Abstract
Background
The normal range of fractional exhaled nitric oxide (FENO) is influenced by demographic factors. However, single, fixed cut-off values are used for clinical interpretation in children despite rapid growth. We aimed to define the normal range of FENO during childhood and evaluate its utility in a diagnostic setting.
Method
FENO percentile charts were developed using data from nonasthmatic children in a population-based birth cohort (Manchester Asthma and Allergy Study). Children were skin prick tested, FENO measured at the ages of 8, 11, 13–16 and 18 years and clinical information collected. This chart was externally validated in the Study of Eczema and Asthma to Observe the Influence of Nutrition (SEATON) cohort before being prospectively tested in symptomatic, treatment-naïve patients with suspected asthma in a diagnostic setting (Rapid Access Diagnostics for Asthma study).
Results
Height, weight, body mass index and age were predictive of FENO in univariate analysis using 1220 FENO measurements. Only height remained significant after adjustment in the overall, nonatopic and atopic populations, and was included in the predictive equations for 50th, 75th 90th and 98th percentiles. The proposed percentile lines corresponded to the 57th (95% CI 53rd–61st), 80th (76th–83rd), 90th (87th–92nd) and 98th (96th–99th) percentiles in the SEATON cohort (660 measurements). When tested in 73 symptomatic treatment-naïve children and young adults (median (interquartile range) age: 11 (8–14) years), an FENO >90th percentile gave a 96% specificity and positive predictive value of 97%, identifying 59% of children who were subsequently diagnosed with asthma after extensive testing.
Conclusion
We developed a height-based FENO percentile chart which quantifies the probability of asthma in symptomatic children and merits further validation towards clinical implementation.
Short abstract
As height increases rapidly and substantially during childhood, a single FENO cut-off for asthma diagnosis may not be appropriate in children. A validated height-adjusted FENO centile chart can be easily and effectively used in the asthma diagnostic process. https://bit.ly/3nQs2PS
Introduction
The rate of asthma misdiagnosis is substantial, with up to one third of patients being over-treated, whilst others go undiagnosed [1, 2]. The use of fractional exhaled nitric oxide (FENO) is recommended to facilitate the diagnosis of asthma in clinical practice guidelines, where it is used as a dichotomous outcome (“positive” or “negative”). However, the cut-off values are inconsistent between guidelines; in the National Institute for Health and Care Excellence (NICE) guidance [3] the paediatric threshold is 35 ppb whereas in the European Respiratory Society (ERS) guidance it is 25 ppb [4]. There is a significant overlap in FENO levels between healthy and asthmatic populations, and the range is wide [5]. Whilst a single diagnostic cut-off value may be easy for clinicians to implement in practice, it is clear that the clinical probability of asthma increases with increasing FENO levels above the recommended cut-off [4, 5], and collapsing this continuous variable into a dichotomous outcome loses a large amount of information [6]. The normal ranges of FENO are influenced by factors such as sex, age, height, ethnicity and allergic sensitisation [3, 7–12]. Moreover, pubertal growth spurts influence FENO trajectory, potentially further affecting the diagnostic accuracy within this age group when a single fixed cut-off value is used [13]. Currently, none of these factors are considered when FENO levels are interpreted in practice [3, 4, 14]. Other tests used in asthma diagnosis, such as spirometry, are interpreted in relation to predicted values calculated based on individual patient characteristics (age, height, sex and ethnicity) and automatically calculated by the measuring device.
The aim of the current study was to develop individualised normal ranges of FENO in growing children and to evaluate its utility in symptomatic, treatment-naïve children who have suspected asthma.
Methods
To define what constitutes the upper limit of normal for FENO, we developed an FENO percentile chart within nonasthmatic children using data from the Manchester Asthma and Allergy Study (MAAS) [15]. We externally validated the percentile chart in the Study of Eczema and Asthma to Observe the Influence of Nutrition (SEATON) cohort [16], before testing in a diagnostic setting (Rapid Access Diagnostics for Asthma (RADicA) study) [17].
Population 1: chart development cohort (MAAS)
MAAS is a population-based birth cohort and described in detail elsewhere [15]. In brief, children were recruited before birth and followed prospectively. The predominant ethnic group was white British. We analysed data from validated, interviewer-administered questionnaires on parentally reported (age: 8, 11, 13–16 years) or self-reported (age: 18 years) symptoms, physician-diagnosed diseases and asthma medication. Pubertal status was measured at the ages of 11 and 13–16 years (Tanner scale [18, 19]). Allergic sensitisation to common aeroallergens was ascertained using skin prick tests (SPTs).
FENO was measured at each follow-up clinic using either a chemiluminescence analyser (NIOX, Aerocrine, Sweden) or an electrochemical analyser (NIOX Mino, Aerocrine, Sweden), changed on 4 May 2012 (devices gave comparable results in previous studies [20, 21]). We calculated the predicted 50th, 75th, 90th and 98th percentiles in nonasthmatic children (defined as no self-reported wheeze over the past 12 months, no history of physician diagnosed asthma and no asthma medication use) (Table E1).
