Abstract
The aims and objectives of the study were to evaluate the antiParkinson’s (PD) potential of B cernua (BCE). B cernua (Poir.) Müll. Arg. (B cernua) is a member of the Phyllanthaceae family. HPLC revealed the presence of various phytochemicals. Study was conducted for 40 days. After PD induction by paraquat behavioural studies were carried out. Biochemical parameters such as DPPH, NO-scavenging, Ferrous reducing power, MDA, GSH, CAT, SOD, acetylcholinesterase (AChE), neurotransmitter estimation and TNF-α and IL-6 levels were determined. DPPH, NO-scavenging and Ferrous reducing power assays showed 78.02%, 48.05% and 71.45% inhibitions, respectively. There was significant improvement in motor functions and coordination in a dose-dependent manner (50 < 250 < 500 mg/kg) in PD rat model. Biochemical markers; SOD, CAT, GPx and GSH showed significant restoration (P < .001) while MDA showed significant decrease (P < .05). The AChE level was significantly reduced (P < .05) at 500 mg/kg while neurotransmitters were significantly improved (P < .001) in a dose-dependent fashion. The ELISA results showed significant (P < .001) down-regulation of IL-6 and TNF-α level. In conclusion, it is suggested that BCE has the potential to reduce the symptoms of PD.
Keywords: Breynia cernua, phyllanthaceae, Parkinson’s disease, paraquat, glycosides, saponins
Introduction
Parkinson disease (PD) is an age-dependent neurodegenerative disorder, characterized by increasing extrapyramidal motor dysfunctions caused by selective depletion of dopamine in striatum and substantia nigra pars compacta region. 1 The pathogenesis of PD is influenced by protein aggregation, excitotoxicity, inflammation and inhibition of mitochondrial complex-1. PD is characterized by motor function abnormality, involuntary facial movement, protruding tongue, dyskinesia and akinesia, etc.2,3 Dopamine levels in basal ganglia and caudate are reduced, which leads to pathological conditions (inclusions of intracellular lewy bodies, neuronal degeneration and depigmentation). Oxidative stress plays a main role in the aetiology and progression of PD. 4 Dopamine is an unstable chemical that easily undergoes auto-oxidation in the cytoplasm. Any alteration that increases cytoplasmic dopamine can also increase dopamine autoxidation, reactive oxygen species (ROS) production, and ultimately the development of Parkinson’s disease (PD). 5
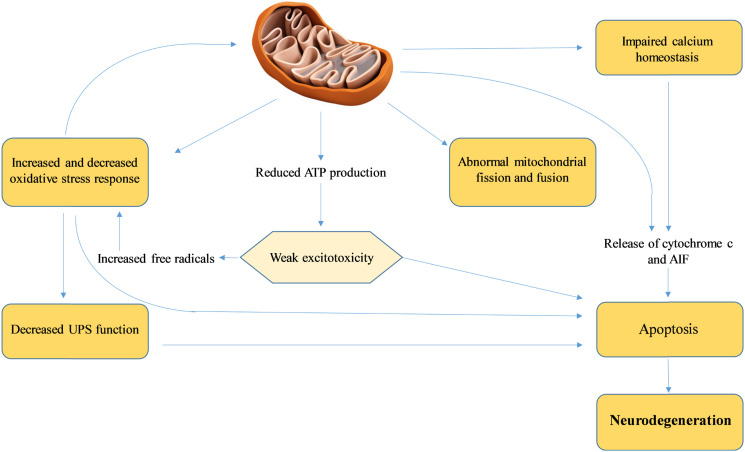
Mitochondrial dysfunction increases the ROS. This causes oxidative stress and damage proteins, lipids, and DNA and reduces the glutathione (antioxidant) level. Oxidative stress causes lewy body protein aggregation, α-synuclein aggregation and protein degradation (characteristic of PD). 6 General mitochondrial stress mechanism is mentioned in Figure 1.
Figure 1.
Mitochondrial stress mechanisms: Parkinson disease has been associated with aberrant mitochondrial morphology, poor metabolic function and defective fission-fusion balance. Increased reactive oxygen species diminished the ubiquitin-proteasomal system function. When intracellular calcium levels rise as a result of excitotoxicity, mitochondria also sequesters calcium. Impaired mitochondrial ATP production decreases the threshold for ecotoxicity. Cell death is the result of cytochrome c and other ‘pro-apoptotic factors’, including apoptosis initiating factor, being released by the mitochondria into the cytoplasm, causes neurodegeneration.
Treatments that currently available are levodopa, carbidopa, dopamine agonist, etc., can reverse the PD symptoms by enhancing dopamine neurotransmission, but no effect on disease progression and the chronic administration also causes hypertension, depression, ulcer and various more toxicities. 7 Therefore, it is necessary to find out novel therapeutic option that not only provide symptomatic relief but also slows down the progression of the disease.
Exposure to herbicides, pesticides and heavy metals may have increased incidence of PD in humans, according to epidemiological research. PQ2+, N, N′-dimethyl-4-4′-bipiridinium (Paraquat; PQ) is one such environmental toxin. It is a divalent cation, converted to a monovalent cation by redox cycling with cellular diaphorases (NADPH oxidase and nitric oxide synthase). Superoxide radical is produced as a result of this redox cycle, resulting in oxidative stress–related cytotoxicity. 8 The reactive O2- triggers a well-known chain of events that produces various ROS, including hydrogen peroxide (H2O2) and the hydroxyl radical (OH−). These ROS promote mitochondrial malfunction, which gradually triggers the death of dopaminergic neurons. It is believed that this procedure triggers the development of PD. 9 PQ causes particular neurodegeneration in the substantia nigra region, enters the CNS by neutral amino acid transporter. 10
Natural compounds originating from plants serve an essential part in human health maintenance. With the advancement of research on more scientific grounds and progress in naturopathy, there is increased use of natural products from organic origin.11-15 Breynia species are primarily distributed in Vietnam, Australia, Malaysia and some areas of India. 16 The majority of Phyllanthaceae family plants are shrubs, herbs or trees, few of them are climbers and one species is aquatic. 17 Traditionally, the plants of this genus are used for the treatment of haemorrhages, tonsillitis, malaria, fevers, dysentery, cough, inflammation, diabetes, cancer, kidney disorders and skin disorders.
Phytochemical screening of B cernua shows that it contains secondary metabolites in the form of glycosides, alkaloids, hydrocarbons, triterpenoids, flavonoids, saponins, tannins and sterol.18,19 Metals such as Cl, K, Mg and Ca in high percentage and Cr, Fe, Co and Ni in lower percentage, indicated elemental concentrations in different cellular structures of plant. 20 Traditionally used for the treatment of swollen legs, sores, ulcers, body pains, coughs, dysentery, malaria and diarrhoeal complications. B cernua shows broad spectrum of antimicrobial activity, and the n-hexane fraction of B cernua shows cytotoxicity against MCF-7 breast cancer cells. 18
The genus Breynia has gained significance for medicinal use since it contains secondary metabolites such as glycosides, lignins, steroids, terpenoids, alkaloids, nucleosides, tannins and phenolic compounds. B cernua phytochemicals are least reported with their medicinal uses against CNS disorders. Therefore, this research was designed to authenticate the existence of valuable compounds in B cernua extract holding neuroprotective potential. Until now, the neuropharmacological effects of B cernua are not well documented in previous literature. B cernua extract was further investigated in paraquat induced PD animal model after Fourier transform infrared spectroscopy (FTIR) and HPLC analysis and estimation of in-vitro antioxidant assays.
Material and Methods
Plant Collection and Extraction
B.cernua was collected from Lahore (from a nursery). Washed with water, separated the aerial parts from root and dried under shade for 15 days. Extraction was done by simple maceration technique. In 2000 mL of ethanol 700 g of plant material was suspended, allowed to stand for 3 days at room temperature with infrequent shaking, every day filtered the material with Whatman filter paper. After 3 days, menstruum was placed in rotatory evaporator. Percentage yield was calculated by using following formula
Phytochemical Analysis
The phytochemical analysis of B cernua ariel part extract to determine the reducing sugars, proteins, fats, saponins, steroidal glycosides, tannins, anthraquinone glycosides, resins and alkaloids were performed by following previously described methods. 21
FTIR Analysis
The plant extract was used for the qualitative analysis by FTIR fingerprinting. One mg of B cernua extract was combined with 100 mg of KBr and ground into a fine powder. This powder was added to a die, which was then compacted into a disc using a hydraulic press. From the disc, FTIR spectrum was obtained at 4000–400 cm−1. This method was repeated 3 times, with peaks in various places compared to standard organic compound peaks.
