Abstract
This systematic review investigates complications and recurrence of Dupuytren’s contracture in metacarpophalangeal joints (MCPJs) and/or proximal interphalangeal joints (PIPJs) of fingers treated with collagenase clostridium histolyticum (CCH). A review of the literature on Dupuytren’s disease was performed using PRISMA guidelines. Included publications described complications and/or recurrences for contractures ≥20° in MCPJs and/or PIPJs treated with CCH. Successful treatments reduced contractures to ≤5° immediately. Treatment-related adverse events (AEs) were classified as minor, major surgical, and major nonsurgical. Contracture recurrence involved return of fixed-flexion contracture ≥20° in a successfully treated finger in patients with ≥12 months of follow-up. Of 2675 patients (3753 joints), 94% experienced ≥1 treatment-related AE, most commonly peripheral edema (64%), pain in extremity (53%), and contusion (51%). Major surgical complications occurred in 9 patients (1.0%). Major nonsurgical complications occurred in 2 patients, specifically nonrupture tendon injury and anaphylaxis. Of 1488 patients (2069 joints), recurrences were reported in 23% of successfully treated joints (n = 466; 20% MCPJs, 28% PIPJs), on average 12 to 24 months after treatment. MCPJs achieved greater success than PIPJs in initial contracture reduction (77% versus 36%). CCH is a safe, effective treatment to improve hand function in Dupuytren’s contracture. Most AEs are minor and self-resolving, although the risk of major AEs still exists. Following treatment, 23% of successfully treated joints experience recurrence, typically within 12 to 24 months but sometimes as early as 6 months. Surgeons are encouraged to discuss these risks with patients for shared decision-making regarding optimal treatment modalities.
Keywords: Dupuytren, contracture, CCH, collagenase, hand, anatomy
Introduction
Dupuytren’s contracture is a benign, progressive connective tissue disorder that can lead to severe deformity and impaired function of the hand. 1 Prevalence of Dupuytren’s contracture in Western countries ranges from 0.6% to 31.6%, and this rate increases with patient age. 2 While the exact mechanism of the disease is unknown, Dupuytren’s contracture classically affects men of northern European descent over age 50 and is associated with risk factors such as smoking, alcohol consumption, diabetes mellitus, dyslipidemia, epilepsy, anticonvulsant and antiretroviral use, and hand trauma.3,4
Surgical treatments for Dupuytren’s contracture commonly include fasciotomy, percutaneous needle fasciotomy (PNF), fasciectomy, and dermofasciectomy, while nonsurgical treatments involve collagenase clostridium histolyticum (CCH) followed by passive joint extension to induce cord rupture. In extreme circumstances, failure to effectively treat Dupuytren’s contracture may lead patients to undergo elective finger amputation. 3
In a review of CCH for treatment of Dupuytren’s contracture, Smeraglia et al 1 found that CCH treatment results in better outcomes with fewer complications and side effects than open fasciectomy. Due to its noninvasive application and rapid administration, the use of CCH to treat Dupuytren’s contracture has increased in recent years; 1 however, there are limited long-term outcome data reporting complications and recurrences following CCH treatment. This systematic review of the literature specifically investigates treatment efficacy, complications, and recurrence of contracture in patients originally presenting with contractures of ≥20° in the metacarpophalangeal joints (MCPJs) and/or proximal interphalangeal joints (PIPJs) of nonthumb fingers treated with CCH.
Materials and Methods
Literature Search
This systematic review employed a search of articles in Medline (PubMed), Web of Science, and Scopus databases using the keywords “Dupuytren collagenase” and “Dupuytren clostridium histolyticum” 1 and of articles published between October 12, 2015 and April 1, 2019 in the Medline (PubMed) database with the same keywords.
Study Selection
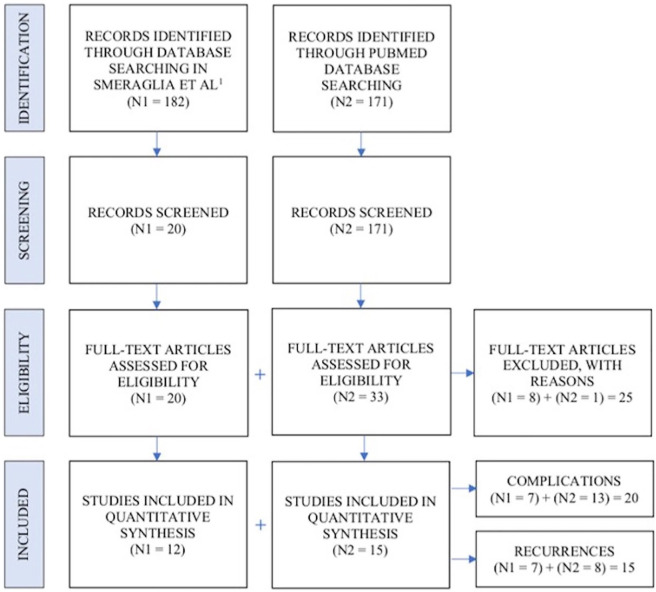
Abstracts were manually screened by investigators prior to inclusion. Eligible studies for inclusion reported complications and incidences of recurrence, were either published in English or English translations were available, and were not biomechanical, conducted on animals or cadavers, technical notes, letters to the editor, or instructional courses. Studies that were retracted from journals for lack of ethical approval or recurrence studies that had follow-up periods of less 1 year were excluded from review. For each study that met the inclusion criteria, a full-text version of the publication was downloaded and reviewed by investigators. A total of 353 articles were identified in the database search and ultimately 17 were included in quantitative synthesis (Figure 1). Data on patient demographics, study characteristics, follow-up periods, Dupuytren’s contracture patterns pre- and posttreatment, CCH therapy outcomes, CCH therapy adverse events (AEs), and contracture recurrences were collected and recorded for analysis.
