Abstract
Objective
This paper reviews the research of platelet-rich plasma (PRP) in articular cartilage injury repair, to assess the mechanism, utilization, and efficacy of PRP in the treatment of articular cartilage injury, hoping to provide a theoretical basis for the clinical application of PRP in the future.
Materials and Methods
A comprehensive database search on PRP applications in cartilage repair was performed. Among them, the retrieval time range of PRP in clinical trials of repairing knee cartilage injury was from January 1, 2021 to January 1, 2022. Non-clinical trials and studies unrelated to cartilage injury were excluded.
Result
PRP can affect inflammation, angiogenesis, cartilage protection, and cellular proliferation and differentiation after articular cartilage injury through different pathways. In all, 13 clinical trials were included in the analysis.
Conclusion
PRP is an emergent therapeutic approach in tissue engineering. Most studies reported that PRP has a positive effect on cartilage injury, improving the joint function, meanwhile there is a lack of standardized standards. The technology of PRP in the repair and treatment of articular cartilage injury is worthy of further research.
Keywords: platelet rich plasma, articular cartilage, growth factor
Introduction
Articular cartilage relieves stress and lubricates joints, playing a crucial role in joint movement. It is mainly composed of chondrocytes and cartilage matrix. When cartilage is damaged, it secretes metalloproteinase and inflammatory factors. The composition of extracellular matrix changes, and the cartilage synthesis and catabolism are imbalanced, resulting in the degradation of the cartilage matrix, as well as articular cartilage lesions are formed. 1 Once articular cartilage is damaged, it is difficult to recover, mainly for 2 reasons: first, articular cartilage has no nerves and blood vessels, and its nutrients must be obtained from articular fluid, which can provide very limited nutrition, and second, articular chondrocytes have poor proliferation, differentiation, and migration ability and are difficult to aggregate to the injured site for articular cartilage repair. 2
Articular cartilage injury will affect the structure and function of the joint. If not treated in time, it will cause joint pain and movement disorder, eventually lead to osteoarthritis. 3 It is reported that people with knee cartilage injury are 7.4 times more likely to suffer from osteoarthritis than those without cartilage injury. 4
So far, the treatment methods for articular cartilage injury mainly include conservative treatment and surgical treatment. However, these treatment methods can only relieve pain, delay lesions, or partially repair cartilage injury, and it is difficult to fundamentally solve the problem of poor regeneration ability of articular cartilage. 5 At present, tissue engineering provides a new choice for the repair of articular cartilage injury. Growth factor, 1 of the 3 elements of tissue engineering, is produced by tissue and cells, which has a significant regulatory effect on cell growth and differentiation.
Platelet-rich plasma (PRP) is a platelet concentrate obtained by whole blood centrifugation. Its platelet concentration is higher than that of normal whole blood and contains a variety of growth factors. PRP can reduce the occurrence of inflammation, improved angiogenesis, and promote the proliferation and differentiation of chondrocytes by secreting a huge amount of cytokines, chemokines, and growth factors, so as to promote the healing of bone and cartilage injuries, which has been widely used in orthopedic injury-–related diseases.6,7 Riewruja et al. found that the levels of cytokines in PRP were significantly higher than those in platelet-poor plasma (PPP) in vitro. In further clinical studies, patients with knee osteoarthritis treated with PRP showed improvement in physical function and pain, suggesting that intra-articular injection of PRP may be a potential therapeutic strategy for relieving knee pain. 8 In addition to these growth factors, platelets can also secrete exosomes, which have a similar effect to their parent material. 9 It has been reported that PRP exosomes also have protective effects on articular cartilage recently. 10 The main advantages of PRP for treatment of articular cartilage injury are simple production, high safety, minimal adverse reactions, and no foreign body rejection. 11
Through the efforts of many graduate students, although PRP has made some achievements in the repair and treatment of articular cartilage injury, there are still some disputes about its mechanism of action, clinical treatment effect and preparation method, because of its complex component. Based on this, in this review, we focus on the research progress of PRP in the repair and treatment of articular cartilage injury.
Materials and Methods
Literature search was performed using the databases of PubMed and “Clinical Trials. gov.” The literature was searched using the keywords “platelet rich plasma OR PRP,” “cartilage injury AND platelet rich plasma,,” “cartilage injury AND PRP”, “cartilage AND platelet rich plasm,,” “osteoarthritis AND platelet rich plasm,,” and “cartilage injury AND platelet rich plasm AND treatment.”
Inclusion criteria were as follows: articles related to the preparation, mechanism, and application of PRP. Experimental results of PRP for the treatment of articular and osteochondral defects in human and animal studies. The article exploring the clinical effects of PRP was only included in the human clinical trial, and the time range was from January 1, 2021 to January 1, 2022.
We excluded articles unrelated to the topic of this article, and excluded all reviews, duplicates, and non-English studies. A review of the abstracts of all the identified articles was performed. If necessary, the full article was obtained for further evaluation of inclusion and exclusion criteria. In addition, all references included in the study were reviewed to verify that no relevant articles were omitted from the review.
Results
Preparation of PRP
PRP can be obtained from autologous or allogeneic sources. At present, most clinical studies are treated with autologous PRP,12-15 probably with a consideration that autologous PRP has less rejection and less possibility of adverse reactions. However, there were still a small number of studies that had used allogeneic PRP for treatment, due to the poor health of patients, blood collection restrictions, and other reasons. PRP obtained from blood transfusions in good health has more stable and reliable performance. 16 In vitro, some researchers have studied whether allogeneic PRP affects the differentiation of peripheral blood monocytes into dendritic cells, and tested the immune response and tissue repair ability of allogeneic PRP. The results show that allogeneic PRP has low immune response, which can promote the differentiation of monocytes into anti-inflammatory cells and is conducive to wound healing. 17 An animal study injected allogeneic PRP into the radius defect model of New Zealand rabbits, observing and comparing the therapeutic effect of allogeneic PRP in repairing bone defects. With a result that allogeneic PRP had good application prospect in the treatment of bone defects, with low immunogenicity, good healing effect, and would not bring additional health burden to patients. 18 A study using autologous, allogeneic, and xenogeneic PRP to treat wounds found that it can improve and accelerate the wound healing process regardless of the source of PRP. 19 In a prospective, case-control cohort clinical trial, there are 75 diabetic patients divided into autologous PRP group and allogeneic PRP group according to their wishes. After treatment, there was no statistically significant difference between the 2 groups, suggesting that allogeneic PRP could be used as a feasible, effective, and safe therapy when autologous PRP is limited. 20 In another clinical trial, 60 people with chronic wounds were treated in groups. The control group only received standard care, while the PRP group was treated with allogeneic PRP combined with a standard care. The results indicated that the combination of standard care and allogeneic PRP could significantly shorten the healing time of chronic wounds, suggesting that allogeneic PRP is an effective and safe adjuvant treatment of chronic wounds. 21 In most cases, it has not been determined that allogeneic PRP has significant side effects or adverse reactions, that is, autologous/allogeneic sterile PRP are clinically safe. Allogeneic PRP provides a safe alternative when autologous PRP is inappropriate. 16 However, it is uncertain whether PRP preparations (autologous or allogeneic) are clinically useful in tissue regenerative techniques, besides, there is no standardized scheme for the preparation of PRP. 22
The preparation methods include commercial preparation system and centrifugation, which is mainly prepared by centrifugation. 23 The basic principle of preparing PRP by centrifugation is that the sedimentation coefficients of platelets, plasma and blood cells are different. The density of platelets is between plasma and blood cells. After centrifugation, the whole blood is separated into 3 layers: upper plasma layer, middle platelet, and leukocyte layer and lower erythrocyte layer. Platelet concentrate in plasma can be collected from the middle layer, that is, PRP. 24 Due to the large centrifugal force and long duration of centrifugal force, a layer of white membrane formed by platelets and leukocytes cannot separate leukocytes alone. Although leukocytes play a key role in tissue repair and provide ideal protection against infectious factors, their pro-inflammatory and immune effects can also cause adverse local reactions, which will weaken the role of platelets, that is, low leukocyte PRP has greater benefits.25-27 Therefore, the centrifugal conditions for the preparation of PRP need to be optimized.
Centrifugation can be divided into primary centrifugation, secondary centrifugation, and so on. Anitua method is the first reported and recognized method for preparing PRP by 1-step centrifugation. 28 At present, the mainstream PRP preparation method is the secondary centrifugation method. The secondary centrifugation method is to obtain PRP through twice centrifugation. According to different centrifugal force and time, it can be divided into Landesberg method, Okuda method, Petrungaro method, and so on.29-33 Researchers have different opinions on which method to prepare PRP is more efficient. For example, Sabarish et al. 34 believe that the PRP platelet count and enrichment percentage obtained by Marx method are higher, while Efeoglu et al. 35 prove that the PRP prepared by Landesberg method has the best quality. Table 1 summarizes several common centrifugal methods.
Table 1.
Several Common Centrifugal Methods with Their Speed and Time.
| Method | First Centrifugation | Second Centrifugation |
|---|---|---|
| Anitua 28 | 160xg, 6 min | — |
| Petrungaro 29 | 500xg, 6 min | 1000xg, 6 min |
| Landesberg et al. 30 and Efeoglu et al. 35 | 200xg, 10 min | 200xg, 10 min |
| Aghaloo et al. 33 | 215xg, 10 min | 863xg, 10 min |
| Marx et al. 31 and Sabarish et al. 34 | 1,000 rpm, 4 min | 800 rpm, 9 min |
| Okuda et al. 32 | 2,400 rpm, 10 min | 3,600 rpm, 15 min |
To sum up, the preparation of PRP has not formed a clear standard scheme so far. Centrifugation is more widely used because of its relatively low feasibility and cost. Among them, secondary centrifugation can obtain PRP with higher platelet concentration, but there is no best scheme for its specific centrifugation speed and centrifugation time. Platelet concentration is directly related to blood collection volume and equipment centrifugal force. Due to the great heterogeneity between PRP separation methods, its preparation method must be further standardized.
