Abstract
Biogenic amines (BAs) are a group of molecules naturally present in foods that contain amino acids, peptides, and proteins as well as in biological systems. In foods, their concentrations typically increase during processing and storage because of exposure to microorganisms that catalyze their formation by releasing amino acid decarboxylases. The concentrations of BAs above certain values are indicative of unsafe foods due to associate neuronal toxicity, allergenic reactions, and increase risks of cardiovascular diseases. There are therefore various strategies that focus on the control of BAs in foods mostly through elimination, inactivation, or inhibition of the growth of microorganisms. Increasingly, there are works on bioactive compounds that can decrease the concentration of BAs through their antimicrobial activity as well as the inhibition of decarboxylating enzymes that control their formation in foods or amine oxidases and N-acetyltransferase that control the degradation in vivo. This review focusses on the role of food-derived bioactive compounds and the mechanism by which they regulate the concentration of BAs. The findings are that most active molecules belong to polyphenols, one of the largest groups of plant secondary metabolites, additionally other useful +compounds are present in extracts of different herbs and spices. Different mechanisms have been proposed for the effects of polyphenols depending on the model system. Studies on the effects in vivo are limited and there is a lack of bioavailability and transport data which are important to assess the importance of the bioactive molecules.
Keywords: Biogenic amines, Polyphenols, Phytochemicals, Monoamine oxidase, Decarboxylases
Biogenic amines; Polyphenols; Phytochemicals; Monoamine oxidase; Decarboxylases.
1. Introduction
It is an established fact that foods provide nutrients that nourish our body and keep our system in proper working conditions. Meanwhile, from early civilization, certain foods are known to confer additional health benefits to human like the prevention and treatment of diseases [1, 2]. Today, foods with beneficial effects are commonly referred to as functional foods, and although no consensus definition exists, they are generally recognised to provide a health benefit beyond basic nutrition [3]. Most research works on the health-promoting effects of active food-derived molecules have focused on chronic conditions such as hypertension, diabetes, cardiovascular diseases, or cancer [4, 5, 6, 7]. There are also increasing works on their role in neuronal and mental health [8, 9]. One of the areas of interests is the role bioactive compounds may have in the control of substances whose presence or certain concentrations may be detrimental to either the quality of foods or to human health. Biogenic amines (BAs) fall within this category. BAs are nitrogen containing molecules found in raw or processed foods and in biological systems [10, 11]. In foods and beverages, they can be formed during fermentation processes via a decarboxylation of the amino acid functional group. In biological systems, the concentration of BAs is dependent on the consumed amount from foods, the activity of decarboxylases and the overall metabolism that involves catechol-O-methyltransferase, N-acetyltransferase, and amine oxidases [12, 13]. Antioxidative properties have been reported for some BAs [14, 15]. The complexation of caffeic acid with spermine and spermidine for example, increases its ability to scavenge ABTS radicals while also decreasing the growth of tumor cells by 15–25% [16]. Meanwhile, increased concentrations in foods are related to spoilage which is caused by an increase activity of microbial decarboxylases and subsequently toxicity associated for example with higher concentrations of histamine, tyramine, and nitrosamines [17, 18, 19]. Strategies to control the formation or regulate the concentration of BAs are therefore necessary to improve the quality and safety of foods, and to improve human health. This review is intended to summarize different types of biogenic amines in food and biological systems, their functions, and strategies to control the amount with a focus on the use functional ingredients and food-related bioactive compounds. This is different from other reviews that focus on the control of BAs using mostly temperature, antimicrobials, irradiation, high hydrostatic pressure, starter culture, or non-producing decarboxylating microorganisms [20, 21, 22].
2. Biogenic amines
Biogenic amines (BAs) are low molecular weight nitrogen-containing organic compounds. They are formed by the action of proteases secreted by living micro-organisms, during the storage of foods or processes such as fermentations. There is a diversity in structures of BAs dependent on whether the substrate is an amino acid, an aldehyde, or a general nitrogen-containing compound. Their formation typically proceeds via a decarboxylation, amination or transamination reactions catalysed by enzymes (decarboxylases, aminases) produced by microorganisms in foods and during normal metabolic processes in living cells [23].
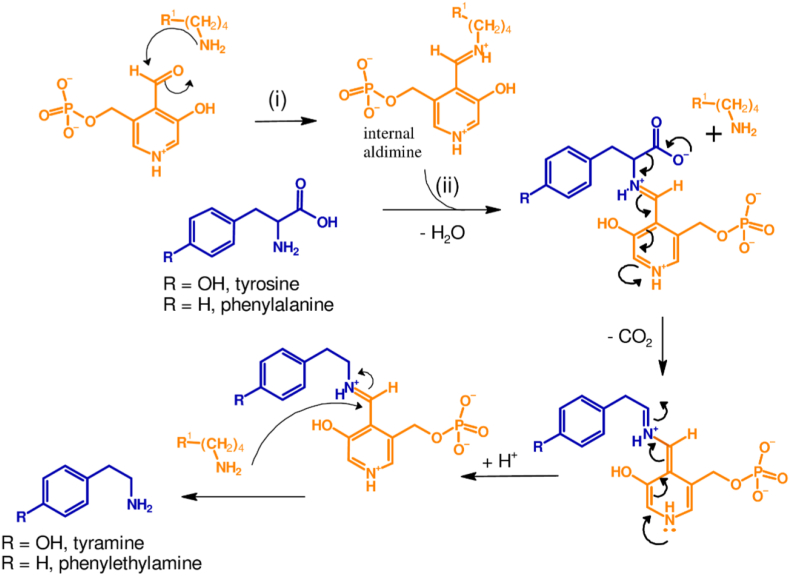
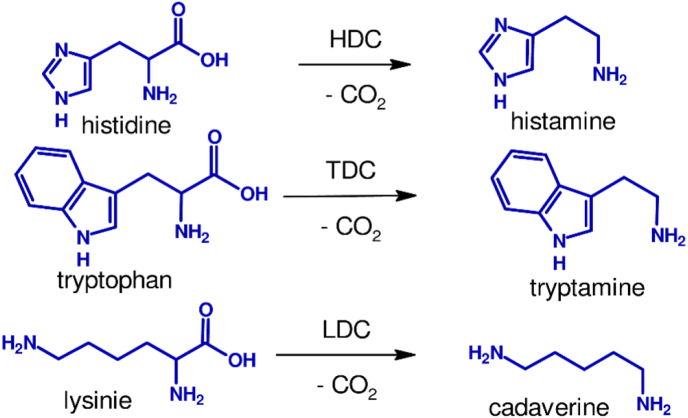
The vast majority of biogenic amines are derived from amino acids and depending on the substrate, they are classified into mono-, di-, and polyamines (Figure 1). Monoamines of interest tyramine and phenylethylamine are produced from tyrosine and phenylalanine by the action of tyrosine and phenylalanine decarboxylase, respectively (Figure 1). Diamines, histamine, tryptamine and cadaverine are produced from direct decarboxylation of histidine, tryptophan, and lysine, respectively (Figure 2). In contrast, the other diamine putrescine is formed through complex metabolic pathways that can involve up to eight different enzymes depending on the type of microorganisms involved in its formation [24]. In some bacteria for example, putrescine is formed from arginine by the action of arginine decarboxylase followed by that of Agmatinase and putrescine carbamoyl transferase, or from the action of arginase followed by ornithine decarboxylase. A summary of the formation of putrescine is shown in Figure 3.
Figure 1.
Formation of tyramine and phenylethylamine. (i): In the resting state of the enzyme, the co-factor, pyridoxal 5′-phosphate of tyrosine or phenylalanine decarboxylase forms an internal aldimine (or Schiff base intermediate); (ii): The activated intermediate then reacts with the amine of the amino acid to form an intermediate structure, which then decarboxylates and generate the biogenic amine via transaldimination.
Figure 2.
Formation of diamines histamine, tryptamine, and cadaverine by pyridoxal 5′-phosphate dependent histidine (HDC), tryptophan (TDC), and lysine decarboxylase, respectively.
Figure 3.
Formation of the aliphatic diamine putrescine from arginine by the action of arginine decarboxylase (ADC), agmatine deiminase (AgDI) and putrescine carbamoyl transferase (PCT), and via arginase (ARG) and ornithine decarboxylase (ODC).
The three common polyamines are agmatine, spermidine and spermine. Agmatine synthesized from arginine via the action of arginine decarboxylase is also an intermediate in the synthesis of putrescine (Figure 4). Methionine provides the aminopropyl groups necessary for the formation of spermidine and spermine from putrescine [25] as illustrated in Figure 4. The action of adenosyl transferase followed by that of S-adenosylmethionine (SAM) decarboxylase converts methionine to decarboxylated S-adenosyl-methionine. In the next step, a transfer of an aminopropyl from decarboxylated S-adenosyl-methionine to putrescine generates the tri amine spermidine while another transfer leads to spermine (Figure 4).
Figure 4.
Scheme of the formation of spermidine and spermine from putrescine by the action of S-adenosine methionine (SAM). The reaction of SAM-3-aminopropyl methyl sulfate with either putrescine to form spermidine (i) or with spermidine to form spermine (ii).
Many literature works have reported that BAs can also be formed by amination and transamination of carbonyl compounds (aldehydes, ketones). However, the search of the literature did not identify any biogenic anime in foods or in biological systems that are formed from an aldehyde or a ketone. Transamination reactions in the literature are instead related to reversible amination and deamination that mediate the redistribution of amino groups amongst amino acids with an α-keto acid serving as an amino acceptor. In human, there are other BAs (dopamine, norepinephrine or noradrenaline, epinephrine or adrenaline, and serotonin or 5-hydroxytryptamine) that act as neurotransmitters and their formation was summarized by Purves et al. [26]. In brief, hydroxylation of tyrosine forms dihydroxyphenylalanine (DOPA) which upon the action of DOPA decarboxylase yields dopamine, subsequent action of dopamine β-hydroxylase catalyzes the formation of norepinephrine while further action of phenylethanolamine-N-methyltransferase leads to the formation of adrenaline. Serotonin is formed from tryptophan via a two-step process catalyzed by tryptophan-5-hydroxylase and a decarboxylase.
3. Analysis of biogenic amines
There are several procedures to analyse BAs which have been documented in literature review publications [27, 28, 29]. They include gas chromatography, thin layer chromatography, high-performance liquid chromatography (HPLC) with precolumn or post column derivatization, capillary electrophoresis as well biosensors [29, 30]. The chromatographic system can be coupled to a mass spectrometer for enhanced precision and selectivity. The first step is typically a sample processing to remove interfering molecules, a pre-concentration of the analyte, or the derivatization of the analyte for improved detection and selectivity. The derivatizing agents commonly used are dansyl chloride and ortho-phthaldehyde for either ultraviolet or fluorescent detection but, 6-aminoquinolyl-N-hydroxysuccinimidyl carbamate, 9-Fluorenylmethoxycarbonyl, benzoyl chloride are also used [5]. Some derivatizing reagents such as dansyl chloride can react with phenols, alcohols and secondary amines which then can interfere with the analysis of BAs. Stable isotope dilution procedures to quantity BAs by HPLC coupled to tandem mass spectrometers can overcome the issues associated with the non-specificity of the derivatizing reagent [31, 32].