Figure 1.
PRISMA flow diagram.
Note. PRISMA = Preferred Reporting Items for Systematic Reviews and Meta-Analyses.
Definitions
Dupuytren’s contracture was defined as a fixed-flexion contracture in the MCPJ or PIPJ of ≥20° in at least one nonthumb finger.1,5-25 Clinical success was defined as a reduction in contracture to ≤5° immediately following CCH treatment.5-12,14,16-18,20,21,23-29
Major surgical complications were defined as treatment-related AEs that required or were candidates for surgical correction. Major nonsurgical complications were defined as nontransient treatment-related AEs that were not treated with surgery. Minor complications were defined as transient treatment-related AEs without long-term consequences.
Minor AEs reported by number of patients were included in analysis, while studies reporting minor AEs by joints treated and/or injections administered were excluded from analysis in total to preserve comparability. Recurrence of contracture was defined as a return of fixed-flexion contracture of ≥20° in a previously successfully treated finger in patients with at least 12 months of follow-up.1,6,9,11,12,15-17,19,22-26,28
Statistical Analysis
Summary measures included estimate means with 95% confidence intervals (CIs). Statistical analysis was performed using a random model to account for both between-study and within-study variation. Between-study heterogeneity was subsequently evaluated with I2 values and total variance with Cochran’s Q P-values. Inferential statistics were used to compare contracture patterns and treatment success between and within complications and recurrence studies included. Analysis was performed according to the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines.
Results
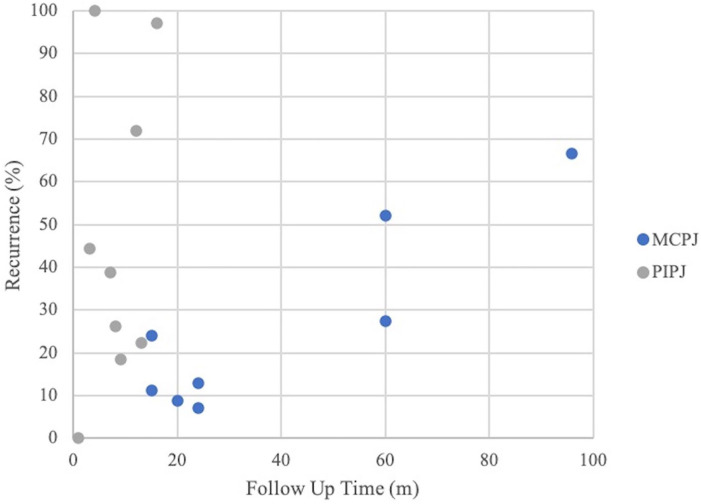
Of 353 studies identified by the search process, 191 abstracts were screened and 53 full-text articles were assessed (Figure 1). Complications were reviewed in 2675 patients and 3753 joints (MCPJ = 51.9%, PIPJ = 48.1%) (Supplementary Table 1) with a mean follow-up time of 16.8 months (CI = 8.6, 25.0), mean age of 64.0 years (CI = 62.5, 65.4), and an 84% male distribution.5-19,26-28,30 Recurrences were reviewed in 1488 patients and 2069 joints (MCPJ = 63.2%, PIPJ = 36.8%) (Supplementary Table 1) with a mean follow-up time of 34.6 months (CI = 22.2, 47.0) (Figure 2), mean age of 65.1 years (CI = 64.1, 66.2), and 86% male distribution.9,11,15-17,19-26,28,29
Figure 2.
Contracture recurrence versus study follow-up.
Note. MCPJ = metacarpophalangeal joint; PIPJ = proximal interphalangeal joint.
Complications
Twenty studies were used in analysis of complications (Table 1). The mean initial contracture was 49.9° (CI = 44.1, 55.8) for MCPJs and 49.5° (CI = 44.7, 54.4) for PIPJs.5,7,10,11,13,16,19,28,30 MCPJs were reduced to a mean of 7.4° (CI = 5.3, 9.5) and PIPJs to 15.2° (CI = 8.5, 21.9) within 1 month of the date of last injection.7,13,16 Average change in range of motion (ROM) was 36.6° (CI = 27.8, 45.4) for MCPJs and 31.5° (CI = 26.0, 37.1) for PIPJs.7,12,16 Clinical success was achieved in 76.1% (CI = 69.9, 81.3) of MCPJs and 41.3% (CI = 33.6, 49.5) of PIPJs.4,6-12,14-19,26,27 MCPJs reached clinical success more often than PIPJs (P < .001) (Table 2; Supplementary Table 1; Supplementary Figures 1 and 2).
Table 1.
