Abstract
Background
Artificial Intelligence (AI) has improved the way of looking at technological challenges. Today, we can afford to see many of the problems as just an input-output system rather than solving from the first principles. The field of Orthopaedics is not spared from this rapidly expanding technology. The recent surge in the use of AI can be attributed mainly to advancements in deep learning methodologies and computing resources. This review was conducted to draw an outline on the role of AI in orthopaedics.
Methods
We developed a search strategy and looked for articles on PubMed, Scopus, and EMBASE. A total of 40 articles were selected for this study, from tools for medical aid like imaging solutions, implant management, and robotic surgery to understanding scientific questions.
Results
A total of 40 studies have been included in this review. The role of AI in the various subspecialties such as arthroplasty, trauma, orthopaedic oncology, foot and ankle etc. have been discussed in detail.
Conclusion
AI has touched most of the aspects of Orthopaedics. The increase in technological literacy, data management plans, and hardware systems, amalgamated with the access to hand-held devices like mobiles, and electronic pads, augur well for the exciting times ahead in this field. We have discussed various technological breakthroughs in AI that have been able to perform in Orthopaedics, and also the limitations and the problem with the black-box approach of modern AI algorithms. We advocate for better interpretable algorithms which can help both the patients and surgeons alike.
Keywords: Artificial intelligence, Deep learning, Machine learning, Orthopaedics, Trauma, Arthroplasty
1. Introduction
Artificial intelligence (AI) are machines and algorithms which are supervised, simulate intellectuality like a human, and have rational thinking and self-rectifying potential.1 One of the major responsibilities in the health delivery system is providing a better quality of care. Artificial intelligence has a broad spectrum of applications in medical science, helping in providing improved healthcare delivery and minimizing medical errors. In orthopaedics, AI helps in the careful evaluation of radiological images, training of surgical residents, and excellent performance of machine-assisted surgeries.2,3
In 1956, Prof. John McCarthy put forward the idea of AI, to reproduce the intelligence of humans using a machine/computer with minimal human involvement.1,4 AI was possible with the help of matching the patterns, algorithms, machine learning, and cognitive computing. It includes information, reasoning, and self-correction capability. With the recent advances in affordable computational power and rapid growth in unusual enormous data sets (“Big Data”), AI has blossomed from mere hypothesis to unquestionable implementation on an exceptional stratum.4
AI has a broad spectrum of subcomponents, including machine learning (ML), which consists of a subdivision called deep learning (DL).5,6 ML is an advanced subset of AI that uses complex computer-based algorithms to model complex relationships between variables.7 DL is a subset of AI that uses a network of neurons.8 It uses a combination of units wherein each unit is a neuron cell. With the evolution of technology and the growing use of AI, we feel AI is the future of patient management in Orthopaedics and trauma. This technology has been used in various subspecialties of Orthopaedics successfully. Numerous papers have explained the use of AI. However, only a few systematic reviews highlight AI's role in Orthopaedics and Trauma.9,10 These have been highly focused studies that have not delineated the complete scope of AI in the vast specialty of Orthopaedics. Hence, this study was conducted to outline the role of AI in the different subspecialities of orthopedics with relevant examples, and state their specific merits and demerits, if any.
2. Methodology
This review was conducted in accordance with PRISMA guidelines.
2.1. Sources of information and literature search
To understand and review the role of AI in Orthopaedics, a thorough search was conducted in Scopus, Embase, and PubMed databases using keywords as summarized in Table 1.
Table 1.
Search strategy and keywords used.
| Search strategy | Keywords and MeSH terms |
|---|---|
| 1 |
artificial intelligence artificial intelligence"[MeSH Terms] OR (“artificial"[All Fields] AND “intelligence"[All Fields]) OR “artificial intelligence"[All Fields] |
| 2 |
machine learning “machine learning"[MeSH Terms] OR (“machine"[All Fields] AND “learning"[All Fields]) OR “machine learning"[All Fields] |
| 3 |
deep learning “deep learning"[MeSH Terms] OR (“deep"[All Fields] AND “learning"[All Fields]) OR “deep learning"[All Fields] |
| 4 |
((artificialintelligence) OR (machinelearning)) OR (deeplearning) “artificialintelligence"[All Fields] OR “machinelearning"[All Fields] OR “deeplearning"[All Fields] |
| 5 | (orthopaedics) OR (orthopedics) |
| 6 |
(((artificialintelligence) OR (machinelearning)) OR (deeplearning)) AND ((orthopaedics) OR (orthopedics)) (“artificialintelligence"[All Fields] OR “machinelearning"[All Fields] OR “deeplearning"[All Fields]) AND (“orthopaedic"[All Fields] OR “orthopedics"[MeSH Terms] OR “orthopedics"[All Fields] OR “orthopedic"[All Fields] OR “orthopedical"[All Fields] OR “orthopedical"[All Fields] OR “orthopaedics"[All Fields] OR (“orthopaedic"[All Fields] OR “orthopedics"[MeSH Terms] OR “orthopedics"[All Fields] OR “orthopedic"[All Fields] OR “orthopedical"[All Fields] OR “orthopedical"[All Fields] OR “orthopaedics"[All Fields])) |
2.2. Selection of studies
Inclusion Criteria:
-
•
Studies which have reported the use of AI in the diagnosis of Orthopaedic diseases.