Solid food samples (e.g., fish, meat, cheese, sausage, vegetables) for HPLC and capillary electrophoresis are often solubilized in acidic solvents (0.1–0.6 M) made of trichloroacetic acid, perchloric acid or hydrochloric acid to precipitate and remove proteins. The extracts are then derivatized and analyzed directly or clean-up with solid phase extraction cartridges before the analysis. Beverages such as wines and beers can be analyzed with or without derivatization but treatment with acid is important for dairy beverages and juices. Treatments of wine samples with polyvinylpolypyrolidone to remove phenolic components have been shown to be sufficient for the detection of BAs without any further processing. Biological samples (urine, plasma) are often acidified to about pH 2 to precipitate the proteins followed by their removal by centrifugation. The deproteinated biological sample can be analyzed either directly [33], after clean-up with solid phase extraction cartridges [34] or after derivatization [35].
A review on methods to analyse BAs in foods concluded that, out of seven hundred publications, 72% of procedures were based on HPLC, 18% on electrophoresis and 10% on gas chromatography [29]. The popularity of HPLC methods is due to the diversity of derivatizing reagents, the separation efficiency (many liquid and solid options) and their coupling to high performance mass spectrometers. The less use of gas chromatography can be attributed to the low volatility of BAs.
4. Biogenic amines in foods
In theory, they can be found in all foods that contain amino acids, peptides or proteins and which are exposed to conditions enabling the production of required enzymes as well as conditions for transamination reactions [36]. The nature of the BAs and their concentrations typically vary with the type of foods, and the processing or storing conditions. There are therefore differences in BAs present for example in meat versus fish, dairy, beverage or vegetable products which have been summarized in review articles [11, 37]. These reviews showed that food commonly investigated for BAs in terms of frequency are fish, meat, wine, and cheese products while vegetable products are less frequently investigated. The determination of BAs is important because they can serve as markers of food freshness, and indicators of improper processing and storage of foods.
4.1. Biogenic amines in seafoods
In seafood products, the most commons BAs are histamine, tyramine, cadaverine, and putrescine [38]. Their concentrations are affected by the conditions under which they are stored (temperature, time duration) because of a direct relationship with the growth of microorganisms [39]. The most abundant BA in seafood products is histamine followed by phenylethylamine due to higher concentrations of free histidine and phenylamine, respectively. The amounts of histamine in freshly captured fish are generally less than 1 mg/kg [40]. Meanwhile, toxic levels of histamine more than 10 time the FDA (Food and Drug Administration) regulatory limit (50 mg/kg [41] has caused the condition known as fish scombroid syndrome. Morganella psychrotoleran and Photobacterium phosphoreum microorganisms are commonly associated with the production of histamine in seafood products [42, 43]. A review by Visciano et al., summarized the content of biogenic amines in raw and processed seafood stored under different conditions [10]. In raw products, the concentrations range from not detected to 49.3 mg/kg with spermine and spermidine generally being the most abundant, histamine up to 19.5 and tyramine up to 46.9 mg/kg. In processed concentrations of tryptamines as high as 5487 mg/kg were reported in fish paste [10].
4.2. Biogenic amines in meat and dairy products
Fermented meats as well as other fermented food products typically contain higher amounts of BAs due to an increase concentration of free amino acids, the main precursors of their formation during the fermentation process. In dry sausages, tyramine (up to 600 mg/kg) and putrescine (up to 450 mg/kg) are the most abundant BAs [44]. There are reviews on biogenic amines in meat products [45, 46, 47]. In raw fresh meats, concentrations of BAs ranged from not detectable to the following values for individual BAs [47]. Histamine (0.5–27 mg/kg), tyramine (0.0–15.6 mg/kg), spermine (0.1–42 mg/kg), spermidine (0.0–10 mg/kg) and putrescine (0.5–12 mg/kg). There is a variation in concentration depending on the meat type (e.g. beef versus pork or poultry) but also on the animal part (e.g. leg versus breast). Storage and processing significantly increase the concentrations.
In cheese, native microorganisms with decarboxylase activities are responsible for the generation of low amount BAs but BA concentrations typically increase during ripening because of partial proteolytic cleavage of casein and the subsequent increase in free amino acids with histamine being the BA of concern [48]. In raw or pasteurized cow milk, BAs are low and often not detected. In cheese concentration of tyramine can increase to more than 830 mg/kg and that of histamine to 260 mg/kg [48]. A recent review article reported the concentration of histamine in other fermented milk products as 7 mg/kg in sour cream, 13–21 mg/kg in yogurt and 4 mg/kg in kefir [49].
4.3. Biogenic amines in plant-based foods
In plant-based foods, those that are fermented are the most studied for the presence of biogenic amines. Examples include fermented soy products (e.g. tofu, sauces) in which BAs over the regulatory limit have been detected. In fermented soy products, the hydrolysis of proteins provides the required substrate for the synthesis of BAs. A study by Yongmei et al. reported that tyramine was the most prevailing amine in soy sauce followed by spermidine, histamine, and cadaverine with a total content of 41.7–1357 mg/L [50]. Other works have reported total content of 56–634 mL/L for soy sauce [51]; 30–82 mg/kg for tofu with spermidine being the most abundant [52]. Fermented cabbage known as sauerkraut also contains BAS specifically, histamine, tyramine, putrescine, cadaverine, spermine, and spermidine [53]. The BAs in wine are believed to be influenced by both the nature of grapes and the vinification process. For example, only putrescine and tyramine were found in musts from sterilised grapes while musts from non-sterilised grapes contained cadaverine, tyramine and agmatine [54]. This is likely because sterilization inactivates some enzymes that can release peptides and amino acids or act as decarboxylases. Putrescine has been reported to be the most abundant BA in wine [55] while spermidine and putrescine have been identified as two dominant BAs in grapes and must [56].
5. Properties and functions of biogenic amines
5.1. Role in food quality and safety
In foods, the concentration of BAs is mainly associated with quality such as freshness, acceptability indicators, storage conditions and safety [57, 58]. In foods like fish, meat, and wines BAs are often determined a part of a quality control process because their excess amounts are associated with toxicological issues mostly with the nervous or vascular systems [59, 60]. The legal limit of BAs in food varies depending on the type of food, the chemical nature, and the country. In the USA for example, the limit of histamine is 50 mg/kg of flesh meat or fish, compared to 100 mg/kg in the European Union and South Africa, or 200 mg/kg in Australia and New Zealand [40]. The relationship between types of BAs was used to generate a chemical index for tuna decomposition in which, the sum of histamine, putrescine, cadaverine, and tyramine below 50 mg/kg is considered an acceptable sensory characteristic of tuna products [61]. In the case of meat products, the biogenic amine index (BAI) that consists of the sum of putrescine, cadaverine, histamine, and tyramine is instead recommended as a quality indicator [62, 63]. In that index, a value below 5 mg/kg is equivalent to good quality (i.e. fresh), between 5–20 mg/kg acceptable, 20–50 mg/kg low quality, and above 50 mg/kg spoiled meats [63, 64]. In beer beverages, Loret et al. [65] proposed a different index based on the ratio of BAs mostly produced by bacterial decarboxylases (tyramine, putrescine, cadaverine, histamine, phenylethylamine, and tryptamine) to the agmatine that mostly originates from the raw malt material [66]. They concluded that an index below 1.5 was of high quality while a value above 10 was a consequence of a poor hygienic fermentation process (i.e., presence of high level of decarboxylating bacteria).
5.2. Role in foods as bioactive molecules
Although, the presence of BAs is mainly used as quality indicators, they may provide some functionalities to foods specifically, those with aromatic moieties or proton donating abilities in their structures [67]. In a linoleic acid model system for example, tyramine, tryptamine and phenylethylamine inhibited the formation of hydroperoxide [14] indicating that in foods BAs can have antioxidant activities. Higher amounts of serotonin, putrescine and spermidine for example in fruits (e.g. grapes, bananas, plums) increase their resistance to oxidative damage which then enhances their quality postharvest by preserving the integrity of cell membranes during storage [68, 69, 70]. The concentration of dopamine in banana peel was 8-fold higher compared to naringin, the second most abundant molecule [71]. Dopamine was then identified as the most antioxidant molecule in those banana peels. In grapes that contained higher concentrations of putrescine, there was less incidence of decay, browning, and cracking during cold storage [72]. The conjugation of BAs with other molecules in foods can enhance their biological activity. In goji berries for example, N-feruloyl tyramine dimer and N-trans-and N-cis-feruloyl tyramine were identified as molecules that contributed to the ability of the extract to scavenge 2,2-diphenyl-1-picrylhydrazyl (DPPH) radicals [73]. The N-trans-feruloyl tyramine scavenged 12% more radical than butylated hydroxyanisole, a reference antioxidant molecule [73]. The presence of amide compounds is not limited to the berries or to tyramine. These types of amides are formed by reactions between esters of hydroxycinnamic acids and the BAs. Tyramyl amides have been reported in fruits, in garlic, and in onion [74, 75] but also in pepper and tomatoes [76] where they may contribute to antioxidant (scavenge radicals, inhibit lipid oxidation) and amicrobial activities. N-E-coumaroyl tyramine and N-E-feruloyl tyramine both from Lycium barbarum fruits scavenged 96% of DPPH radicals and inhibited lipid induced oxidation (83–96%) in rat liver microsomes at 0.1 mM concentrations [74]. Putrescine, spermidine and agmatine amides which are in most cases, coumaroyl, feruloyl, and sinapoyl-substituted amines have been purified from corn bran, corn bread and other cereal products [77, 78]. Many have been found to possess radical scavenging and anti-inflammatory activities [79, 80, 81]. For example, N,N‘-diferuloyl-putrescine obtained from corn bran showed potent inhibition of DPPH radicals (IC50 = 38.46 μM) and superoxide anion radicals (IC50 = 291.62 μM) [80]. These data suggest that aromatic BAs at the safe concentration level as well as hydrocinnamic derivates of both aromatic and aliphatic BAs may provide some stability to foods by acting as antioxidant or antimicrobial agents. It will be interesting to establish the functional relation between foods and BAs in the presence or absence of contaminating microorganisms.
5.3. Role of biogenic amines in human
Biogenic amines at the right concentration are important sources of nitrogenous compounds for human with the most active ones being histamine and tyramine. The concentration of BAs in a biological system is dependent on dietary sources, endogenous synthesis, and metabolic degradation. A total concentration of BAs of safe foods should not exceed 1000 mg/kg with threshold values of 50, 100, 30 mg/kg for histamine, tyramine, and phenylethylamine [52, 59, 82]. Tyramine and histamine are involved for example in the control of circadian rhythm, attention, gastric acid secretion, cell growth and differentiation [28, 83]. Polyamines, putrescine, spermine and spermidine also play essential roles in cell growth and differentiation [38, 84]. The polyamines are positively charged and can affect the functions of nucleic acids (DNA, RNA) via strong ionic interactions.
The concentrations of BAs above safe values are linked to various issues in the nervous, vascular and lung systems [23, 64]. There many are reviews dealing with the toxicological effects and poisoning related to the consumption of poorly processed or stored foods (fish, cheeses) that contain high concentrations of BAs. Total BAs concentration above 1000 mg/kg in the body system can cause either a mild allergic reaction or serious impairment of the respiratory, cardiovascular, and nervous systems [23, 59]. Physiological symptoms of the two most toxic BAs, histamine and tyramine include headache, migraine, hypertension, and respiratory disorders but similar symptoms have also been reported for phenylethylamine and tryptamine [85]. The most frequent BAs intoxication involved histamine. Its higher intake from poorly conserved fish products leads to what is known as scombroid fish poisoning because products are often derived from fishes of the Scombridae and Scomberesocidae families [58], while intake of tyramine poisoning from the consumption of unsafe cheese products is characterized as a cheese reaction [33]. In human, aliphatic BAs (putrescine and cadaverine, spermidine and spermine) are generally not directly toxic but they can enhance the toxicity of tyramine and histamine due to their inhibition of oxidases and N-methyltransferase involved in their metabolic turnover [86, 87].
