Abstract
The current COVID-19 global pandemic caused by SARS-CoV-2 has claimed more than 6 million lives since its emergence in December 2019. The first oral coronavirus main protease inhibitor, nirmatrelvir, was granted Emergency Use Authorization by the U.S. FDA in December 2021, with a twice-daily dosing regimen in combination with ritonavir. In March 2022, Shionogi & Co. announced their single-agent, once-daily oral SARS-CoV-2 main protease inhibitor, ensitrelvir, was granted approval for global phase 3 clinical trials. Unlike nirmatrelvir, ensitrelvir is a nonpeptidic, noncovalent, small molecule. This Patent Highlight describes key structures and their inhibitory activities in Shionogi & Co.’s and Hokkaido University’s patent WO 2022/138987 A1.
Important Compound Classes
Title
Triazine Derivative Having Virus Propagation Inhibitory Effect, and Pharmaceutical Composition Containing Same
Patent Publication Number
WO 2022/138987 A1
URL: https://patentscope.wipo.int/search/en/detail.jsf?docId=WO2022138987
Publication Date
June 30, 2022
Priority Application
JP 2021-068672
Priority Date
April 14, 2021
Inventors
Tachibana, Y.; Uehara, S.; Unoh, Y.; Nakahara, K.; Taoda, Y.; Kasamatsu, K.; Yamatsu, Y.; Ando, S.; Iimuro, A.; Suto, T.; Sasaki, M.
Assignee Company
Shionogi & Co., Ltd.; National University Corporation, Hokkaido University
Disease Area
COVID-19
Biological Target
SARS-CoV-2 main protease
Summary
First reported in Wuhan, China, in December 2019, coronavirus disease 2019 (COVID-19), caused by the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2), rapidly escalated into a global pandemic. Common symptoms include sore throat, dry cough, headache, fever, fatigue, muscle ache, ageusia and anosmia. More severe symptoms include breathing difficulties and chest pains which can become fatal. By July 15, 2022, the World Health Organization (WHO) reported more than 555 million infection cases and more than 6.3 million deaths worldwide. The SARS-CoV-2 main protease (Mpro), also known as 3 chymotrypsin-like protease (3CLpro), is deemed an ideal drug target due to its role in viral polyprotein processing, which is required for virus propagation and pathogenesis. Indeed, the first and currently only approved oral Mpro inhibitor, nirmatrelvir, developed by Pfizer, was granted Emergency Use Authorization (EUA) by the United States Food and Drug Administration (FDA) on Dec 22, 2021, for treating COVID-19 patients in combination with ritonavir in a twice-daily dosing regimen. Nirmatrelvir is a tripeptide valine-leucine-glutamine mimic designed to bind specifically to the active site of Mpro. The latter is subsequently inhibited when nirmatrelvir’s C-terminal electrophilic nitrile functional group forms a covalent bond to a cysteine residue in the active site.
On Feb 17, 2022, Shionogi & Co., together with Hokkaido University, filed an international patent application (WO 2022/138987 A1) describing a family of small-molecule Mpro inhibitors containing a central 1,3,5-triazine-2,4-dione scaffold. Unlike nirmatrelvir, these compounds are nonpeptidic and noncovalent Mpro inhibitors, initially discovered by virtual screening followed by biological screening of an in-house compound library. On March 16, 2022, Shionogi & Co. announced that their forerunner candidate, S-217622/ensitrelvir/Xocova, was granted approval by the FDA to enter phase 3 clinical trials as a single agent with a once-daily oral dosing regimen (ClinicalTrials.gov Identifier: NCT05305547).
