Summary
Abnormal Uterine Bleeding (AUB) is a common debilitating condition that significantly reduces quality of life of women across the reproductive age span. AUB creates significant morbidity, medical, social, and economic problems for women, their families, workplace, and health services. Despite the profoundly negative effects of AUB on public health, advancement in understanding the pathophysiology of AUB and the discovery of novel effective therapies is slow due to lack of reliable pre-clinical models.
This review discusses currently available laboratory-based pre-clinical scientific models and how they are used to study AUB. Human and animal in vitro, ex vivo, and in vivo models will be described along with advantages and limitations of each method.
Keywords: Abnormal uterine bleeding (AUB), Endometrium, Pre-clinical models, Epithelial organoids, Endometrial co-cultures, Adenomyosis, Leiomyoma (uterine fibroids)
Abnormal uterine bleeding (AUB) definition, diagnosis, and need for pre-clinical models
Endometrial function through the menstrual, proliferative, and secretory phases is tightly regulated by hormonal endocrine signalling. Circulating ovarian sex steroids oestrogen and progesterone are crucial for this regulation. Oestrogen stimulates endometrial cell proliferation and regeneration following menstruation; progesterone opposes oestrogen-stimulated proliferation and stimulates cell differentiation in preparation for implantation. If pregnancy does not occur, progesterone is withdrawn, and this triggers menstruation as the new menstrual cycle begins.1 Dysregulation of these processes may lead to abnormalities in endometrial breakdown, bleeding, repair, and regeneration.
Normal uterine bleeding is defined using four parameters: frequency, duration, volume, and regularity. A typical menstrual cycle (i) has a frequency of 24–38 days, (ii) a regularity (cycle to cycle variation) of 2–20 days, (iii) has a duration of 4.5–8 days, and (iv) has a volume of 5–80 millilitres.
Abnormal Uterine Bleeding (AUB) is a highly prevalent symptom of an underlying condition/s experienced by one in three women of reproductive age. AUB, which includes heavy menstrual bleeding (HMB), causes significant morbidity and affects every aspect of the sufferer's life. A clear definition, description of the causes, and signs of AUB has been established, which “should assist in providing a solid basis for the standardisation of international research and clinical manuscripts addressing the diagnosis, pathogenesis, and management of AUB”.2,3 The International Federation of Obstetrics and Gynaecology (FIGO) Menstrual Disorders Committee (MDC) published the two FIGO systems (terminology; System 1 and classification; System 2) in 2011 with revisions in 2018.4
System 1 defines AUB as a symptom presenting values of menstrual bleeding frequency, duration, volume, and regularity outside of the normal range described above.2 Acute AUB is an episode of heavy menstrual bleeding (HMB) that requires immediate intervention. Chronic AUB is AUB present for most of the past 6 months that does not require urgent medical attention.3
FIGO system 2 classifies AUB using the acronym PALM-COEIN [pahm-koin] describing two groups of AUB aetiologies – “PALM” describes the structural, and “COEIN” - the non-structural causes (Figure 1).
Figure 1.
FIGO classification of abnormal uterine bleeding (AUB) – structural (PALM) and non-structural (COEIN) causes (4).
Abnormal uterine bleeding can be caused by none, one, or multiple PALM-COEIN aetiologies. Some may be present but asymptomatic (AUB-L, see Figure 1), and others can only be diagnosed by exclusion of other identifiable factors (such as underlying endometrial anomaly, AUB-E).4 This classification system is essential for scientific and clinical progress in this area. Non-structural causes such as AUB-E require delineation of mechanism of action for treatment improvement, whereas structural causes require delineation of endometrial impact as a cause (or lack) of AUB. Only by gaining understanding of the above, can we then address the complexity of the presence of more than one cause of AUB in a single patient.
Advancement in our understanding of endometrial pathophysiology has been possible through use of modern cellular and molecular techniques, as well as animal models. This has facilitated investigation of therapeutic targets and new treatment options in the wider goal of improving precision for patient management.1
Definition, purpose, and validity of a pre-clinical model
Broadly, pre-clinical modelling is any experimental preparation in a laboratory setting which enables an in-depth investigation of a complex biological phenomenon.
Essential to the development of a pre-clinical model is the consideration of the model's intended purpose. This will establish validation requirements of the model. In the context of studying human menstrual disorders, i.e., AUB and endometrial function, a pre-clinical model must present the same normal physiological response to sex steroid hormones as is observed physiologically. To study endometrial cell-cell interactions, the model needs construction with endometrium-derived cells with preserved phenotype when extracted from their natural microenvironment. In other cases, the model requires the use of undifferentiated progenitor cells, and their phenotype must be induced in vitro to reflect the tissue of origin. In vitro models have advanced to offer sophisticated approaches to mimic normal endometrial physiology in 3D environments. This makes them a preferred method when studying cell interactions and function as their scientific potential lies in the deconvolution of the system and ease of manipulation. If the purpose of the model, however, is to explore physiological functions or systemic interactions between organs, whole organism models such as mouse or non-human primates may be better suited or complement the in vitro systems.
Uterine composition as a basis for designing a pre-clinical model to study AUB
The first step in designing a pre-clinical model to study AUB is an understanding of the individual components of the uterus and the endometrium.
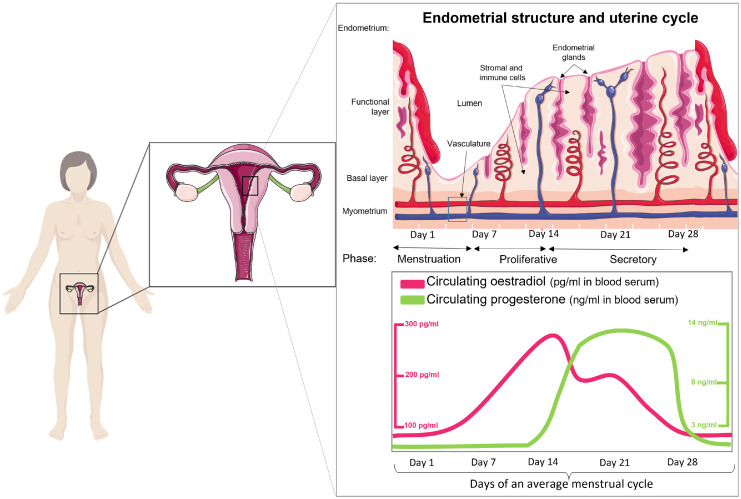
The uterus comprises the outer serosa, myometrium, and the endometrium. The myometrium is a smooth muscle cell multilayer that undergoes contraction during labour and is the site of the structural causes of AUB, adenomyosis and fibroids (also known as leiomyomata or leiomyomas).4 The endometrium is composed of two layers: the basal layer, containing progenitor cells, and a functional layer, which serves as the site for embryo implantation if pregnancy occurs (Figure 2, top). The function of both layers is regulated by circulating oestrogen and progesterone (Figure 2, bottom). In the absence of pregnancy, the functional layer of the endometrium sheds during menstruation and is then rapidly repaired through a complex sequence of events involving inflammation, angiogenesis, and tissue remodelling.1
Figure 2.
The menstrual cycle comprises three functionally distinct phases – proliferative, secretory, and menstrual, that are regulated by sex steroid hormones – oestrogen and progesterone.
The endometrium is a multicellular tissue comprising stromal, epithelial, endothelial, and immune cells including uterine natural killer cells (uNK), neutrophils and macrophages.1,5 Modern cellular techniques employ these endometrial cells for the development of several pre-clinical models to delineate endometrial pathophysiology.
Current pre-clinical models to study human endometrium and AUB
Human in vitro models
The most widely used in vitro models for the study of endometrial function include primary endometrial cell cultures (stromal and epithelial cells) and organotypic cultures of epithelial cells. These may be obtained from a human endometrial biopsy or menstrual fluid, or the non-invasive alternative of using immortalised endometrial cell lines. Endometrial cell culture models offer a unique opportunity to study functional differences between the different endometrial cell subpopulations under strictly modulated growth and hormone stimuli.
Primary endometrium-derived stromal cells (ESCs)
Isolation and culture of primary endometrial stromal cells is a one of the biggest advancements in the efforts of delineating endometrial physiology.
One of the earliest reports to describe the preparation of an endometrial stromal cell suspension was published in 1973, in which the authors used a tissue digestion medium with trypsin, collagenase, and dispase for the dissociation of rat endometrial cells from the whole tissue fragment.6 Soon after, the negative impact of trypsin on cell microtubules and cytoskeletal elements was demonstrated and another method was developed using manual endometrial scraping.7,8 The cell yield with the latter method, however, was significantly lower and the utility of the earlier described trypsin-free collagenase and dispase digestion medium has remained as the gold standard of human endometrial stromal cell isolation (Figure 3, Figure 4a).9,10 Over time, culture conditions have been optimised with growth factors and serum to reflect the natural microenvironment of the endometrium. Stromal cells respond to ovarian hormonal stimuli, and this has allowed for in vitro modelling of decidualisation.9 Literature on endometrial-derived stromal cells is now extensive with over 2300 publications in the last 10 years.
Figure 3.
Current standard endometrial cell isolation method. Enzymatic digestion was performed on an endometrial biopsy from consented individuals. To separate single cell suspension, containing stromal cells, innate immune cells, and endothelial cells, from epithelial glandular fragments, the digest was flushed through a 40 µm sieve. The single cell suspension was pelleted, and epithelial glandular fragments back flushed and pelleted separately. Only stromal cells remain attached to the dish, forming a monolayer monoculture.
Figure 4.
Models of endometrial stromal and epithelial cell cultures in isolation and in a co-culture. a) Following isolation of single cell suspension, containing stromal cells, innate immune cells, and endothelial cells, the cells were plated on a tissue culture petri dish, where only the stromal cells remain attached to the dish as they form a monolayer monoculture. b) The epithelial glandular fragments can be pelleted, re-suspended in culture medium, and plated on a tissue culture petri dish or embedded in a hydrogel droplet and cultured with a specialised medium to stimulate organoid formation.35,36 c) Epithelial cells are grown in a hydrogel to form organoids, while stromal cells are seeded and cultured in a Petri dish in a monolayer. When cells have reached confluence, they are lifted and combined as a co-culture within a hydrogel. d) Endometrial organ-on-a-chip system which can combine multiple endometrial cell types and create a complex endometrial microenvironment ex utero.
As primary cells, they are non-transformed and non-immortalised which enables for closer simulation of the molecular and biochemical processes to endometrial stromal cells in vivo. Primary ESCs are a widely used model for studying endometrial stromal-specific cell function, morphology, and differentiation.9,11, 12, 13, 14, 15 Understanding these processes establishes a more solid foundation for comparison with pathological cells and may elucidate therapeutic targets for AUB. For example, recent publications show their value for studying angiogenesis in vitro.16 Angiogenesis is the physiological process of vascular repair following menstruation. It is fundamental for adequate endometrial regeneration and its dysregulation may result in the symptom of AUB.17 This model also allows for ethical experimentation on human-derived tissue while reducing the need for animal models.
