Abstract
Background
Opioid-induced immunomodulation may be important in colon adenocarcinoma, where tumour DNA mismatch repair (MMR) can determine the level of immune activation with consequences for therapeutic response and prognosis. We evaluated the relationship between intraoperative opioid exposure, MMR subtype, and oncological outcomes after surgery for colon adenocarcinoma.
Methods
Intraoperative opioid use (standardised by calculating morphine milligram equivalents) during stage I–III colon adenocarcinoma resection was reviewed retrospectively. Tumours were classified as DNA mismatch repair deficient (dMMR) or proficient (pMMR) by immunohistochemistry. The primary outcome was local tumour recurrence, distant tumour recurrence, or both (multivariable analysis). The exposures of interest were intraoperative analgesia and tumour subtype. Opioid-related gene expression was analysed using The Cancer Genome Atlas Colon Adenocarcinoma transcriptomic data.
Results
Clinical and pathological data were analysed from 1157 subjects (median age, 60 [51–70] yr; 49% female) who underwent curative resection for stage I–III colon adenocarcinoma. Higher intraoperative opioid doses were associated with reduced risk of tumour recurrence (hazard ratio=0.92 per 10 morphine milligram equivalents; 95% confidence interval [95% CI], 0.87–0.98; P=0.007), but not with overall survival. In tumours deficient in DNA MMR, tumour recurrence was less likely (HR=0.38; 95% CI, 0.21–0.68; P=0.001), with higher opioid dose associated with eightfold lower recurrence rates. Gene expression related to opioid signalling was different between dMMR and pMMR tumours.
Conclusions
Higher intraoperative opioid dose was associated with a lower risk of tumour recurrence after surgery for stage I–III colon adenocarcinoma, but particularly so in tumours in which DNA MMR was deficient.
Keywords: colon adenocarcinoma, DNA mismatch repair, gene expression, immunomodulation, opioids, surgery
Editor's key points.
-
•
Opioid-induced immunomodulation may alter recurrence, survival after surgery, or both for colon adenocarcinoma.
-
•
The impact of opioids on cancer progression may also be influenced by the genomic landscape of tumours.
-
•
Higher intraoperative opioid use was associated with lower tumour recurrence, particularly in tumours with deficient DNA mismatch repair.
-
•
Onco-anaesthesia may benefit from a personalised medicine approach that incorporates tumour genomics.
Perioperative opioids may have a deleterious effect in cancer1, 2, 3, 4 through multiple mechanisms,5,6 but clinical evidence is more nuanced.7,8 The impact of perioperative exposure to opioids, and other analgesic drugs,6,9, 10, 11, 12 on cancer progression may also be influenced by the genomic landscape of tumours.9,10,13
The DNA mismatch repair (MMR) system maintains genomic integrity by identifying and repairing mismatched nucleotides that occur during genetic recombination or because of damage.14 When one or more enzymes in the MMR system are altered, the tumour is referred to as MMR deficient (dMMR); when unaltered, it is referred to as MMR proficient (pMMR).15 Around 15% of early-stage, non-metastatic colon adenocarcinoma (COAD) tumours harbour dMMR alterations that cause high microsatellite instability (MSI-H) (as opposed to pMMR tumors which are microsatellite low (MSI-L) or stable (MSS)).15 dMMR tumours generate neoantigens, resulting in immune activation and recruitment of tumour-infiltrating lymphocytes (TILs).16,17 dMMR tumours are less responsive to traditional chemotherapy15,18 but more responsive to immunotherapy19 and are associated with improved stage-specific prognosis.20
Given that both MMR subtype and opioids may affect cancer progression through immunomodulation,21 we hypothesised that tumour MMR subtype may interact with intraoperative opioid dose, other analgesic drugs, or both. We further explored mechanisms for our findings, based both on data in our clinical cohort and using transcriptomic data in The Cancer Genome Atlas for COAD.22
Methods
Study design
After obtaining institutional review board approval from Memorial Sloan Kettering Hospital, we performed a retrospective review from March 1, 2010 to December 31, 2018 (follow-up updated in July 2020).
Inclusion criteria
Patients with stage I–III COAD who underwent curative resection at Memorial Sloan Kettering were eligible for analysis.
Exclusion criteria
Patients were excluded if their DNA MMR subtype was unknown, had received neoadjuvant treatment, had a rare histological subtype, or another invasive cancer within 5 yr before colectomy (Supplementary Fig. S1).
Intraoperative analgesia
Intraoperative doses of fentanyl, hydromorphone, and morphine were extracted from the electronic anaesthesia records. Total doses were converted to oral morphine milligram equivalents (MMEs); 10 MMEs equal 50 μg i.v. fentanyl. Intraoperative administration of ketorolac, ketamine, and dexmedetomidine was also recorded.
Mismatch repair subtype
pMMR was defined by immunohistochemical (IHC) staining for the proteins MLH1 (MutL homolog 1), MSH2 (MutS homolog 2), MSH6 (MutS homolog 6), and PMS2 (Postmeiotic Segregation Increased 2) in the pretreatment biopsy or the resected specimen.23 dMMR was defined by the absence of one or more of these proteins.
