Abstract
The viabilities of five strains of Vibrio vulnificus were evaluated during the storage of the organisms in sterile seawater at 5°C. The number of CFU was measured by plate count methods on rich media. The total cell numbers were determined by direct microscopic count methods. The titer of CFU declined logarithmically to undetectable levels over a period of 2 to 3 weeks, while the total cell numbers were unchanged. Midway through each study, higher culturable cell counts began to be observed on plates containing catalase or sodium pyruvate; during the latter stages of the study, the plate counts on such media were up to 1,000-fold higher than those on unsupplemented plates. Because autoclaving is known to generate hydrogen peroxide in rich media, and because catalase and sodium pyruvate are known to eliminate hydrogen peroxide, it appears that the conditions of the experiments led to the selection of a hydrogen peroxide-sensitive culturable cell subpopulation. At the time of the final stage of the decline in viability of each culture, hydrogen peroxide-sensitive cells were the only culturable cells present. Warming samples of the cultures to room temperature led to the growth of these residual culturable cells, utilizing nutrients provided by the nonculturable cells. The cells that grew recovered hydrogen peroxide resistance. When mixtures of culturable and nonculturable cells were diluted to the point where only nonculturable cells were present, or when the hydrogen peroxide-sensitive culturable cells had declined to undetectable levels, warming had no effect; no culturable cells were recovered. Warming has been reported to “resuscitate” nonculturable cells. Recognition of the existence of hydrogen peroxide-sensitive culturable cell populations, as well as their ability to grow to high levels in the warmed seawater microcosms, leads instead to the conclusion that while warming permits culturable cells to grow, it has no effect on nonculturable cells.
When many species of bacteria which are readily culturable in standard laboratory media are subjected to prolonged incubation in sterile water, the culturable-cell counts decline over time to undetectable levels. The total cell count usually remains constant at the initial level, so that as the culturable-cell count declines, the fraction of nonculturable cells increases (1, 2, 5, 6, 13, 24). The simplest explanation of these results is that nonculturable cells are dead (5, 6), and indeed, this is the basic presumption of standard plate count methods for enumerating readily culturable bacteria (13). An alternative explanation that has been advanced posits that the nonculturable cells have entered a state in which they remain viable but cannot be cultured on standard microbiological media (1, 2, 13, 24). Such cells are said to be in the “viable but nonculturable” (VBNC) state.
Confirmation of the VBNC hypothesis would require recovery of culturable cells from a population of nonculturable cells. There have been numerous reports of the appearance of large numbers of culturable cells after the addition of nutrients to populations of nonculturable cells in a process termed “resuscitation” (reviewed in references 1, 2, 13, and 24). However, a critical review of this literature indicates that such observations could be attributable to the presence of a low level of residual culturable cells that are able to respond to the addition of nutrient, thus giving the appearance of resuscitation (1, 2, 13). Extensive nutrient addition experiments with nonculturable populations of various species of enteric bacteria have indicated that no culturable cells could be recovered (5, 6).
One explanation of these results is that the nonculturable population is composed of dead cells. However, proponents of the VBNC hypothesis have offered two alternative explanations. First, it has been suggested that the presence of culturable cells is required for the recovery of nonculturable cells, perhaps owing to a factor produced by culturable cells that triggers resuscitation in nonculturable cells (12, 26, 33). This possibility could neither be confirmed nor ruled out in pure-culture studies, since it was not possible to determine whether the additional culturable cells were new cells or resuscitated nonculturable cells. This question was addressed by the development of a method, termed “mixed-culture recovery” (MCR), in which mixtures of easily distinguishable culturable and nonculturable cells were used to determine whether only culturable cells or both culturable and nonculturable cells had responded to nutrient addition. In repeated applications of the MCR method to mixed populations of culturable and nonculturable cells of several species of enteric bacteria, only the culturable cells responded to the addition of nutrient. The nonculturable cells were dead (5).
Second, it has been suggested that something in rich media may inhibit the recovery of culturable cells from nonculturable cells (37). This would imply that the earlier reports of resuscitation following nutrient addition should be reinterpreted simply as the growth of residual culturable cells. It also raises the question of the nature of the inhibitory component of rich media. More importantly, experimental support for this modified VBNC hypothesis requires the establishment of methods which can recover culturable cells from nonculturable cells without the addition of any rich nutrients. An answer to this question has been proposed by Whitesides and Oliver (37), who reported that nonculturable cells of Vibrio vulnificus in sterile seawater could be returned to culturability by a temperature upshift in the absence of any added rich nutrient.
The observations of Whitesides and Oliver (37) are consistent with the modified VBNC hypothesis. However, in a related study we reported that a temperature upshift had no effect on nonculturable cells of five other species of enteric bacteria (5). In the present study, we have repeated and extended the experiments of Whitesides and Oliver (37). Our results lead to the conclusion that the effects of a temperature upshift on V. vulnificus are attributable to a previously unrecognized population of hydrogen peroxide-sensitive culturable cells.
MATERIALS AND METHODS
Bacterial strains.