Complication Studies.
| Authors | Year | Follow-up (m) | Patients (n) | MCPJ (n) | PIPJ (n) | Type of study | Level of evidence |
|---|---|---|---|---|---|---|---|
| Arora et al 5 | 2016 | 3 | 120 | — | — | Case series | 4 |
| Badalamente et al 6 | 2015 | 3 | 506 | — | 644 | Analysis of prospective, randomized, double-blind, placebo-controlled | 2 |
| Coleman et al 7 | 2014 | 1 | 60 | 75 | 45 | Prospective case series | 4 |
| Gaston et al 8 | 2015 | 2 | 715 | 896 | 552 | Prospective case series | 4 |
| Gilpin et al 9 | 2010 | 12 | 45 | 20 | 25 | Prospective, randomized, double-blinded, placebo-controlled trial | 1 |
| Grandizio et al 10 | 2017 | 1 | 31 | 34 | 34 | Prospective case series | 4 |
| Hansen et al 11 | 2017 | 15 | 212 | 170 | 65 | Prospective cohort | 2 |
| Hurst et al 12 | 2009 | 3 | 203 | 133 | 70 | Prospective, randomized, double-blind, placebo-controlled trial | 1 |
| Hwee et al 26 | 2017 | 60 | 113 | 72 | 74 | Retrospective case series | 4 |
| Leclere et al 30 | 2018 | 24 | 38 | 39 | 29 | Retrospective case series | 4 |
| Malafa et al 27 | 2016 | 6 | 36 | 40 | 7 | Retrospective case series | 4 |
| McMahon et al 20 | 2013 | 15 | 48 | 46 | 18 | Retrospective case series | 4 |
| Murphy et al 13 | 2017 | 23 | 20 | 19 | 17 | Prospective, observational case series | 4 |
| Sanjuan-Cerverò et al 4 | 2013 | 12 | 43 | 31 | 31 | Retrospective case control | 3 |
| Sanjuan-Cerverò et al 14 | 2018 | 1 | 151 | 138 | 70 | Prospective cohort | 2 |
| Simon-Perez et al 15 | 2017 | 48 | 71 | 67 | 4 | Prospective case series | 4 |
| Skov et al 16 | 2017 | 29 | 29 | 0 | 50 | Prospective, independent, open-label, randomized control trial | 1 |
| Strömberg et al 17 | 2017 | 12 | 20 | 20 | 0 | Prospective case series in subpopulation of randomized controlled trial | 4 |
| Verstreken et al 18 | 2016 | 1 | 110 | 73 | 38 | Prospective, observational, multicenter, pharmaco-epidemiological study | 4 |
| Werlinrud et al 19 | 2018 | 60 | 104 | 73 | 34 | Prospective cohort | 2 |
| Total | — | — | 2675 | 1946 | 1807 | — | — |
Note. MCPJ = metacarpophalangeal joint; PIPJ = proximal interphalangeal joint.
Table 2.
Complication Studies: Treatment Efficacy.
| Complication | Estimate (95% CI) | I2 (95% CI) | Cochran’s Q P value |
|---|---|---|---|
| MCPJ | |||
| Initial MCPJ contracture (°) | 50 (44, 56) | 87 (75, 93) | <.001 |
| Post CCH MCPJ contracture (°) | 7 (5, 10) | — | .644 |
| MCPJ success (%) | 76 (70, 81) | 80 (67, 88) | <.001 |
| PIPJ | |||
| Initial PIPJ contracture (°) | 50 (45, 54) | 73 (45, 87) | <.001 |
| Post CCH PIPJ contracture (°) | 15 (9, 22) | 72 (6, 92) | .273 |
| PIPJ success (%) | 31 (34, 50) | 86 (78, 91) | <.001 |
Note. CI = confidence interval; MCPJ = metacarpophalangeal joint; CCH = collagenase clostridium histolyticum; PIPJ = proximal interphalangeal joint.
Treatment-related AEs were common, with 94% (CI = 86, 97) of patients reporting at least one AE. Minor AEs that were observed in a majority of patients included peripheral edema (64%, CI = 49, 76), pain in extremity (53%, CI = 36, 70), and contusion (51%, CI = 34, 68). Major nonsurgical AEs included nonrupture tendon injury and anaphylaxis, each observed in 1 patient. Major surgical AEs, observed in 9 patients, included tendon and pulley rupture (0.98%, CI = 0.58, 1.65) (Table 3).
Table 3.
Complication Frequencies.
| Complication | Percent affected (95% CI) | I2 (95% CI) | Cochran’s Q P value |
|---|---|---|---|
| Patients with at least 1 treatment-related AE | 94% (86, 97) | 84% (71, 91) | <.001 |
| Minor AE | |||
| Peripheral edema | 64% (49, 76) | 95% (92, 97) | <.001 |
| Contusion | 51% (34, 68) | 94% (89, 97) | <.001 |
| Pain in extremity | 53% (36, 70) | 96% (93, 97) | <.001 |
| Ecchymosis | 41% (11, 80) | 99% (98, 99) | <.001 |
| Injection site pain | 33% (20, 50) | 97% (95, 98) | <.001 |
| Tenderness | 28% (16, 44) | 88% (65, 96) | <.001 |
| Skin laceration | 27% (21, 33) | 86% (79, 91) | .0013 |
| Injection site swelling | 24% (7.7, 54) | 99% (98, 99) | <.001 |
| Injection site hematoma | 23% (6.3, 58) | 97% (96, 98) | <.001 |
| Pruritus | 20% (13, 28) | 90% (81, 94) | <.001 |
| Injection site hemorrhage | 19% (8.4, 37) | 96% (94, 98) | <.001 |
| Lymphadenopathy | 15% (11, 20) | 83% (72, 90) | <.001 |
| Blood blister | 11% (5.9, 20) | 91% (88, 96) | <.001 |
| Axillary pain | 6.2% (4.5, 8.5) | 25% (0, 70) | .2523 |
| Injection site vesicles | 2.6% (1.1, 5.6) | — | .3416 |
| Major nonsurgical AE | |||
| Nonrupture tendon injury | 0.62% (0.32, 1.21) | 0% (0, 15) | .906 |
| Anaphylaxis | 0.61% (0.31, 1.19) | 0% (0, 17.1) | .896 |
| Major surgical AE | |||
| Tendon and pulley rupture | 0.98% (0.58, 1.65) | 0% (0, 44.4) | .612 |
Note. CI = confidence interval; AE = adverse events.