-
•
Studies that have investigated the use of AI in Orthopaedic surgeries.
Exclusion Criteria:
-
•
Studies in languages other than English.
-
•
Studies that did not report the use of AI.
-
•
Review articles
-
•
Studies reporting the use of AI in fields other than Orthopaedics and trauma.
Deduplicated results from the search were reviewed independently by all the authors. Titles were screened first, followed by the screening of the abstracts. Then the full texts of the shortlisted articles were obtained. Studies were included only if they met the prespecified inclusion criteria. If there was a conflict regarding the inclusion of a particular study, it was resolved by mutual consent.
After finalizing eligible studies, data was extracted by two independent reviewers into pre-defined spreadsheets. Data extracted included the name of the first author, study design, number of cases and demographic data, the subspeciality where AI was used, and relevant outcome parameters. Since the data was found to be heterogenous in nature, a formal meta-analysis could not be conducted, and only qualitative synthesis was done.
3. Results
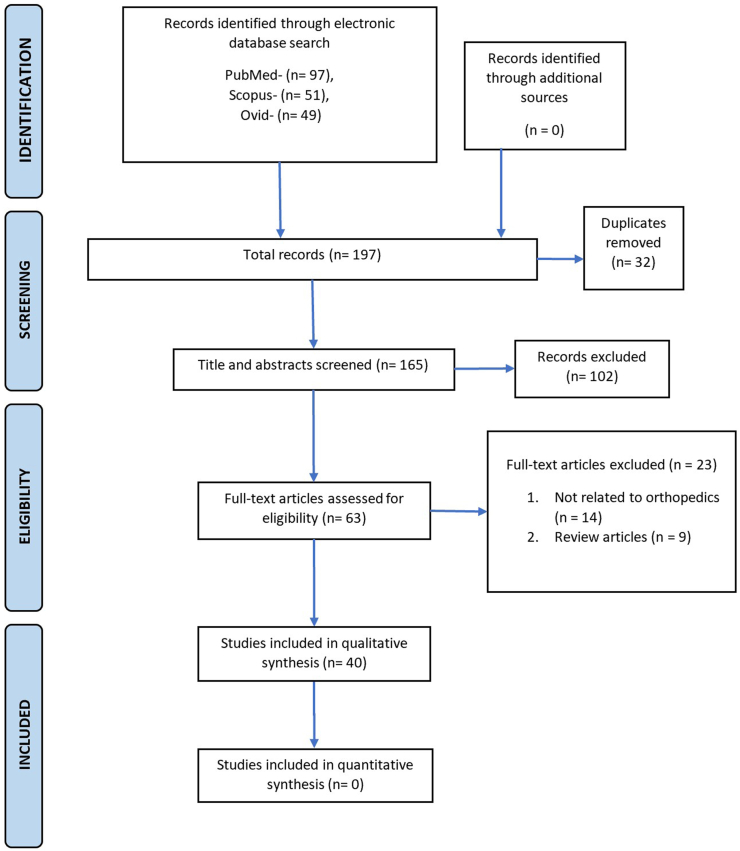
Our primary and secondary searches yielded 197 results. After an initial assessment, we zeroed down on 63 studies. The full texts of these files were obtained. Out of the 63, we included 40 articles in this study. The full text of the articles were reviewed to summarize the current status and role of AI in Orthopaedics.
