Abstract
COVID-19 is a highly infectious disease caused by SARS-CoV-2. First reported in December 2019, it rapidly escalated into a global pandemic, resulting in over 6.3 million fatalities by July 4, 2022. The first oral coronavirus main protease inhibitor, nirmatrelvir, was granted Emergency Use Authorization by the U.S. FDA in December 2021. It is a tripeptide incorporated with a C-terminal nitrile designed to bind and form a covalent attachment to the SARS-CoV-2 main protease. Shortly after nirmatrelvir’s approval, Enanta Pharmaceuticals’ peptidomimetic SARS-CoV-2 main protease inhibitor entered clinical trials in February 2022. This patent highlight reports key structures of di- and tripeptide inhibitors described in Enanta Pharmaceuticals’ patent WO 2022/020242 A1.
Important Compound Classes
Title
Functionalized Peptides As Antiviral Agents
Patent Publication Number
WO 2022/020242 A1
URL: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2022020242&_fid=DK365316736
Publication Date
January 27, 2022
Priority Application
US 63/054,048
Priority Date
July 20, 2020
Inventors
Panarese, J. D.; Davis, D.; Kenton, N. T.; Bartlett, S.; Rafferty, S.; Or, Y. S.
Assignee Company
Enanta Pharmaceuticals, Inc., USA
Disease Area
COVID-19
Biological Target
SARS-CoV-2 main protease
Summary
Coronavirus disease 2019 (COVID-19) is a highly infectious disease caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). First reported in Wuhan, China, in December 2019, it rapidly escalated into a global pandemic by March 2020. Common symptoms include sore throat, dry cough, pyrexia, headache, fatigue, anosmia and ageusia. More serious symptoms include dyspnea and chest pains which can become fatal. By July 4, 2022, the World Health Organization reported more than 545 million infection cases and more than 6.3 million deaths globally. The SARS-CoV-2 main protease (Mpro), also known as 3 chymotrypsin-like protease (3CLpro), is involved in viral polyprotein processing and plays an essential role in the virus life cycle. Its substrate recognition sequence, Val-Leu-Gln, has not been observed for human proteases, making Mpro a good drug target. Indeed, the first (and currently only) Mpro inhibitor, nirmatrelvir, was granted Emergency Use Authorization (EUA) by the U.S. Food and Drug Administration (FDA) on December 22, 2021, for treating COVID-19 in combination with ritonavir (known collectively as Paxlovid). Nirmatrelvir is a tripeptide Val-Leu-Gln mimic specifically designed to bind to Mpro. The viral protease is inhibited when the inhibitor’s C-terminal nitrile forms a covalent bond to Mpro’s active site Cys145.
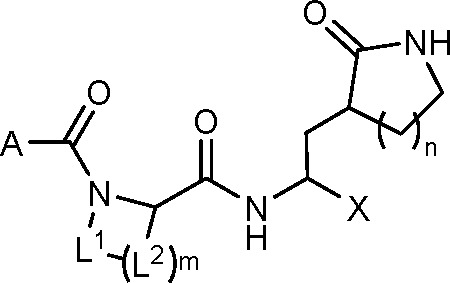
Enanta Pharmaceuticals filed an international patent application (WO 2022/020242 A1) on July 19, 2021, near Pfizer’s application on August 6, 2021 (WO 2021/250648 A1). The former describes 324 SARS-CoV-2 Mpro di- and tripeptide inhibitors incorporated with various C-terminal electrophilic moieties including aldehydes, halomethylketones, hydroxymethylketones, ketoamides and nitriles. As in nirmatrelvir, the reported tripeptide peptidomimetics were designed to mimic the Val-Leu-Gln recognition sequence by incorporating a lactam glutamine mimic, a cyclic leucine-proline hybrid and a tert-butyl glycine at the P1, P2, and P3 positions, respectively. It is noteworthy that the reported nitrile tripeptides were highly potent SARS-CoV-2 Mpro inhibitors (IC50 < 100 nM), with forerunner candidate EDP-235 entering a phase 1 clinical trial in February 2022 with a once-a-day oral dosing regimen (ClinicalTrials.gov Identifier: NCT05246878).
