Abstract
Helicobacter pylori is a human-pathogenic bacterial species that is subdivided geographically, with different genotypes predominating in different parts of the world. Here we test and extend an earlier conclusion that metronidazole (Mtz) resistance is due to mutation in rdxA (HP0954), which encodes a nitroreductase that converts Mtz from prodrug to bactericidal agent. We found that (i) rdxA genes PCR amplified from 50 representative Mtzr strains from previously unstudied populations in Asia, South Africa, Europe, and the Americas could, in each case, transform Mtzs H. pylori to Mtzr; (ii) Mtzr mutant derivatives of a cultured Mtzs strain resulted from mutation in rdxA; and (iii) transformation of Mtzs strains with rdxA-null alleles usually resulted in moderate level Mtz resistance (16 μg/ml). However, resistance to higher Mtz levels was common among clinical isolates, a result that implicates at least one additional gene. Expression in Escherichia coli of frxA (HP0642; flavin oxidoreductase), an rdxA paralog, made this normally resistant species Mtzs, and frxA inactivation enhanced Mtz resistance in rdxA-deficient cells but had little effect on the Mtz susceptibility of rdxA+ cells. Strains carrying frxA-null and rdxA-null alleles could mutate to even higher resistance, a result implicating one or more additional genes in residual Mtz susceptibility and hyperresistance. We conclude that most Mtz resistance in H. pylori depends on rdxA inactivation, that mutations in frxA can enhance resistance, and that genes that confer Mtz resistance without rdxA inactivation are rare or nonexistent in H. pylori populations.
Helicobacter pylori is a gram-negative microaerophilic bacterium that chronically infects human gastric epithelial cell surfaces and the overlying gastric mucin, a niche that few if any other microbes can occupy. It is carried by more than half of all people worldwide and is an important human pathogen: a major cause of peptic ulcer disease, and a contributor to other illnesses, ranging from childhood malnutrition to gastric cancer, and to increased susceptibility to other food- and water-borne pathogens (7, 8, 32, 38, 47). There is great intrinsic and public health interest in fully elucidating H. pylori's metabolic pathways and how H. pylori maintains its redox balance during microaerobic growth. Such knowledge should help us to understand the extraordinary chronicity of H. pylori infection and factors that determine whether a given infection will be benign or virulent, elucidate mechanisms of drug susceptibility and resistance, and identify potential targets for new effective antimicrobial agents.
Here we focus on mechanisms of susceptibility and resistance of H. pylori to metronidazole (Mtz), a synthetic nitroimidazole that is a key component of popular and affordable anti-H. pylori therapies worldwide and that is also widely used against various anaerobic and parasitic infections (13, 36, 45). Resistance to Mtz is common among H. pylori strains, with frequencies among clinical isolates ranging from 10 to >90%, depending on geographic region and patient group (17, 29, 30). Much of this is attributable to the repeated use of Mtz against other (non-Helicobacter) infections in regimens that are only partially inhibitory, leading to selection for resistance to H. pylori. This is important clinically because Mtz resistance in H. pylori markedly decreases the efficiency of Mtz-based eradication therapy and the cure of associated disease (15, 28).
We had traced the resistance of a Mtzr clinical isolate to a loss-of-function mutation in rdxA (HP0954), a chromosomal gene for an oxygen-insensitive NADPH nitroreductase, and then identified equivalent rdxA mutations in 15 other Mtzr strains from North and South America, Australia, and Europe (10, 16). Our experiments also showed that (i) mutational inactivation of rdxA was sufficient to cause Mtz resistance in an Mtzs reference strain (26695); (ii) expression of rdxA from Mtzs H. pylori strains in Escherichia coli rendered this normally Mtzr species Mtzs; (iii) expression of a functional rdxA+ allele on a shuttle plasmid restored Mtz susceptibility to an Mtzr H. pylori strain; and (iv) new mutations in rdxA, not gene transfer from unrelated lineages, were often responsible for Mtz resistance in clinical isolates (16). In confirmation, rdxA mutations were found in 25 of 27 Mtzr derivatives of strain SS1 obtained from infected Mtz-treated mice (22) and in 12 of 13 Mtzr clinical isolates from France and North Africa (42) (the bases of resistance in the unusual Mtzr strains with apparently intact rdxA genes were not determined). Consideration of enzyme mechanisms had indicated that Mtz activation by the RdxA nitroreductase generates nitroso- and hydroxylamine-related compounds that should be mutagenic and bactericidal (16). Mtz-induced mutation has been documented in H. pylori and also in E. coli carrying an expressed rdxA gene (39). Thus, recurrent exposure of resident H. pylori strains to Mtz, an inadvertent consequence of therapy against other common infections, may induce as well as select for Mtz resistance in this gastric pathogen.
Following our report linking rdxA inactivation and Mtz resistance (16), several researchers suggested that other mechanisms (presumably rdxA independent) might often also cause Mtz resistance (18, 27). This was based in part on observations that nominally Mtzr clinical isolates differ in the levels of Mtz that they tolerate (resistance level, or MIC), and also fit with precedents of multiple mechanisms of drug resistance in other bacterial species (9, 33, 37). In principle, resistance might also result from (i) diminished Mtz uptake or its active export (26, 40), (ii) more efficient DNA damage repair (6, 43), or (iii) enhanced scavenging of oxygen radicals that are produced according to certain models of Mtz activation (23, 41). Of particular note are plasmid- and transposon-borne nim genes in certain Mtzr strains of Bacteroides fragilis that promote conversion of nitroimidazoles from prodrug to harmless amino derivatives, rather than to toxic nitroso radicals, and that thus confer resistance without loss of chromosomal nitroreductase gene function (5, 46).
DNA fingerprint and sequence analyses have indicated that each H. pylori clinical isolate differs genetically from most other independent isolates (1, 2). Superimposed on this great general diversity, we and others have identified several subpopulations of H. pylori that are relatively distinct genetically, with each specific to a different geographic region or human ethnic group: one in southwest Europe (Spain); a second in East Asia; and a third in Calcutta, India (1, 21, 24, 30, 31). The strains of South and Central America seemed most closely related to southwest European (Spanish) strains, not Asian strains, as are many strains from Africa and the United States (24). Most or all strains whose Mtz resistance has been studied to date are probably of the European type; the possibility of alternative resistance genes being abundant in the gene pools of non-Western H. pylori strains remains to be tested.
Here we describe functional and sequence analyses that (i) establish that mutational inactivation of the rdxA nitroreductase gene is critically involved in primary Mtz resistance in most or all strains from South and East Asia and sub-Saharan Africa, as well as from the West; (ii) demonstrate that the resistance of Mtzr (rdxA-deficient) strains can be increased by mutation in other genes, including frxA (flavin nitroreductase; HP0642 in reference 42); and (iii) show that frxA does not contribute to the normal Mtzs phenotype of wild-type H. pylori strains. No evidence of determinants that bypass the need for rdxA inactivation in the development of clinically significant Mtz resistance was found.
MATERIALS AND METHODS
Bacterial strains and culture conditions.
The H. pylori strains used in this study were clinical isolates from diverse parts of the world, and most have been described in references 24 and 30. The recent clinical isolates from Alaska are from Native peoples in Anchorage and other sites around the state. Strains 26695 and J99 are Mtzs reference strains whose complete genome DNA sequences have been determined (3, 44). Each strain is from an ethnic European patient: 26695 from the United Kingdom (44), and J99 from an ethnic European in Pulaski, Tenn. (T. L. Cover, personal communication).
H. pylori strains were grown on brain heart infusion agar (Difco) supplemented with 7% horse blood, 0.4% IsoVitaleX and the antibiotics amphotericin B (8 μg/ml), trimethoprim (5 μg/ml), and vancomycin (6 μg/ml) (BHI agar). Mtz was added to this medium when needed at a concentration appropriate for the experiment, as detailed below. The plates were incubated at 37°C under microaerobic conditions (5% O2, 10% CO2, 85% N2). H. pylori transformation (electroporation) was carried out as described elsewhere (34).
E. coli DH5α was grown on Luria-Bertani medium. The small multicopy Ampr plasmid vector pBluescript SK− (pBS) was used as a cloning vector, and cells carrying it were selected on medium with 50 μg of ampicillin/ml.
DNA methods.
