Abstract
Hereditary leiomyomatosis and renal cell cancer (HLRCC) is a rare genetic disorder characterised by a germline mutation of the fumarate hydratase (FH) gene, in which affected individuals have a high likelihood of developing cutaneous leiomyomas, uterine leiomyomas and renal cell cancer (RCC). HLRCC-associated RCC is characterised by presentation at a younger age than the sporadic form, its aggressive nature and rapid metastatic potential. We present the case of a 50 year old woman with FH mutation, a history of early onset symptomatic uterine leiomyomas, and RCC with the first reported case of an isolated metastasis to the pituitary gland.
Keywords: Hereditary leiomyomatosis and renal cell cancer, Pituitary gland, Metastasis
Abbreviations: HLRCC, Hereditary Leiomyomatosis and renal cell cancer; RCC, Renal cell carcinoma; IVC, Inferior vena cava
1. Introduction
Hereditary leiomyomatosis and renal cell cancer (HLRCC) is an autosomal dominant syndrome, caused by heterozygous germline mutation in the tumour suppressor gene fumarate hydratase (FH) and phenotypically characterised by the development of cutaneous leiomyomas, uterine leiomyomas and renal cell carcinoma (RCC). Approximately 20% of affected individuals develop HLRCC-associated RCC, an aggressive variant with propensity for early metastasis.1,2 HLRCC-RCC typically presents early, diagnosed on average at 36-years compared to 67-years for sporadic RCC,2 and has poor prognosis with 5-year survival of 31%, and a median survival of 18 months for metastatic disease.3 Unlike sporadic RCC, intracranial metastases have not been reported for HLRCC-RCC. We report a novel case of HLRCC–RCC with metastasis to the pituitary gland.
2. Case presentation
A 50-year old female, non-smoker, presented with one month of left flank pain, bilateral lower limb swelling and dyspnoea. Medical background included subtotal hysterectomy for multiple large uterine leiomyomas at 37-years. No cutaneous leiomyomas were revealed in past history or physical examination. At presentation, CT demonstrated a 129 × 83mm renal mass, replacing the entirety of the left kidney, with tumour thrombus invading the left renal vein and extending into right atrium via the inferior vena cava (IVC). PET scan demonstrated markedly increased metabolism in the renal mass consistent with high grade tumour with extensive caval infiltration, and slightly increased metabolism in ethmoid sinus which was thought secondary to inflammation (Fig. 1).
Fig. 1.
Imaging at initial diagnosis. (A) CT Abdomen and (B) MRI Renal demonstrating large infiltrating left renal mass with associated tumour thrombus extending into the left renal vein, into the IVC and up to the right atrium. There is also extension into the left gonadal vein (C) PET Scan demonstrates markedly enlarged Left kidney (12cm in coronal plane) with increased metabolism and SUV 14.3 consistent with an aggressive neoplasm. The abnormal metabolism extends into the left renal vein, across the midline and into the inferior vena cava, where it extends inferiorly for 29mm, and superiorly to the right atrium. There are no abnormalities in abdominal or pelvic lymph nodes or liver. There is slightly increased metabolism in the left side of the ethmoid sinus which at the time was thought to reflect inflammation.
Patient underwent tumour resection in a multi-team procedure. Right atriotomy under deep hypothermic circulatory arrest allowed for excision of the atrial mass, which extended into the ventricle. Tumour was also present at the ostia of hepatic veins, which was carefully removed. Left nephrectomy with enbloc resection of infrahepatic IVC and left renal vein removed the bulk of the tumour, while the right kidney was autotransplanted into the right iliac fossa as the IVC required complete resection due to the bulky tumour thrombus. The renal vessels were anastamosed to the external iliac vessels with a warm ischemia time of 18 minutes. The IVC was reconstructed using bovine pericardium. Post-operatively the patient had intermittent vasopressor requirements due to right ventricular dysfunction which gradually improved. She was discharged to home day 17. Macroscopically the resected kidney was enlarged at 170 × 100 × 85mm, with virtually no normal kidney parenchyma appreciable. The tumour invaded through capsule of the kidney and into the perinephric fat. The adrenal gland was not involved. Histopathology was consistent with papillary type 2 RCC, with loss of FH immunostaining (Fig. 2). Tumour stage was pT3cN0M0.
Fig. 2.