The reaction of nitrates with cadaverine leads to N-nitrosopiperidine, while putrescine, spermine and spermidine are converted to N-nitrosopyrrolidine which are carcinogenic compounds [88, 89]. The above compounds and other N-nitrosylated compounds are synthetized in dried salted foods (meat and fish) but also come from cigarette's smoke [90, 91]. N-nitrosopiperidine is an oesophageal carcinogen while N- nitrosopyrrolidine is an inducer of liver tumours as found in mice and rats [88]. A review by Gushgari et Halden [92] estimated human exposure to total nitrosamines from foods and drinks to be 1.9 ± 0.38 μg/day) which is below the tolerable value of exposure in humans, 5–10 μg/kg of body weight per day [93]. N-nitrosopiperidine and N-nitrosopyrrolidine are classified as possibly carcinogenic to humans by the International Agency for Research on Cancer [92] meaning that despite animal data, quantitative human data are still not available.
6. Role of functional ingredients and bioactive compounds in regulating the concentration of biogenic amines
The intake of a small concentration of BAs through the diet is normally not harmful to health because the human body has a detoxification system to take care of these BAs very quickly in the intestine through the action of enzymes such as monoamine oxidase (MAO), diamine oxidase (DAO), and polyamine oxidase (PAO) which can metabolize dietary BAs in a healthy person. However, when a quantity in food is too high or if these enzymes are dysfunctional either genetically or due to the intake of inhibitors such as alcohol or certain antidepressant drugs, this mechanism may be hampered and allow greater concentration of BAs to enter the systemic circulation and exert a toxic effect on different organs [94, 95]. Bioactive compounds to control the concentration of BAs can work via several mechanisms, including the inhibition of their formation (e.g. inhibition of decarboxylases) or increase the metabolism of biogenic amines (e.g. greater expression of amine oxidases). Other methods to control BAs include the use of starter cultures that lack decarboxylating enzymes and additives that can act as antimicrobials.
6.1. Reduction of biogenic amines in foods
The concentration of biogenic amines in foods can be controlled by a strict adherence to cold chain because their formation is temperature dependent and decreases at low temperature through the inhibition of microbial growth and the reduction of the activity of decarboxylating enzymes [96, 97, 98]. Temperature control meanwhile is not enough since some bacteria produce BAs at temperatures below 5 °C. Methods that consist of modified atmosphere packaging, high hydrostatic pressure, or irradiation have developed and are summarized in the literature [21]. Other methods to control BAs include the use of starter cultures that lack decarboxylating enzymes but can also degrade for example histamine [99, 100].
The other alternative is the use of phytochemicals (secondary metabolites) in purified forms or as extracts to regulate the concentration of BAs in foods (Table 1). Grape juices made with purple-fleshed sweet potato (10 and 30%) lower serotonin and dopamine concentrations with a concomitant decrease of tyramine, histamine with the benefits being attributed to the presence of anthocyanins and catechins [101]. The addition of tea polyphenol extracts to freeze-chilled shrimps (immersed in 1% polyphenol solution for 30 min, −20 °C for 2 weeks and then 4 °C for 6 days) decreased the concentration of putrescine from 13.76 and 7.57 mg/kg meanwhile, there was no significant change in histamine concentrations [102]. A polyphenol rich-extract from cava lees, a winery by-product added to fermented sausages decreased cadaverine and putrescine concentrations by 21 and 40%, respectively [103]. There are studies conducted on the role of extract from spices such as garlic, onion, cinnamon, or ginger. Garlic extract applied during the ripening of a Korean seafood (anchovy) product reduced by 8.7% the total concentration of biogenic amines [104]. The mechanism was suggested to be due to the inhibition of the growth of Bacillus licheniformis, one of the known BAs producing microorganisms in the tested food [104]. The addition 0.3 mg/g extract of star anise, cassia, nutmeg, or cinnamon, during the preparation of dry fermented sausages showed a reduction of 19–28% in histamine, tryptamine, putrescine, spermidine, tyrosamine, and phenylethylamine concentrations [105, 106]. Possible mechanisms of extracts vary as they contain different molecules. The effects of cassia and fennel extracts were suggested to be due to high contents of estragole and trans-anethole which can affect the integrity of microbe cell membranes while that of clove is believed to be associated with the antimicrobial activity of the most abundant compound, eugenol. Another mechanism is the greater decrease of pH in the presence of species extracts [106] that will decrease microbial growth or inhibit the activity of decarboxylases. Polyphenols extracted from green tea and grape seeds as well as α-tocopherol, each evaluated at 300 mg/kg reduced the concentration of putrescine, cadaverine, histamine, tyramine and spermine in cured meats with tea extract having the greater which was associated with the inhibition of Enterobacteriaceae populations during ripening and storage [107]. Glucono-δ-lactone (1%) decreased histamine and putrescine in meat through a pH reduction [108]. Small organic acids like citric acid, succinic acid, and malic acid inhibited decarboxylase activity and the result was a decrease of histamine formation [21, 109]. Marination of pork with black currant juice decreased by 22.7–66.6% the concentration of total BAs, putrescine, cadaverine, or tyramine during storage (10 days) at refrigerator temperatures [110].
Table 1.
Effect of bioactive compounds and extracts on biogenic amines (BAs) in foods.
| Food | Treatment | Duration | BA detected | Effect on treatment | Reference |
|---|---|---|---|---|---|
| Sausage | 0.3 mg/g ethanol extract of star anise, clove, cassia, fennel, bay leaf, or nutmeg (74 volatile compounds) | Fermented, 8 days | tryptamine, putrescine, Spermidine, Phenylethylamine, Tyrosamine, and Histamine | 18–28% reduction | [105] |
| Fish sauces | Ginger, anise, garlic, or cinnamon ethanol extract (2%) | Fermented 15 days 30 °C | Histamine, Putrescine, Tyramine, Spermidine | 18%–37% reduction | [136] |
| Cereal based fermented food | 0.5–2% commercial pomegranate seed extract powder | 15 days 30 °C | Total BAs (putrescine, cadaverine, spermidine, spermine, histamine, tyramine) | 36–53% reduction | [137] |
| Shrimp paste | Commercial tea polyphenols (0.3%) | Stored 120 days 25 °C | Putrescine | 54–68% reduction | [138] |
| Cadaverine | |||||
| Histamine | |||||
| Phenylethylamine | |||||
| Tryptamine | |||||
| Tyramine | |||||
| Fermented pork product (Nham) | Ginger extract | 7 days 30–50 °C | Histamine | 65% reduction total BAs | [139] |
| Tyramine and others | |||||
| Atlantic salmon fillets | Phlorentin 2–4 mg/ml | Stored 3 days 4 °C | Histamine | 10–16% reduction | [114] |
| Putrescine | |||||
| Cadaverine | |||||
| Tyramine | |||||
| Mackerel | Gallic acid (5%) | Stored 12 days, 4 °C | Histamine | 63–84% reduction | [113] |
| Putrescine | |||||
| Cadaverine | |||||
| Cured Bacons | Commercial green Tea Polyphenol 300 mg/kg | 12 days 13–31 °C | Putrescine | 23–71% reduction | [107] |
| Cadaverine | |||||
| Spermine | |||||
| Tyramine | |||||
| Histamine | |||||
| Sauerkraut (shredded and salted cabbage) fermentation | Shredded onion (10 g/kg), or caraway seed (350 g/kg) | Fermented 14 days (18 °C or 31 °C) Stored 12 wk (4 °C) | Cadaverine | 2–3 fold reduction | [53] |
| Spermine | |||||
| Spermidine | |||||
| Histamine | |||||
| Dry-fermented sausage | green tea extract (0.15 and 0.3 g/kg) | 15 days 18–26 °C | Putrescine Histamine Tyramine | 34–36% reduction | [140] |
| Camel meat | 0.5–2.5% Gingerol | 30 min, Room temperature | Total BAs (cadaverine, Histamine, Putrescine, Spermine, Tyramine) | 47–91 reduction | [141] |
| Fish (Rainbow rout) | 3% rosemary oil | Stored 9 days, 4 °C | Histamine, tyramine, cadaverine, putrescine | 86%–94% reduction | [142] |
| Sardine fillets | Rosemary ethanol extract (1%) | Stored 20 days, 3 °C | Histamine, cadaverine, putrescine | 18–25% reduction | [143] |
| Harbin dry sausage | Spices ethanol extract 0.3 g/kg (cinnamon, clove, or anise) | Fermented, 9 days, | cadaverine, putrescine, tyrosamine, phenylethylamine, histamine, tryptamine | 11–22% reduction | [106] |
| Sardine fillets | Mentha spicata Mint ethanol extract (1%) | 21 days 3 °C | Putrescine | 27–66% reduction | [144] |
| Cadaverine | |||||
| Spermidine | |||||
| Histamine | |||||
| Tyramine |
Polyphenols are one of the groups of bioactive molecules used to control the formation of BAs in food. The inclusion of ferulic acid and its complex to smoked horsemeat sausages decreased the formation of tyramine via the inhibition of tyrosine decarboxylase and tyrosine/tyramine antiporter genes expression [111]. The addition of a mixture of quercetin, 4-hexylresorcinol, and cinnamic acid coupled with modified atmosphere packaging had a significant effect on the reduction of tyramine in pacific shrimp stored at 4 °C but not on putrescine and cadaverine [112]. A coating of mackerel with a solution of gallic acid (5%) reduced histamine concentration from 529 to 83 mg/kg after 12 days storage at 4 °C which was associated with reduce microbial growth [113]. In a related work, phloretin (4 mg/mL), a polyphenol (flavonoid) from apples reduced tyramine and histamine values from 21 and 16 mg/kg to 0.50 and 0.43 mg/kg, respectively in Atlantic salmon fillets [114]. In wine, the addition of naringenin (0.3 mg/mL), a polyphenol present in fruits and grains reduced by 71% the concentration of histamine. In the studies of polyphenols, the mechanism of BAs reduction is generally associated with their antimicrobial activity. Catechins such as epicatechin, epigallocatechin, epicatechin gallate, and epigallocatechin gallate have been shown to reduce by 60–76% the concentration of putrescine and cadaverine in fermented soy foods [115]. Additionally, in many cases, there were reports of increased oxidative stability characterized by inhibition of the oxidation of lipids.
6.2. Reduction of biogenic amines in biological systems
The control of BAs in biological systems is associated with both synthesis and metabolic pathways leading to their utilisation, degradation, and elimination. Functional ingredients can then target the decarboxylases or monoamine and polyamine oxidases. Apart from BAs commonly found in foods, two other ones are important in biological systems. They are dopamine formed via hydroxylation of tyrosine followed by a decarboxylation; and serotonin or 5-hydroxyltrypamine which is formed via hydroxylation of tryptophan followed by a decarboxylation [26]. Biogenic amines functions include their beneficial role in cellular communications, neurotransmission or as sources of amines. They also possess pathological roles in stress, allergy, neurological and cardiovascular disorders. Research on the effect on BAs in vivo are then often associated with these conditions.