HPLC
A .45 μm Millex-HV PVDF (Millipore, New Bedford, MA, USA) membrane was used to filter the sample. Using an analytical column (Phenomenex® ODS 100 A 250 mm × 4.60 mm, 5 μm) accompanied by a C18 guard column (2.0 cm 4.0 mm; 5 m), HPLC analysis was carried out on a Shimadzu chromatographer outfitted with a ternary pump (Shimadzu LC-20AT) and diode assay detector (Shimadzu SPD-M20A, Kyoto, Japan). Data processing was carried out using LC solutions software (version 1.25). Acetonitrile and water were used as the mobile phase in a gradient chromatography procedure, with the initial acetonitrile/water ratio being 2:8 v/v and the final acetonitrile/water ratio being 8:2 v/v at 1 mL/min flow rate over the course of 30 min with 25oC column temperature and 20 μL injection volume. Every day, the mobile phase was prepared and sonicated to remove gas before usage. The UV spectra were observed between 450 and 200 nm. All of the standard solutions and extracts were made with ethanol. The concentration of the solution chemical was 2000 g/mL, whereas the reference solutions were employed in 6 different concentrations (12.5, 25.0, 100.0, 150.0, 250.0, and 500.0 g/mL). 22
In Vitro Antioxidant Activity
DDPH scavenging, ferrous reducing power and NO-scavenging assays
The antioxidant potential of ethanol extract of B cernua was determined by DPPH radical scavenging assay suggested by Sultan, Bhatti and Iqbal 23 Ferrous reducing power assay as described by Yen, Duh and Chuang 24 and NO-scavenging activity as described by Francis M. Awah and Andrew W. Verla. 25 Dose inhibitory curve and IC50 value was calculated by using Graph Pad Prism software version 8.0.2.
Total phenolic content
Total phenolic content (TPC) of Breynia cernua (BCE) was calculated by following procedure: .5 mL of GC (in 5 mL of DW (distilled water) .05 g of extract), Folin-ciocalteu reagent (.5 mL) and DW (7.5 mL) were added in a falcon tube, placed at 25oC for 10 min. After 10 min, 20% of Na2CO3 (1.5 mL) is added, place the solution in dark for 40 min and absorbance was recorded at 725 nm. To construct the standard curve, Gallic acid is used. Total phenolic content was measured as GAE (Gallic acid equivalent), results are expressed in mg/kg of extract 13
Total flavonoid content
Total flavonoid content was measured by using quercetin as standard (standard curve at 10–130 ppm). For this method, .5 mL of GC solution kept at room temperature for 5 min. Then .6 mL of 10% AlCl3 (Aluminium chloride) were added in the tube and wait for 5 min by keeping at room temperature. After that 1 M NaOH (sodium hydroxide) (2 mL) was added in above solution, 2.4 mL of DW was added for making a final volume. At 510 nm, absorbance was calculated by using spectrophotometer. TFC was measured as quercetin equivalent (CE) 26
Antiparkinsonian Activity
Experimental Study Design
A total of 30 healthy male (5 in each group) Wistar rats, weight about 150 g ± 10 g were chosen for the experiment. All animals were placed in separate cages under conventional laboratory temperature and humidity settings, with easy food and water access. Animals are used in the study according to the (guide for the Care and use of Laboratory Animals 8th edition) published by National Research Council. Approval was obtained by Ethical Review Committee of Government College University, Faisalabad, Pakistan. Animals were randomly grouped into following groups, such as:
1. Group 1: Negative control (NC) treated with vehicle orally.
2. Group 2: Standard L-Dopa + 10 mg/kg paraquat orally.
3. Group 3: Disease control (DC) 10 mg/kg paraquat orally.
4. Group 4: BCE 50 mg/kg + 10 mg/kg paraquat orally.
5. Group 5: BCE 250 mg/kg + 10 mg/kg paraquat orally.
6. Group 6: BCE 500 mg/kg + 10 mg/kg paraquat orally.
Literature shows that Paraquat (PQ) has a strong effect on brain functioning by forming the ROS in the brain. The disease was confirmed by motor incoordination and few extrapyramidal symptoms, such as stiff muscle, tremors and abnormal facial movements. This confirms the PD for the evaluation of study.27,28 This detail is also added in the manuscript. The doses were prepared in sterilized saline solution. Study was conducted for 40 days. At the beginning and end of the experiment, behavioural and physical evaluations were conducted. After behavioural experiments, by cervical dislocation animals were euthanized. Whole brains of the experimental animals were collected and washed with phosphate buffer solution (PBS) and preserved at temperature −80°C.
Behavioural Studies
Open field test
In this experiment, animal mobility, exploration and anxiety were simultaneously examined. It was constructed of plywood and had walls that were 36 cm thick and measured 72 cm by 72 cm, look like a white painted hollow square chamber, divided the floor into 16 squares. Animals were handled by tail and placed at the centre of chamber and observed (total distance travelled and total line crossed) for 10 min, after each trial apparatus was rinsed with methanol. In 10 min, first 5 min were given to rat to move freely and after 5 min time spend in central area, postures, defecation, freezing, rearing, grooming and stretches attend were observed. 29
Wire hanging test
This test was performed for measurement of neuromuscular stretches. Apparatus was set as described by Tillerson and Miller 2003. 30 It is a non-invasive, affordable and straight forward tool for assessing rodents' grip strength and neuromuscular coordination. Rats were trained to hold the wire prior to the experiment. After that rats were placed on wire and measured the latency time (falling down from wire to surface in seconds) for 120 seconds. The hanging time of the animals is used to compare results with BCE-treated groups since animals with PD symptoms have inadequate neuromuscular power. 31
Hole board test
In animals, this test is used to assess anxiety, stress, neophilia and emotionality. Apparatus is made from Plexiglas material having 25 × 25 × 30 cm high walls, 16 equidistant holes at floor and hole board apparatus was positioned 1.5 m high. Total number of head dipping were calculated for 8 min. 30
Y maze test
This test aimed to determine short-term memory, cognitive and spatial memory deficiencies in rats. The apparatus has 3 arms (35 × 25 × 10 cm triangular central area) attached at 120° in the shape of Y. Rats were positioned at the arm’s one end, and number of entries in each arm were recorded for 8 min to determine spontaneous alterations. The following formula was used to calculate the amount of spontaneous alterations 32
Elevated plus-maze test
In mouse models of CNS diseases, the anxiety related behaviours are assessed by this test. Two closed arms (oppositely positioned) of 50 × 40 × 10 cm dimension, two open arms (oppositely positioned) with 50 × 10 cm dimension, and central area of 10 × 10 cm dimension make up this ‘+’-shaped maze that is elevated above the ground. Rat was positioned at one end of an open arm and transfer latency (time taken by animal to move from open arm to closed arm) were noted. 33
Histopathological analysis
The animals were euthanized according to the guidlines as already described.sacrificed. The cerebellum and hippocampus area of brain were isolated and stored in PBS after being rinsed with ice-cold normal saline. Slides were prepared by haematoxylin and eosin staining and observed under microscope at 10X magnification.