Nonetheless, utility of primary ESCs carries some limitations. Primary cells are derived from differentiated tissue biopsies and have finite cell passage capacity (reliably cultured for up to six consecutive passages) before they acquire senescent phenotype, leading to irreversible cell cycle arrest.18,19 This limits experimental time frames and requires constant availability of endometrial biopsies. Cells isolated from different donors may also behave differently, which requires increased sample size for each experiment. Finally, caution should be taken when obtaining results from stromal monolayer monocultures. These cells are extracted from their natural microenvironment, natural cues and cell-network communication are removed, and cells are grown on plastic or glass surface, which may alter their natural response to external influences such as hormonal treatments.
Secondary cell lines as a model for AUB due to endometrial malignancy or hyperplasia
Endometrial cell lines differ from primary cell culture as most are derived from endometrial adenocarcinomas. Cell lines are a widely accepted model to study cellular function in malignant endometrium in vitro. Most well-known endometrial epithelial cell lines include Ishikawa, derived from a 39-year-old Japanese patient with a well-differentiated tumour,20 and HEC-1-A and HEC-1-B, derived from a 71-year-old patient with a poorly differentiated tumour.21 The molecular characteristics of these cell lines represent the features of grade 1 and grade 2 endometrial cancers, respectively, making them suitable to investigate cellular mechanisms underpinning AUB, including when associated with malignancy - “AUB-M”.22,23 Both cell lines are oestrogen and progesterone responsive, which makes them ideal candidates for studies of steroid-dependent gene expression patterns, cell signalling pathways, and receptor activity.20,21 In addition, they have provided insights on the importance of adiponectin,24 fibronectin25 and bone morphogenetic protein (BMPs) signalling26 in endometrial cancer pathogenesis, as well as oestrogen-induced angiogenesis in adenomyosis development.27
Human endometrial stromal cell (HESC) lines are also available. Introduction of telomerase into cultured HESCs by transfection prevents the natural chromosomal telomere shortening during mitosis, making the HESCs immortal.28 The telomerase transfected HESCs (THESCs) present similar morphological and functional features as the primary parent stromal cells and have been useful in studying the role of UV light application as a treatment option for endometrial hyperplasia and the therapy's effect on endometrial cell apoptosis.28,29 While telomerase transfection does not alter the biochemical endpoints of cell decidualisation (e.g., increased production of prolactin, insulin growth factor binding protein-1, and fibronectin), it does decrease the quantity of these proteins detected in THESCs compared to primary HESCs.28
Due to their immortalised phenotype, cell lines provide unlimited supply of material, making them cost effective and easy to use. They also offer highly reproducible results and bypass the ethical concerns that come with experimentation on animal and human tissue.
Like other models, however, cell lines have limitations. To be immortalised, the cells undergo genetic modification that may also alter their native function and response to stimuli. Continuous passaging may also cause genetic variation and a drift from original phenotype; hence, they cannot adequately reflect primary cell behaviour. Cell lines also carry a higher risk of cross-contamination with other cell lines or mycoplasma.30 The HeLa cell line, isolated from an aggressive cervical adenocarcinoma, is the most frequent contaminator of mammalian cell lines including the human endometrial epithelial cell line HES.31,32 Thus, working with cell lines requires continuous diligence and cell authentication that ensures cell culture purity. Although they are a powerful tool in research, cell line results should ideally be confirmed with primary cell culture experiments.33
Primary endometrial gland-derived epithelial cells: a two-dimensional culture
Human endometrial epithelial cells can also be isolated from an endometrial biopsy following the same isolation procedure as primary stromal cells (Figure 3). The method was developed in 1970s and has been updated for maximum efficiency over succeeding years.34, 35, 36 The optimised technique uses trypsin-free digestion medium to obtain epithelial fragments and single cell suspension, which are separated using a 40 µm filter. While the stromal cell suspension flows through the filter, the epithelial glandular fragments remain on the filter until they are reverse washed. To eliminate contamination with stromal cells, the isolation technique includes a “selective attachment” step, which incubates the reverse washed cell suspension with serum-rich medium. During this step any stromal cells become attached to the petri dish, while the epithelial fragments are aspirated and plated in a separate synthetic extracellular matrix-coated cell culture plate (Figure 4b).37
Gland-derived endometrial epithelial cells (EECs) cultured in a two-dimensional system have been a valuable model for studying the regenerative capacity of the human endometrium. Cervelló et. al. reports that EEC cultures contain a unique somatic stem cell population hypothesised to be responsible for endometrial regeneration following menstruation.38 In addition, EECs have been invaluable for studying the cell molecular signature during the implantation window.39,40
Endometrial epithelial cells offer the same benefits to research as primary stromal cells but carry some major limitations. While the method incorporates techniques to limit presence of stromal cells, contamination is common and often stromal cell growth overtakes the epithelial cell cultures.37 In addition, two-dimensional (2D) in vitro EEC cultures do not reproduce the full complexity of the natural topographical environment that supports glandular function.41 Grown in 2D and on cell culture appropriate plastic ware, these EEC cultures degenerate after 10-15 days, making them unviable for passaging, culture propagation, and model standardisation.34 These cultures are also unresponsive to oestrogen and progesterone, making them unable to differentiate and incompetent for functional studies.34,35 Hence, new strategies were employed to create a three-dimensional (3D) culture system, which was transformative for the field – the development of epithelial organoids.
Primary endometrial gland-derived epithelial organoids: a three-dimensional culture
Following the identification of adult stem cells in variety of adult organs, in 2009 Sato et al described the generation of a 3D intestinal culture system (organoids) that has thereafter led to the establishment of organoids from multiple other tissues.42 Since 2009, over 8500 papers have been published that make use of organoids, demonstrating their applicability to a wide range of studies, including of the endometrium.43, 44, 45
Endometrial epithelial organoids (EEOs) are self-organising epithelial 3D culture systems that retain functional and morphological features of the endometrial glands in vitro, while allowing flexibility for experimental manipulation.43,44 For organoid development, isolated EECs are embedded into an extracellular matrix such as the commercially available Matrigel®, an Engelbreth-Holm-Swarm mouse sarcoma-derived gelatinous protein mixture (Figure 4b).43,44 The EECs contain populations of progenitor stem cells and differentiated epithelial cells. These cells are then cultured in basal medium supplemented with a cocktail of growth and signalling factors that support organoid formation, growth, and culture expansion.43,44,46 Recently, Cindrova-Davies et al, using similar methods as derivation from endometrial biopsies demonstrated that menstrual flow has potential as an alternative, non-invasive source of primary sample/tissue/effluent, offering a more accessible resource to establish endometrial epithelial organoid cultures.47
Three-dimensional EEO cultures are widely accepted to accurately represent functional and structural features of in vivo endometrial glands. The 3D model allows experimental observation of cell self-organisation, cell-cell communication, and cell-matrix interaction in a physiologically relevant environment in all three dimensions that has not been possible with two-dimensional alternatives. EEOs respond to hormonal stimulation in vitro to acquire secretory or ciliated differentiation, and allow the study of cell lineage relationships and the regenerative ability of epithelial cells within the endometrium.43,47,48
EEOs have also been used for endometrial cancer characterisation and drug-response studies.49,50 In such studies, EEOs were derived from pathological endometrium, including from uterine pathologies that present with AUB i.e., hyperplasia and carcinoma (AUB-M). These EEOs accurately reproduce endometrial cancer subtypes, mutational landscape, and demonstrate patient-specific treatment responses in vitro.51,52 The establishment of such pathological models allows direct comparison between malignant-derived organoids and normal tissue-derived organoids from the same patient, while eliminating the “noise” from the patient's biological background.51
Endometrial epithelial organoids have also advanced our understanding of the pathogenesis of adenomyosis, which commonly presents with the symptom of AUB (AUB-A). For example, disruption of cell polarity, the asymmetric organisation of cellular components, has been hypothesised as a key mechanism of adenomyosis pathogenesis.53 With the use of the EEO model, it was found that oestrogen could disrupt the apical-basal polarity of epithelial cells, which may lead to myometrial cell invasion and establishment of an adenomyosis lesion.53
Like other cell culture models, EEOs have limitations. Firstly, the total cost of cell culture is increased due to increased experimental time for epithelial cells to self-organise and grow into organoids, and the multiple medium reagents that are required. Secondly, this model only assesses epithelial cell-specific function and does not consider the influence that stromal or immune cells may have upon epithelial cell function. Next, embedding cells in extracellular matrix naturally favours the organoids to form with the apical side, carrying the microvilli and cilia, facing towards the lumen of the organoid, limiting the accessibility of cell secretions. The extracellular matrix is also a limiting factor, as the most widely used Matrigel® is not chemically defined, has batch-to-batch variability, and may itself interfere with epithelial cell function. Lastly, the model lacks automation which could be useful for controlled and gradual administration of hormones to mimic the ever-changing in vivo endometrial environment.