H. pylori genomic DNAs were isolated from confluent cultures grown on BHI agar using a Qiamp tissue kit (Qiagen Corporation, Chatsworth, Calif.) or the cetyltrimethylammonium bromide-phenol method (4). PCR was carried out in 20-μl volumes containing 10 ng of genomic DNA, 10 pmol of each primer, 1 U of Taq DNA polymerase (Promega) or high-fidelity Taq (Boehringer Mannheim), and 0.25 mmol of each deoxynucleoside triphosphate in standard PCR buffer. Reaction mixtures were preincubated for 2 min at 94°C and then used in 30 cycles of 94°C for 40 s, 58°C for 40 s, and 72°C for 1 min per kb, with the final elongation step of 72°C for 10 min. PCR fragments were purified for sequencing by QIAquik PCR purification kit (Qiagen). Sequencing reactions were carried out using a Big Dye terminator cycle sequencing kit (PE Applied BioSystems, Foster City, Calif.), and products were run on ABI automated sequencers in the Washington University molecular microbiology core facility. The primers used are listed in Table 1.
TABLE 1.
Primers used
| Primer | Sequencea | Remark |
|---|---|---|
| Inward facing | ||
| rdxA-F | 5′-GCAGGAGCATCAGATAGTTCT-3′ | 886-bp product |
| rdxA-R | 5′-GGGATTTTATTGTATGCTACAA-3′ | |
| rdxA-F1 | 5′-CGGACTCATGGAATTGCTCCAT-3′ | 1,343-bp product |
| rdxA-R1 | 5′-GGCAAATCATAGGCATTATGGTG-3′ | |
| Outward facing | ||
| rdxA-F2 | 5′-ATGGAAAAATTTCATTGATTTTCC-3′ | 601-bp deletion |
| rdxA-R2 | 5′-GCGATTACTTGGTTGTGATTAA-3′ | |
| rdxA-F3 | 5′-GTCATCTAGACCTGGCGATTTCAGCGATTTCT-3′ | 111-bp deletion |
| rdxA-R3 | 5′-GTCATCTAGAAGCGCTTCAGCGTTAATGGTGGT-3′ | |
| Inward facing | ||
| frxA-F1 | 5′-GCGCTTCAAAGCTTGGGTTACCA-3′ | 1,383-bp product |
| frxA-R1 | 5′-GCCTTCAATGTTGCGCTCTTTGT-3′ | |
| Outward facing | ||
| frxA-F2 | 5′-GGCGTGTCGGTAATGGCTTGTT-3′ | 523-bp deletion |
| frxA-R2 | 5′-GGTGCTGTAAAGCAACCACTTGT-3′ | |
| Inward facing | ||
| 1508-F1 | 5′-CGGATCCAGCCACTCTAGCCA-3′ | 1,980-bp product |
| 1508-R1 | 5′-GCACGCAAGACATTCGTTGCG-3′ | |
| Outward facing | ||
| 1508-F2 | 5′-CCAAGGCTAGTGGTGATGA-3′ | 1,108-bp deletion |
| 1508-R2 | 5′-GCACCAAGTGAGGATTGAAG-3′ | |
| Inward facing | ||
| 588-F1 | 5′-GCGATTAGAGCCGTGATCTTC-3′ | 976-bp product |
| 588-R1 | 5′-CGCATCACATCAGCGATCACT-3′ | |
| Outward facing | ||
| 588-F2 | 5′-CATGCTCTTAGAAGAGACTA-3′ | 537-bp deletion |
| 588-R2 | 5′-CCTTACACCTGTCTTCATTC-3′ | |
| Inward facing | ||
| cagA2143F | 5′-GCAGAAGAAACGCTAAAAGCCCTT-3′ | 1,370-bp product |
| cagA3512R | 5′-CTTCCCACATTATGCGCAACTAT-3′ | |
| Outward facing | ||
| cagA2900F | 5′-CGATTGATGATCTCGGCGGACCTTT-3′ | 226-bp deletion |
| cagA2673R | 5′-GGGTTCGTTTTCGAGTCCATTATTGT-3′ |
Underline indicates XbaI site.
Determination of Mtz sensitivity and resistance.
Frozen H. pylori cultures were streaked onto Mtz-free BHI agar and incubated for 3 days; then bacterial growth was respread on fresh Mtz-free BHI agar and incubated for 1 day. The resulting young exponentially growing cells were suspended in phosphate-buffered saline; a series of 10-fold dilutions of these suspensions was then prepared, and 10 μl of each dilution was spotted on freshly prepared BHI agar containing appropriate concentrations of Mtz (variously, 0, 0.2, 0.5, 1.5, 3, 8, 16, 32, and 64 μg/ml). When the frequency of cells that formed colonies on Mtz-containing media was very low (<10−6), estimates of viability and mutant frequency were made more accurate by spreading aliquots of cultures on the entire surface of a BHI agar petri plate, instead of spotting aliquots in small areas. A strain was considered to be susceptible to concentrations of Mtz that decreased its efficiency of colony formation at least 10-fold. This quantitative procedure was more sensitive than conventional MIC determinations, which typically estimate concentrations of antibiotic needed to block growth of denser bacterial suspensions. In particular, this procedure minimizes complications that could stem from the mutagenicity of Mtz for H. pylori (39), which would be exacerbated if H. pylori stressed by DNA-damaging agents tended to enter a hypermutable state (35).
New Mtzr mutants.
Mtzr mutant derivatives of strain 26695 that may have induced as well as selected by Mtz (39) were obtained by spreading 108 bacterial cells from young cultures (as above) on BHI agar containing Mtz at 3 μg/ml. Individual colonies were streaked on BHI agar with the same Mtz concentration and then tested on BHI agar containing higher concentrations of Mtz (8, 16, 32, and 64 μg/ml).
Engineered H. pylori strains. (i) rdxA mutants.
The rdxA::cam allele, which contains a cam cassette in the rdxA gene (16), was moved to the chromosome of H. pylori strains by DNA transformation and selection on BHI agar with 15 μg of chloramphenicol (Cam)/ml. Transformants were checked to verify that they had resulted from allelic replacement by PCR using primers rdxA-F and rdxA-R, which generates products of 2 kb in cases of replacement and 886 bp in cases of retention of the original rdxA allele (Table 1).
A deletion of nearly all of rdxA (601 bp of the 630-bp open reading frame) (rdxAΔ601) was engineered as follows. A 1,343-bp PCR product was generated by amplification of strain 26695 genomic DNA with oligonucleotide primers specific for genes that flank rdxA (primers rdxA-F1 and rdxA-R1) and cloned into the EcoRV site of a pBS plasmid vector. A second PCR was carried out using the rdxA plasmid clone with outward facing primers specific for sites near the 5′ and 3′ ends of rdxA (primers rdxA-F2 and rdxA-R2), and the linear products were ligated and recovered as circular plasmids in E. coli DH5α. DNA containing this rdxAΔ601 allele was introduced into Mtzs H. pylori strains by electroporation, with selection for Mtz resistance on BHI agar with 3 or 8 μg of Mtz/ml, with equivalent results. Mtzr transformants were tested by PCR with primers rdxA-F1 and rdxA-R1 to see if they had resulted from the desired allelic exchange; a product of 742 bp, rather than 1,343 bp, indicated replacement.
A 111-bp in-frame deletion in rdxA (rdxAΔ111) was engineered, essentially as with rdxAΔ601, using outward-facing primers containing XbaI sites near their 5′ ends (rdxA-F3 and rdxA-R3). XbaI digestion of the linear PCR product, ligation, recovery in DH5α, transformation into H. pylori with selection for Mtz resistance, and PCR verification were carried out as described above.
(ii) frxA gene cloning and construction of frxA::cam and frxA::kan insertion/deletion mutants.
The frxA flavin nitroreductase gene segment (HP0642 in the strain 26695 genome [44]) was PCR amplified from H. pylori genomic DNAs using primers frxA-F1 and frxA-R1 or, in some experiments, frxA-F and frxA-R. The amplified DNAs were cloned into the EcoRV site of plasmid pBS and recovered after transformation of E. coli DH5α.
A marked 523-bp deletion in frxA from strain 26695 was generated by PCR using outward-facing frxA primers frxA-F2 and frxA-R2, followed by ligation with a cam cassette (as in reference 16) or a kan cassette (25). Mutant plasmids were recovered in DH5α using selection for Camr (20 μg/ml) or Kanr (20 μg/ml). The frxA::cam and frxA::kan alleles were introduced into H. pylori by transformation and selection for Camr (15 μg/ml) or Kanr (20 μg/ml), as appropriate; the structures of transformants were verified by PCR with primers frxA-F1 and frxA-R1, as above.
