Abstract
Infection by Salmonella Typhimurium, a food-borne pathogen, can reduce the poultry production efficiency. The objective of this study was to investigate the effects of tannic acid (TA) supplementation on growth performance, Salmonella colonization, gut barrier integrity, serum endotoxin levels, antioxidant capacity, gut health, and immune function in broilers infected with the Salmonella enterica serovar Typhimurium nalidixic acid resistant strain (STNR). A total of 546 one-day-old broilers were arbitrarily allocated into 6 treatments including 1) Sham-challenged control (SCC; birds fed a basal diet and administrated peptone water); 2) Challenged control (CC; birds fed a basal diet and inoculated with 108 STNR); 3) Tannic acid 0.25 (TA0.25; CC + 0.25 g/kg TA); 4) TA0.5 (CC + 0.5 g/kg TA); 5) TA1 (CC + 1 g/kg TA); and 6) TA2 (CC + 2 g/kg TA). On D 7, supplemental TA linearly reduced STNR colonization in the ceca (P < 0.01), and TA1 and TA2 group had significantly lower reduced STNR colonization in the ceca (P < 0.01). On D 7 to 21, average daily gain tended to be linearly increased by supplemental TA (P = 0.097). The serum endotoxin levels were quadratically decreased by supplemental TA on D 21 (P < 0.05). Supplemental TA quadratically increased ileal villus height (VH; P < 0.05), and the TA0.25 group had higher ileal VH compared to the CC group (P < 0.05). Supplemental TA linearly increased percentage of peripheral blood CD8+ T cells on D 18 (P < 0.01). The TA0.5 group had significantly lower lymphocyte numbers compared to the CC groups (P < 0.05). The abundance of monocytes linearly increased with TA supplementation (P < 0.01). Therefore, broilers fed TA had reduced STNR colonization, increased growth performance, decreased serum endotoxin levels, enhanced gut health in the broilers, and stimulated the immune system in broilers infected with STNR. Supplementation of TA (1–2 g/kg) enhanced growth performance and gut health via antimicrobial and immunostimulatory effects in broilers infected with STNR.
Key words: tannic acid, Salmonella Typhimurium, antimicrobials, immunity, feed additive
INTRODUCTION
Salmonella enterica serovar Typhimurium is one of the most costly pathogens to poultry production because it threatens public health as a food-borne pathogen and reduces poultry production efficiency (Mughini-Gras et al., 2014). Because young broilers have an underdeveloped immune system, S. Typhimurium infection primarily impacts this age group, compromising their growth performance, gut health, and immunity (Alkie et al., 2019; Ibrahim et al., 2021). Impaired growth and gut health in the early broiler stage due to S. Typhimurium infection can negatively affect flock performance (Martínez et al., 2021). While S. Typhimurium infection in the older broilers can remain asymptomatic, S. Typhimurium contaminated poultry products can infect humans. This can induce severe gastrointestinal and systemic symptoms and even mortality in select susceptible human cohorts (Wilson et al., 2016). S. Typhimurium infection has been reported to cause 500,000 to 600,000 deaths in humans worldwide annually (Bula-Rudas et al., 2015).
To decrease the occurrence of Salmonella infection and to ameliorate the negative effects of Salmonella infection in broilers, antibiotic growth promotors (AGP) have been traditionally used in poultry production (Gut et al., 2018). However, due to the concern of spreading resistant bacteria and their resistant genes, some countries or institutions have restricted or strictly banned (EU) the use of AGP in poultry production (Abudabos et al., 2019). Therefore, cost-effective alternatives for AGP are required to control Salmonella infection and to improve growth performance in broiler production (Yang et al., 2015).
Tannins, polyphenol compounds that can precipitate proteins, were recently considered as potential alternatives for AGP mainly due to their strong antimicrobial effects, although tannins can show antinutritional effects at high doses in broilers (Choi and Kim, 2020). Tannic acid (TA) is considered as the standard of hydrolysable tannins, and chestnut TA is a by-product of the wood industry (Mannelli et al., 2019). In many in vitro studies, TA was shown to have strong antimicrobial effects against Salmonella spp. (Van Parys et al., 2010, Graziani et al., 2006; Sivasankar et al., 2020). A previous study by Ramah et al. (2020) reported that appropriate dosages of TA enhanced broiler immunity. Therefore, the hypothesis of the study was that supplemental TA may improve growth performance and gut health in broilers infected with S. Typhimurium via its antimicrobial and immunomodulatory effects. The purpose of the study was to investigate the effects of supplemental TA on growth performance, Salmonella colonization, gut barrier integrity, serum endotoxin levels, liver and intestinal antioxidant capacity, ileal morphology, ileal brush border digestive enzymes activities, serum alkaline phosphatase activities, and peripheral immune system in broilers infected S. Typhimurium.
MATERIALS AND METHODS
Preparation of Salmonella Typhimurium Inoculum
The inoculum of Salmonella enterica serovar Typhimurium nalidixic acid resistant strain (STNR) was prepared according to Yadav et al. (2022). Briefly, a single colony of in vitro passaged STNR was cultured on the brilliant green sulfa (BGS; Difco, Detroit, MI) agar containing 200 μg/mL nalidixic acid (NA; Sigma-Aldrich Co., St Louis, MO). It was then streaked in tryptic soy broth (TSB; Sigma-Aldrich Co. St Louis, MO) containing 200 μg/mL of nalidixic acid (NA) and grown aerobically at 35°C. After 24 h incubation, the culture was washed by centrifuging at 7,000 × g for 10 min and adding phosphate buffered saline (PBS) twice. The bacterial cell optical density (OD) was measured at 600 nm (OD600) using a UV-Vis spectrometer (Genesys 10S UV-Vis, Thermo Fisher Scientific, Waltham, MA) to make 1010 per mL by adding peptone water (0.1%; Fisher Scientific, Fair Lawn, NJ) according to the standard curve generated previously. Afterwards, the 1010 CFU/mL bacterial solution was diluted to 108 CFU/mL by adding peptone water.
Experimental Design and Growth Performance
The study was reviewed and approved by the Institutional Animal Care and Use Committee at the University of Georgia, Athens, GA, USA. A total of 546 one-day-old Cobb 500 broilers were randomly assigned to 6 treatment groups with 7 replicates of 13 birds. The 6 treatments included 1) Sham-challenged control (SCC; birds fed a basal diet and administrated peptone water); 2) Challenged control (CC; birds fed a basal diet and inoculated with 108 STNR); 3) Tannic acid 0.25 (TA0.25; CC + 0.25 g/kg TA); 4) Tannic acid 0.5 (TA0.5; CC + 0.5 g/kg TA); 5) Tannic acid 1 (TA1; CC + 1 g/kg TA); and 6) Tannic acid 2 (TA2; CC + 2.0 g/kg TA). The basal diet was formulated to meet or exceed energy and nutrient requirements according to Cobb Broiler Management Guide (Table 1; Cobb Vantress 2018Cobb, 2018). The TA (Sigma-Aldrich Co. St Louis, MO) was added into the filler part with sand to acquire the designated concentrations for each dietary treatment. Broilers were fed ad-libitum during the whole experimental period (21 D), and temperature and light were maintained according to Cobb Broiler Management Guide (Cobb Vantress 2018Cobb, 2018). One-day-old birds received either 0.5 mL peptone water for the SCC group or 0.5 mL of 108 STNR in peptone water for the infected group. On D 7 and 21, body weight (BW) and feed disappearance were recorded to calculate average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR).
Table 1.
Ingredients and nutrient compositions of basal diets (As-fed basis).1
| Items | D 0 to 7 | D 7 to 21 |
|---|---|---|
| Ingredients | ||
| Corn | 606.61 | 647.1 |
| Soybean meal (480 g crude protein/kg) | 330.76 | 291.18 |
| Dicalcium phosphate | 16.57 | 15.26 |
| Filler1 | 10 | 10 |
| Soybean oil | 12.62 | 14.15 |
| Limestone | 11.7 | 11.11 |
| DL-Methionine 99% | 3.19 | 2.93 |
| L-Lysine HCl 78% | 2.51 | 2.5 |
| Common Salt | 3.5 | 3.51 |
| L-threonine | 1.2 | 0.77 |
| Mineral Premix3 | 0.8 | 0.8 |
| Vitamin Premix2 | 0.5 | 0.5 |
| Choline Cl -70% | 0.04 | 0.2 |
| Total | 1,000 | 1,000 |
| Calculated energy and nutrient value, % | ||
| Metabolizable energy, Mcal/kg | 2,975 | 3,025 |
| Crude protein | 21.89 | 20.28 |
| SID4 Methionine | 0.63 | 0.58 |
| SID4 Total sulfur amino acids | 0.91 | 0.85 |
| SID4 Lysine | 1.22 | 1.12 |
| SID4 Threonine | 0.83 | 0.73 |
| Total calcium | 0.9 | 0.84 |
| Available phosphate | 0.45 | 0.42 |
Sand and tannic acid were added to obtain desired tannic acid dosages in the feed as follows: Sham-challenged control (SCC) and challenged control (CC): sand 10 g/kg + tannic acid 0 g/kg; Tannic acid 0.25 (TA0.25): sand 9.75 g/kg + tannic acid 0.25 g/kg; Tannic acid 0.5 (TA0.5): sand 9.5 g/kg + tannic acid 0.5 g/kg; Tannic acid 1 (TA1): sand 9 g/kg + tannic acid 1 g/kg; and Tannic acid 2 (TA2): sand 8 g/kg + tannic acid 2 g/kg. Tannic acid was purchased from Sigma–Aldrich (St Louis, MO).
Vitamin mix provided the following in mg/100 g diet: thiamine-HCl, 1.5; riboflavin 1.5; nicotinic acid amide 15; folic acid 7.5; pyridoxine-HCl, 1.2; d-biotin 3; vitamin B-12 (source concentrations, 0.1%) 2; d-calcium pantothenate 4; menadione sodium bisulfite, 1.98; α-tocopherol acetate (source 500,000 IU/g), 22.8; cholecalciferol (source 5,000,000 IU/g) 0.09; retinyl palmitate (source 500,000 IU/g), 2.8; ethoxyquin, 13.34; I-inositol, 2.5; and dextrose, 762.2.
Mineral mix provided the following in g/100 g diet: Ca(H2PO4)2 · H2O, 3.62; CaCO3, 1.48; KH2PO4, 1.00; Na2SeO4, 0.0002; MnSO4 · H2O, 0.035; FeSO4 · 7H2O, 0.05; MgSO4 · 7H2O, 0.62; KIO3, 0.001; NaCl, 0.60; CuSO4 · 5H2O, 0.008; ZnCO3, 0.015; CoCl2 · 6H2O, 0.00032; NaMoO4 · 2H2O, 0.0011; KCl, 0.10; dextrose, 0.40.
SID: standard ileal digestible.
Colonization Analysis of Ceca, Liver, and Spleen and Sample Collection
On D 4, 7, 14, and 21, one bird per pen was euthanized via cervical dislocation, and the ceca, liver (without gall bladder), and spleen samples were collected using aseptic technique. Ceca samples were collected in sterile Whirl-Pak bags (Nasco, Fort Atkinson, WI), and liver and spleen samples were collected in the sterile sample bags (VWR International, Radnor, PA). The samples were stored in ice before being delivered to the lab. In the lab, bags containing the ceca were weighed and manually squeezed and homogenized using a paddle lab blender (Masticator Silver Panoramic, Neutec Group Inc, Farmingdale, NY) after adding 10 mL of buffered peptone water (BPW; HiMedia, Mumbai, India) containing 200 mg/kg NA. The cecal content homogenate was transferred to a 10 mL sterile glass tube and diluted to 10−6. Next, 100 µL of diluted samples were plated on the prepared BGS agar containing 200 mg/kg NA followed by at 35°C for 24 h. After the incubation, the number of STNR colonies was counted. To the sample bags containing liver and spleen samples, 10 mL and 5 mL of BPW containing 200 mg/kg NA were added, respectively and homogenized thoroughly by using hands and the paddle lab blender (Masticator Silver Panoramic, Neutec Group Inc). The homogenized liver and spleen samples were incubated at 35°C for 24 h and plated on the BGS containing 200 mg/kg NA using sterile swabs and incubated at 35°C for 24 h. Afterward, the presence or absence of STNR was determined.
On D 7 and 21, mid-ileal tissue (half point of ileo-ceco-rectal junction and Meckel's diverticulum) samples were washed with PBS to remove digesta. For intestinal morphology analysis, ileal samples were immersed in 10% formalin for fixation. For analyses by real-time reverse transcription (RT)-PCR, intestinal antioxidant capacity, and ileal brush border digestive enzyme activities, ileal tissue samples were snap-frozen in the liquid nitrogen. On D 21, liver samples were collected and snap-frozen in liquid nitrogen for liver antioxidant capacity analyses. Blood samples were collected on D 7 and D 21 by heart puncture after euthanizing, and serum was recovered from clotted blood by centrifugation at 1,500 × g for 12 min. Serum samples and snap-frozen samples were stored at −80°C for further analyses.
