Abstract
BACKGROUND.
Microsatellite instability (MSI-H)/mismatch repair-deficiency (dMMR) is a biomarker for response to immune-checkpoint inhibitors (ICIs). Whether mechanisms underlying MSI alter responses to ICIs is unclear. We report data from a prospective phase II pilot study (NCT02899793) of pembrolizumab in recurrent MSI-H endometrial cancer (EC) patients analyzed by whole exome sequencing (WES) and potential mechanisms of primary/secondary ICI resistance.
METHODS.
Patients with measurable, MSI-H/dMMR EC confirmed by polymerase chain reaction/immunohistochemistry were evaluated by WES and received pembrolizumab 200 mg every 3 weeks for ≤2 years. The primary end point was objective response rate (ORR). Secondary endpoints included progression-free survival (PFS) and overall survival (OS).
RESULTS.
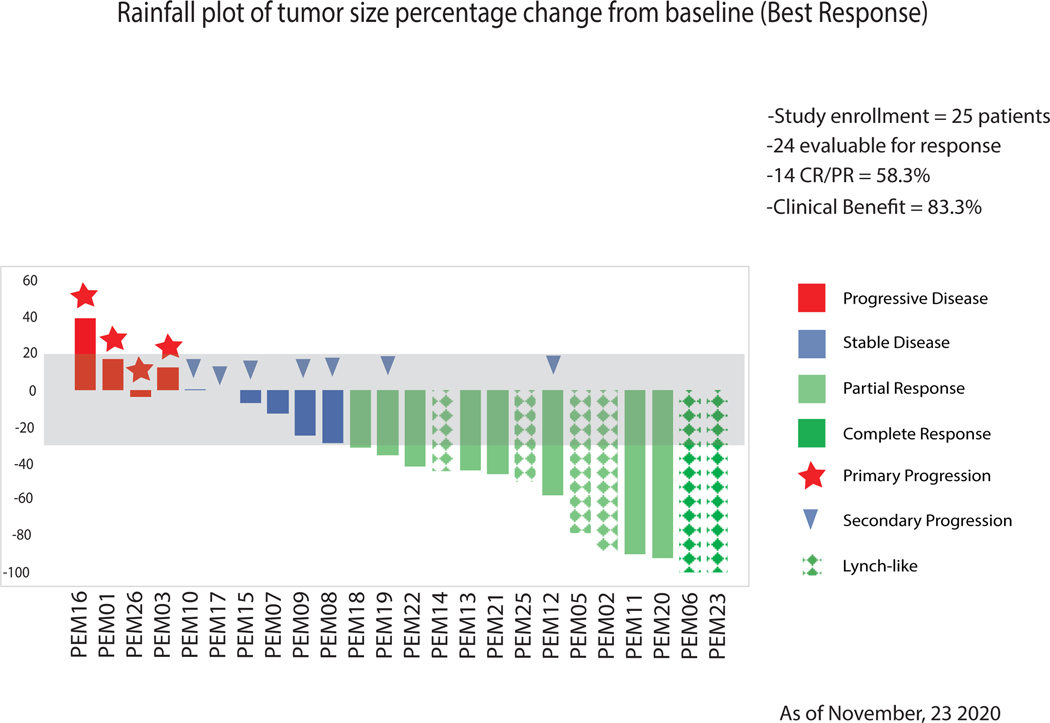
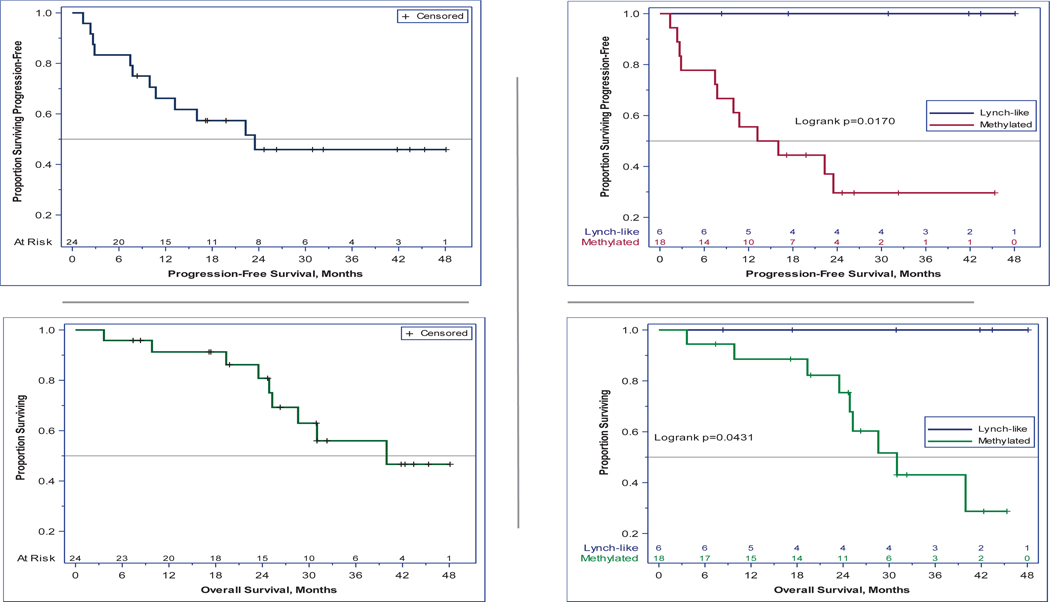
Twenty-five patients (24 evaluable) were treated. Six (25%) patients harbored Lynch/Lynch-like tumors while 18 (75%) had sporadic EC. Tumor mutational burden (TMB) was higher in Lynch-like (median 2939 mutations/megabase [Mut/Mb], IQR:867-5108) versus sporadic tumors (median 604 Mut/Mb, IQR:411-798) (P=0.0076). ORR was 100% in Lynch/Lynch-like but only 44% in sporadic patients (P=0.024). The 3-year PFS/OS proportions were 100% versus 30% (P=0.017) and 100% versus 43% (P=0.043), respectively.
CONCLUSIONS.
Our study suggests prognostic significance of Lynch-like versus sporadic MSI-H/dMMR EC on ORR, PFS and OS when treated with pembrolizumab. Larger, confirmatory studies in EC and other MSI-H/dMMR tumors are necessary. Defective antigen processing/presentation and deranged induction in interferon responses served as mechanisms of resistance in sporadic MSI-H EC. Oligo-progression in MSI-H/dMMR patients appeared salvageable by surgical resection and/or local treatment and continuation of pembrolizumab off-study. Clinical studies evaluating separate subtypes of MSI-H/dMMR EC treated with ICIs are warranted.
Keywords: endometrial cancer, Gynecologic Oncology, Gynecologic Cancers, Immunotherapy/checkpoint blockade, clinical trial results, Phase II clinical trial
Precis:
Our study suggests prognostic significance of Lynch-like versus sporadic MSI-H EC on ORR, PFS and OS when treated with pembrolizumab. Defective antigen processing/presentation and deranged induction in interferon responses served as mechanisms of resistance in sporadic MSI-H EC, and clinical studies evaluating separate subtypes of MSI-H EC treated with ICIs are warranted.
INTRODUCTION
An estimated 66,570 women in the United States will be diagnosed with uterine cancer, and 12,940 women will die of disease in 2021.1 About one-third of endometrial cancer (EC) patients and 15–20% of advanced colorectal and gastric cancers are characterized by mismatch repair deficiency (dMMR) leading to a microsatellite instability-high (MSI-H) phenotype, high tumor mutational burden (TMB) and lymphocytic infiltration.2 It is estimated that 8% of stage I-III and 4% of stage IV cancers have MSI-H/dMMR- characteristics. This represents roughly 40,000 stage I-III and 20,000 stage IV diagnoses annually in the United States alone.3
dMMR/MSI-H tumors originate from different molecular pathways including germline mutations in canonical MMR genes (MLH1, MSH2, MSH6 and PMS2) (ie, Lynch syndrome), somatically acquired MMR gene mutations (ie, Lynch-like), or homozygous methylation of the MLH1 gene promoter (ie, sporadic).4 Microsatellite instability is almost exclusively a feature of endometrioid tumors and remains rare in other endometrial subtypes. In a study of 307 endometrioid, 53 serous and 13 mixed serous/endometrioid tumors, MSI was present in 40% of endometrioid tumors and only 2% of serous tumors.5 In another study of 473 endometrial cancers, MSI was present in 23% of endometrioid, 4% serous, 6% clear cell and 40% undifferentiated tumors.6 Although recent studies have reported significant differences in the immune cell microenvironment infiltrating dMMR/MSI-H tumors, whether Lynch/Lynch-like MSI-H cancers differ from sporadic MLH-1-methylated EC with respect to tumor mutation burden (TMB) and/or clinical response to immune check-point inhibitors (ICI) is currently unknown.4
Pembrolizumab is a monoclonal anti-programmed cell death 1 (PD-1) antibody blocking the interaction between PD-1 and its ligands PD-L1/PD-L2. In clinical studies (ie, KEYNOTE-016, 164, 012, 028, and 158 trials) pembrolizumab provided an objective response rate (ORR) of 40–71% in patients with progressive dMMR and/or MSI-H metastatic colorectal and non-colorectal cancers. 7 Additional biomarkers to better identify subsets of MSI-H/dMMR patients that fail to respond to anti-PD-1 ICIs are essential.