Estimation of Biochemical Markers
Brain homogenate preparation
For the preparation of brain homogenate, small pieces of brain tissue were sliced and placed in a falcon tube, and then 10 X volumes of phosphate buffer (.1 mmol/L having pH 7.4) was added into it. Homogenised with homogenizer, then centrifuged at 6000 r/min for 10 min. Separated the supernatant, and by using the isolated supernatant, antioxidant enzymes were determined. 31
Estimation of glutathione reduced activity
In homogenate (1 mL) 10% of trichloroacetic acid (TCA) (1 mL), .1 M phosphate buffer (pH 7.4) (4 mL) and DTNB (prepared by dissolving 29.78 mg in 25 mL of methanol) (.5 mL) was added in a falcon tube. At 412 nm absorbance was calculated, the following formula was used for the determination of GSH level
BT (tissue homogenate), Vu (volume of aliquots), Y (absorbance), DF (dilution factor i-e., 1). 31
Estimation of glutathione peroxidase activity
In .1 mL of brain homogenate, .2 mL of sodium azide, .2 mL of EDTA and .2 mL of H2O2 were added in falcon’s tube. After few minutes, reaction was stopped by adding TCA, and then centrifuged for 10 min at 2000 r/min. Supernatant was separated and merged with disodium hydrogen phosphate (4 mL) and DTNB (.5 mL). At 420 nm, absorbance was measured. 29
Estimation of superoxide dismutase activity
In brain homogenate (.1 mL), .05 M sodium phosphate buffer (pH 8.3) (1.2 mL), nitro blue tetrazolium (.3 mL), phenazine methosulfate (.1 mL) and triton X (.2 mL) were added in a falcon’s tube. Then incubated for 90 min at 30oC. After that, for the termination of chemical reaction, glacial acetic acid was added. Then with constant stirring 4 mL of n-butanol was added, allowed to stand for 10 min and centrifuged at 1000 r/min for 10 min. Supernatant was separated and at 560 nm absorbance was calculated. By using following formula, superoxide dismutase (SOD) level was calculated 29
Estimation of catalases activity
In brain homogenate (50 μL), 50 mM phosphate buffer (pH 7.4) (1.95 mL) and 30 mM H2O2 (1 mL) were added in a falcon’s tube. Optical density was noted at 240 nm, CAT activity was determined by using the following formula
E (extinction coefficient of H2O2) is .071 mmol/cm and (absorbance shift per minute). 29
Estimation of malondialdehyde activity
In brain homogenate (.2 mL), sodium dodecyl sulphate (8.1%) (.2 mL), .8% TBA (1.5 mL) and 20% acetic acid (1.5 mL) were mixed and transferred into DW (4 mL). Then on water bath at 100oC the mixture was heated for 60 min, cooled and blended with n-butanol (5 mL) and DW (1 mL). Wait for the appearance of yellow colour, yellow colour appeared after 120 min; then for 10 min the solution was centrifuged at 4000 r/min, and overlying top later was separated and at 532 nm absorbance was measured. 29 By using following formula Malondialdehyde (MDA) level was calculated
Vt (total volume of assay mixture), Vu (volume of aliquots), Y (absorbance at 532 nm), 1.56 × 105 (molar extinction coefficient) and Wt (weight of brain). 31
Estimation of AChE activity
In a falcon tube, phosphate buffer (pH 8.0) (2.6 mL), DTNB (.1 mL) and acetylthiocholine iodide (20 μl) were added. An aliquot of tissue homogenate (.4 mL) was added to the reaction mixture. Yellow colour will appear, and then at 412 nm absorbance was recorded 34
A (change in absorbance/min), Co (tissue homogenate’s initial concentration) and R (rate in moles of substrate hydrolysed/minute/g tissue).
Estimation of protein level
In a falcon tube, brain homogenate (.2 mL) and reagent A (composed of 2% of sodium carbonate, .1 N NaOH (48 mL), 1% sodium potassium titrate (1 mL) in DW and .5% CuSO4 (1 mL)) (4.5 mL) were added, stayed for 10 min. Then reagent B (prepared by 1 part of H2O and 2 parts of 2 N Folin-Phenol) (.5 mL) was added and incubated at 37oC for 30 min. After that at 660 nm absorbance was calculated. By using BSA as standard, protein level was calculated. 35
Biochemical Analysis
Estimation of Neurotransmitters level
Preparation of tissue extracts
50–75 mg of brain tissues were homogenized with HCl-Butanol (37% HCl (.425 mL) in n-butanol (50 mL)) (5 mL) for 1 min. Then centrifuged for 10 min at 2000 r/min, supernatant was separated and mixed with heptane (2.5 mL) and .1 M HCl (.31 mL), shaking vigorously and again centrifuged for 10 min at 2000 r/min. Upper aqueous layer was separated and lower organic layer was discarded. 36
Estimation of noradrenaline and dopamine
For the estimation of noradrenaline and dopamine aqueous phase (.2 mL), .4 M HCl (.05 mL) and EDTA (.1 mL) were added in a falcon tube. Then for oxidation, iodine solution (.1 mL) was added. After 2 min reaction was stopped by adding Na2SO3 solution (.1 mL). Then after 1.5 min Acetic acid (.1 mL) is added, heated for 6 min at 100oC. The excitation and emission spectra from the spectrofluorometer were read when the solution reached room temperature. For dopamine readings were taken at 330–375 nm and for adrenaline 395–485 nm. 36 By using following formula dopamine level was calculated
Estimation of serotonin
For the estimation of serotonin, the aqueous extract (.2 mL) and O-phthaldialdehyde (OPT) (.25 mL) reagent were added in a falcon tube. Heated at 100oC for 10 min fluorophore was developed. When sample reached at equilibrium reading were taken at 360–470 nm in Spectro fluorometer. 36
Estimation of inflammatory biomarkers by ELISA
The elabscience ELISA kit of IL-6 and TNF-α was used, protocol is followed according to the kit description.
Statistical analysis
The data was presented as Mean ± SD. The statistical analysis was done by using Graph Pad Prism software version 8.0.2. One-way Analysis of Variance (ANOVA) by followed by Turkeys multiple compartment tests as well as Dunnett’s multiple compartment tests were employed to find out the variation between the groups.
Results
Percentage Yield of Ethanolic Extract Breynia cernua
The percentage yield of ethanolic extract of B cernua found to be 2%. Total dry weight of extract was 14 gm.
Phytochemical Analysis Ethanolic Extract of Breynia cernua
Qualitative analysis
For primary phytochemicals, proteins, carbohydrates and lipids were present. Secondary metabolites, alkaloids, glycosides, tannins, saponins, terpenoids and resins were identified. Results are mentioned in Table 1.
Table 1.
Phytochemical Analysis of Plant Constituents Breynia cernua (BCE) Whole Plant Extract.
| Constituents | Tests performed | Ethanolic extract |
|---|---|---|
| Reducing sugars | Fehling’s tests | +++ |
| Proteins | Ninhydrin test | _ |
| Fats | Soap formation test | _ |
| Saponins | Foam test | ++ |
| Steroidal glycosides | Keller Killani test | +++ |
| Tannins | Ferric chloride test | ++ |
| Anthraquinones glycosides | Bontrager’s test | +++ |
| Resins | Acid anhydride test | _ |
| Alkaloids | Dragendroff test | + |
Quantitative analysis
FTIR spectrum
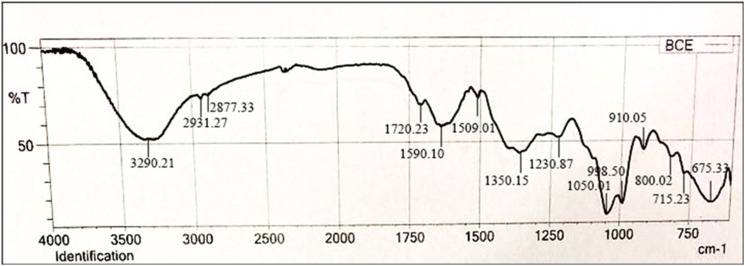
The FTIR analysis of B cernua shows the presence of many pharmacologically active constituents. The FTIR spectra of B cernua showed peaks mentioned in Table 2. These peaks reveal the C–H stretch (alkanes), O–H stretch (carboxylic acid), N–H stretch (primary ammonium salts), C–O–H bend (carboxylic acid and alcohols), C–O stretch (primary alcohols) and C = C stretch (alkyl part of fatty acid chain). FTIR spectrum is shown in Figures 2 and 3.
Table 2.
Phytochemical Composition of Ethanolic Extract of Breynia cernua (BCE) Whole Plant Extract Through FTIR Analysis.
| Wave number (cm−1) | Intensity of estimation | Group or functional group class |
|---|---|---|
| 3290.21 | S | O–H stretch (alcohols) |
| 2931.27 | W | C–H stretch (Alkanes) N–H stretch (amine salt) |
| 2877.33 | W | C–H stretch (alkanes) |
| 1720.23 | M | C=O stretch (Aldehyde) |
| 1590.10 | M | C=C stretch (α, β-unsaturated ketone) |
| 1509.01 | W | N–O stretch (nitro compounds) |
| 1350.15 | M | O–H bend (carboxylic acid and alchohols) |
| 1230.87 | M | C–O stretch (alkyl aryl ethers) |
| 1050.01 | S | C–O stretch (primary alcohols) |
| 998.50 | S | C=C bending (monosubstituted alkenes) |
| 910.05 | M | C=C bend (alkenes like vanylidine) |
| 800.02 | M | C=C bend (trisubstituted alkenes) |
| 715.23 | M | C=C bend (cis trisubstituted alkenes) |
| 675.33 | S | C–X stretch (halo compounds) |
Figure 2.
Fourier transform infrared spectroscopy spectrum of ethanolic extract of Breynia cernua.
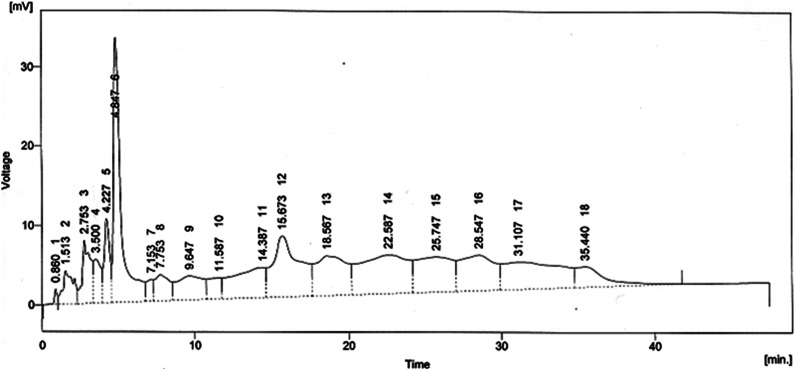
Figure 3.