Recent advances in developing 3D cell culture methods have demonstrated the importance of the nature of the scaffold used for this in vitro model of endometrial epithelial glands. Natural and semi-natural hydrogels have gained strong interest as alternatives to Matrigel® for epithelial organoids and other endometrial cell populations. For example, both fibrin and collagen, of which gelatine is a derivative, are proteins that regulate angiogenesis, making these gels suitable for in vitro studies on endothelial cell function and neovessel formation.54,55 Increased angiogenesis has been identified in patients suffering from adenomyosis.56 In this context, the fibrin and collagen-derived hydrogels can be applied to studies that investigate the exact role of angiogenesis in the aetiology of adenomyosis and in relation to AUB. While these natural biomaterials are well-suited for some studies, they may have batch-to-batch variability; their stiffness and degradation kinetics are not tuneable; may present immunologic reaction in studies involving more complex culturing system; and can only be culture short-term.55,57
Cook et al have described a well-defined, synthetic hydrogel, based on polyethylene glycol (PEG) that provides reliability and reproducibility of experimental analysis comparable to the widely used Matrigel®. This PEG hydrogel is supplemented with specific peptides that allow for dynamic response to endometrial cell secretions and cell-specific local ECM modulation.58 By systematically varying the components of this hydrogel, Hernandez-Gordillo et al recognised that the incorporation of the collagen-derived peptide GFOGER contributes to the robust success of epithelial organoid growth and reproducibility of results, demonstrating the advantage of working with a chemically defined hydrogel. Hernandez-Gordillo et al also showed the applicability of this synthetic ECM to wider fields, including the generation of intestinal and endometrial epithelial organoids.59 Recently, Gnecco et al generated an endometrial stromal cell and epithelial organoid co-culture model in this PEG-based hydrogel [pre-print].60 By dissecting the molecular and phenotypic consequences of the cross talk between cells, the authors demonstrated that this gel can sustain the dynamic changes of the endometrial microenvironment and can be applied when studying uterine health and disease [pre-print].60
Endometrium-like stromal-epithelial co-culture models
The interdependence of stromal-epithelial interaction on endometrial function has been studied extensively through a 3D co-culture model, where both stromal and epithelial cells are embedded in a 3D matrix (Figure 4c). In vivo, aberration of intercellular communication may lead to endometrial dysregulation and the clinical complaint of AUB. Co-culture models are an essential tool for the investigation of cell communication. Key co-culture findings include the stromal oestrogen receptor mediation of epithelial mitogenesis,61 and the importance of stromal-epithelial crosstalk for epithelial secretion of metalloproteinases, which are essential for extracellular matrix remodelling during endometrial breakdown and regeneration.62 In addition, stromal-secreted decidualisation markers, for example, prolactin and IGFBP-1, also stimulate epithelial gland differentiation and secretion as demonstrated by co-culture systems.63
With the establishment of co-culture models, there has been an increasing interest in modelling gynaecological pathologies, such as adenomyosis, that presents with the symptom of AUB. Adenomyosis lesions have been difficult to model in a 2D environment due to their complex pathological processes and biological components. Co-culturing of endometrial stromal, epithelial, immune, and endothelial cells in combination with myometrial smooth muscle cells have enabled the generation of such a sophisticated model of adenomyosis lesion microenvironment in vitro. The prospects for applying such physiomimetic modelling to adenomyosis has been extensively reviewed by Gnecco et al in 2020.64
Co-culturing of endometrial cells can be a useful model to study cell-cell interactions in vitro, which could be perturbed in patients suffering with AUB. However, like other models, co-cultures can have key limitations that must be considered. Like the popularity of using Matrigel® for growing epithelial organoids, Matrigel® is also widely used for stromal-epithelial co-cultures. It has been previously reported that stromal cells embedded in Matrigel® lose their proliferative capability compared to stromal cells grown on plastic as a monolayer.65 In addition, the inversed cell polarity of epithelial organoids means there is limited access to cell secretions, as well as inability to generate a model of the lumenal portion of the endometrium to investigate cell function. Establishment of lumenal-only epithelium and stromal cell co-culture has been demonstrated using porous collagen scaffold. While this collagen-based scaffold preserved stromal and epithelial cell responsiveness to hormones, Abbas et al recognise that directing epithelial cells to form gland-like extension into the gel is still challenging.66 Further research efforts into the establishment of an improved and more physiological scaffold for stromal-epithelial co-cultures have led to the generation of a porcine-derived endometrial extracellular matrix (EndoECM).67 EndoECM is described to support stromal and epithelial cell co-culture, cell viability, ECM remodelling, and to improve stromal cell proliferation compared to collagen-based and Matrigel® co-cultures.67
Finally, tissue-derived organoids from kidney, stomach, and intestine have been used as a co-culture model in immunology studies of epithelial-immune cell interaction in tissue development, and has the prospect for applications in endometrial-specific research.68
Endometrial organ-on-a-chip system
A step towards more “near-to-in vivo” studies is the establishment of an organ-on-a-chip model based on a microfluidic system. A microfluidic chip is a polymer device with moulded or engraved micro channels and chambers, through which fluids are injected, directed, mixed, or manipulated before evacuating the chip. Different designs of microfluidic chips exist, presenting various possibilities for experimentation with cells, organoids, and/or co-cultures.
A microfluidic model of the female reproductive tract was described in 2017 revealing a powerful tool to study hormonal signalling as a phenocopy of the menstrual cycle.69 In addition, Gnecco et al designed a microfluidic model of endometrial perivascular stromal cells and observed cellular response to changing hormonal stimulation. After simulation of laminar perfusion and haemodynamic forces, typical of endometrial blood flow, they found increased endothelial cell secretion of prostanoids and enhanced perivascular decidualisation of stromal cells.70
Microfluidic models offer an unprecedented opportunity to study endometrial physiology that is not possible with other pre-clinical models. They are permeable to gas, and accommodate dynamic flow of media and hormonal gradients at a microscale. This allows for the use of minimal volume of cells, reagents, and chemicals, making the system cheaper than conventional cell culturing models. In addition, they can be automated, where minimal manual handling is applied, reducing the risks of human error. The users may also have high experimental control by setting multiple mechanical, optical, chemical, and electrical biosensors to simultaneously measure cell and tissue function in real-time. They offer faster reaction time, enhanced analytical sensitivity, portability, and do not require costly equipment.
This experimental model also presents some challenges. Re-locating cells from standard cell culture dishes to microfluidic chips require revision of culture protocols to account for the reduced medium volume and different polymer surfaces. There is growing evidence for differing cell responses between macro and micro cultures in consideration of change in growth surface physical properties, cell numbers, volume densities, nutrient consumption, and pH regulation.71 Next, the effect of structural abnormalities such as the presence of large uterine fibroids in vivo, cannot be studied using this model. Finally, the lack of adipose cells to absorb lipophilic chemicals may also alter the results in drug discovery experiments, which is particularly important when studying drug distribution. Similarly, the most widely used silicon-based polydimethylsiloxane (PDMS) to construct microfluidic devices can release uncured PDMS into the culture medium and can absorb lipophilic molecules, which alters the experimental results.72 Emerging technologies are addressing such fundamental issues and novel microfluidic platforms that become available have overcome some of these limitations.73
Human ex vivo model
Organotypic ex vivo models to study AUB and uterine fibroids (leiomyoma)
Another approach for studying AUB is the use of an ex vivo model, usually thinly cut whole tissue slices, mounted onto a porous membrane, and cultured under controlled conditions. This “in vivo-like” model, preserves the natural architecture, cellular diversity and networks, cell viability, systemic gene and protein expression patterns, and pathway activity of the tissue of origin.74 Often, this technique is employed for studying tumours of lung, prostate, gut, and breast, however, recent advancement also shows establishment of uterine fibroid (leiomyoma) organotypic cultures.75 Salas et al describe uterine leiomyoma slices cultured on an alginate scaffold that respond to hormonal stimuli and express genes associated with leiomyoma development. The alginate scaffold has been constructed to contain microspheres with encapsulated drug, which offers a novel approach to investigate patient-specific drug responses.75
The limitation of this model is accessibility to human-derived whole tissue biopsy and short culture maintenance of up to five days.74 Despise this, the potential of organotypic ex vivo constructs as a personalised pre-clinical model for cytotoxicity studies to targeted therapies is widely recognised.
Current pre-clinical models to study non-human endometrium and AUB
Non-human in vivo models of menstruation
The regulation and mechanism of menstruation is ideally studied in humans. However, human heterogeneity and the inevitable disruption of endometrial architecture during tissue sampling may limit findings. Animal models of menstruation provide genetic homogeneity and allow genetic, environmental, and pharmacological manipulation to determine causation.
Non-human primates
Non-human primates menstruate and undergo spontaneous endometrial decidualisation. Rhesus macaques have similar uterine morphology and length of menstrual cycle to humans.76 Both species display tightly co-ordinated spatial and temporal regulation of endometrial physiology at menstruation, e.g., increased MMPs and VEGF, vasoconstriction of spiral arterioles, and local tissue hypoxia.77,78 These observations are preceded by progesterone withdrawal.77,79,80 Macaques are reported to experience menstrual abnormalities (e.g. heavy menstrual bleeding) and may be fitted with tampons, and are excellent candidates for evaluating therapies for menstrual disorders.78 Despite menstruating naturally, macaques may undergo ovariectomy and treatment with oestrogen and progesterone to create artificial menstrual cycles and accurate timing of endometrial sampling. Use of this model requires large experimental groups and long experimental times, resulting in significant costs. Therefore, many researchers now preferentially use rodent models to study menstruation.
Mouse models of simulated menses
The mouse model of simulated menstruation was initially described in 198481 and further optimised in the 2000’s82 (Figure 5). Mice are ovariectomised and supplemented with exogenous oestrogen and progesterone to mimic the human hormonal endometrial environment. The model requires artificial induction of decidualisation, via a transcervical or surgical intrauterine injection of oil, reinforcing the importance of a decidualisation step prior to progesterone withdrawal in the physiology of menstruation. Once decidualisation has taken place, progesterone withdrawal results in histological and molecular changes analogous to those observed in the human endometrium at menstruation, with recruitment of leukocytes,83 shedding of the luminal endometrium, and visible menstrual-like bleeding.84 Endometrial tissue is then repaired and remodelled. Alternatively, simulation of menses may be achieved by inducing pseudopregnancy. In this model, female mice are mated with vasectomised males to mimic fertilisation events. Progesterone withdrawal may occur naturally, by ovariectomy, or by administration of a progesterone antagonist.85
Figure 5.
Mouse model of simulated menses. Ovariectomy is performed on female mice to deplete endogenous levels of ovarian hormones. After allowing 7 days for surgery recovery, mice are given daily subcutaneous injections of oestradiol (E2) for three days (days 1-3). Seven days after the first E2 injection, a progesterone (P4) implant is subcutaneously inserted (day 7) along with a lower dose of E2 (days 7-9). In order to trigger decidualisation, oil is transcervically administered at day 9. The removal of the P4 implant (t0) recapitulates menstrual events, with mice bleeding 8h after P4 withdrawal (t8). Twenty-four hours after P4 withdrawal (t24), endometrial regeneration events can be observed.81,82
Despite endocrine (e.g., a shorter length of cycle and lack of spontaneous decidualisation) and immunological differences between mice and humans,86 exogenous hormonal supplementation permits recapitulation of the events of human menstruation and decidualisation in this murine model.5,87,88 Consistent with findings in the human endometrium,89 the mouse model also has a critical period of progesterone withdrawal, where replacement of progesterone early after its withdrawal prevented menstrual-like bleeding and endometrial shedding. However, progesterone replacement at later time-points did not suppress menstruation.90 It is also a popular model to investigate the dynamics of endometrial breakdown and repair and has been used to manipulate inflammation91 and the endocrine environment92 at menstruation, and to pharmacologically and genetically alter key factors involved at menses, for example, hypoxia93,94
Xenograft mouse model
The xenograft mouse model provides an alternative model for in vivo examination of menstrual physiology and pathology.95, 96, 97 In this model, fragments of human functional endometrium are xenografted to ovariectomised, immune-deficient mice, most commonly the severe combined immunodeficiency (SCID) mouse. Treatment with oestrogen and progesterone followed by removal of ovarian steroids resulted in menstrual breakdown of the xenografted human endometrium. Xenograft menstruation studies have examined endometrial regeneration and the role of ovarian steroids in regulating this process.95,98 The advantages of this model are the ability to standardise ovarian hormone variations, manipulate the endometrium in a way that would be unethical in humans, and examine the local versus systemic leukocyte response by identification of human and mouse cell contributions. Limitations of the model include significant compromise of endometrial tissue architecture following transplantation, which may alter vascular and cellular responses. In addition, the necessary immunosuppressed state of the recipient mice may alter physiological inflammatory events in the menstrual endometrium. However, the commonly used SCID model aims to suppress T and B-cell mediated xenograft rejection without substantially affecting the innate immune response, and may be more relevant than other immunocompromised recipient mice.95
Spiny mouse
The common spiny mouse (Acomys cahirinus) is the only known rodent to display spontaneous decidualisation and natural menstruation, with menstrual duration of 3 days and frequency of 6-10 days.99,100 This rodent has previously been studied as an animal model for obesity and diabetes mellitus, and therefore, some laboratory reagents are currently available. The spiny mouse uterus is anatomically different to the human, but has physiological similarities, such as spiral arteriole remodelling in the perimenstrual phase.99 In addition, endometrial decidualisation is tightly controlled, not compromising the structural integrity of the endometrial glands or the myometrium, as observed in other rodent models. Like women, the spiny mouse produces cortisol as its circulating glucocorticoid, as opposed to corticosterone in standard laboratory mice. This rodent may permit study of multiple successive menstrual cycles and examination of any pre-conditioning effects that menstrual cycles will have on endometrial physiology. Disadvantages include the variability of natural cycles, the current inability to genetically manipulate the model, limiting definitive mechanistic studies, and lack of specific antibodies and molecular biological reagents (e.g., primers).
