Abstract
Bacterial infections can compromise the physical and biological functionalities of humans and pose a huge economical and psychological burden on infected patients. Nitric oxide (NO) is a broad-spectrum antimicrobial agent, whose mechanism of action is not affected by bacterial resistance. S-nitrosoglutathione (GSNO), an endogenous donor and carrier of NO, has gained increasing attention because of its potent antibacterial activity and efficient biocompatibility. Significant breakthroughs have been made in the application of GSNO in biomaterials. This review is based on the existing evidence that comprehensively summarizes the progress of antimicrobial GSNO applications focusing on their anti-infective performance, underlying antibacterial mechanisms, and application in anti-infective biomaterials. We provide an accurate overview of the roles and applications of GSNO in antibacterial biomaterials and shed new light on the avenues for future studies.
Keywords: Infection, S-nitrosoglutathione, Antibacterial property, Bacterial resistance, Biomaterials
Abbreviations: GSNO, S-Nitrosoglutathione; GSNOR, S-Nitrosoglutathione Reductase; w, week; d, day; h, hour; min, minute; M.Tuberculosis, Mycobacterium tuberculosis; NA, Not available; L.major, Leishmania major; L.amazonensis, Leishmania amazonensis; S.pneumoniae, Streptococcus pneumoniae; P.aeruginosa, Pseudomonas aeruginosa; E. coli, Escherichia coli; DPA, Diethylenetriamine pentaacetic acid; E.tenella, Eimeria tenella; P.berghei, Plasmodium berghei; S.aureus, Staphylococcus aureus; K.Pneumonia, Klebsiella Pneumonia; C.albicans, Candida albicans; DMSO, Dimethyl sulfoxide; M.smegmatis, Mycobacterium smegmatis; ECM, Experimental cerebral malaria; PCVAD, Porcine circovirus-associated disease; A.baumannii, Acinetobacter baumannii; PVC, poly(vinyl chloride); PVA, poly(vinyl alcohol); HUVEC, Human umbilical vein endothelial cells; PVA/PEG, poly(vinyl alcohol)/poly(ethylene glycol); AgNPs, Silver nanoparticles; HCC, Human cervical carcinoma; HDFs, Human dermal fibroblasts; PCL, Polycaprolactone; P.mirabilis, Proteus mirabilis; CS/GE, Chitosan/gelatin; PDA-GSNO NPs, Polydopamine nanoparticles containing GSNO; PTT, Photothermal therapy; PEG, polyethylene glycol; MRSA, Methicillin resistant Staphylococcus aureus; MRPA, Multidrug-resistant Pseudomonas aeruginosa; NO-np, NO-releasing nanoparticulate platform; TMOS, Tetramethylorthosilicate; pSiNPs, porous silicon nanoparticles; PLGA, poly(lactic-co-glycolic acid); ICR, Imprinted control region; PHB, polyhydroxybutyrate; PLA, polylactic acid; Cu, copper; NP, Nanoparticle; ECC, Extracorporeal circulation; ZnO, Zinc oxide; S.epidermidis, Staphylococcus epidermidis; Se, Selenium; MOF, Metal–organic framework; TDC, Tunneled dialysis catheters; PDAM@Cu, polydopamine based copper coatings; S. typhimurium, Salmonella typhimurium; Sp3, Specificity proteins 3; cftr, cystic fibrosis transmembrane conductance regulatory gene; N.meningitidis, Neisseria meningitidis; N. gonorrhoeae, Neisseria gonorrhoeae; H.pylori, Helicobacter pylori; SCI, Spinal cord slices; SAKI, Septic acute kidney injury; BMSCs, Bone marrow stem cells
Graphical abstract
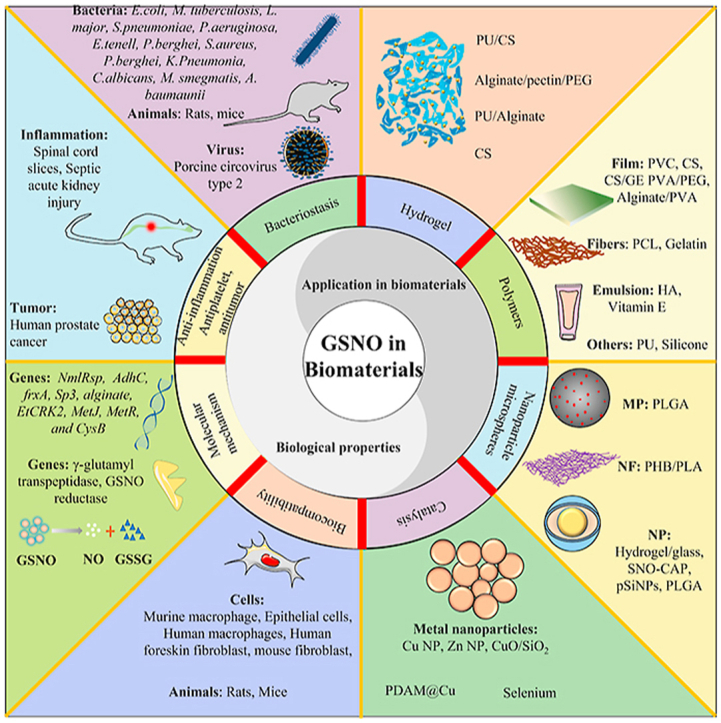
Schematic illustrating role and application of S-nitrosoglutathione in antibacterial biomaterials. H.pylori: Helicobacter pylori; P. aeruginosa: Pseudomonas aeruginosa; M.Tuberculosis: Mycobacterium tuberculosis; L.amazonensis: Leishmania amazonensis; S.pneumoniae: Streptococcus pneumoniae; E. coli: Escherichia coli; S.aureus: Staphylococcus aureus; K.Pneumonia: Klebsiella Pneumonia; M.smegmatis: Mycobacterium smegmatis; P.aeruginosa: Pseudomonas aeruginosa; E.coli: Escherichia coli; MRSA: Methicillin resistant S. aureus; K.Pneumonia: Klebsiella Pneumonia; PVA/PEG: poly(vinyl alcohol)/poly(ethylene glycol); PCL: Polycaprolactone; CS/GE: Chitosan/gelatin; PHB: polyhydroxybutyrate; pSiNPs: porous silicon nanoparticles; TDC: Tunneled dialysis catheters; PDAM@Cu: polydopamine based copper coatings; MOF: Metal–organic framework; MP: Microparticles; NF: Nanofibers; Cu: copper; NP: Nanoparticle; ECC: Extracorporeal circulation; ZnO: Zinc oxide; PLGA: poly(lactic-co-glycolic acid); PLA: polylactic acid. PVC: poly(vinyl chloride); Se: Selenium; HUVEC: Human umbilical vein endothelial cells; HCC: Human cervical carcinoma; HDFs: Human dermal fibroblasts; Sp3: Specificity proteins 3; cftr: cystic fibrosis transmembrane conductance regulatory gene; SCI: Spinal cord slices, SAKI: Septic acute kidney injury.
1. Introduction
Nitric oxide (NO) is an endogenous gaseous molecule produced from arginine by nitric oxide synthase (eNOS, nNOS, or iNOS) [1]. NO is involved in multiple physiological processes, including angiogenesis [2], antiapoptosis, anti-inflammation [[3], [4], [5]], platelet activation [6,7], antithrombosis [8], vasodilation [[9], [10], [11]], and immune response [[12], [13], [14]]. Additionally, NO exhibits broad-spectrum antibacterial activities via the formation of reactive nitrogen species (RNS), including, but not limited to, peroxynitrile, dinitrogen trioxide, and nitrogen dioxide. These RNS molecules can interact with and inactivate bacterial DNA, proteins, and enzymes, thereby contributing to bacterial death [15,16]. Moreover, the characteristic antibacterial mechanisms of NO significantly diminish the risk of developing bacterial resistance, which is a predominant threat for conventional antibacterial drugs [15,16]. Therefore, NO is a promising therapeutic agent for a variety of diseases [[17], [18], [19], [20]].
Despite its desirable properties, the extremely short half-life of gaseous NO (3–4 s) restricts its therapeutic applications, which requires sustainable NO release [21,22]. NO is covalently bound to the sulfur atom of compounds in a C–S–NO pattern and can be released during the heterolytic or homolytic cleavage of the S–N bond [23]. Mammalian glyceryl trinitrate S-nitrosothiols (RSNOs), including S-nitrosoalbumin, glyceryl trinitrate (GTN), S-nitrosohemoglobin, and S-nitrosoglutathione (GSNO), have been confirmed as effective endogenous NO donors and carriers [[23], [24], [25], [26]]. In particular, GSNO has attracted much attention owing to its several advantages over RSNOs [27]. First, as an endogenous RSNO, GSNO is relatively stable and decomposes into oxidized glutathione (GSSG) and glutathione (GSH), which are abundant and biocompatible in animal cells [28]. Second, in addition to its antibacterial activity, GSNO plays a critical role in inflammation and host defense mediated by macrophages [29]. Third, endogenous GSNO was demonstrated to promote the production of vascular endothelial growth factor (VEGF), regulate angiogenesis, and prevent embolization of the vasculature [[30], [31], [32]]. Fourth, GSNO differs from most of the other RSNOs because of its effective direct contribution to the S-nitrosation of enzymes containing sulfhydryl, during which the thiol groups are blocked on the enzymes reversibly [33,34], and lastly GSNO is relatively easier to synthesize and purify [27]. Therefore, GSNO is a desirable agent for endogenous NO production.
GSNO is metabolized in vivo by the conserved enzyme GSNO reductase (GSNOR) [35,36]. In human blood, GSNO is present at a concentration ranging from 0.02 to 0.20 μM and functions as an NO carrier [[37], [38], [39]]. Pharmacological approaches for increasing GSNO have been applied in clinical practice; for example, inhibiting the activity of GSNOR with the first-in-class inhibitor of GSNOR (N6022, N30 Pharmaceuticals) has been used to treat cystic fibrosis patients [35,[40], [41], [42], [43]]. In vitro, GSNO is chemically synthesized in a fast, efficient, and productive manner via an equimolar S-nitrosation reaction between nitrous acid and reduced GSH [27]. Briefly, after cooling in ice bath and purging under nitrogen, the GSH dissolved in aqueous HCl is mixed with equal-molar sodium nitrite followed by stirring in the absence of light. The formed GSNO is precipitated using acetone, separated by vacuum filtration, and washed using ice-cold water and acetone. Finally, the prepared GSNO is stored at −20 °C after drying under vacuum at room temperature [30]. The amount of GSNO was quantified using ultraviolet–visible spectrophotometry at 335 nm [44].
Therefore, GSNO has attracted increasing attention from researchers; in particular, it has become a hotspot for biomaterials in the last decade, such as hydrogels, polymers, films, nanoparticles, microspheres, implantable devices, and blood-contacting devices. This review summarizes the progress made in the applications of GSNO in anti-infective biomaterials based on existing evidence (Graphical abstract), and mainly includes the biological properties of GSNO and its application in biomaterials and provides a useful beacon for future research.
2. Antibacterial property of GSNO and associated mechanisms
2.1. Antibacterial performance of GSNO
GSNO possesses antibacterial activity owing to its ability in the formation of NO. This activity is influenced by various factors, including the bacterial strain, GSNO dose and concentration, treatment duration, and NO release kinetics. Numerous studies have been conducted to evaluate the antibacterial properties of GSNO in various diseases (Table 1), including pulmonary infection [40,[45], [46], [47]], cutaneous infection [23,48,49], cerebral malaria [50], cystic fibrosis [51,52], and digestive tract disease [53] (Fig. 1).
Table 1.
Antibacterial properties of S-nitrosoglutathione and associated mechanisms.
| Author | Year | Disease | Solute/GSNO Concentration | Bacterial/Strain Number | Treatment Time | Cells/Number | Animals | Findings |
|---|---|---|---|---|---|---|---|---|
| Marcinkiewicz, J. [65] | 1997 | NA | NA/0.1-10 mM | E.coli/ATCC 25922 | 1 h | NA | NA | GSNO strongly affected the cytotoxic activity of neutrophils and macrophages. |
| Venketaraman, V. [58] | 2005 | Tuberculosis | PBS/5 mM | M. tuberculosis/H37Rv | 1 and 72 h | Murine macrophage cell line/J774.1 | NA | GSNO at a 5 mM concentration was bactericidal for H37Rv via nitrite and NO. |
| Dayaram, Y. K. [75] | 2006 | Tuberculosis | PBS/5 mM | M. tuberculosis/H37Rv | 5 d | Murine macrophage cell line/J774.1 | NA | γ-glutamyl transpeptidase mutant was resistant to the antibacterial properties of GSH/GSNO |
| de Souza, G. F. [23] | 2006 | Cutaneous leishmaniasis | PBS/10−2 M | L. major | 24 h | NA | NA | GSNO had leishmanicidal activity via S-nitrosation reactions, especially the cysteine proteases. |
| L. amazonensis | ||||||||
| Nahrevanian, H. [49] | 2007 | Cutaneous leishmaniasis | DMSO/olive oil/10 mg/kg | L.major/IR/75 | 6, 8 and 10 w | NA | Balb/c mice | Both SNOG and DMSO/olive oil had leishmanicidal activity. |
| Stroeher, U. H. [47] | 2007 | Pneumonia | PBS/2.5 mM | S.pneumoniae/D39 | 1 h | NA | CD1 mice | Both NmlRsp and AdhC were required for survival in blood |
| Wood, S. R. [56] | 2007 | Cystic fibrosis | NaCl solution/5 mM | P.aeruginosa/PAO578II | 0.5 h | Epithelial Cells/IB3-1 and S9 | NA | GSNO may inhibited the conversion of P. aeruginosa via suppress alginate biosynthesis. |
| Jarboe, L. R. [53] | 2008 | Digestive disease | DPA/0.5 mM | E.coli/BW25113 | 1 h | NA | NA | GSNO altered regulatory activity of MetJ, MetR, and CysB |
| Li, J. [80] | 2010 | NA | NA/20 mM | E.tenella/Yangzhou | 12 h | NA | NA | The inhabitation of GSNO on oocysts was mediated by expression of EtCRK2 mRNA. |
| Zanini, G. M. [50] | 2012 | Malaria | NA/0.035-3.5 mg/mouse | P.berghei/ANKA | 8 d | NA | C57Bl/6 mice | GSNO prevented experimental cerebral malaria development in a wide range of doses |
| Costa, I. S. [48] | 2013 | Localized cutaneous leishmaniasis | NA/0.1-0.3 mM | L. major | 5 w | Human THP-1 macrophages | BALB/c mice | GSNO suppressed lesion growth, reduced the parasite load, and induced healing. |
| L. braziliensis | C57BL/6 mice | |||||||
| Nobre, L. S. [46] | 2013 | NA | NA/0.05-0.15 mM | S.aureus/NCTC 8325 | 2 h | NA | NA | GSNO significantly altered the expression of regulatory function-associated genes. |
| Ong, P. K. [63] | 2013 | Malaria | NA/NA | P.berghei/ANKA | 6 d | NA | C57BL/6 mice | Pial arterioles dilated in exposure to GSNO |
| Tang, C. H. [40] | 2013 | Pneumonia | NA/NA | K.Pneumonia/ATCC® 43816™ | NA | NA | C57BL/6 mice | GSNOR-Deficient Mice had increased susceptibility to K. Pneumonia and Mortality |
| Liao, Z. [79] | 2016 | Fungal infection | DMSO/0.6 mM | C.albicans/SC5314 | 1 h | NA | NA | GSNO and l-arginine could enhance the antifungal activity of Shikonin. |
| C.albicans/SN250 | ||||||||
| C.albicans/cta4Δ/Δ | ||||||||
| C.albicans/yhb1Δ/Δ | ||||||||
| Neufeld, B. H. [57] | 2016 | Infection | NA/5–25 mM | P.aeruginosa/PAO | 24 h | NA | NA | The GSNO concentration causing at least a 90% reduction in bacterial biofilm viability was 10 mM |
| Vargas, D. [64] | 2016 | NA | NA/NA | M. smegmatis/mc2155 | 60 h | NA | NA | GSNOR were required for normal biofilm formation |
| Elphinstone, R. E. [62] | 2017 | Cerebral malaria | NA/NA | P.berghei/ANKA | NA | NA | C57BL/6 mice | GSNOR deficiency was associated with better survival in ECM. |
| Liu, C. [67] | 2017 | PCVAD | NA/10 mM | Porcine circovirus type 2 | In-vitro 72 h In-vivo 7 d | PK-15 cells | BALB/c mice | GSNO suppresses PCV2 infection in PK-15 cells and BALB/c mice. |
| Das, T. [45] | 2019 | Chronic Lung Infection | NA/10 mM | P. aeruginosa/PAO1 | 24 h | Human foreskin fibroblast/HFF-1 | NA | GSNO disrupted biofilms of both P. aeruginosa PAO1 and multidrug resistant A. baumaunii |
| A. baumaunii/MRAB 015069 |
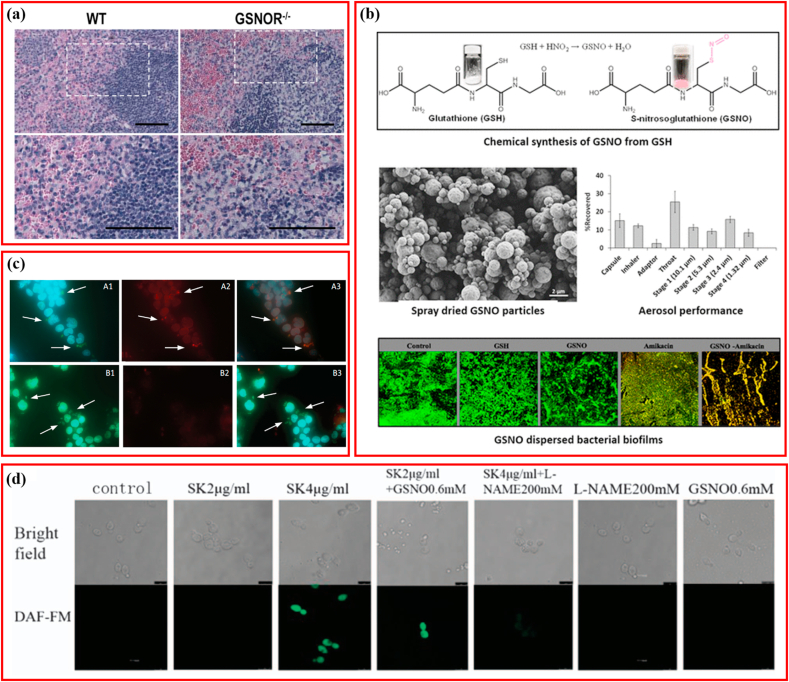
Fig. 1.
