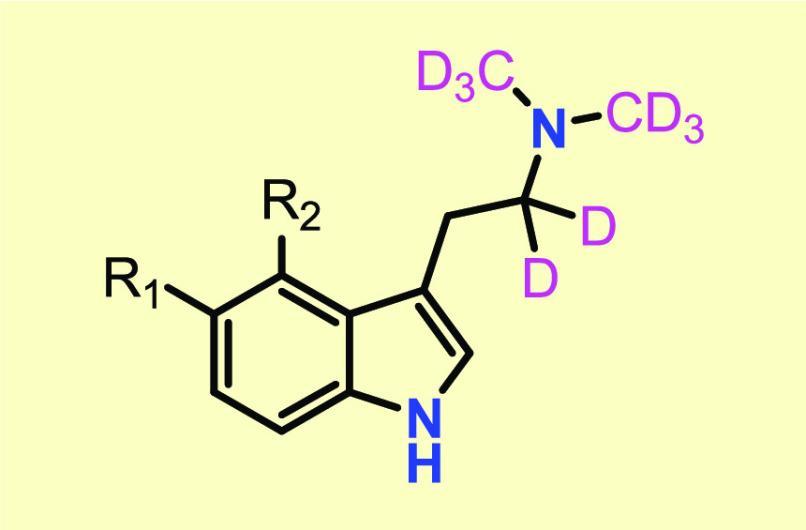
Important Compound Classes
Titles
Deuterated or Partially Deuterated N,N-Dimethyltryptamine Compounds; Inhalable Formulations; and Aerosol Generating Device and Method
Patent Publication Numbers
WO 2022/117359 A1 (URL: https://patents.google.com/patent/WO2022117359A1/en?oq=WO+2022%2f117359+A1)
WO 2022/117640 A1 (URL: https://patents.google.com/patent/WO2022117640A1/en?oq=WO+2022%2f117640+A1)
WO 2022/140292 A1 (URL: https://patents.google.com/patent/WO2022140292A1/en?oq=WO+2022%2f140292+A1)
Publication Dates
June 9, 2022; June 9, 2022; June 30, 2022
Priority Applications
US17/320,155; US17/320,155; US63/254,561
Priority Dates
May 13, 2021; May 13, 2021; October 12, 2021
Inventors
Rands, P.; James, E.; Benway, T.; Joel, Z.; Layzell, M. (WO 2022/117359 A1 and WO 2022/117640 A1); Signor, D. V.; Camacho, C. (WO 2022/140292 A1)
Assignee Companies
Small Pharma Ltd [GB/GB], 6-8 Bonhill Street, London EC2A 4BX, Great Britain (WO 2022/117359 A1 and WO 2022/117640 A1); Breatheasy Co. [US/US], 405 North King Street, Wilmington, Delaware 19801, United States (WO 2022/140292 A1)
Disease Area
Psychiatric and neurological disorders
Biological Target
5-HT2A receptor
Summary
In 2019, just prior to the COVID-19 pandemic, nearly 50 million (19.86%) American adults experienced a mental illness and over 2.5 million youth had severe depression, of which multiracial youth were at greatest risk (https://www.mhanational.org/issues/state-mental-health-america, accessed July 13, 2022). Anxiety and mood disorders are among the leading causes of disability worldwide, and antidepressants are one of the most prescribed kinds of medications in the United States.
The current therapeutic interventions and strategies to treat these disorders are slow acting, lack durability, and may prove to be ineffective for many patients. The current pandemic may only exacerbate the state of mental health worldwide. As a result, there is an urgent need to develop new treatment, new technology, and diversified approaches not only in the design of the clinical trials but also in the delivery of care.
For centuries, humans have consumed N,N-dimethyltryptamine (DMT) as a key ingredient in various tisanes and snuffs used in religious ceremonies, during which users of these botanical concoctions were left feeling peaceful and enlightened, most likely due to the profound psychoactive effects of the chemical constituents. DMT is often extracted together with N-methyltryptamine (NMT) and 5-MeO-DMT from the bark, shoots, and leaves of several plant genera.