Food-derived molecules can affect the concentration of BAs in biological systems through several mechanisms as summarized in Table 2. The inhibition of decarboxylases is a common target, specifically for polyphenol-type compounds. Epigallocatechin gallate (main polyphenol in green tea) decreased the formation of histamine by inhibiting histamine producing mast cells degranulation and tyrosine phosphorylation which was then linked to up to 60% inhibition of the activity of histidine decarboxylase [116, 117, 118]. These were cellular cultures or ex vivo works, and so, the true effects of the catechins will depend on their absorption and bioavailability. The inhibition of kidney dopa-decarboxylase which is responsible for the formation of dopamine and serotonin from 3-hydroxytyrosine and 5-hydroxytryptophan, respectively has been reported [119]. The protective effect of epigallocatechin gallate was further demonstrated in mice, where it reduced skin tumor through an interaction with the higher concentrations of polyamines (putrescine, spermidine and spermine) produced in tumor cells contribute to growth and differentiation [120, 121]. Epigallocatechin gallate reduces the skin tumor by inhibiting (50%) the activity of ornithine decarboxylase. Mangiferin, a polyphenol from mango fruits is believed to be neuroprotective because it can reduce by 13% the concentration of dopamine in aged mice, restore brain acetylcholinesterase to normal level, and protect neurons and oligodendrocytes (myelinating cells of the central nervous system) from excitotoxicity [122, 123]. In spontaneously hypertensive rats, the antihypertensive activity of orally administered flavodilol, a flavone derivative was associated with up to 75% depletion of biogenic amines including serotonin in heart tissues and a 20% decrease of BAs in the brain after acute or chronic treatment with 35–75 mg/kg [124].
Table 2.
Effects of bioactive compounds and extracts on biogenic amines (BAs) in biological systems.
| Model | Sample | Benefits | BA, or enzyme measured | Effect on BA or enzyme | Reference |
|---|---|---|---|---|---|
| Mast cell model: rat basophilic leukemia (RBL-2H3) | Epigallocatechin gallate (100, 200 μM) | Prevent histamine release from the cells | Histamine (antigen stimulated release) | 61–89% reduction | [116] |
| Rat RBL-2H3 cells | Epigallocatechin gallate (100 μM) | Not determined | Histamine decarboxylase | 57% inhibition | [117] |
| transgenic mouse: spontaneous skin tumors due to over-expression of ODC | Epigallocatechin gallate (0.045%) in drinking water | Reduction of tumor | Ornithine decarboxylase (ODC) | 50% inhibition of ODC | [120] |
| Polyamines (putrescine, spermine, spermidine) | No change in polyamines | ||||
| Mice, topical application | Green tea extract, Epigallocatechin gallate, EGC, EC, ECG (2.0 mg sample in 0.2 mL acetone) | Reduction of tumor | Epidermal ornithine decarboxylase (tumor induced) | 17–51% inhibition | [121] |
| Mice—induced ageing | Mangiferin, (polyphenol mango fruits, 10–40 mg/kg) | Improved learning and retention of learned memory | Brain dopamine (ageing induced increase) | Reverse the 10% dopamine increase | [122] |
| Spontaneously hypertensive rats | Flavodilol 35–75 mg/kg | Decline of blood pressure | Serotonin | 70–80% reduction of serotonin in the spleen | [124] |
| 15–20% reduction in brain | |||||
| Mice—induced depression | Oleuropein (8–32 mg/kg), olive polyphenol | Less depression-like behaviors, reduced serotonin and dopamine | serotonin and dopamine | Reverse the 27% decrease of serotonin and dopamine to 10% | [125] |
| Rat brain—Fluoride induced toxicity | Resveratrol | Reduced oxidative damage to brain tissues | Dopamine | The decrease 66–74% of serotonin and dopamine was improved to 89–97% | [126] |
| 20 mg/kg (Maintain BAs in brain region) | Serotonin | ||||
| Mice, Parkinson's disease model | Curcumin (80 mg/kg), Tetrahydro-curcumin (60 mg/kg) | Neuro-protection | Dopamine | Improve brain dopamine from a 73% decrease to 25–30% | [131] |
| Monoamine oxidase-B (MAO-B) | Inhibit brain MAA-B (30–35% | ||||
| Stressed rats | Curcumin (20 and 40 mg/kg) | Anti-depressant | Dopamine, Serotonin, Monoamine oxidase (MAO) | Reverse depletion of brain serotonin and dopamine | [132] |
| Inhibit MAO (30–50%) | |||||
| Human, healthy young adults | blackcurrant berry polyphenol extracts (8.75 mg/kg bodyweight as part of a drink) | Cognitive benefits | Monoamine oxidase-B (MAO-B) | inhibition platelet MAO-B (96%) | [133] |
| Human colon cancer-derived metastatic cells | Procyanidins from apples (95% (−) epicatechin and 4% (+) catechin). Assayed at 50 μg/mL | Chemoprevention, growth inhibition | Ornithine decarboxylase (ODC) and S-adenosyl-L-methionine decarboxylase (AdoMetDC) N-acetyltransferase | Reduced activities of ODC and AdoMetDC were reduced by 38–50% | [134] |
| Increase putrescine (25%) | |||||
| Decrease of spermine (20%) and spermidine (10%) | |||||
| Increase of N-acetylspermidine (15%) |
The mechanisms of several neurotoxic molecules include lowering the concentration of the hormonal biogenic amines (dopamine, serotonin, noradrenaline). As such, food bioactive compounds that can counter the effects of the neurotoxins and prevent the decrease of the neurotransmitter BAs are considered beneficial. Intraperitoneal (i.p.) admiration to mice of oleuropein (8–32 mg/kg), a main bioactive phenolic compound in olives reverses corticosterone-induced depression [125] while in rats, resveratrol (20 mg/kg body weight) prevented fluoride-induced brain toxicity [126]. Oleuropein totally prevented the decrease of dopamine and serotonin while resveratrol maintained 89–97% of their values depending on the brain region compared to 66–74% only in its absence. Chronic administration of catechin (20 mg/kg daily for 28 days) improved the monoaminergic system in old rats with or without exercise ([127, 128]. The benefits were characterized by increased dopamine (25%–26%), increase visuo-spatial working and episodic memory [127]; increase in motor coordination, spatial memory, and serotonin (72%) [128]. In addition to maintaining the right concentrations of BAs, other possible mechanisms of the polyphenols can include inhibition of the synthesis or the activity of decarboxylases, dopamine β-hydroxylase or tyrosine hydroxylase. Alternatively, the polyphenols can scavenge free radicals thereby, prevent them from lowering the BAs through oxidative damage.
Enzymes such as amine oxidases are also important in the physiological regulation of biogenic amines and in their metabolic turnover. The most common are mono amine oxidases A and B. Mono- and poly-amine oxidases are flavoenzymes responsible for the oxidative deamination of BAs into corresponding imine followed by non-enzymatic hydrolysis to waste products (carbonyl compounds and ammonia) [129, 130]. In the case of biogenic amine food intoxication, a greater activity of these enzymes is beneficial for the BAs turnover while in the case of neurotoxicity from other molecules or in the case of neurodegeneration, inhibition of the transferase and amine oxidases will prevent the decrease of BAs and maintain optimum physiological levels. For neuronal diseases such as depression and neurodegeneration, inhibitors of the amine oxidases will provide some benefices. In a Parkinson's disease mouse model for example, neurodegeneration was associated with an increase in activity of monoamine oxidase-B and a concurrent decrease of dopamine and, when curcumin (80 mg/kg i. p.) and tetrahydrocurcumin (60 mg/kg i.p.) were administered they protected the neurons through a significant prevention the depletion of dopamine due the inhibition of the activity of the amine oxidase [131]. In a related work, curcumin 40 mg/kg i.p.) and its combination with piperine (2.5 mg/kg) attenuated chronic stress by reversing the increase in activity of both monoamine oxidases A and B in the brain with concomitant reversal of the decrease of serotonin and dopamine concentrations [132]. Berry extracts, standardized in a drink and given to young adults (8.75 mg of polyphenols/kg of bodyweight) inhibited blood monoamine oxidase-A which then limited the conversion of noradrenaline to 3,4-dihydroxyphenylglycol although, there were no changes in dopamine and serotonin contents in the blood [133].
Polyamines are poly-cationic molecules, and their function is associated with ionic interactions. A physiological way to regulate the concentration of polyamines is through their conversion to neutral molecules or to decrease their positive charge via acetylation reactions. Active ingredients that inhibit either polyamine oxidase or N-acetyltransferase have then been investigated for their role in the regulation of polyamine concentrations. In a work by Gossé et al. [134] on human colon cancer-derived metastatic cells, apple procyanidins decreased the formation of polyamines (spermidine, spermine) by inhibiting the activity of ornithine decarboxylase and S-adenosyl-L-methionine decarboxylase while also inducing the expression of N-acetyltransferase. The overall consequence was an increase concentration of polyamines together with an increase apoptosis. There are other data on the inhibition of amine oxidases and transferases by bioactive foods molecules, but they lack the correlation with biogenic amine contents which is the subject of this paper. Inhibitors of monoamine oxidases are considered neuroprotective agents in neurodegeneration because of their ability to prevent the decrease of BAs while the inhibition of polyamines oxidases can be useful to control tumours. Neurodegeneration and tumour development are however complex issues with unclarified aetiologies and associations with other factors such as elevated oxidative stress, inflammation, apoptosis, dysfunction of mitochondria, excitotoxicity, impaired ubiquitine-proteasome system [135].
7. Conclusion and perspectives
Biogenic amines are a group of amine compounds with important functions in plants and humans. High concentrations in food, specifically those of monoamines are however associated with toxicological effects in human. The control of biogenic amines is done through optimization of handling and processing conditions, the use of antimicrobials, inhibitors of decarboxylates, amine oxidases, and N-transferases. Bioactive compounds mostly polyphenols have been proven useful in food products due to their antimicrobial and decarboxylase inhibitory properties.
In foods, the bioactive compounds tested are combined with temperature control meanwhile it may be of interest to also determine their effects in combination with other conditions such as modified atmosphere packaging and high hydrostatic pressure. In addition, the standardization of polyphenol extracts is important to determine the contribution of individual molecules. In biological systems, the bioactive compounds have a potential, but their effects are going to be limited by their absorption and bioavailability. More studies are needed in human or biological systems. Additionally, not all cells are able to produce biogenic amines, hence the need to develop approaches that can target the bioactive compound to the specified cells which might be challenging.
Declarations
Author contribution statement
All authors listed have significantly contributed to the development and the writing of this article.
Funding statement
Dr Apollinaire Tsopmo was supported by Natural Sciences and Engineering Research Council of Canada [371908].
Data availability statement
Data included in article/supp. material/referenced in article.
Declaration of interests statement
The authors declare the following conflict of interests: Apollinaire Tsopmo; [is a member of the Food Science and Nutrition editorial team.]
Additional information
No additional information is available for this paper.
