Abstract
Bacteriophage K139 was recently characterized as a temperate phage of O1 Vibrio cholerae. In this study we have determined the phage adsorption site on the bacterial cell surface. Phage-binding studies with purified lipopolysaccharide (LPS) of different O1 serotypes and biotypes revealed that the O1 antigen serves as the phage receptor. In addition, phage-resistant O1 El Tor strains were screened by using a virulent isolate of phage K139. Analysis of the LPS of such spontaneous phage-resistant mutants revealed that most of them synthesize incomplete LPS molecules, composed of either defective O1 antigen or core oligosaccharide. By applying phage-binding studies, it was possible to distinguish between receptor mutants and mutations which probably caused abortion of later steps of phage infection. Furthermore, we investigated the genetic nature of O1-negative strains by Southern hybridization with probes specific for the O antigen biosynthesis cluster (rfb region). Two of the investigated O1 antigen-negative mutants revealed insertions of element IS1004 into the rfb gene cluster. Treating one wbeW::IS1004 serum-sensitive mutant with normal human serum, we found that several survivors showed precise excision of IS1004, restoring O antigen biosynthesis and serum resistance. Investigation of clinical isolates by screening for phage resistance and performing LPS analysis of nonlysogenic strains led to the identification of a strain with decreased O1 antigen presentation. This strain had a significant reduction in its ability to colonize the mouse small intestine.
Vibrio cholerae strains from serogroups O1 and O139 are the etiologic agents of cholera, a life-threatening acute diarrhea. The O1 serogroup is divided into the main serotypes Inaba and Ogawa, and O1 is subdivided into two distinct biotypes, designated classical and El Tor (22). Lipopolysaccharide (LPS) is the major integral component of the outer membrane and chemically consists of an O antigen, a core oligosaccharide, and lipid A. The O antigen of O1 V. cholerae consists of a homopolymer of approximately 18 (1→2) linked linear 4-(3-deoxy-l-glycero-tetronamido)-4,6-dideoxy-d-mannose) (23, 36). The LPS also contains the carbohydrate quinovosamine, which at the present time cannot be precisely defined as a component of either the O antigen or the core oligosaccharide (45). The Ogawa and Inaba serotypes differ by the presence of a 2-O-methyl group in the nonreducing terminal carbohydrate in the Ogawa O antigen (19, 21). It was shown that Ogawa and Inaba O1 LPS can interconvert and that this serotype variation is due to spontaneous mutations in the wbeT gene (47). Strains of the serogroup O139 contain only a short O antigen but, in contrast to O1 strains, are encapsulated (51). Molecular and epidemiological analyses as well as phage typing revealed that O139 strains are very similar to O1 El Tor strains (2, 17, 18). One characteristic difference is the replacement of the 22-kb O1 rfb region with a 35-kb DNA fragment encoding the O139 O antigen and capsule (4, 5, 10, 48). Both regions are associated with insertion sequence (IS) elements. IS1358 was found in both O antigen biosynthesis clusters, and an incomplete IS1004 was found in the O1 rfb region (4, 11, 44).
Temperate bacteriophage K139 was originally isolated from an O139 isolate and was identified as belonging to the kappa phage family (37). Further analysis revealed that this phage is widely distributed among clinical O1 El Tor strains and can also be found as a defective prophage in O1 classical strains (34, 37). Since only nonlysogenic O1 El Tor strains could be infected with K139, it was predicted that the O1 antigen serves as the specific phage adsorption site. The O1 antigen is known as the receptor for two other Vibrio phages, CP-T1, which infects O1 classical and El Tor strains (16), and VcII, a phage specific to O1 classical strains (32, 53).
In this study, data are presented which identify the O1 antigen as the receptor for phage K139. Furthermore, we describe the isolation of spontaneous phage K139-resistant O1 El Tor strains with altered LPS patterns. For two of the isolates, it is shown that transposition of element IS1004 is responsible for the selective loss of the O1 side chain. In addition, we describe a clinical isolate of O1 El Tor Ogawa with an altered O1 antigen synthesis, leading to phage resistance, and an impaired colonization phenotype.
MATERIALS AND METHODS
Bacterial strains and media.
V. cholerae strains used in this study are listed in Table 1. Escherichia coli strain LE392 (F− supF supE hsdR galK trpR metB lacY tonA) (41) was used as the recipient strain for the construction of plasmids pJNwbeW and pJNmanB. All strains were grown in Luria-Bertani (LB) broth at 37°C. Antibiotics were used to select for V. cholerae and E. coli at the following concentrations: kanamycin, 50 μg/ml; chloramphenicol, 2 and 30 μg/ml; ampicillin, 100 μg/ml; and streptomycin, 100 μg/ml.
TABLE 1.
V. cholerae strains
| Strain | Genotype and/or phenotype | Serogroup, serotype, biotype | Reference | Sensitivity to K139.cm9a |
|---|---|---|---|---|
| AI1838 | O139 | 52 | R | |
| MO10 | K139 lysogenic | O139 | 52 | R |
| O395 | O1, Ogawa, classical | 30 | R | |
| O395R-1 | gmd::Tn5lac, O1 negative | 51 | R | |
| MAK757 | O1, Ogawa, El Tor | 30 | S | |
| MAKres3 | manB::IS1004, O1 negative | This study | R | |
| P27459 | O1, Inaba, El Tor | 35 | S | |
| P27459-S | Spontaneous Smr | This study | S | |
| P27lacZ | lacZ::pMD13 | This study | S | |
| P27res30 | wbeW::IS1004, O1 negative | This study | R | |
| P27res30rev | O1, Inaba, El Tor | This study | S | |
| P27res144 | Short O1 antigen | This study | R | |
| P27res118 | Core defect, O1 negative | This study | R | |
| P27res29 | Core defect, O1 positive | This study | R | |
| P27res108 | This study | R | ||
| CO966 | O1, Ogawa, El Tor | J. J. Mekalanos | R |
R, resistant; S, sensitive.
Oligonucleotides, PCR, and DNA sequencing.
