Abstract
Objectives
The aims of the study were to evaluate the external contamination of hazardous drug vials used in Chinese hospitals and to compare environmental contamination generated by a robotic intelligent dispensing system (WEINAS) and a manual compounding procedure using a biological safety cabinet (BSC).
Methods
Cyclophosphamide, fluorouracil, and gemcitabine were selected as the representative hazardous drugs to monitor surface contamination of vials. In the comparative analysis of environmental contamination from manual and robotic compounding, wipe samples were taken from infusion bags, gloves, and the different locations of the BSC and the WEINAS robotic system. In this study, high-performance liquid chromatography coupled with double mass spectrometer (HPLC-MS/MS) was employed for sample analysis.
Results
(1) External contamination was measured on vials of all three hazardous drugs. The contamination detected on fluorouracil vials was the highest with an average amount up to 904.33 ng/vial, followed by cyclophosphamide (43.51 ng/vial), and gemcitabine (unprotected vials of 5.92 ng/vial, protected vials of 0.66 ng/vial); (2) overall, the environmental contamination induced by WEINAS robotic compounding was significantly reduced compared to that by manual compounding inside the BSC. Particularly, compared with manual compounding, the surface contamination on the infusion bags during robotic compounding was nearly nine times lower for cyclophosphamide (10.62 ng/cm2 vs 90.43 ng/cm2), two times lower for fluorouracil (3.47 vs 7.52 ng/cm2), and more than 23 times lower for gemcitabine (2.61 ng/cm2 vs 62.28 ng/cm2).
Conclusions
The external contamination occurred extensively on some hazardous drug vials that commonly used in Chinese hospitals. Comparison analysis for both compounding procedures revealed that robotic compounding can remarkably reduce environmental contamination.
Keywords: Hazardous drugs, occupational exposure, drug contamination, biological safety cabinet, robotic system
Introduction
As one of most common strategies of cancer treatment, chemotherapy exhibits efficacy on malignancies by delivering one or more hazardous drugs throughout the entire body to target and kill the fast-dividing cells (such as cancer cells). In chemotherapy, hazardous drugs (HDs) affect all cells in the body, not only destroy the cancer cells and prevent them from metastasis, but also damage the normal cells that divide rapidly, thereby inducing severe side effects such as hair loss, 1 infertility, 2 immunosuppression, teratogenicity, 3 and even carcinogenicity. 4 Therefore, exposure to HDs in healthy individuals who are not cancer patients will cause adverse health effects. In the last two decades, numerous studies reported the extensive contamination with HDs in pharmacies and nurse stations in hospitals, and revealed the risk of exposure to HDs among pharmacy staff and nurses who handle these drugs during their daily practice.5 –13 Respecting this risk, the National Institute for Occupational Safety and Health (NIOSH, USA) 14 has issued an alert for preventing occupational exposures to cytotoxic agents. Subsequently, some health care communities including International Society of Oncology Pharmacy Practitioners 15 (ISOPP), the American Society of Health-System Pharmacists 16 (ASHP, USA) and the Association paritaire pour la santé et sécurité du travail du secteur affaires sociales (ASSTSAS, Canada), published the guidelines and practicing standards on safely handing of cytotoxic drugs and recommended the strategies to prevent occupational exposure to HDs.
In recent years, as more than three million new cancer patients are diagnosed annually in China, 17 the administration of HDs has been increased tremendously, which led to a remarkable increase in the risk of occupational exposure to HDs. As such, there are urgent needs to evaluate environmental contamination of HDs in the Chinese market and to develop the effective prevention strategies for protecting Chinese healthcare workers from occupational exposure. Thus, many Chinese hospitals established the unit of pharmacy intravenous admixture service (PIVAS) to centralize the preparation and reconstitution of HDs. The PIVAS units commonly use biological safety cabinets (BSC) and personal protective equipment (PPE) to prepare infusions of HDs in an isolated clean room by well-trained pharmacists. While the application of the PIVAS has resulted in an apparent reduction of occupational exposure of HDs, the surface contamination with HDs is still frequently observed in the working areas of pharmacies. 18 , 19
Most recently, the robot-assisted compounding systems have been introduced to pharmacies based on the advantages of robotic compounding over manual compounding, such as safety improvement of healthcare worker and patient, enhancement of workflow efficiency, and complete accountability of the compounding process. 20 Inspired by the findings that compounding HDs with intelligent robotic systems (such as APOTECAchemo, ARCT, CytoCare, KIRO etc) could dramatically reduce environmental contamination,21 –26 several leading Chinese hospitals have implemented the robotic systems for preparing HDs to further improve their working environment. However, there is no report investigating the environmental contamination generated with such robot-assisted systems in China.
In the present study, with cyclophosphamide, fluorouracil, and gemcitabine as the representative HDs, we measured the residual active ingredients on the three HDs vials to evaluate external contamination of HDs that commonly used in the Chinese hospitals. We further investigated the contamination in our PIVAS center induced both by manual and robotic compounding of HDs through monitoring and comparing the environmental contamination arising from the manual procedure taking place in the BSC and the robotic procedure utilizing the WEINAS robotic dispensing system. As such, our study will not only help for the development of preventive strategies to reduce/or to eliminate occupational exposure, but also provides the first line of evidence in establishing regulations and practice guidelines for automated compounding in China.
Methods
The detection of external contamination on the surface of HDs injection vials
Sample collecting
To investigate the contamination of HDs on injection vials, we selected injections of cyclophosphamide, fluorouracil, and gemcitabine as the representative drugs in the study, based on the fact that they are all the most commonly used HDs in Chinese hospitals. 27 The different resources of the three HDs vials were chosen according the fact that they are frequently used commercial HDs and the preference of oncologists in our hospital. There are two types of gemcitabine injections used in our hospital (e.g. type A of gemcitabine injection (0.2 g/vial) with a protective film covering the vial, and type B of gemcitabine injection (0.2 g/vial) without the protective film), thus this study includes four injection vials of three HDs (Table 1). For each type of injection, we randomly took three different batches and ten vials from each batch injection for monitoring the external contamination.
Table 1.
Information of the tested antineoplastic drugs.
| Drugs | Brand name | Manufacturer | Specification | Batch number |
|---|---|---|---|---|
| Cyclophosphamide | Anderson® | Baxter Oncology GmbH, Germany | 200 mg | 8D231A, 8E240A, 8E237B |
| Fluorouracil | Tianjin Jinyao, China | 10 ml/250 mg | 1805231, 1805261, 1806101 | |
| Gemcitabine (protected vials) | Gem® | Eli Lilly, France | 200 mg | 187424A, C816256A, 187300A |
| Gemcitabine (unprotected vials) | Zephyr® | Jiangsuhausen, China | 200 mg | 619190904, 181103, 619190806 |
Sample processing
The sample processing was performed as described in the publication by Yoshida et al., 28 with a slight modification. In brief, each selected injection vial was placed in a 50 ml glass beaker and methanol was added into the beaker to the height of the lower edge of the vial metal cap. Then the beaker was treated with an ultrasonic cleaner for ten minutes to elute the external contamination from the surface of the vial. After sonication, the eluting was filtered and transferred to a single-necked distillation flask to concentrate the sample on a rotary evaporator. The concentrated sample was then evaporated under nitrogen gas at 60°C and dissolved in 500 µl of 50% methanol. The dissolved sample was centrifuged at 1400 r/min/min for 10 min, and the supernatant was the experimental sample for HPLC-MS/MS analysis.
