Abstract
Elesclomol is an anticancer drug that targets mitochondrial metabolism. In the past, elesclomol was recognized as an inducer of oxidative stress, but now it has also been found to suppress cancer by inducing cuproptosis. Elesclomol’s anticancer activity is determined by the dependence of cancer on mitochondrial metabolism. The mitochondrial metabolism of cancer stem cells, cancer cells resistant to platinum drugs, proteasome inhibitors, molecularly targeted drugs, and cancer cells with inhibited glycolysis was significantly enhanced. Elesclomol exhibited tremendous toxicity to all three kinds of cells. Elesclomol's toxicity to cells is highly dependent on its transport of extracellular copper ions, a process involved in cuproptosis. The discovery of cuproptosis has perfected the specific cancer suppressor mechanism of elesclomol. For some time, elesclomol failed to yield favorable results in oncology clinical trials, but its safety in clinical application was confirmed. Research progress on the relationship between elesclomol, mitochondrial metabolism and cuproptosis provides a possibility to explore the reapplication of elesclomol in the clinic. New clinical trials should selectively target cancer types with high mitochondrial metabolism and attempt to combine elesclomol with platinum, proteasome inhibitors, molecularly targeted drugs, or glycolysis inhibitors. Herein, the particular anticancer mechanism of elesclomol and its relationship with mitochondrial metabolism and cuproptosis will be presented, which may shed light on the better application of elesclomol in clinical tumor treatment.
Keywords: Elesclomol, Cuproptosis, Mitochondrial metabolism, Cancer, Anticancer drugs, Cancer stem cells, Drug safety, Clinical trials
Background
In the past, aerobic glycolysis was thought to be the primary energy source for cancer cells because many cancers showed enhanced glycolysis even when oxygen was abundant [1]. This phenomenon is known as the Warburg effect [1]. However, increasing evidence challenges this view since mitochondrial metabolism has also been found to be a crucial energy source for some cancer cells [2]. The spontaneous enhancement of mitochondrial metabolism has been found in various cancers, including melanoma, breast cancer and leukemia [2]. Additionally, the survival of drug-resistant cancer cells and cancer stem cells is also heavily reliant on energy provided by mitochondrial respiration [2, 3]. In fact, mitochondrial metabolism plays a critical role in tumorigenesis, proliferation, metastasis, and drug resistance [4]. Therefore, targeting mitochondrial metabolism is an effective strategy for cancer suppression.
Inhibitors of oxidative phosphorylation (OXPHOS) have been shown to be effective cancer suppressors that rely on mitochondrial metabolism [3]. However, OXPHOS inhibitors may also inhibit immune cells with the same metabolic profile as cancer cells [2]. Considering the critical role of immune mechanisms in the body's anticancer efforts, the blind use of mitochondrial respiration-inhibiting drugs may be counterproductive [5, 6]. Moreover, some classical mitochondrial toxic drugs lead to unacceptable side effects, which are also problematic in targeting mitochondrial metabolism for cancer therapy [3]. We noticed a small molecule compound called elesclomol, which is an anticancer drug that induces oxidative stress in cancer cells, and that enhanced mitochondrial metabolism makes cancer cells sensitive to elesclomol [7]. Given that several clinical trials have validated the safety of elesclomol and the cytotoxicity of elesclomol selectively targets cancer cells [6], it is expected to be a safe and effective anticancer agent. It is worth noting that eleslcomol did not induce significant reduction in basal or adenosine triphosphate (ATP)-linked respiration by targeting the electron transport chain (ETC) directly, but rather significantly reduced the spare capacity of respiration by inhibiting components of the tricarboxylic acid (TCA) cycle [7].
Elesclomol is a chemotherapeutic adjuvant developed by Synta Pharmaceuticals and originally developed for treating metastatic melanoma [8]. In subsequent clinical trials, a sodium salt formulation of elesclomol was developed for use in combination with paclitaxel or alone for treating multiple solid tumors and acute myeloid leukemia [5, 9–11] (Table 1). Published data show a favorable safety profile for elesclomol alone or in combination with paclitaxel, but patients are less sensitive to elesclomol. Except for a small phase II trial in patients with stage IV melanoma, which found that elesclomol was effective in prolonging progression-free survival (PFS), elesclomol did not produce a desired clinical response [5, 9–11]. While a subsequent phase III trial demonstrated that elesclomol combined with paclitaxel did not prolong PFS, this study found that patients with low lactate dehydrogenase (LDH) levels may be more sensitive to elesclomol [12]. The elevated serum LDH level in tumor is usually related to hypoxia, which forces tumor cells to supply energy through enhanced glycolysis [13]. This also strongly hints at the relationship between cancer sensitivity to elesclomol and cellular metabolism. In the last two years, there has been renewed interest in elesclomol [14], whose specific transport function of copper ions to cellular mitochondria suggests its potential therapeutic for rare diseases of copper deficiency, such as the Menkes disease [15]. Here, we propose that the prospect of elesclomol in cancer metabolic therapy deserves special attention.
Table 1.
Elesclomol-related clinical trials
| Clinical Trial Subjects | Staging of Clinical Trials | Recruitment Status | Drug use | Results | References |
|---|---|---|---|---|---|
| Acute myeloid leukemia | Phase I | Unknown | Elesclomol sodium | Elesclomol has a good clinical safety profile, but patients have not developed clinical responses to elesclomol | 26732437 |
| Solid tumors | Phase I | Completed | Elesclomol sodium, Paclitaxel | The combination of Elesclomol and paclitaxel was well tolerated by patients and the toxicity profile of elesclomol was similar to that of single agent paclitaxel | 17255281 |
| Melanoma | Phase I/II | Completed | Elesclomol sodium, Paclitaxel | The combination of elesclomol and paclitaxel resulted in a statistically significant doubling of median PFS, with an acceptable toxicity profile and encouraging OS | 19826135 |
| Melanoma | Phase III | Terminated | Elesclomol sodium, Paclitaxel | The combination of elesclomol and paclitaxel did not significantly improve PFS | 23401447 |
| Recurrent or persistent ovarian epithelial cancer, fallopian tube cancer, primary peritoneal cancer | Phase II | Completed | Elesclomol sodium, Paclitaxel | The combination of elesclomol and paclitaxel was well tolerated by patients but the proportion responding was low | 30309721 |
| Solid tumors | Phase I | Suspended | Elesclomol sodium | Unpublished | - |
| Prostate cancer | Phase I | Completed | Elesclomol sodium, Docetaxel | Unpublished | - |
| Stage IIIB/IV non-small cell lung cancer | Phase I/II | Completed | Elesclomol sodiuml, Paclitaxel, Carboplatin | Unpublished | - |
| Soft tissue sarcoma | Phase II | Completed | Elesclomol sodium, taxane | Elesclomol enhanced taxane efficacy by induction of Hsp70 | 16784029 |
Elesclomol-related clinical trials were searched using the ClinicalTrials.gov platform. Nearly a dozen clinical trials related to elesclomol treatment have been conducted with the estimated enrollment of a thousand patients with different types of cancers, including melanoma, ovarian cancer, and acute myeloid leukemia. Unfortunately, according to published results, the clinical anticancer efficacy of elesclomol is suboptimal, but patients have good tolerance to elesclomol
PFS Progression-free survival, OS Overall survival
The anticancer mechanism of elesclomol has long been interpreted as promoting the accumulation of intracellular reactive oxygen species and ultimately inducing oxidative stress, which involves multiple molecular targets that range from mitochondria to nuclear DNA [6, 16–24]. Meanwhile, the anticancer effect of elesclomol has been found to rely on its ability to transport extracellular copper ions [6, 7] as well as the mitochondrial metabolic intensity of cancer cells [17, 20, 24–29], but a specific mechanism to explain this phenomenon is lacking. Recently, cuproptosis has been proposed as a novel form of cell death, and soon after, elesclomol was found to be a cuproptosis inducer [7, 28]. Excitingly, the occurrence of cuproptosis is also related to mitochondrial metabolism, and the proposed concept of cuproptosis emerged as a distinct mechanism to explain the cancer suppressive effect of elesclomol [7]. The association between elesclomol and cuproptosis also enables people to re-explore the clinical anticancer potential of elesclomol. This review will emphasize the anticancer mechanism of elesclomol and the clinical application value of elesclomol via its targeting of mitochondrial metabolism.