3.1. Artificial intelligence in orthopaedics
AI has also been used in the medical field in upgrading professionals and their clinical performance, individualizing management and care of the patients, and advancing the scope of research.11,12 The research centers on two data set types: single-level (individual) and community-based (population) datasets. Individual based datasets are acquired from research on the analysis of gait by tracking skeletal kinematics and sophisticated radiological evaluation to forecast the early onset of degeneration of major joints (hip and knee). Here AI has the possibility of predicting the early onset of disease, earliest detection of disease, determining disease evolution and course, assisting in treatment protocol with higher performance. At a community level, there has been great accessibility of information (National Joint Registry, National Hip Fracture Database, etc.) which increases the probability of projecting orthopaedics specific patient results including implant longevity, functional and radiological follow-up scores, the possibility of drawbacks/disabilities, duration of hospital stay, and many more. The objective of such research is two-folded: to advise higher quality of shared decision making, and at a radical and financial level, to ensure the efficacious coordination and execution of health-related undertakings.
Rather than replacing treating physicians, AI assists/helps in increasing the accuracy of diagnosis and minimizing medical errors. AI has been used widely in medical imaging, from the acquisition of data, analysis, and interpretation.2 By feeding the particulars/data available in the patient's health document/record (clinical presentation, clinical signs, blood tests), AI suggests appropriate management like required radiological investigations and treatment guidelines.13 AI is helpful in swift data acquisition of magnetic resonance imaging (MRI) and reducing the radiation dose from computed tomography (CT).14
AI algorithms have been applied in orthopaedic surgery in various conditions, including diagnosis of fractures, osteoarthritis of hip and knee, and determining the age and strength of bone.2 Apart from diagnosis, AI has higher accuracy (95.6%) in labeling, and detection and helps in the grading of conditions, like intervertebral disc prolapse, using classification systems on MRI.15 AI also provides the advantage of improving quantitative image analysis by allowing automatic segmentation of the area of interest; however, it requires vast information/data for training, which is expensive and reduces service equality. One of the major challenges faced during clinical practice is risk assessment and predicting outcomes, AI provides a different approach that helps in overcoming the challenges during clinical practice. ML can be used to guide the management of patients by providing a patient-specific post-operative complication following lumbar surgery.16
AI technique assists the treating physician in making a decision or diagnosis for the patients. The cognitive computing system has used ML approaches to create a decision support system for the treatment of cancer in the western world. Clinical decision support helps in the diagnosis and providing treatment of patients with low back pain.17 These systems help find faults in the existing system and environment and provide reasonable approaches and information to help in minimizing human errors.
The main areas in Orthopaedics where AI has played a role:
-
1
. Arthroplasty: The first use of this technology dates back to 1992, when ROBODOC was used in Total Hip Arthroplasty (THA) for making femoral cuts.18 Various other systems like CASPAR and RIO have been used to make cuts in Total Knee Arthroplasty (TKA). These tools have not only made the surgery more accurate but also have reduced surgical time and blood loss. Borjali et al. created an algorithm that can diagnose implant loosening after THA.19 Recently, an AI-based model was designed that could help diagnose a prosthetic joint infection.20 Nowadays, the arthroplasty registries are also maintained with the help of AI, which has improved the data storage and patient monitoring and follow-up.21
-
2.
Ligament injury: Labbe et al. developed a support vector machine that could grade the pivot shift phenomenon using sensors placed on the iliac crest, femur, and tibia.22 Bien et al. developed an algorithm that could diagnose intra-articular pathology in the knee like meniscal injuries and cruciate injuries.23 Several algorithms have been developed that can diagnose injuries to cruciate ligaments based on MRI images with great accuracy.24, 25, 26 The results of these algorithms are easily reproducible and reliable. Li et al. developed another model that could diagnose ACL injuries based on plantar pressure monitoring.27 Jonmohamadi et al. created an algorithm called U-NET, which utilizes the arthroscopy video to create a segmented real-time image of structures seen by the surgeon.28 This could help human surgical training and tool tracking in future robotic arthroscopic applications.28
-
3.
Trauma: Beyaz et al. created a Convolutional Neural Network (CNN) model-based deep learning algorithm to diagnose femoral neck fractures.29 Bae et al. used residual neural network (ResNet) 18 with convolutional block attention module (CBAM) + + to detect the femoral neck fractures in radiographs.30 A similar CNN model was developed by Sato et al. for detecting adult hip fractures.3 Langerhuizen et al. created a deep learning model for the diagnosis of occult scaphoid fractures and the results were encouraging.31 Li et al. developed an artificial intelligence-based model for detecting fractures in the lumbar spine. The model demonstrated excellent accuracy, sensitivity, and specificity.32In 2016, a multi-indication Orthopaedics surgical robot, TianJi, was used for multiple levels of spine fixation, fractures of the pelvis, acetabulum, and long bones.33, 34, 35 Lind et al. designed a ResNet-based neural network that could not only identify injuries around the knee joint but also classify them based on AO/OTA classification system.36 There have also been algorithms that can diagnose and classify calcaneal fractures.37
-
4.