Population 2: chart validation cohort (SEATON)
To externally validate the percentile chart, we analysed data collected from children who attended follow-up clinics at the ages of 10 and 15 years within the SEATON birth cohort [16] (Table E1). Validated questionnaires were parentally administered at the age of 10 years and self-administered at 15 years. Children were skin prick tested for common inhaled allergens and FENO (NIOX chemiluminescence analyser at 10 years old and NIOX MINO (both Aerocrine, Sweden) at 15 years old) were measured. The percentages of children above and below the corresponding proposed percentile lines were calculated.
Population 3: symptomatic and treatment-naïve patients (RADicA)
We prospectively tested our newly proposed percentiles of FENO for the probability of asthma in symptomatic children and young adults in the RADicA study (ISRCTN 11676160; www.radica.org.uk) described in detail elsewhere [17]. Briefly, symptomatic (wheeze, shortness of breath, chest tightness or cough), treatment-naïve patients with a clinician suspicion of asthma were recruited from primary care. Clinical history, examination and asthma diagnostic tests were performed before a trial of inhaled corticosteroids (ICS) was given. Symptom control was measured using an asthma control questionnaire (ACQ) [22]. The diagnostic tests and ACQ were repeated after 8 weeks of treatment before a diagnosis was established following an expert panel objective evidence review (EPOER) comprising a minimum of two senior asthma physicians (Table E2).
RADicA study procedures
Diagnostic procedures are described in detail elsewhere [17]. Briefly, FENO was measured using NIOX VERO (Circassia, UK) in accordance with international guidelines [23]. Spirometry was measured (JAEGER™ Vyntus™ PNEUMO, Vyaire medical, USA) before and after bronchodilator use; reversibility was calculated as percentage change of forced expiratory volume in 1 s 15 min after administration of 400 µg of salbutamol via a large volume spacer. SPTs for common inhalant allergens were performed and blood eosinophil levels measured. Peak expiratory flow variability was measured using an eMini Wright digital flow meter (Clement Clarke Ltd. Harlow, UK). Methacholine (Vyaire medical, USA) and mannitol (Osmohale, Pharmaxis Pharmaceuticals Limited, Ireland) bronchial challenges were performed (Table E2).
All study protocols were approved by the local research ethics committee, all parents gave written informed consent and children gave assent.
Statistical analysis
Data were analysed using paired or unpaired Student t-tests for parametric data and the Mann–Whitney U test for nonparametric data, as appropriate. As FENO is log-normally distributed, the geometric mean was presented. For cross-sectional data, correlations between the absolute FENO levels and other associated variables were calculated using Spearman's rank test; for longitudinal data, the correlation with the repeated measurement function was used. For model development, longitudinal data were analysed using mixed-effect quantile regressions for the predictions of percentile equations using the absolute FENO levels (without log transformation) for ease of clinical interpretation. Quantile regression models do not assume normal data distributions and have been previously used to model FENO percentiles [7, 24]. Bootstrapping with 1000 iterations was used to calculate the 95% confidence interval. Within the RADicA dataset, the diagnostic probability of FENO above each percentile line was evaluated using sensitivity, specificity, positive and negative predictive values and positive (+LR, sensitivity/(1-specificity)) and negative likelihood ratios (−LR, (1-sensitivity)/specificity) [6]. All analyses were performed using RStudio (version 1.4.1106) and R (version 4.1.1).
Results
Chart development (MAAS)
Baseline characteristics
From the MAAS, 840 children had one or more FENO reading during follow-up, totalling 1954 measurements (Tables E3–4, Figures E1–2). After exclusions (Figure E2) 1474 FENO measurements were available for further analysis, of which 254 were from children with asthma.
FENO levels were significantly higher in children with current wheeze, physician-diagnosed asthma, current asthma medication use, SPT sensitisation and current hay fever (Table E5). Whilst the FENO levels of atopic nonasthmatic and asthmatic children were substantially higher than those in healthy nonatopic children, there was significant overlap between the groups (Figures E3 and E4).
FENO measurements from children with current wheeze, history of doctor-diagnosed asthma or current asthma medication use were excluded from the model development. Asymptomatic nonasthmatic children who had complete datasets were included in the development of the FENO percentile chart (table 1).
TABLE 1.
Demographic data for included nonasthmatic children in the Manchester Asthma and Allergy Study cohort.
| Age 8 years (n=275) | Age 11 years (n=387) | Age 13–16 years (n=309) | Age 18 years (n=249) | |
| Sex, male, n (%) | 135 (49.1) | 175 (45.2) | 147 (47.6) | 109 (43.8) |
| Age (years), mean (sd), (range) | 8.0 (±0.1) (7.1–8.6) | 11.5 (±0.5) (10.0–12.8) | 16.1 (±0.5) (14.2–17) | 19.4 (±0.7) (18.0–21.8) |
| Ethnicity, white, n (%) | 266 (97.8) | 370 (97.4) | 292 (96.7) | 233 (97.1) |
| Height (cm), mean (sd), (range) | 128 (±5.0) (116–145) | 149 (±7.2) (130–170) | 170.1 (±8.6) (150–196) | 171.7 (±9.5) (151–198) |
| Weight (kg), mean (sd), (range) | 28 (±5.1) (20–51) | 42.7 (±9.9) (23.6–91.4) | 63.3 (±11.6) (42.3–116) | 69.6 (±13.5) (46.3–138) |
| Body mass index (kg·m−2), mean (sd), (range) | 16.9 (±2.2) (13.1–26.3) | 19.1 (±3.3) (12.9–40.8) | 21.9 (±3.4) (16.2–37.4) | 23.5 (±4.0) (16.6–41.3) |
| SPT sensitisation, n (%) | 64 (23.3) | 93 (24) | 141 (45.6) | 114 (45.8) |
| Current eczema (self-reported), n (%) | 36 (13.2) | 59 (15.6) | 28 (9.1) | 28 (11.3) |
| Current hay fever (self-reported) n(%) | 30 (11.4%) | 71 (18.8%) | 97 (32.4%) | 90 (36.1%) |
SPT: skin prick test.