Mitochondrial metabolism affects the sensitivity of cancer cells to elesclomol
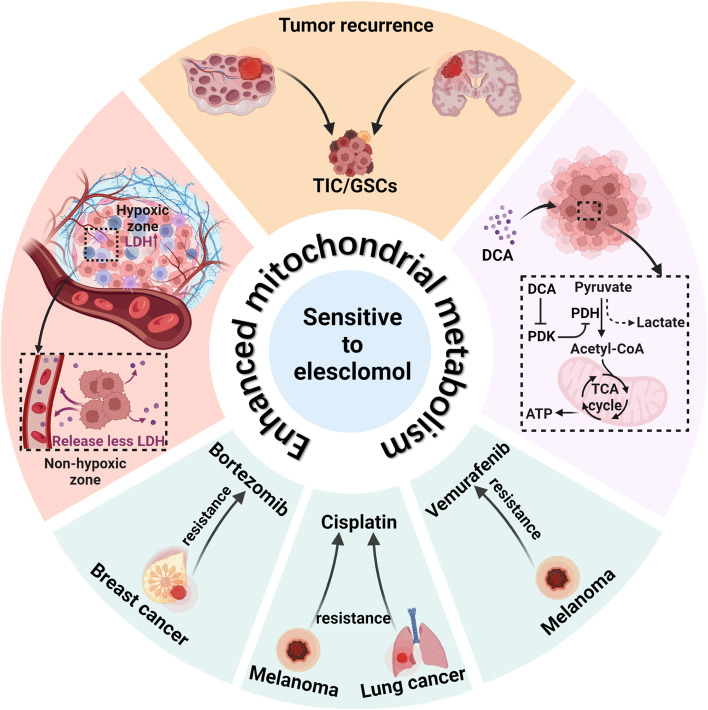
The anticancer activity of elesclomol is related to the mitochondrial metabolic state of cancer cells. The higher the reliance on mitochondrial metabolism is, the higher the sensitivity of cancer cells to elesclomol (Fig. 1).
Fig. 1.
Enhanced mitochondrial metabolism sensitizes cancer cells to elesclomol. Cancer cells highly dependent on mitochondrial metabolism are sensitive to elesclomol, including cancer stem cells, drug-resistant cancer cells, and glycolysis-inhibiting cancer cells. Both TICs in ovarian cancer and GSCs in glioblastoma are associated with cancer recurrence and are highly dependent on mitochondrial metabolism. Drug-resistant cancer cells, including bortezomib-resistant breast cancer cells, cisplatin-resistant melanocytes and lung cancer cells, and vemurafenib-resistant melanocytes, increase their dependence on mitochondrial metabolism in the development of drug resistance. PDK inhibitor DCA enhances mitochondrial metabolism in melanoma cells by shifting their metabolism from glycolysis to oxidative phosphorylation. In addition, hypoxia in solid tumors reduces the intensity of mitochondrial metabolism, and the degree of tumor hypoxia is positively correlated with the serum LDH levels of the patients. Patients with low serum LDH levels are sensitive to elesclomol. ATP: adenosine triphosphate; DCA: dichloroacetate; GSCs: glioblastoma stem cells; LDH: lactate dehydrogenase; PDH: pyruvate dehydrogenase; PDK: pyruvate dehydrogenase kinases; TICs: tumor-initiating cells
Cancer stem cells are sensitive to elesclomol
The stem-like tumor-initiating cells (TICs) in ovarian cancer (OC) are associated with the recurrence of ovarian cancer after chemotherapy, which ultimately leads to an abysmal prognosis in OC patients [30]. Using high-throughput drug screening, researchers tested the sensitivity of OC cells cultured under TIC-enriched conditions [31] to 1,978 compounds, of which elesclomol proved to be one of the potent anticancer compounds [29]. Under TIC-enriched conditions, elesclomol treatment reduced the sphere formation [29] of OC cells, a phenotype favorable to TICs [32]. OC cells with a high expression of CD133 and high aldehyde dehydrogenase (ALDH), which are markers of TICs [33], were also reduced [29]. Notably, disulfiram (DSF), another copper ionophore, was also screened as an effective drug [29]. At the same time, OC TICs tended to supply energy by increasing mitochondrial respiration, which may explain their sensitivity to copper ionophores, elesclomol, or DSF.
In addition, some studies have reported that glioblastoma stem cells (GSCs) are also heavily reliant on mitochondrial respiration for energy supply [34]. A drug screen for GSCs revealed that elesclomol at submicromolar concentrations was extremely potent in killing GSCs [24]. Elesclomol treatment resulted in an increase in the mitochondrial membrane potential of GSCs accompanied by an increase in reactive oxygen species (ROS) [24], which are mainly concentrated in mitochondria rather than the cytoplasm, suggesting that mitochondria are potential targets of elesclomol [24].
Drug-resistant cancer cells are sensitive to elesclomol
Platinum-resistant cancer cells
Platinum is currently the first-line drug for clinical cancer treatment, but platinum resistance in cancer cells has greatly limited its clinical application [35]. Drug resistance inevitably occurs in the advanced treatment of melanoma patients, especially when traditional platinum is used [36]. A series of studies has shown that the activation of mitochondrial metabolism is closely related to the development of cisplatin resistance in melanoma [37]. In recent years, it has also been reported that the development of cisplatin resistance in lung cancer cells is associated with metabolic reprogramming, which involves the transformation of glycolysis metabolism to mitochondrial metabolism [26, 38].
Proteomic analysis has identified a group of slow-cycling cells [39, 40] in melanoma that rely on mitochondrial respiration and whose replenishment of the tumor underlies the development of drug resistance and tumor maintenance [27]. This group of cells is characterized by the high expression of Jumonji AT-rich interactive domain 1B (JARID1B) [27]. When melanoma cells were treated with elesclomol for 24 h, the specific subpopulation with high JARID1B expression almost disappeared [27]. In addition, the combination of elesclomol with cisplatin also reversed the enrichment of high JARID1B cells caused by cisplatin monotherapy [27]. Compared to the control group, cisplatin treatment resulted in an abrupt increase in the high JARID1B expression subpopulation from 5 to 30%, while the combination of elesclomol dose-dependently reduced the percentage of this subpopulation [27]. This was also confirmed by Western blotting results, where the expression of JARID1B was downregulated in adherent cells and upregulated in dead cells in the supernatant after elesclomol treatment, indicating that elesclomol is somewhat selective for cells with a high expression of JARID1B. The dependence of this group of cells on oxidative ATP production may be the basis of their sensitivity to elesclomol [41].
The downregulation of thioredoxin-1 (TRX-1) expression and activation of mitochondrial respiration in cisplatin-resistant lung cancer cells have been found, the latter including increased cellular oxygen consumption and enhancement of mRNA of mitochondrial respiration-related enzymes such as argininosuccinate synthetase and fumarase [26]. In addition, cisplatin-resistant cells were more sensitive to elesclomol than parental cells were [26]. When TRX-1 expression was upregulated in cisplatin-resistant cells, the mitochondrial respiration intensity appeared to decrease, accompanied by the remission of cisplatin resistance [26]. However, more importantly, the sensitivity of cisplatin-resistant cells to elesclomol disappeared with the decrease in mitochondrial respiration intensity [26]. Thus, the sensitivity of cisplatin-resistant lung cancer cells to elesclomol is closely related to mitochondrial metabolism.
Elesclomol therapy increased ROS in cisplatin-resistant lung cancer cells [17, 26], a phenomenon partially reversed by the antioxidant N-acetylcysteine (NAC). The elesclomol treatment-induced intracellular ROS levels may be related to the decompensation of the intracellular antioxidant system. Oxidative damage caused by ROS ultimately leads to cell death [17].
Proteasome inhibitor-resistant cancer cells
On the one hand, massive protein synthesis is the basis for the exuberant proliferation of cancer cells [42]. On the other hand, a protein quality control system is crucial for cancer cells to relieve intracellular proteotoxic stress [42]. Proteasome inhibitors (PIs) are reasonable cancer therapeutic drugs that exhibit potent cancer suppression under in vitro culture conditions [42]. Unfortunately, tumors in vivo show strong adaptability to PI, which significantly limits the clinical application of PI [43].
Functional genomics analysis demonstrated that the generation of PI resistance is related to mitochondrial metabolism [28]. Mitochondrial OXPHOS was significantly enriched in PI-resistant cells [28]. Replacing glucose with galactose in culture medium resulted in an enhanced reliance on mitochondrial metabolism in cells [44]. The investigators screened 549 cancer cell lines for PI resistance in a mitochondrial metabolism-reliant state using PRISM, a barcoding method [28]. Despite the reduced viability of some cells due to galactose, the survival rate of cells in the mitochondrial metabolism-reliant state after PI treatment was significantly higher than that of the control group [28]. Subsequently, elesclomol emerged as one of the only three effective drugs in the screening of anticancer drugs for PI-resistant cells [28]. PI-resistant cells showed sensitivity to elesclomol, and elesclomol treatment in turn enhanced the sensitivity of PI-resistant cells to PI [28]. The results of subsequent animal experiments also validated the therapeutic effect of elesclomol on PI-resistant cancer cells [28]. Here, elesclomol was found to induce copper-dependent death, and the copper chelator tetrathiomolybdate (TTM), rather than the apoptosis inhibitor or ferroptosis inhibitor, reversed the cell death induced by elesclomol [28].
Molecularly targeted agent-resistant cancer cells
Vemurafenib is an anticancer agent targeting the BRAFV600E mutation in melanoma cells [45]. Its emergence has changed the clinical treatment paradigm for melanoma but is threatened by the rapid development of drug resistance in clinical practice [45].