Detection of environmental contamination generated during compounding of HDs
Study sites
The study was conducted at the PIVAS Center of People's Hospital of Sichuan Province in Chengdu, China. As the first and largest intelligent drug dispensing center in the central and western regions of China, the PIVAS center is equipped with three sets of robotic intelligent systems of WEINAS (Weibang Technology, China) to compound the intravenous HDs for treating almost 50,000 cancer patients annually. Since the implementation of WEINAS robotic systems in 2015, the PIVAS center has provided approximately 159,000 i.v preparations (daily average: 536 preparations) with 40 hazardous drugs every year.
Compounding procedures
Intelligent dispensing system working procedure
The intelligent dispensing system of WEINAS is a robotic system used to prepare hazardous injectable drugs such as antineoplastic drugs. The robotic system is installed in a cleanroom and the internal space of the system is characterized by a negative pressure gradient, laminar airflow, and grade A air quality. According to the system’s instruction, a pharmacist is needed for loading the initial materials and unloading the final products. First, the robotic system automatically identifies the components of a prescription, which have been loaded by the pharmacist, through means of a vision system, optical sensors, and a barcode reader. Then, the robot transfers and positions all the needed components into the compounding area, which is a closed sterile area. Inside the area, the robot prepares the medication according to the prescription data and mechanically repeats the preparative procedure (such as handling vials, syringes and bags; reconstituting and dosing drugs and solvents). After labeling the finished products, the dispensing is completed. The robotic system (WEINAS) contains an exhaust system and a purification equipment that is composed of an HEPA H14 high efficiency filter and an activated carbon HDsorption apparatus. As the internal space of the robotic system is maintained in negative pressure of 5/10 Pa, the harmful air generated during compounding will be sucked into the exhaust system, and then safely discharged into atmosphere after treated with the purification equipment. The purified air is 100 grade and meets the requirement of Comprehensive Air Pollutant Emission Standard (GB16297-199, China). The entire preparation process, including all personnel actions, drug label images, materials in and out, is continuously verified and recorded for traceability.
BSC working procedure
The manual preparation of HDs is conducted in a biosafety cabinet (BSC, II class and A2 type) and requires two pharmacists: one pharmacist performs the preparation work inside the cabinet, and other is responsible for coordinating the operation steps outside the cabinet. Under the laminar flow of the BSC, the pharmacist first dissolves HDs with their corresponding solvents and then dispenses these drugs by using a syringe to mix and transfer the liquid medicine to the infusion bags. After labeling the finished products, the dispensing is completed.
Sample collecting
To evaluate the potential exposure risk of pharmacy staff during robotic compounding with WEINAS robotic system and the manual preparation inside the BSC, we measured the environmental contamination induced by the two working procedures. The samples from both the robotic system and the BSC were obtained every other day over a six-day period, and the samples from the two working procedures were collected by the same pharmacist. Based on risk considerations, the sampling locations were selected in a way as to involve the entire workflow of compounding procedures and the waste transfer channel in PIVAS. The selected monitored sites are seen in Figures 1 and 2.
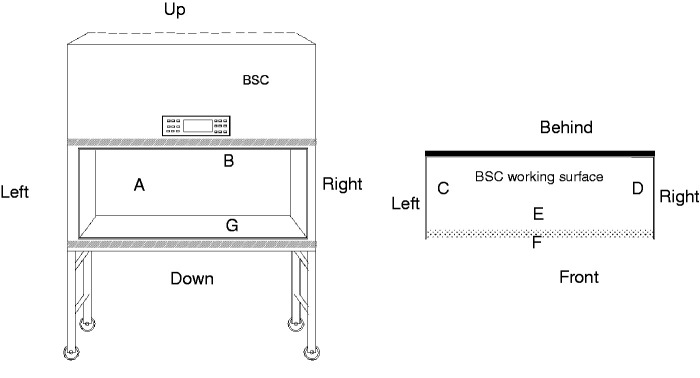
Figure 1.
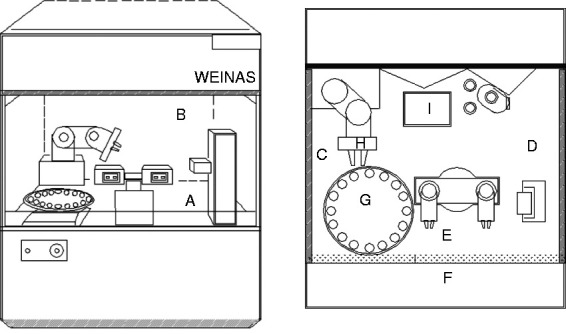
Selected monitored sites at the WEINAS system: (a) surface of internal warehouse substrate; (b) top surface of internal high efficiency filter; (c) internal left surface; (d) internal right surface; (e) internal surface of the front window glass; (f) external surface of the front window glass; (g) surface of the disc; (h) clamping arm; (i) waste inlet.
Figure 2.
Selected monitored sites at the BSC: (a) opposite surface of operating area-opposite plane; (b) top surface of operating area; (c) internal left surface; (d) internal right surface; (e) internal surface of the front window glass; (f) external surface of the front window glass; (g) PHDs of BSC working surface.
Wipe sampling
The medical HDsorbent gauze pieces (5 × 5 cm2, PIAOAN Group, China) were used as the wipes for wiping samples on different locations (Figures 1 and 2), and methanol as the solvent. Before wiping, a piece of gauze was moistened with 10 ml of methanol, and the sample collection was performed by wiping a location with two different directions (up and down, right and left). The wiping samples were placed in the borosilicate glass bottles (50 ml) and stored at 4°C until sample preparation.
Gloves sampling
To protect the skin, the pharmacists must put on the highly impervious gloves used specifically for HDs preparations and cleaning. Gloves were collected and analyzed for contamination after completing the preparation task. As for the preparation in the BSC, only the gloves from the pharmacist who directly performed the preparation were taken for analysis. The sampling area of a pair of gloves was about 600 cm2.
Infusion bags sampling
The outer surfaces of the infusion bags prepared by the BSC and the robotic system were wiped to detect possible contamination. The sampling area of an infusion bag was about 200 cm2.
Sample processing
The each collected sample was placed in a 200 ml glass beaker and 100 ml methanol was added into the beaker for extraction of contaminated drugs. The extraction procedure was identical to the sample processing for detection of surface contamination on injection vials.
Daily cleaning
In our pharmacy, we perform the cleaning procedures on the robotic systems and the BSC at end of compounding. The daily routine cleaning protocol includes three steps: (1) by using a gauze wetted with 5% sodium hypochlorite, wiping the all parts and the surfaces of the robotic system, as well as the surfaces of the BSC; (2) rinsing the surfaces parts with deionized water and a new gauze, and then wiping to dry; (3) wiping with isopropylalcohol. No contamination was detected in the parts and surfaces after cleaning.
Sample analysis
Quantification and detection of HDs in the sample extracts were conducted by a high-performance liquid chromatography (HPLC, 1260, Agilent, America) coupled with a triple quadrupole mass spectrometer (AB-5500Q-TRAP, Waters Corporation, Milford, MA, USA). The HPLC was equipped with an analytical column of Poroshell 120 EC-C18 (2.1 mm × 50 mm, 2.7 μm, Agilent) and a guard column of Poroshell 120 EC-C18 (2.1 mm × 5 mm, 2.7 μm, Agilent). The mobile phase was 0.1% formic acid water: methanol (7:3) for cyclophosphamide and gemcitabine, and 0.1% formic acid water: methanol (3:7) for fluorouracil. Injection volume is 2 μl, column temperature is 35°C.