Serum Endotoxin Concentrations and Gut Permeability Analyses
Serum endotoxin concentrations were quantified using a Pierce LAL Chromogenic Endotoxin Quantitation Kit (Thermo Fisher Scientific, Waltham, MA) according to the manufacturer's protocol using a 1:9 dilution in endotoxin free water. On D 14 and 21, gut permeability was measured according to Choi et al. (2021). Briefly, 2.2 mg/mL fluorescein isothiocyanatedextran 4 kDa (FITC-D4; Sigma-Aldrich Co. St Louis, MO) were dissolved in PBS. One milliliter of the FITC solution was administrated to one bird per pen via oral gavage. After 2 h, birds were euthanized via cervical dislocation, and blood was collected from heart. The samples were stored in a dark container at room temperature to allow for clotting. Serum was recovered by centrifugation at 1,500 × g for 15 min. Serum, 100 µL/well, was aliquoted to duplicate wells of a dark 96 well plate (Greiner Bio-one, Monroe, NC). The degree of fluorescence was enumerated using an excitation wavelength of 485 nm and an emission wavelength of 528 nm using a ICTOR3 Multilabel Plate Reader (Perkin Elmer, Waltham, MA). The fluorescence levels were expressed as relative to the SCC group.
Ileal Morphology and Goblet Cell Counting
Alcian blue/period acid-schiff (AB/PAS) staining was used to assess villus height (VH), crypts depth (CD), VH:CD ratio as well as, to enumerate goblet cell number per 100 μm VH and 100 μm CD in the ileum. The ileum samples were cut by blades and put it in cassettes. Each section was stained with alcian blue for 15 min and washed with distilled water. Samples were treated with periodic acid for 5 min and washed with distilled water. Subsequently, the samples were stained with Schiff's reagents for 10 min and washed with distilled water. Samples were counterstained in haematoxylin for 1 min and washed and dehydrated. The stained sections were viewed with a BZ microscope (BZ-X810; Keyence, Osaka, Japan). The captured images (4 ×) were analyzed using ImageJ (National Institutes of Health, Bethesda, MD).
Activities of Ileal Brush Border Digestive Enzymes and Serum Alkaline Phosphatase
Mid-ileal tissue samples, 100 mg, were homogenized in 2 mL PBS using a beads beater (Biospec Products, Bartlesville, OK). The supernatant of homogenized samples after the centrifugation at 4°C and 12,000 × g for 15 min was collected to analyze protein content using Pierce BCA Protein Assay Kits (Thermo Fisher Scientific, Waltham, MA) after 10-fold sample dilution. Activities of maltase and sucrase were determined according to Fan et al. (2004). Activities of alanine-aminopeptidase (APN) were analyzed according to Maroux et al. (1973). Activities of serum alkaline phosphatase and intestinal alkaline phosphatase were determined according to Lackeyram et al. (2010). Lipase activities were determined according to Elgharbawy et al. (2018). The activities of digestive enzymes were shown as values per mg protein.
Total Antioxidant Capacity, Total Glutathione, Oxidized GSH, and Superoxidase Dismutase Measurement
Liver samples (100 mg each) were homogenized in 1 mL of designated solution using a beads beater (Biospec Products, Bartlesville, OK) for each analysis including total antioxidant capacity (TAC), glutathione (GSH) and oxidized GSG (GSSG), and superoxide dismutase (SOD). Total antioxidant capacity of the liver tissues was measured using a commercial kit (QuantiCromAntioxidant Assay Kit; DTAC-100, BioAssay Systems, Hayward, CA) after 2-time sample dilution. Total GSH and GSSG (D 21 liver tissues) were analyzed after diluting 20 times and 2 times, respectively, by using Caymans GSH assay kits (Cayman Chemical, Ann Arbor, MI). The activities of SOD (D 21 liver tissues) were analyzed using Caymans GSH assay kits (Cayman Chemical, Ann Arbor, MI) after 400 times dilution. Aliquots of the supernatants were collected for analysis of protein content using Pierce BCA Protein Assay Kits (Thermo Fisher Scientific, Waltham, MA) with 1:9 dilution. The TAC, GSH, and GSSG concentrations, and SOD activities were expressed as values per mg protein.
RNA Extraction and Real-Time RT-PCR Analysis
Approximately, 100 mg of the mid-ileal samples were homogenized in QIAzol lysis reagents (Qiagen, Valencia, CA) using a bead beater (Biospec Products, Bartlesville, OK), and RNA was extracted according to the manufacturer's procedure. RNA quantity and purity were checked using a NanoDrop 2000 spectrophotometer (Thermo Fisher Scientific, Waltham, MA). One μg RNA was used to produce the first-strand cDNA using high-capacity cDNA synthesis kits (Applied Biosystems, Foster City, CA). Primers used in the study are shown in Table 2. Real-time RT-PCR was performed using SYBR Green Master Mix with a Step One thermocycler (Applied Biosystems, Foster City, CA). The final PCR volume (10 μL) contained 5 μL of SYBR Green Master Mix, 1.5 μL of cDNA, 0.5 μL of forward and reverse primers (10 μM), and 2.5 μL of water. Thermal cycle conditions for all reactions were as follows: 95°C denature for 10 min, 40 cycles at 95°C for 15 s and 60°C for 1 min, 95°C for 15 s, 60°C for 1 min and 95°C for 15 s. Several PCR products from each gene were stained with 6 × DNA loading dye (Thermo Fisher Scientific, Waltham, MA), electrophoresed on a 3% agarose gel in a Tris-acetate-EDTA buffer, and visualized by adding ethidium bromide, and the melting curve of each gene was then checked to confirm the specificity of each PCR product. Beta-actin and Glyceraldehyde 3-phosphate dehydrogenase (GAPDH) were used as the housekeeping genes (reference genes). The target mRNA abundance was normalized with geometric means of housekeeping genes (Vandesompele et al., 2002). Relative mRNA abundance was determined by using the 2–∆∆Ct method (Livak and Schmittgen, 2001). The negative control, containing no cDNA, was included in each run, and each sample was run in duplicate.
Table 2.
Primers used in the study.
| Genes1 | Sequence, 5′ to 3′ | Amplicon |
|---|---|---|
| Beta actin | F: CAACACAGTGCTGTCTGGTGGTA | 205 |
| R: ATCGTACTCCTGCTTGCTGATCC | ||
| GAPDH | F: GCTAAGGCTGTGGGGAAAGT | 161 |
| R: TCAGCAGCAGCCTTCACTAC | ||
| TLR2 | F: CGGTGGAAAGGGAGAAAG | 103 |
| R: CTTGCCACATCAGCTTCATT | ||
| TLR4 | F: CCTGGACTTGGACCTCAGTT | 110 |
| R: TTGTATGGATGTGGCACCTT | ||
| TLR5 | F: CGTTAGTGAGAATGGCTGGA | 106 |
| R: TGAGCCCATTGTATGAGAGC | ||
| NFκB | F: GAAGGAATCGTACCGGGAACA | 131 |
| R: CTCAGAGGGCCTTGTGACAGTAA | ||
| IL1b | F: TGCCTGCAGAAGAAGCCTCG | 204 |
| R: GACGGGCTCAAAAACCTCCT | ||
| IL2 | F: CGTAAGTGGATGGTTTTCCTCT | 161 |
| R: GGCTAAAGCTCACCTGGGTC | ||
| IL6 | F: ATAAATCCCGATGAAGTGG | 146 |
| R: CTCACGGTCTTCTCCATAAA | ||
| IL10 | F: CTGTCACCGCTTCTTCACC | 85 |
| R: CCCGTTCTCATCCATCTTCT | ||
| CD36 | F: CTGGGAAGGTTACTGCGATT | 109 |
| R: GGATCTGCAAATGTCAGAGG | ||
| CD74 | F: GAAATCAGACCCCAGGAAGA | 109 |
| R: GGTCTCAAAATCCTGCCAGT | ||
| CD80 | F: ACCCTCTTTGTTACCGCTGA | 118 |
| R: GTTTGGGAAAACCTCCATGA | ||
| SGLT1 | F: GCC ATG GCC AGG GCT TA | 66 |
| R: CAATAACCTGATCTGTGCACCAGTA | ||
| PepT1 | F: CCCCTGAGGAGGATCCTT | 66 |
| R: CAAAAGAGCAGCAACGA | ||
| EAAT3 | F: TGCTGCTTTGGATTCCAGTGT | 79 |
| R: AGCAATGACTGTAGTGCAGAAGTAATATATG | ||
| B0AT1 | F: GGGTTTTGTGTTGGCTTAGGAA | 60 |
| R: TCCATGGCTCTGGCAGAGAT | ||
| MUC2 | F: ATGCGATGTTAACACAGGACTC | 110 |
| R: GTGGAGCACAGCAGACTTTG | ||
| IAP | F: CTTCCTCGGAGATGGATTTG | 123 |
| R: AGAGCCACATAGGGGAAAGA |
GAPDH, Glyceraldehyde 3-phosphate dehydrogenase; TLR, toll like receptor; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; IL, interleukin; CD, cluster of differentiation; SGLT1, sodium glucose transporter 1; PepT1, peptide transporter 1; EAAT3, excitatory amino acid transporter 3; B0AT1, Sodium-dependent neutral amino acid transporter 1; MUC2, mucin 2; IAP, intestinal alkaline phosphatase.
Peripheral Blood Leukocyte Enrichment
On D18, blood samples (10 mL) were collected from one bird per pen after euthanizing via cervical dislocation. The blood samples were collected using 18 g needles (1.5 inches; World Precision Instruments, Inc., Sarasota, FL) with syringes and transferred into the Na-heparin 10 mL glass tubes (Grainer Bio-One, Kremsmuenster, Austria). To collect buffy coat, blood samples were centrifuged at 20 × g, 23°C for 10 min with the acceleration and brake set to 0. The buffy coat was carefully collected by gently stirring the top plasma layer using an electronic pipette equipped with a sterile 9” glass pipette and transferred to a sterile 15 mL conical tube (Thermo Fisher Scientific, Waltham, MA) to create a liquid funnel. This technique lifted the leukocyte fraction into the plasma layer to facilitate collection. The samples were centrifuged at 250 × g, 23°C for 10 min to collect leukocytes, and the supernatant was discarded, and sterile PBS was added. Leukocyte cell recovery and viability were measured on a Nexcelom Cell Counter (Nexcelom Bioscience, Lawrence, MA). The cell gates were set between 5 to 30 µm, and viability was measured via trypan blue exclusion. For the cell phenotyping and proliferation assays, the cells were diluted to 4.0 × 106/mL in complete RPMI medium (Gibco, Grand Island, NY) media containing 10% FBS (heat inactivated, USDA Approved; Gibco, Grand Island, NY), 2 mM L-glutamine (Gibco, Grand Island, NY), 50 IU/mL penicillin (HyClone Lab, Inc.; Logan, UT), and 50 μg/mL streptomycin (HyClone Lab, Inc.; Logan, UT).
Peripheral T Cell Phenotyping Using Flow Cytometry
Flow cytometry analysis was conducted according to Krunkosky et al. (2020) with some modifications. A cell suspension of 100 μL (4.0 × 105) was aliquoted into wells followed by 100 μL /well of CD4 (Mouse Anti-Chicken CD4-PE), CD8 (Mouse Anti-Chicken CD8a-FITC), and CD44 (Mouse Anti-Chicken CD44-PE) antibodies purchased from Southern Biotech (Birmingham, AL). The final antibody concentrations per reaction of PE anti-CD4 (clone CT-4), FITC anti-CD8 (clone CD8alpha), and PE anti-CD44 (clone CD44) were set to 0.5 μg/4.0 × 105 cells. After the addition of the conjugated antibodies, the samples were incubated for 30 min at 4°C on an orbital mixer (New Brunswick Scientific, New Brunswick, NJ). The cells were then washed with 200 μL FACS-PBS buffer and centrifuged at 250 × g, 4°C for 10 min. The supernatant was carefully aspirated from each well, and 100 μL FACS-PBS buffer and 100 μL flow fixing solution (Thermo Fisher Scientific, Waltham, MA) were added. The stained cell suspension was then transferred to individual flow tubes. The stained cell suspensions were analyzed on a BD Accuri C6 Flow Cytometer (San Jose, CA). Gating was set according to a previously determined P1 gate (FSCA vs. FSC-H) of enriched peripheral blood leukocytes (>90% lymphocytes) and the P2 gate (FSC-A vs. SSCA). Gate setting is shown in Figure 1. A total of 10,000 events were obtained per sample in the lymphocyte gate. Values were reported as percentage of expression of gated leukocyte population.
Figure 1.
Gate setting for flow cytometry.
Peripheral Blood Lymphocyte Proliferation Assay Measure by Alamar Blue
Lymphocyte proliferation by using concanavalin A (Con A) was analyzed according to Ahmed et al. (1994) with modifications. Aliquots of cells, 100 μL/well (4.0 × 105) in complete RPMI 1640, were transferred in the sterile 96 wells of round bottom plates to triplicate wells containing 0.0, 0.1, 1, 10, 20 μg/well of Con A. The plates were incubated for 48 h under humidified conditions with 95% O2 and 5% CO2 at 37°C. After 48 h of culture, 20 μL/well of Alamar blue (Thermo Fisher Scientific, Waltham, MA) were added, and the plates were incubated an additional 24 h. After 72 h of culture, the plates were placed in a BioTek Synergy 4 plate reader (Biotek, Winooski, VT), and the absorbances at 570 and 600 nm were measured to assess cell proliferative response. The absorbance at 600 nm was subtracted from the absorbance at 570 nm. The slope was calculated as follows: average of differences between 570 nm and 600 nm/the 4 different amounts (0.0, 0.1, 1, 10, 20 μg per well) of Con A, and the slope was used as the value to represent peripheral blood lymphocyte proliferation by using Con A.