Vemurafenib was found to significantly induce mitochondrial metabolism in BRAFV600E mutant melanoma cells, presenting as elevated basal and higher maximum respiratory capacity [20]. The investigators exposed melanocytes to different concentrations of vemurafenib for 2–3 months to obtain vemurafenib-resistant subcellular lines, and it was found that the vemurafenib-resistant cells also showed an elevation of mitochondrial metabolism, accompanied by the structural and functional complexity of mitochondria, which included an increase in the number of cristae in the mitochondria and an expansion of the cristae space [20]. Subsequently, it was found that vemurafenib-resistant cells exhibited a high degree of sensitivity to elesclomol compared to parental cells, consistent with its sensitivity to KCN [20], a mitochondrial complex IV inhibitor. These experiments demonstrated that vemurafenib-resistant cells are more sensitive to agents that target the key regulators of mitochondrial metabolism.
Cancer cells with lower glycolytic activity are sensitive to elesclomol
Hypoxia is a characteristic of solid tumors. Tumors face the challenge of hypoxia in the process of either onset or metastasis [46]. Metabolic plasticity enables cancer cells to increase their reliance on anaerobic glycolytic metabolism in response to hypoxia [47]. Among many regulatory molecules, hypoxia-inducible factor-1α (HIF-1α) and its downstream protein pyruvate dehydrogenase kinase-3 (PDK3) are important factors in the metabolic plasticity induced by hypoxia [47].
Nucleus accumbens-1 is an upstream regulator of HIF-1α, and its induction of PDK3 enhances the glycolysis reliance of the ovarian cancer SKOV3 cell line and cervical cancer HeLa cell line [48]. After the silencing of intracellular nucleus accumbens-1 expression by exogenous plasmids, mitochondrial respiration was activated in both SKOV3 and HeLa cancer cells, which was more pronounced in hypoxia [48]. Meanwhile, nucleus accumbens-1 silencing enhanced the anticancer activity of elesclomol in vitro and in vivo [48].
Dichloroacetate (DCA) is a small molecule that targets PDK to shift cellular metabolism from glycolysis to mitochondrial metabolism [49]. Treatment with DCA is predicted to increase the reliance of cancer cells on mitochondrial metabolism. The combination of DCA with elesclomol has a synergistic inhibitory effect on melanoma cells cultured in vitro [25]. The combination also significantly retarded tumor growth in a preclinical model of human melanoma HBL cell line tumor grafts in mice [25].
Cancer patients with low serum LDH levels are sensitive to elesclomol
LDH participates in the anaerobic glycolysis of cells, and its transcription is regulated by HIF-1α [50]. In cancer patients, elevated serum LDH levels are associated with poor prognosis [13] because serum LDH levels arise partly from cancer cells under hypoxia [13]. Hypoxia induces increased glycolytic metabolism in cancer cells, ultimately leading to cancer progression [51].
A phase III clinical trial of metastatic melanoma demonstrated that the serum LDH levels of patients correlate with sensitivity to elesclomol [12]. The purpose of this trial was to evaluate whether the addition of elesclomol to paclitaxel therapy prolongs PFS in patients with advanced melanoma [12]. The results showed that the addition of elesclomol was ineffective in patients with high serum LDH levels, but those patients with low serum LDH levels had a 1.6-month increase in median PFS [12]. The investigators speculate that this is because of the characteristics of high mitochondrial metabolism in patients with low serum LDH levels, which make them more sensitive to elesclomol [12]. Thus, serum LDH levels are expected to serve as a marker for whether patients are sensitive to elesclomol [12]. However, another study on acute myeloid leukemia suggested that hematologic tumors differed from solid tumors in that elevated LDH was not strongly associated with hypoxia [10]. In clinical trials of elesclomol therapy, serum LDH levels should not be applied for screening patients with hematologic tumors [10].
The anticancer mechanism of elesclomol
There is no definite conclusion on the cancer-suppressive mechanism of elesclomol. The mainstream view is that elesclomol is an oxidative stress inducer. Excessive ROS in cells may induce cell apoptosis [16, 22]. However, some studies have also found that cellular damage induced by elesclomol also involves DNA damage and cell cycle arrest [52]. In addition, it was reported that elesclomol induces ferroptosis [53]. Recently, elesclomol has also been found to be a cuproptosis inducer [7]. Cuproptosis has just been discovered as a new form of cell death [7]. We believe that the induction of cuproptosis may be a novel and reasonable explanation for the anticancer mechanism of elesclomol (Fig. 2).
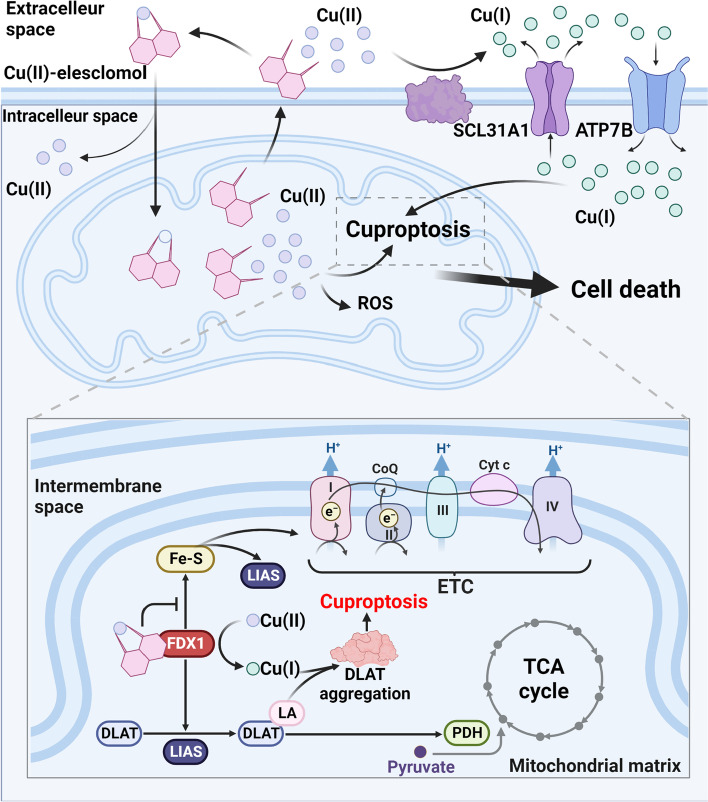
Fig. 2.
Schematic diagram of the mechanism of elesclomol-induced cell death. Elesclomol shuttles inside and outside the cell to selectively transport extracellular Cu(II) to mitochondria, where Cu(II) accumulated in mitochondria induces ROS production and triggers cuproptosis. FDX1, a critical enzyme in the occurrence of pyroptosis, reduces Cu(II) to Cu(I) in mitochondria while promoting the lipoylation of DLAT, an enzyme participating in the formation of the PDH complex and affecting the mitochondrial TCA cycle. The reduced Cu(I) binds to lipoylated DLAT to promote its oligomerization, ultimately leading to the occurrence of cuproptosis. FDX1 also promotes Fe-S synthesis, while Cu(I) inhibits this process. Fe-S is an essential component of LIAS and ETC, the key enzymes in DLAT lipoylation, but its relationship with cuproptosis is unclear. In addition, the regulation of intracellular Cu(I) levels by membrane copper ionophores, such as SCL31A1 and ATP7B, is also associated with the occurrence of cuproptosis. DLAT: dihydrolipoamide S-acetyltransferase; ETC: electron transfer chain; FDX1: ferredoxin 1; Fe-S: iron-sulfur proteins; LA: lipoamide; LIAS: lipoyl synthase; PDH: pyruvate dehydrogenase; ROS: reactive oxygen species; TCA: tricarboxylic acid cycle
The anticancer effect of elesclomol is heavily reliant on copper ions
Although the anticancer mechanism of elesclomol is still controversial, there is no doubt that the anticancer effect of elesclomol depends on the existence of copper ions in the external cellular environment [6, 7].
Extracellularly, elesclomol can form a stable 1:1 complex with Cu(II) [54]. Afterward, elesclomol shuttles back and forth between the inside and outside of the cell to transport copper ions into the cell [6]. Notably, unlike other copper ionophores, such as DSF, cellular copper levels are selectively enriched in mitochondria after elesclomol treatment. Elesclomol induces a much higher rise in intracellular copper ions than DSF at the same concentration [6]. Meanwhile, the use of elesclomol has been reported to degrade copper-transporting ATPase 1 (ATP7A) in colon cancer cells, a protein that mediates intracellular copper export [55]. The degradation of ATP7A by elesclomol further leads to the enrichment of copper ions in the mitochondria of cancer cells [53].
The toxicity of elesclomol originates from the enrichment of copper ions in mitochondria, not from the complex of elesclomol with Cu(II) or elesclomol itself [6]. As early as 2012, researchers found that the killing effect of elesclomol on MDA-MB435 melanoma cells was completely lost when cells were cultured without serum, which was the only source of copper in the culture medium [6]. The addition of copper to a serum-free medium rescued the anticancer effect of elesclomol, whereas iron, manganese, and zinc did not contribute to the rescue of elesclomol’s effect [6]. A recent report on cuproptosis has obtained consistent experimental results in monocytes and lung cancer NCIH2030 cells [7]. In addition, some researchers paired elesclomol with redox inert metals Ni(II) and Pt(II) [56]. They found that compared to Cu(II)-elesclomol, the killing effect of Ni(II)-elesclomol and Pt(II)-elesclomol on K562 leukemia cells decreased 34 times and 1040 times, respectively [56]. Therefore, the toxicity of elesclomol to cancer cells is exerted mainly through copper ions.