Non-human in vivo models of AUB
Adenomyosis models
Adenomyosis occurs spontaneously in different mammals, including non-human primates,101 mice, rats and rabbits. While these animal reports have proven to be useful for studying the development of adenomyosis, timing of onset and variability in the numbers affected limit use as a pre-clinical model for therapeutic intervention. In the 1980s, hormonal carcinogenic studies on mice revealed that exposure to certain endocrine-disrupting chemicals increased the risk of developing adenomyosis, alongside other uterine abnormalities such as cervical abnormalities, mammary carcinomas102 and endometriosis.103 Pre-natal oral exposure to diethylstilbestrol (DES),102 dioxins104 or phthalates105 generated adenomyosis. However, the incidence is strain-dependent, with DES-exposed CD-1 mice being protected from developing adenomyosis, but prone to uterine malignant tumours,106 and BALB/c mice showing complete protection from any uterine abnormalities.102 Post-natal DES administration also displayed varying results depending on administration route. For example, using the same mouse strain, intraperitoneal administration of DES generated adenomyosis,107 while subcutaneous administration showed development of uterine carcinomas.108 Therefore, induction of adenomyosis using endocrine-disrupting chemicals is limited by lack of specificity and a long induction time (6 to 20 months after treatment) that introduces variability with age.
One interesting finding in the DES model was the increased incidence of hyperprolactinemia observed in mice developing adenomyosis.102 This observation triggered the development of adenomyosis models relying on hyperprolactinemia replication via implantation of pituitary grafts from other mice or pharmacological induction with dopamine agonists, or selective serotonin reuptake inhibitors.109,110 Interestingly, the number of pituitary glands implanted, and the donor-recipient match/mismatch did not influence adenomyosis incidence.109 However, the site of implantation did affect incidence, with transvaginal111 or surgical uterine lumen implantation112 displaying increased adenomyosis rates than kidney capsule implantation.113,114 As in previous models, adenomyosis incidence was strongly strain-dependent, with BALB/c mice being protected compared to other strains.115 In contrast to the endocrine-disrupting chemical models, this pituitary grafting model displays a shorter endpoint (∼3 months) and has also been validated in rats.116 This model has contributed to research on novel therapeutics117,118 and the study of molecular aspects of adenomyosis.113,119 Limitations include the significant variability in adenomyosis induction rates, even within the same research group.114,120 In addition, specific induction of adenomyosis remains elusive, with co-development of other uterine abnormalities.112
The most popular current model of adenomyosis is the tamoxifen model.121 Oral administration of this selective oestrogen receptor modulator from day 2 to 5 after birth results in an adenomyosis induction rate of 100%.121 One of the reasons for its success is the short induction time of the lesions: at days 5-10 there is early evidence of adenomyosis121 and by day 42 from birth, the model is completely established.122 Despite its time advantage, the tamoxifen model presents similar limitations to the previous models, displaying strain and route of administration dependency with C57/BL6J mice protected123, and subcutaneous injection resulting in uterine carcinoma development.123
Very recently, Hao et al. have developed two mouse models of adenomyosis by disrupting the interface between the endometrium and the myometrium.123 This disruption is successfully achieved by either a mechanical or a thermal stimulus. With mechanical induction, a microcatheter is inserted into a uterine horn and the tip used to damage the endometrial-myometrial interface. For thermal induction, the microcatheter is attached to an electrosurgical scalpel, generating disruption by electrocoagulation. Although technically more complex, this model seems to be strain independent and provides an opportunity to adjust the severity of the lesions by increasing the damage generated.123
Uterine fibroid (Leiomyoma) models
Uterine fibroid (leiomyoma) formation in rodents occurs following exposure to endocrine-disrupting chemicals.124 Rat neonatal exposure to DES, bisphenol A,125 or tributyltin126 promotes the development of leiomyoma, as well as other uterine abnormalities such as glandular hyperplasia127 or adenomyosis.107 Additionally, some mouse strains also report leiomyoma incidence when prenatally or neonatally exposed to some of these compounds.128 Despite the non-specificity of their phenotype, these models are still in use for the exploration of potential treatments against leiomyoma formation and development.129 However, genetic models such as the Eker rat model have gained popularity.
Eker rats, which are defective for the tumour suppressor gene Tsc2, spontaneously develop uterine fibroids in approximately 65% of cases.130 Alterations in this gene are also observed in some women with leiomyoma.131 The course and composition of the lesions in rats are similar but not identical to human lesions.132 Eker rats have proven useful to study the hormonal influence on the development of leiomyomas.133 In mice, the Tsc2 mutation phenotype is not replicated but mutations in the Med12 gene do generate leiomyomas.134 This gene is affected in up to 80% of women with leiomyoma,135 offering another potential model of genetic predisposition. The most common critique for both these genetic models is that a single mutation may oversimplify this highly complex uterine condition. As a refinement option, the Eker rat model can be combined with endocrine-disrupting chemicals. Neonatal exposure to DES in Eker rats significantly increased leiomyoma formation from 65% to a 100%.136 This combined model has been used to study the molecular triggers of uterine leiomyoma development as well as potential treatments.137
The mouse xenograft model is an established pre-clinical model for developing novel therapeutics to suppress leiomyoma growth.138,139 In the most conventional model, leiomyoma tissue derived from patients is cut in sections or disaggregated and further implanted on different immunosuppressed mice strains. The preferred graft location tends to be subcutaneous140, although some reports suggest that sub-renal implantation displays better engraftment.141 Intrauterine transplantation has also been tested but displays less leiomyoma formation than other routes.142 Tissue sections and freshly isolated cells derived from tissue disaggregation display similar reproducibility and level of engraftment.143 Selection of recipient mice remains controversial, with some studies claiming that the optimal engraftment is achieved using SCID mice,132 and other reports favour lymphocyte T, B, and NK cells-deficient mice (NOD/SCID mice).141 The xenograft model has also been tested on rats where, rather than generating a genetic immunodeficiency, animals are treated with mycophenolate mofetil to avoid transplant rejection.144 In every model, hormonal supplementation is essential for graft survival, with administration of both oestrogen and progesterone displaying the best results.143 This xenograft model has been used to transfect tissue (or cells) with plasmids,145 as well as microRNAs to study the impact on their development.146 Very recently, a new xenograft model was validated, where human leiomyoma cell lines were 3D-cultured prior to subcutaneous implantation in immunosuppressed mice.147 This removes the requirement for fresh tissue samples, reduces donor intra-variability, and showed longer graft survival (over 8 weeks).132
Outstanding questions
The evolution of in vitro models of endometrial-derived, multi-cellular, and three-dimensional constructs have shown their potential for uncovering the function of individual cell types, inter-cellular interaction, and cell interaction with its microenvironment. The advancement of the complexity of these cultures has served as a proof-of-concept that establishment of an in vivo-like endometrial microenvironment is possible. Forward-looking, modern gene and protein discovery techniques can now begin to describe in a non-biased manner the key cellular and molecular responses of the uterus that result in structural and non-structural causes of AUB. Non-structural causes such as AUB-E require delineation of mechanism of action for treatment improvement, whereas structural causes such as uterine fibroids require delineation of endometrial impact as a cause (or lack) of fibroid-associated AUB and vice versa. Gaining such understanding can be possible with the emerging in vitro models, which can help address the complexity of the presence of more than one cause of AUB in a single patient. In addition, in vivo models are still necessary to study the organ-to-organ interactions which may equally impact normal and abnormal uterine function.
Concluding remarks
Pre-clinical models have significantly advanced our understanding of endometrial function and AUB. While every pre-clinical model offers research advantages, each has specific limitations as discussed. For this reason, careful consideration and selection criteria are required when choosing the right model system or combination of models for a specific research question. Correct research approaches can answer scientific questions, lead to therapeutic target identification/validation and inform clinical trial design. It is an exciting time for research in the reproductive field and recent advancements have the potential to identify new approaches to ameliorate abnormal uterine bleeding (AUB).
Search strategy and selection criteria
Data for this Review were identified by searches of the National Institute for Health and Care Excellence website, PubMed, Scopus, ScienceDirect and references from relevant articles using search terms “abnormal uterine bleeding”, “endometrial stromal/epithelial cell cultures”, “endometrial organoids”, “endometrial co-cultures”, “microfluidic”, “adenomyosis”, “leiomyoma “, and “models, animals”. Boolean operator “AND” and truncation strategies were used. Only articles published in English were included with focus on publications between 2015 and 2022. There are several exclusions where description of model development is discussed, and publication year precedes this period.
Contributions
All authors made significant contributions to manuscript preparation and read and approved the final versions and revisions of the manuscript.
Aleksandra O Tsolova: literature search, conceptualisation, figures, writing – original draft, and writing – review & editing.
Rocío Martínez Aguilar: literature search, figures, writing – original draft, and writing – review & editing.
Jacqueline A Maybin: writing – original draft, and writing – review & editing.
Hilary OD Critchley: conceptualisation, original draft; writing — review & editing.
Funding
Hilary OD Critchley receives support from the Medical Research Council Centre for Reproductive Health (MRC CRH) Grant MR/N022556/1; and from Biotechnology and Biological Sciences Research Council (BBSRC; BB/S002995/1)
Aleksandra O Tsolova receives support from the Medical Research Council Centre for Reproductive Health (MRC CRH) Grant MR/N022556/1 and student grant MR/P502030/1
Jacqueline A Maybin receives support from Wellcome Trust Fellowship 209589/Z/17/Z and from the Royal Society of Edinburgh 1077.