We reported in preliminary form the results of this study earlier this year.8 Herein, we describe fully the findings of this pilot phase II trial (NCT02899793)9 separately evaluating the role of pembrolizumab in patients with recurrent Lynch/Lynch-like and sporadic MSI-H EC patients fully characterized by TMB and genetic signatures via whole exome sequencing (WES). We also leveraged the collection of fresh ICI-resistant MSI-H EC samples to investigate whether alterations in genes encoding HLA Class I antigen processing and presentation machinery and interferon signaling play a role in acquired resistance to pembrolizumab.
METHODS
Patient Population.
NCT02899793 is an Investigator-initiated phase II trial evaluating the safety and efficacy of pembrolizumab in patients with recurrent dMMR and/or MSI-H EC identified by conventional immunohistochemistry (IHC) or polymerase chain reaction (PCR) and comprehensively evaluated using whole exome sequencing (WES) for TMB and genetic signatures, as described previously.10,11 Key eligibility criteria included histologically documented, metastatic/recurrent EC that progressed after standard therapy. All EC subtypes were allowed; sarcoma or mesenchymal tumors were excluded. Patients were required to be age ≥18 years, have measurable disease at baseline on the basis of Response Evaluation Criteria in Solid Tumors (RECIST) v1.1,12 have an Eastern Cooperative Oncology Group13 performance status of 0 or 1, and demonstrate adequate organ function within 10 days of treatment initiation. Key exclusion criteria included prior anticancer monoclonal antibody therapy ≤4 weeks prior to treatment initiation; prior chemotherapy, targeted small-molecule therapy, or radiation therapy within 2 weeks of treatment initiation; prior treatment with an anti-PD-1, anti-PD-L1, or anti-PD-L2 therapy or other ICI (full protocol in Supplementary Appendix).
End Points.
The primary efficacy endpoint was objective response (OR) in patients with persistent, recurrent or metastatic endometrial cancer harboring an ultra-mutated or hyper-mutated (MMR gene-defective) phenotype. OR is defined as best complete (CR) or partial response (PR) per RECIST v1.1. The primary safety endpoint was toxicity as assessed by National Cancer Institute Common Terminology Criteria for Adverse Events (CTCAE) v.4.0.14 Secondary endpoints included progression-free survival (PFS; time from allocation to first disease progression or death resulting from any cause), and overall survival (OS; time from allocation to death from any cause or loss to follow-up). Efficacy and safety were assessed in all patients who received ≥1 dose. Exploratory objectives included the (1) prospective comparison of WES relative to IHC/PCR for identification of MSI-H patients; (2) correlation of TMB and somatic mutations in MSI-H patients identified by WES with objective response, PFS and OS (3) characterization of immune infiltrates and PDL-1 expression within MSI-H subtypes versus OR, PFS and OS in pembrolizumab-treated patients.
Trial Oversight.
This was an Investigator-initiated pilot study supported in part by Merck (Kenilworth, New Jersey), who supplied pembrolizumab. The company had no influence on the study design, the data derived, or the analyses and interpretations. The trial was approved by the Institutional Review Board at Yale University. All authors had access to the data and participated in the writing, reviewing and editing of the manuscript. All authors attest that the trial was conducted in accordance with the protocol and amendments with Good Clinical Practice standards in accordance with the Declaration of Helsinki. Patients provided written informed consent.
Study Design.
This was a single-arm, open-label phase II pilot study using the optimal flexible two-stage design of Chen et al15 to evaluate the efficacy of the study regimen. The targeted accrual for the first stage was 12 eligible and evaluable patients but was allowed to range from 8–15. The study would stop early if no ORs occurred among ≤13 first-stage subjects or one OR occurred among 14–15 such subjects; otherwise, accrual to the second stage would proceed. The cumulative accrual to both stages had a target of 22 with an allowable range of 18–25. The treatment regimen was considered successful if ≥2 ORs among 18 subjects or ≥3 ORs among 19–25 subjects were observed. The operating characteristics were as follows: with a null-hypothesis response rate of 5%, the study’s true alpha has an average of 9.5% with a 64.9% average probability of early termination; with an alternative-hypothesis response rate of 25%, the study’s average power is 90.0% (ie, 90% average chance of correctly classifying the study regimen as interesting if the true probability of tumor response is 25%). Additional design details are presented in the protocol (see Supplementary Appendix).
Statistical Analyses.
The ORR for the entire study was reported with exact binomial 95% confidence intervals and was also compared between MSI-H subgroups using Fisher’s exact test. PFS/OS were analyzed using the Kaplan-Meier method, and compared between subgroups using log-rank tests. Safety data were summarized using descriptive statistics. The Wilcoxon rank-sum (WRS) test was used to compare MSI-H subgroups for difference in mutational burden, whereas the Cochran-Armitage Trend test was used to compare them for differences in average IHC-intensity score. WRS tests were also used on patients who achieved OR to compare MSI-H subgroups for differences in time to achieve OR and in baseline tumor size. All statistical tests employed two-sided alpha=0.05 significance levels.
Treatment and Assessments.
Patients received pembrolizumab 200 mg as a flat dose intravenously over 30 minutes every 3 weeks for a maximum of 24 months or until disease progression, intolerable toxicity, death, withdrawal of consent, or investigator decision. Safety was assessed throughout the study via history, physical examination, and laboratory assessment before each cycle and for 30 days after discontinuation (90 days for serious adverse events [AEs] and immune-mediated AEs). Immune-mediated AEs were defined as events with potentially drug-related immunologic causes that were consistent with an immune phenomenon, regardless of attribution to treatment or immune relatedness by the Investigator. For patients exhibiting prespecified treatment-related toxicities, treatment could be withheld and permanently discontinued if toxicity did not resolve to grade 0–1 within 12 weeks of the last pembrolizumab dose. Those with a persistent grade 2 laboratory AE after 12 weeks could continue with Sponsor and Investigator approval only if asymptomatic and controlled. Tumor response was assessed using computed tomography or magnetic resonance imaging at 6 weeks, 12 weeks, and every 12 weeks thereafter. If imaging indicated progressive disease (PD), a confirmatory assessment was required ≥4 weeks later. Patients could continue receiving study treatment during this period. If the repeat scan confirmed progression, study treatment was discontinued.
MSI/dMMR and WES.
Tumors were classified as MSI-H/dMMR when expression by IHC of at least one MMR protein (MLH1/MSH2/MSH6/PMS2) was absent, and/or when the allelic pattern shift involved ≥ 2 loci among 5 microsatellite markers (BAT25, BAT26, NR21, NR24, Mono27) were detected by PCR. All tumors were sequenced at the Foundation Medicine central laboratory (Cambridge, MA, USA) using FoundationOne®, a test sequencing the coding region of 324 genes plus introns from 28 genes to a median depth of coverage of >500x16 and using WES at the West Campus Genomic Facility at Yale University, as previously described by our research group.17,10 Briefly, DNA was isolated from matched normal DNA samples (peripheral blood) and EC samples using RecoverAll Total Nucleic Acid Isolation Kits (Ambion, Austin, TX) and analyzed using standardized protocols. Mutational signature analyses were performed as described by Alexandrov et al.18 while neoantigen load (the number of peptides predicted to bind with major histocompatibility complex (MHC) proteins was identified based on HLA types derived from sequencing data as previously described by our group.19 The COSMIC databse was queried for pathogenicity of mutations.20,21,22
IHC.