HPLC chromatogram of ethanolic extract of Breynia cernua.
HPLC analysis
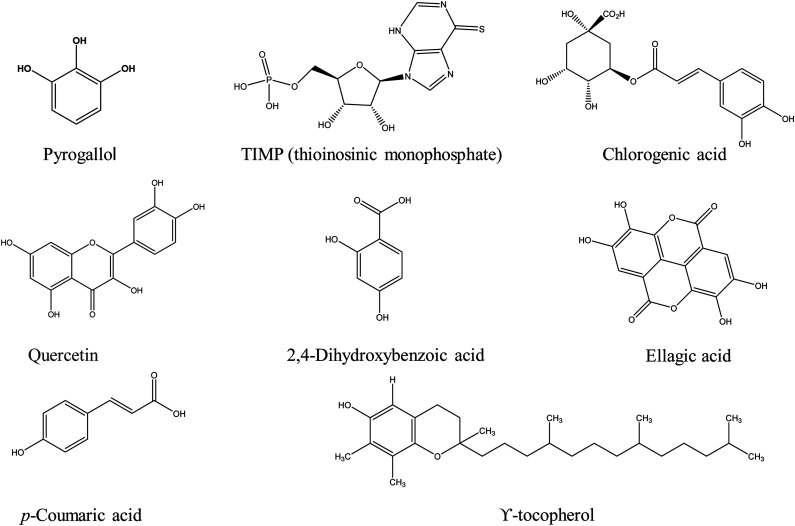
The HPLC analysis of BCE (Figure 2) noticeably reveals the presence of 15 compounds, there structure and names are mentioned in Figure 4 and Table 3. Phytochemicals pyrogallol, propionic acid, N-methyl-11-acetoxyhuperzine B, chlorogenic acid, quercetin, catechol, p-coumaric acid and ϒ-tocopherol are shown with moderate peaks. The thioinosinic monophosphate is separated with a sharp peak.
Figure 4.
Structures of compounds elucidated by HPLC from ethanolic extract of Breynia cernua.
Table 3.
Phytochemicals Identified by HPLC from Ethanolic Extract of Breynia cernua (BCE), their Retention Time.
| Sr No | Retention time (min) | Compounds detected |
|---|---|---|
| 1 | .860 | Pyridine |
| 2 | 2.753 | Pyrogallol |
| 3 | 3.50 | Propionic acid |
| 4 | 4.847 | TIMP (thioinosinic monophosphate) |
| 5 | 7.753 | N-methyl-11-acetoxyhuperzine B |
| 6 | 9.647 | 4-Hydroxybenzoic acid |
| 7 | 11.587 | Chlorogenic acid |
| 8 | 14.387 | Quercetin |
| 9 | 15.673 | Catechol |
| 10 | 18.567 | 2,4-dihydroxybenzoic acid |
| 11 | 22.587 | Ellagic acid |
| 12 | 25.747 | Anisic acid |
| 13 | 28.547 | p-coumaric acid |
| 14 | 31.107 | ϒ-tocopherol |
| 15 | 35.440 | Cimicifugic acid F |
In Vitro antioxidant activity
The in vitro antioxidant DPPH assay shows 78.02% inhibition at .5 mg/mL of BCE with 3.4 μg IC50 value, NO-scavenging activity shows 48.05% inhibition at .25 mg/mL of BCE, and ferrous reducing power assay shows 71.45% inhibition at .5 mg/mL of BCE. Results are mentioned in Table 4. The results of these assays suggest that B cernua exhibited potential antioxidant activity. In Ferrous reducing power assay, the ferric of potassium ferricyanate is reduced to ferrous by the antioxidants of plant.
Table 4.
Effect of Ethanolic Extract of Breynia cernua (BCE) on Antioxidant Biomarkers.
| Plant name | DPPH activity | NO-scavenging activity | Ferrous reducing power | |
|---|---|---|---|---|
| (%) inhibition at .5 mg/mL | IC50 (μg) | (%) inhibition at .25 mg/mL | Vit. C % equivalent at .5 mg/mL | |
| BCE | 78.02 ± .015 | 3.40 ± .60 | 48.45 ±.11 | 71.45 ± .03 |
Total phenolic and flavonoid content
Among secondary metabolites the phenolic compounds are highest in concentration, that is, 17.23% as compared to flavonoid compounds, that is, 10.23% in ethanol extract of B cernua. Linear regression equation Y = .0716X - .0135 with R2 = .9915 obtained from standard curve of Gallic acid is used for the quantification of TPC were 17.23%. The regression equation Y = .0021X + .0149 with R2 = .9857 obtained from the standard curve of quercetin is used for the quantification of total flavonoid content that were 10.23%.
Effect of ethanolic extract of Breynia cernua on behavioural studies
Open field test
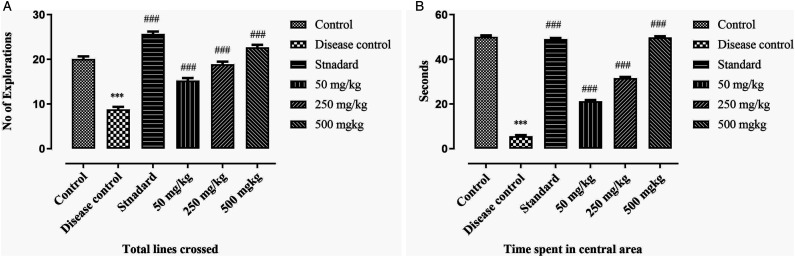
The results of open field test shows that the number of crossed lines and time spending in central area is significantly lower in disease control group as compare to BCE-treated groups (P < .001). It recommends that rats are typically terrified of open spaces, but once they become accustomed to them, they begin to explore the area in quest of food. The exploratory behaviour of rats was significantly increased by the treatment with BCE (dose dependent effect). Results are mentioned in Figure 5A and 5B.
Figure 5.
Effect of ethanolic extract of Breynia cernua on open field test in paraquat induced parkinson disease model. (A) Number of lines crossed and (B) Time spent in central area. Values are shown as the mean ± SEM, (n = 5), *** (P < .001) compared with control, and ### (P < .001) compared with disease control. Disease control: Paraquat intoxication, Standard: (L-dopa + carbidopa).
Wire hanging test
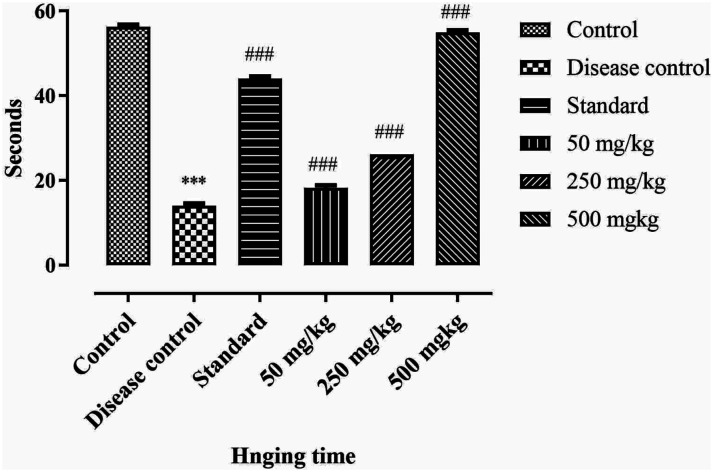
Neuromuscular strength of rats is investigated by wire hanging test. Results shows that the neuromuscular strength in disease control is significantly reduced (P < .001) as compare to control group. The BCE extract showed significant (P < .001) improvement in hanging time in a dose-dependent manner with following order 500 > 250 > 50 mg/kg. So high dose of extract can improve the neuromuscular strength of rats. Results are depicted in Figure 6.
Figure 6.
Effect of ethanolic extract of Breynia cernua on wire hanging test in paraquat induced parkinson disease model. Values are shown as the mean ± SEM, (n = 5), *** (P < .001) compared with control, and ### (P < .001) compared with disease control. Disease control: Paraquat intoxication, Standard: (L-dopa + carbidopa).