Schematic illustrating the antibacterial activity of S-nitrosoglutathione. (a) The antibacterial property of GSNO for K. Pneumonia. Adapted with permission from Ref. [40], copyright 2013 Elsevier Ltd. (b) The antibacterial property of spray-dried GSNO particles for P. aeruginosa and multidrug resistant A. baumaunii. Adapted with permission from Ref. [45], copyright 2019 American Chemical Society. (c) The antibacterial property of GSNO for L. major and L. braziliensis. Adapted with permission from Ref. [48], copyright 2013 Oxford University Press. (d) The antifungal property of GSNO for C. albicans. Adapted with permission from Ref. [79], copyright 2016 Springer Nature.
The lungs are one of the most susceptible organs to bacteria, and bacterial lung infections result in more than 4 million deaths worldwide every year, and with the emerging antibiotic resistance it necessitates the development of novel therapeutic approaches [54]. Great efforts have been made to evaluate the therapeutic efficacy of GSNO in pulmonary infections [40,[45], [46], [47]]. Low levels of GSNO were insufficient to suppress the biosynthesis of alginate and exacerbation of cystic fibrosis in lungs infected with Pseudomonas aeruginosa [55,56]. Under normal culturing conditions (nutrient broth at 37 °C), the concentration of GSNO required to generate adequate NO to reduce >90% P. aeruginosa biofilm on medical-grade polyurethane was determined to be 10 mM, which amounted to 2.73 mM NO [57]. GSNO at a concentration of 5 mM was demonstrated to be bactericidal against Mycobacterium tuberculosis in the detection of colony-forming units and determination of optical density [58]. As previously mentioned, GSNO is metabolized by GSNOR in vivo. One study explored the antibacterial activity of GSNO via gene editing, and found that mice deficient in GSNOR (GSNOR−/−) were more susceptible to Klebsiella pneumoniae derived pulmonary infection, indicating that GSNOR inhibitor should be applied carefully when monitoring infection in clinical trials [40]. The representative histological section staining of K. pneumoniae-infected spleen of wild-type and GSNOR−/− mice is shown in Fig. 1a (The bottom row represents magnification of the images in the top row. Bar = 50 μm) [40]. Using spray drying, inhalable GSNO powders possessing aerosol properties were produced that demonstrated higher efficiency in disrupting biofilms of multidrug-resistant Acinetobacter baumannii and P. aeruginosa, compared to GSH alone (Fig. 1b) [45]. Additionally, GSNO displayed a synergistic effect with amikacin in inhibiting A. baumannii, and also enhanced the growth and confluence of human foreskin fibroblasts (HFF-1).
Numerous species of Leishmania can survive as intracellular non-flagellated amastigotes in macrophages, resulting in infectious cutaneous lesions. Localized cutaneous leishmaniasis (LCL) of a single ulcerated lesion dominates as the most common clinical presentation, followed by mucosal/mucocutaneous patterns, and diffuse cutaneous leishmaniasis [49]. GSNO has been shown to possess leishmanicidal activity, suggesting its potential as a promising alternative for the management of cutaneous leishmaniasis [23,48,49]. The in vitro 50% inhibitory concentrations (IC50) of GSNO against Leishmania amazonensis and Leishmania major were 68.9 ± 7.9 and 68.8 ± 22.86 μmol L−1, respectively [23]. Additionally, reducing agents (ascorbic acid and dithiothreitol) were able to revert protein S-nitrosation induced by GSNO, and decompose GSNO directly, which resulted in the reversal of GSNO's antibacterial activity against Leishmania, indicating that GSNO functions via transnitrosation of proteins [23]. Balb/c mice infected with L. major MRHO/IR/75/ER were treated with the NO inducer lipopolysaccharide (LPS) and GSNO simultaneously; LPS was beneficial for elevating plasma reactive nitrogen intermediates (RNI) and reducing the proliferation of amastigotes inside macrophages, but exhibited no effect on lesion size. The same results of decreasing lesion size and eliminating amastigotes within lesions were obtained on treatment with GSNO and its drug vehicle (DMSO–olive oil), indicating that these performances were associated more with the drug vehicle than with the GSNO [49]. For interferon-γ-knockout mice infected with L. braziliensis, topical application of GSNO suppressed lesion growth notably. The topical application of GSNO showed comparable therapeutic efficiency to intravenous administration of amphotericin B (AMB) for BALB/c mice infected with L. major [48]. In addition, in vitro immunofluorescence staining demonstrated that treatment with GSNO could result in significant protein S-nitrosation in L. major-infected THP-1 cultures with the arrows pointed to intracellular parasites, indicating that the anti-infective effect of GSNO may be mediated by S-nitrosation ( × 60 objective lens, red fluorescence: antinitrocysteine antibody indicating S-nitration; green fluorescence: anti-Leishmania antibody indicating Leishmania; blue fluorescence: DAPI indicating cell nuclei) (Fig. 1c) [48].
Cerebral malaria (CM) is a lethal encephalopathy caused by the malaria parasite and is one of the leading causes of death in children under the age of five years [59,60]. Experimental cerebral malaria (ECM) induced using Plasmodium berghei ANKA (PbA) is distinguished by complications of brain microcirculation, such as decreased blood flow and resultant vascular collapse [50]. Although administration of dipropylenetriamine-NONOate (DPTANO) notably prevented the progression of ECM in mice, the necessary high dose (2 mg/mouse/day) resulted in undesired potent side-effects, such as hypotension [50,61]. In mice infected with PbA, prophylactic application of GSNO suppressed the growth of parasites and reduced ECM incidence in a wide dose range from 0.035 mg to 3.5 mg, with milder side effects [50]. Moreover, among mice infected with PbA, the mortality of the GSNOR knockout (KO) group was significantly delayed compared to that in the wild-type (WT) group; however, the former group possessed a higher parasite load [62]. Additionally, after treatment with the antimalarial drug (artesunate), GSNOR-KO mice showed notably better survival than the WT group. Interestingly, in mice with ECM, treatment with GSNO superfusion activated the pial arteriole response [63]. In addition to the bacteria described above, GSNO has also been reported to show latent antibacterial activity against Streptococcus pneumoniae [47], Staphylococcus aureus [46], Mycobacterium smegmatis [64], Escherichia coli [53,65], Helicobacter pylori [66], and porcine circovirus type 2 [67].
2.2. Underlying mechanisms contributing to GSNO's antimicrobial properties
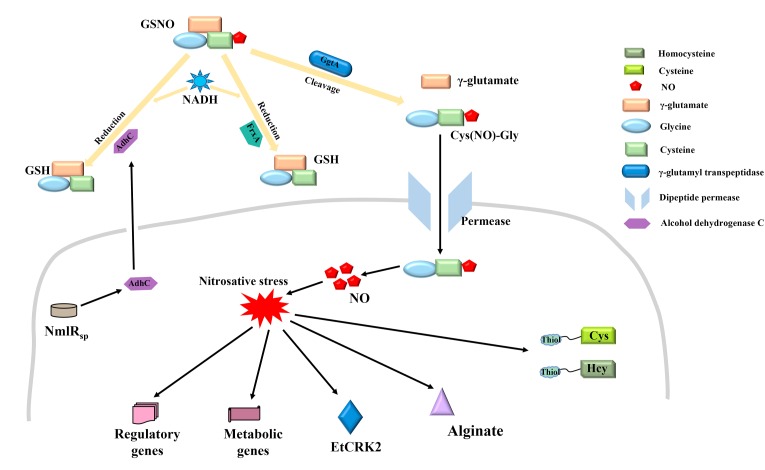
NO is known to play an antibacterial role via the formation of RNS and induction of nitrosative stress, which mediates the bacteriostatic properties of GSNO [68]. However, the underlying molecular mechanisms were not clarified completely (Fig. 2). In addition, the specific mechanisms accounting for the antimicrobial performance of GSNO may vary among different bacteria, especially mutant bacteria. Several critical genes and proteins have been demonstrated to be crucial for the antibacterial activity of GSNO, because the expression of their products could influence multiple processes associated with GSNO, including transport, decomposition, and action (Fig. 2).
Fig. 2.
Schematic illustrating antibacterial mechanisms of S-nitrosoglutathione.
Alginate is a critical independent virulence factor that contributes to the formation of a viscous polysaccharide biofilm-like matrix, making bacteria resistant to pharmacological attack (antibiotics) and host antibacterial innate-immunity (RNS) [51,52,56,[69], [70], [71]]. Alginate promotes the conversion of P. aeruginosa to mucoid phenotypes in cystic fibrosis, resulting in a poor clinical prognosis [56,72]. GSNO induced nitrosative stress downregulates the expression of most genes related to alginate synthesis and reduces alginate protein production in mucoid P. aeruginosa, indicating a viable strategy to suppress P. aeruginosa [56]. GSH and GSNO, tripeptides comprising γ-glutamate-cysteine-glycine, cannot access the bacteria directly. Glutamate is first cleaved from GSNO by γ-glutamyl transpeptidase (GgtA), and the resultant Cys(NO)-Gly dipeptide is transported into bacteria via the dipeptide permease, which is a multicomponent ABC transporter [73,74]. In M. tuberculosis, GgtA mutants may contribute to GSNO resistance and improve survival inside macrophages compared to the wild-type strain [75]. Neisseria merR-like regulator (NmlR) is a novel transcriptional regulator. NmlR is cotranscribed with and manages the expression level of AdhC (alcohol dehydrogenase in class 3), which is capable of metabolizing GSNO using NADH as a reducing agent [76,77]. In S. pneumoniae, mutations in NmlRsp and AdhC weakened the AdhC-derived oxidoreductase activity and became more sensitive to GSNO compared with wild-type bacteria, indicating an NmlRsp-AdhC resistance system to GSNO [47]. Additionally, mutational analysis showed that GSNO oxidoreductase was AdhC-dependent in N. meningitidis but not in N. gonorrhoeae [78].
Similarly, another NADH-dependent oxidoreductase, FrxA, was shown to reduce GSNO, in addition to metabolizing metronidazole and nitrofurans in H. pylori [66]. Mutation of the FrxA-coding gene enhances the susceptibility of H. pylori to GSNO, and FrxA was shown to contribute to the infection of macrophages and mice. Whole-genome microarray detection revealed that GSNO-derived nitrosative stress had a notable impact on the transcription of genes associated with regulatory function. Thus, suggesting a key role for GSNO in inhibiting bacterial respiration, interfering with zinc metabolism, and unbalancing intracellular potassium levels in S. aureus [46]. Interestingly, the combination of GSNO and hydrogen peroxide affects the transcription of genes involved in iron metabolism and the cell envelope [46]. One study mapped the response network of E. coli to GSNO using a novel integrated biochemical analysis and found that GSNO mainly targeted thiols-containing homocysteine (Hcy) and cysteine (Cys) rather than the metal groups, thereby primarily modifying the metabolic and regulatory schemes [53]. GSNO may enhance the antifungal capability of shikonin which exhibits antibacterial activity through inducing NO to regulate YHB1 and CTA4 intracellularly in Candida albicans [79]. As shown in Fig. 1d, significant induction of NO production (green fluorescence) can be seen in the suspension cells incubated with 2 μg/mL SK and 0.6 mM GSNO compared to 2 μg/mL SK alone under confocal laser scanning microscope (Bar = 7.5 μm). However, the association between GSNO and YHB1/CTA4 was not further investigated. GSNO showed an inhibitory effect on Eimeria tenella, although limited to early-stage sporulation, primarily by suppressing the transcriptional expression of cyclin-dependent kinase related protein-2 (EtCRK2) [80]. Additionally, GSNO has been reported to result in oxygen independent cytostasis, which may be most correlated with the antimicrobial or antiproliferative performance of NO [73]. Additionally, heterolytic NO+ transfer and not homolytic NO- release, accounted for the cytostasis [73]. Some researchers proposed that the function of GSNO was concentration-dependent, since low-concentrations of GSNO facilitates Sp3 to bind to ‘housekeeping’ genes like cftr, whereas nitrosative stress induced concentration GSNO cut off Sp3-dependent transcription [81].
Overall, GSNO exerts an antibacterial effect via NO release. Although it was reported that GSNO can affect bacterial activity via regulation of bacterial genes and proteins, this regulatory effect was also mediated by the NO-induced nitrosative stress.
3. Application of GSNO in antibacterial biomaterials
Among critically ill patients, 47.4% of hospital-acquired infections (HAIs) result from medical device-associated infections (MDAI) [82,83]. The high incidence of MDAI necessitates the antibacterial modification of medical devices, especially in the case of implantable devices. However, the direct application of GSNO exhibits an unpredictable decomposition rate in the aqueous phase [84,85]. Thus, encapsulating GSNO in devices exhibited dual effects by enhancing the antibacterial activity of medical devices and slowing GSNO decomposition. Recent studies have made great breakthroughs in functionalizing medical devices and pharmaceutical preparations by loading GSNO.