The old view—induction of oxidative stress for cancer suppression
Elesclomol was found to enhance oxidative stress and induce ROS production in various cells, including melanoma cells [6, 16, 18–20, 23], lung cancer cells [17, 22], GSCs [24], and 14 types of gynecological tumor cells [21]. Among them, melanoma cells have spontaneous enhancement of mitochondrial metabolism [18]; lung cancer cells and melanoma cells enhance mitochondrial metabolism in the process of drug resistance [17, 20]; and GSCs heavily reliant on mitochondrial metabolism [24]. ROS is an inevitable side-product of redox reactions. The aerobic characteristics of mitochondrial metabolism make it a major source of intracellular ROS [57]. The enhanced mitochondrial metabolism in these cells undoubtedly leads to increased ROS production, which is further strengthened by using elesclomol, ultimately leading to decompensation of the intracellular antioxidant system. The induction of intracellular ROS by elesclomol may account for the sensitivity of these cells to elesclomol.
In addition, elesclomol has a certain effect on the intracellular antioxidant system. Elesclomol has been reported to downregulate TRX in cisplatin-resistant lung cancer cells [17]. TRX is a key molecule in maintaining the intracellular reduction reaction [58]. Moreover, elesclomol also downregulates glutathione (GSH) in cisplatin-resistant lung cancer cells [17]. GSH is an essential member of the intracellular antioxidant system that facilitates the clearance of intracellular ROS [59]. Elesclomol not only induces the production of intracellular ROS but also impedes the clearance of ROS.
The induction of ROS by elesclomol seems uncontroversial, but is ROS production necessary for elesclomol to exert cytotoxicity? Several studies have attempted to reverse the induction of ROS by elesclomol using the ROS scavenger N-acetylcysteine (NAC); only in some cells did the use of NAC reverse ROS production [16, 60]. The effect of NAC on the toxic effects of elesclomol is controversial. On the one hand, studies have shown that the use of 0.1 mM and 10 mM NAC can reverse the cytotoxic effects of elesclomol on multiple small cell lung cancer (SCLC) cell lines, including SCLC 1, SCLC SR2, SCLC B, and SCLC BC [17] and non-small cell lung cancer cells A549 [22]. On the other hand, in GSCs, 5 mM NAC did not help to reverse the cytotoxicity of elesclomol, while 10 mM NAC had only a partial reversal effect [24]. Another study also showed that 5 mM NAC only slightly alleviated the loss of activity of three cell lines, NCIH2030, A549, and HCC4009, in response to elesclomol [7]. Thus, the anticancer effect of elesclomol is partly related to its induction of ROS, but there should be a more critical mechanism to explain the cancer cytotoxicity of elesclomol.
A new view—induction of cuproptosis for cancer suppression
The latest view is that elesclomol can also induce a specific copper-dependent cell death, namely, cuproptosis [7]. None of the currently known inhibitors of other forms of cell death can reverse elesclomol-induced cell death, including the apoptosis inhibitors Z-VAD-FMK and Boc-D-FMK [7, 24], the ferroptosis inhibitor ferrostatin-1 [7, 24], the necrotrophic apoptosis inhibitor necrostatin-1 [7, 24] and the autophagy inhibitor 3-methyladenine [24].
Elesclomol-induced cuproptosis is associated with mitochondrial metabolism [7]. Cells that primarily relied on mitochondrial metabolism for energy production were 1000 times more sensitive to elesclomol than cells that primarily relied on anaerobic glycolysis [7]. At the same time, metabolomic analysis revealed that ABC1, an elesclomol-sensitive human lung cancer adenocarcinoma cell, exhibited dysregulation of TCA-related metabolites after elesclomol treatment, which included the upregulation of glutamine, α-ketoglutarate (α-KG), succinate, citric acid, cis-aconitate, and sedoheptulose 7-phosphate [7]. In contrast, there was no change in TCA metabolites in elesclomol-resistant lung cancer cells A549 [7], suggesting that the occurrence of elesclomol-induced cuproptosis is associated with the TCA cycle. In addition, the mitochondrial stress test showed that elesclomol treatment had no effect on basal respiration but significantly inhibited maximal respiration in ABC1 cells [7]. Additionally, the use of elesclomol did not affect the production of ATP [7], which is consistent with the finding that the mitochondrial OXPHOS uncoupler carbonyl cyanide-p-trifluoromethoxyphenylhydrazone (FCCP) did not affect the cytotoxicity of elesclomol [7]. Therefore, the authors speculate that elesclomol does not directly inhibit the electron transport chain.
Elesclomol-induced cuproptosis mainly depends on the oligomerization of lipoylated dihydrolipoamide S-acetyltransferase (DLAT) [7]. The occurrence of cuproptosis is dependent on FDX1. Elesclomol specifically transfers extracellular Cu(II) to mitochondria, after which FDX1 reduces Cu(II) to Cu(I), a more toxic form of copper ion [7]. Additionally, FDX1 is an upstream regulator of mitochondrial protein lipoylation [7]. Protein lipoylation is a highly conserved posttranslational modification process of lysine [61]. As an essential component subunit of the pyruvate dehydrogenase (PDH) complex, lipoylation of DLAT is required for the PDH complex to regulate pyruvate entry into the TCA cycle [61]. The enrichment of Cu(I) in mitochondria caused by elesclomol and FDX1 ultimately leads to oligomerization of DLAT in mitochondria, which depends on the direct binding of Cu(I) to lipoylated DLAT [7].
Elesclomol treatment also results in the loss of iron-sulfur (Fe-S) cluster proteins in mitochondria [7, 28]. Fe-S cluster protein synthesis requires FDX1 as a reducing agent [28], and the specific binding of Cu(II)-elesclomol to FDX1 inhibits the synthesis process [28]. Fe-S cluster proteins are involved in the synthesis of ETC in mitochondria; whereas, elesclomol-induced cuproptosis and ETC do not seem to be directly related [7]. The role of Fe-S cluster proteins in elesclomol-induced cuproptosis remains to be further investigated.
Clinical application prospects of elesclomol targeting mitochondrial metabolism in cancer therapy
Elesclomol treatment for cancers with high mitochondrial metabolism
In some types of cancer, the enhancement of mitochondrial metabolism is spontaneous, which may be driven by genetic mutations. Meanwhile, enhanced mitochondrial metabolism is only shown in some subtypes in other types of cancer [3].
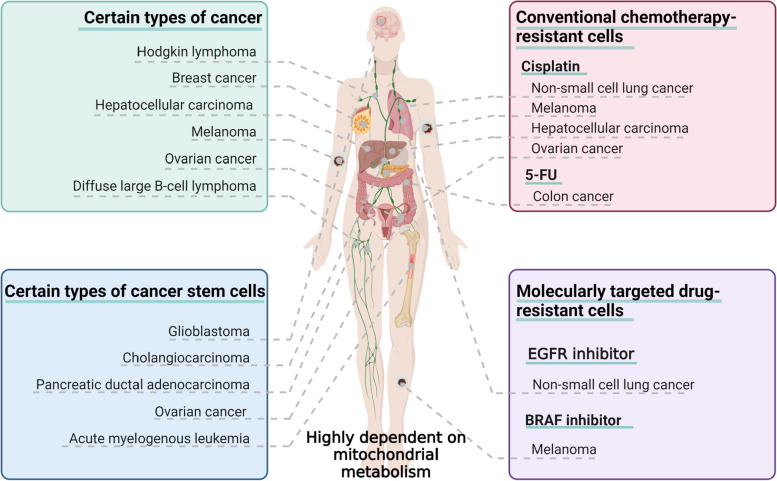
Melanoma [18, 62], breast cancer [63], Hodgkin's lymphoma [64], and hepatocellular carcinoma cells [65] show a marked increase in mitochondrial metabolism, whereas high-grade serous ovarian cancer [66] and diffuse large B-cell lymphoma [67] show metabolic heterogeneity; that is, enhanced mitochondrial metabolism is only observed in some subsets. In addition, cancer stem cells from solid tumors, such as pancreatic ductal adenocarcinoma [68], glioblastoma [34], ovarian cancer [69] and cholangiocarcinoma [70], as well as stem cells of acute myeloid leukemia [71], also show a heavy reliance on mitochondrial metabolism (Fig. 3).
Fig. 3.