Four μm sections were cut from formalin-fixed paraffin-embedded blocks of all 24 evaluable MSI-H patients and stained with the following antibodies according to the manufacturers’ instructions: CD3 (clone 2GV6, Ventana), CD68 (clone PG-M1, DAKO), CD20 (clone L26, 1:200, DAKO), and PD-L1 (clone E1L3N, 1:200, Cell Signaling). Intensity of infiltration for CD3-, CD68-, and CD20-positive cells was scored as none=0, mild=1, moderate=2 and marked=3. Tumor-cell PD-L1 expression was defined as the percentage of tumor cells exhibiting membrane staining at any intensity. Combined Positive Score (CPS) was defined as the number of PD-L1-positive cells (including tumor cells, lymphocytes, and macrophages) divided by the total number of viable tumor cells × 100 for a post hoc analysis. Either primary or metastatic tumor site could be utilized.
RESULTS
Patients.
From September 2016-March 2018, 15 patients were enrolled in the first stage. Accrual to the second stage commenced in April 2018 after 5 responses were observed among 11 response-evaluable first-stage subjects. Accrual continued until March 2020, when 25 patients were enrolled. One patient was subsequently excluded due to MSI-low status upon confirmatory analyses triggered by WES/FoundationOne® analysis. Data cutoff was November 23rd, 2020. Patient characteristics are shown in Table 1. Mean age (SD) was 69.0 (10.2) with a range of 51–86 years. Race was reported as White (N=23, 96%) and Black (N=1, 4%). Two (8%) participants in the study also identified as Hispanic/Latino. Per the inclusion criteria, 100% of evaluable patients had received at least one prior chemotherapy, with a median of 1 (range: 1–5) prior lines consisting of chemotherapy in 32 and hormone therapy in 5 instances. Fifteen patients (62.5%) received radiation, including 12 instances of vaginal brachytherapy, 4 instances of whole pelvic radiotherapy with or without cisplatin sensitization, and 5 instances of palliative radiotherapy to liver, bone or pelvic metastases. Six patients underwent interval surgical debulking other than staging hysterectomy for recurrence.
Table 1.
Patient and clinical characteristics.
| MEAN AGE ± SD, years (range) | 69 ± 10.2 (51–86) | |
|---|---|---|
| MEDIAN LINES OF CHEMOTHERAPY (range) | 1 (1–5) | |
| n | % | |
| RACE | ||
| White | 23 | 96 |
| Black | 1 | 4 |
| ETHNICITY | ||
| Non-Hispanic | 22 | 92 |
| Hispanic | 2 | 8 |
| PRIOR SYSTEMIC THERAPY (37 instances) | ||
| carboplatin/paclitaxel | 18 | 48.6 |
| carboplatin/paclitaxel or nab-paclitaxel/bevacizumab | 4 | 10.8 |
| carboplatin | 2 | 5.4 |
| carboplatin/topotecan | 1 | 2.7 |
| cisplatin/topotecn | 1 | 2.7 |
| carboplatin/pegylated liposomal doxorubicin | 1 | 2.7 |
| cisplatin/doxorubicin | 1 | 2.7 |
| pegylated liposomal doxorubicin | 2 | 5.4 |
| gemcitabine | 1 | 2.7 |
| bevacizumab/nab paclitaxel | 1 | 2.7 |
| aromatase inhibitor | 5 | 13.5 |
| PRIOR RADIOTHERAPY | ||
| no | 9 | 37.5 |
| yes (21 instances) | 15 | 62.5 |
| vaginal brachytherapy | 12 | 57.1 |
| whole pelvis ± cisplatin sensitization | 4 | 19.0 |
| palliative radiation to liver, bone, or pelvic metastases | 5 | 23.8 |
| SURGICAL DEBULKING FOLLOWING STAGING HYSTERECTOMY | ||
| no | 18 | 75 |
| yes | 6 | 25 |
| FIGO STAGE AT DIAGNOSIS | ||
| I | 12 | 50.0 |
| II | 1 | 4.2 |
| III | 8 | 33.3 |
| IV | 3 | 12.5 |
| HISTOLOGY/GRADE | ||
| EAC G1 | 2 | 8.3 |
| EAC G2 | 9 | 37.5 |
| EAC G3* | 13 | 54.2 |
G3 tumors included 9 endometrial endometrioid adenocarcinomas (EAC), 2 uterine serous carcinomas (1 pure and 1 mixed), 1 clear cell carcinoma (CC) and 1 carcinosarcoma (CS)
Conventional Determination of MSI/dMMR versus WES.
For all patients the same tumor tissue block from the primary or metastatic tumor site was used for both MSI/dMMR testing and genetic analysis (WES). All patients were confirmed to be MSI-H by PCR and/or dMMR by IHC. Nineteen patients demonstrated MLH1 promoter methylation, and 6 patients demonstrated somatic loss of MMR proteins by IHC; one participant exhibited both MLH1 methylation and loss of an MMR protein (Supplementary Table 1). WES of this EC showed a deleterious somatic mutation in the MSH6 gene (ie, F1088sfs2) with loss of MSH6 protein expression by IHC, a tumor immunophenotype previously reported in a subset of Lynch patients.23,24,25 There were no deleterious germline Lynch patients, though 5 patients each harbored one germline mutation in Lynch genes: two variants of uncertain significance in MSH2 (p.L488V) and PMS2 (p.A317G), as well as two benign germline mutations in PMS2 (p.D60E), and EPCAM (p.V51I). WES identified somatic variants in canonical Lynch-associated genes but also numerous additional genes (eg, PMS1, POLE, POLD1, MSH3, MLH3) known to contribute to a hypermutated phenotype (Supplementary Table 1). In our study, 2 of 8 mutations in POLE (M1998V, V411L), 1 of 3 mutations in PMS1 (T112M), 3 of 11 mutations in POLD1 (D877N, D316N, R224C), 0 of 3 mutations in MLH3, and 0 of 2 mutations in MSH3 were predicted to be pathogenic by the COSMIC database20 (FATHMM score >0.5).22,21 One POLE mutant (V922I) was predicted to be non-pathogenic. Predictions were not available for any of the other mutations. Most (n=7) tumors harbored a single defect in non-Lynch hypermutation genes (e.g., PMS1, POLE, POLD1, MSH3, MLH3), but three tumors contained 2 mutations; one tumor possessed 6 and another tumor demonstrated 8 mutations. All of the pathogenic POLE/POLD1 mutations were identified in Lynch-like patients. Both pathogenic POLE mutations and one of the pathogenic mutations in POLD1 (R224C) were associated with a PR; the remaining pathogenic POLD1 mutation (D316N) was associated with a CR. Six copy number variants (CNV) (deletions) occurred in the methylated group compared to one in the Lynch-like cohort. TMB was significantly higher in the Lynch-like compared to sporadic methylated cohort (mean±SD= 4386±5045, median 2939, interquartile range (IQR) 867–5108 Mut/Mb versus 608±241, median 604, IQR 411–798 Mut/Mb, P=0.0076) (Supplementary Figure 1). TMB estimates by WES correlated well (Pearson’s r2 = 0.98) with commercially available testing (ie, FoundationOne®) (Supplementary Figure 2). Copy number variants (CNVs) were identified by comparing coverage depth of individual capture intervals from tumor and normal samples. Deletions were evident in POLE and PMS2, but amplifications also occurred in MSH2, MSH6, MLH1, MSH3, EPCAM, MSH6 (Supplementary Table 1).
Primary Endpoint: OR Rate and Safety.
Median follow-up was 25.8 months. Among all patients, best response was CR (n=2, 8.3%), PR (n=12, 50%), SD (n=6, 25%), or PD (n=4, 16.6%). Responses in relationship to molecular characteristics are summarized in Supplementary Table 1. ORR was 58% (95% CI, 37%−78%) (Figure 1). Disease control (PR+CR+SD) was achieved in 20 of the 24 evaluable patients (83.3%; 95% CI, 68% to 97%). Among Lynch-like patients, OR rate was 100% but only 44% (8/18) in MLH1-methylated patients (P=0.024). Both CRs were observed among Lynch-like patients. Amongst the 14 patients who achieved PR/CR (all confirmed), the median time to achieve it was 62 days (IQR: 53–75 days) among the 6 Lynch-like patients, versus 177 days (IQR: 86–460 days) among the 8 responders with methylated MLH-1 (P=0.020). Importantly, the two groups of responders had nearly equal tumor sizes at baseline. The medians (IQRs) of tumor size were 42.0 mm (21.4–47.5 mm) for Lynch-like responders versus 46.4 mm (35.5–61.8 mm) for MLH-1-methylated responders (P=0.61), with negligible correlation between baseline tumor size and time to PR (Spearman’s rho=4.2%; P=0.89).
Figure 1.
Waterfall plot showing distribution of the best percentage change in the sum of target lesion size from baseline for an individual patient. The lines (–30 and + 20%) indicate the region with change from baseline that typically represent stable disease based on RECIST guidelines.
