Abstract
The peroxide response-inducible genes ahpCF, dps, and katB in the obligate anaerobe Bacteroides fragilis are controlled by the redox-sensitive transcriptional activator OxyR. This is the first functional oxidative stress regulator identified and characterized in anaerobic bacteria. oxyR and dps were found to be divergently transcribed, with an overlap in their respective promoter regulatory regions. B. fragilis OxyR and Dps proteins showed high identity to homologues from a closely related anaerobe, Porphyromonas gingivalis. Northern blot analysis revealed that oxyR was expressed as a monocistronic 1-kb mRNA and that dps mRNA was approximately 500 bases in length. dps mRNA was induced over 500-fold by oxidative stress in the parent strain and was constitutively induced in the peroxide-resistant mutant IB263. The constitutive peroxide response in strain IB263 was shown to have resulted from a missense mutation at codon 202 (GAT to GGT) of the oxyR gene [oxyR(Con)] with a predicted D202G substitution in the OxyR protein. Transcriptional fusion analysis revealed that deletion of oxyR abolished the induction of ahpC and katB following treatment with hydrogen peroxide or oxygen exposure. However, dps expression was induced approximately fourfold by oxygen exposure in ΔoxyR strains but not by hydrogen peroxide. This indicates that dps expression is also under the control of an oxygen-dependent OxyR-independent mechanism. Complementation of ΔoxyR mutant strains with wild-type oxyR and oxyR(Con) restored the inducible peroxide response and the constitutive response of the ahpCF, katB, and dps genes, respectively. However, overexpression of OxyR abolished the catalase activity but not katB expression, suggesting that higher levels of intracellular OxyR may be involved in other physiological processes. Analysis of oxyR expression in the parents and in ΔoxyR and overexpressing oxyR strains by Northern blotting and oxyR′::xylB fusions revealed that B. fragilis OxyR does not control its own expression.
The human intestinal obligate anaerobe Bacteroides fragilis possesses a complex oxidative stress response mechanism which is required to maintain extended aerotolerance compared to control cultures (24). A set of approximately 28 proteins are synthesized in response to treatment with hydrogen peroxide or oxygen exposure, but other proteins are also down regulated following a shift to aerobic conditions, and their role in the physiological adaptation to this adverse environment still remains unclear (24). The catalase gene katB is typical of the B. fragilis oxidative stress genes and is induced in mid-log phase following the addition of hydrogen peroxide or exposure to molecular oxygen or after entering the stationary phase (25). A katB mutant was found to be more sensitive to exogenous hydrogen peroxide under anaerobic conditions than was the parent strain, but aerotolerance in the presence of atmospheric oxygen was not significantly altered (24). The studies on resistance to peroxides led to the isolation of a KatB-overproducing mutant, IB263, with constitutive high resistance to hydrogen peroxide and organic peroxides but not atmospheric oxygen (26). Two other antioxidant proteins, AhpCF and Dps, were also constitutively expressed in the B. fragilis peroxide-resistant strain (26), and mutants with mutations in AhpCF were more sensitive to mutagenesis and killing by organic peroxides than was the parent strain (27). Further studies have revealed that katB, ahpCF, and dps are coordinately regulated at the transcriptional level, suggesting that these peroxide response genes were under the control of a common regulator (26, 27).
Recently, several other genes have been characterized as part of the oxidative stress response in B. fragilis, but these were not part of the peroxide regulon. The genes encoding ribonucleotide diphosphate reductase, nrdA, a pyridoxal 5′-phosphate binding protein, oip-1, and superoxide dismutase, sod, were induced by a peroxide-independent oxygen-dependent mode, whereas recA and malonyl coenzyme A-acyl carrier protein transacylase mRNAs were down regulated following an oxidative stress insult in B. fragilis (D. J. Smalley, E. R. Rocha, and C. J. Smith, Abstr. 97th Gen. Meet. Am. Soc. Microbiol. 1997, abstr. k-141, p. 365, 1997). Thus, these studies confirm that the physiological response of the anaerobe B. fragilis to oxidative stress is not a simple adaptation to an adverse environment but that instead there are multiple regulatory mechanisms that control specific aspects of the response.
Similarly, the peroxide and superoxide stress responses in Escherichia coli and Salmonella enterica serovar Typhimurium are independent, and numerous studies have shown that they are controlled at the transcriptional level by two major regulators, OxyR, and SoxRS, respectively (34, 35). In contrast, very little is known about how anaerobic bacteria control the expression of genes involved in the oxidative stress response, and no regulatory genes have been found. Thus, based on the experimental evidence for the presence of oxidative stress regulators in B. fragilis mentioned above, we used the peroxide-resistant strain as a genetic tool to identify the mechanism controlling the peroxide response in B. fragilis. In this paper we report on the identification and characterization of an OxyR homologue and show that a mutated oxyR gene is responsible for the constitutive expression of the peroxide response in the peroxide-resistant mutant.
MATERIALS AND METHODS
Strains and growth conditions.
The B. fragilis strains and plasmids used in this study are listed in Table 1. All strains were grown anaerobically in brain heart infusion broth supplemented with hemin, cysteine, and NaHCO3 (BHIS) for routine cultures and genetic procedures (32). Cysteine was omitted in some experiments where indicated, and 20 μg of rifampin per ml, 50 μg of gentamicin per ml, 5 μg of tetracycline per ml, 10 μg of erythromycin per ml, and/or 25 μg of cefoxitin per ml were added to the medium when required.
TABLE 1.
Relevant characteristics of B. fragilis strains and plasmids used in this study
| Strain or plasmid | Phenotypea | Reference |
|---|---|---|
| Strains | ||
| 638R | Clinical isolate, Rif | 23 |
| IB263 | 638R hydrogen peroxide-resistant oxyR(Con) Rif | 27 |
| IB272 | 638R katB′::xylB bglA Rif Erm | Smalley et al., abstract |
| IB277 | 638R ahpC′::xylB Rif Erm | 27 |
| IB278 | IB263 ahpC′::xylB Rif Erm | 27 |
| IB294 | 638R oxyR′::xylB bglA Rif Erm | This study |
| IB295 | 638R dps′::xylB bglA Rif Erm | This study |
| IB296 | IB263 oxyR′::xylB bglA Rif Erm | This study |
| IB297 | IB263 dps′::xylB bglA Rif Erm | This study |
| IB298 | 638R ΔoxyR::tetQ Rif Tet | This study |
| IB299 | IB263 ΔoxyR(Con)::tetQ Rif Tet | This study |
| IB300 | 638R ΔoxyR::tetQ ahpC′::xylB Rif Erm Tet | This study |
| IB301 | IB263 ΔoxyR(Con)::tetQ ahpC′::xylB Rif Erm Tet | This study |
| IB307 | 638R ΔoxyR::tetQ oxyR′::xylB bglA Rif Erm Tet | This study |
| IB308 | IB263 ΔoxyR(Con)::tetQ oxyR′::xylB bglA Rif Erm Tet | This study |
| IB309 | 638R ΔoxyR::tetQ dps′::xylB bglA Rif Erm Tet | This study |
| IB310 | IB263 ΔoxyR(Con)::tetQ dps′::xylB bglA Rif Erm Tet | This study |
| Plasmids | ||
| pFD288 | Shuttle vector, oriT, pUC19::pBI143 chimera, (Sp) Erm | 33 |
| pFD516 | Suicide vector, derived from deletion of pBI143 in pFD288 (TetX) (Sp) Erm | 33 |
| pFD697 | Reporter gene vector, 1.2-kb EcoRI fragment with promoterless β-xylosidase/arabinosidase (xylB) cloned into EcoRI site of pFD516 with a 600-bp TaqI internal fragment of bglA in the ClaI site, (Sp) Erm | Smalley et al., abstract |
| pFD770 | 1.34-kb oxyR(Con) fragment cloned into the SmaI site of pFD288 containing a 2.4-kb blunted BamHI-NarI cfxA fragment into the blunted EcoRI-NarI sites, (Sp) Erm Cfx | This study |
| pFD772 | 1.34-kb oxyR fragment cloned into the SmaI site of pFD288 containing a 2.4-kb blunted BamHI-NarI cfxA fragment cloned into the blunted EcoRI-NarI sites, (Sp) Erm Cfx | This study |
Erm, erythromycin resistance; Cfx, cefoxithine resistance; Rif, rifampin resistance; Tet, tetracycline resistance; Sp, spectinomycin resistance. Parentheses indicate antibiotic resistance expression in E. coli.