Key Structures
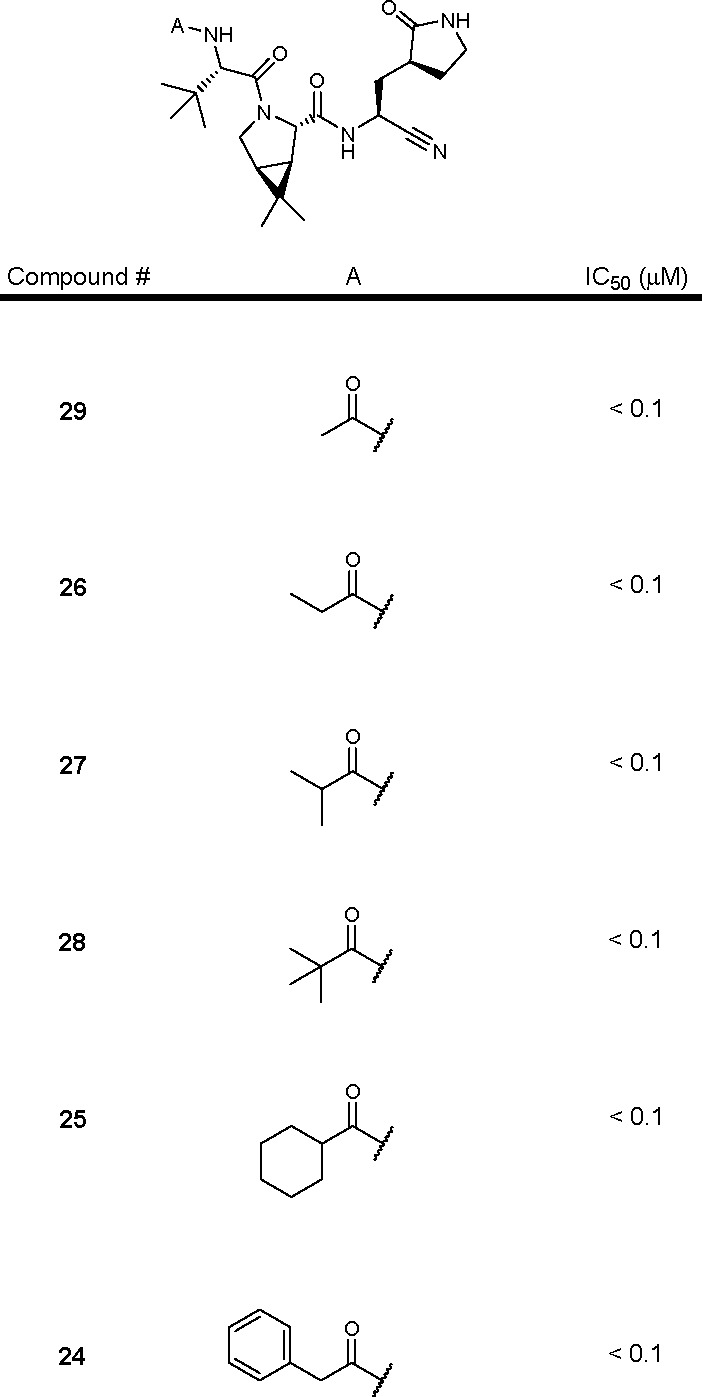
The patent describes 324 structures of di- and tripeptides with C-terminal electrophilic warheads, along with their synthesis protocols. Key exemplified structures and their corresponding SARS-CoV-2 Mpro inhibitory activities (IC50 values) are tabulated below.
Biological Assay
Inhibitory activities were determined in a biochemical SARS-CoV-2 Mpro fluorescence resonance energy transfer assay where the activities of exemplified compounds were determined in a dose–response titration with SARS-CoV-2 Mpro and a fluorogenic peptide substrate, TAMRA-SITSAVLQSGFRKMK-DABCYL-OH.