(iii) HP1508::cam insertion/deletion mutation.
The HP1508 gene, which encodes a ferredoxin-like protein of unknown function, was PCR amplified from strain 26695 DNA, using primers 1508-F1 and 1508-R1, and similarly cloned into pBS. A marked 1,108-bp internal deletion was generated using PCR with outward-facing primers 1508-F2 and 1508-R2, followed by ligation with a cam cassette, as above. The structures of Camr transformants were verified by PCR with primers 1508-F1 and 1508-R1.
(iv) oorD::cam insertion/deletion mutation.
The oorD (HP0588) gene, which encodes the ferridoxin component of the multisubunit oxoglutarate oxidoreductase enzyme (20), was PCR amplified from strain 26695 DNA, using primers 588-F1 and 588-R1, and cloned into pBS. A marked 537-bp internal deletion was generated using PCR with outward facing primers 588-F2 and 588-R2, followed by ligation with a cam cassette, as above. The structure of the one H. pylori transformant obtained (see Results) was verified by PCR with primers 588-F1 and 588-R1.
(v) Addition of a functional rdxA gene to the H. pylori genome.
A functional rdxA gene, PCR amplified from strain 26695 with primers rdxA-F1 and rdxA-R1, and the cam cassette were cloned into the EcoRV and SmaI sites, respectively, of plasmid pBS (each gene transcribed toward the other). In parallel, a 1.37-kb segment of cagA, PCR amplified from strain NCTC11637 using primers cagA2143F and cagA3512R, was cloned into the SmaI site of pBS. The resulting plasmid was linearized by PCR with outward-facing primers cagA2673R and cagA2900F, which are specific to sites 531 and 613 bp from the two ends of the cloned cagA DNA fragment. The rdxA-cam segment was then PCR amplified from the pBS-rdxA-cam construct, using pBS-specific primers M13F and M13R, and cloned between the 531- and 613-bp fragments of the cagA gene. An isolate in which rdxA was oriented in the same direction as cagA was used to transform NCTC11637 to Camr. The structures of resultant transformants were verified by PCR using primers cagA2143F and cagA3512R.
RESULTS
Intrinsic Mtz susceptibility or resistance of H. pylori reference strains and clinical isolates.
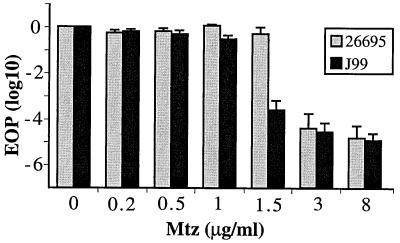
To determine the lowest concentrations of Mtz that permitted survival of reference strains 26695 and J99, young cultures were diluted serially, aliquots of dilutions were spotted on Mtz-containing BHI agar, and numbers of colonies formed at appropriate dilutions were determined. Strain 26695 exhibited an efficiency of plating (EOP) of ∼1 on BHI agar with up to 1.5 μg of Mtz/ml and ∼10−4 on BHI agar with 3 or 8 μg of Mtz/ml (phenotype designated 1.5R 3S). Reference strain J99 was somewhat more susceptible, exhibiting EOPs of ∼1 and 10−3 on BHI agar with 1 and 1.5 μg of Mtz/ml, respectively (phenotype designated 1R 1.5S) and 10−4 on BHI agar with 3 or 8 μg of Mtz/ml (Fig. 1).
FIG. 1.
Profiles of intrinsic susceptibility and resistance to Mtz of reference strains 26695 and J99. Young exponentially growing cultures were diluted, aliquots were spotted on BHI agar with indicated concentrations of Mtz, and surviving colonies were counted from appropriate dilutions, as detailed in Materials and Methods. Presented are the average and range of results with two single colony isolates (cultures) of each strain, with assays repeated three times with each culture.
H. pylori strains from patients from five continents, chosen to represent much of the diversity of this pathogen worldwide, were divided into two groups based on a first-pass test of susceptibility or resistance to Mtz, defined as inability or ability to grow on BHI agar containing Mtz at 8 μg/ml (a concentration generally used clinically as a threshold for significant resistance). The levels of Mtz just sufficient to kill representative strains from each group were then determined more precisely, as with the reference strains above. The Mtzs strains included 20 from Japan, a society in which Mtz use is rare and hence in which H. pylori strains should have had little inadvertent exposure to this drug, as well as strains from societies in which Mtz use is common and in which more than half of strains are resistant (India, Peru, and South Africa). Forty-eight of these 61 strains tested were like strain 26695 in phenotype (1.5R 3S), and another seven were like strain J99 (1R 1.5S) (Table 2). Two were more susceptible (0.5R 1.0S), and four were marginally more resistant (3R 8S).
TABLE 2.
Distribution of MICs among Mtzs H. pylori isolatesa
| Geographic region | No. of strains | Mtz phenotype
|
||||
|---|---|---|---|---|---|---|
| 0.2R 0.5S | 0.5R 1S | 1R 1.5S | 1.5R 3S | 3R 8S | ||
| Japan | 20 | 0 | 0 | 0 | 20 | 0 |
| China | 9a | 0 | 1 | 0 | 6 | 2 |
| India | 4 | 0 | 0 | 2 | 1 | 1 |
| South Africa | 8 | 0 | 1 | 1 | 5 | 1 |
| Alaska | 5 | 0 | 0 | 2 | 3 | 0 |
| Spain | 10 | 0 | 0 | 1 | 9 | 0 |
| Peru | 5 | 0 | 0 | 1 | 4 | 0 |
| Total | 61 | 0 | 2 | 7 | 48 | 4 |
Five strains from Shenyang; four strains (including one with 0.5R 1S phenotype) from Hong Kong.
In equivalent characterizations of 55 representative Mtzr clinical isolates, nearly 40% were resistant to just 16 μg/ml (16R 32S; 21 of 55 strains), another 40% were resistant to just 32 μg/ml (32R 64S; 22 of 55 strains), and 16% exhibited higher resistance (64R; 9 strains). Just 3 of the 55 strains exhibited lower resistance (8R 16S) (Table 3).
TABLE 3.
Distribution of MICs among Mtzr H. pylori isolates
| Geographic region | No. of strains | Mtz phenotype
|
|||
|---|---|---|---|---|---|
| 8R 16S | 16R 32S | 32R 64S | 64R | ||
| Chinaa | 6 | 2 | 3 | 1 | 0 |
| India | 20 | 0 | 8 | 6 | 6 |
| South Africa | 7 | 0 | 2 | 2 | 3 |
| Alaska | 11 | 0 | 5 | 6 | 0 |
| Peru | 11 | 1 | 3 | 7 | 0 |
| Total | 55 | 3 | 21 | 22 | 9 |
Each of the Chinese strains is from Shenyang.
New Mtzr mutants generated in culture.
To test our inference that Mtz resistance generally involves decreased rdxA function, new mutant Mtzr derivatives of reference strain 26695 were selected on BHI agar containing just 3 μg/ml, the lowest concentration of Mtz that allowed Mtzr mutants to emerge cleanly from background growth. Such mutants were obtained at frequencies of about 10−4 in cultures from different single-colony isolates, as noted above (Fig. 1).
Only 13 of these 149 mutants selected for resistance to at least 3 μg/ml were susceptible to Mtz at 8 μg/ml (phenotype designated 3R 8S). Each of the other 137 mutants was resistant to at least 8 μg/ml. Of these, 39 were unable to grow on BHI agar with 16 μg/ml (8R 16S phenotype), 97 grew well with 16 but not 32 μg/ml (16R 32S phenotype), and one exceptional mutant (mutant 0161) grew well with 32 μg of Mtz/ml (32R 64S phenotype). The differences in distributions of levels of Mtz resistance among clinical isolates versus newly arisen mutants (≥32R phenotype in 56% of clinical isolates versus <1% of newly arisen mutants; conversely, 8R 16S phenotype in only 10% of clinical isolates versus 29% of new mutants) suggested both that H. pylori is often exposed to relatively high concentrations of Mtz during human infection and that high-level resistance (≥32 μg/ml) might arise in several steps.
Nature of newly arisen Mtzr mutants.