Mechanistic analyses
Tumour-infiltrating lymphocytes
We examined the relationship between opioid use, tumour type, and TILs, the increased presence of which is associated with a lower risk of recurrence.24 For 1010 (87%) of the 1157 patients in the cohort, surgical pathology reports explicitly noted the presence or absence of increased numbers of TILs. A tumour was classified as having increased TILs if the mean number of lymphocytes per high-powered field was ≥4, averaged from five consecutive high-powered fields in an area determined to have the highest concentration of TILs by examination of the entire tumour. The relationships of TILs, intraoperative opioids, and recurrence and OS outcomes were explored using cumulative incidence functions and Kaplan–Meier estimates, respectively.
Opioid signalling transcriptomic analysis
We used the TCGA-COAD cohort22 to also examine gene expression related to opioid signalling and function in dMMR (MSI-H) vs pMMR tumour (MSS and MSI-L) tumours, compared with normal tissue using bulk RNA sequencing data from 358 patients (Supplementary material).
Differential gene expression analysis was performed using the R package DESeq2 (R Foundation for Statistical Computing, Vienna, Austria).25 P-values were adjusted using Benjamini–Hochberg correction for multiple hypothesis testing. An absolute fold change of 2 and an adjusted P-value cut-off of 0.05 defined statistical significance. The canonical opioid receptors (mu [OPRM1], delta [OPRD1], kappa [OPRK1]) plus 430 genes broadly related to opioid signalling and function was generated using Geneshot.26 This list was subsequently refined to only include those genes determined to be differentially expressed between MSI and MSS tumours. This was further divided into lists of up- and downregulated genes, where up and down are in reference to expression in MSI vs MSS, and referred to as ‘Opioid∗MSI’ and ‘Opioid∗MSS’, respectively. For example, the gene CCK (Cholecystokinin) is upregulated in MSI compared to MSS and as such is in the Opioid∗MSI list. Single-sample gene set enrichment analysis (SSGSEA) was used to correlate pathways and immune cell types with the Opioid∗MSI and Opioid∗MSS lists. Pathways included the 50 ‘Hallmark’ gene lists (representing well-defined biological processes),27 whereas 25 immune cell types were represented by specific gene signatures.28
Primary outcome
The primary outcome was local tumour recurrence, distant tumour recurrence, or both. Death without recurrence was treated as a competing event. Recurrence was calculated from time of surgery to recurrence if a patient experienced the event, until death if a patient experienced the competing event, or it was censored at last follow-up.
Secondary outcome
The secondary outcome was overall survival (OS), calculated from time of surgery to death from any cause.
Exposures of interest
MMR and analgesic dose were the exposures of interest in relation to tumour recurrence and overall survival.
Statistical analyses
The relationship between intraoperative opioids and MMR subtype on recurrence was summarised using cumulative incidence functions and quantified using competing risk regression models. The relationship between intraoperative opioids and MMR subtype on OS was summarised using the Kaplan–Meier approach and quantified using Cox proportional hazards regression models. In all models, intraoperative MMEs were treated as a continuous variable, and administration of adjuncts and ketorolac as categorical variables. For multivariable analyses, a set of factors selected a priori (intraoperative MMEs, MMR subtype, adjunct, and ketorolac) were included. Backward regression was used to determine additional adjusting baseline factors, starting with a model including all factors with P<0.1 in the univariable models for each endpoint. Associations quantified via regression modelling were presented as hazard ratios (HRs) and 95% confidence intervals (CIs). Handling of missing covariate data is described in the Supplementary methods.
To explore the potential interaction between MMR subtype and MMEs on oncological outcomes, we calculated the Kaplan–Meier and cumulative incidence functions separately for dMMR and pMMR patients, stratified by MME at the median (see Supplementary methods for further details). Statistical tests were two-sided with P<0.05 indicating statistical significance. All analyses were performed using R software version 4.1.1 (R Core Team, Vienna, Austria) with the mice v3.13.0 package for multiple imputation.29
Results
Study participants
A total of 1157 patients (median age, 60 [51–70] yr) met the inclusion criteria (Supplementary Fig. S1), of whom 282 (24%) had dMMR tumours (Table 1). Patients received general anaesthesia, which generally involved induction with propofol and maintenance with sevoflurane. The median opioid dose was 60 MMEs (inter-quartile range [IQR], 44–90), with fewer than 50% patients receiving ketamine, dexmedetomidine, and/or ketorolac (Table 1). Regional analgesia (epidural or transversus abdominis plane block) was associated with lower intraoperative MMEs (Supplementary Table S1). The median follow-up duration was 3.0 yr (95% CI, 2.8–3.2).
Table 1.