Among children without asthma, FENO increased with age in nonatopic and atopic children (table 2). Age, height, weight and body mass index were correlated with FENO in children with and without atopy (Table E6). Boys had higher FENO than girls beyond the age of 13–16 years, coinciding with the age when boys outgrow girls in height (Tables E7–8, Figures E5). Tanner scales at the ages of 11 and 13–16 years were associated with increased FENO in longitudinal univariate analysis, but this was no longer significant after adjustment for height (Tables E9–10).
TABLE 2.
The distribution of fractional exhaled nitric oxide (FENO) in nonatopic and atopic children without asthma across age groups.
| Follow-up clinics | FENO geometric mean (geometric sd) (ppb) | 5th and 95th percentile (ppb) |
| Healthy nonatopic children | ||
| 8 years (n=211) | 8.7 (1.6) | 4.6–16.4 |
| 11 years (n=294) | 8.9 (1.6) | 4.7–20 |
| 13–16 years (n=168) | 13.7 (1.6) | 7–32 |
| 18 years (n=135) | 12.1 (1.6) | 6–27 |
| Atopic children without asthma | ||
| 8 years (n=64) | 15.3 (2.3) | 5.3–57.9 |
| 11 years (n=93) | 17.3 (2.4) | 4.3–70.0 |
| 13–16 years (n=141) | 23.3 (2.2) | 7–90 |
| 18 years (n=114) | 19.7 (2.1) | 7–77 |
As FENO is not normally distributed, the geometric mean and the 5th and 95th percentiles are presented in the table. sd: standard deviation
Model development for FENO percentiles
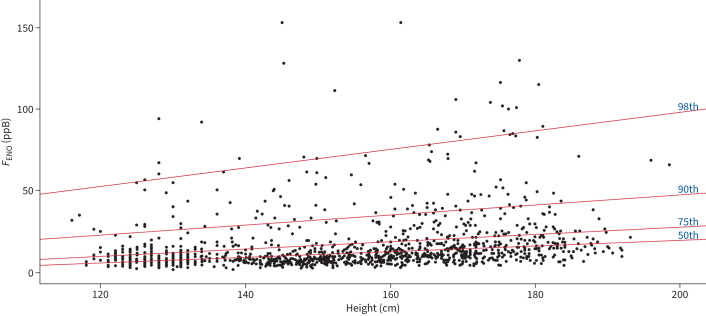
To establish the percentile lines of FENO in children without asthma (regardless of atopic status), univariate analysis (including age, height, weight and sex as predictors) for the 50th, 75th, 90th and 98th percentiles were performed. In the quantile multivariate backwards regression model, predictors with p<0.05 in the univariate analysis (sex for the 50th percentile, height, weight and age) were included, but only height remained significant and was included in the final model (Table E11, table 3, figure 1). Log transformation of FENO did not affect the results. The ERS (25 ppb) and NICE (35 ppb) cut-off values intercept the 90th percentile line at the heights of 127 cm and 158 cm, respectively.
TABLE 3.
Intercepts and regression coefficient for children without current asthma (regardless of atopic status) for 50th, 75th, 90th and 98th percentiles.
| Percentile | RC | RC bootstrap (95% CI) | Intercept | Intercept bootstrap (95% CI) | Equations |
| 50th | 0.18 | (0.12–0.23) | −15.56 | (−23.21–−7.45) | 0.18×height (cm)−15.56 |
| 75th | 0.22 | (0.16–0.27) | −15.56 | (−23.21–−7.45) | 0.22×height (cm)−15.56 |
| 90th | 0.32 | (0.25–0.38) | −15.56 | (−23.21–−7.45) | 0.32×height (cm)−15.56 |
| 98th | 0.57 | (0.49–0.66) | −15.56 | (−23.21–−7.45) | 0.57×height (cm)−15.56 |
CI: confidence interval; RC: regression coefficient.
FIGURE 1.
Percentile chart for nonasthmatic children and adolescents.
As FENO is also associated with atopic status, we used the same model to develop the percentile equations for nonatopic and atopic populations separately (Tables E12–E16, Figure E6).
External validation (SEATON cohort)
External validation of the percentiles developed from the MAAS was performed within the SEATON cohort where FENO measurements were available in 419 children at the age of 10 years and 462 children at 15 years, of which 372 (88.8%) and 379 (82.0%), respectively, had information to determine if they had current asthma or not. Of these, 332 (89.2%) at 10 years and 328 (86.5%) at 15 years were asymptomatic nonasthmatics (regardless of atopy) and were included in the analysis (Table E16). The MAAS percentile lines correlated well with the SEATON percentile lines (table 4, Table E17).