Spine surgery: Renaissance and the Rosa robots used in spinal surgeries have shown higher accuracy of pedicle screw placement and limited radiation exposure to the patients and operating team. These systems can assist the surgeons in placing pedicle screws in deformed cases also.
-
5.
Musculoskeletal Oncology: Lodwick et al. made the first attempt to diagnose bone tumors using a computer program in 1963.38 Since then, numerous advancements have taken place in the field of artificial intelligence to improve the diagnosis and treatment of musculoskeletal oncology. Nevertheless, the use of AI is still limited to the diagnosis of tumors only. Burns et al. successfully identified sclerotic lesions in the thoracolumbar spine using Computer-Aided Design (CADe) techniques on CT scan.39 Wang et al. created a CNN-based model that could detect spinal metastasis.40 Bandyopadhyay et al. created a CADx-based model that could accurately predict bone-destruction pattern, stage, and grade of cancer in 85% of cases.41 Do et al. created an AI-based model that accurately detects the presence of bone tumors on a radiograph in most instances.42 Han et al. designed a model to predict survival in patients with synovial sarcoma.43
-
6.
Osteoarthritis (OA): Xue et al. created a CNN model to diagnose OA. This model predicted hip arthritis with great sensitivity and specificity.44 Conrozier et al. described an automated computer-based method to measure joint space width for early diagnosis of OA in radiographs.45 This method had excellent reproducibility and sensitivity.
-
7.
Femoroacetabular impingement (FAI): Nepple et al. built a computer-assisted model for diagnosing FAI and hip dysplasia.46 This model accurately predicted the change in articular surfaces and the arthritis onset.
-
8.
Foot And Ankle disorders: Ashkani-Eshfahani et al. used 2 deep convolutional neural network (Inception V3 and Renet 50) to detect ankle fractures using radiographs.47 Chae et al. developed a model for classifying foot types using the image and numerical foot pressure data.48 Day et al. described an AI-based tool to evaluate a new artificial intelligence (AI)-based automatic measurement for the M1-M2 intermetatarsal angle (IMA) in hallux valgus (HV) in patients undergoing weight-bearing cone beam CT scan. They compared them with manually measured IMA on digital radiographs.49 Guss et al. assessed the accuracy, sensitivity, and specificity of 3D volume WBCT evaluation using DCNN algorithms in patients with subtle syndesmotic instability.50 Merrill et al. used machine learning to predict the outcomes of ankle fractures post open reduction and internal fixation.51
3.2. Limitations of AI
An algorithm is as good as the data it is trained upon. AI only learns from the given data of the patient, and therefore accuracy of surgery depends upon the data given. AI technology only does what it has been programmed to do and these machines do not understand emotions, human thought, and reasons to make an accurate decision. It cannot fulfil the requirement of creative thinking in Orthopaedics like a human being because a human being can feel, think, and make a valuable decision. It requires supervision and data capturing during treatment and only reliable data can provide satisfactory results.
‘Explainable AI’ is one of the hottest topics and emerging concepts and it refers to an AI system in which the developers can understand the results of the solution. This is in contrast to ‘Blackbox machine learning, wherein the developer cannot explain how the machine arrived at the results nor analyze the concept behind arriving at the solution. One of the most important aspects that have gained ground in recent times is the effort toward developing an algorithm or system that can help achieve the AI system's needs in medical applications. It is essential for majorly two reasons, first, it helps in designing better algorithms and understanding the science behind them, and second, it helps in conveying the exact reasons for the consultative decisions to the ever-inquisitive and knowledgeable patient, (e.g., an algorithm clearly explaining the possible course of actions in a possible decision is both reassuring to the patient and the doctor). The explanation gains more importance in the case of deep learning (DL) algorithms where a large number of parameters are involved. DL algorithms have been successfully used in critical Orthopaedic applications such as detection of implants mechanical loosening,19 bone tumor diagnosis,42 bone disease stage grading.44 Although the DL algorithms have been shown to outperform the traditional ML algorithms, interpretability is a significant challenge. Recent algorithms such as shap, lime, and DeepShap are a step in this direction.52, 53, 54 The interpretability here is a major challenge and need of the hour because the DL algorithms are effective, and the AI field has gravitated towards this in cases where the exact reasons and causes can be affixed. Finally, there is a need for open repositories and crowdsourcing of the stakeholders (e.g., Computer Scientists and Doctors) to work in this direction so that algorithms, procedures, and legal aspects can be ironed out in case of AI algorithms making or aiding decisions.