Primary clinical resistance to initial therapy with pembrolizumab (ie, PD on first study scan), was noted in 4 patients (16.6%) (Figure 1). One of these patients (PEM03) demonstrated a mixed response, in which all measurable lesions except one decreased in diameter. This patient underwent surgical resection of the single growing lesion in the lung (Supplementary Figure 3). The patient then continued treatment with single-agent pembrolizumab off-protocol and is alive and progression-free at the time of this writing, 41 months from study discontinuation. In accordance with study design, this patient is listed as having PD (ie, primary resistance) (Figure 1). Seven cases of secondary resistance to pembrolizumab were noted, where patients developed PD after an initial OR or SD. Like patient PEM03 described above, PEM08 exhibited secondary progression limited to a single lesion in the abdomen, and the metastatic pembrolizumab-resistant tumor was surgically resected. After the procedure, the patient was continued on single-agent pembrolizumab off-protocol for a total of 9 cycles and she remains alive with no evidence of disease at the time of this writing, 42 months from study discontinuation. Two of 7 patients with secondary pembrolizumab resistance developed progression when pembrolizumab was suspended while the patients received high dose steroids to treat grade 3 diarrhea or grade 2 pneumonitis. Seven patients with confirmed PR but residual disease by imaging and one of two patients who achieved a CR completed therapy after reaching the 2-year milestone and no recurrence/progression has been observed as of the data cutoff.
There were 3 serious adverse events (SAEs) attributable to study drug, including grade 3 diarrhea (n=2) and hyperthyroidism (N=1). Twenty patients experienced 177 AEs classified as possibly, probably, or definitely attributable to study drug. These 177 AEs consisted of mostly grade 1 (N=120, 67.8%) or grade 2 (N=45, 25.4%) toxicities. Only twelve (6.8%) of the treatment-related AEs were grade 3 or 4 (Table 2), and they occurred among eight study subjects. The most common AEs were diarrhea (N=22, 12.4%), non-specific skin or subcutaneous tissue disorders (N=14, 7.9%), fatigue (N=12, 6.8%), and infusion reaction (N=10, 5.6%).
Table 2.
Distribution of 177 adverse events possibly, probably, or definitely related to study drug by grade and system.
| Grade (CTCAE v4.0) | All Grades | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | |||||||
| AEs | Patients | AEs | Patients | AEs | Patients | AEs | Patients | AE total | ||
| System Organ Class (Category) | CTCAE Term | . | . | 1 | 1 | . | . | . | . | 1 |
| Blood and lymphatic system disorders | Anemia | |||||||||
| Cardiac disorders | Atrial fibrillation | . | . | 1 | 1 | . | . | . | . | 1 |
| Palpitations | . | . | 1 | 1 | . | . | . | . | 1 | |
| Endocrine disorders | Endocrine disorders - Other, specify | 2 | 2 | . | . | . | . | . | . | 2 |
| Hyperthyroidism | 1 | 1 | . | . | 1 | 1 | . | . | 2 | |
| Hypothyroidism | 4 | 4 | 2 | 2 | . | . | . | . | 6 | |
| Eye disorders | Dry eye | 3 | 3 | . | . | . | . | . | . | 3 |
| Eye disorders - Other, specify | 4 | 3 | . | . | . | . | . | . | 4 | |
| Gastrointestinal disorders | Constipation | 1 | 1 | . | . | . | . | . | . | 1 |
| Diarrhea | 12 | 8 | 7 | 3 | 3 | 3 | . | . | 22 | |
| Dry mouth | 1 | 1 | . | . | . | . | . | . | 1 | |
| Nausea | 3 | 3 | 1 | 1 | . | . | . | . | 4 | |
| Rectal hemorrhage | 1 | 1 | . | . | . | . | . | . | 1 | |
| Vomiting | 1 | 1 | . | . | . | . | . | . | 1 | |
| General disorders and administration site conditions | Chills | 1 | 1 | . | . | . | . | . | . | 1 |
| Edema face | 1 | 1 | . | . | . | . | . | . | 1 | |
| Fatigue | 5 | 5 | 6 | 6 | 1 | 1 | . | . | 12 | |
| Fever | 2 | 2 | . | . | . | . | . | . | 2 | |
| General disorders and administration site conditions - Other, specify | 8 | 5 | . | . | . | . | . | . | 8 | |
| Pain | 2 | 2 | . | . | . | . | . | . | 2 | |
| Edema limbs | 2 | 2 | . | . | . | . | . | . | 2 | |
| Infusion related reaction | 8 | 6 | 2 | 2 | . | . | . | . | 10 | |
| Infections and infestations | Infections and infestations - Other, specify | 2 | 2 | . | . | . | . | . | . | 2 |
| Mucosal infection | 1 | 1 | . | . | . | . | . | . | 1 | |
| Investigations | Alanine aminotransferase increased | . | . | 1 | 1 | . | . | . | . | 1 |
| Alkaline phosphatase increased | 2 | 2 | 1 | 1 | 1 | 1 | . | . | 4 | |
| Aspartate aminotransferase increased | . | . | 1 | 1 | . | . | . | . | 1 | |
| Cholesterol high | 1 | 1 | . | . | . | . | . | . | 1 | |
| Creatinine increased | 1 | 1 | 1 | 1 | . | . | . | . | 2 | |
| Platelet count decreased | 1 | 1 | . | . | . | . | . | . | 1 | |
| Weight gain | 1 | 1 | . | . | . | . | . | . | 1 | |
| Weight loss | 1 | 1 | . | . | . | . | . | . | 1 | |
| Metabolism and nutrition disorders | Anorexia | 1 | 1 | . | . | . | . | . | . | 1 |
| Dehydration | 1 | 1 | 1 | 1 | . | . | . | . | 2 | |
| Hyperglycemia | . | . | 3 | 3 | 3 | 3 | 1 | 1 | 7 | |
| Hyperkalemia | 1 | 1 | . | . | . | . | . | . | 1 | |
| Hypokalemia | . | . | . | . | 1 | 1 | . | . | 1 | |
| Hypomagnesemia | 3 | 2 | . | . | . | . | . | . | 3 | |
| Hyponatremia | 1 | 1 | 1 | 1 | . | . | . | . | 2 | |
| Musculoskeletal and connective tissue disorders | Arthralgia | 3 | 3 | 2 | 2 | . | . | . | . | 5 |
| Back pain | 1 | 1 | 1 | 1 | . | . | . | . | 2 | |
| Musculoskeletal and connective tissue disorder - Other, specify | 7 | 7 | . | . | . | . | . | . | 7 | |
| Myalgia | 2 | 2 | 2 | 2 | . | . | . | . | 4 | |
| Pain in extremity | 1 | 1 | . | . | . | . | . | . | 1 | |
| Nervous system disorders | Dysgeusia | 1 | 1 | . | . | . | . | . | . | 1 |
| Headache | 3 | 3 | 1 | 1 | . | . | . | . | 4 | |
| Peripheral sensory neuropathy | 1 | 1 | . | . | . | . | . | . | 1 | |
| Respiratory, thoracic and mediastinal disorders | Cough | 1 | 1 | . | . | . | . | . | . | 1 |
| Pneumonitis | 1 | 1 | 1 | 1 | . | . | . | . | 2 | |
| Skin and subcutaneous tissue disorders | Bullous dermatitis | . | . | 1 | 1 | . | . | . | . | 1 |
| Dry skin | 2 | 2 | . | . | . | . | . | . | 2 | |
| Pruritus | 5 | 5 | . | . | . | . | . | . | 5 | |
| Rash maculo-papular | 4 | 3 | 2 | 1 | . | . | . | . | 6 | |
| Skin and subcutaneous tissue disorders - Other, specify | 8 | 6 | 5 | 4 | 1 | 1 | . | . | 14 | |
| Vascular disorders | Hot flashes | 1 | 1 | . | . | . | . | . | . | 1 |
| 120 | 106 | 45 | 39 | 11 | 11 | 1 | 1 | 177 | ||
CTCAE, Common Terminology Criteria for Adverse Events; AE adverse events
Secondary Endpoints: PFS, OS.
Twelve PFS events (9 deaths) were observed during 642.2 person-months of follow-up; individual follow-up durations had a median (range) of 25.8 (3.6–48.1) months. Among all patients, median PFS was 23.5 months (95% CI, 10.7-not reached [NR]); at 1 and 3 years, 66% and 46% remained progression-free. At 1 and 3 years, 100% of Lynch-like patients remained alive and progression-free; among methylated patients, only 56% and 30% were progression-free (log-rank P=0.017). Median PFS within the methylated cohort was 14.6 months (95% CI, 7.8-NR) (Figure 2-top). Among all patients, median OS was 40.0 months (95% CI, 25.3-NR); at 1 and 3 years, overall survival proportions were 91% and 56%. Among methylated patients, median OS was 31.0 months (95% CI, 24.9-NR) and overall survival proportions at 1 and 3 years were 88.6% and 43.1% (log-rank P=0.043) (Figure 2-bottom).