All oligonucleotides used for PCR and DNA sequencing are listed in Table 2. PCR was performed as described by Mullis and Faloona (33). DNA sequencing was performed by the dideoxy nucleotide chain termination method of Sanger et al. (40), and the cycling reaction was performed as specified by Amersham Life Sciences. DNA separation and data collection were performed with the LiCor automatic sequencing system (MWG Biotech GmbH, Ebersberg, Germany).
TABLE 2.
Oligonucleotides used in this study
| Primer | Sequence (5′ to 3′) | GenBank accession no. or reference |
|---|---|---|
| O1 | CGCCGACATAAACGAAATCA | X59554 |
| O2 | ACTTGCTGATTCTTTCCAAC | X59554 |
| O3 | GGAGACTCCTTACGAAAAAT | X59554 |
| O4 | ATTGTCTAGGAGCTATTACA | X59554 |
| O5 | GAGGTAGTAATGAAACATCT | X59554 |
| O6 | GTGATGAACCACTTCCATGT | Y07788 |
| manB1 | CGGGATCCTGATGTAGTACGTTTCGAGGa | X59554 |
| manB2 | TACAGGTCGACCCGCTAGATAAGAACCATCT | X59554 |
| manBseq | GCCCCGGATATTAGCTTATC | X59554 |
| wbeW1 | AAAACTGCAGAAAAACACTACACTGGTCGCC | X59554 |
| wbeWseq | AAAAACACTACACTGGTCGCC | X59554 |
| IS1004 | CTGCTCTTGCTCAAGCTCTT | Z67733 |
| 10 | ATTGTCATCCCTAAACCACC | 6 |
| IS1004seq | AAGAGCTTGAGCAAGAGCAG | Z67733 |
Underlined nucleotides are not exact matches to the sequence and were introduced to add restriction enzyme sites.
Construction of complementing plasmids.
Oligonucleotides manB1 and manB2 were designed to introduce BamHI and SalI sites at the 5′ and 3′ ends of manB. Following PCR amplification, the product was digested with BamHI and SalI and subsequently ligated into the BamHI- and SalI-digested plasmid pACYC184 (39). The resulting plasmid (pJNmanB) expresses manB from the tet promoter. The PCR-amplified fragment obtained from the wbeW1 and O6 primers was digested with PstI and XmnI and ligated into the PstI- and FspI-digested plasmid pACYC177 (38), resulting in plasmid pJNwbeW, which expresses wbeW under the control of the bla promoter.
Construction of bacterial strains.
To construct a V. cholerae strain containing a mutation in lacZ, plasmid pMD13 (12) was mated by conjugation from E. coli SM10λpir (31) into V. cholerae P27459-S, with selection for streptomycin and ampicillin resistance. The resulting strain had a chromosomal insertion caused by integration of the plasmid through homologous recombination via the internal lacZ fragment.
Isolation of phage-resistant cells.
MAK757 phage-resistant mutants were isolated after cross-streaking against the lytic phage derivative K139.cm9 (34). Starting with a single colony, phage-resistant cells of strain P27459 (K139 nonlysogenic; isolated in Bangladesh in 1976) were isolated, diluted in LB broth (about 10 to 20 cells), and incubated at 37°C. At early, mid-log, and late growth phases (with optical densities of 0.05, 0.6, and 2), samples were taken and phage K139.cm9 was added (with a multiplicity of infection from 2 to 10) in Top agar. The bacterium-phage mixture was then plated on L agar and incubated overnight. To test for phage sensitivity, colonies were picked, purified, and cross-streaked against K139.cm9.
Isolation of chromosomal DNA and LPS.
To obtain chromosomal DNA and LPS, we modified the method of Grimberg et al. (15). Five-milliliter overnight cultures were collected by centrifugation, washed in 1 ml of TNE (10 mM Tris [pH 8], 10 mM NaCl, 10 mM EDTA), and resuspended in 540 μl of TNEX (TNE–1% Triton X-100). Sixty microliters of lysozyme (5 mg/ml; Sigma) was added, and the mixture was incubated for 20 min at 37°C. Prior to phenol extraction, 30 μl of proteinase K (20 μg/ml; Sigma) was added, and the mixture was incubated for 2 h at 65°C. The aqueous phase was divided into two halves; one half was used for the preparation of chromosomal DNA, and 20 μl of the other half served for analyzing the pattern of the LPS on a 15% polyacrylamide gel. LPS for the phage neutralization studies (plaque inhibition assays) was prepared by using the hot phenol-water method of Slauch et al. (42). LPS from V. cholerae 569B and Salmonella enterica serovar Typhimurium was purchased from Sigma.
Southern hybridization.
Southern blotting was performed according to the method of Southern (43). Chromosomal DNA was digested with appropriate restriction enzymes. DNA was fractionated on a 0.7% agarose gel and transferred to a Hybond N+ membrane (Amersham, Little Chalfont, United Kingdom). Hybridization with horseradish peroxidase-labeled probe and detection of hybridizing bands was carried out according to the procedure provided by the manufacturer of the ECL system (Amersham).
SDS-PAGE and Western blotting.
LPS was separated by sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) (27) and either silver stained as described by Tsai and Frasch (50) or transferred to a nitrocellulose membrane (49). The membrane was incubated with polyclonal anti-O1 serum (O1 polyvalent; Difco) and a secondary antibody (anti-rabbit conjugated with horseradish peroxidase). After incubation with the ECL reagent (Amersham), the signals were detected.
Serum resistance assay.
Normal human serum (NHS) was obtained and pooled from four healthy lab volunteers who had never been infected with V. cholerae. Cells were grown to mid-exponential phase in LB broth, washed, and mixed to a final concentration of either 50% NHS or 50% heat-inactivated NHS in phosphate-buffered saline (PBS) with 0.1% peptone. After incubation at 37°C for 1 h, the cells were harvested, washed, and resuspended in PBS–0.1% peptone. The number of viable cells was determined by serial dilution of samples and subsequent plating on L agar.
Phage inactivation by LPS (plaque inhibition assay).
The phage-neutralizing capacity of purified LPS was determined by incubating 104 PFU of K139.cm9 with various concentrations of LPS. Experiments were done in 1 ml of LB broth–10 mM CaCl2 at 37°C for 60 min. Five, 10, and 50 μl of this mixture were added to 100-μl aliquots of a MAK757 overnight culture in Top agar and plated on L agar. Plaques were counted after incubation for 6 h at 37°C.