Mechanisms of PRP
PRP contains a variety of growth factors (Table 2), such as platelet-derived growth factor (PDGF) and transforming growth factor β1 (TGF-β1), insulin-like growth factor 1 (IGF-1), fibroblast growth factor 2 (FGF-2), hepatocyte growth factor (HGF), and vascular endothelial growth factor A (VEGF-A). 36 The beneficial effects of PRP therapy are mainly mediated by growth factors, which are mainly present in the α granules of platelets. 37 When platelets are activated, growth factors are transformed into active state. Through endocrine, autocrine, paracrine, and other methods, they can improve the inflammatory response of patients with articular chondropathy, promote cell proliferation and differentiation, angiogenesis and tissue regeneration, promote the repair of articular cartilage injury, and then improve the clinical symptoms of patients. 38 Therefore, the mechanism of PRP in cartilage injury and repair is divided into 4 aspects: anti-inflammation, angiogenesis, cartilage protection, and cellular proliferation and differentiation.
Table 2.
Partial Growth Factors in Platelet Rich Plasma and Their Role in Articular Cartilage Injury Repair.
| Growth Factors | Role in Articular Cartilage Injury Repair | References |
|---|---|---|
| Platelet-derived growth factor (PDGF) | Promotes mitosis of mesenchymal stem cells and osteoblasts, regulate collagen synthesis, and improve vascular formation. | Civinini et al. 39 and Giusti et al. 40 |
| Transforming growth factor (TGF)-β1, β2 | Promotes the proliferation and differentiation of chondrocytes, enhances the expression of matrix metalloproteinase inhibitor protein, inhibits the interference of interleukin-1β to chondrocytes, prevents chondrocyte apoptosis, and promote angiogenesis. | Xie et al. 41 and Zhai et al. 42 |
| Vascular endothelial growth factor (VEGF) | Increases angiogenesis, vascular permeability, and stimulates endothelial cell mitosis | Deppermann and Kubes 43 and Apte et al. 44 |
| Fibroblast growth factor (FGF) | Accelerates the differentiation of mesenchymal stem cells into chondrocytes, stimulates blood vessel formation, increases collagen production. | Chen et al. 45 and Jia et al. 46 |
| Insulin-like growth factor (IGF) | Regulates cell proliferation and differentiation, promotes the secretion of proteoglycan, collagen proteins, inhibits the pro-inflammatory NFκB pathway, and stimulates the proliferation and differentiation of osteoblasts and chondrocytes. | Everts et al. 47 and Berke et al. 48 |
| Hepatocyte growth factor (HGF) | Regulates the growth and motility of epithelial/endothelial cells and enhances the formation of blood vessels. | Yu et al. 49 |
| Connective tissue growth factor (CTGF) | Promotes platelet adhesion, leukocyte migration and angiogenesis, and regulates collagen synthesis. | Ornetti et al. 50 and Xu et al. 51 |
| Epidermal growth factor (EGF) | Regulates cell proliferation, apoptosis, promote the migration of fibroblasts and epithelialization. | Ai et al. 52 |
Anti-inflammation
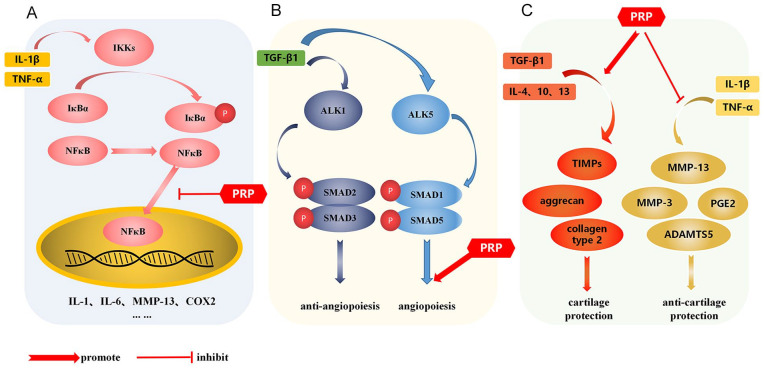
Wound healing can be divided into 3 stages: inflammation, proliferation, and remodeling. The initial inflammatory stage is characterized by hemostasis, platelets forming blood clots, release of growth factors, and activation and attraction of inflammatory cells, such as neutrophils and macrophages, to the injury site. 53 In the early stages of articular cartilage injury, inflammatory cells often produce proinflammatory cytokines, such as interleukin-1β (IL-1β) and tumor necrosis factor-alpha (TNF-α). 54 These proinflammatory cytokines are mediated by a variety of pathways, the most important of which is nuclear factor kappa-B (NFκB) signaling pathway; once NFκB inhibitor kinase (IKK) is activated by cytokines, NFκB inhibitor is αphosphorylated(p-IκBα) and activates NFκB translocation into nucleus, NFκB then mediates the expression of a variety of inflammatory genes, including IL-1β, interleukin-6 (IL-6), interleukin-8 (IL-8), matrix metalloproteinase (MMPs), and so on (Fig. 1A). These catabolic and inflammatory factors further strengthen inflammation and stimulate tissue destruction. Inhibiting inflammation can improve symptoms and joint function in patients with articular cartilage injury.48,55,56
Figure 1.
Mechanism of PRP. (A) PRP inhibits NFκB signaling and improves inflammatory response after articular cartilage injury. (B) TGF-β1 has a dual effect on angiogenesis. When TGF-β1 binds ALK1, it induces phosphorylation of SMAD1/SMAD5, leading to angiogenesis. On the other hand, when TGF-β1 binds ALK5, SMAD2/SMAD3 is phosphorylated and angiogenesis is inhibited. (C) PRP promotes chondrocyte secretion of a variety of anabolic factors (TGF-β1, IL-4, IL-10 and IL-13) to stimulate the synthesis of extracellular matrix, thereby promoting cartilage repair. Meanwhile, PRP inhibits the increased expression of MMP-1, MMP-3 and so on caused by inflammatory factors, reducing the degradation of extracellular matrix, thus improving articular cartilage injury. PRP = platelet rich plasma; TGF-β1 = transforming growth factor β1; IL-4 = interleukin-4; IL-10 = interleukin-10; IL-13 = interleukin-13; MMP = matrix metalloproteinase; IKK = inhibitor kinase; TNF-α = tumor necrosis factor-alpha; TIMPs = tissue inhibitors of metalloproteinases.
In recent years, studies have found that PRP can interfere with NFκB signaling pathway plays an anti-inflammatory role. Xu et al. cultured PRP with bone marrow mesenchymal stem cells. PRP release can reduce IL-1β induced NFκB is activated, thereby inhibiting the inflammatory process of chondrocytes. 57 Xin et al. 58 founded that PRP or PRP combined with alendronate inhibited NFκB signaling pathway can reduce MMP-13, inflammatory factors IL-18 and IL-1β, TNF-α mRNA expression and p-IκBα/IκBα, p-p65/p65 protein expression, while increasing type II collagen, thus promoting chondrocyte proliferation and improving cartilage matrix metabolism. Zhao et al. 59 reported that PRP inhibited the imbalance of matrix-related factors induced by adriamycin, reduced the protein level of adriamycin-induced inflammatory markers, and blocked adriamycin-induced articular chondrocyte IκBα and NFκB phosphorylation can improve chondrocyte apoptosis and weaken cartilage injury.
Angiogenesis
Angiogenesis is closely related to the occurrence of chronic inflammation, and inflammatory mediators can directly or indirectly stimulate angiogenesis.60,61 In the inflammation of articular cartilage injury, inflammatory cells not only secrete inflammatory mediators to induce angiogenesis, but also secrete factors to stimulate endothelial cells and fibroblasts to produce angiogenic factors. 62 Angiogenesis is regulated by a variety of activators and inhibitors, and PRP contains a variety of angiogenesis promoting factors, such as VEGF, FGF, PDGF, HGF, and TGF-β.63,64 VEGF is the most effective angiogenesis promoting growth factor described so far. It binds to receptors and stimulates endothelial cell proliferation, migration, and tube formation.43,44 FGF is a stronger angiogenic factor than VEGF, which stimulates angiogenesis and fibroblast proliferation and can promote angiogenesis. Studies have proved that FGF-2 promotes angiogenesis by activating serine arginine-rich splicing factor 1/ serine arginine-rich splicing factor 3/ serine arginine-rich protein specific kinase 1 (SRSF1/SRSF3/SRPK1) network.46,65,66 HGF stimulates endothelial cell proliferation and migration through its specific receptor cellular-mesenchymal epithelial transition factor (c-Met) and promotes angiogenesis. 49 TGF-β1 has a dual effect on angiogenesis (Fig. 1B), when TGF-β1 binds to activator receptor like kinase 1 (ALK1) and induces recombinant mothers against decapentaplegic homolog 1/ recombinant mothers against decapentaplegic homolog 5(SMAD1/SMAD5) phosphorylation, resulting in angiogenesis. On the other hand, when TGF-β1 combined with ALK5, SMAD2/SMAD3 is phosphorylated and angiogenesis is inhibited.67-69 A study proved that PRP can induce the activation of extracellular matrix cells and activate endothelial progenitor cells through intercellular interaction, so as to promote the regeneration and angiogenesis of endothelial cells. 63 Xu et al. founded in the SD rat model of steroid-related necrosis of the femoral head (SANFH), PRP significantly up regulates the expression of angiogenesis marker VEGF and platelet endothelial cell adhesion molecule-1 (CD31).
PRP can improve blood supply and promote angiogenesis, but the exact mechanism of its effect on the level of various factors is unclear. 64 PRP contains a variety of growth factors. Each growth factor has different effects on angiogenesis. The effect of PRP on angiogenesis is still unclear. Further research is needed to better clarify the mechanism of PRP.
Cartilage protection
In order to inhibit the progression of articular cartilage, the stability of the internal environment of the joint should be maintained and the microenvironment of the joint should be improved. Articular cartilage is mainly composed of chondrocytes and extracellular matrix, and the balance control of cells and their extracellular matrix by cartilage synthesis and catabolic factors plays a key role in articular cartilage regeneration.70,71 Chondroprotection is thought to occur through 2 main mechanisms (Fig. 1C): (1) by increasing major players in chondroprotection, such as TGF-β1 and (2) by reducing markers of cartilage degradation, such as MMPs. 72 Chondrocytes secrete various anabolic factors (such as TGF-β1, IL-4, IL-10, and IL-13) to stimulate the synthesis of extracellular matrix, thereby promoting cartilage repair. 73 MMPs have extensive in proteolytic capacity and are involved in remodeling the extracellular matrix of connective tissue, and inhibition of MMPs has been shown to reduce the severity of cartilage degradation in osteoarthritis. 56 Lu et al. 74 founded that PRP could improve articular cartilage damage by inhibiting the toll-like receptor-2(TLR2)-mediated signaling pathway, inhibiting the expression of MMP-1, MMP-3, and MMP-13, reducing the degradation of extracellular matrix in articular cartilage. Moussa et al. 72 demonstrated that PRP significantly reduced MMP3, MMP13, aggrecanase-2(ADAMTS-5), IL-6, Cyclooxygenase-2(COX-2), TGF-β, aggrecan, collagen type 2, tissue inhibitors of metalloproteinases (TIMPs), and intracellular IL-4, IL-10, and IL-13 increased significantly, that is, PRP significantly increased osteoarthritis chondrocyte proliferation and decreased apoptosis. Sun et al. 75 treated PRP in a rat model of cartilage injury in vitro and in vivo and found PRP could reduce inflammatory factors such as MMP-13, IL-1β, IL-18, and TNF-α by increasing the expression levels of miR-337 and miR-375 mRNA expression levels, while increasing the expression levels of type II collagen and anti-apoptotic B-cell lymphoma-2(Bcl-2), play a chondroprotective role in articular cartilage injury.