4. Future directions
AI needs to meet the ever-increasing demands of an orthopaedic surgeon, and AI is needed in every field, right from diagnosis to treatment to detection of outcomes. Until now, most of the work in the field of AI has been on the diagnostic aspect. There is a need to build an algorithm to help a surgeon detect and intervene with unfavourable outcomes in the intraoperative period. Since most of the surgeries in Orthopaedics involving Artificial Intelligence and machines need human assistance, we can modify them and make them more independent. Using AI protocols in the intraoperative period will help a less experienced surgeon perform more complex surgeries with ease and minimal mistakes. In the field of oncology, a device should be able to detect the tumor margins to enable an R0 resection. In the future, we can also think of an AI that can interview patients, make a diagnosis, conduct relevant tests and reveal results.
5. Limitations of our study
We acknowledge the fact that there are limitations to our study. First and foremost, our search strategy included only three databases (PubMed, Scopus, and EMBASE); therefore, it is possible that some studies which were not indexed by these databases could have been missed. However, we chose PubMed and EMBASE databases as it has been shown that searching these two databases can yield more than 93% of the relevant literature.55 Moreover, increasing the number of databases also increases the number of records to be screened and may therefore not be practical.(Figure. 1)
Figure 1.
PRISMA Flowchart.
6. Conclusion
AI can aid in advancing the research and patient management in Orthopaedics significantly. Whatever the misgivings regarding interpretability, job loss, mechanization, etc., we believe that this novel technology is here to stay, and researchers, practitioners globally have started accepting it, driven either by the governments or private companies. AI currently plays a role in various subspecialties such as arthroplasty, spine surgery, oncology, trauma, foot and ankle and various other areas, which have been discussed in detail. However, it should be kept in mind that human interaction should not be drowned out in the glittering of technology.
Contribution of each author
1. Vishal Kumar: Proof reading.
2. Sandeep Patel: Conceptualized the idea, structure of manuscript.
3. Vishnu Baburaj: Wrote the manuscript.
4. Aditya Vardhan: Wrote the manuscript.
5. Prasoon Kumar Singh: Article search.
6. Raju Vaishya: Proof reading.
Declaration of competing interest
None.
Footnotes
This research did not receive any specific grant from public, commercial, or not-for-profit funding agencies.
Contributor Information
Vishal Kumar, Email: drkumarvishal@gmail.com.
Sandeep Patel, Email: sandeepdrpatelortho@gmail.com.
Vishnu Baburaj, Email: drvbms@gmail.com.
Aditya Vardhan, Email: vardhanguduru@gmail.com.
Prasoon Kumar Singh, Email: drprasoonksingh@gmail.com.
Raju Vaishya, Email: raju.vaishya@gmail.com.
References
- 1.Hamet P., Tremblay J. Artificial intelligence in medicine. Metabolism. 2017;69:S36–S40. doi: 10.1016/j.metabol.2017.01.011. [DOI] [PubMed] [Google Scholar]
- 2.Gyftopoulos S., Lin D., Knoll F., Doshi A.M., Rodrigues T.C., Recht M.P. Artificial intelligence in musculoskeletal imaging: current status and future directions. Am J Roentgenol. 2019;213:506–513. doi: 10.2214/AJR.19.21117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sato Y., Takegami Y., Asamoto T., et al. Artificial intelligence improves the accuracy of residents in the diagnosis of hip fractures: a multicenter study. BMC Muscoskel Disord. 2021:22. doi: 10.1186/s12891-021-04260-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Topol EJ. Deep Medicine: How Artificial Intelligence Can Make Healthcare Human Again. New York: Basic Books First edition.378 p.