Recurrences
Of the 15 studies assessing recurrences with a follow-up time of at least 1 year (Table 4), 11 reported at least 1 recurrence.11,15,19-21,23-26,28,29 Follow-up times for recurrence ranged from 12 to 96 months (Figure 2). Earliest time to recurrence ranged from 6 to 24 months with a majority occurring between 12 and 24 months after treatment11,15,16,20,21,23,24,26,28 (Figure 2). The mean initial contracture for joints experiencing a recurrence was 51.0° (CI = 45.7, 56.3) for MCPJs and 56.0° (CI = 46.7, 65.2) for PIPJs.11,16,19-21,24,28 Studies reported contractures of 3.6° (CI = 0.52, 6.7) 26 and 13.4° (CI = 6.1, 20.7) for MCPJs and PIPJs, respectively, 1 month after last injection.16,22 Clinical success was reached in 78% (CI = 71.4, 83.7) of MCPJs and 40% (CI = 31.3, 50.2) of PIPJs.9,11,17,19-26 While recurrences were reported in 23% (n = 466) of successfully treated joints, PIPJ contractures recurred more frequently than MCPJ contractures, with rates of 28% (n = 213) versus 20% (n = 264), respectively, in studies with at least 12 months of follow-up. Recurrence contractures averaged 24° (SD = 5.1°) and 45° (SD = 13°) for MCPJs and PIPJs, respectively, but heterogeneity analysis was limited by unreported measures of variance (Table 5; Supplementary Table 1; Supplementary Figures 3 and 4).15,20,21,29
Table 4.
Recurrence Studies.
| Authors | Year | Follow-up (m) | Patients (n) | MCPJ (n) | PIPJ (n) | Type of study | Level of evidence |
|---|---|---|---|---|---|---|---|
| Badalamente and Hurst 20 | 2000 | 20 | 35 | 34 | 9 | Prospective case series | 4 |
| Badalamente and Hurst 21 | 2007 | 24 | 35 | 14 | 9 | Prospective, randomized, double-blinded, placebo-controlled trial | 1 |
| Gilpin et al 9 | 2010 | 12 | 45 | 20 | 25 | Prospective, randomized, double-blinded, placebo-controlled trial | 1 |
| Hansen et al 11 | 2017 | 15 | 212 | 170 | 65 | Prospective cohort | 2 |
| Hwee et al 26 | 2017 | 60 | 113 | 72 | 74 | Retrospective case series | 4 |
| McMahon et al 28 | 2013 | 15 | 48 | 46 | 18 | Retrospective case series | 4 |
| Naam 22 | 2013 | 24 | 25 | 21 | 11 | Retrospective case control | 2 |
| Peimer et al 23 | 2015 | 60 | 644 | 648 | 433 | Retrospective case series | 4 |
| Simon-Perez et al 15 | 2017 | 48 | 71 | 67 | 4 | Prospective case series | 4 |
| Skov et al 16 | 2017 | 29 | 29 | 0 | 50 | Prospective, independent, open-label, randomized control trial | 1 |
| Strömberg et al 24 | 2018 | 24 | 78 | 78 | 27 | Prospective, randomized, single-blinded, controlled trial | 1 |
| Strömberg et al 17 | 2017 | 12 | 20 | 20 | 0 | Prospective case series in subpopulation of randomized controlled trial | 4 |
| Vanek et al 25 | 2018 | 24 | 39 | 39 | 0 | Prospective cohort | 2 |
| Watt et al 30 | 2009 | 96 | 8 | 6 | 2 | Retrospective case series | 4 |
| Werlinrud et al 19 | 2018 | 60 | 104 | 73 | 34 | Prospective cohort | 2 |
| Total | — | — | 1506 | 1308 | 761 | — | — |
Note. MCPJ = metacarpophalangeal joint; PIPJ = proximal interphalangeal joint.
Table 5.
Recurrence Studies: Treatment Efficacy.
| Complication | Percent affected (95% CI) | I2 (95% CI) | Cochran’s Q P value |
|---|---|---|---|
| MCPJ | |||
| Initial MCPJ contracture (°) | 51 (46, 56) | 84 (66, 92) | <.001 |
| Post CCH MCPJ contracture (°) | 3.6 (0.52, 6.7) | — | — |
| MCPJ success (%) | 78 (71, 84) | 70 (44, 84) | <.001 |
| Number of MCPJ recurrences | 264/1308 (20%) | — | — |
| PIPJ | |||
| Initial PIPJ contracture (°) | 56 (47, 56) | 90 (82, 94) | <.001 |
| Post CCH PIPJ contracture (°) | 13 (6.1, 21) | 73 (0, 94) | .056 |
| PIPJ success (%) | 40 (31, 50) | 73 (49, 86) | <.001 |
| Number of PIPJ recurrences | 213/761 (28%) | — | — |
Note. CI = confidence interval; MCPJ = metacarpophalangeal joint; CCH = collagenase clostridium histolyticum; PIPJ = proximal interphalangeal joint.
Among studies reporting on recurrences, 63% reported 155 interventions following recurrence, split between 53% (n = 32) medical and 47% (n = 28) surgical.11,16,19,21,23,28,29 Specific medical interventions were rarely specified, although pain management and hand therapy were reported. Surgical interventions included cord-releasing procedures such as PNF, fasciotomy, fasciectomy, and dermofasciectomy or re-injection of CCH.