Cellular proliferation and differentiation
Once articular cartilage injury, due to the limitation of the anatomical structure of the knee joint, the cartilage defect area lacks undifferentiated cells that can migrate and proliferate. Intra-articular injection PRP is a promising method to solve this problem. The role of PRP can be summarized in 2 aspects. (1) PRP has the ability to promote chondrogenic differentiation of stem cells.76-78 When PRP and stem cells are co-transplanted into the knee joint, a variety of growth factors can stimulate and induce stem cells to differentiate into chondrocytes, and then promote the recovery of cartilage injury. In an animal experiment, PRP and human dental pump stem cells were transplanted into the rabbit full-thickness articular cartilage defect model. Compared with the control group, co-transplanted of PRP and stem cells, the symptoms of synovitis decreased, furthermore, the differentiation of stem cells into hyaline cartilage increased, which can improve the full-thickness cartilage defect. 79 Similarly, adipose-derived mesenchymal stem cells and PRP were injected into the joints to observe their effects on lameness, pain, and quality of life in osteoarthritis dogs. The results showed that the combined injection significantly improved the symptoms of osteoarthritis dogs, also controlled the local microenvironment through anti-inflammatory and immunosuppressive factors, so as to protect the cartilage from further tissue damage. 80 What’s more, there is a study that reported that combining PRP and autologous human granulocyte colony-stimulating factor (hG-CSF) into peripheral blood stem cells (AAPBSC) can further stimulate AAPBSC to proliferate toward a chondrocyte phenotype, which in turn promotes cartilage repair. 81 In the same way, Xiao et al. proved that the combination of PRP and mesenchymal stem cell can have a positive effect on articular cartilage injury by stimulating extracellular matrix synthesis and chondrocyte proliferation and inhibiting inflammatory response.82-84 (2) PRP can recruit stem cells to the injury and then induce them to differentiate into chondrocytes. In an in vitro study, human PRP was added to human subchondral progenitor cells cultured in vitro to observe its effect on the migration and differentiation of human subchondral progenitor cells. As a result, PRP could stimulate the migration of human subchondral progenitor cells and promote their chondrogenic differentiation, but the adipogenic or osteogenic differentiation was not obvious, suggesting that PRP injected into the articular cartilage defect can stimulate the migration of human subchondral progenitor cells located below the defect area, recruit it to the defect area, and promote its differentiation into cartilage, subsequently repairing the cartilage damage. 85 The study of Vinod et al. 86 reached a similar conclusion that culturing chondroprogenitor cells with PRP can promote chondrogenic differentiation of chondroprogenitor cells, PRP intra-articular injection can be used as an effective biological scaffold for cartilage healing. In addition, studies have transplanted PRP and synovial mesenchymal stem cells into the articular cartilage injury model of New Zealand rabbits. This is in good agreement that PRP can inhibit the expression of MMP-3 and promote the expression of type II collagen and SOX9. At the same time, it can induce synovial mesenchymal stem cells to differentiate into chondrocytes, increase the generation of cartilage markers, and then improve joint function.87,88 Whether PRP injected into human joints can play the same role needs further clinical experimental research.
Taken together, PRP can improve cartilage damage by inhibiting inflammation, promoting angiogenesis, enhancing cartilage protection, and improving cellular proliferation and differentiation. Although PRP has attracted the attention of clinicians in skeletal muscle regenerative medicine and has become an exciting frontier field in cartilage injury repair, the exact mechanism of action of PRP in cartilage injury repair remains unclear and requires further in-depth research. The main reason is that PRP contains a large number of proteins, including growth factors, cytokines, chemokines, and so on; furthermore, the binding of these proteins may lead to complex and even contradictory interactions, which makes it challenging to elucidate the exact mechanism of action of PRP.
Delivery of PRP
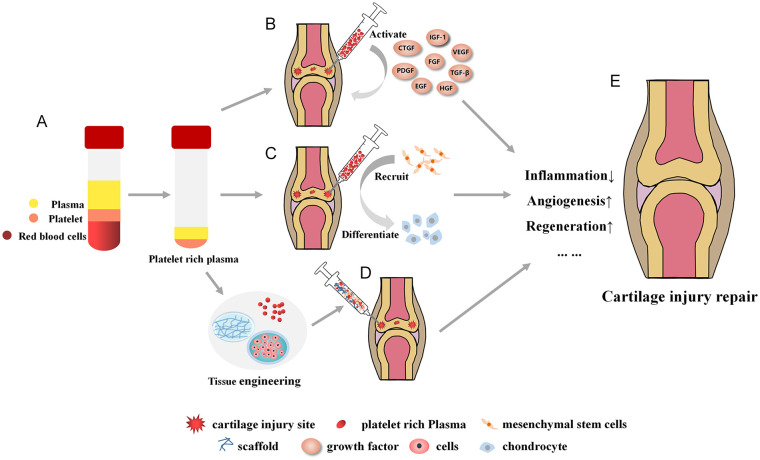
There are many ways of delivery of PRP in the repair of articular cartilage injury (Fig. 2), such as direct injection, combined stem cell or chondrocyte injection, combined scaffold, and so on.82,89-91 Different delivery methods have different advantages and disadvantages, as well as different scope of application.
Figure 2.
Schematic diagram of PRP in the treatment of articular cartilage injury. (A) Preparation of PRP. (B) Intra articular injection of PRP. After activation of PRP, it can release various growth factors, which can inhibit intra-articular inflammation, promote angiogenesis and effectively restore articular cartilage injury. (C) Intra articular injection of PRP, which can recruit intra-articular mesenchymal stem cells (such as synovial mesenchymal stem cells) to cartilage injury site. Furthermore, growth factor can induce them to differentiate into chondrocytes, enhance cartilage regeneration, and then repair articular cartilage injury. (D) PRP combined with cells and scaffolds to form cartilage tissue engineering scaffolds. The 3 complement each other and promote the recovery of articular cartilage injury when transplanting to the injured site together. (E) PRP can improve articular cartilage injury through a variety of ways and mechanisms. PRP = platelet rich plasma; IGF-1 = insulin-like growth factor 1; VEGF = vascular endothelial growth factor; TGF-β = transforming growth factor-β; HGF = hepatocyte growth factor; EGF = Epidermal growth factor; PDGF = platelet-derived growth factor; CTGF = Connective tissue growth factor.
PRP combined with cells and scaffold is often used in in vitro experiments. The regeneration ability of chondrocytes after articular cartilage injury is poor. Some studies have used autologous chondrocytes proliferated in vitro to repair articular cartilage defects in vivo. However, due to the limited proliferation ability of chondrocytes, the chondrocytes obtained by repeated subculture in order to obtain a sufficient amount of chondrocytes have the problems of aging and dedifferentiation. 92 Because of its strong differentiation ability, stem cells can differentiate into chondrocytes, which have gradually replaced chondrocytes and become a more promising treatment method after articular cartilage injury.93,94 Internationally, there are approved stem cell products for the treatment of articular cartilage injury. Whereas, there is often an inflammatory pathological microenvironment in the injured joint, which is not conducive to the survival of transplanted cells. The addition of PRP can inhibit inflammation.54,57 In addition, due to the special structure of the joint cavity and the simple injection of stem cells to repair specific damaged parts, the joint may only experience the benefits of “flushing..” 95 The selection of appropriate scaffolds can make the cells evenly and persistently distributed in the injury site. The combination of the 3 is more beneficial to the repair of articular cartilage injury in vitro experiments.
In another aspect, the safety of scaffolds to human body needs to be demonstrated. Scaffolds can be divided into natural materials and chemical materials, especially scaffolds prepared with chemical materials, which cannot completely remove the side effects caused by chemicals, will endanger human health in serious cases. In addition, cells will cause human immune response. 96 Therefore, in clinical trials, most of them are simply injected with PRP. Simple injection of PRP generally requires multiple injections because it cannot be distributed at the injured site for a long time, and the experimental results show that the effect of multiple injections is better than that of single injection.97,98 PRP alone has fewer side effects than combining that with stem cells and/or vectors. In short, the use of PRP should choose the appropriate mode of delivery according to the situation. In clinical trials, safety is most important.
Clinical Effect of PRP in the Repair of Articular Cartilage Disease
PRP treatment has been reported in clinical practice in 1980s and 1990s. It has been popular in regenerative medicine and other specialties. Its application can involve in dentistry, maxillofacial surgery, diabetes, and delaying senility.99-101 Nowadays, in musculoskeletal therapy, PRP has become a very attractive option because of its influence in repairing injured tissues and accelerating recovery.7,102 Using the “Clinical Trials. gov” database to count the number and national distribution of clinical research projects currently using PRP in the treatment of cartilage injury repair or osteoarthritis. Taking the keywords “platelet rich plasma” and “osteoarthritis” as examples, the search shows that up to now, a total of 134 clinical research projects have been registered in the world, mainly distributed in North America, United States, and Europe. By searching the keywords “platelet rich plasma” and “cartilage investigation,,” it can be seen that up to now, there are only 2 clinical research projects registered in the world. Osteoarthritis is often caused by “wear” and degradation of articular cartilage. In addition, patients with osteoarthritis often have different degrees of articular cartilage damage. It is reported that clinicians found cartilage damage (defect not penetrating subchondral bone) and osteochondral damage (defect penetrating subchondral bone) in knee arthroscopy of 61% of patients with osteoarthritis. 103 The following describes the clinical application and effect of PRP in the repair treatment group of knee arthritis cartilage injury in the recent year (January 1, 2021 to January 1, 2022) (Table 3).
Table 3.