3.1. Polymers loading GSNO for antibacterial application
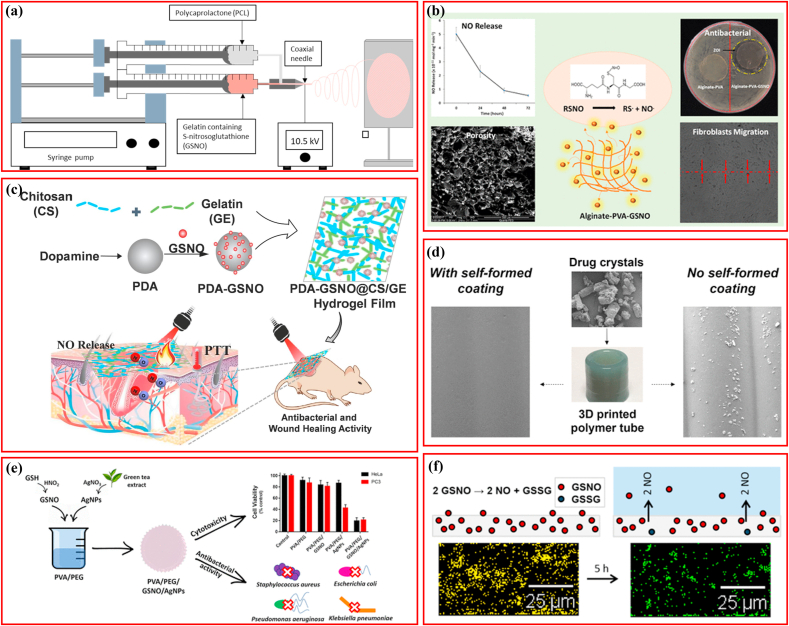
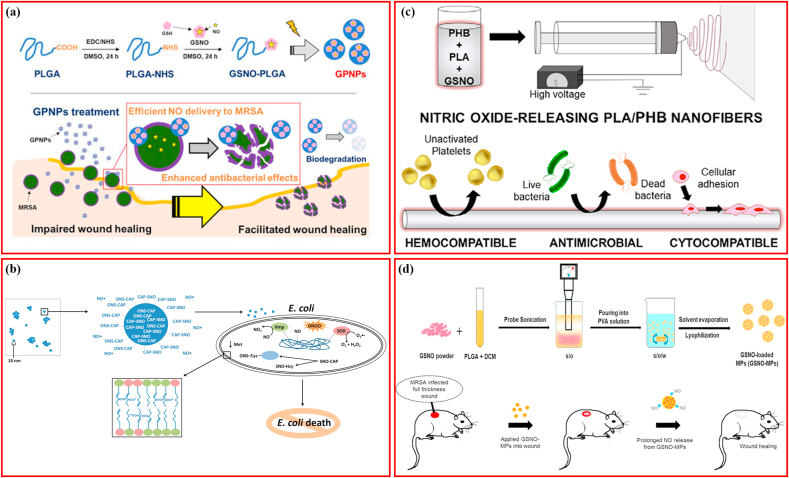
Wound healing is a well-coordinated process comprising inflammation, cellular proliferation, deposition of extracellular matrix, and tissue remodeling. Bacterial infections represent a common menace for wounds, which usually disrupt the healing process [22,86]. The polymers loaded with GSNO are listed in Table 2. Fiber scaffolds comprising gelatin, GSNO, and polycaprolactone (PCL) were manufactured via electrospinning for wound healing and pathogen elimination, wherein GSNO was encapsulated in shells (Fig. 3a) [44]. A consistent and prolonged NO release profile >4 days was identified in the coaxial fibers, whereas large GSNO release and leaching was detected in the blended fibers. Both fibers contributed to a 3-log reduction in S. aureus viability without any in vitro cytotoxicity to the mouse fibroblast cells. GSNO was integrated with chitosan (CS) to produce an NO-releasing chitosan film (CS/NO film) for the treatment of full-thickness wounds, in which the NO-releasing feature followed the Korsmeyer–Peppas model with Fickian diffusion kinetics [22]. In addition, this CS/NO film showed more potent bactericidal properties against S. aureus and P. aeruginosa than the CS film, and accelerated re-epithelialization and healing of full-thickness wounds favorably. In an extended study, the CS/NO film demonstrated anti-biofilm activity against MRSA and promoted wound healing in MRSA-infected diabetic mice, with CS cooperating with NO to accelerate healing synergistically [21]. Similar to the CS/NO films, hybrid wound dressings comprising poly vinyl alcohol (PVA) (synthetic polymer), alginate (natural polymer), and GSNO were prepared through cross-linking, were able to eradicate P. aeruginosa and S. aureus with an efficiency of 98.93 ± 0.69% and 99.89 ± 0.40%, respectively; and enhanced proliferation of human endothelial cells by 3-fold in comparison to the control without GSNO, indicating a considerable potential for angiogenesis (Fig. 3b) [30]. Moreover, this alginate−PVA−GSNO dressing promoted a notable proliferation and migration of mouse fibroblasts. To further increase GSNO use and efficiency, one study covalently bound GSNO with polydopamine nanoparticles (PDA-GSNO NPs) to minimize NO loss in aqueous conditions. The PDA-GSNO NPs were then embedded into biocompatible CS/gelatin (GE) composite films possessing favorable hydrophilicity and mechanical-adhesive properties, which were able to release NO for killing bacteria under photo-thermal stimulation (Fig. 3c) [87]. The PDA used is a near-infrared (NIR) light-responsive material, which can transform NIR light into heat efficiently [88,89], provided two benefits for the PDA-SGNO NPs: first, the photothermal conversion of PDA NPs could be an energy source for accelerating controlled NO release; and second, NIR light irradiation-derived local heat conversion could activate the bacterial lysis, also known as photothermal therapy (PTT) [90,91]. The results indicated that the CS/GE/PAD-GSNO composite films possessed potent antimicrobial activities against E. coli and S. aureus in vitro and promoted wound healing in vivo under NIR light irradiation with good biocompatibility, which was similar to some extent to the NO enhanced photodynamic therapy reported recently [20].
Table 2.
Polymers loading S-nitrosoglutathione for antibacterial application.
| Author | Year | Diseases | Polymers | Methods | GSNO | NO releasing | Bacterial/ID | Cells/ID | Animals | Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Joslin, J. M. [134] | 2013 | Devices-associated blood clot and infection | PVC films | Adding GSNO to the Tygon solution and drying | 5-30 w/w% | 0.64 ± 0.5 × 10−10 mol cm−1 min−1 | NA | NA | NA | GSNO-incorporated Tygon film was constructed and concentration of GSNO within the film does not influence NO release profiles. |
| Kim, J. O. [22] | 2015 | Wound infection | Chitosan film | Mixing | 2.5–20 wt% | NA | P. aeruginosa/PAO1 | NA | Sprague–Dawley rats | CS/NO film showed a stronger antibacterial activity and accelerated wound healing |
| S.aureus/RN4220 | ||||||||||
| Pant, J. [30] | 2019 | Skin infections | Alginate/PVA | Cross-linking | NA | 5.01 ± 0.49 × 10-10 mol cm−1 min−1 | S.aureus/ATCC 5538 | Mouse fibroblast cell/ATCC 1658 | NA | Alginate-PVA-GSNO dressings eradicated bacteria and enhanced cellular proliferation and migration. |
| P.aeruginosa/ATCC 27853 | HUVEC | |||||||||
| Rolim, W. R. [95] | 2019 | Infection and tumor | AgNPs-containing PVA/PEG Films | Solvent casting | 2.5 wt% | 4-7 μmol NO/cm2 | E.coli/ATCC 25922 | Human prostate cancer cell/PC3 | NA | GSNO-containing PVA/PEG films demonstrated toxicity toward tumor cells but had no antibacterial effect. |
| S.aureus/ATCC 29213 | ||||||||||
| K.pneumoniae/ATCC 700603 | human foreskin fibroblast cell/HFF-1 | |||||||||
| P.aeruginosa/ATCC-27853 | ||||||||||
| Yapor, J. P. [14] | 2019 | Infection | Emulsion containing HA and vitamin E | Mixing | 1.72 w/w % | 46 ± 4 μmol g−1 | NA | HDFs | NA | GSNO-containing emulsion was produced and showed no cytotoxicity to HDFs |
| Hopkins, S. P. [44] | 2020 | NA | Fibers comprising PCL and gelatin | Electrospinning | 20 wt % | 3.91 ± 0.96 × 10−10 mol mg−1 min−1 | S.aureus/ATCC 5538 | Mouse fibroblast cells/ATCC 1658 | NA | PCL/gelatin fiber containing GSNO demonstrated antibacterial activity and no cytotoxic response. |
| Li, W. [8] | 2021 | Devices-associated inflammation and infection | Silicone | 3D printing | 2 wt % | >10 × 10−10 mol cm−2 min−1 | P.mirabilis/ATCC 29906 | Murine fibroblast cell/L929 | NA | GSNO embedded in the printed silicone matrix released NO about one month. |
| Wang, W. [87] | 2022 | Cutaneous wound infection | CS/GE films | Mixing | NA | NA | E.coli | NA | BALB/c mice | CS/GE films containing GSNO functionalized PDA nanoparticles demonstrated antimicrobial efficacy. |
| S.aureus |
Fig. 3.
Schematic illustrating polymers loading S-nitrosoglutathione for antibacterial application. (a) PCL fiber loading GSNO. Adapted with permission from Ref. [44], copyright 2020 American Chemical Society. (b) PVA loading GSNO. Adapted with permission from Ref. [30], copyright 2019 American Chemical Society. (c) Chitosan/gelatin hydrogel films GSNO. Adapted with permission from Ref. [87], copyright 2022 Elsevier Ltd. (d) 3D-printing silicone loading GSNO. Adapted with permission from Ref. [8], copyright 2021 American Chemical Society. (e) PVA/PEG films loading GSNO. Adapted with permission from Ref. [95], copyright 2019 American Chemical Society. (f) Tygon materials loading GSNO. Adapted with permission from Ref. [134], copyright 2013 American Chemical Society.
In addition to wound healing, the application of GSNO-loaded polymers in other diseases have also been developed. The advantage of personalized device design via 3D printing has garnered increasing attention [92]. A previous study reported a one-step printing strategy for fabricating NO-eluting devices possessing drug-free coating and drug-loaded substrate, with high-viscosity silicone as the 3D printing ink, crystalline-forms of GSNO as the model drug, and low-viscosity silicone as the outermost layer, and the printed devices had a smooth surface coating because of the low-viscosity silicone diffused outward upon deposition (Fig. 3d) [8]. The GSNO-embedded silicone matrix could release NO for up to one month under physiological conditions showed strong antibacterial performance against P. mirabilis and adequate biosafety for murine fibroblast cells. Selenium (Se) is an essential trace element that plays a critical role in multiple Se- enzyme-dependent physiological processes, including endogenous production of NO from RSNO [82,93,94]. One study designed a hierarchical S-nitroso-N-acetylpenicillamine (SNAP)-doped polymer with a blended Se-interface [82]. This polymer composite was able to release NO from the SNAP reservoir and generate NO via the incorporated Se explicitly, resulting in antibacterial activity against S. aureus and E. coli. To make use of the antimicrobial effect GSNO and silver nanoparticles (AgNPs) synergistically, researchers incorporated both into PVA/polyethylene glycol (PVA/PEG) polymeric solid films for topical application (Fig. 3e) [95]. Unexpectedly and inconsistent with previous studies [21,30,87], this film showed no antibacterial properties against S. aureus, E. coli, P. aeruginosa, and K. pneumoniae, but were toxic to human prostate cancer and cervical carcinoma cells [95].
3.2. Hydrogels loading GSNO for antibacterial application
Hydrogel-based medical dressings are ideal for accelerating wound healing because they can build moist environments and are usually injectable [96]. GSNO loading significantly enhanced the anti-infective ability of the hydrogel and provided a novel source for the treatment of infected wounds, as listed in Table 3.
Table 3.
Hydrogels loading S-nitrosoglutathione for antibacterial application.
| Author | Year | Diseases | Hydrogels | Methods | GSNO | NO releasing | Bacterial/ID | Cells/ID | Animals | Findings |
|---|---|---|---|---|---|---|---|---|---|---|
| Pelegrino, M. T. [97] | 2018 | Infection | Pluronic F-127/Chitosan hydrogel | Incorporating | 50 mmol L−1 | 36 mmol L−1 | P.aeruginosa/ATCC 27853 | Vero cells/CCIAL-057 | NA | GSNO-PL/CS hydrogel demonstrated antibacterial effect and no toxicity to the Vero mammalian cell. |
| Lee, J. [15] | 2019 | Cutaneous wound infections | Alginate/pectin/PEG hydrogel | Blending and micronizing | 4 wt % | NA | MRSA/USA 300 | NA | Mice | NO/GP remained stable over four months, which absorbed wound fluid and immediately converted to a hydrogel showing potent antibacterial activity against MRSA. |
| P.aeruginosa/PAO1 | ||||||||||
| Cao, J. [84] | 2020 | Infected wounds | Pluronic/alginate hydrogel | Gelation | NA | >7 days | MRPA/3089 | Mouse fibroblasts/L929 | Mice | GSNO-PL/AL thermoresponsive hydrogel exhibited potent bactericidal activity and accelerated infected wounds healing. |
| MRSA/2200 | ||||||||||
| Choi, M. [21] | 2020 | Infected wounds | Chitosan hydrogel | Incorporating | NA | >3 days | MRSA/FPR3757 | Mouse fibroblasts/L929 | Diabetic mice | NO-releasing chitosan film inhibited bacteria significantly. |
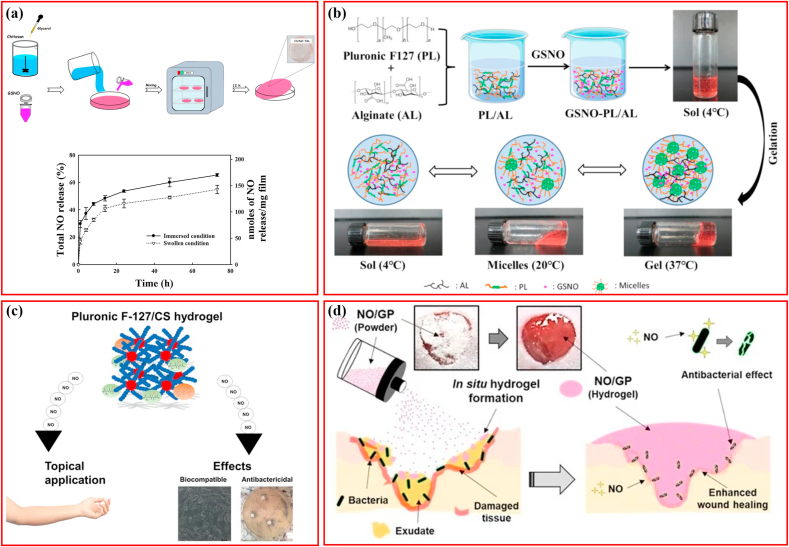
As described previously, CS/NO film fabricated via GSNO and chitosan mixing followed by drying showed anti-biofilm activity against MRSA, and CS in cooperation with NO synergistically accelerated wound healing (Fig. 4a) [21]. Through the incorporation of GSNO into the pluronic F127/alginate (PL/AL) mixture at a given temperature, the thermoresponsive GSNO-PL/AL hydrogel was prepared (Fig. 4b), which actively responded to heat and released NO sustainably for seven days. It was demonstrated to be biocompatible with L929 mouse fibroblasts, bactericidal to MRSA and multidrug-resistant P. aeruginosa (MRPA), and facilitated healing of MRPA-infected wounds, indicating a promising option for dealing with infectious wounds [84]. Considering the intrinsic anti-infective property of CS and thermal reactivity of PL, researchers merged GSNO in PL/CS mixture to fabricate the GSNO-PL/CS hydrogel (Fig. 4c). The hydrogel benefited from the thermoresponsive behavior of PL, controlled release of NO, and antibacterial activity of CS, with a low minimum inhibitory concentration (MIC) of 0.5 μg mL−1 against P. aeruginosa (Fig. 4c) and biocompatibility with the Vero mammalian cells [97]. Despite these advantages, the clinical application of hydrogels loaded with GNSO was flawed by the time-consuming process, unmatched shapes of gels corresponding to the wounds and unsatisfactory storage stability. In contrast, in situ hydrogel-forming powders could benefit from the advantages of both hydrogels and powders, such as high storage stability, plastic shapes fitting the wound surface, application even in the bendable areas of the body, protecting the wound area from the external environment, and maintaining a moist environment by absorbing fluid and converting it to a hydrogel [[98], [99], [100]]. In addition, fluid absorption during hydrogel formation is beneficial for bacterial elimination and wound healing [[101], [102], [103]]. An on-site hydrogel-forming powder comprising GSNO pectin, alginate, and PEG was manufactured through blending and micronizing, which remained stable for >4 months under 4° or 37 °C (Fig. 4d) [15]. When absorbing fluid, the powders were converted to hydrogels that possessed adhesive properties similar to those of commercial-Vaseline petroleum jelly (Fig. 4d). The formed hydrogel could result in 6-log reduction in colony formation of MRSA and P. aeruginosa and accelerate the healing of bacterial-infected full-thickness wounds.
Fig. 4.
Schematic illustrating hydrogels loading S-nitrosoglutathione for antibacterial application. (a) Chitosan hydrogel loading GSNO. Adapted with permission from Ref. [21], copyright 2020 Elsevier Ltd. (b) Pluronic F127/alginate hydrogel loading GSNO. Adapted with permission from Ref. [84], copyright 2020 MDPI (Basel, Switzerland). (c) Pluronic F-127/Chitosan hydrogel loading GSNO. Adapted with permission from Ref. [97], copyright 2018 MDPI (Basel, Switzerland). (d) In situ hydrogel-forming powder containing GSNO. Adapted with permission from Ref. [15], copyright 2019 MDPI (Basel, Switzerland).