Hole board test
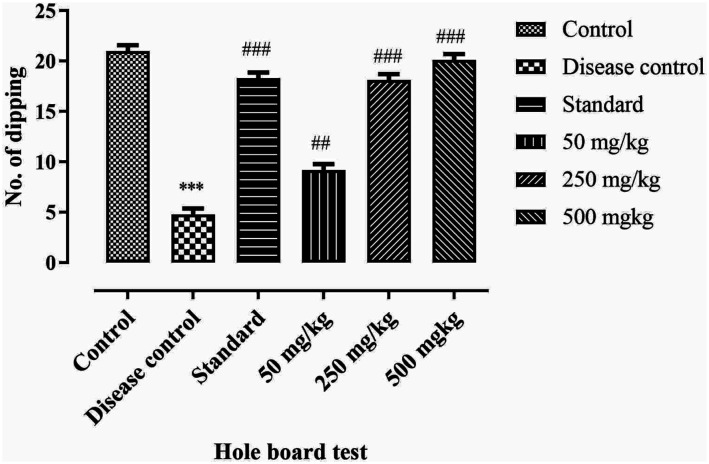
This test is used to study the learning and spatial performance in rats or any other rodents. Number of head dipping are calculated. Results shows that the number of head dipping in disease control group was significantly reduced (P < .001) as compare to standard and control. The BCE treatments groups shows dose dependent effect as the dose increases the cognitive and memory functioning was significantly increased (P < .001). Results are mentioned in Figure 7.
Figure 7.
Effect of ethanolic extract of Breynia cernua on hole board test in paraquat induced parkinson disease model. Number of head drippings in holes, Values are shown as the mean ± SEM, (n = 5) *** (P < .001) compared with control and ### (P < .001) compared with disease control. Disease control: Paraquat intoxication, Standard: (L-dopa + carbidopa).
Y maze test
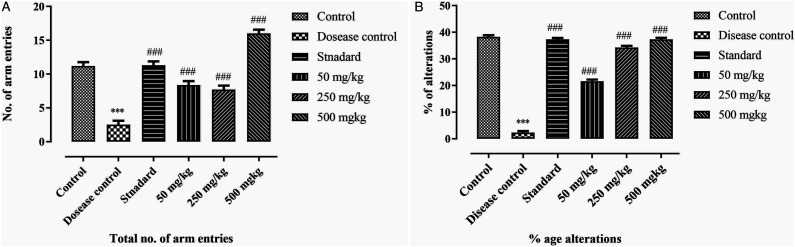
The exploratory behaviour of rats is determined by the help of Y-maze. With the help of this maze, the cognitive deficit in rats is measured. Results of our study shows that the % alteration and total number of arm entries in disease control group was significantly reduced (P < .001) as compare to control. The BCE-treated groups show dose dependent improvement in percentage alteration and total number of arm entries. The high dosage group (500 mg/kg) shows maximum memory regain. The exploratory behaviour of rats was significantly increased (P > .001) in all BCE-treated groups. Results are mentioned in Figure 8A and 8B.
Figure 8.
Effect of ethanolic extract of Breynia cernua on Y-maze test in paraquat induced parkinson disease model. (A) total number of arm entries and (B) %age alterations, Values are shown as the mean ± SEM, (n = 5) *** (P < .001) compared with control, and ### (P < .001) compared with disease control. Disease control: Paraquat intoxication, Standard: (L-dopa + carbidopa).
Effect of ethanolic extract of Breynia cernua on histopathological analysis
The histopathological examination of brain showed that the brain tissues of control group are normal as compared to disease control. There is a significant neuronal damage, haemorrhage, pigmentation, large intracellular spaces, high amount of lewy bodies and cell death is observed in disease control group. In standard group (levodopa plus carbidopa) there is a significant reduction in neuronal damage. When compared to BCE-treated groups, 500 mg/kg shows significant reversal of neuronal damage as compare to 250, and 50 mg/kg. Results are mentioned in Figure 9.
Figure 9.
Brain histopathological analysis. Haematoxylin and eosin staining of transverse brain section at 10X magnification. Control: showed the normal architecture of control group. Paraquat: Nft (neurofibrillary tangles), IP (intracellular plaques), AP (amyloid beta plaques), LB (Lewy bodies) and PG (pigmentation). It indicates the neurodegeneration, inflammation and vacuolated cytoplasm in disease control group. Nft, IP, AP and LB were less in standard group. Neurodegeneration was slightly improved in 50 mg/kg ethanolic extract of Breynia cernua treated group. Neuronal loss was improved in 250 mg/kg BCE-treated group. 500 mg/kg BCE-treated group significantly improved the architecture of transverse brain section. Disease control: Paraquat (PQ) intoxication, Standard: (L-dopa + carbidopa).
Effect of Ethanolic Extract of Breynia cernua on Biochemical Parameters
Estimation of GSH, GPx, SOD, CAT and MDA
The biochemical parameters (GSH, GPx, SOD and CAT) were significantly reduced in disease control group (treated with paraquat), but the MDA level (lipid peroxidation) was increased significantly (P < .001) in disease control group compared to control. BCE (250 and 500 mg/kg) and standard shows significant increase in GSH, GPx, SOD and CAT and significantly reduced the MDA level. Data is mentioned in Table 5.
Table 5.
Effect of Ethanolic Extract of Breynia cernua (BCE) on MDA, GPx, CAT, SOD and GSH.
| Groups | MDA (TBA mg/ml) | GPx (μg/mg protein) | CAT (IU/μl) | SOD (IU/μl) | GSH (μg/mg protein) |
|---|---|---|---|---|---|
| Control | 73.13 ± .57735 | 9.25 ± .57735 | .72 ± .058 | .08 ± .006 | .732 ± .012 |
| Disease control | 98.04 ± .57735*** | 3.23 ± .57735*** | .55 ± .057*** | .025 ± .005*** | .248 ± .009*** |
| Standard | 70.45 ± .57735### | 8.66 ± .57735### | .715 ± .057ns | .058 ± .003## | .688 ± .009### |
| 50 mg/kg | 75.57667 ± .58### | 4.46 ± .57735ns | .672 ± .056ns | .032 ± .004ns | .379 ± .015ns |
| 250 mg/kg | 61.62 ± .57735### | 10.49 ± .57735### | .698 ± .058ns | .034 ± .006ns | .57 ± .02## |
| 500 mg/kg | 46.25 ± .57735### | 10.35 ± .57735### | .711 ± .059ns | .061 ± .007## | .717 ± .009### |
Values are shown as the mean ± SEM, (n = 5), *** (P < .001) compared with control, and ### (P < .001), ns (non-significant) compared with disease control. Disease control: Paraquat (PQ) intoxication, Standard: (L-dopa + carbidopa).
Estimation of neurotransmitter level
In Table 6, the level of dopamine and noradrenaline in each group of animals is mentioned. Results show a significant decrease in the level of dopamine in disease control group than control group. The treatment groups showed dose dependent effect (increasing the concentration of extract level of dopamine is also increased). Noradrenaline shows significant (P < .001) variation and improvement in each group.
Table 6.
Effect of Ethanolic Extract of Breynia cernua (BCE) on Neurotransmitters Level: Dopamine, Noradrenaline, and Serotonin.
| Groups | Dopamine (μg/μg of bt) | Noradrenaline (μg/μg of bt) | Serotonin (μg/μg of bt) |
|---|---|---|---|
| Control | .34 ± .05 | .06 ± .005 | .65 ± .05 |
| Disease control (paraquat) | .12 ± .05*** | .05 ± .005 | .27 ± .05*** |
| Standard (L-dopa + carbidopa) | .58 ± .05### | .07 ± .005 | .58 ± .05### |
| 50 mg/kg | .36 ± .05ns | .04 ± .005 | .36 ± .05ns |
| 250 mg/kg | .42 ± .05### | .04 ± .005 | .44 ± .05ns |
| 500 mg/kg | .54 ± .05### | .06 ± .005 | .56 ± .05### |
Values are shown as the mean ± SEM, (n = 5), *** (P < .001) compared with control, and ### (P < .001), ns (non-significant) compared with disease control. Disease control: Paraquat (PQ) intoxication, Standard: (L-dopa + carbidopa).
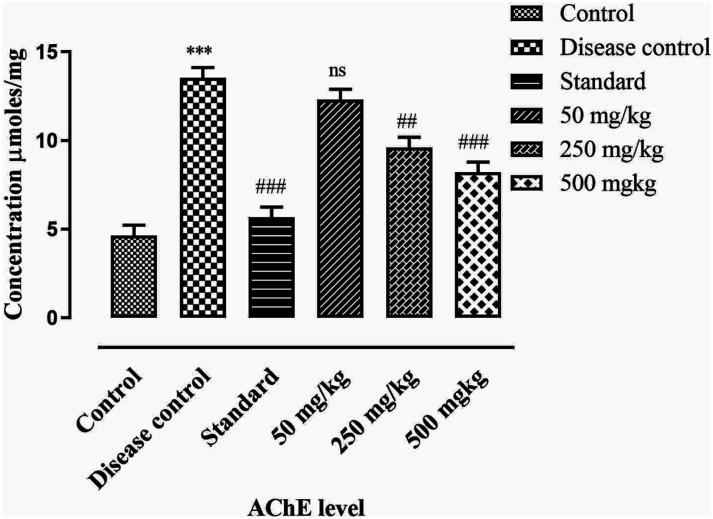
Estimation of AChE
In PD, the level of acetylcholine is decreased due to the breakdown of choline acetyltransferases, which is associated with cognitive impairment. BCE showed significant (P < .001) protective activity for acetylcholine by inhibiting the acetylcholinesterase (AChE). The level of AChE was significantly increased (P < .05) in disease control compared to control. In BCE-treated groups the AChE level was significantly reduced (P < .001) in a dose-dependent manner compared to disease control. Results are mentioned in Figure 10.