Clinical Outcome Studies of PRP Use in Cartilage Disease.
| References | Study design | Method | PRP count | Result | Conclusion |
|---|---|---|---|---|---|
| Tucker et al. 104 | Randomized controlled trial Level of evidence:1 |
Group A:11 patients, single PRP injection Group B:7 patients, single saline injection |
PLT count 158% ± 67% of whole blood values Leukocyte quality: N/S |
WOMAC scores declined for up to 3 months from baseline levels and remained low at 6 and 12 months in the PRP group. In contrast, WOMAC scores for patients receiving the saline injection were relatively unchanged for up to 12 months. | PRP modulates the local knee synovial environment by altering the inflammatory milieu, matrix degradation, and angiogenic growth factors. |
| Dório et al. 105 | Randomized controlled trial Level of evidence:1 |
Group A:20 patients, PRP injection Group B:21 patients, plasma injection Group C:21 patients, saline injection All treated with 2 injections |
PLT count 300% of whole blood values Leukocyte quality: poor |
Change in pain from baseline at week 24 were -2.9, -2.4 and -3.5 cm for PRP, plasma and saline. There were no differences between the 3 groups at weeks 6 and 12. The PRP group showed higher frequency of adverse events, mostly mild transitory increase in pain. | PRP and plasma were not superior to placebo for pain and function improvement in knee osteoarthritis over 24 weeks. The PRP group had a higher frequency of mild transitory increase in pain. |
| Ngarmukos et al. 106 | Prospective cohort study, with control group Level of evidence:2 |
Group A:51patients, 2 PRP injections Group B:43patients, 4 PRP injections injection (every 6 months) |
PLT count: 430000/ L Leukocyte quality: 200/L |
There were no changes of synovial inflammatory cytokines, anti-inflammatory cytokines and growth factors before 6 weeks. Both groups had significantly improved clinical outcomes from 6 weeks including VAS, patient-reported outcome measures,WOMAC and Short Form-12. | Two- or 4-PRP intra-articular injection at a 6-week interval for knee OA demonstrated no changes of synovial cytokines and growth factors but similarly improved clinical outcomes from 6 weeks until 1 year. |
| Dulic et al. 107 | Prospective cohort study, with control group Level of evidence:2 |
Group A:111 patients, BMAC injection Group B:34 patients, PRP injection Group C:30 patients, HA injection (3 does weekly) |
PLT count 600% of whole blood values Leukocyte quality: rich |
The mean VAS scores showed significant differences between groups with a drop of VAS in all groups but with a difference in the BMAC group in comparison to other groups. There were no differences between the HA and PRP groups, although PRP showed a higher level of clinical improvement. | BMAC could be better in terms of clinical improvements in the treatment of knee osteoarthritis than PRP and HA up to 12 months. PRP provides better outcomes than HA during the observation period, but these results are not statistically significant. |
| Park et al. 108 | Randomized controlled trial Level of evidence:1 |
Group A:55 patients, single PRP injection Group B:55 patients, single HA injection |
PLT count not reported Leukocyte quality: N/S |
PRP showed significantly improvement in IKDC subjective scores and the Patient Global Assessment score at 6 months. Within the PRP group, the concentrations of platelet-derived growth factors were high in patients with a score above the MCID for VAS at 6 months. The incidence of adverse events did not differ between the groups. | PRP had better clinical efficacy than HA. High concentrations of growth factors were observed in patients who scored above the MCID for clinical outcomes in the PRP group. |
| Bansal et al. 109 | Prospective cohort study, with control group Level of evidence:2 |
Group A:75 patients, single PRP injection Group B:75 patients, single HA injection |
PLT count:10 billion in 8 mL Leukocyte quality: poor |
Significant improvements in WOMAC, IKDC scores, 6-min pain free walking distance persisted in PRP compared to HA group at 1 year. Significant decline IL-6 and TNF-α levels observed in PRP group compared to HA at 1 month. | An absolute count of 10 billion platelets is crucial in a PRP formulation to have long sustained chondroprotective effect up to 1 year in moderate knee osteoarthritis. |
| Raeissadat et al. 110 | Randomized controlled trial Level of evidence:1 |
Group A:59 patients, HA injection (3 does
weekly) Group B:59 patients, PRP injection (2 does weekly) Group C:60 patients, PRGF injection (2 does weekly) Group D:60 patients, Ozone injection (3 does weekly) |
PLT count 400% of whole blood
values Leukocyte quality: N/S |
In 2 months, significant improvement was seen in all groups, but the ozone group had the best results. In 6 month, HA, PRP, and PRGF groups demonstrated better therapeutic effects in all scores in comparison with ozone. At the end of the 12th month, PRGF and PRP groups had better results versus HA and ozone groups in scores of VAS, WOMAC, and Lequesne index. | Patients in PRP and PRGF groups improved symptoms persisted for 12 months. Therefore, these products could be the preferable choices for long-term management. |
| Xu et al. 111 | Prospective cohort study, with control group Level of evidence:2 |
Group A:34 patients, HA injection Group B:40 patients, PRP injection Group C:48 patients PRP+HA injection All treated with 3 intra-articular injection (every half a month) |
PLT count not reported Leukocyte quality: poor |
At 24 months, pain and function scores in the PRP+HA group were better than those in the HA and PRP alone groups. At 6 and 12 months, synovial hyperplasia in the PRP and PRP+HA groups was improved. After 6 and 12 months, the PSV, synovial EDV, S/D and RI were improved in the PRP+HA group. Complications were highest in the PRP group. | PRP combined with HA is more effective than PRP or HA alone at inhibiting synovial inflammation and can effectively improve pain and function and reduce adverse reactions. Its mechanism involves changes in the synovium and cytokine content. |
| Sun et al. 112 | Prospective cohort study, with control group Level of evidence:2 |
Group A:43 patients, single HYAJOINT Plus followed by
PRP Group B:42 patients, single PRP injection |
PLT count 240% of whole blood
values Leukocyte quality: poor |
Patients receiving a single PRP experienced significantly greater improvements in VAS pain than patients receiving combined injections at 1-month follow-up. However, at 6-month follow-up, the combined-injection group achieved significantly better VAS pain reduction | Combined injections of HYAJOINT Plus and PRP achieved better VAS pain reduction than a single PRP at 6 months. The long -term benefit effect of the combination in a knee osteoarthritis need to be replicated in larger trials. |
| Louis et al. 113 | Prospective cohort study, with control group Level of evidence:2 |
Group A:10 patients, MF-Saline injection Group B:10 patients, MF-PRP LD injection Group C:10 patients, MF-PRP HD injection |
Group B PLT count: 1000 × 109/L Group C PLT count: 3000 × 109/L Leukocyte quality: poor |
A significant decrease observed at 6 months on all scores. MF-PRP HD at 3 months and significantly higher compared to MF-PRP LD. Half of the injected PRP in the MF-PRP LD group displayed red blood cell contamination of over 8%, which was correlated with an impairment of T2max. | A single intra-articular injection of MF with or without PRP is safe and may offer significant clinical improvement for patients with osteoarthritis. High concentrations of PRP were more effective in improving articular cartilage function. |
| Wu et al. 114 | Retrospective study, with control group Level of evidence:4 |
Group A:12 patients, LCG Group B:12 patients, MCG Group C:13 patients, HCG |
Group A PLT count: 1000-1400 × 109/L Group B PLT count: 1400-1800 × 109/L Group C PLT count: 1800-2100 × 109/L Leukocyte quality: poor |
After 2 months, compared with the HCG, articular cartilage thickness of the medial and lateral femur of the diseased side in the MCG was obviously higher. After treatment, compared with the LCG, knee joint function scores of the MCG and HCG were obviously better. Compared with the HCG, levels of MMPs and inflammatory factor in the MCG were obviously lower. | PRP combined with quadriceps training can accelerate cartilage repair and reduce inflammatory factor levels and levels of MMPs, but the treatment effect of PRP depends on platelet concentration, with the best range of 1400 1800 109/L. Too high or too low platelet concentrations will affect recovery of knee function. |
| Elawamy et al. 115 | Randomized controlled trial Level of evidence:1 |
Group PRF:100 patient, pulsed radiofrequency Group PRP:100 patient, PRP injection (repeated 1 month later) |
PLT count >300% of whole blood values Leukocyte quality: N/S |
Visual analog scale was significantly lower in the PRF group compared to the PRP group at 6 and 12 months, respectively. Regarding to the postinterventional index of severity of osteoarthritis, it was significantly lower in the PRF group than the PRP group at follow-up. | Pulsed radiofrequency of the genicular nerves can be considered superior to knee intraarticular platelet-rich plasma injection for sustained pain relief and the lower severity index in patients with chronic knee osteoarthritis. |
| Danieli et al. 116 | Prospective cohort study, with control group Level of evidence:2 |
Group A:31 patient, no PRP injection Group B:33 patient, PRP injection |
PLT count not reported Leukocyte quality: poor |
IKDC and KOOS scores showed increase at each evaluation time points after surgery in both groups, but the Group B showed a higher increase with statistically significant difference. | Those patients affected by ICRS grade III chondral injuries under-going arthroscopic chondroplasty who were also treated with PRP showed better and faster outcomes than the control group. |
PRP = platelet rich plasma; PLT: Platelet count; WOMAC: Western Ontario and McMaster Universities Osteoarthritis Index; VAS: visual analog scale; OA = osteoarthritis; BMAC: Bone Marrow Aspirate Concentrate; HA: Hyaluronic acid; IKDC: International Knee Documentation Committee; MCID: minimal clinically important difference; IL-6 = interleukin-6; TNF-α = tumor necrosis factor-alpha; PRGF: plasma rich in growth factors; PSV: peak systolic velocity; EDV: end diastolic velocity; S/D: systolic/diastolic ratio; RI: resistance index; MF: microfat; LD: Low Dose; HD: High Dose; LCG: low concentration group; MCG: medium concentration group; HCG: high concentration group; MMP: matrix metalloproteinases; PRF: Pulsed radiofrequency; KOOS: Knee injury and Osteoarthritis Outcome Score; ICRS: International Cartilage Repair Society.