Clinical and pathological characteristics. Data are presented as number and frequency for categorical variables and median, interquartile range, and range for continuous variables. P-values are calculated with Wilcoxon rank-sum test, Pearson's χ2 test, and Fisher's exact test. CEA, carcinoembryonic antigen; COAD, colon adenocarcinoma; EBL, estimated blood loss; IQR, interquartile range; MMEs, oral morphine milligram equivalents; MMR, mismatch repair; TAP, transversus abdominis plane.
| Characteristic | Overall n=1157 |
MMR |
P-value | |
|---|---|---|---|---|
| Proficient, n=875 (76%) |
Deficient, n=282 (24%) |
|||
| Intraoperative MMEs | 0.5 | |||
| Median (IQR) | 60 (44, 90) | 62 (47, 90) | 59 (40, 92) | |
| [Range] | [0, 473] | [0, 323] | [0, 473] | |
| Adjunct | 0.025 | |||
| None | 630 (54%) | 457 (52%) | 173 (61%) | |
| Dexmedetomidine | 161 (14%) | 130 (15%) | 31 (11%) | |
| Ketamine | 366 (32%) | 288 (33%) | 78 (28%) | |
| Ketorolac use | 0.5 | |||
| No ketorolac | 930 (80%) | 699 (80%) | 231 (82%) | |
| Yes ketorolac | 227 (20%) | 176 (20%) | 51 (18%) | |
| Regional anaesthesia | 0.006 | |||
| None | 583 (50%) | 429 (49%) | 154 (55%) | |
| Epidural | 269 (23%) | 200 (23%) | 69 (24%) | |
| TAP block | 303 (26%) | 246 (28%) | 57 (20%) | |
| Both | 2 (0.2%) | 0 (0%) | 2 (0.7%) | |
| Patient age (yr) | <0.001 | |||
| Median (IQR) | 60 (51, 70) | 58 (50, 68) | 66 (53, 77) | |
| [Range] | [24, 96] | [25, 93] | [24, 96] | |
| Sex | 0.14 | |||
| Male | 594 (51%) | 460 (53%) | 134 (48%) | |
| Female | 563 (49%) | 415 (47%) | 148 (52%) | |
| Race | 0.002 | |||
| White | 937 (84%) | 688 (82%) | 249 (91%) | |
| Black | 67 (6.0%) | 58 (6.9%) | 9 (3.3%) | |
| Other | 105 (9.5%) | 90 (11%) | 15 (5.5%) | |
| Unknown | 48 | 39 | 9 | |
| Ethnicity | 0.6 | |||
| Hispanic | 70 (6.1%) | 51 (5.9%) | 19 (6.8%) | |
| Not Hispanic | 1071 (94%) | 810 (94%) | 261 (93%) | |
| Unknown | 16 | 14 | 2 | |
| Pathological T stage | 0.2 | |||
| 1 | 153 (13%) | 112 (13%) | 41 (15%) | |
| 2 | 161 (14%) | 120 (14%) | 41 (15%) | |
| 3 | 701 (61%) | 526 (60%) | 175 (62%) | |
| 4 | 142 (12%) | 117 (13%) | 25 (8.9%) | |
| Pathological N stage | <0.001 | |||
| 0 | 706 (61%) | 497 (57%) | 209 (74%) | |
| 1 | 313 (27%) | 261 (30%) | 52 (18%) | |
| 2 | 138 (12%) | 117 (13%) | 21 (7.4%) | |
| Tumour location | <0.001 | |||
| Right | 579 (50%) | 370 (42%) | 209 (74%) | |
| Left | 488 (42%) | 443 (51%) | 45 (16%) | |
| Mid-transverse | 90 (7.8%) | 62 (7.1%) | 28 (9.9%) | |
| CEA (ng ml−1) | 0.3 | |||
| Median (IQR) | 3 (2, 6) | 3 (2, 6) | 3 (2, 6) | |
| [Range] | [0, 1360] | [0, 1360] | [1, 836] | |
| Unknown | 144 | 106 | 38 | |
| Conversion | >0.9 | |||
| No | 1088 (94%) | 823 (94%) | 265 (94%) | |
| Yes | 69 (6.0%) | 52 (5.9%) | 17 (6.0%) | |
| Synchronous COAD | 0.6 | |||
| No | 1122 (97%) | 850 (97%) | 272 (96%) | |
| Yes | 35 (3.0%) | 25 (2.9%) | 10 (3.5%) | |
| Type of resection | 0.003 | |||
| Open | 192 (17%) | 137 (16%) | 55 (20%) | |
| Laparoscopic | 298 (26%) | 209 (24%) | 89 (32%) | |
| Robotic | 667 (58%) | 529 (60%) | 138 (49%) | |
| Extent of resection | 0.001 | |||
| Extended | 69 (6.0%) | 41 (4.7%) | 28 (9.9%) | |
| Segmental | 1088 (94%) | 834 (95%) | 254 (90%) | |