Cloning and DNA sequencing of oxyR.
All DNA modifications and manipulations were carried out by standard methods (4, 28). In an effort to amplify oxyR homologues from the B. fragilis chromosome, oligonucleotide primers were designed based on conserved amino acid sequences adjacent to the DNA binding motif MNIR(Q)D(Q)LE(K)YL(I)V(A)A and the functional cysteine residue conserved-region consensus E(D)E(D)GHCL(F)RD(N)Q of bacterial OxyR proteins available in the database. The sense and antisense oligonucleotide sequences are 5′-ATG AAY ATH MRI SAI YTI RAR TAY HTI GYI GC and 5′-TGR TRI CKI ARR CAR TGI CCI TCI TC, respectively. A 600-bp fragment was then amplified by Taq polymerase using a PCR amplification kit (Qiagen, Valencia, Calif.). The thermocycling conditions were set using touchdown annealing temperatures as follows: 5 cycles at 50°C, 5 cycles at 45°C, 5 cycles at 45°C, and 25 cycles at 35°C. The denaturing and extension temperatures for all reaction cycles were set at 94°C for 15 s and 72°C for 1 min, respectively. The amplified fragment was extracted from an agarose gel, ligated into cloning vector pGEM-T (Promega, Madison, Wis.), and electrotransformed into E. coli DH10B, resulting in pFD726. Southern blot hybridization analysis using the cloned fragment as a probe revealed homology to 2.5-kb EcoRV and 5-kb PstI DNA fragments in the B. fragilis chromosome. Then, using inverse PCR (13), the 2.5-kb EcoRV and 5-kb PstI fragments were amplified by Platinum Taq High Fidelity DNA polymerase (Life Technologies, Rockville, Md.) using the specific oligonucleotide primers 5′-CGG TAA CAC TGC CAA TCG GAA TG and 5′-GCT GGA TGA TGC CGC ATT AAC GG, based on known sequences. The amplified fragments were then cloned into the pGEM-T vector for further nucleotide sequencing. The procedure to isolate the oxyR gene from the peroxide-resistant strain IB263 was carried out by inverse PCR as above using the IB263 chromosome as template. Automated nucleotide sequencing was performed on double-stranded DNA templates (Molecular Biology Resource Facility, University of Tennessee, Knoxville, Tenn.). Additional oligonucleotide primers were designed based on available sequence information to extend and confirm the existing sequence.
RNA extraction, Northern blot hybridization, and primer extension.
Total RNA extraction and Northern blot analysis of mRNA were carried out as previously described (25). Internal fragments of dps and oxyR were used as specific probes. Densitometry analysis of the autoradiograph was normalized to the relative intensity of total 23S and 16S rRNA detected on the ethidium bromide-stained agarose gel to correct for any loading differences.
Primer extension analysis was performed on total RNA obtained from mid-log-phase cells of B. fragilis 638R and IB263 grown anaerobically and then subjected to oxidative stress conditions as described previously (25). A dps-specific oligonucleotide, 5′-GAT GTT CCA GTG AAA TCC TCT CAG GTT TGC, complementary to nucleotides 97 to 137 of the dps coding region and an oxyR-specific oligonucleotide, 5′-CAG TTT CAC CCC CAA TTC GTC TTC CAG CTT CTG G, complementary to nucleotides 108 to 141 of the oxyR coding region were labeled with [γ-32P]ATP and used as primers for the reverse transcriptase reaction as described previously (25). The extended labeled product was electrophoresed on 8% polyacrylamide gels containing urea. A nucleotide sequence ladder was prepared with Sequenase (USB, Cleveland, Ohio) using a template covering the transcription start site region with the same oligonucleotides that were used for the reverse transcription reactions.
Construction of oxyR deletion mutants.
Briefly, a 2.7-kb chromosome fragment containing the oxyR region was amplified by PCR with two oligonucleotides containing nucleotide modifications to create sites for EcoRI and BamHI. The amplified fragment was then cloned into the EcoRI and BamHI sites of the suicide vector pFD516 (33) to create pFD750. Subsequently, an internal 652-bp SalI-NdeI (blunted) fragment from the oxyR gene was removed and replace with a SalI-SmaI tetQ fragment to construct pFD754 (ΔoxyR::tetQ). pFD754 was mobilized from E. coli DH10B into both B. fragilis 638R and IB263 strains by triparental matings (31), and exconjugants were selected on BHIS agar plates containing 20 μg of rifampin per ml, 100 μg of gentamicin per ml, and 5 μg of tetracycline per ml. Determination of sensitivity to erythromycin and Southern blot analysis of chromosomal DNA were carried out to confirm the double-crossover genetic allele exchange of pFD754 into the B. fragilis chromosome to create the oxyR deletion mutants 638R ΔoxyR::tetQ (IB298) and IB263 ΔoxyR::tetQ (IB299).
Construction of oxyR′ and dps′ β-xylosidase (xylB) transcriptional fusions.
A 187-bp DraI-HincII fragment from pFD750 was cloned into the SmaI site of pUC19 in both orientations. Then SphI-SstI fragments from both constructs were cloned into the SphI-SstI sites of pFD700 containing a 600-bp fragment from B. fragilis bglA as a target for integration into the B. fragilis chromosome (Smalley et al., Abstr. 97th ASM Meet.). A 1.2-kb EcoRI fragment from pXA1 containing the promoterless xylosidase/arabinosidase (xylB) bifunctional reporter gene (42) was cloned into the unique EcoRI site of the new construct. Restriction analysis were used to confirm the orientation of the new constructs, pFD752 and pFD753, containing the oxyR′::xylB and dps′::xylB transcriptional fusions, respectively. pFD752 and pFD753 were mobilized from E. coli DH10B into B. fragilis strains by triparental matings, and they integrated into the bglA gene.
Enzyme assays.
β-Xylosidase and catalase activity assays were carried out in bacterial crude extracts as described previously (27). Cell crude extracts were obtained from mid-log-phase anaerobic cultures of B. fragilis in BHIS without cysteine supplementation. The cultures were treated with 50 μM hydrogen peroxide for 15 min or by exposure to atmospheric oxygen for 1 h as described previously (24).
Complementation of oxyR mutants with oxyR and oxyR(Con).