Rocío Martínez Aguilar receives salary support from Wellcome Trust Grant 209589/Z/17/Z.
The funders had no role in manuscript design, data collection, data analysis, interpretation, writing of this manuscript.
Declaration of interests
Hilary Critchley (HC) has received clinical research support for laboratory consumables and staff from Bayer AG and provides consultancy advice (paid to Institution) for Bayer AG, Gedeon Richter, Vifor Pharma UK Ltd,; Myovant Sciences GmbH. HC has received royalties from UpToDate for article on abnormal uterine bleeding.
Jacqueline A Maybin receives Wellcome Trust Clinical Research Career Development Fellowship (salary support) - To institution; Tenovus Scotland - To institution; Royal Society of Edinburgh - To institution; Wellbeing of Women - To institution.
Acknowledgments
Authors wish to thank Tatiana White, MRC Centre for Reproductive Health, University of Edinburgh for assistance with finalisation of manuscript preparation and submission.
References
- 1.Critchley Maybin JA, Armstrong GM, Williams ARW. Physiology of the endometrium and regulation of menstruation. Physiol Rev. 2020;100(3):1149–1179. doi: 10.1152/physrev.00031.2019. [DOI] [PubMed] [Google Scholar]
- 2.Fraser IS, Critchley HO, Munro MG, Broder M. Can we achieve international agreement on terminologies and definitions used to describe abnormalities of menstrual bleeding? Hum Reprod. 2007;22(3):635–643. doi: 10.1093/humrep/del478. [DOI] [PubMed] [Google Scholar]
- 3.Munro MG, Critchley HO, Fraser IS. The FIGO classification of causes of abnormal uterine bleeding: malcolm G. Munro, Hilary O.D. Crithcley, Ian S. Fraser, for the FIGO working group on menstrual disorders. Int J Gynaecol Obstet. 2011;113(1):1–2. doi: 10.1016/j.ijgo.2011.01.001. [DOI] [PubMed] [Google Scholar]
- 4.Munro MG, Critchley HOD, Fraser IS, Committee tFMD Corrigendum to “The two FIGO systems for normal and abnormal uterine bleeding symptoms and classification of causes of abnormal uterine bleeding in the reproductive years: 2018 revisions” [Int J Gynecol Obstet 143(2018) 393–408] Int J Gynecol Obstetr. 2018;144(2):237. doi: 10.1002/ijgo.12709. [DOI] [PubMed] [Google Scholar]
- 5.Armstrong GM, Maybin JA, Murray AA, et al. Endometrial apoptosis and neutrophil infiltration during menstruation exhibits spatial and temporal dynamics that are recapitulated in a mouse model. Sci Rep. 2017;7(1):17416. doi: 10.1038/s41598-017-17565-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Williams D, Gorski J. Preparation and characterization of free cell suspensions from the immature rat uterus. Biochemistry. 1973;12(2):297–306. doi: 10.1021/bi00726a019. [DOI] [PubMed] [Google Scholar]
- 7.Revel JP, Hoch P, Ho D. Adhesion of culture cells to their substratum. Exp Cell Res. 1974;84(1):207–218. doi: 10.1016/0014-4827(74)90398-x. [DOI] [PubMed] [Google Scholar]
- 8.Pietras RJ, Szego CM. Steroid hormone-responsive, isolated endometrial cells. Endocrinology. 1975;96(4):946–954. doi: 10.1210/endo-96-4-946. [DOI] [PubMed] [Google Scholar]
- 9.Irwin JC, Kirk D, King RJ, Quigley MM, Gwatkin RB. Hormonal regulation of human endometrial stromal cells in culture: an in vitro model for decidualization. Fertil Steril. 1989;52(5):761–768. doi: 10.1016/s0015-0282(16)61028-2. [DOI] [PubMed] [Google Scholar]
- 10.Tseng L. Effect of estradiol and progesterone on human endometrial aromatase activity in primary cell culture. Endocrinology. 1984;115(2):833–835. doi: 10.1210/endo-115-2-833. [DOI] [PubMed] [Google Scholar]
- 11.Bell SC, Jackson JA, Ashmore J, Zhu HH, Tseng L. Regulation of insulin-like growth factor-binding protein-1 synthesis and secretion by progestin and relaxin in long term cultures of human endometrial stromal cells. J Clin Endocrinol Metab. 1991;72(5):1014–1024. doi: 10.1210/jcem-72-5-1014. [DOI] [PubMed] [Google Scholar]
- 12.Evron A, Goldman S, Shalev E. Effect of primary human endometrial stromal cells on epithelial cell receptivity and protein expression is dependent on menstrual cycle stage. Human Reproduction (Oxford) 2011;26(1):176–190. doi: 10.1093/humrep/deq296. [DOI] [PubMed] [Google Scholar]
- 13.Huang JR, Tseng L, Bischof P, Jänne OA. Regulation of prolactin production by progestin, estrogen, and relaxin in human endometrial stromal cells. Endocrinology. 1987;121(6):2011–2017. doi: 10.1210/endo-121-6-2011. [DOI] [PubMed] [Google Scholar]
- 14.Tseng L. Effect of estradiol and progesterone on human endometrial aromatase activity in primary cell culture. Endocrinology. 1984;115(2):833–835. doi: 10.1210/endo-115-2-833. [DOI] [PubMed] [Google Scholar]
- 15.Zhu HH, Huang JR, Mazela J, Elias J, Tseng L. Progestin stimulates the biosynthesis of fibronectin and accumulation of fibronectin mRNA in human endometrial stromal cells. Hum Reprod. 1992;7(2):141–146. doi: 10.1093/oxfordjournals.humrep.a137606. [DOI] [PubMed] [Google Scholar]
- 16.Qi QR, Lechuga TJ, Patel B, et al. Enhanced stromal Cell CBS-H2S production promotes estrogen-stimulated human endometrial angiogenesis. Endocrinology. 2020;161(11):1–14. doi: 10.1210/endocr/bqaa176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Maybin J, Critchley H. Repair and regeneration of the human endometrium. Expert Rev Obstetr Gynecol. 2009;4(3):283–298. [Google Scholar]
- 18.Campisi J, d'Adda di Fagagna F. Cellular senescence: when bad things happen to good cells. Nat Rev Mol Cell Biol. 2007;8(9):729–740. doi: 10.1038/nrm2233. [DOI] [PubMed] [Google Scholar]
- 19.Queckbörner S, Syk Lundberg E, Gemzell-Danielsson K, Davies LC. Endometrial stromal cells exhibit a distinct phenotypic and immunomodulatory profile. Stem Cell Res Therapy. 2020;11(1):15. doi: 10.1186/s13287-019-1496-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Nishida M, Kasahara K, Kaneko M, Iwasaki H, Hayashi K. Establishment of a new human endometrial adenocarcinoma cell line, Ishikawa cells, containing estrogen and progesterone receptors. Nihon Sanka Fujinka Gakkai Zasshi. 1985;37(7):1103–1111. [PubMed] [Google Scholar]
- 21.Kurarmoto H, Hamano M, Imai M. HEC-1 cells. Hum Cell. 2002;15(2):81–95. doi: 10.1111/j.1749-0774.2002.tb00103.x. [DOI] [PubMed] [Google Scholar]
- 22.Reno EM, Haughian JM, Dimitrova IK, Jackson TA, Shroyer KR, Bradford AP. Analysis of protein kinase C delta (PKC delta) expression in endometrial tumors. Hum Pathol. 2008;39(1):21–29. doi: 10.1016/j.humpath.2007.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Albitar L, Pickett G, Morgan M, Davies S, Leslie KK. Models representing type I and type II human endometrial cancers: Ishikawa H and Hec50co cells. Gynecol Oncol. 2007;106(1):52–64. doi: 10.1016/j.ygyno.2007.02.033. [DOI] [PubMed] [Google Scholar]
- 24.Ciortea R, Şuşman S, Măluţan AM, et al. Mesenchymal stem cells derived from adipose tissue and Ishikawa cells co-culture highlight the role of adiponectin in endometrial cancer pathogenesis. Rom J Morphol Embryol. 2018;59(4):1165–1172. [PubMed] [Google Scholar]
- 25.Yoshida S, Asanoma K, Yagi H, et al. Fibronectin mediates activation of stromal fibroblasts by SPARC in endometrial cancer cells. BMC Cancer. 2021;21(1):156. doi: 10.1186/s12885-021-07875-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Fukuda T, Fukuda R, Miyazono K, Heldin CH. Tumor promoting effect of BMP signaling in endometrial cancer. Int J Mol Sci. 2021;22(15) doi: 10.3390/ijms22157882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Huang TS, Chen YJ, Chou TY, et al. Oestrogen-induced angiogenesis promotes adenomyosis by activating the Slug-VEGF axis in endometrial epithelial cells. J Cell Mol Med. 2014;18(7):1358–1371. doi: 10.1111/jcmm.12300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Krikun G, Mor G, Alvero A, et al. A novel immortalized human endometrial stromal cell line with normal progestational response. Endocrinology. 2004;145(5):2291–2296. doi: 10.1210/en.2003-1606. [DOI] [PubMed] [Google Scholar]
- 29.Stanojevic-Pirkovic M, Andelkovic M, Lukovic J, et al. UV irradiation induces apoptosis in the human endometrial stromal cell line (ThESC) J BUON. 2020;25(3):1541–1546. [PubMed] [Google Scholar]
- 30.Capes-Davis A, Theodosopoulos G, Atkin I, et al. Check your cultures! A list of cross-contaminated or misidentified cell lines. Int J Cancer. 2010;127(1):1–8. doi: 10.1002/ijc.25242. [DOI] [PubMed] [Google Scholar]
- 31.Lucey BP, WA Nelson-Rees, Hutchins GM. Henrietta Lacks, HeLa cells, and cell culture contamination. Arch Pathol Lab Med. 2009;133(9):1463–1467. doi: 10.5858/133.9.1463. [DOI] [PubMed] [Google Scholar]
- 32.Kniss DA, Summerfield TL. Discovery of HeLa Cell Contamination in HES cells: call for cell line authentication in reproductive biology research. Reproduct Sci. 2014;21(8):1015–1019. doi: 10.1177/1933719114522518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kaur G, Dufour JM. Cell lines: valuable tools or useless artifacts. Spermatogenesis. 2012;2(1):1–5. doi: 10.4161/spmg.19885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kirk D, King RJ, Heyes J, Peachey L, Hirsch PJ, Taylor RW. Normal human endometrium in cell culture. I. Separation and characterization of epithelial and stromal components in vitro. In Vitro. 1978;14(8):651–662. doi: 10.1007/BF02616162. [DOI] [PubMed] [Google Scholar]
- 35.Kirk D, Irwin JC. Normal human endometrium in cell culture. Methods Cell Biol. 1980;21b:51–77. doi: 10.1016/s0091-679x(08)60678-0. [DOI] [PubMed] [Google Scholar]