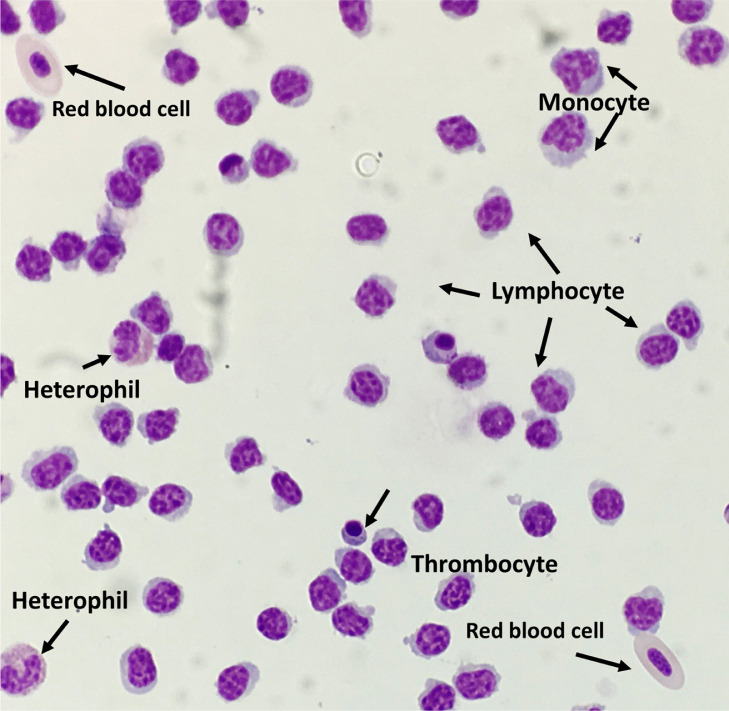
Peripheral Blood Leukocyte Cytology
Cell suspensions, 20 μL (approximately 8 × 104 cells), were aliquoted into individual cytospin slide chambers containing 180 μL of Ca free/Mg free PBS (Fisher Scientific, Fair Lawn, NJ). Slide chambers were centrifuged at 34 × g for 3 min at 23°C using a 7150 Hematology Slide-Stainer Cytocentrifuge (Wescor, Logan, UT). Slides were then stained with Wright-Giemsa (Sigma-Aldrich, St. Louis, MO) using a 10 min full stain/10 min diluted stain protocol. Stained slides were evaluated on an Olympus CX43 compound light microscope (Olympus America Inc., Center Valley, PA) under 600 × magnification. A total of 200 leukocytes across a minimum of 10 fields were enumerated to determine lymphocyte purity percentage. Examples of stained leukocytes are shown in Figure 2. Values are expressed as mean %.
Figure 2.
Different leukocytes in the peripheral blood.
Statistical Analyses
Statistical analyses were performed using SAS (version 9.4; SAS Inst. Inc., Cary, NC). Data normality was checked using proc univariate except for the results of presence of STNR in the spleen and liver. The presence of STNR in the spleen and liver was analyzed by chi-square analyses. The effects of STNR inoculation (SCC vs. CC) were evaluated by the unpaired t-test. Challenged groups (CC, TA0.25, TA0.5, TA1, and TA2) were compared using PROC MIXED in a completely randomized design followed by the Tukey's comparison test. Orthogonal polynomial contrasts were utilized to evaluate the significance of linear or quadratic effects of different dosages of TA within the treatments infected with STNR. Statistical significance was set at P < 0.05, and trends (0.05 ≤ P ≤ 0.1) were also presented.
RESULTS
Growth Performance
Infection with STNR tended to reduce BW (P = 0.096) and ADG (P = 0.098) and significantly decreased ADFI (P < 0.05) on D 7 (Table 3). Supplemental TA tended to linearly reduce BW (P = 0.087) and ADG (P = 0.074) in broilers infected with STNR on D 7. In the grower phase (D 7–21), ADG showed a linear increasing trend in TA supplemented groups (P = 0.097).
Table 3.
Effects of supplemental tannic acid on the growth performance [body weight (BW), average daily gain (ADG), average daily feed intake (ADFI), and feed conversion ratio (FCR)] of broilers infected with Salmonella Typhimurium nalidixic acid resistant strain (STNR) in the starter phase (D 0 to 7), grower phase (D 7 to 21), and whole phase (D 0 to 21).
| STNR-challenged2 |
Polynomial contrast |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Items | SCC1 | CC | TA0.25 | TA0.5 | TA1 | TA2 | SEM | P value | Linear | Quadratic |
| Initial BW, g | 49.61 | 49.51 | 49.55 | 49.59 | 49.51 | 49.61 | 0.21 | 0.61 | ||
| Starter phase (D 0 to 7) |
||||||||||
| BW, g | 168.8* | 162.9 | 163.8 | 159.7 | 159.4 | 156 | 8.56 | 0.471 | 0.087 | 0.850 |
| ADG, g/d | 16.22* | 16.20 | 16.32 | 15.73 | 15.68 | 15.18 | 1.21 | 0.425 | 0.074 | 0.855 |
| ADFI, g/d | 19.48** | 17.97 | 18.11 | 17.34 | 17.81 | 16.81 | 1.63 | 0.570 | 0.160 | 0.809 |
| FCR, g/g | 1.15 | 1.11 | 1.11 | 1.10 | 1.13 | 1.11 | 0.05 | 0.863 | 0.887 | 0.479 |
| Grower phase (D 7 to 21) | ||||||||||
| BW, g | 543.6 | 491 | 525.24 | 538.35 | 540.72 | 541.65 | 61.58 | 0.513 | 0.215 | 0.268 |
| ADG, g/d | 27.20 | 23.47 | 25.91 | 27.17 | 27.34 | 27.61 | 4.03 | 0.312 | 0.097 | 0.204 |
| ADFI, g/d | 47.33 | 42.71 | 43.78 | 46.45 | 45.25 | 46.73 | 5.63 | 0.639 | 0.224 | 0.569 |
| FCR, g/g | 1.98 | 1.98 | 1.78 | 1.79 | 1.71 | 1.81 | 0.26 | 0.421 | 0.402 | 0.122 |
| Whole phase (D 0 to 21) | ||||||||||
| ADG, g/d | 17.85 | 15.78 | 17.03 | 17.52 | 17.59 | 17.6 | 2.18 | 0.494 | 0.215 | 0.250 |
| ADFI, g/d | 38.05 | 34.47 | 35.22 | 36.75 | 36.1 | 36.76 | 4.04 | 0.796 | 0.345 | 0.575 |
| FCR, g/g | 1.7 | 1.69 | 1.55 | 1.56 | 1.52 | 1.57 | 0.18 | 0.477 | 0.423 | 0.149 |
SCC (sham-challenged control): broilers fed a basal diet and challenged with peptone water; CC (challenged control): broilers fed a basal diet and challenged with 0.5 mL of 1 × 108 CFU/mL of STNR; TA0.25 (tannic acid 0.25 g/kg): CC + 0.25 g/kg of tannic acid; TA0.5 (tannic acid 0.5 g/kg): CC + 0.5 g/kg of tannic acid; TA1 (tannic acid 1 g/kg): CC + 1 g/kg of tannic acid; and TA2 (tannic acid 2 g/kg): CC + 2 g/kg of tannic acid.
SCC versus CC (unpaired t test): *0.05 < P < 0.10, **P < 0.05, ***P < 0.01.
STNR infected groups (CC, TA0.25, TA0.5, TA1, and TA2) were compared by PROC MIXED followed by the Tukey's multiple comparison test.
Colonization of STNR in the Ceca, Liver, and Spleen
Colonization of STNR was measured in the ceca, liver, and spleen in broilers infected with STNR (Table 4). On D 7, TA supplementation linearly (P < 0.01) and quadratically (P < 0.05) reduced STNR colonization in the ceca, and the TA1 and TA2 groups had significantly lower STNR colonization in the ceca compared to the CC group (P < 0.05). On D 14, supplemental TA significantly modulated STNR colonization in the liver (P < 0.05).
Table 4.
Effects of supplemental tannic acid on the colonization of Salmonella Typhimurium nalidixic acid resistant strain (STNR) in the cecal content (log10), liver (infected birds per treatment), and spleen (infected birds per treatment) in broilers infected with STNR on D 4, 7, 14, and 21.1
| STNR-challenged |
Polynomial contrast |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Items | SCC | CC | TA0.25 | TA0.5 | TA1 | TA2 | SEM | P value | Linear | Quadratic |
| Cecal content1 | ||||||||||
| D 4 | Not detected | 5.86 | 6.96 | 6.01 | 5.94 | 6.37 | 1.25 | 0.460 | 0.931 | 0.755 |
| D 7 | 6.23a | 4.8ab | 5.11ab | 2.59b | 3.45b | 1.66 | 0.002 | 0.002 | 0.018 | |
| D 14 | 3.12 | 0.79 | 3.44 | 3.01 | 1.20 | 2.21 | 0.101 | 0.314 | 0.294 | |
| D 21 | 1.30 | 2.13 | 2.60 | 2.78 | 1.96 | 2.26 | 0.762 | 0.736 | 0.208 | |
| Liver2 | ||||||||||
| D 4 | 3/7 | 5/7 | 5/7 | 3/7 | 3/7 | 0.598 | ||||
| D 7 | 4/7 | 6/7 | 2/7 | 4/7 | 1/7 | 0.069 | ||||
| D 14 | 4/7 | 0/7 | 0/7 | 4/7 | 1/7 | 0.013 | ||||
| D 21 | 3/7 | 5/7 | 5/7 | 3/7 | 3/7 | 0.598 | ||||
| Spleen3 | ||||||||||
| D 4 | 2/7 | 2/7 | 3/7 | 2/7 | 1/7 | 0.844 | ||||
| D 7 | 2/7 | 1/7 | 1/7 | 1/7 | 1/7 | 0.938 | ||||
| D 14 | 3/7 | 0/7 | 0/7 | 1/7 | 1/7 | 0.136 | ||||
| D 21 | 2/7 | 2/7 | 3/7 | 2/7 | 1/7 | 0.844 | ||||
SCC (sham-challenged control): broilers fed a basal diet and challenged with peptone water; CC (challenged control): broilers fed a basal diet and challenged with 0.5 mL of 1 × 108 CFU/mL of STNR; TA0.25 (tannic acid 0.25 g/kg): CC + 0.25 g/kg of tannic acid; TA0.5 (tannic acid 0.5 g/kg): CC + 0.5 g/kg of tannic acid; TA1 (tannic acid 1 g/kg): CC + 1 g/kg of tannic acid; and TA2 (tannic acid 2 g/kg): CC + 2 g/kg of tannic acid.
STNR infected groups (CC, TA0.25, TA0.5, TA1, and TA2) were compared by PROC MIXED followed by the Tukey's multiple comparison test for cecal samples. Means within the same row with different superscripts (a,b) differ significantly (P < 0.05).
For the presence of STNR in the liver and spleen, STNR infected groups (CC, TA0.25, TA0.5, TA1, and TA2) were compared by chi-square test for independence.
FITC-D4 Flux and Serum Endotoxin Levels
The FITC-D4 flux levels were analyzed on D 14 and 21, and serum endotoxin levels were analyzed on D 7 and 21 (Table 5). On D 14, infection with STNR significantly increased FITC-D4 flux, and the TA0.5 group had the higher FITC-D4 flux compared to the CC and TA2 (P < 0.05). On D 21, STNR infection significantly increased serum endotoxin levels (P < 0.05), and serum endotoxin levels were quadratically decreased by the supplementation of TA (P < 0.05).
Table 5.
Effects of supplemental tannic acid on the gut permeability [flux of fluorescein isothiocyanate–dextran (FITC-D4; average mol wt: 4000); D 14 and 21] and serum endotoxin concentrations (EU/mL; D 7 and 21) in broilers infected with Salmonella Typhimurium nalidixic acid resistant strain (STNR).
| STNR-challenged2 |
Polynomial contrast |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Items | SCC1 | CC | TA0.25 | TA0.5 | TA1 | TA2 | SEM | P value | Linear | Quadratic |
| FITC-D4 flux relative to the SCC group | ||||||||||
| D 14 | 1** | 1.54b | 1.89ab | 2.33a | 1.82ab | 1.58b | 0.46 | 0.022 | 0.348 | 0.022 |
| D 21 | 1 | 1.1 | 1.03 | 1.04 | 1.13 | 1.14 | 0.2 | 0.768 | 0.377 | 0.841 |
| Serum endotoxin | ||||||||||
| D 7 | 3.43 | 3.56 | 3.43 | 3.98 | 3.82 | 3.62 | 0.53 | 0.352 | 0.781 | 0.168 |
| D 21 | 2.97*** | 3.28 | 3.11 | 3.12 | 3.02 | 3.34 | 0.25 | 0.136 | 0.385 | 0.018 |
SCC (sham-challenged control): broilers fed a basal diet and challenged with peptone water; CC (challenged control): broilers fed a basal diet and challenged with 0.5 mL of 1 × 108 CFU/mL of STNR; TA0.25 (tannic acid 0.25 g/kg): CC + 0.25 g/kg of tannic acid; TA0.5 (tannic acid 0.5 g/kg): CC + 0.5 g/kg of tannic acid; TA1 (tannic acid 1 g/kg): CC + 1 g/kg of tannic acid; and TA2 (tannic acid 2 g/kg): CC + 2 g/kg of tannic acid.
SCC versus CC (unpaired t test): *0.05 < P < 0.10, **P < 0.05, ***P < 0.01.
STNR infected groups (CC, TA0.25, TA0.5, TA1, and TA2) were compared by PROC MIXED followed by the Tukey's multiple comparison test. Means within the same row with different superscripts (a,b) differ significantly (P < 0.05).