Eight low-level Mtzr mutants (3R 8S) were characterized by sequencing. Three contained mutations in or immediately upstream of rdxA, whereas the other five did not (Table 4, group A); the mutations that caused the weak Mtz resistance of these latter five isolates have not been identified. The rdxA genes of four independent Mtzr mutants with the more common higher-level Mtz resistance were also sequenced (two 8R 16S, one 16R 32S, and one 32R 64S). Simple point mutations in rdxA were found in each case (Table 4, group B), as expected (16).
TABLE 4.
Spontaneous mutation in rdxA and amino acid substitutions in H. pylori 26695
| Mutant | Mtz phenotype | Substitution
|
|
|---|---|---|---|
| Nucleotide | Amino acida | ||
| Group A, 3R 8S | |||
| 202 | 8R 16S | CGC→TGC | Arg(16)→Cys |
| 407 | 3R 8S | CCA→CTA | Pro(96)→Leu |
| 301 | 3R 8S | A→G | 37 bp upstream from start codon (ATG) |
| 201 | 3R 8S | ||
| 116 | 3R 8S | ||
| 515 | 3R 8S | ||
| 619 | 3R 8S | ||
| 251 | 3R 8S | ||
| Group B, ≥8R | |||
| 501 | 8R 16S | GCA→GAA | Ala(67)→Glu |
| 621 | 8R 16S | GAT→GGT | Asp(23)→Gly |
| 111 | 16R 32S | GCT→GTT | Ala(80)→Val |
| 161 | 32R 64S | GAG→TAG | Glu(75)→stop |
| Group C, 3R 8S→8R | |||
| 1001 | 32R 64S | CGC→AGC | Arg(16)→Ser |
| 1161 | 16R 32S | CGC→CAC | Arg(16)→His |
| 2002 | 16R 32S | CTA→-TA | Frameshift from Leu(42) |
| 5151 | 16R 32S | GAG→TAG | Glu(75)→stop |
| 1162 | 16R 32S | GAG→TAG | Glu(175)→stop |
| 2001 | 16R 32S | AAGGAA→AAAGAA | Shine-Dalgarno ribosome-binding site |
Position of the amino acid substitution is given in parentheses.
The possibility of clinically significant resistance to Mtz arising by stepwise accumulation of mutations in loci other than rdxA (without rdxA inactivation) was tested using three of the weakly Mtzr mutants (3R 8S phenotype), in which resistance was due to mutation outside of rdxA. Five of six derivatives selected on BHI agar with Mtz at 8 μg/ml had a 16R 32S phenotype, and the sixth had a 32R 64S phenotype. Each of these six contained a new point mutation, either in the rdxA open reading frame (five cases) or in the Shine-Dalgarno sequence just upstream of rdxA (one case) (Table 4, group C). These results support the conclusion that resistance to the more clinically significant levels of Mtz usually involves rdxA inactivation.
Loss-of-function mutations in rdxA associated with Mtzr worldwide.
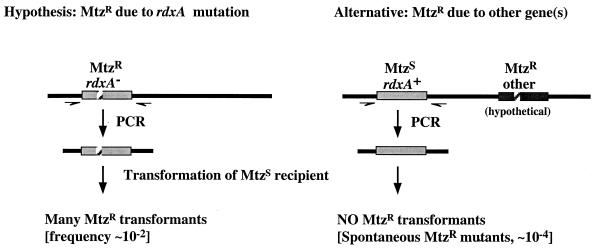
The idea that rdxA inactivation is critically involved in most or all clinically significant cases of Mtz resistance was tested against an alternative possibility, that Mtz resistance in certain geographic regions might often result from auxiliary (e.g., plasmid or transposon) resistance genes that are uncommon in Western H. pylori strains and that bypass the need for rdxA inactivation. This entailed PCR amplification of a segment containing rdxA from Mtzr strains from various representative populations (12 Chinese, 12 Indian, 11 Alaska Native, 9 Peru Native, and 6 South African), electroporation of the Mtzs strain 26695 with these PCR-amplified rdxA DNAs, and quantitation of the yield of Mtzr transformants on BHI agar with 8 μg of Mtz/ml (Fig. 2). Mtzr transformants were obtained with frequencies of about 10−2, using rdxA DNA amplified from each of the 50 Mtzr clinical isolates tested and also from strain SS1rdxAΔ111, as a positive control. This frequency was 100-fold higher than the yield of Mtzr colonies obtained with PCR products from each of six control Mtzs strains (∼10−4) (two Alaska Native, two South African, and two Indian), indicating that each of the 50 Mtzr strains contained mutant alleles of rdxA. It is also noteworthy that each of the 50 rdxA mutant PCR products was of the size expected (∼890 bp), indicating that each contained a point mutation, not an insertion or deletion, in rdxA. These results showed that rdxA inactivation is critically involved in most cases of Mtz resistance worldwide and ruled out a model (14) in which changes in regulatory genes that affect expression of rdxA and/or other reductase genes would be responsible for most Mtz resistance in clinical isolates.
FIG. 2.
DNA transformation strategy for testing involvement of rdxA gene mutation in Mtzr clinical isolates.
RdxA as principal determinant of Mtz susceptibility of most wild-type strains.
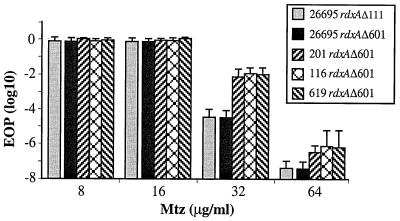
Initial tests had shown that transformants of the Mtzs strain 26695 obtained using an rdxA::cam cassette and selected for Camr were Mtzr in phenotype (16). In the present, more quantitative tests, this rdxA::cam mutant strain exhibited a 16R 32S phenotype, as did most newly arisen Mtzr mutants. Equivalent 16R 32S phenotypes were exhibited by derivatives of strain 26695 containing unmarked rdxA deletion alleles (rdxAΔ601 or rdxAΔ111) (Fig. 3), in each case selected after DNA transformation and selection for resistance to just 3 μg of Mtz/ml. These results showed that rdxA encodes the only nitroreductase sufficiently active to confer an Mtzs phenotype on this reference strain.
FIG. 3.
Mtz resistance profiles of rdxAΔ111 or rdxAΔ601 deletion mutant transformants of strain 26695 and of rdxAΔ601-containing transformants of three 26695 derivatives that had exhibited an unusual 3R 8S phenotype that was not attributable to mutation in rdxA (201, 116, and 619 [Table 4]). Transformants containing the rdxAΔ111 and rdxAΔ601 alleles were selected using BHI agar with 8 μg of Mtz/ml. Presented are the average and range of results with three single colony isolates (cultures) of each strain, with each culture assayed two times.
The generality of these results was tested by transformation using the rdxA::cam allele and a sampling of strains with normal Mtzs phenotypes (1.0R 1.5S or 1.5R 3S) (i) from a society in which Mtz use is uncommon and Mtz resistance is rare (29) and (ii) from societies in which Mtz use is more common and many (in some countries, most) strains are Mtzr. Inactivation of rdxA led to an Mtzr phenotype in 26 of the 28 strains tested (8 from Japan, 5 of 6 from South Africa, 5 of 6 from Hong Kong, 3 each from Peru and Spain, and 2 from India). rdxA inactivation led to a 16R 32S phenotype in 24 of these strains and an 8R 16S (nearly as resistant) phenotype in the other two. Further analysis of the other two (most interesting) exceptions, implicating RdxA and one other expressed nitroreductase in their unusual susceptibility to Mtz, is presented below.
Mutations in other genes can affect the level of Mtz resistance.
Three sets of results established that the level of resistance of a typical Mtzr (rdxA-deficient) H. pylori strain can be affected by other genetic determinants. First, introduction of the rdxAΔ601 deletion into three derivatives of strain 26695 with weak Mtzr phenotypes (3R 8S) that were ascribed to unknown sequence changes outside rdxA (see above) resulted in 16R 32S phenotypes in each case. However, the EOP of these transformants on BHI agar with 32 μg of Mtz/ml was reproducibly ∼10−2—that is, several hundred-fold higher than that of control rdxAΔ601 transformants of 26695 wild type, selected in parallel (Fig. 3). This suggests that the mutations responsible for the 3R 8S phenotype may have affected process(es) distinct from those controlled by RdxA nitroreductase itself.