The strain of V. vulnificus used in the Whitesides and Oliver study (37), designated C7184-opaque, was obtained from J. D. Oliver, University of North Carolina at Charlotte, Charlotte. Four other strains of V. vulnificus were also employed, an attenuated seawater isolate designated MLT367, an attenuated oyster isolate designated MLT403, and two virulent clinical isolates from patients who died of sepsis following ingestion of contaminated oysters, designated VV1009 and 2400-112 (31a, 32); these strains were obtained from P. A. Gulig, University of Florida, Gainesville. All five strains were confirmed to be V. vulnificus on a Vitek system (bioMerieux, Hazelwood, Mo.) and by fatty acid methyl ester analysis performed by two laboratories, Microbe Inotech (St. Louis, Mo.) and Microcheck, Inc. (Northfield, Vt.). Microbe Inotech also identified the strains as V. vulnificus on a Biolog (Hayward, Calif.) system. All five strains were strongly catalase positive.
Media and chemicals.
Heart infusion (HI) broth and agar, Bacto Peptone, Bacto Proteose Peptone, lactose, and Bacto Agar were obtained from Difco Laboratories (Detroit, Mich.). Acridine orange, neutral red, sodium pyruvate, 3% hydrogen peroxide, sodium chloride, potassium chloride, calcium chloride dihydrate, magnesium chloride hexahydrate, magnesium sulfate heptahydrate, and sodium bicarbonate were obtained from Sigma Chemical Co. (St. Louis, Mo.). Beef liver catalase (65,000 U per mg) was obtained from Boehringer Mannheim (Indianapolis, Ind.). Neutral red lactose plates consisted of 17 g of Bacto Peptone, 3 g of Bacto Proteose Peptone, 5 g of sodium chloride, 0.03 g of neutral red, 15g of Bacto Agar, and 10 g of lactose per liter of distilled water; before autoclaving, the pH of the medium was adjusted to 7.1. HI plate and broth media were prepared according to instructions provided by the supplier and were sterilized by being autoclaved for 30 min. HI-80 plates were prepared by surface spreading a filter-sterilized solution containing 80 mg of sodium pyruvate onto each HI plate. Individual plates contained about 30 ml of HI agar medium.
Seawater microcosms.
Artificial seawater (ASW) (38) contained 24.7 g of sodium chloride, 0.67 g of potassium chloride, 1.36 g of calcium chloride dihydrate, 4.66 g of magnesium chloride hexahydrate, 6.29 g of magnesium sulfate heptahydrate, and 0.18 g of sodium bicarbonate per liter of distilled water. The ASW was sterilized by being autoclaved for 30 min.
The strains were grown in HI broth at 37°C and, in mid-logarithmic growth phase, were washed twice with ASW and then used to inoculate duplicate 1-liter flasks of ASW to an initial concentration of about 107 CFU per ml. The inoculated flasks of ASW were placed in a 5°C refrigerator with no agitation.
Colony and cell counting.
Plate counts were performed by diluting samples in ASW and then spread plating 0.1-ml aliquots in duplicate. The plates were incubated at 37°C for 24 h or at room temperature (about 22°C) for 48 h prior to being counted. Longer periods of incubation, 5 days at 37°C or 10 days at room temperature, never yielded any additional colonies. All of the colonies on plates containing fewer than 300 colonies were added and divided by the total volume plated to estimate CFU per milliliter; a limit of detection of 1 CFU per ml was obtained by plating five 0.2-ml aliquots. Acridine orange direct counts (AODC) were performed by the method of Hobbie et al. (10), i.e., staining with 0.01% acridine orange at room temperature for 15 min; this indicated the total number of cells per milliliter, regardless of whether they were able to form a visible colony. A second direct-count method, the “live/dead” kit of Molecular Probes, Inc. (Eugene, Oreg.), was employed using instructions supplied by the company; this kit utilizes a mixture of the stains SYTO 9 and propidium iodide to evaluate cell membrane integrity (15). A Nikon Optiphot fluorescence microscope with an HBO-100 light source was used for the examination of the preparations at a magnification factor of 1,000. Most-probable-number (MPN) estimates were performed with tubes containing 10 ml of ASW microcosm supernatant, which was prepared by centrifuging samples of ASW microcosms at 3,000 × g followed by passage through a 0.2-μm-pore-size filter to remove the cells. A 10-tube procedure and probability table (9) were employed; the tubes were incubated at room temperature (about 22°C) and scored after 3 days by HI and HI-80 plate counts.
RESULTS
Decline of the strains in cold seawater and development of hydrogen peroxide-sensitive cells.
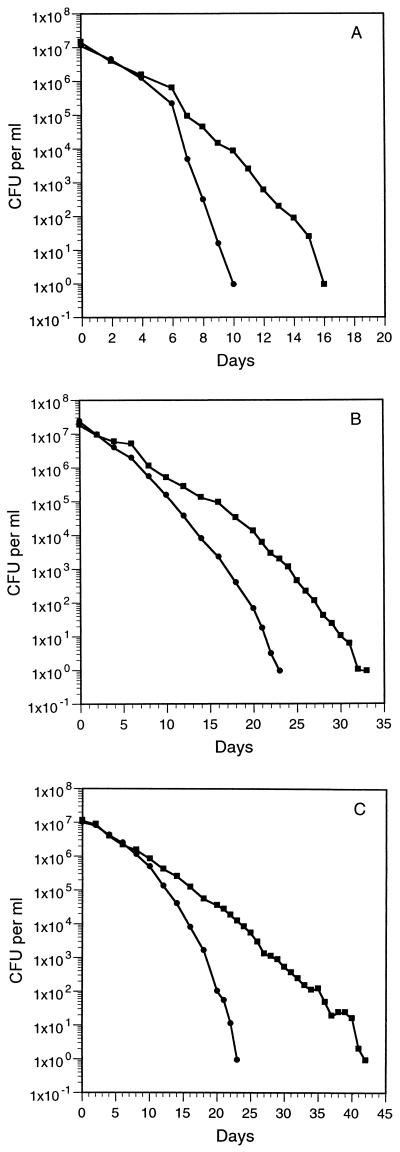
Figure 1 shows the responses of V. vulnificus strains to long-term starvation in ASW at 5°C. Three different methods were used to monitor the cells in the cold ASW: plate counts, AODC, and SYTO 9-propidium iodide staining. Total cell counts remained constant at the initial levels throughout the experiments; with the SYTO 9-propidium iodide staining technique, all of the cells remained fluorescent green, indicating that the cells retained intact membranes (15). The HI plate counts declined gradually (over 10 to 23 days) to undetectable levels (<1 CFU per ml). For the entire strain C7184-opaque study shown in Fig. 1, duplicate sets of plates were incubated at 37°C and at room temperature. The incubation temperature did not affect the plate counts.