Figure 2.
Top: Progression-free survival (PFS). A total of 12 PFS events were observed during 475.7 person-months. Left: Among all patients, median PFS was 23.5 months (95% CI 10.7-not reached [NR]); at 1 and 3 years, 66% and 46% remained progression-free. Right: At 1 and 3 years, 100% of Lynch-like patients remained progression-free; among methylated patients, only 55.6% and 30% were progression-free (p=0.02). Median PFS within the methylated cohort was 14.6 months (95% CI 7.8-NR). Bottom: Overall survival (OS). Left: Among all patients, median OS was 40 months (95% CI 25.3-NR); at 1 and 3 years, overall survival proportions were 91% and 56%. Right: Among methylated patients, median OS was 31.0 months (95% CI 24.9-NR) and overall survival proportions at 1 and 3 years were 88.6% and 43.1% (p=0.04). All Lynch-like patients remained alive at those timepoints.
Comparison of Immune Cell Populations and PDL1 Expression in Lynch-like versus Sporadic MSI-H EC.
Tumor tissue was evaluated for CD3, CD68, CD20 and PD-L1 expression. As shown in Supplementary Figure 4, Lynch-like MSI-H EC cases demonstrated a significantly higher infiltration when compared to the sporadic cases for CD68+ macrophages in both tumor and adjacent stroma. Average CD68+ IHC scores were 2.8 (mostly marked) for Lynch-like cases versus 2.1 (moderate) for sporadic cases (P=0.022). CD3+ T cells showed an average IHC score of 2.5 (moderate-to-marked) in Lynch-like versus 1.9 (moderate) in sporadic (p=0.18). No significant differences were noted in CD20+ B cells infiltration between the two cohorts. PDL-1 membrane staining ≥1% in tumor cells was identified in 4 (16.7%) of the patients while 17 (70.8%) demonstrated PD-L1 expression in tumor-associated immune cells (Supplementary Table 1). When PD-L1 expression was assessed in both tumor cells and tumor-associated immune cells (ie, CPS ≥1), a total of 17 MSI-H patients (70.8%) had quantifiable PD-L1 expression. CPS scores ranged from 0–60. CPS ≥1 was detected in 5 out of 6 (83.3%) of Lynch-like patients but only 12 out of 18 (66.7%) of the methylated MSI-H patients. This difference was not statistically significant (Fisher’s exact P=0.63).
Conventional MSI/dMMR Testing versus Genetic Signature by WES.
All enrolled 25 EC patients demonstrated either microsatellite instability-high (MSI-H) by PCR and/or mismatch repair-deficiency (dMMR) by IHC at the time of initial pathology review (Supplementary Table 1). In contrast, mutational signature analysis using SigProfiler13 of the WES tumor-normal pair results revealed that only 24 of the 25 samples had an MSI-H/dMMR signature (ie, enrichment in signatures 6, 20, 26 and 53) (Supplementary Figure 5). This unexpected finding prompted us to re-review the initial PCR results for the PEM24 sample which unequivocally demonstrated the overcall of an MSI-Low tumor phenotype as only one allelic loci size was shifted. Accordingly, this patient was removed from the trial and began lenvatinib with pembrolizumab. While all remaining MSI-H EC samples were concordant between IHC/PCR and WES genetic signatures, 3 out of 6 of the Lynch-like samples (PEM23, PEM02, and PEM14) generated a sub-cluster due to a slightly different mutational signature, for which conventional MMR analysis alone cannot discriminate (Supplementary Figure 5).
WES and Antigenicity in MSI-H Tumors with Primary and Secondary Resistance to Pembrolizumab.
Since mechanisms of primary and secondary resistance to ICI in MSI-H EC are poorly understood, we employed WES to investigate whether alterations in genes encoding HLA Class I antigen processing and presentation machinery components or interferon signaling contribute. The lung lesion obtained from PEM03 (primary resistance) and the abdominal metastases obtained from PEM08 (secondary resistance) were utilized. In PEM03, the primary EC and lung metastasis shared 149 nonsynonymous somatic mutations, providing unequivocal evidence that the metastasis was derived from the primary EC rather than from an independent lung tumor. Moreover, the metastasis harbored 894 nonsynonymous new mutations absent from the primary tumor, while the primary tumor harbored 50 mutations absent from the metastasis (Supplementary Figure 6). In PEM08, the primary EC and the upper abdomen recurrence shared 106 nonsynonymous somatic mutations, similarly providing unequivocal evidence of a genetic relationship between the two lesions. The abdominal metastasis harbored 557 nonsynonymous mutations absent from the primary EC, while the primary tumor harbored 260 mutations absent from the metastasis. Importantly, the lung metastasis from PEM03 but not the primary EC contained an in-frame deleterious mutation (EYACRVNM96del) in the B2M gene (β2-microglobulin, a protein required for antigen presentation).26 Consistent with WES results, flow cytometric experiments of fresh tumor cells obtained from the PEM03 biopsy harboring the B2M mutation demonstrated lack of surface expression of HLA Class I antigen. In the case of PEM08, the pembrolizumab-resistant abdominal metastasis acquired a truncating mutation (G422fs) in the JAK3 gene (encoding for a tyrosine kinase that belongs to the Janus family of kinases including JAK1, JAK2 and TYK2). Mutation in JAK3 did not affect HLA Class I expression in PEM08 (Supplementary Figure 6), but mutations in other JAK family members have been implicated in the development of resistance to ICIs secondary to a defective response to induction of type I and type II interferons.27 Finally, when we evaluated the matched specimens for potential differences in antigenicity, we found the distribution of neoantigens predicted to have strong (IC50≤ 50 nmol/L), intermediate (intermediate: 50 nmol/L< IC50≤150 nmol/L), and weak (150 nmol/L< IC50≤ 500 nmol/L) binding affinities for their corresponding HLA I alleles to be similar in pretreatment and ICI-resistant specimens (Supplementary Figure 6).
DISCUSSION
In May 2017, the FDA granted accelerated approval for pembrolizumab as the first tumor-agnostic drug for MSI-H/dMMR cancers.28 In the original supporting studies, the response rate for MSI-H/dMMR EC (N=14) was 36% (95% CI 13–65%) with a duration of response of 4.2 to 17.3 months. In June 2020, the FDA also authorized use of pembrolizumab in TMB-high tumors (>10 Mut/Mb).29
In the present phase II study of MSI-H/dMMR ECs previously treated with platinum (100%) and radiation (62.5%), pembrolizumab achieved an ORR of 58.3% (95% CI, 36.6–77.9%) with an acceptable toxicity profile, with only 12 of 177 AEs representing grade 3 or 4 toxicity. Notably, we observed an ORR of 100% in Lynch-like patients but only 44% in MLH1-methylated patients (p=0.03), establishing for the first time in a prospective investigation the prognostic significance of subcategorization of MSI-H/dMMR ECs by mechanism of MSI. Remarkably, at 3 years, 100% of Lynch-like patients versus 30% of methylated patients (P=0.02) remained progression-free. Consistent with this finding and models by others that predict the neoantigen load of MSI tumors to be 7-fold higher than MS-stable tumors,30 Lynch-like patients exhibited a significantly greater average TMB, highlighting the importance of this characteristic in eliciting response to ICIs. The present study is limited by small numbers; the intriguing results demand larger confirmatory studies in endometrial as well as other disease sites. Response rates to checkpoint inhibition among MSI-H tumors across different primaries can vary greatly,31 and the influence of other elements of tumor biology remain relevant. In KEYNOTE-158,32 a phase II study of efficacy of pembrolizumab in non-colorectal MSI-H cancers, the OR rate ranged from 0% (brain) to 57.1% (endometrial); OR rate was 40.9, 45.8, 42.1, 33.3 and 18.2% among cholangiocarcinomas, gastric cancers, small intestine cancers, ovarian and pancreatic cancers, respectively. In colorectal carcinoma, BRAFV600E mutations are linked to sporadic MSI and rarely found in Lynch syndrome.33 CheckMate-142 was a phase II study of nivolumab in patients with metastatic DNA mismatch repair deficient/microsatellite instability–high colorectal cancer.34 Among 74 MSI-H patients, 16% were BRAF-mutated. Response rate among BRAF-mutated MSI-H cancers was 25%, compared to 41% among BRAF wild-type tumors. Among responders, the rate of disease control at ≥12 weeks was 75% and 79%, respectively.