Second, mutant derivatives of 26695 carrying an rdxA deletion (16R 32S phenotype) that could grow on BHI agar with 32 or 64 μg of Mtz/ml were selected. These mutants were obtained at frequencies of about 10−4 and 10−7 (selection at 32 and 64 μg of Mtz/ml, respectively), using strains carrying the rdxAΔ111 or rdxAΔ601 deletion allele. In contrast, such hyperresistant (64R) mutants were not obtained from 26695 wild type (rdxA+) (frequency, <10−9). Thus, the enhanced resistance that these additional mutations confer depended on rdxA inactivation: these mutations did not bypass the need to mutate rdxA in order to develop a resistant phenotype.
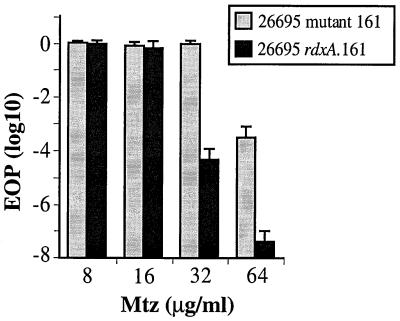
Third, more than half of the 55 Mtzr clinical isolates that we screened had 32R or 64R phenotypes (Table 3). Transformants of strain 26695 made with rdxA genes from two hyperresistant Indian strains and selected on BHI agar with just 8 μg of Mtz/ml were examined carefully. Each exhibited a 16R 32S (moderate resistance) phenotype, not the 64R (hyperresistance) phenotype of their DNA donor parents. Similarly, transformants made with the rdxA gene from a mutant derivative of 26695 that was unusual in exhibiting a 32R 64S phenotype (161 [Table 4, group B]) were also only 16R 32S in phenotype (Fig. 4). Thus, clinical isolates and laboratory mutants with very high level resistance must have contained an additional mutation that enhanced the moderate resistance conferred by simple point mutations in rdxA.
FIG. 4.
Mtz resistance profiles of the unusual highly resistant mutant of strain 26695 (designated 26695 mutant 161 [Table 4]) and also a 26695 derivative that contains only the rdxA gene from this mutant (designated rdxA.161). The rdxA.161 transformant was selected on BHI agar with 8 μg of Mtz/ml. The presence of the expected GAG-to-TAG change in rdxA (Table 4) was verified by DNA sequencing. Presented are the average and range of results with five single colony isolates (cultures) from each strain, with each culture assayed once.
Flavin nitroreductase (frxA gene product) contributes to residual Mtz susceptibility of rdxA mutant strains.
Theoretical considerations had suggested that frxA [HP0642, encoding NAD(P)H-flavin oxidoreductase; an rdxA paralog] might contribute to Mtz susceptibility in H. pylori (16). Although one frxA gene clone did not make E. coli susceptible to Mtz (16), further studies identified an frxA-containing cosmid clone from an H. pylori strain with a high-level Mtzr phenotype (32R) (strain 439 in reference 16) that increased the yield of Mtzr H. pylori transformant colonies when mixed with rdxA mutant DNA but did not transform Mtzs H. pylori to Mtzr when used alone. Given these various results, we elected to reexamine the possibility of a role for frxA in Mtz susceptibility and resistance. First, we sought to again PCR amplify and clone frxA-containing DNA segments from several different Mtzs H. pylori strains, but using a high-fidelity Taq polymerase formulation to minimize mutation during PCR. Four of ten independent frxA-containing pBS plasmid clones that were recovered in E. coli DH5α (two from 26695; one each from SS1 and HP500) resulted in susceptibility to Mtz. In quantitative determinations using frxA clones from 26695, the EOPs were about 0.01 and 0.001 on L agar with 1 and 2.5 μg of Mtz/ml, respectively, whereas the parental E. coli strain (lacking frxA) exhibited an EOP of 1 on L agar with 50 μg of Mtz/ml. It was also noted that E. coli carrying cloned functional frxA genes tended to make small colonies on Mtz-free L agar. This result suggested that the poor yield of frxA-containing clones that rendered E. coli Mtzs (only 4 of 10) might be due to some toxicity of frxA when hyperexpressed and that the initial lack of Mtz susceptibility associated with frxA cloning (16) was a spurious result, perhaps reflecting mutation during PCR or cloning and unwitting selection of a healthy (frxA mutant) transformant colony.
In a second test, frxA was sequenced from three strains that were resistant to high levels of Mtz (≥32 μg/ml). An ATG-to-ATA change was found in the start codon of frxA in a highly resistant mutant derivative of an rdxA-deficient transformant of strain 26695 (64R instead of 16R 32S in phenotype); similarly, −1 frameshift mutations were found in poly(A) tracts at nucleotide positions 48 and 310 of frxA in two highly resistant (32R) derivatives of SS1 that also carried an rdxA-null mutation. In accord with this are recent descriptions of rdxA and frxA from the type strain of H. pylori, NCTC11637, which also exhibits a 32R phenotype: it contains a mini-IS605 insertion and adjacent deletion in rdxA (10) and also a frameshift mutation in frxA (D. H. Kwon et al., GenBank accession no. AF225923).
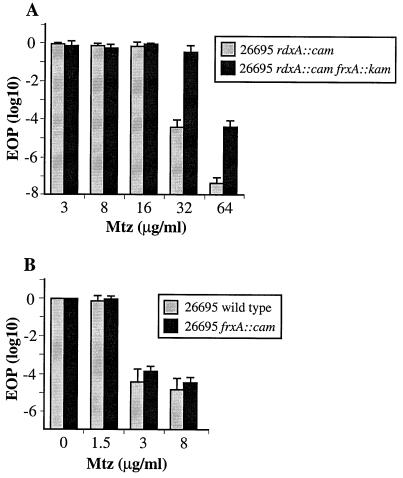
In a third test, frxA::kan transformant derivatives of rdxA::cam (16R phenotype) derivatives of three normal Mtzs H. pylori strains were constructed: 26695, HUP-B57, and HK192, from England, Spain, and Hong Kong, respectively. Each rdxA frxA double mutant exhibited a 32R 64S phenotype (Fig. 5A). In contrast, frxA inactivation in strains with functional rdxA+ genes had little if any effect on their intrinsic susceptibility to Mtz (1.5R 3S phenotype) (Fig. 5B). Similarly, inactivation of frxA in each of five other new Mtzs clinical isolates did not affect their intrinsic Mtz susceptibility (phenotypes 1.5R 3S in two Hong Kong and two Spanish strains; 1R 1.5S in one Peruvian strain). In accord with this, incorporation of a functional rdxA gene into the chromosome (as a rdxA+ cam cassette flanked by segments of the cagA gene) of the rdxA and frxA mutant type strain NCTC11637 caused a change in its phenotype from 32R 64S to 0.5R 1S. In sum, these studies showed that loss of frxA function contributes significantly to Mtz resistance in H. pylori, but generally only if rdxA is also mutant. The near absence of effect of frxA inactivation on the Mtzs phenotype of rdxA+ strains is in agreement with findings that rdxA inactivation is generally sufficient to cause an Mtzr phenotype.
FIG. 5.
Effect of frxA inactivation on Mtz susceptibility and resistance depends on whether rdxA is functional or not. (A) Profile of Mtz susceptibility of an frxA-null mutant of an rdxA::cam strain and of its frxA+ parent; (B) profile of Mtz susceptibility of an frxA-null mutant derivative of 26695 (rdxA+) and of its wild-type parent. Presented are the average and range of results with three single colony isolate cultures of each strain, with each culture assayed two times.
The two unusual clinical isolates that had remained Mtzs after rdxA inactivation (described above) were then studied further. Transformation using frxA::kan DNA of derivatives of these two strains that already carried rdxA::cam alleles resulted in a 32R 64S Mtzr phenotype in each case, whereas equivalent transformation of the original rdxA+ parental strains with the frxA::kan allele resulted in retention of Mtzs phenotypes. Thus, these two clinical isolates were unusual in requiring inactivation of both frxA and rdxA to achieve a clinically significant Mtzr phenotype. Although the basis of their unusual FrxA activity (e.g., high expression of the frxA gene versus unusually high specific activity of the FrxA product) is currently under study, these two exceptions also reinforce the sense that mutation in rdxA and frxA are each important in the development of resistance to levels of Mtz higher than can be achieved by rdxA inactivation alone.
Other possible contributors to resistance.