TABLE 4.
Proposed fractional exhaled nitric oxide (FENO) percentile developed from the Manchester Asthma and Allergy Study (MAAS) cohort correlated well with percentile within nonasthmatic children within the Study of Eczema and Asthma To Observe the influence of Nutrition (SEATON) cohort.
| MAAS FENO percentile in nonasthmatic children | Number of SEATON participants with FENO above MAAS-defined percentile, n (% (95%CI)) | Equivalent percentile in nonasthmatic children from the SEATON cohort (95% CI) |
| 50th | 282/660 (43 (39–47) | 57th (53rd–61st) |
| 75th | 134/660 (20 17–24) | 80th (76th–83rd) |
| 90th | 67/660 (10 8–13) | 90th (87th–92nd) |
| 98th | 15/660 (2 1–4) | 98th (96th–99th) |
Using the FENO percentile chart for asthma diagnosis in symptomatic children (RADicA study)
Of 214 symptomatic and treatment-naïve patients referred to the RADicA study from primary care for possible asthma, 73 (median (interquartile range) age: 11 (8–14) years, 47.9% male, 54.8% white) were aged 22 years or younger (age-matching for chart development cohort), had a definitive diagnostic outcome (51 had asthma, 22 did not have asthma) and a measurement of FENO before treatment was initiated (Table E18). Data from these participants were used to assess sensitivity and specificity of FENO percentile charts for asthma diagnosis. Children with an unclassified diagnostic outcome were excluded from the analysis. The distribution of FENO in relation to height, atopic and asthmatic status is shown in Figure E7.
As expected, sensitivity decreased with increasing FENO percentiles whilst the specificity improved (table 5). Where FENO levels fell above the 90th percentile, 97% were diagnosed with asthma, with a +LR of 13.1, corresponding to a large increase in post-test probability after taking into account asthma prevalence [6]. For individuals with FENO above the 98th percentile (accounting for over a third of patients who were subsequently diagnosed with asthma), asthma diagnosis can be confidently made (100% specificity). The negative likelihood ratios for all percentiles were poor, indicating insufficient power to exclude asthma.
TABLE 5.
Asthma risk stratification based on percentile cut-off.
| Percentile | Sensitivity, % (n) | Specificity, % (n) | PPV, % (n) | NPV, % (n) | +LR # | −LR ¶ |
| >50th | 78.4 (40/51) | 40.9 (9/22) | 75.5 (40/53) | 45.0 (9/20) | 1.3 | 0.5 |
| >75th | 72.5 (37/51) | 77.3 (17/22) | 88.1 (37/42) | 54.8 (17/31) | 3.2 | 0.4 |
| >90th | 58.8 (30/51) | 95.5 (21/22) | 96.8 (30/31) | 50.0 (21/42) | 13.1 | 0.4 |
| >98th | 33.3 (17/51) | 100 (22/22) | 100 (17/17) | 39.3 (22/56) | ∞ | 0.7 |
#: +LR: sensitivity/(1-specificity). ¶: −LR: (1-sensitivity)/specificity. LR: likelihood ratio; NPV: negative predictive value; PPV: positive predictive value.
Using charts stratified by atopic status, for symptomatic and nonatopic children, an FENO >98th percentile gave a 100% specificity and 21% sensitivity; in symptomatic and atopic children, an FENO level of more than the 90th percentile on the atopic percentile chart gave a sensitivity of 46% with 100% specificity (Table E19).
Discussion
Using two population-based birth cohorts, we have confirmed that height is the key independent predictor of FENO in childhood. As height increases rapidly and substantially during childhood, a single cut-off for asthma diagnosis may not be appropriate in this age group. To define the normal ranges, we developed and validated a height-adjusted percentile chart for FENO, covering childhood and adolescence. Within a prospective, symptomatic and untreated cohort of children and young adults who had undergone a detailed diagnostic work up to confirm or refute an asthma diagnosis, we demonstrated that the use of height-adjusted FENO percentile charts can be effectively used in the asthma diagnostic process.
The percentile chart may provide more information than the current dichotomous approach and may be more clinically useful, allowing the identification of individuals with FENO measurements that are well above the norm for their height. For example, at a height of 125 cm, an FENO value of 25 ppb would be at the 90th percentile, giving a high probability of asthma (+LR of 13), whereas at a height of 185 cm the same value would fall below the 75th percentile with a poor diagnostic value (+LR of 3). We postulate that those with asthma-like symptoms and a very high FENO for height (i.e. >98th percentile) could be confidently diagnosed with asthma. In contrast, for individuals who have only a moderately elevated FENO for their height, the probability of an asthma diagnosis is lower and appropriate clinical decisions regarding further diagnostic tests should be made. Whilst the interpretation of spirometry is moving away from a single standard for all [25], the height-adjusted centile charts also provide individualised ways to define what is a “normal” FENO, especially during adolescence. Notably, it will particularly benefit children with heights towards the lower and upper extremes, ensuring that the diagnostic process is inclusive for those who are much shorter or taller than average.