Discussion
The purpose of this systematic review is to investigate CCH treatment-related AEs and recurrence for Dupuytren’s contracture in MCPJs and PIPJs. Key findings include differences in clinical success of treatment between MCPJs and PIPJs, high frequency of minor AEs but low frequency of major AEs, and high rates of recurrence (23%). Many studies identify CCH as a safe, effective, and minimally invasive treatment that improves hand function in Dupuytren’s patients.5,6,8,9,11,12,16,18,20-22,26,29 The results of this study can be used to advise patients about treatment-related AEs and potential for contracture recurrence following CCH, and will assist physicians in counseling patients on the successes and drawbacks of this treatment modality.
Treatment Success
Similar trends in reaching clinical success were observed in both studies including complications (76% of MCPJs versus 31% of PIPJs) and studies including recurrence rates (78% of MCPJs versus 40% of PIPJs). Certain factors may predispose patients to CCH success, such as contracture limited to a single cord in the MCPJ and joints with less severe initial contractures.12,16 Procedural technique and familiarity may reduce risk of major complications and play a role in achieving successful contracture reduction;6,8,10,11,20,27 for example, a study by Malafa et al 27 on CCH treatment with a hand therapist-based protocol notes that a prone-oriented, patient-controlled wrist extension may prevent manipulators from prematurely stopping rupture upon seeing a skin tear, resulting in greater rupture of residual cord and increased potential for a higher quality outcome. 27 We observed that major AEs only occurred in studies published in 2015 and earlier, which is within 5 years of U.S. Food and Drug Administration approval of CCH treatment; however, studies that included major AEs tended to have larger sample sizes, making them more likely to observe rare events.
Complications
At least one treatment-related AE occurred in the vast majority of patients treated with CCH; however, major surgical (n = 9/2300) or nonsurgical (n = 2/2300) treatment-related AEs were rare. As compared to placebo treatment, patients treated with CCH experienced higher rates of contracture reduction and ROM but experienced significantly more mild or moderate AEs. 31 Trends in complications were notable for marked similarities between studies, and AEs described are typically minor, self-resolving, and limited to the injection site.5,9,11,16,22 There is no indication that minor AE incidence is related to treatment effectiveness. 14 There is inconsistent evidence as to whether multiple CCH injections in the same hand increases risk of minor AE’s, with Coleman et al 7 describing increased incidence of pruritis, lymphadenopathy, blood blister, and skin lacerations with multiple joints and Gaston et al 8 describing no significant differences with multiple joints aside from skin laceration.7,8
Our study found the occurrence of major AEs to be rare, and previous studies similarly indicate that serious AEs occur more commonly after dermofasciectomy and fasciectomy as compared to CCH.1,4,13,15,26,32 Regardless, previous systematic review has demonstrated increased relative risk of AEs in CCH versus PNF 32 and previous studies have similarly found higher rates of total AEs following CCH. 33 A systematic review by Krefter et al 33 noted complication rates of 17.4%, 18.9%, and 11.6% for fasciectomy, PNF, and dermofasciectomy, respectively, versus 78% for CCH. 33 While surgery is considered the gold standard for Dupuytren’s contracture treatment, a number of studies identify CCH as a viable alternative with a lower risk of complications and shorter recovery time.13,27,30 Other studies report comparable outcomes between CCH and fasciectomy as well as CCH and PNF.22,24,32 Since surgery is not always viable due to patient comorbidities, CCH represents a nonsurgical alternative that can reduce the number of patients requiring surgery.4,12 While dissatisfaction with collagenase is greater in patients who have a poor outcome early on following the procedure, dissatisfaction is not necessarily greater in patients who experience an initial AE from CCH. 34 Collagenase clostridium histolyticum is widely associated with high rates of patient satisfaction, which Leclère et al 30 attribute to procedural simplicity, decreased pain, and shorter recovery times compared to surgery.2,7-9,16,18,20,22,28,29,30
Recurrence
Approximately one quarter (23%) of successfully treated joints that received CCH treatment in either MCPJs or PIPJs experience recurrence, with more PIPJs experiencing recurrence than MCPJs. This discrepancy may affect treatment and patient education decisions; for example, Werlinrud et al 19 conclude that while recurrence rates following CCH treatment are acceptable in MCPJs and PIPJs, the higher recurrence in PIPJs warrants patient education on the greater likelihood for recurrence and possibly repeated treatment for PIPJs. 30 As compared to other treatments, Arora et al 5 and Hansen et al 11 cite higher contracture recurrence following CCH as compared to limited fasciectomy, while Chen et al 35 cite lower recurrence in CCH. Other studies cite lower recurrence with CCH versus PNF.4,5,9,11,26 There is variability in literature as to how recurrence following CCH compares to surgical treatment options, with no clear consensus as to which method provides lowest recurrence rates.4,6
Recurrence rates occur proportionally to length of follow-up, and many studies call for longer follow-up times and analysis of recurrence to further elucidate the true rate of recurrance.4,6-8,20,26,27,30 Additionally, limiting the study population to successful treatments means that failed treatments are not accounted for in the overall recurrence rate. Patients with recurrence are not necessarily dissatisfied with CCH treatment; 4 six out of eight patients in a study by Watt et al 29 experienced recurrence, yet seven out of eight indicated that they would again consider CCH for treating recurrence or disease progression. 29 However, Bradley and Warwick 34 note that patient satisfaction with CCH and willingness to receive a second CCH treatment decrease as recurrence increases.