| Adjuvant chemotherapy | <0.001 | |||
| No | 610 (54%) | 405 (48%) | 205 (75%) | |
| Yes | 511 (46%) | 443 (52%) | 68 (25%) | |
| Unknown | 36 | 27 | 9 | |
| Surgery time (min) | <0.001 | |||
| Median (IQR) | 183 (137, 232) | 186 (144, 239) | 168 (126, 222) | |
| [Range] | [49, 620] | [49, 620] | [58, 600] | |
| Smoking history | 0.7 | |||
| Never used | 656 (57%) | 502 (58%) | 154 (55%) | |
| Past smoker | 401 (35%) | 298 (34%) | 103 (37%) | |
| Current smoker | 92 (8.0%) | 69 (7.9%) | 23 (8.2%) | |
| Unknown | 8 | 6 | 2 | |
| Albumin (g dl−1) | <0.001 | |||
| Median (IQR) | 4.20 (3.90, 4.40) | 4.20 (4.00, 4.40) | 4.10 (3.80, 4.30) | |
| [Range] | [1.70, 5.20] | [1.70, 5.20] | [2.40, 4.90] | |
| Unknown | 6 | 3 | 3 | |
| ASA physical status | 0.024 | |||
| 1/2 | 401 (35%) | 319 (36%) | 82 (29%) | |
| 3/4 | 756 (65%) | 556 (64%) | 200 (71%) | |
| van Walraven score | 0.8 | |||
| Median (IQR) | 12.0 (8.0, 12.0) | 12.0 (8.0, 12.0) | 12.0 (7.0, 12.0) | |
| [Range] | [0.0, 46.0] | [0.0, 46.0] | [0.0, 33.0] | |
| BMI (kg m−2) | 0.4 | |||
| Median (IQR) | 27.5 (24.2, 32.4) | 27.7 (24.3, 32.7) | 27.4 (24.2, 31.4) | |
| [Range] | [15.8, 62.5] | [15.8, 62.5] | [16.3, 53.9] | |
| Unknown | 3 | 2 | 1 | |
| EBL (ml) | 0.016 | |||
| EBL <100 ml | 834 (72%) | 615 (70%) | 219 (78%) | |
| EBL ≥100 ml | 323 (28%) | 260 (30%) | 63 (22%) | |
Primary outcome
Tumour recurrence and intraoperative analgesia
Tumour recurrence (19 local, 107 distant) occurred in in 126 patients (Fig. 1a). Higher intraoperative opioid dose was associated with lower rates of tumour recurrence in both univariable (HR=0.93 per 10 MME; 95% CI, 0.88–0.98; P=0.008) and multivariable (HR=0.92 per 10 MME; 95% CI, 0.87–0.98; P=0.007) analyses. Ketamine, dexmedetomidine, and ketorolac were not associated with recurrence in either univariable or multivariable analysis.
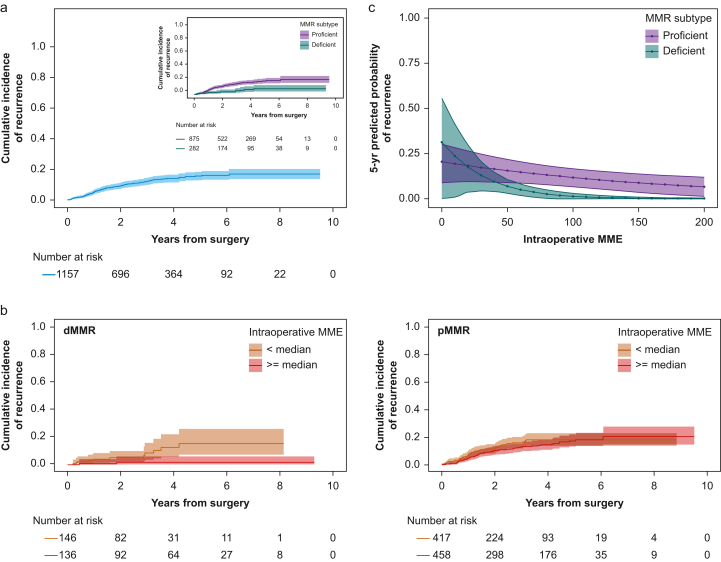
Fig 1.
Associations between intraoperative opioid dose, DNA mismatch repair subtype. and recurrence. Cumulative incidence functions of recurrence for (a) all patients, (inset) stratified by MMR subtype, and (b) stratified by intraoperative opioid dose (<median: below vs ≥ median: above median) for (l) dMMR and (r) pMMR patients separately. (c) Model-estimated probability of recurrence at 5 yr after surgery over a range of intraoperative MME values based on MMR subtype (for a hypothetical patient with these MVA factor values: T stage=3, N stage=0, BMI=27.5, no adjuvant chemotherapy, no adjunct, and no ketorolac). Shaded area represents 95% confidence intervals for all panels. dMMR, MMR deficient; MME, intraoperative oral morphine milligram equivalent; MVA, multivariable analysis; pMMR, MMR proficient.
Tumour recurrence and mismatch repair
The dMMR tumour subtype was associated with lower rates of tumour recurrence (HR= 0.38; 95% CI, 0.21–0.68; P=0.001; Fig. 1a). An eightfold greater decrease of recurrence was associated with high vs low opioid dose for dMMR, compared with pMMR (P=0.016; Fig. 1b and c). Adjuvant chemotherapy, tumour stage, and BMI were also independently associated with tumour recurrence (Table 2; Supplementary Table S2).
Table 2.