As the atopic status of most children is not known at the time of measuring the FENO, we have developed a percentile chart for all children regardless of atopic status. We also developed separate charts adjusted for atopic status and note that they are quite different, highlighting the importance of assessing atopic status in FENO interpretation. Atopic and symptomatic children have increased FENO (94% of those with FENO above 98th percentile line on the atopic/nonatopic combined chart were atopic in the RADicA cohort), consistent with previous reports [26, 27]. The nonatopic asthma phenotype is uncommon in children, accounting for 27% in our symptomatic cohort. Whilst the role of FENO in nonatopic children is less clear [28], we have found that FENO may still be useful for risk stratification in this group in diagnostic settings. However, it is important to note that the sample size was small (n=14) and future research is needed to further elucidate the role of FENO in such groups of children.
Although we acknowledge not all children can master FENO measurements, spirometry tests can also be challenging, and many struggle to perform forced expiratory manoeuvres to residual volume, resulting in variable quality and difficult interpretation. Using nonaerosol-generating procedures (such as FENO, SPT or immunoglobulin E) to facilitate asthma diagnosis may be beneficial during a pandemic (such as coronavirus disease 2019) when access to procedures with aerosol-generating potential is limited and thus may avoid delays in commencing treatment in some children [17]. However, we acknowledge the role of spirometry-based tests in demonstrating airflow obstruction and variability over time and following bronchodilator therapy, as well as during bronchial challenge testing, and spirometry remains an essential part of the asthma diagnostic armamentarium. Nevertheless, the current study provides useful information to facilitate future work in the determination of the diagnostic algorithm, taking into account different clinical probabilities and health-economic scenarios.
Many studies evaluating the diagnostic efficiency of FENO were limited by heterogeneity of the children and adult participants, use of ICS and potential selection bias of the study populations [27–31]. We note that no studies to date have assessed the diagnostic efficiency of using height-adjusted values.
Like other measures of pulmonary function, FENO is influenced by demographic factors in both healthy and asthmatic children (particularly height [8, 11, 32]), and it seems something of an anomaly that clinicians use height- (as well as age- and sex-) adjusted values for spirometry, with results usually presented as % predicted (as well as the absolute value), but this has not been the case for FENO. The association between height and FENO may be particularly important in childhood, with a steep growth trajectory occurring between 5 and 16 years old [33]. Indeed, our data suggest that differences in height (>80 cm range in both the MAAS and the SEATON cohorts) markedly influence FENO, particularly in relation to the higher percentile lines. Consistent with this, Garcia et al. [13] used repeated measures within children going through puberty and reported a substantial increase in FENO between 8 and 16 years old, with tracking of personalised FENO measurements, and highlighted the limitations of fixed FENO reference values. Whilst using a fixed single FENO cut-off may seem easier for clinicians to implement, technology allowing input of demographic data and automatic calculation of % predicted values (as for spirometry) would streamline the utility of a height-adjusted approach in practice. Furthermore, to meaningfully reduce the misdiagnosis rate in asthma at a population level, it may be that the diagnostic process should occur in “diagnostic hubs” where key tests (including FENO and assessment for atopy) can be performed before a diagnosis is confirmed or refuted by asthma specialists. Such a healthcare infrastructure should be subjected to future research and health-economics evaluation.
Strength and limitations
To our knowledge, our study is the first to develop and externally validate a height-adjusted percentile chart for FENO in children. Both MAAS and SEATON are unselected population-based birth cohorts; however, they are predominantly (>95%) of white ethnicity, which although reflecting the local population at the time of recruitment, is not representative of current UK residents. Further external validation in ethnically diverse populations may be necessary.
It is also important to highlight that despite there being a good correlation between the chemiluminescent and electrochemical analysers, the agreement and technical reproducibility vary between the two [34]. As the MAAS and SEATON birth cohorts span >20 years, chemiluminescent analysers were used before electrochemical analysers became available. Furthermore, even among electrochemical analysers, devices produced by different manufacturers demonstrate differences in FENO levels [35]. Therefore, the types and manufacturers of the analysers used should be taken into consideration in future work.
Moreover, it is well-established that FENO demonstrates diurnal and day-to-day variabilities in patients with asthma [36]. Whilst diurnal variation may be predictable in some patients, the day-to-day and longer-term variations may be less so. In the current study, we focused on factors which contribute to the predictable longer-term variations of FENO (e.g. height, weight, age) in growing children, but did not adjust for time-of-the-day factors for diurnal variability and other factors such as allergen exposure or disease severity around the time of testing. We tested the percentile chart in a diagnostic setting using FENO measurements taken in symptomatic children and young adults before they commenced ICS treatment (RADicA study). The RADicA study participants all underwent extensive assessment and all information was assessed by an expert panel comprising a minimum of two senior asthma physicians (EPOER). An EPOER designation of “asthma” or “not asthma” was used as the gold standard against which the performance of the FENO percentile chart was assessed. Like many studies evaluating the diagnostic role of FENO in asthma [28, 30, 37, 38], our symptomatic and untreated patient cohort had high pre-test probability (70%). The performance in probability stratification remains uncertain in children with low pre-test probably where an alternative diagnosis is more likely and therefore should not be extrapolated to this population. The sample size for the symptomatic, untreated patient cohort was limited, particularly in children with extreme heights (in whom the height-adjusted equation is likely to be more advantageous than any unified cut-off values) and therefore it was not possible to compare the diagnostic efficiency of the current approach with established guidelines. It is imperative that our study findings are externally validated in larger cohorts of patients in a diagnostic setting.