A 1.34-kb oxyR fragment was amplified by PCR with Platinum Taq High Fidelity DNA polymerase from the 638R and IB263 chromosomes. The oxyR(Con) and oxyR fragments were cloned into the SmaI site of shuttle vector pFD288 (33). Also, a 2.4-kb blunted BamHI-NarI DNA fragment containing a cefoxitin (cfxA) cassette was cloned into the blunted EcoRI-NarI sites to produce pFD770[oxyR(Con)] and pFD772(oxyR), respectively. pFD770 and pFD772 were mobilized into B. fragilis strains by triparental matings. Transconjugants were selected on BHIS agar plates containing 20 μg of rifampin per ml, 100 μg of gentamicin per ml, 10 μg of erythromycin per ml, and 25 μg of cefoxitin per ml.
DNA sequence analysis and database comparison.
Computer analysis of nucleotide and amino acid sequence data was performed using the University of Wisconsin Genetics Computer Group DNA sequence analysis software (version 10) (11). Phylogenetic relationships were inferred by the parsimony method with the PHYLIP phylogeny inference package (version 3.5) (14) from a multiple amino acid sequence alignment generated by Pileup. A consensus tree was constructed from 100 bootstrap replications.
Other gene sequences (and their products) used for the analysis, together with their respective GenBank accession numbers, are as follows: Bacillus subtilis metalloregulation DNA-binding protein MrgA (P37960), B. subtilis general stress protein GSP20U (P80879), Borrelia burgdoferi neutrophil-activating protein A (NapA) (AE001169), E. coli OxyR (P11721), E. coli Dps (P27430), Erwinia chrysanthemi OxyR (AJ005255), Haemophilus influenzae OxyR (P44418) and H. influenzae hypothetical protein HI1349 (P45173), Helycobacter pylori neutrophil-activating protein A (NapA) (U16121), Listeria innocua non-heme-iron-containing ferritin (P80725), Mycobacterium leprae OxyR (P52678), Rickettsia prowazekii unknown protein (AJ235273), Streptococcus pneumoniae unknown protein (AF055720), Synechocystis strain PCC6803 hypothetical 17.8-kDa protein YI94 (P73321), and Xanthomonas campestris pv. phaseoli OxyR (U94336). Porphyromonas gingivalis OxyR and Dps preliminary sequence data were obtained from The Institute for Genomic Research website at http://www.tigr.org. Bordetella pertussis OxyR and Neisseria meningitidis OxyR preliminary sequence data were obtained from the Sequencing Group at Sanger Center. Pseudomonas aeruginosa OxyR preliminary sequence data were obtained from the Pseudomonas Genome Project.
Nucleotide sequence accession numbers.
The nucleotide sequences of the B. fragilis 638R oxyR and dps genes and the IB263 oxyR(Con) gene have been deposited in GenBank under accession numbers AF206033 and AF206034, respectively.
RESULTS
Analysis of the oxyR and dps nucleotide sequences.
Previous work on the regulation of katB (25) and ahpCF (27) and the phenotype of a hydrogen peroxide-resistant mutant (26) suggested that there was coordinate regulation of at least some oxidative stress genes in B. fragilis. The possibility that this control was mediated by OxyR was strengthened by the observation of an oxyR homologue in the genome sequence of a closely related anaerobe, P. gingivalis. Thus, using a PCR approach with primers based on the conserved regions of all known OxyR proteins (described in Materials and Methods), we cloned the B. fragilis 638R oxyR gene. This gene is composed of an open reading frame containing 927 nucleotides, and the deduced amino acid sequence revealed a 308-amino-acid peptide with significant homology to OxyR and other members of the LysR-type family of transcriptional activators in the databases. As expected, B. fragilis OxyR had the highest homology (58.6% identity and 66% similarity) to a hypothetical OxyR found in P. gingivalis. However, compared to other facultative and aerobic organisms, this similarity was greatly reduced to about 40% identity. The alignment of OxyR amino acid sequences is shown in Fig. 1A. The helix-turn-helix motif region for DNA binding and promoter recognition present at the N-terminal domain of LysR-type regulators (19, 29) and the functional cysteine residues (C199 and C208) essential for the redox activity in E. coli OxyR (18, 45) are highly conserved in B. fragilis OxyR (Fig. 1A).
FIG. 1.
Multiple alignment of the B. fragilis (Bf) deduced amino acid sequences for OxyR (A) and Dps (B) with other bacterial OxyR and Dps homologue amino acid sequences, respectively. E. coli (Ec), B. burgdorferi (Bb), B. pertussis (Bp), B. subtilis (Bs), E. chrysanthemi (Ech), H. influenzae (Hi), H. pylori (Hp), L. innocua (Li), M. leprae (Ml), N. miningitis (Nm), P. gingivalis (Pg), P. aeruginosa (Pa), R. prowazekii (Rp), S. pneumoniae (Sp), Synechocystis sp. (Sy), and Xanthomonas campestris (Xc) sequences were used. Lines drawn above and below the amino acid sequences indicate the LyR helix-turn-helix DNA-binding nucleotide sequence motif and functional redox-active cysteine residues of E. coli OxyR C199 and C208 (29, 45) in panel A and the Dps protein signature consensus pattern associated with DNA binding properties (6, 43) in panel B. Consensus of at least 50% identical amino acid residues are labeled with black boxes. Conserved amino acid substitutions are depicted by grey boxes. The respective protein descriptions and GenBank accession numbers for the sequences are listed in Materials and Methods.
The phylogenetic relationship of 22 bacterial OxyR and 5 members of the LysR-type family of transcriptional regulators was determined from a progressive multiple alignment of the amino acid sequences followed by parsimony analysis (data not shown). This comparison clearly shows that the obligate anaerobes B. fragilis and P. gingivalis were clustered in a branch separated from other gram-negative eubacteria.
When the nucleotide sequence upstream of oxyR was analyzed, it revealed an ORF containing 474 nucleotides oriented in the opposite direction from oxyR translational start codon (Fig. 1B and 2). The deduced amino acid sequence revealed a peptide with homology to Dps, a bacterial oxidative stress and stationary-phase nonspecific DNA binding protein (1, 2). The first 30 N-terminal amino acids showed 100% identity to the N-terminal sequence previously obtained by Edman degradation of a protein in the peroxide-resistant mutant IB263 (26). This therefore confirms the presence of a dps gene in B. fragilis and the expression of its respective product. Alignment of the B. fragilis Dps with other Dps homologues revealed high homology to the putative P. gingivalis Dps (47.5% identity, 60% similarity) and the putative H. influenzae Dps (45% identity, 56% similarity). The alignment of the B. fragilis Dps sequence with other bacterial Dps homologues revealed the presence of the carboxylated amino acids presumed to be involved in iron withholding in L. innocua Ftn and H. pylori NapA (6, 39) and the DNA binding motif (Fig. 1B). The phylogenetic relationship between B. fragilis Dps and 19 bacterial Dps homologues was determined from a progressive multiple alignment of the amino acid sequences followed by parsimony analysis. Interestingly, B. fragilis Dps was clustered with H. influenzae and P. gingivalis Dps in a branch also containing B. burgdoferi NapA (data not shown).