Multivariable competing risk regression analysis for tumour recurrence. Estimates are pooled from 10 imputed datasets. Model includes clinical factors of interest (intraoperative MMEs, MMR subtype, adjunct, and ketorolac) and statistically significant factors from the univariable analysis for adjusting baseline factors, followed by backwards selection on the adjusting factors. CI, confidence interval; HR, hazard ratio; MMEs, oral morphine milligram equivalents; MMR, mismatch repair.
| Characteristic | HR | 95% CI | P-value |
|---|---|---|---|
| Intraoperative MMEs (per 10) | 0.92 | 0.87–0.98 | 0.007 |
| MMR subtype | |||
| Proficient | – | – | |
| Deficient | 0.38 | 0.21–0.68 | 0.001 |
| Adjunct | |||
| None | – | – | |
| Dexmedetomidine | 1.02 | 0.56–1.87 | >0.9 |
| Ketamine | 0.90 | 0.59–1.38 | 0.6 |
| Ketorolac use | |||
| No ketorolac | – | – | |
| Yes ketorolac | 1.41 | 0.93–2.15 | 0.10 |
| Pathological T stage | |||
| 1 | – | – | |
| 2 | 1.13 | 0.25–5.00 | 0.9 |
| 3 | 4.62 | 1.43–14.88 | 0.010 |
| 4 | 10.0 | 2.94–34.21 | <0.001 |
| Pathological N stage | |||
| 0 | – | – | |
| 1 | 2.96 | 1.62–5.40 | <0.001 |
| 2 | 4.34 | 2.23–8.44 | <0.001 |
| BMI (kg m−2) | 1.03 | 1.01–1.06 | 0.009 |
| Adjuvant chemotherapy | |||
| No | – | – | |
| Yes | 0.49 | 0.27–0.90 | 0.022 |
Secondary outcome: overall survival
Seventy-six patients died during follow-up, with 37 deaths occurring after tumour recurrence. Estimated 3- and 5-yr OS probability was 95% (95% CI, 93–96%) and 90% (95% CI, 88–93%), respectively, which were similar for pMMR and dMMR. There was no relationship between any intraoperative analgesic agents and OS (Supplementary Tables S3 and S4).
Exploratory analyses
Tumour infiltrating lymphocytes
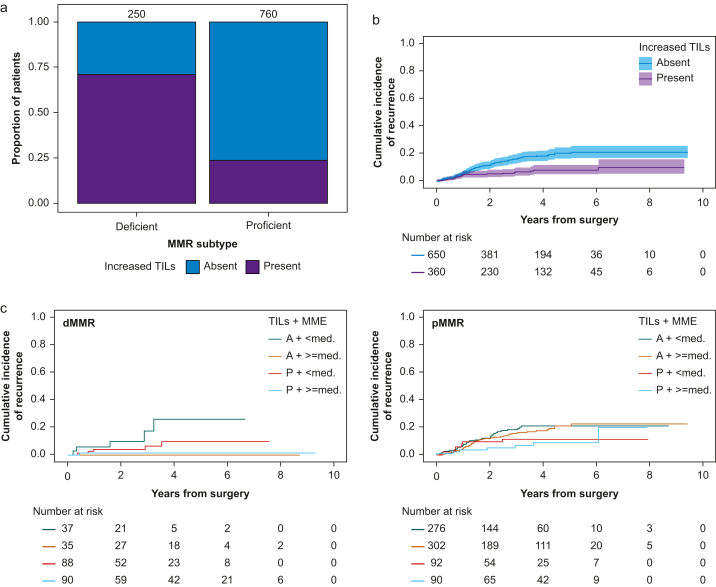
Increased numbers of TILs were reported in 360/1010 (36%) specimens, with the majority (71%) present in dMMR (Fig. 2a). Increased TILs were associated with a lower risk of recurrence (Fig. 2b), but higher opioid doses were associated with lower risk of recurrence in dMMR tumours independent of whether increased TILs were present (Fig. 2c; Supplementary Figs S2 and S3).
Fig 2.
Associations between intraoperative opioid dose, tumour-infiltrating lymphocytes, and recurrence. (a) Distribution of absent vs present increased TILs by DNA mismatch repair subtype. Cumulative incidence function of recurrence (b) stratified by absent vs present increased TILs. Shaded area represents 95% confidence intervals. (c) Cumulative incidence function of recurrence stratified by increased TILs (A [absent] vs P [present]) and intraoperative opioid dose (<med: below vs ≥ med: above median) for (l) dMMR and (r) pMMR patients separately. dMMR, MMR deficient; MME, intraoperative oral morphine milligram equivalents; MMR, mismatch repair; pMMR, MMR proficient; TILs, tumour-infiltrating lymphocytes.