Mouse colonization assays.
The infant mouse colonization assay has been described previously (26). Briefly, strain CO966 (Lac+) was mixed with strain P27lac (Lac−) and given in a peroral inoculum ratio of approximately 106 CFU of CO966 to 106 CFU of P27lac to 5- to 6-day-old CD-1 suckling mice. After a 24-h period of colonization, intestinal homogenates were collected and the ratio of mutant to wild-type colonies was determined by plating dilutions on LB agar containing streptomycin and X-Gal (5-bromo-4-chloro-3-indolyl-β-d-galactopyranoside).
Immunogold electron microscopy.
Immunogold labeling was performed using a method adapted from that of Levine et al. (28). Plastic-coated nickel grids were placed facedown on 40 μl of a PBS-washed bacterial suspension. Excess liquid was removed and the grids were placed coated-side down on a drop of preadsorbed polyclonal anti-Ogawa antiserum (Difco) in PBS–1% bovine serum albumin for 20 min. After thorough washing, the grids were placed on drops of a solution containing a 12-nm gold-conjugated secondary antibody (diluted 1:10 in PBS–1% bovine serum albumin; Dianova, Hamburg, Germany). After further washing, the grids were examined with a Zeiss EM 900 electron microscope using an accelerating voltage of 50 kV.
RESULTS AND DISCUSSION
Identification of V. cholerae O1 antigen as K139 bacteriophage receptor.
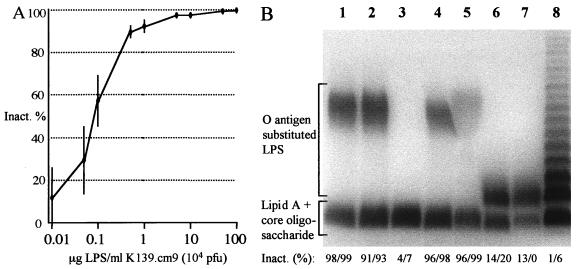
Earlier results (37) suggested that serogroup specificity contributes to phage K139 susceptibility. To test this hypothesis, a plaque inhibition assay was performed as described in Materials and Methods. In this assay the lytic phage K139.cm9, a clear plaque mutant (34), was incubated with LPS preparations prior to infection with reference strain MAK757. The plaque number decreased when phages were titrated out or were inactivated by LPS fractions. As shown in Fig. 1A, purified O1 LPS of strain 569B, in the range between 1 and 100 μg/ml, produced a significant reduction in plaque formation, indicating that interaction between O1 LPS and phage K139 takes place.
FIG. 1.
(A) Inactivation of bacteriophage K139.cm9 with purified LPS derived from V. cholerae O1 classical Inaba strain 569B (Sigma). The inhibition data represent the means ± the standard errors of three independent experiments. (B) SDS-PAGE pattern of purified LPS after silver staining. Strains used for LPS preparation for lanes were as follows: 1, 569B; 2, O395; 3, O395R-1; 4, MAK757; 5, P27459; 6, AI1838; 7, MO10; 8, Salmonella serovar Typhimurium. The inactivation of phage K139.cm9 was determined in plaque inhibition assays and is indicated as a percentage (mean values of at least two independent experiments); the first and second values in each set were obtained using 10 and 100 μg of purified LPS per ml, respectively.
To verify that the O1 antigen alone, not the core oligosaccharides or both, served as the receptor, phage adsorption to other types of LPS was investigated. For these experiments, LPS was extracted from several strains (Fig. 1B), including different serogroups (O1 and O139), serotypes (Inaba and Ogawa), and biotypes (classical and El Tor) and a mutant strain lacking the O1 antigen (O395R-1) (51). For the plaque inhibition assays, 10 and 100 μg of LPS per ml was used, and both concentrations were sufficient to inactivate the phage (Fig. 1A). For a negative control we used purified LPS from Salmonella serovar Typhimurium. As shown in Fig. 1B, only LPS with the O1 antigen, regardless of the serotype or biotype, was able to inhibit the plaque formation of K139.cm9. This reveals that the phage specificity is determined by the O1 antigen and that the terminal methylation of the perosamine of the Ogawa serotype is not recognized. The LPS of the K139 donor strain O139 (MO10) was also not capable of inhibiting plaque formation (Fig. 1B, lane 7). This suggests that this O139 isolate was probably derived from a former O1 El Tor K139 lysogenic isolate. This is in agreement with the finding of several investigators, who presented evidence that O139 strains developed out of O1 El Tor strains by exchange in the O antigen biosynthetic gene cluster (4, 5, 10, 48). It is also possible but less likely that the K139 phage genome could have been transferred into O139 strains by another horizontal transfer event, e.g., by another generalized transducing phage or by in trans conjugation.
Characterizing phage K139-resistant El Tor V. cholerae isolates.
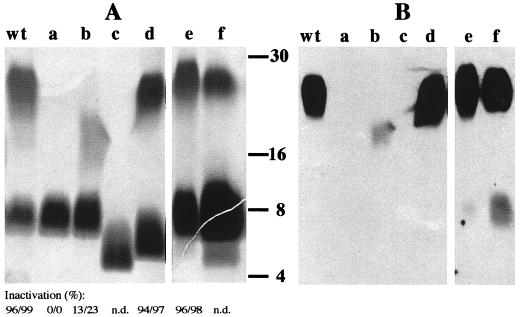
The identity of the phage receptor and the ability to use highly lytic phage derivative K139.cm9 prompted us to investigate spontaneous phage-resistant mutants. The clinical O1 El Tor isolate P27459 was chosen for these experiments. We started with a single culture inoculated with about 10 to 20 cells and collected phage-resistant mutants at different time points during culture (see Materials and Methods). For further analysis, 100 colonies were picked and purified. These isolates were then grouped into five types according to their LPS patterns on silver-stained polyacrylamide gels. Cross-streak and Southern blot analysis confirmed all isolates to be phage resistant and nonlysogenic (data not shown). In Fig. 2, representative isolates are shown by their LPS patterns in SDS-PAGE and Western blot analysis. Mutants were classified into five groups according to their LPS features as follows: group a, loss of the O1 antigen; group b, altered O1 antigen (the O antigen of this isolate migrates faster in a polyacrylamide gel than the wild-type O1 antigen and is only weakly exposed by silver staining, which correlates with attenuated O1 antibody recognition); group c, lack of O1 antigen as well as a defect in the core oligosaccharide; group d, altered core oligosaccharide structure with intact O1 antigen; and group e, no visible differences in the LPS pattern compared to the wild type. In summary, among the mutants isolated we found that the most abundant mutants were from group a, whereas mutants from groups b to e were quite rare. This observation indicates that several different mutations were generated; however, some of them could have had clonal origins, especially if mutations occurred early in the growth culture.