As noted above, the 26695 rdxA frxA double mutant had a 32R 64S phenotype. Derivatives with higher resistance (64 instead of 32 μg of Mtz/ml) were recovered at a frequency of ∼10−4, in contrast to ∼10−7 in the case of frxA+ rdxA-deficient strains (Fig. 5A). This indicated that resistance can be enhanced by mutation in at least one additional locus.
HP1508, which encodes a putative ferredoxin-like protein of unknown function, was tested for possible effects on Mtz susceptibility or resistance after transformation with a HP1508::cam insertion allele. Camr transformant derivatives of strain 26695 made in the rdxA+ background had a normal 1.5R 3S phenotype; those in a rdxAΔ111 background had a normal 16R 32S phenotype; and those in an rdxAΔ111 frxA::kan background had a normal 32R 64S phenotype. The generality of this result was tested by transforming the HP1508::cam insertion allele into six other Mtz-susceptible clinical isolates (two from Hong Kong; one each from India, Peru, Spain, and South Africa). No effect of HP1508 inactivation on intrinsic Mtz susceptibility was detected in any of these six strains.
Equivalent tests were also attempted with cam insertion alleles of HP0558, which encodes the ferridoxin component of the multisubunit oxoglutarate oxidoreductase, an enzyme considered to be essential for viability (20). One Camr colony was obtained in several attempts to transform strain 26695 with HP0558::cam insertion mutant DNA under conditions that normally yield hundreds or thousands of transformants. PCR tests confirmed that this one exceptional Camr colony contained a replacement of the wild-type allele with the HP0588::cam insertion allele (data not shown). However, attempts to transform 26695 wild type with genomic DNA from this HP0588::cam sibling strain were also unsuccessful. Assuming that this gene is normally essential for viability (20), the one exceptional transformant obtained is inferred to contain a bypass-suppressor mutation at another locus, sufficient to allow survival without HP0588 function. In terms of the present Mtz resistance studies, it is noteworthy that the 26695 HP0588::cam strain exhibited a normal Mtzs phenotype (1.5 3S) and that transformation of the rdxAΔ111 allele into this strain resulted in a normal 16R 32S Mtzr phenotype. Thus, if it is assumed that the putative suppressor mutation does not affect Mtz reduction, these results would suggest that the HP0588-encoded ferredoxin does not contribute to the Mtz susceptibility of wild-type H. pylori or to the residual Mtz susceptibility of rdxA mutants.
DISCUSSION
We have studied mechanisms of susceptibility and resistance to Mtz in H. pylori strains from diverse parts of the world, motivated in part by recent findings that different H. pylori genotypes predominate in East Asia, South Asia, and Europe, and that Latin American and African and many U.S. strains tend to be most closely related to those of Europe, not Asia (24, 30, 31). Here we present mutational, gene cloning, and sequence analyses that confirm and extend our initial conclusions (10, 16) in establishing (i) that the lethality of Mtz to wild-type H. pylori depends primarily on the activity of an oxygen-insensitive NADPH nitroreductase encoded by the rdxA (HP0954) gene, which mediates conversion of Mtz from harmless prodrug to toxic and mutagenic product (16, 39); and (ii) that Mtz resistance generally results, at least in part, from mutations that inactivate rdxA.
In the present studies of East Asian (China and Japan), South Asian (Calcutta), South African, and Alaska Native strains, as well as Western (Spain and Amerindian Peruvian) strains, we found (i) that a functional rdxA nitroreductase gene is primarily responsible for the high susceptibility to Mtz of most or all wild-type H. pylori strains; (ii) that clinically significant Mtz resistance generally requires mutation in rdxA; and (iii) that the level of Mtz resistance that a strain exhibits can be further enhanced by additional changes elsewhere in its genome, but only if it is already mutant in rdxA. With only a few possible exceptions (discussed below), no evidence of auxiliary resistance genes that confer clinically significant Mtz resistance without rdxA inactivation was found in any population. This is noteworthy, because many of the strains examined came from societies in which H. pylori infection and Mtz usage are frequent—conditions that would have favored the spread of any plasmid- or transposon-borne auxiliary resistance determinants.
That additional genes might also be important is emphasized by the finding that more than half of Mtzr clinical isolates were resistant to levels of Mtz higher than can be obtained by inactivation of rdxA alone (Table 3). Further analyses indicated that this enhanced resistance can occur stepwise, by mutation in at least two other loci in strains already mutant in rdxA. First, mutational inactivation of frxA (HP0642), an rdxA homolog that encodes a related reductase (24% amino acid sequence identity), increased the resistance of rdxA-deficient H. pylori from 16 to 32 μg/ml. However, frxA inactivation, by itself, had little effect on the intrinsic Mtz susceptibility of Mtzs strains. This is in accord with evidence that rdxA inactivation is sufficient to render Mtzs strains Mtzr. In this context, our finding that cloned frxA+ genes from each of several Mtzs H. pylori strains made E. coli highly susceptible to Mtz suggests that synthesis or activity of the FrxA reductase may be down-regulated in H. pylori. In accord with this view, we found two unusual clinical isolates that became Mtzr only if rdxA and frxA were each inactivated. In parallel studies, we have also found that the special mouse-adapted strain SS1 also requires inactivation of both frxA and rdxA to achieve an Mtzr phenotype and that frxA mRNA levels in this strain are higher than in reference strains (J. Y. Jeong and D. E. Berg, unpublished data). We have begun to search for the putative regulatory gene(s) and/or site(s) that may be mutant in these strains and to test the possibility that unusually high FrxA activity may contribute to bacterial fitness in certain hosts.
Even higher-level resistance (64R phenotype) is common among clinical isolates and is readily obtained in culture, starting with an rdxA frxA double-mutant strain. Hence, at least one other gene must be involved in residual Mtz susceptibility and the emergence of hyperresistance. The involvement of other reductase enzymes in susceptibility is suggested by our finding that Mtz can be mutagenic even for hyperresistant H. pylori strains, since Mtz-promoted mutagenesis reflects enzymatic reduction of Mtz (39). The gene responsible for this third incremental component of hyperresistance has not yet been defined.
The multiplicity of metabolic and housekeeping functions that potentially can affect Mtz susceptibility and resistance is further illustrated by our finding that five of the eight derivatives of strain 26695, selected for very slight decreases in susceptibility to Mtz (3R instead of 1.5R phenotype), had resulted from mutation outside of rdxA. One explanation invokes polar mutations in upstream sequences that simply decrease rdxA expression. This explanation seems unlikely, however, since the level of Mtz resistance achieved after transformation of these mutants with an rdxA deletion allele was slightly but reproducibly higher than in their isogenic parent (Fig. 3). This implies that the 3R 8S and rdxA mutations affect quite different processes or pathways. The mutations are also unlikely to be in frxA: their enhancement of Mtz resistance in strain 26695 wild type, although slight, was greater than that conferred by an frxA-null allele, whereas they had less effect on Mtz resistance than the frxA-null allele in the rdxA-null background. Given the mutagenic and DNA-damaging effects of products of Mtz activation (39) and the dramatic increase in Mtz susceptibility caused by recA gene inactivation (43), these subtle mutant phenotypes might be ascribed to changes in genes affecting DNA replication or repair (6, 43), or equally to changes in efficiency of Mtz uptake (26) or efficiency of physiologic adaptation to growth with Mtz (19).
Also meriting further study are a few exceptional Mtzr strains that were reported by others to contain normal rdxA sequences: 2 of 27 Mtzr variants recovered from mice infected with strain SS1 and treated subtherapeutically with Mtz (22), and 1 of 13 Mtzr strains from France and North Africa (42). It should now be possible to learn if any of these unusual mutants have decreased RdxA synthesis or activity, or if any of them result from an alternative, but still rare, mechanism for Mtz resistance that bypasses the need for rdxA inactivation.
The distribution of various levels of Mtz resistance among clinical isolates differs markedly from that obtained by one-step forward mutation to Mtzr in culture. We propose that this distribution reflects a complex dynamic, including (i) the mutagenic effects of Mtz activation; (ii) the intensity of selection for Mtzr phenotypes during Mtz-based therapy, which is dictated by amounts of Mtz administered, frequency and duration of treatment, and gastric acidity or physiologic parameters that affect drug potency in H. pylori's mucosal niche; and (iii) possible effects of resistance on H. pylori fitness during periods between therapy. Given the diversity among H. pylori strains and their human hosts, the evolutionary cost of a given level of Mtz resistance may depend on various aspects of bacterial genotype that affect the overall flow of metabolites during growth, and also on aspects of human genotype and physiology that affect human susceptibility to a given H. pylori strain (11, 12). Many of these issues should soon be clarified through high-resolution H. pylori molecular genetics and use of appropriate in vitro culture strategies and well-chosen experimental animal infection models.