Opioid receptor/signalling transcriptomics
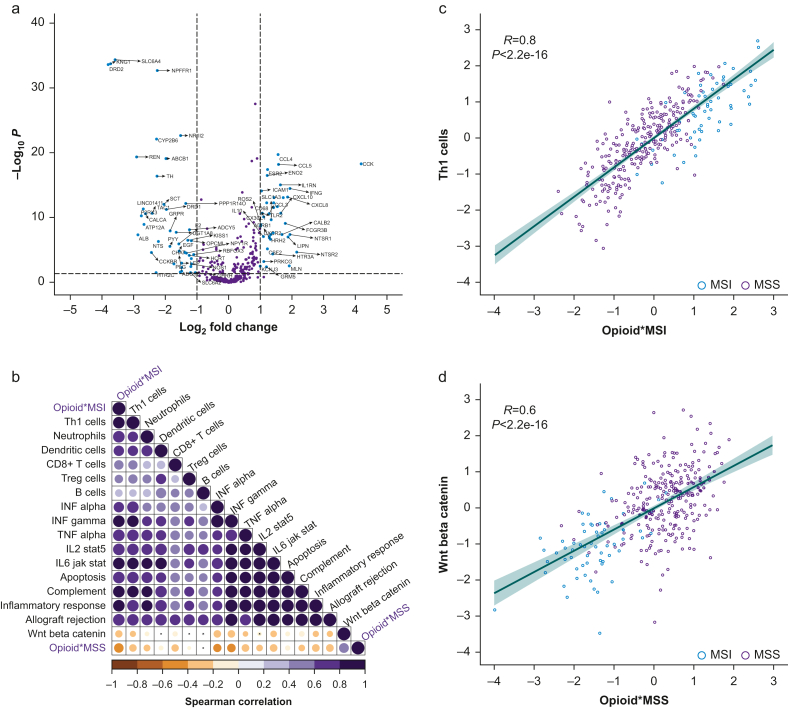
Opioid receptors were differentially expressed between tumour and normal tissue but not between dMMR and pMMR subtypes (Supplementary Fig. S4). Overall, 83 of 430 genes regulating opioid signalling and function were differentially expressed between dMMR and pMMR tumours (Fig. 3a; Supplementary Table S5), which correlated with immune pathways that modulate the tumour microenvironment (Fig. 3b–d).
Fig 3.
Differential expression of opioid-related genes in colon adenocarcinoma. Transcriptomic data from The Cancer Genome Atlas Colon Adenocarcinoma (TCGA-COAD) database was analysed. (a) Volcano plot showing differential expression of opioid genes for MSI (dMMR) vs MSS (pMMR) tumours. –Log10 P-value (adjusted for multiple testing) is plotted against log2 (fold change). The horizontal dotted line represents P=0.05. Vertical lines dotted at an absolute fold change of 2. Genes with absolute fold change higher than 2 and P-value<0.05 are coloured blue. (b) SSGSEA analysis correlation plot highlighting major pathways and cell types correlated with MSI upregulated (Opioid∗MSI) and downregulated (Opioid∗MSS) opioid genes. For each pairwise correlation, the colour and the size of the circle denote the Spearman correlation. (c) SSGSEA correlation of MSI upregulated opioid genes and Th1 immune signature and (d) MSI downregulated opioid genes and Wnt beta-catenin pathway; each patient sample is labelled as MSI (blue) or MSS (purple) and is plotted by normalised SSGSEA scores for the relevant gene sets on the x- and y-axes. Shaded area represents the 95% confidence interval around the best fit line. MSI, microsatellite instability; MSS, microsatellite stability; SSGSEA, single-sample gene set enrichment analysis.
Discussion
The main finding of this study is that intraoperative opioids are associated with a lower hazard of recurrence in a large cohort of non-metastatic patients with COAD who underwent primary tumour resection. The associated anti-tumour effect of opioids was amplified in patients with dMMR tumours compared with pMMR tumours, suggesting that tumour genomics (in this case, the DNA MMR system) may interact with intraoperative opioid dose to modify recurrence risk.
In principle, information pertaining to individual tumour genomics is attainable before primary resection (e.g. from IHC staining or next-generation sequencing of a preoperative biopsy specimen, or from plasma-derived cell-free DNA). It may therefore be possible to develop a precision approach to analgesia in COAD patients based on tumour genomics. The current findings may be relevant beyond the perioperative period, given recent evidence that opioids may affect the efficacy of immunotherapy30 and recent work suggesting that although chronic opioid use may increase the risk of a cancer diagnosis generally, colorectal cancer was one of a few cancer types where this risk may be decreased.31
Exploratory analysis to elucidate contributory factors underlying decreased recurrence with increased opioid dose in COAD reveals that opioids may promote anti-tumour TILs. However, although increased TILs are more prominent in dMMR, this alone cannot explain amplification of recurrence risk reduction in dMMR at higher opioid dose, which was still present in dMMR tumours without increased TILs (even compared with pMMR tumours with increased TILs). Differences in gene expression at the intersection of opioid and MMR signalling may explain this amplification but not at the level of the opioid receptors (although the pattern of receptor differential expression in tumour vs normal is similar to that observed in triple-negative breast cancer, another cancer type where TILs recruitment is prognostic for improved survival, where opioids were found to improve recurrence-free survival,7 and where, as a subset of breast cancer more generally, chronic opioid use may actually decrease the risk of diagnosis).31 Instead, genes more broadly involved in opioid signalling and differentially expressed between dMMR and pMMR correlated with specific pathways and immune cell types, suggesting that dMMR amplification may involve opioid interaction with the Th1 immune response (known to be relevant to survival differences between dMMR and pMMR)17,32 and Wnt signalling.9 Opioid interaction with MMR subtype (and with COAD more generally) may rely on both TILs-mediated (Th1 immune response) and on-tumour (Wnt signalling) effects.
The strengths of the study include its use of opioid dose as a continuous variable and the detailed clinicopathologic features including MMR subtype and characterisation of TILs. However, the study is limited by its retrospective design, lack of detailed data on postoperative opioid use, and the use of bulk, rather than single-cell, sequencing data from an external cohort. Although it is possible that different opioids may have variable effects on oncological outcomes, fentanyl accounted for the overwhelming majority of opioids used in this study (Supplementary Figure S6).