FIG. 2.
(A) Analysis of LPS from parent and mutant strains. Lanes a to e represent LPS patterns of phage-resistant P27459 mutants and lane f represents the LPS pattern of the clinical isolate CO966. LPS was prepared from strains P27459 (wt), P27res30 (a), P27res118 (b), P27res29 (c), P27res144 (d), P27res108 (e), and CO966 (f). The inactivation of phage K139.cm9 was determined in plaque inhibition assays and is indicated as a percentage; the first and second values in each set were obtained using 10 and 100 μg of purified LPS per ml, respectively. n.d., not done. (B) Western blot analysis using a polyclonal antiserum against O1 LPS (O1 common; Difco) corresponding to the samples from panel A. The molecular size standard is indicated in kilodaltons according to the Kaleidoscope polypeptide standard (Bio-Rad).
Next, we examined whether phage resistance was caused by a lack of receptor binding or because of a defect in one of the later steps of infection. We purified LPS (types a, b, d, and e) and used it in plaque inhibition analysis. As expected, the phage could not be inactivated with LPS lacking O1 antigen (Fig. 2A, lane a). There was only limited interaction between type b LPS and the phage, indicating that the specific receptor recognition site is absent. LPS of types d and e was able to produce significant plaque inhibition (Fig. 2). We assume that type d mutants had gained a mutation(s) in the core region, altering the integrity of the outer membrane, which might be deleterious to secondary phage infection processes. Type e LPS is apparently not affected by the LPS structure and possibly represents a class of phage-resistant mutations which are not associated with LPS synthesis. Alternatively, an outer membrane or associated protein which is crucial for outer membrane integrity or secondary phage infection steps might be mutated.
Identification of IS1004 insertions in the rfb gene cluster and selection of O1-positive revertant strains.
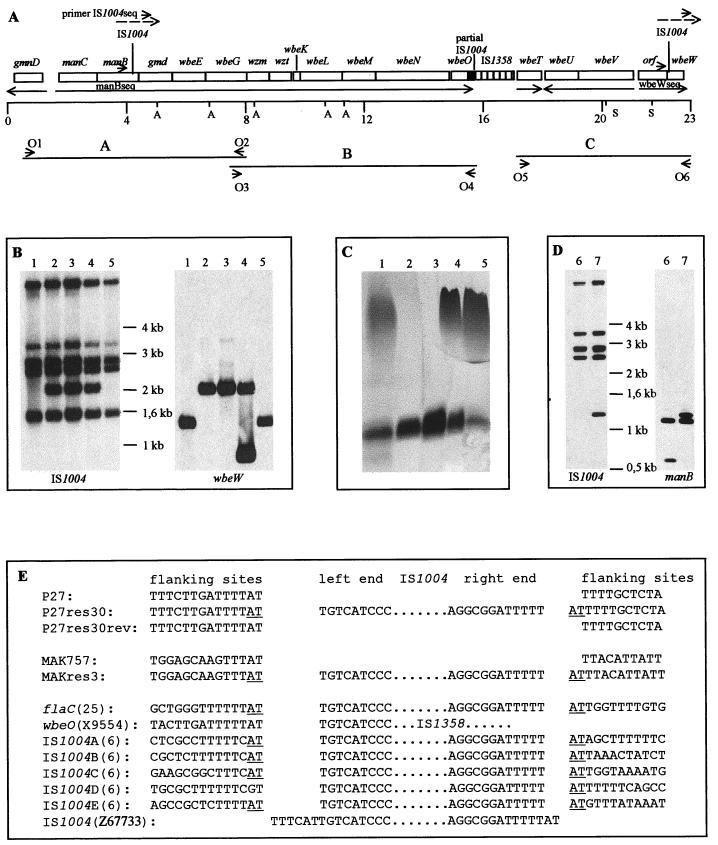
To learn more about the nature of the spontaneous mutations, we analyzed the LPS mutants, focusing on those mutants that had lost the ability to synthesize the O1 antigen. To search for DNA alterations in the characterized rfb region (45), AvaI- and SacI-digested chromosomal DNA from 45 isolates of group a were analyzed by Southern hybridization using PCR-generated fragments which covered most of the rfb region (Fig. 3A). The isolate P27res30 showed a different restriction fragment pattern from that of the wild-type strain by hybridization with probe C (data not shown). PCR analysis of this strain with oligonucleotides O5 and O6 (Fig. 3A) and subsequent digestion with a combination of SacI and BssHI confirmed a fragment shift of about 600 bp linked with wbeW (data not shown). DNA sequence determination by utilizing a specific sequencing oligonucleotide (wbewseq) (Table 2) and the O5- and O6-amplified PCR fragment revealed an IS1004 insertion into wbeW at bp 25 of the encoding gene (Fig. 3A and E).
FIG. 3.