Conclusion
We have defined the normal range of FENO using height-adjusted percentile charts in nonasthmatic children from two UK birth cohorts. By applying the FENO percentile charts in a diagnostic setting, in symptomatic, treatment-naïve young people with clinician-suspected asthma, we identified one third of those who were subsequently diagnosed with asthma without the need for further tests. Our height-adjusted percentile chart may facilitate the development of a more personalised asthma diagnostic algorithm. Further external validation in a larger cohort of patients in a diagnostic setting is warranted.
Supplementary material
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00319-2022.SUPPLEMENT (564KB, pdf)
Figure E1 00319-2022.FIGUREE1 (52.3KB, tif)
Figure E2 00319-2022.FIGUREE2 (188.8KB, tif)
Figure E3A 00319-2022.FIGUREE3A (77.6KB, jpg)
Figure E3B 00319-2022.FIGUREE3B (240KB, tif)
Figure E4 00319-2022.FIGUREE4 (82.3KB, jpg)
Figure E5 00319-2022.FIGUREE5 (346KB, tif)
Figure E6A 00319-2022.FIGUREE6A (605KB, tif)
Figure E6B 00319-2022.FIGUREE6B (674.3KB, tif)
Figure E7 00319-2022.FIGUREE7 (255KB, tif)
Acknowledgement
We would like to thank Emma Barrett in providing statistical advice and support. We would like to thank all staff of the MAAS, SEATON and RADicA study teams for assistance with participant recruitment and data collection. The authors would like to thank the MAAS study participants and their parents for their continued support and enthusiasm. The SEATON research team express their thanks to the participants and parents for their ongoing enthusiasm for the study. We would like to thank the RADicA study participants for their time and commitment to the study and the RADicA study team for help with data collection.
Footnotes
Provenance: Submitted article, peer reviewed.
Author contributions: R. Wang, S.J. Fowler, C.S. Murray and A. Simpson conceived and designed the study, collated the data and wrote the manuscript. R. Wang, S. Drake, L. Healy, L. Lowe, H. Wardman and M. Bennett recruited participants and collected data from the RADicA study. S.W. Turner and C.S. Murray are principal investigators for the SEATON and MAAS birth cohorts respectively. R. Wang performed the statistical analysis with advice and support from E. Barrett (acknowledged). All authors contributed to the revising the manuscript.
Conflict of interest: R. Wang has nothing to disclose.
Conflict of interest: S.J. Fowler has nothing to disclose.
Conflict of interest: S.W. Turner has nothing to disclose.
Conflict of interest: S. Drake has nothing to disclose.
Conflict of interest: L. Healy has nothing to disclose.
Conflict of interest: L. Lowe has nothing to disclose.
Conflict of interest: H. Wardman has nothing to disclose.
Conflict of interest: M. Bennett has nothing to disclose.
Conflict of interest: A. Custovic has nothing to disclose.
Conflict of interest: A. Simpson has nothing to disclose.
Conflict of interest: C.S. Murray has nothing to disclose.
Support statement: MAAS was supported by Asthma UK grants 301 (1995–1998), 362 (1998–2001), 01/012 (2001–2004) and 04/014 (2004–2007), BMA James Trust (2005) and The J.P. Moulton Charitable Foundation (2004 to current), The North West Lung Centre Charity (1997 to current), and Medical Research Council (MRC) grants G0601361 (2007–2012), MR/K002449/1 (2013–2014) and MR/L012693/1 (2014–2018), and MR/S025340/1 UNICORN (Unified Cohorts Research Network: Disaggregating asthma; 2020–2024). The SEATON study was made possible by funding from Asthma UK and MRC. The RADicA study was supported by the National Institute for Health Research (NIHR) Manchester Biomedical Research Centre (BRC) (grant number BRC-1215–20007) and Asthma UK/Innovate (grant number AUK-PG-2018–406). The funders have no role in the study design, data collection, analysis or design of the manuscript. This manuscript reports independent research supported by NIHR Manchester BRC. The views expressed in this publication are those of the authors and not necessarily those of the NHS, the NIHR or the Department of Health. Angela Simpson, Stephen Fowler and Clare Murray are supported by the NIHR Manchester BRC. Funding information for this article has been deposited with the Crossref Funder Registry.
