagahama M., Sakurai J. Signal transduction mechanism Involved in Clostridium perfringens alpha-toxin-induced superoxide anion generation in rabbit neutrophils. Infect. Immun. 2006;74:2876–2886. doi: 10.1128/IAI.74.5.2876-2886.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oda M., Kabura M., Takagishi T., Suzue A., Tominaga K., Urano S., Nagahama M., Kobayashi K., Furukawa K., Furukawa K., Sakurai J. Clostridium perfringens alpha-toxin recognizes the GM1a-TrkA complex. J. Biol. Chem. 2012;287:33070–33079. doi: 10.1074/jbc.M112.393801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paiva D., McElroy A. Necrotic enteritis: applications for the poultry industry. J. Appl. Poult. Res. 2014;23:557–566. [Google Scholar]
- Porter A.G., Jänicke R.U. Emerging roles of caspase-3 in apoptosis. Cell Death Differ. 1999;6:99–104. doi: 10.1038/sj.cdd.4400476. [DOI] [PubMed] [Google Scholar]
- Rossi B., Toschi A., Piva A., Grilli E. Single components of botanicals and nature-identical compounds as a non-antibiotic strategy to ameliorate health status and improve performance in poultry and pigs. Nutr. Res. Rev. 2020;33:218–234. doi: 10.1017/S0954422420000013. [DOI] [PubMed] [Google Scholar]
- Saleh H.A., Yousef M.H., Abdelnaser A. The anti-inflammatory properties of phytochemicals and their effects on epigenetic mechanisms involved in TLR4/NF-κB-mediated inflammation. Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.606069. https://www.frontiersin.org/article/10.3389/fimmu.2021.606069 (Verified April 5, 2022) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takehara M., Kobayashi K., Nagahama M. Toll-like receptor 4 protects against Clostridium perfringens infection in mice. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.633440. https://www.frontiersin.org/articles/10.3389/fcimb.2021.633440/full (Verified June 14, 2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Timbermont L., Haesebrouck F., Ducatelle R., Immerseel F.V. Necrotic enteritis in broilers: an updated review on the pathogenesis. Avian Pathol. 2011;40:341–347. doi: 10.1080/03079457.2011.590967. [DOI] [PubMed] [Google Scholar]
- Toschi A., Rossi B., Tugnoli B., Piva A., Grilli E. Nature-identical compounds and organic acids ameliorate and prevent the damages induced by an inflammatory challenge in Caco-2 cell culture. Molecules. 2020;25:4296. doi: 10.3390/molecules25184296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandeplas S., Dauphin R.D., Beckers Y., Thonart P., Théwis A. Salmonella in chicken: current and developing strategies to reduce contamination at farm level. J. Food Prot. 2010;73:774–785. doi: 10.4315/0362-028x-73.4.774. [DOI] [PubMed] [Google Scholar]
- Wade B., Keyburn A. The true cost of necrotic enteritis. World Poult. 2015;31:16–17. [Google Scholar]
- Wang L., Luo J., Fu Y., He S. Induction of interleukin-8 secretion and activation of ERK1/2, p38 MAPK signaling pathways by thrombin in dermal fibroblasts. Int. J. Biochem. Cell Biol. 2006;38:1571–1583. doi: 10.1016/j.biocel.2006.03.016. [DOI] [PubMed] [Google Scholar]
- Wu L., Feng B.-S., He S.-H., Zheng P.-Y., Croitoru K., Yang P.-C. Bacterial peptidoglycan breaks down intestinal tolerance via mast cell activation: the role of TLR2 and NOD2. Immunol. Cell Biol. 2007;85:538–545. doi: 10.1038/sj.icb.7100079. [DOI] [PubMed] [Google Scholar]
- Yamamura K., Ashida H., Okano T., Kinoshita-Daitoku R., Suzuki S., Ohtani K., Hamagaki M., Ikeda T., Suzuki T. Inflammasome activation induced by perfringolysin O of Clostridium perfringens and its involvement in the progression of gas gangrene. Front. Microbiol. 2019;10 doi: 10.3389/fmicb.2019.02406. https://www.ncbi.nlm.nih.gov/pmc/articles/PMC6823607/ (Verified June 14, 2021) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang W.-Y., Lee Y.-J., Lu H.-Y., Branton S.L., Chou C.-H., Wang C. The netB-positive Clostridium perfringens in the experimental induction of necrotic enteritis with or without predisposing factors. Poult. Sci. 2019;98:5297–5306. doi: 10.3382/ps/pez311. [DOI] [PubMed] [Google Scholar]
- Zhang G., Darius S., Smith S.R., Ritchie S.J. In vitro inhibitory effect of hen egg white lysozyme on Clostridium perfringens type A associated with broiler necrotic enteritis and its alpha-toxin production. Lett. Appl. Microbiol. 2006;42:138–143. doi: 10.1111/j.1472-765X.2005.01812.x. [DOI] [PubMed] [Google Scholar]
- Zwietering M.H., Jongenburger I., Rombouts F.M., van ’t Riet K. Modeling of the bacterial growth curve. Appl. Environ. Microbiol. 1990;56:1875–1881. doi: 10.1128/aem.56.6.1875-1881.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]