Findings from resection of primary tumour (A) Left kidney in perirenal fat, renal vein and length of IVC (B) Bivalved specimen demonstrating tumour replacing majority of normal kidney parenchyma with only remnant being 5mm cortex in upper zone. (C) Histopathology demonstrates renal cell carcinoma, papillary type 2 with (D) loss of fumarate hydratase immunostaining. (E and F) Histologically the tumour has uniform appearance, and consists of closely packed bulky papillae, with fibrovascular cores showing variable hyalinization and myxoid change. Composed of large cells, with discrete nucleoli.
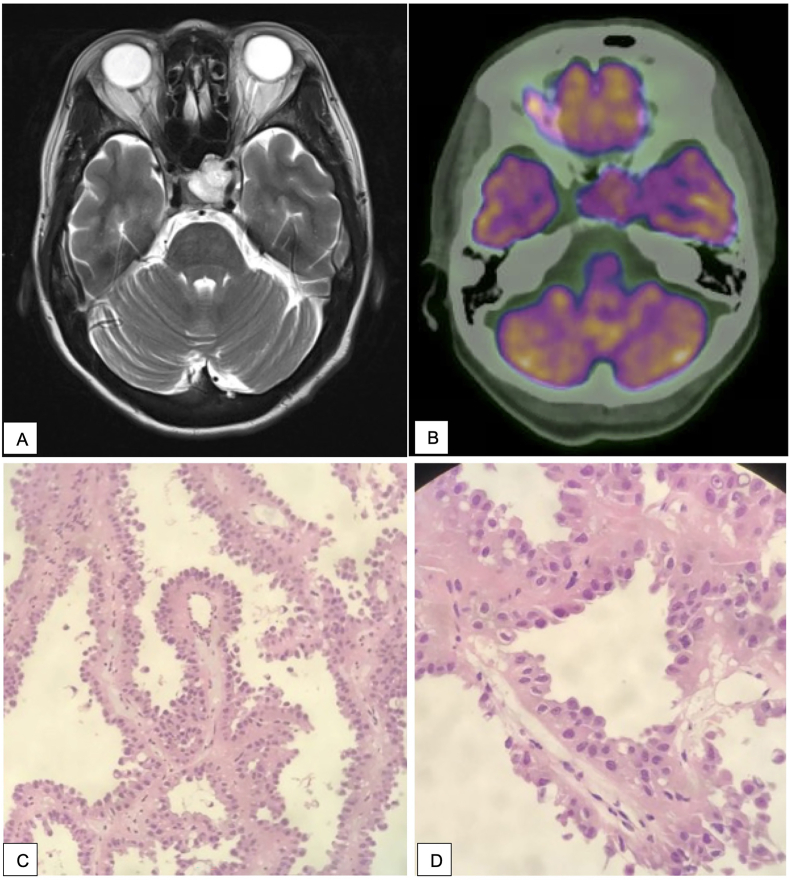
Eleven months postoperatively, the patient presented with headaches, left sided visual disturbance and cranial nerve III palsy (dilated left pupil, left ptosis, ophalmoplegia and diplopia). MRI brain demonstrated a mass in the pituitary gland. PET scan did not demonstrate other areas of disease and she underwent endoscopic transphenoidal debulking of the pituitary tumour, with histopathology consistent with metastatic HLRCC lesion (Fig. 3). Four weeks postoperatively progress MRI demonstrated re-growth of the tumour, and the patient was referred for radiotherapy where she completed 30Gy/10 fractions. Post-radiotherapy MRI demonstrated the pituitary lesion to be stable in size. Four months post-resection of pituitary metastasis, new hepatic metastases were radiologically diagnosed, and the patient was commenced systemic therapy with Bevacizumab and Erlotinib. Progress MRI at six months post-radiotherapy demonstrated marked decrease in the size of the pituitary mass, with a small residual rind of enhancing tissue, and concurrent improvement in symptoms; resolution of headaches, and improvement in ptosis and diplopia.
Fig. 3.
Representation 11 months post nephrectomy. (A) MRI Brain demonstrates a lobulated T2 hyperintense 23 × 15 × 23mm mass lesion expanding the pituitary, with superior extension displacing the optic chiasm and extension into the left cavernous sinus with encasement of the cavernous and supraclinoid segments of the internal carotid arteries. The mass extended to the left orbital apex and contacted the intracranial portions of the optic nerves bilaterally. Inferiorly it invaded through the pituitary fossa floor into the left sphenoid sinus. (B) PET Scan demonstrated moderate FDG avidity (C, D) Histopathology of resected pituitary mass demonstrates identical morphology to renal mass.