The mass spectrometer (MS) analysis was carried out on a triple quadrupole mass spectrometer equipped with an electrospray ionization source and operated in multiple reaction monitoring (MRM) mode: Cyclophosphamide : positive ion detection, detection ion pairs of CP (Cyclophosphamide, Sigma, C3250000) and the internal standard antipyrine (Sigma, A5882); Fluorouracil : negative ion detection, detection ion pairs of 5-Fu (5-Fluorouracil, Solarbio, IF0170) and the internal standard 5-bromouracil (5-BrU; Sigma, 8,52,473); Gemcitabine : positive ion detection, detection ion pairs of GEM (Gemcitabine, China Inspection Institute, YZ-100622) and the internal standard Cefaclor Monohydrate (Solarbio, C7350).
In the concentration range of 1.25–500 ng/mL, the standard curve equations for the three drugs: y = 0.00215x + 0.000589, r = 0.9998 (cyclophosphamide); y = 0.036x + 0.0511, r = 0.9993 (fluorouracil); y = 0.0371x + 0.0151, r = 0.9992 (gemcitabine). The lowest limit of quantification of cyclophosphamide was 0.34 ng/ml, fluorouracil was 0.027 ng/ml, and gemcitabine was 0.036 ng/ml. The total sample recovery was 97.09–102.04% for cyclophosphamide, 97.28–102.30% for fluorouracil, and 96.39–102.46% for gemcitabine. Precision: cyclophosphamide <1.76%, fluorouracil <1.94%, gemcitabine <1.89%.
Statistical analysis
Statistical analysis was performed using SAS Statistics Version 9.2 for Windows.
Results
External contamination was detected on the surface of every vial of all three HDs injections, and the protective film resulted in remarkable reduction of contamination
Previous studies revealed that external contamination on HDs vials is a global issue,29 –34 thus we used an analytical method 28 based on HPLC-MS/MS to investigate the external contamination of HDs vials that commonly used in Chinese hospitals, with the three representative HDs. We first examined the external contaminations on the vial surfaces of cyclophosphamide and fluorouracil, the two most frequently used HDs in chemotherapy. We separately collected 10 samples of each drug for each batch, totally with three batches. As reported in Table 2, all tested samples displayed the contamination with the different amounts of active ingredients. The contamination on cyclophosphamide vials were detected with an average amount of 43.51 ng/vial, and fluorouracil vials with an average of 904.33 ng/vial. We did not observe the significant differences in the level of contamination between batches of vials for the two HDs (p > 0.05, Table 2). Of note, we have found that external contamination on some vials of fluorouracil of batch 2 (Lot 2) were much higher than that of Lot 1 and 3. As fluorouracil vial in used this study was one kind of clear glass vial (Tianjin Jinyao, China), we speculate that some vials of Lot 2 experienced accidental leakage during transport and distribution, and therefore contaminated other vials.
Table 2.
External contamination measured on cyclophosphamide and fluorouracil vials.
| No. of samples |
Amount (ng) |
||
|---|---|---|---|
| LOT 1 | LOT 2 | LOT 3 | |
| Cyclophosphamide | |||
| 01 | 41.67 | 36.92 | 33.68 |
| 02 | 38.83 | 39.38 | 31.72 |
| 03 | 47.23 | 32.46 | 44.15 |
| 04 | 46.27 | 30.24 | 46.75 |
| 05 | 43.90 | 38.54 | 44.32 |
| 06 | 44.76 | 42.69 | 48.93 |
| 07 | 41.34 | 39.77 | 43.75 |
| 08 | 59.63 | 41.18 | 51.54 |
| 09 | 49.59 | 47.94 | 43.69 |
| 10 | 56.78 | 43.88 | 53.77 |
| Total (vial × batches) | 30 (10 × 3) | 1305.3 ng | |
| Average | 43.51 ng/vial | ||
| Fluorouracil | |||
| 01 | 917.4 | 803.4 | 633.2 |
| 02 | 933.6 | 819.6 | 677.8 |
| 03 | 815.3 | 823.1 | 646.3 |
| 04 | 803.7 | 853.9 | 632.7 |
| 05 | 693.9 | 3122.1 | 738.1 |
| 06 | 731.2 | 968.3 | 758.1 |
| 07 | 704.9 | 1009.6 | 743.8 |
| 08 | 1006.4 | 1047.9 | 695.7 |
| 09 | 998.7 | 1077.2 | 719.1 |
| 10 | 1004.9 | 1054.9 | 695.2 |
| Total (vial × batches) | 30 (10 × 3) | 27,130 ng | |
| Average | 904.33 ng/vial | ||
With the same sampling approach, we then analyzed and compared the external contamination between the unprotected and the protected gemcitabine injection vials, the two types of gemcitabine injections used in our hospital. The results from Table 3 confirm that every sample was contaminated with gemcitabine, there is, however, a dramatic statistic difference in external contamination between the two types of gemcitabine vials (P < 0.001), with a high contamination of 5.92 ng/vial for the unprotected vials, and in contrast, a very low contamination of 0.66 ng/vial for the gemcitabine vials covered with the protective films. Unexpectedly, we also detected a high contamination (5.40 ng) on one protected gemcitabine vial of batch 2, which may be related to the damage of its protective film during transport and distribution. This result demonstrates that even though contamination cannot be completely eliminated, the protective film covering significantly reduces the external contamination.
Table 3.
External contamination measured on gemcitabine vials.
| No. of samples |
Amount (ng) |
P-value | ||
|---|---|---|---|---|
| LOT 1 | LOT 2 | LOT 3 | ||
| Gemcitabine (protected vials) | <0.001 | |||
| 01 | 0.37 | 0.41 | 0.71 | |
| 02 | 0.48 | 0.46 | 0.73 | |
| 03 | 0.38 | 0.39 | 0.56 | |
| 04 | 0.41 | 0.43 | 0.49 | |
| 05 | 0.68 | 0.49 | 0.43 | |
| 06 | 0.67 | 5.40 | 0.38 | |
| 07 | 0.64 | 0.52 | 0.32 | |
| 08 | 0.36 | 0.73 | 0.38 | |
| 09 | 0.39 | 0.69 | 0.33 | |
| 10 | 0.35 | 0.77 | 0.37 | |
| Total (vial × batches) | 30 (10 × 3) | 19.72 ng | ||
| Average | 0.66 ng/vial | |||
| Gemcitabine (unprotected vials) | ||||
| 01 | 7.87 | 5.13 | 6.06 | |
| 02 | 7.91 | 5.17 | 6.34 | |
| 03 | 8.11 | 5.03 | 5.79 | |
| 04 | 8.21 | 4.87 | 5.91 | |
| 05 | 5.65 | 4.67 | 6.09 | |
| 06 | 6.17 | 4.77 | 6.28 | |
| 07 | 5.88 | 4.96 | 5.93 | |
| 08 | 6.09 | 5.50 | 5.41 | |
| 09 | 6.14 | 5.79 | 5.37 | |
| 10 | 5.97 | 5.61 | 5.02 | |
| Total (vial × batches) | 30 (10 × 3) | 177.7 ng | ||
| Average | 5.92 ng/vial | |||
Taken together, our results indicate that external contamination extensively exists on some HDs vials that commonly used in Chinese hospitals.