A randomized double-blinded study of 18 total patients by Tucker et al. 104 demonstrated that in the PRP group, the Western Ontario and McMaster Universities Osteoarthritis Index (WOMAC) scores declined for up to 3 months from baseline levels and remained low at 6 and 12 months. While WOMAC scores for patients receiving the saline injection were relatively unchanged for up to 12 months. In contrast, Dorio et al. 105 made a randomized controlled trial in which 62 patients were divided into 3 groups by injection of PRP, plasma, and saline. Pain and adverse effects were assessed at 6, 12, and 24 weeks, respectively. The result showed that PRP and plasma were not superior to placebo in terms of improvement in pain and function in knee arthritis cartilage injury patients at 24 weeks. Increased frequency of mild transient pain was higher in the PRP group. Therefore, the authors question the efficacy of PRP. However, Ngarmukos et al. 106 took a different view, and a study of 94 patients who received either 2 or 4 injections of PRP found 2- or 4-PRP intra-articular injection at a 6-week interval for knee OA demonstrated no changes of synovial cytokines and growth factors but improved clinical outcomes from 6 weeks until 1 year.
Dulic et al. 107 compared bone marrow aspirate concentrate bone marrow derived stroma cell (BMAC) to PRP, hyaluronic acid (HA) injections in the treatment of knee chondropathy or osteoarthritis, with a result that BMAC could be better in terms of clinical improvements in the treatment of knee arthritis cartilage injury than PRP and HA up to 12 months. PRP provides better outcomes than HA during the observation period, but these results are not statistically significant. By contrast, significantly improvement in International Knee Documentation Committee (IKDC) subjective scores and Patient Global Assessment score in the PRP group. The incidence of adverse events did not differ between the groups, as per the findings of Park et al. 108 Besides, they discovered the concentrations of platelet-derived growth factors were high in patients with a score above the minimal clinically important difference (MCID) for visual analogue scale (VAS) at 6 months in the PRP group, which can promote the recovery of articular cartilage. Both Bansal et al. 109 and Raeissadat et al. 110 showed that symptom improvement in patients in the PRP injection group lasted for 12 months. Therefore, PRP may play an important role in long-term cartilage protection. In addition, Xu et al. 111 prospectively followed 122 patients who received a total of 3 injections of PRP, HA, or PRP+HA within an interval of half a month, and found that PRP combined with HA is more effective than PRP or HA alone at inhibiting synovial inflammation and can effectively improve pain and function and reduce adverse reactions. Its mechanism involves changes in the synovium and cytokine content. At the same time, a prospective cohort study of 85 patients by Sun et al. 112 similarly reported that patients who combined injections of crosslinked Hyaluronan (HYAJOINT) Plus and PRP achieved better VAS pain reduction than a single PRP at 6 months. This also needs to be replicated in larger trials for indicating a long-term benefit of the combination of HYAJOINT Plus and PRP in improving knee functional status.
In a prospective cohort study of Louis et al, 113 30 patients were randomly divided into 3 groups with injections microfat (MF)+ Saline, MF+ Low Dose PRP (PRP LD, 1000*109/L), or MF+ High Dose PRP (PRP HD, 3000*109/L). All treatments were effective in improving knee functional status and reducing symptoms, with significant reductions in all scores observed at 6 months. At 3 months, MF + PRP HD was significantly higher than MF + PRP LD. In the MF+PRP LD group, half of patients injected with PRP had more than 8% red blood count (RBC) contamination. The results showed that a single intra articular injection of MF was safe, and high concentrations of PRP were more effective in improving articular cartilage function. In a retrospective analysis of 37 patients, there were different results. 114 The patients received high (1800-2100 * 109/L), medium (1400-1800 * 109/L), or low (1000-1400 * 109/L) concentrations of PRP respectively. All patients received quadriceps femoris training. After 2 months of treatment, the knee function score of the medium concentration group was significantly better than that of the high- and low-concentration groups. PRP combined with quadriceps training can promote cartilage repair and reduce the level of inflammatory factors and MMPs in patients, but the therapeutic effect of PRP depends on platelet concentration, and the optimal range is 1400 to 1800 109/L. Too high or too low platelet concentration will affect the recovery of knee function. Therefore, there is no optimal standard for the optimal concentration of PRP injection, and more experimental verification is needed. Despite the results of a randomized controlled trial by Elawamy et al, 115 knee nerve pulsed radiofrequency (PRF) therapy can be considered superior to intraarticular PRP injection for sustained pain relief and reduced severity indices in patients with chronic knee osteoarthritis, with an experimental result that visual analog scale was significantly lower in the PRF group compared to the PRP group at 6 and 12 months, respectively. Regarding the postinterventional index of severity of osteoarthritis, it was significantly lower in the PRF group than the PRP group at follow-up. However, a prospective trial of Danieli et al. 116 showed that patients with International Cartilage Repair Society (ICRS) grade III cartilage injury who underwent arthroscopic chondroplasty in combination with PRP performed better and faster than the one who only received an arthroscopic chondroplasty. PRP can be used in conjunction with other cartilage procedures to treat osteochondral diseases. Although there is a relative lack of literature in human clinical studies, animal studies have evaluated PRP recommendations for focal osteochondral defects.
Problems and Prospects
Some scholars have observed that intra-articular injection of PRP can inhibit and produce local adverse reactions, including local swelling and pain, but it will be relieved after a short time, which may be related to the dose and method of PRP injection and the content of leukocytes in PRP. 105 ,117-119 Most studies have proved that PRP injection has little adverse effect on patients and can be alleviated in a very short time.111,120 In addition, there are the following problems in intra-articular injection of PRP: (1) lack of standard guidelines for PPP preparation and injection methods, (2) there is a certain bias in the research results, (3) the clinical application of PPP needs to be standardized, (4) lack of unified clinical efficacy evaluation method, (5) lack of clinical long-term follow-up study, and (6) expensive, and so on. 121 , 122 At present, there are few research literatures published in this field at home and abroad. More research is needed to prove the safety, effectiveness, and practicality of PRP application, and further determine the standard scheme of PRP application. Nevertheless, the application of PRP in cartilage injury repair tissue engineering still has broad prospects.
Conclusion
The paper systematically evaluated the mechanism and clinical therapeutic effect of PRP in the repair of articular cartilage disease by analyzing the literature at home and abroad in recent years. A large number of basic scientific and clinical studies have shown that PRP contains a variety of growth factors, which can affect inflammation, angiogenesis, cartilage protection and cellular proliferation and differentiation after articular cartilage injury through different pathways. Although the clinical efficacy of PRP in the treatment of articular cartilage injury has not been uniformly reported, most studies reported that PRP has a positive effect on cartilage injury, improving the joint function. In addition, many studies found that the best curative effect of intra-articular injection of PRP is about 1 year. PRP is very promising in the treatment of articular cartilage injury, because of its low rate of adverse events observed in many clinical studies. At the same time, it is found that there is a lack of standardization in the preparation, use methods and curative effect observation of PRP, which will also become the research direction of researchers in the future. The technology of PRP in the repair and treatment of articular cartilage injury is worthy of further research, which will benefit the majority of patients with articular cartilage injury.
Footnotes
Author Contributions: Conceptualization, Y.L., J.L. and Y.W.; methodology, Y.W. and L.C.; resources, J.C. and H.W.; data curation, Y.L., J.L. and J.H.; writing original draft preparation, Y.L., J.L., Y.W., J.H. and L.C.; writing review and editing, Y.L., J.L., J.C. and H.W.; project administration, Y.L., J.L., J.C. and H.W. All authors have read and agreed to the published version of the manuscript.
Acknowledgments and Funding: This research was funded by the National Natural Science Foundation of China (grant numbers 82071374), Discipline construction project of Guangdong Medical University (grant numbers1.13), College Students Innovative Experimental Project in Guangdong Medical University (grant numbers FYDB015, ZCDS001, ZYDB016, ZZDI001) and College Students’ Science and Technology Innovation Training Project (grant numbers GDMU2020194, GDMU2020195, GDMU2021021, GDMU2021023, GDMU2021091, GDMU2021111). Special fund for Affiliated Hospital of Guangdong Medical University “Clinical Medicine +” CnTech Co-construction Platform (grant numbers CLP2021A001).
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Ethical Approval: Not applicable.