Cancers highly dependent on mitochondrial metabolism. Cancers such as melanoma, breast cancer, and ovarian cancer show spontaneous enhancement of mitochondrial metabolism. Cancer stem cells of glioblastoma, ovarian cancer, cholangiocarcinoma, and other cancers highly depend on mitochondrial metabolism. Increased dependence on mitochondrial metabolism is seen in drug-resistant cancer cells generated in some anticancer treatments, including cisplatin-resistant melanoma and hepatocellular carcinoma from conventional chemotherapies and 5-FU-resistant colon cancer, BRAF inhibitor-resistant melanoma from molecularly targeted drugs, and EGFR inhibitor-resistant non-small cell lung cancer. 5FU: 5-Fluorouracil; BRAF: v-Raf murine sarcoma viral oncogene homolog B1; EGFR: epidermal growth factor receptor
Based on the specific relationship between elesclomol and cancer metabolism, selecting cancers with high mitochondrial metabolism for clinical trials seems more likely to yield desirable results. Hypoxia leads to tumor progression and induces glycolytic metabolism, which reduces the sensitivity to elesclomol. With this understanding, hypoxic biological markers can be used to screen for patients sensitive to elesclomol. For example, LDH is a biomarker of tumor hypoxia [13], and patients with low LDH levels are more sensitive to elesclomol [12]. Genomic studies help identify new tumor hypoxia biomarkers [72] and hypoxia-related genes [73]. It is important to screen specific populations for elesclomol treatment with the guidance of genomics and to carry out precise therapy.
Cancer stem cells play a crucial role in cancer development, drug resistance, and recurrence. They offer self-renewal properties to cancer and facilitate long-term cancer survival [74, 75]. Due to the stronger reliance of cancer stem cells on mitochondrial metabolism, elesclomol therapy may reduce the ability of cancer cells to adapt to the harsh environment, delay recurrence, and improve the survival of patients.
Elesclomol treatment for drug-resistant cancer cells
Studies have found that cancer cells resistant to anticancer drugs, including conventional chemotherapeutic drugs [76, 77] and molecularly targeted drugs [40, 78], are associated with metabolic reprogramming.
Elesclomol has been found to have a potent killing effect on cisplatin-resistant lung cancer cells [17, 26] and melanoma cells [27], vemurafenib-resistant melanoma cells [20], and PI inhibitor-resistant breast cancer cells [28], which is associated with upregulation of mitochondrial metabolism in these cells. In addition, mitochondrial metabolism was also enhanced in 5-fluorouracil-resistant colon cancer cells [76] and cisplatin-resistant hepatocellular carcinoma [79], ovarian cancer cells [77]. The epidermal growth factor receptor (EGFR) inhibitor erlotinib, a key drug in the clinical treatment of small cell lung cancer [80], was found to have an off-target effect in activating mitochondrial metabolic function in non-small cell lung cancer [78]. It is of great clinical significance to study the inhibitory effect of elesclomol on these drug-resistant cells that show changes in mitochondrial function.
Furthermore, using elesclomol as an adjuvant in chemotherapy in combination with other drugs seems to be a viable strategy. Treatment with chemotherapeutic drugs forces cancer cells to selectively alter their metabolic patterns, increasing their reliance on mitochondrial metabolism to adapt to the changes brought about by the drugs. However, cells with high mitochondrial metabolism are sensitive to elesclomol [16]. Under the dual stress of both chemotherapeutic drugs and elesclomol, cancer cells face opposite pressure, which may help prevent the development of drug resistance in cancer cells.
Combination of elesclomol with glycolysis inhibitors for cancer therapy
Glycolysis has been proposed as a target for cancer therapy for many years [81]. A number of small molecules with glycolysis inhibitory activity have shown satisfactory anticancer activity in vivo and in vitro. For instance, 2-Deoxy-d-glucose (2-DG) is a compound that blocks glycolysis by competitively inhibiting the rate-limiting enzyme hexokinase intracellularly and leading to inhibition of glycolysis [82]. 2-DG is routinely used as a radioactive tracer in clinical practice and has been found to be safe [82]. Based on its glycolytic inhibitory effect, the role of 2-DG in cancer therapy is gaining attention [82]. Recently, glycolysis inhibitors such as 6-aminonicotinamide [83] and 3-bromopyruvic [84] have gradually shown new therapeutic value in cancer treatment. Several clinical trials have also been carried out [81].
The combination of the above glycolytic inhibitors with elesclomol may be an effective solution. Although there are no reports on the combination of elesclomol and glycolysis inhibitors in the treatment of cancer, the related investigation predicts that the combination of these two will achieve good therapeutic effects. In preclinical models, promising results have been achieved with the combination of elesclomol and the PDK inhibitor DCA, a small molecule that shift cellular metabolism from glycolysis to mitochondrial metabolism [25]. Cancer metabolic plasticity enables cells to increase their reliance on mitochondrial metabolism in the face of glycolytic inhibition, which facilitates the function of elesclomol.
Safety of elesclomol in cancer therapy
The safety of drugs is also an issue worthy of further attention. Some studies have reported that elesclomol's cytotoxicity seems to be selective for cancer cells [6]. Human peripheral blood mononuclear cells (PMBCs) were unaffected at concentrations that had a significant killing effect on cancer cells, and elesclomol could not induce copper ion enrichment in PMBCs [6]. However, some studies have reported that elesclomol has certain effects on mitochondrial function in normal mammalian cells, and the treatment of CV-1 cells with more than 40 μM elesclomol increases the production of ROS in mitochondria while significantly reducing the mitochondrial membrane potential [85].
According to existing reports, nearly one thousand patients have received high doses of elesclomol in clinical trials. Good tolerance of elesclomol by patients is a common feature of these trials. For example, the maximum tolerated dose of elesclomol was up to 438 mg/m2 in patients with solid tumors in a phase I trial [9]. In past clinical trials, no patients have been reported to develop elesclomol-related organic or functional impairment [5, 9–11]. Therefore, elesclomol treatment has a high safety profile.
Conclusion
Elesclomol targets mitochondrial metabolism, and its induction of cuproptosis is a crucial discovery in cancer research. Cuproptosis, a novel form of cell death, has made an essential supplement to the specific anticancer mechanism of elesclomol. In turn, further understanding the mechanism of its cancer suppression will help in understanding the exact process of cuproptosis.
Cancer cells that heavily rely on mitochondrial metabolism are extremely sensitive to elesclomol [7]. Existing studies have reported significant inhibitory effects of elesclomol on a variety of cells, including cancer stem cells [24], drug-resistant cells [27], and cells with lower glycolytic activity [25], due to enhanced mitochondrial metabolism. In addition, many clinical trials have been conducted to ensure the safety of elesclomol in clinical application. Serum LDH levels may be a potential biomarker for assessing patients’ sensitivity to elesclomol [12].
Based on the characteristics of cancer cells in which those with high mitochondrial metabolism are much more sensitive to elesclomol, further research is suggested to identify cancer types or subtypes sensitive to elesclomol, to validate the efficacy of elesclomol combined with chemotherapeutic drugs or glycolysis inhibitors in preclinical models and to seek the clinical application value of such combinations. In clinical trials, screening populations susceptible to elesclomol based on serum LDH levels or other biological markers of hypoxia would be more conducive to obtaining desirable trial results.
Acknowledgements
Figures were created through BioRender.com and Fig. 1 was modified using Adobe Photoshop after exporting from BioRender.com. We thank Jinze Shen for his help in the creation of the figures.
Abbreviations
- ATP
Adenosine triphosphate
- ALDH
Aldehyde dehydrogenase
- FCCP
Carbonyl cyanide-p-trifluoromethoxyphenylhydrazone
- DCA
Dichloroacetate
- DLAT
Dihydrolipoamide S-acetyltransferase
- DSF
Disulfiram
- ETC
Electron transport chain
- EGFR
Epidermal growth factor receptor
- GSCs
Glioblastoma stem cells
- GSH
Glutathione
- HIF-1α
Hypoxia-inducible factor-1α
- Fe-S
Iron-sulfur proteins
- JARID1B
Jumonji AT-rich interactive domain 1B
- LDH
Lactate dehydrogenase
- NAC
N-Acetyl-L-cysteine
- OC
Ovarian cancer
- OXPHOS
Oxidative phosphorylation
- PMBCs
Peripheral blood mononuclear cells
- PFS
Progression-free survival
- PI
Proteasome inhibitorprotease inhibitor
- PDH
Pyruvate dehydrogenase
- PDK3
Pyruvate dehydrogenase kinase-3
- ROS
Reactive oxygen species
- SCLC
Small cell lung cancer
- TTM
Tetrathiomolybdate
- TRX-1
Thioredoxin-1
- TCA
Tricarboxylic acid cycle
- TICs
Tumor-initiating cells
- α-KG
α-Ketoglutarate
- 2-DG
2-Deoxy-d-glucose
Authors’ contributions
PZ and YD contributed to the conception, design, and final approval of the submitted version. Literature was collected and analyzed by PZ and YD. PZ, LL and YD contributed to the manuscript writing. PZ, CZ, BL and YD contributed to the graphic design, and all authors conceived and approved the final manuscript.
Funding
This research was funded by the Zhejiang Provincial Public Welfare Technology Research Program/Experimental Animal Project (LGD19H090008).
Availability of data and materials
Not applicable.
Declarations
Ethics approval and consent to participate
Not applicable.
Consent for publication
The Authors confirm: (1) that the work described has not been published before; (2) that it is not under consideration for publication elsewhere; (3) that its publication has been approved by all coauthors, if any; (4) that its publication has been approved (tacitly or explicitly) by the responsible authorities at the institution where the work is carried out.