FIG. 1.
Decline of V. vulnificus strains in sterile seawater at 5°C. Colony counts on HI plates (●) and on HI-80 plates (■) are shown. (A) Decline of strain MLT403; (B) decline of strain C7184-opaque; (C) decline of strain VV1009. The declines of strains MLT367 and 2400-112 were very similar to the declines of strains C7184-opaque and VV1009, respectively (data not shown). Each point is the mean of values from duplicate microcosms. The standard errors averaged 20% of the presented values when the CFU per milliliter were greater than 100.
The addition of sodium pyruvate was found to increase the HI plate counts of samples taken during the latter stages of the studies. The maximum effect of sodium pyruvate was achieved with 40 mg of sodium pyruvate per plate; higher levels of sodium pyruvate were tested, up to 320 mg per plate, without additional benefit. The amount of sodium pyruvate added to each HI plate for the remainder of the studies reported here was 80 mg per plate, yielding a medium designated HI-80.
Since one known effect of sodium pyruvate on microbiological media is to degrade hydrogen peroxide (3, 7, 14, 16, 17, 18, 29), the possibility that a hydrogen peroxide-sensitive population of culturable cells had formed in the cold ASW was investigated. Catalase, an enzyme which breaks down hydrogen peroxide, was added to HI plates by surface spreading. The presence of as little as 130 U of catalase per HI plate yielded counts identical to those on HI-80 plates; higher levels of catalase (up to 13,000 U per plate) had no additional benefit. Heat-inactivated catalase (held at 80°C for 30 min) did not increase the HI plate counts.
In subsequent experiments (Fig. 1), samples were plated at each time point on HI and HI-80 plates. The results show the development of a hydrogen peroxide-sensitive population of culturable cells, which by the later stage of each study became the only culturable cell type in the ASW microcosms.
The development of hydrogen peroxide sensitivity in the ASW microcosm populations was further characterized by measuring their hydrogen peroxide resistances relative to that of fresh cells. Various amounts of a 3% solution of hydrogen peroxide were diluted to a convenient volume and spread on HI plates, which were then inoculated with samples of the five V. vulnificus strains. Cells obtained from fresh HI broth cultures of the five strains formed colonies on plates supplemented with up to 20 μl of 3% hydrogen peroxide but did not form colonies on plates supplemented with 40 μl of 3% hydrogen peroxide. Samples of each strain taken from the ASW microcosms at a time point about midway through the studies formed colonies on plates supplemented with up to 2.5 μl of 3% hydrogen peroxide but did not form colonies on plates supplemented with 5 μl of 3% hydrogen peroxide.
Effect of a temperature upshift.
Starting about midway through the studies, 10-ml samples were removed on a daily basis from the ASW microcosms and placed at room temperature without agitation. The plate counts in these samples declined about 10-fold during the first 12 h at room temperature and then increased gradually, reaching maximum levels after 3 days; in samples monitored for much longer periods (up to 30 days) there were no further increases in the plate counts. For the strains MLT367, MLT403, VV1009, and 2400-112, the maximum plate counts observed were about 1.5- to 3-fold higher than the plate counts from the start of the study. For strain C7184-opaque, the maximum plate counts observed were about 10-fold lower than the plate counts from the start of the study. The plate counts at these maximums were essentially identical on HI and HI-80 plates.
After HI plate counts had declined to <1 CFU per ml, but while there were still cells present capable of forming colonies on HI-80 plates, warming samples to room temperature still resulted in increases in the colony counts (Table 1). As the HI-80 plate counts declined to about 250 CFU per ml or less, however, the initial decline observed after shifting a sample to room temperature occasionally drove these plate counts to <1 CFU per ml with no subsequent recovery. When the HI-80 plate counts had declined to less than 80 CFU per ml, the temperature upshift always resulted in the complete loss of CFU on HI-80 plates (Table 1).
TABLE 1.