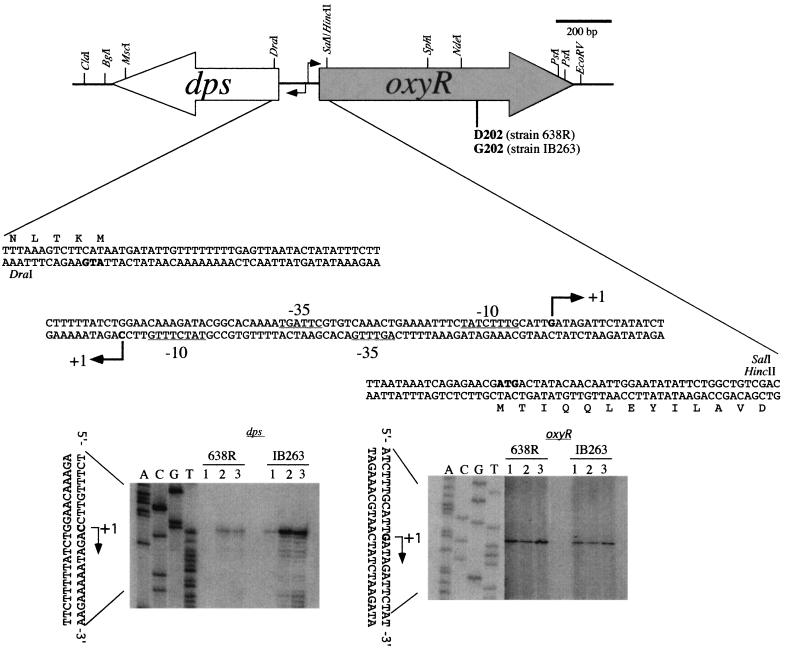
FIG. 2.
Diagram of oxyR and dps genetic organization and structure of the promoter regions. The open and grey long arrows indicate the open reading frames and their respective direction of transcription. The dps-oxyR intergenic nucleotide sequence region and the first 5 codons of dps and 13 codons of oxyR also are shown. A partial restriction endonuclease map of the sequenced genes is indicated. The dark arrowheads indicate the transcription initiation nucleotide for dps and oxyR mRNAs. Based on a B. fragilis consensus (D. P. Bayley and C. J. Smith, unpublished data), the predicted −10 and −35 promoter region for each gene is underlined. The bottom panels show the primer extension autoradiographs used to determine the transcription start sites for dps and oxyR mRNAs. To the left of each panel is a DNA sequencing ladder generated with the same primer used for primer extension reactions. The following treatments were used as described in the Materials and Methods: anaerobic growth (lane 1), hydrogen peroxide treatment (lane 2), and oxygen exposure (lane 3).
Identification of an oxyR mutation in the constitutive peroxide-resistant mutant IB263.
To investigate whether the constitutive peroxide-resistant strain IB263 had an altered OxyR regulator, the entire IB263 oxyR operon was sequenced. Analysis of the nucleotide sequence revealed a single-base substitution (A to G) at codon 202 (GAT to GGT), leading to a D202G amino acid substitution in the IB263 OxyR protein compared to the parent. No other base substitution was found in the promoter and coding regions of oxyR (data not shown). To confirm this point mutation, three independent PCR amplifications of the IB263 and 638R chromosomal oxyR regions were performed using High Fidelity proofreading Taq polymerase. All amplified IB263 oxyR nucleotide sequences obtained showed the same single-nucleotide base substitution at codon 202 compared to 638R oxyR sequences. This confirms that a point mutation had occurred in the IB263 oxyR gene, which is hereafter named oxyR(Con). D202 is in a highly conserved region around the functional cysteine C199 region at the C-terminal domain of the OxyR protein.
Regulation of dps and oxyR mRNA expression by oxidative stress.
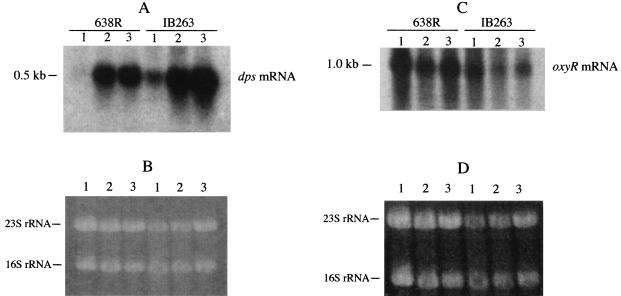
To investigate the expression of dps and oxyR, total RNA extracted from mid-log-phase cells exposed to different oxidative stress conditions was probed with specific internal DNA fragments. Northern blot hybridization analysis revealed that expression of dps mRNA was regulated at the transcriptional level. Transcripts of approximately 0.5 kb were detected, suggesting that dps was transcribed as a monocistronic mRNA (Fig. 3A). Densitometric analysis of the Northern blots showed approximately a 500-fold increase in the level of dps mRNA in cultures treated with H2O2 or exposed to oxygen compared to that in anaerobic cultures. dps mRNA was constitutively expressed anaerobically (250-fold increase) in IB263 compared to anaerobic cultures of the parent 638R (Fig. 3A). When Northern blots were probed with the oxyR fragment, the autoradiographs revealed an mRNA of approximately 1.0 kb, suggesting that oxyR was also transcribed as a monocistronic oxyR mRNA (Fig. 3C). In contrast to dps, oxyR mRNA levels were not significantly altered after treatment with hydrogen peroxide and exposure to oxygen in the parent or the oxyR(Con) mutant strain.
FIG. 3.
(A and C) Autoradiographs of Northern hybridization membranes of total RNA from mid-log-phase B. fragilis 638R and IB263 following exposure to different oxidative stress conditions. The probe was a dps (A) or oxyR (C) internal gene fragment. Lanes: 1, anaerobic growth; 2, cultures treated with hydrogen peroxide; 3, cultures exposed to oxygen. The approximate sizes of the transcripts are indicated. (B and D) Respective ethidium bromide-stained agarose gels loaded with approximately 30 μg total RNA in each lane. The 23S and 16S rRNAs are also indicated.
Primer extension analysis of dps and oxyR mRNAs showed that dps mRNA starts at a cytosine nucleotide 49 bp upstream of the dps translation start codon whereas oxyR mRNA starts at guanine nucleotide 34 bp upstream of the oxyR translation start codon in the opposite strand. The dps and oxyR intergenic region was 142 nucleotides in length, and the predicted −10 and −35 promoter regions for both genes were found overlapped. A diagram of the dps and oxyR −10 and −35 promoter regions and transcription start nucleotides is shown in Fig. 2. These findings indicate that the nonspecific DNA binding dps is strongly upregulated by oxidative stress while oxyR transcription levels are not altered following oxidative stress.
oxyR-dependent control of the oxidative stress response genes katB, aphCF, and dps.