Cost-Effectiveness
A number of studies have identified CCH as a cost-effective treatment for Dupuytren’s contracture,1,4,13 and Sanjuan Cerveró et al 3 report a 29% to 51.5% reduction in associated healthcare costs. PNF is considered the cheapest treatment option, 31 and Strömberg et al 17 found the use of CCH to be 43 times more expensive than PNF. 17 Some studies note that CCH is not superior to PNF and is considerably more expensive, while others consider the high rates of recurrence with PNF unacceptable and postulate that the increased cost offsets the lower risk of recurrence.4,16 One study found that patients returned to work 1.9 days after CCH versus 37.4 days after open surgery. 22 Contributory factors to the long recovery period from surgical treatment may stem from complications such as tendon rupture in 0.2% of patients, digital nerve injury in 1.7% to 7.8% of patients, digital artery injury in 1.9% to 9.7% of patients, and infection in 1.0% to 10.6% of patients, as well as the general greater need for hand therapy. 9 Studies that endorse cost-effectiveness typically attribute it factors such as fewer hospitalizations, 4 decreased time with a hand therapist, and faster return to work. 22
Limitations
Different methods of tracking and measuring angles of contracture posttreatment, including successful versus unsuccessful correction, degree of correction, and change in ROM at different follow-up intervals, limit study comparability for treatment efficacy. Specifically, a lack of reported postoperative follow-up times limits standardization in analyzing when recurrences occurred. The heterogeneity of reporting and measuring outcomes is an existing trend in Dupuytren’s research. 36 The lack of reported analyses of variance also limited measurement of heterogeneity between studies. By excluding articles from recurrence rates with follow-up times of less than 1 year, contractures that recur shortly after CCH treatment are overlooked; conversely, the lack of long-term follow-up also presents challenges in effectively tracking recurrences. Certain studies also note atypical demographics: Grandizio et al 10 describe a limitation in racial distribution, as Dupuytren’s contracture is predominantly see in Caucasians, and McMahon et al 28 describe an unusually high population of females. Authors investigating this topic are encouraged to report longer follow-up data as well as analyses of variance to facilitate future meta analyses and comparisons of CCH to other treatment methods.
Conclusion
Overall, CCH is a safe, minimally invasive and effective means by which to improve hand function in patients with Dupuytren’s contracture. This modality, however, is not without risk, and many patients experience recurrence at rates proportional to length of follow-up. Surgeons are encouraged to discuss these risks with patients, and to come to a shared decision regarding the optimal treatment modality.
Supplemental Material
Supplemental material, sj-docx-1-han-10.1177_1558944720974119 for Treatment of Dupuytren’s Contracture With Collagenase: A Systematic Review by Alexis B. Sandler, John P. Scanaliato, Thomas Dennis, Gilberto A. Gonzalez Trevizo, Sorana Raiciulescu, Leon Nesti and John C. Dunn in HAND
Supplemental material, sj-jpg-1-han-10.1177_1558944720974119 for Treatment of Dupuytren’s Contracture With Collagenase: A Systematic Review by Alexis B. Sandler, John P. Scanaliato, Thomas Dennis, Gilberto A. Gonzalez Trevizo, Sorana Raiciulescu, Leon Nesti and John C. Dunn in HAND
Supplemental material, sj-jpg-2-han-10.1177_1558944720974119 for Treatment of Dupuytren’s Contracture With Collagenase: A Systematic Review by Alexis B. Sandler, John P. Scanaliato, Thomas Dennis, Gilberto A. Gonzalez Trevizo, Sorana Raiciulescu, Leon Nesti and John C. Dunn in HAND
Supplemental material, sj-jpg-3-han-10.1177_1558944720974119 for Treatment of Dupuytren’s Contracture With Collagenase: A Systematic Review by Alexis B. Sandler, John P. Scanaliato, Thomas Dennis, Gilberto A. Gonzalez Trevizo, Sorana Raiciulescu, Leon Nesti and John C. Dunn in HAND
Supplemental material, sj-jpg-4-han-10.1177_1558944720974119 for Treatment of Dupuytren’s Contracture With Collagenase: A Systematic Review by Alexis B. Sandler, John P. Scanaliato, Thomas Dennis, Gilberto A. Gonzalez Trevizo, Sorana Raiciulescu, Leon Nesti and John C. Dunn in HAND
Supplemental material, sj-pdf-1-han-10.1177_1558944720974119 for Treatment of Dupuytren’s Contracture With Collagenase: A Systematic Review by Alexis B. Sandler, John P. Scanaliato, Thomas Dennis, Gilberto A. Gonzalez Trevizo, Sorana Raiciulescu, Leon Nesti and John C. Dunn in HAND
Footnotes
Ethical Approval: This study was exempt from the Institutional review board approval.
Statement of Human and Animal Rights: This study does not involve any human or animal subjects.
Statement of Informed Consent: Informed consent was not indicated since this study did not involve subjects.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
ORCID iDs: Alexis B. Sandler  https://orcid.org/0000-0002-5784-9473
https://orcid.org/0000-0002-5784-9473
John C. Dunn  https://orcid.org/0000-0002-2292-8227
https://orcid.org/0000-0002-2292-8227
Supplemental material: Supplemental material is available in the online version of the article.