References
- 1.Martins N., Morales P., Barros L., Ferreira I.C.F.R. The increasing demand for functional foods. Wild Plants, Mushrooms Nuts Funct. Food Prop. Appl. 2017 [Google Scholar]
- 2.Murray M.T., Pizzorno J. Atria Book; New York, USA: 2005. The Encyclopedia of Healing Foods. [Google Scholar]
- 3.Institute of Food Technologists . March 2005. Functional Foods: Opportunities and Challenges. [Google Scholar]
- 4.Khuhawar M.Y., Qureshi G.A. Polyamines as cancer markers: applicable separation methods. J. Chromatogr. B Biomed. Sci. Appl. 2001;764(1–2):385–407. doi: 10.1016/s0378-4347(01)00395-4. [DOI] [PubMed] [Google Scholar]
- 5.Larqué E., Sabater-Molina M., Zamora S. Biological significance of dietary polyamines. Nutrition. 2007;23:87–95. doi: 10.1016/j.nut.2006.09.006. [DOI] [PubMed] [Google Scholar]
- 6.Lozanov V., Benkova B., Mateva L., Petrov S., Popov E., Slavov C., et al. Liquid chromatography method for simultaneous analysis of amino acids and biogenic amines in biological fluids with simultaneous gradient of pH and acetonitrile. J. Chromatogr., B: Anal. Technol. Biomed. Life Sci. 2007;860(1):92–97. doi: 10.1016/j.jchromb.2007.10.020. [DOI] [PubMed] [Google Scholar]
- 7.Ming Mao H., Guan Chen B., Ming Qian X., Liu Z. Simultaneous determination of twelve biogenic amines in serum by high performance liquid chromatography. Microchem. J. 2009;91(2):176–180. [Google Scholar]
- 8.Vermeiren Y., Le Bastard N., Van Hemelrijck A., Drinkenburg W.H., Engelborghs S., De Deyn P.P. Behavioral correlates of cerebrospinal fluid amino acid and biogenic amine neurotransmitter alterations in dementia. Alzheimer's Dementia. 2013 Sep;9(5):488–498. doi: 10.1016/j.jalz.2012.06.010. [DOI] [PubMed] [Google Scholar]
- 9.Id A.B., Meinitzer A., Rothenhä Usler H.-B., Amouzadeh-Ghadikolai O., Lewinski D.V., Breitenecker R.J., et al. 2018. Metabolomics Approach in the Investigation of Depression Biomarkers in Pharmacologically Induced Immune-Related Depression. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Visciano P., Schirone M., Tofalo R., Suzzi G. Biogenic amines in raw and processed seafood. Front Microbiol. 2012;3:188. doi: 10.3389/fmicb.2012.00188. https://www.frontiersin.org/article/10.3389/fmicb.2012.00188 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Doeun D., Davaatseren M., Chung M.-S. Biogenic amines in foods. Food Sci. Biotechnol. 2017;26(6):1463–1474. doi: 10.1007/s10068-017-0239-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pugin B., Barcik W., Westermann P., Heider A., Wawrzyniak M., Hellings P., et al. A wide diversity of bacteria from the human gut produces and degrades biogenic amines. Microb. Ecol. Health Dis. 2017 Jan 1;28(1) doi: 10.1080/16512235.2017.1353881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Slominski A.T., Zmijewski M.A., Skobowiat C., Zbytek B., Slominski R.M., Steketee J.D. Sensing the Environment: Regulation of Local and Global Homeostasis by the Skin’s Neuroendocrine System [Internet] Springer; 2012. Biogenic amines in the skin; pp. 7–26.http://link.springer.com/10.1007/978-3-642-19683-6_2 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Yen G.-C., Kao H.-H. Antioxidative effect of biogenic amine on the peroxidation of linoleic acid. Biosci. Biotechnol. Biochem. 1993 Jan;57(1):115–116. doi: 10.1271/bbb.57.115. [DOI] [PubMed] [Google Scholar]
- 15.Tan D.-X., Manchester L.C., Burkhardt S., Sainz R.M., Mayo J.C., Kohen R., et al. N 1-acetyl-N 2-formyl-5-methoxykynuramine, a biogenic amine and melatonin metabolite, functions as a potent antioxidant. Faseb. J. 2001 doi: 10.1096/fj.01-0309fje. [DOI] [PubMed] [Google Scholar]
- 16.Mude H., Balapure A., Thakur A., Ganesan R., Ray Dutta J. Enhanced antibacterial, antioxidant and anticancer activity of caffeic acid by simple acid-base complexation with spermine/spermidine. Nat. Prod. Res. 2022 Feb;10:1–6. doi: 10.1080/14786419.2022.2038597. [DOI] [PubMed] [Google Scholar]
- 17.De Mey E., De Klerck K., De Maere H., Dewulf L., Derdelinckx G., Peeters M.-C., et al. The occurrence of N-nitrosamines, residual nitrite and biogenic amines in commercial dry fermented sausages and evaluation of their occasional relation. Meat Sci. 2014;96(2, Part A):821–828. doi: 10.1016/j.meatsci.2013.09.010. https://www.sciencedirect.com/science/article/pii/S0309174013005287 Available from: [DOI] [PubMed] [Google Scholar]
- 18.Bulushi I Al, Poole S., Deeth H.C., Dykes G.A. Biogenic amines in fish: roles in intoxication, spoilage, and nitrosamine formation—a review. Crit. Rev. Food Sci. Nutr. 2009 Feb 24;49(4):369–377. doi: 10.1080/10408390802067514. [DOI] [PubMed] [Google Scholar]
- 19.Mah J.-H., Yoon M.-Y., Cha G.-S., Byun M.-W., Hwang H.-J. Influence of curing and heating on formation of N-nitrosamines from biogenic amines in food model system using Korean traditional fermented fish product. Food Sci. Biotechnol. 2005;14(1):168–170. [Google Scholar]
- 20.Emer C.D., Marques S., Colla L.M., Reinehr C.O. Biogenic amines and the importance of starter cultures for malolactic fermentation. Aust. J. Grape Wine Res. 2021 Jan 1;27(1):26–33. [Google Scholar]
- 21.Naila A., Flint S., Fletcher G., Bremer P., Meerdink G. Control of biogenic amines in food—existing and emerging approaches. J. Food Sci. 2010 Sep 1;75(7):R139–R150. doi: 10.1111/j.1750-3841.2010.01774.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Wang J., Qu Y., Liu Z., Zhou H. Formation, analytical methods, change tendency, and control strategies of biogenic amines in canned aquatic products: a systematic review. J. Food Prot. 2021;84(11):2020–2036. doi: 10.4315/JFP-21-120. https://www.scopus.com/inward/record.uri?eid=2-s2.0-85119179561&doi=10.4315%2FJFP-21-120&partnerID=40&md5=2d7d925335ba1f8c19592fc14d9b3caa Available from: [DOI] [PubMed] [Google Scholar]
- 23.Erdag D., Merhan O., Yildiz B. In: Biogenic Amines [Internet] Proestos C., editor. IntechOpen; Rijeka: 2019. Biochemical and pharmacological properties of biogenic amines; pp. 63–69.https://www.intechopen.com/books/biogenic-amines/biochemical-and-pharmacological-properties-of-biogenic-amines Available from: [Google Scholar]
- 24.Romano A., Trip H., Lonvaud-Funel A., Lolkema J.S., Lucas P.M. Evidence of two functionally distinct ornithine decarboxylation systems in lactic acid bacteria. Appl. Environ. Microbiol. 2012;78(6):1953–1961. doi: 10.1128/AEM.07161-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kalač P., Krausová P. A review of dietary polyamines: formation, implications for growth and health and occurrence in foods. Food Chem. 2005;90(1–2):219–230. [Google Scholar]
- 26.Purves D., Augustine G., Fitzpatrick D., Katz L.C., LaMantia A.S., McNamara J.O., et al. second ed. Sinauer Associates; Sunderland, MA, USA: 2001. Neuroscience.https://www.ncbi.nlm.nih.gov/books/NBK11035/ Available from: [Google Scholar]
- 27.Bedia Erim F. Recent analytical approaches to the analysis of biogenic amines in food samples. TrAC Trends Anal. Chem. 2013;52:239–247. https://www.sciencedirect.com/science/article/pii/S0165993613001854 Available from: [Google Scholar]
- 28.Mohammed G.I., Bashammakh A.S., Alsibaai A.A., Alwael H., El-Shahawi M.S. A critical overview on the chemistry, clean-up and recent advances in analysis of biogenic amines in foodstuffs. TrAC Trends Anal. Chem. 2016;78:84–94. https://www.sciencedirect.com/science/article/pii/S0165993615301539 Available from: [Google Scholar]
- 29.Jain A., Verma K.K. Strategies in liquid chromatographic methods for the analysis of biogenic amines without and with derivatization. TrAC Trends Anal. Chem. 2018;109:62–82. https://www.sciencedirect.com/science/article/pii/S0165993618304655 Available from: [Google Scholar]
- 30.Torre R., Costa-Rama E., Nouws H.P.A., Delerue-Matos C. Screen-printed electrode-based sensors for food spoilage control: bacteria and biogenic amines detection. Biosensors. 2020;10(10) doi: 10.3390/bios10100139. https://www.mdpi.com/2079-6374/10/10/139 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Cai Y., Sun Z., Chen G., Liu X., You J., Zhang C. Rapid analysis of biogenic amines from rice wine with isotope-coded derivatization followed by high performance liquid chromatography–tandem mass spectrometry. Food Chem. 2016;192:388–394. doi: 10.1016/j.foodchem.2015.07.024. https://www.sciencedirect.com/science/article/pii/S0308814615010298 Available from: [DOI] [PubMed] [Google Scholar]
- 32.Mayr C.M., Schieberle P. Development of stable isotope dilution assays for the simultaneous quantitation of biogenic amines and polyamines in foods by LC-MS/MS. J. Agric. Food Chem. 2012 Mar 28;60(12):3026–3032. doi: 10.1021/jf204900v. [DOI] [PubMed] [Google Scholar]
- 33.Gosetti F., Mazzucco E., Gennaro M.C., Marengo E. Simultaneous determination of sixteen underivatized biogenic amines in human urine by HPLC-MS/MS. Anal. Bioanal. Chem. 2013;405(2):907–916. doi: 10.1007/s00216-012-6269-z. [DOI] [PubMed] [Google Scholar]
- 34.Konieczna L., Roszkowska A., Synakiewicz A., Stachowicz-Stencel T., Adamkiewicz-Drożyńska E., Bączek T. Analytical approach to determining human biogenic amines and their metabolites using eVol microextraction in packed syringe coupled to liquid chromatography mass spectrometry method with hydrophilic interaction chromatography column. Talanta. 2016;150:331–339. doi: 10.1016/j.talanta.2015.12.056. https://www.sciencedirect.com/science/article/pii/S0039914015305853 Available from: [DOI] [PubMed] [Google Scholar]
- 35.Sakaguchi Y., Yoshida H., Hayama T., Itoyama M., Todoroki K., Yamaguchi M., et al. Selective liquid-chromatographic determination of native fluorescent biogenic amines in human urine based on fluorous derivatization. J. Chromatogr. A. 2011;1218(33):5581–5586. doi: 10.1016/j.chroma.2011.05.076. https://www.sciencedirect.com/science/article/pii/S0021967311007370 Available from: [DOI] [PubMed] [Google Scholar]
- 36.Halász A., Baráth Á., Simon-Sarkadi L., Holzapfel W. Biogenic amines and their production by microorganisms in food. Trends Food Sci. Technol. 1994;5(2):42–49. http://www.sciencedirect.com/science/article/pii/0924224494900701 Available from: [Google Scholar]
- 37.Papageorgiou M., Lambropoulou D., Morrison C., Kłodzińska E., Namieśnik J., Płotka-Wasylka J. Literature update of analytical methods for biogenic amines determination in food and beverages. TrAC Trends Anal. Chem. 2018;98:128–142. https://www.sciencedirect.com/science/article/pii/S0165993617303990 Available from: [Google Scholar]