Detection of environmental contamination generated by different preparation methods
To assess the contamination induced by the two compounding procedures and to understand which procedure reduces the contamination more efficiently, we compounded the three representative HDs (cyclophosphamide, fluorouracil, and gemcitabine) continuously three days using both the WEINAS robotic system and the BSC with a comparable amount of the HDs (4.33 ± 0.58 g/day vs 4.27 ± 0.58 g/day for cyclophosphamide; 3.00 ± 0 g/day vs 2.58 ± 0.38 g/day for fluorouracil, and 2.20 ± 0.35 g/day vs 2.67 ± 0.35 g/day for gemcitabine, all P > 0.05). Of note, in the experimental setting, we used the unprotected gemcitabine vials (Jiangsuhausen China) for the investigation since the oncologists in our hospital prefer to use the unprotected gemcitabine vials in chemotherapy in order to reduce the financial burden of cancer patients. We subsequently analyzed the contamination patterns from the two compounding procedures. Tables 4 and 5 summarize the contamination from the two working procedures.
Table 4.
Contamination measured in WEINAS robotic system.
| WEINAS |
Mean (/day) |
|||
|---|---|---|---|---|
| Cyclophosphamide (ng/cm2) | Fluorouracil (ng/cm2) | Gemcitabine (ng/cm2) | ||
| Locations | Clamping arma | 31933.33 ± 3356.09 | 680.0 ± 74.36 | 608.67 ± 76.46 |
| Disc | 36.89 ± 3.43 | 0.20 ± 0.025 | 8.87 ± 1.71 | |
| Waste inlet | 36.74 ± 7.53 | 10.26 ± 0.695 | 12.42 ± 0.81 | |
| Surfaces | A (Internal) | 0.60 ± 0.10 | 0.049 ± 0.0078 | 0.087 ± 0.025 |
| B (Internal) | 0.32 ± 0.02 | 0.009 ± 0.001 | 0.0097 ± 0.0015 | |
| C (Internal) | 0.22 ± 0.19 | 0.028 ± 0.0021 | 0.049 ± 0.005 | |
| D (Internal) | 0.4 ± 0.044 | 0.024 ± 0.0035 | 0.029 ± 0.0045 | |
| E (Internal) | 0.44 ± 0.095 | 0.087 ± 0.0035 | 0.032 ± 0.005 | |
| F (External) | 0.13 ± 0.029 | 0.024 ± 0.0036 | 0.012 ± 0.0026 | |
| Gloves | 14.02 ± 0.90 | 2.24 ± 0.090 | 0.63 ± 0.021 | |
| Infusion bags | 10.62 ± 3.09 | 3.47 ± 0.41 | 2.61 ± 0.88 | |
aThe clamping arm is a leak point, not a flat surface.
Table 5.
Contamination measured in BSC.
| BSC |
Mean (/day) |
|||
|---|---|---|---|---|
| Cyclophosphamide (ng/cm2) | Fluorouracil (ng/cm2) | Gemcitabine (ng/cm2) | ||
| Locations | Pad | 103.33 ± 0.38 | 5.24 ± 0.074 | 1.41 ± 0.035 |
| Surfaces | A (Internal) | 1.27 ± 0.0945 | 2.80 ± 0.18 | 0.093 ± 0.007 |
| B (Internal) | 0.11 ± 0.0058 | 0.032 ± 0.0036 | 0.011 ± 0.0012 | |
| C (Internal) | 1.15 ± 0.0458 | 0.29 ± 0.060 | 0.075 ± 0.0059 | |
| D (Internal) | 0.64 ± 0.0586 | 0.36 ± 0.055 | 0.059 ± 0.002 | |
| E (Internal) | 0.13 ± 0.035 | 0.24 ± 0.027 | 0.044 ± 0.0012 | |
| F (External) | 0.58 ± 0.0361 | 0.13 ± 0.015 | 0.069 ± 0.0035 | |
| Gloves | 30.04 ± 0.63 | 9.55 ± 0.33 | 8.56 ± 0.23 | |
| Infusion bags | 90.43 ± 47.43 | 7.52 ± 1.39 | 62.28 ± 19.51 | |
Environmental contamination in the WEINAS robotic system
During the three days of robotic production, we collected a total of 33 wipe samples from different locations of the system (see Figure 1), infusion bags, and gloves. HPLC-MS/MS analysis revealed that all the wipe samples contained contamination of the active ingredients. The details of contamination are seen in Table 4 and Supplemental Table 1.
Among the three HDs, cyclophosphamide contamination was the highest, followed by fluorouracil and gemcitabine. As expected, the highest contamination occurred on the robotic clamping arm, with cyclophosphamide an amount of up to 31933.33 ± 3356.09 ng/day, fluorouracil of 680.0 ± 74.36 ng/day, and gemcitabine of 608.67 ± 76.46 ng/day. High levels of contamination were observed at the disc area (36.89 ± 3.43 ng/cm2/day for cyclophosphamide, 8.87 ± 1.71 ng/cm2/day for gemcitabine, and 0.20 ± 0.025 ng/cm2/day for fluorouracil). Moreover, at the waste inlet, we measured considerable contamination with cyclophosphamide (36.74 ± 7.53 ng/cm2/day), gemcitabine (12.42 ± 0.81 ng/cm2/day), and fluorouracil (10.26 ± 0.695 ng/cm2/day). In addition, we also observed the low level of contamination (<1 ng/cm2/day) on the inside surfaces, and a very low-level contamination on the external of front glass window (<0.2 ng/cm2/day).
Contamination was also detected on infusion bags with the following amounts: cyclophosphamide (10.62 ± 3.09 ng/cm2), fluorouracil (3.47 ± 0.41 ng/cm2), and gemcitabine (2.61 ± 0.88 ng/cm2). In particularly, we detected a mean level of contamination on the gloves used by the pharmacist who only conducted the initial loading and final unloading, with active ingredients at various levels: cyclophosphamide (14.02 ± 0.90 ng/cm2/day on average), fluorouracil (2.24 ± 0.090 ng/cm2/day), and gemcitabine (0.63 ± 0.021 ng/cm2/day).
Environmental contamination in the BSC
A total of 27 wipe samples were collected during the three days of compounding inside the BSC. HPLC-MS/MS analysis indicates that all the samples were contaminated with the three HDs. Similar to the results from the robotic compounding, the contamination level of cyclophosphamide was the highest (Table 5). Unsurprisingly, the pad in working area was the most contaminated location with an amount of 103.33 ± 0.38 ng/cm2/day for cyclophosphamide, 5.24 ± 0.074 ng/cm2/day for fluorouracil, and 1.41 ± 0.035 ng/cm2/day for gemcitabine, respectively.
As seen in Table 5 and supplemental Table 2, low levels of contaminations (<3 ng/cm2/day) were measured on the five inside surfaces. Among them, location A is the most contaminated area (2.80 ± 0.18 ng/cm2/day, 1.27 ± 0.0945 ng/cm2/day, and 0.093 ± 0.007 ng/cm2/day, for fluorouracil, cyclophosphamide, and gemcitabine, respectively). We also detected the residual contamination (<0.6 ng/cm2/day) on the outside surface of the front glass window.