At the time of the study cut-off, 4 (16.6%) and 7 (29.1%) of the MSI-H methylated patients have developed primary and secondary resistance to pembrolizumab, respectively, compared to none of the Lynch-like patients. Importantly, oligoprogression (ie, progression of a single metastatic lesion) in MSI-H methylated patients appeared salvageable by surgical resection and continuation of pembrolizumab off-study to achieve either a complete response (PEM 03, 42 months) or a PR with durable PFS (PEM08, 41 months). These results are consistent with recent data reported in melanoma patients35 and strongly suggest that for selected MSI-H EC patients who progress on ICI there may be a role for local therapy to induce durable PFS in combination with pembrolizumab.
To gain further insight into the mechanism of primary and secondary resistance to pembrolizumab in the sporadic MSI-H cohort we took advantage of the surgically resected oligometastatic sites of two patients. Using WES, we investigated whether alterations in genes encoding HLA Class I antigen processing and presentation machinery components or interferon signaling play a role in MSI-H EC resistance to pembrolizumab. In the case with primary ICI resistance, we found acquired homozygous loss of B2M that caused lack of cell-surface HLA Class I expression in the lung metastatic tumor and in its matched freshly established primary cell line. In the case with secondary ICI resistance, we found acquired homozygous loss of JAK3. Loss of function in this Janus kinase receptor, similarly to inactivating mutations in JAK1 and JAK2, may cause a defective response to induction with type I and type II interferons.27 WES demonstrated a larger number of mutations in both cases of ICI MSI-H resistant tumors relative to pretreatment samples, yet the distribution of neoantigens for their corresponding HLA I alleles was similar, suggesting defective antigen processing pathways and cytokine responses may represent major mechanisms of primary and secondary resistance to pembrolizumab in MSI-H EC, similar to observations in lung cancer and melanoma patients treated with ICIs36
The pathophysiology underlying the response advantage for Lynch-like tumors is likely complex and multifactorial. A retrospective study of EC4 compared enrichment of the tumor microenvironment with immunologically active populations across Lynch-related MSI-H, sporadic MSI-H, and MS-stable EC.4 Lynch-related MSI-H EC exhibited increased CD8+ cytotoxic T lymphocytes in stroma. These tumors also harbored reduced numbers of CD68+ macrophages, in the stromal and tumor compartments compared with sporadic MSI-H endometrial cancer; CD68+ macrophages have been associated in some studies with poor prognostic features among a variety of cancers37,38. Our IHC results demonstrated only a trend in increased infiltration in CD3+ T lymphocytes in Lynch-like versus sporadic MSI-H patients (most likely due to the low number of Lynch-like cases), while a significantly higher infiltration in CD68+ macrophages was detected in Lynch-like patients. There was no significant difference in PD-L1+ cells between Lynch-related and sporadic MSI-H. Others have found conflicting data to suggest macrophage infiltration or expression of PD-L1 to be significantly higher in immune cells of hypermutated tumors compared to MSS tumors,30,39 though there was no distinction made between Lynch-like and sporadic subtypes. Similar to previous studies of EC and colorectal MSI-H cancers4,40 we found significant immune cell expression of PD-L1 and very little tumor PD-L1 expression.
WES identified several genomic alterations in genes associated with hypermutated phenotypes beyond the canonical Lynch-associated alterations (Supplementary Table 1). Approximately 30% of ECs exhibit a hypermutated phenotype.41 POLE mutations were identified in 10% of endometrial cancers by The Cancer Genome Atlas network and conferred a hypermutated phenotype with favorable prognosis.5 In an analysis of 78,452 adult and 2,885 childhood tumors, Campbell and colleagues found that most POLE mutated tumors are microsatellite-stable, and hypothesized that this stemmed from late loss of MMR proficiency.42 In our study restricted to EC, we captured pathogenic mutations in POLE and POLD1 in Lynch-like patients, all of whom experienced a PR or CR to pembrolizumab. Copy number variants (CNV) (deletions) were almost exclusive to the methylated group. The CNV deletions in the methylated group were associated with 2 instances of PD, 3 SD, and 1 PR. With larger studies, such data may contribute to development of more sophisticated algorithms to predict biologic behavior. PCR and IHC techniques are current standard for the identification of MSI-H/dMMR patients but they may yield false positive or negative rates up to 15%.43,44 WES may offer a superior approach to identify candidates for ICI treatment since it may allow enhanced detection of MSI-H patients through recognition of robust and pathognomonic signatures of dMMR,13 as well as comprehensive information regarding SNV, CNV and TMB. In our study, we found higher TMB in the MSI-H Lynch-like versus the methylated group of EC patients. Importantly, WES genetic signature analysis identified a false positive MSI-H patient erroneously defined as MSI-H by conventional analyses. Larger studies are required to confirm whether a mutational threshold exists or if the MSI-H/dMMR mutational signatures used in this study may serve as a new standard for the identification of MSI-H patients most likely to respond to ICI treatment.
The significance of MSI heterogeneity in other disease sites such as colorectal cancer has been investigated.45 In this study, we have shown that the mechanism underlying MSI in EC may predict response to pembrolizumab, possibly through distinct genetic characteristics as well as alterations in the immune microenvironment. The refinement of MSI-H/dMMR prognostic groups may allow the identification of certain patients that may not respond (primary resistance) or rapidly experience progression after an initial response (secondary resistance) to single-agent pembrolizumab and could potentially benefit from novel combination strategies.46 Clinical studies evaluating separately Lynch/Lynch-like versus sporadic MSI-H patients treated with ICIs may be warranted. Pembrolizumab remains a highly effective therapy for Lynch-like EC with high TMB.
Supplementary Material
Supplementary Figure 1. Differences in tumor mutational burden (Mut/Mb) among Lynch-like and methylated cohorts.
Supplementary Figure 2. Correlation of tumor mutational burden determined by whole-exome sequencing compared to commercially available testing (FoundationOne®).
Supplementary Figure 3. Characteristics of treatment responses in a representative MSI-H methylated patient (PEM03) demonstrating primary resistance to pembrolizumab (ie, mixed tumor response). Please note the continued growth at both the Time 1 and Time 2 CT scan times points of the metastatic EC lung lesion versus the progressive decrease in the diameter of the abdominal metastatic tumor deposits (ie, carcinomatosis).
Supplementary Figure 4. Immunohistochemistry (IHC) results for CD3 and CD68 expression in representative Lynch-like (PEM25) and methylated (PEM22) MSI-H patients. Left Upper and Lower Panels: summary expression results for CD3 and CD68 biomarkers in all 24 evaluable patients by IHC. Middle Upper Panel: marked CD3 expression in a Lynch-like case. Right Upper Panel: mild CD3 expression in a methylated case. Middle Lower panel: marked CD68 in Lynch-like; Right Lower Panel: mild CD68 expression in methylated case. Main images at 100x, inserts at 400x original magnification.
Supplementary Figure 5. Mutational signature hierarchical clustering of Lynch-Like and methylated MSI-H patients. Please note that with the single exception of PEM24 (a tumor initially reported as MSI-H by PCR) all remaining 24 MSI-H EC samples were concordant between IHC/PCR and WES genetic signatures. Of interest, 3 out of 6 of the Lynch-like samples (PEM23, PEM02, PEM14) generated a sub-cluster due to a slightly different mutational signature.
Supplementary Figure 6. Representative TMB (ie, total number of SNV) and Antigenicity (number of shared epitopes) results in two matched MSI-H tumors with primary (PEM03) and secondary (PEM08) resistance to pembrolizumab. Upper panel: Venn diagrams comparing SNV (left upper and middle panels) and Antigenicity (right upper and middle panels) in matched PEM03 and PEM08 tumors. Briefly, antigenicity evaluation was performed using a Python pipeline to identify 9/10/11-mers encompassing somatic mutations and frameshift alterations from NGS. Peptides were considered eligible for HLA presentation, using netMHC software, after patient genotyping analysis using the OptiType algorithm. Figure depicts total number of SNV and epitopes unique to primary/recurrent tumors or shared between the matched tumors. An affinity of 500 nM was used as a threshold for peptide selection. Lower panel: flow cytometry analysis of HLA Class I expression in PEM03 and PEM08 primary cell lines. Briefly, flow cytometry was performed using fluorochrome-conjugated MAbs directed against the HLA Class I antigens (W6/32) and analyzed on a FACScan (Becton Dickinson) on primary cell lines established from fresh tumor tissue specimens. Please note the lack of HLA Class I expression in PEM03 cells harboring a B2M inactivating mutation while HLA Class I expression is preserved in PEM08 cells harboring a JAK3 truncating mutation.