Initial scientific studies on DMT conducted in the late 20th century suggested it had mood-elevating and calming properties. This has garnered significant interest, as it is reasoned that DMT and related alkaloids might be used to treat depression and other neuropsychiatric disorders. These compounds are potent psychoplastogens, and their effects on neural plasticity have been used to explain their long-lasting behavioral effects on anxiety and mood disorders. DMT has a simplistic structural characteristic, including low molecular weight, is hydrophobic, and has characteristics that enable it to readily cross the blood–brain barrier (BBB). DMT binds with high affinity to a variety of neuroreceptors and elicits robust behavioral responses. Unmetabolized DMT reaching the brain interacts with various receptors, including a large number of serotonin receptors.
DMT binds with nanomolar affinities to the 5-HT1A, 5-HT1B, 5-HT1D, 5-HT2A, 5-HT2B, 5-HT2C, 5-HT6, and 5-HT7 receptors. Genetic experiments and a variety of pharmacological experiments have shown that many of DMT’s biological effects are mediated, in part, by the 5-HT2A, 5-HT1A, and 5-HT2C receptors, where it may act as an agonist or partial agonist. However, the interoceptive and hallucinogenic effects of DMT are believed to result primarily from agonism of the 5-HT2A receptor and are modulated by mGlu2/3 receptors.
The 5-HT2A receptor does not desensitize to DMT over time, which perhaps explains why tolerance to DMT does not develop in humans. Structure–activity relationship (SAR) studies have demonstrated that the relatively small methyl groups of DMT are critical for achieving high affinity for the 5-HT2A receptor, as N-substituents larger than isopropyl drastically reduced 5-HT2A receptor affinity.
The sigma-1 receptor is well studied due to its potential role in the treatment of depression and anxiety. However, relatively few endogenous ligands of the sigma-1 receptor are known. DMT is one of the only known endogenous sigma-1 agonists (Kd = 15 μM), but the affinity of DMT for sigma-1 receptors is 100-fold lower than that for 5-HT2A receptors. Through activation of the sigma-1 receptor, DMT inhibits the production of pro-inflammatory cytokines while enhancing the secretion of IL-10, an anti-inflammatory cytokine. Sigma-1 agonists like DMT might also prove useful for treating neurodegenerative disorders by reducing inflammation. DMT has demonstrated potent anti-inflammatory properties.
Trace amine-associated receptor 1 (TAAR1) has also been suggested as a target of DMT. DMT activates TAAR1 to increase cAMP production in a TAAR1-expressing HEK293 cell line. DMT and several other trace amines, psychedelics, and psychostimulants have been shown to bind to and activate TAAR1 to a greater extent than traditional neurotransmitters like serotonin, dopamine, or norepinephrine. Furthermore, DMT may act as a substrate, rather than an inhibitor, for the serotonin transporter (SERT) and vesicular monoamine transporter (VMAT) via an active-transport mechanism. In contrast, the binding affinity of DMT for dopamine receptors is quite low (Ki ≈ 5 μM) compared to that of ergolines such as LSD (Ki ≈ 20 nM).
The metabolism and pharmacokinetics of DMT play prominent roles in how it is typically administered and in the qualitatively different experience compared to other psychedelics. The subjective effects of DMT administered to humans via i.v. injection are rapid and transient, peaking at 5 min, but may cease after 30 min, which is similar to effects observed when it is smoked. Following systemic administration, DMT quickly crosses the BBB and concurrently is rapidly metabolized by MAO-A as well as liver enzymes. It is not orally active due to its rapid degradation by MAO-A in the gut and liver.