- 5.A machine learning primer for clinicians–Part 1 – HIStalk. https://histalk2.com/2018/10/17/a-machine-learning-primer-for-clinicians-part-1/ [cited 2022 Jan 11]. Available from:
- 6.Cunha L. Deep learning with Python (2a ed) - françois chollet - Manning. outubro. 2021:504. Interações: Sociedade e as novas modernidades. 2022:113-115. [Google Scholar]
- 7.Martin I., Jakob M., Schäfer D., Dick W., Spagnoli G., Heberer M. Quantitative analysis of gene expression in human articular cartilage from normal and osteoarthritic joints. Osteoarthritis Cartilage. 2001;9:112–118. doi: 10.1053/joca.2000.0366. [DOI] [PubMed] [Google Scholar]
- 8.Kalmet P.H.S., Sanduleanu S., Primakov S., et al. Deep learning in fracture detection: a narrative review. Acta Orthop. 2020;91:215–220. doi: 10.1080/17453674.2019.1711323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ren M., Yi P.H. Artificial intelligence in orthopedic implant model classification: a systematic review. Skeletal Radiol. 2021:1–10. doi: 10.1007/s00256-021-03884-8. [DOI] [PubMed] [Google Scholar]
- 10.Langerhuizen D.W., Janssen S.J., Mallee W.H., et al. What are the applications and limitations of artificial intelligence for fracture detection and classification in orthopaedic trauma imaging? A systematic review. Clin Orthop Relat Res. 2019;477:2482. doi: 10.1097/CORR.0000000000000848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Panchmatia J.R., Visenio M.R., Panch T. The role of artificial intelligence in orthopaedic surgery. Br J Hosp Med. 2018;79:676–681. doi: 10.12968/hmed.2018.79.12.676. [DOI] [PubMed] [Google Scholar]
- 12.Tack C. Artificial intelligence and machine learning | applications in musculoskeletal physiotherapy. Musculoskeletal Science and Practice. 2019;39:164–169. doi: 10.1016/j.msksp.2018.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Lakhani P., Prater A.B., Hutson R.K., et al. Machine learning in radiology: applications beyond image interpretation. J Am Coll Radiol. 2018;15:350–359. doi: 10.1016/j.jacr.2017.09.044. [DOI] [PubMed] [Google Scholar]
- 14.Hammernik K., Klatzer T., Kobler E., et al. Learning a variational network for reconstruction of accelerated MRI data. Magn Reson Med. 2017;79:3055–3071. doi: 10.1002/mrm.26977. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jamaludin A., Lootus M., Kadir T., et al. Issls prize in bioengineering science 2017: automation of reading of radiological features from magnetic resonance images (MRIs) of the lumbar spine without human intervention is comparable with an expert radiologist. Eur Spine J. 2017;26:1374–1383. doi: 10.1007/s00586-017-4956-3. [DOI] [PubMed] [Google Scholar]
- 16.Kim J.S., Arvind V., Oermann E.K., et al. Predicting surgical complications in patients undergoing elective adult spinal deformity procedures using machine learning. Spine Deformity. 2018;6:762–770. doi: 10.1016/j.jspd.2018.03.003. [DOI] [PubMed] [Google Scholar]
- 17.Hill J.C., Dunn K.M., Lewis M., et al. A primary care back pain screening tool: identifying patient subgroups for initial treatment. Arthritis Rheum. 2008;59:632–641. doi: 10.1002/art.23563. [DOI] [PubMed] [Google Scholar]
- 18.Lang J.E., Mannava S., Floyd A.J., et al. Robotic systems in orthopaedic surgery. J Bone Joint Surg. 2011;93-B:1296–1299. doi: 10.1302/0301-620X.93B10.27418. [DOI] [PubMed] [Google Scholar]
- 19.Borjali A., Chen A.F., Muratoglu O.K., Morid M.A., Varadarajan K.M. Detecting total hip replacement prosthesis design on plain radiographs using deep convolutional neural network. J Orthop Res. 2020;38:1465–1471. doi: 10.1002/jor.24617. [DOI] [PubMed] [Google Scholar]
- 20.Klemt C., Laurencin S., Uzosike A.C., et al. Machine learning models accurately predict recurrent infection following revision total knee arthroplasty for periprosthetic joint infection. Knee Surg Sports Traumatol Arthrosc. 2021;30:2582–2590. doi: 10.1007/s00167-021-06794-3. [DOI] [PubMed] [Google Scholar]
- 21.Ramkumar P.N., Haeberle H.S., Bloomfield M.R., et al. Artificial intelligence and arthroplasty at a single institution: real-world applications of machine learning to big data, value-based care, mobile health, and remote patient monitoring. J Arthroplasty. 2019;34:2204–2209. doi: 10.1016/j.arth.2019.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Labbe D.R., de Guise J.A., Mezghani N., et al. Objective grading of the pivot shift phenomenon using a support vector machine approach. J Biomech. 2011;44:1–5. doi: 10.1016/j.jbiomech.2010.08.012. [DOI] [PubMed] [Google Scholar]