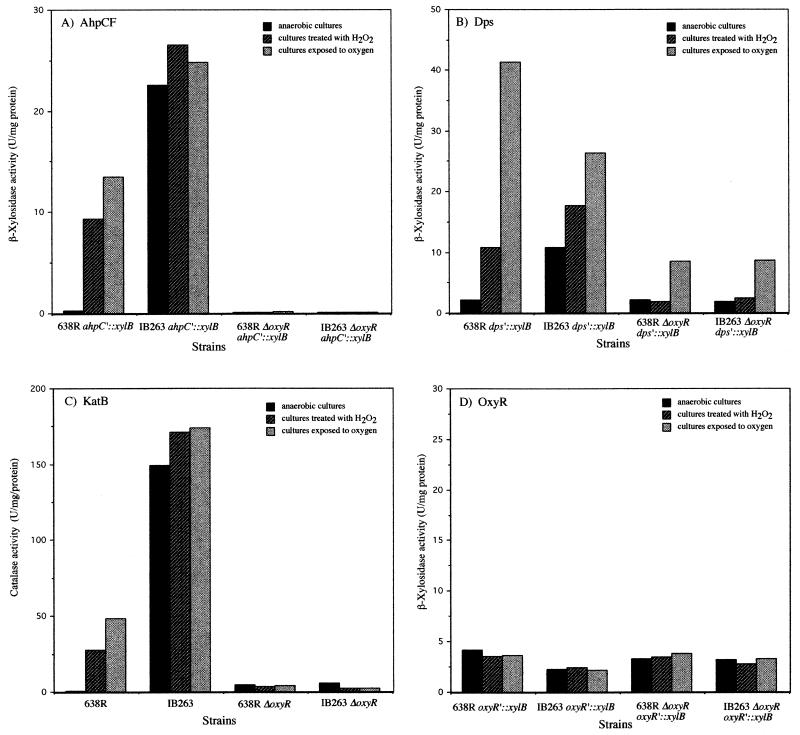
To investigate the role of OxyR in expression of the peroxide regulon, oxyR deletion mutants of the parent strain and the hydrogen peroxide-resistant mutant (IB263) were constructed by double-crossover allelic gene exchange. Preliminary characterization of the mutants showed that while they were highly sensitive to hydrogen peroxide killing, their aerotolerance was just marginally decreased, as indicated by viable-cell counts (data not shown). The effect of the oxyR deletion on gene expression as measured by analysis of β-xylosidase transcriptional fusions and catalase activity following oxidative stress is shown in Fig. 4. The induction of katB and ahpCF by both oxygen and hydrogen peroxide was nearly abolished in oxyR mutants compared to the parent strains. This indicates that a functional OxyR is essential for induction of these stress response proteins (Fig. 4A and C). Moreover, it also confirms that the oxyR(Con) is responsible for the constitutive peroxide response phenotype in IB263. In contrast to ahpCF and katB, dps expression was still significantly induced (fourfold) following oxygen exposure in both 638R ΔoxyR and IB263 ΔoxyR, suggesting that dps may be under dual regulation by OxyR and an oxygen-dependent, OxyR-independent mechanism (Fig. 4B). In addition, dps expression under anaerobic conditions was not altered in the ΔoxyR mutants, indicating that there may also be a growth-dependent regulation. The levels of oxyR expression were not affected by deletion of the oxyR gene from the parent strains, as determined by using the transcriptional reporter fusion oxyR′::xylB (Fig. 4D).
FIG. 4.
Expression of peroxide-inducible genes in oxyR mutants. Determination of β-xylosidase (A, B, and D) and catalase (C) activities in crude extracts of mid-log-phase cells of B. fragilis parent and ΔoxyR mutant strains is shown. Bacteria were grown in BHIS and exposed to different oxidative stress conditions as indicated.
Genetic complementation of oxyR in ΔoxyR mutants with pFD770[oxyR(Con)] and pFD772(oxyR).
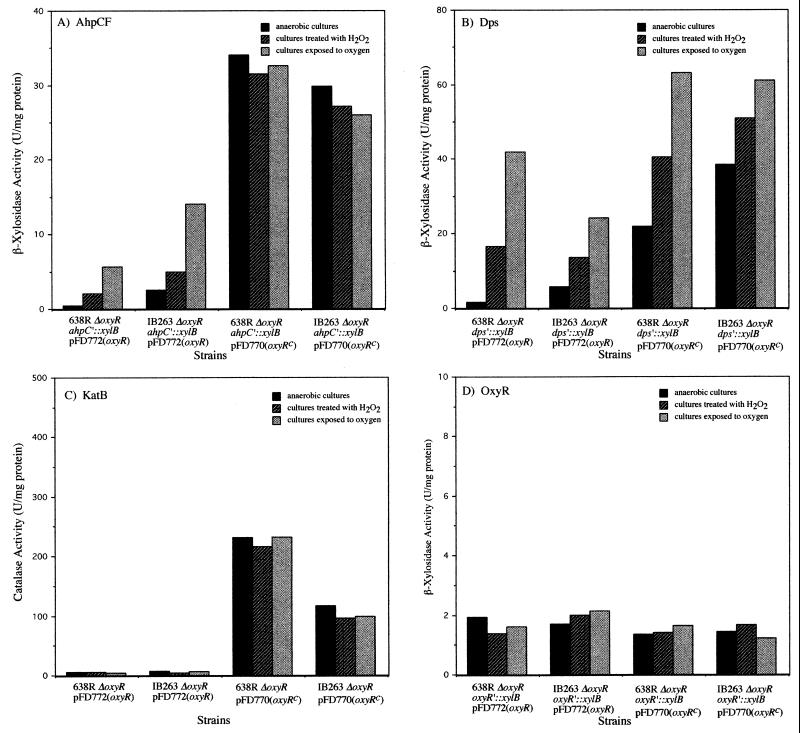
Restoration of the OxyR phenotype in ΔoxyR strains was investigated by complementation with plasmids pFD770[oxyR(Con)] and pFD772(oxyR) (Fig. 5). The copy number of these plasmid constructs is estimated at 15 to 20 copies per cell based on the parent replicon pIB143 (33). Considering the lack of oxyR autoregulation, this would suggest that there was a 15 to 20-fold overexpression of these genes during the complementation experiments. The presence of the wild-type oxyR gene restored induction of ahpCF and dps expression during treatment with hydrogen peroxide or oxygen exposure, while complementation of ΔoxyR with oxyR(Con) restored the constitutive regulation of ahpCF and dps expression compared to anaerobic culture controls (Fig. 5A and B). These findings establish that OxyR(Con) is responsible for the constitutive regulation of the peroxide response in IB263. Figure 4D also shows that genetic complementation of the ΔoxyR strains with both oxyR and oxyR(Con) had no effect on the expression levels of oxyR compared to those in the parent strains (Fig. 5D). Taken together with data presented in Fig. 3C and 4D, these results demonstrate that B. fragilis OxyR is not involved in its own regulation.
FIG. 5.
Complementation of oxyR mutations. Determination of β-xylosidase (A, B, and D) and catalase (C) activities in crude extracts of mid-log-phase cells of B. fragilis ΔoxyR mutant strains completed with constitutive OxyR(Con), pFD770[oxyR(Con)], or wild-type OxyR, pFd772(oxyR) is shown. oxyRc is equivalent to oxyR(Con). Bacteria were grown in BHIS and exposed to different oxidative stress conditions as indicated.
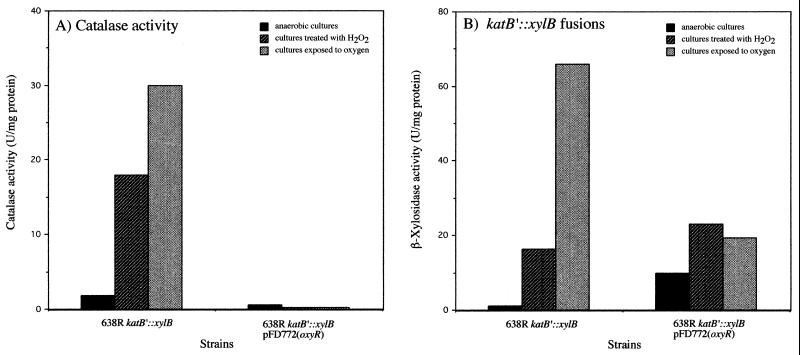
Surprisingly, when ΔoxyR mutants were complemented with the wild-type oxyR gene, there was no induction of catalase activity by treatment with hydrogen peroxide or oxygen exposure compared to anaerobic cultures (Fig. 5C). In contrast, complementation of B. fragilis 638R ΔoxyR and IB263 ΔoxyR strains with oxyR(Con) (Fig. 5C) resulted in the constitutive expression of catalase activity, although there was no further induction by H2O2 treatment as seen with IB263 (Fig. 4C). The catalase activity was also abolished in the ahpC, dps, and oxyR β-xylosidase fusion strains when complemented with pFD772 following oxidative stress (data not shown). Moreover, constitutive expression of catalase activity was detected in all the β-xylosidase fusion strains complemented with pFD770 (data not shown). These finding indicated that multicopy oxyR was having an unexpected posttranscriptional effect on catalase. To test this hypothesis, pFD772 was mobilized into the 638R katB′::xylB strain (IB272), which carries the katB fusion integrated into the bglA gene and has a single copy of chromosomal oxyR. Then the β-xylosidase and catalase activities in the crude extracts of the new construct 638R katB′::xylB pFD772 were determined following oxidative stress (Fig. 6). The results show that induction of catalase activity was abolished in the strain carrying multicopy oxyR (Fig. 6A) while katB transcription was normally regulated as determined by katB′::xylB fusions (Fig. 6B). This suggests that multiple copies of oxyR in B. fragilis have a posttranscriptional effect on KatB when exposed to oxidative stress.