- 38.Houicher A., Bensid A., Regenstein J.M., Özogul F. Control of biogenic amine production and bacterial growth in fish and seafood products using phytochemicals as biopreservatives: a review. Food Biosci. 2021;39 https://www.sciencedirect.com/science/article/pii/S2212429220311457 Available from: [Google Scholar]
- 39.Lehane L., Olley J. Histamine fish poisoning revisited. Int. J. Food Microbiol. 2000;58(1):1–37. doi: 10.1016/s0168-1605(00)00296-8. https://www.sciencedirect.com/science/article/pii/S0168160500002968 Available from: [DOI] [PubMed] [Google Scholar]
- 40.Auerswald L., Morren C., Lopata A.L. Histamine levels in seventeen species of fresh and processed South African seafood. Food Chem. 2006;98(2):231–239. https://www.sciencedirect.com/science/article/pii/S0308814605005042 Available from: [Google Scholar]
- 41.Administration F and D . US Department of Health and Human Services Food and Drug Administration; 2011. Fish and Fishery Products Hazards and Controls Guidance. [Google Scholar]
- 42.Ucak I., Gokoglu N., Toepfl S., Galanakis C.M. Inhibitory effects of high pressure processing on Photobacterium phosphoreum and Morganella psychrotolerans in vacuum packed herring (Clupea harengus) J. Food Saf. 2018 Dec 1;38(6) [Google Scholar]
- 43.Mercogliano R., Santonicola S. Scombroid fish poisoning: factors influencing the production of histamine in tuna supply chain. A review. LWT. 2019;114 https://www.sciencedirect.com/science/article/pii/S0023643819307169 Available from: [Google Scholar]
- 44.Suzzi G., Gardini F. Biogenic amines in dry fermented sausages: a review. Int. J. Food Microbiol. 2003;88(1):41–54. doi: 10.1016/s0168-1605(03)00080-1. https://www.sciencedirect.com/science/article/pii/S0168160503000801 Available from: [DOI] [PubMed] [Google Scholar]
- 45.Ruiz-Capillas C., Jiménez-Colmenero F. Biogenic amines in meat and meat products. Crit. Rev. Food Sci. Nutr. 2005 Feb 10;44(7–8):489–599. doi: 10.1080/10408690490489341. [DOI] [PubMed] [Google Scholar]
- 46.Jairath G., Singh P.K., Dabur R.S., Rani M., Chaudhari M. Biogenic amines in meat and meat products and its public health significance: a review. J. Food Sci. Technol. 2015 Nov 7;52(11):6835–6846. [Google Scholar]
- 47.Schirone M., Esposito L., D’Onofrio F., Visciano P., Martuscelli M., Mastrocola D., et al. Biogenic amines in meat and meat products: a review of the science and future perspectives. Foods. 2022;11 doi: 10.3390/foods11060788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Linares D.M., del Río B., Ladero V., Martínez N., Fernández M., Martín M.C., et al. Factors influencing biogenic amines accumulation in dairy products. Front Microbiol. 2012;3:180. doi: 10.3389/fmicb.2012.00180. https://www.frontiersin.org/article/10.3389/fmicb.2012.00180 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Moniente M., García-Gonzalo D., Ontañón I., Pagán R., Botello-Morte L. Histamine accumulation in dairy products: microbial causes, techniques for the detection of histamine-producing microbiota, and potential solutions. Compr. Rev. Food Sci. Food Saf. 2021 Mar;20(2):1481–1523. doi: 10.1111/1541-4337.12704. [DOI] [PubMed] [Google Scholar]
- 50.Yongmei L., Xiaohong C., Mei J., Xin L., Rahman N., Mingsheng D., et al. Biogenic amines in Chinese soy sauce. Food Control. 2009;20(6):593–597. https://www.sciencedirect.com/science/article/pii/S0956713508002442 Available from: [Google Scholar]
- 51.Li J., Zhou L., Feng W., Cheng H., Muhammad A.I., Ye X., et al. Comparison of biogenic amines in Chinese commercial soy sauces. Molecules. 2019;24 doi: 10.3390/molecules24081522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Byun B.Y., Bai X., Mah J.-H. Occurrence of biogenic amines in doubanjiang and tofu. Food Sci. Biotechnol. 2013;22(1):55–62. [Google Scholar]
- 53.Majcherczyk J., Surówka K. Effects of onion or caraway on the formation of biogenic amines during sauerkraut fermentation and refrigerated storage. Food Chem. 2019;298 doi: 10.1016/j.foodchem.2019.125083. https://www.sciencedirect.com/science/article/pii/S0308814619311872 Available from: [DOI] [PubMed] [Google Scholar]
- 54.Cecchini F., Morassut M. Effect of grape storage time on biogenic amines content in must. Food Chem. 2010;123(2):263–268. https://www.sciencedirect.com/science/article/pii/S0308814610004826 Available from: [Google Scholar]
- 55.Kántor A., Kačániová M., Pachlová V. Biogenic amines content in different wine samples. J. Microbiol. Biotechnol. Food Sci. 2015;4(Special issue 01):37–40. [Google Scholar]
- 56.Henríquez-Aedo K., Vega M., Prieto-Rodríguez S., Aranda M. Evaluation of biogenic amines content in chilean reserve varietal wines. Food Chem. Toxicol. 2012;50(8):2742–2750. doi: 10.1016/j.fct.2012.05.034. [DOI] [PubMed] [Google Scholar]
- 57.Sirocchi V., Caprioli G., Cecchini C., Coman M.M., Cresci A., Maggi F., et al. Biogenic amines as freshness index of meat wrapped in a new active packaging system formulated with essential oils of Rosmarinus officinalis. Int. J. Food Sci. Nutr. 2013 Dec 1;64(8):921–928. doi: 10.3109/09637486.2013.809706. [DOI] [PubMed] [Google Scholar]
- 58.Wójcik W., Łukasiewicz M., Puppel K. Biogenic amines: formation, action and toxicity – a review. J. Sci. Food Agric. 2021 May 1;101(7):2634–2640. doi: 10.1002/jsfa.10928. [DOI] [PubMed] [Google Scholar]
- 59.Sivamaruthi B.S., Kesika P., Chaiyasut C. A narrative review on biogenic amines in fermented fish and meat products. J. Food Sci Technol. 2021;58(5):1623–1639. doi: 10.1007/s13197-020-04686-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Preti R., Vinci G. Biogenic amine content in red wines from different protected designations of origin of southern Italy: chemometric characterization and classification. Food Anal. Methods. 2016;9(8):2280–2287. [Google Scholar]
- 61.Mietz J.L., Karmas E. Chemical quality index of canned tuna as determined by high-pressure liquid chromatography. J. Food Sci. 1977 Jan 1;42(1):155–158. [Google Scholar]
- 62.Triki M., Herrero A.M., Jiménez-Colmenero F., Ruiz-Capillas C. Quality assessment of fresh meat from several species based on free amino acid and biogenic amine contents during chilled storage. Foods. 2018;7 doi: 10.3390/foods7090132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hernández-Jover T., Izquierdo-Pulido M., Veciana-Nogués M.T., Vidal-Carou M.C. Ion-pair high-performance liquid chromatographic determination of biogenic amines in meat and meat products. J. Agric. Food Chem. 1996 Jan 1;44(9):2710–2715. [Google Scholar]
- 64.Ruiz-Capillas C., Herrero A.M. Impact of biogenic amines on food quality and safety. Foods. 2019;8 doi: 10.3390/foods8020062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Loret S., Deloyer P., Dandrifosse G. Levels of biogenic amines as a measure of the quality of the beer fermentation process: data from Belgian samples. Food Chem. 2005;89(4):519–525. https://www.sciencedirect.com/science/article/pii/S0308814604002365 Available from: [Google Scholar]
- 66.Kalac P., Krízek M. A review of biogenic amines and polyamines in beer. J. Inst. Brew. 2003 Jan 1;109(2):123–128. [Google Scholar]
- 67.Esfandi R., Walters M.E., Tsopmo A. Antioxidant properties and potential mechanisms of hydrolyzed proteins and peptides from cereals. Heliyon. 2019;5(4) doi: 10.1016/j.heliyon.2019.e01538. http://www.sciencedirect.com/science/article/pii/S2405844018368919 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Gomes E.P., Vanz Borges C., Monteiro G.C., Filiol Belin M.A., Minatel I.O., Pimentel Junior A., et al. Preharvest salicylic acid treatments improve phenolic compounds and biogenic amines in ‘Niagara Rosada’ table grape. Postharvest. Biol. Technol. 2021;176 https://www.sciencedirect.com/science/article/pii/S0925521421000442 Available from: [Google Scholar]
- 69.Borges C.V., Belin M.A.F., Amorim E.P., Minatel I.O., Monteiro G.C., Gomez Gomez H.A., et al. Bioactive amines changes during the ripening and thermal processes of bananas and plantains. Food Chem. 2019;298 doi: 10.1016/j.foodchem.2019.125020. https://www.sciencedirect.com/science/article/pii/S0308814619311227 Available from: [DOI] [PubMed] [Google Scholar]
- 70.Luo Z., Chen C., Xie J. Effect of salicylic acid treatment on alleviating postharvest chilling injury of ‘Qingnai’ plum fruit. Postharvest. Biol. Technol. 2011;62(2):115–120. https://www.sciencedirect.com/science/article/pii/S0925521411001293 Available from: [Google Scholar]
- 71.Kanazawa K., Sakakibara H. High content of dopamine, a strong antioxidant, in cavendish banana. J. Agric. Food Chem. 2000 Mar 1;48(3):844–848. doi: 10.1021/jf9909860. [DOI] [PubMed] [Google Scholar]
- 72.Shiri M.A., Ghasemnezhad M., Bakhshi D., Sarikhani H. Effect of postharvest putrescine application and chitosan coating on maintaining quality of table grape cv. “Shahroudi” during long-term storage. J. Food Proc. Preserv. 2013 Oct 1;37(5):999–1007. [Google Scholar]
- 73.Forino M., Tartaglione L., Dell’Aversano C., Ciminiello P. NMR-based identification of the phenolic profile of fruits of Lycium Barbarum (Goji Berries). Isolation and structural determination of a novel N-Feruloyl tyramine dimer as the most abundant antioxidant polyphenol of goji berries. Food Chem. 2016;194:1254–1259. doi: 10.1016/j.foodchem.2015.08.129. https://www.sciencedirect.com/science/article/pii/S0308814615013382 Available from: [DOI] [PubMed] [Google Scholar]
- 74.Gao K., Ma D., Cheng Y., Tian X., Lu Y., Du X., et al. Three new dimers and two monomers of phenolic amides from the fruits of Lycium barbarum and their antioxidant activities. J. Agric. Food Chem. 2015 Feb 4;63(4):1067–1075. doi: 10.1021/jf5049222. [DOI] [PubMed] [Google Scholar]
- 75.Leonard W., Zhang P., Ying D., Fang Z. Tyramine-derived hydroxycinnamic acid amides in plant foods: sources, synthesis, health effects and potential applications in food industry. Crit. Rev. Food Sci. Nutr. 2020;18:1–18. doi: 10.1080/10408398.2020.1845603. [DOI] [PubMed] [Google Scholar]
- 76.Ly D., Kang K., Choi J.-Y., Ishihara A., Back K., Lee S.-G. HPLC analysis of serotonin, tryptamine, tyramine, and the hydroxycinnamic acid amides of serotonin and tyramine in food vegetables. J. Med. Food. 2008 Jun 1;11(2):385–389. doi: 10.1089/jmf.2007.514. [DOI] [PubMed] [Google Scholar]
- 77.Li Z., Zhao C., Zhao X., Xia Y., Sun X., Xie W., et al. Deep annotation of hydroxycinnamic acid amides in plants based on ultra-high-performance liquid chromatography–high-resolution mass spectrometry and its in silico database. Anal. Chem. 2018 Dec 18;90(24):14321–14330. doi: 10.1021/acs.analchem.8b03654. [DOI] [PubMed] [Google Scholar]