Effect of shift to room temperature on ASW microcosm samples
| Strain | Day | CFU/ml on HI-80 plates
|
|||
|---|---|---|---|---|---|
| Flask 1a
|
Flask 2a
|
||||
| Initial | 3 days after temperature shift | Initial | 3 days after temperature shift | ||
| MLT403 | 12b | 640 | 3.2 × 107 | 510 | 2.9 × 107 |
| 13 | 230 | <1 | 150 | 3.1 × 107 | |
| 14 | 78 | <1 | 90 | 3.2 × 107 | |
| 15c | 24 | <1 | 23 | <1 | |
| C7184-opaque | 25b | 480 | 2.4 × 106 | 370 | 2.3 × 106 |
| 26 | 170 | 2.4 × 106 | 240 | <1 | |
| 27 | 80 | 2.1 × 106 | 130 | <1 | |
| 28c | 25 | <1 | 54 | <1 | |
| MLT367 | 26b | 460 | 2.5 × 107 | 350 | 2.6 × 107 |
| 27 | 120 | 3.1 × 107 | 80 | 3.0 × 107 | |
| 28 | 90 | <1 | 100 | 2.8 × 107 | |
| 29c | 42 | <1 | 54 | <1 | |
| 2400-112 | 27b | 310 | 3.8 × 107 | 300 | 3.8 × 107 |
| 28 | 150 | 3.9 × 107 | 140 | 4.2 × 107 | |
| 29 | 100 | <1 | 89 | 4.3 × 107 | |
| 30c | 52 | <1 | 45 | <1 | |
| VV1009 | 31b | 390 | 2.4 × 107 | 330 | 2.0 × 107 |
| 32 | 200 | 2.0 × 107 | 270 | 2.3 × 107 | |
| 33 | 120 | <1 | 170 | <1 | |
| 34c | 92 | <1 | 120 | <1 | |
Flask 1 and flask 2 are duplicate microcosms.
Samples from all prior days exhibited regrowth.
Samples from all subsequent days did not exhibit regrowth.
Increases in colony counts after temperature upshift are attributable to growth of culturable cells.
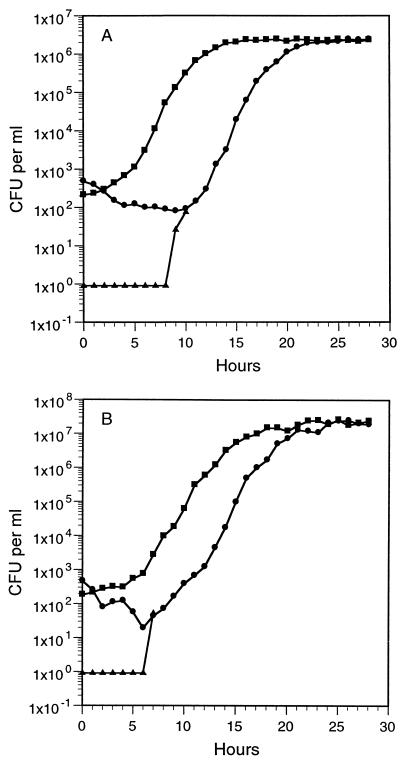
More-detailed experiments were performed to characterize the observed increases in plate counts following the shift to room temperature. When the ASW microcosm HI-80 plate counts had declined to about 1,000 to 2,000 CFU per ml (at which point the HI plate counts were <1 CFU per ml), samples were placed at room temperature, and HI and HI-80 plate counts were performed every hour (Fig. 2). The HI plate counts remained at undetectable levels for several hours before increasing rapidly to the level of the HI-80 plate counts, after which the two plate counts increased in essentially identical fashions.
FIG. 2.
Growth curves (■) of V. vulnificus strains C7184-opaque (A) and MLT367 (B). Also shown are the plate counts for the two strains on HI plates (▴) and HI-80 plates (●) after cold ASW microcosm samples had been shifted to room temperature. The strain C7184-opaque sample was taken on day 25, and the strain MLT367 sample was taken on day 26. Strains MLT403, VV1009, and 2400-112 exhibited similar responses (data not shown). After the HI plate counts increased to the level of the HI-80 plate counts, the counts were essentially identical, and only the HI-80 plate counts are shown past that point.
The HI-80 plate counts exhibited an initial decline, after which they recovered and began increasing in a fashion which resembled a growth curve (Fig. 2). To test the possibility that ASW microcosm cell suspensions could support the growth of V. vulnificus, 5 days after the ASW microcosm HI-80 plate counts had declined to <1 CFU per ml, samples of these completely nonculturable ASW microcosm cell suspensions were inoculated with about 200 cells per ml from a fresh HI broth culture and placed at room temperature, and HI plate counts were performed every hour. The growth curves obtained (Fig. 2) were very similar to the curves from the plate counts of the temperature upshift experiment described above; the doubling times for both sets of cultures ranged from 30 to 35 min. This experiment was repeated with the nonculturable cell suspensions centrifuged and filtered through 0.2-μm-pore-size filters to remove all of the cells prior to inoculation with culturable cells; similar growth curves were obtained, with the only difference being that these cultures reached final cell levels which were about half of the levels shown in Fig. 2. In a third experiment, the test medium was composed of the nonculturable cell suspension pellets resuspended in fresh ASW prior to inoculation with culturable cells; again, similar growth curves and final cell levels of half those shown in Fig. 2 were obtained. Finally, the nonculturable ASW microcosms were autoclaved and then reinoculated with culturable cells; growth curves essentially identical to those shown in Fig. 2 were obtained. When the cells which had grown in the medium with ASW plus nonculturable cells were passaged 10 more times in this medium, the same growth profile was obtained each time.
MPN estimates and MCR tests.
Toward the end of each study, MPN estimates were made with ASW microcosm supernatant as the MPN growth medium and with the tubes incubated at room temperature (Table 2). Independently, HI broth (plus and minus sodium pyruvate) MPN counts were determined to be similar to the HI and HI-80 plate counts. The ASW microcosm supernatant MPN counts were lower than the HI-80 plate counts and sometimes were completely negative when the HI-80 plate counts were low, presumably due to the initial loss of culturable cells when ASW microcosm samples were warmed to room temperature (as described above). The MPN counts were extended past the point when the culturable cells in the ASW microcosms had declined to undetectable levels; no culturable cells were detected in any of these later MPN counts (Table 2).