(A) The rfb region of V. cholerae O1. The representation is based on the sequences submitted to GenBank (see text) and of Stroeher et al. (45) and Bik et al. (4). The transcriptional directions are indicated by arrows. Probes A to C, used for Southern hybridization, are indicated by horizontal lines. The scales are indicated in kilobases. A, AvaI; S, SacI; O1 to O6, primers used to amplify the probes. (B) Southern blot analysis of HpaII-digested chromosomal DNA with IS1004- and wbeW-specific DNA probes. Lanes 1, P27459; lanes 2, P27res30; lanes 3, P27res30(pACYC177); lanes 4, P27res30(pJNwbeW); lanes 5, P27res30rev. The additional band that hybridizes with the wbeW probe in P27res30(pJNwbeW) (lane 4) is due to the digestion of the complementing plasmid containing wbeW. (C) LPS pattern after SDS-PAGE and silver staining (lanes contain samples from the strains listed for panel B. (D) Southern blot analysis of HpaII-digested chromosomal DNA with IS1004- and manB-specific DNA probes. Lanes 6, MAK757; lanes 7, MAKres3. (E) DNA sequences at sites of IS1004 insertions. PCR fragments amplified by primers O5 and O6 out of strains P27459 (P27), P27res30, and P27res30rev were sequenced with sequencing primer wbeWseq, and P27res30 was sequenced additionally with primer IS1004seq (panel A). Sequence analysis of PCR fragments generated with primers manB1 and manB2 out of strains MAK757 and MAKres3 was performed with manBseq and IS1004seq (MAKres3 only) primers (panel A). Sequences for the other IS1004 insertions have been retrieved from the literature or GenBank. Possible duplicated AT base pairs at the insertion site are underlined.
Similar investigations were performed with four strains from another pool of K139.cm9-resistant isolates of O1 El Tor Ogawa strain MAK757. The isolate MAKres3 showed an IS1004 insertion in the gene manB at bp 1194. The function of the proteins encoded by manB and wbeW were predicted by homology analysis. ManB appears to be involved in the biosynthesis of perosamine (46), and WbeW is likely to encode a glycosyltransferase (13). For both genes, mutations which resulted in a defective O1 antigen biosynthesis were characterized (13, 20).
To confirm that the phenotypes (phage resistance, loss of the O1 antigen) of P27res30 and MAKres3 are due to the IS1004 insertions in these particular genes, we constructed plasmids containing either wbeW or manB. Both plasmids could complement the mutations in trans, resulting in strains MAKres3(pJNmanB) and P27res30(pJNwbeW), which are phage sensitive and able to express the O1 antigen (Fig. 3C).
In a further characterization, we tested strain P27res30 (wbeW::IS1004) for its ability to switch back to intact LPS production. Since O1-negative V. cholerae cells show a significantly increased serum sensitivity (8, 51), we treated strain P27res30 with 50% NHS (see Materials and Methods). Approximately 108 cells were used in each assay, and the surviving cells (about 2.3 × 10−4% ± 2.5 × 10−4%) from four independent assays were tested for phage K139.cm9 sensitivity. It was found that 23 of 76 analyzed surviving cells showed a phage-sensitive phenotype. The wbeW loci of eight isolates were characterized by Southern blot analysis, which confirmed that the isolates had lost IS1004 in wbeW (data not shown). Analysis of the LPS patterns by SDS-PAGE and silver staining revealed that all revertants had restored O1 antigen biosynthesis (data not shown). The Southern blot analysis failed to detect the mutations of the other spontaneous O1-negative mutants. Such mutations could be caused by various events, such as base pair substitutions or frameshift mutations.
IS1004 transposition.
IS1004 (V. cholerae) is grouped together in a family with IS605 (Helicobacter pylori) and IS200 (Salmonella serovar Typhimurium, Shigella spp., Clostridium spp., and Streptococcus pneumoniae) (29). For V. cholerae, the IS1004 element has been exclusively described as an epidemiological marker in molecular typing (6, 7). It was reported that El Tor strains contain 5 to 6 copies (6) and classical strain 569B contains 10 copies (7). To obtain additional information about the role of IS1004 transposition, the distribution of IS1004 copies was determined in the chromosome of the wild-type strain, the wbeW::IS1004 and manB::IS1004 mutants, and one revertant. We performed Southern hybridization according to the method of Bik et al. (6) and used chromosomal DNA, which was digested with HpaII, and an IS1004-specific DNA hybridization probe. The results showed that the wild-type strain P27459 harbors five copies of IS1004 (Fig. 3B, lane 1) and MAK757 harbors four copies (Fig. 3D, lane 6). The IS1004 insertion mutants contained one additional copy (Fig. 3B, lane 2, and D, lane 7), whereas the wbeW::IS1004 revertant showed the same pattern as the wild-type strain (Fig. 3B, lane 5) (also observed for seven other revertants [data not shown]). Rehybridization of the same blot with wbeW- or manB-specific probes confirmed that insertions of IS1004 had taken place in wbeW and manB (Fig. 3B and D). Additionally, the chromosomal DNA of P27459, P27res30, and P27res30rev was digested with the enzymes EcoRI, HindIII, and PstI (restriction enzymes that do not cut in IS1004) and hybridized with the IS1004 probe; this study revealed no additional IS1004 copies (data not shown). The presence of an additional IS1004 fragment in the wbeW::IS1004 and manB::IS1004 mutants implies that a replication of IS1004 probably occurred during the course of the transposition process. In the case of the revertant, it seems that precise excision of the IS element took place. We suggest that this reflects the loss of the element; however, sometimes Southern blot analysis is not sufficiently sensitive to determine the exact numbers of insertion sequences. Further investigations are necessary to clarify the transposition mechanism of this IS element.
The data presented in this work strongly suggest that IS1004 is an active mobile genetic element which is able to transpose in V. cholerae O1 El Tor strains. From the sequence data of five cloned IS1004 copies and their flanking regions, it was concluded that the IS1004 element comprises 628 bp, with no terminal inverted repeats and no evidence of target sequence duplication (6). The sequence data presented here and summarized in Fig. 3E revealed that the first 6 bp on the left end of the published sequence (6) (GenBank accession no. Z67733) are not IS1004 specific. We hypothesized that the left end of the IS element starts with TGTCAT. Comparing all published IS1004 flanking sequences with our sequence data (Fig. 3E), we concluded that this insertion element inserts preferentially into AT-rich sequences. Furthermore, it seems that insertion is favored if 5′-TTTAT or 5′-TTCAT sequences are present. This specificity may result from initial recognition of the potentially bent feature of the AT-rich DNA; this mechanism is also predicted for other IS elements, including IS200 and IS605 (3, 14, 24). In addition, most of the flanking sequences at the site of IS1004 insertion show two AT pairs, one at each side of the element (Fig. 3E), revealing that insertion caused a 2-bp duplication. The absence of AT in the left flanking site of IS1004D does not invalidate the hypothesis of duplication upon insertion, because events subsequent to insertion could lead to a different flanking end.