In summary, our study provides the rationale for a prospective study focused on perioperative opioid dosing and tumour subtypes in patients undergoing surgery for COAD.
Authors' contributions
Study conception and design: JBY, GWF, JG-A, JSM
Data acquisition: JBY, HMT, FSV, PJM, JS, MRW, JSM
Data analysis: JBY, JL, FW, HVG, TI, JRS, JJS, FS-V, ST, JSM
Drafting of the manuscript: JBY, JL, FW, FS-V, ST, GWF, JG-A, JSM
Revision and approval of the manuscript: all authors
Acknowledgements
JSM and HVG acknowledge John Chodera of the Sloan Kettering Institute for his support and insightful discussions. JSM also acknowledges Sahrena London for helpful conversations. JBY acknowledges Yael Renert-Yuval for helpful conversations. The authors thank Olga Rukovets for editing the manuscript.
Handling editor: Gareth Ackland
Footnotes
Supplementary data to this article can be found online at https://doi.org/10.1016/j.bja.2022.04.024.
Declarations of interest
PJM's spouse has an equity interest in Johnson & Johnson. GWF is on the speaker's bureau and serves as a consultant for Edwards Lifesciences. JG-A has received support from Medtronic, Johnson & Johnson, and Intuitive Surgical. Dr Smith has served as a clinical advisor to Guardant Health Inc. (2019) and received travel support from Intuitive Surgical Inc. for fellow education (2015). JS is a consultant for Paige AI.
Funding
The National Institutes of Health/National Cancer Institute Memorial Sloan Kettering Cancer Center Support Grant (P30 CA008748). JBY's research fellowship at Memorial Sloan Kettering was funded in part by a grant from the National Cancer Institute (T32 CA009501). Marie-Josée and Henry R. Kravis Center for Molecular Oncology.
Appendix A. Supplementary data
The following are the Supplementary data to this article:
References
- 1.Maher D.P., Wong W., White P.F., et al. Association of increased postoperative opioid administration with non-small-cell lung cancer recurrence: a retrospective analysis. Br J Anaesth. 2014;113(Suppl 1):i88–94. doi: 10.1093/bja/aeu192. [DOI] [PubMed] [Google Scholar]
- 2.Cata J.P., Zafereo M., Villarreal J., et al. Intraoperative opioids use for laryngeal squamous cell carcinoma surgery and recurrence: a retrospective study. J Clin Anesth. 2015;27:672–679. doi: 10.1016/j.jclinane.2015.07.012. [DOI] [PubMed] [Google Scholar]
- 3.Cata J.P., Keerty V., Keerty D., et al. A retrospective analysis of the effect of intraoperative opioid dose on cancer recurrence after non-small cell lung cancer resection. Cancer Med. 2014;3:900–908. doi: 10.1002/cam4.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Silagy A.W., Hannum M.L., Mano R., et al. Impact of intraoperative opioid and adjunct analgesic use on renal cell carcinoma recurrence: role for onco-anaesthesia. Br J Anaesth. 2020;125:e402–e404. doi: 10.1016/j.bja.2020.06.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hiller J.G., Perry N.J., Poulogiannis G., Riedel B., Sloan E.K. Perioperative events influence cancer recurrence risk after surgery. Nat Rev Clin Oncol. 2018;15:205–218. doi: 10.1038/nrclinonc.2017.194. [DOI] [PubMed] [Google Scholar]
- 6.Wall T., Sherwin A., Ma D., Buggy D.J. Influence of perioperative anaesthetic and analgesic interventions on oncological outcomes: a narrative review. Br J Anaesth. 2019;123:135–150. doi: 10.1016/j.bja.2019.04.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Montagna G., Gupta H.V., Hannum M., et al. Intraoperative opioids are associated with improved recurrence-free survival in triple-negative breast cancer. Br J Anaesth. 2021;126:367–376. doi: 10.1016/j.bja.2020.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Du K.N., Feng L., Newhouse A., et al. Effects of intraoperative opioid use on recurrence-free and overall survival in patients with esophageal adenocarcinoma and squamous cell carcinoma. Anesth Analg. 2018;127:210–216. doi: 10.1213/ANE.0000000000003428. [DOI] [PubMed] [Google Scholar]
- 9.Connolly J.G., Tan K.S., Mastrogiacomo B., et al. Intraoperative opioid exposure, tumour genomic alterations, and survival differences in people with lung adenocarcinoma. Br J Anaesth. 2021;127:75–84. doi: 10.1016/j.bja.2021.03.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Connolly J.G., Scarpa J.R., Gupta H.V., et al. Intraoperative ketorolac may interact with patient-specific tumour genomics to modify recurrence risk in lung adenocarcinoma: an exploratory analysis. Br J Anaesth. 2021;127:e82–e85. doi: 10.1016/j.bja.2021.05.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lavon H., Matzner P., Benbenishty A., et al. Dexmedetomidine promotes metastasis in rodent models of breast, lung, and colon cancers. Br J Anaesth. 2018;120:188–196. doi: 10.1016/j.bja.2017.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Wang C., Datoo T., Zhao H., et al. Midazolam and dexmedetomidine affect neuroglioma and lung carcinoma cell biology in vitro and in vivo. Anesthesiology. 2018;129:1000–1014. doi: 10.1097/ALN.0000000000002401. [DOI] [PubMed] [Google Scholar]