- 36.Zhang L, Rees MC, Bicknell R. The isolation and long-term culture of normal human endometrial epithelium and stroma. Expression of mRNAs for angiogenic polypeptides basally and on oestrogen and progesterone challenges. J Cell Sci. 1995;108(Pt 1):323–331. doi: 10.1242/jcs.108.1.323. [DOI] [PubMed] [Google Scholar]
- 37.Chen JC, Johnson BA, Erikson DW, et al. Seminal plasma induces global transcriptomic changes associated with cell migration, proliferation and viability in endometrial epithelial cells and stromal fibroblasts. Human Reproduction. 2014;6(29):1255–1270. doi: 10.1093/humrep/deu047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Cervelló I, Gil-Sanchis C, Mas A, et al. Human endometrial side population cells exhibit genotypic, phenotypic and functional features of somatic stem cells. PLoS One. 2010;5(6):e10964. doi: 10.1371/journal.pone.0010964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Dominguez F, Gadea B, Mercader A, Esteban FJ, Pellicer A, Simón C. Embryologic outcome and secretome profile of implanted blastocysts obtained after coculture in human endometrial epithelial cells versus the sequential system. Fertil Steril. 2010;93(3):774–782.e1. doi: 10.1016/j.fertnstert.2008.10.019. [DOI] [PubMed] [Google Scholar]
- 40.Evans GE, Martínez-Conejero JA, Phillipson GT, et al. Gene and protein expression signature of endometrial glandular and stromal compartments during the window of implantation. Fertil Steril. 2012;97(6):1365–1373. doi: 10.1016/j.fertnstert.2012.03.007. e1-2. [DOI] [PubMed] [Google Scholar]
- 41.Baker BM, Chen CS. Deconstructing the third dimension – how 3D culture microenvironments alter cellular cues. J Cell Sci. 2012;125(13):3015–3024. doi: 10.1242/jcs.079509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sato T, Vries RG, Snippert HJ, et al. Single Lgr5 stem cells build crypt-villus structures in vitro without a mesenchymal niche. Nature. 2009;459(7244):262–265. doi: 10.1038/nature07935. [DOI] [PubMed] [Google Scholar]
- 43.Turco MY, Gardner L, Hughes J, et al. Long-term, hormone-responsive organoid cultures of human endometrium in a chemically defined medium. Nat Cell Biol. 2017;19(5):568–577. doi: 10.1038/ncb3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boretto M, Cox B, Noben M, et al. Development of organoids from mouse and human endometrium showing endometrial epithelium physiology and long-term expandability. Development. 2017;144(10):1775–1786. doi: 10.1242/dev.148478. [DOI] [PubMed] [Google Scholar]
- 45.Fujii M, Sato T. Somatic cell-derived organoids as prototypes of human epithelial tissues and diseases. Nat Mater. 2021;20(2):156–169. doi: 10.1038/s41563-020-0754-0. [DOI] [PubMed] [Google Scholar]
- 46.Gu Z-Y, Jia S-Z, Liu S, Leng J-H. Endometrial organoids: a new model for the research of endometrial-related diseases†. Biol Reprod. 2020;103(5):918–926. doi: 10.1093/biolre/ioaa124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cindrova-Davies T, Zhao X, Elder K, et al. Menstrual flow as a non-invasive source of endometrial organoids. Commun Biol. 2021;4(1):651. doi: 10.1038/s42003-021-02194-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Garcia-Alonso L, Handfield L-F, Roberts K, et al. Mapping the temporal and spatial dynamics of the human endometrium in vivo and in vitro. Nat Genet. 2021;53(12):1698–1711. doi: 10.1038/s41588-021-00972-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.van de Wetering M, Francies HE, Francis JM, et al. Prospective derivation of a living organoid biobank of colorectal cancer patients. Cell. 2015;161(4):933–945. doi: 10.1016/j.cell.2015.03.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sachs N, de Ligt J, Kopper O, et al. A living biobank of breast cancer organoids captures disease heterogeneity. Cell. 2018;172(1):373–386. doi: 10.1016/j.cell.2017.11.010. e10. [DOI] [PubMed] [Google Scholar]
- 51.Boretto M, Maenhoudt N, Luo X, et al. Patient-derived organoids from endometrial disease capture clinical heterogeneity and are amenable to drug screening. Nat Cell Biol. 2019;21(8):1041–1051. doi: 10.1038/s41556-019-0360-z. [DOI] [PubMed] [Google Scholar]
- 52.Maru Y, Tanaka N, Itami M, Hippo Y. Efficient use of patient-derived organoids as a preclinical model for gynecologic tumors. Gynecol Oncol. 2019;154(1):189–198. doi: 10.1016/j.ygyno.2019.05.005. [DOI] [PubMed] [Google Scholar]
- 53.Jin Z, Liu H, Xu C. Estrogen degrades Scribble in endometrial epithelial cells through E3 ubiquitin ligase HECW1 in the development of diffuse adenomyosis†. Biol Reprod. 2020;102(2):376–387. doi: 10.1093/biolre/ioz194. [DOI] [PubMed] [Google Scholar]
- 54.Kroon ME, van Schie ML, van der Vecht B, van Hinsbergh VW, Koolwijk P. Collagen type 1 retards tube formation by human microvascular endothelial cells in a fibrin matrix. Angiogenesis. 2002;5(4):257–265. doi: 10.1023/a:1024540701634. [DOI] [PubMed] [Google Scholar]
- 55.Zambuto SG, Clancy KBH, Harley BAC. A gelatin hydrogel to study endometrial angiogenesis and trophoblast invasion. Interface Focus. 2019;9(5) doi: 10.1098/rsfs.2019.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Harmsen MJ, Wong CFC, Mijatovic V, et al. Role of angiogenesis in adenomyosis-associated abnormal uterine bleeding and subfertility: a systematic review. Hum Reprod Update. 2019;25(5):647–671. doi: 10.1093/humupd/dmz024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Calderon GA, Thai P, Hsu CW, et al. Tubulogenesis of co-cultured human iPS-derived endothelial cells and human mesenchymal stem cells in fibrin and gelatin methacrylate gels. Biomaterials Science. 2017;5(8):1652–1660. doi: 10.1039/c7bm00223h. [DOI] [PubMed] [Google Scholar]
- 58.Cook CD, Hill AS, Guo M, et al. Local remodeling of synthetic extracellular matrix microenvironments by co-cultured endometrial epithelial and stromal cells enables long-term dynamic physiological function. Integr Biol (Camb) 2017;9(4):271–289. doi: 10.1039/c6ib00245e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hernandez-Gordillo V, Kassis T, et al. Fully synthetic matrices for in vitro culture of primary human intestinal enteroids and endometrial organoids. Biomaterials. 2020;254 doi: 10.1016/j.biomaterials.2020.120125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gnecco JS, Brown A, Buttrey K, et al. Organoid co-culture model of the cycling human endometrium in a fully-defined synthetic extracellular matrix reveals epithelial-stromal crosstalk. bioRxiv. 2022:2021.09.30.462577. [pre-print] [DOI] [PMC free article] [PubMed]
- 61.Cooke PS, Buchanan DL, Young P, et al. Stromal estrogen receptors mediate mitogenic effects of estradiol on uterine epithelium. Proc Natl Acad Sci U S A. 1997;94(12):6535–6540. doi: 10.1073/pnas.94.12.6535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Osteen KG, Rodgers WH, Gaire M, Hargrove JT, Gorstein F, Matrisian LM. Stromal-epithelial interaction mediates steroidal regulation of metalloproteinase expression in human endometrium. Proc Natl Acad Sci U S A. 1994;91(21):10129–10133. doi: 10.1073/pnas.91.21.10129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Maslar IA, Riddick DH. Prolactin production by human endometrium during the normal menstrual cycle. Am J Obstet Gynecol. 1979;135(6):751–754. doi: 10.1016/0002-9378(79)90386-7. [DOI] [PubMed] [Google Scholar]
- 64.Gnecco JS, Brown AT, Kan EL, et al. Physiomimetic Models of Adenomyosis. Semin Reprod Med. 2020;38(2-03):179–196. doi: 10.1055/s-0040-1719084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Arnold JT, Kaufman DG, Seppälä M, Lessey BA. Endometrial stromal cells regulate epithelial cell growth in vitro: a new co-culture model. Hum Reprod. 2001;16(5):836–845. doi: 10.1093/humrep/16.5.836. [DOI] [PubMed] [Google Scholar]
- 66.Abbas Y, Brunel LG, Hollinshead MS, et al. Generation of a three-dimensional collagen scaffold-based model of the human endometrium. Interface Focus. 2020;10(2) doi: 10.1098/rsfs.2019.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.López-Martínez S, Campo H, de Miguel-Gómez L, et al. A natural xenogeneic endometrial extracellular matrix hydrogel toward improving current human in vitro models and future in vivo applications. Front Bioeng Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.639688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Bar-Ephraim YE, Kretzschmar K, Clevers H. Organoids in immunological research. Nat Rev Immunol. 2020;20(5):279–293. doi: 10.1038/s41577-019-0248-y. [DOI] [PubMed] [Google Scholar]
- 69.Xiao S, Coppeta JR, Rogers HB, et al. A microfluidic culture model of the human reproductive tract and 28-day menstrual cycle. Nat Commun. 2017;8:14584. doi: 10.1038/ncomms14584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Gnecco JS, Ding T, Smith C, Lu J, Bruner-Tran KL, Osteen KG. Hemodynamic forces enhance decidualization via endothelial-derived prostaglandin E2 and prostacyclin in a microfluidic model of the human endometrium. Hum Reprod. 2019;34(4):702–714. doi: 10.1093/humrep/dez003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Halldorsson S, Lucumi E, Gómez-Sjöberg R, Fleming RMT. Advantages and challenges of microfluidic cell culture in polydimethylsiloxane devices. Biosens Bioelectron. 2015;63:218–231. doi: 10.1016/j.bios.2014.07.029. [DOI] [PubMed] [Google Scholar]