Clinical O1 El Tor V. cholerae isolates with LPS alterations.
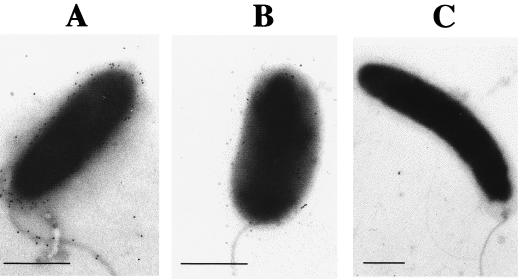
To test whether the approach of screening for phage resistance coupled with LPS analysis could allow the identification of natural V. cholerae cells with altered LPS, we investigated some clinical isolates. First, cross-streaking led to the identification of phage-resistant strains. Second, these strains were analyzed for phage K139 lysogeny, and the LPS patterns of nonlysogenic strains were further investigated by SDS-PAGE. As a result, one O1 Ogawa strain (CO966; isolated in India in 1994) was identified which showed a decreased amount of O1 antigen. To detect the O antigen of strain CO966 on a silver-stained polyacrylamide gel, it was necessary to load more LPS (Fig. 2, lane f; note the altered proportion of O antigen to lipid A plus core between this LPS and the wild-type LPS [lane wt]). This observation was confirmed by the specific detection of O1 antigen on whole cells with immunogold-conjugated antibodies, as analyzed by electron microscopy (Fig. 4). To our knowledge, this phenotype has not been described previously for V. cholerae. Since LPS is a known virulence factor which participates in the colonization process of V. cholerae (1, 9, 20, 51), this isolate was further investigated in perorally infected CD-1 suckling mice. It was found that the colonization behavior of CO966 was significantly attenuated, with a competition index of 0.0312 (n = 7, P < 0.01 by Student's two-tailed t test), compared to reference strain P27459. These results suggest that the low levels of O1 expression on CO966 may lead to lower levels of intestinal colonization. However, strains P27459 and CO966 are not isogenic; therefore, it is also likely that other strain characteristics could contribute to the attenuated colonization phenotype of CO966.
FIG. 4.
Immunogold detection of O1 antigen. V. cholerae O1 Ogawa strains were stained with anti-Ogawa O1 antiserum and anti-rabbit immunoglobulin-gold conjugate and visualized as electron-dense particles in electron micrographs. (A) The O antigen is present over the whole cell surface of strain MAK757, including the LPS-sheathed flagellum. (B) Strain CO966 possesses only a small number of O antigen-containing LPS molecules. (C) No O1 antigen could be detected in the mutant MAKres3 (manB::IS1004). These results confirmed the LPS analysis by SDS-PAGE and silver staining (Fig. 2A). Bars indicate 0.5 μm.
In conclusion, we have provided data for the specificity of the host receptor for Vibrio phage K139, identified as the O1 antigen. Applying hypervirulent phage K139.cm9 to O1 El Tor strains allowed us to identify different phage-resistant mutant groups which express different LPS mutations. Interestingly, mutants were isolated which were linked not with the O1 antigen but with the core structure. Such mutants indirectly implicate the core region of the LPS in secondary phage infection steps. Among the O1 antigen-defective mutants, we identified IS1004 insertion and subsequent excision events in O1 biosynthetic genes. These findings demonstrate that IS1004 is a mobile element in V. cholerae and is able to insert into the rfb region. It should be noted that this element could potentially contribute to rearrangements in and instability of the rfb gene region, facilitating further O antigen variation.
ACKNOWLEDGMENTS
We thank J. Schmidt-Brauns for many helpful comments, critical reading, and suggestions. For the clinical V. cholerae strains used in this study, we thank J. J. Mekalanos. We also thank M. Waldor for the O1 side chain mutant and W. Brabetz for his help in LPS handling.
This work was funded by BMBF grant 01KI8906 and NIH grant AI43486 to K.E.K.
REFERENCES
- 1.Baselski V S, Parker C D. Intestinal distribution of Vibrio cholerae in orally infected infant mice: kinetics of recovery of radiolabel and viable cells. Infect Immun. 1978;21:518–525. doi: 10.1128/iai.21.2.518-525.1978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berche P, Poyart C, Abachin E, Lelievre H, Vandepitte J, Dodin A, Fournier J M. The novel epidemic strain O139 is closely related to the pandemic strain O1 of Vibrio cholerae. J Infect Dis. 1994;170:701–704. doi: 10.1093/infdis/170.3.701. [DOI] [PubMed] [Google Scholar]
- 3.Beuzon C R, Casadesus J. Conserved structure of IS200 elements in Salmonella. Nucleic Acids Res. 1997;25:1355–1361. doi: 10.1093/nar/25.7.1355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bik E M, Bunschoten A E, Gouw R D, Mooi F R. Genesis of the novel epidemic Vibrio cholerae O139 strain: evidence for horizontal transfer of genes involved in polysaccharide synthesis. EMBO J. 1995;14:209–216. doi: 10.1002/j.1460-2075.1995.tb06993.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bik E M, Bunschoten A E, Willems R J L, Chang A C Y, Mooi F R. Genetic organization and functional analysis of the otn DNA essential for cell-wall polysaccharide synthesis in Vibrio cholerae O139. Mol Microbiol. 1996;20:799–811. doi: 10.1111/j.1365-2958.1996.tb02518.x. [DOI] [PubMed] [Google Scholar]