Figure 10.
Effect of ethanolic extract of Breynia cernua on acetylcholinesterase level in brain, values are shown as the mean ± SEM, (n = 5), *** (P < .001) compared with control, and ### (P <.001), ns (non-significant) compared with disease control. Disease control: Paraquat intoxication, Standard: (L-dopa + carbidopa).
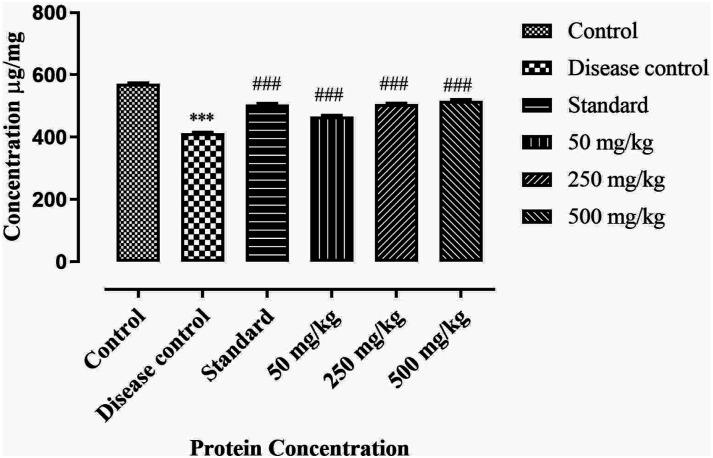
Estimation of protein level
Protein level is calculated by linear regression equation obtained for bovine albumin serum (BSA) as
Results suggests that protein level was decreased significantly (P < .001) in disease control when compared with control. The BCE-treated groups show dose dependent effect, as 500 mg/kg shows high normal protein level as compare to low dosage group. Treatment groups show significant increase (P < .001) in protein level in brain of rats compared with disease control. Results are mentioned in Figure 11.
Figure 11.
Effect of ethanolic extract of Breynia cernua on level of protein in rat’s brain. Values are shown as the mean ± SEM, (n = 5), *** (P < .001) compared with control, and ### (P < .001) compared with disease control. Disease control: Paraquat intoxication, Standard: (L-dopa + carbidopa).
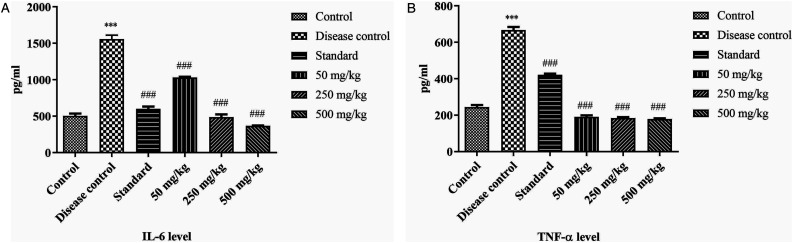
Estimation of inflammatory biomarkers by ELISA
The results suggested that the level of IL-6 and TNF-α was significantly high (P > .001) in disease control as compared to control group. The BCE-treated groups showed dose dependent effect. The treatment group of 500 mg/kg significantly decreased (P > .001) the level of IL-6 and TNF-α compared to disease control group. Results are shown in Figure 12.
Figure 12.
Effect of ethanolic extract of Breynia cernua on inflammatory markers: IL-6 and TNF-α level. Values are shown as the mean ± SEM, (n = 5), *** (P < .001) compared with control, and ### (P < .001) compared with disease control. Disease control: Paraquat intoxication, Standard: (L-dopa + carbidopa).
Discussion
Neurodegenerative diseases such as; PD and alzheimer disease prevalence are increased now a days. Most of the neurodegenerative disorders have a close relation with age and age dependency is an important factor in their pathogenesis.37,38 The importance of research in novel herbal neuroprotective compounds is extremely important because there is lack of specific treatment, increased morbidity and expensive pharmaceuticals for neurodegenerative diseases.
Phytochemical analyses of B cernua showed its pharmacological importance due to the presence of alkaloids, flavonoids, glycosides, lignans, saponins and polyphenolic. Flavonoids-rich food supplements and plant extracts have the ability to slow neurodegeneration and also improving memory, cognitive function and learning. Plant extracts rich in flavonoids are beneficial for neurodegenerative disorders because they modulate intracellular signalling pathways that enhance cell survival and age-related neuronal activities. B cernua contains a good number of flavonoids and polyphenolics,39-41which are powerful antioxidants with anti-ischaemic action and can be used as precursor for the development of new neuroprotective medicines. Flavonoids exert their neuroprotective effects primarily via promoting neuronal regeneration and reducing oxidative stress. 37 So, the flavonoids present in B cernua extract are the main compounds for neuroprotective agent’s development. Our findings of FTIR and HPLC analysis provided the evidence for neuroprotective and antioxidant potential of B cernua. HPLC analysis showed the presence of fifteen different compounds. Majority of them are phenolic and flavonoid compounds. Phenolic acids like chlorogenic acid, p-coumaric acid and ellagic acid are best antioxidant compounds found in B cernua with strong peaks. Previous studies suggested that these compounds show anti-Parkinson activity by the reduction of TNF-α, pro-inflammatory cytokines, MDA level and increased SOD level and neurons number in the brain of rats. They reduced the ROS in brain so act as potent antioxidant compounds. 42 According to the one previous study, the lycodine-type alkaloids from Lycopodiastrum casuarinoides showed strong AChE inhibitory activity by inhibiting the ROS the N-methyl-11-acetoxyhuperzine B is elucidated with sharp peak in B cernua. 43 Another study suggests that quercetin is a potent antioxidant compound, represses TNF-α, reduces pro-inflammatory cytokines, decreases AChE level in brain and shows strong anti-neuroinflammatory properties in PD.35,44 Due to the presence of all these compounds in B cernua, the DPPH, NO-scavenging and ferrous reducing power assays shows highest radical scavenging potential and inhibitory activity. Therefore, B cernua can play a pivotal role in neurodegenerative diseases induced by oxidative stress such as PD. As, it is already reported and also established that AChE inhibition is important in relation to motor function and its coordination. AChE inhibition enhances the learning and memory. Additionally, it is responsible for betterment of cholinergic transmission that enhances the motor coordination and movements. Thus, AChE estimation is very important parameter for PD induction and management. 35
Paraquat induced neurodegeneration in the SN region by entering in the CNS via the neutral amino acid transporter in the disease control group. The wire hanging and hole board test results suggest that BCE improves neuromuscular coordination and reduces the depression and psychiatric issues related to PD by increases the hanging time and head dipping in a dose-dependent manner (50 < 250 < 500 mg/kg) in treatment groups. Thus, the wire hanging test findings were according to the previous study by Jay Parkash and Satyndra Kumar Yadav, in which the Withania somnifera root extracts significantly improve the hanging time and decrease the oxidative stress in the paraquat–induced PD model. 10 The hole board and Y maze test findings were also harmonious with the previous studies.45,46
Oxidative stress and mitochondrial dysfunction are the main genetic form of abnormalities that are found in PD. 47 Multiple biological functions are impaired by disruptions in the physiological regulation of the redox potential in neurons, which eventually results in cell death. 48 Body combats against the effects of free radicals by a defensive system of antioxidant enzymes (SOD, CAT, GSH, and GPx). Redox cycling compound (paraquat) increases ROS, mitochondrial damage and ultimately increases the oxidative stress by disturbing the mitochondrial function. Thus, mitochondrial dysfunctionality caused an imbalance of MDA (lipid peroxidation) level paraquat treated group. 49 In this study, the level of MDA is increased in disease control group and decreased in a dose-dependent manner in BCE-treated groups due to presence of flavonoids, phenols, alkaloids and saponins. The endogenous antioxidant and homoeostasis of cells depends on the antioxidant enzyme SOD, CAT, GSH and GPx level. 50 In the present project, the level of these antioxidant enzymes is significantly reduced (P < .001) while BCE treatment significantly improved these antioxidant markers in a dose-dependent manner as compare to disease control. The level of protein is decreased in diseased model due to the formation of ROS that causes oxidative stress. 51 The study suggested that the high dose of BCE treated (500 mg/kg) showed significance increase (P < .001) in protein level as compare to the disease control group.