References
- 1.Aaron SD, Boulet LP, Reddel HK,et al. Underdiagnosis and overdiagnosis of asthma. Am J Respir Crit Care Med 2018; 198: 1012–1020. doi: 10.1164/rccm.201804-0682CI [DOI] [PubMed] [Google Scholar]
- 2.Aaron SD, Vandemheen KL, FizGerald JM,et al. Reevaluation of diagnosis in adults with physician-diagnosed asthma. JAMA 2017; 317: 269–279. doi: 10.1001/jama.2016.19627 [DOI] [PubMed] [Google Scholar]
- 3.National Institute for Health and Care Excellence . Asthma: diagnosis, monitoring and chronic asthma management. www.nice.org.uk/guidance/ng80 Date last accessed: 11 May 2022. Date last updated: 22 March 2021. [PubMed]
- 4.Gaillard EA, Kuehni CE, Turner S,et al. European Respiratory Society clinical practice guidelines for the diagnosis of asthma in children aged 5–16 years. Eur Respir J 2021; 58: 2004173. doi: 10.1183/13993003.04173-2020 [DOI] [PubMed] [Google Scholar]
- 5.Dweik RA, Boggs PB, Erzurum SC,et al. An official ATS clinical practice guideline: interpretation of exhaled nitric oxide levels (FENO) for clinical applications. Am J Respir Crit Care Med 2011; 184: 602–615. doi: 10.1164/rccm.9120-11ST [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Grimes DA, Schulz KF. Refining clinical diagnosis with likelihood ratios. Lancet 2005; 365: 1500–1505. doi: 10.1016/S0140-6736(05)66422-7 [DOI] [PubMed] [Google Scholar]
- 7.Torén K, Murgia N, Schioler L,et al. Reference values of fractional excretion of exhaled nitric oxide among non-smokers and current smokers. BMC Pulm Med 2017; 17: 118. doi: 10.1186/s12890-017-0456-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cho HJ, Jung YH, Yang SI,et al. Reference values and determinants of fractional concentration of exhaled nitric oxide in healthy children . Allergy Asthma Immunol Res 2014; 6: 169–174. doi: 10.4168/aair.2014.6.2.169 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Suksawat Y, Pacharn P, Jirapongsananuruk O,et al. Determination of fractional exhaled nitric oxide (FENO) reference values in healthy Thai population. Asian Pac J Allergy Immunol 2017; 35: 127–131. doi: 10.12932/AP0840 [DOI] [PubMed] [Google Scholar]
- 10.Rouatbi S, Alqodwa A, Mdella SB,et al. Fraction of exhaled nitric oxide (FeNO) norms in healthy North African children 5–16 years old. Pediatr Pulmonol 2013; 48: 981–995. doi: 10.1002/ppul.22721 [DOI] [PubMed] [Google Scholar]
- 11.Malmberg LP, Petays T, Haahtela T,et al. Exhaled nitric oxide in healthy nonatopic school-age children: determinants and height-adjusted reference values. Pediatr Pulmonol 2006; 41:635–642. doi: 10.1002/ppul.20417 [DOI] [PubMed] [Google Scholar]
- 12.Ma'pol A, Hashim JH, Norback D,et al. FeNO level and allergy status among school children in Terengganu, Malaysia. J Asthma 2020; 57: 842–849. doi: 10.1080/02770903.2019.1614614 [DOI] [PubMed] [Google Scholar]
- 13.Garcia E, Zhang Y, Rappaport EB,et al. Patterns and determinants of exhaled nitric oxide trajectories in schoolchildren over a 7-year period. Eur Respir J 2020; 56: 2000011. doi: 10.1183/13993003.00011-2020 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.National Institute for Health and Care Excellence . Asthma: diagnosis and monitoring of asthma in adults, children and young people. www.nice.org.uk/guidance/ng80/documents/draft-guideline Date last accessed: 11 May 2022. Date last updated: July 2017. [PubMed]
- 15.Custovic A, Simpson BM, Murray C,et al. The National Asthma Campaign Manchester Asthma and Allergy Study. Pediatr Allergy Immunol 2002; 13: 32–37. doi: 10.1034/j.1399-3038.13.s.15.3.x [DOI] [PubMed] [Google Scholar]
- 16.Martindale S, McNeill G, Devereux G,et al. Antioxidant intake in pregnancy in relation to wheeze and eczema in the first two years of life. Am J Respir Crit Care Med 2005; 171:121–128. doi: 10.1164/rccm.200402-220OC [DOI] [PubMed] [Google Scholar]
- 17.Drake S, Wang R, Healy L,et al. Diagnosing asthma with and without aerosol generating procedures. J Allergy Clin Immunol Pract 2021: 9:4243–4251. doi: 10.1016/j.jaip.2021.07.006 [DOI] [PubMed] [Google Scholar]
- 18.Marshall WA, Tanner JM. Variations in the pattern of pubertal changes in boys. Arch Dis Child 1970; 45: 13–23. doi: 10.1136/adc.45.239.13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Marshall WA, Tanner JM. Variations in pattern of pubertal changes in girls. Arch Dis Child 1969; 44: 291–303. doi: 10.1136/adc.44.235.291 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.McGill C, Malik G,, Turner SW, Validation of a hand-held exhaled nitric oxide analyzer for use in children. Pediatr Pulmonol 2006; 41: 1053–1057. doi: 10.1002/ppul.20491 [DOI] [PubMed] [Google Scholar]