- 6.Bik E M, Gouw R D, Mooi F R. DNA fingerprinting of Vibrio cholerae strains with a novel insertion sequence element: a tool to identify epidemic strains. J Clin Microbiol. 1996;34:1453–1461. doi: 10.1128/jcm.34.6.1453-1461.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chatterjee S, Mondal A K, Begum N A, Roychoudhury S, Das J. Ordered cloned DNA map of the genome of Vibrio cholerae 569B and localization of genetic markers. J Bacteriol. 1998;180:901–908. doi: 10.1128/jb.180.4.901-908.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chiang S L, Mekalanos J J. rfb mutations in Vibrio cholerae do not affect surface production of toxin-coregulated pili but still inhibit intestinal colonization. Infect Immun. 1999;67:976–980. doi: 10.1128/iai.67.2.976-980.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chiang S L, Mekalanos J J. Use of signature-tagged transposon mutagenesis to identify Vibrio cholerae genes critical for colonization. Mol Microbiol. 1998;27:797–805. doi: 10.1046/j.1365-2958.1998.00726.x. [DOI] [PubMed] [Google Scholar]
- 10.Comstock L E, Johnson J A, Michalski J M, Morris J G, Jr, Kaper J B. Cloning and sequence of a region encoding a surface polysaccharide of Vibrio cholerae O139 and characterization of the insertion site in the chromosome of Vibrio cholerae O1. Mol Microbiol. 1996;19:815–826. doi: 10.1046/j.1365-2958.1996.407928.x. [DOI] [PubMed] [Google Scholar]
- 11.Dumontier S, Trieu-Cuot P, Berche P. Structural and functional characterization of IS1358 from Vibrio cholerae. J Bacteriol. 1998;180:6101–6106. doi: 10.1128/jb.180.23.6101-6106.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Dziejman M, Mekalanos J J. Analysis of membrane protein interaction: ToxR can dimerize the amino terminus of phage lambda repressor. Mol Microbiol. 1994;13:485–494. doi: 10.1111/j.1365-2958.1994.tb00443.x. [DOI] [PubMed] [Google Scholar]
- 13.Fallarino A, Mavrangelos C, Stroeher U H, Manning P A. Identification of additional genes required for O-antigen biosynthesis in Vibrio cholerae O1. J Bacteriol. 1997;179:2147–2153. doi: 10.1128/jb.179.7.2147-2153.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Galas D J, Chandler M. Bacterial insertion sequences. In: Berg D E, Howe M M, editors. Mobile DNA. Washington, D.C.: American Society for Microbiology; 1989. pp. 109–162. [Google Scholar]
- 15.Grimberg J, Maguire S, Belluscio L. A simple method for the preparation of plasmid and chromosomal E. coli DNA. Nucleic Acids Res. 1989;17:8893. doi: 10.1093/nar/17.21.8893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guidolin A, Manning P A. Bacteriophage CP-T1 of Vibrio cholerae: identification of the cell surface receptor. Eur J Biochem. 1985;153:89–94. doi: 10.1111/j.1432-1033.1985.tb09271.x. [DOI] [PubMed] [Google Scholar]
- 17.Hall R H, Khambaty F M, Kothary M, Keasler S P. Non-O1 Vibrio cholerae. Lancet. 1993;342:430. doi: 10.1016/0140-6736(93)92839-l. [DOI] [PubMed] [Google Scholar]
- 18.Higa N, Honma Y, Albert J M, Iwanga M. Characterization of Vibrio cholerae O139 synonym Bengal isolated from patients with cholera-like disease in Bangladesh. Microbiol Immunol. 1993;37:971–974. doi: 10.1111/j.1348-0421.1993.tb01731.x. [DOI] [PubMed] [Google Scholar]
- 19.Hisatsune K, Kondo S, Isshiki Y, Iguchi T, Haishima Y. Occurrence of 2-O-methyl-N-(3-deoxy-l-glycero-tetronyl)-d-perosamine (4-amino-4,6-dideoxy-d-manno-pyranose) in lipopolysaccharide from Ogawa but not from Inaba O forms of O1 Vibrio cholerae. Biochem Biophys Res Commun. 1993;190:302–307. doi: 10.1006/bbrc.1993.1046. [DOI] [PubMed] [Google Scholar]
- 20.Iredell J R, Stroeher U H, Ward H M, Manning P A. Lipopolysaccharide O-antigen expression and the effect of its absence on virulence in rfb mutants of Vibrio cholerae O1. FEMS Immunol Med Microbiol. 1998;20:45–54. doi: 10.1111/j.1574-695X.1998.tb01110.x. [DOI] [PubMed] [Google Scholar]
- 21.Ito T, Higuchi T, Hirobe M, Hiramatsu K, Yokota T. Identification of a novel sugar, 4-amino-4,6-dideoxy-2-O-methylmannose in the lipopolysaccharide of Vibrio cholerae O1 serotype Ogawa. Carbohydr Res. 1994;256:113–128. doi: 10.1016/0008-6215(94)84231-0. [DOI] [PubMed] [Google Scholar]
- 22.Kay B A, Bopp C A, Wells J G. Isolation and identification of Vibrio cholerae O1 from fecal specimens. In: Wachsmuth I K, Blake P A, Olsvik Ø, editors. Vibrio cholerae and cholera: molecular to global perspectives. Washington, D.C.: ASM Press; 1994. pp. 3–25. [Google Scholar]
- 23.Kenne L, Lindberg B, Unger P, Gustafsson B, Holme T. Structural studies of the Vibrio cholerae O-antigen. Carbohydr Res. 1982;100:341–349. doi: 10.1016/s0008-6215(00)81047-2. [DOI] [PubMed] [Google Scholar]
- 24.Kersulyte D, Akopyants N S, Clifton S W, Roe B A, Berg D E. Novel sequence organization and insertion specificity of IS605 and IS606: chimaeric transposable elements of Helicobacter pylori. Gene. 1998;223:175–186. doi: 10.1016/s0378-1119(98)00164-4. [DOI] [PubMed] [Google Scholar]
- 25.Klose K E, Mekalanos J J. Differential regulation of multiple flagellins in Vibrio cholerae. J Bacteriol. 1998;180:303–316. doi: 10.1128/jb.180.2.303-316.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Klose K E, Mekalanos J J. Distinct roles of an alternative sigma factor during both free-swimming and colonizing phases of the Vibrio cholerae pathogenic cycle. Mol Microbiol. 1998;28:501–520. doi: 10.1046/j.1365-2958.1998.00809.x. [DOI] [PubMed] [Google Scholar]
- 27.Laemmli U K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970;227:680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- 28.Levine M M, Ristaino P, Marley G, Smyth C, Knutton S, Boedeker E, Black R, Young C, Clements M L, Cheney C, Patnaik R. Coli surface antigens 1 and 3 of colonization factor antigen II-positive enterotoxigenic Escherichia coli: morphology, purification, and immune responses in humans. Infect Immun. 1984;44:409–420. doi: 10.1128/iai.44.2.409-420.1984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Mahillon J, Chandler M. Insertion sequences. Microbiol Mol Biol Rev. 1998;62:725–774. doi: 10.1128/mmbr.62.3.725-774.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mekalanos J J. Duplication and amplification of toxin genes in Vibrio cholerae. Cell. 1983;35:253–263. doi: 10.1016/0092-8674(83)90228-3. [DOI] [PubMed] [Google Scholar]