Competing interests
The authors declare that the study was conducted in the absence of any business or financial relationship that could be interpreted as a potential conflict of interest.
Footnotes
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Koppenol WH, Bounds PL, Dang CV. Otto Warburg's contributions to current concepts of cancer metabolism. Nat Rev Cancer. 2011;11(5):325–337. doi: 10.1038/nrc3038. [DOI] [PubMed] [Google Scholar]
- 2.Porporato PE, Filigheddu N, Pedro JMB, Kroemer G, Galluzzi L. Mitochondrial metabolism and cancer. Cell Res. 2018;28(3):265–280. doi: 10.1038/cr.2017.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ashton TM, McKenna WG, Kunz-Schughart LA, Higgins GS. Oxidative Phosphorylation as an Emerging Target in Cancer Therapy. Clin Cancer Res. 2018;24(11):2482–2490. doi: 10.1158/1078-0432.CCR-17-3070. [DOI] [PubMed] [Google Scholar]
- 4.Ghosh P, Vidal C, Dey S, Zhang L. Mitochondria Targeting as an Effective Strategy for Cancer Therapy. Int J Mol Sci. 2020;21(9):336. doi: 10.3390/ijms21093363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Monk BJ, Kauderer JT, Moxley KM, Bonebrake AJ, Dewdney SB, Secord AA, et al. A phase II evaluation of elesclomol sodium and weekly paclitaxel in the treatment of recurrent or persistent platinum-resistant ovarian, fallopian tube or primary peritoneal cancer: An NRG oncology/gynecologic oncology group study. Gynecol Oncol. 2018;151(3):422–427. doi: 10.1016/j.ygyno.2018.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Nagai M, Vo NH, Shin Ogawa L, Chimmanamada D, Inoue T, Chu J, et al. The oncology drug elesclomol selectively transports copper to the mitochondria to induce oxidative stress in cancer cells. Free Radical Biol Med. 2012;52(10):2142–2150. doi: 10.1016/j.freeradbiomed.2012.03.017. [DOI] [PubMed] [Google Scholar]
- 7.Tsvetkov P, Coy S, Petrova B, Dreishpoon M, Verma A, Abdusamad M, et al. Copper induces cell death by targeting lipoylated TCA cycle proteins. Science (New York, NY) 2022;375(6586):1254–1261. doi: 10.1126/science.abf0529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Chen S, Sun L, Koya K, Tatsuta N, Xia Z, Korbut T, et al. Syntheses and antitumor activities of N'1, N'3-dialkyl-N'1, N'3-di-(alkylcarbonothioyl) malonohydrazide: the discovery of elesclomol. Bioorg Med Chem Lett. 2013;23(18):5070–5076. doi: 10.1016/j.bmcl.2013.07.032. [DOI] [PubMed] [Google Scholar]
- 9.Berkenblit A, Eder JP, Jr, Ryan DP, Seiden MV, Tatsuta N, Sherman ML, et al. Phase I clinical trial of STA-4783 in combination with paclitaxel in patients with refractory solid tumors. Clin Cancer Res. 2007;13(2 Pt 1):584–590. doi: 10.1158/1078-0432.CCR-06-0964. [DOI] [PubMed] [Google Scholar]
- 10.Hedley D, Shamas-Din A, Chow S, Sanfelice D, Schuh AC, Brandwein JM, et al. A phase I study of elesclomol sodium in patients with acute myeloid leukemia. Leuk Lymphoma. 2016;57(10):2437–2440. doi: 10.3109/10428194.2016.1138293. [DOI] [PubMed] [Google Scholar]
- 11.O'Day S, Gonzalez R, Lawson D, Weber R, Hutchins L, Anderson C, et al. Phase II, randomized, controlled, double-blinded trial of weekly elesclomol plus paclitaxel versus paclitaxel alone for stage IV metastatic melanoma. J Clin Oncol. 2009;27(32):5452–5458. doi: 10.1200/JCO.2008.17.1579. [DOI] [PubMed] [Google Scholar]
- 12.O'Day SJ, Eggermont AM, Chiarion-Sileni V, Kefford R, Grob JJ, Mortier L, et al. Final results of phase III SYMMETRY study: randomized, double-blind trial of elesclomol plus paclitaxel versus paclitaxel alone as treatment for chemotherapy-naive patients with advanced melanoma. J Clin Oncol. 2013;31(9):1211–1218. doi: 10.1200/JCO.2012.44.5585. [DOI] [PubMed] [Google Scholar]
- 13.Koukourakis MI, Giatromanolaki A, Sivridis E, Bougioukas G, Didilis V, Gatter KC, et al. Lactate dehydrogenase-5 (LDH-5) overexpression in non-small-cell lung cancer tissues is linked to tumour hypoxia, angiogenic factor production and poor prognosis. Br J Cancer. 2003;89(5):877–885. doi: 10.1038/sj.bjc.6601205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Gohil VM. Repurposing elesclomol, an investigational drug for the treatment of copper metabolism disorders. Expert Opin Investig Drugs. 2021;30(1):1–4. doi: 10.1080/13543784.2021.1840550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Guthrie LM, Soma S, Yuan S, Silva A, Zulkifli M, Snavely TC, et al. Elesclomol alleviates Menkes pathology and mortality by escorting Cu to cuproenzymes in mice. Science (New York, NY) 2020;368(6491):620–625. doi: 10.1126/science.aaz8899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kirshner JR, He S, Balasubramanyam V, Kepros J, Yang CY, Zhang M, et al. Elesclomol induces cancer cell apoptosis through oxidative stress. Mol Cancer Ther. 2008;7(8):2319–2327. doi: 10.1158/1535-7163.MCT-08-0298. [DOI] [PubMed] [Google Scholar]
- 17.Wangpaichitr M, Wu C, You M, Maher JC, Dinh V, Feun LG, et al. N', N'-Dimethyl-N', N'-bis(phenylcarbonothioyl) Propanedihydrazide (Elesclomol) Selectively Kills Cisplatin Resistant Lung Cancer Cells through Reactive Oxygen Species (ROS) Cancers. 2009;1(1):23–38. doi: 10.3390/cancers1010023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barbi de Moura M, Vincent G, Fayewicz SL, Bateman NW, Hood BL, Sun M, et al. Mitochondrial respiration–an important therapeutic target in melanoma. PloS One. 2012;7(8):e40690. doi: 10.1371/journal.pone.0040690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blackman RK, Cheung-Ong K, Gebbia M, Proia DA, He S, Kepros J, et al. Mitochondrial electron transport is the cellular target of the oncology drug elesclomol. PLoS One. 2012;7(1):e29798. doi: 10.1371/journal.pone.0029798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Corazao-Rozas P, Guerreschi P, Jendoubi M, André F, Jonneaux A, Scalbert C, et al. Mitochondrial oxidative stress is the Achille's heel of melanoma cells resistant to Braf-mutant inhibitor. Oncotarget. 2013;4(11):1986–1998. doi: 10.18632/oncotarget.1420. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kwan SY, Cheng X, Tsang YT, Choi JS, Kwan SY, Izaguirre DI, et al. Loss of ARID1A expression leads to sensitivity to ROS-inducing agent elesclomol in gynecologic cancer cells. Oncotarget. 2016;7(35):56933–56943. doi: 10.18632/oncotarget.10921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee JH, Cho YS, Jung KH, Park JW, Lee KH. Genipin enhances the antitumor effect of elesclomol in A549 lung cancer cells by blocking uncoupling protein-2 and stimulating reactive oxygen species production. Oncol Lett. 2020;20(6):374. doi: 10.3892/ol.2020.12237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Wong HN, Lewies A, Haigh M, Viljoen JM, Wentzel JF, Haynes RK, et al. Anti-Melanoma Activities of Artemisone and Prenylated Amino-Artemisinins in Combination With Known Anticancer Drugs. Front Pharmacol. 2020;11:558894. doi: 10.3389/fphar.2020.558894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Buccarelli M, D'Alessandris QG, Matarrese P, Mollinari C, Signore M, Cappannini A, et al. Elesclomol-induced increase of mitochondrial reactive oxygen species impairs glioblastoma stem-like cell survival and tumor growth. J Exp Clin Cancer Res. 2021;40(1):228. doi: 10.1186/s13046-021-02031-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kluza J, Corazao-Rozas P, Touil Y, Jendoubi M, Maire C, Guerreschi P, et al. Inactivation of the HIF-1α/PDK3 signaling axis drives melanoma toward mitochondrial oxidative metabolism and potentiates the therapeutic activity of pro-oxidants. Can Res. 2012;72(19):5035–5047. doi: 10.1158/0008-5472.CAN-12-0979. [DOI] [PubMed] [Google Scholar]