On the surfaces of infusion bags, we observed the high level of contamination with the average amounts of cyclophosphamide up to 90.425 ± 47.43 ng/cm2, gemcitabine of 62.28 ± 19.51 ng/cm2, and fluorouracil of 7.52 ± 1.39 ng/cm2. Moreover, we also observed a mean amount of contamination on the gloves, with the highest of cyclophosphamide, next by fluorouracil and then gemcitabine (average of 30.04 ± 0.63 ng/cm2/day, 9.55 ± 0.33 ng/cm2/day, and 8.56 ± 0.23 ng/cm2/day, respectively).
Comparison of contamination induced by the two compounding methods
Given that surface contamination was detected in all wiping samples collected from both compounding procedures, we then conducted a comparative analysis to assess the reduction level of contamination between the robotic and manual production. Table 6 shows the average contamination concentrations of cyclophosphamide, fluorouracil, and gemcitabine measured at the examined sites during the two compounding procedures. The overall comparison revealed that the WEINAS robotic system considerably reduced the environmental contamination compared to the BSC. Relative to the manual production, the robotic production not only generated less contamination on the external surface of the equipment, but also significantly decreased contamination on the gloves, and especially in the infusion bags, showing a near nine-times reduction for cyclophosphamide, two times for fluorouracil, and more than twenty-three times for gemcitabine.
Table 6.
Comparison of contamination between WEINAS and BSC.
|
Robotic (WEINAS) |
BSC |
P-value | |||
|---|---|---|---|---|---|
| Detection rate (%) | Mean (ng/cm2) | Detection rate (%) | Mean (ng/cm2) | ||
| External surface | |||||
| Cyclophosphamide | 100 | 0.13 ± 0.029 | 100 | 0.58 ± 0.0361 | <0.001 |
| Fluorouracil | 100 | 0.024 ± 0.0036 | 100 | 0.13 ± 0.015 | <0.001 |
| Gemcitabine | 100 | 0.012 ± 0.0026 | 100 | 0.069 ± 0.0035 | <0.001 |
| Gloves | |||||
| Cyclophosphamide | 100 | 14.02 ± 0.90 | 100 | 30.04 ± 0.63 | <0.001 |
| Fluorouracil | 100 | 2.24 ± 0.090 | 100 | 9.55 ± 0.33 | <0.001 |
| Gemcitabine | 100 | 0.63 ± 0.021 | 100 | 8.56 ± 0.23 | <0.001 |
| Infusion bags | |||||
| Cyclophosphamide | 100 | 10.62 ± 3.09 | 100 | 90.43 ± 47.43 | 0.044 |
| Fluorouracil | 100 | 3.47 ± 0.41 | 100 | 7.52 ± 1.39 | 0.008 |
| Gemcitabine | 100 | 2.61 ± 0.88 | 100 | 62.28 ± 19.51 | 0.006 |
Contamination on the surface of the waste transfer channel
We also examined the residual contamination on the wiping samples collected from the surface of waste transfer channel (total area of 17,062 cm2), with the concentration of 0.41 ± 0.021 ng/cm2/day for cyclophosphamide, 0.015 ± 0.002 ng/cm2/day for fluorouracil, and 0.16 ± 0.017 ng/cm2/day for gemcitabine.
Discussion
In general, occupational exposure to HDs is caused through directly skin contact, inhalation, ingestion, and accidental injection.35 –38 The first resource for exposure risk comes from the healthcare workers routinely handling HDs vials that have already been contaminated during the manufacturing or packaging. Previous studies worldwide identified the extensive contamination on the surfaces of commercially HDs vials and packaging including cyclophosphamide, fluorouracil, gemcitabine, ifosfamide, cisplatin, carboplatin, methotrexate, doxorubicin, vincristine, docetaxel, paclitaxel, irinotecan, and other HDs.29 –34, 39 For example, in the U.S., Connor et al showed the contamination ranging from 88 to 69,800 ng/vial on cyclophosphamide vials, and with up to 630,650 ng/vial on fluorouracil packaging; 34 and the Bussieres' group from Canada observed the external contamination on the surfaces of nine frequently used HDs vials, with a maximum of 272 ng/vial on gemcitabine container (vials). 31 In this study, we found contamination on every vial of the three representative HDs, and a particular high amount of 904.33 ng/vial contamination detected on fluorouracil vials, indicating that external contamination might be extensive on HDs vials that commonly used in Chinese hospitals.
Given that the prevalence of external contamination, some manufacturers have developed the strategies that either cover HDs vial with a protective film or load the whole vial into a break-proof plastic container at the end of the filling process to prevent the external contamination. 12 , 39 , 40 By measuring the residual of active ingredients on vial surfaces of two types of gemcitabine injections, we observed that the contamination was dramatically reduced on the protected vials compared to the unprotected vials (0.66 vs 5.92 ng/vial, P < 0.01, Table 3). Consistent with our observation, Schierl et al reported that the contamination on the HDs vials using a safety sheeting system was decreased ten times more compared to the unprotected vials. 40 However, low amounts of contaminations were still detected on the protected vials in the two studies, suggesting that just application of one protective approach is not sufficient to completely eliminate external contamination. One study from Gesy group 13 utilized a combination strategy of decontamination method and a protective sheathing/sleeve had successfully eliminated the external contamination on vials of 5-fluorouracil, cisplatin and methotrexate, indicating the importance of multiple protective approaches in the prevention of occupational exposure of HDs. All the above findings clearly indicate that contamination may have been existed before HDs entered the hospitals, which jeopardizes health care workers with potential exposure risk even when they receive and unpackage HDs. Therefore, healthcare workers should be very cautious for handing HDs vials and packaging. At the same time, these findings should also prompt the manufactures to take more effective measures to eliminate external contamination.
Another main source for occupational exposure is the contamination created during the compounding of HDs. The harmful outcome of this type of exposure risk has been well documented.41–43 Even though many effective safety precautions such as BSC and closed system transfer devices (CSTD) have been adopted in pharmacies to reduce the exposure, environmental contamination is still detected.44–46 Recently, the robot-assisted compounding systems have been introduced to pharmacies to further improve the working environment of compounding HDs. In this study, our results demonstrate that compounding HDs with the WEINAS robotic system created substantially safer working environment over the manual compounding inside the BSC. This conclusion is supported by the following evidence: (1) limiting the exposure to HDs. In the robotic system, compounding HDs is completed by a robotic system in a tightly sealed space, with the pharmacist only conducting simple tasks of loading initial materials and unloading final products. Conversely, in the manual compounding, the pharmacist has to be directly involved in the entire compounding process inside the BSC; (2) a much lower level of contamination was detected on the external surfaces during the robotic compounding compared to that by the BSC compounding (see Table 6); (3) compared with the BSC, much lower amounts of contamination on gloves and infusion bags were measured in the WEINAS robotic system (Table 6). The above evidence strongly demonstrates that the robotic compounding greatly decreased the exposure risk of pharmacy staff. It is necessary to point out that even compounding with the WEINAS robotic system, the contamination on the surfaces of infusion bags and gloves could not be completely eliminated (although it is significantly decreased compared to that with the BSC compounding). The possible sources causing the low level of contamination (<11 ng/cm2, Table 4) may include the contamination that had spread from the already contaminated vials when the pharmacists handled these initial materials, as well as the contamination generated during the robotic compounding. Therefore, pre-cleaning process (such as washing the vials with water) prior to compounding, and replacing new gloves frequently during compounding process should be the effective ways to reduce the contamination. Since skin contact is considered as the main route of occupational exposure, it is imperative to require healthcare workers such as nurses to always use PPE when administrating these infusion bags to cancer patients.