Ileal Morphology and Number of the Goblet Cells in the Ileum
Ileal morphology and the number of goblet cells in the ileum were evaluated on D 7 and 21 (Table 6). On D 7, the STNR infection tended to decrease ileal VH (P = 0.084) and VH:CD (P = 0.082). No significant differences in ileal morphology and number of goblet cells in the ileum were observed across the treatment groups. On D 21, STNR infection significantly reduced ileal VH and VH:CD. Supplemental TA quadratically increased ileal VH (P < 0.05), and the TA0.25 group had higher ileal VH compared to the CC group (P < 0.05). Goblet cells per 100 μm VH were also linearly increased due to supplementation of TA (P < 0.05), and the TA2 group had significantly a higher numbers of goblet cells per 100 μm VH compared to the CC group (P < 0.05).
Table 6.
Effects of supplemental tannic acid on the ileal morphology [villus height (VH, μm), crypts depth (CD, μm), and VH:CD] and number of goblet cells per 100 μm VH and CD in the ileum of broilers infected with Salmonella Typhimurium nalidixic acid resistant strain (STNR) on D 7 and 21.
| STNR-challenged2 |
Polynomial contrast |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Items | SCC1 | CC | TA0.25 | TA0.5 | TA1 | TA2 | SEM | P value | Linear | Quadratic |
| D 7 | ||||||||||
| VH | 560.1* | 495.3 | 488.1 | 503.2 | 497.8 | 535.2 | 54.78 | 0.545 | 0.787 | 0.916 |
| CD | 132.1 | 135.3 | 124.5 | 131.4 | 138.6 | 134.2 | 16.87 | 0.601 | 0.425 | 0.270 |
| VH:CD | 4.34* | 3.78 | 4.04 | 3.96 | 3.73 | 4.17 | 0.59 | 0.629 | 0.712 | 0.343 |
| Goblet cells per 100 μm VH | 9.45 | 9.78 | 9.41 | 9.30 | 10.30 | 8.87 | 1.12 | 0.200 | 0.355 | 0.124 |
| Goblet cells per 100 μm CD | 9.92 | 10.30 | 11.44 | 10.39 | 10.72 | 10.54 | 1.12 | 0.349 | 0.905 | 0.665 |
| D 21 | ||||||||||
| VH | 581.5** | 454.1b | 568.9a | 506.9ab | 484.2ab | 491.9ab | 65.41 | 0.036 | 0.825 | 0.037 |
| CD | 113.9 | 112.6 | 127.1 | 116.3 | 115.0 | 121.9 | 16.49 | 0.486 | 0.710 | 0.385 |
| VH:CD | 5.18** | 4.21 | 4.57 | 4.44 | 4.29 | 4.14 | 0.53 | 0.565 | 0.945 | 0.289 |
| Goblet cells per 100 μm VH | 11.7 | 11.14b | 9.70b | 11.69ab | 11.91ab | 13.54a | 1.47 | 0.001 | 0.052 | 0.498 |
| Goblet cells per 100 μm CD | 12.78 | 12.21 | 11.62 | 12.17 | 12.71 | 12.44 | 1.28 | 0.597 | 0.268 | 0.386 |
SCC (sham-challenged control): broilers fed a basal diet and challenged with peptone water; CC (challenged control): broilers fed a basal diet and challenged with 0.5 mL of 1 × 108 CFU/mL of STNR; TA0.25 (tannic acid 0.25 g/kg): CC + 0.25 g/kg of tannic acid; TA0.5 (tannic acid 0.5 g/kg): CC + 0.5 g/kg of tannic acid; TA1 (tannic acid 1 g/kg): CC + 1 g/kg of tannic acid; and TA2 (tannic acid 2 g/kg): CC + 2 g/kg of tannic acid.
SCC versus CC (unpaired t test): *0.05 < P < 0.10, **P < 0.05, ***P < 0.01.
STNR infected groups (CC, TA0.25, TA0.5, TA1, and TA2) were compared by PROC MIXED followed by the Tukey's multiple comparison test. Means within the same row with different superscripts (a,b) differ significantly (P < 0.05).
Activities of Ileal Brush Border Digestive Enzymes and Serum Alkaline Phosphatase
Activities of ileal brush border digestive enzymes and serum alkaline phosphatase were evaluated on D 7 and 21 (Table 7). On D 7, aminopeptidase activities were significantly increased, and lipase activities were significantly decreased in STNR infected broilers compared to the SCC group. The TA fed broilers had linearly decreased maltase activities (P < 0.05), with the TA0.5 group having significantly higher maltase activities compared to the TA2 group (P < 0.05). The TA supplementation tended to enhance lipase activities (P = 0.055). On D 21, activities of serum alkaline phosphate were quadratically increased due to supplementation of TA in broilers infected with STNR (P < 0.05). However, no differences were observed in the ileal brush border digestive enzymes on D 21 (P > 0.1).
Table 7.
Effects of supplemental tannic acid on the activities of ileal brush border digestive enzymes [maltase (nmol glucose released/mg protein/min), sucrase (nmol glucose released/mg protein/min), aminopeptidase (nmol p-nitroaniline liberated/mg protein/min), lipase (mmol p-nitrophenyl phosphate liberated/mg protein/min), intestinal alkaline phosphatase (μmol p-nitrophenol liberated/mg protein/min), and serum alkaline phosphatase (μmol p-nitrophenol liberated/mL serum/min)] of broilers infected with Salmonella Typhimurium nalidixic acid resistant strain (STNR) on D 7 and 21.
| STNR-challenged2 |
Polynomial contrast |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Items | SCC1 | CC | TA0.25 | TA0.5 | TA1 | TA2 | SEM | P value | Linear | Quadratic |
| D 7 | ||||||||||
| Sucrase | 0.81 | 1.30 | 1.11 | 1.42 | 1.08 | 1.76 | 0.69 | 0.359 | 0.169 | 0.276 |
| Maltase | 94.48 | 150.84ab | 109.35ab | 178.41a | 110.76ab | 78.08b | 62.33 | 0.045 | 0.026 | 0.555 |
| Aminopeptidase | 38.90** | 44.52 | 52.59 | 50.77 | 44.46 | 53.61 | 14.61 | 0.639 | 0.515 | 0.731 |
| Lipase | 0.92** | 0.58 | 0.62 | 0.67 | 0.54 | 0.85 | 0.23 | 0.141 | 0.055 | 0.231 |
| Intestinal alkaline phosphatase | 0.15 | 0.18 | 0.22 | 0.21 | 0.18 | 0.29 | 0.21 | 0.482 | 0.148 | 0.475 |
| Serum alkaline phosphatase | 1.57 | 1.75 | 1.98 | 1.76 | 1.46 | 1.69 | 0.56 | 0.607 | 0.465 | 0.459 |
| D 21 | ||||||||||
| Sucrase | 0.90 | 0.53 | 1.24 | 0.45 | 1.38 | 0.77 | 1 | 0.340 | 0.780 | 0.306 |
| Maltase | 176.12 | 127.71 | 148.29 | 118.96 | 268.75 | 170.79 | 196.48 | 0.631 | 0.504 | 0.360 |
| Aminopeptidase | 44.80 | 52.40 | 57.86 | 45.82 | 73.87 | 57.90 | 34.23 | 0.640 | 0.596 | 0.485 |
| Lipase | 1.34 | 1.62 | 2.57 | 1.67 | 2.08 | 2.17 | 2.09 | 0.917 | 0.805 | 0.943 |
| Intestinal alkaline phosphatase | 0.26 | 0.34 | 0.54 | 0.29 | 0.65 | 0.38 | 0.39 | 0.415 | 0.884 | 0.294 |
| Serum alkaline phosphatase | 1.97 | 1.83 | 2.17 | 2.01 | 2.29 | 1.88 | 0.42 | 0.233 | 0.906 | 0.047 |
SCC (sham-challenged control): broilers fed a basal diet and challenged with peptone water; CC (challenged control): broilers fed a basal diet and challenged with 0.5 mL of 1 × 108 CFU/mL of STNR; TA0.25 (tannic acid 0.25 g/kg): CC + 0.25 g/kg of tannic acid; TA0.5 (tannic acid 0.5 g/kg): CC + 0.5 g/kg of tannic acid; TA1 (tannic acid 1 g/kg): CC + 1 g/kg of tannic acid; and TA2 (tannic acid 2 g/kg): CC + 2 g/kg of tannic acid.
SCC versus CC (unpaired t test): *0.05 < P < 0.10, **P < 0.05, ***P < 0.01.
STNR infected groups (CC, TA0.25, TA0.5, TA1, and TA2) were compared by PROC MIXED followed by the Tukey's multiple comparison test. Means within the same row with different superscripts (a,b) differ significantly (P < 0.05).
Total Antioxidant Capacity, Total Glutathione, Oxidized GSH, and Superoxidase Dismutase
The total antioxidant capacity (TAC), total glutathione (GSH), oxidized GSH (GSSG), and superoxidase dismutase (SOD) were evaluated in the ileum and liver on D 7 and 21 (Table 8). No differences were observed across the treatments in the ileal TAC on D 7 and 21 (P > 0.1). Liver GSH concentrations and SOD activities were not different among the treatments on D 7 and 21.
Table 8.
Effects of supplemental tannic acid on the total antioxidant capacity (TAC; µM Trolox Equivalents/mg protein; D 7 and 21) of the ileum and TAC, activities of superoxide dismutase (SOD; U/mg protein), and concentrations of glutathione (GSH; µM/mg protein), oxidized GSH (GSSG; µM/mg protein), reduced GSH (µM/mg protein), and reduced GSH:GSSG of the liver in D 21 broilers infected with Salmonella Typhimurium nalidixic acid resistant strain (STNR).
| STNR-challenged2 |
Polynomial contrast |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Items | SCC1 | CC | TA0.25 | TA0.5 | TA1 | TA2 | SEM | P value | Linear | Quadratic |
| Ileum TAC | ||||||||||
| D 7 | 56.08 | 57.38 | 56.31 | 57.28 | 63.06 | 58.63 | 6.59 | 0.356 | 0.376 | 0.209 |
| D 21 | 68.41 | 60.22 | 74.35 | 65.48 | 61.87 | 61.67 | 14.14 | 0.354 | 0.471 | 0.745 |
| D 21 Liver | ||||||||||
| TAC | 100 | 104.65 | 105.32 | 103.74 | 108.10 | 101.88 | 9.66 | 0.814 | 0.643 | 0.411 |
| SOD | 19.08 | 21.23 | 22.59 | 25.64 | 21.55 | 20.93 | 6.14 | 0.608 | 0.543 | 0.456 |
| GSH | 36.13 | 35.96 | 30.96 | 30.51 | 34.60 | 29.67 | 5.45 | 0.180 | 0.202 | 0.995 |
| GSSG | 1.28 | 1.62 | 1.27 | 1.34 | 1.68 | 1.48 | 0.46 | 0.418 | 0.766 | 0.948 |
| Reduced GSH3 | 28.14 | 32.71 | 28.41 | 27.84 | 31.23 | 26.71 | 4.87 | 0.172 | 0.140 | 0.995 |
| Reduced GSH:GSSG | 23.36 | 20.24 | 22.62 | 21.27 | 20.04 | 19.11 | 4.27 | 0.608 | 0.272 | 0.725 |
SCC (sham-challenged control): broilers fed a basal diet and challenged with peptone water; CC (challenged control): broilers fed a basal diet and challenged with 0.5 mL of 1 × 108 CFU/mL of STNR; TA0.25 (tannic acid 250 mg/kg): CC + 250 mg/kg of tannic acid; TA0.5 (tannic acid 500 mg/kg): CC + 500 mg/kg of tannic acid; TA1 (tannic acid 1,000 mg/kg): CC + 1,000 mg/kg of tannic acid; and TA2 (tannic acid 2,000 mg/kg): CC + 2,000 mg/kg of tannic acid.
SCC versus CC (unpaired t test): *0.05 < P < 0.10, **P < 0.05, ***P < 0.01.
STNR infected groups (CC, TA0.25, TA0.5, TA1, and TA2) were compared by PROC MIXED followed by the Tukey's multiple comparison test.
Reduced GSH = total GSH – 2 × GSSG.
Relative mRNA Expression of Genes Related to the Immune System and Nutrient Transporters
The mRNA expression of genes related to immunity and nutrient transporters in the ileum was evaluated on D 7 and 21 (Table 9). On D 7, STNR infection significantly reduced mRNA expression of toll like receptor 2 (TLR2; P < 0.05) and increased mRNA expression of nuclear factor kappa-light-chain-enhancer of activated B cells (NFκB; P = 0.067). The STNR infection increased mRNA expression of mucin 2 (MUC2; P = 0.061). The TA supplementation tended to linearly decrease mRNA expression of excitatory amino acid transporter 3 (EAAT3; P = 0.075). On D 21, the CC group had significantly decreased mRNA expression of TLR2 (P < 0.01) and TLR4 (P < 0.05) compared to the SCC group. The mRNA expression of interleukin 6 (IL6) was quadratically decreased in TA supplemented birds (P < 0.05). The mRNA expression of cluster of differentiation 36 (CD36), CD74, and sodium glucose transporter 1 (SGLT1) was significantly decreased in the CC group compared to the SCC group (P < 0.05). The TA supplementation trended toward a linear increase in mRNA expression of peptide transporter 1 (PepT1; P = 0.095). The STNR infected birds had significantly decreased mRNA expression of EAAT3 and sodium-dependent neutral amino acid transporter (B0AT1), while supplementation with TA yielded a linear increase in EAAT3 expression (P < 0.05). On D 21, STNR infection trended toward a decrease in mRNA expression of intestinal alkaline phosphatase (P = 0.063).
Table 9.