DMT is often administered with MAO inhibitors (MAOIs) to prevent inactivation of the compound before it reaches the target site in the body, which allows for a prolonged and increased exposure to the compound. However, MAOIs can cause high blood pressure when taken with certain foods or medications, and the use of MAOIs by a patient typically requires restrictions in the diet and avoidance of some other medications.
When DMT is administered in controlled clinical settings where participants are carefully screened for factors that could predispose them to long-term adverse psychological effects, DMT appears to be exceptionally safe. In general, psychedelics are not considered to be addictive and are substantially safer than drugs like alcohol or nicotine.
The DMT core structure is a prominent feature of a class of medications known as the triptans and related to the natural compounds of serotonin and melatonin; however, the effects of DMT on the central nervous system are quite distinct from the effects of these other compounds. Triptan drugs (Sumatriptan, Rizatriptan, and Zolmitriptan) are vasoconstrictors that are used to treat migraines and cluster headaches, and other DMT analogues have shown promise for treating Alzheimer’s disease and other ailments in preclinical models.
Naturally occurring hydrogen contains about 0.02 molar % deuterium and 99.98% protium. Differences in physical and chemical properties between protium and deuterium are small but measurable. For example, deuterium is slightly less lipophilic than protium and has a smaller molar volume, and carbon–deuterium bonds are shorter than carbon–protium bonds. These properties may indicate that the incorporation of deuterium into DMT could be expected to progressively reduce lipophilicity and increase basicity in a non-additive manner while retaining the biochemical potency and selectivity of the parent compound. In addition, the enrichment of DMT hydrogen atoms with deuterium is expected to cause a shift in the compound’s stability, which could be measured as the deuterium kinetic isotope effect (DKIE).
DMT is metabolized very quickly in the human body and has a clearance rate of 24 483 mL/min, which equates to 350 mL/min/kg based on a 70 kg person. The average human liver blood flow is estimated to be 20 mL/min/kg, with a cardiac output of 71 mL/min/kg. This implies that DMT is largely metabolized before reaching the human liver.
Given the therapeutic potential of DMT and its substituted analogues, there remains a need for alternative compounds with improved bioavailability, extended or modified pharmacokinetics, and modified pharmacodynamics, for use in clinical applications involving psychedelic-assisted psychotherapy. The disclosure in patent WO 2022117359 A1 addresses this need.
Similarly, the disclosure in patent WO 2022117640 A1 relates to inhalable formulations comprising a free base of a deuterium-substituted DMT with a biocompatible excipient that could be used in the treatment of psychiatric or neurological disorders. The deuterium-substituted DMT compounds may be metabolized more slowly than their protio analogues, which may allow longer lasting therapeutic effects.
Finally, the disclosure in patent WO 2022/140292 A1 provides an aerosol delivery system that is intended to be inhaled by a person and used for the administration of medicine and novel formulations across a wide range of medical and consumer indications.