ORCID iD: Liji Chen  https://orcid.org/0000-0002-5810-1723
https://orcid.org/0000-0002-5810-1723
References
- 1. Tang Q, Lim T, Shen LY, Zheng G, Wei XJ, Zhang CQ, et al. Well-dispersed platelet lysate entrapped nanoparticles incorporate with injectable PDLLA-PEG-PDLLA triblock for preferable cartilage engineering application. Biomaterials. 2021;268:120605. [DOI] [PubMed] [Google Scholar]
- 2. Carballo CB, Nakagawa Y, Sekiya I, Rodeo SA. Basic science of articular cartilage. Clin Sports Med. 2017;36(3):413-25. [DOI] [PubMed] [Google Scholar]
- 3. Liu YL, Yen CC, Liu TT, Chang CH, Shih TT, Wang JH, et al. Safety and efficacy of kartigen® in treating cartilage defects: a randomized, controlled, phase I trial. Polymers (Basel). 2021;13(18):3029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hjelle K, Solheim E, Strand T, Muri R, Brittberg M. Articular cartilage defects in 1,000 knee arthroscopies. Arthroscopy. 2002;18(7):730-4. [DOI] [PubMed] [Google Scholar]
- 5. Simon TM, Jackson DW. Articular cartilage: injury pathways and treatment options. Sports Med Arthrosc Rev. 2018;26(1):31-9. [DOI] [PubMed] [Google Scholar]
- 6. Gilat R, Haunschild ED, Knapik DM, Evuarherhe A, Jr, Parvaresh KC, Cole BJ. Hyaluronic acid and platelet-rich plasma for the management of knee osteoarthritis. Int Orthop. 2021;45(2):345-54. [DOI] [PubMed] [Google Scholar]
- 7. Everts PA, van Erp A, DeSimone A, Cohen DS, Gardner RD. Platelet rich plasma in orthopedic surgical medicine. Platelets. 2021;32(2):163-74. [DOI] [PubMed] [Google Scholar]
- 8. Riewruja K, Phakham S, Sompolpong P, Reantragoon R, Tanavalee A, Ngarmukos S, et al. Cytokine profiling and intra-articular injection of autologous platelet-rich plasma in knee osteoarthritis. Int J Mol Sci. 2022;23(2):890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Tao SC, Yuan T, Rui BY, Zhu ZZ, Guo SC, Zhang CQ. Exosomes derived from human platelet-rich plasma prevent apoptosis induced by glucocorticoid-associated endoplasmic reticulum stress in rat osteonecrosis of the femoral head via the Akt/Bad/Bcl-2 signal pathway. Theranostics. 2017;7(3):733-50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Zhang Y, Wang X, Chen J, Qian D, Gao P, Qin T, et al. Exosomes derived from platelet-rich plasma administration in site mediate cartilage protection in subtalar osteoarthritis. J Nanobiotechnology. 2022;20(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Pogozhykh O, Prokopyuk V, Figueiredo C, Pogozhykh D. Placenta and placental derivatives in regenerative therapies: experimental studies, history, and prospects. Stem Cells Int. 2018;2018:4837930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Gupta V, Parihar AS, Sharma VK, Jain S, Singh V, Khanna N. Evaluation of platelet-rich plasma on hair regrowth and lesional T-cell cytokine expression in alopecia areata: a randomized observer-blinded, placebo-controlled, split-head pilot study. J Am Acad Dermatol. 2021;84(5):1321-8. [DOI] [PubMed] [Google Scholar]
- 13. Keene DJ, Alsousou J, Harrison P, Hulley P, Wagland S, Parsons SR, et al. Platelet rich plasma injection for acute Achilles tendon rupture: PATH-2 randomised, placebo controlled, superiority trial. BMJ. 2019;367:l6132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Shapiro J, Ho A, Sukhdeo K, Yin L, Lo Sicco K. Evaluation of platelet-rich plasma as a treatment for androgenetic alopecia: a randomized controlled trial. J Am Acad Dermatol. 2020;83(5):1298-303. [DOI] [PubMed] [Google Scholar]
- 15. Kwong CA, Woodmass JM, Gusnowski EM, Bois AJ, Leblanc J, More KD, et al. Platelet-rich plasma in patients with partial-thickness rotator cuff tears or tendinopathy leads to significantly improved short-term pain relief and function compared with corticosteroid injection: a double-blind randomized controlled trial. Arthroscopy. 2021;37(2):510-7. [DOI] [PubMed] [Google Scholar]
- 16. Akbarzadeh S, McKenzie MB, Rahman MM, Cleland H. Allogeneic platelet-rich plasma: is it safe and effective for wound repair? Eur Surg Res. 2021;62(1):1-9. [DOI] [PubMed] [Google Scholar]
- 17. Papait A, Cancedda R, Mastrogiacomo M, Poggi A. Allogeneic platelet-rich plasma affects monocyte differentiation to dendritic cells causing an anti-inflammatory microenvironment, putatively fostering wound healing. J Tissue Eng Regen Med. 2018;12(1):30-43. [DOI] [PubMed] [Google Scholar]
- 18. Zhang ZY, Huang AW, Fan JJ, Wei K, Jin D, Chen B, et al. The potential use of allogeneic platelet-rich plasma for large bone defect treatment: immunogenicity and defect healing efficacy. Cell Transplant. 2013;22(1):175-87. [DOI] [PubMed] [Google Scholar]
- 19. Barrionuevo DV, Laposy CB, Abegão KG, Nogueira RM, Nai GA, Bracale BN, et al. Comparison of experimentally-induced wounds in rabbits treated with different sources of platelet-rich plasma. Lab Anim. 2015;49(3):209-14. [DOI] [PubMed] [Google Scholar]
- 20. He M, Guo X, Li T, Jiang X, Chen Y, Yuan Y, et al. Comparison of allogeneic platelet-rich plasma with autologous platelet-rich plasma for the treatment of diabetic lower extremity ulcers. Cell Transplant. 2020;29:963689720931428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Liao X, Liang JX, Li SH, Huang S, Yan JX, Xiao LL, et al. Allogeneic platelet-rich plasma therapy as an effective and safe adjuvant method for chronic wounds. J Surg Res. 2020;246:284-91. [DOI] [PubMed] [Google Scholar]
- 22. Harrison P, Subcommittee on Platelet Physiology. The use of platelets in regenerative medicine and proposal for a new classification system: guidance from the SSC of the ISTH. J Thromb Haemost. 2018;16(9):1895-900. [DOI] [PubMed] [Google Scholar]
- 23. Yin W, Xu H, Sheng J, Zhu Z, Jin D, Hsu P, et al. Optimization of pure platelet-rich plasma preparation: a comparative study of pure platelet-rich plasma obtained using different centrifugal conditions in a single-donor model. Exp Ther Med. 2017;14(3):2060-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Bos-Mikich A, De Oliveira R, Frantz N. Platelet-rich plasma therapy and reproductive medicine. J Assist Reprod Genet. 2018;35(5):753-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Muthu S, Patel S, Selvaraj P, Jeyaraman M. Comparative analysis of leucocyte poor vs leucocyte rich platelet-rich plasma in the management of lateral epicondylitis: systematic review & meta-analysis of randomised controlled trials. J Clin Orthop Trauma. 2021;19:96-107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Lim W, Park SH, Kim B, Kang SW, Lee JW, Moon YL. Relationship of cytokine levels and clinical effect on platelet-rich plasma-treated lateral epicondylitis. J Orthop Res. 2018;36(3):913-20. [DOI] [PubMed] [Google Scholar]
- 27. Sunwoo JY, Eliasberg CD, Carballo CB, Rodeo SA. The role of the macrophage in tendinopathy and tendon healing. J Orthop Res. 2020;38(8):1666-75. [DOI] [PubMed] [Google Scholar]
- 28. Anitua E. Plasma rich in growth factors: preliminary results of use in the preparation of future sites for implants. Int J Oral Maxillofac Implants. 1999;14(4):529-35. [PubMed] [Google Scholar]
- 29. Petrungaro PS. Using platelet-rich plasma to accelerate soft tissue maturation in esthetic periodontal surgery. Compend Contin Educ Dent. 2001;22(9):729-46. [PubMed] [Google Scholar]
- 30. Landesberg R, Roy M, Glickman RS. Quantification of growth factors levels using a simplified method of platelet rich plasma gel preparation. J Oral Maxillofac Surg. 2000;58:297-300. [DOI] [PubMed] [Google Scholar]
- 31. Marx RE, Carlson ER, Eichstaedt RM, Schimmele SR, Strauss JE, Georgeff KR. Platelet-rich plasma: growth factor enhancement for bone grafts. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85(6):638-46. [DOI] [PubMed] [Google Scholar]
- 32. Okuda K, Kawase T, Momose M, Murata M, Saito Y, Suzuki H, et al. Platelet-rich plasma contains high levels of platelet-derived growth factor and transforming growth factor-beta and modulates the proliferation of periodontally related cells in vitro. J Periodontol. 2003;74(6):849-57. [DOI] [PubMed] [Google Scholar]
- 33. Aghaloo TL, Moy PK, Freymiller EG. Investigation of platelet-rich plasma in rabbit cranial defects: a pilot study. J Oral Maxillofac Surg. 2002;60(10):1176-81. [DOI] [PubMed] [Google Scholar]
- 34. Sabarish R, Lavu V, Rao SR. A comparison of platelet count and enrichment percentages in the platelet rich plasma (PRP) obtained following preparation by three different methods. J Clin Diagn Res. 2015;9(2):ZC10-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Efeoglu C, Akcay YD, Erturk S. A modified method for preparing platelet-rich plasma: an experimental study. J Oral Maxillofac Surg. 2004;62(11):1403-7. [DOI] [PubMed] [Google Scholar]
- 36. Tang R, Wang S, Yang J, Wu T, Fei J. Application of platelet-rich plasma in traumatic bone infections. Expert Rev Anti Infect Ther. 2021;19(7):867-75. [DOI] [PubMed] [Google Scholar]
- 37. Masuki H, Okudera T, Watanebe T, Suzuki M, Nishiyama K, Okudera H, et al. Growth factor and pro-inflammatory cytokine contents in platelet-rich plasma (PRP), plasma rich in growth factors (PRGF), advanced platelet-rich fibrin (A-PRF), and concentrated growth factors (CGF). Int J Implant Dent. 2016;2(1):19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Andia I, Maffulli N. A contemporary view of platelet-rich plasma therapies: moving toward refined clinical protocols and precise indications. Regen Med. 2018;13(6):717-28. [DOI] [PubMed] [Google Scholar]
- 39. Civinini R, Nistri L, Martini C, Redl B, Ristori G, Innocenti M. Growth factors in the treatment of early osteoarthritis. Clin Cases Miner Bone Metab. 2013;10(1):26-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Giusti I, D’Ascenzo S, Macchiarelli G, Dolo V. In vitro evidence supporting applications of platelet derivatives in regenera-tive medicine. Blood Transfus. 2020;18(2):117-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Xie X, Zhang C, Tuan RS. Biology of platelet-rich plasma and its clinical application in cartilage repair. Arthritis Res Ther. 2014;16(1):204-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Zhai G, Dore J, Rahman P. TGF-beta signal transduction pathways and osteoarthritis. Rheumatol Int. 2015;35(8):1283-92. [DOI] [PubMed] [Google Scholar]