Effects of supplemental tannic acid on the relative mRNA expression (2–∆∆Ct) of genes related to immune system and nutrient transporters in the ileum of broilers infected with Salmonella Typhimurium nalidixic acid resistant strain (STNR) on D 7 and 211.
| STNR-challenged3 |
Polynomial contrast |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Items | SCC2 | CC | TA0.25 | TA0.5 | TA1 | TA2 | SEM | P value | Linear | Quadratic |
| D 7 | ||||||||||
| TLR2 | 1.04⁎⁎ | 0.69 | 0.68 | 0.77 | 0.73 | 0.70 | 0.27 | 0.968 | 0.932 | 0.624 |
| TLR4 | 1.08 | 1.15 | 0.99 | 1.12 | 1.04 | 1.05 | 0.36 | 0.924 | 0.777 | 0.823 |
| TLR5 | 1.17 | 1.18 | 1.06 | 1.12 | 0.81 | 0.96 | 0.48 | 0.625 | 0.303 | 0.354 |
| NFκB | 1.04* | 1.46 | 1.28 | 1.33 | 1.06 | 1.18 | 0.35 | 0.276 | 0.123 | 0.167 |
| IL1b | 1.14 | 1.19 | 1.26 | 1.28 | 0.73 | 1.23 | 0.58 | 0.365 | 0.722 | 0.193 |
| IL2 | 2 | 1.91 | 1.03 | 0.88 | 1.77 | 0.77 | 1.67 | 0.593 | 0.445 | 0.937 |
| IL6 | 1.27 | 1.14 | 0.57 | 0.59 | 0.79 | 0.51 | 0.84 | 0.635 | 0.376 | 0.683 |
| IL10 | 1.14 | 0.97 | 1.06 | 1.71 | 1.08 | 0.66 | 0.92 | 0.336 | 0.302 | 0.215 |
| CD36 | 1.12 | 0.87 | 0.37 | 0.90 | 0.65 | 0.76 | 0.62 | 0.517 | 0.885 | 0.752 |
| CD74 | 1.16 | 1.12 | 1.08 | 1.15 | 1.03 | 0.98 | 0.41 | 0.930 | 0.440 | 0.950 |
| CD80 | 1.03 | 1.25 | 1.25 | 1.25 | 1.25 | 1.25 | 0.56 | 0.953 | 0.589 | 0.572 |
| SGLT1 | 1.01 | 1.07 | 1.22 | 1.22 | 1.00 | 1.50 | 0.57 | 0.533 | 0.235 | 0.427 |
| PepT1 | 1.05 | 0.86 | 0.93 | 0.74 | 0.82 | 0.98 | 0.34 | 0.706 | 0.518 | 0.365 |
| EAAT3 | 1.22 | 1.44 | 1.24 | 1.80 | 0.78 | 0.70 | 0.98 | 0.214 | 0.075 | 0.991 |
| MUC2 | 1.11* | 1.54 | 1.87 | 1.71 | 1.58 | 2.00 | 0.6 | 0.578 | 0.304 | 0.611 |
| B0AT1 | 1.08 | 1.40 | 1.39 | 1.30 | 0.99 | 1.19 | 0.39 | 0.294 | 0.166 | 0.160 |
| IAP | 1.05 | 0.93 | 1.16 | 1.43 | 1.29 | 1.44 | 0.44 | 0.188 | 0.072 | 0.269 |
| D 21 | ||||||||||
| TLR2 | 1.02⁎⁎⁎ | 0.60 | 0.75 | 0.83 | 0.63 | 0.82 | 0.49 | 0.849 | 0.604 | 0.983 |
| TLR4 | 1.01⁎⁎ | 0.70 | 0.68 | 0.91 | 0.64 | 0.90 | 0.32 | 0.364 | 0.325 | 0.688 |
| TLR5 | 1.08 | 1.64 | 0.85 | 1.35 | 1.22 | 1.28 | 0.86 | 0.601 | 0.869 | 0.569 |
| NFκB | 1.01 | 0.99 | 1.09 | 1.18 | 1.16 | 1.28 | 0.36 | 0.656 | 0.169 | 0.698 |
| IL1b | 1.06 | 0.78 | 1.15 | 1.15 | 1.00 | 1.30 | 0.58 | 0.523 | 0.223 | 0.863 |
| IL2 | 1.23 | 1.46 | 0.57 | 0.57 | 0.89 | 0.67 | 1 | 0.429 | 0.444 | 0.403 |
| IL6 | 1.01 | 1.55 | 0.86 | 0.98 | 0.82 | 1.20 | 0.73 | 0.337 | 0.750 | 0.081 |
| IL10 | 1.57 | 1.18 | 1.47 | 1.45 | 1.11 | 1.08 | 0.86 | 0.852 | 0.488 | 0.828 |
| CD36 | 1.17⁎⁎ | 0.47 | 0.44 | 0.84 | 0.44 | 0.51 | 0.37 | 0.298 | 0.905 | 0.549 |
| CD74 | 1.03⁎⁎ | 0.74 | 0.80 | 0.89 | 0.79 | 1.04 | 0.33 | 0.485 | 0.118 | 0.760 |
| CD80 | 1.13 | 0.91 | 1.24 | 1.13 | 0.94 | 0.90 | 0.56 | 0.731 | 0.519 | 0.693 |
| SGLT1 | 1.09⁎⁎ | 0.53 | 0.83 | 0.88 | 0.65 | 0.83 | 0.34 | 0.285 | 0.416 | 0.633 |
| PepT1 | 1.24 | 1.01 | 0.78 | 1.55 | 1.15 | 1.65 | 0.79 | 0.227 | 0.095 | 0.956 |
| MUC2 | 1.48 | 1.14 | 1.42 | 1.23 | 1.04 | 1.46 | 0.57 | 0.606 | 0.545 | 0.419 |
| EAAT3 | 1.07⁎⁎ | 0.65 | 0.67 | 0.87 | 0.73 | 1.18 | 0.48 | 0.237 | 0.039 | 0.628 |
| B0AT1 | 1.04⁎⁎ | 0.68 | 0.67 | 0.97 | 0.75 | 1.12 | 0.51 | 0.378 | 0.101 | 0.831 |
| IAP | 1.05* | 0.69 | 0.99 | 1.10 | 0.78 | 1.12 | 0.4 | 0.202 | 0.225 | 0.955 |
SCC (sham-challenged control): broilers fed a basal diet and challenged with peptone water; CC (challenged control): broilers fed a basal diet and challenged with 0.5 mL of 1 × 108 CFU/mL of STNR; TA0.25 (tannic acid 0.25 g/kg): CC + 0.25 g/kg of tannic acid; TA0.5 (tannic acid 0.5 g/kg): CC + 0.5 g/kg of tannic acid; TA1 (tannic acid 1 g/kg): CC + 1 g/kg of tannic acid; and TA2 (tannic acid 2 g/kg): CC + 2 g/kg of tannic acid.
GAPDH, Glyceraldehyde 3-phosphate dehydrogenase; TLR, toll like receptor; NFκB, nuclear factor kappa-light-chain-enhancer of activated B cells; IL, interleukin; CD, cluster of differentiation; SGLT1, sodium glucose transporter 1; PepT1, peptide transporter 1; EAAT3, excitatory amino acid transporter 3; B0AT1, Sodium-dependent neutral amino acid transporter 1; MUC2, mucin 2; IAP, intestinal alkaline phosphatase
SCC versus CC (unpaired t test): *0.05 < P < 0.10, **P < 0.05, ***P < 0.01.
STNR infected groups (CC, TA0.25, TA0.5, TA1, and TA2) were compared by PROC MIXED followed by the Tukey's multiple comparison test.
Lymphocyte Proliferation and Immune Cell Phenotyping of Enriched Peripheral Blood Leukocytes
On D18, peripheral blood leukocytes were enriched and assessed for T cell and pan-leukocyte phenotype as well as capacity to respond to a pan T cell mitogen, Con A (Table 10). Average lymphocyte purity and viability were 87% and >89% across all treatment groups, respectively. Enriched peripheral blood leukocytes generated a Con A proliferation curve that was comparable across all treatment groups. However, no differences were observed among the treatments in the Con A proliferation rate (P > 0.1). When these same cells were phenotyped with pan-leukocyte cell marker CD44+, the gated cell fraction averaged ≥ 95% leukocytes across all treatment groups, and the percentage of CD4+ was comparable across all treatment groups. However, the percentage of CD8+ cells was linearly significantly increased due to supplemental TA (P < 0.01), resulting in a decrease in the ratio of CD4:CD8 cells (P < 0.05).
Table 10.
Effects of supplemental tannic acid on the T cell proliferation by concanavalin A (Con A), proportion of CD4, CD8, and CD44 in the serum leukocytes of broilers infected with Salmonella Typhimurium nalidixic acid resistant strain (STNR) on D 18.
| STNR-challenged2 |
Polynomial contrast |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Items | SCC1 | CC | TA0.25 | TA0.5 | TA1 | TA2 | SEM | P value | Linear | Quadratic |
| T cell proliferation by Con A (Slope3) | 31.92 | 35.09 | 30.74 | 33.88 | 34.56 | 33.51 | 6.26 | 0.731 | 0.921 | 0.986 |
| Leukocyte cell Phenotype | ||||||||||
| CD4+ (%) | 54.86 | 52.99 | 50.96 | 53.88 | 50.86 | 51.01 | 5.92 | 0.852 | 0.540 | 0.925 |
| CD8+ (%) | 22.90 | 22.69 | 21.94 | 20.02 | 27.09 | 28.01 | 4.95 | 0.033 | 0.006 | 0.935 |
| CD4:CD8 | 2.51 | 2.66 | 2.39 | 2.85 | 1.92 | 1.92 | 0.8 | 0.162 | 0.042 | 0.708 |
| CD44+ (%) | 95.70 | 95.46 | 92.07 | 91.78 | 95.24 | 95.00 | 2.93 | 0.072 | 0.269 | 0.355 |
SCC (sham-challenged control): broilers fed a basal diet and challenged with peptone water; CC (challenged control): broilers fed a basal diet and challenged with 0.5 mL of 1 × 108 CFU/mL of STNR; TA0.25 (tannic acid 0.25 g/kg): CC + 0.25 g/kg of tannic acid; TA0.5 (tannic acid 0.5 g/kg): CC + 0.5 g/kg of tannic acid; TA1 (tannic acid 1 g/kg): CC + 1 g/kg of tannic acid; and TA2 (tannic acid 2 g/kg): CC + 2 g/kg of tannic acid.
SCC versus CC (unpaired t test): *0.05 < P < 0.10, **P < 0.05, ***P < 0.01.
STNR infected groups (CC, TA0.25, TA0.5, TA1, and TA2) were compared by PROC MIXED followed by the Tukey's multiple comparison test.
The slope was calculated as follows: average of differences between 570 nm and 600 nm / the four different amounts (0.0, 0.1, 1, 10, 20 μg per well) of Con A.
Cytology and Morphology of Peripheral Blood Leukocytes
Cytologic assessment of the enriched peripheral blood leukocytes via cytospins revealed that the mean sample number across all treatments contained >95.0% mononuclear cells with lymphocytes comprising ≥89.0% of the cells. As shown in Table 11, supplemental TA quadratically reduced lymphocyte abundance (P < 0.05), and the TA0.5 group had significantly lower lymphocytes compared to the CC groups (P < 0.05). Heterophils were the only other leukocyte enumerated from this enriched leukocyte fraction and ranged from 2 to 11%, except for 2 slide samples where chicken red blood cells averaged less than 4.0%. When the results were compared across the challenged treatment groups, % of monocytes was linearly increased by supplemental TA (P < 0.01).
Table 11.
Effects of supplemental tannic acid on enriched peripheral blood leukocytes (lymphocytes, monocytes, and heterophils) in broilers infected with Salmonella Typhimurium nalidixic acid resistant strain (STNR) on D 18.
| STNR-challenged2 |
Polynomial contrast |
|||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Items | SCC1 | TA0 | TA0.25 | TA0.5 | TA1 | TA2 | SEM | P value | Linear | Quadratic |
| Lymphocytes | 89.67 | 91.50a | 92.50a | 83.25b | 87.67ab | 88.75ab | 3.3 | 0.015 | 0.301 | 0.037 |
| Monocytes | 4.33 | 5 | 3 | 6.5 | 7.67 | 8.5 | 2.52 | 0.062 | 0.016 | 0.523 |
| Heterophils | 0.67 | 1.25 | 1.75 | 2.5 | 2 | 1 | 1.08 | 0.364 | 0.443 | 0.111 |
SCC (sham-challenged control): broilers fed a basal diet and challenged with peptone water; CC (challenged control): broilers fed a basal diet and challenged with 0.5 mL of 1 × 108 CFU/mL of STNR; TA0.25 (tannic acid 0.25 g/kg): CC + 0.25 g/kg of tannic acid; TA0.5 (tannic acid 0.5 g/kg): CC + 0.5 g/kg of tannic acid; TA1 (tannic acid 1 g/kg): CC + 1 g/kg of tannic acid; and TA2 (tannic acid 2 g/kg): CC + 2 g/kg of tannic acid.
SCC versus TA0 (unpaired t test): *0.05 < P < 0.10, **P < 0.05, ***P < 0.01.
STNR infected groups (TA0, TA0.25, TA0.5, TA1, and TA2) were compared by PROC MIXED followed by the Tukey's multiple comparison test. Means within the same row with different superscripts (a,b) differ significantly (P < 0.05).