The important neuromodulators in basal ganglia are dopamine, noradrenaline, serotonin and acetylcholine which regulates the motor and cognitive abilities. The level of these neurotransmitters decreased due to the oxidative stress. The level of acetylcholine is decreased due to deterioration of choline acetyltransferase, causes cognitive debilities in PD. Apoptosis causes the loss of dopaminergic neurons in pars compacta of PD patients and AChE is the key regulator of apoptosis. AChE interacts with apoptotic protease activating factor-1 and cytochrome c cause cell death. AChE inhibitors have a role in preventing neurodegeneration by preventing cell death. 52 The BCE treated rat’s group showed a significant increment (P < .001) in the level of these neurotransmitters by showing antioxidant property due to the presence of phenolic compounds. The high dose of BCE (500 mg/kg) showed significantly improved acetylcholine levels by inhibiting the AChE because this enzyme breakdown the acetylcholine to its by-products, that is, acetic acid and choline.
In a previous study, Ashwagandha root extract demonstrated antiparkinsonian propensity by reducing the duration of catalepsy and improving oxidative stress by reducing lipid peroxidation in the Paraquat induced experimental model and one of our study results were consistent with these findings. 53 Therefore, results suggested that BCE improved the behavioural parameters which are evident with improved muscular strength, locomotion, memory and reduced oxidative stress.
Parkinson disease pathogenesis is strongly linked to increased expression of pro-inflammatory cytokines like TNF-α and Interleukin-6. Increased level of TNF-α has an ability to enhance the levels of various cytokines which might cause neurodegeneration.54,55 The higher level of pro-inflammatory cytokines (TNF-α and IL-6) causes the dopaminergic cell death in PD brain. TNF-α and IL-6 are strong mediators of microglial functions, involved in the dopaminergic neuronal cell death. 56 Recent studies reported that neurodegenerative disorders can be treated by anti-inflammatory agents, present in plants. The IL-6 decreased the level of NF-KB and upregulated the level of MAPKA pathway, resulting in the neuro-inflammation. 57 In the present study, TNF-α and IL-6 is increased in disease control group due to damage in basal ganglia region of brain. The BCE showed dose dependent decremental effects on inflammatory biomarkers level. The most significant (P < .001) effect was seen at BCE 500 mg/kg dose group.
The majority of the phytoconstituents have been found to activate neuroprotective, controlled cell-stress response pathways. As a result, we came to the conclusion that BCE acts as a modulator against the behavioural and biochemical changes caused by Paraquat-induced neurotoxicity, and it showed a good potential in amelioration of the PD progression and symptomatology.
Conclusion
The phytochemical screening of B cernua reveals the presence of both primary and secondary metabolites. Thus, it provides the rationale for its folkloric uses in inflammatory and neurodegenerative conditions of the brain. But more detailed molecular mechanism is still warranted to authenticate the study at clinical level.
Footnotes
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iD
Liaqat Hussain https://orcid.org/0000-0001-7171-5917
References
- 1.Olanow CW, Stern MB, Sethi K. The scientific and clinical basis for the treatment of Parkinson disease (2009). Neurology. 2009;72(21 suppl 4):S1-S136. [DOI] [PubMed] [Google Scholar]
- 2.Ríos J-L, Onteniente M, Picazo D, Montesinos M-C. Medicinal plants and natural products as potential sources for antiparkinson drugs. Planta Med. 2016;82(11/12):942-951. [DOI] [PubMed] [Google Scholar]
- 3.Chaudhuri KR, Schapira AH. Non-motor symptoms of Parkinson's disease: dopaminergic pathophysiology and treatment. Lancet Neurol. 2009;8(5):464-474. [DOI] [PubMed] [Google Scholar]
- 4.Ingale SP, Kasture SB. Antioxidant and antiparkinsonian activity of Passiflora incarnata leaves. Oriental Pharmacy and Experimental Medicine. 2014;14(3):231-236. [Google Scholar]
- 5.Weng M, Xie X, Liu C, Lim K-L, Zhang C-w, Li L. The sources of reactive oxygen species and its possible role in the pathogenesis of Parkinson’s disease. Parkinson’s disease. 2018;2018:1-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Henchcliffe C, Beal MF. Mitochondrial biology and oxidative stress in Parkinson disease pathogenesis. Nat Clin Pract Neurol. 2008;4(11):600-609. [DOI] [PubMed] [Google Scholar]
- 7.Olanow CW. The pathogenesis of cell death in Parkinson's disease–2007. Mov Disord: Official J Mov Disord Soc. 2007;22(S17):S335-S342. [DOI] [PubMed] [Google Scholar]
- 8.Rappold PM, Cui M, Chesser AS, et al. Paraquat neurotoxicity is mediated by the dopamine transporter and organic cation transporter-3. Proc Natl Acad Sci USA. 2011;108(51):20766-20771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhang X-f, Thompson M, Xu Y-h. Multifactorial theory applied to the neurotoxicity of paraquat and paraquat-induced mechanisms of developing Parkinson’s disease. Lab Invest. 2016;96(5):496-507. [DOI] [PubMed] [Google Scholar]
- 10.Prakash J, Yadav SK, Chouhan S, Singh SP. Neuroprotective role of Withania somnifera root extract in Maneb–Paraquat induced mouse model of parkinsonism. Neurochem Res. 2013;38(5):972-980. [DOI] [PubMed] [Google Scholar]
- 11.Zeng L-H, Rana S, Hussain L, et al. Polycystic ovary syndrome: a disorder of reproductive age; its pathogenesis and a discussion on emerging role of herbal remedies. Front Pharmacol. 2022;13:874914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Iqbal SM, Hussain L, Hussain M, et al. Nephroprotective potential of a standardized extract of bambusa arundinacea: in vitro and in vivo studies. ACS Omega. 2022;7(21):18159-18167. doi: 10.1021/acsomega.2c02047 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Younas A, Hussain L, Shabbir A, Asif M, Hussain M, Manzoor F. Effects of fagonia indica on letrozole-induced polycystic ovarian syndrome (PCOS) in young adult female rats. Evid base Compl Alternative Med. 2022;2022:1397060. doi: 10.1155/2022/1397060 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Manzoor F, Nisa MU, Shakoor A, Hussain L, Mahmood A, Younas a. Effect of sodium alginate supplementation on weight management and reproductive hormones in polycystic females. Food Funct. 2022. doi: 10.1039/D2FO01539K [DOI] [PubMed] [Google Scholar]
- 15.Zeng L-H, Rana S, Hussain L, et al. Polycystic ovary syndrome: a disorder of reproductive age, its pathogenesis, and a discussion on the emerging role of herbal remedies. review. Front Pharmacol. 2022;13:874914. doi: 10.3389/fphar.2022.874914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hoffmann P, Kathriarachchi H, Wurdack KJ. A phylogenetic classification of Phyllanthaceae (Malpighiales; Euphorbiaceae sensu lato). Kew Bull. 2006;61(1):37-53. [Google Scholar]
- 17.Vorontsova MS, Hoffmann P. A phylogenetic classification of tribe Poranthereae (Phyllanthaceae, Euphorbiaceae sensu lato). Kew Bull. 2008;63(1):41-59. [DOI] [PubMed] [Google Scholar]
- 18.Dirgantara S, Tanjung RH, Maury HK, Meiyanto E. Cytotoxic activity and phytochemical analysis of breynia cernua from Papua. Indones J Pharm Sci Technolo. 2018;1(1):31-36. [Google Scholar]
- 19.Khan M, Omoloso A. Antibacterial and antifungal activities of Breynia cernua. Fitoterapia. 2008;79(5):370-373. [DOI] [PubMed] [Google Scholar]
- 20.Gotera KMC, Claveria RJR, Doronila AI, Perez TR. Localization of nickel in the hyperaccumulator plant Breynia cernua (Poir.) Mull. Arg. discovered in the nickeliferous laterites of Zambales, the Philippines. Int J Phytoremediation. 2020;22(2):127-133. [DOI] [PubMed] [Google Scholar]
- 21.Le BaoDuy N, Trang DTD, Trang NPM. Preliminary phytochemical analysis of leaf extracts ofthuja orientalis (l.) Endl. Int J Res Sci Manag . 2015;2(1):21. [Google Scholar]