Despite the overall lower contamination observed in the robotic compounding when compared to the manual compounding, we have also monitored the high concentration of the three HDs in the compounding area of the WEINAS robotic system, especially in the robotic clamping arm (Table 6 and Figure 1). The high contamination in the clamping arm was most likely caused by the spillage at the connector of a transfer device. The workflow of the WEINAS robotic compounding includes the steps for reconstitution (for powder drugs), dilution, withdrawals, and transfers. These steps are completed by a transfer device that is composed of a needle, a plastic tube, and a connector, with the clamping arm being a holder to fix the connector. During the compounding process, reconstitution and dilution of HDs created the positive pressure inside the vials that led to the spilling of HDs at the connector, thereby contaminating the clamping arm with high amounts of HDs. As the robotic compounding was performed in a tightly sealed space, the other internal components such as the disc and the waste inlet were contaminated by the spilling as well. The high contaminations in the clamping arm and other internal locations reflect the weakness of the robotic system, and consequently, the manufacture should further improve their design to minimize the contamination. Alternatively, the pre-removing air from infusion bags will be one of effective ways to decrease the positive pressure inside vials so that to decrease the spilling at the connector, thereby reducing the contamination on the clamping arm.
We and others 47 , 48 have observed that cyclophosphamide, relative to fluorouracil and gemcitabine, causes more serious surface contamination regardless of the compounding methods used. This phenomenon may be correlated with the physicochemical characteristics of cyclophosphamide. Compared to fluorouracil and gemcitabine, cyclophosphamide not only has a higher water solubility (43 vs 5.86 mg/ml and <1.2 mg/ml), but also possesses the much lower melting and boiling points (53°C vs 283°C and 168.64°C; 336.1°C vs 561.9°C and 482.7°C, respectively).49 –52 The above properties confer cyclophosphamide is prone to be volatile and aerosolized during compounding operations, which contributes its contamination easily and results in difficult to removal of the contamination. 53 In addition, cyclophosphamide is provided in powder form and needs to be dissolved and diluted before administration. Thus, the additional manipulation also increases the risk of contamination.
It is noteworthy that we not only detected the contamination on every wipe sample, but we also found the residual HDs active ingredients on the waste transfer channel, indicating that low-level contamination exists in our PIVAS center. Compared to other studies, the contamination levels generated with our robotic system is considerably higher than that of other groups. A study using APOTECAchemo reported a much lower contamination of cyclophosphamide on surfaces of infusion bags, compared to our results (0.294 vs 10.26 ng/cm2). 21 The following reasons might correlate with the higher contamination level in our study: (1) different robotic dispensing systems (WEINAS vs APOTECAchemo) used; (2) different regulatory standards and practice guidelines employed; (3) much larger amount of daily drug prepared (536 vs 104 preparations); and (4) most probably, in our PIVAS center, a total of three sets of WINAS robotic systems are installed to meet the needs of chemotherapy for a larger number of cancer patients. Our study was conducted mainly on one set of the robotic equipment because other two sets are required for routine HDs compounding. Therefore, the relative higher contamination, especially in the infusion bags and outside of the equipment, may reflect the total contamination at our PIVAS center generated by all the three sets of robotic systems. The higher contamination related to our robotic compounding highlights the importance of re-establishing the compounding workflow and the primary physical facility considerations during the shift from manual to robotic compounding. 54 In response to the higher contamination in our compounding process with the WINAS system (comparing other robotic systems), a project that aims to reduce/rule out the robotic environmental contamination is under investigation at our PIVAS center.
Due to the repeatability, traceability, accuracy, great productivity, and especially for its high efficiency in the reduction of occupational exposure, using robotics to automate i.v. production has been considered by more and more hospitals and cancer treatment centers worldwide. 54 With millions of new cancer patients are diagnosed every year in China, it is certain that both HDs compounding and occupational exposure risk in Chinese pharmacies will be greatly increased. Thus, the implementation of robotic system for HDs preparation will be the inevitable for healthcare communities to meet the tremendous requirements of HDs preparation. Currently, several leading hospitals in China have already started utilizing robotic intelligent dispensing systems for preparing i.v. infusions of HDs. However, the practice guidelines for the implementation of robotics in i.v. compounding have not been established among the academic communities of Chinese hospitals, and there is also no report to summarize the efficiency and safety of robotic compounding of HDs. At our PIVAS center, we have utilized the three sets of robotic system of WEINAS for compounding of HDs since 2015, and therefore our current results which demonstrate that robotic compounding of HDs generates substantially lower contamination over manual compounding, together with our cumulative experiences in robotic compounding, will be the first line of evidence and foundation for the establishment of regulatory standards and practice guidelines of robotic i.v. compounding in China.
Conclusion
The present study demonstrates that the external contamination occurred extensively on HDs vials that commonly used in Chinese hospitals. Importantly, the study further indicates the compounding HDs with the WEINAS robotic system can dramatically reduce environmental contamination over manual compounding with the BSC. Hence our results will provide the first line of evidence to establish standards and guidelines of automate HDs compounding in Chinese pharmacies.
Supplemental Material
Supplemental material, sj-pdf-1-opp-10.1177_10781552211023571 for Evaluation of external contamination on the vial surfaces of some hazardous drugs that commonly used in Chinese hospitals and comparison between environmental contamination generated during robotic compounding by IV: Dispensing robot vs. manual compounding in biological safety cabinet by Hao ML, Wang T, Zhu JQ, Song YJ, Gong TJ, Zou LK, Liu J and Yan JF in Journal of Oncology Pharmacy Practice
Supplemental material, sj-pdf-2-opp-10.1177_10781552211023571 for Evaluation of external contamination on the vial surfaces of some hazardous drugs that commonly used in Chinese hospitals and comparison between environmental contamination generated during robotic compounding by IV: Dispensing robot vs. manual compounding in biological safety cabinet by Hao ML, Wang T, Zhu JQ, Song YJ, Gong TJ, Zou LK, Liu J and Yan JF in Journal of Oncology Pharmacy Practice
Supplemental material, sj-pdf-3-opp-10.1177_10781552211023571 for Evaluation of external contamination on the vial surfaces of some hazardous drugs that commonly used in Chinese hospitals and comparison between environmental contamination generated during robotic compounding by IV: Dispensing robot vs. manual compounding in biological safety cabinet by Hao ML, Wang T, Zhu JQ, Song YJ, Gong TJ, Zou LK, Liu J and Yan JF in Journal of Oncology Pharmacy Practice
Footnotes
Authors’ contributions: Yan JF conceived and designed the study, interpreted data, and finalized the manuscript; Hao ML performed most experiments and data analysis; Wang T contributed significantly to drug analysis and established methodology of analysis; Zhu JQ, Gong TJ, Song YJ, Zou LK, and Liu J provided assistance in some experiments; Hao ML and Yan JF were involved in manuscript writing; All authors read and approved the manuscript.
Declaration of conflicting interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: Science and Technology Achievement Transformation Project of Sichuan Science and Technology Department (grant number No. 30504010366).
ORCID iD: Junfeng Yan https://orcid.org/0000-0002-1812-6328
Supplemental material: Supplemental material for this article is available online.