DISCUSSION
The purpose of the study was to investigate the effects of supplemental TA on growth performance, Salmonella colonization, serum endotoxin levels, ileal and hepatic antioxidant capacity, ileal morphology, gut barrier integrity, activities of ileal brush border digestive enzymes, serum alkaline phosphatase activities, and immunity in broilers infected with S. Typhimurium. The STNR infection model was implemented by administrating 0.5 mL of 108 STNR in peptone water on D 0, with STNR selected as the S. Typhimurium infection model in broilers. The NA is an antibiotic that has been commonly used in poultry production (Antunes et al., 2003). However, NA resistant strains of S. Typhimurium are adversely affecting broiler and human health (Campioni et al., 2017). There are already many antibiotic resistant strains of Salmonella spp. including tetracycline, ciprofloxacin, and ampicillin-resistant ones, and thus, novel strategies are needed to control the spread of these antibiotic resistant Salmonella spp. strains (Yuan and Guo, 2017). Furthermore, the NA resistant strains of Salmonella spp. have been frequently being used as a research model due to their easy management and analyses (Adhikari et al., 2018). Inoculation was conducted on D 0 because egg contamination from layers is the one of the common ways for STNR to infect young broilers (De Buck et al., 2004). On D 4 and 7, STNR concentrations in the ceca peaked, and after D 7, STNR concentrations decreased in the ceca of broilers.
Young broilers (newly hatched; D 0–7) are more vulnerable to the Salmonella infection because their heterophils, one of the predominant immune cells in the young birds, have lower phagocytic ability, degranulation, and oxidative burst compared to the mature broilers (Alkie et al., 2019). In the current study, D 0 to 7 and D 7 to 21 was considered as acute infection and chronic infection period, respectively. The STNR infection reduced BW and ADG on D 7 but did not affect growth performance on D 21. Decreased ADG on D 0 to 7 because of STNR infection would be mainly due to decreased ADFI in the current study. Reducing feed intake is one of the pathways pathogenic bacteria employ to decrease growth performance in broilers (Remus et al., 2014), which is consistent with our current study. Still, it is possible that STNR-induced fever as well as local and systemic stress may have contributed to a reduced appetite in these infected birds. Moreover, colonized STNR may have deprived the chicken host gut of nutrients (especially proteins) and beneficial microbes. Further, the STNR colonization can limit attachment sites for beneficial microbes (Nisbet et al., 1996).
The lower section of the gastrointestinal tract (GI) tract, where STNR mainly colonize, is the primary site to produce microbial metabolites (e.g., volatile fatty acids), which are important nutrients for gut development and energy homeostasis (Choi et al., 2021). Deficiency of nutrients and energy due to inadequate production of volatile fatty acids may lead to poor gut development, which can decrease feed intake in broilers (Allameh and Toghyani, 2019). Because STNR concentrations in the ceca of young broilers were higher in D 4 and 7 compared to those in D 14 and 21 in the current study, the effects of STNR colonization on growth performance would have been more severe in young broilers compared to the older broilers. In the current study, supplemental TA linearly decreased ADG on D 0 to 7, and supplemental TA linearly increased ADG on D 7 to 21 in STNR infected broilers. Young broilers may have been sensitive to dietary TA levels, which is consistent with a previous study (Chang and Fuller, 1964) reporting that tannins can severely limit nutrient digestion and show toxicity in young broilers compared to mature broilers. This would be because young broilers have unmature gastrointestinal tract (e.g., lower enzyme activities, shorter passaging time, etc.) (Sell, 1996), which may lead to aggravate antinutritional effects (e.g., precipitation of nutrients and endogenous enzymes) of dietary TA in the gastrointestinal tract. Increased ADG in TA supplemented groups in the grower phase could be due to higher resistance to TA in the mature broilers as well as reduced cecal STNR concentrations on D 7 in the present study. Although supplemental TA negatively affected growth performance of broilers on D 7, TA supplementation in the grower phase can be beneficial to improve growth performance from D 7 to 21, as evident by an increased antimicrobial effect of TA against STNR on D 7.
The antimicrobial effects of TA against diverse pathogens have been demonstrated in previous in vitro studies (Kim et al., 2010, Graziani et al., 2006; Sivasankar et al., 2020). However, the antimicrobial effects of TA under in vivo conditions have not been reported, because TA cannot exhibit antimicrobial effects if it forms complexes with nutrients or reach the lower GI tract in sufficient concentrations, where many pathogens propagate (Van Parys et al., 2010). In the present study, supplemental TA linearly reduced cecal STNR load on D 7 and 1 and 2 g/kg supplementation of TA significantly reduced cecal STNR load compared to the CC group but did not display antimicrobial effects on D 4. In other words, TA did not inhibit the initial colonization of STNR, but gradually reduced Salmonella colonization via exhibiting bacteriostatic and bactericidal effects against STNR. This observation implies that at least 1 g/kg of dietary TA should be supplemented in the feed to guarantee antimicrobial effects against STNR in broilers, and active forms (pure or partially degraded forms) of TA were able to seed the lower GI tract of the STNR-infected broilers. According to Choi and Kim (2020), potential mode of actions of antimicrobial effects of TA are 1) increasing membrane permeability of bacteria by interacting with components of cell walls; 2) reducing activities of microbial enzymes via precipitation; and 3) depriving essential nutrients (e.g., iron) for STNR via precipitation.
Our current study reported that TA supplementation modulated colonization of STNR in the liver of broilers infected with STNR. S. Typhimurium that enters the blood stream mainly by invading enterocytes of the distal ileum or ceca to infect the internal organs of broilers (Lawhon et al., 2002). The invasion and colonization by STNR requires swarming motility and biofilm formation, which are controlled by cell communications (e.g., quorum sensing) (Kearns, 2010; Merino et al., 2019). Sivasankar et al. (2020) reported that TA showed antiquorum sensing and antivirulence activities under in vitro conditions, which could explain the reduced liver colonization of STNR, without affecting cecal colonization, that was observed on D 14 in the current study.
Gut barrier integrity, otherwise known as gut permeability, is a defensive mechanism against pathogenic bacteria and their toxins in the gut (Choi et al., 2020). The FITC-D4 flux assay method is useful to measure gut permeability in broilers (Liu et al., 2021). In the present study, FITC-D4 flux was measured on D 14 and 21 rather than D 7 and 21 because the FITC-D4 method was previously optimized for only D 14 to 21 (Teng et al., 2020). On D 14, STNR infection increased gut permeability, and supplemental TA quadratically increased gut permeability in broilers infected with STNR. A study reported that Salmonella infection caused disruption of tight junctions, which would suggest altered gut permeability (Wang et al., 2018), whereas other studies have reported that TA supplementation enhanced gut barrier integrity in animals (Scaldaferri et al., 2014; Lopetuso et al., 2015; Yu et al., 2020). Thus, the observed aggravated gut barrier integrity in the TA fed birds is likely due to epithelial cell toxicity in the GI tract as the dosages of TA were higher for these young broilers. Moreover, these gut permeability results suggest a possible TA contribution to the reduced growth performance.
The serum endotoxin levels (e.g., lipopolysaccharides; LPS) in broilers, produced by gram-negative bacteria during their growth, division, and death in the intestine, are affected by microbiota composition and activities, and gut permeability. Once endotoxins enter the blood circulation, they stimulate inflammatory reactions, which can lead to bird sepsis (Reisinger et al., 2020). In the current study, serum endotoxin levels were not modulated due to STNR infection, whereas it was confirmed that there were around log 6.23 CFU/g content of STNR in the ceca on D 7. However, whereas there were only log 1.3 CFU/g content of STNR in the ceca on D 21, the serum endotoxin levels were increased on D 21 due to STNR infection. Other than Salmonella spp., diverse gram-negative bacteria such as Escherichia coli, Pseudomonas hydrophila, Aeromonas hydrophila, and Vibrio damsel produce endotoxins (El-Naggar et al., 2019). Moreover, no differences were observed in the gut permeability on D 21 in this study. Therefore, STNR was not the main intestinal microbe producing LPS in broilers in the present study. Continuous infection of STNR potentially altered the entire microbiota and their activities, which leads to increased serum endotoxin concentrations regardless of STNR concentrations in the ceca. In the current study, TA supplementation quadratically decreased serum endotoxin levels on D 21. This would suggest that TA favorably modified the gut microbiota and altered endotoxin production, which is supported in a previous study by Diaz Carrasco et al. (2018) reporting that TA modulated the gut microbiome of broilers. Alkaline phosphatase has an important role detoxify LPS in the blood (Yang et al., 2012), and TA supplementation quadratically increased activities of serum alkaline phosphatase on D 21 in consistent with the serum endotoxin level. Potentially, activates of serum alkaline phosphatase was increased due to higher production of short chain fatty acids by cecal microbiota (Koyama and Ono, 1976) and/or developed gastrointestinal tract in broilers fed supplemental TA (Yang et al., 2012). Moreover, according to Osete-Alcaraz et al. (2020), TA interacts with polysaccharides, which would infer that TA can interact with LPS.
Intestinal morphology is an important indicator to reflect the capacity of nutrient digestion and absorption in broilers. The ileal section was selected for analyses for intestinal morphology and other gut health parameters because STNR inhabits in the lower intestine (ileum and ceca), and the ileum still has functions of nutrient digestion and absorption in broilers. Hence, we hypothesized that more differences would be observed in the ileal section in the current study. Results showed the STNR infection reduced ileal morphology in the ileum on D 7 and 21, which would be associated with induced inflammation due to STNR infection (Wu et al., 2018). The mRNA expression of TLR2 (an activator of the innate immune system) and NFκB (proinflammatory transcription factor related to inflammation, immune response, and cell apoptosis) were reduced due to STNR infection D 7 (Jin et al., 2020). While a statistical significance was not achieved (P = 0.123), supplementation of TA linearly reduced mRNA expression of NFκB in the ileum, which supports that TA has an anti-inflammatory function (Huang et al., 2020). However, this did not lead to enhancement in ileal morphology on D 7. On D 21, STNR infection reduced mRNA expression of TLR2, TLR4, CD36, and CD74, which are related to innate immunity (Liu et al., 2017) and B cell activation (Gore et al., 2008). Huang et al. (2020) reported infection of S. Typhimurium inhibited the innate immune system and promoted cell apoptosis. On D 21, TA supplementation quadratically increased ileal morphology, and TA0.25 group significantly higher VH compared to the CC group. Supplemental TA quadratically decreased mRNA expression of IL6 (a proinflammatory cytokine) in the ileum on D 21 in the current study, which suggested that supplemental TA enhanced ileal morphology by showing anti-inflammatory effects. Moreover, mRNA expression of nutrient transporters including SGLT1 and EAAT3 was linearly enhanced by supplemental TA, which potentially explains linearly improved growth performance in the TA groups during the grower phase. Collectively, anti-inflammatory effects of TA may lead to improved ileal morphology and nutrient absorption of broilers infected with STNR.
Activities of ileal brush border enzymes are crucial indicators to reflect gut development and capacity of nutrient digestion in broilers. In the current study, STNR infection reduced lipase activities on D 7. Secreted lipase from pancreas is trapped in the mucus layer and digests lipids (Song et al., 2018), and lipase activities can be measured in the ileal tissue. Reduced lipase due to STNR infection in the current study is potentially because STNR infection impaired pancreas function by inducing system inflammation (Wu et al., 2019). Increased lipase activities by supplemental TA might be the result of supplemental TA ameliorating impaired pancreas functions due to STNR infection in the current study. This is supported by Majumdar and Moudgal (1994) reported that TA showed beneficial effects on activities of pancreatin enzymes in broilers. In contrast, STNR infection increased aminopeptidase activities on D 7 in the current study. Enzyme activities are mainly affected by number of enzymes, and Carroll et al. (2012) and Cheng et al. (2015) reported that aminopeptidase can provide colonization areas for bacteria. This possibly implies that aminopeptidase also can provide colonization areas for STNR, and STNR increased the number of aminopeptidases for their proliferation, which led to increased aminopeptidase activities in the present study. Supplemental TA reduced maltase activities on D 7 potentially because of forming a complex with maltase. This can partially support that supplemental TA can restrict nutrient digestion in the GI tract and show toxicity in young broilers infected with STNR.
Intestinal mucus, which is produced by mucin-secreting goblet cells in the epithelial layer, plays a crucial role as an innate defense against pathogens, supporting the colonization of commensal bacteria and facilitating nutrient digestion and absorption (Duangnumsawang et al., 2021). Increased mucus production can serve either as an indicator of bacterial infections or for enhanced gut health of broilers. In the current study, STNR infection increased mRNA expression of MUC2, which is the dominant mucin in the intestine (Yamashita and Melo, 2018). This result is in agreement with a previous study (Fasina et al., 2010) demonstrating that STNR infection increased mucus production in broilers. However, on D 21, supplemental TA increased goblet cell density in broilers infected with STNR along with enhanced ADG in the current study. Potentially, on D 21, increased mucus production by supplemental TA might have facilitated nutrient transportation, which led to increased growth performance. Increased mRNA expression of nutrient transporters (e.g., SGLT1 and EAAT3) by supplemental TA might be associated with enhanced mucus production on D 21 in the current study.
Regarding the timeline to assess the chicken host immune response, D 18 was selected as birds infected with STNR were previously reported to have impaired immunity and growth performance in broilers (Adhikari et al., 2020). In the current study, the percentage of peripheral blood CD8+ cells and the CD4:CD8 ratio were linearly increased and decreased, respectively along with enhanced ADG with increased supplementation of TA in D 18 birds. An elevated percentage of CD8+ cells is correlated with host immune response to bacterial infection (Jarosz et al., 2016) through enhanced T cell signaling and cytotoxicity (Fresnay et al., 2016). Thus, in the presence of an STNR infection, dietary supplementation with TA appears to favorably enhance cytotoxic CD8 mobility inferring some level of host cell mediated response. The number of lymphocytes, which are responsible for adaptive immunity, was quadratically decreased, and the TA0.5 group had the lower number of lymphocytes compared to the TA0 group. A decreased number of lymphocytes indicate attenuated inflammation (Guyton and Hall, 1996). Consistent with the present study, Ramirez and Roa Jr (2003) reported that tannin extract showed gastroprotective effects via a reduction in lymphocyte numbers in rats.