Biological Data
The SARS-CoV-2 Mpro inhibitory data of key inhibitors are summarized in the following
tables.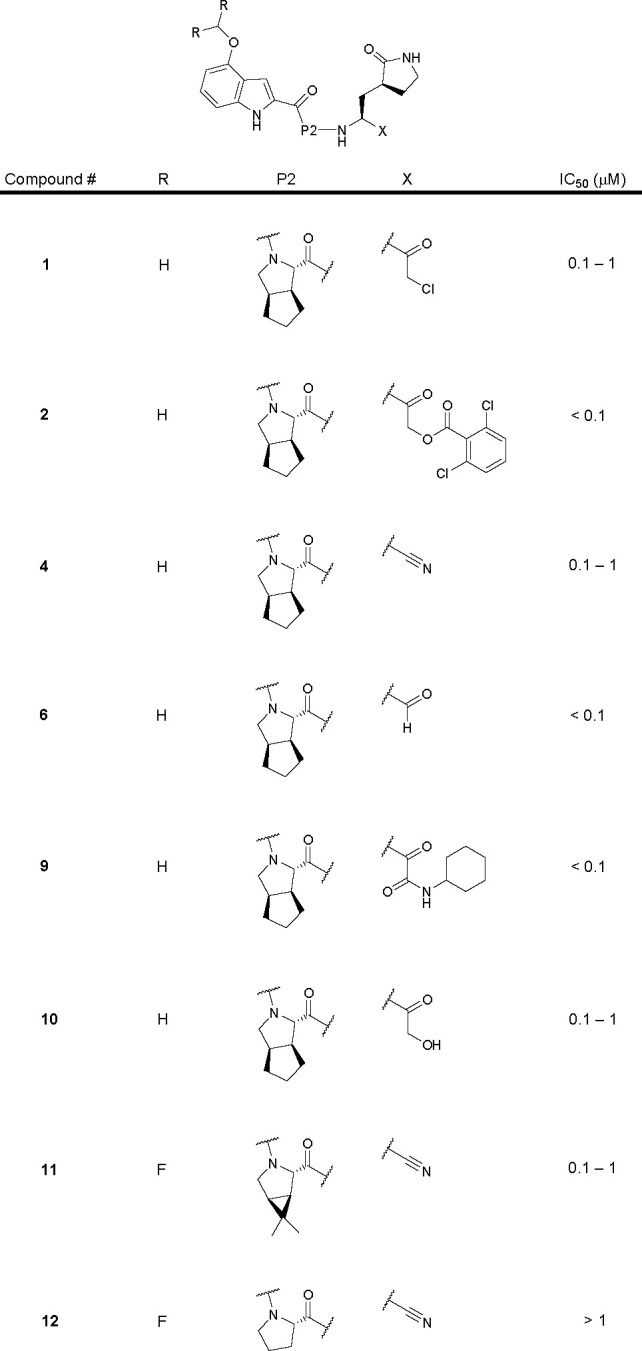
Acknowledgments
The authors thank the Agency for Science, Technology and Research (ASTAR), Singapore, for funding this Patent Review.
The authors declare no competing financial interest.
Recent Review Articles. References
- Banerjee R.; Perera L.; Tillekeratne L. M. V. Potential SARS-CoV-2 main protease inhibitors. Drug Discovery Today 2021, 26, 804. 10.1016/j.drudis.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D. R.; Allerton C. M. N.; Anderson A. S.; Aschenbrenner L.; Avery M.; Berritt S.; Boras B.; et al. An oral SARS-CoV-2 M pro inhibitor clinical candidate for the treatment of COVID-19. Science 2021, 374, 1586. 10.1126/science.abl4784. [DOI] [PubMed] [Google Scholar]
- Chia C. S. B. Novel Nitrile Peptidomimetics for Treating COVID-19. ACS Med. Chem. Lett. 2022, 13, 330. 10.1021/acsmedchemlett.2c00030. [DOI] [PMC free article] [PubMed] [Google Scholar]