ACKNOWLEDGMENTS
This work was supported in part by NIH grants AI38166, DK53727, and TW00611 to D.E.B. and P30 DK52574 to Washington University and by grants from MRC (R-14292), from AstraZeneca Canada, and from Romark Laboratories to P.S.H.
REFERENCES
- 1.Achtman M, Azuma T, Berg D E, Ito Y, Morelli G, Pan Z J, Suerbaum S, Thompson S A, Van Der Ende A, Van Doorn L J. Recombination and clonal groupings within Helicobacter pylori from different geographical regions. Mol Microbiol. 1999;32:459–470. doi: 10.1046/j.1365-2958.1999.01382.x. [DOI] [PubMed] [Google Scholar]
- 2.Akopyanz N, Bukanov N O, Westblom T U, Kresovich S, Berg D E. DNA diversity among clinical isolates of Helicobacter pylori detected by PCR-based RAPD fingerprinting. Nucleic Acids Res. 1992;20:5137–5142. doi: 10.1093/nar/20.19.5137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Alm R A, Ling L S, Moir D T, King B L, Brown E D, Doig P C, Smith D R, Noonan B, Guild B C, deJonge B L, Carmel G, Tummino P J, Caruso A, Uria-Nickelsen M, Mills D M, Ives C, Gibson R, Merberg D, Mills S D, Jiang Q, Taylor D E, Vovis G F, Trust T J. Genomic-sequence comparison of two unrelated isolates of the human gastric pathogen Helicobacter pylori. Nature. 1999;397:176–180. doi: 10.1038/16495. [DOI] [PubMed] [Google Scholar]
- 4.Ausubel F M, et al., editors. Current protocols in molecular biology. Vol. 1. New York, N.Y: Wiley Interscience; 1998. p. 1.1.1. [Google Scholar]
- 5.Carlier J P, Sellier N, Rager M N, Reysset G. Metabolism of a 5-nitroimidazole in susceptible and resistant isogenic strains of Bacteroides fragilis. Antimicrob Agents Chemother. 1997;41:1495–1499. doi: 10.1128/aac.41.7.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chang K C, Ho S W, Yang J C, Wang J T. Isolation of a genetic locus associated with metronidazole resistance in Helicobacter pylori. Biochem Biophys Res Commun. 1997;236:785–788. doi: 10.1006/bbrc.1997.7050. [DOI] [PubMed] [Google Scholar]
- 7.Clemens J, Albert M J, Rao M, Qadri F, Huda S, Kay B, van Loon F P, Sack D, Pradhan B A, Sack R B. Impact of infection by Helicobacter pylori on the risk and severity of endemic cholera. J Infect Dis. 1995;171:1653–1656. doi: 10.1093/infdis/171.6.1653. [DOI] [PubMed] [Google Scholar]
- 8.Dale A, Thomas J E, Darboe M K, Coward W A, Harding M, Weaver L T. Helicobacter pylori infection, gastric acid secretion, and infant growth. J Pediatr Gastroenterol Nutr. 1998;26:393–397. doi: 10.1097/00005176-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 9.Davies J, Wright G D. Bacterial resistance to aminoglycoside antibiotics. Trends Microbiol. 1997;5:234–240. doi: 10.1016/S0966-842X(97)01033-0. [DOI] [PubMed] [Google Scholar]
- 10.Debets-Ossenkopp Y J, Pot R G, van Westerloo D J, Goodwin A, Vandenbroucke-Grauls C M, Berg D E, Hoffman P S, Kusters J G. Insertion of mini-IS605 and deletion of adjacent sequences in the nitroreductase (rdxA) gene cause metronidazole resistance in Helicobacter pylori NCTC11637. Antimicrob Agents Chemother. 1999;43:2657–2662. doi: 10.1128/aac.43.11.2657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dubois A, Berg D E, Incecik E T, Fiala N, Heman-Ackah L M, Perez-Perez G I, Blaser M J. Transient and persistent experimental infection of nonhuman primates with Helicobacter pylori: implications for human disease. Infect Immun. 1996;64:2885–2891. doi: 10.1128/iai.64.8.2885-2891.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dubois A, Berg D E, Incecik E T, Fiala N, Heman-Ackah L M, Del Valle J, Yang M, Wirth H P, Perez-Perez G I, Blaser M J. Host specificity of Helicobacter pylori strains and host responses in experimentally challenged nonhuman primates. Gastroenterology. 1999;116:90–96. doi: 10.1016/s0016-5085(99)70232-5. [DOI] [PubMed] [Google Scholar]
- 13.Edwards D I. Nitroimidazole drugs—action and resistance mechanisms. I. Mechanisms of action. J Antimicrob Chemother. 1993;31:9–20. doi: 10.1093/jac/31.1.9. [DOI] [PubMed] [Google Scholar]
- 14.el-Zaatari F A, Kwon D H, Dore M P, Graham D Y, Osato M S. Reply to Rappuoli, Pizza and Covacci. Clin Infect Dis. 1999;28:938–939. [Google Scholar]
- 15.Goddard A F, Logan R P. Antimicrobial resistance and Helicobacter pylori. J Antimicrob Chemother. 1996;37:639–643. doi: 10.1093/jac/37.4.639. [DOI] [PubMed] [Google Scholar]
- 16.Goodwin A, Kersulyte D, Sisson G, Veldhuyzen van Zanten S J O, Berg D E, Hoffman P S. Metronidazole resistance in Helicobacter pylori is due to null mutations in a gene (rdxA) that encodes an oxygen-insensitive NADPH nitroreductase. Mol Microbiol. 1998;28:383–393. doi: 10.1046/j.1365-2958.1998.00806.x. [DOI] [PubMed] [Google Scholar]
- 17.Glupczynski Y. Antimicrobial resistance in Helicobacter pylori: a global overview. Acta Gastroenterol Belg. 1998;61:357–366. [PubMed] [Google Scholar]
- 18.Graham D Y. Antibiotic resistance in Helicobacter pylori: implications for therapy. Gastroenterology. 1998;115:1272–1277. doi: 10.1016/s0016-5085(98)70100-3. [DOI] [PubMed] [Google Scholar]
- 19.Hoffman P S, Goodwin A, Johnsen J, Magee K, Veldhuyzen van Zanten S J O. Metabolic activities of metronidazole-sensitive and -resistant strains of Helicobacter pylori: repression of pyruvate oxidoreductase and expression of isocitrate lyase activity correlate with resistance. J Bacteriol. 1996;178:4822–4829. doi: 10.1128/jb.178.16.4822-4829.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hughes N J, Clayton C L, Chalk P A, Kelly D J. Helicobacter pylori porCDAB and oorDABC genes encode distinct pyruvate:flavodoxin and 2-oxoglutarate:acceptor oxidoreductases which mediate electron transport to NADP. J Bacteriol. 1998;180:1119–1128. doi: 10.1128/jb.180.5.1119-1128.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ito Y, Azuma T, Ito S, Suto H, Miyaji H, Yamazaki Y, Kohli Y, Kuriyama M. Full-length sequence analysis of the vacA gene from cytotoxic and noncytotoxic Helicobacter pylori. J Infect Dis. 1998;178:1391–1398. doi: 10.1086/314435. [DOI] [PubMed] [Google Scholar]
- 22.Jenks P J, Ferrero R L, Labinge A. The role of the rdxA gene in the evolution of metronidazole resistance in Helicobacter pylori. J Antimicrob Chemother. 1999;43:753–758. doi: 10.1093/jac/43.6.753. [DOI] [PubMed] [Google Scholar]
- 23.Jorgensen M A, Manos J, Mendz G L, Hazell S L. The mode of action of metronidazole in Helicobacter pylori: futile cycling or reduction? J Antimicrob Chemother. 1998;41:67–75. doi: 10.1093/jac/41.1.67. [DOI] [PubMed] [Google Scholar]