FIG. 6.
Comparison of KatB enzyme activity to the expression of katB transcriptional fusion in OxyR-overproducing strains. Determination of catalase (A) and β-xylosidase (B) activities in crude extracts of mid-log-phase cells of B. fragilis 638R katB′::xylB transformed with pFd772(oxyR) is shown. Bacteria were grown to mid-log phase in BHIS and exposed to different oxidative stress conditions as indicated.
DISCUSSION
Previous reports have shown that an oxidative stress response in the obligate anaerobe B. fragilis is inducible following treatment with hydrogen peroxide or oxygen exposure (22, 24, 30). In this study we show that the redox-sensitive transcriptional activator OxyR, a member of the LysR-type family of bacterial transcriptional activators (9, 36), is responsible for the control of the peroxide response regulon in this anaerobic microorganism. This is the first description of a functional oxidative stress response regulator in obligate anaerobic bacteria and in one so greatly diverged from the main line of eubacterial descent (41). We also show that deletion of oxyR resulted in loss of the peroxide-inducible response in both the parent strain and a constitutive peroxide-resistant mutant. Moreover, complementation of ΔoxyR with the oxyR gene restored the positive transcriptional activation of the peroxide response genes investigated and a point mutation in oxyR was linked to constitutive expression of these genes. Characteristic of the LysR-type family of transcriptional activators where the regulator is divergently transcribed from a gene it activates (29), oxyR was divergently transcribed from dps, a nonspecific DNA binding protein.
Strong evidence for control of the peroxide regulon by OxyR in B. fragilis is provided by the finding that the constitutive peroxide resistance phenotype of IB263 (26) is due to a mutated oxyR gene (GAT to GGT) at codon 202. It is likely that the D202G amino acid substitution near the redox-active C199 residue in IB263 resulted in a conformational change leading to a permanently activated form of OxyR, which is responsible for the constitutive expression of KatB, AhpC, and Dps. Other studies performed with E. coli have found that the A233V mutation is responsible for the constitutive oxyR2 phenotype (9, 18). In another study, randomly mutagenized oxyR genes mapped to amino acid substitutions at the OxyR C-terminal domain conferred a permanent “locked” oxidized form of the protein. These mutations constitutively induced transcription under both reduced and oxidized conditions due to permanently induced cooperative binding of RNA polymerase (19, 38). It is interesting that mutation in the B. fragilis oxyR gene involved a C · G-to-T · A modification as occurred in all of the constitutive E. coli oxyR mutants investigated (18). This type of transition base substitution mutation is typical following oxidative DNA damage (17, 44), which probably occurred during the selection of IB263 for its increased hydrogen peroxide resistance.
As mentioned above, B. fragilis OxyR positively regulates the expression of the antioxidants KatB, AhpCF, and Dps as components of a set of approximately 28 oxidative stress proteins induced by hydrogen peroxide or oxygen exposure. These findings are similar to the peroxide response present in E. coli and Senterica serovar Typhimurium, where hydrogen peroxide induces the expression of a set of approximately 30 proteins (8, 10). Among these proteins, OxyR positively activates the transcription of nine antioxidant proteins including KatG, AhpCF, Dps, and GorA and a small regulatory RNA encoded by oxyS (2, 3, 8). Consistent with this role, preliminary experiments showed that the B. fragilis peroxide response protected primarily against peroxides, since there was only a small effect on the aerotolerance of ΔoxyR mutants (data not shown). Likewise, the oxyR(Con) mutant showed a much greater increase in its resistance to hydrogen peroxide killing than in its resistance to oxygen killing (26). It is interesting that although B. fragilis is an obligate anaerobic bacterium which cannot shift to an aerobic metabolism, it possesses a highly regulated peroxide response similar to the peroxide response reported to occur in aerobic and facultative bacteria.
In contrast to E. coli OxyR, which represses its own expression whether it is in the reduced or oxidized form (9, 37), we have found in this study that deletion of oxyR did not significantly alter the level of oxyR expression. Either B. fragilis oxyR is constitutively expressed or the autoregulatory mechanism does not allow sufficient alteration in oxyR expression levels to be detected by our oxyR′::xylB fusions. However, we think the evidence suggests that OxyR does not repress its own expression and is constitutively expressed. This is based on the facts that (i) basal levels of oxyR expression were not altered in the oxyR deletion mutants IB298 and IB299, (ii) oxyR expression was not altered following oxidative stress compared to anaerobic culture controls, and (iii) oxyR expression was not altered by complementation of ΔoxyR mutation with oxyR and oxyR(Con) genes.
The P. gingivalis genomic database revealed that this phylogenetically related anaerobe also contained oxyR and dps and that these genes were closely related to the B. fragilis homologues. However, the genes are differently organized, with the B. fragilis dps and oxyR being divergently transcribed while the P. gingivalis oxyR is found in a head-to-tail arrangement with genes encoding an exodeoxyribonuclease and a single strand DNA binding protein (sequence data were obtained from the Institute for Genomic Research website at http://www.tigr.org). It is common for OxyR-regulated genes to be located adjacent to oxyR, but this is not always observed (9, 12, 15, 21, 37).
The role of OxyR in the control of the oxidative stress is well established, but there is some evidence that OxyR may be involved in other regulatory pathways which are apparently not involved directly in either scavenging oxygen radicals or repairing oxidative damage (5, 16, 40). In this regard, our finding that strains overproducing OxyR have repressed levels of catalase activity but not repressed transcription may suggest another role of OxyR in B. fragilis. That is, OxyR may be involved with iron or heme uptake, leading to the inactive catalase. Recent studies have shown that the H. influenzae oxyR mutant was unable to utilize protoporphyrin IX and had a reduced ability to incorporate heme (20). In E. coli, OxyR and SoxRS activate the expression of Fur, the global regulator of ferric iron uptake, suggesting that iron metabolism is coordinately regulated with the oxidative stress defenses (46). In this regard, the B. fragilis ferritin (ftn) gene was cloned and sequenced, and ftn expression was found to be up regulated in the parent strain and down regulated in a ΔoxyR mutant following oxidative stress (E. R. Rocha and C. J. Smith, unpublished results). This reinforces the idea that OxyR is involved in the mobilization of intra-cellular iron.