In the current study, there was also a linear increase in the number of monocytes in the enriched leukocyte fraction. Monocytes belong to innate immunity and play an important role in rapid response to pathogenic infection by producing cytokines, exhibiting phagocytic activities, and presenting antigens for further immune response (Stabler et al., 1994). Therefore, increased numbers of monocytes may represent enhanced innate immunity against pathogenic infection (Shang et al., 2015). These findings are supported by Ramah et al. (2020), who observed that dietary TA supplementation enhanced broiler immunity. Overall, TA supplementation was demonstrated to positively affect innate and adaptive immunity in broilers.
The effects of different doses of supplemental TA on broilers infected with STNR were summarized in Table 12. The TA can show either negative or positive effects (e.g., hormesis) on broiler growth and health depends on the dosages. Our current study showed that 1 to 2 g/kg supplemental TA exhibited beneficial effects in reducing STNR colonization in the ceca and stimulating immune system, which potentially resulted in improved growth performance in broilers infected with STNR. Our previous study showed that 0.5 to 2.75 g/kg supplemental TA had potential to be an anticoccidial agent in broilers infected with Eimeria maxima (Choi et al., 2022a). However, supplemental TA higher than 1 g/kg exhibited antinutritional effects on broilers under nonchallenge conditions (Choi et al., 2022b). Therefore, 1 g/kg supplemental TA would be appropriate would be appropriate to be an alternative for AGP in broiler production.
Table 12.
Summary of effects of supplemental tannic acid on broilers infected with Salmonella Typhimurium nalidixic acid resistant strain (STNR).
| Treatment | Observations |
|---|---|
| TA0.25 | Decrease growth performance D 7 |
| Decrease STNR liver colonization D 14 | |
| Decrease serum endotoxin D 21 | |
| Increase ileal villus height D 21 | |
| TA0.5 | Decrease growth performance D 7 |
| Increase gut permeability D 14 | |
| Increase growth performance D 21 | |
| Decrease STNR salmonella liver colonization D 14 | |
| Decrease serum endotoxin D 21 | |
| Increase serum alkaline activities D 21 | |
| TA1 | Decrease growth performance D 7 |
| Increase growth performance D 21 | |
| Decrease STNR salmonella cecal colonization D 7 | |
| Decrease serum endotoxin D 21 | |
| Increase serum alkaline activities D 21 | |
| Stimulate immune system D 21 | |
| TA2 | Decrease growth performance D 7 |
| Increase growth performance D 21 | |
| Decrease STNR salmonella cecal colonization D 7 | |
| Increase goblet cell density D 21 | |
| Increase serum alkaline activities D 21 | |
| Increase gene expression of nutrient transporters D 21 | |
| Stimulate immune system D 21 |
TA0.25 (tannic acid 0.25 g/kg): CC + 0.25 g/kg of tannic acid; TA0.5 (tannic acid 0.5 g/kg): CC + 0.5 g/kg of tannic acid; TA1 (tannic acid 1 g/kg): CC + 1 g/kg of tannic acid; and TA2 (tannic acid 2 g/kg): CC + 2 g/kg of tannic acid.
CONCLUSION
STNR infection resulted in decreased growth performance, increased gut permeability, increased the serum endotoxin concentrations, negatively affected immune parameters, decreased ileal morphology, modulated mucus production, and decreased nutrient absorption in broilers. However, supplemental TA led to reduced STNR colonization, increased growth performance, decreased the serum endotoxin concentrations, improved immune responses that were diminished by STNR, enhanced ileal morphology, increased mucus production, and increased nutrient absorption in broilers infected with STNR. Therefore, supplemental TA (1–2 g/kg) increased growth performance and gut health via antimicrobial and immunostimulatory effects in broilers infected with STNR. Supplemental TA can therefore be a potential alternative to AGP in broiler production.
Disclosures
The authors announce that the research was conducted in the absence of any commercial or financial relationships.
REFERENCES
- Abudabos A.M., Ali M.H., Nassan M.A., Saleh A.A. Ameliorative effect of Bacillus subtilis on growth performance and intestinal architecture in broiler infected with Salmonella. Animals. 2019;9:190. doi: 10.3390/ani9040190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adhikari P., Cosby D.E., Cox N.A., Franca M.S., Williams S.M., Gogal R.M., Jr, Ritz C.W., Kim W.K. Effect of dietary fructooligosaccharide supplementation on internal organs Salmonella colonization, immune response, ileal morphology, and ileal immunohistochemistry in laying hens challenged with Salmonella enteritidis. Poult. Sci. 2018;97:2525–2533. doi: 10.3382/ps/pey101. [DOI] [PubMed] [Google Scholar]
- Adhikari P., Yadav S., Cosby D.E., Cox N.A., Jendza J.A., Kim W.K. Research Note: effect of organic acid mixture on growth performance and Salmonella Typhimurium colonization in broiler chickens. Poult. Sci. 2020;99:2645–2649. doi: 10.1016/j.psj.2019.12.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahmed S.A., Gogal R.M., Jr, Walsh J.E. A new rapid and simple non-radioactive assay to monitor and determine the proliferation of lymphocytes: an alternative to [3H] thymidine incorporation assay. J. Immunol. Methods. 1994;170:211–224. doi: 10.1016/0022-1759(94)90396-4. [DOI] [PubMed] [Google Scholar]
- Alkie T.N., Yitbarek A., Hodgins D.C., Kulkarni R.R., Taha-Abdelaziz K., Sharif S. Development of innate immunity in chicken embryos and newly hatched chicks: a disease control perspective. Avian Dis. 2019;48:288–310. doi: 10.1080/03079457.2019.1607966. [DOI] [PubMed] [Google Scholar]
- Allameh S., Toghyani M. Effect of dietary valine supplementation to low protein diets on performance, intestinal morphology and immune responses in broiler chickens. Livest. Sci. 2019;229:137–144. [Google Scholar]
- Antunes P.c., Réu C., Sousa J.C., Peixe L.s., Pestana N. Incidence of Salmonella from poultry products and their susceptibility to antimicrobial agents. Int. J. Food Microbiol. 2003;82:97–103. doi: 10.1016/s0168-1605(02)00251-9. [DOI] [PubMed] [Google Scholar]
- Bula-Rudas F.J., Rathore M.H., Maraqa N.F. Salmonella infections in childhood. Adv. Pediatr. 2015;62:29–58. doi: 10.1016/j.yapd.2015.04.005. [DOI] [PubMed] [Google Scholar]
- Campioni F., Souza R.A., Martins V.V., Stehling E.G., Bergamini A.M.M., Falcao J.P. Prevalence of gyrA mutations in nalidixic acid-resistant strains of Salmonella Enteritidis isolated from humans, food, chickens, and the farm environment in Brazil. Microb. Drug Resist. 2017;23:421–428. doi: 10.1089/mdr.2016.0024. [DOI] [PubMed] [Google Scholar]
- Carroll R.K., Robison T.M., Rivera F.E., Davenport J.E., Jonsson M., Florczyk D., Tarkowski A., Potempa J., Koziel J., Shaw L.N. Identification of an intracellular M17 family leucine aminopeptidase that is required for virulence in Staphylococcus aureus. Microbes Infect. 2012;14:989–999. doi: 10.1016/j.micinf.2012.04.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang S.I., Fuller H. Effect of tannin content of grain sorghums on their feeding value for growing chicks. Poult. Sci. 1964;43:30–36. [Google Scholar]
- Cheng C., Wang X., Dong Z., Shao C., Yang Y., Fang W., Fang C., Wang H., Yang M., Jiang L. Aminopeptidase T of M29 family acts as a novel intracellular virulence factor for Listeria monocytogenes infection. Sci. Rep. 2015;5:1–12. doi: 10.1038/srep17370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Kim W.K. Dietary application of tannins as a potential mitigation strategy for current challenges in poultry production: a review. Animals. 2020;10:2389. doi: 10.3390/ani10122389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Ko H., Tompkins Y.H., Teng P.-Y., Lourenco J.M., Callaway T.R., Kim W.K. Effects of eimeria tenella infection on key parameters for feed efficiency in broiler chickens. Animals. 2021;11:3428. doi: 10.3390/ani11123428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Tompkins Y.H., Teng P.-Y., Gogal R.M., Jr, Kim W.K. Effects of tannic acid supplementation on growth performance, oocyst shedding, and gut health of in broilers infected with eimeria maxima. Animals. 2022;12:1378. doi: 10.3390/ani12111378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., Wang L., Liu S., Lu P., Zhao X., Liu H., Lahaye L., Santin E., Liu S., Nyachoti M. Effects of a microencapsulated formula of organic acids and essential oils on nutrient absorption, immunity, gut barrier function, and abundance of enterotoxigenic Escherichia coli F4 in weaned piglets challenged with E. coli F4. J. Anim. Sci. 2020;98:skaa259. doi: 10.1093/jas/skaa259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi J., S. Yadav, J. Wang, B.J. Lorentz, J.M. Lourenco, T.R. Callaway, and W.K. Kim. 2022b. Effects of supplemental tannic acid on growth performance, gut health, microbiota, and fat accumulation and optimal dosages of tannic acid in broilers [e-pub ahead of print]. Front. Physiol. 13:912797. doi: 10.3389/fphys.2022.912797, accessed August 26, 2022. [DOI] [PMC free article] [PubMed]
- Cobb Vantress 2018Cobb Vantress. 2018. COBB broiler management guide. Accessed Jan. 2020. https://cobbstorage.blob.core.windows.net/guides/5fc96620-0aba-11e9-9c88-c51e407c53ab.
- De Buck J., Van Immerseel F., Haesebrouck F., Ducatelle R. Colonization of the chicken reproductive tract and egg contamination by Salmonella. J. Appl. Microbiol. 2004;97:233–245. doi: 10.1111/j.1365-2672.2004.02294.x. [DOI] [PubMed] [Google Scholar]
- Diaz Carrasco J.M., Redondo E.A., Pin Viso N.D., Redondo L.M., Farber M.D., Fernandez Miyakawa M.E. Tannins and bacitracin differentially modulate gut microbiota of broiler chickens. Biomed Res. Int. 2018;2018 doi: 10.1155/2018/1879168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duangnumsawang Y., Zentek J., Boroojeni F.G. Development and functional properties of intestinal mucus layer in poultry [e-pub ahead of print] Front. Immunol. 2021;12 doi: 10.3389/fimmu.2021.745849. accessed August 26, 2022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elgharbawy A.A., Hayyan A., Hayyan M., Rashid S.N., Nor M.R.M., Zulkifli M.Y., Alias Y. Shedding light on lipase stability in natural deep eutectic solvents. Chem. Biochem. Eng. Q. 2018;32:359–370. [Google Scholar]
- El-Naggar M.Y., Hamdan A.M., Beltagy E.A., Ibrahim H.A., Moustafa M.M. Endotoxin production by pseudomonas aeruginosa ATCC 9027 with potential medical applications. J. Pure Appl. Microbiol. 2019;13:97–107. [Google Scholar]
- Fan M.Z., Matthews J.C., Etienne N.M., Stoll B., Lackeyram D. Expression of apical membrane L-glutamate transporters in neonatal porcine epithelial cells along the small intestinal crypt-villus axis. Am. J. Physiol. Gastrointest. 2004;287:G385–G398. doi: 10.1152/ajpgi.00232.2003. [DOI] [PubMed] [Google Scholar]
- Fasina Y., Hoerr F., McKee S., Conner D. Influence of Salmonella enterica serovar Typhimurium infection on intestinal goblet cells and villous morphology in broiler chicks. Avian Dis. 2010;54:841–847. doi: 10.1637/9055-090809-Reg.1. [DOI] [PubMed] [Google Scholar]
- Fresnay S., McArthur M.A., Magder L., Darton T.C., Jones C., Waddington C.S., Blohmke C.J., Angus B., Levine M.M., Pollard A.J. Salmonella Typhi-specific multifunctional CD8+ T cells play a dominant role in protection from typhoid fever in humans. J. Transl. Med. 2016;14:1–14. doi: 10.1186/s12967-016-0819-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gore Y., Starlets D., Maharshak N., Becker-Herman S., Kaneyuki U., Leng L., Bucala R., Shachar I. Macrophage migration inhibitory factor induces B cell survival by activation of a CD74-CD44 receptor complex. J. Biol. Chem. 2008;283:2784–2792. doi: 10.1074/jbc.M703265200. [DOI] [PubMed] [Google Scholar]
- Graziani, R., G. Tosi, and R. Denti. Year. In vitro antimicrobial activity of SILVA FEED ENC® on bacterial strains of poultry origin. Proc. EPC 2006–12th European Poultry Conference. 2006. Verona, Italy.