3.3. Micro/nano-scale structures loading GSNO for antibacterial application
In recent years micro/nanoscale structures have been applied in multiple clinical applications [104], and nanoparticle-based drug delivery systems have shown distinct advantages in increasing drug delivery efficiency and improving biocompatibility [105]. GSNO-loaded micro/nanoscale structures inaugurated new possibilities for the application of GSNO, as listed in Table 4.
Table 4.
Micro/nano-scale structures loading S-nitrosoglutathione.
| Author | Year | Diseases | NP | Methods | Bacterial/ID | Cells/ID | Animals | Findings |
|---|---|---|---|---|---|---|---|---|
| Friedman, A. J. [33] | 2011 | Infection | Hydrogel/glass NO-np | Forming GSNO in the presence of GSH | P. aeruginosa/Clinical isolates | NA | NA | Combination of NO-np with GSHcould generate GSNO and had potent antimicrobial therapy. |
| K. pneumoniae/Clinical isolates | ||||||||
| E. coli/Clinical isolates | ||||||||
| MRSA/Clinical isolates | ||||||||
| Chouake, J. [107] | 2012 | Infected wounds | Hydrogel/glass NO-np | Forming GSNO in the presence of GSH | P. aeruginosa | NA | Mice | NO-np + GSH accelerated wound closure in P aeruginosaeinfected wounds |
| Mordorski, B. [108] | 2015 | Infection | SNO-CAP-np | Modified TMOS-based sol–gel method | E. coli/Clinical isolates | NA | zebrafish embryos | SNOCAP-np exhibited increased GSNO formation. |
| MRSA/Clinical isolates | ||||||||
| Hasanzadeh Kafshgari, M. [111] | 2016 | Infection | pSiNPs | GSNO was conjugated on the surface of pSiNPs | E.coli | 3T3 fibroblast cells | NA | pSiNPs conjugated with GSNO could release NO, which was boosted in the presence of ascorbic acid. |
| S.aureus | ||||||||
| Hlaing, S. P. [28] | 2018 | MRSA infections | PLGA microparticles | Solid-in-oil-in-water emulsion solvent evaporation method | MRSA | L929 mouse fibroblast cells | ICR mice | NO releasing prolonged over 7 days and exerted antibacterial activity against MRSA |
| Lee, J. [106] | 2020 | MRSA-infected cutaneous wounds | PLGA NP | EDC/NHS coupling reaction | MRSA/USA300 | NA | ICR mice | GSNO-conjugated PLGA nanoparticles could deliver NO more efficiently, enhanced antibacterial effects against MRSA, facilitated infected wound healing. |
| Douglass, M. [110] | 2021 | Thrombosis and infection | PHB/PLA nanofibers | Electrospinning | S. aureus/ATCC 5538 | 3T3 mouse fibroblasts/ATCC 1658 | NA | GSNO-based PHB/PLA nanofibers reduced bacterial adhesion and platelet adhesion. |
Binding hydrophilic GSNO onto hydrophobic polylactic-co-glycolic acid (PLGA) covalently through an EDC/NHS coupling reaction is a feasible method to generate GSNO-conjugated PLGA nanoparticles (GSNO-PLGA; GPNPs), which minimizes the loss of GSNO during fabrication (Fig. 5a) [106]. The prepared GPNPs displayed higher efficiency in delivering NO and improved antibacterial activity against MRSA compared to that of GSNO and facilitated the healing of full-thickness wounds in MRSA challenged mice. In addition to loading the prepared GSNO directly, GSNO can also be synthesized at the site. Inspired by the formation of GSNO via the reaction between NO and GSH, researchers mixed an antibacterial NO-releasing nanoparticulate platform (NO-np) with GSH to produce GSNO, demonstrating that the rapidly produced GSNO was more active against MRSA, P. aeruginosa, E. coli, and K. pneumoniae than the NO-np [33]. In addition, NO-np plus GSH completely inhibited P. aeruginosa within one day and accelerated healing of the infected wounds [107]. To take advantage of this finding, researchers incorporated thiol-containing captopril into NO-np to form SNO-CAP-np, which could release NO with S-nitrosocaptopril providing thiol to facilitate transnitrosylation (Fig. 5b) [108]. Upon exposure to GSH, SNO-CAP-np exhibited stronger transnitrosylation activity and increased GSNO formation compared to that of NO-np, and showed potent antimicrobial properties against E. coli and MRSA in a dose-dependent manner. It was concluded that transnitrosylation was responsible for resolving the issue of E. coli resistance, rather than free NO. Sterically stabilized cationic liposomes (SSCL), cationic liposomes containing phospholipid-conjugated polyethylene glycol (PEGylated phospholipid), are prone to be recognized by and target macrophages via mechanisms associated with endocytic clathrin and caveolae [29,109]. GSNO-loaded SSCL was demonstrated to improve the intracellular NO level by a magnitude of 20, confirming the macrophage-targeting capacity of SSCL [29]. GSNO-loaded SSCL also possessed strong bacteriostatic activity against S. aureus and P. aeruginosa, indicating that liposomal GSNO is a promising anti-infective approach. GSNO was blended with polylactic acid (PLA) and polyhydroxybutyrate (PHB) to fabricate antibacterial nanofibers with different GSNO concentrations ranging from 10 to 20 wt % (Fig. 5c), and the manufactured nanofibers released NO in a GSNO concentration-dependent mode [110]. Moreover, the nanofiber successfully reduced ∼80% viability of S. aureus within 24 h, showed no obvious signs of cytotoxicity, and facilitated the adhesion and functionality of NIH/3T3 mouse fibroblasts, thereby providing an adequate coating to blood-contacting medical devices. NO release from GSNO conjugated to the surface of porous silicon nanoparticles (pSiNPs) can be increased by ascorbic acid, which then eliminates the growth of S. aureus and E. coli within a 2-h incubation, suggesting that NO-releasing pSiNPs are a potential formulation for antibacterial applications [111]. Similar to the GPNPs, GSNO-loaded PLGA microparticles (GSNO-MPs) fabricated through the solvent evaporation method (Fig. 5d) can prolong NO release by more than a week, display antimicrobial activity against MRSA in a time- and concentration-dependent manner, and accelerate healing of MRSA-infected wounds [28].
Fig. 5.
Schematic illustrating micro/nano-scale structures loading S-nitrosoglutathione for antibacterial application. (a) PLGA nanoparticles loading GSNO. Adapted with permission from Ref. [106], copyright 2020 MDPI (Basel, Switzerland). (b) SNO-CAP-nanoparticles loading GSNO. Adapted with permission from Ref. [108], copyright 2015 Future Medicine Ltd. (c) PLA/PHB nanofiber loading GSNO. Adapted with permission from Ref. [110], copyright 2021 WILEY-VCH Verlag GmbH & Co. KGaA, Weinheim. (d) PLGA microparticles loading GSNO. Adapted with permission from Ref. [28], copyright 2018 Elsevier Ltd.
3.4. Catalytic modification promoting NO release from GSNO
Generally, NO-releasing (NOrel) materials release NO under favorable physiological circumstances in a limited manner, which restricts their bactericidal properties as sustained and sufficient NO is necessary for killing bacteria [82]. In contrast, NO-generating (NOgen) materials can generate NO locally and catalytically by triggering natural enzymes or utilizing metal ions such as Cu2+, Zn2+, Fe2+, Ni2+, Co2+ [82,112]. As a NOgen, GSNO can generate NO in a controlled manner. The S–NO bond binds NO to the RSNO species, the disruption of which mediates the production of NO from GSNO and can occur via various mechanisms, such as metal ion catalysis [[113], [114], [115]]. Inspired by this, numerous attempts have been made to promote NO production from GSNO using metal nanoparticles or ions [53,113,115], as listed in Table 5.
Table 5.
Nanoparticles catalyzing NO releasing from S-nitrosoglutathione.
| Author | Year | Disease | NP | Target Materials | Methods | NO flux | Bacterial/ID | Cells/ID | Animals | Findings | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Douglass, M. E. [113] | 2019 | Thrombosis and infection in ECC | Cu NP | Tygon PVC tubing | Coating | 7.1 ± 0.4 × 10−10mol cm−2min−1 | P. aeruginosa/ATCC 27853 | Mouse 3T3 fibroblast cells/ATCC 1658 | Rabbit | Cu NPs promoted NO releasing from GSNO and increased antimicrobial properties of ECC loops without cytotoxicity | |
| S. aureus/ATCC 5538 | |||||||||||
| Doverspike, J. C. [37] | 2019 | Infected wounds | ZnO NP | Vaseline | Mixing | NA | S. aureus/ATCC 25923 | NA | NA | ZnO NPs promoted NO releasing from GSNO and increased antimicrobial properties | |
| S. epidermidis/ATCC 12228 | |||||||||||
| P. aeruginosa/ATCC 27853 | |||||||||||
| E.coli/ATCC 11303 | |||||||||||
| Doverspike, J. C. [126] | 2020 | Blood stream infections | ZnO NP | TDC | Dispensing | NA | P. aeruginosa/ATCC 27853 | NA | Sheep | ZnO NPs promoted NO releasing from GSNO and increased antimicrobial properties | |
| S.aureus/ATCC 25923 | |||||||||||
| Kulyk, K. [120] | 2020 | Thrombosis and inflammation | CuO/SiO2 | NA | Mixing | NA | NA | NA | NA | CuO/SiO2 promoted NO releasing from GSNO | |
| Mendhi, J. [121] | 2021 | Titanium implant | PDAM@Cu | Implant-associated infection | Dip coating | NA | Saliva | NA | NA | PDAM@Cu coatings with NO generating surfaces have a dual anti-biofilm function. | |
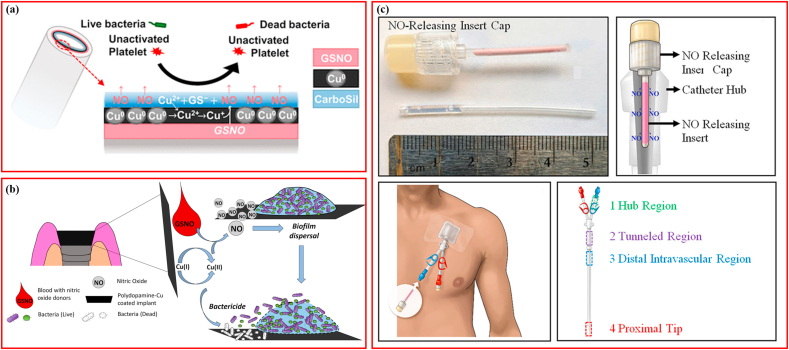
Copper has been demonstrated to mediate GSNO decomposition via the interaction between Cu+2-derived Cu+1 and nitrosothiol, ultimately promoting the release of NO [116]. Therefore, Cu nanoparticles (Cu-NPs) provide an excellent option for the ion catalysis of GSNO. It is worth noting that Cu-NPs not only benefits GSNO decomposition and resultant NO production, but also interacts with other endogenous RSNOs in the blood [117] and exhibits intrinsic antimicrobial activity [118,119]. When using Cu-NPs to modify NO release from GSNO coated on polyvinyl chloride (PVC) (tubing for extracorporeal circulation) (Fig. 6a), the addition of Cu-NPs increased NO flux five-fold within the same release duration, resulting in a 3-log and 1-log reduction in bacterial adhesion of S. aureus and P. aeruginosa, respectively [113]. Additionally, in vitro and in vivo experiments using mouse fibroblasts and rabbits revealed that Cu-NPs plus GSNO improved hemocompatibility, with no cytotoxicity. Copper (II) oxide/silica nanoparticles (CuO/SiO2 NP) prepared through chemisorption of Cu2+ acetylacetonate on nanosilica and subsequent calcination were proven to be capable of catalyzing NO generation from GSNO, and this catalytic activity depended on surface CuO exposure and material surface area but not on the bulk content of CuO [120]. One study fabricated a polydopamine copper (PDAM@Cu) coating on titanium surfaces to modify NO generation using a dip-coating technique (Fig. 6b), and demonstrated that the novel coatings promoted NO production from GSNO and possessed synergistic functions of both biofilm dispersal from NO and bactericidal performance from Cu ions [121]. In blood plasma, GSNO can be catalyzed to be decomposed by a heterogeneous catalyst, CuBTTri, which is a copper-containing metal-organic framework (MOF) [122]. However, neither CuBTTri- nor copper ion-catalyzed GSNO decomposition in the blood could be monitored precisely making it difficult to compare their efficiencies [123]. In response, researchers developed a novel 1H nuclear magnetic resonance (1H NMR) method, which was able to monitor the concentrations of GSH, GSSG, and GSNO in water simultaneously, providing a determined stoichiometry for comparison between copper ions and CuBTTri in catalyzing GSNO decomposition [123].
Fig. 6.
Schematic illustrating catalytic modification promoting NO releasing from S-nitrosoglutathione. (a) Cu nanoparticles catalyzing GSNO. Adapted with permission from Ref. [113], copyright 2019 American Chemical Society. (b) ZnO nanoparticles catalyzing GSNO. Adapted with permission from Ref. [126], copyright 2020 American Chemical Society. (c) PDA-Cu coatings catalyzing GSNO. Adapted with permission from Ref. [121], copyright 2021 Elsevier Ltd.
Additionally, Zinc (Zn) ions are a promising option for catalyzing NO generation, and also possess antibacterial properties [124]. Patients using tunneled dialysis catheters for hemodialysis treatment are usually threatened by bloodstream infections [125]. Modifying the catheter hubs using GSNO and zinc oxide nanoparticles (ZnO-NPs) with ZnO-NPs accelerating NO release from GSNO could yield a reduction of >6.6-log for S. aureus and P. aeruginosa (Fig. 6c) [126]. Similarly, an NO-releasing cream consisting of ZnO-NPs, GSNO, and Vaseline petroleum jelly, designed for infected wounds treatment, showed potent bactericidal properties against S. epidermidis, S. aureus, and P. aeruginosa, which were dependent on the final concentration of GSNO [37].
4. Application of GSNO in antithrombosis and antiinflammation
NO plays a key role in a number of biological activities involved in thrombosis, including inhibiting adhesion and aggregation of platelets, vasodilation, and preventing the proliferation of smooth muscle cells, and therefore, GSNO also regulates thrombus formation [127]. Additionally, the most abundant intracellular antioxidant, GSH, is also produced during GSNO decomposition [128], endowing GSNO with anti-inflammation and anti-oxidation functions (Table 6). However, because of their potent antibacterial activity, the above two functions of GSNO have not received considerable attention.
Table 6.
Other studies about S-nitrosoglutathione.
| Author | Year | Diseases | Bacterial/ID | Cells/ID | Animals | Findings |
|---|---|---|---|---|---|---|
| De Groote, M. A. [73] | 1995 | NA | S. typhimur | NA | NA | GSNO caused oxygenindependent cytostasis. |
| Zaman, K. [81] | 2004 | NA | NA | Type II alveolar epithelial/A549 | NA | Low concentrations of GSNO promoted Sp3 binding to cftr, whereas nitrosative stress-associated GSNO shut off Sp3-dependent transcription. |
| Drosophila SL2 cells | ||||||
| Romero, J. M. [135] | 2006 | Spinal cord slices | NA | NA | Sprague-Dawley rats | Extracellular GSNO mediated protein S-nitrosation in spinal cord slices |
| Potter, A. J. [78] | 2007 | Meningitis and septicemia | N. gonorrhoeae/1291 | NA | NA | NADH GSNO oxidoreductase activity was associated with adhC in N.meningitidis but not in N.gonorrhoea. |
| N.meningitidis/MC58 ¢3 | ||||||
| Justino, M. C. [66] | 2014 | Gastric pathologies | H.pylori/26695 | Murine macrophage/ATCC Tib71 | C57BL/6J mice | NADH-flavin oxidoreductase FrxA of H. pyloripossessed GSNO reductase activity and contributed to the chronic colonization. |
| H.pylori/B128 | ||||||
| Tuttle, R. R. [123] | 2019 | NA | NA | NA | NA | A direct 1H NMR method was designed, which simultaneously monitors GSNO, GSH, and GSSG in water. |
| Fan, H. [5] | 2021 | Septic acute kidney injury | NA | NA | Sprague-Dawley rats | GSNO attenuated Septic acute kidney injury of rats via inhibiting apoptosis, inflammation, and oxidation. |
| Mondal, A. [82] | 2019 | Thrombosis and infection | S.aureus/ATCC 6538 | 3T3 mouse fibroblast cells/ATCC 1658 | NA | The Se interface was able to generate NO in the presence of GSNO |
| Zhang, Q. [133] | 2020 | Thrombus and inflammation | NA | Human monocyte-derived macrophage/THP-1 | NA | GSNO and CD47 modification could enhanced anti-inflammatory/anti-platelet effects. |
Various blood-contacting devices such as sensors, extracorporeal circuits, and intravascular catheters are used to treat thrombosis caused by platelet activation [129,130]. Owing to its antiplatelet and anti-inflammatory properties, NO has become an attractive substance for improving the biocompatibility of these blood-contacting devices [131]. However, it has been demonstrated that physiological NO fluxes comparable to the concentrations necessary for IV devices derived from endothelial cells (0.5–4.0 × 10−10 mol cm−2 min−1) were difficult to maintain [132,133]. The application of GSNO can provide alternatives to resolve the disparities in the field. CD47, a transmembrane protein with anti-platelet properties, can complement NO and improve longevity and effectiveness [133]. Silicone tubing with a CD47 surface was filled with GSNO/ascorbic acid solution, the CD47 coating and NO release worked synergistically and significantly improved the anti-inflammatory and anti-platelet properties of the blood-contacting surface [133]. One study incorporated GSNO into Tygon, a PVC polymer used as an extracorporeal circuit [134]. SEM observation and corresponding energy dispersive spectrometer maps detecting S-containing phase revealed significant alteration after soaking for 5 h in PBS at 37 °C (Fig. 3f). The prepared films could produce an NO release covering the lowest endothelium NO flux, and concentrations of GSNO ranging from 5 to 30 w/w% did not impact the release profiles of NO, indicating the potential of the GSNO-incorporated films in blood-contacting applications [134]. Similarly, the SNAP-Se-1 polymer was also reported to inhibit platelet adhesion by 85.5% in comparison to their respective controls [82].