TABLE 2.
MPN and MPN-MCR test results
| Strain (flask no.) | Day | CFU/ml on HI-80 platesb | No. of positive tubes at dilutiona:
|
MPN estimate of viable cells/ml | ||||
|---|---|---|---|---|---|---|---|---|
| 100 | 10−1 | 10−2 | 10−3 | 10−4 | ||||
| C1784-opaque (1) | 25 | 480 | 10 | 10 | 6 | 0 | 0 | 79 |
| 10c | 10 | 4 | 0 | 0 | 49 | |||
| 27 | 80 | 9 | 4 | 0 | 0 | 0 | 3 | |
| 10c | 2 | 0 | 0 | 0 | 3 | |||
| 33 | <1 | 0 | 0 | 0 | 0 | 0 | <1 | |
| 0c | 0 | 0 | 0 | 0 | <1 | |||
| 2400-112 (1) | 26 | 650 | 10 | 5 | 2 | 0 | 0 | 86 |
| 10c | 6 | 3 | 0 | 0 | 120 | |||
| 29 | 100 | 10 | 10 | 1 | 0 | 0 | 28 | |
| 10c | 9 | 0 | 0 | 0 | 17 | |||
| 36 | <1 | 0 | 0 | 0 | 0 | 0 | <1 | |
| 0c | 0 | 0 | 0 | 0 | <1 | |||
| MLT403 (2) | 12 | 510 | 10 | 10 | 4 | 1 | 0 | 56 |
| 14 | 90 | 9 | 0 | 0 | 0 | 0 | 2 | |
| 16 | <1 | 0 | 0 | 0 | 0 | 0 | <1 | |
| MLT367 (1) | 26 | 460 | 10 | 10 | 4 | 0 | 0 | 49 |
| 28 | 90 | 0 | 0 | 0 | 0 | 0 | <1 | |
| 35 | <1 | 0 | 0 | 0 | 0 | 0 | <1 | |
| VV1009 (1) | 28 | 900 | 10 | 10 | 4 | 0 | 0 | 49 |
| 34 | 92 | 0 | 0 | 0 | 0 | 0 | <1 | |
| 42 | <1 | 0 | 0 | 0 | 0 | 0 | <1 | |
There were 10 tubes at each dilution.
HI-80 plate counts in the ASW microcosms are shown. The HI plate counts were <1 CFU/ml at all of these time points.
MPN-MCR test; scored as positive if they contained both Lac+ and Lac− colonies (see the text).
Combination MPN and MCR tests (5) were performed with strain C7184-opaque, which was lactose positive, and strain 2400-112, which was lactose negative. About 200 culturable cells of the lactose-negative strain 2400-112, obtained from an ASW microcosm, were used to inoculate each tube in a second set of strain C7184-opaque MPN tubes. The converse experiment was performed with a second set of strain 2400-112 MPN tubes, each inoculated with culturable cells of strain C7184-opaque. The inoculated tubes were placed at room temperature and scored after 3 days by colony counts on neutral red lactose plates. Since all of the MPN tubes initially contained at least one type of culturable cell (having all been inoculated with culturable cells of one lactose phenotype and also potentially containing culturable cells of the other lactose phenotype), the MPN-MCR tubes were scored as positive if they yielded colonies of both lactose phenotypes and negative if they yielded only colonies with the lactose phenotype of the inoculated cells. These combination MPN-MCR estimates were essentially the same as the standard MPN estimates (Table 2).
DISCUSSION
Five different strains of V. vulnificus, inoculated at high levels into sterile seawater at 5°C and monitored for nearly 50 days, displayed declining numbers of CFU and increasing numbers of nonculturable cells. The total cell counts remained constant at the initial level, while plate counts indicated that the number of culturable cells dropped to less than 1 CFU per ml (Fig. 1).
After the plate counts had declined by about 100- to 1,000-fold (Fig. 1), subpopulations of hydrogen peroxide-sensitive culturable cells developed. A plating assay revealed that the culturable cells from middle time points in the studies were much more sensitive to hydrogen peroxide than fresh HI broth-derived cells. Supplementation of HI plates with either sodium pyruvate or catalase, agents which degrade hydrogen peroxide (3, 7, 14, 16, 17, 18, 29), yielded higher colony counts. However, there was also a continued decline to complete loss of these culturable cells (Fig. 1).
Later in the studies, the hydrogen peroxide-sensitive culturable cells were the only culturable cells remaining in the ASW microcosms. Warming samples of the ASW microcosms to room temperature resulted at first in a slight decline and then in an increase in plate counts (Fig. 2). Several lines of evidence indicated that the increased plate counts were due to the growth of the residual hydrogen peroxide-sensitive culturable cells in the warmed ASW microcosms.