- 13.Scarpa J.R., DiNatale R.G., Mano R., et al. Identifying clear cell renal cell carcinoma coexpression networks associated with opioid signaling and survival. Cancer Res. 2021;81:1101–1110. doi: 10.1158/0008-5472.CAN-20-1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Zhao P., Li L., Jiang X., Li Q. Mismatch repair deficiency/microsatellite instability-high as a predictor for anti-PD-1/PD-L1 immunotherapy efficacy. J Hematol Oncol. 2019;12:54. doi: 10.1186/s13045-019-0738-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Popat S., Hubner R., Houlston R.S. Systematic review of microsatellite instability and colorectal cancer prognosis. J Clin Oncol. 2005;23:609–618. doi: 10.1200/JCO.2005.01.086. [DOI] [PubMed] [Google Scholar]
- 16.Lee H., Sha D., Foster N.R., et al. Analysis of tumor microenvironmental features to refine prognosis by T, N risk group in patients with stage III colon cancer (NCCTG N0147) (Alliance) Ann Oncol. 2020;31:487–494. doi: 10.1016/j.annonc.2020.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Llosa N.J., Cruise M., Tam A., et al. The vigorous immune microenvironment of microsatellite instable colon cancer is balanced by multiple counter-inhibitory checkpoints. Cancer Discov. 2015;5:43–51. doi: 10.1158/2159-8290.CD-14-0863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Ribic C.M., Sargent D.J., Moore M.J., et al. Tumor microsatellite-instability status as a predictor of benefit from fluorouracil-based adjuvant chemotherapy for colon cancer. N Engl J Med. 2003;349:247–257. doi: 10.1056/NEJMoa022289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Le D.T., Uram J.N., Wang H., et al. PD-1 blockade in tumors with mismatch-repair deficiency. N Engl J Med. 2015;372:2509–2520. doi: 10.1056/NEJMoa1500596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Thibodeau S.N., Bren G., Schaid D. Microsatellite instability in cancer of the proximal colon. Science. 1993;260:816–819. doi: 10.1126/science.8484122. [DOI] [PubMed] [Google Scholar]
- 21.Franchi S., Moschetti G., Amodeo G., Sacerdote P. Do All opioid drugs share the same immunomodulatory properties? A review from animal and human studies. Front Immunol. 2019;10:2914. doi: 10.3389/fimmu.2019.02914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cancer Genome Atlas Network Comprehensive molecular characterization of human colon and rectal cancer. Nature. 2012;487:330–337. doi: 10.1038/nature11252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vilar E., Gruber S.B. Microsatellite instability in colorectal cancer—the stable evidence. Nat Rev Clin Oncol. 2010;7:153–162. doi: 10.1038/nrclinonc.2009.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Weiser M.R., Hsu M., Bauer P.S., et al. Clinical calculator based on molecular and clinicopathologic characteristics predicts recurrence following resection of stage I–III colon cancer. J Clin Oncol. 2021;39:911–919. doi: 10.1200/JCO.20.02553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Love M.I., Huber W., Anders S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014;15:550. doi: 10.1186/s13059-014-0550-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lachmann A., Schilder B.M., Wojciechowicz M.L., et al. Geneshot: search engine for ranking genes from arbitrary text queries. Nucleic Acids Res. 2019;47:W571–W577. doi: 10.1093/nar/gkz393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liberzon A., Birger C., Thorvaldsdottir H., Ghandi M., Mesirov J.P., Tamayo P. The Molecular Signatures Database (MSigDB) hallmark gene set collection. Cell Syst. 2015;1:417–425. doi: 10.1016/j.cels.2015.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Bindea G., Mlecnik B., Tosolini M., et al. Spatiotemporal dynamics of intratumoral immune cells reveal the immune landscape in human cancer. Immunity. 2013;39:782–795. doi: 10.1016/j.immuni.2013.10.003. [DOI] [PubMed] [Google Scholar]
- 29.van Buuren S., Groothuis-Oudshoorn K.M.I.C.E. Multivariate imputation by chained equations in R. J Stat Softw. 2011;45:1–67. [Google Scholar]
- 30.Botticelli A., Cirillo A., Pomati G., et al. The role of opioids in cancer response to immunotherapy. J Transl Med. 2021;19:119. doi: 10.1186/s12967-021-02784-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sun M., Chang C.L., Lu C.Y., Zhang J., Wu S.Y., Effect of opioids on cancer survival in patients with chronic pain: a propensity score-matched population-based cohort study Br J Anaesth. 2022;128:708–717. doi: 10.1016/j.bja.2021.12.051. [DOI] [PubMed] [Google Scholar]
- 32.Tosolini M., Kirilovsky A., Mlecnik B., et al. Clinical impact of different classes of infiltrating T cytotoxic and helper cells (Th1, th2, treg, th17) in patients with colorectal cancer. Cancer Res. 2011;71:1263–1271. doi: 10.1158/0008-5472.CAN-10-2907. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.