Key Structures
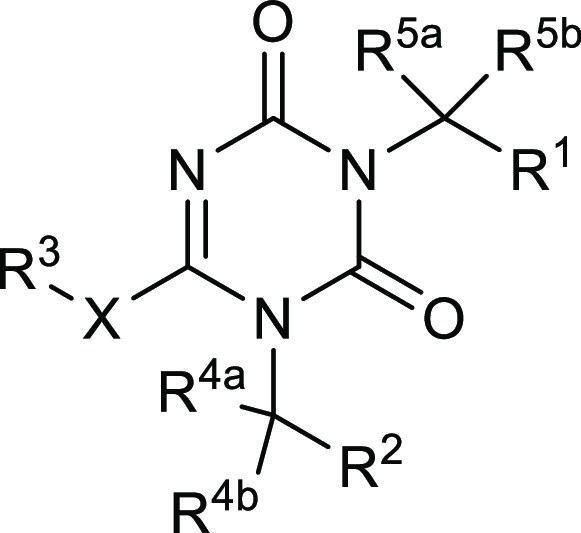
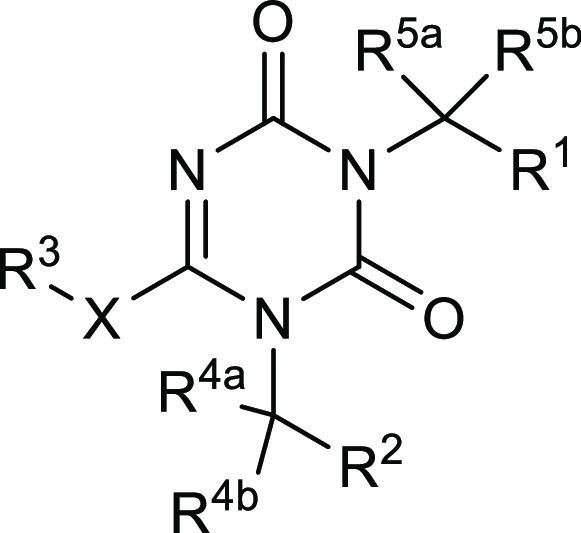
The patent describes 1090 structures along with their synthetic procedures. Key exemplified structures and their biological activities are tabulated vide infra.
Biological Assays
Inhibitory activities (IC50 values) were determined in a biochemical fluorescence resonance energy transfer (FRET) assay using dose–response titration with recombinant SARS-CoV-2 Mpro. Test compounds were challenged with a fluorogenic peptide substrate, DABCYL-KTSAVLQSGFRKME(EDANS)-NH2 (DABCYL = 4-dimethylaminoazobenzene-4-carboxylic acid; EDANS = 5-[(2-aminoethylamino)]naphthalene-1-sulfonic acid). EC50 values were determined in a cell-based assay using SARS-CoV-2-infected VeroE6 cells expressing human transmembrane protease serine 2 (TMPRSS2; JCRB1819). Cell viability was measured using CellTiter-Glo 2.0 (Promega) to quantify cellular ATP levels.
Biological Data
IC50 and EC50 values of key exemplified structures are summarized
in the following table.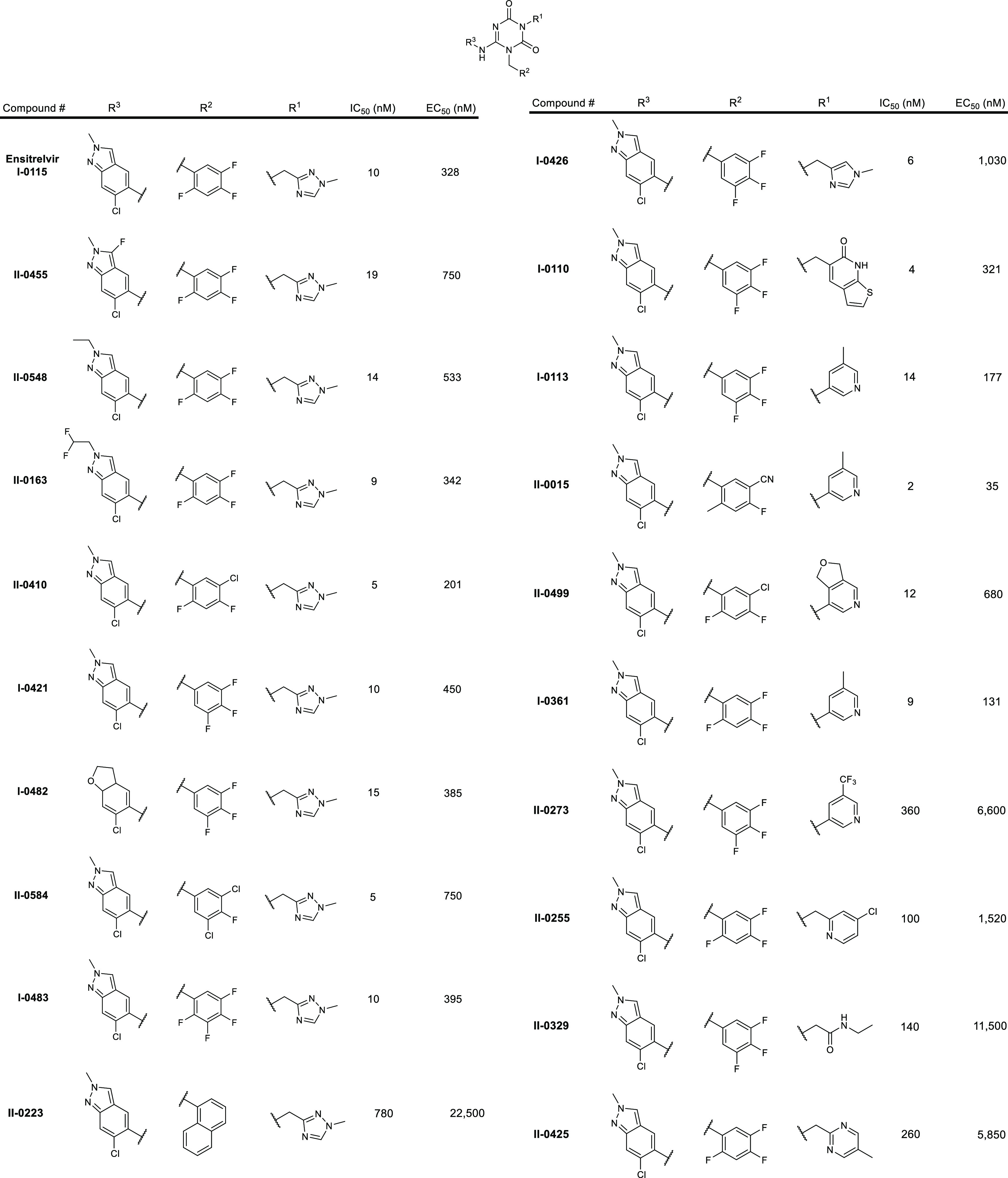
Acknowledgments
The authors thank the Agency for Science, Technology and Research (A*STAR), Singapore, for funding this Patent Review.
The authors declare no competing financial interest.
Recent Review Articles. References