First, regardless of how the ASW microcosm samples were treated, additional culturable cells were observed only when there were residual hydrogen peroxide-sensitive culturable cells initially present in the samples. Immediately after the HI-80 plate counts had declined to <1 CFU per ml, none of the recovery methods yielded any culturable cells. Second, with the strains MLT367, MLT403, VV1009, and 2400-112, the increased plate counts reached levels of about 1.5 × 107 to 4.0 × 107 CFU per ml (Table 1), higher than their initial levels of about 1 × 107 CFU per ml; clearly, some additional growth had occurred. Apparently, a mid-logarithmic-phase HI broth-derived cell of these strains contains enough nutrient to support the formation of more than one progeny cell. Third, the plate counts increased in a manner which resembled the growth of these strains in this medium (Fig. 2). The fact that the medium with ASW plus nonculturable cells contained nutrients capable of supporting the observed growth was demonstrated by similar growth profiles of the strains in the cell-free component of the medium, in the nonculturable-cell component, and in autoclaved flasks of the medium. The strains were growing on nutrients on the medium rather than by “reductive division,” since repeated passages yielded the same growth profile. Finally, the MPN and MCR results indicated that the nonculturable cells were not affected by a temperature upshift, even in the presence of culturable cells (Table 2).
The suggestion that a temperature upshift could resuscitate cells of V. vulnificus that had become nonculturable in cold ASW (23) initiated a debate over whether the observation was due to the regrowth of residual culturable cells. Supporting the regrowth explanation were observations with V. vulnificus that are similar to some of the findings reported in this paper. For example, it was reported that recovery occurred for only a limited time after the microcosms reached complete nonculturability (4, 36) and that nonculturable cells provided sufficient nutrient for considerable growth of culturable cells (8, 36). In agreement with our SYTO 9-propidium iodide staining results, others have shown that nonculturable cells retained an intact morphology (36) but appeared to lose intact nucleic acids (35). Antibiotics were shown to inhibit the increase in culturable cell numbers (23). In a paper which foreshadowed our recognition of hydrogen peroxide-sensitive culturable-cell populations, Weichart and Kjelleberg (34) suggested that “injured subpopulations may partly explain the resuscitation of a fraction of the cold-incubated populations.” The debate turned in favor of the resuscitation explanation with reports which employed diluted cell suspensions clearly free of culturable cells able to form colonies on HI plates and yet retaining virulence towards mice (25) and the capacity to respond to a temperature upshift (37).
In the Whitesides and Oliver study (37) with strain C7184-opaque held in cold ASW to the point of complete nonculturability on HI plates, warming a sample of the ASW microcosm to room temperature for 7 h resulted in the subsequent appearance of CFU on HI plates at a rate exceeding the growth rate. These authors concluded that they had observed rapid resuscitation of large numbers of nonculturable cells. Given the data presented, this was a reasonable conclusion. The results we present here, from studies designed to replicate the experimental conditions of the Whitesides and Oliver study, suggest that hydrogen peroxide-sensitive culturable-cell populations could be the basis of their observations as well as of the in vivo-virulence observations (25).
Resuscitation of nonculturable cells, which is the keystone of the VBNC hypothesis, is operationally defined as the conversion of nonculturable cells into culturable cells without any change in cell numbers due to regrowth. The results presented here suggest instead that additional culturable cells observed following a temperature upshift originated only from the growth of residual culturable cells. Furthermore, our results indicate that the rapid increase in HI plate counts after the temperature upshift, at a rate exceeding the growth rate, was due merely to the recovery of hydrogen peroxide resistance in the growing population of cells (Fig. 2).
While our results would seem to invalidate the suggestion that a temperature upshift rapidly resuscitates large populations of nonculturable cells, they also raise the question of the nature of the small transient populations of hydrogen peroxide-sensitive culturable cells which were observed. The transient nature of the hydrogen peroxide-sensitive culturable cells suggests a model in which cells of V. vulnificus inoculated into cold ASW gradually degenerate, passing through a hydrogen peroxide-sensitive injured state as they die. Injury in bacteria has been defined by respected workers in the field, such as Speck and McFeters, as an increased sensitivity to components of growth media which are not normally inhibitory (11, 19, 20, 27, 28, 30, 31); the injured state is transient, resulting from cumulative damage, and death in bacteria has been defined by these workers as the point “where injury extends beyond the ability of a cell to multiply and form a colony” (28, 31). Amendment of plate count media with catalase or sodium pyruvate has long been employed as a means to recover injured bacterial cells (3, 7, 14, 16, 17, 18, 29). It could be argued that our results support the VBNC hypothesis, in that the cells have entered a state in which they are viable but nonculturable on HI plates. Rather than exhibiting long-term survival, we note that the hydrogen peroxide-sensitive populations continued to decline to complete loss of culturability (Fig. 1). Furthermore, we feel that such a definition of the VBNC state does not distinguish it from established views of injured bacteria (1, 2, 11, 19, 20, 27, 28, 30, 31), failing to describe a specific program of differentiation into a long-term survival state (akin to spore formation) as opposed to degeneration into a short-term injured state followed by further degeneration to death. The continued decline of hydrogen peroxide-sensitive cells has also been noted with Escherichia coli (21), with complete loss of culturability of such cells obtained in studies with Vibrio parahaemolyticus (22) and V. vulnificus (L. Sides, M. F. Hite, and J. D. Oliver, Abstr. 99th Gen. Meet. Am. Soc. Microbiol., abstr. Q-129, 1999).
We also considered the possibility that the amended HI plates enumerated only a fraction of the residual culturable cells. Indeed, the AODC and SYTO 9-propidium iodide staining results of the nonculturable cells obtained in the present work have been interpreted by others to indicate that such cells were still viable. For readily culturable species of bacteria, we equate viability with culturability and contend that nonculturable cells which stain the same as culturable cells have not been shown to be alive. Indeed, we observed a tight correlation between loss of CFU on HI-80 plates (and loss of viable cells by MPN counts) and disappearance of any response to a temperature upshift or other attempts to demonstrate culturability. We conclude that a temperature upshift only affected the residual culturable cells and, through MCR tests, that the residual culturable cells did not have any effect on the remaining nonculturable cells. Equating viability with culturability for readily culturable species of bacteria, it is our contention that nonculturable cells of V. vulnificus unable to form progeny should be considered dead.