In septic rats, pretreatment with GSNO significantly decreased mortality, reduced pathological damage to the kidneys, lowered the levels of inflammatory indices, including serum creatinine, IL-1β, and TNF-α, and downregulated the expression of iNOS, COX-2, NO, and PGE2 in the kidneys [5]. GSNO integrated into hyaluronic acid and vitamin E to prepare an emulsion with excellent viscosity, pH, and biocompatibility, allowed moisture retention in dermal tissue and provided a potential platform for wound healing [14]. However, another study indicated that GSNO, rather than S-nitrosocysteine or N2O3, is an effective S-nitrosating agent in the spinal cord slices of rats, and when exposed to high-concentration GSNO, the viable proteins for S-nitrosation were limited, revealing the critical role of GSNO in inflammation status [135].
5. Discussion
Infections associated with respiration [136], medical devices [137], and wounds [138] have resulted in tremendous challenges to human health and socio-economy. In particular, the overuse of antibiotics have resulted in the emergence of bacterial resistance, which is considered as one of the most challenging public health concerns in the 21st century [28,111]. Bacteria secrete and embed within the extracellular polymeric substances (EPS) to form complex communities called biofilms [139]. Mature biofilms build protective microenvironments that shield bacteria from phagocytosis by immune cells, and make them resistant to conventional antibiotics [[140], [141], [142]]. Therefore, there is an urgent need to develop more effective treatment options against bacterial infections.
NO is a readily diffusible, highly reactive, gaseous molecule with powerful inherent oxidant activities. This compound is synthesized via three distinct sub-type NO synthases (NOS) with notably different expressional modes and functional performances: neuronal NOS (nNOS or NOS1), inducible NOS (iNOS or NOS2), and endothelial NOS (eNOS or NOS3) [[143], [144], [145], [146]]. In response to infections induced by various microbial pathogens, both innate and adaptive immune responses promote NO generation by macrophages [64,147,148]. In the early 1990s, the critical role of NO in combating pathogen infection was reported for the first time [111,149]. In the same period, the crucial role of NO in preventing endothelial cell related thrombosis was recognized [120]. As mentioned previously, NO has attracted increasing attention as a novel therapeutic agent for infection owing to its powerful broad-spectrum anti-infective properties, facilitating cellular proliferation and tissue remodeling, and promoting wound healing [15]. It was also reported that NO could prevent biofilm formation and dispersion via cyclic di-GMP by initiating the functions of the effector and triggering biofilm disruption [21,150]. Most importantly, these antibacterial mechanisms are different from conventional antibacterial agents, because NO is not affected by bacterial resistance [28,111].
NO exerts its antibacterial effects through multiple mechanisms. First, NO can react with superoxide anions (O2−) to form cytotoxic peroxynitrite (OONO−), and the highly reactive OONO− oxidizes several cellular targeting molecules, thus collaborating with the respiratory burst of phagocytic cells [151,152]. Second, NO can interact with intracellular metal ions, inactivate essential enzymes, or exhaust cellular iron storage [153]. Third, reactions between NO and thiols can catalyze the formation of disulfide bonds and dysregulate protein function [108]. Fourth, NO may react with DNA directly, leading to deamination [154]. Finally, NO can obtain indirect antibacterial properties by upregulating gamma interferon (IFN-γ) [155] and accelerating the release of superoxide and hydrogen peroxide in neutrophils [156]. In addition, the hydrophobic nature of NO makes it ready to access the membranes [108]. Central to its mechanism of action is the reducing power of radical NO, which subsequently leads to lipid peroxidation and bacterial cell wall rupture [111]. However, some scholars have proposed that it was the transfer of the nitrosonium group (NO+) rather than the NO itself, which accounted for damage to pathogen DNA or enzymes, ultimately inhibiting microbial proliferation [33].
Since endogenous NO was not sufficient and sustainable for antibacterial performance, supplementation with NO was considered. However, the toxicity of the byproducts formed during the generation of NO has emerged as a major concern for the anti-infective application of NO donors, including nitrite, N-diazeniumdiolates (NONOate), and RSNO [37]. Moreover, NONOates have been reported to form N-nitrosamines through a back reaction, and most nitrosamines are carcinogenic [157]. In contrast, RSNO is the most biocompatible NO-releasing agent [37]. As an important NO donor, RSNO exists in the plasma at a concentration of 7 × 10−6 M, including S-nitrosoalbumin and GSNO [120]. The relatively stable GSNO is regarded as an ideal NO carrier for the fabrication of antibacterial biomaterials. NO generated from GSNO was primarily detected using the Griess assay [158], and another product, GSH, was assayed via spectrophotometry and monochlorobimane staining [58,159].
Based on the existing evidence summarized in this review article, we highlighted the potent antibacterial properties of GSNO. Although very few exceptions have been reported, GSNO-modified materials show no significant antimicrobial activity [95]. The reason for this inconsistency may be that the GSNO concentration was too low to generate sufficient NO, or that the GSNO was blocked in the materials during processing and could not act efficiently. The phenomenon of the change in GSNO function in a dose-dependent manner [81] may be explained by the concentration-dependent effect of NO [97]. Low concentrations of NO ranging from picomoles to nanomoles promote vasodilation, cellular proliferation, wound healing, and favorably reduce adhesion and aggregation of platelets [160]. In contrast, high-concentration NO ranging from micromolar to millimolar concentrations exhibits antibacterial and antitumor properties [103]. NO release eliminates the antibacterial mechanisms of GSNO [87,106,107,113]. However, GSNO could also exert anti-infective activity through other biochemical mechanisms, although some scholars proposed that it was the direct transnitrosation and S-thiolation of thiol groups that damaged pathogen DNA or enzymes primarily, ultimately inhibiting microbial proliferation [33,53]. GSNO enhanced the antifungal activity of shikonin, and shikonin inhibited the expression of YHB1 and CAT4 [79]. Considering that both shikonin and GSNO properties are functions of NO production, it is possible that GSNO also plays a role in orchestrating the expression of YHB1 and CAT4. Despite the numerous advantages, GSNO possesses few inevitable weaknesses. First, GSNO is not very stable and usually stored in the dark, where it can degrade and become ineffective under long-time exposure to light, limiting its further application to some extent. Second, although the GSNO can be synthesized efficiently, its production cost is relatively high. Finally, the effect of GSNO, at effetive antimicrobial-concentrations, on critical normal cells, especially bone marrow stem cells (BMSCs) remain unknown.
Considerable achievements have been made in the application of GSNO in antibacterial biomaterials, including hydrogels, nanoparticles, microspheres, and polymer films. Polymers are attractive biomaterials due to their wide source and cost effectiveness, but it has the disadvantage of unsatisfactory biodegradability. Hydrogels benefit from injectable property/plasticity but are criticized due to their weak strength, which does not provide sufficient mechanical support in some specific cases. The advantage of loading GSNO using micro/nano-scale structure is recognized by the sustained controlled-release, however, low drug-loading efficiency and instability remain its major disadvantages. Catalyses using metal ion modification could felicitate the NO release from GSNO positively, however, the uncertainty of biological effects of metallic ions on human body is still a factor that has to be considered. Additionally, favorable anti-thrombosis, anti-inflammation, and antitumor properties are also demonstrated by GSNO. Importantly, light decomposition, metal ion catalysis, and thermal decomposition are powerful accelerators of NO generation from GSNO [[113], [114], [115]]. Photocatalysis showed that 336- and 545-nm GSNO-absorbance irradiation could cleave the S–NO bond photolytically, but the requirement of obvious light exposure restricted its application [113]. Thermal decomposition is difficult to achieve at ambient temperatures, and excessive temperatures may also be harmful to normal tissues [113]. Considering these drawbacks, researchers have integrated metal ions into GSNO for NO-releasing catalysis, compensating for its shortcomings in decomposition [37,82,113,120,121,126]. However, the intrinsic functional activities of the metal particles have not been fully utilized or studied when applied to catalyzing GSNO in biomaterials, such as the osteogenic activity of Zn2+ [161].
Currently, impressive results have been achieved in the research of GSNO, specifically: 1) It was demonstrated that GSNO showed potent antibacterial properties against both gram-negative and gram-positive bacteria; 2) The underlying mechanisms involved in the antibacterial effect were elucidated partly; 3) GSNO has been designed and applied to various antibacterial biomaterials widely and dexterously. While, the application of GSNO in double- and multi-functional biomaterials is rather rare and more efforts should be directed in this filed. In future studies, it would be useful to apply GSNO to biomaterials used in implantable devices, including load-bearing orthopedic scaffolds, thrombosis-challenging intravascular catheters, and coronary stents. In addition to obliterating the common infection challenge, GSNO can also provide unique biochemical effects for individual devices, including NO-inducing vascularization for orthopedic scaffolds [162], NO-derived antithrombosis and antiinflammation for coronary stents [130], and intravascular catheters [163].
6. Conclusion
GSNO has demonstrated significant antibacterial activity against multiple initial and antibiotic-resistant bacteria by generating NO or by transnitrosation. Considerable progress has been made in the application of GSNO in antibacterial biomaterials, and biomaterials incorporating GSNO have shown potent anti-infective performance in vitro and in vivo without apparent cytotoxicity. Further studies are needed to elucidate the antimicrobial mechanisms of GSNO and take advantage of these mechanisms in a more effective and novel manner.
Funding
This study was supported by the Scientific and Technological projects of Zunyi City (Grant No. HZ2020207); Collaborative Innovation Center of Chinese Ministry of Education (2020-39).
Author contributions
JA conceived the original ideas of this manuscript and reviewed the finished manuscript and executed supervision throughout the process. HQ and LP prepared the manuscript, tables, and figures. ZY made critical revision of the manuscript. All authors have read and approved the manuscript.
Declaration of competing interest
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Acknowledgement
None.
References
- 1.Robbins R.A., Grisham M.B. Nitric oxide. Int. J. Biochem. Cell Biol. 1997;29(6):857–860. doi: 10.1016/s1357-2725(96)00167-7. [DOI] [PubMed] [Google Scholar]
- 2.Fukumura D., Gohongi T., Kadambi A., Izumi Y., Ang J., Yun C.O., Buerk D.G., Huang P.L., Jain R.K. Predominant role of endothelial nitric oxide synthase in vascular endothelial growth factor-induced angiogenesis and vascular permeability. Proc. Natl. Acad. Sci. U. S. A. 2001;98(5):2604–2609. doi: 10.1073/pnas.041359198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chung H.T., Choi B.M., Kwon Y.G., Kim Y.M. Interactive relations between nitric oxide (NO) and carbon monoxide (CO): heme oxygenase-1/CO pathway is a key modulator in NO-mediated antiapoptosis and anti-inflammation. Methods Enzymol. 2008;441:329–338. doi: 10.1016/S0076-6879(08)01218-4. [DOI] [PubMed] [Google Scholar]
- 4.Corti A., Franzini M., Scataglini I., Pompella A. Mechanisms and targets of the modulatory action of S-nitrosoglutathione (GSNO) on inflammatory cytokines expression. Arch. Biochem. Biophys. 2014;562:80–91. doi: 10.1016/j.abb.2014.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Fan H., Le J.W., Sun M., Zhu J.H. Pretreatment with S-nitrosoglutathione attenuates septic acute kidney injury in rats by inhibiting inflammation, oxidation, and apoptosis. BioMed Res. Int. 2021;2021 doi: 10.1155/2021/6678165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gkaliagkousi E., Douma S., Zamboulis C., Ferro A. Nitric oxide dysfunction in vascular endothelium and platelets: role in essential hypertension. J. Hypertens. 2009;27(12):2310–2320. doi: 10.1097/HJH.0b013e328330e89a. [DOI] [PubMed] [Google Scholar]
- 7.Nong Z., Hoylaerts M., Van Pelt N., Collen D., Janssens S. Nitric oxide inhalation inhibits platelet aggregation and platelet-mediated pulmonary thrombosis in rats. Circ. Res. 1997;81(5):865–869. doi: 10.1161/01.res.81.5.865. [DOI] [PubMed] [Google Scholar]
- 8.Li W., Yang Y., Ehrhardt C.J., Lewinski N., Gascoyne D., Lucas G., Zhao H., Wang X. 3D printing of antibacterial polymer devices based on nitric oxide release from embedded S-nitrosothiol crystals. ACS Appl. Bio Mater. 2021;4(10):7653–7662. doi: 10.1021/acsabm.1c00887. [DOI] [PubMed] [Google Scholar]
- 9.Liu T., Zhang M., Mukosera G.T., Borchardt D., Li Q., Tipple T.E., Ishtiaq Ahmed A.S., Power G.G., Blood A.B. L-NAME releases nitric oxide and potentiates subsequent nitroglycerin-mediated vasodilation. Redox Biol. 2019;26 doi: 10.1016/j.redox.2019.101238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Marques A.A.M., da Silva C.H.F., de Souza P., de Almeida C.L.B., Cechinel-Filho V., Lourenço E.L.B., Gasparotto Junior A. Nitric oxide and Ca(2+)-activated high-conductance K(+) channels mediate nothofagin-induced endothelium-dependent vasodilation in the perfused rat kidney. Chem. Biol. Interact. 2020;327 doi: 10.1016/j.cbi.2020.109182. [DOI] [PubMed] [Google Scholar]