- 26.Wangpaichitr M, Sullivan EJ, Theodoropoulos G, Wu C, You M, Feun LG, et al. The relationship of thioredoxin-1 and cisplatin resistance: its impact on ROS and oxidative metabolism in lung cancer cells. Mol Cancer Ther. 2012;11(3):604–615. doi: 10.1158/1535-7163.MCT-11-0599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cierlitza M, Chauvistré H, Bogeski I, Zhang X, Hauschild A, Herlyn M, et al. Mitochondrial oxidative stress as a novel therapeutic target to overcome intrinsic drug resistance in melanoma cell subpopulations. Exp Dermatol. 2015;24(2):155–157. doi: 10.1111/exd.12613. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Tsvetkov P, Detappe A, Cai K, Keys HR, Brune Z, Ying W, et al. Mitochondrial metabolism promotes adaptation to proteotoxic stress. Nat Chem Biol. 2019;15(7):681–689. doi: 10.1038/s41589-019-0291-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Harrington BS, Ozaki MK, Caminear MW, Hernandez LF, Jordan E, Kalinowski NJ, et al. Drugs Targeting Tumor-Initiating Cells Prolong Survival in a Post-Surgery, Post-Chemotherapy Ovarian Cancer Relapse Model. Cancers. 2020;12(6):1645. doi: 10.3390/cancers12061645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Meng E, Long B, Sullivan P, McClellan S, Finan MA, Reed E, et al. CD44+/CD24- ovarian cancer cells demonstrate cancer stem cell properties and correlate to survival. Clin Exp Metas. 2012;29(8):939–948. doi: 10.1007/s10585-012-9482-4. [DOI] [PubMed] [Google Scholar]
- 31.House CD, Hernandez L, Annunziata CM. In vitro enrichment of ovarian cancer tumor-initiating cells. J Vis Exp. 2015;(96):52446. [DOI] [PMC free article] [PubMed]
- 32.Kim B, Jung JW, Jung J, Han Y, Suh DH, Kim HS, et al. PGC1α induced by reactive oxygen species contributes to chemoresistance of ovarian cancer cells. Oncotarget. 2017;8(36):60299–60311. doi: 10.18632/oncotarget.19140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.House CD, Jordan E, Hernandez L, Ozaki M, James JM, Kim M, et al. NFκB Promotes Ovarian Tumorigenesis via Classical Pathways That Support Proliferative Cancer Cells and Alternative Pathways That Support ALDH(+) Cancer Stem-like Cells. Can Res. 2017;77(24):6927–6940. doi: 10.1158/0008-5472.CAN-17-0366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sighel D, Notarangelo M, Aibara S, Re A, Ricci G, Guida M, et al. Inhibition of mitochondrial translation suppresses glioblastoma stem cell growth. Cell Rep. 2021;35(4):109024. doi: 10.1016/j.celrep.2021.109024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Lv P, Man S, Xie L, Ma L, Gao W. Pathogenesis and therapeutic strategy in platinum resistance lung cancer. Biochim Biophys Acta. 2021;1876(1):188577. doi: 10.1016/j.bbcan.2021.188577. [DOI] [PubMed] [Google Scholar]
- 36.Rossi A, Roberto M, Panebianco M, Botticelli A, Mazzuca F, Marchetti P. Drug resistance of BRAF-mutant melanoma: Review of up-to-date mechanisms of action and promising targeted agents. Eur J Pharmacol. 2019;862:172621. doi: 10.1016/j.ejphar.2019.172621. [DOI] [PubMed] [Google Scholar]
- 37.Bristot IJ, Kehl Dias C, Chapola H, Parsons RB, Klamt F. Metabolic rewiring in melanoma drug-resistant cells. Crit Rev Oncol Hematol. 2020;153:102995. doi: 10.1016/j.critrevonc.2020.102995. [DOI] [PubMed] [Google Scholar]
- 38.Cruz-Bermúdez A, Laza-Briviesca R, Vicente-Blanco RJ, García-Grande A, Coronado MJ, Laine-Menéndez S, et al. Cisplatin resistance involves a metabolic reprogramming through ROS and PGC-1α in NSCLC which can be overcome by OXPHOS inhibition. Free Radical Biol Med. 2019;135:167–181. doi: 10.1016/j.freeradbiomed.2019.03.009. [DOI] [PubMed] [Google Scholar]
- 39.Roesch A, Fukunaga-Kalabis M, Schmidt EC, Zabierowski SE, Brafford PA, Vultur A, et al. A temporarily distinct subpopulation of slow-cycling melanoma cells is required for continuous tumor growth. Cell. 2010;141(4):583–594. doi: 10.1016/j.cell.2010.04.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roesch A, Vultur A, Bogeski I, Wang H, Zimmermann KM, Speicher D, et al. Overcoming intrinsic multidrug resistance in melanoma by blocking the mitochondrial respiratory chain of slow-cycling JARID1B(high) cells. Cancer Cell. 2013;23(6):811–825. doi: 10.1016/j.ccr.2013.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Livingstone E, Swann S, Lilla C, Schadendorf D, Roesch A. Combining BRAF(V) (600E) inhibition with modulators of the mitochondrial bioenergy metabolism to overcome drug resistance in metastatic melanoma. Exp Dermatol. 2015;24(9):709–710. doi: 10.1111/exd.12718. [DOI] [PubMed] [Google Scholar]
- 42.Adams J, Palombella VJ, Sausville EA, Johnson J, Destree A, Lazarus DD, et al. Proteasome inhibitors: a novel class of potent and effective antitumor agents. Can Res. 1999;59(11):2615–2622. [PubMed] [Google Scholar]
- 43.Orlowski RZ, Kuhn DJ. Proteasome inhibitors in cancer therapy: lessons from the first decade. Clin Cancer Res. 2008;14(6):1649–1657. doi: 10.1158/1078-0432.CCR-07-2218. [DOI] [PubMed] [Google Scholar]
- 44.Marroquin LD, Hynes J, Dykens JA, Jamieson JD, Will Y. Circumventing the Crabtree effect: replacing media glucose with galactose increases susceptibility of HepG2 cells to mitochondrial toxicants. Toxicol Sci. 2007;97(2):539–547. doi: 10.1093/toxsci/kfm052. [DOI] [PubMed] [Google Scholar]
- 45.Karoulia Z, Gavathiotis E, Poulikakos PI. New perspectives for targeting RAF kinase in human cancer. Nat Rev Cancer. 2017;17(11):676–691. doi: 10.1038/nrc.2017.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.McGuirk S, Audet-Delage Y, St-Pierre J. Metabolic Fitness and Plasticity in Cancer Progression. Trends in cancer. 2020;6(1):49–61. doi: 10.1016/j.trecan.2019.11.009. [DOI] [PubMed] [Google Scholar]
- 47.Xie H, Simon MC. Oxygen availability and metabolic reprogramming in cancer. J Biol Chem. 2017;292(41):16825–16832. doi: 10.1074/jbc.R117.799973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Ren YJ, Wang XH, Ji C, Guan YD, Lu XJ, Liu XR, et al. Silencing of NAC1 Expression Induces Cancer Cells Oxidative Stress in Hypoxia and Potentiates the Therapeutic Activity of Elesclomol. Front Pharmacol. 2017;8:804. doi: 10.3389/fphar.2017.00804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Tataranni T, Piccoli C. Dichloroacetate (DCA) and Cancer: An Overview towards Clinical Applications. Oxid Med Cell Longev. 2019;2019:8201079. doi: 10.1155/2019/8201079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Semenza GL. HIF-1: using two hands to flip the angiogenic switch. Cancer Metastasis Rev. 2000;19(1–2):59–65. doi: 10.1023/A:1026544214667. [DOI] [PubMed] [Google Scholar]
- 51.Wu Z, Zuo M, Zeng L, Cui K, Liu B, Yan C, et al. OMA1 reprograms metabolism under hypoxia to promote colorectal cancer development. EMBO Rep. 2021;22(1):e50827. doi: 10.15252/embr.202050827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hasinoff BB, Wu X, Yadav AA, Patel D, Zhang H, Wang DS, et al. Cellular mechanisms of the cytotoxicity of the anticancer drug elesclomol and its complex with Cu(II) Biochem Pharmacol. 2015;93(3):266–276. doi: 10.1016/j.bcp.2014.12.008. [DOI] [PubMed] [Google Scholar]
- 53.Gao W, Huang Z, Duan J, Nice EC, Lin J, Huang C. Elesclomol induces copper-dependent ferroptosis in colorectal cancer cells via degradation of ATP7A. Mol Oncol. 2021;15(12):3527–3544. doi: 10.1002/1878-0261.13079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wu L, Zhou L, Liu DQ, Vogt FG, Kord AS. LC-MS/MS and density functional theory study of copper(II) and nickel(II) chelating complexes of elesclomol (a novel anticancer agent) J Pharm Biomed Anal. 2011;54(2):331–336. doi: 10.1016/j.jpba.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 55.Fukai T, Ushio-Fukai M, Kaplan JH. Copper transporters and copper chaperones: roles in cardiovascular physiology and disease. Am J Physiol Cell Physiol. 2018;315(2):C186–c201. doi: 10.1152/ajpcell.00132.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Yadav AA, Patel D, Wu X, Hasinoff BB. Molecular mechanisms of the biological activity of the anticancer drug elesclomol and its complexes with Cu(II), Ni(II) and Pt(II) J Inorg Biochem. 2013;126:1–6. doi: 10.1016/j.jinorgbio.2013.04.013. [DOI] [PubMed] [Google Scholar]