- 22.Saleem M, Javed F, Asif M, Kashif Baig M, Arif M. HPLC analysis and in vivo renoprotective evaluation of hydroalcoholic extract of Cucumis melo seeds in gentamicin-induced renal damage. Medicina. 2019;55(4):107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sultan M, Bhatti HN, Iqbal Z. Chemical analysis of essential oil of ginger (Zingiber officinale). Pakistan J Biol Sci. 2005;8(11):1576-1578. [Google Scholar]
- 24.Yen G-C, Duh P-D, Chuang D-Y. Antioxidant activity of anthraquinones and anthrone. Food Chem. 2000;70(4):437-441. [Google Scholar]
- 25.Awah F, Wirnkor V. Antioxidant activity, nitric oxide scavenging activity and phenolic content of Ocimum gratissimum leaf extract. J Med Plant Res. 2010;4:2479-2487. doi: 10.5897/JMPR10.262 [DOI] [Google Scholar]
- 26.Chang C-C, Yang M-H, Wen H-M, Chern J-C. Estimation of total flavonoid content in propolis by two complementary colometric methods. J Food Drug Anal. 2002;10(3):3. [Google Scholar]
- 27.Lama J, Buhidma Y, Fletcher EJ, Duty S. Animal models of Parkinson’s disease: a guide to selecting the optimal model for your research. %J Neuronal Signaling. 2021;5(4):NS20210026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Duty S, Jenner P. Animal models of Parkinson's disease: a source of novel treatments and clues to the cause of the disease. Br J Pharmacol. 2011;164(4):1357-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Saleem U, Chauhdary Z, Raza Z, et al. Anti-Parkinson’s activity of tribulus terrestris via modulation of AChE, α-synuclein, TNF-α, and IL-1β. ACS Omega. 2020;5(39):25216-25227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tillerson JL, Caudle W, Reveron M, Miller G. Exercise induces behavioral recovery and attenuates neurochemical deficits in rodent models of Parkinson's disease. Neuroscience. 2003;119(3):899-911. [DOI] [PubMed] [Google Scholar]
- 31.Saleem U, Shehzad A, Shah S, et al. Antiparkinsonian activity of Cucurbita pepo seeds along with possible underlying mechanism. Metab Brain Dis. 2021;36(6):1231-1251. [DOI] [PubMed] [Google Scholar]
- 32.Aydin E, Hritcu L, Dogan G, Hayta S, Bagci E. The effects of inhaled Pimpinella peregrina essential oil on scopolamine-induced memory impairment, anxiety, and depression in laboratory rats. Mol Neurobiol. 2016;53(9):6557-6567. [DOI] [PubMed] [Google Scholar]
- 33.Parle M, Vasudevan M, Singh N. Swim everyday to keep dementia away. J Sports Sci Med. 2005;4(1):37-46. [PMC free article] [PubMed] [Google Scholar]
- 34.Pickering C, Pickering R. Methods for the estimation of acetylcholinesterase activity in the plasma and brain of laboratory animals given carbamates or organophosphorus compounds. Arch Toxicol. 1971;27(3-4):292-310. [DOI] [PubMed] [Google Scholar]
- 35.Vannur A, Biradar PR, Patil V. Experimental validation of Vitex negundo leaves hydroalcoholic extract for neuroprotection in haloperidol induced Parkinson’s disease in rat. Metab Brain Dis. 2022;37(2):411-426. [DOI] [PubMed] [Google Scholar]
- 36.Schlumpf M, Lichtensteiger W, Langemann H, Waser PG, Hefti F. A fluorometric micromethod for the simultaneous determination of serotonin, noradrenaline and dopamine in milligram amounts of brain tissue. Biochem Pharmacol. 1974;23(17):2437-2446. [DOI] [PubMed] [Google Scholar]
- 37.Luo Y, Smith JV, Paramasivam V, et al. Inhibition of amyloid-β aggregation and caspase-3 activation by the Ginkgo biloba extract EGb761. Proc Natl Acad Sci USA. 2002;99(19):12197-12202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Rizzo G, Copetti M, Arcuti S, Martino D, Fontana A, Logroscino G. Accuracy of clinical diagnosis of Parkinson disease: a systematic review and meta-analysis. Neurology. 2016;86(6):566-576. [DOI] [PubMed] [Google Scholar]
- 39.Hussain L, Ikram J, Rehman K, Tariq M, Ibrahim M, Akash MSH. Hepatoprotective effects of Malva sylvestris L. against paracetamol-induced hepatotoxicity. Turk J Biol. 2014;38(3):396-402. [Google Scholar]
- 40.Ali M, Qadir MI, Saleem M, et al. Hepatoprotective potential of Convolvulus arvensis against paracetamol-induced hepatotoxicity. Bangladesh J Pharmacol 2013;8(3):300-304. [Google Scholar]
- 41.Imran I, Hussain L, Zia-Ul-Haq M, Janbaz KH, Gilani AH, De Feo V. Gastrointestial and respiratory activities of Acacia leucophloea. J Ethnopharmacol. 2011;138(3):676-682. [DOI] [PubMed] [Google Scholar]
- 42.Szwajgier D, Borowiec K, Pustelniak K. The neuroprotective effects of phenolic acids: molecular mechanism of action. Nutrients. 2017;9(5):477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhang D-B, Chen J-J, Song Q-Y, Zhang L, Gao K. Lycodine-type alkaloids from Lycopodiastrum casuarinoides and their acetylcholinesterase inhibitory activity. Molecules. 2014;19(7):9999-10010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Islam MS, Quispe C, Hossain R, et al. Neuropharmacological effects of quercetin: a literature-based review. Front Pharmacol. 2021;12:665031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Bagewadi HG, Khan AA. Investigation of antiparkinsonian effect of Aloe vera on haloperidol induced experimental animal model. Indian J Pharmaceut Biol Res. 2015;3(1):108-113. [Google Scholar]
- 46.Ogunnoiki AO, Ishola IO, Akinleye MO, Adeyemi OO. Musa acuminata Colla Millsp ameliorates paraquat-induced memory deficit and oxidative stress in murine Parkinson disease model. Niger J Pharm. 2022;56(1):136-143. 10.51412/psnnjp.2022.15 [DOI] [Google Scholar]
- 47.Tanner CM, Kamel F, Ross GW, et al. Rotenone, paraquat, and Parkinson’s disease. Environmental health perspectives. 2011;119(6):866-872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Swanson MD, Winter HC, Goldstein IJ, Markovitz DM. A lectin isolated from bananas is a potent inhibitor of HIV replication. J Biol Chem. 2010;285(12):8646-8655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Opara J, Małecki A, Małecka E, Socha T. Motor assessment in Parkinsons disease. Ann Agric Environ Med. 2017;24(3):411-415. [DOI] [PubMed] [Google Scholar]
- 50.Chinta SJ, Woods G, Demaria M, et al. Cellular senescence is induced by the environmental neurotoxin paraquat and contributes to neuropathology linked to Parkinson’s disease. Cell Rep. 2018;22(4):930-940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Jenner P. Oxidative stress in Parkinson's disease. Ann Neurol: Official J Am Neurol Assoc Child Neurol Soc. 2003;53(S3):S26-S38. [DOI] [PubMed] [Google Scholar]
- 52.Zhang X, Lu L, Liu S, Ye W, Wu J, Zhang X. Acetylcholinesterase deficiency decreases aposis in dopaminergic neurons in the neurotoxin model of Parkinson's disease. Int J Biochem Cell Biol. 2013;45(2):265-272. [DOI] [PubMed] [Google Scholar]
- 53.Vegh C, Wear D, Okaj I, et al. Combined Ubisol-Q10 and ashwagandha root extract target multiple biochemical mechanisms and reduces neurodegeneration in a paraquat-induced rat model of Parkinson’s disease. Antioxidants. 2021;10(4):563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Mogi M, Togari A, Tanaka K-i, Ogawa N, Ichinose H, Nagatsu T. Increase in level of tumor necrosis factor-α in 6-hydroxydopamine-lesioned striatum in rats is suppressed by immunosuppressant FK506. Neurosci Lett. 2000;289(3):165-168. [DOI] [PubMed] [Google Scholar]
- 55.Mogi M, Harada M, Kondo T, et al. Interleukin-1β, interleukin-6, epidermal growth factor and transforming growth factor-α are elevated in the brain from parkinsonian patients. Neurosci Lett. 1994;180(2):147-150. [DOI] [PubMed] [Google Scholar]
- 56.Boka G, Anglade P, Wallach D, Javoy-Agid F, Agid Y, Hirsch E. Immunocytochemical analysis of tumor necrosis factor and its receptors in Parkinson's disease. Neurosci Lett. 1994;172(1-2):151-154. [DOI] [PubMed] [Google Scholar]
- 57.Jung HW, Son HY, Minh CV, Kim YH, Park YK. Methanol extract of Ficus leaf inhibits the production of nitric oxide and proinflammatory cytokines in LPS‐stimulated microglia via the MAPK pathway. Phytother Res. 2008;22(8):1064-1069. [DOI] [PubMed] [Google Scholar]