- 23.Bien N., Rajpurkar P., Ball R.L., et al. Deep-learning-assisted diagnosis for knee magnetic resonance imaging: development and retrospective validation of MRNet. PLoS Med. 2018;15 doi: 10.1371/journal.pmed.1002699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chang P.D., Wong T.T., Rasiej M.J. Deep learning for detection of complete anterior cruciate ligament tear. J Digit Imag. 2019;32:980–986. doi: 10.1007/s10278-019-00193-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Liu F., Guan B., Zhou Z., et al. Fully automated diagnosis of anterior cruciate ligament tears on knee MR images by using deep learning. Radiology: Artif Intell. 2019;1 doi: 10.1148/ryai.2019180091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Štajduhar I., Mamula M., Miletić D., Ünal G. Semi-automated detection of anterior cruciate ligament injury from MRI. Comput Methods Progr Biomed. 2017;140:151–164. doi: 10.1016/j.cmpb.2016.12.006. [DOI] [PubMed] [Google Scholar]
- 27.Li X., Huang H., Wang J., Yu Y., Ao Y. The analysis of plantar pressure data based on multimodel method in patients with anterior cruciate ligament deficiency during walking. BioMed Res Int. 2016;2016:1–12. doi: 10.1155/2016/7891407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Jonmohamadi Y., Takeda Y., Liu F., et al. Automatic segmentation of multiple structures in knee arthroscopy using deep learning. IEEE Access. 2020;8:51853–51861. [Google Scholar]
- 29.Beyaz S. Femoral neck fracture detection in X-ray images using deep learning and genetic algorithm approaches. Joint Diseases and Related Surgery. 2020;31:175–183. doi: 10.5606/ehc.2020.72163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bae J., Yu S., Oh J., et al. External validation of deep learning algorithm for detecting and visualizing femoral neck fracture including displaced and non-displaced fracture on plain X-ray. J Digit Imag. 2021;34:1099–1109. doi: 10.1007/s10278-021-00499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Langerhuizen D.W.G., Bulstra A.E.J., Janssen S.J., et al. Is deep learning on par with human observers for detection of radiographically visible and occult fractures of the scaphoid? Clin Orthop Relat Res. 2020;478:2653–2659. doi: 10.1097/CORR.0000000000001318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y.-C., Chen H.-H., Horng-Shing Lu H., Hondar Wu H.-T., Chang M.-C., Chou P.-H. Can a deep-learning model for the automated detection of vertebral fractures approach the performance level of human subspecialists? Clin Orthop Relat Res. 2021;479:1598–1612. doi: 10.1097/CORR.0000000000001685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Han X., Tian W., Liu Y., et al. Safety and accuracy of robot-assisted versus fluoroscopy-assisted pedicle screw insertion in thoracolumbar spinal surgery: a prospective randomized controlled trial. J Neurosurg Spine. 2019;30:615–622. doi: 10.3171/2018.10.SPINE18487. [DOI] [PubMed] [Google Scholar]
- 34.Tian W. Robot-assisted posterior C1–2 transarticular screw fixation for atlantoaxial instability. Spine. 2016;41:B2–B5. doi: 10.1097/BRS.0000000000001674. [DOI] [PubMed] [Google Scholar]
- 35.Tian W., Fan M.-X., Liu Y.-J. Robot-assisted percutaneous pedicle screw placement using three-dimensional fluoroscopy. Chinese Med J. 2017;130:1617–1618. doi: 10.4103/0366-6999.208251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Lind A., Akbarian E., Olsson S., et al. Artificial intelligence for the classification of fractures around the knee in adults according to the 2018 AO/OTA classification system. PLoS One. 2021;16 doi: 10.1371/journal.pone.0248809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Aghnia Farda N., Lai J.-Y., Wang J.-C., Lee P.-Y., Liu J.-W., Hsieh I.H. Sanders classification of calcaneal fractures in CT images with deep learning and differential data augmentation techniques. Injury. 2021;52:616–624. doi: 10.1016/j.injury.2020.09.010. [DOI] [PubMed] [Google Scholar]
- 38.Lodwick G.S., Haun C.L., Smith W.E., Keller R.F., Robertson E.D. Computer diagnosis of primary bone tumors. Radiology. 1963;80:273–275. [Google Scholar]