- 31.Miller V L, Mekalanos J J. A novel suicide vector and its use in construction of insertion mutations: osmoregulation of outer membrane proteins and virulence determinants in Vibrio cholerae requires toxR. J Bacteriol. 1988;170:2575–2583. doi: 10.1128/jb.170.6.2575-2583.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mukerjee S. Identification of S-R dissociation of the strains of Vibrio cholerae by group II bacteriophages. Ann Biochem Exp Med. 1959;19:9–12. [Google Scholar]
- 33.Mullis K B, Faloona F. Specific synthesis of DNA in vitro via a polymerase chain reaction. Methods Enzymol. 1987;155:335–340. doi: 10.1016/0076-6879(87)55023-6. [DOI] [PubMed] [Google Scholar]
- 34.Nesper J, Blaß J, Fountoulakis M, Reidl J. Characterization of the major control region of Vibrio cholerae bacteriophage K139: immunity, exclusion, and integration. J Bacteriol. 1999;181:2902–2913. [Google Scholar]
- 35.Pearson G D N, Woods A, Chiang S L, Mekalanos J J. CTX genetic element encodes a site-specific recombination system and an intestinal colonization factor. Proc Natl Acad Sci USA. 1993;90:3750–3754. doi: 10.1073/pnas.90.8.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Redmond J W. The structure of the O-antigenic side chain of the lipopolysaccharide of Vibrio cholerae 569B (Inaba) Biochim Biophys Acta. 1979;584:346–352. doi: 10.1016/0304-4165(79)90280-0. [DOI] [PubMed] [Google Scholar]
- 37.Reidl J, Mekalanos J J. Characterization of Vibrio cholerae bacteriophage K139 and use of a novel mini transposon to identify a phage-encoded virulence factor. Mol Microbiol. 1995;18:685–701. doi: 10.1111/j.1365-2958.1995.mmi_18040685.x. [DOI] [PubMed] [Google Scholar]
- 38.Rose R E. The nucleotide sequence of pACYC177. Nucleic Acids Res. 1988;16:356. doi: 10.1093/nar/16.1.356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Rose R E. The nucleotide sequence of pACYC184. Nucleic Acids Res. 1988;16:355. doi: 10.1093/nar/16.1.355. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Sanger F, Nicklen S, Coulson A R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci USA. 1977;74:5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Silhavy T J, Berman M L, Enquist L W. Experiments with gene fusions. Cold Spring Harbor, N.Y: Cold Spring Harbor Laboratory; 1984. [Google Scholar]
- 42.Slauch J M, Mahan M J, Michetti P, Neutra M R, Mekalanos J J. Acetylation (O-factor 5) affects the structural and immunological properties of Salmonella typhimurium lipopolysaccharide O antigen. Infect Immun. 1995;63:437–441. doi: 10.1128/iai.63.2.437-441.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Southern E M. Detection of specific sequences among DNA fragments separated by gel electrophoresis. J Mol Biol. 1975;98:503–517. doi: 10.1016/s0022-2836(75)80083-0. [DOI] [PubMed] [Google Scholar]
- 44.Stroeher U H, Jedani K E, Dredge B K, Morona R, Brown M H, Karageorgos L E, Albert M J, Manning P A. Genetic rearrangements in the rfb regions of Vibrio cholerae O1 and O139. Proc Natl Acad Sci USA. 1995;92:10374–10378. doi: 10.1073/pnas.92.22.10374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Stroeher U H, Jedani K E, Manning P A. Genetic organization of the regions associated with surface polysaccharide synthesis in Vibrio cholerae O1, O139 and Vibrio anguillarum O1 and O2: a review. Gene. 1998;223:269–282. doi: 10.1016/s0378-1119(98)00407-7. [DOI] [PubMed] [Google Scholar]
- 46.Stroeher U H, Karageorgos L E, Brown M H, Morona R, Manning P A. A putative pathway for perosamine biosynthesis is the first function encoded within the rfb region of Vibrio cholerae O1. Gene. 1995;166:33–42. doi: 10.1016/0378-1119(95)00589-0. [DOI] [PubMed] [Google Scholar]
- 47.Stroeher U H, Karageorgos L E, Morona R, Manning P A. Serotype conversion in Vibrio cholerae O1. Proc Natl Acad Sci USA. 1992;89:2566–2570. doi: 10.1073/pnas.89.7.2566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Stroeher U H, Parasivam G, Dredge B K, Manning P A. Novel Vibrio cholerae O139 genes involved in lipopolysaccharide biosynthesis. J Bacteriol. 1997;179:2740–2747. doi: 10.1128/jb.179.8.2740-2747.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Tsai C M, Frasch C E. A sensitive silver stain for detecting lipopolysaccharides in polyacrylamide gels. Anal Biochem. 1982;119:115–119. doi: 10.1016/0003-2697(82)90673-x. [DOI] [PubMed] [Google Scholar]
- 51.Waldor M K, Colwell R, Mekalanos J J. The Vibrio cholerae O139 serogroup antigen includes an O-antigen capsule and lipopolysaccharide virulence determinants. Proc Natl Acad Sci USA. 1994;91:11388–11392. doi: 10.1073/pnas.91.24.11388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Waldor M K, Mekalanos J J. ToxR regulates virulence gene expression in non-O1 strains of Vibrio cholerae that cause epidemic cholera. Infect Immun. 1994;62:72–78. doi: 10.1128/iai.62.1.72-78.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ward H M, Manning P A. Mapping of chromosomal loci associated with lipopolysaccharide synthesis and serotype specificity in Vibrio cholerae O1 by transposon mutagenesis using Tn5 and Tn2680. Mol Gen Genet. 1989;218:367–370. doi: 10.1007/BF00331294. [DOI] [PubMed] [Google Scholar]