- 78.Dong X., Gao Y., Chen W., Wang W., Gong L., Liu X., et al. Spatiotemporal distribution of phenolamides and the genetics of natural variation of hydroxycinnamoyl spermidine in rice. Mol. Plant. 2015;8(1):111–121. doi: 10.1016/j.molp.2014.11.003. https://www.sciencedirect.com/science/article/pii/S1674205214000045 Available from: [DOI] [PubMed] [Google Scholar]
- 79.Bento-Silva A., Duarte N., Mecha E., Belo M., Vaz Patto M.C., Bronze M.D. Hydroxycinnamic acids and their derivatives in Broa, a traditional ethnic maize bread. Foods. 2020;9 doi: 10.3390/foods9101471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Choi S.W., Lee S.K., Kim E.O., Oh J.H., Yoon K.S., Parris N., et al. Antioxidant and antimelanogenic activities of polyamine conjugates from corn bran and related hydroxycinnamic acids. J. Agric. Food Chem. 2007 May 1;55(10):3920–3925. doi: 10.1021/jf0635154. [DOI] [PubMed] [Google Scholar]
- 81.Kim E.O., Min K.J., Kwon T.K., Um B.H., Moreau R.A., Choi S.W. Anti-inflammatory activity of hydroxycinnamic acid derivatives isolated from corn bran in lipopolysaccharide-stimulated Raw 264.7 macrophages. Food Chem. Toxicol. 2012;50(5):1309–1316. doi: 10.1016/j.fct.2012.02.011. https://www.sciencedirect.com/science/article/pii/S0278691512000944 Available from: [DOI] [PubMed] [Google Scholar]
- 82.ten Brink B., Damink C., Joosten H.M.L.J., Huis in ’t Veld J.H.J. Occurrence and formation of biologically active amines in foods. Int. J. Food Microbiol. 1990;11(1):73–84. doi: 10.1016/0168-1605(90)90040-c. https://www.sciencedirect.com/science/article/pii/016816059090040C Available from: [DOI] [PubMed] [Google Scholar]
- 83.Ladero V., Calles-Enriquez M., Fernandez M., Alvarez M A. Toxicological effects of dietary biogenic amines. Curr. Nutr. Food Sci. 2010;6:145–156. http://www.eurekaselect.com/node/86193/article Available from: [Google Scholar]
- 84.Moinard C., Cynober L., de Bandt J.-P. Polyamines: metabolism and implications in human diseases. Clin. Nutr. 2005;24(2):184–197. doi: 10.1016/j.clnu.2004.11.001. https://www.sciencedirect.com/science/article/pii/S0261561404001967 Available from: [DOI] [PubMed] [Google Scholar]
- 85.Nakamura Y., Ishimaru K., Shibata S., Nakao A. Regulation of plasma histamine levels by the mast cell clock and its modulation by stress. Sci. Rep. 2017;7:1–12. doi: 10.1038/srep39934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sagratini G., Fernández-Franzón M., De Berardinis F., Font G., Vittori S., Mañes J. Simultaneous determination of eight underivatised biogenic amines in fish by solid phase extraction and liquid chromatography–tandem mass spectrometry. Food Chem. 2012;132(1):537–543. doi: 10.1016/j.foodchem.2011.10.054. https://www.sciencedirect.com/science/article/pii/S0308814611015068 Available from: [DOI] [PubMed] [Google Scholar]
- 87.Wunderlichová L., Buňková L., Koutný M., Jančová P., Buňka F. Formation, degradation, and detoxification of putrescine by foodborne bacteria: a review. Compr. Rev. Food Sci. Food Saf. 2014 Sep 1;13(5):1012–1030. [Google Scholar]
- 88.Wong H.L., Murphy S.E., Wang M., Hecht S.S. Comparative metabolism of N- nitrosopiperidine and N- nitrosopyrrolidine by rat liver and esophageal microsomes and cytochrome P450 2A3. Carcinogenesis. 2003 Feb 1;24(2):291–300. doi: 10.1093/carcin/24.2.291. [DOI] [PubMed] [Google Scholar]
- 89.Drabik-Markiewicz G., Dejaegher B., De Mey E., Kowalska T., Paelinck H., Vander Heyden Y. Influence of putrescine, cadaverine, spermidine or spermine on the formation of N-nitrosamine in heated cured pork meat. Food Chem. 2011;126(4):1539–1545. doi: 10.1016/j.foodchem.2010.11.149. https://www.sciencedirect.com/science/article/pii/S0308814610015773 Available from: [DOI] [PubMed] [Google Scholar]
- 90.Wu Y., Qin L., Chen J., Wang H., Liao E. Nitrite, biogenic amines and volatile N-nitrosamines in commercial Chinese traditional fermented fish products. Food Addit. Contam. Part B. 2021 Aug;24:1–10. doi: 10.1080/19393210.2021.1971303. [DOI] [PubMed] [Google Scholar]
- 91.Sallan S., Kaban G., Şişik Oğraş Ş., Çelik M., Kaya M. Nitrosamine formation in a semi-dry fermented sausage: effects of nitrite, ascorbate and starter culture and role of cooking. Meat Sci. 2020;159 doi: 10.1016/j.meatsci.2019.107917. https://www.sciencedirect.com/science/article/pii/S0309174019300397 Available from: [DOI] [PubMed] [Google Scholar]
- 92.Gushgari A.J., Halden R.U. Critical review of major sources of human exposure to N-nitrosamines. Chemosphere. 2018;210:1124–1136. doi: 10.1016/j.chemosphere.2018.07.098. https://www.sciencedirect.com/science/article/pii/S0045653518313626 Available from: [DOI] [PubMed] [Google Scholar]
- 93.Giménez-Campillo C., Pastor-Belda M., Campillo N., Hernández J.D., Guillén I., Vizcaíno P., et al. Ultrasound assisted extraction approach to test the effect of elastic rubber nettings on the N-nitrosamines content of Ham meat samples. Foods. 2021;10 doi: 10.3390/foods10112564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Önal A. A review: current analytical methods for the determination of biogenic amines in foods. Food Chem. 2007;103(4):1475–1486. [Google Scholar]
- 95.Biji K.B., Ravishankar C.N., Venkateswarlu R., Mohan C.O., Gopal T.K.S. Biogenic amines in seafood: a review. J. Food Sci. Technol. 2016;53(5):2210–2218. doi: 10.1007/s13197-016-2224-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Sun Q., Sun F., Zheng D., Kong B., Liu Q. Complex starter culture combined with vacuum packaging reduces biogenic amine formation and delays the quality deterioration of dry sausage during storage. Food Cont. 2019;100:58–66. https://www.sciencedirect.com/science/article/pii/S0956713519300155 Available from: [Google Scholar]
- 97.Sun Q., Chen Q., Li F., Zheng D., Kong B. Biogenic amine inhibition and quality protection of Harbin dry sausages by inoculation with Staphylococcus xylosus and Lactobacillus plantarum. Food Cont. 2016;68:358–366. https://www.sciencedirect.com/science/article/pii/S0956713516301918 Available from: [Google Scholar]
- 98.Mah J.-H., Park Y., Jin Y., Lee J.-H., Hwang H.-J. Bacterial production and control of biogenic amines in asian fermented soybean foods. Foods. 2019 Feb 25;8(2):85. doi: 10.3390/foods8020085. https://www.mdpi.com/2304-8158/8/2/85 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Xia X., Luo Y., Zhang Q., Huang Y., Zhang B. Mixed starter culture regulates biogenic amines formation via decarboxylation and transamination during Chinese rice wine fermentation. J. Agric. Food Chem. 2018 Jun 27;66(25):6348–6356. doi: 10.1021/acs.jafc.8b01134. [DOI] [PubMed] [Google Scholar]
- 100.Zhao J., Niu C., Du S., Liu C., Zheng F., Wang J., et al. Reduction of biogenic amines formation during soybean paste fermentation by using staphylococcus Carnosus M43 and Pediococcus acidilactici M28 as starter culture. LWT. 2020;133 https://www.sciencedirect.com/science/article/pii/S0023643820309063 Available from: [Google Scholar]
- 101.Basílio L.S.P., Vanz Borges C., Minatel I.O., Vargas P.F., Tecchio M.A., Vianello F., et al. New beverage based on grapes and purple-fleshed sweet potatoes: use of non-standard tubers. Food Biosci. 2022;47 https://www.sciencedirect.com/science/article/pii/S2212429222000852 Available from: [Google Scholar]
- 102.Li Y., Lei Y., Tan Y., Zhang J., Hong H., Luo Y. Efficacy of freeze-chilled storage combined with tea polyphenol for controlling melanosis, quality deterioration, and spoilage bacterial growth of pacific white shrimp (Litopenaeus Vannamei) Food Chem. 2022 doi: 10.1016/j.foodchem.2021.130924. https://www.sciencedirect.com/science/article/pii/S0308814621019300 370:130924. Available from: [DOI] [PubMed] [Google Scholar]
- 103.Hernández-Macias S., Martín-Garcia A., Ferrer-Bustins N., Comas-Basté O., Riu-Aumatell M., López-Tamames E., et al. Inhibition of biogenic amines formation in fermented foods by the addition of cava lees. Front Microbiol. 2022:12. doi: 10.3389/fmicb.2021.818565. https://www.frontiersin.org/article/10.3389/fmicb.2021.818565 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Mah J.-H., Kim Y.J., Hwang H.-J. Inhibitory effects of garlic and other spices on biogenic amine production in Myeolchi-jeot, Korean salted and fermented anchovy product. Food Cont. 2009;20(5):449–454. https://www.sciencedirect.com/science/article/pii/S0956713508001783 Available from: [Google Scholar]
- 105.Jia W., Zhang R., Shi L., Zhang F., Chang J., Chu X. Effects of spices on the formation of biogenic amines during the fermentation of dry fermented mutton sausage. Food Chem. 2020;321 doi: 10.1016/j.foodchem.2020.126723. https://www.sciencedirect.com/science/article/pii/S0308814620305859 Available from: [DOI] [PubMed] [Google Scholar]
- 106.Sun Q., Zhao X., Chen H., Zhang C., Kong B. Impact of spice extracts on the formation of biogenic amines and the physicochemical, microbiological and sensory quality of dry sausage. Food Cont. 2018;92:190–200. https://www.sciencedirect.com/science/article/pii/S0956713518302275 Available from: [Google Scholar]
- 107.Wang Y., Li F., Zhuang H., Chen X., Li L., Qiao W., et al. Effects of plant polyphenols and α-tocopherol on lipid oxidation, residual nitrites, biogenic amines, and N-nitrosamines formation during ripening and storage of dry-cured bacon. LWT--Food Sci. Technol. 2015;60(1):199–206. https://www.sciencedirect.com/science/article/pii/S0023643814005775 Available from: [Google Scholar]
- 108.Maijala R.L., Eerola S.H., Aho M.A., Hirn J.A. The Effect of GDL-induced pH decrease on the formation of biogenic amines in meat. J Food Prot [Internet] 1993 Feb 1;56(2):125–129. doi: 10.4315/0362-028X-56.2.125. [DOI] [PubMed] [Google Scholar]
- 109.Shalaby A.R. Significance of biogenic amines to food safety and human health. Food Res. Int. 1996;29(7):675–690. https://www.sciencedirect.com/science/article/pii/S096399699600066X Available from: [Google Scholar]
- 110.Cho J., Kim H.-J., Kwon J.-S., Kim H.-J., Jang A. Effect of marination with black currant juice on the formation of biogenic amines in pork belly during refrigerated storage. Food Sci. Anim. Resour. 2021 Sep;41(5):763–778. doi: 10.5851/kosfa.2021.e34. https://pubmed.ncbi.nlm.nih.gov/34632397 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Huang Y., Yu H., Lu S., Zou L., Tang Z., Zeng T., et al. Effect and mechanism of ferulic acid inclusion complexes on tyramine production by Enterobacter hormaechei MW386398 in smoked horsemeat sausages. Food Biosci. 2022;46 https://www.sciencedirect.com/science/article/pii/S2212429221006453 Available from: [Google Scholar]