- 57.Foo BJ, Eu JQ, Hirpara JL, Pervaiz S. Interplay between Mitochondrial Metabolism and Cellular Redox State Dictates Cancer Cell Survival. Oxid Med Cell Longev. 2021;2021:1341604. doi: 10.1155/2021/1341604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Bian M, Fan R, Zhao S, Liu W. Targeting the Thioredoxin System as a Strategy for Cancer Therapy. J Med Chem. 2019;62(16):7309–7321. doi: 10.1021/acs.jmedchem.8b01595. [DOI] [PubMed] [Google Scholar]
- 59.Niu B, Liao K, Zhou Y, Wen T, Quan G, Pan X, et al. Application of glutathione depletion in cancer therapy: Enhanced ROS-based therapy, ferroptosis, and chemotherapy. Biomaterials. 2021;277:121110. doi: 10.1016/j.biomaterials.2021.121110. [DOI] [PubMed] [Google Scholar]
- 60.Rushworth GF, Megson IL. Existing and potential therapeutic uses for N-acetylcysteine: the need for conversion to intracellular glutathione for antioxidant benefits. Pharmacol Ther. 2014;141(2):150–159. doi: 10.1016/j.pharmthera.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 61.Rowland EA, Snowden CK, Cristea IM. Protein lipoylation: an evolutionarily conserved metabolic regulator of health and disease. Curr Opin Chem Biol. 2018;42:76–85. doi: 10.1016/j.cbpa.2017.11.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Harel M, Ortenberg R, Varanasi SK, Mangalhara KC, Mardamshina M, Markovits E, et al. Proteomics of Melanoma Response to Immunotherapy Reveals Mitochondrial Dependence. Cell. 2019;179(1):236–50.e18. doi: 10.1016/j.cell.2019.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jones RA, Robinson TJ, Liu JC, Shrestha M, Voisin V, Ju Y, et al. RB1 deficiency in triple-negative breast cancer induces mitochondrial protein translation. J Clin Investig. 2016;126(10):3739–3757. doi: 10.1172/JCI81568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Birkenmeier K, Dröse S, Wittig I, Winkelmann R, Käfer V, Döring C, et al. Hodgkin and Reed-Sternberg cells of classical Hodgkin lymphoma are highly dependent on oxidative phosphorylation. Int J Cancer. 2016;138(9):2231–2246. doi: 10.1002/ijc.29934. [DOI] [PubMed] [Google Scholar]
- 65.Zhang Z, Li TE, Chen M, Xu D, Zhu Y, Hu BY, et al. MFN1-dependent alteration of mitochondrial dynamics drives hepatocellular carcinoma metastasis by glucose metabolic reprogramming. Br J Cancer. 2020;122(2):209–220. doi: 10.1038/s41416-019-0658-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Gentric G, Kieffer Y, Mieulet V, Goundiam O, Bonneau C, Nemati F, et al. PML-Regulated Mitochondrial Metabolism Enhances Chemosensitivity in Human Ovarian Cancers. Cell Metab. 2019;29(1):156–73.e10. doi: 10.1016/j.cmet.2018.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Caro P, Kishan AU, Norberg E, Stanley IA, Chapuy B, Ficarro SB, et al. Metabolic signatures uncover distinct targets in molecular subsets of diffuse large B cell lymphoma. Cancer Cell. 2012;22(4):547–560. doi: 10.1016/j.ccr.2012.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Viale A, Pettazzoni P, Lyssiotis CA, Ying H, Sánchez N, Marchesini M, et al. Oncogene ablation-resistant pancreatic cancer cells depend on mitochondrial function. Nature. 2014;514(7524):628–632. doi: 10.1038/nature13611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Pastò A, Bellio C, Pilotto G, Ciminale V, Silic-Benussi M, Guzzo G, et al. Cancer stem cells from epithelial ovarian cancer patients privilege oxidative phosphorylation, and resist glucose deprivation. Oncotarget. 2014;5(12):4305–4319. doi: 10.18632/oncotarget.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Raggi C, Taddei ML, Sacco E, Navari N, Correnti M, Piombanti B, et al. Mitochondrial oxidative metabolism contributes to a cancer stem cell phenotype in cholangiocarcinoma. J Hepatol. 2021;74(6):1373–1385. doi: 10.1016/j.jhep.2020.12.031. [DOI] [PubMed] [Google Scholar]
- 71.Lagadinou ED, Sach A, Callahan K, Rossi RM, Neering SJ, Minhajuddin M, et al. BCL-2 inhibition targets oxidative phosphorylation and selectively eradicates quiescent human leukemia stem cells. Cell Stem Cell. 2013;12(3):329–341. doi: 10.1016/j.stem.2012.12.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Gui CP, Wei JH, Chen YH, Fu LM, Tang YM, Cao JZ, et al. A new thinking: extended application of genomic selection to screen multiomics data for development of novel hypoxia-immune biomarkers and target therapy of clear cell renal cell carcinoma. Brief Bioinform. 2021;22(6):bbab173. doi: 10.1093/bib/bbab173. [DOI] [PubMed] [Google Scholar]
- 73.Zhang B, Tang B, Gao J, Li J, Kong L, Qin L. A hypoxia-related signature for clinically predicting diagnosis, prognosis and immune microenvironment of hepatocellular carcinoma patients. J Transl Med. 2020;18(1):342. doi: 10.1186/s12967-020-02492-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Bajaj J, Diaz E, Reya T. Stem cells in cancer initiation and progression. J Cell Biol. 2020;219(1):e201911053. doi: 10.1083/jcb.201911053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Clara JA, Monge C, Yang Y, Takebe N. Targeting signalling pathways and the immune microenvironment of cancer stem cells - a clinical update. Nat Rev Clin Oncol. 2020;17(4):204–232. doi: 10.1038/s41571-019-0293-2. [DOI] [PubMed] [Google Scholar]
- 76.Denise C, Paoli P, Calvani M, Taddei ML, Giannoni E, Kopetz S, et al. 5-fluorouracil resistant colon cancer cells are addicted to OXPHOS to survive and enhance stem-like traits. Oncotarget. 2015;6(39):41706–41721. doi: 10.18632/oncotarget.5991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Matassa DS, Amoroso MR, Lu H, Avolio R, Arzeni D, Procaccini C, et al. Oxidative metabolism drives inflammation-induced platinum resistance in human ovarian cancer. Cell Death Differ. 2016;23(9):1542–1554. doi: 10.1038/cdd.2016.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.De Rosa V, Iommelli F, Monti M, Fonti R, Votta G, Stoppelli MP, et al. Reversal of Warburg Effect and Reactivation of Oxidative Phosphorylation by Differential Inhibition of EGFR Signaling Pathways in Non-Small Cell Lung Cancer. Clin Cancer Res. 2015;21(22):5110–5120. doi: 10.1158/1078-0432.CCR-15-0375. [DOI] [PubMed] [Google Scholar]
- 79.Sun Y, Xu H, Chen X, Li X, Luo B. Inhibition of mitochondrial respiration overcomes hepatocellular carcinoma chemoresistance. Biochem Biophys Res Commun. 2019;508(2):626–632. doi: 10.1016/j.bbrc.2018.11.182. [DOI] [PubMed] [Google Scholar]
- 80.Lim SM, Syn NL, Cho BC, Soo RA. Acquired resistance to EGFR targeted therapy in non-small cell lung cancer: Mechanisms and therapeutic strategies. Cancer Treat Rev. 2018;65:1–10. doi: 10.1016/j.ctrv.2018.02.006. [DOI] [PubMed] [Google Scholar]
- 81.Pelicano H, Martin DS, Xu RH, Huang P. Glycolysis inhibition for anticancer treatment. Oncogene. 2006;25(34):4633–4646. doi: 10.1038/sj.onc.1209597. [DOI] [PubMed] [Google Scholar]
- 82.Zhang D, Li J, Wang F, Hu J, Wang S, Sun Y. 2-Deoxy-D-glucose targeting of glucose metabolism in cancer cells as a potential therapy. Cancer Lett. 2014;355(2):176–183. doi: 10.1016/j.canlet.2014.09.003. [DOI] [PubMed] [Google Scholar]
- 83.Varshney R, Dwarakanath B, Jain V. Radiosensitization by 6-aminonicotinamide and 2-deoxy-D-glucose in human cancer cells. Int J Radiat Biol. 2005;81(5):397–408. doi: 10.1080/09553000500148590. [DOI] [PubMed] [Google Scholar]
- 84.Shoshan MC. 3-Bromopyruvate: targets and outcomes. J Bioenerg Biomembr. 2012;44(1):7–15. doi: 10.1007/s10863-012-9419-2. [DOI] [PubMed] [Google Scholar]
- 85.Modica-Napolitano JS, Bharath LP, Hanlon AJ, Hurley LD. The Anticancer Agent Elesclomol Has Direct Effects on Mitochondrial Bioenergetic Function in Isolated Mammalian Mitochondria. Biomolecules. 2019;9(8):298. doi: 10.3390/biom9080298. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
Not applicable.