ACKNOWLEDGMENTS
We thank James D. Oliver and Paul A. Gulig for gifts of strains, Steve Denham for assistance with statistical analysis, and Michael R. Barer, Ronald L. Somerville, Thomas C. White, and Wesley E. Workman for critically reviewing the manuscript.
REFERENCES
- 1.Barer M R, Gribbon L T, Harwood C R, Nwoguh C E. The viable but non-culturable hypothesis and medical bacteriology. Rev Med Microbiol. 1993;4:183–191. [Google Scholar]
- 2.Barer M R, Harwood C R. Bacterial viability and culturability. Adv Microb Physiol. 1999;41:93–137. doi: 10.1016/s0065-2911(08)60166-6. [DOI] [PubMed] [Google Scholar]
- 3.Barry V C, Conalty M L, Denneny J M, Winder F. Peroxide formation in bacteriological media. Nature. 1956;178:596–597. doi: 10.1038/178596a0. [DOI] [PubMed] [Google Scholar]
- 4.Biosca E G, Amaro C, Marco-Noales E, Oliver J D. Effect of low temperature on starvation-survival of the eel pathogen Vibrio vulnificus biotype 2. Appl Environ Microbiol. 1996;62:450–455. doi: 10.1128/aem.62.2.450-455.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bogosian G, Morris P J L, O'Neil J P. A mixed culture recovery method indicates that enteric bacteria do not enter the viable but nonculturable state. Appl Environ Microbiol. 1998;64:1736–1742. doi: 10.1128/aem.64.5.1736-1742.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bogosian G, Sammons L E, Morris P J L, O'Neil J P, Heitkamp M A, Weber D B. Death of the Escherichia coli K-12 strain W3110 in soil and water. Appl Environ Microbiol. 1996;62:4114–4120. doi: 10.1128/aem.62.11.4114-4120.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Calabrese J P, Bissonnette G K. Improved detection of acid mine water stressed coliform bacteria on media containing catalase and sodium pyruvate. Can J Microbiol. 1990;36:544–550. doi: 10.1139/m90-095. [DOI] [PubMed] [Google Scholar]
- 8.Firth J R, Diaper J P, Edwards C. Survival and viability of Vibrio vulnificus in seawater monitored by flow cytometry. Lett Appl Microbiol. 1994;18:268–271. [Google Scholar]
- 9.Halvorson H O, Ziegler N R. Application of statistics to problems in bacteriology. I. A means of determining bacterial population by the dilution method. J Bacteriol. 1933;25:101–121. doi: 10.1128/jb.25.2.101-121.1933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hobbie J E, Daley R J, Jasper S. Use of nucleopore filters for counting bacteria by fluorescence microscopy. Appl Environ Microbiol. 1977;33:1225–1228. doi: 10.1128/aem.33.5.1225-1228.1977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Johnson M, Ray B, Speck M L. Freeze-injury in cell wall and its repair in Lactobacillus acidophilus. Cryo-Letters. 1984;5:171–176. [Google Scholar]
- 12.Kaprelyants A S, Mukamolova G V, Kell D B. Estimation of dormant Micrococcus luteus cells by penicillin lysis and by resuscitation in cell-free spent culture medium at high dilution. FEMS Microbiol Lett. 1994;115:347–352. [Google Scholar]
- 13.Kell D B, Kaprelyants A S, Weichart D H, Harwood C R, Barer M R. Viability and activity in readily culturable bacteria: a review and discussion of the practical issues. Antonie Leeuwenhoek. 1998;73:169–187. doi: 10.1023/a:1000664013047. [DOI] [PubMed] [Google Scholar]
- 14.Lee R M, Hartman P A. Optimal pyruvate concentration for the recovery of coliforms from water. J Food Prot. 1989;52:119–121. doi: 10.4315/0362-028X-52.2.119. [DOI] [PubMed] [Google Scholar]
- 15.Lloyd D, Hayes A J. Vigour, vitality and viability of microorganisms. FEMS Microbiol Lett. 1995;133:1–7. [Google Scholar]
- 16.Mackey B M, Seymour D A. The effect of catalase on recovery of heat-injured DNA-repair mutants of Escherichia coli. J Gen Microbiol. 1987;133:1601–1610. doi: 10.1099/00221287-133-6-1601. [DOI] [PubMed] [Google Scholar]
- 17.Martin S E, Flowers R S, Ordal Z J. Catalase: its effect on microbial enumeration. Appl Environ Microbiol. 1976;32:731–734. doi: 10.1128/aem.32.5.731-734.1976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.McDonald L C, Hackney C R, Ray B. Enhanced recovery of injured Escherichia coli by compounds that degrade hydrogen peroxide or block its formation. Appl Environ Microbiol. 1983;45:360–365. doi: 10.1128/aem.45.2.360-365.1983. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.McFeters G A. Enumeration, occurrence, and significance of injured indicator bacteria in drinking water. In: McFeters G A, editor. Drinking water microbiology. New York, N.Y: Springer-Verlag; 1990. pp. 478–492. [Google Scholar]
- 20.Mipuri R G, Jones W L, McFeters G A, Ridgway H F. Physiological stress in batch cultures of Pseudomonas putida 54G during toluene degradation. J Ind Microbiol Biotechnol. 1997;18:406–413. doi: 10.1038/sj.jim.2900407. [DOI] [PubMed] [Google Scholar]