- 11.Wotherspoon F., Browne D.L., Meeking D.R., Allard S.E., Munday L.J., Shaw K.M., Cummings M.H. The contribution of nitric oxide and vasodilatory prostanoids to bradykinin-mediated vasodilation in Type 1 diabetes. Diabet. Med. : J. Br. Diabet. Assoc. 2005;22(6):697–702. doi: 10.1111/j.1464-5491.2005.01493.x. [DOI] [PubMed] [Google Scholar]
- 12.Bogdan C. Nitric oxide synthase in innate and adaptive immunity: an update. Trends Immunol. 2015;36(3):161–178. doi: 10.1016/j.it.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 13.García-Ortiz A., Serrador J.M. Nitric oxide signaling in T cell-mediated immunity. Trends Mol. Med. 2018;24(4):412–427. doi: 10.1016/j.molmed.2018.02.002. [DOI] [PubMed] [Google Scholar]
- 14.Yapor J.P., Gordon J.L., Henderson C.N., Reynolds M.M. Nitric oxide-releasing emulsion with hyaluronic acid and vitamin E. RSC Adv. 2019;9(38):21873–21880. doi: 10.1039/c9ra03840j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee J., Hlaing S.P., Cao J., Hasan N., Ahn H.J., Song K.W., Yoo J.W. In situ hydrogel-forming/nitric oxide-releasing wound dressing for enhanced antibacterial activity and healing in mice with infected wounds. Pharmaceutics. 2019;11(10) doi: 10.3390/pharmaceutics11100496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones M.L., Ganopolsky J.G., Labbé A., Wahl C., Prakash S. Antimicrobial properties of nitric oxide and its application in antimicrobial formulations and medical devices. Appl. Microbiol. Biotechnol. 2010;88(2):401–407. doi: 10.1007/s00253-010-2733-x. [DOI] [PubMed] [Google Scholar]
- 17.Barnes M., Brisbois E.J. Clinical use of inhaled nitric oxide: local and systemic applications. Free Rad. Biol. Med. 2020;152:422–431. doi: 10.1016/j.freeradbiomed.2019.11.029. [DOI] [PubMed] [Google Scholar]
- 18.Paul S., Pan S., Mukherjee A., De P. Nitric oxide releasing delivery platforms: design, detection, biomedical applications, and future possibilities. Mol. Pharm. 2021;18(9):3181–3205. doi: 10.1021/acs.molpharmaceut.1c00486. [DOI] [PubMed] [Google Scholar]
- 19.Wu M., Lu Z., Wu K., Nam C., Zhang L., Guo J. Recent advances in the development of nitric oxide-releasing biomaterials and their application potentials in chronic wound healing. J. Mater. Chem. B. 2021;9(35):7063–7075. doi: 10.1039/d1tb00847a. [DOI] [PubMed] [Google Scholar]
- 20.Sun Y., Wen R.L., Yu D., Zhu Y.W., Zheng L., Liu X.D., Wang H.R., Yu B.R., Xu F.J. Flexible electrostatic hydrogels from marine organism for nitric oxide-enhanced photodynamic therapy against multidrug-resistant bacterial infection. Sci. China Mater. 2022 [Google Scholar]
- 21.Choi M., Hasan N., Cao J., Lee J., Hlaing S.P., Yoo J.W. Chitosan-based nitric oxide-releasing dressing for anti-biofilm and in vivo healing activities in MRSA biofilm-infected wounds. Int. J. Biol. Macromol. 2020;142:680–692. doi: 10.1016/j.ijbiomac.2019.10.009. [DOI] [PubMed] [Google Scholar]
- 22.Kim J.O., Noh J.K., Thapa R.K., Hasan N., Choi M., Kim J.H., Lee J.H., Ku S.K., Yoo J.W. Nitric oxide-releasing chitosan film for enhanced antibacterial and in vivo wound-healing efficacy. Int. J. Biol. Macromol. 2015;79:217–225. doi: 10.1016/j.ijbiomac.2015.04.073. [DOI] [PubMed] [Google Scholar]
- 23.de Souza G.F., Yokoyama-Yasunaka J.K., Seabra A.B., Miguel D.C., de Oliveira M.G., Uliana S.R. Leishmanicidal activity of primary S-nitrosothiols against Leishmania major and Leishmania amazonensis: implications for the treatment of cutaneous leishmaniasis. Nitric Oxide : Biol. Chem. 2006;15(3):209–216. doi: 10.1016/j.niox.2006.01.011. [DOI] [PubMed] [Google Scholar]
- 24.Hogg N. Biological chemistry and clinical potential of S-nitrosothiols. Free Rad. Biol. Med. 2000;28(10):1478–1486. doi: 10.1016/s0891-5849(00)00248-3. [DOI] [PubMed] [Google Scholar]
- 25.Giustarini D., Milzani A., Colombo R., Dalle-Donne I., Rossi R. Nitric oxide and S-nitrosothiols in human blood. Clin. Chim. Acta Int. J. Clin. Chem. 2003;330(1–2):85–98. doi: 10.1016/s0009-8981(03)00046-9. [DOI] [PubMed] [Google Scholar]
- 26.Clancy R.M., Levartovsky D., Leszczynska-Piziak J., Yegudin J., Abramson S.B. Nitric oxide reacts with intracellular glutathione and activates the hexose monophosphate shunt in human neutrophils: evidence for S-nitrosoglutathione as a bioactive intermediary. Proc. Natl. Acad. Sci. U. S. A. 1994;91(9):3680–3684. doi: 10.1073/pnas.91.9.3680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Broniowska K.A., Diers A.R., Hogg N. S-nitrosoglutathione. Biochim. Biophys. Acta. 2013;1830(5):3173–3181. doi: 10.1016/j.bbagen.2013.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hlaing S.P., Kim J., Lee J., Hasan N., Cao J., Naeem M., Lee E.H., Shin J.H., Jung Y., Lee B.L., Jhun B.H., Yoo J.W. S-Nitrosoglutathione loaded poly(lactic-co-glycolic acid) microparticles for prolonged nitric oxide release and enhanced healing of methicillin-resistant Staphylococcus aureus-infected wounds. Eur. J. Pharm. Biopharm. : Off. J. Arbeitsgemeinschaft Pharm. Verfahrenstechnik e.V. 2018;132:94–102. doi: 10.1016/j.ejpb.2018.09.009. [DOI] [PubMed] [Google Scholar]
- 29.Diab R., Virriat A.S., Ronzani C., Fontanay S., Grandemange S., Elaissari A., Foliguet B., Maincent P., Leroy P., Duvaj R.E., Rihn B.H., Joubert O. Elaboration of sterically stabilized liposomes for S-nitrosoglutathione targeting to macrophages. J. Biomed. Nanotechnol. 2016;12(1):217–230. doi: 10.1166/jbn.2016.2130. [DOI] [PubMed] [Google Scholar]
- 30.Pant J., Pedaparthi S., Hopkins S.P., Goudie M.J., Douglass M.E., Handa H. Antibacterial and cellular response toward a gasotransmitter-based hybrid wound dressing. ACS Biomater. Sci. Eng. 2019;5(8):4002–4012. doi: 10.1021/acsbiomaterials.9b00737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Kaposzta Z., Baskerville P.A., Madge D., Fraser S., Martin J.F., Markus H.S. L-arginine and S-nitrosoglutathione reduce embolization in humans. Circulation. 2001;103(19):2371–2375. doi: 10.1161/01.cir.103.19.2371. [DOI] [PubMed] [Google Scholar]
- 32.Lima B., Lam G.K., Xie L., Diesen D.L., Villamizar N., Nienaber J., Messina E., Bowles D., Kontos C.D., Hare J.M., Stamler J.S., Rockman H.A. Endogenous S-nitrosothiols protect against myocardial injury. Proc. Natl. Acad. Sci. U. S. A. 2009;106(15):6297–6302. doi: 10.1073/pnas.0901043106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Friedman A.J., Blecher K., Schairer D., Tuckman-Vernon C., Nacharaju P., Sanchez D., Gialanella P., Martinez L.R., Friedman J.M., Nosanchuk J.D. Improved antimicrobial efficacy with nitric oxide releasing nanoparticle generated S-nitrosoglutathione. Nitric Oxide : Biol. Chem. 2011;25(4):381–386. doi: 10.1016/j.niox.2011.09.001. [DOI] [PubMed] [Google Scholar]
- 34.Butler A.R., Flitney F.W., Williams D.L. NO, nitrosonium ions, nitroxide ions, nitrosothiols and iron-nitrosyls in biology: a chemist's perspective. Trends Pharmacol. Sci. 1995;16(1):18–22. doi: 10.1016/s0165-6147(00)88968-3. [DOI] [PubMed] [Google Scholar]
- 35.Barnett S.D., Buxton I.L.O. The role of S-nitrosoglutathione reductase (GSNOR) in human disease and therapy. Crit. Rev. Biochem. Mol. Biol. 2017;52(3):340–354. doi: 10.1080/10409238.2017.1304353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Li B., Sun C., Lin X., Busch W. The emerging role of GSNOR in oxidative stress regulation. Trends Plant Sci. 2021;26(2):156–168. doi: 10.1016/j.tplants.2020.09.004. [DOI] [PubMed] [Google Scholar]
- 37.Doverspike J.C., Zhou Y., Wu J., Tan X., Xi C., Meyerhoff M.E. Nitric oxide releasing two-part creams containing S-nitrosoglutathione and zinc oxide for potential topical antimicrobial applications. Nitric Oxide : Biol. Chem. 2019;90:1–9. doi: 10.1016/j.niox.2019.05.009. [DOI] [PubMed] [Google Scholar]
- 38.Liu X., Miller M.J., Joshi M.S., Thomas D.D., Lancaster J.R., Jr. Accelerated reaction of nitric oxide with O2 within the hydrophobic interior of biological membranes. Proc. Natl. Acad. Sci. U. S. A. 1998;95(5):2175–2179. doi: 10.1073/pnas.95.5.2175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kelm M. Nitric oxide metabolism and breakdown. Biochim. Biophys. Acta. 1999;1411(2–3):273–289. doi: 10.1016/s0005-2728(99)00020-1. [DOI] [PubMed] [Google Scholar]
- 40.Tang C.H., Seeley E.J., Huang X., Wolters P.J., Liu L. Increased susceptibility to Klebsiella pneumonia and mortality in GSNOR-deficient mice. Biochem. Biophys. Res. Commun. 2013;442(1–2):122–126. doi: 10.1016/j.bbrc.2013.11.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Khan M., Qiao F., Islam S.M.T., Dhammu T.S., Kumar P., Won J., Singh A.K., Singh I. GSNOR and ALDH2 alleviate traumatic spinal cord injury. Brain Res. 2021;1758 doi: 10.1016/j.brainres.2021.147335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim J., Islam S.M.T., Qiao F., Singh A.K., Khan M., Won J., Singh I. Regulation of B cell functions by S-nitrosoglutathione in the EAE model. Redox Biol. 2021;45 doi: 10.1016/j.redox.2021.102053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Saxena N., Won J., Choi S., Singh A.K., Singh I. S-nitrosoglutathione reductase (GSNOR) inhibitor as an immune modulator in experimental autoimmune encephalomyelitis. Free Rad. Biol. Med. 2018;121:57–68. doi: 10.1016/j.freeradbiomed.2018.04.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Hopkins S.P., Pant J., Goudie M.J., Nguyen D.T., Handa H. Electrospun bioabsorbable fibers containing S-nitrosoglutathione for tissue engineering applications. ACS Appl. Bio Mater. 2020;3(11):7677–7686. doi: 10.1021/acsabm.0c00862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Das T., Choong H.J., Kwang Y.C., Chan H.K., Manos J., Kwok P.C.L., Duong H.T.T. Spray-dried particles of nitric oxide-modified glutathione for the treatment of chronic lung infection. Mol. Pharm. 2019;16(4):1723–1731. doi: 10.1021/acs.molpharmaceut.9b00080. [DOI] [PubMed] [Google Scholar]
- 46.Nobre L.S., Saraiva L.M. Effect of combined oxidative and nitrosative stresses on Staphylococcus aureus transcriptome. Appl. Microbiol. Biotechnol. 2013;97(6):2563–2573. doi: 10.1007/s00253-013-4730-3. [DOI] [PubMed] [Google Scholar]
- 47.Stroeher U.H., Kidd S.P., Stafford S.L., Jennings M.P., Paton J.C., McEwan A.G. A pneumococcal MerR-like regulator and S-nitrosoglutathione reductase are required for systemic virulence. J. Infect. Dis. 2007;196(12):1820–1826. doi: 10.1086/523107. [DOI] [PubMed] [Google Scholar]
- 48.Costa I.S., de Souza G.F., de Oliveira M.G., Abrahamsohn Ide A. S-nitrosoglutathione (GSNO) is cytotoxic to intracellular amastigotes and promotes healing of topically treated Leishmania major or Leishmania braziliensis skin lesions-authors' response. J. Antimicrob. Chemother. 2014;69(8):2302–2303. doi: 10.1093/jac/dku223. [DOI] [PubMed] [Google Scholar]
- 49.Nahrevanian H., Farahmand M., Aghighi Z., Assmar M., Amirkhani A. Pharmacological evaluation of anti-leishmanial activity by in vivo nitric oxide modulation in Balb/c mice infected with Leishmania major MRHO/IR/75/ER: an Iranian strain of cutaneous leishmaniasis. Exp. Parasitol. 2007;116(3):233–240. doi: 10.1016/j.exppara.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 50.Zanini G.M., Martins Y.C., Cabrales P., Frangos J.A., Carvalho L.J. S-nitrosoglutathione prevents experimental cerebral malaria. J. Neuroimmune Pharmacol. : Off. J. Soc. NeuroImmune Pharmacol. 2012;7(2):477–487. doi: 10.1007/s11481-012-9343-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Govan J.R., Deretic V. Microbial pathogenesis in cystic fibrosis: mucoid Pseudomonas aeruginosa and Burkholderia cepacia. Microbiol. Rev. 1996;60(3):539–574. doi: 10.1128/mr.60.3.539-574.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin D.W., Schurr M.J., Mudd M.H., Govan J.R., Holloway B.W., Deretic V. Mechanism of conversion to mucoidy in Pseudomonas aeruginosa infecting cystic fibrosis patients. Proc. Natl. Acad. Sci. U. S. A. 1993;90(18):8377–8381. doi: 10.1073/pnas.90.18.8377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jarboe L.R., Hyduke D.R., Tran L.M., Chou K.J., Liao J.C. Determination of the Escherichia coli S-nitrosoglutathione response network using integrated biochemical and systems analysis. J. Biol. Chem. 2008;283(8):5148–5157. doi: 10.1074/jbc.M706018200. [DOI] [PubMed] [Google Scholar]
- 54.Bouch S., Litvack M.L., Litman K., Luo L., Post A., Williston E., Park A.J., Roach E.J., Berezuk A.M., Khursigara C.M., Post M. Therapeutic stem cell-derived alveolar-like macrophages display bactericidal effects and resolve Pseudomonas aeruginosa-induced lung injury. J. Cell Mol. Med. 2022;26(10):3046–3059. doi: 10.1111/jcmm.17324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Firoved A.M., Wood S.R., Ornatowski W., Deretic V., Timmins G.S. Microarray analysis and functional characterization of the nitrosative stress response in nonmucoid and mucoid Pseudomonas aeruginosa. J. Bacteriol. 2004;186(12):4046–4050. doi: 10.1128/JB.186.12.4046-4050.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wood S.R., Firoved A.M., Ornatowski W., Mai T., Deretic V., Timmins G.S. Nitrosative stress inhibits production of the virulence factor alginate in mucoid Pseudomonas aeruginosa. Free Radic. Res. 2007;41(2):208–215. doi: 10.1080/10715760601052610. [DOI] [PubMed] [Google Scholar]
- 57.Neufeld B.H., Reynolds M.M. Critical nitric oxide concentration for Pseudomonas aeruginosa biofilm reduction on polyurethane substrates. Biointerphases. 2016;11(3) doi: 10.1116/1.4962266. [DOI] [PubMed] [Google Scholar]
- 58.Venketaraman V., Dayaram Y.K., Talaue M.T., Connell N.D. Glutathione and nitrosoglutathione in macrophage defense against Mycobacterium tuberculosis. Infect. Immun. 2005;73(3):1886–1889. doi: 10.1128/IAI.73.3.1886-1889.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Andoh N.E., Gyan B.A. The potential roles of glial cells in the neuropathogenesis of cerebral malaria. Front. Cell. Infect. Microbiol. 2021;11 doi: 10.3389/fcimb.2021.741370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Riggle B.A., Miller L.H., Pierce S.K. Desperately seeking therapies for cerebral malaria. J. Immunol. (Baltimore, Md.: 1950) 2020;204(2):327–334. doi: 10.4049/jimmunol.1900829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Gramaglia I., Sobolewski P., Meays D., Contreras R., Nolan J.P., Frangos J.A., Intaglietta M., van der Heyde H.C. Low nitric oxide bioavailability contributes to the genesis of experimental cerebral malaria. Nat. Med. 2006;12(12):1417–1422. doi: 10.1038/nm1499. [DOI] [PubMed] [Google Scholar]