- Gut A.M., Vasiljevic T., Yeager T., Donkor O.N. Salmonella infection–prevention and treatment by antibiotics and probiotic yeasts: a review. Microbiology. 2018;164:1327–1344. doi: 10.1099/mic.0.000709. [DOI] [PubMed] [Google Scholar]
- Guyton A., Hall J. 9th ed. wb Saunders Company; Philadelphia. PA: 1996. Textbook of Medical Physiology; p. 1006. [Google Scholar]
- Huang T., Jiang C., Yang M., Xiao H., Huang X., Wu L., Yao M. Salmonella enterica serovar Typhimurium inhibits the innate immune response and promotes apoptosis in a ribosomal/TRP53-dependent manner in swine neutrophils. Vet. Res. 2020;51:1–12. doi: 10.1186/s13567-020-00828-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim D., Abdelfattah-Hassan A., Badawi M., Ismail T.A., Bendary M.M., Abdelaziz A.M., Mosbah R.A., Mohamed D.I., Arisha A.H., El-Hamid M.I.A. Thymol nanoemulsion promoted broiler chicken's growth, gastrointestinal barrier and bacterial community and conferred protection against Salmonella Typhimurium. Sci. Rep. 2021;11:1–20. doi: 10.1038/s41598-021-86990-w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarosz Ł., Kwiecień M., Marek A., Grądzki Z., Winiarska-Mieczan A., Kalinowski M., Laskowska E. Effects of feed supplementation with glycine chelate and iron sulfate on selected parameters of cell-mediated immune response in broiler chickens. Res. Vet. Sci. 2016;107:68–74. doi: 10.1016/j.rvsc.2016.04.003. [DOI] [PubMed] [Google Scholar]
- Jin H., Haicheng Y., Caiyun Z., Yong Z., Jinrong W. The expression of NF-kB signaling pathway was inhibited by silencing TGF-b4 in chicken IECs infected with E. tenella. Braz. J. Poultry Sci. 2020;22 doi: 10.1590/1806-9061-2020-1338. [DOI] [Google Scholar]
- Kearns D.B. A field guide to bacterial swarming motility. Nat. Rev. Microbiol. 2010;8:634–644. doi: 10.1038/nrmicro2405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim T., Silva J., Kim M., Jung Y. Enhanced antioxidant capacity and antimicrobial activity of tannic acid by thermal processing. Food Chem. 2010;118:740–746. [Google Scholar]
- Koyama H., Ono T. Induction by short-chain fatty acids of alkaline phosphatase activity in cultured mammalian cells. J. Cell. Physiol. 1976;88:49–56. doi: 10.1002/jcp.1040880107. [DOI] [PubMed] [Google Scholar]
- Krunkosky M., García M., Beltran G., Williams S., Hurley D., Gogal R., Jr Ocular exposure to infectious laryngotracheitis virus alters leukocyte subsets in the head-associated lymphoid tissues and trachea of 6-week-old White Leghorn chickens. Avian Dis. 2020;49:404–417. doi: 10.1080/03079457.2020.1757036. [DOI] [PubMed] [Google Scholar]
- Lackeyram D., Yang C., Archbold T., Swanson K.C. Early weaning reduces small intestinal alkaline phosphatase expression in pigs. J. Nutr. 2010;140:461–468. doi: 10.3945/jn.109.117267. [DOI] [PubMed] [Google Scholar]
- Lawhon S.D., Maurer R., Suyemoto M., Altier C. Intestinal short-chain fatty acids alter Salmonella typhimurium invasion gene expression and virulence through BarA/SirA. Mol. Microbiol. 2002;46:1451–1464. doi: 10.1046/j.1365-2958.2002.03268.x. [DOI] [PubMed] [Google Scholar]
- Liu H., Xu W., Yu Q., Yang Q. 4, 4′-Diaponeurosporene-producing Bacillus subtilis increased mouse resistance against Salmonella typhimurium infection in a CD36-dependent manner. Front. Immunol. 2017;8:483. doi: 10.3389/fimmu.2017.00483. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Teng P.-Y., Kim W.K., Applegate T.J. Assay considerations for fluorescein isothiocyanate-dextran (FITC-d): an indicator of intestinal permeability in broiler chickens. Poult. Sci. 2021 doi: 10.1016/j.psj.2021.101202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livak K.J., Schmittgen T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2− ΔΔCT method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lopetuso L.R., Scaldaferri F., Bruno G., Petito V., Franceschi F., Gasbarrini A. The therapeutic management of gut barrier leaking: the emerging role for mucosal barrier protectors. Eur. Rev. Med. Pharmacol. Sci. 2015;19:1068–1076. [PubMed] [Google Scholar]
- Majumdar S., Moudgal R. Effect of tannic acid on activities of certain digestive enzymes and alkaline phosphatase in intestine and glucose absorption in adult chickens. J. Appl. Anim. Res. 1994;6:105–112. [Google Scholar]
- Mannelli F., Minieri S., Tosi G., Secci G., Daghio M., Massi P., Fiorentini L., Galigani I., Lancini S., Rapaccini S. Effect of chestnut tannins and short chain fatty acids as anti-microbials and as feeding supplements in broilers rearing and meat quality. Animals. 2019;9:659. doi: 10.3390/ani9090659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maroux S., Louvard D. The aminopeptidase from hog intestinal brush border. Biochim. Biophys. Acta. 1973;321:282–295. doi: 10.1016/0005-2744(73)90083-1. [DOI] [PubMed] [Google Scholar]
- Martínez Y., Almendares C.I., Hernández C.J., Avellaneda M.C., Urquía A.M., Valdivié M. Effect of acetic acid and sodium bicarbonate supplemented to drinking water on water quality, growth performance, organ weights, cecal traits and hematological parameters of young broilers. Animals. 2021;11:1865. doi: 10.3390/ani11071865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merino L., Procura F., Trejo F.M., Bueno D.J., Golowczyc M.A. Biofilm formation by Salmonella sp. in the poultry industry: Detection, control and eradication strategies. Food Res. Int. 2019;119:530–540. doi: 10.1016/j.foodres.2017.11.024. [DOI] [PubMed] [Google Scholar]
- Mughini-Gras L., Enserink R., Friesema I., Heck M., van Duynhoven Y., van Pelt W. Risk factors for human salmonellosis originating from pigs, cattle, broiler chickens and egg laying hens: a combined case-control and source attribution analysis. PloS One. 2014;9:e87933. doi: 10.1371/journal.pone.0087933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nisbet D.J., Corrier D.E., Ricke S.C., Hume M., BYRD J.A., Deloach J.R. Maintenance of the biological efficacy in chicks of a cecal competitive-exclusion culture against Salmonella by continuous-flow fermentation. J. Food Prot. 1996;59:1279–1283. doi: 10.4315/0362-028X-59.12.1279. [DOI] [PubMed] [Google Scholar]
- Osete-Alcaraz A., Gómez-Plaza E., Martínez-Pérez P., Weiller F., Schückel J., Willats W.G., Moore J.P., Ros-García J.M., Bautista-Ortín A.B. The impact of carbohydrate-active enzymes on mediating cell wall polysaccharide-tannin interactions in a wine-like matrix. Food Res. Int. 2020;129 doi: 10.1016/j.foodres.2019.108889. [DOI] [PubMed] [Google Scholar]
- Ramah A., Yasuda M., Ohashi Y., Urakawa M., Kida T., Yanagita T., Uemura R., Bakry H.H., Abdelaleem N.M., El-Shewy E.A. Different doses of tannin reflect a double-edged impact on broiler chicken immunity. Vet. Immunol. Immunopathol. 2020;220 doi: 10.1016/j.vetimm.2019.109991. [DOI] [PubMed] [Google Scholar]
- Ramirez R.O., Roa C.C., Jr The gastroprotective effect of tannins extracted from duhat (Syzygium cumini Skeels) bark on HCl/ethanol induced gastric mucosal injury in Sprague-Dawley rats. Clin. Hemorheol. Microcirc. 2003;29:253–261. [PubMed] [Google Scholar]
- Reisinger N., Emsenhuber C., Doupovec B., Mayer E., Schatzmayr G., Nagl V., Grenier B. Endotoxin translocation and gut inflammation are increased in broiler chickens receiving an oral lipopolysaccharide (LPS) bolus during heat stress. Toxins. 2020;12:622. doi: 10.3390/toxins12100622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remus A., Hauschild L., Andretta I., Kipper M., Lehnen C., Sakomura N. A meta-analysis of the feed intake and growth performance of broiler chickens challenged by bacteria. Poult. Sci. 2014;93:1149–1158. doi: 10.3382/ps.2013-03540. [DOI] [PubMed] [Google Scholar]
- Scaldaferri F., Lopetuso L.R., Petito V., Cufino V., Bilotta M., Arena V., Stigliano E., Maulucci G., Papi M., Emiliana C.M. Gelatin tannate ameliorates acute colitis in mice by reinforcing mucus layer and modulating gut microbiota composition: emerging role for ‘gut barrier protectors’ in IBD? United Eur. Gastroenterol. J. 2014;2:113–122. doi: 10.1177/2050640614520867. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sell J.L. Physiological limitations and potential for improvement in gastrointestinal tract function of poultry. J. Appl. Poult. Res. 1996;5:96–101. [Google Scholar]
- Shang Y., Regassa A., Kim J.H., Kim W.K. The effect of dietary fructooligosaccharide supplementation on growth performance, intestinal morphology, and immune responses in broiler chickens challenged with Salmonella Enteritidis lipopolysaccharides. Poult. Sci. 2015;94:2887–2897. doi: 10.3382/ps/pev275. [DOI] [PubMed] [Google Scholar]
- Sivasankar C., Jha N.K., Ghosh R., Shetty P.H. Anti quorum sensing and anti virulence activity of tannic acid and it's potential to breach resistance in Salmonella enterica Typhi/Paratyphi A clinical isolates. Microb. Pathog. 2020;138 doi: 10.1016/j.micpath.2019.103813. [DOI] [PubMed] [Google Scholar]
- Song W., Yang Y., Yu M., Zhu Q., Damaneh M.S., Zhong H., Gan Y. Enhanced digestion inhibition and mucus penetration of F127-modified self-nanoemulsions for improved oral delivery. Asian J. Pharm. Sci. 2018;13:326–335. doi: 10.1016/j.ajps.2018.03.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stabler J., McCormick T., Powell K., Kogut M. Avian heterophils and monocytes: phagocytic and bactericidal activities against Salmonella enteritidis. Vet. Microbiol. 1994;38:293–305. doi: 10.1016/0378-1135(94)90148-1. [DOI] [PubMed] [Google Scholar]
- Teng P.-Y., Yadav S., de Souza Castro F.L., Tompkins Y.H., Fuller A.L., Kim W.K. Graded Eimeria challenge linearly regulated growth performance, dynamic change of gastrointestinal permeability, apparent ileal digestibility, intestinal morphology, and tight junctions of broiler chickens. Poult. Sci. 2020;99:4203–4216. doi: 10.1016/j.psj.2020.04.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Parys A., Boyen F., Dewulf J., Haesebrouck F., Pasmans F. The use of tannins to control Salmonella typhimurium infections in pigs. Zoonoses Pub. Health. 2010;57:423–428. doi: 10.1111/j.1863-2378.2009.01242.x. [DOI] [PubMed] [Google Scholar]
- Vandesompele J., De Preter K., Pattyn F., Poppe B., Van Roy N., De Paepe A., Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:1–12. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Li L., Lv Y., Chen Q., Feng J., Zhao X. Lactobacillus plantarum restores intestinal permeability disrupted by Salmonella infection in newly-hatched chicks. Sci. Rep. 2018;8:1–10. doi: 10.1038/s41598-018-20752-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson K., Bourassa D., Davis A., Freeman M., Buhr R. The addition of charcoals to broiler diets did not alter the recovery of Salmonella Typhimurium during grow-out. Poult. Sci. 2016;95:694–704. doi: 10.3382/ps/pev371. [DOI] [PubMed] [Google Scholar]
- Wu Q., Liu N., Wu X., Wang G., Lin L. Glutamine alleviates heat stress-induced impairment of intestinal morphology, intestinal inflammatory response, and barrier integrity in broilers. Poult. Sci. 2018;97:2675–2683. doi: 10.3382/ps/pey123. [DOI] [PubMed] [Google Scholar]
- Wu Q., Liu Z., Li S., Jiao C., Wang Y. Effects of glutamine on digestive function and redox regulation in the intestines of broiler chickens challenged with Salmonella enteritidis. Braz. J. Poultry Sci. 2019;21 doi: 10.1590/1806-9061-2019-1123. [DOI] [Google Scholar]
- Yadav S., Teng P.-Y., Choi J., Singh A., Vaddu S., Thippareddi H., Kim W. Influence of rapeseed, canola meal and glucosinolate metabolite (AITC) as potential antimicrobials: effects on growth performance, and gut health in Salmonella Typhimurium challenged broiler chickens. Poult. Sci. 2022;101 doi: 10.1016/j.psj.2021.101551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamashita M.S.d.A., Melo E.O. Mucin 2 (MUC2) promoter characterization: an overview. Cell Tissue Res. 2018;374:455–463. doi: 10.1007/s00441-018-2916-9. [DOI] [PubMed] [Google Scholar]
- Yang C., Chowdhury M., Huo Y., Gong J. Phytogenic compounds as alternatives to in-feed antibiotics: potentials and challenges in application. Pathogens. 2015;4:137–156. doi: 10.3390/pathogens4010137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Y., Wandler A.M., Postlethwait J.H., Guillemin K. Dynamic evolution of the LPS-detoxifying enzyme intestinal alkaline phosphatase in zebrafish and other vertebrates. Front. Immunol. 2012;3:314. doi: 10.3389/fimmu.2012.00314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu J., Song Y., Yu B., He J., Zheng P., Mao X., Huang Z., Luo Y., Luo J., Yan H. Tannic acid prevents post-weaning diarrhea by improving intestinal barrier integrity and function in weaned piglets. J. Anim. Sci. Biotechnol. 2020;11:1–11. doi: 10.1186/s40104-020-00496-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuan J., Guo W. Mechanisms of resistance to quinolones in Salmonella Typhimurium from patients with infectious diarrhea. Microbiol. Immunol. 2017;61:138–143. doi: 10.1111/1348-0421.12476. [DOI] [PubMed] [Google Scholar]