- 21.Mizunoe Y, Wai S N, Takade A, Yoshida S. Restoration of culturability of starvation-stressed and low-temperature-stressed Escherichia coli O157 cells by using H2O2-degrading compounds. Arch Microbiol. 1999;172:63–67. doi: 10.1007/s002030050741. [DOI] [PubMed] [Google Scholar]
- 22.Mizunoe Y, Wai S N, Ishikawa T, Takade A, Yoshida S. Resuscitation of viable but nonculturable cells of Vibrio parahaemolyticus induced at low temperature under starvation. FEMS Microbiol Lett. 2000;186:115–120. doi: 10.1111/j.1574-6968.2000.tb09091.x. [DOI] [PubMed] [Google Scholar]
- 23.Nilsson L, Oliver J D, Kjelleberg S. Resuscitation of Vibrio vulnificus from the viable but nonculturable state. J Bacteriol. 1991;173:5054–5059. doi: 10.1128/jb.173.16.5054-5059.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Oliver J D. Formation of viable but nonculturable cells. In: Kjelleberg S, editor. Starvation in bacteria. New York, N.Y: Plenum Press; 1993. pp. 239–272. [Google Scholar]
- 25.Oliver J D, Bockian R. In vivo resuscitation, and virulence towards mice, of viable but nonculturable cells of Vibrio vulnificus. Appl Environ Microbiol. 1995;61:2620–2623. doi: 10.1128/aem.61.7.2620-2623.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ravel J, Knight I T, Monahan C E, Hill R T, Colwell R R. Temperature-induced recovery of Vibrio cholerae from the viable but nonculturable state: growth or resuscitation? Microbiology. 1995;141:377–383. doi: 10.1099/13500872-141-2-377. [DOI] [PubMed] [Google Scholar]
- 27.Ray B, Speck M L. Repair of injury induced by freezing Escherichia coli as influenced by recovery medium. Appl Microbiol. 1972;24:258–263. doi: 10.1128/am.24.2.258-263.1972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ray B, Speck M L. Freeze-injury in bacteria. Crit Rev Clin Lab Sci. 1973;4:161–213. doi: 10.3109/10408367309151556. [DOI] [PubMed] [Google Scholar]
- 29.Rayman M K, Aris B, El Derea H B. The effect of compounds which degrade hydrogen peroxide on the enumeration of heat-stressed cells of Salmonella senftenberg. Can J Microbiol. 1978;24:883–885. doi: 10.1139/m78-146. [DOI] [PubMed] [Google Scholar]
- 30.Scheusner D L, Busta F F, Speck M L. Injury of bacteria by sanitizers. Appl Microbiol. 1971;21:41–45. doi: 10.1128/am.21.1.41-45.1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Speck M L, Ray B. Effects of freezing and storage on microorganisms in frozen foods: a review. J Food Prot. 1977;40:333–336. doi: 10.4315/0362-028X-40.5.333. [DOI] [PubMed] [Google Scholar]
- 31a.Starks, A. M., T. R. Schoeb, M. L. Tamplin, S. Parveen, T. J. Doyle, P. E. Bomeisl, G. M. Escudero, and P. A. Gulig. Pathogenesis of Infection by Clinical and Environmental Strains of Vibrio vulnificus in Iron Dextran-Treated Mice. Infect. Immun., in press. [DOI] [PMC free article] [PubMed]
- 32.Tamplin M L, Jackson J K, Buchrieser C, Murphree R L, Portier K M, Gangr V, Miller L G, Kaspar C W. Pulsed-field gel electrophoresis and ribotype profiles of clinical and environmental Vibrio vulnificus isolates. Appl Environ Microbiol. 1996;62:3572–3580. doi: 10.1128/aem.62.10.3572-3580.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Votyakova T V, Kaprelyants A S, Kell D B. Influence of viable cells on the resuscitation of dormant cells in Micrococcus luteus cultures held in an extended stationary phase: the population effect. Appl Environ Microbiol. 1994;60:3284–3291. doi: 10.1128/aem.60.9.3284-3291.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Weichart D, Kjelleberg S. Stress resistance and recovery potential of culturable and viable but nonculturable cells of Vibrio vulnificus. Microbiology. 1996;142:845–853. doi: 10.1099/00221287-142-4-845. [DOI] [PubMed] [Google Scholar]
- 35.Weichart D, McDougald D, Jacobs D, Kjelleberg S. In situ analysis of nucleic acids in cold-induced nonculturable Vibrio vulnificus. Appl Environ Microbiol. 1997;63:2754–2758. doi: 10.1128/aem.63.7.2754-2758.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Weichart D, Oliver J D, Kjelleberg S. Low temperature induced non-culturability and killing of Vibrio vulnificus. FEMS Microbiol Lett. 1992;100:205–210. doi: 10.1111/j.1574-6968.1992.tb14041.x. [DOI] [PubMed] [Google Scholar]
- 37.Whitesides M D, Oliver J D. Resuscitation of Vibrio vulnificus from the viable but nonculturable state. Appl Environ Microbiol. 1997;63:1002–1005. doi: 10.1128/aem.63.3.1002-1005.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wolf P W, Oliver J D. Temperature effects on the viable but non-culturable state of Vibrio vulnificus. FEMS Microbiol Ecol. 1992;101:33–39. [Google Scholar]