- 62.Elphinstone R.E., Besla R., Shikatani E.A., Lu Z., Hausladen A., Davies M., Robbins C.S., Husain M., Stamler J.S., Kain K.C. S-nitrosoglutathione reductase deficiency confers improved survival and neurological outcome in experimental cerebral malaria. Infect. Immun. 2017;85(9) doi: 10.1128/IAI.00371-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Ong P.K., Melchior B., Martins Y.C., Hofer A., Orjuela-Sánchez P., Cabrales P., Zanini G.M., Frangos J.A., Carvalho L.J. Nitric oxide synthase dysfunction contributes to impaired cerebroarteriolar reactivity in experimental cerebral malaria. PLoS Pathog. 2013;9(6) doi: 10.1371/journal.ppat.1003444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Vargas D., Hageman S., Gulati M., Nobile C.J., Rawat M. S-nitrosomycothiol reductase and mycothiol are required for survival under aldehyde stress and biofilm formation in Mycobacterium smegmatis. IUBMB Life. 2016;68(8):621–628. doi: 10.1002/iub.1524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Marcinkiewicz J. Nitric oxide and antimicrobial activity of reactive oxygen intermediates. Immunopharmacology. 1997;37(1):35–41. doi: 10.1016/s0162-3109(96)00168-3. [DOI] [PubMed] [Google Scholar]
- 66.Justino M.C., Parente M.R., Boneca I.G., Saraiva L.M. FrxA is an S-nitrosoglutathione reductase enzyme that contributes to Helicobacter pylori pathogenicity. FEBS J. 2014;281(19):4495–4505. doi: 10.1111/febs.12958. [DOI] [PubMed] [Google Scholar]
- 67.Liu C., Wen L., Xiao Q., He K. Nitric oxide-generating compound GSNO suppresses porcine circovirus type 2 infection in vitro and in vivo. BMC Vet. Res. 2017;13(1):59. doi: 10.1186/s12917-017-0976-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Poh W.H., Rice S.A. Recent developments in nitric oxide donors and delivery for antimicrobial and anti-biofilm applications. Molecules (Basel, Switzerland) 2022;27(3) doi: 10.3390/molecules27030674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yoon S.S., Hennigan R.F., Hilliard G.M., Ochsner U.A., Parvatiyar K., Kamani M.C., Allen H.L., DeKievit T.R., Gardner P.R., Schwab U., Rowe J.J., Iglewski B.H., McDermott T.R., Mason R.P., Wozniak D.J., Hancock R.E., Parsek M.R., Noah T.L., Boucher R.C., Hassett D.J. Pseudomonas aeruginosa anaerobic respiration in biofilms: relationships to cystic fibrosis pathogenesis. Dev. Cell. 2002;3(4):593–603. doi: 10.1016/s1534-5807(02)00295-2. [DOI] [PubMed] [Google Scholar]
- 70.Hassett D.J. Anaerobic production of alginate by Pseudomonas aeruginosa: alginate restricts diffusion of oxygen. J. Bacteriol. 1996;178(24):7322–7325. doi: 10.1128/jb.178.24.7322-7325.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Nichols W.W., Dorrington S.M., Slack M.P., Walmsley H.L. Inhibition of tobramycin diffusion by binding to alginate. Antimicrob. Agents Chemother. 1988;32(4):518–523. doi: 10.1128/aac.32.4.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Pedersen S.S., Høiby N., Espersen F., Koch C. Role of alginate in infection with mucoid Pseudomonas aeruginosa in cystic fibrosis. Thorax. 1992;47(1):6–13. doi: 10.1136/thx.47.1.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.De Groote M.A., Granger D., Xu Y., Campbell G., Prince R., Fang F.C. Genetic and redox determinants of nitric oxide cytotoxicity in a Salmonella typhimurium model. Proc. Natl. Acad. Sci. U. S. A. 1995;92(14):6399–6403. doi: 10.1073/pnas.92.14.6399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Lu S., Killoran P.B., Fang F.C., Riley L.W. The global regulator ArcA controls resistance to reactive nitrogen and oxygen intermediates in Salmonella enterica serovar Enteritidis. Infect. Immun. 2002;70(2):451–461. doi: 10.1128/IAI.70.2.451-461.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Dayaram Y.K., Talaue M.T., Connell N.D., Venketaraman V. Characterization of a glutathione metabolic mutant of Mycobacterium tuberculosis and its resistance to glutathione and nitrosoglutathione. J. Bacteriol. 2006;188(4):1364–1372. doi: 10.1128/JB.188.4.1364-1372.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Liu L., Hausladen A., Zeng M., Que L., Heitman J., Stamler J.S. A metabolic enzyme for S-nitrosothiol conserved from bacteria to humans. Nature. 2001;410(6827):490–494. doi: 10.1038/35068596. [DOI] [PubMed] [Google Scholar]
- 77.Kidd S.P., Potter A.J., Apicella M.A., Jennings M.P., McEwan A.G. NmlR of Neisseria gonorrhoeae: a novel redox responsive transcription factor from the MerR family. Mol. Microbiol. 2005;57(6):1676–1689. doi: 10.1111/j.1365-2958.2005.04773.x. [DOI] [PubMed] [Google Scholar]
- 78.Potter A.J., Kidd S.P., Jennings M.P., McEwan A.G. Evidence for distinctive mechanisms of S-nitrosoglutathione metabolism by AdhC in two closely related species, Neisseria gonorrhoeae and Neisseria meningitidis. Infect. Immun. 2007;75(3):1534–1536. doi: 10.1128/IAI.01634-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Liao Z., Yan Y., Dong H., Zhu Z., Jiang Y., Cao Y. Endogenous nitric oxide accumulation is involved in the antifungal activity of Shikonin against Candida albicans. Emerg. Microb. Infect. 2016;5(8):e88. doi: 10.1038/emi.2016.87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Li J., Xing T., Wang L., Tao J., Liu Z. Inhibitory effect of S-nitroso-glutathione on Eimeria tenella oocysts was mainly limited to the early stages of sporogony. Vet. Parasitol. 2010;173(1–2):64–69. doi: 10.1016/j.vetpar.2010.06.022. [DOI] [PubMed] [Google Scholar]
- 81.Zaman K., Palmer L.A., Doctor A., Hunt J.F., Gaston B. Concentration-dependent effects of endogenous S-nitrosoglutathione on gene regulation by specificity proteins Sp3 and Sp1. Biochem. J. 2004;380(Pt 1):67–74. doi: 10.1042/BJ20031687. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Mondal A., Douglass M., Hopkins S.P., Singha P., Tran M., Handa H., Brisbois E.J. Multifunctional S-nitroso-N-acetylpenicillamine-Incorporated medical-grade polymer with selenium interface for biomedical applications. ACS Appl. Mater. Interfaces. 2019;11(38):34652–34662. doi: 10.1021/acsami.9b10610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Magill S.S., Edwards J.R., Bamberg W., Beldavs Z.G., Dumyati G., Kainer M.A., Lynfield R., Maloney M., McAllister-Hollod L., Nadle J., Ray S.M., Thompson D.L., Wilson L.E., Fridkin S.K. Multistate point-prevalence survey of health care-associated infections. N. Engl. J. Med. 2014;370(13):1198–1208. doi: 10.1056/NEJMoa1306801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Cao J., Su M., Hasan N., Lee J., Kwak D., Kim D.Y., Kim K., Lee E.H., Jung J.H., Yoo J.W. Nitric oxide-releasing thermoresponsive pluronic F127/alginate hydrogel for enhanced antibacterial activity and accelerated healing of infected wounds. Pharmaceutics. 2020;12(10) doi: 10.3390/pharmaceutics12100926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hornyák I., Marosi K., Kiss L., Gróf P., Lacza Z. Increased stability of S-nitrosothiol solutions via pH modulations. Free Radic. Res. 2012;46(2):214–225. doi: 10.3109/10715762.2011.647692. [DOI] [PubMed] [Google Scholar]
- 86.Diegelmann R.F., Evans M.C. Wound healing: an overview of acute, fibrotic and delayed healing. Front. Biosci. : J. Virt. Libr. 2004;9:283–289. doi: 10.2741/1184. [DOI] [PubMed] [Google Scholar]
- 87.Wang W., Sheng H., Cao D., Zhang F., Zhang W., Yan F., Ding D., Cheng N. S-nitrosoglutathione functionalized polydopamine nanoparticles incorporated into chitosan/gelatin hydrogel films with NIR-controlled photothermal/NO-releasing therapy for enhanced wound healing. Int. J. Biol. Macromol. 2022;200:77–86. doi: 10.1016/j.ijbiomac.2021.12.125. [DOI] [PubMed] [Google Scholar]
- 88.Gao G., Jiang Y.W., Jia H.R., Wu F.G. Near-infrared light-controllable on-demand antibiotics release using thermo-sensitive hydrogel-based drug reservoir for combating bacterial infection. Biomaterials. 2019;188:83–95. doi: 10.1016/j.biomaterials.2018.09.045. [DOI] [PubMed] [Google Scholar]
- 89.Xiong Q., Fang Q., Xu K., Liu G., Sang M., Xu Y., Hao L., Xuan S. Near-infrared light-responsive photothermal α-Fe(2)O(3)@Au/PDA core/shell nanostructure with on-off controllable anti-bacterial effects. Dalton Trans. (Cambridge, England : 2003) 2021;50(40):14235–14243. doi: 10.1039/d1dt02251b. [DOI] [PubMed] [Google Scholar]
- 90.Shang T., Yu X., Han S., Yang B. Nanomedicine-based tumor photothermal therapy synergized immunotherapy. Biomater. Sci. 2020;8(19):5241–5259. doi: 10.1039/d0bm01158d. [DOI] [PubMed] [Google Scholar]
- 91.Zhi D., Yang T., O'Hagan J., Zhang S., Donnelly R.F. Photothermal therapy. J. Contr. Release : off. J. Contr. Release Soc. 2020;325:52–71. doi: 10.1016/j.jconrel.2020.06.032. [DOI] [PubMed] [Google Scholar]
- 92.Qian H., Lei T., Lei P., Hu Y. Additively manufactured tantalum implants for repairing bone defects: a systematic review. Tissue Eng. Part B Rev. 2021;27(2):166–180. doi: 10.1089/ten.TEB.2020.0134. [DOI] [PubMed] [Google Scholar]
- 93.Cai W., Wu J., Xi C., Ashe A.J., 3rd, Meyerhoff M.E. Carboxyl-ebselen-based layer-by-layer films as potential antithrombotic and antimicrobial coatings. Biomaterials. 2011;32(31):7774–7784. doi: 10.1016/j.biomaterials.2011.06.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Rayman M.P. The importance of selenium to human health. Lancet (London, England) 2000;356(9225):233–241. doi: 10.1016/S0140-6736(00)02490-9. [DOI] [PubMed] [Google Scholar]
- 95.Rolim W.R., Pieretti J.C., Renó D.L.S., Lima B.A., Nascimento M.H.M., Ambrosio F.N., Lombello C.B., Brocchi M., de Souza A.C.S., Seabra A.B. Antimicrobial activity and cytotoxicity to tumor cells of nitric oxide donor and silver nanoparticles containing PVA/PEG films for topical applications. ACS Appl. Mater. Interfaces. 2019;11(6):6589–6604. doi: 10.1021/acsami.8b19021. [DOI] [PubMed] [Google Scholar]
- 96.Tao G., Cai R., Wang Y., Zuo H., He H. Fabrication of antibacterial sericin based hydrogel as an injectable and mouldable wound dressing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;119 doi: 10.1016/j.msec.2020.111597. [DOI] [PubMed] [Google Scholar]
- 97.Pelegrino M.T., De Araujo Lima B., Do Nascimento M.H.M., Lombello C.B., Brocchi M., Seabra A.B. Biocompatible and antibacterial nitric oxide-releasing pluronic F-127/Chitosan hydrogel for topical applications. Polymers. 2018;10(4) doi: 10.3390/polym10040452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Amante C., Esposito T., Del Gaudio P., Di Sarno V., Porta A., Tosco A., Russo P., Nicolais L., Aquino R.P. A novel three-polysaccharide blend in situ gelling powder for wound healing applications. Pharmaceutics. 2021;13(10) doi: 10.3390/pharmaceutics13101680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Vasquez J.M., Idrees A., Carmagnola I., Sigen A., McMahon S., Marlinghaus L., Ciardelli G., Greiser U., Tai H., Wang W., Salber J., Chiono V. In situ forming hyperbranched PEG-thiolated hyaluronic acid hydrogels with honey-mimetic antibacterial properties. Front. Bioeng. Biotechnol. 2021;9 doi: 10.3389/fbioe.2021.742135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Zhao C., Chen R., Chen Z., Lu Q., Zhu H., Bu Q., Yin J., He H. Bioinspired multifunctional cellulose nanofibril-based in situ liquid wound dressing for multiple synergistic therapy of the postoperative infected wound. ACS Appl. Mater. Interfaces. 2021;13(43):51578–51591. doi: 10.1021/acsami.1c18221. [DOI] [PubMed] [Google Scholar]
- 101.Ali Zahid A., Chakraborty A., Shamiya Y., Ravi S.P., Paul A. Leveraging the advancements in functional biomaterials and scaffold fabrication technologies for chronic wound healing applications. Mater. Horiz. 2022 doi: 10.1039/d2mh00115b. [DOI] [PubMed] [Google Scholar]
- 102.Verdes M., Mace K., Margetts L., Cartmell S. Status and challenges of electrical stimulation use in chronic wound healing. Curr. Opin. Biotechnol. 2022;75 doi: 10.1016/j.copbio.2022.102710. [DOI] [PubMed] [Google Scholar]
- 103.Carpenter A.W., Schoenfisch M.H. Nitric oxide release: part II. Therapeutic applications. Chem. Soc. Rev. 2012;41(10):3742–3752. doi: 10.1039/c2cs15273h. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mitchell M.J., Billingsley M.M., Haley R.M., Wechsler M.E., Peppas N.A., Langer R. Engineering precision nanoparticles for drug delivery. Nat. Rev. Drug Discov. 2021;20(2):101–124. doi: 10.1038/s41573-020-0090-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Nikezić A.V.V., Bondžić A.M., Vasić V.M. Drug delivery systems based on nanoparticles and related nanostructures. Eur. J. Pharmaceut. Sci. : Off. J. Eur. Fed. Pharm. Sci. 2020;151 doi: 10.1016/j.ejps.2020.105412. [DOI] [PubMed] [Google Scholar]
- 106.Lee J., Kwak D., Kim H., Kim J., Hlaing S.P., Hasan N., Cao J., Yoo J.W. Nitric oxide-releasing S-Nitrosoglutathione-Conjugated poly(lactic-Co-glycolic acid) nanoparticles for the treatment of MRSA-infected cutaneous wounds. Pharmaceutics. 2020;12(7) doi: 10.3390/pharmaceutics12070618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Chouake J., Schairer D., Kutner A., Sanchez D.A., Makdisi J., Blecher-Paz K., Nacharaju P., Tuckman-Vernon C., Gialanella P., Friedman J.M., Nosanchuk J.D., Friedman A.J. Nitrosoglutathione generating nitric oxide nanoparticles as an improved strategy for combating Pseudomonas aeruginosa-infected wounds. J. Drugs Dermatol. JDD : JDD. 2012;11(12):1471–1477. [PubMed] [Google Scholar]
- 108.Mordorski B., Pelgrift R., Adler B., Krausz A., da Costa Neto A.B., Liang H., Gunther L., Clendaniel A., Harper S., Friedman J.M., Nosanchuk J.D., Nacharaju P., Friedman A.J. S-nitrosocaptopril nanoparticles as nitric oxide-liberating and transnitrosylating anti-infective technology. Nanomed. Nanotechnol. Biol. Med. 2015;11(2):283–291. doi: 10.1016/j.nano.2014.09.017. [DOI] [PubMed] [Google Scholar]
- 109.Manea S.A., Vlad M.L., Rebleanu D., Lazar A.G., Fenyo I.M., Calin M., Simionescu M., Manea A. Detection of vascular reactive oxygen species in experimental atherosclerosis by high-resolution near-infrared fluorescence imaging using VCAM-1-targeted liposomes entrapping a fluorogenic redox-sensitive probe. Oxid. Med. Cell. Longev. 2021;2021 doi: 10.1155/2021/6685612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Douglass M., Hopkins S., Pandey R., Singha P., Norman M., Handa H. S-Nitrosoglutathione-Based nitric oxide-releasing nanofibers exhibit dual antimicrobial and antithrombotic activity for biomedical applications. Macromol. Biosci. 2021;21(1) doi: 10.1002/mabi.202000248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Hasanzadeh Kafshgari M., Delalat B., Harding F.J., Cavallaro A., Mäkilä E., Salonen J., Vasilev K., Voelcker N.H. Antibacterial properties of nitric oxide-releasing porous silicon nanoparticles. J. Mater. Chem. 2016;B 4(11):2051–2058. doi: 10.1039/c5tb02551f. [DOI] [PubMed] [Google Scholar]