References
- 1. Smeraglia F, Del Buono A, Maffulli N. Collagenase clostridium histolyticum in Dupuytren’s contracture: a systematic review. Brit Med Bull. 2016;118:149-158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Lanting R, Broekstra DC, Werker PM, et al. A systematic review and meta-analysis on the prevalence of Dupuytren disease in the general population of Western countries. Plast Reconstr Surg. 2014;133(3):593-603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Sanjuan Cerveró R, Franco Ferrando N, Poquet Jornet J. Use of resources and costs associated with the treatment of Dupuytren’s contracture at an orthopedics and traumatology surgery department in Denia (Spain): collagenase clostridium hystolyticum versus subtotal fasciectomy. BMC Musculoskelet Disord. 2013;14:293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Mansur HG, Rodrigues de, Oliveira E, Conçalves CB. Epidemiological analysis of patients with Dupuytren’s disease. Rev Bras Ortop. 2016;53:10-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Arora R, Kaiser P, Kastenberger TJ, et al. Injectable collagenase clostridium histolyticum as a nonsurgical treatment for Dupuytren’s disease. Oper Orthop Traumatol. 2016;28:30-37. [DOI] [PubMed] [Google Scholar]
- 6. Badalamente MA, Hurst LC, Benhaim P, et al. Efficacy and safety of collagenase clostridium histolyticum in the treatment of proximal interphalangeal joints in Dupuytren contracture: combined analysis of 4 phase 3 clinical trials. J Hand Surg Am. 2015;40:975-983. [DOI] [PubMed] [Google Scholar]
- 7. Coleman S, Gilpin D, Tursi J, et al. Multiple concurrent collagenase clostridium histolyticum injections to Dupuytren’s cords: an exploratory study. BMC Musculoskelet Disord. 2012;13:61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Gaston RG, Larsen SE, Pess GM, et al. The efficacy and safety of concurrent collagenase clostridium histolyticum injections for 2 Dupuytren contractures in the same hand: a prospective, multicenter study. J Hand Surg Am. 2015;40(10):1963-1971. [DOI] [PubMed] [Google Scholar]
- 9. Gilpin D, Coleman S, Hall S, et al. Injectable collagenase clostridium histolyticum: a new nonsurgical treatment for Dupuytren’s disease. J Hand Surg Am. 2010;35:2027-2038.e1. [DOI] [PubMed] [Google Scholar]
- 10. Grandizio LC, Akoon A, Heimbach J, et al. The use of residual collagenase for single digits with multiple-joint dupuytren contractures. J Hand Surg Am. 2017;472.e1-472.e6. [DOI] [PubMed] [Google Scholar]
- 11. Hansen KL, Werlinrud JC, Larsen S, et al. Difference in success treating proximal interphalangeal and metacarpophalangeal joints with collagenase: results of 208 treatments. Plast Reconstr Surg Glob Open. 2017;5(4):e1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hurst LC, Badalamente MA, Hentz VR, et al. Injectable collagenase clostridium histolyticum for Dupuytren’s contracture. N Engl J Med. 2009;361:968-979. [DOI] [PubMed] [Google Scholar]
- 13. Murphy LE, Murphy KM, Kilpatrick SM, et al. The use of collagenase clostridium histolyticum in the management of Dupuytren’s contracture-outcomes of a pilot study in a district general hospital setting. Ulster Med J. 2017;86(2):94-98. [PMC free article] [PubMed] [Google Scholar]
- 14. Sanjuan-Cerveró R, Carrera-Hueso FJ, Vazquez-Ferreiro P. Adverse effects associated with collagenase clostridium histolyticum in Dupuytren disease: a prospective study. Orthop Traumatol Surg Res. 2018;104(6):901-905. [DOI] [PubMed] [Google Scholar]
- 15. Simón-Pérez C, Alía-Ortega J, García-Medrano B, et al. Factors influencing recurrence and progression of Dupuytren’s disease treated by Collagenase Clostridium histolitycum. Int Orthop. 2018;42:859-866. [DOI] [PubMed] [Google Scholar]
- 16. Skov ST, Bisgaard T, Søndergaard P, et al. Injectable collagenase versus percutaneous needle fasciotomy for Dupuytren contracture in proximal interphalangeal joints: a randomized controlled trial. J Hand Surg Am. 2017;42:321.e3-328.e3. [DOI] [PubMed] [Google Scholar]
- 17. Strömberg J, Vanek P, Fridén J, et al. Ultrasonographic examination of the ruptured cord after collagenase treatment or needle fasciotomy for Dupuytren’s contracture. J Hand Surg Eur Vol. 2017;42:683-688. [DOI] [PubMed] [Google Scholar]
- 18. Verstreken F, Degreef I, Decramer A, et al. Effectiveness and safety of collagenase Clostridium histolyticum in Dupuytren’s disease: an observational study in Belgium. Acta Orthop Belg. 2016;82(2):397-404. [PubMed] [Google Scholar]
- 19. Werlinrud JC, Hansen KL, Larsen S, et al. Five-year results after collagenase treatment of Dupuytren disease. J Hand Surg Eur Vol. 2018;43(8):841-847. [DOI] [PubMed] [Google Scholar]
- 20. Badalamente MA, Hurst LC. Enzyme injection as nonsurgical treatment of Dupuytren’s disease. J Hand Surg Am. 2000;25:629-636. [DOI] [PubMed] [Google Scholar]
- 21. Badalamente MA, Hurst LC. Efficacy and safety of injectable mixed collagenase subtypes in the treatment of Dupuytren’s contracture. J Hand Surg Am. 2007;32:767-774. [DOI] [PubMed] [Google Scholar]