- 72.Regehr KJ, Domenech M, Koepsel JT, et al. Biological implications of polydimethylsiloxane-based microfluidic cell culture. Lab Chip. 2009;9(15):2132–2139. doi: 10.1039/b903043c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Edington CD, Chen WLK, Geishecker E, et al. Interconnected microphysiological systems for quantitative biology and pharmacology studies. Sci Rep. 2018;8(1):4530. doi: 10.1038/s41598-018-22749-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Vaira V, Fedele G, Pyne S, et al. Preclinical model of organotypic culture for pharmacodynamic profiling of human tumors. Proc Natl Acad Sci. 2010;107(18):8352–8356. doi: 10.1073/pnas.0907676107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Salas A, López J, Reyes R, et al. Organotypic culture as a research and preclinical model to study uterine leiomyomas. Sci Rep. 2020;10(1):5212. doi: 10.1038/s41598-020-62158-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Brenner RM, Slayden OD. Molecular and functional aspects of menstruation in the macaque. Rev Endocr Metab Disord. 2012;13(4):309–318. doi: 10.1007/s11154-012-9225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Nayak NR, Critchley HO, Slayden OD, et al. Progesterone withdrawal up-regulates vascular endothelial growth factor receptor type 2 in the superficial zone stroma of the human and macaque endometrium: potential relevance to menstruation. J Clin Endocrinol Metab. 2000;85(9):3442–3452. doi: 10.1210/jcem.85.9.6769. [DOI] [PubMed] [Google Scholar]
- 78.Brenner RM, Slayden OD. Molecular and functional aspects of menstruation in the macaque. Rev Endocr Metab Disord. 2012;13(4):309–318. doi: 10.1007/s11154-012-9225-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Slayden OD, Nayak NR, Burton KA, et al. Progesterone antagonists increase androgen receptor expression in the rhesus macaque and human endometrium. J Clin Endocrinol Metab. 2001;86(6):2668–2679. doi: 10.1210/jcem.86.6.7606. [DOI] [PubMed] [Google Scholar]
- 80.Slayden OD, Brenner RM. A critical period of progesterone withdrawal precedes menstruation in macaques. Reprod Biol Endocrinol. 2006;4(Suppl 1):S6. doi: 10.1186/1477-7827-4-S1-S6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Finn CA, Pope M. Vascular and cellular changes in the decidualized endometrium of the ovariectomized mouse following cessation of hormone treatment: a possible model for menstruation. J Endocrinol. 1984;100(3):295–300. doi: 10.1677/joe.0.1000295. [DOI] [PubMed] [Google Scholar]
- 82.Brasted M, White CA, Kennedy TG, Salamonsen LA. Mimicking the events of menstruation in the murine uterus. Biol Reprod. 2003;69(4):1273–1280. doi: 10.1095/biolreprod.103.016550. [DOI] [PubMed] [Google Scholar]
- 83.Menning A, Walter A, Rudolph M, Gashaw I, Fritzemeier KH, Roese L. Granulocytes and vascularization regulate uterine bleeding and tissue remodeling in a mouse menstruation model. PLoS One. 2012;7(8):e41800. doi: 10.1371/journal.pone.0041800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cousins FL, Murray A, Esnal A, Gibson DA, Critchley HO, Saunders PT. Evidence from a mouse model that epithelial cell migration and mesenchymal-epithelial transition contribute to rapid restoration of uterine tissue integrity during menstruation. PLoS One. 2014;9(1):e86378. doi: 10.1371/journal.pone.0086378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Rudolph M, Docke WD, Muller A, et al. Induction of overt menstruation in intact mice. PLoS One. 2012;7(3):e32922. doi: 10.1371/journal.pone.0032922. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Mestas J, Hughes CCW. Of mice and not men: differences between mouse and human immunology. J Immunol. 2004;172(5):2731. doi: 10.4049/jimmunol.172.5.2731. [DOI] [PubMed] [Google Scholar]
- 87.Evans J, Kaitu'u-Lino T, Salamonsen LA. Extracellular matrix dynamics in scar-free endometrial repair: perspectives from mouse in vivo and human in vitro studies. Biol Reprod. 2011;85(3):511–523. doi: 10.1095/biolreprod.111.090993. [DOI] [PubMed] [Google Scholar]
- 88.Cousins FL, Kirkwood PM, Saunders PT, Gibson DA. Evidence for a dynamic role for mononuclear phagocytes during endometrial repair and remodelling. Sci Rep. 2016;6:36748. doi: 10.1038/srep36748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Kelly RW, King AE, Critchley HO. Cytokine control in human endometrium. Reproduction. 2001;121(1):3–19. doi: 10.1530/rep.0.1210003. [DOI] [PubMed] [Google Scholar]
- 90.Wang Q, Xu X, He B, Li Y, Chen X, Wang J. A critical period of progesterone withdrawal precedes endometrial breakdown and shedding in mouse menstrual-like model. Hum Reprod. 2013;28(6):1670–1678. doi: 10.1093/humrep/det052. [DOI] [PubMed] [Google Scholar]
- 91.Kaitu'u-Lino TJ, Morison NB, Salamonsen LA. Neutrophil depletion retards endometrial repair in a mouse model. Cell Tissue Res. 2007;328(1):197–206. doi: 10.1007/s00441-006-0358-2. [DOI] [PubMed] [Google Scholar]
- 92.Kaitu'u-Lino TJ, Morison NB, Salamonsen LA. Estrogen is not essential for full endometrial restoration after breakdown: lessons from a mouse model. Endocrinology. 2007;148(10):5105–5111. doi: 10.1210/en.2007-0716. [DOI] [PubMed] [Google Scholar]
- 93.Fan X, Krieg S, Kuo CJ, et al. VEGF blockade inhibits angiogenesis and reepithelialization of endometrium. FASEB J. 2008;22(10):3571–3580. doi: 10.1096/fj.08-111401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Maybin JA, Murray AA, Saunders PTK, Hirani N, Carmeliet P, Critchley HOD. Hypoxia and hypoxia inducible factor-1alpha are required for normal endometrial repair during menstruation. Nat Commun. 2018;9(1):295. doi: 10.1038/s41467-017-02375-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Guo Y, He B, Xu X, Wang J. Comprehensive analysis of leukocytes, vascularization and matrix metalloproteinases in human menstrual xenograft model. PLoS One. 2011;6(2):e16840. doi: 10.1371/journal.pone.0016840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Coudyzer P, Lemoine P, Jordan BF, et al. Hypoxia is not required for human endometrial breakdown or repair in a xenograft model of menstruation. FASEB J. 2013;27(9):3711–3719. doi: 10.1096/fj.13-232074. [DOI] [PubMed] [Google Scholar]
- 97.Masuda H, Maruyama T, Hiratsu E, et al. Noninvasive and real-time assessment of reconstructed functional human endometrium in NOD/SCID/gamma c(null) immunodeficient mice. Proc Natl Acad Sci U S A. 2007;104(6):1925–1930. doi: 10.1073/pnas.0604310104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Coudyzer P, Lemoine P, Po C, et al. Induction of post-menstrual regeneration by ovarian steroid withdrawal in the functionalis of xenografted human endometrium. Hum Reprod. 2015;30(5):1156–1168. doi: 10.1093/humrep/dev043. [DOI] [PubMed] [Google Scholar]
- 99.Bellofiore N, Rana S, Dickinson H, Temple-Smith P, Evans J. Characterization of human-like menstruation in the spiny mouse: comparative studies with the human and induced mouse model. Hum Reprod. 2018;33(9):1715–1726. doi: 10.1093/humrep/dey247. [DOI] [PubMed] [Google Scholar]
- 100.Bellofiore N, Ellery SJ, Mamrot J, Walker DW, Temple-Smith P, Dickinson H. First evidence of a menstruating rodent: the spiny mouse (Acomys cahirinus) Am J Obstet Gynecol. 2017;216(1):40. doi: 10.1016/j.ajog.2016.07.041. e1- e11. [DOI] [PubMed] [Google Scholar]
- 101.Barrier BF, Allison J, Hubbard GB, Dick EJ, Jr Brasky KM, Schust DJ. Spontaneous adenomyosis in the chimpanzee (Pan troglodytes): a first report and review of the primate literature: Case Report. Hum Reprod. 2007;22(6):1714–1717. doi: 10.1093/humrep/dem038. [DOI] [PubMed] [Google Scholar]
- 102.Huseby RA, Thurlow S. Effects of prenatal exposure of mice to “low-dose” diethylstilbestrol and the development of adenomyosis associated with evidence of hyperprolactinemia. Am J Obstet Gynecol. 1982;144(8):939–949. doi: 10.1016/0002-9378(82)90189-2. [DOI] [PubMed] [Google Scholar]
- 103.Nayyar T, Bruner-Tran KL, Piestrzeniewicz-Ulanska D, Osteen KG. Developmental exposure of mice to TCDD elicits a similar uterine phenotype in adult animals as observed in women with endometriosis. Reprod Toxicol. 2007;23(3):326–336. doi: 10.1016/j.reprotox.2006.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Bruner-Tran KL, Duleba AJ, Taylor HS, Osteen KG. Developmental toxicant exposure is associated with transgenerational adenomyosis in a murine model1. Biol Reprod. 2016;95(4):1–10. doi: 10.1095/biolreprod.116.138370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Li K, Liszka M, Zhou C, Brehm E, Flaws JA, Nowak RA. Prenatal exposure to a phthalate mixture leads to multigenerational and transgenerational effects on uterine morphology and function in mice. Reprod Toxicol. 2020;93:178–190. doi: 10.1016/j.reprotox.2020.02.012. [DOI] [PubMed] [Google Scholar]
- 106.Walker BE. Uterine tumors in old female mice exposed prenatally to diethylstilbestrol. J Natl Cancer Inst. 1983;70(3):477–484. [PubMed] [Google Scholar]
- 107.Brody JR, Cunha GR. Histologic, morphometric, and immunocytochemical analysis of myometrial development in rats and mice: II. Effects of DES on development. Am J Anat. 1989;186(1):21–42. doi: 10.1002/aja.1001860103. [DOI] [PubMed] [Google Scholar]
- 108.Newbold RR, Bullock BC, McLachlan JA. Uterine adenocarcinoma in mice following developmental treatment with estrogens: a model for hormonal carcinogenesis. Cancer Res. 1990;50(23):7677–7681. [PubMed] [Google Scholar]
- 109.Mori T, Nagasawa H. Mechanisms of development of prolactin-induced adenomyosis in mice. Acta Anat (Basel) 1983;116(1):46–54. doi: 10.1159/000145724. [DOI] [PubMed] [Google Scholar]
- 110.Sengupta P, Sharma A, Mazumdar G, et al. The possible role of fluoxetine in adenomyosis: an animal experiment with clinical correlations. J Clin Diagn Res. 2013;7(7):1530–1534. doi: 10.7860/JCDR/2013/5654.3128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Koujyo T, Hatakeyama S, Yamada H, et al. Induction of endometriosis and adenomyosis by transvaginal pituitary transplantation in mice with and without natural killer cell activity. Am J Reprod Immunol. 1998;40(6):441–446. doi: 10.1111/j.1600-0897.1998.tb00431.x. [DOI] [PubMed] [Google Scholar]