- 21.Murray C, Foden P, Lowe L,et al. Diagnosis of asthma in symptomatic children based on measures of lung function: an analysis of data from a population-based birth cohort study. Lancet Child Adolesc Health 2017; 1: 114–123. doi: 10.1016/S2352-4642(17)30008-1 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Juniper EF, O'Byrne PM, Guyatt GH,et al. Development and validation of a questionnaire to measure asthma control. Eur Respir J 1999; 14: 902–907. doi: 10.1034/j.1399-3003.1999.14d29.x [DOI] [PubMed] [Google Scholar]
- 23.American Thoracic Society , European Respiratory Society . ATS/ERS recommendations for standardized procedures for the online and offline measurement of exhaled lower respiratory nitric oxide and nasal nitric oxide, 2005. Am J Respir Crit Care Med 2005; 171: 912–930. doi: 10.1164/rccm.200406-710ST [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Berhane K, Eckel SP,et al. Determinants of children's exhaled nitric oxide: new insights from quantile regression. PLoS One 2015; 10: e0130505. doi: 10.1371/journal.pone.0130505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stanojevic S, Kaminsky DA, Miller M,et al. ERS/ATS technical standard on interpretive strategies for routine lung function tests. Eur Respir J 2022; 60: 2101499. doi: 10.1183/13993003.01499-2021 [DOI] [PubMed] [Google Scholar]
- 26.Sonnappa S, Bastardo CM, Wade A,et al. Symptom-pattern phenotype and pulmonary function in preschool wheezers. J Allergy Clin Immunol 2010; 126: 519–526. doi: 10.1016/j.jaci.2010.04.018 [DOI] [PubMed] [Google Scholar]
- 27.Cordeiro D, Rudolphus A, Snoey E,et al. Utility of nitric oxide for the diagnosis of asthma in an allergy clinic population. Allergy Asthma Proc 2011; 32: 119–126. doi: 10.2500/aap.2011.32.3419 [DOI] [PubMed] [Google Scholar]
- 28.Woo SI, Lee J, Kim H,et al. Utility of fractional exhaled nitric oxide (F(E)NO) measurements in diagnosing asthma. Respir Med 2012; 106: 1103–1109. doi: 10.1016/j.rmed.2012.03.022 [DOI] [PubMed] [Google Scholar]
- 29.Heffler E, Guida G, Marsico P, et al. Exhaled nitric oxide as a diagnostic test for asthma in rhinitic patients with asthmatic symptoms. Respir Med 2006; 100: 1981–1987. doi: 10.1016/j.rmed.2006.02.019 [DOI] [PubMed] [Google Scholar]
- 30.Voutilainen M, Malmberg LP, Vasankari T,et al. Exhaled nitric oxide indicates poorly athlete's asthma. Clin Respir J 2013; 7: 347–353. doi: 10.1111/crj.12014 [DOI] [PubMed] [Google Scholar]
- 31.Sivan Y, Gadish T, Fireman E,et al. The use of exhaled nitric oxide in the diagnosis of asthma in school children. J Pediatr 2009; 155: 211–216. doi: 10.1016/j.jpeds.2009.02.034 [DOI] [PubMed] [Google Scholar]
- 32.Zhu Z, Xia S, Chen X,et al. Factors associated with exhaled nitric oxide in children with asthma and allergic rhinitis. Clin Respir J 2020; 14: 9–15. doi: 10.1111/crj.13093 [DOI] [PubMed] [Google Scholar]
- 33.Yoshii K, Tanaka T, Establishment of a longitudinal growth chart corresponding to pubertal timing. Clin Pediatr Endocrinol 2018; 27: 215–224. doi: 10.1297/cpe.27.215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Tanabe Y, Harada N, Ito J,et al. Difference between two exhaled nitric oxide analyzers, NIOX VERO electrochemical hand-held analyzer and NOA280i chemiluminescence stationary analyzer. J Asthma 2019; 56: 167–172. doi: 10.1080/02770903.2018.1439953 [DOI] [PubMed] [Google Scholar]
- 35.Saito J, Kikuchi M, Fukuhara A,et al. Comparison of fractional exhaled nitric oxide levels measured by different analyzers produced by different manufactures. J Asthma 2020; 57: 1216–1226. doi: 10.1080/02770903.2019.1642351 [DOI] [PubMed] [Google Scholar]
- 36.Wang R, Murray CS, Fowler SJ,et al. Asthma diagnosis: into the fourth dimension. Thorax 2021; 76: 624–631. doi: 10.1136/thoraxjnl-2020-216421 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Grzelewski T, Witkowski K, Makandjou-Ola E,et al. Diagnostic value of lung function parameters and FeNO for asthma in schoolchildren in large, real-life population. Pediatr Pulmonol 2014; 49: 632–640. doi: 10.1002/ppul.22888 [DOI] [PubMed] [Google Scholar]
- 38.de Jong C, Pedersen E, Mozun R,et al. Diagnosis of asthma in children: findings from the Swiss Paediatric Airway Cohort. Eur Respir J 2020; 56: 2000132. doi: 10.1183/13993003.00132-2020 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Please note: supplementary material is not edited by the Editorial Office, and is uploaded as it has been supplied by the author.
Supplementary material 00319-2022.SUPPLEMENT (564KB, pdf)
Figure E1 00319-2022.FIGUREE1 (52.3KB, tif)
Figure E2 00319-2022.FIGUREE2 (188.8KB, tif)
Figure E3A 00319-2022.FIGUREE3A (77.6KB, jpg)
Figure E3B 00319-2022.FIGUREE3B (240KB, tif)
Figure E4 00319-2022.FIGUREE4 (82.3KB, jpg)
Figure E5 00319-2022.FIGUREE5 (346KB, tif)
Figure E6A 00319-2022.FIGUREE6A (605KB, tif)
Figure E6B 00319-2022.FIGUREE6B (674.3KB, tif)
Figure E7 00319-2022.FIGUREE7 (255KB, tif)