- 22. Naam NH. Functional outcome of collagenase injections compared with fasciectomy in treatment of Dupuytren’s contracture. Hand (N Y). 2013;8(4):410-416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Peimer CA, Blazar P, Coleman S, et al. Dupuytren contracture recurrence following treatment with collagenase clostridium histolyticum (CORDLESS study): 5-year data. J Hand Surg Am. 2015;40:1597-1605. [DOI] [PubMed] [Google Scholar]
- 24. Strömberg J, Sörenson AL, Fridén J. Percutaneous needle fasciotomy versus collagenase treatment for Dupuytren contracture. J Bone Joint Surg Am. 2018;100:1079-1086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Vanek P, Strömberg J, Fridén J, et al. Morphological patterns of the pretendinous cord in Dupuytren’s disease: a predictor of clinical outcome? J Plast Surg Hand Surg. 2018;52:240-244. [DOI] [PubMed] [Google Scholar]
- 26. Hwee YK, Park D, Vinas M, et al. Outcome of Dupuytren contractures after collagenase clostridium histolyticum injection: a single-institution experience. Ann Plast Surg. 2017; 79(2):145-148. [DOI] [PubMed] [Google Scholar]
- 27. Malafa MM, Lehrman C, Criley JW, et al. Collagenase Dupuytren contracture: achieving single treatment success with a hand therapist-based protocol. Plast Reconstr Surg Glob Open. 2016;4(2):e629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. McMahon HA, Bachoura A, Jacoby SM, et al. Examining the efficacy and maintenance of contracture correction after collagenase clostridium histolyticum treatment for Dupuytren’s disease. Hand (NY). 2013;8:261-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Watt AJ, Curtin CM, Hentz VR. Collagenase injection as nonsurgical treatment of Dupuytren’s disease: 8-year follow-up. J Hand Surg Am. 2010;35(4):534-539, e1. [DOI] [PubMed] [Google Scholar]
- 30. Leclère FM, Kohl S, Varonier C, et al. Range of motion, postoperative rehabilitation and patient satisfaction in MCP and PIP joints affected by Dupuytren Tubiana stage 1-3: collagenase enzymatic fasciotomy or limited fasciectomy? a clinical study in 52 patients. Arch Orthop Trauma Surg. 2018;138(11):1623-1631. [DOI] [PubMed] [Google Scholar]
- 31. Brazzelli M, Cruickshank M, Tassie E, et al. Collagenase clostridium histolyticum for the treatment of Dupuytren’s contracture: systematic review and economic evaluation. Health Technol Assess. 2015;19(90):1-202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Soreide E, Murad MH, Denbeigh JM, et al. Treatment of Dupuytren’s contracture: a systematic review. Bone Joint J. 2018;100-B:1138-1145. [DOI] [PubMed] [Google Scholar]
- 33. Krefter C, Marks M, Hensler S, et al. Complications after treating Dupuytren’s disease. A systematic literature review. Hand Surg Rehabil. 2017;36(5):322-329. [DOI] [PubMed] [Google Scholar]
- 34. Bradley J, Warwick D. Patient satisfaction with collagenase. J Hand Surg Am. 2016;41:689-697. [DOI] [PubMed] [Google Scholar]
- 35. Chen NC, Srinivasan RC, Shauver MJ, et al. A systematic review of outcomes of fasciotomy, aponeurotomy, and collagenase treatments for Dupuytren’s contracture. Hand (N Y). 2011;6(3):250-255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Karpinski M, Moltaji S, Baxter C, et al. A systematic review identifying outcomes and outcome measures in Dupuytren’s disease research. J Hand Surg Eur Vol. 2020; 45(5): 513-520. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental material, sj-docx-1-han-10.1177_1558944720974119 for Treatment of Dupuytren’s Contracture With Collagenase: A Systematic Review by Alexis B. Sandler, John P. Scanaliato, Thomas Dennis, Gilberto A. Gonzalez Trevizo, Sorana Raiciulescu, Leon Nesti and John C. Dunn in HAND
Supplemental material, sj-jpg-1-han-10.1177_1558944720974119 for Treatment of Dupuytren’s Contracture With Collagenase: A Systematic Review by Alexis B. Sandler, John P. Scanaliato, Thomas Dennis, Gilberto A. Gonzalez Trevizo, Sorana Raiciulescu, Leon Nesti and John C. Dunn in HAND
Supplemental material, sj-jpg-2-han-10.1177_1558944720974119 for Treatment of Dupuytren’s Contracture With Collagenase: A Systematic Review by Alexis B. Sandler, John P. Scanaliato, Thomas Dennis, Gilberto A. Gonzalez Trevizo, Sorana Raiciulescu, Leon Nesti and John C. Dunn in HAND
Supplemental material, sj-jpg-3-han-10.1177_1558944720974119 for Treatment of Dupuytren’s Contracture With Collagenase: A Systematic Review by Alexis B. Sandler, John P. Scanaliato, Thomas Dennis, Gilberto A. Gonzalez Trevizo, Sorana Raiciulescu, Leon Nesti and John C. Dunn in HAND
Supplemental material, sj-jpg-4-han-10.1177_1558944720974119 for Treatment of Dupuytren’s Contracture With Collagenase: A Systematic Review by Alexis B. Sandler, John P. Scanaliato, Thomas Dennis, Gilberto A. Gonzalez Trevizo, Sorana Raiciulescu, Leon Nesti and John C. Dunn in HAND
Supplemental material, sj-pdf-1-han-10.1177_1558944720974119 for Treatment of Dupuytren’s Contracture With Collagenase: A Systematic Review by Alexis B. Sandler, John P. Scanaliato, Thomas Dennis, Gilberto A. Gonzalez Trevizo, Sorana Raiciulescu, Leon Nesti and John C. Dunn in HAND