Although dps was divergently transcribed from oxyR and was controlled in large part by OxyR, we found that its regulation was more complex than expected, since it was up regulated by an oxygen-dependent, OxyR-independent mechanism in mid-log-phase cells (Fig. 4B) and by a different stationary-phase mechanism as determined by incorporation of radiolabeled methionine after 24 h of anaerobic growth (data not shown). This suggests that B. fragilis dps expression is under a multiregulatory network that is able to activate the expression of this protein under different growth conditions. This seems to be a common characteristic in the regulation of dps in different organisms. In E. coli, dps expression is under the control of OxyR following the oxidative stress response and under the control of ςS and integration host factor in the stationary phase (2). Dps also may be a link between oxidative stress and iron metabolism, as shown for B. subtilis (7). Thus, the presence of Dps in B. fragilis may be part of an important strategy to protect DNA under different environmental stress conditions.
It is worthwhile to note that OxyR and Dps from B. fragilis and P. gingivalis were clustered in the phylogenetic parsimony analysis in a branch separated from aerobic organisms, which suggests that many of the genes involved in the oxidative stress response were present in this anaerobic bacterium prior to its earlier diversion from other eubacteria (41). These two opportunistic human pathogenic anaerobic bacteria are well adapted to the strictly anaerobic environments of the human lower intestinal tract and gingival crevice, respectively; therefore, one must question the role of a complex oxidative stress response in these organisms. Perhaps it is a transitional mechanism used during the process of leaving their natural anaerobic environment to infect and colonize more oxygenated tissues as well as providing resistance to the oxidative burst of human phagocytes until appropriate anaerobic conditions are established at the site of infection.
ACKNOWLEDGMENTS
This work was supported in part by PHS grant AI-40588. E. R. Rocha thanks East Carolina University School of Medicine for research grant SRG-14:99, which supported part of this work.
We thank the Sequencing Group at Sanger Center and the Pseudomonas Genome Project for the release of unpublished sequence data. Preliminary P. gingivalis sequence data was obtained from The Institute for Genomic Research website at http://www.tigr.org. The P. gingivalis genome project at TIGR and Forsyth Dental Institute was supported by USPHS grant DE-12082 from The National Institute of Dental and Craniofacial Research.
REFERENCES
- 1.Almirón M, Link A J, Furlong D, Kolter R. A novel DNA-binding protein with regulatory and protective roles in starved Escherichia coli. Genes Dev. 1992;6:2646–2654. doi: 10.1101/gad.6.12b.2646. [DOI] [PubMed] [Google Scholar]
- 2.Altuvia S, Almirón M, Huisman G, Kolter R, Storz G. The dps promoter is activated by OxyR during growth and by IHF and ςS in stationary phase. Mol Microbiol. 1994;13:265–272. doi: 10.1111/j.1365-2958.1994.tb00421.x. [DOI] [PubMed] [Google Scholar]
- 3.Altuvia S, Weinstein-Fischer D, Zhang A, Postow L, Storz G. A small, stable RNA induced by oxidative stress: role as a pleiotropic regulator and antimutator. Cell. 1997;90:43–53. doi: 10.1016/s0092-8674(00)80312-8. [DOI] [PubMed] [Google Scholar]
- 4.Ausubel F M, Brent R, Kingston R E, Moore D D, Seidman J G, Smith J A, Struhl K. Current protocols in molecular biology. New York, N.Y: John Wiley & Sons, Inc.; 1987. [Google Scholar]
- 5.Bolker M, Kahmann R. The Escherichia coli regulatory protein OxyR discriminates between methylated and unmethylated states of the phage Mu mom promoter. EMBO J. 1989;8:2403–2410. doi: 10.1002/j.1460-2075.1989.tb08370.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bozzi M, Mignogna G, Stefanini S, Barra D, Longhi C, Valenti P, Chiancone E. A novel non-heme iron-binding ferritin related to the DNA-binding proteins of the Dps family in Listeria innocua. J Biol Chem. 1997;272:3259–3265. doi: 10.1074/jbc.272.6.3259. [DOI] [PubMed] [Google Scholar]
- 7.Bsat N, Herbig A, Casillas-Martinez L, Setlow P, Helmann J D. Bacillus subtilis contains multiple Fur homologues: identification of the iron uptake (Fur) and peroxide regulon (PerR) repressors. Mol Microbiol. 1998;29:189–98. doi: 10.1046/j.1365-2958.1998.00921.x. [DOI] [PubMed] [Google Scholar]
- 8.Christman M F, Morgan R W, Jacobson F S, Ames B N. Positive control of a regulon for defenses against oxidative stress and some heat-shock proteins in Salmonella typhimurium. Cell. 1985;41:753–762. doi: 10.1016/s0092-8674(85)80056-8. [DOI] [PubMed] [Google Scholar]
- 9.Christman M F, Storz G, Ames B N. OxyR, a positive regulator of hydrogen peroxide-inducible genes in Escherichia coli and Salmonella typhimurium is homologous to a family of bacterial regulatory proteins. Proc Natl Acad Sci USA. 1989;86:3484–3488. doi: 10.1073/pnas.86.10.3484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Demple B, Halbrook J. Inducible repair of oxidative damage in Escherichia coli. Nature. 1983;304:466–468. doi: 10.1038/304466a0. [DOI] [PubMed] [Google Scholar]
- 11.Devereux J, Haeberli P, Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acid Res. 1984;12:387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dhandayuthapani S, Mudd M, Deretic V. Interactions of OxyR with the promoter region of the oxyR and ahpC genes from Mycobacterium leprae and Mycobacterium tuberculosis. J Bacteriol. 1997;179:2401–2409. doi: 10.1128/jb.179.7.2401-2409.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dieffenbach C W, Dveksler G S. PCR primer: a laboratory manual. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1995. [Google Scholar]
- 14.Felsenstein J. PHYLIP-phylogeny inference package (version 3.2) Cladistics. 1989;5:164–166. [Google Scholar]
- 15.Geißdorf W, Kok R G, Ratajczak A, Hellingwerf K J, Hillen W. The genes rubA and rubB for alkane degradation in Acinetobacter sp. strain ADP1 are in an operon with estB, encoding an esterase, and oxyR. J Bacteriol. 1999;181:4292–4298. doi: 10.1128/jb.181.14.4292-4298.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Henderson I R, Meehan M, Owen P. Antigen 43, a phase-variable bipartite outer membrane protein, determines colony morphology and auto-aggregation in Escherichia coli. FEMS Microbiol Lett. 1997;149:115–120. doi: 10.1111/j.1574-6968.1997.tb10317.x. [DOI] [PubMed] [Google Scholar]