- 43. Deppermann C, Kubes P. Start a fire, kill the bug: the role of platelets in inflammation and infection. Innate Immun. 2018;24(6):335-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Apte RS, Chen DS, Ferrara N. VEGF in signaling and disease: beyond discovery and development. Cell. 2019;176(6):1248-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Chen W, He S, Xiang D. Hypoxia-induced retinal pigment epithelium cell-derived bFGF promotes the migration and angiogenesis of HUVECs through regulating TGF-beta1/smad2/3 pathway. Gene. 2021;790:145695. [DOI] [PubMed] [Google Scholar]
- 46. Jia T, Jacquet T, Dalonneau F, Coudert P, Vaganay E, Exbrayat-Héritier C, et al. FGF-2 promotes angiogenesis through a SRSF1/SRSF3/SRPK1-dependent axis that controls VEGFR1 splicing in endothelial cells. BMC Biol. 2021;19(1):173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Everts P, Onishi K, Jayaram P, Lana JF, Mautner K. Platelet-rich plasma: new performance understandings and therapeutic considerations in 2020. Int J Mol Sci. 2020;21(20):7794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Berke IM, Jain E, Yavuz B, McGrath T, Chen L, Silva MJ, et al. NF-kappaB-mediated effects on behavior and cartilage pathology in a non-invasive loading model of post-traumatic osteoarthritis. Osteoarthritis Cartilage. 2021;29(2):248-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Yu Z, Zeng J, Wang J, Cui Y, Song X, Zhang Y, et al. Hepatocyte growth factor-regulated tyrosine kinase substrate is essential for endothelial cell polarity and cerebrovascular stability. Cardiovasc Res. 2021;117(2):533-46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Ornetti P, Nourissat G, Berenbaum F, Sellam J, Richette P, Chevalier X, et al. Does platelet-rich plasma have a role in the treatment of osteoarthritis? Joint Bone Spine. 2016;83(1):31-6. [DOI] [PubMed] [Google Scholar]
- 51. Xu PC, Xuan M, Cheng B. Effects and mechanism of platelet-rich plasma on military drill injury: a review. Mil Med Res. 2020;7(1):56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Ai G, Shao X, Meng M, Song L, Qiu J, Wu Y, et al. Epidermal growth factor promotes proliferation and maintains multipotency of continuous cultured adipose stem cells via activating STAT signal pathway in vitro. Medicine (Baltimore). 2017;96(30):e7607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Periayah MH, Halim AS, Mat Saad AZ. Mechanism action of platelets and crucial blood coagulation pathways in he-mostasis. Int J Hematol Oncol Stem Cell Res. 2017;11(4):319-27. [PMC free article] [PubMed] [Google Scholar]
- 54. Li M, Yin H, Yan Z, Li H, Wu J, Wang Y, et al. The immune microenvironment in cartilage injury and repair. Acta Biomater. 2022;140:23-42. [DOI] [PubMed] [Google Scholar]
- 55. Early JO, Fagan LE, Curtis AM, Kennedy OD. Mitochondria in injury, inflammation and disease of articular skeletal joints. Front Immunol. 2021;12:695257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Tang Q, Feng Z, Tong M, Xu J, Zheng G, Shen L, et al. Piceatannol inhibits the IL-1beta-induced inflammatory response in human osteoarthritic chondrocytes and ameliorates osteoarthritis in mice by activating Nrf2. Food Funct. 2017;8(11):3926-37. [DOI] [PubMed] [Google Scholar]
- 57. Xu Z, Yin W, Zhang Y, Qi X, Chen Y, Xie X, et al. Comparative evaluation of leukocyte- and platelet-rich plasma and pure platelet-rich plasma for cartilage regeneration. Sci Rep. 2017;7:43301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Xin F, Wang H, Yuan F, Ding Y. Platelet-rich plasma combined with alendronate reduces pain and inflammation in induced osteoarthritis in rats by inhibiting the nuclear factor-Kappa B signaling pathway. Biomed Res Int. 2020;2020:8070295. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 59. Zhao H, Zhu W, Mao W, Shen C. Platelet-rich plasma inhibits Adriamycin-induced inflammation via blocking the NF-kappaB pathway in articular chondrocytes. Mol Med. 2021;27(1):66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. MacDonald IJ, Liu SC, Su CM, Wang YH, Tsai CH, Tang CH. Implications of angiogenesis involvement in arthritis. Int J Mol Sci. 2018;19(7):2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61. Lee HJ, Hong YJ, Kim M. Angiogenesis in chronic inflammatory skin disorders. Int J Mol Sci. 2021;22(21):12035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62. Leblond A, Allanore Y, Avouac J. Targeting synovial neoangiogenesis in rheumatoid arthritis. Autoimmun Rev. 2017;16(6):594-601. [DOI] [PubMed] [Google Scholar]
- 63. Sidiropoulou S, Papadaki S, Tsouka AN, Koutsaliaris IK, Chantzichristos VG, Pantazi D, et al. The effect of platelet-rich plasma on endothelial progenitor cell functionality. Angiology. 2021;72(8):776-86. [DOI] [PubMed] [Google Scholar]
- 64. Xu HH, Li SM, Fang L, Xia CJ, Zhang P, Xu R, et al. Platelet-rich plasma promotes bone formation, restrains adipogenesis and accelerates vascularization to relieve steroids-induced osteonecrosis of the femoral head. Platelets. 2021;32(7):950-9. [DOI] [PubMed] [Google Scholar]
- 65. Liu Y, Liu Y, Deng J, Li W, Nie X. Fibroblast growth factor in diabetic foot ulcer: progress and therapeutic prospects. Front Endocrinol (Lausanne). 2021;12:744868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Zubair M, Ahmad J. Role of growth factors and cytokines in diabetic foot ulcer healing: a detailed review. Rev Endocr Metab Disord. 2019;20(2):207-17. [DOI] [PubMed] [Google Scholar]
- 67. Zhang L, Wei W, Ai X, Kilic E, Hermann DM, Venkataramani V, et al. Extracellular vesicles from hypoxia-preconditioned microglia promote angiogenesis and re-press apoptosis in stroke mice via the TGF-beta/Smad2/3 pathway. Cell Death Dis. 2021;12(11):1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Cao SJ, Hong L, Li XQ. Mechanistic studies on the role of TGF-β1 in angiogenesis through EndMT. Vascular. 2021;29(3):442-50. [DOI] [PubMed] [Google Scholar]
- 69. Chen Z, Chen Y, Li Y, Lian W, Zheng K, Zhang Y, et al. Prrx1 promotes stemness and angiogenesis via activating TGF-beta/smad pathway and up-regulating proangiogenic factors in glioma. Cell Death Dis. 2021;12(6):615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Korotkyi O, Huet A, Dvorshchenko K, Kobyliak N, Falalyeyeva T, Ostapchenko L. Probiotic composition and chondroitin sulfate regulate TLR-2/4-mediated NF-kappaB inflammatory pathway and cartilage metabolism in experimental osteoarthritis. Probiotics Antimicrob Proteins. 2021;13(4):1018-32. [DOI] [PubMed] [Google Scholar]
- 71. Zheng L, Zhang Z, Sheng P, Mobasheri A. The role of metabolism in chondrocyte dysfunction and the progression of osteoarthritis. Ageing Res Rev. 2021;66:101249. [DOI] [PubMed] [Google Scholar]
- 72. Moussa M, Lajeunesse D, Hilal G, El Atat O, Haykal G, Serhal R, et al. Platelet rich plasma (PRP) induces chondroprotection via increasing autophagy, anti-inflammatory markers, and decreasing apoptosis in human osteoarthritic cartilage. Exp Cell Res. 2017;352(1):146-56. [DOI] [PubMed] [Google Scholar]
- 73. Wojdasiewicz P, Poniatowski LA, Szukiewicz D. The role of inflammatory and anti-inflammatory cytokines in the pathogenesis of osteoarthritis. Mediators Inflamm. 2014;2014:561459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Lu HT, Lu JW, Lee CH, Peng YJ, Lee HS, Chu YH, et al. Attenuative effects of platelet-rich plasma on 30 kDa fibronectin fragment-induced MMP-13 expression associated with TLR2 signaling in osteoarthritic chondrocytes and synovial fibroblasts. J Clin Med. 2021;10(19):4496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Sun X, Mi L, Du G, Sun C, He S. Platelet-rich plasma treatment alleviates osteoarthritis-related pain, inflammation, and apoptosis by upregulating the expression levels of microRNA-375 and microRNA-337. Immunopharmacol Immunotoxicol. 2022;44(1):87-98. [DOI] [PubMed] [Google Scholar]
- 76. Wang Z, Wang Z, Zhang B, Zhao Q, Liu Y, Qi W. Effect of activated platelet-rich plasma on chondrogenic differentiation of rabbit bone marrow-derived mesenchymal stem cells. Stem Cells Int. 2021;2021:9947187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77. Jiang G, Li S, Yu K, He B, Hong J, Xu T, et al. A 3D-printed PRP-GelMA hydrogel promotes osteochondral regeneration through M2 macrophage polarization in a rabbit model. Acta Biomater. 2021;128:150-62. [DOI] [PubMed] [Google Scholar]
- 78. Lee JS, Guo P, Klett K, Hall M, Sinha K, Ravuri S, et al. VEGF-attenuated platelet-rich plasma improves therapeutic effect on cartilage repair. Biomater Sci. 2022;10(9):2172-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Yanasse RH, De Lábio RW, Marques L, Fukasawa JT, Segato R, Kinoshita A, et al. Xenotransplantation of human dental pulp stem cells in platelet-rich plasma for the treatment of full-thickness articular cartilage defects in a rabbit model. Exp Ther Med. 2019;17(6):4344-56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Sanghani-Kerai A, Black C, Cheng SO, Collins L, Schneider N, Blunn G, et al. Clinical outcomes following intra-articular injection of autologous adipose-derived mesenchymal stem cells for the treatment of osteoarthritis in dogs characterized by weight-bearing asymmetry. Bone Joint Res. 2021;10(10):650-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Turajane T, Thitiset T, Honsawek S, Chaveewanakorn U, Aojanepong J, Papadopoulos KI. Assessment of chondrogenic differentiation potential of autologous activated peripheral blood stem cells on human early osteoarthritic cancellous tibial bone scaffold. Musculoskelet Surg. 2014;98(1):35-43. [DOI] [PubMed] [Google Scholar]
- 82. Xiao WF, Yang YT, Xie WQ, He M, Liu D, Cai ZJ, et al. Effects of platelet-rich plasma and bone marrow mesenchymal stem cells on meniscal repair in the white-white zone of the meniscus. Orthop Surg. 2021;13(8):2423-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Ahmad MR, Khan MA, Mahmood A, Latif N, Iqbal T, et al. Combination of preconditioned adipose-derived mesenchymal stem cells and platelet-rich plasma improves the repair of osteoarthritis in rat. Regen Med. 2020;15(11):2285-95. [DOI] [PubMed] [Google Scholar]
- 84. Vinod E, Amirtham SM, Kachroo U, Goyal A, Ozbey O, James JV, et al. Articular chondroprogenitors in platelet rich plasma for treatment of osteoarthritis and osteochondral defects in a rabbit knee model. Knee. 2021;30:51-62. [DOI] [PubMed] [Google Scholar]