- Banerjee R.; Perera L.; Tillekeratne L. M. V. Potential SARSCoV-2 main protease inhibitors. Drug Discovery Today 2021, 26, 804. 10.1016/j.drudis.2020.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Unoh Y.; Uehara S.; Nakahara K.; Nobori H.; Yamatsu Y.; Yamamoto S.; Maruyama Y.; Taoda Y.; Kasamatsu K.; Suto T.; Kouki K.; Nakahashi A.; Kawashima S.; Sanaki T.; Toba S.; Uemura K.; Mizutare T.; Ando S.; Sasaki M.; Orba Y.; Sawa H.; Sato A.; Sato T.; Kato T.; Tachibana Y. Discovery of S-217622, a noncovalent oral SARS-CoV-2 3CL protease inhibitor clinical candidate for treating COVID-19. J. Med. Chem. 2022, 65, 6499. 10.1021/acs.jmedchem.2c00117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen D. R.; Allerton C. M. N.; Anderson A. S.; Aschenbrenner L.; Avery M.; Berritt S.; Boras B.; Cardin R. D.; Carlo A.; Coffman K. J.; Dantonio A.; Di L.; Eng H.; Ferre R. A.; Gajiwala K. S.; Gibson S. A.; Greasley S. E.; Hurst B. L.; Kadar E. P.; Kalgutkar A. S.; Lee J. C.; Lee J.; Liu W.; Mason S. W.; Noell S.; Novak J. J.; Obach R. S.; Ogilvie K.; Patel N. C.; Pettersson M.; Rai D. K.; Reese M. R.; Sammons M. F.; Sathish J. G.; Singh R. S. P.; Steppan C. M.; Stewart A. E.; Tuttle J. B.; Updyke L.; Verhoest P. R.; Wei L.; Yang Q.; Zhu Y. An oral SARS-CoV-2 Mpro inhibitor clinical candidate for the treatment of COVID-19. Science 2021, 374, 1586. 10.1126/science.abl4784. [DOI] [PubMed] [Google Scholar]
- Chia C. S. B. Novel nitrile peptidomimetics for treating COVID-19. ACS Med. Chem. Lett. 2022, 13, 330. 10.1021/acsmedchemlett.2c00030. [DOI] [PMC free article] [PubMed] [Google Scholar]