References
- 1.Chadha V, Shenoi SD. Hair loss in cancer chemotherapeutic patients. Ind J Dermatol Venereol Leprol 2003; 69: 131–132. [PubMed] [Google Scholar]
- 2.Brydoy M, Fossa SD, Dahl O, et al. Gonadal dysfunction and fertility problems in cancer survivors. Acta Oncol 2007; 46: 480–489. [DOI] [PubMed] [Google Scholar]
- 3.Arnon J, Meirow D, Lewis-Roness H, et al. Genetic and teratogenic effects of cancer treatments on gametes and embryos. Hum Reprod Update 2001; 7: 394–403. [DOI] [PubMed] [Google Scholar]
- 4.Hijiya N, Hudson MM, Lensing S, et al. Cumulative incidence of secondary neoplasms as a first event after childhood acute lymphoblastic leukemia. JAMA 2007; 297: 1207–1215. [DOI] [PubMed] [Google Scholar]
- 5.Touzin K, Bussieres JF, Langlois E, et al. Evaluation of surface contamination in a hospital hematology–oncology pharmacy. J Oncol Pharm Pract 2009; 15: 53–61. [DOI] [PubMed] [Google Scholar]
- 6.Mason HJ, Blair S, Sams C, et al. Exposure to antineoplastic drugs in two UK hospital pharmacy units. Ann Occup Hyg 2005; 49: 603–610. [DOI] [PubMed] [Google Scholar]
- 7.Sessink PJ, Boer KA, Scheefhals AP, et al. Occupational exposure to antineoplastic agents at several departments in a hospital. Environmental contamination and excretion of cyclophosphamide and ifosfamide in urine of exposed workers. Int Arch Occup Environ Health 1992; 64: 105–112. [DOI] [PubMed] [Google Scholar]
- 8.Sessink PJ, Wittenhorst BC, Anzion RB, et al. Exposure of pharmacy technicians to antineoplastic agents: reevaluation after additional protective measures. Arch Environ Health 1997; 52: 240–244. [DOI] [PubMed] [Google Scholar]
- 9.Connor TH, Anderson RW, Sessink PJ, et al. Surface contamination with antineoplastic agents in six cancer treatment centers in Canada and the United States. Am J Health Syst Pharm 1999; 56: 1427–1432. [DOI] [PubMed] [Google Scholar]
- 10.Hall AL, Demers PA, Astrakianakis G, et al. Estimating national-level exposure to antineoplastic agents in the workplace: CAREX Canada findings and future research needs. Ann Work Expo Health 2017; 61: 656–658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McDevitt JJ, Lees PS, McDiarmid MA. Exposure of hospital pharmacists and nurses to antineoplastic agents. J Occup Med 1993; 35: 57–60. [PubMed] [Google Scholar]
- 12.Cotteret C, Secretan PH, Gilles-Afchain L, et al. External contamination of antineoplastic drug vials: an occupational risk to consider. Eur J Hosp Pharm 2020; 0: 1–3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Power LA, Sessink PJ, Gesy K, et al. Hazardous drug residue on exterior vial surfaces: evaluation of a commercial manufacturing process. Hosp Pharm 2014; 49: 355–362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Alert N. Preventing occupational exposures to antineoplastic and other hazardous drugs in healthcare settings. U.S. Department of Health and Human Services, Public Health Service, Centers for Disease Control and Prevention, National Institute for Occupational Safety and Health, DHHS (NIOSH) Publication No 2004-165, 2004.
- 15.ISOPP standards of practice. Safe handling of cytotoxics. J Oncol Pharm Pract 2007; 13: 1–81. [DOI] [PubMed] [Google Scholar]
- 16.Power LA, Coyne JW. ASHP guidelines on handling hazardous drugs. Am J Health Syst Pharm 2018; 75: 1996–2031. [DOI] [PubMed] [Google Scholar]
- 17.Feng RM, Zong YN, Cao SM, et al. Current cancer situation in China: good or bad news from the 2018 global cancer statistics? Cancer Commun (Lond) 2019; 39: 22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang J, Bao J, Wang R, et al. A multicenter study of biological effects assessment of pharmacy workers occupationally exposed to antineoplastic drugs in pharmacy intravenous admixture services. J Hazard Mater 2016; 315: 86–92. [DOI] [PubMed] [Google Scholar]
- 19.Zhang X, Zheng Q, Lv Y, et al. Evaluation of adverse health risks associated with antineoplastic drug exposure in nurses at two Chinese hospitals: the effects of implementing a pharmacy intravenous admixture service. Am J Ind Med 2016; 59: 264–273. [DOI] [PubMed] [Google Scholar]
- 20.Rodriguez-Gonzalez CG, Herranz-Alonso A, Escudero-Vilaplana V, et al. Robotic dispensing improves patient safety, inventory management, and staff satisfaction in an outpatient hospital pharmacy. J Eval Clin Pract 2019; 25: 28–35. [DOI] [PubMed] [Google Scholar]
- 21.Schierl R, Masini C, Groeneveld S, et al. Environmental contamination by cyclophosphamide preparation: comparison of conventional manual production in biological safety cabinet and robot-assisted production by APOTECAchemo. J Oncol Pharm Pract 2016; 22: 37–45. [DOI] [PubMed] [Google Scholar]
- 22.Sessink PJ, Leclercq GM, Wouters DM, et al. Environmental contamination, product contamination and workers exposure using a robotic system for antineoplastic drug preparation. J Oncol Pharm Pract 2015; 21: 118–127. [DOI] [PubMed] [Google Scholar]
- 23.Bhakta SB, Colavecchia AC, Coffey W, et al. Implementation and evaluation of a sterile compounding robot in a satellite oncology pharmacy. Am J Health Syst Pharm 2018; 75: S51–S57. [DOI] [PubMed] [Google Scholar]
- 24.Deljehier T, Bougueon G, Heloury J, et al. Simulation program of a cytotoxic compounding robot for monoclonal antibodies and anti-infectious sterile drug preparation. J Oncol Pharm Pract 2019; 25: 1873–1890. [DOI] [PubMed] [Google Scholar]
- 25.Jobard M, Brandely-Piat ML, Chast F, et al. Qualification of a chemotherapy-compounding robot. J Oncol Pharm Pract 2020; 26: 312–324. [DOI] [PubMed] [Google Scholar]
- 26.Buning AW, Geersing TH, Crul M. The assessment of environmental and external cross-contamination in preparing ready-to-administer cytotoxic drugs: a comparison between a robotic system and conventional manual production. Int J Pharm Pract 2020; 28: 66–74. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ferrario A, Stephens P, Guan X, et al. Sales of anti-cancer medicines; China, Indonesia, Kazakhstan, Malaysia, Philippines and Thailand. Bull World Health Org 2020; 98: 467–474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida AT, Hanada Y, Morichika T, et al. Study on surface contamination of antitumor drug containers based on the findings from a questionnaire survey for pharmaceutical companies. Med Pharm (Jpn) 2015; 41: 163–172. [Google Scholar]
- 29.Naito T, Osawa T, Suzuki N, et al. Comparison of contamination levels on the exterior surfaces of vials containing platinum anticancer drugs in Japan. Biol Pharm Bull 2012; 35: 2043–2049. [DOI] [PubMed] [Google Scholar]