- 85. Krüger JP, Hondke S, Endres M, Pruss A, Siclari A, Kaps C. Human platelet-rich plasma stimulates migration and chondrogenic differentiation of human subchondral progenitor cells. J Orthop Res. 2012;30(6):845-52. [DOI] [PubMed] [Google Scholar]
- 86. Vinod E, Vinod Francis D, Manickam Amirtham S, Sathishkumar S, Boopalan PRJVC. Allogeneic platelet rich plasma serves as a scaffold for articular cartilage derived chondroprogenitors. Tissue Cell. 2019;56:107-13. [DOI] [PubMed] [Google Scholar]
- 87. Nabavizadeh SS, Talaei-Khozani T, Zarei M, Zare S, Hosseinabadi OK, Tanideh N, et al. Attenuation of osteoarthritis progression through intra-articular injection of a combination of synovial membrane-derived MSCs (SMMSCs), platelet-rich plasma (PRP) and conditioned me-dium (secretome). J Orthop Surg Res. 2022;17(1):102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88. Lee JC, Min HJ, Park HJ, Lee S, Seong SC, Lee MC. Synovial membrane-derived mesenchymal stem cells supported by platelet-rich plasma can repair osteochondral defects in a rabbit model. Arthroscopy. 2013;29(6):1034-46. [DOI] [PubMed] [Google Scholar]
- 89. Slimi F, Zribi W, Trigui M, Amri R, Gouiaa N, Abid C, et al. The effectiveness of platelet-rich plasma gel on full-thickness cartilage defect repair in a rabbit model. Bone Joint Res. 2021;10(3):192-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90. Singh BN, Nallakumarasamy A, Sinha S, Rastogi A, Mallick SP, Divakar S, et al. Generation of hybrid tissue engineered construct through embedding autologous chondrocyte loaded platelet rich plasma/alginate based hydrogel in porous scaffold for cartilage regeneration. Int J Biol Macromol. 2022;203:389-405. [DOI] [PubMed] [Google Scholar]
- 91. Pan X, Yuan S, Xun X, Fan Z, Xue X, Zhang C, et al. Long-term recruitment of endogenous M2 macrophages by platelet lysate-rich plasma macroporous hydrogel scaffold for articular cartilage defect repair. Adv Healthc Mater. 2022;11(6):e2101661. [DOI] [PubMed] [Google Scholar]
- 92. Schuette HB, Kraeutler MJ, McCarty EC. Matrix-assisted autologous chondrocyte transplantation in the knee: a systematic review of mid- to long-term clinical outcomes. Orthop J Sports Med. 2017;5(6):2325967117709250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Jiang S, Tian G, Yang Z, Gao X, Wang F, Li J, et al. Enhancement of acellular cartilage matrix scaffold by Wharton’s jelly mesenchymal stem cell-derived exosomes to promote osteochondral regeneration. Bioact Mater. 2021;6(9):2711-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94. Wang HC, Lin TH, Hsu CC, Yeh ML. Restoring osteochondral defects through the differentiation potential of cartilage stem/progenitor cells cultivated on porous scaffolds. Cells. 2021;10(12):3536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Liu M, Zeng X, Ma C, Yi H, Ali Z, Mou X, et al. Injectable hydrogels for cartilage and bone tissue engineering. Bone Res. 2017;5:17014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Ruiz-Alonso S, Lafuente-Merchan M, Ciriza J, Saenz-Del-Burgo L, Pedraz JL. Tendon tissue engineering: cells, growth factors, scaffolds and production techniques. J Control Release. 2021;333:448-86. [DOI] [PubMed] [Google Scholar]
- 97. Yurtbay A, Say F, Çinka H, Ersoy A. Multiple platelet-rich plasma injections are superior to single PRP injections or saline in osteoarthritis of the knee: the 2-year results of a randomized, double-blind, placebo-controlled clinical trial. Arch Orthop Trauma Surg. Published online October 27, 2021. doi:10.1007/s00402-021-04230-2. [DOI] [PubMed] [Google Scholar]
- 98. Görmeli G, Görmeli CA, Ataoglu B, Çolak C, Aslantürk O, Ertem K. Multiple PRP injections are more effective than single injections and hyaluronic acid in knees with early osteoarthritis: a randomized, double-blind, placebo-controlled trial. Knee Surg Sports Traumatol Arthrosc. 2017;25(3):958-65. [DOI] [PubMed] [Google Scholar]
- 99. de Miguel-Gómez L, López-Martínez S, Campo H, Francés-Herrero E, Faus A, Díaz A, et al. Comparison of different sources of platelet-rich plasma as treatment option for infertility-causing endometrial pathologies. Fertil Steril. 2021;115(2):490-500. [DOI] [PubMed] [Google Scholar]
- 100. Anitua E, Fernandez-De-Retana S, Alkhraisat MH. Platelet rich plasma in oral and maxillofacial surgery from the perspective of composition. Platelets. 2021;32(2):174-82. [DOI] [PubMed] [Google Scholar]
- 101. Zotti F, Albanese M, Rodella LF, Nocini PF. Platelet-rich plasma in treatment of temporomandibular joint dysfunctions: narrative review. Int J Mol Sci. 2019;20(2):277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102. Van Lieshout EMM, Den Hartog D. Effect of platelet-rich plasma on fracture healing. Injury. 2021;52(Suppl 2):S58-66. [DOI] [PubMed] [Google Scholar]
- 103. Curl WW, Krome J, Gordon ES, Rushing J, Smith BP, Poehling GG. Cartilage injuries: a review of 31,516 knee arthroscopies. Arthroscopy. 1997;13(4):456-60. [DOI] [PubMed] [Google Scholar]
- 104. Tucker JD, Goetz LL, Duncan MB, Gilman JB, Elmore LW, Sell SA, et al. Randomized, placebo-controlled analysis of the knee synovial environment following platelet-rich plasma treatment for knee osteoarthritis. PM R. 2021;13(7):707-19. [DOI] [PubMed] [Google Scholar]
- 105. Dório M, Pereira RMR, Luz AGB, Deveza LA, de Oliveira RM, Fuller R. Efficacy of platelet-rich plasma and plasma for symptomatic treatment of knee osteoarthritis: a double-blinded placebo-controlled randomized clinical trial. BMC Musculoskelet Disord. 2021;22(1):822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106. Ngarmukos S, Tanavalee C, Amarase C, Phakham S, Mingsiritham W, Reantragoon R, et al. Two or four injections of platelet-rich plasma for osteoarthritic knee did not change synovial biomarkers but similarly improved clinical outcomes. Sci Rep. 2021;11(1):23603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. Dulic O, Rasovic P, Lalic I, Kecojevic V, Gavrilovic G, Abazovic D, et al. Bone marrow aspirate concentrate versus platelet rich plasma or hyaluronic acid for the treatment of knee osteoarthritis. Medicina (Kaunas). 2021;57(11):1193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108. Park YB, Kim JH, Ha CW, Lee DH. Clinical Efficacy of platelet-rich plasma injection and its association with growth factors in the treatment of mild to moderate knee osteoarthritis: a randomized double-blind controlled clinical trial as compared with hyaluronic acid. Am J Sports Med. 2021;49(2):487-96. [DOI] [PubMed] [Google Scholar]
- 109. Bansal H, Leon J, Pont JL, Wilson DA, Bansal A, Agarwal D, et al. Author correction: plateletrich plasma (PRP) in osteoarthritis (OA) knee: correct dose critical for long term clinical efficacy. Sci Rep. 2021;11(1):18612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Raeissadat SA, Ghazi Hosseini P, Bahrami MH, Salman Roghani R, Fathi M, Gharooee Ahangar A, et al. The comparison effects of intra-articular injection of Platelet Rich Plasma (PRP), Plasma Rich in Growth Factor (PRGF), Hyaluronic Acid (HA), and ozone in knee osteoarthritis; a one year randomized clinical trial. BMC Musculoskelet Disord. 2021;22(1):134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Xu Z, He Z, Shu L, Li X, Ma M, Ye C. Intra-articular platelet-rich plasma combined with hyaluronic acid injection for knee osteoarthritis is superior to platelet-rich plasma or hyaluronic acid alone in inhibiting inflammation and improving pain and function. Arthroscopy. 2021;37(3):903-15. [DOI] [PubMed] [Google Scholar]
- 112. Sun SF, Lin GC, Hsu CW, Lin HS, Liou IS, Wu SY. Comparing efficacy of intraarticular single crosslinked Hyaluronan (HYAJOINT Plus) and platelet-rich plasma (PRP) versus PRP alone for treating knee osteoarthritis. Sci Rep. 2021;11(1):140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113. Louis ML, Dumonceau RG, Jouve E, Cohen M, Djouri R, Richardet N, et al. Intra-articular injection of autologous microfat and platelet-rich plasma in the treatment of knee osteoarthritis: a double-blind randomized comparative study. Arthroscopy. 2021;37(10):3125-37.e3. [DOI] [PubMed] [Google Scholar]
- 114. Wu Z, Yin J, Yue Y, Zhang Y. Application effect of different concentrations of platelet-rich plasma combined with quadriceps training on cartilage repair of knee osteoarthritis. J Healthc Eng. 2022;2022:7878064. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 115. Elawamy A, Kamel EZ, Mahran SA, Abdellatif H, Hassanien M. Efficacy of genicular nerve radiofrequency ablation versus intra-articular platelet rich plasma in chronic knee osteoarthritis: a single-blind randomized clinical trial. Pain Physician. 2021;24(2):127-34. [PubMed] [Google Scholar]
- 116. Danieli MV, Guerreiro JPF, Queiroz AO, da Rosa Pereira H, Cataneo DC. Leucocyte-poor-platelet-rich plasma intra-operative injection in chondral knee injuries improve patients outcomes. A prospective randomized trial. Int Orthop. 2021;45(2):463-71. [DOI] [PubMed] [Google Scholar]
- 117. Chen P, Huang L, Ma Y, Zhang D, Zhang X, Zhou J, et al. Intra-articular platelet-rich plasma injection for knee osteoarthritis: a summary of meta-analyses. J Orthop Surg Res. 2019;14(1):385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 118. Kon E, Di Matteo B, Delgado D, Cole BJ, Dorotei A, Dragoo JL, et al. Platelet-rich plasma for the treatment of knee osteoarthritis: an expert opinion and proposal for a novel classification and coding system. Expert Opin Biol Ther. 2020;20(12):1447-60. [DOI] [PubMed] [Google Scholar]
- 119. Chen Z, Deng Z, Ma Y, Liao J, Li Q, Li M, et al. Preparation, procedures and evaluation of platelet-rich plasma injection in the treatment of knee osteoarthritis. J Vis Exp. 2019;(143): e57700. doi:10.3791/57700. [DOI] [PubMed] [Google Scholar]
- 120. Arora G, Arora S. Platelet-rich plasma-where do we stand today? A critical narrative review and analysis. Dermatol Ther. 2021;34(1):e14343. [DOI] [PubMed] [Google Scholar]
- 121. Andia I, Maffulli N. A contemporary view of platelet-rich plasma therapies: moving toward refined clinical protocols and precise indications. Regen Med. 2018;13(6):717-28. [DOI] [PubMed] [Google Scholar]
- 122. Southworth TM, Naveen NB, Tauro TM, Leong NL, Cole BJ. The use of platelet-rich plasma in symptomatic knee osteoarthritis. J Knee Surg. 2019;32(1):37-45. [DOI] [PubMed] [Google Scholar]