Key Structures
Biological Assay
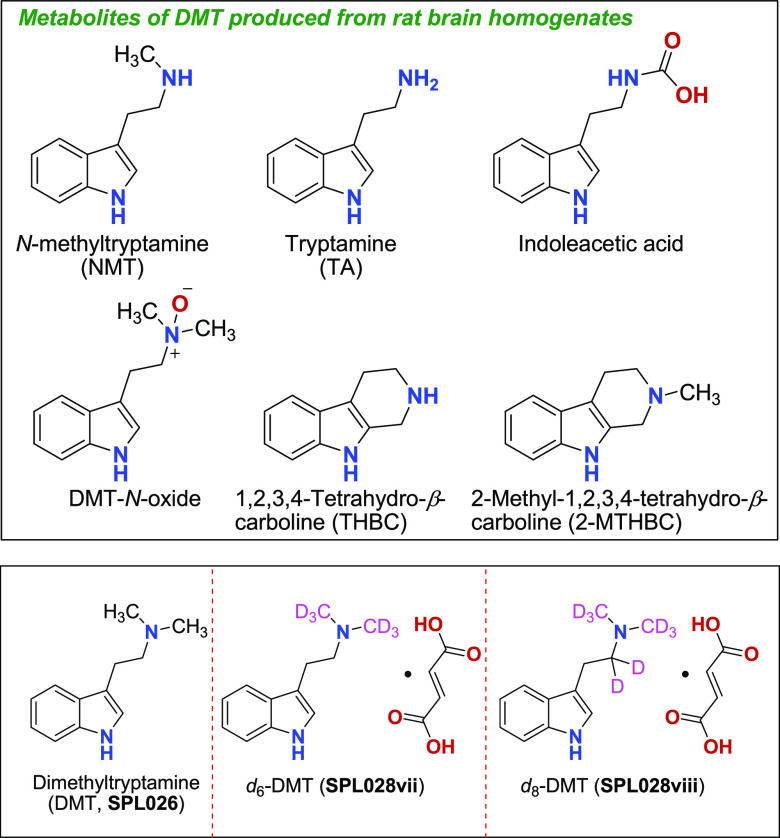
A series of in vitro drug metabolism and pharmacokinetics (DMPK) experiments on deuterated DMT derivatives in human hepatocyte assay and animal tissue as a proxy of in vivo clearance.
Biological Data
The table below shows results from human hepatocytes assays conducted
with D6-DMT and D8-DMT, which indicated that
intrinsic clearance of D8-DMT displayed a 2.1 change from
that of DMT (SPL026).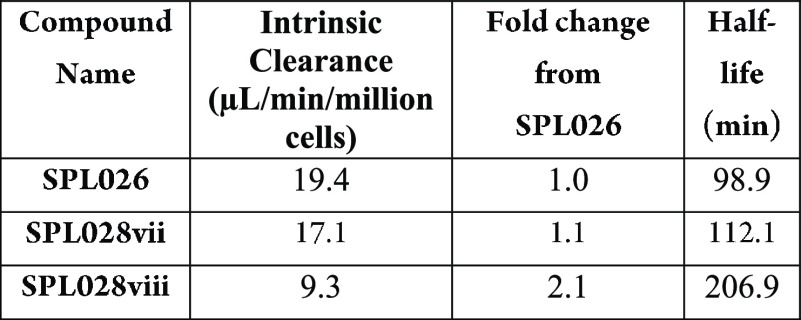
The author declares no competing financial interest.
Recent Review Articles. References
- James E.; Keppler J.; L Robertshaw T.; Sessa B. N,N-Dimethyltryptamine and Amazonian ayahuasca plant medicine. Hum. Psychopharmacol. 2022, 37, e2835. 10.1002/hup.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker S. A. Administration of N,N-dimethyltryptamine (DMT) in psychedelic therapeutics and research and the study of endogenous DMT. Psychopharmacology 2022, 239, 1749. 10.1007/s00213-022-06065-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClure-Begley T. D.; Roth B. L. The promises and perils of psychedelic pharmacology for psychiatry. Nat. Rev. Drug Discovery 2022, 21, 463. 10.1038/s41573-022-00421-7. [DOI] [PubMed] [Google Scholar]
- Vorobyeva N.; Kozlova A. A. Three naturally occurring psychedelics and their significance in the treatment of mental health disorders. Front. Pharmacol. 2022, 13, 927984. 10.3389/fphar.2022.927984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron L. P.; Benson C. J.; DeFelice B. C.; Fiehn O.; Olson D. E. Chronic, intermittent microdoses of the psychedelic N,N-dimethyltryptamine (DMT) produce positive effects on mood and anxiety in rodents. ACS Chem. Neurosci. 2019, 10, 3261. 10.1021/acschemneuro.8b00692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cameron L. P.; Olson D. E. Dark classics in chemical neuroscience: N,N-dimethyltryptamine (DMT). ACS Chem. Neurosci. 2018, 9, 2344. 10.1021/acschemneuro.8b00101. [DOI] [PubMed] [Google Scholar]