- 112.McCarthy C.W., Guillory R.J., 2nd, Goldman J., Frost M.C. Transition-metal-mediated release of nitric oxide (NO) from S-nitroso-N-acetyl-d-penicillamine (SNAP): potential applications for endogenous release of NO at the surface of stents via corrosion products. ACS Appl. Mater. Interfaces. 2016;8(16):10128–10135. doi: 10.1021/acsami.6b00145. [DOI] [PubMed] [Google Scholar]
- 113.Douglass M.E., Goudie M.J., Pant J., Singha P., Hopkins S., Devine R., Schmiedt C.W., Handa H. Catalyzed nitric oxide release via Cu nanoparticles leads to an increase in antimicrobial effects and hemocompatibility for short term extracorporeal circulation. ACS Appl. Bio Mater. 2019;2(6):2539–2548. doi: 10.1021/acsabm.9b00237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Seabra A.B., De Oliveira M.G. Poly(vinyl alcohol) and poly(vinyl pyrrolidone) blended films for local nitric oxide release. Biomaterials. 2004;25(17):3773–3782. doi: 10.1016/j.biomaterials.2003.10.035. [DOI] [PubMed] [Google Scholar]
- 115.Heikal L., Martin G.P., Dailey L.A. Characterisation of the decomposition behaviour of S-nitrosoglutathione and a new class of analogues: S-Nitrosophytochelatins. Nitric Oxide : Biol. Chem. 2009;20(3):157–165. doi: 10.1016/j.niox.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 116.Frost M.C., Reynolds M.M., Meyerhoff M.E. Polymers incorporating nitric oxide releasing/generating substances for improved biocompatibility of blood-contacting medical devices. Biomaterials. 2005;26(14):1685–1693. doi: 10.1016/j.biomaterials.2004.06.006. [DOI] [PubMed] [Google Scholar]
- 117.Tao W., Yerbulekova A., Moore C.E., Shafaat H.S., Zhang S. Controlling the direction of S-nitrosation versus denitrosation: reversible cleavage and formation of an S-N bond within a dicopper center. J. Am. Chem. Soc. 2022;144(7):2867–2872. doi: 10.1021/jacs.1c12799. [DOI] [PubMed] [Google Scholar]
- 118.Duvanova E., Krasnou I., Krumme A., Mikli V., Radio S., Rozantsev G.M., Karpichev Y. Development of functional composite Cu(II)-Polyoxometalate/PLA with antimicrobial properties. Molecules (Basel, Switzerland) 2022;27(8) doi: 10.3390/molecules27082510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Zhang Z.Y., An Y.L., Wang X.S., Cui L.Y., Li S.Q., Liu C.B., Zou Y.H., Zhang F., Zeng R.C. In vitro degradation, photo-dynamic and thermal antibacterial activities of Cu-bearing chlorophyllin-induced Ca-P coating on magnesium alloy AZ31. Bioact. Mater. 2022;18:284–299. doi: 10.1016/j.bioactmat.2022.01.050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Kulyk K., Azizova L., Cunningham J.M., Mikhalovska L., Borysenko M., Mikhalovsky S. Nanosized copper(ii) oxide/silica for catalytic generation of nitric oxide from S-nitrosothiols. J. Mater. Chem. B. 2020;8(19):4267–4277. doi: 10.1039/d0tb00137f. [DOI] [PubMed] [Google Scholar]
- 121.Mendhi J., Ramachandra S.S., Prasadam I., Ivanovski S., Yang Y., Xiao Y. Endogenous nitric oxide-generating surfaces via polydopamine-copper coatings for preventing biofilm dispersal and promoting microbial killing. Mater. Sci. Eng. C Mater. Biol. Appl. 2021;128 doi: 10.1016/j.msec.2021.112297. [DOI] [PubMed] [Google Scholar]
- 122.Harding J.L., Reynolds M.M. Metal organic frameworks as nitric oxide catalysts. J. Am. Chem. Soc. 2012;134(7):3330–3333. doi: 10.1021/ja210771m. [DOI] [PubMed] [Google Scholar]
- 123.Tuttle R.R., Rubin H.N., Rithner C.D., Finke R.G., Reynolds M.M. Copper ion vs copper metal-organic framework catalyzed NO release from bioavailable S-Nitrosoglutathione en route to biomedical applications: direct (1)H NMR monitoring in water allowing identification of the distinct, true reaction stoichiometries and thiol dependencies. J. Inorg. Biochem. 2019;199 doi: 10.1016/j.jinorgbio.2019.110760. [DOI] [PubMed] [Google Scholar]
- 124.Rodwihok C., Suwannakeaw M., Charoensri K., Wongratanaphisan D., Woon Woo S., Kim H.S. Alkali/zinc-activated fly ash nanocomposites for dye removal and antibacterial applications. Bioresour. Technol. 2021;331 doi: 10.1016/j.biortech.2021.125060. [DOI] [PubMed] [Google Scholar]
- 125.Rupp M.E., Karnatak R. Intravascular catheter-related bloodstream infections. Infect. Dis. Clin. North Am. 2018;32(4):765–787. doi: 10.1016/j.idc.2018.06.002. [DOI] [PubMed] [Google Scholar]
- 126.Doverspike J.C., Mack S.J., Luo A., Stringer B., Reno S., Cornell M.S., Rojas-Pena A., Wu J., Xi C., Yevzlin A., Meyerhoff M.E. Nitric oxide-releasing insert for disinfecting the hub region of tunnel dialysis catheters. ACS Appl. Mater. Interfaces. 2020;12(40):44475–44484. doi: 10.1021/acsami.0c13230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Gresele P., Momi S., Guglielmini G. Nitric oxide-enhancing or -releasing agents as antithrombotic drugs. Biochem. Pharmacol. 2019;166:300–312. doi: 10.1016/j.bcp.2019.05.030. [DOI] [PubMed] [Google Scholar]
- 128.Asantewaa G., Harris I.S. Glutathione and its precursors in cancer. Curr. Opin. Biotechnol. 2021;68:292–299. doi: 10.1016/j.copbio.2021.03.001. [DOI] [PubMed] [Google Scholar]
- 129.Jaffer I.H., Fredenburgh J.C., Hirsh J., Weitz J.I. Medical device-induced thrombosis: what causes it and how can we prevent it? J. Thromb. Haemostasis : JTH. 2015;13(Suppl 1):S72–S81. doi: 10.1111/jth.12961. [DOI] [PubMed] [Google Scholar]
- 130.Ullrich H., Münzel T., Gori T. Coronary stent thrombosis- predictors and prevention. Deutsches Arzteblatt Int. 2020;117(18):320–326. doi: 10.3238/arztebl.2020.0320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Loscalzo J. Nitric oxide insufficiency, platelet activation, and arterial thrombosis. Circ. Res. 2001;88(8):756–762. doi: 10.1161/hh0801.089861. [DOI] [PubMed] [Google Scholar]
- 132.Wu Y., Zhou Z., Meyerhoff M.E. In vitro platelet adhesion on polymeric surfaces with varying fluxes of continuous nitric oxide release. J. Biomed. Mater. Res., Part A. 2007;81(4):956–963. doi: 10.1002/jbm.a.31105. [DOI] [PubMed] [Google Scholar]
- 133.Zhang Q., Stachelek S.J., Inamdar V.V., Alferiev I., Nagaswami C., Weisel J.W., Hwang J.H., Meyerhoff M.E. Studies of combined NO-eluting/CD47-modified polyurethane surfaces for synergistic enhancement of biocompatibility. Colloids Surf. B Biointerfaces. 2020;192 doi: 10.1016/j.colsurfb.2020.111060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Joslin J.M., Lantvit S.M., Reynolds M.M. Nitric oxide releasing Tygon materials: studies in donor leaching and localized nitric oxide release at a polymer-buffer interface. ACS Appl. Mater. Interfaces. 2013;5(19):9285–9294. doi: 10.1021/am402112y. [DOI] [PubMed] [Google Scholar]
- 135.Romero J.M., Bizzozero O.A. Extracellular S-nitrosoglutathione, but not S-nitrosocysteine or N(2)O(3), mediates protein S-nitrosation in rat spinal cord slices. J. Neurochem. 2006;99(4):1299–1310. doi: 10.1111/j.1471-4159.2006.04180.x. [DOI] [PubMed] [Google Scholar]
- 136.Mizgerd J.P. Acute lower respiratory tract infection. N. Engl. J. Med. 2008;358(7):716–727. doi: 10.1056/NEJMra074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Stewart P.S., Bjarnsholt T. Risk factors for chronic biofilm-related infection associated with implanted medical devices. Clin. Microbiol. Infect.: Off. Publ. Eur. Soc. Clin. Microbiol. Infect. Dis. 2020;26(8):1034–1038. doi: 10.1016/j.cmi.2020.02.027. [DOI] [PubMed] [Google Scholar]
- 138.Li S., Renick P., Senkowsky J., Nair A., Tang L. Diagnostics for wound infections. Adv. Wound Care. 2021;10(6):317–327. doi: 10.1089/wound.2019.1103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Tolker-Nielsen T. Biofilm development. Microbiol. Spectr. 2015;3(2) doi: 10.1128/microbiolspec.MB-0001-2014. Mb-0001-2014. [DOI] [PubMed] [Google Scholar]
- 140.Bjarnsholt T., Kirketerp-Møller K., Jensen P., Madsen K.G., Phipps R., Krogfelt K., Høiby N., Givskov M. Why chronic wounds will not heal: a novel hypothesis. Wound Repair Regen.: Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2008;16(1):2–10. doi: 10.1111/j.1524-475X.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- 141.Davis S.C., Ricotti C., Cazzaniga A., Welsh E., Eaglstein W.H., Mertz P.M. Microscopic and physiologic evidence for biofilm-associated wound colonization in vivo. Wound Repair Regen.: Off. Publ. Wound Heal. Soc. Eur. Tissue Repair Soc. 2008;16(1):23–29. doi: 10.1111/j.1524-475X.2007.00303.x. [DOI] [PubMed] [Google Scholar]
- 142.Seabra A.B., Duran N. Nanoparticulated nitric oxide donors and their biomedical applications. Mini Rev. Med. Chem. 2017;17(3):216–223. doi: 10.2174/1389557516666160808124624. [DOI] [PubMed] [Google Scholar]
- 143.Cyr A.R., Huckaby L.V., Shiva S.S., Zuckerbraun B.S. Nitric oxide and endothelial dysfunction. Crit. Care Clin. 2020;36(2):307–321. doi: 10.1016/j.ccc.2019.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Calabrese V., Mancuso C., Calvani M., Rizzarelli E., Butterfield D.A., Stella A.M. Nitric oxide in the central nervous system: neuroprotection versus neurotoxicity. Nat. Rev. Neurosci. 2007;8(10):766–775. doi: 10.1038/nrn2214. [DOI] [PubMed] [Google Scholar]
- 145.Chen Y. Recent developments of fluorescent probes for detection and bioimaging of nitric oxide. Nitric Oxide : Biol. Chem. 2020;98:1–19. doi: 10.1016/j.niox.2020.02.002. [DOI] [PubMed] [Google Scholar]
- 146.Marletta M.A. Nitric oxide: biosynthesis and biological significance. Trends Biochem. Sci. 1989;14(12):488–492. doi: 10.1016/0968-0004(89)90181-3. [DOI] [PubMed] [Google Scholar]
- 147.Bogdan C. Nitric oxide and the immune response. Nat. Immunol. 2001;2(10):907–916. doi: 10.1038/ni1001-907. [DOI] [PubMed] [Google Scholar]
- 148.Chan E.D., Chan J., Schluger N.W. What is the role of nitric oxide in murine and human host defense against tuberculosis?Current knowledge. Am. J. Respir. Cell Mol. Biol. 2001;25(5):606–612. doi: 10.1165/ajrcmb.25.5.4487. [DOI] [PubMed] [Google Scholar]
- 149.Ghaffari A., Miller C.C., McMullin B., Ghahary A. Potential application of gaseous nitric oxide as a topical antimicrobial agent. Nitric Oxide : Biol. Chem. 2006;14(1):21–29. doi: 10.1016/j.niox.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 150.Barraud N., Schleheck D., Klebensberger J., Webb J.S., Hassett D.J., Rice S.A., Kjelleberg S. Nitric oxide signaling in Pseudomonas aeruginosa biofilms mediates phosphodiesterase activity, decreased cyclic di-GMP levels, and enhanced dispersal. J. Bacteriol. 2009;191(23):7333–7342. doi: 10.1128/JB.00975-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.De Groote M.A., Fang F.C. NO inhibitions: antimicrobial properties of nitric oxide. Clin. Infect. Dis.: Off. Publ. Infect. Diseases Soc. Am. 1995;21(Suppl 2):S162–S165. doi: 10.1093/clinids/21.supplement_2.s162. [DOI] [PubMed] [Google Scholar]
- 152.Jourd'heuil D., Kang D., Grisham M.B. Interactions between superoxide and nitric oxide: implications in DNA damage and mutagenesis. Front. Biosci. : J. Virt. Libr. 1997;2:d189–d196. doi: 10.2741/a182. [DOI] [PubMed] [Google Scholar]
- 153.Crack J.C., Green J., Thomson A.J., Le Brun N.E. Iron-sulfur clusters as biological sensors: the chemistry of reactions with molecular oxygen and nitric oxide. Acc. Chem. Res. 2014;47(10):3196–3205. doi: 10.1021/ar5002507. [DOI] [PubMed] [Google Scholar]
- 154.Nguyen T., Brunson D., Crespi C.L., Penman B.W., Wishnok J.S., Tannenbaum S.R. DNA damage and mutation in human cells exposed to nitric oxide in vitro. Proc. Natl. Acad. Sci. U. S. A. 1992;89(7):3030–3034. doi: 10.1073/pnas.89.7.3030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Diefenbach A., Schindler H., Röllinghoff M., Yokoyama W.M., Bogdan C. Requirement for type 2 NO synthase for IL-12 signaling in innate immunity. Science (New York, N.Y.) 1999;284(5416):951–955. doi: 10.1126/science.284.5416.951. [DOI] [PubMed] [Google Scholar]
- 156.Lee C., Miura K., Liu X., Zweier J.L. Biphasic regulation of leukocyte superoxide generation by nitric oxide and peroxynitrite. J. Biol. Chem. 2000;275(50):38965–38972. doi: 10.1074/jbc.M006341200. [DOI] [PubMed] [Google Scholar]
- 157.Maragos C.M., Morley D., Wink D.A., Dunams T.M., Saavedra J.E., Hoffman A., Bove A.A., Isaac L., Hrabie J.A., Keefer L.K. Complexes of .NO with nucleophiles as agents for the controlled biological release of nitric oxide. Vasorelaxant effects. J. Med. Chem. 1991;34(11):3242–3247. doi: 10.1021/jm00115a013. [DOI] [PubMed] [Google Scholar]
- 158.Sundaram M.K., Khan M.A., Alalami U., Somvanshi P., Bhardwaj T., Pramodh S., Raina R., Shekfeh Z., Haque S., Hussain A. Phytochemicals induce apoptosis by modulation of nitric oxide signaling pathway in cervical cancer cells. Eur. Rev. Med. Pharmacol. Sci. 2020;24(22):11827–11844. doi: 10.26355/eurrev_202011_23840. [DOI] [PubMed] [Google Scholar]
- 159.Venketaraman V., Dayaram Y.K., Amin A.G., Ngo R., Green R.M., Talaue M.T., Mann J., Connell N.D. Role of glutathione in macrophage control of mycobacteria. Infect. Immun. 2003;71(4):1864–1871. doi: 10.1128/IAI.71.4.1864-1871.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Seabra A.B., Justo G.Z., Haddad P.S. State of the art, challenges and perspectives in the design of nitric oxide-releasing polymeric nanomaterials for biomedical applications. Biotechnol. Adv. 2015;33(6 Pt 3):1370–1379. doi: 10.1016/j.biotechadv.2015.01.005. [DOI] [PubMed] [Google Scholar]
- 161.Guo H., Xia D., Zheng Y., Zhu Y., Liu Y., Zhou Y. A pure zinc membrane with degradability and osteogenesis promotion for guided bone regeneration: in vitro and in vivo studies. Acta Biomater. 2020;106:396–409. doi: 10.1016/j.actbio.2020.02.024. [DOI] [PubMed] [Google Scholar]
- 162.Kusumbe A.P., Ramasamy S.K., Adams R.H. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323–328. doi: 10.1038/nature13145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Brisbois E.J., Davis R.P., Jones A.M., Major T.C., Bartlett R.H., Meyerhoff M.E., Handa H. Reduction in thrombosis and bacterial adhesion with 7 Day implantation of S-nitroso-N-acetylpenicillamine (SNAP)-Doped Elast-eon E2As catheters in sheep. J. Mater. Chem. B. 2015;3(8):1639–1645. doi: 10.1039/C4TB01839G. [DOI] [PMC free article] [PubMed] [Google Scholar]