Supplementary Table 1. Molecular characterization of tumor: PD-L1 combined positive score, conventional microsatellite classification, and genomic analyses by whole-exome sequencing. For POLE and POLD mutants, pathogenicity predictions are based on COSMIC database predictions (FATHMM), in which scores above 0.5 are presumed to be deleterious).
Supplementary Appendix 1. Full protocol.
FUNDING DISCLOSURES:
This work was supported in part by grants from NIH U01 CA176067-01, the Tina Brozman Foundation, the Guido Berlucchi Foundation and Gilead Sciences Inc., Foster City, CA to Alessandro Santin. This investigation was also supported by NIH Research Grant CA-16359 from NCI and Stand-up-to-cancer (SU2C) convergence grant 2.0 to Alessandro Santin. We would like to thank Merck-US for its industry support.
Footnotes
AUTHOR DISCLOSURES: The authors have declared no conflicts of interest.
REFERENCES
- 1.Siegel RL, Miller KD, Fuchs HE, Jemal A. Cancer Statistics, 2021. CA Cancer J Clin. 2021;71(1):7–33. doi: 10.3322/caac.21654 [DOI] [PubMed] [Google Scholar]
- 2.Hause RJ, Pritchard CC, Shendure J, Salipante SJ. Classification and characterization of microsatellite instability across 18 cancer types. Nat Med. 2016;22(11):1342–1350. doi: 10.1038/nm.4191 [DOI] [PubMed] [Google Scholar]
- 3.Le DT, Durham JN, Smith KN, et al. Mismatch repair deficiency predicts response of solid tumors to PD-1 blockade. Science. 2017;357(6349):409–413. doi: 10.1126/science.aan6733 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pakish JB, Zhang Q, Chen Z, et al. Immune Microenvironment in Microsatellite-Instable Endometrial Cancers: Hereditary or Sporadic Origin Matters. Clin Cancer Res Off J Am Assoc Cancer Res. 2017;23(15):4473–4481. doi: 10.1158/1078-0432.CCR-16-2655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Levine DA. Integrated genomic characterization of endometrial carcinoma. Nature. 2013;497(7447):67–73. doi: 10.1038/nature12113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Black D, Soslow RA, Levine DA, et al. Clinicopathologic Significance of Defective DNA Mismatch Repair in Endometrial Carcinoma. J Clin Oncol. Published online September 21, 2016. doi: 10.1200/JCO.2005.04.1574 [DOI] [PubMed] [Google Scholar]
- 7.Lemery S, Keegan P, Pazdur R. First FDA Approval Agnostic of Cancer Site - When a Biomarker Defines the Indication. N Engl J Med. 2017;377(15):1409–1412. doi: 10.1056/NEJMp1709968 [DOI] [PubMed] [Google Scholar]
- 8.Bellone S, Roque DM, Siegel ER, et al. A phase II evaluation of pembrolizumab in recurrent microsatellite instability-high (MSI-H) endometrial cancer patients with Lynch-like versus MLH-1 methylated characteristics (NCT02899793). Ann Oncol Off J Eur Soc Med Oncol. 2021;32(8):1045–1046. doi: 10.1016/j.annonc.2021.04.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Pembrolizumab in Ultramutated and Hypermutated Endometrial Cancer (NCT02899793). Accessed January 2, 2021. https://clinicaltrials.gov/ct2/show/NCT02899793
- 10.Zhao S, Bellone S, Lopez S, et al. Mutational landscape of uterine and ovarian carcinosarcomas implicates histone genes in epithelial-mesenchymal transition. Proc Natl Acad Sci U S A. 2016;113(43):12238–12243. doi: 10.1073/pnas.1614120113 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Santin AD, Deng W, Frumovitz M, et al. Phase II evaluation of nivolumab in the treatment of persistent or recurrent cervical cancer (NCT02257528/NRG-GY002). Gynecol Oncol. 2020;157(1):161–166. doi: 10.1016/j.ygyno.2019.12.034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer Oxf Engl 1990. 2009;45(2):228–247. doi: 10.1016/j.ejca.2008.10.026 [DOI] [PubMed] [Google Scholar]
- 13.Oken MM, Creech RH, Tormey DC, et al. Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol. 1982;5(6):649–655. [PubMed] [Google Scholar]
- 14.Common Terminology Criteria for Adverse Events (CTCAE) - CTCAE_4.03_2010–06-14_QuickReference_5×7.pdf. Accessed October 3, 2016. https://evs.nci.nih.gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_QuickReference_5×7.pdf
- 15.Chen TT, Ng TH. Optimal flexible designs in phase II clinical trials. Stat Med. 1998;17(20):2301–2312. doi: [DOI] [PubMed] [Google Scholar]
- 16.Frampton GM, Fichtenholtz A, Otto GA, et al. Development and validation of a clinical cancer genomic profiling test based on massively parallel DNA sequencing. Nat Biotechnol. 2013;31(11):1023–1031. doi: 10.1038/nbt.2696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Zhao S, Choi M, Overton JD, et al. Landscape of somatic single-nucleotide and copy-number mutations in uterine serous carcinoma. Proc Natl Acad Sci U S A. 2013;110(8):2916–2921. doi: 10.1073/pnas.1222577110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Alexandrov LB, Kim J, Haradhvala NJ, et al. The repertoire of mutational signatures in human cancer. Nature. 2020;578(7793):94–101. doi: 10.1038/s41586-020-1943-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bellone S, Buza N, Choi J, et al. Exceptional Response to Pembrolizumab in a Metastatic, Chemotherapy/Radiation-Resistant Ovarian Cancer Patient Harboring a PD-L1-Genetic Rearrangement. Clin Cancer Res Off J Am Assoc Cancer Res. 2018;24(14):3282–3291. doi: 10.1158/1078-0432.CCR-17-1805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tate JG, Bamford S, Jubb HC, et al. COSMIC: the Catalogue Of Somatic Mutations In Cancer. Nucleic Acids Res. 2019;47(D1):D941–D947. doi: 10.1093/nar/gky1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Shihab HA, Rogers MF, Gough J, et al. An integrative approach to predicting the functional effects of non-coding and coding sequence variation. Bioinformatics. 2015;31(10):1536–1543. doi: 10.1093/bioinformatics/btv009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Shihab HA, Gough J, Cooper DN, et al. Predicting the functional, molecular, and phenotypic consequences of amino acid substitutions using hidden Markov models. Hum Mutat. 2013;34(1):57–65. doi: 10.1002/humu.22225 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yokoyama T, Takehara K, Sugimoto N, et al. Lynch syndrome-associated endometrial carcinoma with MLH1 germline mutation and MLH1 promoter hypermethylation: a case report and literature review. BMC Cancer. 2018;18(1):576. doi: 10.1186/s12885-018-4489-0 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rahner N, Friedrichs N, Steinke V, et al. Coexisting somatic promoter hypermethylation and pathogenic MLH1 germline mutation in Lynch syndrome. J Pathol. 2008;214(1):10–16. doi: 10.1002/path.2263 [DOI] [PubMed] [Google Scholar]
- 25.Hagen CE, Lefferts J, Hornick JL, Srivastava A. “Null pattern” of immunoreactivity in a Lynch syndrome-associated colon cancer due to germline MSH2 mutation and somatic MLH1 hypermethylation. Am J Surg Pathol. 2011;35(12):1902–1905. doi: 10.1097/PAS.0b013e318237c6ab [DOI] [PubMed] [Google Scholar]
- 26.Zaretsky JM, Garcia-Diaz A, Shin DS, et al. Mutations Associated with Acquired Resistance to PD-1 Blockade in Melanoma. N Engl J Med. 2016;375(9):819–829. doi: 10.1056/NEJMoa1604958 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Shin DS, Zaretsky JM, Escuin-Ordinas H, et al. Primary Resistance to PD-1 Blockade Mediated by JAK1/2 Mutations. Cancer Discov. 2017;7(2):188–201. doi: 10.1158/2159-8290.CD-16-1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Pembrolizumab prescribing information. Accessed December 20, 2020. https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/125514s014lbl.pdf
- 29.Subbiah V, Solit DB, Chan TA, Kurzrock R. The FDA approval of pembrolizumab for adult and pediatric patients with tumor mutational burden (TMB) ≥10: a decision centered on empowering patients and their physicians. Ann Oncol Off J Eur Soc Med Oncol. 2020;31(9):1115–1118. doi: 10.1016/j.annonc.2020.07.002 [DOI] [PubMed] [Google Scholar]
- 30.Howitt BE, Shukla SA, Sholl LM, et al. Association of Polymerase e-Mutated and Microsatellite-Instable Endometrial Cancers With Neoantigen Load, Number of Tumor-Infiltrating Lymphocytes, and Expression of PD-1 and PD-L1. JAMA Oncol. 2015;1(9):1319–1323. doi: 10.1001/jamaoncol.2015.2151 [DOI] [PubMed] [Google Scholar]