- 24.Kersulyte D, Mukhopadhyay A K, Velapatiño B, Su W W, Pan Z J, Garcia C, Hernandez V, Valdez Y, Mistry R S, Gilman R H, Yuan Y, Gao H, Alarcon T, Lopez Brea M, Nair G B, Chowdhury A, Datta S, Shirai M, Nakazawa T, Ally R, Segal I, Wong B C Y, Lam S K, Olfat F, Boren T, Engstrand L, Torres O, Schneider R, Thomas J E, Czinn S, Berg D E. Differences in genotypes of Helicobacter pylori from different human populations. J Bacteriol. 2000;182:3210–3218. doi: 10.1128/jb.182.11.3210-3218.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Labigne-Roussel A, Courcoux P, Tompkins L. Gene disruption and replacement as a feasible approach for mutagenesis of Campylobacter jejuni. J Bacteriol. 1988;170:1704–1708. doi: 10.1128/jb.170.4.1704-1708.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lacey S L, Moss S F, Taylor G W. Metronidazole uptake by sensitive and resistant isolates of Helicobacter pylori. J Antimicrob Chemother. 1993;32:393–400. doi: 10.1093/jac/32.3.393. [DOI] [PubMed] [Google Scholar]
- 27.Megraud F. Epidemiology and mechanism of antibiotic resistance in Helicobacter pylori. Gastroenterology. 1998;115:1278–1282. doi: 10.1016/s0016-5085(98)70101-5. [DOI] [PubMed] [Google Scholar]
- 28.Megraud F, Lehn N, Lind T, Bayerdorffer E, O'Morain C, Spiller R, Unge P, van Zanten S Y, Wrangstadh M, Burman C F. Antimicrobial susceptibility testing of Helicobacter pylori in a large multicenter trial: the MACH 2 study. Antimicrob Agents Chemother. 1999;43:2747–2752. doi: 10.1128/aac.43.11.2747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miyaji H, Azuma T, Ito S, Suto H, Ito Y, Yamazaki Y, Sato F, Hirai M, Kuriyama M, Kato T, Kohli Y. Susceptibility of Helicobacter pylori isolates to metronidazole, clarithromycin and amoxycillin in vitro and in clinical treatment in Japan. Aliment Pharmacol Ther. 1997;11:1131–1136. doi: 10.1046/j.1365-2036.1997.00258.x. [DOI] [PubMed] [Google Scholar]
- 30.Mukhopadhyay A K, Kersulyte D, Jeong J Y, Datta S, Ito Y, Chowdhury A, Chowdhury S, Santra A, Bhattacharya S K, Azuma T, Nair G B, Berg D E. Distinctiveness of genotypes of Helicobacter pylori in Calcutta, India. J Bacteriol. 2000;182:3219–3227. doi: 10.1128/jb.182.11.3219-3227.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pan Z-J, Berg D E, van der Hulst R W M, Su W-W, Raudonikiene A, Xia S-D, Dankert J, Tytgat G N J, van der Ende A. High prevalence of vacuolating cytotoxin production and unusual distribution of vacA alleles in Helicobacter pylori from China. J Infect Dis. 1998;178:220–226. doi: 10.1086/515601. [DOI] [PubMed] [Google Scholar]
- 32.Parsonnet J. Helicobacter and gastric adenocarcinoma. In: Parsonnet J, editor. Microbes and malignancy: infection as a cause of human cancers. New York, N.Y: Oxford University Press; 1999. pp. 372–408. [Google Scholar]
- 33.Rasmussen B A, Bush K, Tally F P. Antimicrobial resistance in anaerobes. Clin Infect Dis. 1997;24(Suppl. 1):S110–S120. doi: 10.1093/clinids/24.supplement_1.s110. [DOI] [PubMed] [Google Scholar]
- 34.Raudonikiene A, Zakharova N, Su W W, Jeong J Y, Bryden L, Hoffman P S, Berg D E, Severinov K. Helicobacter pylori with separate beta- and beta′-subunits of RNA polymerase is viable and can colonize conventional mice. Mol Microbiol. 1999;32:131–138. doi: 10.1046/j.1365-2958.1999.01336.x. [DOI] [PubMed] [Google Scholar]
- 35.Rosenberg S M, Thulin C, Harris R S. Transient and heritable mutators in adaptive evolution in the lab and in nature. Genetics. 1999;148:1559–1566. doi: 10.1093/genetics/148.4.1559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Samuelson J. Why metronidazole is active against both bacteria and parasites. Antimicrob Agents Chemother. 1999;43:1533–1541. doi: 10.1128/aac.43.7.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shaw K J, Rather P N, Hare R S, Miller G H. Molecular genetics of aminoglycoside resistance genes and familial relationships of the aminoglycoside-modifying enzymes. Microbiol Rev. 1993;57:138–163. doi: 10.1128/mr.57.1.138-163.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shahinian M L, Passaro D J, Swerdlow D L, Mintz E D, Rodriguez M, Parsonnel J. Helicobacter pylori and epidemic Vibrio cholerae O1 infection in Peru. Lancet. 2000;355:377–378. doi: 10.1016/s0140-6736(99)05143-0. [DOI] [PubMed] [Google Scholar]
- 39.Sisson G, Jeong J-Y, Goodwin A, Bryden L, Rossler N, Lim-Morrison S, Raudonikiene A, Berg D E, Hoffman P S. Metronidazole activation is mutagenic and causes DNA fragmentation in Helicobacter pylori and in Escherichia coli containing a cloned H. pylori rdxA+ (nitroreductase) gene. J Bacteriol. 2000;182:5091–5096. doi: 10.1128/jb.182.18.5091-5096.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Smith M A, Edwards D I. The influence of microaerophilia and anaerobiosis on metronidazole uptake in Helicobacter pylori. J Antimicrob Chemother. 1995;36:453–461. doi: 10.1093/jac/36.3.453. [DOI] [PubMed] [Google Scholar]
- 41.Smith M A, Edwards D I. Oxygen scavenging, NADH oxidase and metronidazole resistance in Helicobacter pylori. J Antimicrob Chemother. 1997;39:347–353. doi: 10.1093/jac/39.3.347. [DOI] [PubMed] [Google Scholar]
- 42.Tankovic J, Lamarque D, Delchier J C, Soussy C J, Labigne A, Jenks P J. Frequent association between alteration of the rdxA gene and metronidazole resistance in french and North African isolates of Helicobacter pylori. Antimicrob Agents Chemother. 2000;44:608–613. doi: 10.1128/aac.44.3.608-613.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Thompson S A, Blaser M J. Isolation of the Helicobacter pylori recA gene and involvement of the recA region in resistance to low pH. Infect Immun. 1995;63:2185–2193. doi: 10.1128/iai.63.6.2185-2193.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tomb J F, White O, Kerlavage A R, Clayton R A, Sutton G G, Fleischmann R D, Ketchum K, Klenk H, Gill S, Dougherty B, Nelson K, Quackenbush J, Zhou L, Kirkness E, Peterson S, Loftus B, Richardson D, Dodson R, Khalak H, Glodek A, McKenney K, Fitzegerald L, Lee N, Adams M, Hickey E, Berg D, Gocayne J, Utterback T, Peterson J, Kelley J, Cotton M, Weidman J, Fujii C, Bowman C, Watthey L, Wallin E, Hayes W, Borodovsky M, Karp P, Smith H, Fraser C, Venter J. The complete genome sequence of the gastric pathogen Helicobacter pylori. Nature. 1997;388:539–547. doi: 10.1038/41483. [DOI] [PubMed] [Google Scholar]
- 45.Townson S M, Boreham P F L, Upcroft P, Upcroft J A. Resistance to the nitroheterocyclic drugs. Acta Trop. 1994;56:173–194. doi: 10.1016/0001-706x(94)90062-0. [DOI] [PubMed] [Google Scholar]
- 46.Trinh S, Haggoud A, Reysset G. Conjugal transfer of the 5- nitroimidazole resistance plasmid pIP417 from Bacteroides vulgatus BV-17: characterization and nucleotide sequence analysis of the mobilization region. J Bacteriol. 1996;178:6671–6676. doi: 10.1128/jb.178.23.6671-6676.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Westblom T U, Czinn S J, Nedrud J G, editors. Gastroduodenal disease and Helicobacter pylori: pathophysiology, diagnosis and treatment. Current topics in microbiology and immunology. Vol. 241. Berlin, Germany: Springer-Verlag; 1999. [Google Scholar]