- 17.Kreutzer D A, Essigmann J M. Oxidized, deaminated cytosines are a source of C→T transitions in vivo. Proc Natl Acad Sci USA. 1998;95:3578–3582. doi: 10.1073/pnas.95.7.3578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kullik I, Toledano M B, Tartaglia L A, Storz G. Mutational analysis of the redox-sensitive transcriptional regulator OxyR: regions important for oxidation and transcriptional activation. J Bacteriol. 1995;177:1275–1284. doi: 10.1128/jb.177.5.1275-1284.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kullik I, Stevens J, Toledano M, Storz G. Mutational analysis of the redox-sensitive transcriptional regulator OxyR: regions important for DNA binding and multimerization. J Bacteriol. 1995;177:1285–1291. doi: 10.1128/jb.177.5.1285-1291.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maciver I, Hansen E J. Lack of expression of the global regulator OxyR in Haemophilus influenzae has a profound effect on growth phenotype. Infect Immun. 1996;64:4618–4629. doi: 10.1128/iai.64.11.4618-4629.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mongkolsuk S, Loprasert S, Whangsuk W, Fuangthong M, Atichartpongkun S. Characterization of transcription organization and analysis of unique expression patterns of an alkyl hydroperoxide reductase C gene (ahpC) and the peroxide regulator operon ahpF-oxyR-orfX from Xanthomonas campestris pv. phaseoli. J Bacteriol. 1997;179:3950–3955. doi: 10.1128/jb.179.12.3950-3955.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Privalle C T, Gregory E M. Superoxide dismutase and O2 lethality in Bacteroides fragilis. J Bacteriol. 1979;138:139–145. doi: 10.1128/jb.138.1.139-145.1979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Privitera G, Dublanchet A, Sebald M. Transfer of multiple antibiotic resistance between subspecies of Bacteroides fragilis. J Infect Dis. 1979;139:97–101. doi: 10.1093/infdis/139.1.97. [DOI] [PubMed] [Google Scholar]
- 24.Rocha E R, Selby T, Coleman J P, Smith C J. The oxidative stress response in an anaerobe, Bacteroides fragilis: a role for catalase in protection against hydrogen peroxide. J Bacteriol. 1996;178:6895–6903. doi: 10.1128/jb.178.23.6895-6903.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rocha E R, Smith C J. Regulation of Bacteroides fragilis katB mRNA expression by oxidative stress and carbon limitation. J Bacteriol. 1997;179:7033–7039. doi: 10.1128/jb.179.22.7033-7039.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rocha E R, Smith C J. Characterization of a peroxide-resistant mutant of the anaerobic bacterium Bacteroides fragilis. J Bacteriol. 1998;180:5906–5912. doi: 10.1128/jb.180.22.5906-5912.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rocha E R, Smith C J. Role of the alkyl hydroperoxide reductase (ahpCF) gene in oxidative stress defense of the obligate anaerobe Bacteroides fragilis. J Bacteriol. 1999;181:5701–5710. doi: 10.1128/jb.181.18.5701-5710.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sambrook J, Fritsch E F, Maniatis T. Molecular cloning: a laboratory manual. 2nd ed. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1989. [Google Scholar]
- 29.Schell M A. Molecular biology of the LysR family of transcriptional regulators. Annu Rev Microbiol. 1993;47:597–626. doi: 10.1146/annurev.mi.47.100193.003121. [DOI] [PubMed] [Google Scholar]
- 30.Schumann J P, Jones D T, Woods D R. Induction of proteins during phage reactivation induced by UV irradiation, oxygen and peroxide in Bacteroides fragilis. FEMS Microbiol Lett. 1984;23:131–135. [Google Scholar]
- 31.Shoemaker N B, Getty C, Gardner J F, Salyers A A. Tn4351 in Bacteroides spp. mediates the integration of plasmid R751 into the Bacteroides chromosome. J Bacteriol. 1986;165:929–936. doi: 10.1128/jb.165.3.929-936.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Smith C J. Characterization of Bacteroides ovatus plasmid pBI136 and structure of its clindamycin resistance region. J Bacteriol. 1985;161:1069–1073. doi: 10.1128/jb.161.3.1069-1073.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith C J, Rollins L A, Parker A C. Nucleotide sequence determination and genetic analysis of the Bacteroides plasmid, pBI143. Plasmid. 1995;34:211–222. doi: 10.1006/plas.1995.0007. [DOI] [PubMed] [Google Scholar]
- 34.Storz G, Jamieson D J. Transcriptional regulators of oxidative stress responses. In: Scandalios J G, editor. Oxidative stress and the molecular biology of antioxidant defenses. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory Press; 1997. pp. 91–115. [Google Scholar]
- 35.Storz G, Imlay J A. Oxidative stress. Curr Opin Microbiol. 1999;2:188–194. doi: 10.1016/s1369-5274(99)80033-2. [DOI] [PubMed] [Google Scholar]
- 36.Tao K, Makino K, Yonei S, Nakata A, Shinagawa H. Molecular cloning and nucleotide sequencing of oxyR, the positive regulatory gene of a regulon for an adaptative response to oxidative stress in Escherichia coli: homologies between OxyR protein and a family of bacterial activator proteins. Mol Gen Genet. 1989;218:371–376. doi: 10.1007/BF00332397. [DOI] [PubMed] [Google Scholar]
- 37.Tao K, Makino K, Yonei S, Nakata A, Shinagawa H. Purification and characterization of the Escherichia coli OxyR protein, the positive regulator for a hydrogen peroxide-inducible regulon. J Biochem. 1991;109:262–266. [PubMed] [Google Scholar]
- 38.Tao K, Fujita N, Ishihama A. Involvement of the RNA polymerase α subunit C-terminal region in co-operative interaction and transcriptional activation with OxyR protein. Mol Microbiol. 1993;7:859–864. doi: 10.1111/j.1365-2958.1993.tb01176.x. [DOI] [PubMed] [Google Scholar]
- 39.Tonello F, Dundon W G, Satin B, Molinari M, Tognon G, Grandi G, Giudice G D, Rappuoli R, Montecucco C. The Helicobacter pylori neutrophil-activating protein is an iron-binding protein with dodecameric structure. Mol Microbiol. 1999;34:238–246. doi: 10.1046/j.1365-2958.1999.01584.x. [DOI] [PubMed] [Google Scholar]
- 40.Warne S R, Varley J M, Boulnois G J, Norton M G. Identification and characterization of a gene that controls colony morphology and auto-aggregation in Escherichia coli K12. J Gen Microbiol. 1990;136:455–462. doi: 10.1099/00221287-136-3-455. [DOI] [PubMed] [Google Scholar]
- 41.Weisburg W G, Oyaizu Y, Oyaizu H, Woese C R. Natural relationship between Bacteriodes and flavobacteria. J Bacteriol. 1985;164:230–236. doi: 10.1128/jb.164.1.230-236.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Whitehead T R. Development of a bifunctional xylosidase/arabinosidase gene as a reporter gene for the Gram-negative anaerobes Bacteroides and Porphyromonas, and Escherichia coli. Curr Microbiol. 1997;35:282–286. doi: 10.1007/s002849900255. [DOI] [PubMed] [Google Scholar]
- 43.Wolf S G, Frenkiel D, Arad T, Finkel S E, Kolter R, Minsky A. DNA protection by stress-induced biocrystallization. Nature. 1999;400:83–85. doi: 10.1038/21918. [DOI] [PubMed] [Google Scholar]
- 44.Wood M L, Dizdaroglu M, Gajewski E, Essigmann J M. Mechanistic studies of ionizing radiation and oxidative mutagenesis: genetic effects of a single 8-hydroxyguanine (7-hydro-8-oxoguanine) residue inserted at a unique site in a viral genome. Biochemistry. 1990;29:7024–32. doi: 10.1021/bi00482a011. [DOI] [PubMed] [Google Scholar]
- 45.Zheng M, Aslund F, Storz G. Activation of the OxyR transcriptional factor by reversible disulfide bond formation. Science. 1998;279:1718–721. doi: 10.1126/science.279.5357.1718. [DOI] [PubMed] [Google Scholar]
- 46.Zheng M, Doan B, Schneider T D, Storz G. OxyR and SoxRS regulation of fur. J Bacteriol. 1999;181:4639–4643. doi: 10.1128/jb.181.15.4639-4643.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]