- 30.Palamini M, Gagne S, Caron N, et al. Cross-sectional evaluation of surface contamination with 9 antineoplastic drugs in 93 Canadian healthcare centers: 2019 results. J Oncol Pharm Pract 2020; 26: 1921–1930. [DOI] [PubMed] [Google Scholar]
- 31.Hilliquin D, Bussieres JF. External contamination of antineoplastic drug containers from a Canadian wholesaler. J Oncol Pharm Pract 2020; 26: 423–427. [DOI] [PubMed] [Google Scholar]
- 32.Schierl R, Bohlandt A, Nowak D. Guidance values for surface monitoring of antineoplastic drugs in German pharmacies. Ann Occup Hyg 2009; 53: 703–711. [DOI] [PubMed] [Google Scholar]
- 33.Muller-Ramirez C, Squibb K, McDiarmid M. Measuring extent of surface contamination produced by the handling of antineoplastic drugs in low- to Middle-income country oncology health care settings. Arch Environ Occup Health 2017; 72: 289–298. [DOI] [PubMed] [Google Scholar]
- 34.Connor TH, Sessink PJ, Harrison BR, et al. Surface contamination of chemotherapy drug vials and evaluation of new vial-cleaning techniques: results of three studies. Am J Health Syst Pharm 2005; 62: 475–484. [DOI] [PubMed] [Google Scholar]
- 35.Fransman W, Vermeulen R, Kromhout H. Dermal exposure to cyclophosphamide in hospitals during preparation, nursing and cleaning activities. Int Arch Occup Environ Health 2005; 78: 403–412. [DOI] [PubMed] [Google Scholar]
- 36.Hedmer M, Wohlfart G. Hygienic guidance values for wipe sampling of antineoplastic drugs in Swedish hospitals. J Environ Monit 2012; 14: 1968–1975. [DOI] [PubMed] [Google Scholar]
- 37.McDiarmid MA, Oliver MS, Roth TS, et al. Chromosome 5 and 7 abnormalities in oncology personnel handling anticancer drugs. J Occup Environ Med 2010; 52: 1028–1034. [DOI] [PubMed] [Google Scholar]
- 38.Fransman W, Kager H, Meijster T, et al. Leukemia from dermal exposure to cyclophosphamide among nurses in the Netherlands: quantitative assessment of the risk. Ann Occup Hyg 2014; 58: 271–282. [DOI] [PubMed] [Google Scholar]
- 39.Fleury-Souverain S, Nussbaumer S, Mattiuzzo M, et al. Determination of the external contamination and cross-contamination by cytotoxic drugs on the surfaces of vials available on the Swiss market. J Oncol Pharm Pract 2014; 20: 100–111. [DOI] [PubMed] [Google Scholar]
- 40.Schierl R, Herwig A, Pfaller A, et al. Surface contamination of antineoplastic drug vials: comparison of unprotected and protected vials. Am J Health Syst Pharm 2010; 67: 428–429. [DOI] [PubMed] [Google Scholar]
- 41.Connor TH, DeBord DG, Pretty JR, et al. Evaluation of antineoplastic drug exposure of health care workers at three university-based US cancer centers. J Occup Environ Med 2010; 52: 1019–1027. [DOI] [PubMed] [Google Scholar]
- 42.Sottani C, Porro B, Imbriani M, et al. Occupational exposure to antineoplastic drugs in four Italian health care settings. Toxicol Lett 2012; 213: 107–115. [DOI] [PubMed] [Google Scholar]
- 43.Sabatini L, Barbieri A, Lodi V, et al. Biological monitoring of occupational exposure to antineoplastic drugs in hospital settings. Med Lav 2012; 103: 394–401. [PubMed] [Google Scholar]
- 44.Zock MD, Soefje S, Rickabaugh K. Evaluation of surface contamination with cyclophosphamide following simulated hazardous drug preparation activities using two closed-system products. J Oncol Pharm Pract 2011; 17: 49–54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wick C, Slawson MH, Jorgenson JA, et al. Using a closed-system protective device to reduce personnel exposure to antineoplastic agents. Am J Health Syst Pharm 2003; 60: 2314–2320. [DOI] [PubMed] [Google Scholar]
- 46.Yoshida J, Tei G, Mochizuki C, et al. Use of a closed system device to reduce occupational contamination and exposure to antineoplastic drugs in the hospital work environment. Ann Occup Hyg 2009; 53: 153–160. [DOI] [PubMed] [Google Scholar]
- 47.Chauchat L, Tanguay C, Caron NJ, et al. Surface contamination with ten antineoplastic drugs in 83 canadian centers. J Oncol Pharm Pract 2019; 25: 1089–1098. [DOI] [PubMed] [Google Scholar]
- 48.Harrison BR, Peters BG, Bing MR. Comparison of surface contamination with cyclophosphamide and fluorouracil using a closed-system drug transfer device versus standard preparation techniques. Am J Health Syst Pharm 2006; 63: 1736–1744. [DOI] [PubMed] [Google Scholar]
- 49.ChemSpider. Cyclophosphamide, www.chemspider.com/Chemical-Structure.2804.html (2015, accessed May, 11 2020).
- 50.ChemSpider. Fluorouracil, www.chemspider.com/Chemical-Structure.3268.html (2015, accessed May, 11 2020).
- 51.ChemSpider. Gemcitabine, www.chemspider.com/Chemical-Structure.54753.html (2015, accessed May, 11 2020).
- 52.DrugBank. Fluorouracil, www.drugbank.ca/drugs/DB00544 (2015, accessed May, 11 2020).
- 53.Connor TH, Anderson RW, Sessink PJ, et al. Effectiveness of a closed-system device in containing surface contamination with cyclophosphamide and ifosfamide in an i.v. admixture area. Am J Health Syst Pharm 2002; 59: 68–72. [DOI] [PubMed] [Google Scholar]
- 54.Yaniv AW, Orsborn A, Bonkowski JJ, et al. Robotic i.v. medication compounding: recommendations from the international community of APOTECAchemo users. Am J Health Syst Pharm 2017; 74: e40–e46. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-pdf-1-opp-10.1177_10781552211023571 for Evaluation of external contamination on the vial surfaces of some hazardous drugs that commonly used in Chinese hospitals and comparison between environmental contamination generated during robotic compounding by IV: Dispensing robot vs. manual compounding in biological safety cabinet by Hao ML, Wang T, Zhu JQ, Song YJ, Gong TJ, Zou LK, Liu J and Yan JF in Journal of Oncology Pharmacy Practice
Supplemental material, sj-pdf-2-opp-10.1177_10781552211023571 for Evaluation of external contamination on the vial surfaces of some hazardous drugs that commonly used in Chinese hospitals and comparison between environmental contamination generated during robotic compounding by IV: Dispensing robot vs. manual compounding in biological safety cabinet by Hao ML, Wang T, Zhu JQ, Song YJ, Gong TJ, Zou LK, Liu J and Yan JF in Journal of Oncology Pharmacy Practice
Supplemental material, sj-pdf-3-opp-10.1177_10781552211023571 for Evaluation of external contamination on the vial surfaces of some hazardous drugs that commonly used in Chinese hospitals and comparison between environmental contamination generated during robotic compounding by IV: Dispensing robot vs. manual compounding in biological safety cabinet by Hao ML, Wang T, Zhu JQ, Song YJ, Gong TJ, Zou LK, Liu J and Yan JF in Journal of Oncology Pharmacy Practice