- 112.Mori T, Nagasawa H, Takahashi S. The induction of adenomyosis in mice by intrauterine pituitary isografts. Life Sci. 1981;29(12):1277–1282. doi: 10.1016/0024-3205(81)90234-4. [DOI] [PubMed] [Google Scholar]
- 113.Matsuda M, Sasabe H, Adachi Y, Suzuki T, Mori T. Increased invasion activity of endometrial stromal cells and elevated expression of matrix metalloproteinase messenger RNA in the uterine tissues of mice with experimentally induced adenomyosis. Am J Obstet Gynecol. 2001;185(6):1374–1380. doi: 10.1067/mob.2001.117967. [DOI] [PubMed] [Google Scholar]
- 114.Zhou Y-F, Mori T, Kudo H, Asakai R, Sassa S, Sakamoto S. Effects of angiogenesis inhibitor TNP-470 on the development of uterine adenomyosis in mice. Fertil Steril. 2003;80:788–794. doi: 10.1016/s0015-0282(03)00988-9. [DOI] [PubMed] [Google Scholar]
- 115.Huseby RA, Soares MJ, Talamantes F. Ectopic pituitary grafts in mice: hormone levels, effects on fertility, and the development of adenomyosis uteri, prolactinomas, and mammary carcinomas. Endocrinology. 1985;116(4):1440–1448. doi: 10.1210/endo-116-4-1440. [DOI] [PubMed] [Google Scholar]
- 116.Mori T, Kyokuwa M, Nagasawa H. Animal model of uterine adenomyosis: induction of the lesion in rats by ectopic pituitary isografting. Lab Anim Sci. 1998;48(1):64–68. [PubMed] [Google Scholar]
- 117.Singtripop T, Mori T, Sakamoto S, Sassa S, Park MK, Kawashima S. Suppression of the development of uterine adenomyosis by danazol treatment in mice. Life Sci. 1992;51(14):1119–1125. doi: 10.1016/0024-3205(92)90513-o. [DOI] [PubMed] [Google Scholar]
- 118.Mori T, Kurata Y, Tabata Y, Niho N, Matsuda M, Zhou Y-F. Priming effects of novel nonsteroidal progesterone receptor modulators CP8816 and CP8863 on the development of adenomyosis in the mouse uterus. Life Sci. 2002;71(5):527–535. doi: 10.1016/s0024-3205(02)01727-7. [DOI] [PubMed] [Google Scholar]
- 119.Kawahara R, Matsuda M, Mori T. Increase in the number of integrinβ1-immunoreactive monocyte-lineage cells in experimentally-induced adenomyosis in mice. Life Sci. 2003;73(7):907–916. doi: 10.1016/s0024-3205(03)00350-3. [DOI] [PubMed] [Google Scholar]
- 120.Zhou YF, Mori T, Nagasawa H, Nakayama T, Kubota T, Sakamoto S. Probucol, a hypocholesterolemic agent, prevents the development of uterine adenomyosis induced by pituitary grafting in mice. Anticancer Res. 2004;24(4):2209–2212. [PubMed] [Google Scholar]
- 121.Parrott E, Butterworth M, Green A, White INH, Greaves P. Adenomyosis—A Result of Disordered Stromal Differentiation. Am J Pathol. 2001;159(2):623–630. doi: 10.1016/S0002-9440(10)61733-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Shen M, Liu X, Zhang H, Guo S-W. Transforming growth factor β1 signaling coincides with epithelial–mesenchymal transition and fibroblast-to-myofibroblast transdifferentiation in the development of adenomyosis in mice. Hum Reprod. 2015;31(2):355–369. doi: 10.1093/humrep/dev314. [DOI] [PubMed] [Google Scholar]
- 123.Hao M, Liu X, Guo S-W. Adenomyosis in mice resulting from mechanically or thermally induced endometrial–myometrial interface disruption and its possible prevention. Reprod Biomed Online. 2020;41(5):925–942. doi: 10.1016/j.rbmo.2020.07.023. [DOI] [PubMed] [Google Scholar]
- 124.Katz TA, Yang Q, Treviño LS, Walker CL, Al-Hendy A. Endocrine-disrupting chemicals and uterine fibroids. Fertil Steril. 2016;106(4):967–977. doi: 10.1016/j.fertnstert.2016.08.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Newbold RR, Jefferson WN, Padilla-Banks E. Long-term adverse effects of neonatal exposure to bisphenol A on the murine female reproductive tract. Reprod Toxicol. 2007;24(2):253–258. doi: 10.1016/j.reprotox.2007.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Chen M, Guo J, Ruan J, Yang Z, He C, Zuo Z. Neonatal exposure to environment-relevant levels of tributyltin leads to uterine dysplasia in rats. Sci Total Environ. 2020;720 doi: 10.1016/j.scitotenv.2020.137615. [DOI] [PubMed] [Google Scholar]
- 127.Branham WS, Zehr DR, Chen JJ, Sheehan DM. Uterine abnormalities in rats exposed neonatally to diethylstilbestrol, ethynylestradiol, or clomiphene citrate. Toxicology. 1988;51(2):201–212. doi: 10.1016/0300-483x(88)90150-3. [DOI] [PubMed] [Google Scholar]
- 128.Newbold RR, Moore AB, Dixon D. Characterization of uterine leiomyomas in CD-1 mice following developmental exposure to diethylstilbestrol (DES) Toxicol Pathol. 2002;30(5):611–616. doi: 10.1080/01926230290105839. [DOI] [PubMed] [Google Scholar]
- 129.Yu CH, Zhao JS, Zhao H, et al. Transcriptional profiling of uterine leiomyoma rats treated by a traditional herb pair, Curcumae rhizoma and Sparganii rhizoma. Braz J Med Biol Res. 2019;52(6):e8132. doi: 10.1590/1414-431X20198132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Walker CL, Hunter D, Everitt JI. Uterine leiomyoma in the Eker rat: a unique model for important diseases of women. Genes, Chromosomes Cancer. 2003;38(4):349–356. doi: 10.1002/gcc.10281. [DOI] [PubMed] [Google Scholar]
- 131.Wei J, Chiriboga L, Mizuguchi M, Yee H, Mittal K. Expression profile of tuberin and some potential tumorigenic factors in 60 patients with uterine leiomyomata. Mod Pathol. 2005;18(2):179–188. doi: 10.1038/modpathol.3800283. [DOI] [PubMed] [Google Scholar]
- 132.Fritsch M, Schmidt N, Gröticke I, et al. Application of a patient derived xenograft model for predicative study of uterine fibroid disease. PLoS One. 2015;10(11) doi: 10.1371/journal.pone.0142429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Walker CL. Role of hormonal and reproductive factors in the etiology and treatment of uterine leiomyoma. Recent Prog Horm Res. 2002;57:277–294. doi: 10.1210/rp.57.1.277. [DOI] [PubMed] [Google Scholar]
- 134.Mittal P, Shin Y-h, Yatsenko SA, Castro CA, Surti U, Rajkovic A. Med12 gain-of-function mutation causes leiomyomas and genomic instability. J Clin Invest. 2015;125(8):3280–3284. doi: 10.1172/JCI81534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 135.Mäkinen N, Mehine M, Tolvanen J, et al. MED12, the mediator complex subunit 12 gene, is mutated at high frequency in uterine leiomyomas. Science. 2011;334(6053):252–255. doi: 10.1126/science.1208930. [DOI] [PubMed] [Google Scholar]
- 136.Cook JD, Davis BJ, Goewey JA, Berry TD, Walker CL. Identification of a sensitive period for developmental programming that increases risk for uterine leiomyoma in Eker rats. Reproduct Sci. 2007;14(2):121–136. doi: 10.1177/1933719106298401. [DOI] [PubMed] [Google Scholar]
- 137.Elkafas H, Ali M, Elmorsy E, et al. Vitamin D3 ameliorates DNA damage caused by developmental exposure to endocrine disruptors in the uterine myometrial stem cells of eker rats. Cells. 2020;9(6):1459. doi: 10.3390/cells9061459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Koohestani F, Qiang W, MacNeill AL, et al. Halofuginone suppresses growth of human uterine leiomyoma cells in a mouse xenograft model. Hum Reprod. 2016;31(7):1540–1551. doi: 10.1093/humrep/dew094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Borahay MA, Vincent KL, Motamedi M, et al. Liposomal 2-methoxyestradiol nanoparticles for treatment of uterine leiomyoma in a patient-derived xenograft mouse model. Reprod Sci. 2021;28(1):271–277. doi: 10.1007/s43032-020-00248-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tsuiji K, Takeda T, Li B, Kondo A, Ito M, Yaegashi N. Establishment of a novel xenograft model for human uterine leiomyoma in immunodeficient mice. Tohoku J Exp Med. 2010;222(1):55–61. doi: 10.1620/tjem.222.55. [DOI] [PubMed] [Google Scholar]
- 141.Wang G, Ishikawa H, Sone K, et al. Nonobese diabetic/severe combined immunodeficient murine xenograft model for human uterine leiomyoma. Fertil Steril. 2014;101(5):1485–1492. doi: 10.1016/j.fertnstert.2014.01.054. [DOI] [PubMed] [Google Scholar]
- 142.Drosch M, Bullerdiek J, Zollner TM, Prinz F, Koch M, Schmidt N. A novel mouse model that closely mimics human uterine leiomyomas. Fertil Steril. 2013;99(3):927–935.e6. doi: 10.1016/j.fertnstert.2012.11.032. [DOI] [PubMed] [Google Scholar]
- 143.Ishikawa H, Ishi K, Serna VA, Kakazu R, Bulun SE, Kurita T. Progesterone is essential for maintenance and growth of uterine leiomyoma. Endocrinology. 2010;151(6):2433–2442. doi: 10.1210/en.2009-1225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.de Sousa WB, Garcia JBS, Neto JN, Furtado PGR. Araújo dos Anjos JA. Xenotransplantation of uterine leiomyoma in Wistar rats: a pilot study. Eur J Obstetr Gynecol Reproduct Biol. 2015;190:71–75. doi: 10.1016/j.ejogrb.2015.04.012. [DOI] [PubMed] [Google Scholar]
- 145.Hassan MH, Eyzaguirre E, Arafa HM, Hamada FM, Salama SA, Al-Hendy A. Memy I: a novel murine model for uterine leiomyoma using adenovirus-enhanced human fibroid explants in severe combined immune deficiency mice. Am J Obstet Gynecol. 2008;199(2):156. doi: 10.1016/j.ajog.2008.02.010. e1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Qiang W, Liu Z, Serna VA, et al. Down-regulation of miR-29b is essential for pathogenesis of uterine leiomyoma. Endocrinology. 2014;155(3):663–669. doi: 10.1210/en.2013-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Malik M, Britten J, Catherino WH. Development and validation of hormonal impact of a mouse xenograft model for human uterine leiomyoma. Reproduct Sci. 2020;27(6):1304–1317. doi: 10.1007/s43032-019-00123-3. [DOI] [PubMed] [Google Scholar]