3. Discussion
HLRCC-RCC is an aggressive RCC subtype that typically presents as a unilateral, solitary lesion that metastasises when the tumour is as small as 1cm.1 47% of HLRCC-RCC patients present with metastatic disease,3 compared to one-third of patients with sporadic RCC.4 HLRCC-RCC is known to metastasise to liver, adrenal glands, bone (primarily axial), spleen, peritoneum, and lung.1, 2, 3,5 Approximately 40% of cases have reported local invasion at diagnosis.1,2 Here we describe a novel case of a patient with HLRCC-RCC with metastasis to the pituitary gland.
Sporadic RCC most commonly metastasises to lung (45.2%), bone (29.5%), lymph nodes (21.8%), liver (20.3%), adrenal gland (8.9%) and brain (8.1%), however pituitary metastases are rare and typically have poor prognosis.4 FH mutations increase the likelihood of development of a variety of tumours, and thus could enhance the metastatic potential of HLRCC-RCC, and contribute to tumour aggressiveness. A study of 19 patients with HLRCC-RCC, found that patients with completely resected Stage 1 masses had a favourable prognosis with disease-free survival of 10 years.2 However, a significant proportion of patients developed metastatic disease despite complete initial resection, with 47% of patients developing local recurrence or distant metastasis.2 Involvement of regional lymph nodes is a poor prognostic marker, with 77% individuals with lymph node involvement developing distant metastases, and under 30% of these patients surviving to 15 months.5
Treatment of HLRCC associated kidney masses centres on prompt wide-margin surgical excision, typically complete nephrectomy. In other renal tumours associated with syndromes (eg. von Hippel-Lindau and Birt-Hogg-Dube syndromes), surgical intervention is recommended when tumour diameter exceeds 3cm due to their relative slow growth, however HLRCC-RCC poses a higher metastatic risk2 and should be promptly resected. Systemic treatment of metastatic HLRCC-RCC remains in clinical trial phase, with renewed interest in the role of immunotherapy, with reported success utilising bevacizumab plus erlotinib for HLRCC-RCC with nodal metastases.
4. Conclusion
This case report describes the first intracranial metastasis reported for HLRCC-RCC, with rapid tumour growth in the pituitary gland, which has been treated with tumour debulking surgery, radiotherapy then systemically with Bevacizumab and Erlotinib. This case highlights classic aspects of this rare disease, with patient having a prior hysterectomy for multiple symptomatic uterine leiomyoma at a young age, followed by diagnosis of a large, locally invasive, solitary renal tumour. Despite attempt at complete resection, distant metastasis to the pituitary gland was diagnosed within the year, and further metastases detected 6 months thereafter. With combination therapy, the patient has reduced disease and improved symptoms at 20 months post initial diagnosis.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Consent
Written informed consent was obtained from the patient for publication of this Case Report and any accompanying images.
Declaration of competing interest
The authors declare no potential conflicts of interest.
References
- 1.Merino M.J., Torres-Cabala C., Pinto P., Linehan W.M. The morphologic spectrum of kidney tumors in hereditary leiomyomatosis and renal cell carcinoma (HLRCC) syndrome. Am J Surg Pathol. 2007;31(10):1578–1585. doi: 10.1097/PAS.0b013e31804375b8. [DOI] [PubMed] [Google Scholar]
- 2.Grubb R.L., 3rd, Franks M.E., Toro J., et al. Hereditary leiomyomatosis and renal cell cancer: a syndrome associated with an aggressive form of inherited renal cancer. J Urol. 2007;177(6):2074–2079. doi: 10.1016/j.juro.2007.01.155. ; discussion 9-80. [DOI] [PubMed] [Google Scholar]
- 3.Muller M., Ferlicot S., Guillaud-Bataille M., et al. Reassessing the clinical spectrum associated with hereditary leiomyomatosis and renal cell carcinoma syndrome in French FH mutation carriers. Clin Genet. 2017;92(6):606–615. doi: 10.1111/cge.13014. [DOI] [PubMed] [Google Scholar]
- 4.Gong J., Maia M.C., Dizman N., Govindarajan A., Pal S.K. Metastasis in renal cell carcinoma: biology and implications for therapy. Asian J Urol. 2016;3(4):286–292. doi: 10.1016/j.ajur.2016.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen Y.B., Brannon A.R., Toubaji A., et al. Hereditary leiomyomatosis and renal cell carcinoma syndrome-associated renal cancer: recognition of the syndrome by pathologic features and the utility of detecting aberrant succination by immunohistochemistry. Am J Surg Pathol. 2014;38(5):627–637. doi: 10.1097/PAS.0000000000000163. [DOI] [PMC free article] [PubMed] [Google Scholar]