- 31.Sahin IH. Immune checkpoint inhibitor response in mismatch repair-deficient colorectal cancer and other solid tumors: is it truly disease-agnostic? Colorectal Cancer. 2020;9(4):CRC29. doi: 10.2217/crc-2020-0020 [DOI] [Google Scholar]
- 32.Marabelle A, Le DT, Ascierto PA, et al. Efficacy of Pembrolizumab in Patients With Noncolorectal High Microsatellite Instability/Mismatch Repair-Deficient Cancer: Results From the Phase II KEYNOTE-158 Study. J Clin Oncol Off J Am Soc Clin Oncol. 2020;38(1):1–10. doi: 10.1200/JCO.19.02105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Domingo E, Niessen RC, Oliveira C, et al. BRAF-V600E is not involved in the colorectal tumorigenesis of HNPCC in patients with functional MLH1 and MSH2 genes. Oncogene. 2005;24(24):3995–3998. doi: 10.1038/sj.onc.1208569 [DOI] [PubMed] [Google Scholar]
- 34.Overman MJ, McDermott R, Leach JL, et al. Nivolumab in patients with metastatic DNA mismatch repair deficient/microsatellite instability–high colorectal cancer (CheckMate 142): results of an open-label, multicentre, phase 2 study. Lancet Oncol. 2017;18(9):1182–1191. doi: 10.1016/S1470-2045(17)30422-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Klemen ND, Wang M, Feingold PL, et al. Patterns of failure after immunotherapy with checkpoint inhibitors predict durable progression-free survival after local therapy for metastatic melanoma. J Immunother Cancer. 2019;7(1):196. doi: 10.1186/s40425-019-0672-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Gettinger S, Choi J, Hastings K, et al. Impaired HLA Class I Antigen Processing and Presentation as a Mechanism of Acquired Resistance to Immune Checkpoint Inhibitors in Lung Cancer. Cancer Discov. 2017;7(12):1420–1435. doi: 10.1158/2159-8290.CD-17-0593 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Zhao X, Qu J, Sun Y, et al. Prognostic significance of tumor-associated macrophages in breast cancer: a meta-analysis of the literature. Oncotarget. 2017;8(18):30576–30586. doi: 10.18632/oncotarget.15736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seminerio I, Kindt N, Descamps G, et al. High infiltration of CD68+ macrophages is associated with poor prognoses of head and neck squamous cell carcinoma patients and is influenced by human papillomavirus. Oncotarget. 2018;9(13):11046–11059. doi: 10.18632/oncotarget.24306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bauer K, Michel S, Reuschenbach M, Nelius N, von Knebel Doeberitz M, Kloor M. Dendritic cell and macrophage infiltration in microsatellite-unstable and microsatellite-stable colorectal cancer. Fam Cancer. 2011;10(3):557–565. doi: 10.1007/s10689-011-9449-7 [DOI] [PubMed] [Google Scholar]
- 40.Llosa NJ, Cruise M, Tam A, et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5(1):43–51. doi: 10.1158/2159-8290.CD-14-0863 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kunitomi H, Banno K, Yanokura M, et al. New use of microsatellite instability analysis in endometrial cancer. Oncol Lett. 2017;14(3):3297–3301. doi: 10.3892/ol.2017.6640 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Campbell BB, Light N, Fabrizio D, et al. Comprehensive Analysis of Hypermutation in Human Cancer. Cell. 2017;171(5):1042–1056.e10. doi: 10.1016/j.cell.2017.09.048 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wu Y, Berends MJ, Mensink RG, et al. Association of hereditary nonpolyposis colorectal cancer-related tumors displaying low microsatellite instability with MSH6 germline mutations. Am J Hum Genet. 1999;65(5):1291–1298. doi: 10.1086/302612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Schweizer P, Moisio AL, Kuismanen SA, et al. Lack of MSH2 and MSH6 characterizes endometrial but not colon carcinomas in hereditary nonpolyposis colorectal cancer. Cancer Res. 2001;61(7):2813–2815. [PubMed] [Google Scholar]
- 45.Kim JH, Rhee Y-Y, Bae J-M, et al. Subsets of microsatellite-unstable colorectal cancers exhibit discordance between the CpG island methylator phenotype and MLH1 methylation status. Mod Pathol. 2013;26(7):1013–1022. doi: 10.1038/modpathol.2012.241 [DOI] [PubMed] [Google Scholar]
- 46.Ishizuka JJ, Manguso RT, Cheruiyot CK, et al. Loss of ADAR1 in tumours overcomes resistance to immune checkpoint blockade. Nature. 2019;565(7737):43–48. doi: 10.1038/s41586-018-0768-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Figure 1. Differences in tumor mutational burden (Mut/Mb) among Lynch-like and methylated cohorts.
Supplementary Figure 2. Correlation of tumor mutational burden determined by whole-exome sequencing compared to commercially available testing (FoundationOne®).
Supplementary Figure 3. Characteristics of treatment responses in a representative MSI-H methylated patient (PEM03) demonstrating primary resistance to pembrolizumab (ie, mixed tumor response). Please note the continued growth at both the Time 1 and Time 2 CT scan times points of the metastatic EC lung lesion versus the progressive decrease in the diameter of the abdominal metastatic tumor deposits (ie, carcinomatosis).
Supplementary Figure 4. Immunohistochemistry (IHC) results for CD3 and CD68 expression in representative Lynch-like (PEM25) and methylated (PEM22) MSI-H patients. Left Upper and Lower Panels: summary expression results for CD3 and CD68 biomarkers in all 24 evaluable patients by IHC. Middle Upper Panel: marked CD3 expression in a Lynch-like case. Right Upper Panel: mild CD3 expression in a methylated case. Middle Lower panel: marked CD68 in Lynch-like; Right Lower Panel: mild CD68 expression in methylated case. Main images at 100x, inserts at 400x original magnification.
Supplementary Figure 5. Mutational signature hierarchical clustering of Lynch-Like and methylated MSI-H patients. Please note that with the single exception of PEM24 (a tumor initially reported as MSI-H by PCR) all remaining 24 MSI-H EC samples were concordant between IHC/PCR and WES genetic signatures. Of interest, 3 out of 6 of the Lynch-like samples (PEM23, PEM02, PEM14) generated a sub-cluster due to a slightly different mutational signature.
Supplementary Figure 6. Representative TMB (ie, total number of SNV) and Antigenicity (number of shared epitopes) results in two matched MSI-H tumors with primary (PEM03) and secondary (PEM08) resistance to pembrolizumab. Upper panel: Venn diagrams comparing SNV (left upper and middle panels) and Antigenicity (right upper and middle panels) in matched PEM03 and PEM08 tumors. Briefly, antigenicity evaluation was performed using a Python pipeline to identify 9/10/11-mers encompassing somatic mutations and frameshift alterations from NGS. Peptides were considered eligible for HLA presentation, using netMHC software, after patient genotyping analysis using the OptiType algorithm. Figure depicts total number of SNV and epitopes unique to primary/recurrent tumors or shared between the matched tumors. An affinity of 500 nM was used as a threshold for peptide selection. Lower panel: flow cytometry analysis of HLA Class I expression in PEM03 and PEM08 primary cell lines. Briefly, flow cytometry was performed using fluorochrome-conjugated MAbs directed against the HLA Class I antigens (W6/32) and analyzed on a FACScan (Becton Dickinson) on primary cell lines established from fresh tumor tissue specimens. Please note the lack of HLA Class I expression in PEM03 cells harboring a B2M inactivating mutation while HLA Class I expression is preserved in PEM08 cells harboring a JAK3 truncating mutation.
Supplementary Table 1. Molecular characterization of tumor: PD-L1 combined positive score, conventional microsatellite classification, and genomic analyses by whole-exome sequencing. For POLE and POLD mutants, pathogenicity predictions are based on COSMIC database predictions (FATHMM), in which scores above 0.5 are presumed to be deleterious).
Supplementary Appendix 1. Full protocol.