- 39.Burns J.E., Yao J., Wiese T.S., Muñoz H.E., Jones E.C., Summers R.M. Automated detection of sclerotic metastases in the thoracolumbar spine at CT. Radiology. 2013;268:69–78. doi: 10.1148/radiol.13121351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wang J., Fang Z., Lang N., Yuan H., Su M.-Y., Baldi P. A multi-resolution approach for spinal metastasis detection using deep Siamese neural networks. Comput Biol Med. 2017;84:137–146. doi: 10.1016/j.compbiomed.2017.03.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bandyopadhyay O., Biswas A., Bhattacharya B.B. Bone-cancer assessment and destruction pattern analysis in long-bone X-ray image. J Digit Imag. 2018;32:300–313. doi: 10.1007/s10278-018-0145-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Do B.H., Langlotz C., Beaulieu C.F. Bone tumor diagnosis using a naïve bayesian model of demographic and radiographic features. J Digit Imag. 2017;30:640–647. doi: 10.1007/s10278-017-0001-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Han I., Kim J.H., Park H., Kim H.-S., Seo S.W. Deep learning approach for survival prediction for patients with synovial sarcoma. Tumor Biol. 2018;40 doi: 10.1177/1010428318799264. [DOI] [PubMed] [Google Scholar]
- 44.Xue Y., Zhang R., Deng Y., Chen K., Jiang T. A preliminary examination of the diagnostic value of deep learning in hip osteoarthritis. PLoS One. 2017;12 doi: 10.1371/journal.pone.0178992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Conrozier T., Brandt K., Piperno M., Mathieu P., Merle-Vincent F., Vignon E. Reproducibility and sensitivity to change of a new method of computer measurement of joint space width in hip osteoarthritis. Performance of three radiographic views obtained at a 3-year interval. Osteoarthritis Cartilage. 2009;17:864–870. doi: 10.1016/j.joca.2008.12.003. [DOI] [PubMed] [Google Scholar]
- 46.Nepple J.J., Martell J.M., Kim Y.-J., et al. Interobserver and intraobserver reliability of the radiographic analysis of femoroacetabular impingement and dysplasia using computer-assisted measurements. Am J Sports Med. 2014;42:2393–2401. doi: 10.1177/0363546514542797. [DOI] [PubMed] [Google Scholar]
- 47.Ashkani-Esfahani S., Mojahed-Yazdi R., Bhimani R., et al. Assessment of ankle fractures using deep learning algorithms and convolutional neural network. Foot & Ankle Orthopaedics. 2022;7 doi: 10.1016/j.fas.2022.05.005. 2473011421S2473010009. [DOI] [PubMed] [Google Scholar]
- 48.Chae J., Kang Y.-J., Noh Y. A deep-learning approach for foot-type classification using heterogeneous pressure data. Sensors. 2020;20:4481. doi: 10.3390/s20164481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Day J., de Cesar Netto C., Richter M., et al. Evaluation of a weightbearing CT artificial intelligence-based automatic measurement for the M1-M2 intermetatarsal angle in hallux valgus. Foot Ankle Int. 2021;42:1502–1509. doi: 10.1177/10711007211015177. [DOI] [PubMed] [Google Scholar]
- 50.Guss D., Mojahed-Yazdi R., Ashkani-Esfahani S., et al. Deep learning improves the accuracy of weightbearing CT scan in detecting subtle syndesmotic instability. Foot & Ankle Orthopaedics. 2022;7 2473011421S2473010022. [Google Scholar]
- 51.Merrill R.K., Ferrandino R.M., Hoffman R., Shaffer G.W., Ndu A. Machine learning accurately predicts short-term outcomes following open reduction and internal fixation of ankle fractures. J Foot Ankle Surg. 2019;58:410–416. doi: 10.1053/j.jfas.2018.09.004. [DOI] [PubMed] [Google Scholar]
- 52.Lundberg S., Lee S.-I. A unified approach to interpreting model predictions. http://arxiv.org/abs/1705.07874 arXiv:170507874. 2017 [cited 2022 Jan 11]; Available from:
- 53.Ribeiro M.T., Singh S., Guestrin C. Proceedings of the 22nd ACM SIGKDD International Conference on Knowledge Discovery and Data Mining. ACM; 2016. Why should I trust you? [Google Scholar]
- 54.Fernando Z.T., Singh J., Anand A. Proceedings of the 42nd International ACM SIGIR Conference on Research and Development in Information Retrieval. ACM; 2019. A study on the interpretability of neural retrieval models using DeepSHAP. [Google Scholar]
- 55.Bramer W.M., Giustini D., Kleijnen J., Franco O.H. Searching Embase and MEDLINE by using only major descriptors or title and abstract fields: a prospective exploratory study. Syst Rev. 2018;7 doi: 10.1186/s13643-018-0864-9. [DOI] [PMC free article] [PubMed] [Google Scholar]