- 112.Qian Y., Yang S., Ye J., Xie J. Effect of quercetin-containing preservatives and modified atmospheric packaging on the production of biogenic amines in Pacific white shrimp (Litopenaeus vannamei) Aquac. Fish. 2018;3(6):254–259. https://www.sciencedirect.com/science/article/pii/S2468550X17300928 Available from: [Google Scholar]
- 113.Wu C., Li Y., Wang L., Hu Y., Chen J., Liu D., et al. Efficacy of chitosan-gallic acid coating on shelf life extension of refrigerated pacific mackerel fillets. Food Bioproc. Technol. 2016;9(4):675–685. [Google Scholar]
- 114.Wang J., Fang J., Wei L., Zhang Y., Deng H., Guo Y., et al. Decrease of microbial community diversity, biogenic amines formation, and lipid oxidation by phloretin in Atlantic salmon fillets. LWT. 2019;101:419–426. https://www.sciencedirect.com/science/article/pii/S0023643818309903 Available from: [Google Scholar]
- 115.Lee J.-Y., Kim Y., Her J.-Y., Kim M.K., Lee K.-G. Reduction of biogenic amine contents in fermented soybean paste using food additives. LWT. 2018;98:470–476. https://www.sciencedirect.com/science/article/pii/S0023643818307473 Available from: [Google Scholar]
- 116.Yamashita K., Suzuki Y., Matsui T., Yoshimaru T., Yamaki M., Suzuki-Karasaki M., et al. Epigallocatechin gallate inhibits histamine release from rat basophilic leukemia (RBL-2H3) cells: role of tyrosine phosphorylation pathway. Biochem. Biophys. Res. Commun. 2000;274(3):603–608. doi: 10.1006/bbrc.2000.3200. https://www.sciencedirect.com/science/article/pii/S0006291X00932005 Available from: [DOI] [PubMed] [Google Scholar]
- 117.Rodríguez-Caso C., Rodríguez-Agudo D., Sánchez-Jiménez F., Medina M.A. Green tea epigallocatechin-3-gallate is an inhibitor of mammalian histidine decarboxylase. Cell Mol. Life Sci. C. 2003;60(8):1760–1763. doi: 10.1007/s00018-003-3135-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118.Melgarejo E., Urdiales J.L., Sánchez-Jiménez F., Medina M.Á. Targeting polyamines and biogenic amines by green tea epigallocatechin-3-gallate. Amino Acids. 2010;38(2):519–523. doi: 10.1007/s00726-009-0411-z. [DOI] [PubMed] [Google Scholar]
- 119.Bertoldi M., Gonsalvi M., Borri Voltattorni C. Green tea polyphenols: novel irreversible inhibitors of dopa decarboxylase. Biochem. Biophys. Res. Commun. 2001;284(1):90–93. doi: 10.1006/bbrc.2001.4945. https://www.sciencedirect.com/science/article/pii/S0006291X01949459 Available from: [DOI] [PubMed] [Google Scholar]
- 120.Paul B., Hayes C.S., Kim A., Athar M., Gilmour S.K. Elevated polyamines lead to selective induction of apoptosis and inhibition of tumorigenesis by (−)-epigallocatechin-3-gallate (EGCG) in ODC/Ras transgenic mice. Carcinogenesis. 2005 Jan 1;26(1):119–124. doi: 10.1093/carcin/bgh281. [DOI] [PubMed] [Google Scholar]
- 121.Agarwal R., Katiyar S.K., Zaidi S.I.A., Mukhtar H. Inhibition of skin tumor promoter-caused induction of epidermal ornithine decarboxylase in SENCAR mice by polyphenolic fraction isolated from green tea and its individual epicatechin derivatives. Cancer Res. 1992 Jul 1;52(13):3582. http://cancerres.aacrjournals.org/content/52/13/3582.abstract Available from: [PubMed] [Google Scholar]
- 122.Biradar S.M., Joshi H., Chheda T.K. Neuropharmacological effect of Mangiferin on brain cholinesterase and brain biogenic amines in the management of Alzheimer’s disease. Eur. J. Pharmacol. 2012;683(1):140–147. doi: 10.1016/j.ejphar.2012.02.042. https://www.sciencedirect.com/science/article/pii/S0014299912002063 Available from: [DOI] [PubMed] [Google Scholar]
- 123.Ibarretxe G., Sánchez-Gómez M.V., Campos-Esparza M.R., Alberdi E., Matute C. Differential oxidative stress in oligodendrocytes and neurons after excitotoxic insults and protection by natural polyphenols. Glia. 2006 Jan 15;53(2):201–211. doi: 10.1002/glia.20267. [DOI] [PubMed] [Google Scholar]
- 124.Blosser J.C., McCreedy S., Parker R.B., Kinsolving C.R., Watkins B.E., Wu E.S. Flavodilol: a new antihypertensive agent that preferentially depletes peripheral biogenic amines. J. Cardiovasc. Pharmacol. 1989;14(1):142–156. http://europepmc.org/abstract/MED/2475706 Available from: [PubMed] [Google Scholar]
- 125.Badr A.M., Attia H.A., Al-Rasheed N. Oleuropein reverses repeated corticosterone-induced depressive-like behavior in mice: evidence of modulating effect on biogenic amines. Sci. Rep. 2020;10(1):3336. doi: 10.1038/s41598-020-60026-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Pal S., Sarkar C. Protective effect of resveratrol on fluoride induced alteration in protein and nucleic acid metabolism, DNA damage and biogenic amines in rat brain. Environ. Toxicol. Pharmacol. 2014;38(2):684–699. doi: 10.1016/j.etap.2014.07.009. https://www.sciencedirect.com/science/article/pii/S138266891400163X Available from: [DOI] [PubMed] [Google Scholar]
- 127.Ramis M.R., Sarubbo F., Tejada S., Jiménez M., Esteban S., Miralles A., et al. Chronic polyphenon-60 or catechin treatments increase brain monoamines syntheses and hippocampal SIRT1 levels improving cognition in aged rats. Nutrients. 2020;12 doi: 10.3390/nu12020326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Ramis M.R., Sarubbo F., Moranta D., Tejada S., Lladó J., Miralles A., et al. Neurochemical and cognitive beneficial effects of moderate physical activity and catechin in aged rats. Antioxidants. 2021;10 doi: 10.3390/antiox10040621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Vianello R., Repič M., Mavri J. How are biogenic amines metabolized by monoamine oxidases? European J. Org. Chem. 2012 Dec 1;2012(36):7057–7065. [Google Scholar]
- 130.Agostinelli E., Arancia G., Vedova L.D., Belli F., Marra M., Salvi M., et al. The biological functions of polyamine oxidation products by amine oxidases: perspectives of clinical applications. Amino Acids. 2004;27(3):347–358. doi: 10.1007/s00726-004-0114-4. [DOI] [PubMed] [Google Scholar]
- 131.Rajeswari A., Sabesan M. Inhibition of monoamine oxidase-B by the polyphenolic compound, curcumin and its metabolite tetrahydrocurcumin, in a model of Parkinson’s disease induced by MPTP neurodegeneration in mice. Inflammopharmacology. 2008;16(2):96–99. doi: 10.1007/s10787-007-1614-0. [DOI] [PubMed] [Google Scholar]
- 132.Bhutani M.K., Bishnoi M., Kulkarni S.K. Anti-depressant like effect of curcumin and its combination with piperine in unpredictable chronic stress-induced behavioral, biochemical and neurochemical changes. Pharmacol. Biochem. Behav. 2009;92(1):39–43. doi: 10.1016/j.pbb.2008.10.007. https://www.sciencedirect.com/science/article/pii/S009130570800350X Available from: [DOI] [PubMed] [Google Scholar]
- 133.Watson A.W., Haskell-Ramsay C.F., Kennedy D.O., Cooney J.M., Trower T., Scheepens A. Acute supplementation with blackcurrant extracts modulates cognitive functioning and inhibits monoamine oxidase-B in healthy young adults. J. Funct. Foods. 2015;17:524–539. https://www.sciencedirect.com/science/article/pii/S1756464615002893 Available from: [Google Scholar]
- 134.Gossé F., Roussi S., Guyot S., Schoenfelder A., Mann A., Bergerat J.-P., et al. Potentiation of apple procyanidin-triggered apoptosis by the polyamine oxidase inactivator MDL 72527 in human colon cancer-derived metastatic cells. Int. J. Oncol. 2006 Aug 1;29(2):423–428. http://www.ncbi.nlm.nih.gov/pubmed/16820885 Available from: [PubMed] [Google Scholar]
- 135.Naoi M., Maruyama W. Monoamine oxidase inhibitors as neuroprotective agents in age-dependent neurodegenerative disorders. Curr. Pharmaceut. Des. 2010;16(25):2799–2817. doi: 10.2174/138161210793176527. [DOI] [PubMed] [Google Scholar]
- 136.Zhou X., Qiu M., Zhao D., Lu F., Ding Y. Inhibitory effects of spices on biogenic amine accumulation during fish sauce fermentation. J. Food Sci. 2016 Apr 1;81(4):M913–M920. doi: 10.1111/1750-3841.13255. [DOI] [PubMed] [Google Scholar]
- 137.Erol T., Özdestan Ocak Ö. Influence of pomegranate seed extract on the formation of biogenic amines in a cereal based fermented food: Tarhana. J. Food Sci. Technol. 2020;57(12):4492–4500. doi: 10.1007/s13197-020-04486-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 138.Li J. Application of tea polyphenols in combination with 6-gingerol on shrimp paste of during storage: biogenic amines formation and quality determination. Front Microbiol. 2015:6. doi: 10.3389/fmicb.2015.00981. https://www.frontiersin.org/article/10.3389/fmicb.2015.00981 Available from: [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Kongkiattikajorn J. Effect of ginger extract to inhibit biogenic amines accumulation during nham fermentation. J. Food Chem Nanotechnol. 2015;1(1):15–19. http://unitedscientificgroup.com/journals/ets/articles/v1n1/jfcn-003-jirasak-kongkiattikajorn.pdf Available from: [Google Scholar]
- 140.Bozkurt H. Utilization of natural antioxidants: green tea extract and Thymbra spicata oil in Turkish dry-fermented sausage. Meat Sci. 2006;73(3):442–450. doi: 10.1016/j.meatsci.2006.01.005. https://www.sciencedirect.com/science/article/pii/S0309174006000271 Available from: [DOI] [PubMed] [Google Scholar]
- 141.Tang H., Darwish W.S., El-Ghareeb W.R., Al-Humam N.A., Chen L., Zhong R.-M., et al. Microbial quality and formation of biogenic amines in the meat and edible offal of Camelus dromedaries with a protection trial using gingerol and nisin. Food Sci. Nutr. 2020 Apr 1;8(4):2094–2101. doi: 10.1002/fsn3.1503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Peiretti P.G., Gai F., Ortoffi M., Aigotti R., Medana C. Effects of rosemary oil (Rosmarinus Officinalis) on the shelf-life of minced rainbow trout (Oncorhynchus mykiss) during refrigerated storage. Foods. 2012;1 doi: 10.3390/foods1010028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Özogul F., Kuley E., Kenar M. Effects of rosemary and sage tea extract on biogenic amines formation of sardine (Sardina pilchardus) fillets. Int. J. Food Sci. Technol. 2011 Apr 1;46(4):761–766. [Google Scholar]
- 144.Houicher A., Kuley E., Özogul F., Bendeddouche B. Effect of natural extracts (mentha spicata L. And artemisia campestris) on biogenic amine formation of sardine vacuum-packed and refrigerated (sardina pilchardus) fillets. J. Food Proc. Preserv. 2015 Dec 1;39(6):2393–2403. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Data included in article/supp. material/referenced in article.